Abstract
We previously demonstrated that a single injection of 109 PFU of recombinant adenovirus into patients induces strong vector-specific immune responses (H. Gahéry-Ségard, V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J.-G. Guillet, and F. Farace, J. Clin. Investig. 100:2218–2226, 1997). In the present study we analyzed the mechanism of vector recognition by cytotoxic T lymphocytes (CTL). CD8+ CTL lines were derived from two patients and maintained in long-term cultures. Target cell infections with E1-deleted and E1-plus E2-deleted adenoviruses, as well as transcription-blocking experiments with actinomycin D, revealed that host T-cell recognition did not require viral gene transcription. Target cells treated with brefeldin A were not lysed, indicating that viral input protein-derived peptides are associated with HLA class I molecules. Using recombinant capsid component-loaded targets, we observed that the three major proteins could be recognized. These results raise the question of the use of multideleted adenoviruses for gene therapy in the quest to diminish antivector CTL responses.
Replication-deficient recombinant adenoviruses are being studied widely as therapeutic gene transfer vectors (4, 11, 20, 21, 24, 25, 27, 34). Despite unique advantages, the major limitation of these vectors is the host antiviral immune response, which causes transitory transgene expression in vivo as well as inefficient gene transfer when rechallenged (17, 28–32). In humans, natural infection induces neutralizing antibodies, which were recently correlated with the response elicited by vector administration (12) and may directly impair viral attachment and penetration (8). Cellular cytotoxic responses to the virus and to the transgenic protein were found to be responsible for the destruction of transduced cells and thus for a dramatic reduction in viral genome persistence (17, 26, 28, 30). The first studies with E1-deleted vectors in rodents suggested that de novo-synthesized antigens played a major role in inducing antiviral cytotoxic T lymphocytes (CTL) (31). Indeed, despite the deletion of the E1 region, first-generation vectors continued to express low levels of early and late viral genes. Additional deletions that inactivated E2A or E4 regulatory genes were therefore introduced to reduce such expression. In vivo testing of second-generation vectors showed no diminution in the antiviral immune responses and no improvement of viral genome persistence (15). However, in the absence of a transgene, antiadenovirus CTLs did not appear to be effective in eliminating the transduced cells (15). Transgene expression induced by E1-plus E4-deleted vectors in immunodeficient or recombinant protein transgenic models was also transient, suggesting a critical role for E4 products in the regulation of transgene transcription (1, 3, 6), which was recently confirmed (16). Overall, studies have revealed that the immune mechanism elicited by these vectors is extremely complex. It is noteworthy that its impact on the efficacy of gene transfer in humans remains poorly understood. Its elucidation is nonetheless essential to further improve gene therapy protocols.
We previously reported the analysis of humoral and cellular immune responses to the vector and the β-galactosidase protein in patients with lung cancer who received a single intratumor injection of an E1-deleted recombinant adenovirus encoding the lacZ gene (9, 27). We observed that a strong in vivo vector-specific cytotoxic response was induced by a 109 PFU injection. In the present study, we evaluated the respective contributions of de novo-synthesized antigens and viral input antigens in the CTL response and identified the target proteins recognized in two patients. The results revealed the mechanisms of adenovirus recognition by CTL and have important implications for the use of additionally deleted vectors aimed at diminishing anti-vector-specific responses in gene therapy protocols.
Antiadenovirus CD8+ CTL lines were derived from postinjection peripheral blood mononuclear cells obtained from two patients and expanded in long-term cultures. After 5 to 7 days of stimulation with adenovirus (9), the cells were tested for antiadenovirus cytotoxic activity in a 4-h standard chromium release assay (data not shown). Non-CD4+ and non-CD56+ cells were then negatively selected by immunorosetting, and the CD8+ TcR αβ+ phenotype was verified by flow cytometry (data not shown). The CTLs were then expanded on adenovirus-infected irradiated feeder cells composed of autologous Epstein-Barr virus-immortalized lymphoblastoid cell lines (LCL) (obtained as described in reference 9) and maintained for 2 to 3 weeks. All the CTL lines tested in subsequent experiments displayed 76 to 506 lytic units at 20% lysis of 5 × 103 adenovirus-infected targets per 107 effectors.
