Abstract
Background
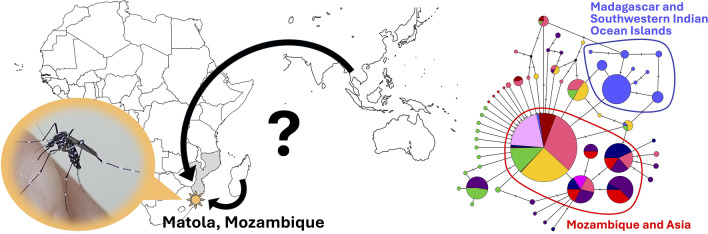
The Aedes albopictus mosquito is of medical concern due to its ability to transmit viral diseases, such as dengue and chikungunya. Aedes albopictus originated in Asia and is now present on all continents, with the exception of Antarctica. In Mozambique, Ae. albopictus was first reported in 2015 within the capital city of Maputo, and by 2019, it had become established in the surrounding area. It was suspected that the mosquito population originated in Madagascar or islands of the Western Indian Ocean (IWIO). The aim of this study was to determine its origin. Given the risk of spreading insecticide resistance, we also examined relevant mutations in the voltage-sensitive sodium channel (VSSC).
Methods
Eggs of Ae. albopictus were collected in Matola-Rio, a municipality adjacent to Maputo, and reared to adults in the laboratory. Cytochrome c oxidase subunit I (COI) sequences and microsatellite loci were analyzed to estimate origins. The presence of knockdown resistance (kdr) mutations within domain II and III of the VSSC were examined using Sanger sequencing.
Results
The COI network analysis denied the hypothesis that the Ae. albopictus population originated in Madagascar or IWIO; rather both the COI network and microsatellites analyses showed that the population was genetically similar to those in continental Southeast Asia and Hangzhou, China. Sanger sequencing determined the presence of the F1534C knockdown mutation, which is widely distributed among Asian populations, with a high allele frequency (46%).
Conclusions
These results do not support the hypothesis that the Mozambique Ae. albopictus population originated in Madagascar or IWIO. Instead, they suggest that the origin is continental Southeast Asia or a coastal town in China.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06375-6.
Keywords: Aedes albopictus, Mozambique, Invasion, COI, Microsatellites, kdr mutation
Background
Globalization is leading to the worldwide expansion of various cosmopolitan insects, including infectious disease vectors such as mosquitoes and ticks. Aedes albopictus is a cosmopolitan mosquito that originated in Asia and which transmits globally prevalent arboviruses such as dengue (DENV), chikungunya (CHIKV) and Zika (ZIKV).
In recent years, the introduction and expansion of Ae. albopictus populations in African countries has become a tremendous public health concern. This mosquito was first reported in the African continent in 1989, in Cape Town, South Africa [1], with immature stages of Ae. albopictus found in tires imported from Japan in 1989 and 1990 [1]. However, effective control prevented mosquito establishment in the country. In 1991, eggs of Ae. albopictus were collected by ovitraps in Delta State, Nigeria, which is the first record of a breeding population in the African continent [2]. Aedes albopictus was also reported from South Africa in 1992, but it failed to establish a population [3]. Since then, Ae. albopictus has established local populations in six West African countries [4–9] and was found to be a principal vector in CHIKV, DENV, and ZIKV outbreaks in Gabon, Cameroon and the Democratic Republic of the Congo (DRC) [10].
Before its introduction to the African continent, Ae. albopictus had established in the islands of the Western Indian Ocean (IWIO), being first reported in Mauritius in 1900, followed by Madagascar in 1904, Seychelles in 1912, La Réunion in 1913, Rodrigues in 1923, Mayotte in 2001 and Glorieuse in 2008 [11]. In IWIO, Chikungunya outbreaks caused by CHIKV occurred from 2005 to 2006, and then co-circulation of DENV was also reported [12–14]. Aedes albopictus has been implicated as the primary vector of these arboviruses in these outbreaks [13, 15–18], as this mosquito has high vector competence for the E1-226V strain of CHIKV, which has an amino acid mutation within the envelope protein [17–23]. However, the vector competence of Ae. albopictus varies among geographical populations and virus species [14, 19, 20, 24–26].
The introduction of a vector population may bring unwelcome traits, and thus identifying vector origins is essential to estimating the risk. Knockdown resistance (kdr) mutations have been reported within the Ae. albopictus population in West Africa, although their frequency is still low among the established populations [27–33]. kdr mutations of this species have been observed at three loci within the voltage-sensitive sodium channel (VSSC) domains II (V1016G in DII) and III (I1532T and F1534C/S/L in DIII). The mutation at codon 1534, which is associated with resistance to pyrethroid insecticides, has often been observed worldwide [29, 30, 32, 34–39], and the introduction of these resistant alleles is of concern for vector control in African countries [40]. If the introduced Ae. albopictus population originated in the temperate region, the overwintering ability of the temperate population may expand its distribution toward cooler areas [41].
Mitochondrial DNA (mtDNA) studies suggest that the West and Central African Ae. albopictus populations have multiple tropical and temperate origins [42–44]. A study using cytochrome c oxidase subunit I (COI) revealed that the Madagascar and the La Réunion populations are close to those of East Asia and North America [45]. In contrast, microsatellite studies have shown that the La Réunion population is similar to those of Southeast Asia and North America [45, 46]. The discrepancy between the two molecular studies of the La Réunion population is still the subject of debate [45].
In 2015, adults of Ae. albopictus were collected in Maputo, the capital of Mozambique [47]. A broader geographical survey in 2016 also found Ae. albopictus in Tete, located in the central region of Mozambique [48]. In 2018, large numbers of this mosquito species were collected using ovitraps at several sites within Matola-Rio, a municipality adjacent to Maputo. These findings confirmed that this species was established in the region, and represented its first recorded first establishment in the southern and eastern African regions.
The aim of the present study was to determine the origin of Ae. albopictus and to identify its insecticide resistance status to better understand the potential health risks associated with this established population. The study hypothesis was that Ae. albopictus originated from established populations in the nearby islands, Madagascar or IWIO. We also examined the occurrence of the kdr mutations in VSSC DII and DIII.
