Abstract
Background
This systematic study aims to assess the global epidemiologic, economic, and humanistic burden of illness associated with all types of hereditary angioedema.
Methods
A systematic search for articles reporting the epidemiologic, economic, and humanistic burden associated with patients with HAE was conducted using English and Chinese literature databases from the inception to May 23, 2022. The selected studies were assessed for their quality and risk of bias. The study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses and registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022352377).
Results
In total, 65 articles that met the search inclusion criteria reported 10,310 patients with HAE, of whom 5861 were female patients. Altogether, 4312 patients (81%) and 479 patients (9%) had type 1 and type 2 HAE, respectively, whereas 422 patients (8%) had HAE-normal C1-INH. The overall prevalence of all types of HAE was between 0.13 and 1.6 cases per 100,000. The mean or median delay from the first onset of a symptom of HAE to confirmed diagnosis ranged from 3.9 to 26 years. The estimated risk of death from asphyxiation was 8.6% for patients with HAE. Hospitalization, medication, unnecessary surgeries, doctor visits, specialist services, and nursing costs are direct expenses that contribute to the growing economic burden. The indirect cost accounted mostly due to missing work ($3402/year) and loss of productivity ($5750/year). Furthermore, impairment of QoL as reported by patient-reported outcomes was observed. QoL measures identified depression, anxiety, and stress to be the most common symptoms for adult patients and children.
Conclusion
This study highlights the importance of early diagnosis and the need for improving awareness among health care professionals to reduce the burden of HAE on patients and society.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-024-03265-z.
Keywords: Hereditary angioedema, Autosomal disorder, Economic cost, Clinical burden, Quality of life
Introduction
Hereditary angioedema (HAE) is a rare, debilitating, life-threatening genetic disorder characterized by recurrent attacks of subcutaneous and/or submucosal angioedema [1]. Several forms of HAE have been defined based on gene mutations: (1) type 1 HAE identified as C1 inhibitor (C1-INH) deficiency with low levels of C1-INH; (2) type 2 HAE identified as C1-INH dysfunction with normal or slightly increased levels of C1-INH but low functional levels, both type 1 and type 2 are due to mutations of the serine protease inhibitor gene 1 (SERPING1); and (3) HAE with normal C1-INH levels (HAE-nC1-INH) including (a) mutations of FXII gene (HAE-FXII), (b) HAE with a mutation in the angiopoietin-1 gene (HAE-ANGPT1), (c) HAE with a mutation in the plasminogen gene (HAE-PLG), (d) HAE with a mutation in the kininogen 1 gene (HAE-KNG1), (e) HAE with a mutation in the myoferlin gene (HAE-MYOF), and (f) HAE with a mutation in the heparan sulfate 3-O-sulfotransferase 6 gene (HAE-HS3ST6); some patients have HAE due to unknown mutations identified as HAE-UNK [2]. C1-INH is an inhibitor of plasma kallikrein and factor XII that are responsible for the generation of bradykinin. An increase in the levels of bradykinin causes extravasation of plasma, which leads to painful swelling. In cases of larynx angioedema, it could be life-threatening to the patients [2].
Hereditary angioedema accounts for approximately 2% of clinical angioedema cases [3]. Although the global prevalence of HAE is estimated at 1:50,000, the true prevalence of HAE remains unclear because the disease is rare [4]. Furthermore, according to the epidemiologic reports, the prevalence of type 1 HAE is observed in the majority of patients (80%-85%), whereas type 2 HAE is present in 15% to 20% of patients [5–8]. HAE-nC1-INH is only accounted for by a minor proportion of patients [9]. An earlier study has observed no major gender or ethnic differences in the HAE type 1/2 [4–7, 10]. However, an analysis reported that HAE-nC1-INH is exclusive to women and postulated it to be related to X-linked dominant mode of inheritance [11]. Likewise, HAE-FXII and HAE-unknown were more pronounced in females with a male to female ration of 1:68 and 1:6.3, respectively [12]. The onset of HAE symptoms varies by age and can occur in children aged < 1 year, with the development of laryngeal attacks occurring usually after the age of 3 years with an increased frequency observed after puberty [13].
Patients with HAE had angioedema attacks including pain and swelling at the extremities, abdomen, genitourinary tract, face, or oropharynx and any other possible site. More often, because of the overlap of clinical symptoms between various forms of angioedema or with other systemic diseases, and the relatively rare of it, HAE remains underreported or misdiagnosed. Consequently, there is a considerable delay in the accurate diagnosis of HAE from the onset of symptoms [1]. This may lead to unnecessary treatments and surgeries further delaying the timely treatment of HAE, which may contribute to a substantial burden in patients with HAE.
HAE attacks are usually variable as well as unpredictable and might be induced by various stimuli. The empirical triggering factors include stress, physical exertion, trauma, infection, hormonal changes, medical interventions, seasonal changes, and the use of certain medicinal products [14]. On average, the frequency of attacks ranges from 1 to 26 per year [10, 15]. But in rare cases, patients have reported 100 attacks per year, which may last up to 5 days [16]. The unpredictability of angioedema attacks, high risk of asphyxia, and the need for emergency intervention often result in a significant burden for patients with C1-INH-HAE[17]. Moreover, the above factors adversely affect the patients’ health-related quality of life (HRQoL) and increase the economic burden.
Many efforts have been taken to quantify the epidemiologic, economic, and humanistic burden of this disease, but the poor comparability between the studies has limited the detection of common issues and real differences. To address this gap, we sought to systematically synthesize the evidence on the epidemiologic, economic, and humanistic burden associated with HAE.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022352377).
Search Strategy
We searched English and Chinese databases for articles related to the epidemiologic, humanistic, and economic burden associated with HAE published from the inception of respective databases until May 23, 2022. The following search terms were used for conducting literature searches: “hereditary angioedema,” “HAE,” “epidemiology,” “prevalence,” “incidence,” “mortality,” “death rate,” “fatality,” “burden of disease,” “healthcare resource utilization,” “cost of illness,” “cost,” “productivity,” “economic,” “economic burden,” “healthcare costs,” “hospitalization,” “direct cost,” “indirect cost,” “quality of life,” “Health-Related Quality Of Life,” “Life Quality,” “activities of daily living,” “patient satisfaction,” “caregiver burden,” “impact of burden,” and “quality adjusted life year.” The search strategies for each database and review process are detailed in the Supplementary file.
Inclusion and exclusion criteria
We included (1) studies with patients suffering from HAE; (2) studies with the following outcomes (a) epidemiology (prevalence, incidence, mortality rates, and diagnosis delay), (b) economic burden (health resource utilization, direct and indirect cost, inpatient and outpatient visit expenses, family care cost, hospitalization cost, and financial burden cost), or (c) humanistic burden (HRQoL measurements with different tools, disability-adjusted life year [DALY], activities of daily living [ADL], quality-adjusted life-year [QALY], patient satisfaction, and caregiver burden); (3) observational studies (prospective and retrospective cohort studies, cross-sectional studies, and case–control studies) and experimental studies (randomized controlled trials [RCTs], single-arm or nonrandomized controlled trials, and cluster trials); and (4) studies that have been published in English or Chinese databases from the inception to May 23, 2022. Studies that reported costs or cost-effectiveness associated with specific treatments of HAE and consisted of study designs, comments, study protocol, editorials, review articles, case reports, and case series were excluded.
Study selection
The preliminary screening was conducted based on the title and abstracts according to the predefined eligibility criteria. The full texts of the included articles were further reviewed and examined for relevant outcome as aligned with the eligibility criteria. A full-text screening was conducted independently by 2 researchers, and any disagreements between the reviewers were resolved by discussing with the third independent reviewer.
Data extraction and quality assessment
Information from the included articles was extracted into a standardized MS Office Excel table. Data related to the author, year of publication, title, study design, demographics of the study population, and outcomes of interest were extracted by 2 independent reviewers with the quality check performed by the third reviewer. Although statistical analysis was not planned, the results were narratively synthesized to identify the common themes and gaps in the evidence. The methodological quality of eligible nonrandomized studies was determined using the Newcastle–Ottawa scale (NOS). The NOS consists of 3 quality parameters with a total of 9 points. Studies with an NOS score of > 6 were considered high-quality studies [18].
Results
Study selection
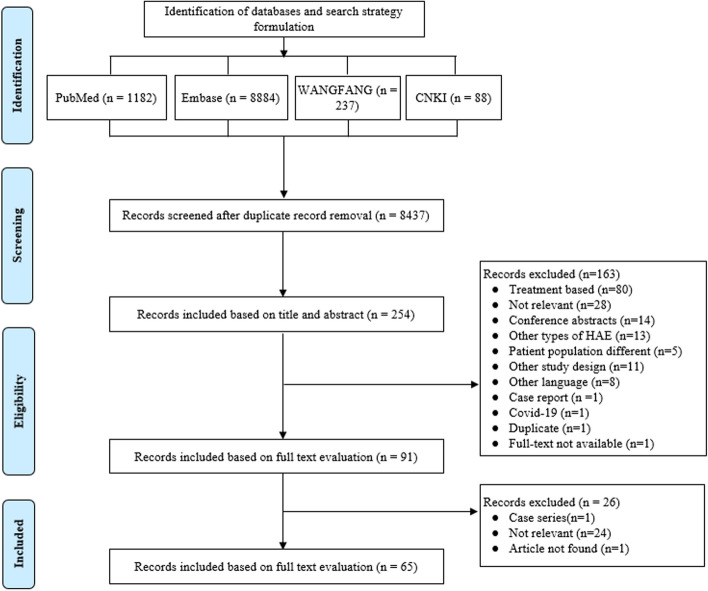
A total of 10,391 articles were identified from the database search. Titles and abstracts of 8437 articles were screened after eliminating duplicate articles. A total of 254 articles were identified for full-text evaluation based on the abstract review. Finally, 65 full-text articles that were assessed to fulfill the study outcomes were included for the evidence synthesis and quality assessment (Fig. 1). The burden of all types of HAE with respect to epidemiology was reported in 39 articles [7, 18–55], whereas the economic burden and humanistic burden of the disease were reported in 16 [51, 52, 56–68], and 23 articles, respectively [17, 50–55, 57–61, 69–79].
