SUMMARY
While many patients are treated beyond progression (TBP), the magnitude and duration of clinical benefit in these patients have not been fully quantified. Data from 799 patients with melanoma (n = 176), non-small cell lung cancer (NSCLC; n = 146), gastric cancer (GC; n = 87), head and neck squamous cell carcinoma (HNSCC; n = 112), clear-cell renal cell carcinoma (ccRCC; n = 51), and urothelial carcinoma (UC; n = 227) TBP were included. Patients had received pembrolizumab beyond confirmed progressive disease (PD) per RECIST v1.1. A subset of patients displays a 30% reduction in the sum of lesion diameters in the post-progression period (melanoma 24.4%, NSCLC 11.6%, 12.6% GC, 8.9% HNSCC, 15.7% ccRCC, and 13.2% UC). Most patients show stable target lesion dynamics in the post-progression period (melanoma, 64.8%; NSCLC, 72.6%; GC, 69.0%, 75.9% HNSCC, 72.5% ccRCC, 75.3% UC). Pembrolizumab generates meaningful efficacy in a subset of patients treated beyond RECIST v1.1 progression.
Graphical Abstract
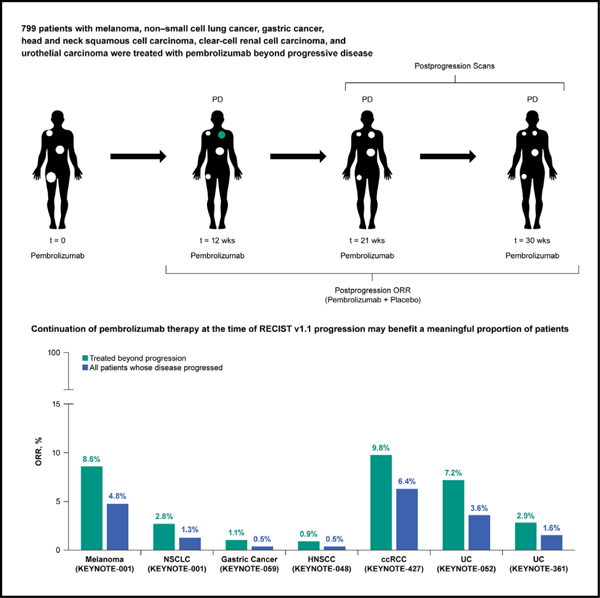
In brief
Topp et al. analyze data of six KEYNOTE clinical trials from patients treated with pembrolizumab beyond cancer progression. They find that in patients with advanced solid tumors, pembrolizumab generates meaningful efficacy in a subset of patients treated beyond disease progression per RECIST v1.1 guidelines.
INTRODUCTION
Treatment with immune checkpoint inhibitors (ICIs) alone or in combination has become standard of care across most solid tumors. While this modality has led to remarkably durable efficacy in some patients, most will experience disease progression. Clinical trials investigating ICIs in combination with other therapies after progression on ICI monotherapy are now common. Traditionally, clinical trials in oncology are conducted to examine a novel therapy and compare tumor responses to historical clinical outcomes in the same setting. However, with the advent of ICI use earlier in therapy and the concurrent testing of ICI-based combinations after progression on ICI monotherapy, determination of the extent of activity and contribution of the novel therapy is challenging in the absence of applicable historical controls. In addition, ICIs after disease progression on ICI-based therapy can be efficacious in some patients,1–4 further complicating assessment of the activity of ICIs in combination with novel agents after progression on ICI monotherapy.
The Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were originally developed and refined (RECIST version 1.1 [v1.1]) to assess tumor response to cytotoxic agents, and they assume that tumor growth or the appearance of new lesions is indicative of progressive disease (PD).5,6 However, patterns of response with ICI therapy differ from cytotoxic agents, with stable disease or PD being noted in some cases before response.7 Several studies have shown that a proportion of patients treated with ICIs who are documented with PD per RECIST v1.1 actually have stable or reduced tumor burden, with PD often being driven by the appearance of new lesions or unequivocal growth of nontarget lesions.7–10 Furthermore, when target lesions are involved in PD, it is often driven by growth of a subset of target lesions, while the remaining lesions remain stable or get smaller in size.9 This suggests that many patients documented with PD per RECIST v1.1 on ICI therapy may have continuing efficacy in a subset of lesions.
Given the known limitations of RECIST v1.1 for assessing response to ICIs, clinical trials have often allowed patients to continue receiving treatment beyond initial PD. Although these patients are not representative of all patients with cancer, they may reflect patients whose overall performance status enables participation in a clinical trial investigating ICI-based combination therapy after documenting PD. In this study, we characterized the efficacy of pembrolizumab in patients with solid tumors who continued to receive pembrolizumab beyond PD per RECIST v1.1. These data will facilitate more accurate assessment of the contribution of novel therapies when investigated in combination with programmed death 1 (PD-1) inhibitors in patients with PD-1 inhibitor-refractory disease.
RESULTS
In this analysis, 799 patients were TBP: 176 with melanoma, 146 with NSCLC, 87 with gastric cancer, 112 with HNSCC, 51 with ccRCC, and 227 with UC (Figure 1). Approximately 50% of patients who developed PD in their parent trial received TBP and were eligible for inclusion in this analysis (Figure S1A). Patients were selected for TBP at the discretion of the treating physician. Post-progression scan intervals were consistent in the pre- and post-progression periods. However, post hoc analysis shows that patients selected for TBP displayed better BOR than patients who were discontinued (Figure S2A).
Figure 1. Study design.