Methods
Mosquito collection
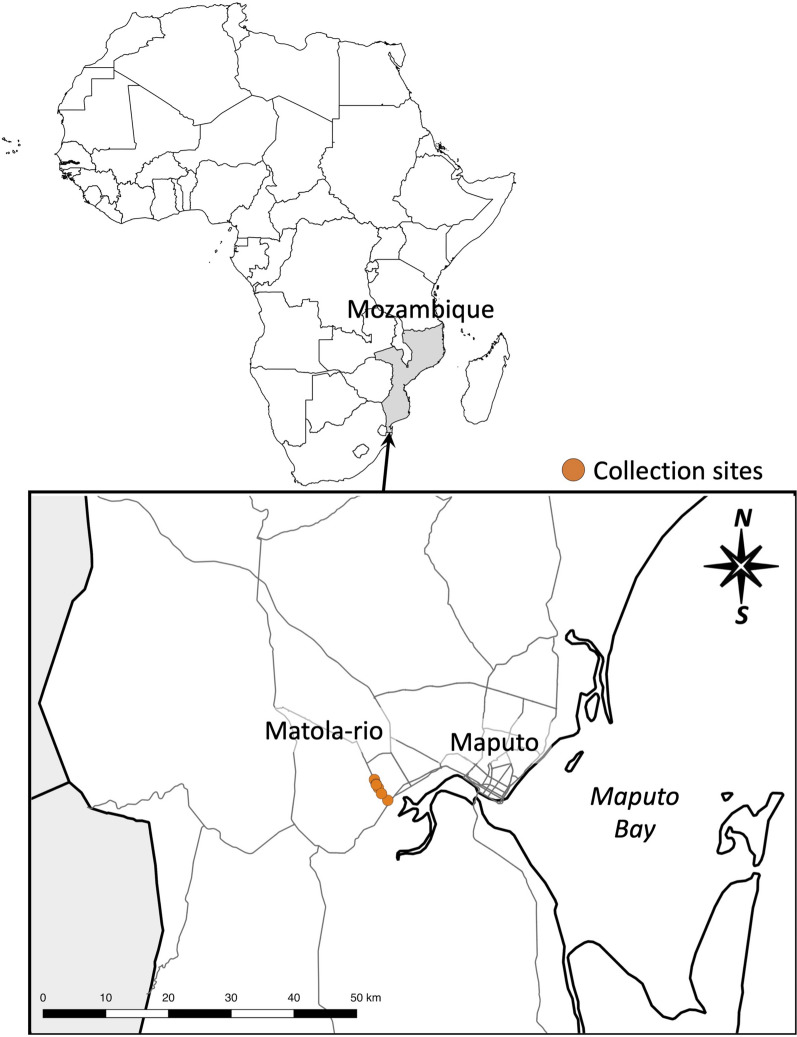
Mosquitoes were sampled in Matola-Rio in Boane district, Maputo province in southern Mozambique (Fig. 1). The area is classified as a savanna climate with the Köppen climate classification [49]. Four to ten ovitraps were placed at each of six sites (4 restaurants and 1 garage) along 4 km of the Mozal Road in February and March 2019. The collected eggs were dried and transferred to the Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan. All collected eggs were mixed and reared together to pupae in an environmental chamber under conditions of 25 °C, 70% relative humidity and a photoregimen of 16/8 h (light/dark). The pupae were divided into separate cages by sex. Emerging adults were morphologically examined, and only Ae. albopictus adults were used in subsequent genetic analyses.
Fig. 1.
Ovitrap sites for Aedes albopictus in Matola-Rio, Boane district in Maputo province in Mozambique
DNA extraction
DNA was extracted from a single leg of randomly selected mosquitoes (25 females, 25 males) using a REDExtract-N-Amp Tissue PCR Kit (Merck KGaA, Darmstadt, Germany) following the manufacturer’s protocol.
Determination of COI sequences and haplotype network
Partial COI sequences were amplified using two sets of primers (albo1454F and albo2160R; albo2027F and albo2886R) [50]. Each PCR analysis was performed in a 10 µl reaction volume containing 1.0 µl of template DNA, 3.6 µl distilled water, 5.0 µl REDExtract-N-AmpTMPCR Reaction Mix and 0.2 µl of each of the forward and reverse primers (mentioned in previous sentence). PCR thermocycling included an initial denaturing step at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, with a final additional extension at 72 °C for 6 min. The amplicons were used for cycle sequencing reaction with the BigDye Terminator v3.1 Kit (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced on an ABI3730 DNA analyzer (Thermo Fisher Scientific). The sequences were aligned with MEGA X software [51] and haplotypes were confirmed. The number of haplotypes, haplotype diversity, nucleotide diversity, Fu’s Fs and Tajima’s D were calculated using the DnaSP version 6.12.03 software package [52]. To estimate the genetic relationship of the global population, we obtained COI haplotypes in IWIO, Madagascar, Oceania, Asia, Americas, the Middle East, the African continent and Europe (Additional file 1: Table S1) [11, 50, 53–66] and constructed TCS haplotype network [67] using PopART-1.7 [68] with longer (1302 bp) and shorter (452 bp) lengths.
Genotyping of microsatellites loci and Bayesian clustering
Thirteen microsatellites loci that had been previously determined [69] were genotyped for the 50 DNA samples following Yang et al. [58]. For all loci, the allelic richness (A), expected heterozygosity (He), observed heterozygosity (Ho) and inbreeding coefficient (Gis) were calculated using GenoDive [70]. The obtained genotype data were analyzed together with previously reported data from Japan, the Philippines and Thailand [58]. The genetic differences between each pair of populations (Fst) were calculated using GenoDive. Bayesian clustering was done with STRUCTURE, and the analyzed results were visualized by CLAMPAC [71]. Based on a pre-run, the number of clusters (K) was set from 1 to 19. Following Yang et al. [58], each run consisted of 200,000 burn-in replications followed by 1,000,000 samplings. Collection locations were used as prior information, and an allele frequency correlated model was applied for 10 independent runs as replication. The best K was determined based on Evanno’s criteria for delta K [72]. The posterior probability of individual assignment to each cluster was rearranged by selecting the best K using DISTRUCT on the CLUMPACK server. Discriminant analysis of principal components (DAPC) was applied for the data using the package ‘adegenet’ of R to show similarity among the populations [73].
