Abstract
RNA-targeting type VI CRISPR-Cas effectors are widely used in RNA applications. Cas13h is a recently identified subtype of Cas13 ribonuclease, with strong RNA cleavage activity and robust in vivo RNA knockdown efficiency. However, little is known regarding its biochemical properties and working mechanisms. Biochemical characterization of Cas13h1 indicated that it lacks in vitro pre-crRNA processing activity and adopts a central seed. The cleavage activity of Cas13h1 is enhanced by a R(G/A) 5′-PFS, and inhibited by tag:anti-tag RNA pairing. We determined the structures of Cas13h1-crRNA binary complex at 3.1 Å and Cas13h1-crRNA-target RNA ternary complex at 3.0 Å. The ternary complex adopts an elongated architecture, and encodes a nucleotide-binding pocket within Helical-2 domain to recognize the guanosine at the 5′-end of the target RNA. Base pairing between crRNA guide and target RNA disrupts Cas13h1-guide interactions, leading to dramatic movement of HEPN domains. Upon target RNA engagement, Cas13h1 adopts a complicated activation mechanism, including separation of HEPN catalytic residues and destabilization of the active site loop and NTD domain, to get activated. Collectively, these insights expand our understanding into Cas13 effectors.
Graphical Abstract
Graphical Abstract.
Introduction
Clustered regularly interspaced short palindromic repeats (CRISPR) and the associated proteins (CRISPR-Cas) are components of the prokaryotic adaptive immune system that protects them from the invasion of mobile genetic elements (MGEs) via RNA-guided cleavage of target nucleic acids (1–5). CRISPR systems contain two classes and six types (6–10). Class 2 CRISPR-Cas system, containing type II, V and VI, encodes a single, multidomain crRNA-binding protein that possesses all the activity required for interference (6), and has been harnessed for genome editing and targeting (11,12). Type VI CRISPR-Cas system encompasses Cas13 effectors that recognize the cognate crRNA for RNA interference (13–17). Cas13 effectors exclusively target RNA, and have been widely used in RNA-related applications, including site-specific RNA editing, RNA knockdown and molecular diagnostics (18,19).
Cas13 ribonucleases are characterized with divergent domain organization and two conserved HEPN (Higher Eukaryotes and Prokaryotes Nucleotide-binding) domains (13,15,17). Cas13 proteins generally possess two enzymatically distinct ribonuclease activities that are required for optimal interference (14–17,20). Besides the target RNA cleavage activity provided by HEPN domains, the pre-crRNA processing activity helps Cas13 proteins form mature interference complexes (14–17,20). However, recent studies indicate some Cas13 subtypes lack in vitro pre-crRNA processing activity (20–23). Besides the programmable cis-cleavage activity, Cas13 proteins exhibit collateral activity upon target RNA engagement, leading to the promiscuous cleavage of environment RNA (18). The collateral activity of Cas13 proteins has been leveraged to develop nucleic acid detection methods, such as SHERLOCK (24). Recent bioinformatic endeavors, with Cas13e-i identified (25), have expanded the number of Cas13 subtypes into 11 (Cas13a-i, Cas13X and Cas13Y) (25–27), of which Cas13a, Cas13c and Cas13d use 5′-DR (Direct Repeat)-containing crRNA, and the rest of the subtypes use a crRNA with 3′-positioned DR.
For some of the type VI CRISPR-Cas systems, PFS (Protospacer Flanking Site) and extended complementarity between crRNA and target RNA beyond guide-target RNA duplex (also called tag:anti-tag RNA pairing) dramatically impact the cleavage activity of Cas13 nucleases, representing potential mechanisms for self/non-self discrimination (13,14,28,29). PFS is required for target cleavage of type VI-A LshCas13a (Leptotrichia shahii Cas13a) (13,18) and type VI-B Cas13b systems (15,19), while PFS is not essential for type VI-A LbuCas13a (Leptotrichia buccalis Cas13a) (14) and type VI-D Cas13d (16). The tag:anti-tag RNA pairing blocks cleavage in LseCas13a (Listeria seeligeri) and LshCas13a, and induces conformational changes that prevent the catalytic residues from HEPN domains getting closer to suppress the cleavage activity (28,29).
Extensive structural work has been dedicated to the mechanistic understanding regarding how Cas13 ribonucleases are activated upon target RNA engagement (22,23,29–38). For Cas13a and Cas13d that utilize 5′-DR crRNA, it was established that the catalytic residues of HEPN domains were drawn closer upon target RNA binding due to conformational changes (31,34). For Cas13b that utilizes 3′-DR crRNA, the activation mechanism remains unknown due to absence of target RNA-bound structure (33,38). For Cas13bt3 (also called Cas13X.1) with 3′-DR crRNA, local disordering of the loop surrounding the active site represents a possible activation mechanism (22).
Cas13h is a recently identified subtype of Cas13 ribonuclease, with strong RNA cleavage activity and robust in vivo RNA knockdown efficiency (25). Eight members, ranging from Cas13h1 to Cas13h8, were categorized as Cas13h subtype (25). The CRISPR-Cas13h1 system in this study was found in soil metagenome (contig accession No: DATCCX010000659) (39). However, except for the conserved HEPN domain motif (25), Cas13h1 shows low sequence similarity with previously reported Cas13 proteins (12.6% with LbuCas13a, 11.0% with Prevotella buccae Cas13b [PbuCas13b], 13.5% with Eubacterium siraeum DSM15702 Cas13d [EsCas13d] and 13.1% with Cas13bt3). Preliminary analysis of Cas13h1 revealed that its structure predicted via AlphaFold2 (40) exhibits a distinct scaffold with little similarity to any reported Cas13 ribonucleases (overall R.M.S.D. >30), implying its structural novelty. Despite the bioinformatic discovery and in vivo activity, little is known regarding most of the biochemical properties of Cas13h, motivating us to investigate the working mechanism of the Cas13h from a biochemical and structural aspect.
Materials and methods
Protein expression and purification
The full-length Cas13h1 gene was synthesized by overlap extension PCR and cloned into a 2CT-10 vector containing a TEV-cleavable N-terminal His10-MBP fusion tag followed by Asn10. The plasmids for expressing LbuCas13a and HheCas13a are from Addgene (#83482 and #91871). The Cas13h1 protein was overexpressed in Escherichia coli BL21(AI) (Gimmico) cells that were induced with 0.33 mM isopropyl-1-thio-b-d-galactopyranoside (IPTG) at 18 °C for 18–20 h until OD600 reached 0.6. The cells were harvested by centrifugation, resuspended in 35 ml buffer A containing 50 mM Tris–HCl, pH 8.0, 500 mM NaCl, 20 mM imidazole, 5 mM β‐mercaptoethanol, 1 mM PMSF and 2% glycerol, and lysed by sonication. After centrifugation, the supernatant was incubated with Ni-NTA agarose, and washed with excess buffer A. Then, the bound protein was eluted with buffer B containing 50 mM Tris–HCl, pH 8.0, 500 mM NaCl, 500 mM imidazole, 5 mM β‐mercaptoethanol and 2% glycerol. The target proteins were then loaded into the Heparin column (Cytiva, Inc) and eluted with a linear NaCl gradient. The Cas13h1 protein was further purified by size-exclusion chromatography (Superdex 200 Increase 10/300, Cytiva, Inc) in buffer C containing 20 mM Tris–HCl, pH 8.0, 200 mM NaCl, 1 mM MgCl2, 5 mM β‐mercaptoethanol. Finally, peak fractions were pooled, concentrated and stocked at −80°C. The purification of LbuCas13a and HheCas13a are identical to that of Cas13h1, except the gel filtration step is omitted.
