Abstract
Presilphiperfolan-8β-ol synthase (BcBOT2), a substrate-promiscuous sesquiterpene cyclase (STC) of fungal origin, is capable of converting two new farnesyl pyrophosphate (FPP) derivatives modified at C7 of farnesyl pyrophosphate (FPP) bearing either a hydroxymethyl group or a methoxymethyl group. These substrates were chosen based on a computationally generated model. Biotransformations yielded five new oxygenated terpenoids. Remarkably, the formation of one of these tricyclic products can only be explained by a cationically induced migration of the methoxy group, presumably via a Meerwein-salt intermediate, unprecedented in synthetic chemistry and biosynthesis. The results show the great principle and general potential of terpene cyclases for mechanistic studies of unusual cation chemistry and for the creation of new terpene skeletons.
Introduction
Sesquiterpene cyclases (STCs), like other terpene cyclases (TCs), use linear, unsaturated, methyl-branched precursors activated as diphosphate monoesters. In the case of STCs, this is farnesyl pyrophosphate (FPP 1), and initially, a more or less prominent cationic allyl intermediate is formed that can react further with distant alkenes to form (oligo)carbocyclic products.1 In recent years, the substrate promiscuity of sesquiterpene cyclases has become an active field of research. In this context, oxygenated FPP derivatives are attractive unnatural candidates,2−4 also because oxyfunctionalized mono- and sesquiterpenes are widely used in the flavor and fragrance industry due to favorable olfactoric properties compared to the hydrocarbons initially formed by STCs.5
Allemann and co-workers chose two related oxygen-functionalized FPP derivatives, the allyl alcohol 2 and the oxirane 3, and investigated their potential as unnatural substrates for different STCs (Scheme 1).6 In particular, germacrene A synthase from Solidago canadensis (GAS) and the germacradiene-4-ol synthase (GdolS) showed promiscuity toward these two substrates, and the product-converging transformations yielded the same macrocyclization product 4.6 Acceptance for FPP 2 and 3 derivatives was also found for aristolochene synthase from Penicilium roqueforti (PR-AS) and amorphadiene synthase (ADS), although with variable and lower yields. A remarkable preparative application of using non-natural FPP derivatives was reported by the same group. The biotransformation of 12-hydroxy-FPP 5 with ADS yielded dihydroartemisinic aldehyde (6, DHAAI) as a suitable precursor for accessing artemisinin.7
Scheme 1. Structure of FPP 1, STC-Promoted Conversions of Oxygenated FPP-Derivatives 2, 3, and 5, of FPP Ether Derivative 7 and Structures of FPP Derivatives 10 and 11 Studied in This Work [OPP = OP2O6H3 (Protonated Form)].
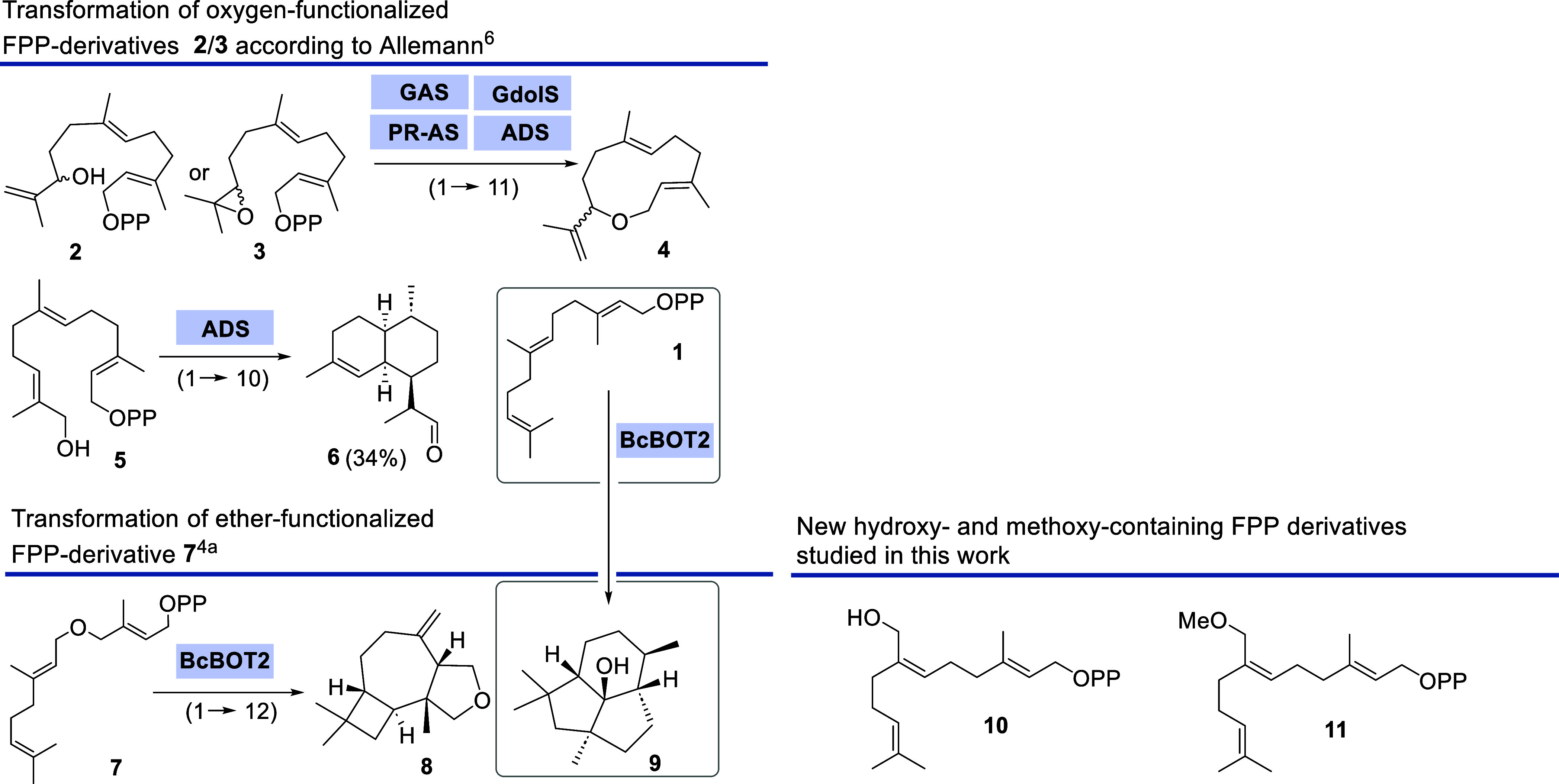
Also, ether and thioether FPP derivatives were harnessed and transformed, and among them, FPP ether 7(2) proved to be a particularly interesting substrate for STCs. With presilphiperfolan-8β-ol synthase (BcBOT2), a fungal sesquiterpene cyclase from Botrytis cinerea,8 the biotransformation yielded the tricyclic terpenoid 8, which displays a significantly altered backbone to the natural cyclization product presilphiperfolan-8β-ol 9, and this new oxysesquiterpene exerts a similar olfactory profile to the sesquiterpene rotundone.4a
The examples described in Scheme 1 show that the initially formed cationic allyl species either allows one to create a C–C bond during macrocyclization or can alternatively be captured by an oxygen nucleophile to form a C–O bond. In essence, TCs show a much broader synthetic potential than is commonly assumed.
