Abstract
Objective
Within the scope of the Exposome Project for Health and Occupational Research on applying the exposome concept to working life health, we aimed to provide a broad overview of the status of knowledge on occupational exposures and associated health effects across multiple noncommunicable diseases (NCDs) to help inform research priorities.
Methods
We conducted a narrative review of occupational risk factors that can be considered to have “consistent evidence for an association,” or where there is “limited/inadequate evidence for an association” for 6 NCD groups: nonmalignant respiratory diseases; neurodegenerative diseases; cardiovascular/metabolic diseases; mental disorders; musculoskeletal diseases; and cancer. The assessment was done in expert sessions, primarily based on systematic reviews, supplemented with narrative reviews, reports, and original studies. Subsequently, knowledge gaps were identified, e.g. based on missing information on exposure–response relationships, gender differences, critical time-windows, interactions, and inadequate study quality.
Results
We identified over 200 occupational exposures with consistent or limited/inadequate evidence for associations with one or more of 60+ NCDs. Various exposures were identified as possible risk factors for multiple outcomes. Examples are diesel engine exhaust and cadmium, with consistent evidence for lung cancer, but limited/inadequate evidence for other cancer sites, respiratory, neurodegenerative, and cardiovascular diseases. Other examples are physically heavy work, shift work, and decision latitude/job control. For associations with limited/inadequate evidence, new studies are needed to confirm the association. For risk factors with consistent evidence, improvements in study design, exposure assessment, and case definition could lead to a better understanding of the association and help inform health-based threshold levels.
Conclusions
By providing an overview of knowledge gaps in the associations between occupational exposures and their health effects, our narrative review will help setting priorities in occupational health research. Future epidemiological studies should prioritize to include large sample sizes, assess exposures prior to disease onset, and quantify exposures. Potential sources of biases and confounding need to be identified and accounted for in both original studies and systematic reviews.
Keywords: aetiology, epidemiology, exposome, occupational health
What’s Important About This Paper?
Many occupational exposures have been studied in relation to noncommunicable diseases, but estimates of the global burden of occupational disease are still largely underestimated due to gaps of knowledge on occupational exposures and related diseases. We here provide a broad overview of known risk factors of occupational NCDs. Bringing together these various health outcomes (including respiratory, cardiovascular, neurodegenerative and musculoskeletal diseases, as well as mental disorders and cancer) provides a much-needed resource for occupational health researchers. This study will help setting priorities in occupational health research for multiple noncommunicable diseases.
In middle- and high-income countries, noncommunicable diseases (NCD) make up nearly all occupational diseases, with common groups being cancers and respiratory, cardiovascular, neurodegenerative and musculoskeletal diseases, as well as mental disorders (Driscoll et al. 2014; Takala et al. 2014; Stanaway et al. 2018; Rehm and Shield 2019; WHO/ILO 2021). It is well-known that external factors play an important role in the causation or exacerbation of NCDs, and various occupational exposures have been studied in relation to NCDs. Examples are dust and chemical exposures linked to respiratory disease and cancer, and heavy lifting and vibrations linked to musculoskeletal diseases. Estimates of the global burden of occupational disease based on these studies vary between 5% and 7% of premature mortality, translating to about 2 million deaths each year (Rushton 2017).
These estimates, which are based on associations between single known risk factors and NCDs (mainly driven by cancer and circulatory diseases), likely underestimate the total burden given that exposure-disease associations may involve multiple exposures from the workplace. For example, the global indicator for the occupational burden of disease, as published in 2023, was based on only 21 pairings of occupational risk factors and diseases (Pega et al. 2023). Most of these well-known associations have been observed in studies in settings with relatively high exposure levels, as the effects of low-level exposures are yet largely unknown. Major data gaps also exist on the exposures with smaller effect magnitudes, which may not have been identified as risk factors due to low study quality and limited exposure assessment. Moreover, not all possibly relevant diseases have been taken into account in the occupational burden of disease estimations, such as neurodegenerative diseases, since evidence of associations with occupational exposures may yet be insufficient. Lastly, knowledge regarding vulnerable groups is limited (Stücker et al. 2017), and the estimated burden of occupational diseases shows that we are still not effectively preventing work-related diseases based on current knowledge.
For implementation of more effective measures to prevent occupational diseases, more holistic and diversity-sensitive risk characterization is needed. The “exposome” incorporates all nongenetic risk factors experienced during a person’s life (Wild 2005), and has been recognized to have a dominant role in the chronic disease burden (Vermeulen et al. 2020). In the Exposome Project for Health and Occupational Research (EPHOR) (Pronk et al. 2022) we apply the exposome concept to working life health to overcome some of the aforementioned challenges.
The objective of this paper was to provide an overview of the status of knowledge on occupational exposures in relation to common NCDs. Via this process, we aim to highlight knowledge gaps to inform priority setting in occupational health research and guide the development of new research questions in the EU-EPHOR project and in occupational health research in general.
Methods
We conducted a narrative review on the status of knowledge in the associations between occupational exposures and their hypothesized health effects. We selected 6 major NCD groups: nonmalignant respiratory diseases; neurodegenerative diseases; cardiovascular and metabolic diseases; mental disorders; musculoskeletal diseases; and cancer. For each disease group, expert sessions were held with occupational health researchers from the EPHOR project. The experts were epidemiologists (both junior and senior researchers) with specific interests and expertise in the respective NCD. Based on occurrence, severity, and the possible importance of work-related exposures, the most relevant diseases and disorders within each group were selected. Regular meetings between the expert group leaders were held to align efforts, coordinate literature searches, and discuss findings.
Given the broad scope of diseases and exposures, a systematic review was not feasible. Hence, we conducted a comprehensive narrative review for each exposure-disease combination, which information was then assessed by the occupational health experts in each NCD group. Systematic reviews and meta-analyses were considered most informative and were therefore, where possible, used as the basis for the current overview. If such information was not available, we included additional sources such as narrative reviews, reports, and original studies. For cancer, a longstanding comprehensive evaluation program exists: the International Agency for Research on Cancer (IARC) monographs on the identification of carcinogenic hazards to humans. Hence, the cancer expert group summarized the IARC evaluations.
The status of knowledge on exposure-disease associations was classified into 2 categories: 1) “consistent evidence for an association” when indicated by state-of-the-art reviews; and 2) “limited or inadequate evidence for an association” if reviews concluded that the evidence for association was weak or insufficient, when only few original studies were published, or when there was disagreement between the reviews regarding the evidence for the observed association. This classification was inspired by the IARC monograph preamble (Samet et al. 2020), and discussions on the classification were held within the NCD expert groups as well as across NCD groups among the expert group leaders.
Based on the reviewed literature, knowledge gaps were identified and described for the various exposure-disease combinations during discussions in the expert sessions. We focused on key topics that would be needed in occupational epidemiological studies to increase the current understanding of the disease aetiology, including exposures-response quantification, sex differences, critical exposure-time-windows, and exposure interactions, as well as improved study designs (including better control for confounders), disease identification, and exposure assessment.
Results
Status of Knowledge on Associations
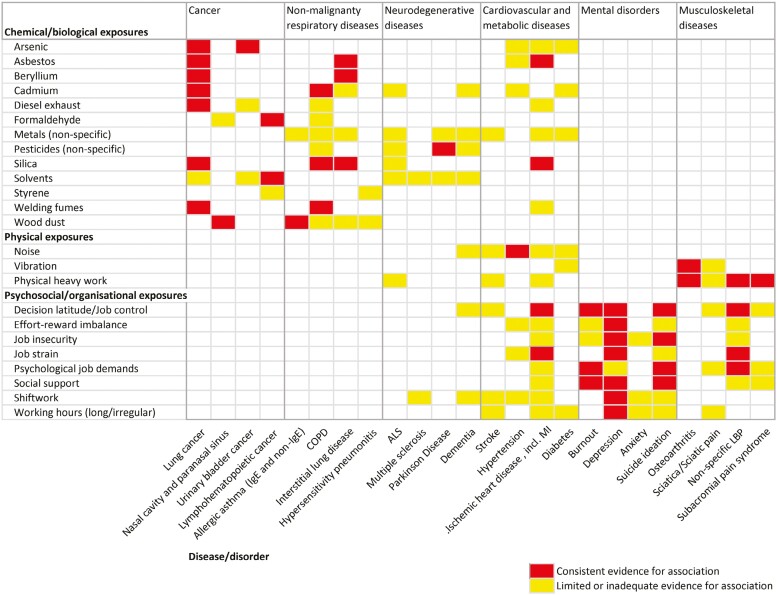
Tables 1–5 present the status of knowledge for the exposures that are suspected to be associated with nonmalignant respiratory diseases; neurodegenerative diseases; cardiovascular and metabolic diseases; mental disorders; and musculoskeletal diseases. For cancer, the overview based on the IARC evaluations is shown in Supplementary Table S1. Below we summarized the findings per disease group, as described in the tables. Supplementary Tables S2–S6 show this information grouped by exposure type (i.e. chemical and biological agents; physical exposures; biomechanical exposures; psychosocial and organizational exposures; and specific occupations, industries, and processes), with the full list of references. In a heatmap, we visualized the evidence for the associations between occupational exposures that were relevant for more than one of the major disease groups, for a selection of NCDs to demonstrate the overlap (Fig. 1).