Mutagenesis
Single-site mutations were introduced by the QuikChange site-directed mutagenesis method. Large-fragment deletion was introduced by ligating inverse PCR-amplified backbone encoding a flexible linker. Group mutations containing multiple sites were introduced by ligating inverse PCR-amplified backbone with mutations bearing DNA oligonucleotides. All mutants were confirmed by DNA sequencing. The mutant proteins were expressed and purified as described for wild-type Cas13h1.
In vitro RNA transcription
Except the 66-nt crRNA, FAM-labeled target RNAs, 31-nt target RNA3, and 31-nt target RNA1 were synthesized from GenScript Biotech Inc., other RNA used in the experiments were transcribed in vitro. DNA templates for RNA transcription were generated by overlap extension PCR. In vitro transcription reactions were carried out using T7 in vitro transcription Kit (K0441, ThermoFisher) according to the manufacturer's protocol. The transcribed RNA was resuspended in DEPC water and stored at −80°C.
Size exclusion chromatography and complex reconstitution
The Cas13h1-crRNA complex was reconstituted by incubating purified wild-type protein and crRNA at the molar ratio of 1:1.1 on ice for 30 min. The resulting complex was purified on a Superdex 200 Increase 10/300 gel filtration column (Cytiva, Inc) in buffer C. The Cas13h1-crRNA-target RNA complex was reconstituted by incubating purified mutant protein (R200A), and crRNA at the molar ratio of 1:1.1 on ice for 30 min before adding target RNA. The mixture was then incubated with target RNA1 (or target RNA3) at a molar ratio of 1:1.1 on ice for 30 min. The resulting complex was purified as described above. The ‘Middle’ region of Cas13h1 (residues 340–930) and the ‘HEPNs’ region of Cas13h1 (residues 1–340 and 930–1111 ligated by glycine/serine linker) from Supplementary Figure S1B were incubated with crRNA at the molar ratio of 1:1.1 on ice for 30 min before adding target RNA, respectively. The mixtures were then incubated with target RNA1 at a molar ratio of 1:1.1 on ice for 30 min. The resulting complex was purified as described above. The truncated proteins and crRNA-target duplex were also subjected to size exclusion chromatography as controls.
Pre-crRNA processing assays
6 μM pre-crRNAs were incubated at 37 °C for 30 min with 8 μM different Cas13 proteins (Cas13h1, LbuCas13a and HheCas13a) in 10 μl reaction buffer containing 20 mM Tris–HCl, pH 8.0, 100 mM KCl, 5 mM MgCl2, 1 mM β‐mercaptoethanol, 5% Glycerol, 10 μg/ml BSA, 10 μg/ml yeast tRNA, and 0.05% Igepal CA-630, respectively. Reactions were stopped by adding 1 μl quench buffer containing 20 mM EDTA, 10 mg/ml proteinase K. The reaction products were denatured with 2X loading buffer at 95 °C for 5 min, and resolved by 15% denaturing (7 M urea) PAGE, stained by ethidium bromide, and then visualized by ultraviolet transilluminator of Bio-Rad imaging system. This experiment was performed at least three times, and representative results were shown. All the RNA sequences are summarized in Supplementary Table S1.
RNA cleavage assays
The cleavage reactions were performed in cleavage buffer containing 20 mM Tris–HCl, pH 8.0, 100 mM KCl, 5 mM MgCl2, 1 mM β‐mercaptoethanol, 5% glycerol with RNA targets. Briefly, 6 μM crRNA was incubated on ice for 30 min with 4 μM Cas13h1 (wild-type or mutant) in cleavage buffer ahead of reactions. For target RNA cleavage assays, 12 μM different target RNAs were added into Cas13h1-crRNA mixture. For collateral RNA cleavage assays, 2.1 μg collateral RNA and 6 μM (final concentration) different target RNAs were added into Cas13h1-crRNA mixture. All reactions were performed at 37°C for 5 min, and then stopped by adding 1 μl quench buffer containing 20 mM EDTA, 10 mg/ml proteinase K. The reaction products were denatured with 2X loading buffer at 95°C for 5 min, and resolved by 15% denaturing (7 M urea) PAGE. FAM-labeled RNA was visualized by ultraviolet transilluminator of Bio-Rad imaging system, and label-free RNA was stained by ethidium bromide before visualized by ultraviolet transilluminator. These experiments were performed at least three times, and representative results were shown. All the RNA sequences are summarized in Supplementary Table S1.
Electrophoretic mobility shift assays
Electrophoretic mobility shift assay (EMSA) was performed in buffer containing 20 mM Tris pH 8.0, 200 mM KCl, 1 mM β‐mercaptoethanol and 5% glycerol. 4 μM Cas13h1 (wild-type or mutant) were incubated on ice for 30 min with 6 μM crRNA in the buffer. The samples were resolved by a 1.5% TAE agarose gel and run at 90 V at 4°C for 40 min. The gels were stained by ethidium bromide, and then visualized by ultraviolet transilluminator of Bio-Rad imaging system. These experiments were performed at least three times, and representative results were shown.
Electron microscopy
4 μl aliquots of Cas13h1-crRNA (3.6 mg/ml) with additional 0.1% DDM and Cas13h1-crRNA-target (0.69 mg/ml) were applied to glow-discharged Quantifoil holey carbon grids (Au, R1.2/1.3, 300 mesh) and UltrAuFoil holey gold grids (R1.2/1.3, 300 mesh), respectively. The grids were blotted with force 3 for 15 s and plunged into liquid ethane using a Vitrobot. Cryo-EM data were collected with a Titan Krios microscope (FEI) operated at 300 kV and images were collected using EPU (41) at a nominal magnification of 105,000x (resulting in a calibrated physical pixel size of 0.85 Å/pixel) with a defocus range from −1.2 μm to −2.2 μm. The images were recorded on a K3 summit electron direct detector in super-resolution mode at the end of a GIF-Quantum energy filter operated with a slit width of 20 eV. A dose rate of 15 electrons per pixel per second and an exposure time of 2.5 s were used, generating 40 movie frames with a total dose of ∼54 electrons/Å2. A total of 2239 and 1670 movie stacks were collected for Cas13h1-crRNA and Cas13h1-crRNA-target RNA respectively (Table 1).
Table 1.
Cryo-EM data collection, refinement and validation statistics
| Cas13h1-crRNA (8WCS, EMD-37448) | Cas13h1-crRNA-target RNA (8WCE, EMD-37439) | |
|---|---|---|
| Data collection and processing | ||
| Magnification | 105 000 | 105 000 |
| Voltage (kV) | 300 | 300 |
| Electron exposure (e–/Å2) | 54 | 54 |
| Defocus range (-μm) | 1.2–2.2 | 1.2–2.2 |
| Pixel size (Å) | 0.85 | 0.85 |
| Symmetry imposed | C1 | C1 |
| Initial particle images (no.) | 5 266 728 | 2 692 221 |
| Final particle images (no.) | 207 575 | 291 461 |
| Map resolution (Å) | 3.10 | 3.0 |
| FSC threshold | 0.143 | 0.143 |
| Map resolution range (Å) | 3.0–5.0 | 2.9–4.5 |
| Refinement | ||
| Initial model used | PDB:8WCE | None |
| Model resolution (Å) | 3.1 | 3.0 |
| FSC threshold | 0.5 | 0.5 |
| Model resolution range (Å) | 3.0–5.0 | 2.9–4.5 |
| Map sharpening B factor (Å2) | –129.3 | –144.4 |
| Model composition | ||
| Non-hydrogen atoms | 10 096 | 10 350 |
| Protein residues | 1044 | 994 |
| Nucleotides | 66 | 97 |
| B factors (Å2) | ||
| Protein | 60.90 | 24.54 |
| Nucleotide | 64.55 | 44.03 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.005 | 0.004 |
| Bond angles (°) | 1.026 | 0.908 |
| Validation | ||
| MolProbity score | 1.75 | 1.72 |
| Clashscore | 4.70 | 4.16 |
| Poor rotamers (%) | 0.74 | 0.33 |
| Ramachandran plot | ||
| Favored (%) | 91.43 | 90.81 |
| Allowed (%) | 8.57 | 9.19 |
| Disallowed (%) | 0.00 | 0.00 |
Image processing
The movie frames were imported to RELION-3 (42), aligned using MotionCor2 with bin 2 (43), and subjected to contrast transfer function (CTF) estimation using Gctf (44) on the fly. For the Cas13h1-crRNA dataset, 5 266 728 particles were picked with a template and extracted from the dose weighted micrographs. 1 038 542 particles were selected from 2D classification. 9000 particles from different views were used to generate initial models in cryoSPARC (45). 3D classification was performed to distinguish different conformational states. 207 575 particles were used for final 3D refinement, converging at 3.1 Å resolution. For the Cas13h1-crRNA-target RNA dataset, 2 692 221 particles were picked with a template and extracted from the dose weighted micrographs. 778 780 particles were selected from 2D classification. 9000 particles from different views were used to generate initial models in cryoSPARC. 3D classification was performed to distinguish different conformational states. 291461 particles were used for final 3D refinement, converging at 3.0 Å resolution. Details of the cryo-EM image processing is summarized in Table 1.