Results and Discussion
As part of a larger research program, we now disclose our findings on the substrate promiscuity of the fungal sesquiterpene cyclase BcBOT2 using oxygenated FPP derivatives 10 and 11. In the list of sesquiterpene cyclases whose substrate promiscuity we have tested in the past, BcBOT2 stands out, both in terms of substrate promiscuity as well as in terms of its efficiency, which allows preparative scalable transformations to be performed with relative ease.
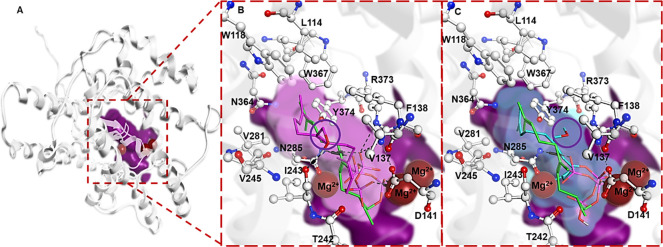
The choice of these substrates 10 and 11 was based on structural rationale. During the course of these studies, the first structural data on BcBOT2 were reported.9 Furthermore, we developed a structural model for BcBOT2 which was predicted using AlphaFold2.10 It was combined with an energy minimization using the AMBER14 force field, the overall RMSD of the Alphafold model used, and the newly reported structure being <1.72 Å (details are found in SI).10 The cavity calculations revealed that the total volume for the active site cavity of BcBOT2 where the substrate binding occurs is 567.8 Å3. As a result, we performed additional molecular docking simulations to assess whether additional substituents could be added to the FPP and whether the new derivatives would still fit into the active site of BcBOT2. Specifically, as we planned to attach an additional hydroxy and a methoxy group, respectively, at the central methyl group at C7 of FPP 1, we calculated the total volume for these two derivatives and found that it was 350 and 369.5 Å3, respectively (Figure 1; details are found in the SI). Given these results, we assumed that the free space should be sufficient for positioning the additional hydroxy and methoxy groups in the active site of BcBOT2. These findings served as the starting point for the present study which was initiated with the synthesis of the two FPP derivatives 10 and 11.
Figure 1.
Structural model of BcBOT2 with FPP 1 and new derivatives 10 and 11. (A) Model is displayed graphically in gray, with the shaded area representing the volume occupied by the three superimposed substrates, while the triad of Mg2+ ions is shown as red spheres. The purple shaded area shows the total volume of the binding pocket, while the magenta and cyan shaded areas indicate the volume of the hydroxyl and methoxy derivatives of FPP 10 and 11, respectively. The substituted group in each of the derivatives is indicated by a purple circle. The FPP molecule is shown in the form of green sticks. Close-up of the binding pocket with FPP derivatives 10 (B) in cyan and 11 (C) in magenta sticks superimposed on FPP 1, respectively.
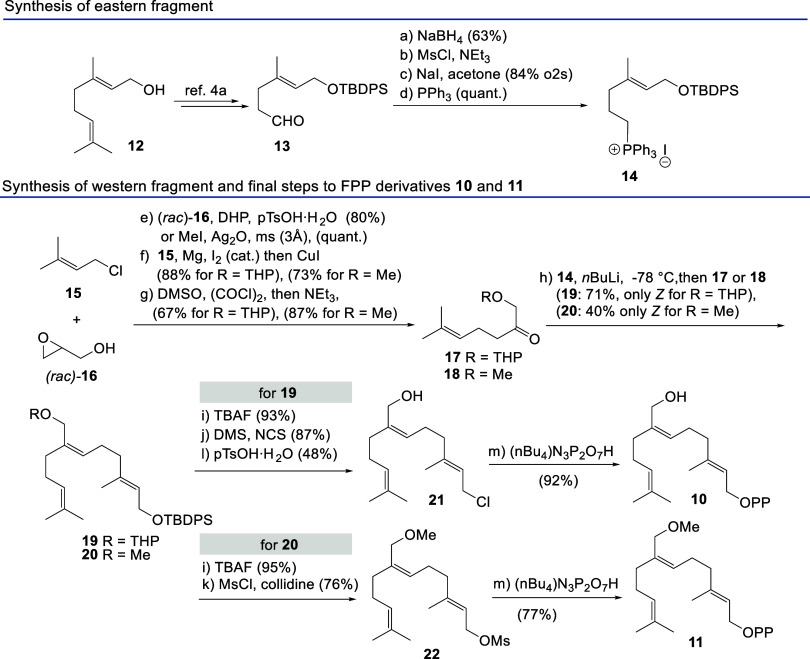
The syntheses for the two FPP derivatives 10 and 11 were carried out quite analogously, except that the two pathways separated at an early stage, namely, when the methoxy group was established (Scheme 2). The western and eastern fragments were merged by a highly Z-selective Wittig olefination of the P-ylid derived from phosphonium salt 14 and ketones 17 and 18,11 respectively.
Scheme 2. Syntheses of FPP Derivatives 10 and 11.
The synthesis of fragment 14 commenced from geraniol 12, which was transformed to aldehyde 13 by a known procedure.4a This was converted to 14 via the alcohol, the mesylate, and hence the intermediate iodide. The syntheses of the two western fragments 17 and 18 utilize prenyl chloride 15 and rac-glycidol 16. The alcohol group was either protected as THP-acetal or O-methylated before being reacted with the Grignard reagent derived from chloride 15.
Both Wittig olefinations that followed proceeded with excellent Z-selectivity and yielded TBDPS-ether 19 and 20. At this stage, the diphosphate moiety was introduced after the removal of the TBDPS protection following a protocol that either proceeds via the intermediate allyl chloride or allyl mesylate.12
Next, the enzyme BcBOT2 was cloned and expressed in E. coli as detailed in the SI. To determine enzyme activity and substrate tolerance, in vitro enzyme assays were conducted with FPP 1 and derivatives 10 and 11 (500 μL scale, 0.1 g/L BcBOT2, 37 °C, 0.5 h). The outcome of biotransformations of FPP derivatives 10 and 11 with BcBOT2 is summarized in Table 1, revealing the formation of several products.
Table 1. Overview of Biotransformations of BcBOT2 Using Diphosphates 10 and 11a.