Table 1.
Occupational risk factors for non-malignant respiratory diseases.
| Disease/health condition | Consistent evidence for association | Limited or inadequate evidence for association |
|---|---|---|
| Chronic obstructive pulmonary disease (COPD), excess lung function decline |
Chemical and biological exposures
Coal mine dust Cadmium fume Farming dust (grain) Respirable crystalline silica Textile dust (cotton, flax, jute) Vapours, gases, dusts or fumes (VGDF, non-specified) Welding fume |
Chemical and biological exposures
Alcohol Asphalt emissions Cement dust Coke oven emissions Disinfectants Endotoxin Engine exhaust, diesel Formaldehyde Glutaraldehyde Hypochlorite bleach Hydrogen peroxide Pesticides Quaternary ammonium compounds Rubber process dust Wood dust Occupation, industry, and process Glass/ceramics production Petroleum production Tunnel work |
| Allergic IgE-mediated asthma |
Chemical and biological exposures
High molecular weight (HMW) allergens (flour fish and animal proteins, enzymes, mites) Some low molecular weight (LMW) allergens (e.g. platinum salts; phthalic anhydride, wood dust from western red cedar and pine) Some wood dusts and plants (e.g. obeche, psyllium, latex) |
Chemical and biological exposures
Drugs (e.g. opiates, antibiotics) Insects Molluscs Moulds Some wood dust and plants (e.g. pine, beech, oak, iroko, tobacco, paprika, coffee) |
| Allergic non IgE-mediated asthma |
Chemical and biological exposures
Endotoxin Isocyanates Western red cedar |
Chemical and biological exposures
Drugs (e.g. opiates, antibiotics) Metals Moulds Some wood dust and plants (e.g. pine, beech, oak, iroko, tobacco, paprika, coffee) |
| Acute irritant induced asthma (formerly known as reactive airways dysfunction syndrome, RADS) |
Chemical and biological exposures
Irritants (gas, smoke, fumes, vapours) |
|
| Low-dose repeated exposures to irritant-induced asthma |
Chemical and biological exposures
Chemicals Chlorine gas Cleaning agents and disinfectants Combustion particles/fumes Fluoride (aluminium production) Irritant gases/fumes |
Chemical and biological exposures
Solvent vapours |
| Interstitial lung disease (pneumoconiosis) |
Chemical and biological exposures
Asbestos Beryllium Coal dust Hard metal (e.g. tungsten carbide, cobalt) respirable crystalline silica |
Chemical and biological exposures
Metals (tin, iron, copper, cadmium, indium) Non-silica coal dust Nylon Talc |
| Interstitial lung disease (idiopathic pulmonary fibrosis) |
Chemical and biological exposures
Metal dust Stone/sand/silica dust VGDF (vapours, gases, dust, and fumes) Wood dust Occupation, industry, and process Agriculture/livestock Hairdressing Raising birds |
|
| Sarcoidosis |
Chemical and biological exposures
Agricultural dust Cotton dust Pesticides Metal dust Mineral fibres Mould/mildew Respirable crystalline silica Wood dust Occupation, industry, and process Automobile manufacturing Fire fighting Military personnel Raising birds |
|
| Hypersensitivity pneumonitis |
Chemical and biological exposures
Metalworking fluids (water-based, microbial contamination) Mould/fungus and mites (e.g. mouldy hay, dry sausage dust, flour dust) Proteins from birds |
Chemical and biological exposures
Fluor Fluorocarbon Isocyanates Marine biomolecules (salmon, shrimp powder) Styrene Waterproofing spray Wood dust (pine, medium density fibreboard (MDF)) |
| Chronic rhinosinusitis |
Chemical and biological exposures
Viral infection Psychosocial exposures Psychosocial factors |
|
| Chronic/irritant/perennial rhinitis |
Chemical and biological exposures
Irritants |
Chemical and biological exposures
Ammonia Bleach Chlorine gas Cleaning agents Hydrochloric acid Hydrogen sulphide Nitrogen hydroxide Solvents |
| Allergic rhinosinusitis/rhinitis and hay fever |
Chemical and biological exposures
Enzymes Fish and animal proteins Flour Mites Some low molecular weight (LMW) allergens (e.g. platinum salts; phthalic anhydride) Some wood dust and plants (e.g. obeche, latex) |
Chemical and biological exposures
Drugs (e.g. opiates, antibiotics) |
Supplementary Tables S2–S6 show this information grouped by exposure type, with the full list of references.
Table 5.
Occupational risk factors for musculoskeletal diseases.
| Disease/ health condition | Consistent evidence for association | Limited or inadequate evidence for association |
|---|---|---|
| Knee osteoarthritis |
Biomechanical exposures
Heavy lifting Kneeling and squatting in combination Physically heavy work |
Biomechanical exposures
Climbing stairs Kneeling Lifting and carrying heavy loads in combination Lifting and kneeling/squatting in combination Standing |
| Hip osteoarthritis |
Physical exposures
Whole body vibration (in men) Biomechanical exposures Heavy lifting |
Biomechanical exposures
Kneeling and squatting in combination Lifting and carrying heavy loads in combination Standing |
| Hand/wrist osteoarthritis |
Physical exposures
Hand-arm vibration Biomechanical exposures Forceful hand movement Highly repetitive hand tasks |
|
| Subacromial pain syndrome |
Biomechanical exposures
Arm elevation (hands at or above shoulder level) Combined biomechanical exposures Forceful shoulder exertion (e.g. lifting/carrying and pushing/pulling) |
Physical exposures
Hand-arm vibration Biomechanical exposures Repetitive shoulder movement Psychosocial exposures Job control/decision latitude Psychosocial job demands Social support |
| Sciatic pain |
Physical exposures
Whole-body vibration, professional driving Biomechanical exposures Kneeling/squatting Manual material handling (lifting, carrying, pushing/pulling) Physically heavy work Sitting at work Spinal loading Trunk flexion, twisting of the trunk Working with hands above shoulder level Psychosocial exposures: Job control Job demands Job satisfaction Social support Underutilization of skills and expertise Organizational exposures Irregular or long working hours |
|
| Sciatica |
Physical exposures
Whole-body vibration, professional driving Biomechanical exposures Kneeling/squatting Lifting and bending of the trunk Lifting and carrying Physically heavy work Sitting at work (not driving) Twisting of the trunk, bending and twisting of the trunk |
|
| Non-specific low back pain |
Physical exposures
Whole-body vibration Biomechanical exposures Awkward trunk posture, including bending Combined biomechanical exposures Heavy lifting Manual material handling/patient handling Psychosocial exposures Job control Job dissatisfaction Job strain Psychosocial job demands |
Biomechanical exposures
Carrying, pushing, or pulling Kneeling/squatting Physically heavy work Repetitive movement Sitting at work Standing (alone or in combination with walking) Psychosocial exposures Effort-reward imbalance Highly monotonous work Job insecurity Social support |
| Carpal tunnel syndrome |
Physical exposures
Hand-arm vibration Biomechanical exposures Hand force Repetitive movement |
Chemical and biological exposures
Chemicals (non-specific) Physical exposures: Working in a cold environment Biomechanical exposures Computer work Extended/flexed wrist Psychosocial exposures Job control Psychosocial work demands |
Supplementary Tables S2–S6 show this information grouped by exposure type, with the full list of references.
Figure 1.
Heatmap of the evidence for a selection of exposure-disease associations, demonstrating the overlap in relevant occupational exposures. (NB: we here visualized the overlap for a selection of the NCDs and exposures that we reviewed, the white spaces do not suggest that there is no association).