Model building, refinement and visualization
For model building of Cas13h1-crRNA-target RNA, the protein and RNA were built from scratch in COOT (46). For model building of Cas13h1-crRNA, the protein and RNA were adjusted manually based on the structure of Cas13h1-crRNA-target RNA. Model refinement was performed using phenix.real_space_refine tool in Phenix (47). The refinement statistics are summarized in Table 1. Figures were generated via PyMOL and UCSF Chimera (48).
Results
Biochemical characterization of Cas13h1
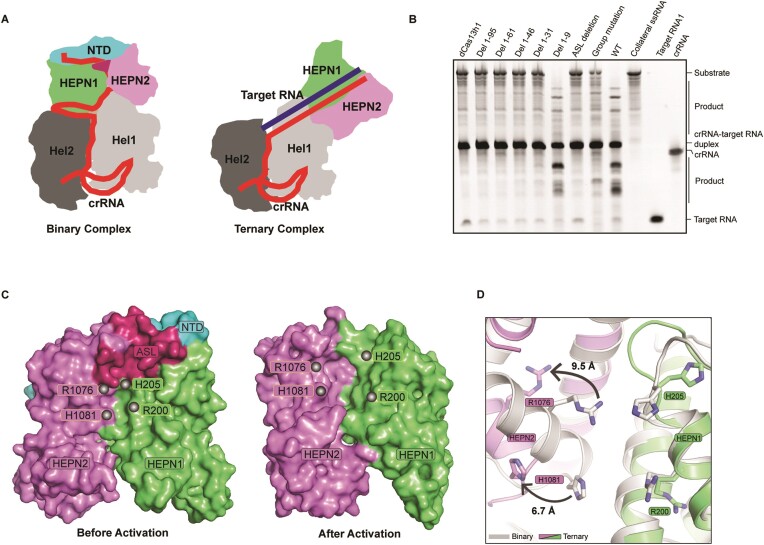
The pre-crRNA processing assay of Cas13h1 was performed under same buffer conditions with previous study, using LbuCas13a as the positive control and HheCas13a (Herbinix hemicellulosilytica Cas13a) as the negative control (20). Cas13h1 failed to cleave ‘spacer-repeat-spacer-repeat’ label-free pre-crRNA, indicating its lack of in vitro pre-crRNA processing activity (Supplementary Figure S1A), similar to Cas13a proteins from Thermoclostridium caenicola (21,23), HheCas13a (20,21) and Cas13bt3 (22). Truncation experiment indicated that the middle region (residues 340–930) of Cas13h1 sufficiently binds crRNA-target RNA duplex, while the HEPN domains (residues 1–340 and 930–1111 ligated by glycine/serine linker) of Cas13h1 fail (Supplementary Figure S1B, E). Mismatch experiment indicated that mismatches between crRNA guide and target RNA ranging from position 13 to 24 impaired the cleavage activity more than the rest of positions (Supplementary Figure S1C), suggesting that Cas13h1, similar to most of other Cas13 ribonucleases, might adopt a ‘central seed’ within its crRNA to initiate the target RNA recognition. In vitro cleavage assay indicated that Cas13h1 exhibited more efficient cleavage activity when the FAM-labeled target RNA is flanked by A/G than flanked by C/U at 5′-end or without flanking nucleotide at 5′-end (Supplementary Figure S1D), suggesting the presence of a R(G/A) 5′-PFS. Extended complementarity between crRNA and target RNA beyond the guide-target RNA duplex robustly inhibits the cleavage activity (Supplementary Figure S1D).
Overall structure of Cas13h1-crRNA and Cas13h1-crRNA-target RNA
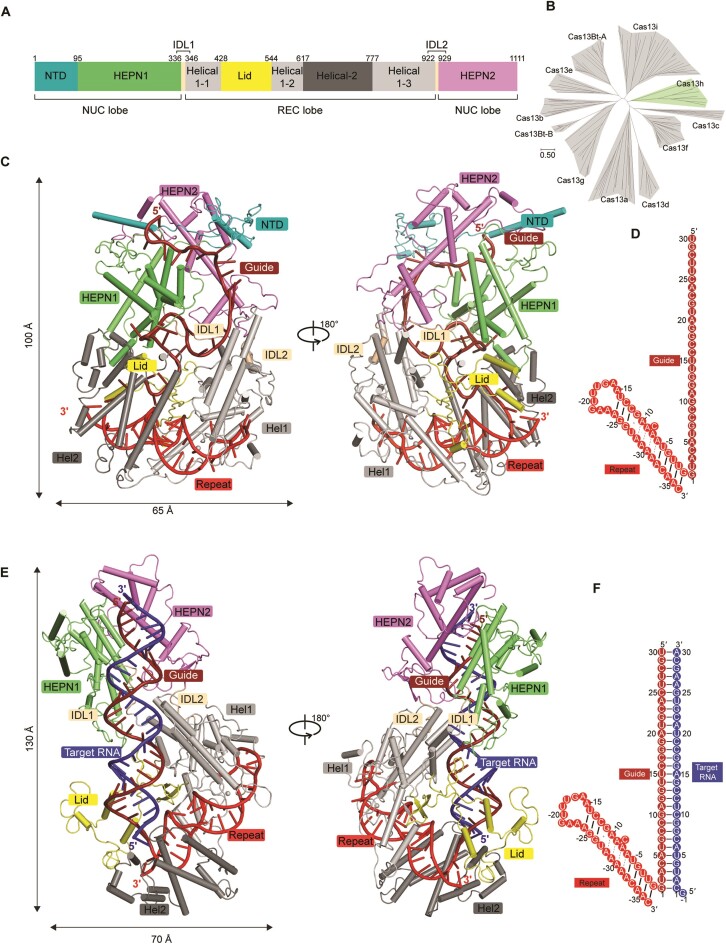
We assembled the Cas13h1-crRNA binary complex by incubating wild-type Cas13h1 with its cognate crRNA (30-nt guide and 36-nt repeat). The structure was determined at 3.1 Å resolution (Figure 1C, D, Table1, Supplementary Figure S2). Most of the amino acids and nucleotides can be modeled except for some protein loops (residues 133–147 and 450–493). The overall structure of binary complex adopts a compact and squashed architecture (∼65 Å in width and ∼100 Å in length), with crRNA buried inside Cas13h1 (Figure 1C). We assembled the ternary complex by incubating the catalytically dead Cas13h1 (R200A), the cognate crRNA (30-nt guide and 36-nt repeat), and a 31-nt target RNA with a 5′-flanking guanosine (Figure 1F). The structure was determined at 3.0 Å resolution (Figure 1E, F, Table 1, Supplementary Figure S3). Most of the amino acids and nucleotides can be modeled except for several protein loops (residues 1–97 and 1024–1049). In contrast, the overall structure of ternary complex adopts an elongated architecture (∼70 Å in width and ∼130 Å in length), with the guide-target RNA duplex adhering to Cas13h1 (Figure 1E).