Fig. 1.
PRISMA flowchart for systematic review. HAE, hereditary angioedema
Clinical characteristics of included studies
The included studies were published from 1997 to 2022, and the study duration ranged from 43 days to 30 years. The studies comprised 7 multinational studies, and the remaining studies included data from 25 countries/regions: the United States (US; n = 11); Brazil (n = 6); Mainland China (n = 6); Germany (n = 3); Canada (n = 3); Denmark (n = 3); Japan (n = 3); Turkey (n = 3); Sweden, France, and Hungary (n = 2 each); and Australia, Belarus, Greece, India, Iran, Italy, New Zealand, Portugal, Puerto Rico, South Africa, South Korea, Switzerland, Taiwan, and the United Kingdom (n = 1 each). The included studies were mostly cross-sectional studies (n = 37) [54, 55], retrospective observational studies (n = 23), and others (n = 5). A total of 10,310 patients were evaluated, of whom 5861 were female patients and 3261 were male patients. In total, 4312 patients (81%) had type 1 HAE and 479 patients (9%) had type 2 HAE, whereas HAE-nC1-INH was reported in 422 patients (8%) and the type of angioedema was not identifiable in 122 patients (2%). The key characteristics of eligible studies and quality assessments are provided in Table 1.
Table 1.
Key characteristics of included studies
| Author name | Year | Country/Region | Study design | Population | Study duration | Numbers | Female (n (%)) | HAE typesa | Prevalence | Diagnosis Delay (mean (SD)) | Deathb | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fragnan NTML et al. | 2018 | Brazil | Retrospective | Children; adult | December 2009—November 2017 | 51 | 38 (74.5) | 49;2;0 | NR | median(range): 13 (0.25–50) | NR | 5 |
| Cui Q et al. | 2022 | China | Retrospective | Adult | January 2019—July 2020 | 2 | 1(50.0) | 2;0;0 | C4 decreased cohort: 2.43/10,000 | 25(NA) | NR | 3 |
| Jones DH et al. | 2022 | US | Retrospective | Adult | NR | 23 | 20(87.0) | 0;0;23 | NR | > 10(NA) | NR | 3 |
| Guryanova I et al. | 2021 | Belarus | Cross-sectional | Children; adult | 2010 | 64 | 39(60.9) | 54;10;0 | 1/148,000 | Median (IQ25-IQ75): 19.3 (2.4) | NR | 5 |
| Ayteki̇n G et al. | 2021 | Turkey | Retrospective | NR | 5-year | 38 | 25 (65.8) | 18;20;0 | NR | 8.84(8.97) | NR | 5 |
| Veronez CL et al. | 2021 | Brazil | Retrospective | Children; adult | March 2012-March 2020 | 425 | 316(73.3) | 116;9;300 | NR |
HAE-C1-INH: 21 (15) HAE-F12: 15 (13) HAE-U: 14 (14) |
NR | 4 |
| Araújo-Simões J et al. | 2021 | Brazil | Retrospective | Children | First clinical evaluation until December 2018 | 95 | 44(46.3) | NR | NR | 3.9(NA) | NR | 4 |
| Jindal AK et al. | 2021 | India | Retrospective | Children | January 1996—December 2019 | 32 | 11(34.4) | 32;0;0 | NR | Median (range): 6.5 (0–28) | 1 | 4 |
| Cao Y et al. | 2021 | China | Cross-sectional | Children; adult | NR | 107 | 58 (54.2) | 103;4;0 | NR | 14.2 (range,0–50) | NR | 3 |
| Ohsawa I et al. | 2021 | Japan | Cross-sectional | Children; adult | June 2019—May 2020 | 68 | 39 (67.2) | NR; NR;10 | NR | 18.8 (range,0–60) | NR | 3 |
| Cao Y et al. | 2020 | China | Cross-sectional | Children; adult | NR | 103 | 56(54.4) | 103;0;0 | NR | Median (IQR): 11 (6–19.5) | NR | 3 |
| Magerl M et al. | 2020 | Germany | Cross-sectional | Adult | July 2017—April 2018 | 81 | 60 (74.1) | NR | NR | 18.1 (14.6) | NR | 3 |
| Alonso MLO et al. | 2019 | Brazil | Cross-sectional | Children; adult | NR | 107 | 72 (67.3) | 105;2;0 | NR | 17.7(12.6) | NR | 4 |
| Schöffl C et al. | 2019 | Austria | Cross-sectional | NR | NR | 137 | 77(56.2) | 77;19;0;41 | 1/64,396 | 15.0 (9.9) | NR | 5 |
| Liu S et al. | 2019 | China | Cross-sectional | Children; adult | NR | 96 | 53 (55.2) | 92;4;0 | NR | Median (IQR):11.04(6.06 –18.27) | NR | 5 |
| Jung JW et al. | 2018 | South Korea | Retrospective | NR | First diagnosed until 2016 | 65 | 44 (67.7) | 59;6;0 | 1.3/1,000,000 | 7.75(10.54) | NR | 3 |
| Coovadia KM et al. | 2018 | South Africa | Retrospective | Adult | 2010–2015 | 43 | 28(65.1) | 43;0;0 | 1/140,000 | NR | 2 | 5 |
| Zanichelli A | 2016 | Austria; Brazil; Denmark; France; Germany; Greece; Israel; Italy; Spain; Sweden; United Kingdom | Retrospective | Children; adult | July 2009—January 2016 | 418 | 243(58.1) | 387;31;0 | NR |
≥ 1 misdiagnoses:15.0 (13.4); without misdiagnosis:7.0 (13.2) |
NR | 3 |
| Deroux A et al. | 2016 | France | Retrospective | Children; adult | Since 2006 | 57 | 46(80.7) | 0;0;57 | NR | 12.7(NA) | NR | 3 |
| Kargarsharif F et al. | 2015 | Iran | Cross-sectional | Children; adult | NR | 51 | 26(51.0) | 33;18;0 | NR | 11.02 (11.60) | 2 | 5 |
| Nanda MK et al. | 2015 | US | Retrospective | Children | 10 years | 21 | 6 (28.6%) | NR | NR |
Without a family history: median 6.0 With family history: median -0.9 |
NR | 4 |
| Kim SJ et al. | 2014 | US | Retrospective | Children; adult | 1999—2010 | 600 | NR | NR | NR | NR | 0.17 (95% CI 0.15–0.18) per million persons per year | 7 |
| Psarros F et al. | 2014 | Greece | Cross-sectional | Children; adult | July 2010—June 2013 | 116 | 55(47.4) | NR | NR | 16.5(NA) | NR | 5 |
| Caballero T et al. | 2014 | Spain; Germany; Denmark | Cross-sectional | NR | May-December 2011 | 186 | 112 (60.2) | NR | NR | 12(15) | NR | 5 |
| Bork K et al. | 2012 | Germany | Partly retrospective and Partly prospective | NR | NR | 728 | 388(53.3) | 682;46;0 | NR | NR | 70 | 3 |
| Kesim B et al. | 2011 | Turkey | Retrospective | NR | NR | 70 | 42 (60.0) | 67;3;0 | NR | 26.0 (14.4) | NR | 4 |
| Bygum A et al. | 2009 | Denmark | Cross-sectional | Children; adult | 2001–2002; 2007–2008 | 82 | 42(51.2) | 77;5;0 | 1.41/100,000 | 16.3 (range, 0–63) | NR | 5 |
| Bork K et al | 2000 | Germany | Retrospective | Children; adult | NR | 153 | NR | 146;7;0 | NR | NR | 24 | 3 |
| Winnewisser J et al. | 1997 | Switzerland | Cross-sectional | NR | NR | 59 | NR | NR | NR | NR | 4 | 3 |
| Sylvestre S et al. | 2021 | US | Cross-sectional | NR | July 2021 | 2122 | 1463(68.9) | NR |
Overall:0.99/100,000 Black patients: 1.64/100,000 White patients:1.47/100,000 Hispanic patients:0.80/100,000 |
NR | NR | 5 |
| Xu YY et al. | 2013 | China | Retrospective | NR | 1982—2011 | 158 | 80(50.6) | 156;2;0 | NR | 12.64(NA) | 18 | 6 |
| Wei-Te Lei et al. | 2011 | Taiwan | Retrospective | NR | 2003—2011 | 19 | 8 (42.10) | 19;0;0 | NR | 8.45(11.04) | 1 | 4 |
| Balla Z et al. | 2021 | Hungary | Retrospective | NR | 1990–2020 | 197 | 109(55.3) | 184;13;0 | NR | NR | 2 | 7 |
| Banerji A et al. | 2020 | US | Cross-sectional | Adult | March 17; 2017—April 28; 2017 | 445 | 348 (78.2) | 349;96;0 | NR | 8.4 (10.6) | NR | 5 |
| Zilberberg MD et al. | 2011 | US | Retrospective | Children; adult | 2006—2007 | NA | NR | NR | NR | NR | NR | 3 |
| Zilberberg MD et al. | 2011 | US | Retrospective | Children; adult | 2004—2007 | NA | NR | NR | NR | NR | All-HAE:145 HAE-PD:9 | 3 |