Nonmalignant Respiratory Diseases
There is consistent evidence that exposures to various types of dust (both mineral and biological) and fumes are risk factors for chronic obstructive pulmonary disease (COPD) (Table 1). For allergic asthma, both high molecular weight (HMW) agents (e.g. flour, fish, and animal proteins) and low molecular weight (LMW) agents (e.g. platinum salts, di-isocyanates, and phthalic anhydride) have been identified as risk factors. Several HMW and LMW agents are also known to be able to cause allergic rhinitis and rhinosinusitis, both in child- and adulthood. Asthma can also be caused by irritant exposures (irritant-induced asthma (IIA)), with strong evidence for new-onset asthma occurring suddenly following exposure to high concentrations of irritants. More recently, evidence has emerged that repeated exposure to irritants at lower levels can also cause IIA, with onset after a latency period (Dumas and Le Moual 2016), most clearly for cleaning agents (Archangelidi et al., 2021; Dumas, 2021).
Interstitial lung disease is a heterogenous group of diseases characterized by inflammation and fibrosis. Inhalation exposure to asbestos, silica, coal dust, beryllium, and hard metals are risk factors with consistent evidence for pneumoconiosis. Hypersensitivity pneumonitis is associated with exposures such as bacteria, fungi, animal and plant proteins, and metals, with various levels of evidence. For idiopathic pulmonary fibrosis (IPF) there is limited/inadequate evidence for associations with exposure to dust from metal, wood, stone, sand, and silica, as well as with exposures related to agriculture and livestock, questioning the classification as idiopathic. Evidence is yet limited/inadequate for associations between sarcoidosis and exposures to various dusts including silica and agricultural dust.
Neurodegenerative Diseases
Few exposures have been reported as occupational risk factors for neurodegenerative diseases with consistent evidence, including extremely low-frequency magnetic fields (ELF-MF) and military service for amyotrophic lateral sclerosis (ALS), pesticides for Parkinson’s disease, and manganese for parkinsonism (Table 2).
Table 2.
Occupational risk factors for neurodegenerative diseases.
| Disease/health condition | Consistent evidence for association | Limited or inadequate evidence for association |
|---|---|---|
| Amyotrophic lateral sclerosis |
Physical exposures
ELF-MF Occupation, industry, and process Military service |
Chemical and biological exposures
Metals (incl. lead, cadmium) Pesticides Respirable crystalline silica Solvents Viral infection Physical exposures Electric shock Trauma Biomechanical exposures: Physical activity |
| Parkinson’s disease |
Chemical and biological exposures
Pesticides (incl. paraquat and rotenone) |
Chemical and biological exposures
Hydrocarbons (incl. solvents, such as trichloroethylene) Metals (incl. lead) Physical exposures ELF-MF Head trauma |
| Parkinsonism |
Chemical and biological exposures
Manganese |
|
| Dementia |
Chemical and biological exposures
Metals (incl. cadmium, aluminium) Pesticides Solvents Physical exposures ELF-MF Head trauma Noise Psychosocial exposures Job complexity Job control Organizational exposures Night shift work |
|
| Multiple sclerosis |
Chemical and biological agents
Organic solvents Organizational exposures Night shift work |
ELF-MF: extremely low-frequency magnetic fields.
Supplementary Tables S2–S6 show this information grouped by exposure type, with the full list of references.
Evidence is mixed for the effect of various metals and solvents on neurodegenerative diseases, possibly because the exposure groups include diverse substances with different properties. Among metals, higher levels of cadmium in urine or blood are associated with Alzheimer’s disease and ALS in the general population. However, it is unknown if this relationship is also present among persons exposed to cadmium in the workplace, as biological cadmium concentrations may be a proxy for smoking. Occupational exposure to lead has been suggested as a risk factor for both ALS and Parkinson’s disease, but results are yet inconclusive.
Risk factors such as head traumas and possibly also night shift work may increase dementia risks via effects on toxic proteins (e.g. hyperphosphorylated tau) in the brain. Some work characteristics, such as high job control and cognitive complexity, are suspected to be protective against dementia. However, more work is needed to disentangle these factors from other socio-economic factors to confirm their individual effects.
Cardiovascular and Metabolic Diseases
There is consistent evidence for the association between noise exposure and hypertension, while evidence is limited/inadequate for associations with job strain, effort-reward imbalance, metals, asbestos, and shift work (Table 3). There is consistent evidence for the association between long working hours and stroke. There is limited/inadequate evidence that workplace exposures to metals, various chemicals, ionizing radiation, noise, physically heavy work, shift work, as well as decision latitude/job control, are associated with stroke.
Table 3.
Occupational risk factors for cardiovascular and metabolic diseases.
| Disease/health condition | Consistent evidence for association | Limited or inadequate evidence for association |
|---|---|---|
| Hypertension |
Physical exposures
Noise |
Chemical and biological exposures
Asbestos Metals (lead, cadmium, arsenic) Psychosocial exposures Effort-reward imbalance Job strain Organizational exposures: Shift work |
| Stroke |
Organizational exposures
Long working hours |
Chemical and biological exposures
Agrochemicals Carbon disulphide Metals (lead, mercury) Phenoxy acids containing TCDD Physical exposures Ionizing radiation Noise Biomechanical exposures Physical activity/physically heavy work Psychosocial exposures: Decision latitude/ job control Organizational exposures Shift work Occupation, industry, and process Electrolytic production of aluminium |
| Ischemic heart disease |
Chemical and biological exposures
Asbestos Carbon disulphide Engine exhaust (incl. diesel) Metals (arsenic, lead, cadmium) Phenoxy acid containing TCDD Respirable crystalline silica Psychosocial exposures Decision latitude/job control Job strain Organizational exposures Long working hours |
Chemical and biological exposures
Benzo(a)pyrene Carbon monoxide Metals (methylmercury) Metalworking fluids Nitroglycerine PAHs Welding fumes Dichloromethane Physical exposures Noise Biomechanical exposures Physical activity/physically heavy work Psychosocial exposures Effort-reward imbalance Job insecurity Psychosocial job demands Social support Organizational exposures Shift work Occupation, industry, and process Electrolytic production of aluminium Fire fighting Paper production (using sulphate pulping process) |
| Diabetes mellitus (type 1 and 2) |
Chemical and biological exposures
Bisphenol A Metals (copper, zinc, arsenic, selenium, molybdenum, cadmium, manganese, barium, lead) Physical exposures Noise Vibration Organizational exposures Long working hours |
|
| Gestational diabetes |
Physical exposures
Noise Vibration Psychosocial and organizational exposures Long working hours |
|
| Obesity |
Organizational exposures
Night shift work |
Biomechanical exposures
Sedentary work Psychosocial exposures: Job strain Organizational exposures Long working hours Shift work (any) |
TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; PAH: polycyclic aromatic hydrocarbons.
Supplementary Tables S2–S6 show this information grouped by exposure type, with the full list of references.
There is consistent evidence for an increased risk of ischemic heart disease (IHD) for workers who experience high job strain, low decision latitude/job control, and long working hours. There is limited/inadequate evidence for an increased IHD risk among workers with physically heavy work, effort-reward imbalance, little social support at work, injustice or insufficient opportunities for personal development, job insecurity, and those who work night shifts, as well as those who are exposed to noise. Consistent evidence for increased risks of IHD was also found for workplace exposure to silica dust, asbestos, engine exhaust (including diesel), metals (lead, arsenic, cadmium), phenoxy acids containing 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD), and carbon disulphide. Limited/inadequate evidence for associations with IHD exists, among others, for exposure to metalworking fluids, carbon monoxide, polycyclic aromatic hydrocarbons, welding fumes, and noise, as well as working with electrolytic production of aluminium or the production of paper when the sulphate pulping process is used.
There is limited/inadequate evidence for associations between exposure to noise, vibration, metals, bisphenol A, and long working hours and an increased risk of type 1, type 2, or gestational diabetes. Obesity, a marker of metabolic disease, has been associated with night shift work with consistent evidence, and there is limited/inadequate evidence for an association with experienced job strain, sedentary work, and long working hours.
Mental Disorders
Anxiety has shown to be consistently associated with bullying, whereas the evidence for an association with violence, job insecurity, temporary agency work, working hours, and shift work is limited/inadequate (Table 4).
Table 4.