Figure 1.
Structures of Cas13h1-crRNA and Cas13h1-crRNA-target RNA. (A) Domain organization of Cas13h1. (B) Phylogenetic tree of Cas13 subtypes. (C) Overall structure of Cas13h1-crRNA, with Cas13h1 domains colored based on (A), crRNA repeat colored in red, and crRNA guide colored in dark red. (D) Schematic of the crRNA sequence used for the binary complex assembly. (E) Overall structure of Cas13h1-crRNA-target RNA, with Cas13h1 domains colored based on (A), crRNA repeat colored in red, crRNA guide colored in dark red, and target RNA colored in blue. (F) Schematic of the crRNA and target RNA sequences used for the ternary complex assembly.
The Cas13h1 protein adopts a bilobed architecture, consisting of REC and NUC lobes (Figure 1). The REC lobe contains Helical-1 (residues 347–428, 545–617 and 778–922), Lid (residues 429–544) and Helical-2 (residues 618–777) domains (Figure 1A). The Helical-1 domain is composed of 10 α-helices and 1 β-sheet, the Helical-2 domain is composed of 10 α-helices, and the Lid domain is composed of 3 α-helices and 4 β-sheets (Supplementary Figure S4). The NUC lobe contains NTD (residues 1–95), HEPN1 (residues 96–336), and HEPN2 (residues 930–1111) domains (Figure 1A). The NTD domain is an N-terminal extended segment that covers both HEPN1 and HEPN2 domains (Supplementary Figure S4). The HEPN1 domain is composed of 9 α-helices, and the HEPN2 domain is composed of 6 α-helices (Supplementary Figure S4). The HEPN1 and HEPN2 domains form extensive interactions in the binary and ternary complexes. The REC and NUC lobes are connected by inter-domain linkers 1 (IDL1, residues 337–346) and 2 (IDL2, residues 923–929) (Figure 1C, D).
DALI search revealed that the structures closely similar to Cas13h1 include Prevotella buccae Cas13b (PDB: 6AAY, z-score = 13.7), Bergeyella zoohelcum Cas13b (PDB: 6DTD, z-score = 13.3), and Cas13bt3 (PDB: 7VTI, z-score = 12.4). Superposition of individual domains of Cas13h1 and PbuCas13b (PDB:6DTD) indicated that HEPN domains and the catalytic residues align well, and the overall folds of Helical domains are similar (Supplementary Figure S4). The NTD domain is absent from Cas13b, and DALI search indicated that Lid domain adopts a novel fold without similarity with any reported structures. Different from Cas13a and Cas13d which utilize 5′-DR, the domain organization of Cas13h1 is similar to those of Cas13b proteins and Cas13bt3 in which the domains from the REC lobe are flanked by HEPN domains (Figure 1A).
Interaction between crRNA repeat and Cas13h1
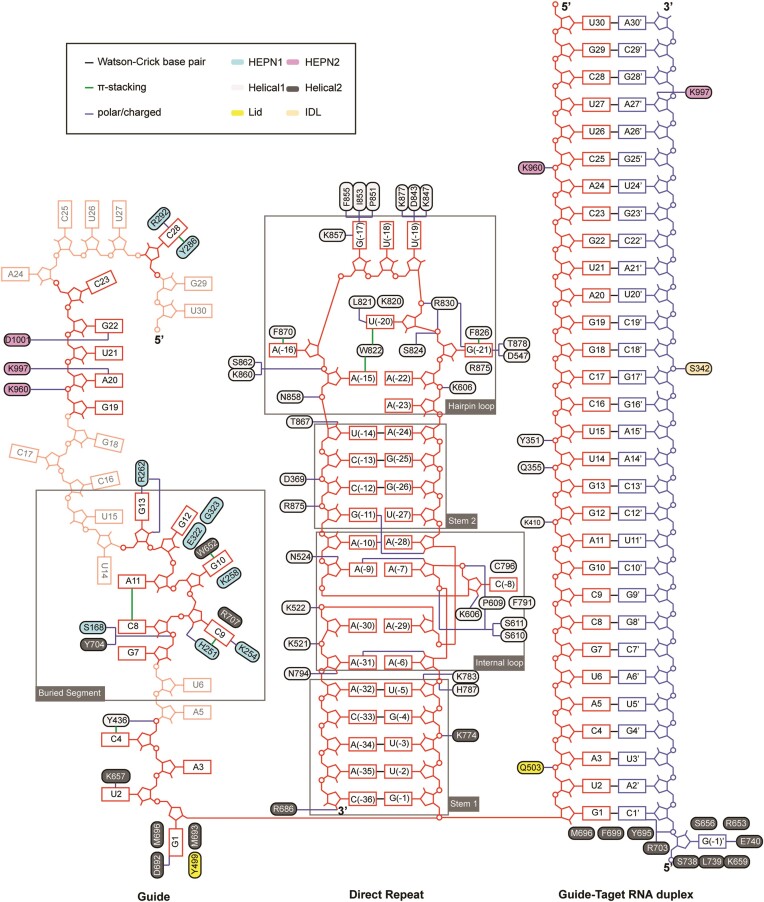
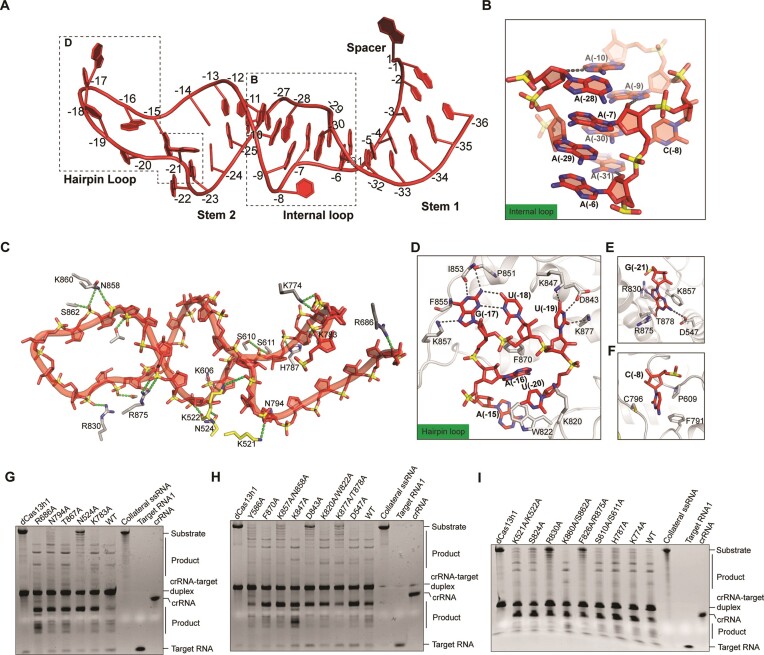
In both binary and ternary complexes, the crRNA repeat is sandwiched between Helical-1 and Helical-2 domains, forming extensive and almost identical interactions (Figure 2). The 36-nt crRNA repeat is divided into four parts, including stem 1 loop, internal loop, stem 2 loop, and hairpin loop. The stem 1 loop contains five Watson–Crick base pairs (G(-1)-C(-36), U(-2)-A(-35), U(-3)-A(-34), G(-4)-C(-33) and U(-5)-A(-32)) (Figure 3A). Eight out of the nine nucleotides (A(-6) to A(-10) and A(-28) to A(-31)) that constitute the internal loop are adenosine, and they adopt an intertwined duplex structure (Figure 3A, B). The stem 2 loop contains a wobble base pair (G(-11)-U(-27)) and 3 Watson–Crick base pairs (C(-12)-G(-26), C(-13)-G(-25) and U(-14)-A(-24)) (Figure 3A). The hairpin loop (A(-15) to A(-23)) protrudes some of the nucleobases to interact with the Helical-1 domain (Figure 3A, D). The sequence-independent interaction with Cas13h1 is mediated by the 2′-OH and the phosphate groups of crRNA repeat, and the sequence-specific interaction is mediated by the nucleobases of the bulge residues of crRNA repeat.