| Zilberberg MD et al. | 2010 | US | Retrospective | NR | 2007 | NA | NR | NR | NR | NR | NR | 3 |
| Wilson DA et al. | 2010 | US | Cross-sectional | Adult | November 2007—January 2009 | 457 | 345(75.5) | NR | NR | NR | NR | 3 |
| Javaud N et al. | 2019 | France | Cluster randomized trial | Adult | March 2013-June 2014 | 200 | 74 (37.0) | 164;14;22 | NR | NR | NR | 5 |
| Mendivil J et al. | 2021 | France; United Kingdom; Spain; Canada; Australia; Switzerland; Germany; Austria | Cross-sectional | Adult | July–October 2018 | 242 | 163 (67.4) | 198;44;0 | NR | 9.3 (11.0) | NR | 4 |
| Hews-Girard J et al. | 2021 | Canada | Cross-sectional | Adult | NR | 17 | 13 (76.5) | 11;6;0 | NR | NR | NR | 5 |
| Nunes FL et al. | 2021 | Brazil | Prospective trial | Children; adult | 14 months | 33 | 18 (54.5) | 33;0;0 | NR | NR | NR | 6 |
| Forjaz MJ et al. | 2021 | Spain; Hungary; Austria; Germany; Argentina; Brazil; Canada; Denmark; Israel; Poland; Romania | Prospective observational | Adult | NR | 290 | 200(69.0) | 232;58;0 | NR | NR | NR | 5 |
| Ohsawa I et al. | 2015 | Japan | Cross-sectional | Children; adult | March—May 2014 | 171 | 117 (68.4) | 99;9;3;60 | NR | 13.8 (range, 0–58) | NR | 5 |
| Aygören-Pürsün E et al. | 2014 | Spain; Germany; Denmark | Cross-sectional | Children; adult | May-December 2011 | 164 | 100 (61.0) | NR | NR | 12(NA) | NR | 3 |
| Jolles S et al. | 2014 | UK | Retrospective | Children; adult | 2010–2012 | 376 | NR | 320;23;4 | NR |
type 1: 10(NA) type 2: 18(NA) |
NR | 5 |
| Lumry WR et al. | 2010 | US | Cross-sectional | Adult | November 2007—January 2008 | 457 | 345 (75.5) | NR | NR | NR | NR | 3 |
| Lindsay K et al. | 2021 | New Zealand | Retrospective | Adult | 1st June 2015-31st December 2019 | 38 | 20 (52.6) | 29;9;0 | NR | Without a family history: 13.2(NA) | NR | 4 |
| Iwamoto K et al. | 2021 | Japan | Cross-sectional | Children; adult | 2016—2017 | 70 | 55(78.6) | NR | NR | 15.6(13.3) | NR | 4 |
| Savarese L et al. | 2021 | Italy | Cross-sectional | Adult | NR | 28 | 20 (71.4) | NR | NR | NR | NR | 4 |
| Balla Z et al. | 2021 | Hungary | Prospective trial | Adult | 2016—2018 | 125 | 72(57.6) | NR | NR | NR | NR | 6 |
| Lee EY et al. | 2021 | Canada | Cross-sectional | Adult | NR | 72 | 53 (73.6) | NR | NR | NR | NR | 3 |
| Kuman Tunçel Ö et al. | 2019 | Turkey | Cross-sectional | Adult | NR | 33 | 19 (57.6) | 30;3 | NR | 19.8(9.4) | NR | 5 |
| Liu S et al. | 2019 | China | Cross-sectional | Adult | NR | 104 | 57 (54.8) | 101;3;0 | NR | NR | NR | 5 |
| Arce-Ayala YM et al. | 2019 | Puerto Rico | Cross-sectional | Children; adult | November 2015-April 2016 | 32 | 25 (83.3) | 13;4;3;11 | NR | NR | NR | 3 |
| Kessel A et al. | 2017 | Hungary; Israel | Cross-sectional | Children | NR | 33 | 19(57.6) | NR | NR | NR | NR | 4 |
| Aabom A et al. | 2017 | Denmark | Cross-sectional | Children | May 2013—August 2014 | 14 | 6(42.9) | 13;1;0 | NR | NR | NR | 2 |
| Nordenfelt P et al. | 2017 | Sweden | Cross-sectional | Adult | May—October 2016 | 64 | 38(59.4) | 60;4;0 | 1.61/100,000 | NR | NR | 5 |
| Engel-Yeger B et al. | 2017 | Israel; Hungary | Cross-sectional | Children | NR | 34 | 19 (55.8) | 34;0;0 | NR | NR | NR | 4 |
| Jindal NL et al. | 2017 | Canada | Cross-sectional | Adult | NR | 21 | 20 (95.2) | NR | NR | NR | NR | 5 |
| Nordenfelt P et al. | 2014 | Sweden | Cross-sectional | Children; adult | NR | 103 | 54(52.4) | NR | 1/66,000 | NR | NR | 4 |
| Gomide MACMS et al. | 2013 | Brazil | Cross-sectional | Children; adult | NR | 35 | 25(71.4) | NR | NR | NR | NR | 2 |
| Luz S et al. | 2011 | Portugal | Cross-sectional | NR | NR | 25 | 17(68.0) | NR | NR | NR | NR | 3 |
| Aabom A et al. | 2015 | Denmark | Cross-sectional | NR | 2009 | 27 | 6(22.2) | NR | NR | NR | NR | 3 |
| Fouche AS et al. | 2014 | US | Cross-sectional | Adult | NR | 26 | 12(46.2) | 22;4;0 | NR | NR | NR | 3 |
HAE Hereditary angioedema
aHAE type: type1; type2; HAE-nC1-INH; non-identification
bDeath due to asphyxia
Epidemiologic burden
Diagnosed prevalence of HAE
As reported in 8 studies, the prevalence of HAE ranged between 0.13 and 1.6 cases per 100,000 (Table 1). The prevalence rates were low across all included studies. In Sweden, the estimated prevalence rates for 2011 and 2016 were 1.5 and 1.6 cases per 100,000, respectively [54, 55]. In South Korea, the prevalence was 0.13 cases per 100,000 [33], whereas in Denmark and Austria, the estimated prevalence was 1.4 and 1.6 cases per 100,000, respectively [31, 44]. Similarly, the estimated prevalence rates in Belarus, South Africa, and the US were between 0.7 and 1.0 cases per 100,000 [21, 34, 47]. A study conducted in the US simultaneously reported the prevalence of HAE among ethnic groups. The prevalence of HAE in Black patients (1.64 cases per 100,000) was almost similar to that of White patients (1.47 cases per 100,000), whereas it was lower among Hispanic patients (0.80 cases per 100,000) [47]. Furthermore, according to a study conducted in Tongji Hospital in China, the prevalence of HAE was reported to be 2.43 cases per 10,000 in patients with decreased complement 4 level [19].
Risk of death in patients with HAE
One study reported a low age-adjusted mortality (the ratio of the number of deaths in a specified time to a given population) of 0.17 (95% CI, 0.15–0.18) per million persons per year for HAE in the US [39]. Additional 9 studies reported 124 deaths caused by asphyxiation (due to laryngeal edema) among 1440 patients, which leads to an estimated 8.6% of risk of death from asphyxiation for patients with HAE. One of the 9 studies reported the lifespan of patients with undiagnosed HAE type 1/2 who died of asphyxiation was shorter than that of patients with undiagnosed HAE type 1/2 who died of other causes (40.8 years vs 72.0 years) [42]. Furthermore, a descriptive epidemiologic study conducted in the US evaluated the death among all HAE hospitalizations and HAE-related hospitalizations [66]. The study observed that 145 deaths occurred during all HAE hospitalizations (n = 10,125) and 9 deaths occurred during 3216 HAE-related hospitalizations (Table 1).
Diagnosis of HAE
The delay in diagnosis was reported by 34 studies with the mean or median range of 3.9 to 26 years from the first onset of HAE symptoms to the confirmed diagnosis (Table 1). In 25 articles, the mean or median delay was reported to be > 10 years, indicating there was a widespread misdiagnosis of HAE globally. One of the studies found that patients without family history had a longer delay than those with family history (6.0 years vs -0.9 years) [38], whereas another study found that the delay in diagnosis was shorter in patients with HAE type 1 than those with HAE type 2 (10 years vs 18 years) [64].