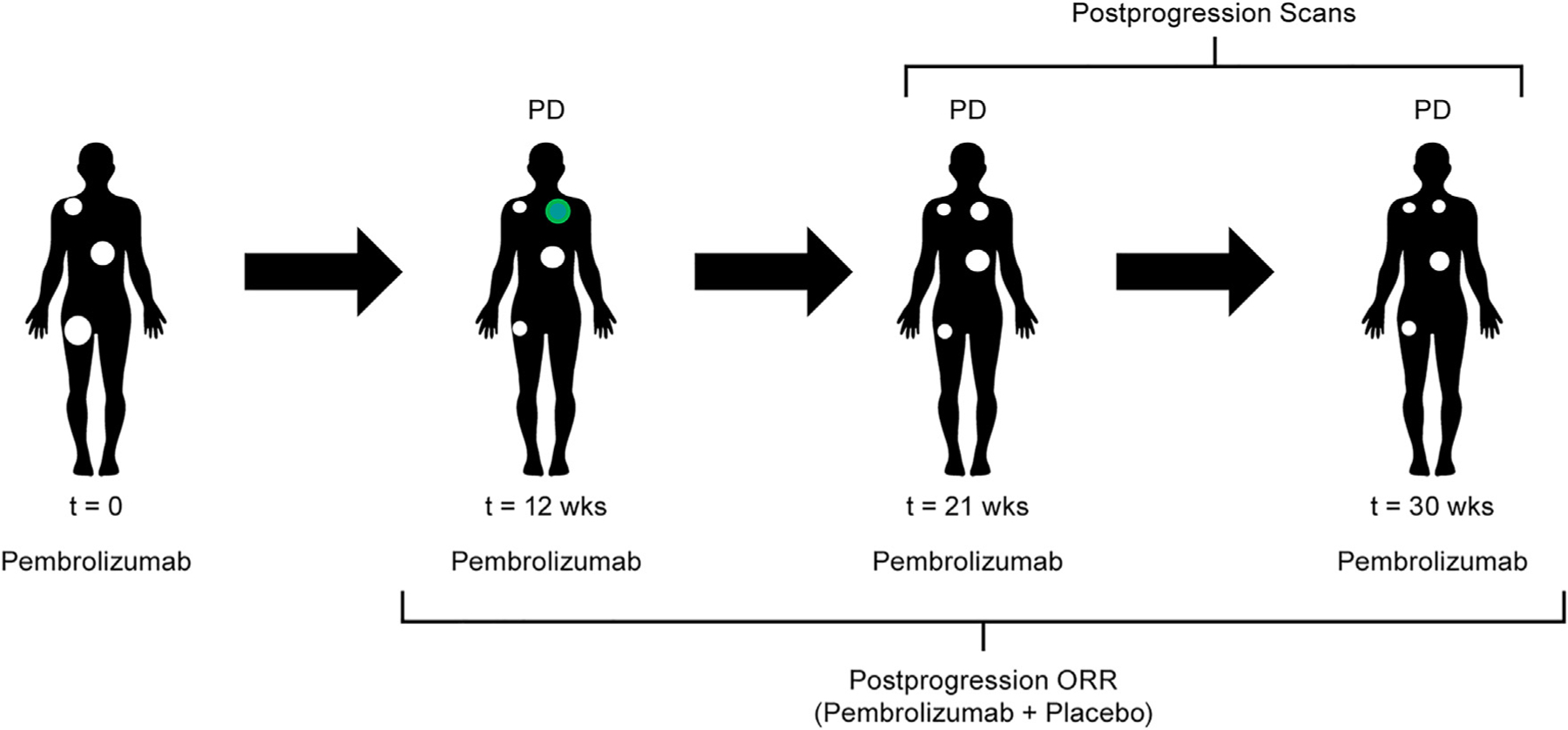
ORR, objective response rate.
Melanoma
Of 313 patients with melanoma who had PD in KEYNOTE-001, 176 were TBP (Figure S1AA). There were 43 patients who had a clinically meaningful reduction (≥30%) in the sum of target lesion diameters in the post-progression period (24.4% of patients TBP; 13.7% of patients with PD) (Table 1 and Figure 2A). The median time to target response was 12 weeks (range, 4.4–120). Of the 43 patients, 15 had a post-progression objective response per RECIST v1.1 (ORR, 8.6% among patients TBP; ORR, 4.8% among patients with PD) (Table 1). Results were similar when ORR was calculated using the confirmatory scan at TBP baseline. The median time to objective response was 12 weeks (range, 8–120). The remaining 28 patients had a BOR of PD because of non-target lesion progression or the appearance of a new metastatic lesion. The median DOR in the post-progression period was 28.1 weeks (range, 5.0–107.8). Of the 176 patients who were TBP, 114 (64.8%) had stable target lesion dynamics while 19 patients (10.7%) had clinically meaningful growth (≥20%) in target lesions during the post-progression period (Figure 2A). The majority of patients (93.8%) displayed at least one stable or shrinking lesion.
Table 1.
Target lesion response and ORR per RECIST v1.1 in the postprogression period among patients who were TBP and among all patients with PD in their parent trial
| Patients Who Were TBP |
All Patients With PD |
|||
|---|---|---|---|---|
| Indication (study name) | Target Lesion Response, % (n/N)a | ORR per RECIST v1.1, % (n/N)b | Target Lesion Response, % (n/N)a | ORR per RECIST v1.1, % (n/N)b |
| Melanoma (KEYNOTE-001) | 24.4 (43/176) | 8.6 (15/176) | 13.7 (43/313) | 4.8 (15/313) |
| NSCLC (KEYNOTE-001) | 11.6 (17/146) | 2.8 (4/146) | 5.7 (17/296) | 1.3 (4/296) |
| Gastric cancer (KEYNOTE-059) | 12.6 (11/87) | 1.1 (1/87) | 5.6 (11/198) | 0.5 (1/198) |
| HNSCC (KEYNOTE-048) | 8.9 (10/112) | 0.9 (1/112) | 4.9 (10/204) | 0.5 (1/204) |
| ccRCC (KEYNOTE-427) | 15.7 (8/51) | 9.8 (5/51) | 10.3 (8/78) | 6.4 (5/78) |
| UC (KEYNOTE-052) | 12.0 (15/125) | 7.2 (9/125) | 6.0 (15/252) | 3.6 (9/252) |
| UC (KEYNOTE-361) | 14.7 (15/102) | 2.9 (3/102) | 7.7 (15/194) | 1.6 (3/194) |
Abbreviations: ccRCC, clear-cell renal cell carcinoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non–small cell lung cancer; ORR, objective response rate; UC, urothelial carcinoma.
A ≥ 30% reduction in the sum of target lesion size from baseline was considered a clinically meaningful reduction.
ORR per RECIST v1.1 was calculated for each patient at each postprogression time point based on the sum of target lesion size (relative to the original time of PD), the appearance of new metastatic lesions, or progression of a nontarget lesion.
Figure 2.
