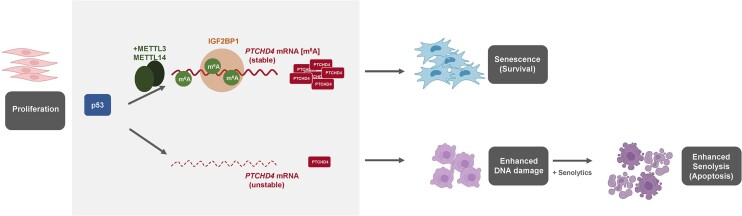
Abstract
RNA modifications, including N6-methyladenosine (m6A), critically modulate protein expression programs in a range of cellular processes. Although the transcriptomes of cells undergoing senescence are strongly regulated, the landscape and impact of m6A modifications during senescence are poorly understood. Here, we report a robust m6A modification of PTCHD4 mRNA, encoding Patched Domain-Containing Protein 4, in senescent cells. The METTL3/METTL14 complex was found to incorporate the m6A modification on PTCHD4 mRNA; addition of m6A rendered PTCHD4 mRNA more stable and increased PTCHD4 production. MeRIP RT-qPCR and eCLIP analyses were used to map this m6A modification to the last exon of PTCHD4 mRNA. Further investigation identified IGF2BP1, but not other m6A readers, as responsible for the stabilization and increased abundance of m6A-modified PTCHD4 mRNA. Silencing PTCHD4, a transmembrane protein, enhanced growth arrest and DNA damage in pre-senescent cells and sensitized them to senolysis and apoptosis. Our results indicate that m6A modification of PTCHD4 mRNA increases the production of PTCHD4, a protein associated with senescent cell survival, supporting the notion that regulating m6A modification on specific mRNAs could be exploited to eliminate senescent cells for therapeutic benefit.
Graphical Abstract
Graphical Abstract.
Introduction
Cellular senescence is defined as a state of indefinite growth arrest following exposure to different harmful stimuli, including telomere shortening, damage to the DNA and other macromolecules, oncogenic signaling and mitochondria dysfunction (1,2). Senescent cells can be identified by an increase in the activity of a senescence-associated beta galactosidase (SA-β-Gal), resistance to apoptosis and elevated expression of the cell cycle inhibitors p21 (CDKN1A), p53 (TP53) and p16 (CDKN2A) (3,4). Despite the arrested division, senescent cells are metabolically active and secrete many cytokines, growth factors, and matrix metalloproteases; this trait is collectively known as the senescence-associated secretory phenotype (SASP). The transient presence of senescent cells and the SASP can be beneficial in specific developmental times, including embryogenesis and wound healing; however, the long-term accumulation of senescent cells and persistent SASP can have detrimental consequences including chronic inflammation, stem cell exhaustion, and tissue dysfunction (5,6). In turn, the accumulation of senescent cells with age exacerbates age-associated neurodegenerative and cardiovascular diseases, diabetes and cancer (2,7,8). In recent years, many strategies have been sought to clear senescent cells and reduce the SASP (through the use of senolytics and senomorphics, respectively) in order to alleviate age-related conditions (9,10).
Cellular senescence is associated with profound alterations in RNA metabolism, including distinct transcription patterns, and changes in RNA composition, processing, and turnover (11,12). In particular, the dynamic and reversible process of RNA methylation at N6-methyladenosine (m6A) is a highly prevalent internal modification of eukaryotic messenger RNA (mRNA) (13–15) that controls gene expression programs in many developmental and disease processes (16,17). Modification at m6A occurs co-transcriptionally and is controlled by a methyltransferase complex composed of several core proteins (‘writers’), including the catalytic subunit methyltransferase-like 3 (METTL3) and the non-catalytic subunit METTL14, which acts as an RNA-binding scaffold and stabilizer of the methyltransferase complex (15,18). A third enzyme, METTL16, was recently described as another novel methyltransferase (15,19). The m6A modification is identified by several RNA-binding proteins (RBPs) known as m6A ‘readers’, which include IGF2BP1/2/3, HNRNPs, YTHDF1/2/3 and ELAVL1; these proteins carry out alternative splicing of m6A-modified pre-mRNA, as well as the nuclear export, stabilization, and translation of m6A-modified mRNA, collectively regulating protein expression patterns (15,20,21). The m6A modification can be removed by the ‘erasers’ FTO and ALKBH5, which oxidatively demethylate m6A (15).
Evidence is accumulating that m6A modification may critically regulate cellular senescence and aging (22,23). Changes in the activities of m6A regulatory enzymes and the m6A modification of specific mRNAs have been reported in several models of senescence. For example, human embryonic fibroblasts displayed unique patterns of RNA methylation upon replicative senescence and H2O2-induced senescence (24). In human mesenchymal stem cells, m6A modification by METTL3 stabilized the MIS12 mRNA, which encodes the proliferative factor MIS12, and thus reduced premature senescence (25). In human intestinal cells, knockdown of METTL14 decreased m6A on lamin B receptor (LBR) mRNA, lowering LBR mRNA stability and accelerating cellular senescence (26). Finally, in human and murine cells, METTL3 and METTL14 were shown to regulate cellular senescence and SASP production, even though this regulation did not appear to be m6A-dependent (27).
Despite its strong influence on gene expression programs, the impact of m6A on cell senescence has not been systematically investigated. Here, we sought to identify those mRNAs that were highly m6A-modified in two different models of senescence of human diploid fibroblasts (HDFs). In one, HDFs were rendered replicatively senescent (RS) by culturing them until they reached ∼50 rounds of division [or population doubling level (PDL) ∼50], during which cells gradually acquire senescence-associated changes including DNA damage from progressively shortened telomeres. In the other, HDFs were subjected to ionizing radiation-induced senescence (IR-IS), which instead caused senescence as a result of acute DNA damage (28,29). In both models of cellular senescence, PTCHD4 mRNA was highly abundant and highly methylated. PTCHD4 mRNA was transcriptionally induced by p53, a master transcription factor in senescence (30–32) and was co-transcriptionally methylated by the complex METTL3/METTL14. Upon methylation, the m6A reader IGF2BP1 was found to bind and stabilize the PTCHD4 mRNA. PTCHD4, a transmembrane protein, suppresses the Hedgehog signaling pathway in colorectal cancer cells (33,34), but its function has not been investigated in senescent fibroblasts. RNA sequencing (RNA-seq) analysis of pre-senescent WI-38 cells indicated that PTCHD4 sustains the expression of components of the Wnt pathway, implicated in cell adhesion, migration, polarity, proliferation, and survival (35,36). Importantly, PTCHD4 silencing in pre-senescent cells accelerated growth arrest and DNA damage, and sensitized cells to apoptosis induced by senolytic compounds. In summary, we describe for the first time the increased production of PTCHD4 in senescent cells via m6A modification of PTCHD4 mRNA, and the function of PTCHD4 in senescent cell survival and senolysis.
Materials and methods
Cell culture treatment and detection of SA-β-Gal
WI-38 human diploid fibroblasts (Coriell Institute for Medical Research: ID AG06814) were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) supplemented with 10% FBS, 1% antibiotics, and 1% non-essential amino acids (Gibco). Cells were maintained in an incubator at 37°C and 5% CO2 and tested negative for mycoplasma. Replicative senescence (RS) of WI-38 fibroblasts was achieved by passaging proliferating cells [typically at population doubling level (PDL) ∼22] until they stopped proliferating (typically ∼PDL50); pre-senescent cells (∼PDL40) displayed markedly reduced proliferation rates. Ionizing radiation-induced senescence (IR-IS) was elicited by exposing WI-38 cells to 10 Gy IR followed by incubation for the times indicated. Etoposide-induced senescence (ETO-IS) was achieved by treating WI-38 cells with 50 μM etoposide for 72 h, followed by incubation in fresh media for the times indicated. Senescence-associated-β-galactosidase (SA-β-Gal) activity was measured following the manufacturer's instructions (Cell Signaling Technology, cat. 9860); SA-β-Gal micrographs were acquired with a digital camera system (Nikon Digital Sight) adapted to a microscope (Nikon Eclipse TS100). SA-β-Gal activity was quantified with the ‘SPiDER-βGal’ Cellular Senescence Plate Assay Kit (Dojindo) following the manufacturer instructions, and was normalized to cell numbers quantified using the Cell Count Normalization Kit (Dojindo).