Occupational risk factors for mental disorders.
| Disease/health condition | Consistent evidence for association | Limited or inadequate evidence for association |
|---|---|---|
| Burnout |
Psychosocial exposures
Aggression by customer Bullying Decision latitude Emotional demands Psychosocial demands Social support Violence, threats |
Psychosocial exposures:
Aggression by co-workers or supervisor Effort-reward imbalance Job insecurity Organisational injustice |
| Depression |
Psychosocial exposures
Bullying Effort-reward imbalance Emotional demands Decision latitude/ Job control Job strain Social support Violence, threats Job insecurity Organizational exposures Shift work Long working hours |
Psychosocial exposures
Development possibilities Procedural injustice Psychosocial demands Relation injustice Skill discretion Workplace conflicts Organizational exposures: Night work Temporary employment |
| Anxiety |
Psychosocial exposures
Bullying |
Psychosocial exposures
Job insecurity Temporary agency work Violence Organizational exposures Shift work Working hours |
| Suicide ideation |
Psychosocial exposures
Bullying Decision latitude Job insecurity Psychosocial demands Social support |
Psychosocial exposures
Effort-reward imbalance Job strain Role conflict Organizational exposures Shift work Working hours |
| Suicide |
Chemical and biological exposures
Pesticides |
Psychosocial exposures
Decision latitude Psychosocial demands Social support |
Supplementary Tables S2–S6 show this information grouped by exposure type, with the full list of references.
There is consistent evidence for an association between burnout for aggression at work by customers, violence or threats, bullying, low decision latitude, high emotional demands, high psychosocial demands, and low social support. There is limited/inadequate evidence for burnout with aggression by co-workers or supervisors, organizational injustice, lack of rewards, and high job insecurity.
The risk factors with consistent evidence for depression are bullying, violence or threats, effort-reward imbalance, high emotional demands, low decision latitude/job control, high job strain, low social support at work, job insecurity, shift work, and long working hours. Associations with job strain and working hours did not differ by age, sex, or socioeconomic position (Madsen et al. 2017; Virtanen et al. 2018; Wong, Chan, and Ngan 2019), whereas stronger associations between job insecurity and depression were reported for younger workers (Kim and von dem Knesebeck 2016). There is limited/inadequate evidence that increased risk of depression is associated with low development possibilities, relational injustice, procedural injustice, high psychosocial demands, low skill discretion, and workplace conflicts as psychosocial factors, as well as for temporary employment and night work.
Several psychosocial work factors (decision latitude, psychosocial job demands, and lack of social support) for which consistent evidence exists on associations with suicide ideation, were also suspected risk factors for actual suicide, but with limited/inadequate evidence. There was consistent evidence for an association between exposure to pesticides and suicide.
Musculoskeletal Diseases
The associations between heavy lifting and osteoarthritis (knee and hip osteoarthritis), and nonspecific low back pain have been consistently reported in the literature (Table 5). So have manual material handling for low back pain and forceful shoulder exertion and arm elevation for subacromial pain syndrome. There is limited or inconsistent evidence for associations with osteoarthritis and nonspecific low back pain for more specific tasks, such as climbing stairs (knee osteoarthritis), standing and walking (knee and hip osteoarthritis, and nonspecific low back pain), kneeling (knee osteoarthritis, nonspecific low back pain), and highly repetitive tasks (hand/wrist osteoarthritis and nonspecific low back pain). There is also limited/inadequate evidence for associations with subacromial pain syndrome for hand-arm-vibration and repetitive shoulder movements.
There are no occupational exposures with consistent evidence for associations with either sciatica or sciatic pain, but there is limited/inadequate evidence for associations with physically heavy work, trunk flexion/twisting, manual material handling, lifting, spinal loading, whole body vibration, working with hands above shoulder level, kneeling/squatting, bending of the trunk, and sitting at work. Hand force, repetitive movements, and hand-arm vibration are associated with carpal tunnel syndrome with consistent evidence. Evidence for associations between carpal tunnel syndrome and extended/flexed wrist, computer work, psychosocial exposures, cold environment, and chemicals is limited/inadequate.
A role of psychosocial exposures (including job demands, job control, and social support) has been suspected for subacromial pain syndrome, sciatic pain, and carpal tunnel syndrome, but with limited/inadequate evidence for an association. For nonspecific low back pain, there is consistent evidence for associations with job control, job dissatisfaction, job strain, and psychosocial job demands.
Cancer
Among the approximately 1000 agents evaluated since 1971, IARC has identified 47 occupational agents with consistent evidence for an association for one or more cancer types (“Group 1 agents”), which are listed in Supplementary Table S1 (Loomis et al. 2018; Marant Micallef et al. 2018; IARC n.d.). Lung cancer has been associated with the largest number of occupational carcinogens (n = 19), followed by cancer of the skin (n = 8), the haematolymphatic system (n = 7), the urinary bladder (n = 6), bone (n = 5), and nasal cavity and paranasal sinus (n = 5). The 47 established carcinogens can be further classified into chemicals (n = 15) and chemical mixtures (n = 4), radiation and radionuclides (n = 12), airborne particles (n = 9), airborne complex mixtures (n = 2), and metals and metal compounds (n = 5). The primary routes of exposure are inhalation and dermal uptake. Additionally, 13 occupations, industries, and processes (e.g. rubber manufacturing or working as a painter) were identified as causally associated with cancer, though the specific agents were not identified. Limited evidence for an association with cancer exists for an additional 19 occupational agents or exposures (classified by IARC as probable carcinogenic to humans [Group 2A]). For these exposures, a positive association has been observed but limitations remain in the overall body of evidence including questions on chance, bias, or confounding (Marant Micallef et al. 2018).
Discussion
More than 200 occupational risk factors (including both specific exposures and broader categories of exposures) have been reported for the 6 major disease groups reviewed in this paper, in total covering over 60 NCDs. Several exposures were identified as possible risk factors for multiple disease groups (a selection is shown in Fig. 1). Examples are diesel engine exhaust and cadmium, for which there is consistent evidence for lung cancer and limited/inadequate evidence for associations with other cancer sites, and with respiratory, neurodegenerative and cardiovascular diseases (Supplementary Table S2). Another example is shift work, which showed consistent evidence for an association with mental disorders, and is also possibly associated with cancer, as well as several neurodegenerative and cardiovascular diseases (Supplementary Table S5). Decision latitude/job control is consistently associated with IHD, burnout, depression, suicide ideation, and nonspecific low-back pain, plus there are possible associations with dementia and stroke, among others (Supplementary Table S5).
More knowledge is warranted for associations between occupational exposures and NCDs at all evidence levels. Improvements in study design, exposure assessment, and case definition would be needed for associations with limited/inadequate evidence, to reach consistent evidence on causation. Regarding occupational risk factors with consistent evidence, the underlying mechanisms can be better understood by improving the study quality to explore exposure–response relationships and critical exposure–time–windows, for example. Furthermore, better understanding of the association will help inform health-based occupational exposure limits. We here describe the needs for further research on occupational risk factors for NCDs.
Study Design
Due to long latency periods, the exact onset is unknown for many NCDs, in particular for cancer and neurodegenerative diseases. The disease process may start much earlier than the initial appearance of symptoms. Due to such preclinical effects, results from case–control studies may also be affected by reverse causation. Parkinson’s disease, e.g. has a long prodromal phase characterized by symptoms, such as constipation and sleep disorders, that might be present up to 20 years before diagnosis (Savica et al. 2009). Such symptoms are likely to affect medication and lifestyle, such as diet and physical activity, but potentially also occupational exposures, such as changes in night shift work (Ascherio and Schwarzschild 2016). The inverse association between high job control and cognitive complexity in dementia may also be driven by reverse causation. The same limitations apply to mental health problems, which usually start in the first half of people’s lives (the median age of first episodes of anxiety is in the teens and for depression in the mid-thirties). Most epidemiological studies on occupational health, however, include middle-aged workers, and reversed causality cannot be ruled out for some exposures and outcomes. For instance, bullying can lead to depression, but depressed people might also be more vulnerable to bullying (Boudrias, Trépanier, and Salin 2021). Hence, a prospective cohort study design would improve the identification of risk factors for many NCDs.
There is a general need for improved harmonization and coordination of epidemiological studies in occupational health (Marant Micallef et al. 2018; Turner and Mehlum 2018). Recently, large-scale pooling efforts have provided larger power to examine cancer risks. Pooling case–control studies and enhanced exposure assessment provided more detailed data on exposure–response relationships, including associations at low levels of exposure, interactions with smoking, and on more specific cancer subtypes (Ge et al. 2020; Hovanec et al. 2021). Furthermore, large-scale and multicentre studies have been used to examine rare outcomes, including male breast cancer (Sritharan et al. 2019) and other rare cancers (Lynge et al. 2020). Such approaches could also be applied to other NCDs, where large numbers may help to detect relatively low-effect risk factors and to enable the investigations of more in-depth analyses of occupational risk factors with consistent evidence of an association.