Figure 2.
Schematic of nucleic acid recognition by Cas13h1 in the binary and ternary complex. Intermolecular contacts between Cas13h1 and crRNA repeat, guide segment, and guide-target RNA duplex. The Cas13h1 residues are colored based on Figure 1A, crRNA repeat colored in red, crRNA guide colored in dark red, and target RNA colored in blue. The guide segments invisible from the structure are shown in transparency.
Figure 3.
Detailed interactions between Cas13h1 and crRNA repeat. (A) Structure of the crRNA repeat in the ternary complex. (B) Close-up view of the internal loop, with the contact between the 2′-OH and the nucleobases shown in black dashed lines. (C) Interaction of Cas13h1 residues with the phosphate groups and with the 2′-OH of the crRNA repeat, with the contacts shown in green dashed lines. (D) Interaction between crRNA hairpin loop and the Helical-1 domain of Cas13h1. (E, F) Interaction between crRNA bulge residues U(-21) (E) and U(-8) (F) and neighboring amino acids. (G–I) Urea-PAGE indicating Cas13h1 RNase activity by either the wild-type Cas13h1 or the mutants of the crRNA repeat interacting residues. dCas13h1, Cas13h1(R200A, H205A, R1076A and H1081A), was used as a negative control. crRNA, target RNA1, and collateral RNA were loaded as markers. The RNAs are label-free and stained by ethidium bromide before visualization.
The 2′-OH of U(-5), C(-8), A(-9), and A(-10), U(-14), A(-31) and A(-36) form polar contacts with the side chain of K783, the main chain of K606, the side chain of N524, the main chain of K522, the side chain of T867, the side chain of N794, and the side chain of R686, respectively (Figures 2, 3C). Besides mediating the interaction with Cas13h1, the 2′-OH also contributes in stabilizing the internal loop. Specifically, 2′-OH of A(-28), A(-7) and A(-6) interacts with the nucleobases of A(-10), A(-9) and A(-31), respectively (Figures 2, 3B). The phosphate groups of G(-4), G(-6), A(-7), C(-8), C(-12), A(-15), A(-16), U(-20), A(-21), A(-30) and A(-31) interacts with the side chains of K774, H787, S611, S610, R875, N858, S862 and K860, R830, S824, K522, and K521, respectively (Figures 2, 3C). Alanine substitution of N524, R830, and F826/R875 abolished the cleavage activity, K521/K522, K774, H787, K857/N858, S824 and N794 impaired the cleavage activity, while K783, S610/S611, K860/S862, T867 and R686 hardly affected the cleavage activity of Cas13h1 (Figure 3G–I).
The bulge residues within crRNA repeat include C(-8), G(-17), U(-18), U(-19), U(-20) and G(-21). The nucleobase of G(-21) is sandwiched by F826 and R875, and forms polar contact with D547 and T878 on Watson–Crick side, and with R830 on the Hoogsteen side (Figure 3E). The nucleobase of U(-20) is sandwiched by the side chains of W822 and K820, and forms polar contact with the main chain of W822 (Figure 3D). The nucleobases of U(-19), U(-18) and G(-17) flip out and form extensive interaction with the Helical-1 domain (Figure 3D). The nucleobase of U(-19) forms polar contact with K877, D843, and K847. The nucleobases of G(-17) and U(-18) form a wobble base pair. G(-17) forms polar contact with the main chains of P851, I853 and F855, and charged interaction with the side chain of K857 (Figure 3D). The nucleobase of A16 forms π–π stacking with F870 (Figure 3D), and the nucleobase of C(-8) is sandwiched by C796, F791, and P609 (Figure 3F). Alanine substitution of F826/R875, R830, and D843 abolished the cleavage activity, K877/T878 and K857/N858 impaired the cleavage activity, while D547, K820/W822, K847 and F870 hardly affected the cleavage activity of Cas13h1 (Figure 3G, H).
Interactions between Cas13h1 and crRNA guide and guide-target duplex
In binary complex, the twisted 30-nt crRNA guide segment is mostly buried inside Cas13h1 (Figure 4A). Most of the nucleobases are sandwiched by neighboring Cas13h1 residues in a sequence-independent manner (Figure 2). The guide segments, U14-G18, A24-U27 and G29-U30, show poor cryo-EM density due to lack of interaction with Cas13h1 (Figure 2, Supplementary Figure S2G). G1 inserts into a pocket formed by M693, M696, D692 and Y499 (Figure 4B). K657 forms cation–π interaction with the nucleobase of U2, and Y436 stacks with the nucleobase of C4 (Figure 4B). The nucleobases of G7, C8 and A11 stack together, while the nucleobase of C9 is sandwiched by H251 (HEPN1) and R707 (Helical-2), and the nucleobase of G10 is sandwiched by K258 (HEPN1) and W652 (Helical-2) (Figure 4C). The nucleobases of G12 and G13 are stabilized by the main chains of E322 and G323 and on one end, and R262 on the other end (Figure 4C). The nucleobases of G19, A20, U21 and G22 stack together and are exposed to the solvent (Figure 4A), and the phosphate groups form polar contact with K960, K997 and D1001 (Figure 4D). Given the mismatch experiment indicated that the mismatch ranging from position 13 to 24 is poorly tolerant (Supplementary Figure S1B), the guide segment G19-G22 might serve as the ‘central seed’ to initiate base pairing with target RNA. At the 5′-end of the guide segment, C28 is sandwiched by Y286 and R292 (Figure 4E). Alanine substitution of K258, K960 and D692/M693/M696 abolished the cleavage activity, H251, Y436, W652, Y286/R292 impaired the cleavage activity, while R262, and K657 hardly affected the cleavage activity of Cas13h1 (Figure 4F).
Figure 4.
Interactions of between Cas13h1 and crRNA guide. (A) Surface representation of Cas13h1 binary complex, with interested guide segments enclosed by rectangles. (B–E) Close-up view of interactions between different guide segments and neighboring Cas13h1. (B) G1-C4; C, G7-G13; (D) G19-G22; (E) C28. (F) Urea–PAGE indicating Cas13h1 RNase activity by either the wild-type Cas13h1 or the mutants of the crRNA guide interacting residues. dCas13h1, Cas13h1(R200A, H205A, R1076A and H1081A), was used as a negative control. crRNA, target RNA1, and collateral RNA were loaded as markers. The RNAs are label-free and stained by ethidium bromide before visualization.