For senolytic treatments, cells were treated for 24 h with 10 μM ABT-737 (Selleckchem, cat. S1002), 20 μM Gingerenone A (Cayman Chemical, cat. 36841), or a cocktail of 20 μM Dasatinib (Selleckchem, cat. S1021) + 8 μM Quercetin (Selleckchem, cat. S2391). Control treatments included 0.1% vehicle (DMSO). The METTL3 inhibitor STM2457 was purchased from MedChemExpress (cat. HY-134836) and was used at a final concentration of 2 μM for up to 48 h.
Transfection and transduction
Silencing interventions were performed by transfecting 50 nM of siRNAs using Lipofectamine (RNAiMax, Invitrogen) following the manufacturer's instructions. A non-targeting siRNA pool was purchased from Horizon Discovery (cat. D-001206-14-20). To silence PTCHD4, we first tested two individual siRNAs, siPTCHD4 #1 (D-032649-02-0020) and siPTCHD4 #2 (D-032649-03-0020); where indicated, the two siRNAs were used as a pool (referred to as siPTCHD4). Other siRNA pools purchased from Horizon Discovery included siRNAs targeting IGF2BP2 (L-017705-00-0005), IGF2BP3 (L-003976-00-0005), HNRNPC (L-011869-03-0005), ELAVL1 (L-003773-00-0005) and YTHDF2 (L-021009-02-0005). For IGF2BP1, we used a pool of 4 siRNAs (L-003977-00-0005) and, where specified, we tested each siRNA individually; for silencing METTL3 and METTL14, we designed siRNAs siMETTL3 (CUGCAAGU AUGUUCACUAUGAdTdT) and siMETTL14 (AAGGAUGAGUUAAUAGCUAAAdTdT), respectively (Horizon Discovery); the p53 siRNA was purchased from Santa Cruz Biotechnology (sc-29435).
For PTCHD4 overexpression, lentiviral vectors were purchased from Genecopoeia. Following transduction for 18 h at an MOI (multiplicity of infection) of 5, media was replaced and 48 h later cultures were selected with puromycin (1 μg/ml) for 7 days, before collection for analysis.
Proliferating WI-38 cells (PDL22) were transfected for 6 h with 25 nM of a siRNA directed at the TP53 mRNA (siTP53) or a scrambled siRNA (siCtrl). Eighteen hours later, cells were transfected for 6 h with 50 ng of specific reporter plasmids, either the empty reporter plasmid (pGL3), a reporter plasmid containing the WT promoter of the PTCHD4 gene [100 bp upstream of the transcription start site, pGL3-PTCHD4prom-WT], or a promoter in which the p53 response element was deleted [pGL3-PTCHD4prom-DEL]. Cells were treated for 18 h with DMSO or Nutlin-3a (10 μM), whereupon lysates were prepared, and firefly luciferase signals were assessed in each sample with a Dual-Glo luciferase assay system (Promega), normalized to total protein concentration in each sample.
RNA isolation and RT-qPCR analysis
Total RNA was extracted using a single-step liquid phase separation procedure and TriPure isolation reagent (Sigma-Aldrich). Labeling of nascent RNA was performed by adding 5-ethynyl uridine (EU) to the cell culture media at a final concentration of 0.2 mM for 6 h, after which nascent transcripts were selectively biotinylated and purified with the Click-iT™ Nascent RNA Capture Kit (Thermo Fisher Scientific) following the manufacturer's instructions. RNA integrity was checked on an Agilent TapeStation using the RNA Screen Tape kit (Agilent). Reverse transcription (RT) of total RNA (500 ng) was performed using the Maxima Reverse Transcriptase protocol (Thermo Fisher Scientific) and real-time, quantitative PCR (qPCR) analysis was performed using the SYBR Green FAST qPCR master mix kit (Kapa Biosystems) on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific). Primer oligos were obtained from IDT and their sequences are listed in Supplementary Table S1. RNA levels were calculated with the 2−ΔΔCt method and were normalized by considering the average Ct values of two or more mRNAs encoding housekeeping proteins or 18S rRNA. In general, ACTB and GAPDH mRNAs were used for normalization; however, given that silencing PTCHD4 influenced the levels of ACTB mRNA and other mRNAs encoding cytoskeletal proteins, in the experiments involving PTCHD4 silencing, GAPDH mRNA and 18S rRNA were used for normalization.
To measure mRNA half-life, cells were treated with Actinomycin D (ActD, 2 μg/ml) to block de novo transcription by RNA polymerase II; after extracting total RNA at different times, mRNA levels were measured by RT-qPCR analysis. The levels of 18S rRNA (whose transcription is not influenced by ActD) were measured in parallel and used to normalize differences in RNA input. Half-life was estimated as the time needed for a given mRNA to reach one-half (50%) of its abundance at time 0 h before adding ActD.
Epitranscriptomic array for m6A detection
The detection of m6A-modified mRNAs in proliferating (PDL22) and senescent (RS PDL50, IR-IS) WI-38 cells was performed using the mRNA&LncRNA Epitranscriptomic Array Service (Arraystar). Briefly, total RNA was immunoprecipitated with anti-N6-methyladenosine (m6A) antibody. The modified RNA was then eluted from the immunoprecipitated magnetic beads (‘IP’ RNA), and the unmodified RNA was recovered from the supernatant (‘Sup’ RNA). The IP and Sup RNAs were labeled with Cy5 and Cy3, respectively, using the Arraystar Super RNA Labeling Kit. The fractions were combined and used to hybridize the Arraystar Human mRNA&lncRNA Epitranscriptomic Microarray (8 × 60K, Arraystar). After washing the slides, the arrays were scanned in two-color channels by an Agilent Scanner G2505C. Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Raw intensities of IP RNA (immunoprecipitated, Cy5-labelled) and Sup RNA (supernatant, Cy3-labelled) were normalized with average of log2-scaled Spike-in RNA intensities. RNAs differentially methylated at m6A between two comparison groups were identified by filtering using fold change and statistical significance (P-value) thresholds reported in the figure legends. Data are deposited in GEO, SuperSeries GSE247621; individual datasets for microarray (GSE246934), m6A-eCLIP-seq (GSE247595) and RNA-seq (GSE247596) data are included within the Superseries.
m6A-eCLIP-seq analysis
Sample preparation for the m6A-eCLIP analysis was performed with the m6A-eCLIP Kit (EclipseBio) following the manufacturer's protocol. Briefly, total RNA from proliferating (∼PDL22) and senescent (10 Gy IR, harvested 10 days later) WI-38 cells was chemically fragmented to sizes ranging from 100 to 200 nt. PolyA-selected RNA was UV-crosslinked to the provided m6A antibody. The antibody:RNA complex was size-separated by electrophoresis through SDS-containing polyacrylamide gels (SDS-PAGE) and transferred to a nitrocellulose membrane to remove any non-crosslinked RNA. The extracted RNA samples were treated with proteinase K to remove all the antibody, except the crosslinked portions. RNA was then isolated and used for library preparation and RNA sequencing (paired-end sequencing, 50 million reads) on an Illumina NovaSeq 6000 system. Data analysis was performed by Eclipsebio. Briefly, UMI-tools was used for trimming the 5′ ends (37), after which Cutadapt was used for removing low-quality reads and adapter sequences from 3′ ends (38). STAR (39) was used to first remove repetitive elements such as rRNA, and then map to the hg38 reference genome. PCR duplicates were removed using UMI-tools. Peak calling was performed using CLIPper (https://github.com/YeoLab/clipper/wiki/CLIPper-Home). Each peak was normalized against the paired input sample. The peak p-values and fold enrichments are based on eCLIP reads per million (RPM) compared to input RPM. Only m6A peaks that met cutoffs of log2 fold change ≥3 and −log10(P-value) ≥3 were considered. Reproducible peaks among triplicates were identified using the tool IDR (https://github.com/nboley/idr). Bigwig files were visualized using pyGenomeTracks (40). Raw files are available in GEO (GSE247621).