Detection of point mutations in the VSSC gene
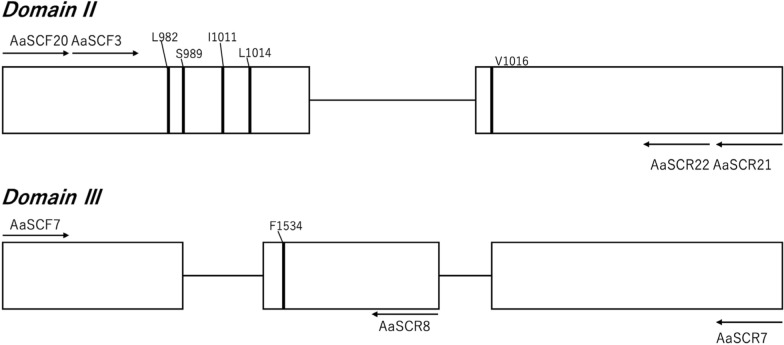
Six amino acid loci were targeted to identify kdr candidate mutations (DII: L982, S989, I1011, L1013, V1016; DIII: F1534). The amplification PCR for DII was performed in a total volume of 10 µl using 6.34 µl of double-distilled water, 0.06 µl of 10× Ex Taq HS (Takara bio, Shiga, Japan), 1.0 µl of 10× Ex Taq Buffer, 0.8 µl of 2.5 mM dNTP Mixture, 0.4 µl of each primer for DII (AaSCF20, 10 µM [5′-GTGGATCGCTTCCC-3′] and AaSCR21, 10 µM [5′-GCAATCTGGGCTTGTTAACTTG-3′] (Fig. 2) and 1.0 µl of DNA template. PCR amplification for DIII was performed using the primer set AaSCF7 (5′-GAGAACTCGCCGATGAACTT-3′) and AaSCR7 (5′-GACGACGAAATCGAACAGGT-3′) [29] (Fig. 2). The PCR amplification regimen consisted of 3 min at 94 ºC, followed by 35 cycles of 94 ºC for 15 s, 55 ºC for 30 s and 72 ºC for 30 s, with a final extension at 72 ºC for 10 min. The PCR products were cleaned using Exo-SAP-IT (Thermo Fisher Scientific) and then sequenced using the BigDye Terminator v3.1 Kit. The reaction mix contained 1.0 µl of the cleaned product, 0.34 µl of Big Dye terminator, 2.0 µl of 5× Sequencing Buffer, 5.66 µl of distilled water and 1.0 µl of one of the three primers (at 10 µM) (Fig. 2): AaSCF3 (5′-GTGGAACTTCACCGACTTCA-3′) and AaSCR22 (5′-TTCACGAACTTGAGCGCGTTG-3′) for DII and AaSCR8 (5′-TAGCTTTCAGCGGCTTCTTC-3′) for DIII. The reaction cycle followed the manufacturer’s protocol. The PCR products were purified by ethanol precipitation, dissolved in Hi-Di Formamide and sequenced on an ABI 3500 sequencer (Thermo Fisher Scientific). The determined sequences were aligned with MEGA X and searched for kdr mutations.
Fig. 2.
Diagram of the location of possible kdr mutations and primer position in domain II and domain III of the voltage-sensitive sodium channel gene. Rectangles indicate exons and solid lines indicate introns. Vertical lines drawn in rectangles indicate kdr mutations found in past studies. Arrows show the direction of the primer with the name written above the arrow. kdr Knockdown resistance
Results
COI sequences and haplotype network
The amplified mitochondrial COI gene spanned an aligned length of 1302 bp, within which seven variable sites were observed, five of which were parsimony-informative sites (GenBank accession numbers: LC726376–LC726425). Eight haplotypes were determined, all of which have been registered in GenBank. The haplotype diversity of the Matola-Rio population was 0.77, and the nucleotide diversity was < 0.01. The values of Tajima’s D and Fu’s Fs were not statistically significant (D = 0.11, P = 0.61, two-tailed test based on the beta distribution; Fs = − 1.14, P = 0.30, coalescent simulation), which indicated no selection pressure on the population.
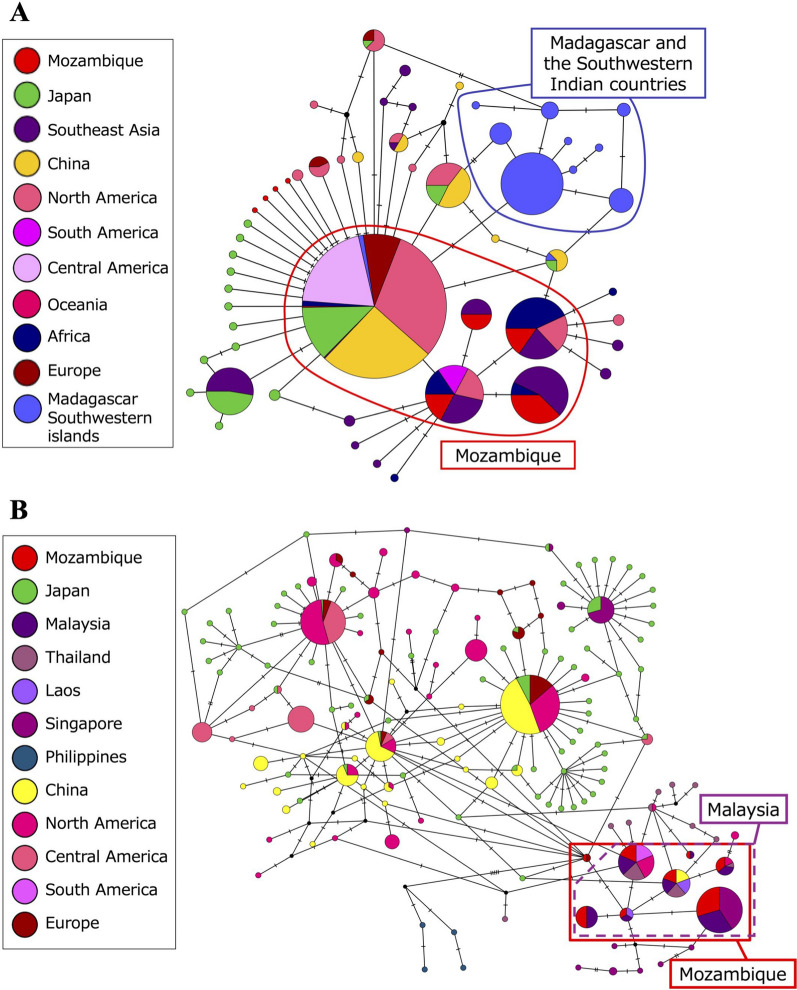
To compare to the Madagascar population, the sequences were shortened, and the eight haplotypes were consolidated into five. Four hyprotypes found in the Matola-Rio population did not match with those found in the Madagascar and IWIO populations (Fig. 3a). One haplotype was shared with those populations, but it was a cosmopolitan haplotype (Fig. 3a). Analysis of the longer sequences revealed that seven of the eight haplotypes matched with those discovered in the Malaysia population (Fig. 3b). Some of these were shared with populations identified in Singapore and Hangzhou, China (Fig. 3b).
Fig. 3.