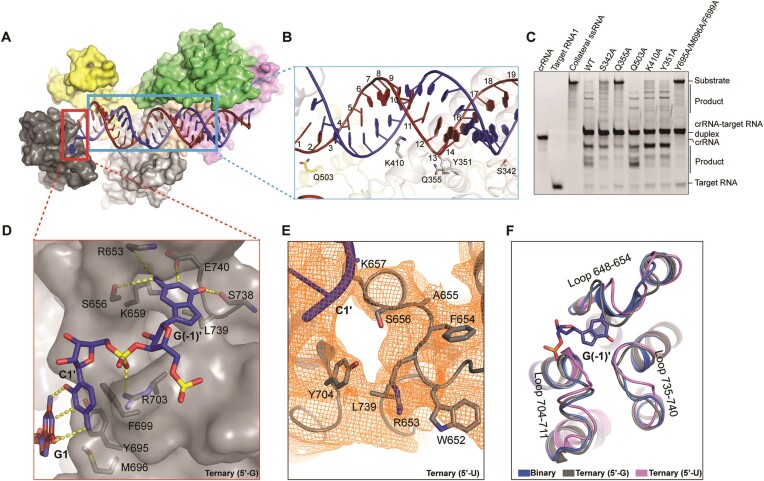
In ternary complex, the guide-target RNA duplex is loosely attached to, and forms few contacts with, the surface formed by HEPN1 and HEPN2 domains, consistent with the biochemical observation that HEPN domains fail to bind the crRNA-target RNA duplex (Figures 1D, 2, 5A, B, Supplementary Figure S1C). These interacting amino acids include K960, Q503, K410, Q355, Y351 and S342 (Figure 5B). Alanine substitution of K960 and Q355 abolished the cleavage activity, S342, Y351 and K410 impaired the cleavage activity, while Q503 hardly affected the cleavage activity of Cas13h1 (Figures 4F, 5C). The HEPN domains interact with the guide-target RNA duplex at position 23–30 (Figure 5A), possibly explaining why shortened guide-target RNA duplex has compromised cleavage activity (25). At the duplex end proximal to crRNA repeat, Y695, M696 and F699 act as a ‘wedge’ to cap the base pairing between guide G1 and target C1’ (Figure 5D). Group alanine substitutions of Y695, M696 and F699 abolished the RNA cleavage activity of Cas13h1 (Figure 5C).
Figure 5.
Interactions of between Cas13h1 and with guide-target duplex. (A) Surface representation of Cas13h1 ternary complex, with interested segments enclosed by rectangles. (B) Interaction details between Cas13h1 and guide-target RNA duplex. (C) Urea–PAGE indicating Cas13h1 RNase activity by either the wild-type Cas13h1 or the mutants of the guide-target duplex interacting residues. crRNA, target RNA1, and collateral RNA were loaded as markers. The RNAs are label-free and stained by ethidium bromide before visualization. (D) Interaction between the terminal base pairs and the ‘wedge’ residues, and interaction between 5′-G and the Heical-2 nucleotide binding pocket. (E) Structure of the 5′-U ternary complex around the 5′-end of target RNA, with cryo-EM density shown in mesh and amino acids around the nucleotide binding pocket shown in stick. (F) Superposition of the Helical-2 domains from the binary complex (blue), and ternary complex with 5′-G (gray), and the ternary complex with 5′-U (pink).
Intriguingly, we found a nucleotide binding pocket which is composed of side chains of K659, L739 and E740, and main chains of S656, R653 and S738 within Helical-2 domain (Figure 5D). These amino acids form extensive contacts with the guanosine at 5′-end of target RNA (Figure 5D). Alanine substitution of K659 and E740 impaired the cleavage activity, and L739 mildly impaired the cleavage activity of Cas13h1 (Supplementary Figure S1D). Glycine substitution of the loop (residues 648–654) that constitute the nucleotide binding pocket mildly impaired the cleavage activity of Cas13h1 (Supplementary Figure S1D). The ternary complex, with the 5′-guanosine of target RNA replaced with uridine, was determined at 3.9 Å resolution, but 5′-U is not observed due to lack of stabilization from neighboring amino acids (Figure 5E). Superposition of Helical-2 domains from the binary complex, ternary complex with 5′-G, and ternary complex with 5′-U revealed that three loops that constitute the nucleotide binding pocket are in slightly different conformation, especially the loop 648–654 (Figure 5F).
Notably, several amino acids play dual roles in binary and ternary complexes. K960 interacts with the phosphate groups of the crRNA A20 and the crRNA C25 in the binary and ternary complexes, respectively (Figure 2). K997 interacts with the crRNA A20 in the binary complex, and interacts with target RNA A27’ in the ternary complex (Figure 2).
Conformational changes of Cas13h1 upon target RNA engagement
Upon target RNA engagement, the crRNA repeat remains unchanged, while the twisted guide segment pairs with target RNA to form an A-form RNA duplex (Figures 1D, E, Supplementary Figure S5C), disrupting most of the Cas13h1-guide interaction observed from the binary complex (Figure 2). Notably, the guide segment G7-G13 tethers HEPN1 and Helical-2 domains together in the binary complex (Figure 4C), while HEPN1 and Helical-2 domains are separated in the ternary complex (Figure 6A). Besides crRNA guide movement, dramatic conformational changes within Cas13h1 were observed upon target RNA engagement. First and foremost, HEPN1 and HEPN2 domains move as far as 85 Å along the guide-target RNA duplex, with IDL1 and IDL2 domains acting as pivot points (Figure 6A, Supplementary Movie S1). Secondly, HEPN2 domain rotates around Helical-1 domain (Figure 6A), forming different inter-domain hydrophobic patches (Supplementary Figure S5D). Group alanine substitution of amino acids that constitute the hydrophobic patches (Y353, L357, W884, W891, F899, F903, I927, Y928, L932, F961, S900 and R901) impaired the cleavage activity (Figure 6B). Thirdly, the Helical-2 domain moves outwards as far as 13.4 Å to accommodate the end of guide-target duplex (Figure 6A, Supplementary Movie S1). Lastly, HEPN domains undergo local rearrangement to get activated (Figure 6C, D).
Figure 6.
Conformational changes of Cas13h1 upon target RNA engagement. (A) Cartoon representation of Cas13h1 binary complex and ternary complex displayed side by side with the same orientation. Individual domains are colored based on Figure 1A. (B) Urea–PAGE indicating Cas13h1 RNase activity by either the wild-type Cas13h1 or mutant Cas13h1. dCas13h1, Cas13h1(R200A, H205A, R1076A and H1081A), was used as a negative control. crRNA, target RNA1 and collateral RNA were loaded as markers. Group mutation: alanine substitution of Y353, L357, W884, W891, F899, F903, I927, Y928, L932, F961, S900 and R901. ASL deletion: substitution of residue 1024–1049 with 7-aa glycine/serine linker. The RNAs are label-free and stained by ethidium bromide before visualization. (C) Surface representation of HEPN domains before activation and after activation. The gray spheres indicate the catalytic residues. (D) Superposition of HEPN domains from the binary complex and ternary complex. The binary complex is colored in gray, and the ternary complex is colored based on Figure 1A. The catalytic residues and their shift are shown.
Local rearrangement within the HEPN domains includes three aspects. Firstly, in the binary complex, the distance between R200 Cα (HEPN1) and H1081 Cα (HEPN2) and the distance between H205 Cα (HEPN1) and R1076 Cα (HEPN2) are 11.57 Å and 11.78 Å respectively, while in the ternary complex, these catalytic residues get farther, with the distances changing into 17.19 Å and 17.39 Å, respectively. On the contrary, the catalytic residues of HEPN domains are drawn closer during the activation of other Cas13 ribonucleases (Supplementary Figure S5A). Secondly, an active site loop (ASL, residues 1025–1049) that covers the HEPN active site in binary complex gets disordered in ternary complex (Figure 6C), similar to the activation of Cas13bt3 (22). ASL deletion abolished the cleavage activity, highlighting the important, yet not well-understood, role of the ASL in the activation and catalysis of HEPN domains (Figure 6B). Lastly, NTD domain wraps around HEPN1 and HEPN2 domains, forms extensive hydrophobic interactions in the binary complex (Supplementary Figure S5B), but cannot be observed from the ternary complex (Figure 6C). Deletion of the entire NTD domain (residues 1–95) abolished the RNA cleavage activity of Cas13h1, and all the region except for the N-terminal region (residues 1–9) is indispensable for the cleavage activity (Figure 6B).