RNA-seq analysis
Total RNA was extracted from WI-38 cells (PDL40), 48 h after transfection with siCtrl, siPTCHD4 #1 or siPTCHD4 #2, and sent to Quick Biology (Quick Biology, Pasadena) for further mRNA purification and analysis. The reads were first mapped to the latest UCSC transcript set using Bowtie2 version 2.1.0, and the levels of different transcripts were estimated using RSEM v1.2.15. Differentially abundant RNAs were identified using the edgeR program. RNAs showing altered expression with P < 0.05 |log2FC| >1.5 were considered differentially abundant. The GOseq package was used to perform GO enrichment analysis, and Kobas was used to perform the pathway analysis. RNA-seq datasets were deposited in GEO (GSE247621).
In vitro transcription and biotin pulldown
RNA was transcribed in vitro using the MEGAscript™ T7 Transcription Kit (Thermo Fisher Scientific). To biotinylate the nascent transcripts, biotin-14-CTP (Thermo Fisher Scientific) was included in the reaction mix, with a 1:4 ratio of biotin-14-CTP to CTP. To generate random m6A sites in RNA probes, m6ATP was added to the reaction mix (1:4 ratio of m6ATP to ATP). RNA was purified with the RNA Clean & Concentrator Kit (Zymo Research). Biotin pulldown and identification of RNA-protein interactions was performed following a previously described protocol (41). Briefly, 1 μg of labeled RNA was incubated with 500 μg of cell lysates from WI-38 cells at the indicated PDL. RNA-protein complexes were pulled down using streptavidin-coated beads, and western blot analysis was performed to detect an interaction between the RNA probes and the indicated RBPs.
Ribonucleoprotein immunoprecipitation (RIP) assays
m6A RNA IP (MeRIP) for validation of the m6A-eCLIP data was performed as previously described (42). Briefly, total RNA was chemically fragmented up to 150–200 nt with the RNA fragmentation kit (Thermo Fisher Scientific, AM8740). Fragmented RNA (5 μg) was incubated for 4 h at 4°C with magnetic protein G Dynabeads previously coated with an anti-m6A antibody (Millipore, MABE1006) or normal mouse IgG (Millipore). The RNA isolated from the IP material was further assessed by RT-qPCR analysis.
For immunoprecipitation of endogenous IGF2BP1, cells were lysed in PEB buffer (20 mM Tris–HCl [pH 7.5], 100 mM KCl, 5 mM MgCl2 and 0.5% NP-40) containing RNase and protease inhibitors for 10 min on ice and centrifuged at 10 000 × g for 15 min at 4°C. The supernatant was incubated with protein A sepharose beads (Invitrogen) coated with anti-IGF2BP1 (Cell Signaling Technology) or Rabbit IgG (Cell Signaling Technology) for 4 h at 4°C. The beads were washed 4× with NT2 buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40), and resuspended in TriPure Isolation Reagent. The RNA extracted from the IP materials was further assessed by RT-qPCR analysis.
RIP using recombinant IGF2BP1 was performed as previously described (43), with some modifications. Briefly, total RNA, including endogenously methylated RNA, was fragmented into segments 100–200 nt long. The fragmented RNA (2 μg) was then incubated with 100 ng of c-Myc-tagged IGF2BP1 human recombinant protein (Origene Technologies); after pulldown using anti-c-Myc-magnetic beads (Pierce, Thermo Fisher Scientific), IGF2BP1-bound RNA fragments were extracted and further assessed by RT-qPCR analysis.
Protein extraction and analysis by western blot, dot blot and immunostaining
To extract total protein, cells were washed twice in 1× PBS and harvested in 2% SDS buffer with 50 mM HEPES and fresh protease/phosphatase inhibitors (Cell Signaling Technology). Protein concentration was quantified with the BCA assay kit (Pierce) and lysates were mixed with 4× SDS Laemmli buffer (Bio-Rad) containing β-mercaptoethanol and heated at 95°C for 5 min. Cell fractionation into nuclei, cytosol and membrane rafts was performed with the Minute Total Lipid Raft Isolation Kit for Mammalian Cells/Tissues (Invent Biotechnologies, cat. LR-039), following the manufacturer's instructions.
For western blot analysis, samples were loaded on Tris-Glycine gels (Bio-Rad), size-separated by SDS-PAGE and transferred to nitrocellulose membranes using the iBlot kit (Invitrogen). Membranes were blocked for 1 h at room temperature with 5% nonfat milk in 1× TBST and incubated for 18 h with primary antibodies at 4°C; a list of the antibodies used for western blot analysis is provided (Supplementary Table S1). Membranes were then washed in 1× TBST and incubated with the secondary antibodies for 1 h at room temperature in 5% nonfat milk. After washes, membranes were incubated with the ECL solution (Kindle Biosciences), and chemiluminescent signals were acquired with a ChemiDoc system (Bio-Rad).
For RNA dot blot analysis, total RNA (200 ng) was spotted on a nylon membrane and UV-crosslinked at 254 nm wavelength. After washing unbound RNA and blocking with 5% BSA, the membrane was incubated overnight with the primary antibody (anti-m6A, Millipore, cat. MABE1006) and developed following the protocol for western blot analysis. A methylene blue solution (0.2% methylene blue in 0.4 M sodium acetate and 0.4 M acetic acid) was used to visualize the spotted RNA.
For protein immunostaining, cells were washed in 1× PBS, fixed in 4% formaldehyde for 15 min, and permeabilized with 0.2% Triton-X100 for 5 min at 25°C. Cells were blocked with 10% goat serum and incubated with primary antibodies for 18 h at 4°C, washed, and incubated with secondary antibodies for 1 h at room temperature. Cells were then washed and stained with DAPI for 10 min in the dark. After the final washes, slides were visualized using a Keyence microscope. To visualize F-actin, fixed cells were stained with rhodamine phalloidin (Thermo Fisher Scientific, R37112) following the manufacturer's instructions. A list of the antibodies used in immunostaining is provided (Supplementary Table S1).
Chromatin immunoprecipitation (ChIP)
ChIP analysis was performed using the MAGnify Chromatin Immunoprecipitation System Kit (Thermo Fisher Scientific). Briefly, WI-38 cells growing in 150-mm culture dishes were crosslinked with 1% formaldehyde for 10 min at room temperature, followed by cell lysis and chromatin isolation. Chromatin was sonicated into 200- to 500-bp fragments and was immunoprecipitated for 18 h with 1 μg of anti-p53 antibody or mouse IgG (Millipore, ChIPAb + p53, cat. 17-613) conjugated to Dynabeads. The DNA associated with p53 was de-crosslinked by heat plus proteinase K treatment, and was used for downstream qPCR analysis. Primer sequences are listed (Supplementary Table S1).
Statistical analysis and graphs
Three or more independent biological repeats were performed for each experiment, unless otherwise stated. Data were tested for normal distribution and statistical significance (*P< 0.05; **P< 0.01; ***P< 0.001) was established using Student's t-test. Graphs were generated using GraphPad Prism 9.
Results
PTCHD4 mRNA is highly abundant and highly methylated in senescent cells
To investigate the role of m6A modification in the senescent transcriptome, we sought to identify m6A-modified RNAs in senescent and proliferating cells. We studied WI-38 human diploid fibroblasts rendered senescent using two previously described methods (28,29). In one, proliferating WI-38 cells at a population doubling level (PDL) of ∼22 were allowed to divide until they reached replicative senescence (RS), at >PDL50. In the other, proliferating (P) WI-38 fibroblasts were treated with 10 Gy of ionizing radiation (IR) and harvested 10 days later to achieve IR-induced senescence (IR-IS). In these two senescence paradigms, as well as in pre-senescent cells (∼PDL40, described later), we found markedly increased SA-β-Gal activity, a widely used marker of cellular senescence (Figure 1A, Supplementary Figure S1A).