Haplotype networks drawn with COI sequences of Aedes albopictus collected in Matola-Rio, Mozambique. A Network constructed with 425-bp sequences, including sequences from Western Indian Ocean Islands and Madagascar populations. B Network constructed with 1302-bp sequences. COI Cytochrome c oxidase subunit I
Microsatellites loci and Bayesian clustering
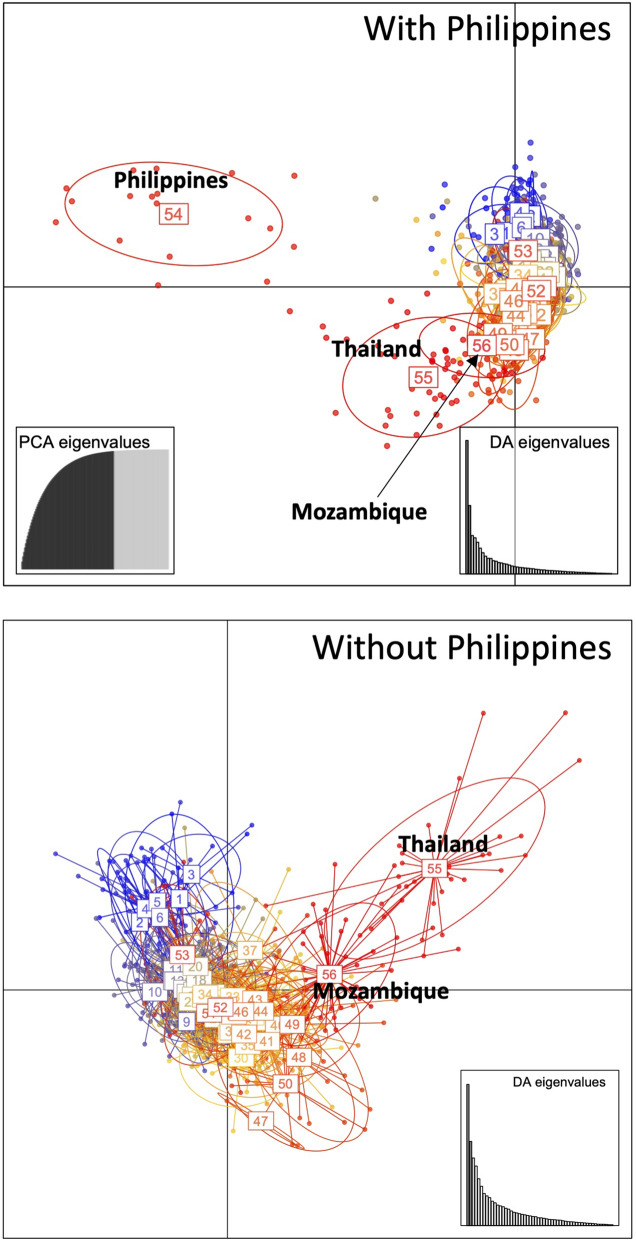
Microsatellite analyses of 13 loci revealed that the values of A, Ho, He and Gis were 5.62, 0.50, 0.65 and 0.24, respectively (Additional file 2: Table S2). Compared to the other populations [58], the Matola-Rio population showed relatively low Gis, suggesting that the inbreeding was not strong. Pairwise Fst between the Matola-Rio population and the other populations was between 0.10 and 0.62, and the Tsushima and Nagasaki populations were relatively similar (Additional file 3: Table S3; Additional file 4: Table S4). The best delta K was 2, but delta Ks of 3, 7 and 10 also showed marked peaks (Fig. 4). The results of clustering with those Ks showed that the Matola-Rio population was: (i) genetically different from the representative populations of temperate regions collected in Fukuoka, Japan (K = 2); (ii) more similar to the Ryukyu and Thailand populations than the Nagasaki, Goto and Tsushima populations; and (iii) closer to the Thailand population than the other populations (Fig. 4). Since the Philippines population was distinct from the other populations in DAPC (Additional file 5: Data S5; Additional file 6: R command S6), the population was removed, and the data reanalyzed (Fig. 5a). The biplot graph showed that the Matola-Rio population was placed between the Thailand and Japanese populations (Fig. 5b).
Fig. 4.
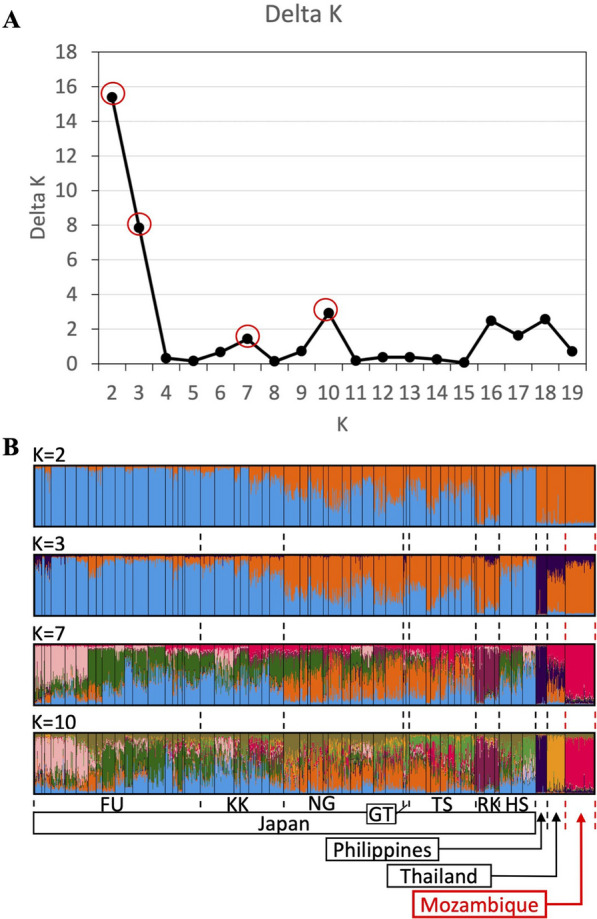
The delta Ks and posterior probability of belonging to an inferred cluster by Bayesian clustering with microsatellite genotypes of Aedes albopictus collected in Matola-Rio, Mozambique. A Delta Ks. B Posterior probability of individuals. The abbreviations FU, KK, NG, GT, TS, RK and HS are population names following Yang et al. [58]
Fig. 5.
Results of DAPC of microsatellites genotypes of Aedes albopictus collected in Matola-Rio, Mozambique. DAPC, Discriminant analysis of principal component
Detection of point mutations in the VSSC gene
A kdr mutation from phenylalanine to cysteine at 1534 in DIII was found in 29 individuals (Table 1). Of 44 samples successfully sequenced, 11 (25%) were found to be homozygous for F1534C. The gene frequency of 1534C was 46% (40/88). Mutations were not found in DII for all 50 individuals (Table 1).
Table 1.