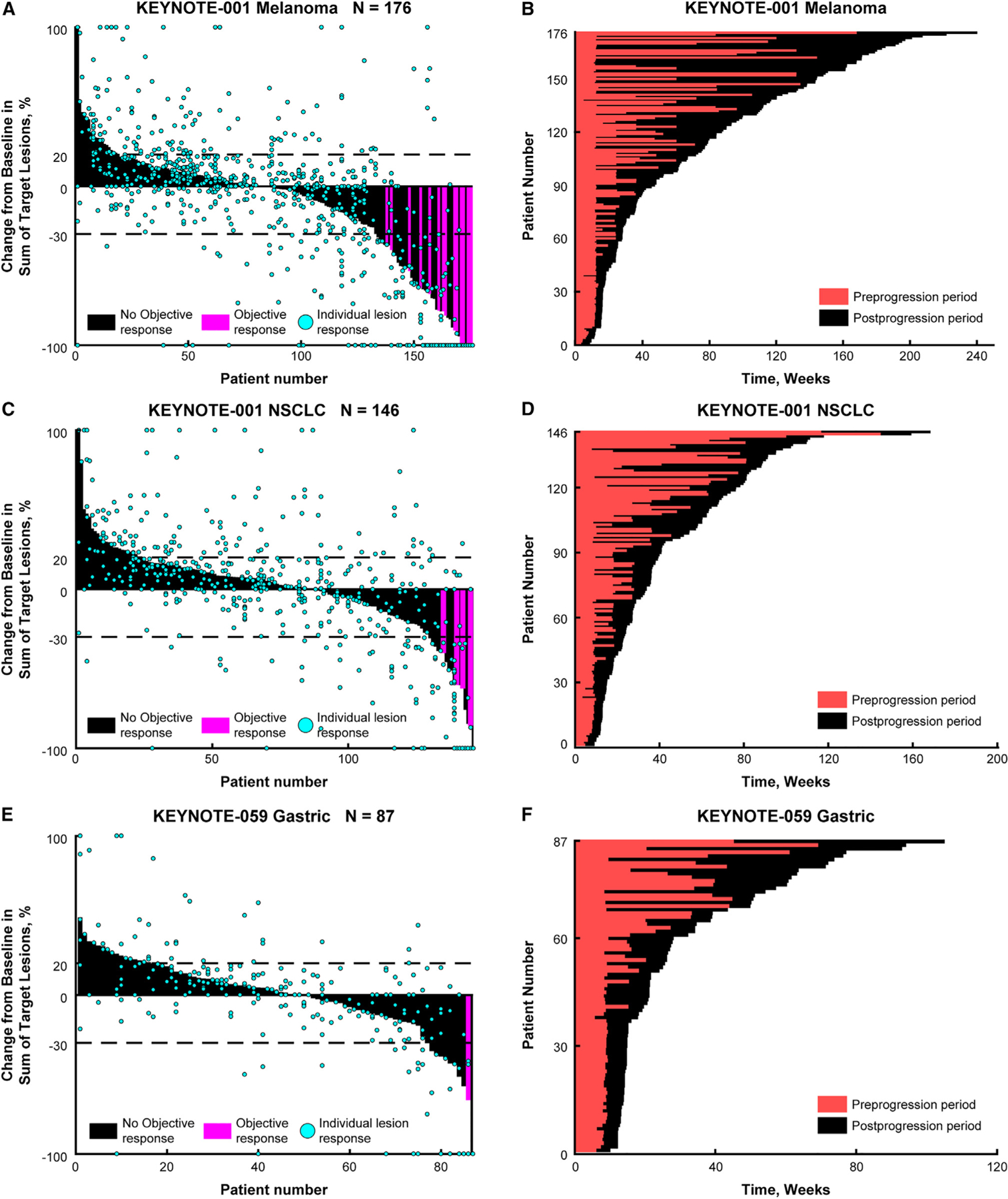
Waterfall and Swim Plots Best overall response and time on trial after progression for patients with (A and B) melanoma, (C and D) NSCLC, and (E and F) gastric cancer. NSCLC, non–small cell lung cancer.
Of the 176 patients TBP, 71 had similar tumor dynamics before and after progression (growth-growth, n = 6; stable-stable, n = 50; shrink-shrink, n = 15) (Figure 3A). The remaining 105 patients showed a change in tumor dynamics after PD diagnosis. The most common transitions were from growing to stable (n = 24) or from shrinking to stable (n = 40) (Figure 3A). Response after a documented period of progression occurred in 4.0% of TBP patients (Figure 3A, grow-shrink). It should also be noted that several patients dropped out at the first post-progression scan and may not have had sufficient time to progress or respond.
Figure 3.
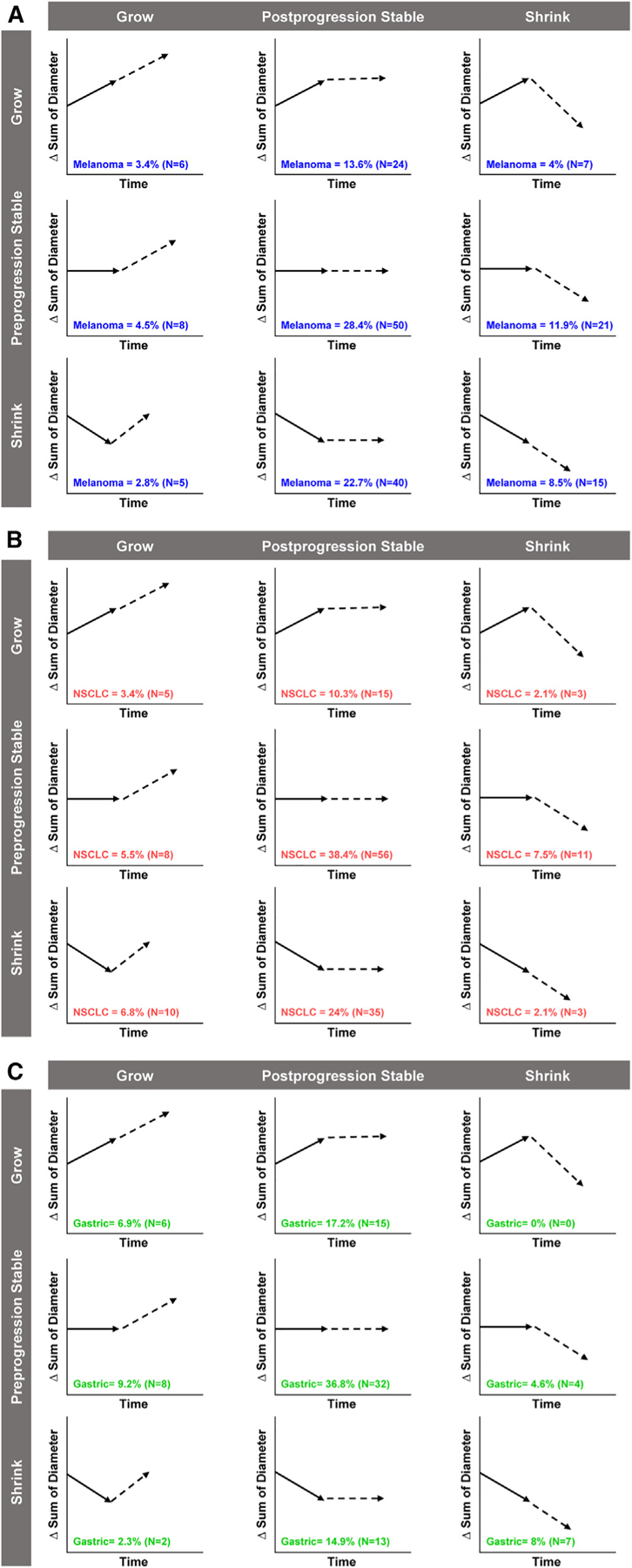
Pre- and Post PD Tumor Dynamics Distribution of target lesion responses in preprogression and postprogression periods for patients with (A) melanoma, (B) NSCLC, and (C) gastric cancer. NSCLC, non-small cell lung cancer.