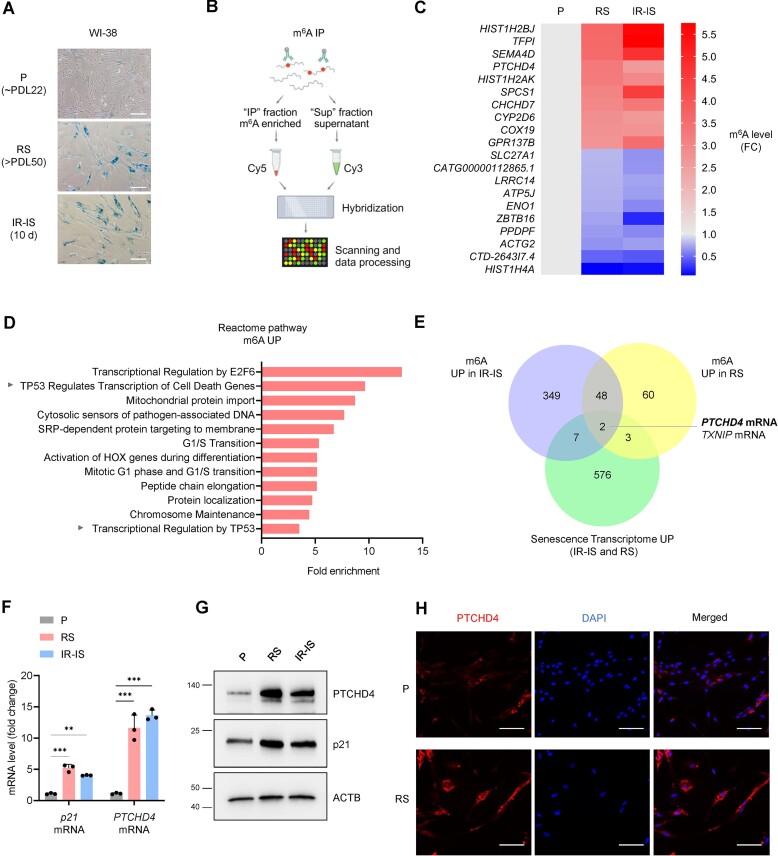
Figure 1.
PTCHD4 mRNA is highly abundant and highly methylated in senescent fibroblasts. (A) Micrographs visualizing SA-β-Gal activity (blue) in WI-38 fibroblasts that were proliferating [P, at population doubling level (PDL) ∼22)], rendered senescent by passaging until replicative exhaustion (RS, ∼PDL50), or rendered senescent by treatment with a single dose of IR (10 Gy) and cultured for an additional 10 days (IR-IS). Scale bar: 100 μm. (B) Schematic (created using BioRender) of the microarray analysis of m6A-modified RNA in proliferating and senescent WI-38 fibroblasts. Total RNA was subjected to m6A immunoprecipitation (m6A IP) using an antibody that recognized m6A, coupled to magnetic beads; the m6A-modified RNAs eluted from the IP fraction were labeled with Cy5, while the RNAs recovered from the supernatant were labeled with Cy3. The two fractions were combined and hybridized to an Arraystar Human m6A epitranscriptomic microarray; the array was scanned and the m6A-labeled vs unlabeled RNA was calculated as the percentage of modification based on the Cy5-labeled and Cy3-labeled normalized intensities. The microarray data are available at GSE247621. (C) Top 20 mRNAs generated from the m6A microarray analysis, showing the transcripts whose m6A levels either increased or decreased in WI-38 cells rendered senescent by RS or IR-IS (as explained in panel A), compared to proliferating (P) cells. The m6A level is expressed as fold change (FC) relative to the proliferating control, set as 1. Ranking criteria: p< 0.05, ranked by the highest fold change in m6A. (D) Reactome pathway analysis of the mRNAs with increased m6A in senescent (RS or IR-IS) relative to were either proliferating (P) or rendered senescent by, processed as explained in (A) and analyzed as explained in (B, C); cutoff: P< 0.05. The analysis was performed with Reactome (45), and the data are summarized in the Supplementary Table S2. (E) Venn diagram showing the overlap among mRNAs with increased methylation level in senescent WI-38 cells in purple (IR-IS versus P), mRNAs with increased m6A level in senescent WI-38 cells in yellow (RS versus P), and mRNAs highly increased in senescent WI-38 cells (RS and IR-IS) compared to P cells from previously datasets (29) in green. All the datasets have cutoffs: FC>2, P<0.05. At the intersection, we identified PTCHD4 mRNA. (F) RT-qPCR analysis of the levels of PTCHD4 mRNA (normalized to the average levels of ACTB and GAPDH mRNAs) in WI-38 cells that were either proliferating (P, ∼PDL22) or senescent (RS at ∼PDL50 or IR-IS). (G) Western blot analysis of the levels of PTCHD4, p21 and loading control ACTB in cells processed as in (F). (H) Immunofluorescence micrographs to visualize PTCHD4 protein (red) in WI-38 cells that were either proliferating (P) or rendered senescent by replicative exhaustion (RS, ∼PDL50). DAPI was used to stain the cell nuclei (blue). Scale bar: 100 μm. Data in (F) represent the means and S.D. from at least three biological replicates; statistical significance (**P< 0.01; ***P< 0.001) was established using Student's t-test. Other data are representative of three or more biological replicates.
To identify the RNAs differentially methylated at m6A between proliferating and senescent cells, we performed m6A epitranscriptomic microarray analysis. Briefly, total RNA extracted from proliferating and senescent cells was subjected to immunoprecipitation (IP) using an antibody that recognized the m6A modification (Figure 1B). The m6A-enriched transcripts present in the pellet (‘IP’ fraction) were labeled with Cy5, while the unbound transcripts present in the supernatant (‘Sup’ fraction) were labeled with Cy3. The labeled samples were then hybridized to a microarray, and the microarrays were scanned and processed (GSE247621) (Supplementary Table S2). Although m6A modification is abundant across eukaryotic coding and noncoding RNAs (44), here we focused on coding transcripts (mRNAs); the top 10 mRNAs with either increased or reduced methylation in both RS and IR-IS relative to proliferating (P) WI-38 cells are indicated (Figure 1C). Reactome pathway analysis (45) of mRNAs differentially methylated in IR-IS and RS (relative to P) cells revealed that proteins encoded by mRNAs that were more methylated during senescence belonged to a network modulated by p53, a master regulator of senescence (30–32) (Figure 1D). Gene ontology (GO) identified a role for the encoded proteins in the regulation of apoptotic signaling (Supplementary Figure S1B), and the MSigDB Hallmark gene set predicted a role for these transcripts in the unfolded protein response, the interferon response, and the p53 pathway (Supplementary Figure S1C) (46).
To identify those mRNAs not only differentially methylated, but also differentially abundant in senescence, we integrated the transcriptomic data obtained from the m6A microarray with a previous transcriptomic signature of senescent cells (29). This analysis uncovered mRNAs differentially expressed in WI-38 fibroblasts rendered senescent by RS or by IR-IS. Due to the inherent heterogeneity across senescence models, we observed only a partial overlap between RS and IR-IS (29,47,48). A further overlap with m6A microarray RNAs revealed PTCHD4 mRNA, encoding Patched Domain Containing Protein 4, as being both highly methylated and highly expressed in senescent WI-38 fibroblasts (all cutoffs: FC>2, P<0.05). (Figure 1E). Complementary analyses among the three datasets are reported in Supplementary Figure S1D (all cutoffs: FC<0.5 for ‘down’ or FC>2 for ‘up’, P<0.05). As reported earlier (29), the expression of PTCHD4 mRNA increased in senescent WI-38 cells and in other models of senescence, including HAECs (human aortic endothelial cells) and HUVECs (human umbilical vein endothelial cells) (Supplementary Figure S1E). In WI-38 cells rendered senescent by RS or IR-IS, we validated the increased expression of PTCHD4 mRNA by reverse transcription followed by quantitative PCR (RT-qPCR) analysis and the increased levels of PTCHD4 protein by western blot analysis and immunostaining (Figure 1F–H). We also measured p21 mRNA and the encoded protein p21, an inhibitor of proliferation and marker of senescence (3,4). Furthermore, we observed a progressive increase in the expression of PTCHD4 mRNA and PTCHD4 protein as senescence was triggered and established (Supplementary Figure S1F). Taken together, PTCHD4 is a highly expressed protein in senescent cells, and it is encoded by PTCHD4 mRNA, an abundant and highly methylated transcript in senescent cells. We thus investigated the mechanisms of PTCHD4 production in this paradigm.