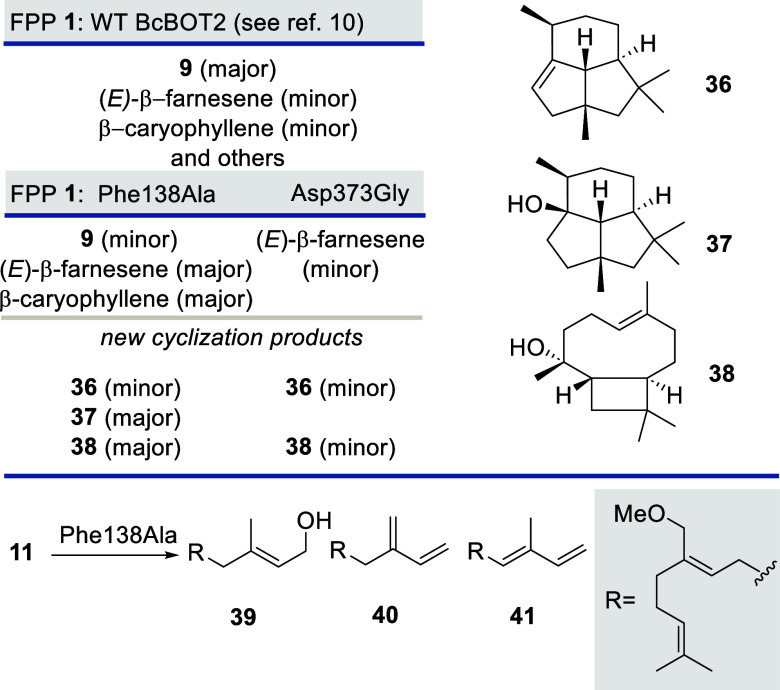
Indeed, transformations with STCs usually do not yield only one product; this is also true for BcBOT2 with the natural substrate FPP 1 which yields presilphiperfolan-8β-ol (9) as major product but also β-caryophyllene, caryophyllene oxide, β-elemene, germacrene A, presilphiperfol-7-ene, (E)-β-farnesene, and (E)-nerolidol (GC: overall A[%] < 5%).10 However, the major product usually predominates to such an extent that it is published alone.
Mass spectrometric analysis of the crude product provides a rapid overview of the product spectrum, including whether the individual product results from the terminal elimination of a proton or the reaction of the final carbocation with the nucleophile water. Incubation of BcBOT2 in the presence of diphosphate 10 only provided one product while diphosphate 11 was transformed into five well-detectable new sesquiterpene derivatives.
Our screening program also included other sesquiterpene cyclases, namely, (+)-caryolan-1-ol synthase (GcoA), viridiflorene synthase (Tps32), epi-isozizaene synthase (Cyc1), and vetispiradiene synthase (Hvs1).13 In most cases, conversion in the presence of FPP derivatives 10 and 11 resulted in new products, as judged by GC-MS analysis. However, a more detailed analysis revealed that only very small amounts were formed, which are not sufficient to practically upscale these transformations. These results support our previous observations on the uniqueness of BcBOT2.
For conducting semipreparative reactions in the following, for the transformations of BcBOT2 with oxygenated FPP derivatives 10 and 11, the conditions were slightly changed (1 mM scale, 0.1 g/L BcBOT2, 37 °C, 24 h). A sufficient amount of material was collected for the purification and structural elucidation of the three main products, namely, the tricyclic oxygenated sesquiterpenes 23, 25, and 26 (Scheme 3, top).
Scheme 3. Left: Transformations of FPP Derivatives 10 and 11 by BcBOT2 (Positions Marked in Grey Are Reference Carbon Atoms for the Absolute Stereochemistry); Right: Details on the Structure Analysis of 23, 25 and 26.
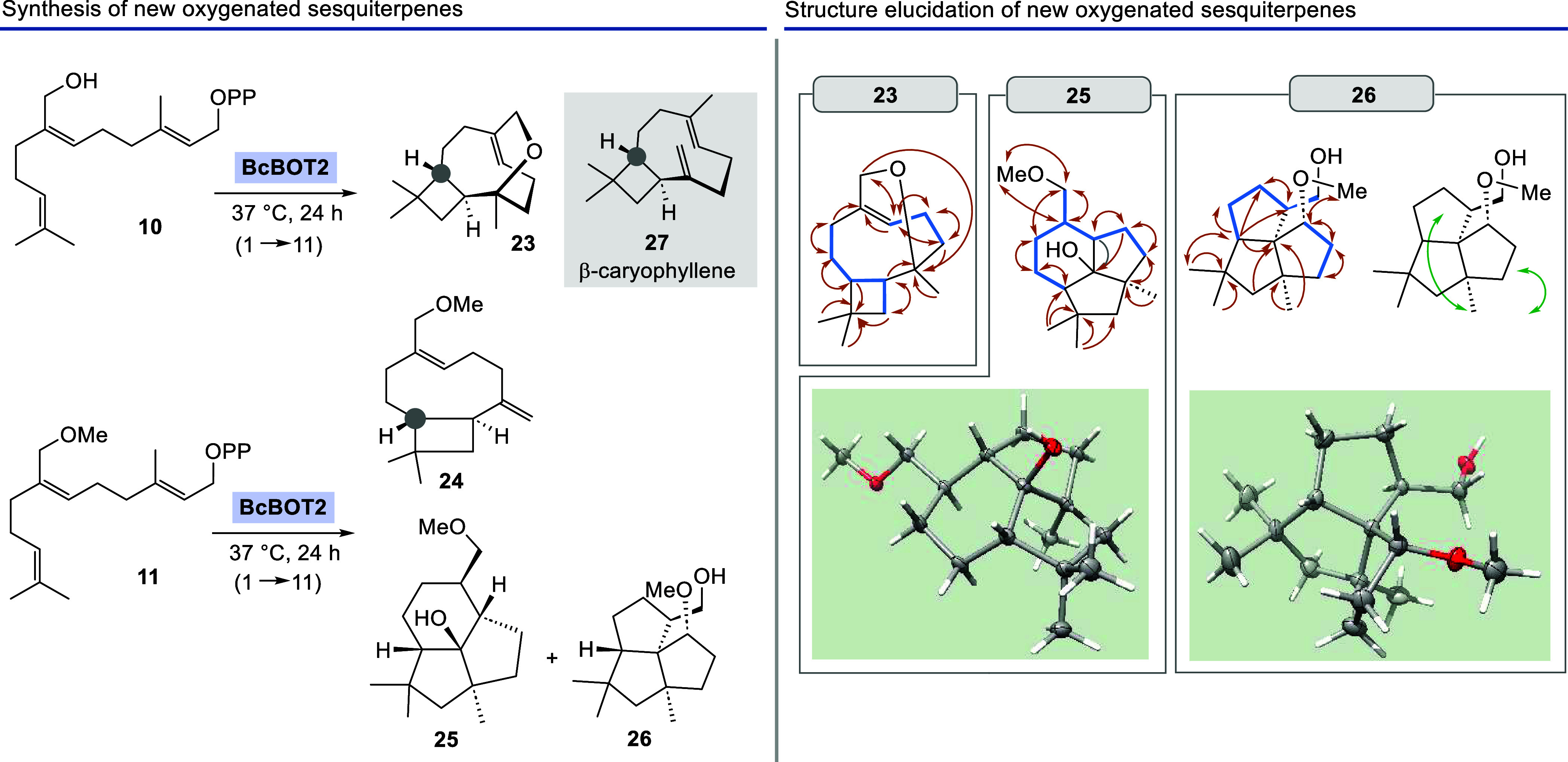
Key correlations of HMBC-spectra (orange arrows), H,H–COSY correlations (blue bonds), key NOEs (areen arrows), and crystal structures as analyzed by X-ray (the absolute configuration was determined via Anormalous Dispersion according to ref (14)).