The number and frequencies of genotypes within the voltage-gated sodium channel gene in Aedes albopictus collected in Mozambique
| Number and frequencies of genotypes | Loci of amino acids of VSSC | |||||||
|---|---|---|---|---|---|---|---|---|
| L982 | S989 | I1011 | L1014 | V1016 | F1534 | |||
| Genotypea | LL | SS | II | LL | VV | FF | FC | CC |
| No. of samples | 50 (50) | 50 (50) | 50 (50) | 50 (50) | 50 (50) | 15 (44) | 18 (44) | 11 (44) |
| Frequency | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.34 | 0.41 | 0.25 |
The numbers in parentheses indicate the total number of successfully sequenced samples
aThe capital letters are abbreviations of amino acids where L = leucine, S = serine, I = isoleucine, V = valine, F = phenylalanine, C = cysteine)
Discussion
The results from the present study do not support the hypothesis that the Matola-Rio Ae. albopictus population was introduced from the long-established population in Madagascar or IWIO [11]. In contrast, the COI analyses showed that the Matola-Rio population shared haplotypes with tropical Asian populations—specifically, Singapore, Malaysia, and China.
The results of the COI network analyses suggested that the Matola-Rio population is closer to the Hangzhou population in the temperate area (Fig. 3). On the other hand, the microsatellite analysis revealed that the Matola-Rio population is not related to the populations in Japan that are also located the temperate area. The microsatellite analyses also showed that the Matola-Rio population is closer to the Thailand population than is the Philippines population. The results from the microsatellite analyses are comparable to ones from the COI analysis; specifically, the Malaysian population shares more haplotypes with the Thailand population than the Philippines population and the Japanese population. These results suggest that the Matola-Rio population is closely related to continental Southeast Asia and a coastal city of China, Hangzhou.
These results from the genetic analyses imply that the Matola-Rio population was introduced by ships from continental Southeast Asia and coastal China. According to the trade statistics of Mozambique in 2019, provided by the Observatory of Economic Complexity (OEC) [74], the largest importing partner (13.3% of total import value) was China, followed by India (12.8%), the DRC (3.8%), Singapore (3.8%), Malaysia (1.6%) and Japan (1.6%) (https://oec.world/en/profile/country/moz?yearlyTradeFlowSelector=flow1&yearSelector1=2019 (accessed 21 Sept 2023). Both Maputo city and Matola-Rio host the country’s largest harbor. These two pieces of information support the introduction of the Matola-Rio Ae. albopictus population from Hangzhou, Singapore and Malaysia. Notably, three haplotypes of the short sequences from the Mozambican population were shared with those from populations in the African continent such as Cameroon and DRC. This also suggests the possibility of introduction from those countries (3.8% of import value from DRC) or the same source of introduction; however, analysis with longer sequences is required.
Sequencing of the VSSC gene revealed the presence of the F1534C kdr mutation at DIII, with an allele frequency of 46% in the Matola-Rio population. The mutation frequency was 2% in 2020 when first reported in Africa, from Cameroon [32]. Since the F1534C kdr mutation was not detected in the study in Cameroon in 2017 [75], this mutation might have developed independently during the 3 years following its introduction as the number of individuals with the mutation needed to be more significant for it to be detected in the earlier study. Similarly, in the present study we analyzed the samples collected 3 years after the first report of Ae. albopictus in Mozambique in 2016 [47]. The earlier survey in 2014 could not find this mosquito in Matola-Rio [76]. A control program using insecticides against Aedes mosquitoes has never been implemented in Mozambique. As Ae. albopictus is a day-biter and exophilic, indoor residual spraying and long-lasting insecticidal nets (LLINs), which are mainly used against anophelines, are less likely affect this mosquito [77, 78] On the other hand, the F1534C mutation has been reported in China and Southeast Asia, where insecticides are intensively used against Aedes mosquitoes [29, 30, 34]. This information suggests that the mutation was already present in the mosquito population introduced from abroad.
DNA samples of Ae. albopictus from IWIO were not available for the present study, which limits the interpretation of the results from the microsatellite analyses. In addition, our ovitrap collection was operated only once in Matola-Rio, which would underestimate the true haplotype diversity of the present Ae. albopictus population in Mozambique.
Conclusions
Despite the limitations, we have proven that the Matola-Rio Ae. albopictus population did not originate from Madagascar or IWIO. Rather, the results from the genetic analyses strongly support the notion that the population was introduced from continental Southeast Asia and a coastal city in China, Hangzhou. The findings of the present study confirmed that the Matola-Rio population had developed the F1534C kdr mutation at DIII. The high kdr mutation frequency also supports the notion that this population was introduced from abroad.
Since Ae. albopictus can adapt to various environments [79], the geographical spread of introduced Ae. albopictus may alter the composition of local vector species and the environment of viral disease transmission [80, 81]. In IWIO, Ae. albopictus has become predominant in domestic and peridomestic areas where Aedes aegypti was once predominant [15, 16, 18, 82]. Although Ae. albopictus is thought to play a role in DENV transmission with less competence than Ae. aegypti [83], it may play an important role in CHIKV outbreaks and co-infection with DENV in the introduced countries [17, 22, 84, 85]. To prevent further expansion, surveillance and integrated vector control programs should be implemented in the mosquito infested and adjacent areas [86].
Supplementary Information
Additional file 1: Table S1. List of the reference sequences included in the network analyses of COI of Aedes albopictus.
Additional file 2: Table S2. Indices of genetic diversity for each microsatellite locus of Aedes albopictus in Mozambique. A: No. of alleles, Ho: Observed heterozygosity, He: Expected heterozygosity, Gis: Inbreeding coefficient.
Additional file 3: Table S3. Microsatellite scores of each locus of Aedes albopictus collected in Mozambique.
Additional file 4: Table S4. Population pairwise differences (Fst) with the Mozambique population (MOZ). The lower diagonal indicates Fst and the upper diagonal indicates statistical significance.
Additional file 5: Data S5. Microsatellite data formats for the analyses used in this study.
Additional file 6: R command S6. R command used for discriminant analysis of principal components (DAPC).
Acknowledgements
The authors thank the vector research unit in Maputo province for their collection of mosquitoes by ovitraps. We also extend our appreciation to the staff of the Department of Vector Ecology & Environment in NEKKEN—in particular to Toshihiko Sunahara and Hitoshi Kawada for their valuable suggestions, Chiaki Tsurukawa and Naomi Sano for their support in mosquito rearing and Junko Sakemoto for her administrative support during our research travel.