PTCHD4 mRNA is transcriptionally induced by p53 in senescent fibroblasts
We first investigated if the rise in PTCHD4 mRNA levels was the result of transcriptional regulation by evaluating changes in the levels of PTCHD4 pre-mRNA, a surrogate measure of de novo transcription, in WI-38 cells rendered senescent by RS and IR-IS (as described in Figure 1A). RT-qPCR analysis indicated that the increase in PTCHD4 mRNA levels was at least in part driven by increased production of PTCHD4 pre-mRNA (Figure 2A). To confirm the transcriptional induction of PTCHD4 mRNA, we incubated cells with 5-ethynyluridine (EU) for 6 h to label nascent RNAs in proliferating and senescent cells. The newly transcribed, EU-modified PTCHD4 mRNA was conjugated to biotin and captured using streptavidin beads; RT-qPCR analysis further confirmed that the rise in PTCHD4 gene transcription was reflected in an increase in PTCHD4 mRNA levels (Figure 2B). The discrepancy in the magnitude of change by these two methods likely reflects the fact that in Figure 2A we measured PTCHD4 pre-mRNA levels before further processing (usually a quick process), while in Figure 2B we assessed the levels of mature PTCHD4 mRNA transcribed over a 6-h period, allowing more time for accumulation. The increased stability of PTCHD4 mRNA in senescence (below), likely further contributed to the differences.
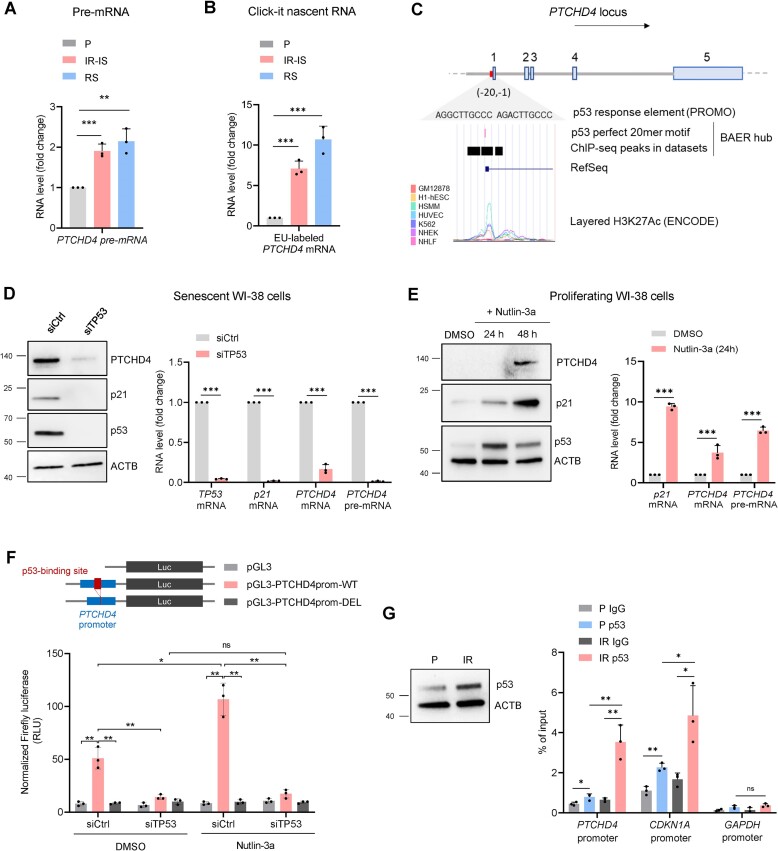
Figure 2.
PTCHD4 mRNA is transcriptionally induced by p53 in senescent fibroblasts. (A) RT-qPCR analysis of the levels of PTCHD4 pre-mRNA in WI-38 cells that were either proliferating (P, ∼PDL22), or rendered senescent by replicative exhaustion (RS, ∼PDL50) or ionizing radiation (IR-IS; 10 Gy IR, cultured for 10 days). (B) WI-38 cells treated as in (A) were incubated with 0.2 mM 5-ethynyl uridine (EU) for 6 h. EU-labeled RNA was then extracted, clicked, reverse-transcribed, and quantified by RT-qPCR analysis following the Click-iT Nascent RNA Capture kit instructions (Materials and methods). (C) Schematic of the PTCHD4 gene locus and PTCHD4 mRNA (Ensembl canonical, RefSeq match NM_001384253.1). The diagram shows a complete p53 response element in the PTCHD4 promoter, localized near the transcription start site (33). The sequence of the p53 binding site was obtained with the online tool Promo (https://alggen.lsi.upc.es/). The p53 RE shows an overlap with the marker of active chromatin H3K27Ac, as retrieved form the UCSC Genome Browser (https://genome.ucsc.edu/). The H3K27Ac mark was reported in 7 cell lines, each one represented by a different color (GM12878, H1-hESC, HSMM, HUVEC, K562, NHEK, NHLF); original data are from the ENCODE Consortium. Enrichment of p53 binding was also confirmed by ChIP-seq in multiple datasets [BAER track hub (76)]. (D) Pre-senescent WI-38 cells (∼PDL40) were transfected for 6 h with a nontargeting siRNA (siCtrl) or a p53 siRNA (siTP53); 48 h later, the levels of p53, p21, PTCHD4 and loading control ACTB in whole-cell lysates were assessed by western blot analysis (left) and the levels of TP53, CDKN1A, and PTCHD4 mRNAs, as well as PTCHD4 pre-mRNA, were assessed by RT-qPCR analysis (right). (E) Proliferating WI-38 cells (∼PDL22) were treated with 10 μM Nutlin-3a or vehicle control DMSO; the levels of p53, p21, PTCHD4, and loading control ACTB in whole-cell lysates were assessed by western blot analysis 24 and 48 h later (left), and the levels of p21 mRNA, PTCHD4 mRNA, and PTCHD4 pre-mRNA were assessed by RT-qPCR analysis 24 h after that (right). (F) Proliferating WI-38 cells (∼PDL22) were transfected for 6 h with 25 nM of a p53-targeting siRNA (siTP53) or a scrambled siRNA (siCtrl). Eighteen hours later, cells were transfected for 6 h with 50 ng of specific reporter plasmids, either containing the WT promoter of the PTCHD4 gene (pGL3-PTCHD4prom-WT), a promoter lacking the p53 response element (pGL3-PTCHD4prom-DEL), or an empty vector (pGL3). Cells were then treated for 18 h with DMSO or Nutlin-3a (10 μM), before lysates were prepared and firefly luciferase signals assessed; luciferase values were normalized to total protein content in the sample. (G) The levels of total p53 and loading control ACTB were assessed by western blot analysis in WI-38 cells that were either proliferating (P) or irradiated (10 Gy IR) and harvested 24 h later (IR). ChIP RT-qPCR analysis was performed on P and IR cells, and the level of p53 binding to different promoters was assessed by designing specific primer pairs targeting the PTCHD4 promoter, the GAPDH promoter (negative control), and the CDKN1A promoter (positive control). Data are presented as percentage of the input. Data in (A–B,D–G) represent the means and S.D. from at least three biological replicates; statistical significance (*P< 0.05; **P< 0.01; ***P< 0.001) was established using Student's t-test. Other data are representative of three or more biological replicates.