The autologous LCL was used in cytotoxicity assays. Since this cell line is poorly permissive to adenovirus, we first determined the viral dose and the infection duration required for optimal sensitization by first-generation vectors. A 24- or 18-h infection was sufficient to sensitize LCL targets, but optimal recognition was obtained only after a 40-h infection (data not shown). The minimal infectious dose was about 600 to 1,000 particles per cell (10 to 13 PFU/cell) with Ad-CFTR (first-generation adenovirus carrying the CFTR gene) and AdE1° (first-generation adenovirus without a transgene), showing that the infectivities of two first-generation adenovirus vectors were similar in a semipermissive cell line (data not shown). Particle number were determined following lysis of the viral suspension by measuring optical density (as described in reference 16). All the experiments reported in this paper were calibrated in particles per cell, since this is a more accurate reflection of the amount of exogenous viral protein provided by the infection.
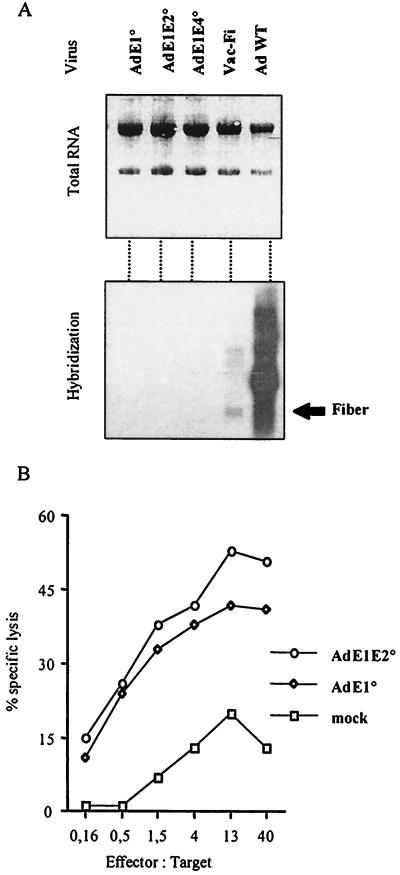
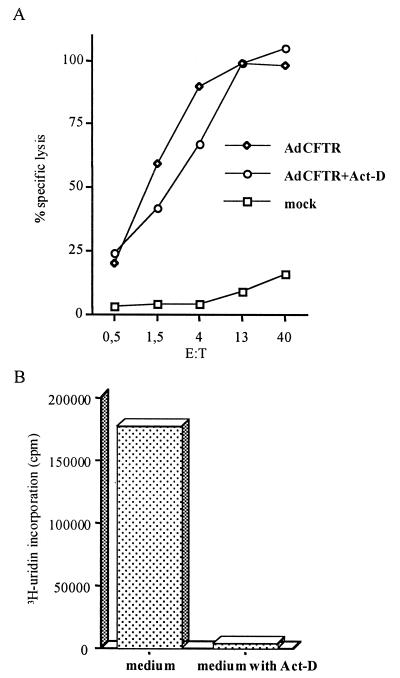
Viral protein expression is markedly reduced or even undetectable in cells infected with second-generation adenovirus vectors (with E1 plus E2 deleted or with E1 plus E4 deleted) compared to that in cells infected with first-generation vectors (E1 deleted) (15). In our study, target cells were infected at a multiplicity of infection (MOI) of 20,000 particles/cell with two isogenic vectors without a transgene (AdE1° and AdE1E2°) and differing only in the E2A deletion. The transcription of early (DNA binding protein) and late (structural proteins) viral antigens was assessed by Northern blot analysis. Despite the high MOI, no viral RNA was detected in either first- or second-generation-vector-infected LCL. Northern blot results obtained by using a probe for the fiber are shown in Fig. 1A. Nevertheless, adenovirus-specific CTLs recognized AdE1°E2°- as well as AdE1°-infected target cells (Fig. 1B), supporting the hypothesis that input viral proteins are recognized by CTLs. This experiment, as well as others described in this report, was successfully repeated at least twice with CTLs from the two patients. However, surprised that Northern blot analysis yielded similar results for first- and second-generation adenoviruses, we decided to further test our findings by using actinomycin D (Act-D), a fungal metabolite, to inhibit RNA transcription. Target cells were treated with Act-D for 30 min before being infected and throughout the chromium release assay, to prevent de novo viral protein synthesis. The transcription block was controlled by [3H]uridine incorporation. Target cells were infected with 12,000 particles of Ad-CFTR per cell for 24 h and were either treated or not treated with Act-D (5 μg/ml before infection, 0.2 μg/ml during and after infection) in parallel with uridine incorporation. As shown in Fig. 2, CTLs lysed Act-D-treated targets as efficiently as they lysed untreated targets whereas uridine incorporation was completely abrogated in Act-D-treated targets. This result confirmed that de novo viral gene transcription is not required for CTL recognition of infected cells. This finding is consistent with a recent report on mice which showed that inactivated adenovirus-infected cells are efficiently recognized by CTLs (14).
FIG. 1.