Exposure Assessment
Overall, the lack of evidence of causality for many possible associations may be attributed to inadequate exposure assessment methods. Better assessment of the suspected causative agent is needed to ensure health-based occupational exposure limits as the basis for implementation of exposure reducing measures, as well as to further characterize the nature of the association.
Many studies rely on self-reported exposures collected via interviews or questionnaires, which is prone to recall bias (Rothman, Greenland, and Lash 2008). Knowledge of disease diagnosis may affect the reporting of exposure, for example when someone has osteoarthritis, carpal tunnel syndrome, or any type of cancer. However, also the disease itself may affect the memory or behaviour of patients. This effect may be particularly true for Alzheimer's and other dementias, but also for patients with depression, Parkinson’s disease, or ALS with co-existing dementias. Future epidemiological studies should strive to assess exposures before the disease onset, preferably quantifying the relevant exposure with respect to duration, frequency, and intensity level of the suspected causative agent. Potential sources of biases and confounding need to be identified and accounted for.
Moreover, similar concepts of exposure measures have been differently operationalized, including reference to different time periods (e.g. from “current” to “in the past year”) and application of different cut-off points. For biomechanical exposures, e.g. there are a limited number of studies with valid, objective physical workload exposure measures repeated over time, and there is large heterogeneity in the measurement and definition of the exposures overall. It is recommended to use validated and comparable exposure measurement methods as well as harmonized definitions of exposure and its metrics.
Longitudinal studies with good exposure data are warranted to fill important knowledge gaps regarding exposure–response relationships, critical exposure–time–windows, and age- and sex-specific associations. Since many occupational exposures co-exist, a major challenge is to disentangle the effect of each as well as estimate the potential interactive effects of combined exposures. For asthma, e.g. there is a special need to address the multitude of exposures present in many work environments, including irritant and sensitizing agents. Few studies have been able to study the potential interaction of these combined exposures; some studies have investigated multiple occupational airborne exposures and asthma or lung function decline separately (Kogevinas et al. 2007; Skaaby et al. 2021), but without an assessment of the joint effect of these exposures.
Some relevant occupational exposures may also exist in residential settings, hence the knowledge gained through environmental studies could be used to interpret associations in occupational settings. Examples include exposure to metals, cleaning products, noise, and physical activity. However, exposure levels as well as mechanisms may differ. For example, exposure to metals in industrial settings will generally be at much higher levels than exposure to drinking water. Furthermore, the health effects of noise exposure (as reported for dementia and mental disorders, for example) go partly through sleep disturbance in residential settings, while in the workplace only an awake response plays a role. Since the relevant levels of exposure, the composition of compounds, and relevant exposure routes and mechanisms may differ between settings, further exploration in occupational settings will be needed for certain suspected occupational risk factors. Such further exploration may also be relevant for mental health, as suggestions for associations with physico-chemical exposures in the general environment have been reported (Dickerson et al. 2020), whereas published occupational studies on these health outcomes are primarily focused on psychosocial and organizational exposures.
Case definition
A major challenge in NCD epidemiology is the various definitions and criteria for the health outcome, particularly for nonmalignant respiratory, neurodegenerative, and musculoskeletal diseases, as well as mental disorders.
For COPD, definitions range from self-reported symptoms to spirometry and physician-diagnosis. Consistently higher risk estimates for occupational airborne agents have been reported for self-reported versus spirometry-defined COPDs (Doney et al. 2019), suggesting bias in self-reported disease outcomes. Collecting and using high-quality data from postbronchodilator spirometry in future studies is therefore important. The clinical, radiological, and pathological findings for interstitial lung diseases may be indistinguishable from one subtype to another, creating the risk of misclassification. Furthermore, the definition of diseases by the presence or absence of causal exposures (as for silicosis) precludes meaningful investigation of the association between exposure and disease. Thus, studies using objective measures of outcome are needed to avoid disease misclassification for meaningful investigations of association.
The diagnosis of neurodegenerative diseases is also complicated, and these diseases are largely under-reported on death certificates. For example, reporting of dementia has improved over time, but still less than half of dementia deaths are reported. Of those that are reported, 70% are recorded as ‘unspecified’ dementia in England and Wales (Gao et al. 2018).
In studies on mental disorders, there are large differences in the measurement of outcomes and cut-off points and these are often not clearly described. For instance, depression covers a broad spectrum, ranging from depressive symptoms measured by a self-administered questionnaire, to clinically diagnosed depression. Moreover, depression can be both episodic (nonchronic) and long-term (chronic), which makes it important to assess the onset as well as the duration of depression.
Osteoarthritis assessment is typically based on valid and reliable methods, such as radiographic or standardized clinical assessments, or a combination of both. However, there is still large heterogeneity across the studies in osteoarthritis outcome definitions in terms of diagnostic criteria and severity, possibly resulting in inconsistent evidence. Likewise, considerable heterogeneity was observed in the definition of subacromial pain syndrome and assessment of carpal tunnel syndrome, which adds challenges to the comparison of exposure-response relationships. Various operationalizations of sciatica and sciatic pain have been used, often involving self-reports. However, more consistent evidence was found for associations of occupational exposures with a more conservative definition of carpal tunnel syndrome (Barcenilla et al. 2012) and clinically defined sciatica and sciatic pain (SBU 2014; Kuijer et al. 2018), indicating the importance of clinical data in the case definition (van der Molen et al. 2021).
Towards an Exposome Approach
The exposome concept, incorporating all nongenetic risk factors experienced during a person’s life, offers a more holistic approach to investigating how occupational exposures may eventually result in disease (Wild 2005). To succeed, it is important to have an emphasis on detailed external exposure characterization across a broad range of occupational and nonoccupational factors over the life course, consideration of critical exposure-time periods and temporal variation of exposures, vulnerable subpopulations (including, but not limited to, young workers, pregnant women, ageing workers, and low-educated workers), and transgenerational health effects (Bessonneau and Rudel 2019; Pronk et al. 2022). Taking an exposome approach requires sufficiently large study populations with detailed information on occupational exposures, as currently pioneered in the EPHOR consortium (Pronk et al. 2022). Given the constant movement of the workforce, the collection of full work histories (i.e. all occupations a person had including time period per occupation) is essential to facilitate studying the role of occupational exposures across the life course. For example, the inclusion of data on individual occupations as a regular data field in population-based health data, such as cancer registry data, would be a major step forward.
Although there are many established associations for cancers (Supplementary Table S1), the effects of age and timing of exposure have virtually only been reported for lung cancer (e.g. for chromium (Gibb et al. 2020) and asbestos (Boffetta et al. 2019)), but not yet for most other cancer sites. Few studies have focused on vulnerable subgroups or populations. For example, workers in lower- and middle-income countries, as well as female workers are typically understudied (Stücker et al. 2017). Some notable exceptions include a study of occupational risk factors for lung cancer in women (Xu, Ho, and Siemiatycki 2021), an investigation into cancer risk due to bystander or spousal exposure to occupational agents (Jackson et al. 2017; Louis et al. 2017), and studies of occupation and breast and prostate cancer in the African continent (Adler et al. 2019; Khalis et al. 2019). Recent studies have further examined individual susceptibility to occupational carcinogens, including genetic susceptibility to radon gas (Rosenberger et al. 2018) and asbestos (Liu et al. 2015). For all NCDs, however, additional epidemiological studies examining potentially susceptible subpopulations and timing of exposure are needed (Kelly and Vineis 2014), both to provide stronger evidence for associations and to improve our understanding of NCD aetiology.
Further research on mixtures and interactions is also warranted (Nagy et al. 2020), as many health outcomes are associated with multiple occupational exposures. Approaches for these investigations are demonstrated by previous studies considering both occupational and environmental sources, including diesel engine exhaust (Vermeulen et al. 2014), asbestos (Visonà et al. 2018; Panou et al. 2019; Huh et al. 2021), and ultraviolet radiation (Vienneau et al. 2017). Recently, the interaction between various occupational lung carcinogens has been described (Olsson et al. 2024). Knowledge of the interrelation between different risk factors (Harvey et al. 2017) is also needed to better understand the role of psychosocial working conditions on mental health. Specifically, research is needed on the effect of multiple adverse exposures, while accounting for favourable factors (social support, reward, development possibilities) which might have a mitigating effect on disease outcome.