We hypothesized that p53 might play a crucial role in the transcriptional increase of PTCHD4 mRNA in senescence based on several pieces of evidence. First, the promoter of the human PTCHD4 gene contains a p53 response element, as predicted using Promo (https://alggen.lsi.upc.es/) and as described previously (33), and this element is associated with markers of active transcription such as H3K27Ac, as reported in the UCSC Genome Browser (https://genome.ucsc.edu/) (Figure 2C). Second, PTCHD4 mRNA was reported to be transcriptionally induced by p53 in colorectal cancer cells (33). Third, p53 is a master transcriptional regulator of the senescence program and enhances the transcription of genes (e.g. CDKN1A, SERPINE1 and PML) encoding senescence proteins p21, PAI-1 and PML, respectively (4,30). Fourth, single-cell (sc)RNA-seq analysis of WI-38 cells rendered senescent after treatment with etoposide (ETO-IS) (49) revealed that PTCHD4 mRNA was highly expressed in a specific cell subpopulation featuring p53-mediated cellular senescence; single-cell RNA sequencing (sc-RNA-seq) analysis revealed that subpopulations of cells expressing PTCHD4 mRNA also expressed the long noncoding RNA NEAT1, an indirect marker of p53 function, as described earlier (49) (Supplementary Figure S2A, B). Finally, Reactome pathway analysis (Figure 1D) suggested that mRNAs highly methylated in cellular senescence belong to a p53-regulated network.
To directly analyze a possible role for p53 on the transcription of PTCHD4 mRNA, we first silenced p53 in pre-senescent WI-38 cells (PDL40); this intervention reduced the levels of p53, p21 and PTCHD4 (Figure 2D). RT-qPCR analysis revealed that the levels of PTCHD4 pre-mRNA were also markedly reduced after p53 silencing (Figure 2D). Extending this possible role of p53 to other models of senescence, we found that silencing p53 in WI-38 cells undergoing IR-IS or ETO-IS similarly reduced PTCHD4 pre-mRNA levels (Supplementary Figure S2C, D).
Further evidence supporting a role for p53 in the induction of PTCHD4 expression in senescence was sought by treating proliferating WI-38 cells (PDL22) with Nutlin-3a, an MDM2 antagonist that inhibits MDM2-p53 interactions and stabilizes the p53 protein (50). As shown, while proliferating WI-38 cells expressed low basal levels of PTCHD4 mRNA and PTCHD4 protein (Figure 1F,G), treatment with Nutlin-3a (10 μM for 48 h) increased PTCHD4 levels (Figure 2E); importantly, Nutlin-3a induced PTCHD4 pre-mRNA levels by 24 h of treatment, supporting a transcriptional role for p53 in driving an early rise in PTCHD4 mRNA levels (Figure 2E). To test the presence of a potential p53-binding site on the PTCHD4 promoter, we used the reporter vector pGL3 to insert the native promoter of the PTCHD4 gene (100 bp from the transcription start site, including the putative p53-binding site) to generate the reporter pGL3-PTCHD4prom-WT. We then modified this reporter by deleting the putative p53-binding site to generate pGL3-PTCHD4prom-DEL. WI-38 cells (PDL22) were first transfected with siTP53 or siCtrl siRNAs, 24 h later they were transfected with each of the three pGL3 vectors, and 6 h after that the transfection media was replaced with media containing either DMSO or Nutlin-3a. Eighteen hours later, luciferase levels were assessed. As shown, luciferase activity from the WT promoter was markedly enhanced by Nutlin-3a treatment and strikingly reduced by silencing of p53 (Figure 2F). In contrast, the mutated promoter lacking the p53-binding site (the pGL3-PTCHD4prom-DEL reporter group) only exhibited background signals, similar to those measured in the empty plasmid (pGL3) reporter group. We then tested if p53 associated with the PTCHD4 promoter after DNA damage by chromatin immunoprecipitation (ChIP) analysis. WI-38 cells that were either left untreated or treated with 10 Gy IR 24 h earlier were collected and p53 levels were assessed (Figure 2G). ChIP assay followed by RT-qPCR analysis using primers spanning the p53-binding region of the PTCHD4 locus revealed increased binding of p53 to the PTCHD4 promoter following IR (Figure 2G). The binding of p53 to the promoter of CDKN1A was assessed as positive control, while binding to the GAPDH promoter was used as negative control (51). Collectively, these results show that p53 directs the transcription of PTCHD4 mRNA in cells undergoing DNA damage-induced senescence.
m6A modification enhances PTCHD4 mRNA stability and abundance in senescent WI-38 cells
In addition to the transcriptional increase in PTCHD4 mRNA levels (Figure 2A, B), we evaluated if post-transcriptional mRNA stabilization was also implicated in the increased expression of PTCHD4 mRNA in senescence (Figure 1F). The stability of PTCHD4 mRNA was tested using Actinomycin D, an inhibitor of the transcriptional function of RNA Polymerase II. As shown (Figure 3A), the half-life of PTCHD4 mRNA in proliferating WI-38 cells (PDL22, t1/2 ∼3 h) was lower than that in pre-senescent WI-38 cells (PDL40, t1/2 > 6 h) or fully senescent cells (RS; PDL50, t1/2> 6 h). As internal controls for the mRNA decay assays, we measured the unstable MYC mRNA and the stable ACTB mRNA. Similarly, the half-life of PTCHD4 mRNA in proliferating cells (PDL22, t1/2 ∼3 h) was lower than that observed in WI-38 cells in IR-IS, assessed 10 days after IR (t1/2> 6 h) (Supplementary Figure S3A). These observations indicated that PTCHD4 mRNA was longer lived in senescent WI-38 cells, and supported the existence of post-transcriptional mechanisms promoting PTCHD4 mRNA stability in senescence. Given that PTCHD4 mRNA was highly abundant and highly methylated in senescent cells (Figure 1), that PTCHD4 mRNA was stabilized in senescent cells, and that m6A modification plays a key role in the stability of several transcripts (52), we hypothesized that m6A methylation could promote a longer half-life for PTCHD4 mRNA in senescence.
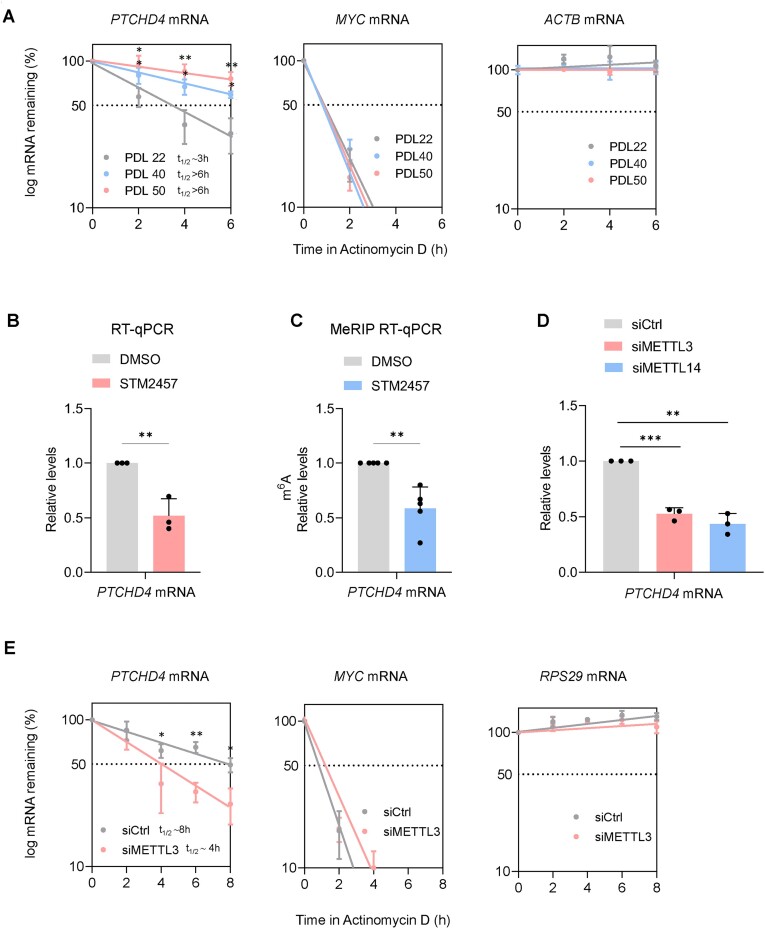
Figure 3.