(A) Northern blot analysis of LCL infected with first- and second-generation vectors. LCL were infected with 20,000 particles of AdE1° (E1 deleted), AdE1E2° (E1 and E2 deleted), or AdE1E4° (E1 and E4 deleted) per cell and 10 PFU of Vac-Fi (recombinant vaccinia virus encoding the adenovirus type 5 fiber) per cell as previously described (9). 293 cells infected with wild-type adenovirus were used as positive controls. All these viruses were produced by Transgene SA. Adenovirus vector construction was described in reference 15. Total RNA was extracted 60 h postinfection for adenovirus vectors and 24 h for Vac-Fi (the delay was used to obtain maximal transcription). RNA (10 μg) was separated on a formaldehyde-agarose gel, transferred to a nitrocellulose membrane, and probed for the fiber with a 32P-labeled fragment of the fiber sequence. (B) CTL recognition of first- and second-generation adenovirus vectors. CTL were assayed in the presence of LCL infected 40 h prior to the test with 20,000 particles of AdE1° or AdE1E2° per cell, or mock infected, in a standard 4-h chromium release assay.
FIG. 2.
Effect of the viral gene transcription block on CTL lysis. (A) LCL treated with Act-D (5 μg/ml) 30 min prior to and during infection or left untreated were infected for 1 h with 12,000 particles of Ad-CFTR per cell and then resuspended in 5 ml of complete medium with or without 0.2 μg of Act-D per ml. After 24 h of culture, CTL recognition was assayed on autologous mock- or adenovirus-infected Act-D-treated and untreated targets in a standard 4-h chromium release assay (a concentration of 0.2 μg of Act-D per ml was maintained throughout the test). (B) In parallel, adenovirus-infected 51Cr-labeled targets were seeded in triplicate in 96-well plates (1.5 × 105 cells/well) and RNA transcription was measured by [3H]uridine (1 μCi/well) incorporation after 18 h of culture. E:T, effector-to-target-cell ratio.
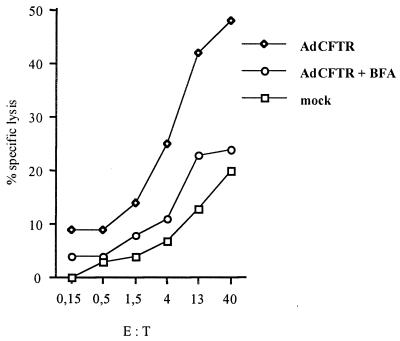
Viral inoculum structural-antigen recognition by human CTLs has been documented with naturally occurring cytomegalovirus and influenza virus infections (19, 33). However, the mechanisms of exogenous protein processing via the class I major histocompatibility complex (MHC) pathway are poorly understood (10). Brefeldin A (BFA) disrupts intracellular membrane traffic by disassembling the Golgi complex, thereby preventing the presentation of intracellularly processed MHC-peptide complexes, whereas complexes already present on the cell surface are not affected by the drug. In our study, target cells were treated by BFA (1 μg/ml) before and during infection (with 1,000 particles of Ad-CFTR or AdE1° per cell for 18 h). A low MOI and a short infection duration were used to facilitate the effect of the drug. In spite of suboptimal conditions, significant lysis of infected cells was achieved, and this was totally blocked by BFA treatment (Fig. 3). In addition, patient 1 CTLs (HLA A2 A23 B44 B49 C7 C8) were tested against an homozygous allogeneic LCL (HLA A2 B60 C10), mismatched for all patient 1 class I HLA restrictions except for HLA A2. For two effector-to-target-cell ratios, the specific lysis against the allogeneic target represented 40% of that obtained against the autologous target (data not shown). Therefore, approximately 40% of Ad-specific lytic activity was mediated by HLA A2-restricted CTLs. Together, these results suggest that CTLs recognize class I MHC peptide complexes resulting from intracellular processing. Internalized virion capsid proteins are therefore processed inside adenovirus-infected cells and serve as a source of peptides for class I MHC molecules.
FIG. 3.
Effect of blockage of viral peptide presentation on CTL lysis. LCL treated with BFA (1 μg/ml 30 mn prior to and during infection) or left untreated were infected for 1 h with 1,000 particles of Ad-CFTR per cell. After 18 h of culture, CTL recognition was assayed on autologous mock- or adenovirus-infected, BFA-treated and untreated targets, in a standard 4-h chromium release assay (a concentration of 1 μg of BFA per ml was maintained throughout the test). E:T, effector-to-target-cell ratio.