Economic burden
Direct costs associated with HAE were reported in 6 studies, which assessed the treatment costs and health care utilization (Table 2). The major components accounting for the increase in economic costs were hospitalization, treatments, unnecessary surgeries, doctors’ visits, specialist services, and nurse costs. Three studies conducted in the US estimated the hospital costs to be $17,335 per year and about $4000 for a single hospitalization [56, 65, 67]. The annual medication costs to reduce the number of attacks and to manage the chronic disease were $235 and $2013, respectively [67]. A study by Javaud et al. conducted in France between 2013 and 2014 reported an increase in the medication cost of €10,038 ± €10,334 and €10,287 ± €8260 at 12 and 24 months, respectively, in patients with HAE [59]. The emergency department (ED) cost for a single visit was $1465 as reported by Zilberberg et al., whereas the annual ED cost was $2603 as reported by Wilson et al. [65, 67]. The other procedural cost accounted for $978 to $3510 among the US patients and €135 among French patients with HAE [59, 67]. According to a study by Wilson et al., outpatient clinic cost comprised the least expenditure with an estimated total cost of $189 [67]. Wilson et al. and Javaud et al. reported the total direct costs with annual medical expenditure of $25,884 and €10,296 for patients from the US and France, respectively [59, 67].
Table 2.
Direct costs related to HAE
| Study name | Country /region | Hospitalization cost | Medication cost | Outpatient’s cost | Emergency visit | Other procedures | Total Direct cost |
|---|---|---|---|---|---|---|---|
| Banerji et al. 2020 [52] | US | NR | NR | NR | NR | $1,000 | NR |
| Zilberberg et al. 2011 [56, 66] | US | NR | NR | NR | HAE-PD: $1,465 | NR | NR |
| Zilberberg et al. 2011 [56, 66] | US | HAE-PD: $4,760 | NR | NR | NR | NR | NR |
| Zilberberg et al. 2010 [65] | US | Around $4,000 for a single hospitalization | NR | NR | NR | NR | NR |
| Wilson et al. 2010 [67] | US | Hospital stays: $17,335 |
Acute attacks: $235 Chronic disease management: $2,013 Total: $2,248 |
Clinic or physician’s office treatment: $189 | $2,603 |
Procedure cost: $978 Routine visit costs: $2,532 Total: $3,510 |
Total direct medical costs of acute attacks: $21,339 Total direct medical costs for chronic disease management (treatment outside acute attacks): $4,545 Total direct medical costs $25,884 annually |
| Nicolas Javaud et al. 2019 [59] | France |
0-to-12-month follow-up: Cost: €122 ± 176 12-to-24-month follow-up Cost: €118 ± 180 (€ = $1.11 in 2015.) |
0-to-12-month follow-up: Drug cost: €10,038 ± 10,334 12- to 24-month follow-up: Drug cost: €10,287 ± 8,260 |
NR | NR |
0-to-12-month follow-up: ED visits + transportation: €99 ± 25 Consultation GP/specialist: €26 ± 10 Nurse: €10 ± 2 12-to-24-month follow-up: ED visits + transportation: €101 ± 24 Consultation GP/specialist: €27 ± 10 Nurse: €10 ± 2 |
Total average health care cos during the first year: €10,296 ± 17,828 Total average health care cos during the second year: €10,544 ± 17,525 |
Abbreviations: ED Emergency department, GP General physician, HAE Hereditary angioedema, PD Principal diagnosis
Twelve studies reported indirect economic burden that included various productivity measures (Table 3). Among them, absenteeism from work was 5.9% to 31.1% because of HAE attacks. The presenteeism among patients who were physically present at work was approximately 20%, ranging from 10% to 24.6%. Furthermore, Wilson et al. reported a loss of $5750 because of decreased productivity at work affecting income. In addition, the study also reported the loss of wages for missed work because of a single attack approximated to be $525. Therefore, missed work because of acute attacks resulted in an income loss of $3402 annually, and for chronic disease management, the income was reduced to almost $6512 because of working fewer hours as compared with working full time [67]. There was variation in the number of days missing from work/school because of different time duration. In general, the average number of missed days in 1 year was between 1.7 and 19.9 days, and in total, the indirect cost was $16,108 annually.
Table 3.
Indirect economic burden of HAE
| Study Name | Absenteeism (mean ± S.D.) | Presenteeism (mean ± S.D.) | Work productivity losses (mean ± S.D.) | Activity impairment (mean ± S.D.) | Loss of income due to productivity loss | Loss of income due to reduced working hours | Number of days lost due to HAE attack | Total indirect cost |
|---|---|---|---|---|---|---|---|---|
| Mendivil et al. 2021 [51] | 7.87% ± NA | 24.59% ± 28.65% | 24.18% ± 30.03 | 33.88% ± 31.20% | NR | NR | NR | NR |
| Hews-Girard et al. 2021 [57] | 31.1% ± NA | 10% ± 18% | 27% ± NA | 20.6% ± 21.1% | NR | NR | NR | NR |
| Leonel Nunes et al. 2021 [58] | 7.29% ± 18.52 | 19.29% ± 28.41 | 21.14% ± 32.39% | 25.00% ± 26.24% | NR | NR | NR | NR |
| Banerji et al. 2020 [52] | 5.9% ± 14.1% | 23.0% ± 25.8% | 25.4% ± 28.1% | 31.8% ± 29.7% | NR | NR | NR | NR |
| Ohsawa et al. 2015 [62] | NR | NR | NR | NR | NR | NR | Mean 1.7 absent days per year | |
| Pürsün et al. 2014 [63] | NR | NR | NR | NR | NR | NR | Days missing from work/school on average per year: 19.9 ± 35.0 day | NR |
| S. Jolles et al. 2014 [64] | NR | NR | NR | NR | NR | NR | Days lost from work/school or where activities of daily living could not be performed: 9 ± 24 days per year | NR |
| Lumry et al. 2010 [60] | 9.4% ± 19.2% | NR | 33.5% ± 25.8% | 45% ± 30.2% | NR | NR |
Numbers of missed day due to most recent attack: Work days 3.3 ± 14.4 school days 1.9 ± 0.8 leisure days 2.7 ± 3.0 |
NR |
| Wilson et al. 2010 [67] | NR | NR | 33.5% ± NA | NR | $5,750 per patient per year |
Average cost of lost wages for missed work due to a single attack (per patient annually): $525 Annual cost of missed work due to acute attacks: $3,402 Annual reduced income because working less than full time: $6,512 |
Numbers of missed day due to most recent attack: Overall: 3.3 ± 14.4 days Mild attack: 2.2 ± 3.3 days Moderate attack: 1.8 ± 1.0 days Severe attack: 5.5 ± 22.9 days |
Total indirect costs: $16,108 |
| Lindsay et al. 2021 [68] | NR | NR | NR | NR | NR | NR | Mean days off work over one year: 16 days (range 1–104) | NR |
| Iwamoto et al. 2021 [69] | NR | NR | NR | NR | NR | NR |
Days absent from work/school in year: 17.5 ± 4.4 days before diagnosis 10.2 ± 3.6 days after diagnosis |
NR |
Abbreviations: HAE Hereditary angioedema, S.D. Standard deviation
Humanistic burden
The quality of life (QoL) was reported in a total of 23 publications. In 9 studies, the most frequently used measure to assess the HRQoL for patient-reported outcome (PRO) was 36-item Short Form health survey (SF-36). Other PRO measures used to assess the HRQoL in patients with HAE included the Angioedema Quality-of-Life Questionnaire (AE-QOL; n = 4); Hereditary Angioedema Quality-of-Life Questionnaire (HAE-QoL; n = 4); the EuroQol-5 Dimension (EQ-5D; n = 3); Pediatric Quality of Life Inventory (Peds-QL; n = 3); 12-item Short Form Health Survey (SF-12; n = 3); Hospital Anxiety and Depression Scale (HADS; n = 2); Visual Analog Scale (VAS; n = 2); Toronto Alexithymia Scale (TAS; n = 1); Emotion Regulation Checklist (ERC; n = 1); Perceived Stress Scale (PSS; n = 1); Depression, Anxiety, Stress Scale-21 (DASS-21; n = 1); State-Trait Anxiety Inventory for Children (STAIC; n = 1); Children's Dermatology Life Quality Index (CDLQI; n = 1); Research and Development-36 (RAND-36; n = 1); Hamilton Depression Inventory-Short Form (HDI-SF; n = 1); and Hamilton Depression Rating Scale (HDRS; n = 1). Table 4 lists the summary of studies reporting humanistic burden in HAE.
Table 4.