Abbreviations
- CHIKV
Chikungunya virus
- COI
Cytochrome c oxidase subunit I
- DII
Domain II
- DIII
Domain III
- DAPC
Discriminant analysis of principal component
- DENV
Dengu virus
- IWIO
Islands in the Western Indian Ocean
- kdr
Knockdown resistance
- mtDNA
Mitochondria DNA
- RH
Relative humidity
- VSSC
Voltage-sensitive sodium channel
- ZIKV
Zika virus
Author contributions
SY: Laboratory work of COI analyses, statistical analyses, writing draft of manuscript. KU: Laboratory work of kdr detection, writing draft of manuscript. CY: Microsatellite data collection and analysis. YH: Microsatellite data collection. NM: Research management, mosquito collection, writing manuscript. NC: Research management, mosquito collection, writing manuscript. KF: planning the research, mosquito collection, rearing mosquitoes, laboratory work of microsatellites analyses, statistical analyses and writing manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by AMED under Grant Number JP15fm0108001.
Availability of data and materials
DNA sequences COI have been submitted to GenBank (LC726376 - LC726425). Microsatellites genotyping data is available in the Additional file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornel AJ, Hunt RH. Aedes albopictus in Africa? first records of live specimens in imported tires in Cape Town. J Am Mosq Control Assoc. 1991;7:107–108. [PubMed] [Google Scholar]
- 2.Savage HM, Ezike VI, Nwankwo AC, Spiegel R, Miller BR. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J Am Mosq Control Assoc. 1992;8:101–103. [PubMed] [Google Scholar]
- 3.Jupp PG, Kemp A. Aedes albopictus and other mosquitoes imported in tires into Durban, South Africa. J Am Mosq Control Assoc. 1992;8:321–322. [PubMed] [Google Scholar]
- 4.Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7:1066–1067. doi: 10.3201/eid0706.010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toto JC, Abaga S, Carnevale P, Simard F. First report of the oriental mosquito Aedes albopictus on the West African island of Bioko. Equatorial Guinea Med Vet Entomol. 2003;17:343–346. doi: 10.1046/j.1365-2915.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- 6.Coffinet T, Mourou JR, Pradines B, Toto JC, Jarjaval F, Amalvict R, et al. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007;23:471–472. doi: 10.2987/5636.1. [DOI] [PubMed] [Google Scholar]
- 7.Diallo M, Laganier R, Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health. 2010;15:1185–1189. doi: 10.1111/j.1365-3156.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 8.Mombouli J-V, Bitsindou P, Elion DOA, Grolla A, Feldmann H, Niama FR, et al. Chikungunya virus infection, Brazzaville, Republic of Congo, 2011. Emerg Infect Dis. 2013;19:1542–1543. doi: 10.3201/eid1909.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobanga T, Moyo M, Vulu F, Irish SR. First Report of Aedes albopictus (Diptera: Culicidae) in the Democratic Republic of Congo. Afr Entomol. 2018;26:234–236. doi: 10.4001/003.026.0234. [DOI] [Google Scholar]
- 10.Ngoagouni C, Kamgang B, Nakouné E, Paupy C, Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors. 2015;8:191. doi: 10.1186/s13071-015-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delatte H, Bagny L, Brengue C, Bouetard A, Paupy C, Fontenille D. The invaders: phylogeography of dengue and chikungunya viruses Aedes vectors, on the South West islands of the Indian Ocean. Infect Genet Evol. 2011;11:1769–1781. doi: 10.1016/j.meegid.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 12.WHO Outbreak news: Chikungunya and dengue, south-west Indian Ocean. Wkly Epidemiol Rec. 2006;81:105–116. [PubMed] [Google Scholar]
- 13.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, et al. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–1137. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazeille M, Dehecq JS, Failloux AB. Vectorial status of the Asian tiger mosquito Aedes albopictus of La Réunion Island for Zika virus. Med Vet Entomol. 2018;32:251–254. doi: 10.1111/mve.12284. [DOI] [PubMed] [Google Scholar]
- 15.Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector Borne Zoonotic Dis. 2008;8:25–34. doi: 10.1089/vbz.2007.0649. [DOI] [PubMed] [Google Scholar]
- 16.Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol. 2009;46:198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- 17.Vazeille M, Mousson L, Martin E, Failloux AB. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis. 2010;4:1–5. doi: 10.1371/journal.pntd.0000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raharimalala FN, Ravaomanarivo LH, Ravelonandro P, Rafarasoa LS, Zouache K, Tran-Van V, et al. Biogeography of the two major arbovirus mosquito vectors, Aedes aegypti and Aedes albopictus (Diptera, Culicidae), in Madagascar. Parasit Vectors. 2012;5:56. doi: 10.1186/1756-3305-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gubler DJ, Rosen L. Variation amond geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am J Trop Med Hyg. 1976;25:318–325. doi: 10.4269/ajtmh.1976.25.318. [DOI] [PubMed] [Google Scholar]
- 20.Tesh RB, Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with Chikungunya virus. Am J Trop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- 21.Vazeille M, Mousson L, Rakatoarivony I, Villeret R, Rodhain F, Duchemin JB, et al. Population genetic structure and competence as a vector for dengue type 2 virus of Aedes aegypti and Aedes albopictus from madagascar. Am J Trop Med Hyg. 2001;65:491–497. doi: 10.4269/ajtmh.2001.65.491. [DOI] [PubMed] [Google Scholar]
- 22.de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:1–6. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lourenço de Oliveira R, Vazeille M, de Filippis AMB, Failloux AB. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil the United States and the Cayman Islands. Am J Trop Med Hyg. 2003;69:105–114. doi: 10.4269/ajtmh.2003.69.105. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, Moi ML, Saito K, Isawa H, Takasaki T, Sawabe K. Aedes albopictus strain and dengue virus serotype in the dengue fever outbreaks in Japan: implications of Wolbachia infection. Jpn J Infect Dis. 2022;75:140–143. doi: 10.7883/yoken.JJID.2021.376. [DOI] [PubMed] [Google Scholar]
- 27.Kawada H, Maekawa Y, Abe M, Ohashi K, Ohba SY, Takagi M. Spatial distribution and pyrethroid susceptibility of mosquito larvae collected from catch basins in parks in Nagasaki City, Nagasaki Japan. Jpn J Infect Dis. 2010;63:19–24. doi: 10.7883/yoken.63.19. [DOI] [PubMed] [Google Scholar]
- 28.Pujiyati E, Kawada H, Sunahara T, Kasai S, Minakawa N. pyrethroid resistance status of Aedes Albopictus (Skuse) collected in Nagasaki City, Japan Japanese. J Environ Entomol Zoolo. 2013;24:143–153. [Google Scholar]
- 29.Kasai S, Ng LC, Lam-Phua SG, Tang CS, Itokawa K, Komagata O, et al. First detection of a putative knockdown resistance gene in major mosquito vector Aedes albopictus. Jpn J Infect Dis. 2011;64:217–221. doi: 10.7883/yoken.64.217. [DOI] [PubMed] [Google Scholar]