m6A modification enhances PTCHD4 mRNA stability and abundance in senescent WI-38 cells. (A) To measure mRNA stability, WI-38 cells that were either proliferating (∼PDL22) or rendered senescent by replicative exhaustion (pre-senescent at ∼PDL40 or fully senescent at ∼PDL50), were treated with the inhibitor of RNA polymerase II Actinomycin D (2 μg/ml), and RNA was collected at the indicated times. RT-qPCR analysis was used to measure the levels of PTCHD4 mRNA, the labile MYC mRNA and the stable ACTB mRNA, and normalized to the levels of 18S rRNA, a highly stable RNA that is not transcribed by RNA polymerase II. The half-lives (t1/2) were estimated as the time needed for each mRNA to reach one-half (50%, discontinuous line) of their abundance at time 0 h. (B, C) Presenescent WI-38 cells (∼PDL40) were incubated with the METTL3/METTL14 inhibitor STM2457 (2 μM) or with vehicle alone (DMSO) for 48 h, whereupon the levels of PTCHD4 mRNA (normalized to the levels or 18S rRNA, which are not influenced by METTL3 silencing) were measured by RT-qPCR analysis (B); or were incubated with STM2457 (2 μM) or DMSO for 24 h and the percentage of m6A methylated PTCHD4 mRNA (relative levels as percentage of the input) was measured by MeRIP-RT-qPCR analysis (C). (D) RT-qPCR analysis of PTCHD4 mRNA levels in WI-38 cells ∼PDL40 48 h after transfection with a control siRNA (siCtrl) or with siRNAs targeting METTL3 (siMETTL3) or METTL14 (siMETTL14). (E) In WI-38 cells at ∼PDL40 that had been transfected for 6 h with Ctrl siRNA or siMETTL3 and 18 h later were treated with Actinomycin D as explained in (A), the stabilities of PTCHD4 mRNA, the labile control MYC mRNA, and the stable control RPS29 mRNA (each normalized to 18S rRNA levels) were assessed by RT-qPCR analysis. Data in (A-E) represent the means and S.D. from at least three biological replicates; statistical significance (**P< 0.01; ***P< 0.001) was established using Student's t-test.
To test this possibility directly, we studied if m6A deposition, which occurs co-transcriptionally by ‘writer’ methyltransferases, could regulate the levels of PTCHD4 mRNA (15,18). Since we observed that PTCHD4 mRNA was stabilized at late PDLs, we investigated this finding using pre-senescent (PDL40) WI-38 fibroblasts. This decision was based on the fact that changes in PTCHD4 mRNA turnover in either direction (increased or decreased stability) would be easier to observe at PDL40, and also because as cells reach PDL50, they become highly refractory to transfection and detach easily, severely limiting the amount of material and interventions we could use experimentally. In further support of this choice, PDL40 and PDL50 cells displayed similar characteristics and markers when compared to proliferating cells, including SA-β-Gal activity levels, PTCHD4 mRNA abundance, and half-life of PTCHD4 mRNA (Figure 3A, Supplementary Figure S1A, F).
We treated WI-38 cells (PDL40) with 2 μM of STM2457, a potent inhibitor of the METTL3-METTL14 complex, responsible for >95% of m6A addition to mRNA (53); 48 h later, RT-qPCR analysis revealed a significant decrease (to 50% of control) in the abundance of PTCHD4 mRNA (Figure 3B). Importantly, PTCHD4 pre-mRNA levels were not reduced by the same treatment (Supplementary Figure S3B), supporting the hypothesis that the decline in PTCHD4 mRNA levels was post-transcriptional. Next, we measured the extent of m6A-modified PTCHD4 mRNA in untreated and STM2457-treated cells. We enriched in m6A-containing transcripts by performing methylated RNA immunoprecipitation (MeRIP) analysis on total RNA using an m6A-recognizing antibody bound to beads. RT-qPCR analysis of PTCHD4 mRNA levels on the beads revealed a significant reduction in the methylation of PTCHD4 mRNA by 24 h after treatment with STM2457 (Figure 3C). To better evaluate the role of the METTL3/METTL14 complex in the regulation of PTCHD4 mRNA levels, we silenced the two enzymes individually in WI-38 cells at PDL40 (Supplementary Figure S3C) and found that each intervention reduced PTCHD4 mRNA levels (Figure 3D). Furthermore, silencing METTL3 reduced the levels of PTCHD4 protein (Supplementary Figure S3D), but PTCHD4 mRNA levels were not significantly reduced after silencing METTL3 in proliferating cells (Supplementary Figure S3E). Finally, to study if increased m6A methylation promotes PTCHD4 mRNA stability in senescent cells, we treated WI-38 cells (PDL40) cells with Actinomycin D after silencing METTL3; as shown, the half-life of PTCHD4 mRNA in the siMETTL3 group (t1/2 ∼4 h) was shorter than that of the control (siCtrl) group (t1/2 ∼8 h). Quantification of the levels of the RPS29 mRNA (54,55), which was poorly methylated in WI-38 cells (Figure 3E, Supplementary Figure S3F), served as a control of a stable transcript that was not influenced by silencing METTL3. In sum, these data indicate that m6A modification enhanced PTCHD4 mRNA stability and abundance in senescent WI-38 cells.