Humanistic burden in HAE
| Study Name | SF-36 | AE-QOL | HAE-QoL | EQ-5D | PedsQL | SF-12 | Other |
|---|---|---|---|---|---|---|---|
| Savarese et al. 2021 [17] | NR | NR | NR | NR | NR | NR |
TAS:43.3 (12.9) ERC:4.4 (0.8) PSS:18.2 (7) |
| Mendivil et al. 2021 [51] | NR | 47.14 (20.69) | NR | NR | NR |
PCS: 49.26 (9.30) MCS: 43.09 (11.23) |
HADS:13.43 (8.17) |
| Hews-Girard and Goodyear 2021 [57] | P < 0.001 compared with Canadian normative data | 39 (18.2) | NR | NR | NR | NR |
DASS-21 depression score:6.8 (10.2) DASS-21 anxiety score: 6.2 (8.2) DASS-21 stress score: 10(10.2) |
| Balla et al. 2021 [50] | NR |
Median (IQR) 20.6 (5.9, 36.8) |
NR | NR | NR | NR | NR |
| Nunes et al. 2021 [58] | NR | NR |
Mean (CrI): Δscore:15.2 (1.23–29.77) at 8 months Δscore:26 (14.56–39.02) at 14 months |
NR | NR | NR | NR |
| Lee et al. 2021 [70] | NR | NR | 102 (23) | NR | NR | NR | NR |
| Banerji et al. 2020 [52] | NR | NR | 93.1 (24.9) | NR | NR |
PCS:48.6 (9.9) MCS: 44.9 (10.9) |
HADS Anxiety 4.3(3.5), depression 2.5(2.9) for Attack-free; anxiety 8.8 (4.7), depression 6.4 (4.8) for 13 or more attacks |
| Kuman Tuncel et al. 2019 [53] | Score of ERF, SF, GH, BP, PRF subscales lower than population norms (P < 0.01) | NR | NR | NR | NR | NR | NR |
| Shuang Liu et al. 2019 [71] |
PCS: 49.81(7.08) MCS: 44.76 (9.18) |
NR | NR | NR | NR | NR | NR |
| Javaud et al. 2019 [59] | NR | NR | NR |
12 months: 0.71(0.12) 24 months: 0.70(0.13) |
NR | NR | NR |
| Arce-Ayala et al. 2019 [59] |
PCS:40.91(NA) MCS: 41.57(NA) |
NR | NR | NR | NR | NR | NR |
| Kessel et al. 2017 [73] | NR | NR | NR | NR |
C1-INH-HAE vs controls Hungary: 81.52(14.18) vs 92.48 (5.54) Israel: 79.93 (11.98) vs 87.42 (8.15) |
NR |
STAIC (HAE vs controls) Anxiety state 44.74(10.56) vs 38.76(10.67) Anxiety trait 29.21(5.16) vs 25.23(4.09) |
| Aabom et al. 2017 [74] | NR | NR | NR | NR |
C1-INH-HAE vs controls Child Self-Report 84.0(18.6) vs 82.9(NA) Parent Proxy-Report 83.4(18.8) vs 81.3(NA) |
NR |
CDLQI: 2.0(5.9) disease-specific questionnaire:5.6(10.0) VAS line: 86.0(23.3) VAS smiley: 84.1(19.8) |
| Nordenfelt et al. 2017 [54] | NR |
Median (range) 36.8 (0–91.7) |
NR | 0.84 (-0.02–1.00) | NR | NR |
VAS: 80 (25–100) RAND-36: The scores of the nine dimensions: 50–100 |
| Engel-Yeger et al. 2017 [75] | NR | NR | NR | NR |
C1-INH-HAE vs controls Hungary: 81.81(13.83) vs 80.22(14.82) Israel: 79.93 (11.98) vs 86.39(5.71) |
NR | NR |
| Jindal et al. 2017 [76] |
PCS: 49.1(NA) MCS: 50.4(NA) |
NR | NR | NR | NR | ||
| Nordenfelt et al. 2014 [55] | NR | NR |
Today vs attack 0.825 (0.207) vs 0.512 (0.299) |
NR | NR | ||
| Gomide et al. 2013 [77] | The scores of the eight dimensions: 51.03 to 75.95 | NR | NR | NR | NR | NR | NR |
| Luz et al. 2011 [78] |
The scores of the eight dimensions: 49.16 to 83.20 vs 55.83 to 75.27 (HAE patient vs Reference) |
NR | NR | NR | NR | NR | NR |
| Lumry et al. 2010 [60] | NR | NR | NR | NR | NR |
HAE patient vs Reference PCS: 43.7(10.2) vs 49.6(9.9) MCS: 42.6(10.1) vs 49.4(9.8) |
HDI-SF 8.1 (6.5) vs 3.1(3.0) (HAE patient vs Reference) |
| Aabom et al. 2015 [79] |
The scores of the eight dimensions: 62.8 to 92.9 vs 58.3 to 87.4 (HAE patient vs Reference) |
NR | NR | NR | NR | NR | NR |
| Fouche et al. 2014 [83] | NR | NR | NR | NR | NR | NR |
17-item HDRS: 7.1 (6.2) vs 3.2 (3.2) for HAE cohort and General population cohort 21-item HDRS: 8 (6.5) 29-item HDRS: 11 (8.9) |
| Forjaz MJ et al. 2021 [61] |
PCS: 49.7(8.8) MCS: 46.2(10.4) |
NR | 95.5(25.5) | NR | NR | NR | NR |
AE-QoL, Angioedema Quality of Life Questionnaire, scores were transformed to a linear scale of 0–100(0 = none; 0–25 = mild; 26–75 =moderate and > 75 = severe). HAE-Qol, Hereditary Angioedema Quality of Life Questionnaire, total score ranges from 25 to 135, higher scores represent better HRQoL. CDLQI, Children's Dermatology Life Quality Index, score range 0-30, higher scores reflect more impaired HRQoL. DASS-21, Depression Anxiety Stress Scale-21, Depression (normal 0-4, mild 5-6, moderate 7-10, severe 11-13, extremely severe ≥14); Anxiety (normal 0-3, mild 4-5, moderate 6-7, severe 8-9, extremely severe ≥10); Stress (normal 0-7, mild 8-9, moderate 10-12, severe 13-16, extremely severe ≥17). ERC, Emotion Regulation Checklist, the mean values for both males and females within the normative samples can be referred as cut-off values for the index. HADS, Hospital Anxiety and Depression Scale, anxiety and depression subscale scores range from 0 to 21, 0-7 = Normal, 8-10 = mild, 11-14=moderate, 15: severe psychological morbidity. HDRS, Hamilton Depression Rating Scale, higher values correlate with more severe depressive symptoms. Peds-QL, Pediatric Quality of Life, 0-100-point scale, a higher total score indicates a better HRQoL. PSS, Perceived Stress Scale, a 10-item self-reported scale, measures the degree to which situations in one’s life are appraised as stressful. Stress was assessed as low with a score < 13, moderate with a score of 14–26, and as highly perceived when the score was > 27. RAND-36, Research and Development, average score between 0 (worst) and 100 (best), a higher total score indicates a better HRQoL. SF-36, 36-item Short Form; average score between 0-100, a higher total score indicates a better HRQoL. SF-12, 12-Item Short Form Health Survey, scores range from 0 to 100 with higher score indicating better physical and mental health. STAIC, State-Trait Anxiety Inventory for Children, total scores for state and trait range from 20 – 80, higher scores indicate greater anxiety. TAS, Toronto Alexithymia Scale, a 20-item self-reported questionnaire which evaluates alexithymia, the difficulty in recognizing and naming and describing one’s own emotions. A score of < 51 indicates absence of alexithymia, a score of 52–60 indicates a possible alexithymia, and a score of > 61 indicates alexithymia. EQ-5D, The EuroQol 5 Dimension, range 0-1, higher scores reflect better health. VAS, Visual Analogue Scale, 0-100, higher scores reflect better general health. HDI-SF, the range of score is 0-33, with higher scores indicating more depression symptom. nonvalidated tool, range 0-52, higher scores reflect more impaired HRQoL
HAE Hereditary angioedema, MCS Mental component summary score, PCS Physical component score, QoL Quality of life, ERF Emotional role functioning, SF Social functioning, GH General health, BP Bodily pain, PRF Physical role functioning
The mean overall summary scores for the physical component summary (PCS) in the SF-36 survey ranged from 40.9 to 49.8 and for the mental component summary (MCS) ranged from 41.6 to 50.4. Three studies from Brazil, Portugal, and Denmark reported the mean scores of 8 domains of SF-36 ranging from 51.0 to 76.0, 49.2 to 83.2, and 62.8 to 92.9 [77–79], respectively. The mean scores in at least 1 dimension were significantly lower for the HAE population compared with the normal population in 5 studies [53, 57, 71, 72, 76], whereas another 2 studies reported that patients with HAE had QoL scores similar to the reference population [78, 79]. Three studies reported the scores of the PCS and MCS in the SF-12 survey, ranging from 43.7 to 49.26 and 42.6 to 44.9, respectively [51, 52, 60]. Nordenfelt et al. evaluated the utility of EQ-5D to describe the current health state and the state during their most recent HAE attack, which indicated an impaired HRQoL for patients with HAE both during and between attacks [55]. Another 2 studies that measured the HRQoL using EQ-5D demonstrated an impairment of QoL as well, with a health utility of 0.7 for French patients and of 0.8 for Swedish patients [54, 59]. The scores of VAS were reported in 2 studies with the value of 80.0 for adult patients and 86.0 for pediatric patients [54, 74].
A disease-specific questionnaire such as AE-QoL was also used to assess the impact of angioedema on daily life for 4 weeks before answering the questionnaires. The instrument involved a 17-item questionnaire assessing the impairment of HRQoL from 4 dimensions (functioning, fatigue/mood, fears/shame, and nutrition), and a higher score indicated the severity of impaired HRQoL. The AE-QoL scores in 4 studies varied from 20.6 to 47.1. HAE-QoL, another 25-item disease-specific questionnaire, assessed the extent to which angioedema has affected daily life for the last 6 months from 7 dimensions (treatment difficulties, physical functioning and health, disease-related stigma, emotional role and social functioning, concern about offspring, perceived control over illness, and mental health), with the higher score representing better HRQoL. The mean scores of the included studies ranged from 93.1 to 102. Nunes et al. showed substantial improvement in HAE-QoL scores at 8 and 14 months compared with baseline because of a systematic intervention (Δ score: 15.2 at 8 months; Δ score: 26.0 at 14 months) [58].