Patients with melanoma remained on treatment for a median of 18.4 weeks (range, 4.0–194.9) after initial PD documentation (Figure 2B). The median time on trial after progression was 15.6 weeks (range, 4.0–194.9) for patients with stable target lesions during the post-progression period (n = 114), 79.0 weeks (range, 4.4–185.0) for patients with a reduction in the sum of target lesion diameters (n = 43), and 95.8 weeks (range, 32.1–140.4) for patients with an objective response (n = 15).
NSCLC
Of 296 patients with NSCLC who had PD in KEYNOTE-001, 146 were TBP (Figure S1AB). There were 17 patients who had a clinically meaningful reduction (≥30%) in the sum of target lesion diameters in the post-progression period (11.6% of patients TBP; 5.7% of patients with PD) (Table 1 and Figure 2C). The median time to target response was 9.4 weeks (range, 7.9–64.1). Of the 17 patients, four had an objective response per RECIST v1.1 (ORR, 2.8% among patients TBP; 1.3% among patients with PD) (Table 1). The median time to objective response was 9.4 weeks (range, 9–18.1). The remaining 13 had a BOR of PD because of non-target progression or the appearance of a new metastatic lesion. Of the 146 patients who were TBP, 106 (72.6%) had stable target lesion dynamics and 23 (15.7%) had clinically meaningful growth (≥20%) in target lesions during the post-progression period (Figure 2C). The median DOR in the post-progression period was 8.9 weeks (range, 8.6–12.2). The majority of patients (94.5%) displayed at least one stable or shrinking lesion.
Of the 146 patients with NSCLC TBP, 64 showed similar tumor dynamics before and after progression (growth-growth, n = 5; stable-stable, n = 56; shrink-shrink, n = 3) (Figure 3B). The remaining 82 patients showed a change in tumor dynamics after initial PD documentation. The most common change was the stabilization of target lesions in the post-progression period (Figure 3B). Response following a documented period of progression occurred in 2.1% of patients receiving TBP (Figure 3B, grow-shrink). It should also be noted that several patients dropped out at the first post-progression scan and may not have had sufficient time to progress or respond.
Patients with NSCLC remained on treatment for a median of 12.5 weeks (range, 4.0–90.4) after initial PD documentation (Figure 2D). The median time on trial after progression was 11.4 weeks (range, 4.0–67.6) for patients with stable target lesions during the post-progression period (n = 106), 43.9 weeks (range, 14.1–90.4) for patients with a reduction in the sum of target lesion diameters (n = 17), and 22.4 weeks (range, 14.6–58.1) for patients with an objective response (n = 4). Five patients with a post-progression reduction in the sum of target lesion diameters who were considered to have PD per RECIST v1.1 because of non-target progression stayed on the treatment for an extended period (range, 55.4–90.4 weeks), contributing to the median time on trial after progression.
Gastric cancer
Of 198 patients with gastric cancer who had PD in KEYNOTE-059, 87 were TBP (Figure S1AC). There were 11 patients who had a clinically meaningful reduction (≥30%) in the sum of target lesion diameters in the post-progression period (12.6% of patients TBP; 5.5% of patients with PD) (Table 1 and Figure 2E). The median time to target response was 12.1 weeks (range, 5.3–54). Of the 11 patients, only one had an objective response per RECIST v1.1 (ORR, 1.1% among patients TBP; ORR, 0.5% among patients with PD) (Table 1). The time to objective response for this patient was 9 weeks. The remaining 10 had a BOR of PD because of non-target progression or the appearance of a new metastatic lesion. Of the 87 patients who were TBP, 60 (68.9%) had stable target lesion dynamics and 16 (18.4%) had clinically meaningful growth (≥20%) in target lesions during the post-progression period (Figure 2E). The DOR in the post-progression period for the one patient with an objective response was 9.0 weeks. Most patients (90.8%) displayed at least one stable or shrinking lesion.
Of the 87 patients with gastric cancer TBP, 45 had similar tumor dynamics before and after progression (growth-growth, n = 6; stable-stable, n = 32; shrink-shrink, n = 7) (Figure 3C). The remaining 42 patients showed a change in tumor dynamics after disease progression. The most common change was stabilization of target lesion dynamics in the post-progression period (Figure 3C). Response following a period of documented progression occurred in 0% of patients receiving TBP (Figure 3C, grow-shrink). It should also be noted that several patients dropped out at the first post-progression scan and may not have had sufficient time to progress or respond.
Patients with gastric cancer remained on treatment for 6.1 weeks (range, 4.0–72.7) after initial PD documentation (Figure 2F). The median time on trial after progression was 6.1 weeks (range, 4.0–47.9) for patients with stable target lesions during the post-progression period (n = 60), 16.1 weeks (range, 5.3–72.7) for patients with a reduction in the sum of target lesion diameters (n = 11), and 9.0 weeks for the one patient with an objective response.
HNSCC, ccRCC, and UC
Similar analyses were performed for patients with HNSCC, ccRCC, and UC, all with qualitatively consistent findings (Table 1, Figures S3A and S4A). Specifically, a clinically meaningful reduction (≥30%) in the sum of target lesion diameters was observed in between 8.9% and 15.7% of patients TBP in the post-progression period (4.9%–10.3% of patients with PD), and objective responses per RECIST v1.1 were observed in 0.9%–9.8% of patients TBP (0.5%–6.4% of patients with PD) (Table 1). The median time to target response was observed in between 11.9 and 18 weeks and between 9.3 and 36 weeks for the time to reach an objective response. Additional results from this analysis can be found in the supplement Table S1A.
Behavior of new metastatic lesions
New metastatic lesions were identified and indexed (measured) in KEYNOTE-001 (melanoma and NSCLC population; Figure 4), KEYNOTE-048 (HNSCC population), KEYNOTE-427 (ccRCC population), KEYNOTE-052 (UC), and KEYNOTE-361 (UC). New metastatic lesions in KEYNOTE-059 (gastric cancer population) were recorded as present or absent but were not measured.
Figure 4.