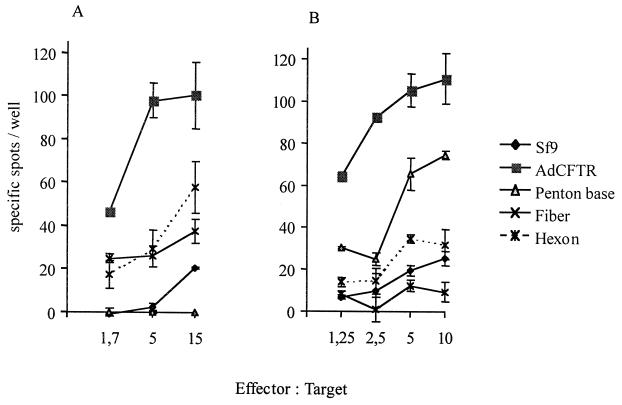
To determine capsid component recognition by CTLs, recombinant adenovirus type 5 hexons (Hx), penton bases (Pb), and fibers (Fi) were produced upon lysis (by successive freezing and thawing) of Sf9 cells infected with recombinant baculoviruses, as previously described (8), and used in gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays. The rationale for using ELISPOT instead of the lysis assay was twofold: (i) assuming that adenovirus-specific lytic activity would be distributed among the three capsid components, we selected the ELISPOT technique, which demonstrated higher sensitivity, and (ii) these experiments were considerably limited by the quantities of CTLs that could be derived from few patient blood samples (ELISPOT required threefold fewer CTL than the chromium release assay did). Recombinant protein extracts were introduced into LCL by hypertonic loading lasting 10 min (18). After 12 h of incubation, cells were used as targets (5,000 cells/well) in an IFN-γ ELISPOT assay (5) at effector-to-target-cell ratios ranging from 15:1 to 1:1. As shown in Fig. 4A, IFN-γ-secreting CTLs from patient 1 were visualized, indicating recognition of recombinant Hx- and Fi-loaded targets but not Pb-loaded targets. In contrast, Pb-loaded cells were targets for CTLs from patient 2 whereas Hx- and Fi-loaded cells were not (Fig. 4B). These results are in agreement with those of a recent study of mice which demonstrated that the CTL response to both adenovirus capsid components and the transgene product varied among mouse strains, indicating that the MHC haplotype has an impact on vector CTL epitope recognition (13).
FIG. 4.
Patient 1 (A) and patient 2 (B) CTL recognition of capsid components. CTLs were tested for 10 min at 37°C by an ELISPOT assay for the secretion of IFN-γ in the presence of autologous LCL (5,000 cells/well) loaded using a hypertonic solution (0.5 M sucrose, 10% polyethylene glycol 1,000, and 10 mM HEPES in RPMI 1640) with 20 μl of recombinant capsid components (penton base, hexon, and fiber) or control Sf9 cell extract (Sf9 plus 500 μl of hypertonic solution). Adenovirus-infected targets (12,000 particles of Ad-CFTR per cell) were used as positive controls, and the results are expressed as the number of specific spots per well after subtracting the background determined with uninfected unloaded LCL.
The mechanism of adenovirus recognition by CTL in humans remains controversial. Using different experimental conditions from ours, Smith et al. showed that de novo viral gene expression was not required to sensitize targets to lysis by adenovirus-specific CTL (23). In contrast, Flomenberg et al. reported that CTLs did not recognize input virion proteins (7). In the present study, we have provided compelling evidence that adenovirus-specific CTL recognition does not require de novo gene expression. In addition, we have demonstrated that capsid components introduced by an exogenous pathway resulted in MHC class I-associated peptide complexes that serve as targets for CTL recognition and have identified these components in two patients. Several experiments with other viral systems have demonstrated that exogenous proteins processed by target cells efficiently induce antiviral CTL responses (2, 19, 33). Moreover, Sigal et al. recently showed that dendritic cells presented antigens from virally infected nonhematopoietic cells, indicating that the exogenous MHC class I pathway is the major mechanism of CTL responses to nonhematopoietic virus-infected cells in vivo (22). Besides contributing to the elucidation of the immune mechanisms elicited by adenovirus in humans, our results have important implications for the design of gene therapy protocols since they demonstrate that the antiviral CTL response will not be reduced by using additionally deleted vectors.
Acknowledgments
This work was supported by grant 9252 from the Association pour la Recherche contre le Cancer (ARC) V.M.-F. was supported initially by a fellowship from ARC and subsequently by the Ligue Nationale contre le Cancer.
We thank Hedi Haddada for critical reading of the manuscript and Lorna Saint Ange for editing.