Peds-QL is the most frequently used tool to evaluate the HRQoL in pediatric patients with HAE, with a higher total score indicating a better HRQoL. Two of the 3 studies measured using Peds-QL demonstrated a lower HRQoL for pediatric patients with HAE than healthy children [73, 75], whereas the third study observed comparable HRQoL scores for pediatric patients with HAE type 1/2 with normal scores for healthy children [74]. Aabom et al. developed a nonvalidated, disease-specific tool using Peds-QL as a structural model to measure the impact of HAE on pediatric patients, with a score of 5.6 ± 10.0. The CDLQI designed to measure physical discomfort, social discomfort, and activity limitation showed a CDLQI score of 2.0 ± 5.9 for pediatric patients [74].
Depression, anxiety, stress, and alexithymia are the most common symptoms for both adult patients and children as proved by DASS-21, PSS, HDI-SF, HDRS, STAIC, TAS, and ERC (see Table 4). Another uncommon instrument has been used to report the QoL in the studies. The RAND-36 is a generic instrument (similar to SF-36) evaluating the HRQoL from 9 dimensions, with 0 for the worst and 100 for the best. Nordenfelt et al. reported median scores of 9 dimensions of RAND-36 ranging from 50 to 100 [54].
Discussion
To the best of our knowledge, this systematic review is the first to provide a comprehensive understanding of the epidemiologic, economic, and humanistic burden of HAE. This review indicates that HAE is associated with a substantial burden and will undoubtedly become more pronounced with rising awareness of the disease globally. At present, because of rarity and limited symptom specificity, HAE is indeed often misdiagnosed, leading to a significant delay (> 10 years) in correct diagnosis. Besides, a lack of awareness among health care professionals, limited availability of diagnostic tests, and incorrect treatment restrict the timely and optimal management of HAE [80, 81]. As a result, it has been found that 8.6% of patients with HAE have experienced laryngeal edema, which has led to death caused by asphyxiation. These concerning statistics emphasize the critical need for early diagnosis and increased awareness of the disease.
The HRQoL is the patients’ perception regarding the multidimensional impact of the disease [82]. Evidence from this review illustrates the negative impact of HAE on the QoL. Twenty-three studies assessed the burden of HAE on the QoL of patients; however, only 3 reported QoL of children aged 2 to 18 years with HAE highlighting a significant knowledge gap [73–75]. In this study, we observed that most patients were provided with an SF-12/SF-36 survey questionnaire or an HAE-QOL/AE-QOL questionnaire. The majority of the studies showed poorer PCS and MCS in patients with HAE relative to the control. There was a significant association of psychological implications such as anxiety and depression with HAE identified by either high score values on rating scales or during conversations with the participants [83]. HAE had a significant negative impact on the QoL both during and between attacks that reflected on absenteeism at work or school [55]. The World Allergy Organization guidelines about C1-INH-HAE suggest considering the HRQoL when determining maintenance treatment, and HAE experts advise to assess the HRQoL annually [2, 84]. Data derived from large populations are necessary to accurately measure the HRQoL in patients with C1-INH-HAE, its trigger factors, and the effects of therapeutic interventions.
To our knowledge, only 2 studies from the US and France have estimated the economic burden associated with HAE. Although the evidence is sparse, the present review identified hospitalization cost, medication cost, and other procedural costs such as surgery, physician visits, and nursing services ($1000-$3510 among the US patients and €135 among French patients with HAE) to be the main components of direct costs, whereas outpatient visits and other outpatient services ($189) were the minimal components of economic burden as reported in the included studies [59, 67]. Economic assessments of HAE indicated decreased work productivity because of disease burden added to the indirect costs. The loss of income because of reduced productivity was $5750 per patient per year as reported by Wilson et al. and lost wages for missed work because of a single attack was estimated to be ~ $525; conversely, the lost wages per annum would be ~ $3402; and the reduction in income because of absenteeism was ~ $6512 [67]. These estimates indicate a considerable economic burden associated with HAE ascertaining the need to prevent functional limitation and improving the QoL for patients is vital in reducing absenteeism.
Meanwhile, there was a huge variation in the reported data pertaining to the cost; few studies provided a detailed breakdown of direct and indirect costs, whereas other studies described only major cost categories, which limited the comparisons between studies. Finally, variability in outcome measures was observed across studies. The study heterogeneity in terms of patient characteristics and study setting (eg, recruitment at secondary or tertiary clinics and claims databases) across the included articles may have contributed to the wide ranges of the observed data in the results. Future studies using standardized approaches to conduct and report the burden of illness would reduce this data heterogeneity and enable better burden comparisons between studies. With the approval of new drugs for HAE globally, there is an urgent need to determine the direct medical costs incurred by patients using these drugs.
Conclusion
The lack of comprehensive epidemiologic data on the incidence of HAE creates a knowledge gap regarding the true overall burden of HAE on society. However, there is considerable evidence indicating that delayed diagnosis of HAE is associated with decreased physical function, increased risk of mortality, negative psychological impact, and higher direct and indirect costs. This review compiles evidence highlighting the need for early diagnosis, improved disease management, and increased awareness among health care professionals to mitigate the excessive burden on patients.
Supplementary Information
Acknowledgements
Takeda Pharmaceutical Company Limited has provided the scientific review of the manuscript. Medical writing assistance was provided by Anwesha Mandal and Roopa Shree Subbaiah, PhD, of Indegene Pvt Ltd and funded by Takeda (China) International Trading Company.
Authors' contributions
XG, YNS, SL, and YXZ contributed to the design of this study. XG, YNS, SL collected the data. SL, MH, and TXC performed the analysis. XG, YNS, and SL prepared the manuscript. YXZ, MH, and TXC helped to revise the manuscript. All authors approved the final version of this study.
Funding
This study was funded by Takeda (China) International Trading Company.
Availability of data and materials
The data included in this report are from the published literature; all articles meeting the search criteria are listed and full publication details are provided.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Yanan Sheng, Miao He, and Tianxiang Chen are employees of Takeda (China) International Trading Co. Ltd. The other authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riedl MA, Lumry WR, Busse P, Levy H, Steele T, Dayno J, et al. Prevalence of hereditary angioedema in untested first-degree blood relatives of known subjects with hereditary angioedema. Allergy Asthma Proc. 2015;36:206–12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4405600/ Cited 2022 Sep 20. [DOI] [PMC free article] [PubMed]
- 2.Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygören-Pürsün E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema – The 2021 revision and update. World Allergy Org J. 2022;15:100627. https://linkinghub.elsevier.com/retrieve/pii/S1939455122000035 Cited 2022 Sep 23. [DOI] [PMC free article] [PubMed]
- 3.Cicardi M, Johnston DT. Hereditary and acquired complement component 1 esterase inhibitor deficiency: a review for the hematologist. Acta Haematol. 2012;127:208–220. doi: 10.1159/000336590. [DOI] [PubMed] [Google Scholar]
- 4.Zanichelli A, Mansi M, Periti G, Cicardi M. Therapeutic management of hereditary angioedema due to C1 inhibitor deficiency. Expert Rev Clin Immunol. 2013;9:477–488. doi: 10.1586/eci.13.22. [DOI] [PubMed] [Google Scholar]
- 5.Pines JM, Poarch K, Hughes S. Recognition and Differential Diagnosis of Hereditary Angioedema in the Emergency Department. J Emerg Med. 2021;60:35–43. doi: 10.1016/j.jemermed.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 6.Ghazi A, Grant JA. Hereditary angioedema: epidemiology, management, and role of icatibant. Biologics. 2013;7:103–113. doi: 10.2147/BTT.S27566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fragnan NTML, Tolentino ALN, Borba GB, Oliveira AC, Simões JA, Palma SMU, et al. Hereditary angioedema with C1 inhibitor (C1-INH) deficit: the strength of recognition (51 cases) Braz J Med Biol Res. 2018;51:e7813. doi: 10.1590/1414-431x20187813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong PFK, Coulter T, El-Shanawany T, Garcez T, Hackett S, Jain R, et al. A National Survey of Hereditary Angioedema and Acquired C1 Inhibitor Deficiency in the United Kingdom. J Allergy Clin Immunol. 2023;11(8):2476–83. doi: 10.1016/j.jaip.2023.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Sinnathamby ES, Issa PP, Roberts L, Norwood H, Malone K, Vemulapalli H, et al. Hereditary Angioedema: Diagnosis, Clinical Implications, and Pathophysiology. Adv Ther. 2023;40:814–827. doi: 10.1007/s12325-022-02401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuraw BL. Clinical practice. Hereditary angioedema N Engl J Med. 2008;359:1027–1036. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 11.Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000;356:213–217. doi: 10.1016/S0140-6736(00)02483-1. [DOI] [PubMed] [Google Scholar]
- 12.Bork K, Wulff K, Witzke G, Hardt J. Hereditary angioedema with normal C1-INH with versus without specific F12 gene mutations. Allergy. 2015;70:1004–1012. doi: 10.1111/all.12648. [DOI] [PubMed] [Google Scholar]
- 13.Bowen T, Cicardi M, Farkas H, Bork K, Longhurst HJ, Zuraw B, et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol. 2010;6:24. doi: 10.1186/1710-1492-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zotter Z, Csuka D, Szabó E, Czaller I, Nébenführer Z, Temesszentandrási G, et al. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet J Rare Dis. 2014;9:44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3977696/ Cited 2022 Sep 23. [DOI] [PMC free article] [PubMed]
- 15.Levy JH, O’Donnell PS. The therapeutic potential of a kallikrein inhibitor for treating hereditary angioedema. Expert Opin Investig Drugs. 2006;15:1077–1090. doi: 10.1517/13543784.15.9.1077. [DOI] [PubMed] [Google Scholar]
- 16.Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379–394. doi: 10.1111/j.1365-2249.2005.02726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savarese L, Bova M, Maiello A, Petraroli A, Mormile I, Cancian M, et al. Psychological processes in the experience of hereditary angioedema in adult patients: an observational study. Orphanet J Rare Dis. 2021;16:23. doi: 10.1186/s13023-020-01643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2 July 2024.