- 30.Kasai S, Caputo B, Tsunoda T, Cuong TC, Maekaw Y, Lam-Phua SG, et al. First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: A new emerging threat to controlling arboviral diseases. Eurosurveillance. 2019;24:1–12. doi: 10.2807/1560-7917.ES.2019.24.5.1700847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Liu L, Cheng P, Yang L, Chen J, Lu Y, et al. Bionomics and insecticide resistance of Aedes albopictus in Shandong, a high latitude and high-risk dengue transmission area in China. Parasit Vectors. 2020;13:1–9. doi: 10.1186/s13071-020-3880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djiappi-Tchamen B, Nana-Ndjangwo MS, Mavridis K, Talipouo A, Nchoutpouen E, Makoudjou I, et al. Analyses of insecticide resistance genes in Aedes aegypti and Aedes albopictus mosquito populations from Cameroon. Genes (Basel) 2021;12:1–13. doi: 10.3390/genes12060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vulu F, Ilombe G, Vizcaino L, Mariën J, Morimoto Y. Insecticide susceptibility of Aedes (Stegomyia) aegypti (Linnaeus, 1762) and Aedes (Stegomyia) albopictus (Skuse, 1894) in Kinshasa, Democratic Republic of the Congo. Biorxiv. 2021;1–23:2021–2111. [Google Scholar]
- 34.Chen H, Li K, Wang X, Yang X, Lin Y, Cai F, et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island China. Infect Dis Poverty. 2016;5:1–8. doi: 10.1186/s40249-016-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Xu J, Zhong D, Zhang H, Yang W, Zhou G, et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasit Vectors. 2018;11:1–10. doi: 10.1186/s13071-017-2581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Bonizzoni M, Zhong D, Zhou G, Cai S, Li Y, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus. PLoS Negl Trop Dis. 2016;10:1–12. doi: 10.1371/journal.pntd.0004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarroll L, Hemingway J. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem Mol Biol. 2002;32:1345–1351. doi: 10.1016/S0965-1748(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre-Obando OA, Martins AJ, Navarro-Silva MA. First report of the Phe1534Cys kdr mutation in natural populations of Aedes albopictus from Brazil. Parasit Vectors. 2017;10:1–10. doi: 10.1186/s13071-017-2089-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Balaska S, Fotakis EA, Kioulos I, Grigoraki L, Mpellou S, Chaskopoulou A, et al. Bioassay and molecular monitoring of insecticide resistance status in Aedes albopictus populations from Greece, to support evidence-based vector control. Parasit Vectors. 2020;13:1–13. doi: 10.1186/s13071-020-04204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15:1–20. doi: 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori A, Oda T, Wada Y. Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Trop Med. 1981;23:79–90. [Google Scholar]
- 42.Kamgang B, Brengues C, Fontenille D, Njiokou F, Simard F, Paupy C. Genetic structure of the tiger mosquito, Aedes albopictus, in Cameroon (Central Africa) PLoS ONE. 2011;6:e20257. doi: 10.1371/journal.pone.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamgang B, Ngoagouni C, Manirakiza A, Nakouné E, Paupy C, Kazanji M. Temporal Patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and Mitochondrial DNA analysis of Ae albopictus in the Central African Republic. PLoS Negl Trop Dis. 2013 doi: 10.1371/journal.pntd.0002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamgang B, Wilson-Bahun TA, Irving H, Kusimo MO, Lenga A, Wondji CS. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018;3:79. doi: 10.12688/wellcomeopenres.14659.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maynard AJ, Ambrose L, Cooper RD, Chow WK, Davis JB, Muzari MO, et al. Tiger on the prowl: invasion history and spatio-temporal genetic structure of the Asian tiger mosquito Aedes albopictus (Skuse 1894) in the Indo-Pacific. PLoS Negl Trop Dis. 2017;11:1–27. doi: 10.1371/journal.pntd.0005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manni M, Guglielmino CR, Scolari F, Vega-Rúa A, Failloux AB, Somboon P, et al. Genetic evidence for a worldwide chaotic dispersion pattern of the arbovirus vector Aedes albopictus. PLoS Negl Trop Dis. 2017 doi: 10.1371/journal.pntd.0005332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kampango A, Abílio AP. The Asian tiger hunts in Maputo city—the first confirmed report of Aedes (Stegomyia) albopictus (Skuse, 1895) in Mozambique. Parasit Vectors. 2016;9:4–7. doi: 10.1186/s13071-016-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abílio AP, Abudasse G, Kampango A, Candrinho B, Sitoi S, Luciano J, et al. Distribution and breeding sites of Aedes aegypti and Aedes albopictus in 32 urban/peri-urban districts of Mozambique: implication for assessing the risk of arbovirus outbreaks. PLoS Negl Trop Dis. 2018;12:1–15. doi: 10.1371/journal.pntd.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z. 2006;15:259–263. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- 50.Zhong D, Lo E, Hu R, Metzger ME, Cummings R, Bonizzoni M, et al. Genetic analysis of invasive Aedes albopictus populations in Los Angeles county, California and its potential public health impact. PLoS ONE. 2013;8:e68586. doi: 10.1371/journal.pone.0068586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 53.Giordano BV, Gasparotto A, Liang P, Nelder MP, Russell C, Hunter FF. Discovery of an Aedes (Stegomyia) albopictus population and first records of Aedes (Stegomyia) aegypti in Canada. Med Vet Entomol. 2020;34:10–16. doi: 10.1111/mve.12408. [DOI] [PubMed] [Google Scholar]
- 54.Hu Y, Xi Z, Liu X, Wang J, Guo Y, Ren D, et al. Identification and molecular characterization of Wolbachia strains in natural populations of Aedes albopictus in China. Parasit Vectors. 2020;13:1–14. doi: 10.1186/s13071-020-3899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Artigas P, Reguera-Gomez M, Valero MA, Osca D, da Silva PR, Rosa-Freitas MG, et al. Aedes albopictus diversity and relationships in south-western Europe and Brazil by rDNA/mtDNA and phenotypic analyses: ITS-2, a useful marker for spread studies. Parasit Vectors. 2021;14:1–23. doi: 10.1186/s13071-021-04829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Futami K, Valderrama A, Baldi M, Minakawa N, Rodríguez RM, Chaves LF. New and common haplotypes shape genetic diversity in Asian tiger mosquito populations from costa Rica and Panamá. J Econ Entomol. 2015;108:761–768. doi: 10.1093/jee/tou028. [DOI] [PubMed] [Google Scholar]
- 57.Adilah-Amrannudin N, Hamsidi M, Ismail NA, Dom NC, Ismail R, Ahmad AH, et al. Aedes albopictus in urban and forested areas of malaysia: A study of mitochondrial sequence variation using the CO1 marker. Trop Biomed. 2018;35:639–652. [PubMed] [Google Scholar]