The constitutions of these new products were elucidated by using various methods of NMR spectroscopy. In particular, the COSY and HMBC correlations played an important role, which were additionally complemented by the determination of selected NOE data (Scheme 3, bottom). These provided information about the preferred conformation of the macrocycles and thus additional information about the relative stereochemistry. The absolute stereochemistry was first determined by assuming a common mechanism for the formation of the four biotransformation products 23-26. The labeled stereogenic center serves as a reference, which is formed right at the beginning of the cation cascade and which is identical to the one in presilphiperfolan-8β-ol (9) at this position (Scheme 4).
Scheme 4. Mechanistic Considerations on the Formation of New Oxygenated Sesquiterpenoids 23–26.
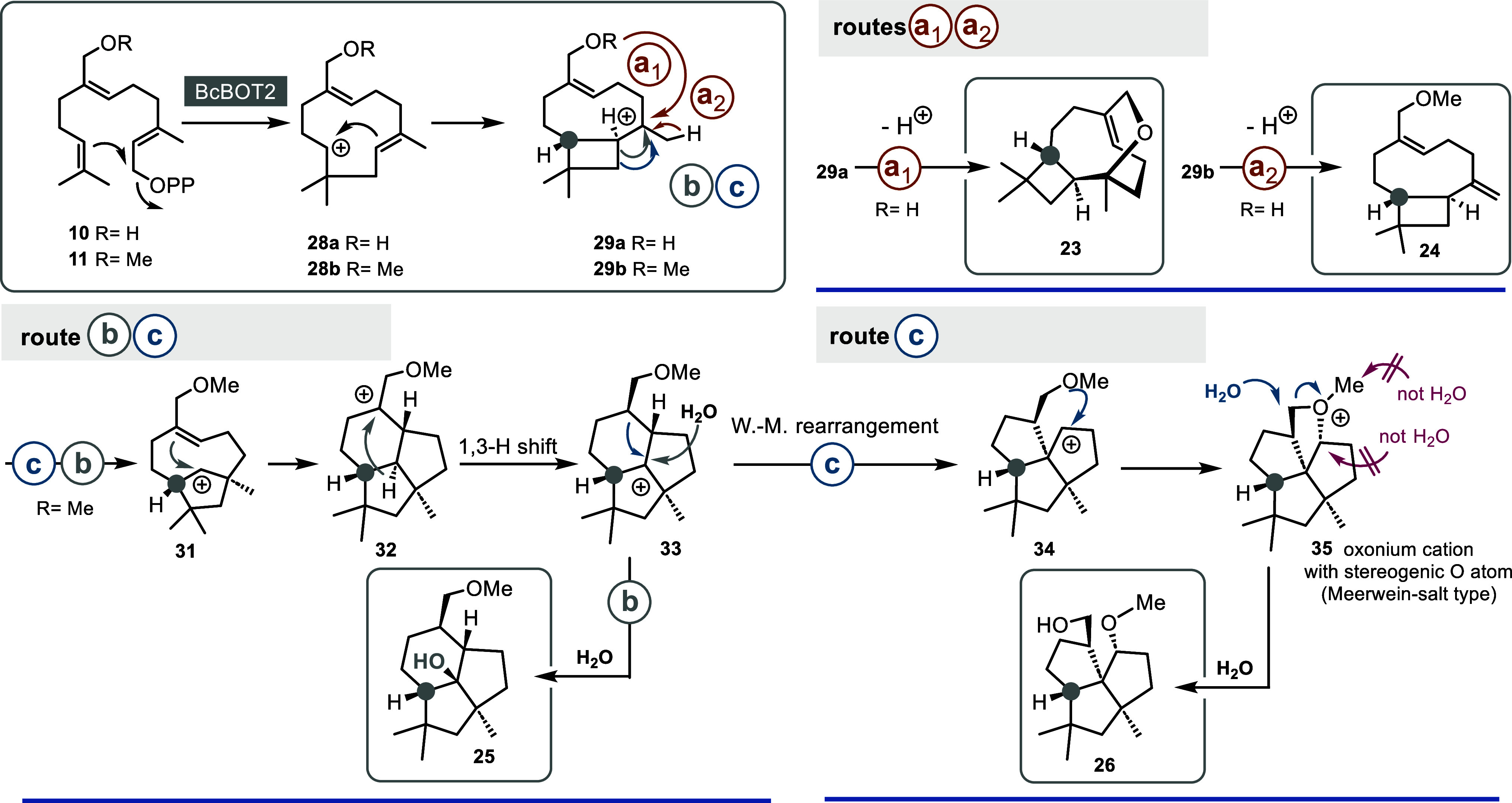
Routes A–C; carbon atom marked in grey acts as a reference point for the absolute stereochemistry of new products.
The following points list key elements of the structure elucidation of oxo-bridged sesquiterpene 23 by NMR analysis. A doublet at δ = 4 ppm indicates the presence of only one CH2–group next to a heteroatom. Following its coupling partners in the HMBC spectrum, it was clear that a macrocyclic ether with a neighboring quaternary carbon atom must have formed. The cyclobutane ring was resolved by identifying the downfield shifted hydrogen atoms of the methylene group. The upper part of 23 was resolved by combining COSY- with HMBC-correlations starting with the signal of the double bond proton. The carbon backbone of oxo-bridged product 23 resembles that of the natural sesquiterpene β-caryophyllene 27.
The alkene and two geminal methyl groups served as the starting point for the structural elucidation of 24 by NMR spectroscopy. COSY, HSQC, DEPT135, and HMBC NMR spectra were primarily used for the assignment and thus for the structure elucidation. The characteristic signals for the 1,1-disubstituted exocyclic double bond (δ = 4.97 and 4.79 ppm) and the presence of the cyclobutane methine groups support the proposed structure. The detection of two sets of signals for the 1,1-disubstituted olefin protons indicates the presence of two different conformers as the GC-MS analysis does not reveal any significant impurities.
The structural elucidation of product 25 was facilitated by direct comparison with the NMR data of presilphiperfolan-8β-ol (9). While the NMR data for the lower part of the two sesquiterpenes are almost identical, a new CH2 group was identified resulting from the presence of the extra OMe group in FPP derivative 11. This additionally led to slight downward shifts of the 1H signals in the neighborhood compared to 9.