- 19.Cui Q, Xu Q, Yang Y, Li W, Huang N, Chen H, et al. The prevalence of hereditary angioedema in a Chinese cohort with decreased complement 4 levels. World Allergy Organ J. 2022;15:100620. doi: 10.1016/j.waojou.2021.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DH, Bansal P, Bernstein JA, Fatteh S, Harper J, Hsu FI, et al. Clinical profile and treatment outcomes in patients with hereditary angioedema with normal C1 esterase inhibitor. World Allergy Organ J. 2022;15:100621. doi: 10.1016/j.waojou.2021.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guryanova I, Suffritti C, Parolin D, Zanichelli A, Ishchanka N, Polyakova E, et al. Hereditary angioedema due to C1 inhibitor deficiency in Belarus: epidemiology, access to diagnosis and seven novel mutations in SERPING1 gene. Clin Mol Allergy. 2021;19:3. doi: 10.1186/s12948-021-00141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayteki̇n G, Yildiz E, Çölkesen F, Akilli NB, Arslan Ş, Çalişkaner AZ. 5-Year Experience at a Single Center: Retrospective Analysis of 38 Patients with Hereditary Angioedema: A Descriptive Study. Turkiye Klinikleri J Med Sci. 2021;41:258–65. https://www.turkiyeklinikleri.com/article/en-5-year-experience-at-a-single-center-retrospective-analysis-of-38-patients-with-hereditary-angioedema-a-descriptive-study-93911.html Cited 2022 Oct 8.
- 23.Veronez CL, Mendes AR, Leite CS, Gomes CP, Grumach AS, Pesquero JB, et al. The Panorama of Primary Angioedema in the Brazilian Population. J Allergy Clin Immunol Pract. 2021;9:2293–2304.e5. doi: 10.1016/j.jaip.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Araújo-Simões J, Boanova AGP, Constantino-Silva RN, Fragnan NTML, Pinto JA, Minafra FG, et al. The Challenges in the Follow-Up and Treatment of Brazilian Children with Hereditary Angioedema. Int Arch Allergy Immunol. 2021;182:585–591. doi: 10.1159/000512944. [DOI] [PubMed] [Google Scholar]
- 25.Jindal AK, Rawat A, Kaur A, Sharma D, Suri D, Gupta A, et al. Novel SERPING1 gene mutations and clinical experience of type 1 hereditary angioedema from North India. Pediatr Allergy Immunol. 2021;32:599–611. doi: 10.1111/pai.13420. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Liu S, Zhi Y. Recurrent and acute abdominal pain as the main clinical manifestation in patients with hereditary angioedema. Allergy Asthma Proc. 2021;42:131–5. doi: 10.2500/aap.2021.42.210001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsawa I, Fukunaga A, Imamura S, Iwamoto K, Tanaka A, Hide M, et al. Survey of actual conditions of erythema marginatum as a prodromal symptom in Japanese patients with hereditary angioedema. World Allergy Org J. 2021;14:100511. https://www.sciencedirect.com/science/article/pii/S1939455121000053 Cited 2022 Oct 8. [DOI] [PMC free article] [PubMed]
- 28.Cao Y, Liu S, Zhi Y. The natural course of hereditary angioedema in a Chinese cohort. Orphanet J Rare Dis. 2020;15:257. doi: 10.1186/s13023-020-01526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magerl M, Gothe H, Krupka S, Lachmann A, Ohlmeier C. A Germany-wide survey study on the patient journey of patients with hereditary angioedema. Orphanet J Rare Dis. 2020;15:221. doi: 10.1186/s13023-020-01506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso MLO, Valle SOR, Tórtora RP, Grumach AS, França AT, Ribeiro MG. Hereditary angioedema: a prospective study of a Brazilian single-center cohort. Int J Dermatol. 2020;59:341–344. doi: 10.1111/ijd.14676. [DOI] [PubMed] [Google Scholar]
- 31.Schöffl C, Wiednig M, Koch L, Blagojevic D, Duschet P, Hawranek T, et al. Hereditary angioedema in Austria: prevalence and regional peculiarities. J Dtsch Dermatol Ges. 2019;17:416–423. doi: 10.1111/ddg.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Wang X, Xu Y, Xu Q, Zhi Y. Risk factors for diagnostic delay in Chinese patients with hereditary angioedema. Allergy Asthma Proc. 2019;40:343–349. doi: 10.2500/aap.2019.40.4234. [DOI] [PubMed] [Google Scholar]
- 33.Jung J-W, Suh DI, Park HJ, Kim S, Kwon HS, Yang MS, et al. Clinical Features of Hereditary Angioedema in Korean Patients: A Nationwide Multicenter Study. Int Arch Allergy Immunol. 2018;176:272–279. doi: 10.1159/000488350. [DOI] [PubMed] [Google Scholar]
- 34.Coovadia KM, Chothia M-Y, Baker SG, Peter JG, Potter PC. Hereditary angio-oedema in the Western Cape Province. South Africa S Afr Med J. 2018;108:283–290. doi: 10.7196/SAMJ.2018.v108i4.12823. [DOI] [PubMed] [Google Scholar]
- 35.Zanichelli A, Longhurst HJ, Maurer M, Bouillet L, Aberer W, Fabien V, et al. Misdiagnosis trends in patients with hereditary angioedema from the real-world clinical setting. Ann Allergy Asthma Immunol. 2016;117:394–398. doi: 10.1016/j.anai.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Deroux A, Boccon-Gibod I, Fain O, Pralong P, Ollivier Y, Pagnier A, et al. Hereditary angioedema with normal C1 inhibitor and factor XII mutation: a series of 57 patients from the French National Center of Reference for Angioedema. Clin Exp Immunol. 2016;185:332–337. doi: 10.1111/cei.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kargarsharif F, Mehranmehr N, Zahedi Fard S, Fazlollahi MR, Ayazi M, Mohammadzadeh I, et al. Type I and Type II Hereditary Angioedema: Clinical and Laboratory Findings in Iranian Patients. Arch Iran Med. 2015;18:425–429. [PubMed] [Google Scholar]
- 38.Nanda MK, Elenburg S, Bernstein JA, Assa’ad AH. Clinical features of pediatric hereditary angioedema. J Allergy Clin Immunol Pract. 2015;3:392–5. doi: 10.1016/j.jaip.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SJ, Brooks JC, Sheikh J, Kaplan MS, Goldberg BJ. Angioedema deaths in the United States, 1979–2010. Ann Allergy Asthma Immunol. 2014;113:630–634. doi: 10.1016/j.anai.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Psarros F, Koutsostathis N, Farmaki E, Speletas MG, Germenis AE. Hereditary angioedema in Greece: the first results of the greek hereditary angioedema registry. Int Arch Allergy Immunol. 2014;164:326–332. doi: 10.1159/000366276. [DOI] [PubMed] [Google Scholar]
- 41.Caballero T, Aygören-Pürsün E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, et al. The humanistic burden of hereditary angioedema: results from the Burden of Illness Study in Europe. Allergy Asthma Proc. 2014;35:47–53. doi: 10.2500/aap.2013.34.3685. [DOI] [PubMed] [Google Scholar]
- 42.Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130:692–697. doi: 10.1016/j.jaci.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 43.Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443–450. doi: 10.1159/000323915. [DOI] [PubMed] [Google Scholar]
- 44.Bygum A. Hereditary angio-oedema in Denmark: a nationwide survey. Br J Dermatol. 2009;161:1153–1158. doi: 10.1111/j.1365-2133.2009.09366.x. [DOI] [PubMed] [Google Scholar]
- 45.Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75:349–354. doi: 10.4065/75.4.349. [DOI] [PubMed] [Google Scholar]
- 46.Winnewisser J, Rossi M, Späth P, Bürgi H. Type I hereditary angio-oedema. Variability of clinical presentation and course within two large kindreds. J Intern Med. 1997;241:39–46. doi: 10.1046/j.1365-2796.1997.76893000.x. [DOI] [PubMed] [Google Scholar]
- 47.Sylvestre S, Craig T, Ajewole O, Craig S, Kaur S, Al-Shaikhly T. Racial and Ethnic Disparities in the Research and Care of Hereditary Angioedema Patients in the United States. J Allergy Clin Immunol Pract. 2021;9:4441–4449.e2. doi: 10.1016/j.jaip.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y-Y, Jiang Y, Zhi Y-X, Yin J, Wang L-L, Wen L-P, et al. Clinical features of hereditary angioedema in Chinese patients: new findings and differences from other populations. Eur J Dermatol. 2013;23:500–504. doi: 10.1684/ejd.2013.2105. [DOI] [PubMed] [Google Scholar]
- 49.Lei W-T, Shyur S-D, Huang L-H, Kao Y-H, Lo C-Y. Type I hereditary angioedema in Taiwan – clinical, biological features and genetic study. Asian Pac J Allergy Immunol. 2011;29:327–331. [PubMed] [Google Scholar]