- 58.Yang C, Sunahara T, Hu J, Futami K, Kawada H, Minakawa N. Searching for a sign of exotic Aedes albopictus (Culicidae) introduction in major international seaports on Kyushu Island Japan. PLoS Negl Trop Dis. 2021;15:1–23. doi: 10.1371/journal.pntd.0009827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motoki MT, Fonseca DM, Miot EF, Demari-Silva B, Thammavong P, Chonephetsarath S, et al. Population genetics of Aedes albopictus (Diptera: Culicidae) in its native range in Lao People’s Democratic Republic. Parasit Vectors. 2019;12:1–13. doi: 10.1186/s13071-019-3740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battaglia V, Gabrieli P, Brandini S, Capodiferro MR, Javier PA, Chen XG, et al. The worldwide spread of the tiger mosquito as revealed by mitogenome haplogroup diversity. Front Genet. 2016;7:1–11. doi: 10.3389/fgene.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook S, Giang Lien N, Mcalister E, Harbach RE. Bothaella manhi, a new species of tribe Aedini (Diptera: Culicidae) from the Cuc Phuong National Park of Vietnam based on morphology and DNA sequence. Zootaxa. 2012;2661:33–46. [PMC free article] [PubMed] [Google Scholar]
- 62.Haddad N, Mousson L, Vazeille M, Chamat S, Tayeh J, Osta MA, et al. Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC Infect Dis. 2012 doi: 10.1186/1471-2334-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennouna A, Balenghien T, El Rhaffouli H, Schaffner F, Garros C, Gardès L, et al. First record of Stegomyia albopicta (= Aedes albopictus) in Morocco: a major threat to public health in North Africa? Med Vet Entomol. 2017;31:102–106. doi: 10.1111/mve.12194. [DOI] [PubMed] [Google Scholar]
- 64.Minard G, Van Tran V, Tran FH, Melaun C, Klimpel S, Koch LK, et al. Identification of sympatric cryptic species of Aedes albopictus subgroup in Vietnam: new perspectives in phylosymbiosis of insect vector. Parasit Vectors. 2017;10:1–14. doi: 10.1186/s13071-017-2202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2018 doi: 10.1371/journal.pntd.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watsenga Tezzo F, Fasine S, Manzambi Zola E, del Marquetti MC, Binene Mbuka G, Ilombe G, et al. High Aedes spp. larval indices in Kinshasa democratic republic of Congo. Parasit Vectors. 2021 doi: 10.1186/s13071-021-04588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clement M, Snell Q, Walke P, Posada D, Crandall K. TCS: Estimating gene genealogies. Proc Int Parallel Dist Proc Symp. 2002;2002:184. [Google Scholar]
- 68.Leigh JW, Bryant D. POPART: full-feature software for haplotype network construction. Methods Ecol Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 69.Beebe NW, Ambrose L, Hill LA, Davis JB, Hapgood G, Cooper RD, et al. Tracing the tiger: population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian region. PLoS Negl Trop Dis. 2013 doi: 10.1371/journal.pntd.0002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meirmans PG. genodive version 3.0: easy-to-use software for the analysis of genetic data of diploids and polyploids. Mol Ecol Resour. 2020;20:1126–1131. doi: 10.1111/1755-0998.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I. CLUMPAK : a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour. 2015;15:1179–1191. doi: 10.1111/1755-0998.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 73.Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 74.Simoes AJG, Hidalgo CA. The economic complexity observatory: an analytical tool for understanding the dynamics of economic development. Workshops at the 25th AAAI Conference on Artificial Intelligence; August 7–11, 2011, San Francisco. AAAI Press: Menlo Park.
- 75.Yougang AP, Kamgang B, Tedjou AN, Wilson-Bahun TA, Njiokou F, Wondji CS. Nationwide profiling of insecticide resistance in Aedes albopictus (Diptera: Culicidae) in cameroon. PLoS ONE. 2020;15:1–14. doi: 10.1371/journal.pone.0234572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higa Y, Abílio AP, Futami K, Lázaro MAF, Minakawa N, Gudo ES. Abundant Aedes (Stegomyia) aegypti mosquitoes in the 2014 dengue outbreak area of Mozambique. Trop Med Health. 2015;43:107–109. doi: 10.2149/tmh.2014-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glunt KD, Abílio AP, Bassat Q, Bulo H, Gilbert AE, Huijben S, et al. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar J. 2015;14:1–7. doi: 10.1186/s12936-015-0807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riveron JM, Huijben S, Tchapga W, Tchouakui M, Wondji MJ, Tchoupo M, et al. Escalation of Pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of piperonyl butoxide-based insecticide-treated nets in Mozambique. J Infect Dis. 2019;220:467–475. doi: 10.1093/infdis/jiz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 80.Lounibos LP, Bargielowski I, Carrasquilla MC, Nishimura N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in peninsular Florida two decades after competitive displacements. J Med Entomol. 2016 doi: 10.1093/jme/tjw122. [DOI] [PubMed] [Google Scholar]
- 81.Vulu F, Futami K, Sunahara T, Mampuya P, Bobanga TL, Mumba Ngoyi D, et al. Geographic expansion of the introduced Aedes albopictus and other native Aedes species in the democratic republic of the Congo. Parasit Vectors. 2024 doi: 10.1186/s13071-024-06137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosquito Cont Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- 83.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010 doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BBB, et al. Recent introduction and rapid dissemination of chikungunya virus and dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central africa. Clin Infect Dis. 2012;55:45–53. doi: 10.1093/cid/cis530. [DOI] [PubMed] [Google Scholar]
- 85.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in central Africa. Vector-Borne and Zoonotic Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 86.Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. List of the reference sequences included in the network analyses of COI of Aedes albopictus.
Additional file 2: Table S2. Indices of genetic diversity for each microsatellite locus of Aedes albopictus in Mozambique. A: No. of alleles, Ho: Observed heterozygosity, He: Expected heterozygosity, Gis: Inbreeding coefficient.
Additional file 3: Table S3. Microsatellite scores of each locus of Aedes albopictus collected in Mozambique.
Additional file 4: Table S4. Population pairwise differences (Fst) with the Mozambique population (MOZ). The lower diagonal indicates Fst and the upper diagonal indicates statistical significance.
Additional file 5: Data S5. Microsatellite data formats for the analyses used in this study.
Additional file 6: R command S6. R command used for discriminant analysis of principal components (DAPC).
Data Availability Statement
DNA sequences COI have been submitted to GenBank (LC726376 - LC726425). Microsatellites genotyping data is available in the Additional file.