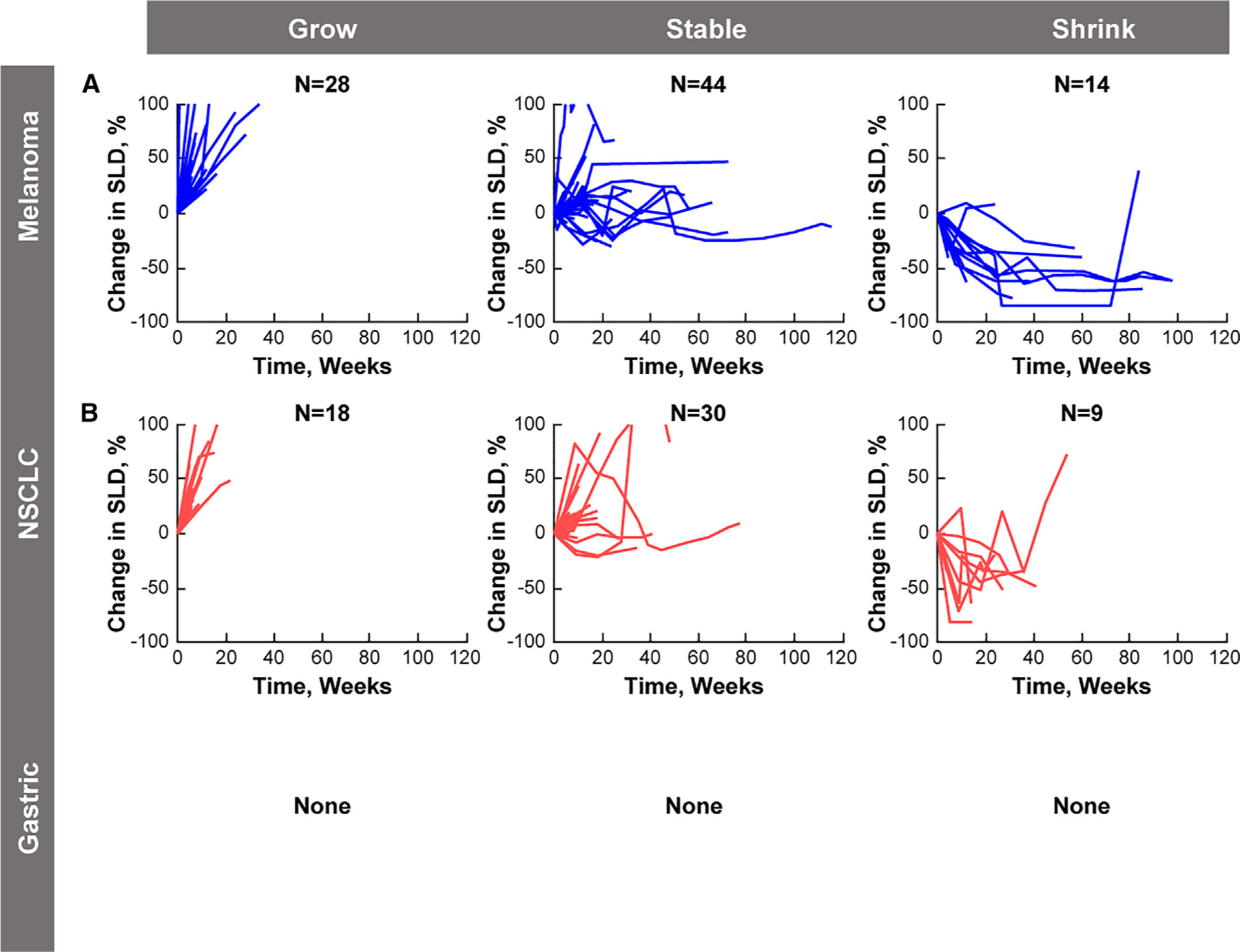
Post-PD Dynamics of New Metastatic Lesions Behavior of new metastatic lesions after initial appearance in patients with (A) melanoma and (B) NSCLC. New metastatic lesions for patients with gastric cancer in KEYNOTE-059 were recorded as present or absent but were not indexed (measured). NSCLC, non-small cell lung cancer; SLD, sum of longest diameter.
New lesions were measured in 86 of 176 patients (48.9%) with melanoma and 57 of 146 patients (39.0%) with NSCLC in KEYNOTE-001. The behavior of these new lesions in the post-progression period relative to size at the time of PD is presented in Figure 4. Approximately half of the patients exhibited stability in the new lesions (melanoma, 44 of 86 [51.2%]; NSCLC, 30 of 57 [52.6%]), and approximately 16% had shrinkage in the new lesions (melanoma, 14 of 86 [16.3%]; NSCLC, nine of 57 [15.8%]). The remaining third of patients exhibited growth in the new lesions (melanoma, 28 of 86 [32.6%]; NSCLC, 18 of 57 [31.6%]). Similar findings were observed in HNSCC, ccRCC, and UC (data not shown).
Additional analyses of post-progression response
Kaplan-Meier plots showing how the duration of treatment differed as a function of tumor reduction (at the time of PD or BOR prior to TBP), the percentage of progressing lesions prior to TBP, PD-L1 levels at baseline, and the presence or absence of liver lesions prior to TBP are were performed. Of the markers tested, only change in tumor size (sum of diameters) prior to TBP showed a trend with duration of TBP.
DISCUSSION
PD-1/L1 inhibitors have now been incorporated into the treatment paradigm for most solid tumors and are being used in progressively earlier stages of disease. The development of therapies for patients who experience disease progression on or after PD-1/L1 inhibitors has become an urgent focus of research in oncology. However, in this setting, it is difficult to define the contribution of components when novel therapies are combined with PD-1/L1 inhibitors. In this analysis, we evaluated post-progression responses among 799 patients treated with pembrolizumab beyond confirmed PD across six tumor types. The results showed that a clinically meaningful reduction (≥30% from the onset of the first PD event) in the sum of target lesion diameters in the post-progression period was observed in between 8.9% and 24.4% of patients TBP, whereas an objective response per RECIST v1.1 was only observed in 0.9%–9.8% of patients TBP (0.5–6.4% of all patients with disease progression on the study, i.e., including those not TBP). The highest response rates were observed in tumor types known to be more ICI responsive, such as melanoma. Notably, the majority of patients (64.8%–76.5%) displayed stable target lesion dynamics in the post-progression period. Growth of target lesions was uncommon, occurring in less than 20% of patients in every tumor population. Time on treatment beyond PD varied, with the longest time on treatment in melanoma (median, 18.4 weeks) and the shortest in gastric cancer (median, 6.1 weeks). Notably, patients with deeper responses tended to show longer duration of treatment. Thus, patients with stable tumor dynamics generally remained on treatment for a shorter period of time than patients with a reduction in the sum of target lesion diameters or an objective response. Although a previous study had shown that the appearance of new metastatic lesions was one of the most common causes of PD per RECIST v1.1,9 the results of the current analysis suggest that one-third of new lesions continue to grow in the post-progression period. Additional analyses suggested that patients with a tumor response prior to TBP remain on TBP longer than patients that did not display a decrease in the sum of target diameters prior to TBP. The results of the current analysis suggest that stable disease or tumor shrinkage should be expected with PD-1 inhibitors in clinical trials involving patients with PD-1 inhibitor-refractory disease. It is important to note, however, that 11–18% of patients experience an increase in their target lesions post-progression, and one-quarter to one-third of new metastatic lesions showed continued growth during the post-progression period.