Discussion
In this study, we determined the structures of the Cas13h1-crRNA binary complex and the Cas13h1-crRNA-target RNA ternary complex. Combined with detailed mutagenesis study, the importance of the amino acids involved in crRNA repeat recognition, guide recognition, guide-target RNA duplex recognition, domain movement, and HEPN activation was validated. Moreover, the biochemical properties of Cas13h1 were characterized. Our results showed that Cas13h1 lacks in vitro pre-crRNA processing activity, contains a central ‘seed’ region in the crRNA guide, encodes a nucleotide binding pocket to recognize 5′-PFS, undergoes dramatic conformational changes for activation, and utilizes a complicated activation mechanism for RNA cleavage.
Despite the poor sequence similarity among different Cas13 subtypes, similarities can be found regarding the domain organization and the structural architecture. For Cas13a and Cas13d that utilize 5′-DR crRNA, the NTD domain recognizes the crRNA repeat, the HEPN1 domain is bipartite, and the guide-target RNA duplex is mostly buried within the protein. For Cas13h1 and Cas13bt3 that utilize 3′-DR crRNA, HEPN domains are flanked by REC lobes, and the guide-target RNA duplex is mostly exposed to the solvent. Surprisingly, Cas13h1 in the ternary complex adopts an unexpected elongated architecture, different from the compact architecture of other Cas13 ternary complexes (22,31,34). Besides, Cas13h1 contains a novel extended N-terminal segment (NTD domain) that wraps around the HEPN domains. These observations highlight the divergence of Cas13 ribonucleases at domain and structure level, and the importance of the directionality of the crRNA repeat in dictating the Cas13 domain organization.
PFS and tag:anti-tag RNA pairing could dramatically impact Cas13 cleavage activity. In this study, we found the cleavage activity of Cas13h1 is enhanced by a R(G/A) 5′-PFS, and inhibited by tag:anti-tag RNA pairing. At the 5′-end of target RNA, the terminal G(-1)’ flips out and inserts into the nucleotide-binding pocket of Helical-2 domain, and this pocket cannot bind uridine. It awaits further study to determine whether Cas13h1 undergoes similar conformational changes to LshCas13a when loaded with crRNA-target RNA duplex with extended complementarity (29). More work needs to be done to test whether the boost and inhibition observed in vitro can be repeated in physiological condition. As for now, it seems controversial whether the self/non-self discrimination is really needed for type VI CRISPR-Cas systems under physiological conditions (28), but at least, investigation into these regulatory elements could facilitate Cas13-based RNA applications.
For Cas9 and Cas12 nucleases, highly detailed working mechanisms have been reported (49,50), while the working mechanism of Cas13 ribonucleases remains less clear. Significant movement of HEPN domains was observed upon target RNA binding; however, the driving force of the domain movement is not well understood for most of the Cas13 ribonucleases. Stabilized by Cas13h1 amino acids, most of the crRNA guide segments can be observed in the Cas13h1 binary complex. The observation that the HEPN1 domain and Helical-2 domain sandwich a 7-nt guide segment (G7–G13) and get separated upon guide-target RNA duplex formation, exemplified how base pairing between crRNA guide and target RNA drives the domain movement required for Cas13 activation.
Last but most importantly, the HEPN domains of Cas13h1 utilize a complicated activation mechanism. Structural analysis into LbuCas13a and EsCas13d indicated that the guide-target RNA duplex formation triggers the HEPN1 domain to move towards the HEPN2 domain and bring the catalytic residues closer. For Cas13b, all the structures are determined in the absence of target RNA. For Cas13bt3, the activation mechanism is limited to the destabilization of the active site loop which can provide access of the ssRNA. The HEPN catalytic residues seems to adopt similar configurations regardless of the target RNA binding (Supplementary Figure S5A). For Cas13h1, the target RNA engagement leads to HEPN1 domain moving away from the HEPN2 domain, with the catalytic residues getting farther, which is opposite to Cas13a and Cas13d (31,34). The ASL loop gets disordered, similar to Cas13bt3; moreover, the NTD domain gets disordered. Therefore, the activation mechanisms of Cas13h1 seems distinct from those of LbuCas13a and Cas13bt3. Although it is tempting to simplify the activation of Cas13h1 HEPN domains into a ‘open/close’ model, recent large-scale molecular dynamics experiments on LbuCas13a indicated that the target RNA binding shifts the dynamics of catalytic cleft and the distance change within catalytic residues differs from previous structural study (31,51). The delicate activation mechanisms of the HEPN domains of Cas13h1 need more biophysical and molecular simulation studies in the future.
Supplementary Material
Acknowledgements
We thank the staff members from Cryo-Electron Microscopy Facility of Hubei University for help on data collection and computation.
Contributor Information
Fugen Chen, State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.
Chendi Zhang, State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.
Jialin Xue, State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.
Feng Wang, State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.
Zhuang Li, State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei 430062, China.
Data availability
Cryo-EM reconstructions of Cas13h1-crRNA and Cas13h1-crRNA-target RNA have been deposited in the Electron Microscopy Data Bank under the accession numbers EMD-37448 and EMD-37439 respectively. Coordinates for atomic models of Cas13h1-crRNA and Cas13h1-crRNA-target RNA have been deposited in the Protein Data Bank under the accession numbers 8WCS and 8WCE respectively.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
Ministry of Science and Technology [2022YFA0911800]; National Natural Science Foundation of China [32201004]; Hubei Provincial Natural Science Foundation [ZRMS2022000096]; Key Science and Technology Innovation Project of Hubei Province [2021BAD001]. Funding for open access charge: Ministry of Science and Technology [2022YFA0911800].
Conflict of interest statement. None declared.
References
- 1. Hille F., Richter H., Wong S.P., Bratovic M., Ressel S., Charpentier E.. The Biology of CRISPR-Cas: backward and Forward. Cell. 2018; 172:1239–1259. [DOI] [PubMed] [Google Scholar]
- 2. Mohanraju P., Makarova K.S., Zetsche B., Zhang F., Koonin E.V., van der Oost J.. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016; 353:aad5147. [DOI] [PubMed] [Google Scholar]
- 3. Wright A.V., Nunez J.K., Doudna J.A.. Biology and applications of CRISPR Systems: harnessing nature's toolbox for genome engineering. Cell. 2016; 164:29–44. [DOI] [PubMed] [Google Scholar]
- 4. Hsu P.D., Lander E.S., Zhang F.. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014; 157:1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Oost J., Westra E.R., Jackson R.N., Wiedenheft B.. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat. Rev. Micro. 2014; 12:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makarova K.S., Wolf Y.I., Iranzo J., Shmakov S.A., Alkhnbashi O.S., Brouns S.J.J., Charpentier E., Cheng D., Haft D.H., Horvath P.et al.. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Micro. 2020; 18:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shmakov S., Smargon A., Scott D., Cox D., Pyzocha N., Yan W., Abudayyeh O.O., Gootenberg J.S., Makarova K.S., Wolf Y.I.et al.. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Micro. 2017; 15:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makarova K.S., Wolf Y.I., Alkhnbashi O.S., Costa F., Shah S.A., Saunders S.J., Barrangou R., Brouns S.J., Charpentier E., Haft D.H.et al.. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Micro. 2015; 13:722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shmakov S., Abudayyeh O.O., Makarova K.S., Wolf Y.I., Gootenberg J.S., Semenova E., Minakhin L., Joung J., Konermann S., Severinov K.et al.. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol. Cell. 2015; 60:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P., Moineau S., Mojica F.J., Wolf Y.I., Yakunin A.F.et al.. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Micro. 2011; 9:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pickar-Oliver A., Gersbach C.A.. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019; 20:490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Komor A.C., Badran A.H., Liu D.R.. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017; 168:20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B., Shmakov S., Makarova K.S., Semenova E., Minakhin L.et al.. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016; 353:aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. East-Seletsky A., O’Connell M.R., Knight S.C., Burstein D., Cate J.H., Tjian R., Doudna J.A.. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature. 2016; 538:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smargon A.A., Cox D.B.T., Pyzocha N.K., Zheng K., Slaymaker I.M., Gootenberg J.S., Abudayyeh O.A., Essletzbichler P., Shmakov S., Makarova K.S.et al.. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Mol. Cell. 2017; 65:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D.. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018; 173:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan W.X., Chong S., Zhang H., Makarova K.S., Koonin E.V., Cheng D.R., Scott D.A.. Cas13d is a compact RNA-targeting type VI CRISPR effector positively modulated by a WYL-domain-containing accessory protein. Mol. Cell. 2018; 70:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., Verdine V., Cox D.B.T., Kellner M.J., Regev A.et al.. RNA targeting with CRISPR-Cas13. Nature. 2017; 550:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox D.B.T., Gootenberg J.S., Abudayyeh O.O., Franklin B., Kellner M.J., Joung J., Zhang F.. RNA editing with CRISPR-Cas13. Science. 2017; 358:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. East-Seletsky A., O’Connell M.R., Burstein D., Knott G.J., Doudna J.A.. RNA targeting by functionally orthogonal type VI-A CRISPR-Cas enzymes. Mol. Cell. 2017; 66:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahas A., Marsic T., Lopez-Portillo Masson M., Wang Q., Aman R., Zheng C., Ali Z., Alsanea M., Al-Qahtani A., Ghanem B.et al.. Characterization of a thermostable Cas13 enzyme for one-pot detection of SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2118260119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakagawa R., Kannan S., Altae-Tran H., Takeda S.N., Tomita A., Hirano H., Kusakizako T., Nishizawa T., Yamashita K., Zhang F.et al.. Structure and engineering of the minimal type VI CRISPR-Cas13bt3. Mol. Cell. 2022; 82:3178–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F., Zhang C., Xu H., Zeng W., Ma L., Li Z.. Structural basis for the ribonuclease activity of a thermostable CRISPR-Cas13a from Thermoclostridium caenicola. J. Mol. Biol. 2023; 435:168197. [DOI] [PubMed] [Google Scholar]
- 24. Kellner M.J., Koob J.G., Gootenberg J.S., Abudayyeh O.O., Zhang F.. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019; 14:2986–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu Y., Chen Y., Xu J., Wang X., Luo S., Mao B., Zhou Q., Li W.. Metagenomic discovery of novel CRISPR-Cas13 systems. Cell Discov. 2022; 8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu C., Zhou Y., Xiao Q., He B., Geng G., Wang Z., Cao B., Dong X., Bai W., Wang Y.et al.. Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes. Nat. Methods. 2021; 18:499–506. [DOI] [PubMed] [Google Scholar]
- 27. Kannan S., Altae-Tran H., Jin X., Madigan V.J., Oshiro R., Makarova K.S., Koonin E.V., Zhang F.. Compact RNA editors with small Cas13 proteins. Nat. Biotechnol. 2022; 40:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meeske A.J., Marraffini L.A.. RNA guide complementarity prevents self-targeting in type VI CRISPR systems. Mol. Cell. 2018; 71:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang B., Zhang T., Yin J., Yu Y., Xu W., Ding J., Patel D.J., Yang H.. Structural basis for self-cleavage prevention by tag:anti-tag pairing complementarity in type VI Cas13 CRISPR systems. Mol. Cell. 2021; 81:1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knott G.J., East-Seletsky A., Cofsky J.C., Holton J.M., Charles E., O’Connell M.R., Doudna J.A.. Guide-bound structures of an RNA-targeting A-cleaving CRISPR-Cas13a enzyme. Nat. Struct. Mol. Biol. 2017; 24:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu L., Li X., Ma J., Li Z., You L., Wang J., Wang M., Zhang X., Wang Y.. The molecular architecture for RNA-guided RNA cleavage by Cas13a. Cell. 2017; 170:714–726. [DOI] [PubMed] [Google Scholar]
- 32. Liu L., Li X., Wang J., Wang M., Chen P., Yin M., Li J., Sheng G., Wang Y.. Two distant catalytic sites are responsible for C2c2 RNase activities. Cell. 2017; 168:121–134. [DOI] [PubMed] [Google Scholar]
- 33. Zhang B., Ye W., Ye Y., Zhou H., Saeed A., Chen J., Lin J., Perculija V., Chen Q., Chen C.J.et al.. Structural insights into Cas13b-guided CRISPR RNA maturation and recognition. Cell Res. 2018; 28:1198–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang C., Konermann S., Brideau N.J., Lotfy P., Wu X., Novick S.J., Strutzenberg T., Griffin P.R., Hsu P.D., Lyumkis D. Structural basis for the RNA-guided ribonuclease activity of CRISPR-Cas13d. Cell. 2018; 175:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meeske A.J., Jia N., Cassel A.K., Kozlova A., Liao J., Wiedmann M., Patel D.J., Marraffini L.A.. A phage-encoded anti-CRISPR enables complete evasion of type VI-A CRISPR-Cas immunity. Science. 2020; 369:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kick L.M., von Wrisberg M.K., Runtsch L.S., Schneider S.. Structure and mechanism of the RNA dependent RNase Cas13a from Rhodobacter capsulatus. Commun. Biol. 2022; 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deng X., Osikpa E., Yang J., Oladeji S.J., Smith J., Gao X., Gao Y.. Structural basis for the activation of a compact CRISPR-Cas13 nuclease. Nat. Commun. 2023; 14:5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slaymaker I.M., Mesa P., Kellner M.J., Kannan S., Brignole E., Koob J., Feliciano P.R., Stella S., Abudayyeh O.O., Gootenberg J.S.et al.. High-resolution structure of Cas13b and biochemical characterization of RNA targeting and cleavage. Cell Rep. 2019; 26:3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma B., Lu C., Wang Y., Yu J., Zhao K., Xue R., Ren H., Lv X., Pan R., Zhang J.et al.. A genomic catalogue of soil microbiomes boosts mining of biodiversity and genetic resources. Nat. Commun. 2023; 14:7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Zidek A., Potapenko A.et al.. Highly accurate protein structure prediction with AlphaFold. Nature. 2021; 596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson R.F., Iadanza M.G., Hesketh E.L., Rawson S., Ranson N.A.. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nat. Protoc. 2019; 14:100–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H.. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018; 7:e42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng S.Q., Palovcak E., Armache J.P., Verba K.A., Cheng Y., Agard D.A.. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017; 14:331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016; 193:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A.. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017; 14:290–296. [DOI] [PubMed] [Google Scholar]
- 46. Emsley P., Lohkamp B., Scott W.G., Cowtan K.. Features and development of Coot. Acta. Crystallogr. D Biol. Crystallogr. 2010; 66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Afonine P.V., Poon B.K., Read R.J., Sobolev O.V., Terwilliger T.C., Urzhumtsev A., Adams P.D.. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol. 2018; 74:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E.. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004; 25:1605–1612. [DOI] [PubMed] [Google Scholar]
- 49. Swarts D.C., Jinek M.. Mechanistic insights into the cis- and trans-acting DNase activities of Cas12a. Mol. Cell. 2019; 73:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang F., Doudna J.A.. CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017; 46:505–529. [DOI] [PubMed] [Google Scholar]
- 51. Sinha S., Molina Vargas A.M., Arantes P.R., Patel A., O’Connell M.R., Palermo G.. Unveiling the RNA-mediated allosteric activation discloses functional hotspots in CRISPR-Cas13a. NucleicAcidsRes. 2023; 52:906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM reconstructions of Cas13h1-crRNA and Cas13h1-crRNA-target RNA have been deposited in the Electron Microscopy Data Bank under the accession numbers EMD-37448 and EMD-37439 respectively. Coordinates for atomic models of Cas13h1-crRNA and Cas13h1-crRNA-target RNA have been deposited in the Protein Data Bank under the accession numbers 8WCS and 8WCE respectively.