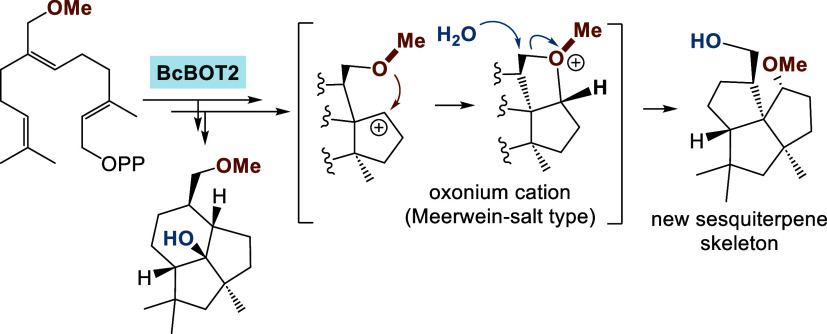
The position of the methoxy group in 26 was first determined by HSQC and HMBC spectra and revealed that it is unexpectedly not attached to a methylene group as in starting FPP derivative 11 but rather bound to a tertiary carbon atom. Obviously, the methoxy group must have undergone migration. Gratifyingly, efforts to crystallize terpenoids 25 and 26 were successful, and X-ray analyses provided final structural proof. These analyses also confirmed the absolute stereochemistry of these new oxygenated terpenoids.
A closer inspection of the backbones of the products reveals that they must have been formed by a complex cationic cascade. This is especially true for tricyclic oxygenated sesquiterpene 26, which has some surprises to offer. Mechanistically, several migrations must have taken place here, and most surprisingly, the methoxy group must have changed its position with respect to the carbon backbone. Given that the caryophyllene cation is an important intermediate on the pathway to presilphiperfolan-8β-ol (9), we propose that the analogous cations 29a,b (from the humulyl-type cations 28a,b) are mechanistically central intermediates from which two main pathways branch mechanistically (a and b, c) that yield the four products 23–26 (Scheme 4). For accessing terpenoid 23, cation 29a is internally trapped by the hydroxyl group (route a1) which creates the oxymethylene bridge which upon deprotonation furnishes sesquiterpene derivative 23. Likewise, the methoxy FPP derivative 11 yields 24 via a similar sequence except that cation 29b is deprotonated in the final step (a2).
Product 25 represents the methoxy derivative of presilphiperfolan-8β-ol (9) and will therefore be formed according to route b by following the mechanism discussed in the literature.4c This route starts with ring expansion of the cyclobutane ring by Wagner-Meerwein rearrangement at the stage of cation 29b. The newly formed cation 31 undergoes another ring closure, and the new carbocation 32 then presumably performs a 1,3-hydride shift until the tertiary cation 33 is intercepted by water and, consequently, 25 is formed.
The proposed formation of terpenoid 26 also starts from intermediate 29b and follows pathway b up to the point of intermediate cation 33. From there, we propose a ring contraction which yields intermediate 34 with a triquinane skeleton. The carbocation and the methoxy group are positioned close in space so that by capturing the cation, the unusual intermediate oxonium ion 35 is formed. Attack by water at the carbon atom where the methoxy group was originally located finally yields terpenoid 26. The oxonium salt 35 represents a complex version of what is called a Meerwein-salt. These are strong alkylating agents.15
In fact, mechanistically, this sequence represents a 1,4-Wagner Meerwein rearrangement. Remarkably, the trialkyloxonium ion 35 bears a stereogenic oxygen formed in a diastereoselective manner. Smith and co-workers only recently presented the first report of a chiral oxonium cation. Its architecture is based on a stable triaryloxonium ion which is embedded in a helical environment.16 The molecular docking of the intermediates in proposed pathway c aligns with findings from previous works on terpenoid cyclases including BcBOT21−4 which has shown that the carbocations formed in the intermediates experience stabilization through π-system interactions mainly through phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp) amino acid residues. As can be seen in Figure 2, such an interaction is also found here with Phe138. An additional interaction between 26 and adjacent Asn285 and Arg373 likely favors the formation of 26.
Figure 2.
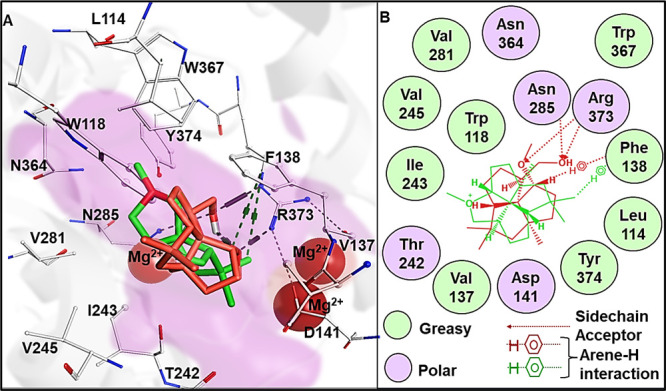
(A) Closeup of the active site of BcBOT2 with the top scoring catalytically competent binding poses from the molecular docking of the reaction intermediate 35 (green) and the product 26 (red) from proposed pathway c, the active site pocket (violet) with interacting residues (white stick and balls) along with the triad of Mg2+ (red spheres) also shown. Side chain acceptor and arene-H interaction depicted as black and green dotted lines, respectively.19 (B) 2D overlay of the ligand interaction diagram of 36 (green) and 26 (red) showing their interactions with the neighboring residues; legend for the ligand interaction diagram shown below. A complete depiction of all the intermediates leading to the formation of 26 by proposed pathway c can be found in SI.
In silico site saturation mutagenesis of these two positions was performed to investigate this hypothesis. All of the variants of Arg373 were predicted not to be stabilizing. The stable variants for Asn285 (N285R, N285F, N285W, and N285Y), all had the critical aryl interaction with Phe138, but the additional interaction was replaced with adjacent Tyr374 for most of 26 (details are found in SI).17 This supports our initial assumption and finds the interaction with Arg373 specifically to be important in the formation of 26.
To corroborate the outcome of the computational studies, we carried out additional biotransformations with two BcBOT2 variants. Specifically, we chose the two Phe138Ala and Arg373Gly variants, which upon incubation with FPP 1 provide new sesquiterpenes 36–38 along with sesquiterpenes also produced by the wildtype enzyme (Scheme 5, top).10 When the FPP derivative 11 was exposed to the Arg373Gly variant, complete suppression of product formation was observed (see the SI). This also includes the cyclization products 25 and 26, as judged by GC-MS. In contrast to this observation, the Phe138Ala variant was able to transform FPP derivative 11, but instead of terpenoids 25 and 26, the formation of the “hydrolysis” product 39 and two minor “elimination” products 40 and 41 were found (Scheme 5, bottom). Both results clearly demonstrate the active involvement of Phe138 and Arg373 in promoting transformations with FPP derivative 11 and the generation of terpenoids 25 and 26.
Scheme 5. Results of Transformations of FPP 1 and FPP Derivative 11 Using BcBOT2 Variants Phe138Ala and Asp373Gly.
Further details using 1 as a substrate are reported in ref (10).
Carbocation-induced migration of methoxy groups is virtually unknown. In rare cases, migrations of anomeric methoxy groups in methyl pyranosides toward ring carbon atoms whose alcohol groups are modified as esters with marked leaving group quality have been reported.18 The resulting oxocarbenium ions at the anomeric center are finally captured by a nucleophile such as water. However, these cases cannot fully be compared with the present case as the oxygen atom of the pyran ring exerts a strong stabilizing effect, which supports such migrations. As such, the migration process described here is unique because it takes place with an isolated methoxy group. It is probably made possible by the protein and its preformed 3D space. This controls the position of the final incoming nucleophile (here water) and the course of the last steps of the cationic cascade, including the methoxy migration in a completely controlled way.