A number of studies have retrospectively quantified antitumor activity and objective responses in patients with solid tumors treated with PD-1 inhibitor therapy beyond PD per RECIST criteria. In a systematic review, 19.7% of patients from 25 trials (total patients TBP, n = 853) who received PD-1/L1-based regimens beyond PD achieved a response after initial RECIST-defined PD.11 Results of other studies have shown that up to 33% of patients TBP exhibit a reduction in target tumor burden of ≥30%, with many more patients having stable target lesion dynamics.12–17 The results of these studies support the findings of the current analysis, which showed that a proportion of patients respond to PD-1 inhibitor therapy beyond progression.
With PD-1/L1 inhibitors becoming first-line therapy in many indications, studies testing PD-1/L1 inhibitors in combination with novel agents in patients with PD-1/L1 inhibitor refractory or resistant disease are becoming increasingly common. Traditionally, any efficacy observed in these studies was attributed to the novel agent, as the patients were considered to no longer benefit from PD-1/L1-inhibitor therapy. However, this study and the retrospective studies described previously, clearly show meaningful efficacy of PD-1/L1 inhibitors in patients whose disease progresses on PD-1/L1 inhibitor therapy. Thus, the efficacy of PD-1/L1 inhibition needs to be accounted for when interpreting combination therapy data in patients who have experienced disease progression on or after PD-1/L1 inhibitors.
This study also has several implications for the advancement of the field’s understanding of the biology of resistance to ICIs. In a recent study by our group, we found that among patients labeled as having primary progression, slightly more than half of the individual target lesions were not themselves progressing.9 This suggests that tissue samples used for biomarker analysis may be derived from individual lesions that are discordant with the overall response status of the patient. The present study advances our understanding by demonstrating that a minority of tumor sites are actually progressing at the time of treatment beyond progression. Indeed, a meaningful proportion of tumors in patients treated beyond progression do not actually display radiographic evidence of resistance: the majority stabilizes and some shrink. Perhaps surprisingly, this finding even applies to new metastatic lesions that accounted for RECIST v1.1-defined progression. Similar results were observed across five solid tumor types (melanoma, NSCLC, gastric cancer, ccRCC, and UC), which may suggest a generalizable principle—although this requires confirmation in additional clinical studies. These findings underscore the importance of tumor-level assessment of both radiographic response and tumor biology in translational studies aiming to understand or treat disease progression on or after ICIs. Studies in the neoadjuvant setting and those generating complementary data from peripheral blood analytes such as circulating tumor DNA may improve the nuances of our understanding of the biology of resistance.18,19
Finally, this study underscores that continuation of pembrolizumab therapy at the time of RECIST v1.1 progression may benefit a meaningful proportion of patients, although the lack of prospective randomization in this study requires note. We had previously shown that most patients with RECIST v1.1 progression display disease control in a subset of lesions.9 Here we show that most patients treated beyond disease progression display stable or shrinking tumors. Notably, we showed that it is common for lesions that are growing at the time of progression to stabilize upon continued therapy. Similar findings were identified for new metastatic lesions, which tend to appear and then stabilize. One-quarter to one-third of new metastatic lesions continued to grow in the post-progression period. Nonetheless, there is a subset of patients who did not benefit from TBP and might have benefited from moving to a new therapy at the time of PD. Such decisions should be made by the patient and their physician considering benefits and risks, including symptoms of tumor, adverse events, and financial considerations. Identifying which patients will or will not benefit from TBP is not yet defined in the literature. One potential approach is lesion-level decision-making. Based on the post-progression waterfall plot, there appear to be three subsets of patients. Patients on the left side of the waterfall plot showed little or no benefit from post-progression treatment and might benefit most from moving to a new therapy. Patients in the middle of the waterfall plot show benefit in a subset of lesions and might benefit from adding a new therapy while maintaining pembrolizumab. Patients on the right of the waterfall plot may benefit from continued pembrolizumab monotherapy. Clearly, additional prospective clinical studies are required to identify patients that may benefit from continued pembrolizumab treatment beyond the time point of RECIST v1.1 progression, including when used in combination with a novel agent.
This study has several limitations. First, the patients included in this analysis were treated with pembrolizumab before and after initial PD documentation; therefore, the tumor behavior described may not apply to other ICI-based therapies. Although studies of pembrolizumab used in combination with chemotherapy (KEYNOTE-361 and KEYNOTE-048) were included, only patients treated with pembrolizumab monotherapy were captured in the current analysis. Second, there is an inherent bias in focusing on patients treated beyond PD because these patients are, by definition, clinically distinct. Such patients may be similar to those who enroll in subsequent clinical trials but are unlikely to represent an all-comers population, many of whom may die due to their disease at or soon after the time of initial progression. Furthermore, treatment beyond progression was at the discretion of the investigator, which may have introduced bias in patient selection. Third, this study does not take into account known biomarkers of response and resistance; the molecular features of patients treated beyond PD may differ from the randomized population. Finally, it should be noted that this is a non-randomized retrospective analysis.
The results of this study provide a baseline expectation per tumor type in patients treated with PD-1 inhibitor therapy beyond PD and confirm that the majority of tumor sites do not demonstrate radiographic evidence of resistance at the time of progression. These data may facilitate more accurate assessment of the responses observed with novel therapies combined with PD-1 inhibitor therapy in clinical studies where applicable historical controls are not available and may lead to a more refined and accurate understanding of the biology underlying resistance to ICIs.