- 50.Balla Z, Andrási N, Pólai Z, Visy B, Czaller I, Temesszentandrási G, et al. The characteristics of upper airway edema in hereditary and acquired angioedema with C1-inhibitor deficiency. Clin Transl Allergy. 2021;11:e12083. doi: 10.1002/clt2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendivil J, Murphy R, de la Cruz M, Janssen E, Boysen HB, Jain G, et al. Clinical characteristics and burden of illness in patients with hereditary angioedema: findings from a multinational patient survey. Orphanet J Rare Dis. 2021;16:94. doi: 10.1186/s13023-021-01717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerji A, Davis KH, Brown TM, Hollis K, Hunter SM, Long J, et al. Patient-reported burden of hereditary angioedema: findings from a patient survey in the United States. Ann Allergy Asthma Immunol. 2020;124:600–607. doi: 10.1016/j.anai.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Kuman Tunçel Ö, Gökmen NM, Demir E, Gülbahar O, Pırıldar Ş. The impact of hereditary angioedema on quality of life and family planning decisions. Int J Psychiatry Med. 2019;54:377–94. doi: 10.1177/0091217419837068. [DOI] [PubMed] [Google Scholar]
- 54.Nordenfelt P, Nilsson M, Lindfors A, Wahlgren C-F, Björkander J. Health-related quality of life in relation to disease activity in adults with hereditary angioedema in Sweden. Allergy Asthma Proc. 2017;38:447–455. doi: 10.2500/aap.2017.38.4087. [DOI] [PubMed] [Google Scholar]
- 55.Nordenfelt P, Dawson S, Wahlgren C-F, Lindfors A, Mallbris L, Björkander J. Quantifying the burden of disease and perceived health state in patients with hereditary angioedema in Sweden. Allergy Asthma Proc. 2014;35:185–190. doi: 10.2500/aap.2014.35.3738. [DOI] [PubMed] [Google Scholar]
- 56.Zilberberg MD, Nathanson BH, Jacobsen T, Tillotson G. Descriptive epidemiology of hereditary angioedema hospitalizations in the United States, 2004–2007. Allergy Asthma Proc. 2011;32:248–254. doi: 10.2500/aap.2011.32.3452. [DOI] [PubMed] [Google Scholar]
- 57.Hews-Girard J, Goodyear MD. Psychosocial burden of type 1 and 2 hereditary angioedema: a single-center Canadian cohort study. Allergy Asthma Clin Immunol. 2021;17:61. doi: 10.1186/s13223-021-00563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nunes FL, Ferriani MPL, Moreno AS, Langer SS, Maia LSM, Ferraro MF, et al. Decreasing Attacks and Improving Quality of Life through a Systematic Management Program for Patients with Hereditary Angioedema. Int Arch Allergy Immunol. 2021;182:697–708. doi: 10.1159/000513896. [DOI] [PubMed] [Google Scholar]
- 59.Javaud N, Bouillet L, Rabetrano H, Bitoun A, Launay D, Lapostolle F, et al. Hereditary angioedema: Clinical presentation and socioeconomic cost of 200 French patients. J Allergy Clin Immunol Pract. 2019;7:328–330. doi: 10.1016/j.jaip.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 60.Lumry WR, Castaldo AJ, Vernon MK, Blaustein MB, Wilson DA, Horn PT. The humanistic burden of hereditary angioedema: Impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010;31:407–414. doi: 10.2500/aap.2010.31.3394. [DOI] [PubMed] [Google Scholar]
- 61.Forjaz MJ, Ayala A, Caminoa M, Prior N, Pérez-Fernández E, Caballero T, et al. HAE-AS: A Specific Disease Activity Scale for Hereditary Angioedema With C1-Inhibitor Deficiency. J Investig Allergol Clin Immunol. 2021;31:246–252. doi: 10.18176/jiaci.0479. [DOI] [PubMed] [Google Scholar]
- 62.Ohsawa I, Honda D, Nagamachi S, Hisada A, Shimamoto M, Inoshita H, et al. Clinical manifestations, diagnosis, and treatment of hereditary angioedema: survey data from 94 physicians in Japan. Ann Allergy Asthma Immunol. 2015;114:492–498. doi: 10.1016/j.anai.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Aygören-Pürsün E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, Wait S, et al. Socioeconomic burden of hereditary angioedema: results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis. 2014;9:99. doi: 10.1186/1750-1172-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jolles S, Williams P, Carne E, Mian H, Huissoon A, Wong G, et al. A UK national audit of hereditary and acquired angioedema. Clin Exp Immunol. 2014;175:59–67. doi: 10.1111/cei.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zilberberg MD, Jacobsen T, Tillotson G. The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007. Allergy Asthma Proc. 2010;31:511–519. doi: 10.2500/aap.2010.31.3403. [DOI] [PubMed] [Google Scholar]
- 66.Zilberberg MD, Nathanson BH, Jacobsen T, Tillotson G. Descriptive epidemiology of hereditary angioedema emergency department visits in the United States, 2006–2007. Allergy Asthma Proc. 2011;32:390–394. doi: 10.2500/aap.2011.32.3478. [DOI] [PubMed] [Google Scholar]
- 67.Wilson DA, Bork K, Shea EP, Rentz AM, Blaustein MB, Pullman WE. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:314–320. doi: 10.1016/j.anai.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 68.Lindsay K, Chua I, Jordan A, Stephens S. National audit of a hereditary and acquired angioedema cohort in New Zealand. Intern Med J. 2022;52(12):2124–9. [DOI] [PubMed]
- 69.Iwamoto K, Yamamoto B, Ohsawa I, Honda D, Horiuchi T, Tanaka A, et al. The diagnosis and treatment of hereditary angioedema patients in Japan: A patient reported outcome survey. Allergol Int. 2021;70:235–243. doi: 10.1016/j.alit.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Lee EY, Hsieh J, Borici-Mazi R, Caballero T, Kanani A, Lacuesta G, et al. Quality of life in patients with hereditary angioedema in Canada. Ann Allergy Asthma Immunol. 2021;126:394–400.e3. doi: 10.1016/j.anai.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Wang X, Xu Y, Xu Q, Zhi Y. Health-related quality of life and its risk factors in Chinese hereditary angioedema patients. Orphanet J Rare Dis. 2019;14:191. doi: 10.1186/s13023-019-1159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arce-Ayala YM, Diaz-Algorri Y, Craig T, Ramos-Romey C. Clinical profile and quality of life of Puerto Ricans with hereditary angioedema. Allergy Asthma Proc. 2019;40:103–110. doi: 10.2500/aap.2019.40.4200. [DOI] [PubMed] [Google Scholar]
- 73.Kessel A, Farkas H, Kivity S, Veszeli N, Kőhalmi KV, Engel-Yeger B. The relationship between anxiety and quality of life in children with hereditary angioedema. Pediatr Allergy Immunol. 2017;28:692–698. doi: 10.1111/pai.12758. [DOI] [PubMed] [Google Scholar]
- 74.Aabom A, Andersen KE, Fagerberg C, Fisker N, Jakobsen MA, Bygum A. Clinical characteristics and real-life diagnostic approaches in all Danish children with hereditary angioedema. Orphanet J Rare Dis. 2017;12:55. doi: 10.1186/s13023-017-0604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engel-Yeger B, Farkas H, Kivity S, Veszeli N, Kőhalmi KV, Kessel A. Health-related quality of life among children with hereditary angioedema. Pediatr Allergy Immunol. 2017;28:370–376. doi: 10.1111/pai.12712. [DOI] [PubMed] [Google Scholar]
- 76.Jindal NL, Harniman E, Prior N, Perez-Fernandez E, Caballero T, Betschel S. Hereditary angioedema: health-related quality of life in Canadian patients as measured by the SF-36. Allergy Asthma Clin Immunol. 2017;13:4. doi: 10.1186/s13223-016-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomide MACMS, Toledo E, Valle SOR, Campos RA, França AT, Gomez NP, et al. Hereditary angioedema: quality of life in Brazilian patients. Clinics. 2013;68:81–3. doi: 10.6061/clinics/2013(01)OA13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luz S, Silva JA, Barbosa F, Santos AS, Ferreira M, Barbosa MP. Quality of life evaluation in patients with hereditary angioedema. Revista Portuguesa de Imunoalergologia. 2011;19:143–149. [Google Scholar]
- 79.Aabom A, Andersen KE, Perez-Fernández E, Caballero T, Bygum A. Health-related quality of life in Danish patients with hereditary angioedema. Acta Derm Venereol. 2015;95:225–226. doi: 10.2340/00015555-1835. [DOI] [PubMed] [Google Scholar]
- 80.Banerji A, Busse P, Christiansen SC, Li H, Lumry W, Davis-Lorton M, et al. Current state of hereditary angioedema management: a patient survey. Allergy Asthma Proc. 2015;36:213–217. doi: 10.2500/aap.2015.36.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agostoni A, Aygören-Pürsün Aygören-Pürsün, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: Problems and progress: Proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51–131. doi: 10.1016/j.jaci.2004.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caballero T, Prior N. Burden of Illness and Quality-of-Life Measures in Angioedema Conditions. Immunol Allergy Clin North Am. 2017;37:597–616. doi: 10.1016/j.iac.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Fouche AS, Saunders EFH, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112:371–375. doi: 10.1016/j.anai.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602–616. doi: 10.1111/all.12380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data included in this report are from the published literature; all articles meeting the search criteria are listed and full publication details are provided.