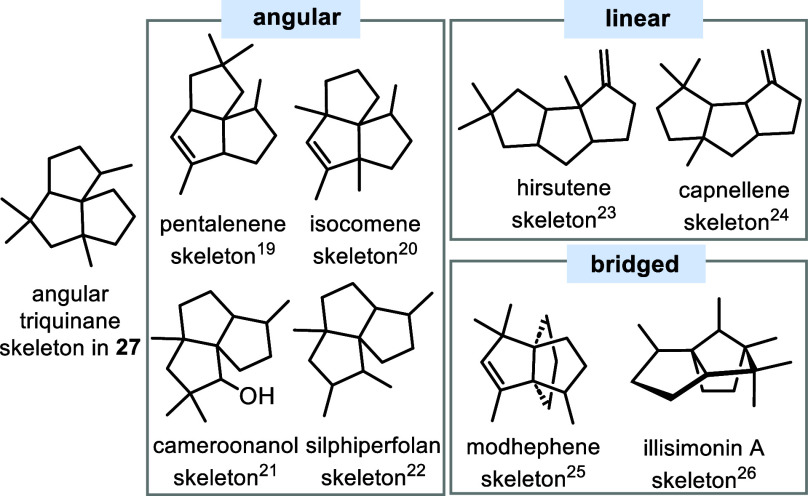
While the carbon skeleton of oxo-bridged terpene 23 and the methoxy terpenoid 24 are akin to that of caryophyllene 27, the relationship of the backbone terpene 26 can be assigned to triquinanes. Tricyclic terpenes composed of three annulated cyclopentane rings are well-known. Angular,19−22 linear,23,24 and bridged25,26 members are listed in Figure 3 and compared, and they reveal that 26 belongs to the group of angular triquinanes. However, the methylation pattern in 26 differs from that found in the known angular triquinane terpenes pentalenan, isocoman, cameroonan, and silphiperfolan carbon backbones.
Figure 3.
Terpene-based triquinane skeletons (angular, linear, bridged) and comparison with the new triquinane backbone 27 reported here (illisimonin A is a highly oxygenated sesquiterpene; for better clarity, only the carbon backbone is shown).
Conclusions
In summary, we report the first example of methoxy migration via an oxonium intermediate mediated by the sesquiterpene cyclase BcBOT2, a sesquiterpene cyclase with unique substrate promiscuity, a process not previously observed in chemical environments. So far, oxonium ions have been proposed as temporary intermediates in a series of biosynthetic27 and synthetic transformations,28,4a usually upon interference of oxirane, furan, or pyran oxygen atoms commonly on nascent bromine or selenonium cations after activation of alkenes.
These results document that not only do small changes in the amino acid composition located in the active site pocket of sesquiterpene cyclases exert large effects on cationic cascade sequences13 but also in native STCs small structural changes in the FPP substrate lead to novel cationic sequences. We show that STCs are able to handle oxonium cations in a highly controlled manner, which was previously unknown, because the natural substrate FPP 1 leads to cationic intermediates in the absence of an oxygen atom. Therefore, the question of whether or how terpene cyclase handles oxonium ions could have never arisen before. We believe that the 3D space formed by the protein in terpene cyclases can thus serve as a chemical laboratory in which a large variety of cationic sequence scenarios become feasible, much more widely than was originally “planned” by Nature. This unique catalytic space should be exploited by chemists to study the scopes of cationic cascade chemistry in confined environments. These may eventually be transferable to chemically designed, catalytically active 3D spaces such as those elegantly described by Tiefenbacher and co-workers in recent years.29
Acknowledgments
We thank Dr. Jörg Fohrer (Leibniz University Hannover) for expert NMR spectroscopic support. The analytical research center (ACR, Symrise AG, Holzminden, Germany) is gratefully acknowledged for NMR and HPLC support. M.D.D. was supported through funds from IPB Halle. Molecular graphics of 25 and 26 were obtained using UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Glossary
ABBREVIATIONS
- TBDPS
tert-butyldiphenylsilyl
- Ms
methylsulfonyl
- DHP
dihydropyrane
- THP
tetrahydropyranyl
- pTsOH
p-tosylsulfonyl
- TBAF
tetra-n-butylammonium fluoride
- DMS
dimethylsulfide
- NCS
N-chlorosuccinimide
- DMSO
dimethyl sulfoxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c03386.
Experimental procedures, characterizations, and analytical data of new compounds, X-ray diffraction data for 25 and 26, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Christianson D. W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. 10.1021/acs.chemrev.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dickschat J. S. Bacterial terpene cyclases. Nat. Prod. Rep. 2016, 33, 87–110. 10.1039/C5NP00102A. [DOI] [PubMed] [Google Scholar]; c Baunach M.; Franke J.; Hertweck C. Terpenoid biosynthesis off the beaten track: unconventional cyclases and their impact on biomimetic synthesis. Angew. Chem., Int. Ed. 2015, 54, 2604–2626. 10.1002/anie.201407883. [DOI] [PubMed] [Google Scholar]
- Harms V.; Kirschning A.; Dickschat J. S. Nature-driven approaches to non-natural terpene analogues. Nat. Prod. Rep. 2020, 37, 1080–1097. 10.1039/C9NP00055K. [DOI] [PubMed] [Google Scholar]
- a Jin Y.; Williams D. C.; Croteau R.; Coates R. M. Taxadiene synthase-catalyzed cyclization of 6-fluorogeranylgeranyl diphosphate to 7-fluoroverticillenes. J. Am. Chem. Soc. 2005, 127, 7834–7842. 10.1021/ja050592r. [DOI] [PubMed] [Google Scholar]; b Cascón O.; Touchet S.; Miller D. J.; Gonzalez V.; Faraldos J. A.; Allemann R. K. Chemoenzymatic preparation of germacrene analogues. Chem. Commun. 2012, 48, 9702–9704. 10.1039/c2cc35542f. [DOI] [PubMed] [Google Scholar]; c Touchet S.; Chamberlain K.; Woodcock C. M.; Miller D. J.; Birkett M. A.; Pickett J. A.; Allemann R. K. Novel olfactory ligands via terpene synthases. Chem. Commun. 2015, 51, 7550–7553. 10.1039/C5CC01814E. [DOI] [PubMed] [Google Scholar]; d Loizzi M.; Millerand D. J.; Allemann R. K. Silent catalytic promiscuity in the high-fidelity terpene cyclase δ-cadinene synthase. Org. Biomol. Chem. 2019, 17, 1206–1214. 10.1039/C8OB02821D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Oberhauser C.; Harms V.; Seidel K.; Schröder B.; Ekramzadeh K.; Beutel S.; Winkler S.; Lauterbach L.; Dickschat J. S.; Kirschning A. Exploiting the synthetic potential of sesquiterpene cyclases for generating unnatural terpenoids. Angew. Chem., Int. Ed. 2018, 57, 11802–11806. 