STAR☆METHODS
RESOURCE AVAILABILITY
Lead contact
Information and requests for resources should be directed to the lead contact, Brian Topp (brian.topp@merck.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. Merck Sharp and Dohme (MSD) is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and European Union (EU) or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Clinical trial identifiers: KEYNOTE-001 (NCT01295827), KEYNOTE-059 (NCT02335411), KEYNOTE-048 (NCT02358031), KEYNOTE-427 (NCT02853344), KEYNOTE-052 (NCT02335424), and KEYNOTE-361 (NCT02853305). Please refer to the key resources table for detailed information regarding the data collected from these clinical trials, which are used for analysis in this study.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
|
| ||
| KEYNOTE-001 (melanoma and non–small cell lung cancer [NSCLC]) | https://doi.org/10.1056/NEJMoa1501824 | NCT01295827 |
| KEYNOTE-059 (gastric cancer) | https://doi.org/10.1001/jamaoncol.2018.0013 | NCT02335411 |
| KEYNOTE-048 (head and neck squamous cell carcinoma [HNSCC]) | https://doi.org/10.1016/S0140-6736(19)32591-7 | NCT02358031 |
| KEYNOTE-427 (clear cell renal cell carcinoma [ccRCC]) | https://doi.org/10.1200/JCO.20.02363 | NCT02853344 |
| KEYNOTE-052 (urothelial carcinoma [UC]) | https://doi.org/10.1200/JCO.19.01213 | NCT02335424 |
| KEYNOTE-361 (UC) | https://doi.org/10.1016/S1470-2045(21)00152-2 | NCT02853305 |
|
| ||
| Software and algorithms | ||
|
| ||
| MATLAB 2021a | MathWorks, Inc | RRID:SCR_001622 |
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Study design and participants
Data for this analysis were included from patients who received pembrolizumab monotherapy in KEYNOTE-001 (NCT01295827; melanoma and non–small cell lung cancer [NSCLC]), KEYNOTE-059 (NCT02335411; gastric cancer), KEYNOTE-048 (NCT02358031; head and neck squamous cell carcinoma [HNSCC]), KEYNOTE-427 (NCT02853344; clear-cell renal cell carcinoma [ccRCC]), KEYNOTE-052 (NCT02335424; urothelial carcinoma [UC]), and KEYNOTE-361 (NCT02853305; UC).
Study oversight
The study protocols for all trials were approved by the appropriate institutional review board or independent ethics committee at each participating institution. The studies were conducted in accordance with the protocols, good clinical practice guidelines, and the principles outlined in the Declaration of Helsinki. All patients had provided written informed consent.
METHOD DETAILS
Replication
Not applicable.
Strategy for randomization and/or stratification
KEYNOTE-001, KEYNOTE-059, KEYNOTE-427, and KEYNOTE-052 are open-label studies.
KEYNOTE-048 and KEYNOTE-61 are randomized, open-label, phase 3 studies. In KEYNTOE-048, participants were stratified by PD-L1 expression, p16 status, and performance status and randomly assigned (1:1:1) to pembrolizumab monotherapy, pembrolizumab plus a platinum and 5-fluorouracil, or cetuximab plus a platinum-based chemotherapy and 5-fluorouracil. Investigators and participants were aware of treatment assignment. In KEYNOTE-361 participants were randomly assigned (1:1:1) via to pembrolizumab 200 plus chemotherapy, pembrolizumab monotherapy, or chemotherapy alone. Participants were stratified by choice of platinum therapy and PD-L1 combined positive score (CPS). Neither patients nor investigators were blinded to the treatment assignment or CPS.
Blinding at any stage of the study
Participants and investigators were not blinded in any study.
Sample-size estimation and statistical method of computation
Not applicable.
Inclusion and exclusion criteria of any data, participants or subjects
To be included in the analysis of patients treated beyond progression (TBP), patients must have received at least one dose of pembrolizumab beyond PD per RECIST v1.1, received at least two doses of pembrolizumab by the time of PD diagnosis, had PD confirmed (per RECIST v1.1 by blinded independent central review except for KEYNOTE-052 where investigator response was used) at least 4 weeks after PD was first documented, and experienced PD less than 12 weeks after the last dose of pembrolizumab. These criteria are similar to those developed by the Society for Immunotherapy of Cancer Immunotherapy Resistance Taskforce for defining tumor resistance to PD-1 pathway blockade.20
QUANTIFICATION AND STATISTICAL ANALYSIS
A total of 1,535 participants whose cancer progressed after receiving pembrolizumab treatment were included in this analysis.
Individual target lesion dynamics in the post-progression period were calculated relative to the lesion size at the time of initial PD. Indexed new metastatic lesions that appeared at the time of PD diagnosis were considered as target lesions in the post-progression period.
Waterfall plots were derived from best overall response (BOR) in the sum of target lesion diameters in the post-progression period. Clinically meaningful growth was defined as a ≥20% increase in the sum of target lesion diameters from the time of initial PD and clinically meaningful shrinkage as a ≥30% reduction in the sum of target lesion diameters from the time of initial PD; anything between was considered stable. Post-progression objective response rate (ORR) per RECIST v1.1 was based on the sum of target lesion diameters (relative to the initial occurrence of PD), the appearance of new metastatic lesions, or progression of a nontarget lesion. Patients who displayed progression of a nontarget lesion or nonindexed new metastatic lesion at the time of the original PD were assumed to display post-progression PD at the first post-progression time point. Target lesion response and ORR per RECIST v1.1 were analyzed among patients who were TBP and among all patients who had PD in the corresponding parent trial. Time on trial was calculated as the time from first dose to the time of the last radiographic scan. Duration of response (DOR) per RECIST v1.1 was also evaluated. Across cohorts, all patients were continuously exposed to pembrolizumab in the pre-progression and post-progression periods.
Kaplan-Meier plots were generated to identify biomarkers that are predictive of the duration of TBP, target lesions response at the time of PD (tPD), best overall response (BOR) before TBP, percentage of growing lesions at tPD, PD-L1 status at baseline (expression ≥1% is positive), and appearance of lesions in liver by tPD were tested for their predictive ability.
All data were analyzed using MATLAB 2021a.
Supplementary Material
Highlights.
~50% of patients received pembrolizumab treatment beyond progression
Most patients treated beyond disease progression have stable or shrinking tumors
Patients with deeper responses tend to show longer duration of treatment
Pembrolizumab has meaningful efficacy in select patients treated beyond progression
ACKNOWLEDGMENTS
Funding for this study was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. We thank the patients and their families and caregivers and all investigators and site personnel. We also thank Kannan Thiagarajan for his scientific contributions. Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Holly C. Cappelli, PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.ccell.2023.08.004.
DECLARATION OF INTERESTS
B.G.T. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and owns stock in Merck & Co., Inc., Rahway, NJ, USA.
M.C. has nothing to disclose.
K.M. is a stockholder of Merck & Co., Inc., Rahway, NJ, USA, and holds a leadership role (Vice President, Clinical Pharmacology, Clinical Development) at Generate Biomedicines.
DPd.A. is a stockholder of Merck & Co., Inc., Rahway, NJ, USA, and holds a leadership role (Senior Vice President, Clinical Drug Development) at Generate Biomedicines.
E.R. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; owns stock in Merck & Co., Inc., Rahway, NJ, USA; and holds a leadership role (Senior Vice President, Clinical Oncology) at Merck & Co., Inc., Rahway, NJ, USA.