Internal exposome data further provide possibilities to investigate biomarkers of occupational exposures and early biological effects to add insight into mechanisms in disease development and understanding of causal pathways (Vermeulen et al. 2020). Triangulation of results from studies using different approaches is gaining increasing importance in causal inference (Lawlor, Tilling, and Davey Smith 2016). The updated preamble to the IARC monograph program also stressed the importance of integrating evidence from different streams to identify the causes of human cancer (Samet et al. 2020).
Furthermore, possible interrelations between health outcomes should be considered. While mental disorders are considered an outcome in the current review, it is well-known that mental health problems can also be a proxy or precursor for other diseases. For example, burnout or depression might act as a proxy or precursor for suicide ideation or suicide, but it is also of interest to investigate the role of mental health in relation to cardiovascular and musculoskeletal diseases. Although evidence suggests a link between mental health and physical disease later in life, there is also a bidirectionality in the relationship (Kivimäki et al. 2020).
Conclusion
We conducted a narrative review, covering a wide range of occupational risk factors for 6 major NCD groups. This work was initiated from the EU-EPHOR project on the working life exposome. While a more systematic approach would have been preferable, the broad scope of occupational exposures and diseases did not allow for a systematic review. Also, the variability in the state of knowledge between the different disease groups hampered a standard approach, e.g. the availability of structured evaluations of the association of various occupational risk factors and cancer, based on dozens to hundreds of studies, compared to very few studies on occupational risk factors for neurodegenerative diseases. Hence, we used discussions in expert sessions to describe the status of knowledge from the literature and to identify knowledge gaps. Nevertheless, we may have missed publications, and we acknowledge that the classification of the association into one of the 2 categories of evidence was not fully standardized across the 6 major disease groups. However, our pragmatic approach allowed us to provide a comprehensive overview for the occupational health research community, describing the status of knowledge of exposure-disease associations across various disease and exposure types, and identifying areas for future research that are relevant for almost all occupational NCDs.
Overall, we identified more than 200 occupational exposures with possible associations with common NCDs. Various areas for improvement have been identified; a prospective cohort design and better specification of exposures have been repeatedly suggested for areas with limited/inadequate causal evidence (Supplementary Tables S2–S6). Obtaining sufficient evidence for suspected exposure-disease associations will importantly improve estimates of the contribution of occupation to the global burden of diseases, whose contribution is currently underestimated. In areas in which there is already consistent evidence for associations between exposures and diseases, further exploration of the exposure–response relationships, possible interactions between exposures, and critical exposure-time-windows will enhance understanding of the aetiologies, which in turn will inform health-based occupational exposure limits. Furthermore, knowledge regarding vulnerable groups is limited, whereas such information is crucial for targeted and effective prevention of work exposures leading to disease.
Finally, the provided overview of identified knowledge gaps in the understanding of adverse health effects of occupational exposures will optimize sound priority setting and recommendations for new research in occupational health.
Supplementary material
Supplementary material is available at Annals of Work Exposures and Health online.
Acknowledgements
EPHOR is funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 874703. KU was supported by a Short-Term Scientific Missions from COST Action CA16216 (OMEGA-NET), supported by COST (European Cooperation in Science and Technology).
Contributor Information
Susan Peters, Institute for Risk Assessment Sciences, Utrecht University, Yalelaan 2, 3584 CM Utrecht, the Netherlands.
Karina Undem, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Svetlana Solovieva, Finnish Institute of Occupational Health, P.O. Box 40 FI-00032 TYÖTERVEYSLAITOS, Finland.
Jenny Selander, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden.
Vivi Schlünssen, Department of Public Health, Research Unit for Environment, Occupation and Health, Danish Ramazzini Centre, Aarhus University, Bartholins Allé 2 DK-8000 Aarhus, Denmark.
Karen M Oude Hengel, Netherlands Organisation for Applied Scientific Research TNO, Princetonlaan 6 3584 CB Utrecht, the Netherlands.
Maria Albin, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden.
Calvin B Ge, Netherlands Organisation for Applied Scientific Research TNO, Princetonlaan 6 3584 CB Utrecht, the Netherlands.
Katarina Kjellberg, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden; Centre for Occupational and Environmental Medicine, Region Stockholm, Torsplan, Solnavägen 4, 113 65 Stockholm, Sweden.
Damien M McElvenny, Institute of Occupational Medicine, Research Ave N, Currie EH14 4AP, Edinburgh, United Kingdom; Centre for Occupational and Environmental Health, University of Manchester, Oxford Rd, Manchester M13 9PL, United Kingdom.
Per Gustavsson, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden.
Henrik A Kolstad, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Anne Mette L Würtz, Department of Public Health, Research Unit for Environment, Occupation and Health, Danish Ramazzini Centre, Aarhus University, Bartholins Allé 2 DK-8000 Aarhus, Denmark.
Bendik C Brinchmann, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Karin Broberg, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden.
Stine Fossum, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Merete Bugge, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Mette Wulf Christensen, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Manosij Ghosh, Department of Public Health and Primary Care, Centre for Environment & Health, KU Leuven, Kapucijnenvoer 7, box 7001 3000 Leuven, Belgium.
David Høyrup Christiansen, Centre of Elective surgery, Region Hospital Silkeborg, Department of Clinical Medicine, Aarhus University, Falkevej 3. 8600 Silkeborg, Denmark.
Suzanne L Merkus, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Lars-Kristian Lunde, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Eira Viikari-Juntura, Finnish Institute of Occupational Health, P.O. Box 40 FI-00032 TYÖTERVEYSLAITOS, Finland.
Annett Dalbøge, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Daniel Falkstedt, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden.
Morten Vejs Willert, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Anke Huss, Institute for Risk Assessment Sciences, Utrecht University, Yalelaan 2, 3584 CM Utrecht, the Netherlands.
Else Toft Würtz, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Orianne Dumas, Université Paris-Saclay, UVSQ, Univ. Paris-Sud, Inserm, Équipe d’Épidémiologie respiratoire intégrative, CESP, 94807, Villejuif, France.
Inge Brosbøl Iversen, Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Mimmi Leite, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Christine Cramer, Department of Public Health, Research Unit for Environment, Occupation and Health, Danish Ramazzini Centre, Aarhus University, Bartholins Allé 2 DK-8000 Aarhus, Denmark; Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99. DK-8200 Aarhus, Denmark.
Jorunn Kirkeleit, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway; Centre for International Health, Department of Global Public Health and Primary Care, University of Bergen, Årstadveien 17 Block D 5009 Bergen, Norway.
Cecilie Svanes, Centre for International Health, Department of Global Public Health and Primary Care, University of Bergen, Årstadveien 17 Block D 5009 Bergen, Norway; Department of Occupational Medicine, Haukeland University Hospital, P.O box 1400 5021 Bergen, Norway.
Håkan Tinnerberg, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden; School of Public Health and Community Medicine, Gothenburg University, Huvudbyggnad Vasaparken, Universitetsplatsen 1, 405 30, Gothenburg, Sweden.
Judith Garcia-Aymerich, Barcelona Institute for Global Health (ISGlobal), C/ Doctor Aiguader 88, 08003 Barcelona, Spain; Universitat Pompeu Fabra (UPF), carrer de la Mercè 12, 08002 Barcelona, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Av. Monforte de Lemos 3-5, 28029 Madrid, Spain.
Anne Vested, Department of Public Health, Research Unit for Environment, Occupation and Health, Danish Ramazzini Centre, Aarhus University, Bartholins Allé 2 DK-8000 Aarhus, Denmark.
Pernilla Wiebert, Institute of Environmental Medicine, Karolinska Institutet, Box 210, SE-171 77 Stockholm, Sweden; Centre for Occupational and Environmental Medicine, Region Stockholm, Torsplan, Solnavägen 4, 113 65 Stockholm, Sweden.
Karl-Christian Nordby, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway.
Lode Godderis, Department of Public Health and Primary Care, Centre for Environment & Health, KU Leuven, Kapucijnenvoer 7, box 7001 3000 Leuven, Belgium.
Roel Vermeulen, Institute for Risk Assessment Sciences, Utrecht University, Yalelaan 2, 3584 CM Utrecht, the Netherlands.
Anjoeka Pronk, Netherlands Organisation for Applied Scientific Research TNO, Princetonlaan 6 3584 CB Utrecht, the Netherlands.