REFERENCES
- 1.Armentano D, Zabner J, Sacks C, Sookedo C, Smith M, St. George J, Wadworth S, Smith A, Gregory R. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 3.Brough D, Hsu C, Kulesa V, Lee G, Cantalupo L, Lizonova A, Kovesdi I. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crystal R, McElvaney N, Rosenfeld M, Chu C, Mastrangeli A, Hay J, Brody S, Jaffe H, Eissa N, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 5.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedieu J, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud J, Aubailly N, Orsini C, Guillaume J, Opolon P, Delaere P, Pericaudet M, Yeh P. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flomenberg P, Piaskowski V, Truitt R L, Casper J T. Human adenovirus-specific CD8+ T-cell responses are not inhibited by E3-19K in the presence of gamma interferon. J Virol 33. 1996;70:6314–6322. doi: 10.1128/jvi.70.9.6314-6322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahery-Ségard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet J-G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahéry-Ségard H, Molinier-Frenkel V, Le Boulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, Le Cesne A, Zitvogel L, Venet A, Schatz C, Courtney M, Le Chevalier T, Tursz T, Guillet J-G, Farace F. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Investig. 1997;100:2218–2226. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gromme M, Uytdehaag F G, Janssen H, Calafat J, van Binnendijk R S, Kenter M J, Tulp A, Verwoerd D, Neefjes J. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA. 1999;96:10326–10331. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habib N A, Hodgson H J, Lemoine N, Pignatelli M. A phase I/II study of hepatic artery infusion with wtp53-CMV-Ad in metastatic malignant liver tumours. Hum Gene Ther. 1999;10:2019–2034. doi: 10.1089/10430349950017383. [DOI] [PubMed] [Google Scholar]
- 12.Harvey B G, Hackett N R, El-Sawy T, Rosengart T K, Hirschowitz E A, Lieberman M D, Lesser M L, Crystal R G. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jooss K, Ertl H, Wilson J M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusky M, Grave L, Dieterle A, Dreyer D, Christ M, Ziller C, Furstenberger P, Kintz J, Ali Hadji D, Pavirani A, Mehtali M. Regulation of adenovirus-mediated transgene expression by the viral E4 gene products: requirement for E4 ORF3. J Virol. 1999;73:8308–8319. doi: 10.1128/jvi.73.10.8308-8319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michou A, Santoro L, Christ M, Juillard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 18.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 19.Riddel S, Rabin M, Geballe A, Britt W, Greenberg P. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 20.Rosenberg S A, Zhai Y, Yang J C, Schwartentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Seipp C A, Einhorn J H, Roberts B, White D E. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuler M, Rochlitz C, Horowitz J A, Schlegel J, Perruchoud A P, Kommoss F, Bolliger C T, Kauczor H U, Dalquen P, Fritz M A, Swanson S, Herrmann R, Huber C. A phase I study of adenovirus-mediated wild-type p53 gene transfer in patients with advanced non-small cell lung cancer. Hum Gene Ther. 1998;9:2075–2082. doi: 10.1089/hum.1998.9.14-2075. [DOI] [PubMed] [Google Scholar]
- 22.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 23.Smith C A, Woodruff L S, Kitchingman G R, Rooney C M. Adenovirus-pulsed dendritic cells stimulate human virus-specific T-cell responses in vitro. J Virol. 1996;70:6733–6740. doi: 10.1128/jvi.70.10.6733-6740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterman D H, Treat J, Litzky L A, Amin K M, Coonrod L, Molnar-Kimber K, Recio A, Knox L, Wilson J M, Albelda S M, Kaiser L R. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998;9:1083–1092. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 25.Stewart A, Lassam N, Quirt I, Bailey D, Rotstein L, Krajden M, Dessureault S, Gallinger S, Cappe D, Wan Y, Addison C, Moen R, Gauldie J, Graham F. Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: results of a phase 1 clinical trial. Gene Ther. 1999;6:350–363. doi: 10.1038/sj.gt.3300833. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 27.Tursz T, Le Cesne A, Baldeyrou P, Gautier E, Opolon P, Schatz C, Pavirani A, Courtney M, Lamy D, Ragot T, Saulnier P, Andremont A, Monier R, Perricaudet M, Le Chevalier T. Phase I study of a recombinant adenoviral-mediated gene transfer in lung cancer patients. J Natl Cancer Inst. 1996;88:1857–1863. doi: 10.1093/jnci/88.24.1857. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Ertl H, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Numes F A, Berencsi K, Furth E E, Gönczöl E, Wilson J M. Cellular immunity to viral antigens limits E1 deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 33.Yewdell J W, Bennink J R, Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988;239:637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]
- 34.Zabner J, Couture L A, Gregory R J, Graham S M, Smith A E, Welsh M J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]