A.S. reports advisory/consultancy to Two River, Inc, holds a leadership role at Generate Biomedicines (CMO), and is an officer/on the board of directors for Navigating Cancer.
J.D.W. reports a role of consultant for Adaptive Biotech, Amgen, Apricity, Ascentage Pharma, Astellas, AstraZeneca, Bayer, Beigene, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, F Star, Imvaq, Kyowa Hakko Kirin, Linneaus, MedImmune, Merck, Neon Therapeutics, Ono, Polaris Pharma, Polynoma, Psioxus, Puretech, Recepta, Takara Bio, Trieza, Truvax, Serametrix, Surface Oncology, Syndax, and Syntalogic; research support from AstraZeneca and Bristol Myers Squibb; and equity in Adaptive Biotechnologies; BeiGene; Imvaq; Linneaus; Potenza Therapeutics; Tizona Pharmaceuticals; and Trieza.
A.R. reports leadership for PACT Pharma, Arcus Biosciences, and Lutris; stock and other ownership interests for Compugen, CytomX Therapeutics, Advaxis, Acrus Biosciences, Tango Therapeutics, PACT Pharma, Merus, ImaginAb, Lutris Pharma, Highlight, MapKure, 4c Biomked, Kite/Gilead, Isoplexis, Appia, Synthekine, Pluto, Inspirna, RAPT Therapeutics, and ImmPACT-Bio; honoraria from Merck Sharp & Dohme, Novartis, Amgen, Chugai/Roche, Genentech/Roche, Sanofi, Vedanta Biosciences, and AstraZeneca; a consulting or advisory role for Merck, Amgen, Novartis, Chugai Pharma, and Sanofi; research funding to institution from Agilent and Bristol Myers Squibb; and patents, royalties, other intellectual property for nonviral gene editing to Arsenal Bio.
REFERENCES
- 1.Giaj Levra M, Cotté FE, Corre R, Calvet C, Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assié JB, and Chouaïd C (2020). Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 140, 99–106. [DOI] [PubMed] [Google Scholar]
- 2.Ravi P, Mantia C, Su C, Sorenson K, Elhag D, Rathi N, Bakouny Z, Agarwal N, Zakharia Y, Costello BA, et al. (2020). Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol 6, 1606–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaremba A, Eggermont AMM, Robert C, Dummer R, Ugurel S, Livingstone E, Ascierto PA, Long GV, Schadendorf D, and Zimmer L (2021). The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur. J. Cancer 155, 268–280. [DOI] [PubMed] [Google Scholar]
- 4.Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, Bajwa R, Momtaz P, Callahan MK, Wolchok JD, et al. (2020). Long-term outcomes and responses to retreatment in patients with melanoma treated With PD-1 blockade. J. Clin. Oncol 38, 1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, and Gwyther SG (2000). New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst 92, 205–216. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. (2009). Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res 15, 7412–7420. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al. (2016). Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol 34, 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topp BG, Thiagarajan K, De Alwis DP, Snyder A, and Hellmann MD (2021). Lesion-level heterogeneity of radiologic progression in patients treated with pembrolizumab. Ann. Oncol 32, 1618–1625. [DOI] [PubMed] [Google Scholar]
- 10.Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, Sridhara R, Goldberg KB, Chuk MK, Chi DC, et al. (2018). Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol 19, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo F, Boutros A, Cecchi F, Croce E, Tanda ET, and Queirolo P (2021). Treatment beyond progression with anti-PD-1/PD-L1 based regimens in advanced solid tumors: a systematic review. BMC Cancer 21, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, Dummer R, Robert C, Márquez-Rodas I, McNeil C, et al. (2017). Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol 3, 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM, and Rini BI (2016). Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a Subgroup analysis of a randomized clinical trial. JAMA Oncol 2, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Necchi A, Joseph RW, Loriot Y, Hoffman-Censits J, Perez-Gracia JL, Petrylak DP, Derleth CL, Tayama D, Zhu Q, Ding B, et al. (2017). Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: post-progression outcomes from the phase II IMvigor210 study. Ann. Oncol 28, 3044–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, et al. (2017). Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur. Urol 72, 368–376. [DOI] [PubMed] [Google Scholar]
- 16.Haddad R, Concha-Benavente F, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Kasper S, Vokes EE, Worden F, et al. (2019). Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: A subgroup analysis of a randomized phase 3 clinical trial. Cancer 125, 3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandara DR, von Pawel J, Mazieres J, Sullivan R, Helland Å, Han JY, Ponce Aix S, Rittmeyer A, Barlesi F, Kubo T, et al. (2018). Atezolizumab treatment beyond progression in advanced NSCLC: Results from the randomized, Phase III OAK Study. J. Thorac. Oncol 13, 1906–1918. [DOI] [PubMed] [Google Scholar]
- 18.Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EHC, Mog BJ, Hou W, Justesen S, Blosser R, et al. (2021). Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 596, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Luo J, Wu S, Si H, Gao C, Xu W, Abdullah SE, Higgs BW, Dennis PA, van der Heijden MS, et al. (2020). Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov 10, 1842–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, Chen HX, Drake CG, Feltquate DM, Ferris RL, et al. (2020). Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 8, e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. Merck Sharp and Dohme (MSD) is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and European Union (EU) or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Clinical trial identifiers: KEYNOTE-001 (NCT01295827), KEYNOTE-059 (NCT02335411), KEYNOTE-048 (NCT02358031), KEYNOTE-427 (NCT02853344), KEYNOTE-052 (NCT02335424), and KEYNOTE-361 (NCT02853305). Please refer to the key resources table for detailed information regarding the data collected from these clinical trials, which are used for analysis in this study.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
|
| ||
| KEYNOTE-001 (melanoma and non–small cell lung cancer [NSCLC]) | https://doi.org/10.1056/NEJMoa1501824 | NCT01295827 |
| KEYNOTE-059 (gastric cancer) | https://doi.org/10.1001/jamaoncol.2018.0013 | NCT02335411 |
| KEYNOTE-048 (head and neck squamous cell carcinoma [HNSCC]) | https://doi.org/10.1016/S0140-6736(19)32591-7 | NCT02358031 |
| KEYNOTE-427 (clear cell renal cell carcinoma [ccRCC]) | https://doi.org/10.1200/JCO.20.02363 | NCT02853344 |
| KEYNOTE-052 (urothelial carcinoma [UC]) | https://doi.org/10.1200/JCO.19.01213 | NCT02335424 |
| KEYNOTE-361 (UC) | https://doi.org/10.1016/S1470-2045(21)00152-2 | NCT02853305 |
|
| ||
| Software and algorithms | ||
|
| ||
| MATLAB 2021a | MathWorks, Inc | RRID:SCR_001622 |