Ingrid Sivesind Mehlum, National Institute of Occupational Health (STAMI), Gydas vei 8, 0363 Oslo, Norway; Institute of Health and Society, University of Oslo, Kirkeveien 166, 0450 Oslo, Norway; Department of Occupational and Environmental Medicine, Bispebjerg and Frederiksberg Hospitals, Copenhagen, Bispebjerg Bakke 23, DK-Copenhagen 2400 NV, Denmark; Department of Public Health, University of Copenhagen, Øster Farimagsgade 5 1353 Copenhagen, Denmark.
Conflict of Interest
None
Ethics Approval
Not applicable
Data Availability
No data were used in this study
References
- Adler C, Friesen MC, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Okyne V, Mensah JE, Truelove A. et al. Usual adult occupation and risk of prostate cancer in West African men: the Ghana Prostate Study. Occup Environ Med. 2019:76(2):71–77. 10.1136/oemed-2018-105391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archangelidi O, Sathiyajit S, Consonni D, Jarvis D, De Matteis S.. Cleaning products and respiratory health outcomes in occupational cleaners: a systematic review and meta-analysis. Occup Environ Med. 2021:78(8):604–617. 10.1136/oemed-2020-106776 [DOI] [PubMed] [Google Scholar]
- Ascherio A, Schwarzschild MA.. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016:15(12):1257–1272. 10.1016/S1474-4422(16)30230-7 [DOI] [PubMed] [Google Scholar]
- Barcenilla A, March LM, Chen JS, Sambrook PN.. Carpal tunnel syndrome and its relationship to occupation: a meta-analysis. Rheumatology (Oxford) 2012:51(2):250–261. 10.1093/rheumatology/ker108 [DOI] [PubMed] [Google Scholar]
- Bessonneau V, Rudel RA.. Mapping the human exposome to uncover the causes of breast cancer. Int J Environ Res Public Health. 2019:17(1):189. 10.3390/ijerph17010189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Donato F, Pira E, Luu HN, La Vecchia C.. Risk of mesothelioma after cessation of asbestos exposure: a systematic review and meta-regression. Int Arch Occup Environ Health. 2019:92(7):949–957. 10.1007/s00420-019-01433-4 [DOI] [PubMed] [Google Scholar]
- Boudrias V, Trépanier S-G, Salin D.. A systematic review of research on the longitudinal consequences of workplace bullying and the mechanisms involved. Aggress Violent Beh. 2021:56:101508. 10.1016/j.avb.2020.101508. [DOI] [Google Scholar]
- Dickerson AS, Wu AC, Liew Z, Weisskopf M.. A scoping review of non-occupational exposures to environmental pollutants and adult depression, anxiety, and suicide. Curr Environ Health Rep. 2020:7(3):256–271. 10.1007/s40572-020-00280-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney B, Kurth L, Halldin C, Hale J, Frenk SM.. Occupational exposure and airflow obstruction and self-reported COPD among ever-employed US adults using a COPD-job exposure matrix. Am J Ind Med. 2019:62(5):393–403. 10.1002/ajim.22958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll T, Jacklyn G, Orchard J, Passmore E, Vos T, Freedman G, Lim S, Punnett L.. The global burden of occupationally related low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014:73(6):975–981. 10.1136/annrheumdis-2013-204631. [DOI] [PubMed] [Google Scholar]
- Dumas O. Cleaners and airway diseases. Curr Opin Allergy Clin Immunol. 2021:21(2):101–109. 10.1097/ACI.0000000000000710 [DOI] [PubMed] [Google Scholar]
- Dumas O, Le Moual N.. Do chronic workplace irritant exposures cause asthma?. Curr Opin Allergy Clin Immunol. 2016:16(2):75–85. 10.1097/ACI.0000000000000247 [DOI] [PubMed] [Google Scholar]
- Gao L, Calloway R, Zhao E, Brayne C, Matthews FE; Medical Research Council Cognitive Function and Ageing Collaboration. Accuracy of death certification of dementia in population-based samples of older people: analysis over time. Age Ageing. 2018:47(4):589–594. 10.1093/ageing/afy068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Peters S, Olsson A, Portengen L, Schüz J, Almansa J, Behrens T, Pesch B, Kendzia B, Ahrens W. et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. A pooled analysis of case-control studies. Am J Respir Crit Care Med. 2020:202(3):412–421. 10.1164/rccm.201910-1926OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb H, Wang J, O’Leary K, Chen C, Bateson TF, Kopylev L.. The effect of age on the relative risk of lung cancer mortality in a cohort of chromium production workers. Am J Ind Med. 2020:63(9):774–778. 10.1002/ajim.23152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SB, Modini M, Joyce S, Milligan-Saville JS, Tan L, Mykletun A, Bryant RA, Christensen H, Mitchell PB.. Can work make you mentally ill? A systematic meta-review of work-related risk factors for common mental health problems. Occup Environ Med. 2017:74(4):301–310. 10.1136/oemed-2016-104015 [DOI] [PubMed] [Google Scholar]
- Hovanec J, Siemiatycki J, Conway DI, Olsson A, Guenel P, Luce D, Jöckel KH, Pohlabeln H, Ahrens W, Karrasch S. et al. Application of two job indices for general occupational demands in a pooled analysis of case-control studies on lung cancer. Scand J Work Environ Health. 2021:47(6):475–481. 10.5271/sjweh.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh DA, Kang MS, Lee J, Choi JY, Moon KW, Lee YJ.. Occupational and environmental asbestos exposure and the risk of lung cancer in Korea: a case-control study in South Chungcheong Province of Korea. PLoS One. 2021:16(4):e0249790. 10.1371/journal.pone.0249790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. n.d. ‘Monographs available’, International Agency for Research on Cancer (IARC) [accessed 2023 July 4]. https://monographs.iarc.who.int/monographs-available/ [Google Scholar]
- Jackson SS, St George DM, Loffredo CA, Amr S.. Nonoccupational exposure to agricultural work and risk of urinary bladder cancer among Egyptian women. Arch Environ Occup Health. 2017:72(3):166–172. 10.1080/19338244.2016.1169155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RS, Vineis P.. Biomarkers of susceptibility to chemical carcinogens: the example of non-Hodgkin lymphomas. Br Med Bull. 2014:111(1):89–100. 10.1093/bmb/ldu015 [DOI] [PubMed] [Google Scholar]
- Khalis M, El Rhazi K, Fort E, Chajès V, Charaka H, Huybrechts I, Moskal A, Biessy C, Romieu I, Abbass F. et al. Occupation and risk of female breast cancer: a case-control study in Morocco. Am J Ind Med. 2019:62(10):838–846. 10.1002/ajim.23027 [DOI] [PubMed] [Google Scholar]
- Kim TJ, von dem Knesebeck O.. Perceived job insecurity, unemployment and depressive symptoms: a systematic review and meta-analysis of prospective observational studies. Int Arch Occup Environ Health. 2016:89(4):561–573. 10.1007/s00420-015-1107-1 [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Batty GD, Pentti J, Shipley MJ, Sipilä PN, Nyberg ST, Suominen SB, Oksanen T, Stenholm S, Virtanen M. et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020:5(3):e140–e149. 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- Kogevinas M, Zock JP, Jarvis D, Kromhout H, Lillienberg L, Plana E, Radon K, Torén K, Alliksoo A, Benke G. et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II). Lancet (London, England). 2007:370(9584):336–341. 10.1016/S0140-6736(07)61164-7. [DOI] [PubMed] [Google Scholar]
- Kuijer P, Verbeek JH, Seidler A, Ellegast R, Hulshof CTJ, Frings-Dresen MHW, Van der Molen HF.. Work-relatedness of lumbosacral radiculopathy syndrome: review and dose–response meta-analysis. Neurology. 2018:91(12):558–564. 10.1212/01.wnl.0000544322.26939.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Tilling K, Davey Smith G.. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016:45(6):1866–1886. 10.1093/ije/dyw314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Stücker I, Chen C, Goodman G, McHugh MK, D’Amelio AM Jr, Etzel CJ, Li S, Lin X, Christiani DC.. Genome-wide gene-asbestos exposure interaction association study identifies a common susceptibility variant on 22q13.31 associated with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2015:24(10):1564–1573. 10.1158/1055-9965.EPI-15-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Guha N, Hall AL, Straif K.. Identifying occupational carcinogens: an update from the IARC Monographs. Occup Environ Med. 2018:75(8):593–603. 10.1136/oemed-2017-104944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis LM, Lerro CC, Friesen MC, Andreotti G, Koutros S, Sandler DP, Blair A, Robson MG, Beane Freeman LE.. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environ Health. 2017:16(1):95. 10.1186/s12940-017-0298-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge E, Kaerlev L, Olsen J, Sabroe S, Afonso N, Ahrens W, Eriksson M, Merletti F, Morales-Suarez-Varelas M, Stengrevics A. et al. ‘Rare cancers of unknown etiology: lessons learned from a European multi-center case-control study’. Eur J Epidemiol. 2020:35(10):937–948. 10.1007/s10654-020-00663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen IEH, Nyberg ST, Magnusson Hanson LL, Ferrie JE, Ahola K, Alfredsson L, Batty GD, Bjorner JB, Borritz M, Burr H. et al. Job strain as a risk factor for clinical depression: systematic review and meta-analysis with additional individual participant data. Psychol Med. 2017:47(8):1342–1356. 10.1017/S003329171600355X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marant Micallef C, Shield KD, Baldi I, Charbotel B, Fervers B, Gilg Soit Ilg A, Guénel P, Olsson A, Rushton L, Hutchings SJ. et al. Occupational exposures and cancer: a review of agents and relative risk estimates. Occup Environ Med. 2018:75(8):604–614. 10.1136/oemed-2017-104858 [DOI] [PubMed] [Google Scholar]