10.1002/anie.201805526. [DOI] [PubMed] [Google Scholar]; b Harms V.; Schröder B.; Oberhauser C.; Tran C. D.; Winkler S.; Dräger G.; Kirschning A. Methyl-Shifted Farnesyldiphosphate Derivatives Are Substrates for Sesquiterpene Cyclases. Org. Lett. 2020, 22, 4360–4365. 10.1021/acs.orglett.0c01345. [DOI] [PubMed] [Google Scholar]; c Harms V.; Ravkina V.; Kirschning A. Mechanistic similarities of sesquiterpene cyclases PenA, Omp6/7, and BcBOT2 are unraveled by an unnatural ″FPP-ether″ derivative. Org. Lett. 2021, 23, 3162–3166. 10.1021/acs.orglett.1c00882. [DOI] [PubMed] [Google Scholar]; d Tran C. D.; Dräger G.; Struwe H.; Siedenberg L.; Vasisth S.; Grunenberg J.; Kirschning A. Terpenoid cyclopropylmethyl diphosphates can serve as a substrate for the sesquiterpene synthase BcBOT2. Org. Biomol. Chem. 2022, 20, 7833–7839. 10.1039/D2OB01279K. [DOI] [PubMed] [Google Scholar]; e Struwe H.; Droste J.; Kirschning A. Chemoenzymatic Synthesis of a New Germacrene Derivative Named Germacrene F. ChemBioChem. 2024, 25, e202300599 10.1002/cbic.202300599. [DOI] [PubMed] [Google Scholar]; f Struwe H.; Schrödter F.; Spinck H.; Kirschning A. New sesquiterpene backbones generated by sesquiterpene cyclases – formation of iso-caryolan-ol and an isoclovane. Org. Lett. 2023, 25, 8575–8579. 10.1021/acs.orglett.3c03383. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Weigel B.; Ludwig J.; Weber R. A.; Ludwig S.; Lennicke C.; Schrank P.; Davari M. D.; Nagia M.; Wessjohann L. A. Heterocyclic and Alkyne Terpenoids by Terpene Synthase-Mediated Biotransformation of Non-Natural Prenyl Diphosphates: Access to New Fragrances and Probes. ChemBioChem. 2022, 23, e2022002 10.1002/cbic.202200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Theime E. T.Fragrance Chemistry: The Science of the Sense of Smell; Elsevier, 2012. [Google Scholar]; (b) Sell C. S.Chemistry and the Sense of Smell; John Wiley & Sons, 2014. [Google Scholar]; (c) Surburg H.; Panten J.. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH Weinheim, 2016. [Google Scholar]
- Huynh F.; Grundy D. J.; Jenkins R. L.; Miller D. J.; Allemann R. K. Sesquiterpene Synthase-Catalysed Formation of a New Medium-Sized Cyclic Terpenoid Ether from Farnesyl Diphosphate Analogues. ChemBioChem. 2018, 19, 1834–1838. 10.1002/cbic.201800218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Tang X.; Demiray M.; Wirth T.; Allemann R. K. Concise synthesis of artemisinin from a farnesyl diphosphate analogue. Bioorg. Med. Chem. 2018, 26, 1314–1319. 10.1016/j.bmc.2017.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Demiray M.; Tang X.; Wirth T.; Faraldos J. A.; Allemann R. K. An efficient chemoenzymatic synthesis of dihydroartemisinic aldehyde. Angew. Chem., Int. Ed. 2017, 56, 4347–4350. 10.1002/anie.201609557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-M.; Hopson R.; Lin X.; Cane D. E. Biosynthesis of the Sesquiterpene Botrydial in Botrytis cinerea. Mechanism and Stereochemistry of the Enzymatic Formation of Presilphiperfolan-8β-ol. J. Am. Chem. Soc. 2009, 131, 8360–8361. 10.1021/ja9021649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou T.; Li A.; Xu H.; Pan J.; Xing B.; Wu R.; Dickschat J. S.; Yang D.; Ma M. Structural Insights into Three Sesquiterpene Synthases for the Biosynthesis of Tricyclic Sesquiterpenes and Chemical Space Expansion by Structure-Based Mutagenesis. J. Am. Chem. Soc. 2023, 145, 8474–8485. 10.1021/jacs.3c00278. [DOI] [PubMed] [Google Scholar]
- Nikolaiczyk V.; Irwan J.; Nguyen T.; Fohrer J.; Elbers P.; Schrank P.; Davari M. D.; Kirschning A. Mutagenic reprogramming of the sesquiterpene synthase BcBOT2 provides new terpenes. Catal. Sci. Technol. 2023, 13, 233–244. 10.1039/D2CY01617F. [DOI] [Google Scholar]
- a Inoue S.; Honda K.; Iwase N.; Sato K. Cis Selective Wittig Olefination of α-Alkoxy Ketones and Its Application to the Stereoselective Synthesis of Plaunotol. Bull. Chem. Soc. Jpn. 1990, 63, 1629–1635. 10.1246/bcsj.63.1629. [DOI] [Google Scholar]; b Löbermann F.; Weisheit L.; Trauner D. Intramolecular Vinyl Quinone Diels–Alder Reactions: Asymmetric Entry to the Cordiachrome Core and Synthesis of (−)-Isoglaziovianol. Org. Lett. 2013, 15, 4324–4326. 10.1021/ol401787n. [DOI] [PubMed] [Google Scholar]
- Woodside A. B.; Huang Z.; Poulter C. D. Trisammonium geranyl diphosphate. Org. Synth. 1987, 66, 211–219. 10.1002/0471264180.os066.27. [DOI] [Google Scholar]
- a Falara V.; Akhtar T. A.; Nguyen T. T. H.; Spyropoulou E. A.; Bleeker P. M.; Schauvinhold I.; Matsuba Y.; Bonini M. E.; Schilmiller A. L.; Last R. L.; Schuurink R. C.; Pichersky E. The Tomato Terpene Synthase Gene Family. Plant Physiol. 2011, 157, 770–789. 10.1104/pp.111.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chappell J. J. Cloning and Bacterial Expression of a Sesquiterpene Cyclase from Hyoscyamus muticus and Its Molecular Comparison to Related Terpene Cyclases. J. Biol. Chem. 1995, 270, 7375–7381. 10.1074/jbc.270.13.7375. [DOI] [PubMed] [Google Scholar]; c Nakano C.; Horinouchi S.; Ohnishi Y. J. Biol. Chem. 2011, 286, 27980–27987. 10.1074/jbc.M111.265652. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lin X.; Hopson R.; Cane D. E. Genome mining in Streptomyces coelicolor: Molecular cloning and characterization of anew sesquiterpene synthase. J. Am. Chem. Soc. 2006, 128, 6022–6023. 10.1021/ja061292s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S.; Flack H. D.; Wagner T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. B 2013, 69, 249–259. 10.1107/S2052519213010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Meerwein H.; Hinz G.; Hofmann P.; Kroning E.; Pfeil E. Über tertiäre Oxoniumsalze I. J. Prakt. Chem. 1937, 147, 257–285. 10.1002/prac.19371471001. [DOI] [Google Scholar]; b Meerwein H.; Bettenberg E.; Pfeil E.; Willfang G. Über tertiäre Oxoniumsalze II. J. Prakt. Chem. 1939, 154, 83–156. 10.1002/prac.19391540305. [DOI] [Google Scholar]
- Smith O.; Popescu M. V.; Hindson M. J.; Paton R. S.; Burton J. W.; Smith M. D. Control of stereogenic oxygen in a helically chiral oxonium ion. Nature 2023, 615, 430–435. 10.1038/s41586-023-05719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- a Buchanan J. G.; Edgar A. R.; Large D. G. Methoxy-group Migration in the Hydrolysis of the 4-Nitrobenzene-p-sulphonates of Methyl β-D-Xylopyranoside and Methyl β-D-Glucopyranoside. J. Chem. Soc. D 1969, 0, 558–559. 10.1039/C29690000558. [DOI] [Google Scholar]; b Gibbs C. F.; Jennings H. J. Methoxy group migrations in the reaction of some methyl-pyranoside chlorosulfate ester derivatives with aluminum chloride. Can. J. Chem. 1970, 48, 2735–2739. 10.1139/v70-461. [DOI] [Google Scholar]; c Tokuyasu T.; Masuyama A.; Nojima M.; McCullough K. J. Halonium Ion-Mediated Reaction of Unsaturated Hydroperoxy Acetals. Competition between the Formation of Cyclic Peroxides and the Migration of the Methoxy (or Hydroxy) Group. J. Org. Chem. 2000, 65, 1069–1075. 10.1021/jo991499m. [DOI] [PubMed] [Google Scholar]
- Cane D. E.; Weiner S. W. Cyclization of farnesyl diphosphate to pentalenene. Orthogonal stereochemistry in an enzyme-catalyzed SE′ reaction?. Can. J. Chem. 1994, 72, 118–127. 10.1139/v94-019. [DOI] [Google Scholar]
- a Zalkow L. H.; Harris R. N. III; Van Derveer D.; Bertrand J. A. Isocomene: a novel sesquiterpene from Isocoma Wrightii. X-Ray crystal structure of the corresponding diol. J. Chem. Soc., Chem. Commun. 1977, 456–457. 10.1039/c39770000456. [DOI] [Google Scholar]; b Bohlmann F.; Le Van N.; Pham C. T. V.; Jacupovics J.; Schuster A.; Zabel V.; Watson W. H. β-Isocomen, ein neues Sesquiterpen aus Berkheya-Arten. Phytochemistry 1979, 18, 1831–1834. 10.1016/0031-9422(79)83063-0. [DOI] [Google Scholar]
- Weyerstahl P.; Marschall H.; Seelmann I.; Jakupovic J. Cameroonane, prenopsane and nopsane, three new tricyclic sesquiterpene skeletons. Eur. J. Org. Chem. 1998, 1998, 1205–1212. . [DOI] [Google Scholar]
- Jakupovic J.; Abraham W.-R.; Bohlmann F. Further silphinene derivatives from Cineraria geifolia var. glabra. Phytochem. 1985, 24, 3048–3050. 10.1016/0031-9422(85)80055-8. [DOI] [Google Scholar]
- Nozoe S.; Furukawa J.; Sankawa U.; Shibata S. Isolation, structure and synthesis of hirsutene, a precursor hydrocarbon of coriolin biosynthesis. Tetrahedron Lett. 1976, 17, 195–198. 10.1016/0040-4039(76)80013-5. [DOI] [Google Scholar]
- Kaisin M.; Sheik Y. M.; Durham L. J.; Djerassi C. Capnellane – a new tricyclic sesquiterpene skeleton from the soft coral Capnella imbicata. Tetrahedron Lett. 1974, 15, 2239–2242. 10.1016/S0040-4039(01)92222-1. [DOI] [Google Scholar]
- a Zalkow L. H.; Harris R. N. III; Van Derveer D. Modhephene: a sesquiterpenoid carbocyclic [3.3.3]propellane. X-Ray crystal structure of the corresponding diol. J. Chem. Soc., Chem. Commun. 1978, 420–421. 10.1039/c39780000420. [DOI] [Google Scholar]; b Bohlmann F.; Zdero C.; Bohlmann R.; King R. M.; Robinson H. Neue Sesquiterpene aus Liabum-arten. Phytochem. 1980, 19, 579–582. 10.1016/0031-9422(80)87019-1. [DOI] [Google Scholar]
- Fukuyama Y.; Huang J. M. Chemistry and neurotrophic activity of seco-prezizaane and anislactone-type sesquiterpenes from Illicium species. Stud. Nat. Prod. Chem. 2005, 32, 395–427. 10.1016/S1572-5995(05)80061-4. [DOI] [Google Scholar]
- a Bonney K. J.; Braddock D. C. A. A Unifying Stereochemical Analysis for the Formation of Halogenated C15-Acetogenin Medium-Ring Ethers From Laurencia Species via Intramolecular Bromonium Ion Assisted Epoxide Ring-Opening and Experimental Corroboration with a Model Epoxide. J. Org. Chem. 2012, 77, 9574–9584. 10.1021/jo301580c. [DOI] [PubMed] [Google Scholar]; b Kikuchi H.; Suzuki T.; Kurosawa E.; Suzuki M. The Structure of Notoryne, a Halogenated C15 Nonterpenoid with a Novel Carbon Skeleton from the Red Alga Laurenda nipponica Yamada. Bull. Chem. Soc. Jpn. 1991, 64, 1763–1775. 10.1246/bcsj.64.1763. [DOI] [Google Scholar]
- a Kim B.; Lee M.; Kim M. J.; Lee H.; Kim S.; Kim D.; Koh M.; Park S. B.; Shin K. J. Biomimetic asymmetric total synthesis of (−)-laurefucin via an organoselenium-mediated intramolecular hydroxyetherification. J. Am. Chem. Soc. 2008, 130, 16807–16811. 10.1021/ja806304s. [DOI] [PubMed] [Google Scholar]; b Zhang Y. A.; Yaw N.; Snyder S. A. General Synthetic Approach for the Laurencia Family of Natural Products Empowered by a Potentially Biomimetic Ring Expansion. J. Am. Chem. Soc. 2019, 141, 7776–7788. 10.1021/jacs.9b01088. [DOI] [PubMed] [Google Scholar]
- a Sokolova D.; Piccini G. M.; Tiefenbacher K. Enantioselective Tail-to-Head Terpene Cyclizations by Optically Active Hexameric Resorcin[4]arene Capsule Derivatives. Angew. Chem., Int. Ed. 2022, 61, e202203384 10.1002/anie.202203384. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Merget S.; Catti L.; Piccini G. M.; Tiefenbacher K. Requirements for Terpene Cyclizations inside the Supramolecular Resorcinarene Capsule: Bound Water and its Protonation Determine the Catalytic Activity. J. Am. Chem. Soc. 2020, 142, 4400–4410. 10.1021/jacs.9b13239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.