- Nagy K, Duca RC, Lovas S, Creta M, Scheepers PTJ, Godderis L, Ádám B.. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ Res. 2020:181:108926. 10.1016/j.envres.2019.108926 [DOI] [PubMed] [Google Scholar]
- Olsson A, Bouaoun L, Schüz J, Vermeulen R, Behrens T, Ge C, Kromhout H, Siemiatycki J, Gustavsson P, Boffetta P. et al. Lung cancer risks associated with occupational exposure to pairs of five lung carcinogens: results from a pooled analysis of case-control studies (SYNERGY). Environ Health Perspect. 2024:132(1):17005. 10.1289/EHP13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panou V, Vyberg M, Meristoudis C, Hansen J, Bøgsted M, Omland O, Weinreich UM, Røe OD.. Non-occupational exposure to asbestos is the main cause of malignant mesothelioma in women in North Jutland, Denmark. Scand J Work Environ Health. 2019:45(1):82–89. 10.5271/sjweh.3756 [DOI] [PubMed] [Google Scholar]
- Pega F, Al-Emam R, Cao B, Davis CW, Edwards SJ, Gagliardi D, Fassa AG, Hassan MN, Hosseinpoor AR, Iavicoli S. et al. New global indicator for workers’ health: mortality rate from diseases attributable to selected occupational risk factors. Bull World Health Organ. 2023:101(6):418–430Q. 10.2471/BLT.23.289703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk A, Loh M, Kuijpers E, Albin M, Selander J, Godderis L, Ghosh M, Vermeulen R, Peters S, Sivesind Mehlum I. et al. ; The EPHOR Consortium. Applying the exposome concept to working life health: the EU EPHOR project. Environ Epidemiol. 2022:6:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD.. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019:21(2):10. 10.1007/s11920-019-0997-0 [DOI] [PubMed] [Google Scholar]
- Rosenberger A, Hung RJ, Christiani DC, Caporaso NE, Liu G, Bojesen SE, Le Marchand L, Haiman CA, Albanes D, Aldrich MC. et al. Genetic modifiers of radon-induced lung cancer risk: a genome-wide interaction study in former uranium miners. Int Arch Occup Environ Health. 2018:91(8):937–950. 10.1007/s00420-018-1334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL.. 2008. Modern epidemiology. Philadelphia USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Rushton L. The global burden of occupational disease. Curr Environ Health Rep. 2017:4(3):340–348. 10.1007/s40572-017-0151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Chiu WA, Cogliano V, Jinot J, Kriebel D, Lunn RM, Beland FA, Bero L, Browne P, Fritschi L. et al. The IARC monographs: updated procedures for modern and transparent evidence synthesis in cancer hazard identification. J Natl Cancer Inst. 2020:112(1):30–37. 10.1093/jnci/djz169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, Bharucha AE, Rocca WA.. ‘Medical records documentation of constipation preceding Parkinson disease: a case-control study’. Neurology. 2009:73(21):1752–1758. 10.1212/WNL.0b013e3181c34af5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBU. 2014. Arbetsmiljöns betydelse för ryggproblem. En systematisk litteraturöversikt [Occupational Exposures and Back Disorders. A Systematic Review]. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU) [Google Scholar]
- Skaaby S, Flachs EM, Lange P, Schlünssen V, Marott JL, Brauer C, Çolak Y, Afzal S, Nordestgaard BG, Sadhra S. et al. Occupational inhalant exposures and longitudinal lung function decline. Eur Respir J. 2021:58(6):2004341. 10.1183/13993003.04341-2020 [DOI] [PubMed] [Google Scholar]
- Sritharan J, MacLeod JS, Dakouo M, Qadri M, McLeod CB, Peter A, Demers PA.. Breast cancer risk by occupation and industry in women and men: results from the Occupational Disease Surveillance System (ODSS). Am J Ind Med. 2019:62(3):205–211. 10.1002/ajim.22942 [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Hassen Abate K, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F. et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018:392(10159):1923–1994. 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stücker I, Martin D, Neri M, Laurent-Puig P, Blons H, Antoine M, Guiochon-Mantel A, Brailly-Tabard S, Canonico M, Wislez M. et al. Women Epidemiology Lung Cancer (WELCA) study: reproductive, hormonal, occupational risk factors and biobank. BMC Public Health. 2017:17(1):324. 10.1186/s12889-017-4191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala J, Hämäläinen P, Leena Saarela K, Yoke Yun L, Manickam K, Jin TW, Heng P, Tjong C, Guan Kheng L, Lim S.. Global estimates of the burden of injury and illness at work in 2012. J Occup Environ Hyg. 2014:11(5):326–337. 10.1080/15459624.2013.863131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Mehlum IS.. Greater coordination and harmonisation of European occupational cohorts is needed. Occup Environ Med. 2018:75(7):475–476. 10.1136/oemed-2017-104955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen HF, Visser S, Alfonso JH, Curti S, Mattioli S, Rempel D, Roquelaure Y, Ppfm K, Tamminga SJ.. Diagnostic criteria for musculoskeletal disorders for use in occupational healthcare or research: a scoping review of consensus- and synthesised-based case definitions. BMC Musculoskelet Disord. 2021:22(1):169. 10.1186/s12891-021-04031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Schymanski EL, Barabási AL, Miller GW.. The exposome and health: where chemistry meets biology. Science. 2020:367(6476):392–396. 10.1126/science.aay3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Silverman DT, Garshick E, Vlaanderen J, Portengen L, Steenland K.. Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ Health Perspect. 2014:122(2):172–177. 10.1289/ehp.1306880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienneau D, Hoogh K. de, Hauri D, Vicedo-Cabrera AM, Schindler C, Huss A, Röösli M.. Effects of radon and UV exposure on skin cancer mortality in Switzerland. Environ Health Perspect. 2017:125(6):067009. 10.1289/EHP825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen M, Ervasti J, Head J, Oksanen T, Salo P, Pentti J, Kouvonen A, Väänänen A, Suominen S, Koskenvuo M. et al. Lifestyle factors and risk of sickness absence from work: a multicohort study. Lancet Public Health. 2018:3(11):e545–e554. 10.1016/S2468-2667(18)30201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visonà SD, Villani S, Manzoni F, Chen Y, Ardissino G, Russo F, Moretti M, Javan GT, Osculati A.. ‘Impact of asbestos on public health: a retrospective study on a series of subjects with occupational and non-occupational exposure to asbestos during the activity of Fibronit plant (Broni, Italy)’. J Public Health Res. 2018:7(3):1519. 10.4081/jphr.2018.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/ILO. 2021. WHO/ILO joint estimates of the work-related burden of disease and injury, 2000–2016: global monitoring report. Geneva: World Health Organization and the International Labour Organization. [Google Scholar]
- Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005:14(8):1847–1850. 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- Wong K, Chan AHS, Ngan SC.. The effect of long working hours and overtime on occupational health: a meta-analysis of evidence from 1998 to 2018. Int J Environ Res Public Health. 2019:16(12):2102. 10.3390/ijerph16122102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Ho V, Siemiatycki J.. Role of occupational exposures in lung cancer risk among women. Occup Environ Med. 2021:78(2):98–104. 10.1136/oemed-2020-106470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were used in this study