IGF2BP1 binds to m6A-modified regions of PTCHD4 mRNA, promotes PTCHD4 mRNA stability
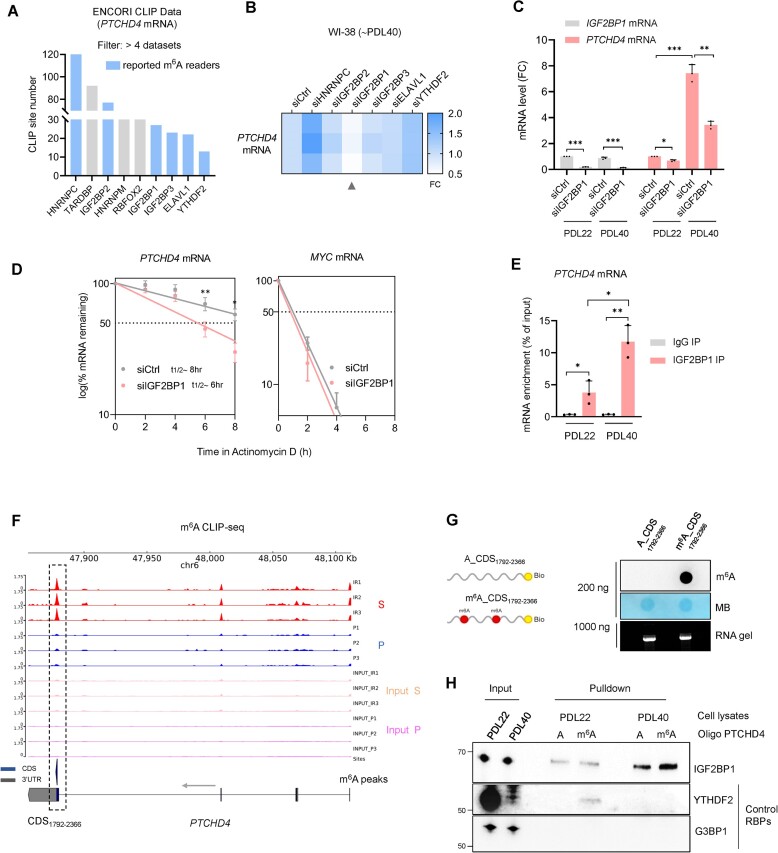
We then set out to identify the RBP(s) that might elicit the stabilization of PTCHD4 mRNA by acting as m6A ‘reader(s)’. The RBPs reported to bind PTCHD4 mRNA were identified from the ENCORI database; among them, only RBPs interacting in more than four datasets across different cell types were considered for subsequent analysis. Out of the nine RBPs identified (Figure 4A), six were known m6A readers (15,20). We individually silenced these six RBPs in WI-38 cells at PDL40 (Supplementary Figure S4A), and found that only IGF2BP1 silencing significantly reduced the levels of PTCHD4 mRNA (Figure 4B); IGF2BP1 silencing also reduced PTCHD4 protein levels (Supplementary Figure S4B). As shown in Figure 4C, the effects of IGF2BP1 silencing were particularly evident in cells at PDL40 (a population in which PTCHD4 mRNA levels were strongly reduced by silencing IGF2BP1) but not in proliferating cells at PDL22, where PTCHD4 mRNA is unstable and in low abundance. To gain further support for the role of IGF2BP1 on the regulation of PTCHD4 mRNA, we silenced IGF2BP1 with each of 4 individual siRNAs (not pooled), and confirmed that each silencing intervention reduced PTCHD4 mRNA levels (Supplementary Figure S4C). We therefore focused on IGF2BP1, previously found to stabilize other mRNAs in cancer in a m6A-dependent manner (21,56,57), as a potential stabilizer and reader of m6A-modified PTCHD4 mRNA. By 24 h after silencing IGF2BP1 in WI-38 cells (PDL40), Actinomycin D treatment followed by mRNA stability analysis revealed a shorter half-life of PTCHD4 mRNA in siIGF2BP1-silenced cells (t1/2 ∼6 h) than in control (siCtrl) cells (t1/2 ∼8 h), supporting the hypothesis that IGF2BP1 stabilized PTCHD4 mRNA. A control labile transcript, MYC mRNA, was included in the analysis (Figure 4D). To assess the level of interaction between IGF2BP1 and PTCHD4 mRNA in WI-38 cells, we performed IGF2BP1 ribonucleoprotein immunoprecipitation (RIP) analysis using cell lysates from PDL22 and PDL40 fibroblasts, and assessed the enrichment of PTCHD4 mRNA. RIP followed by RT-qPCR analysis revealed a stronger interaction between IGF2BP1 and PTCHD4 mRNA in PDL40 cells compared to PDL22 cells (Figure 4E).
Figure 4.
IGF2BP1 binds to m6A-modified regions of PTCHD4 mRNA, promotes PTCHD4 mRNA stability in senescent cells. (A) RNA-binding proteins (RBPs) interacting with PTCHD4 mRNA in at least 4 different m6A-eCLIP-seq datasets (ENCORI database), sorted according to the number of identified binding sites; m6A readers are highlighted in blue. (B) WI-38 cells at ∼PDL40 were transfected for 6 h with siCtrl or siRNAs targeting individual m6A reader RBPs; 48 h later, total RNA was extracted and the levels of PTCHD4 mRNA were quantified by RT-qPCR analysis; the reduction in PTCHD4 mRNA levels after IGF2BP1 silencing is indicated (arrowhead). (C) WI-38 cells at ∼PDL22 or ∼PDL40 were transfected with siCtrl or siRNAs targeting IGF2BP1; 48 h later, total RNA was extracted and the levels of IGF2BP1 and PTCHD4 mRNAs were quantified by RT-qPCR analysis. (D) The stability of PTCHD4 mRNA was measured in WI-38 cells (∼PDL40) that had been transfected for 6 h with siCtrl or siIGF2BP1; 24 h later, cells were treated with 2 μg/ml Actinomycin D, total RNA was collected at the times shown, and mRNA levels were measured by RT-qPCR analysis and normalized to the level of 18S rRNA. Half-lives (t1/2) were estimated as the times needed for PTCHD4 mRNA to reach one-half (50%, discontinuous line) of its abundance at time 0 h in each group. MYC mRNA was included as a control labile transcript. (E) Binding of IGF2BP1 to PTCHD4 mRNA in WI-38 cells (∼PDL22 or ∼PDL40) was verified by RIP analysis. After RIP using IgG or anti-IGF2BP1 antibodies, the presence of PTCHD4 mRNA in the IP material was measured by RT-qPCR analysis and represented as percentage of the input. (F) Total RNA extracted from proliferating and senescent (IR-IS) WI-38 cells was subjected to m6A-eCLIP and RNA-seq analysis to identify m6A peaks on PTCHD4 mRNA. The Genome Browser tracks (using pyGenomeTracks) display the m6A-eCLIP-seq read distributions in PTCHD4 mRNA in proliferating and senescent WI-38 cells. The black dotted line indicates the region with the highest m6A enrichment in senescent cells compared to proliferating cells, located within the coding region (CDS1792-2366). Data are available at GEO (GSE247621). (G) m6A dot blot and RNA electrophoresis of biotinylated in vitro-transcribed RNA corresponding to the CDS1792-2366 of PTCHD4 mRNA, either unmodified or randomly methylated by addition of m6ATP in the reaction mix (A:m6A ratio of 4:1). The m6A dot blot, prepared with 200 ng RNA, was used to monitor the methylation of the in vitro-transcribed RNA. Equal loading of the transcribed RNA molecules was assessed by staining with methylene blue, and their integrity and purity were confirmed by visualization of the RNA after electrophoresis (‘RNA gel’). (H) Whole-cell lysates from WI-38 cells (∼PDL22 or ∼PDL40) were incubated with in vitro-transcribed, biotinylated RNAs corresponding to the CDS1792-2366 of PTCHD4 mRNA, either methylated or unmethylated; the complexes were pulled down using streptavidin beads, and the presence of IGF2BP1 among the bound proteins was determined by western blot analysis. Control proteins G3BP1 (predicted to not bind) and YTHDF2 (predicted to bind) were included. Input, 10 μg of total lysate used in the IP reaction. Data in (C–E) represent the means and S.D. from at least three biological replicates; statistical significance (*P< 0.05; **P< 0.01; ***P< 0.001) was established using Student's t-test. Other data are representative of three or more biological replicates.
Next, we studied whether IGF2BP1 stabilized PTCHD4 mRNA by preferentially binding to the m6A-methylated residues. We first identified the methylated regions of PTCHD4 mRNA by performing m6A-eCLIP-seq analysis on WI-38 senescent cells. This approach uses UV crosslinking to covalently bind an anti-m6A antibody to fragmented poly(A)-selected transcripts containing m6A. The antibody-bound RNA is then purified, processed, and sequenced to identify transcriptome-wide m6A peaks. Because we needed large amounts of total RNA for this analysis, we used WI-38 cells undergoing IR-IS, which allowed us to collect more cells; we later validated the presence of the same m6A peaks in WI-38 cells at PDL40 (GSE247621). The m6A-eCLIP analysis identified differentially methylated peaks in proliferating and senescent WI-38 cells, with a high density of m6A peaks localized on the last exon of the coding sequence (CDS) of PTCHD4 mRNA (Figure 4F), in keeping with earlier documentation of m6A modifications in terminal exons, with potential impact on mRNA stabilization (58,59). Here, the highest concentration of m6A peaks in senescent cells was detected in PTCHD4 CDS1792–2366. This region included three individual m6A peaks at positions 1792–1964, 1956–2199, 2276–2366, which were partially overlapping and shared by all the replicate samples of senescent cells (Supplementary Table S3). m6A IP from total RNA, followed by RT-qPCR analysis in proliferating and PDL40 WI-38 cells was performed to validate the higher methylation of these three regions (Supplementary Figure S4D).
We examined the binding of IGF2BP1 to methylated and non-methylated forms of CDS1792–2366. We transcribed biotinylated fragments of CDS1792–2366in vitro in the presence of unmodified rNTPs or a mixture of ATP:m6ATP (ratio 4:1) to randomly incorporate m6A into the newly synthetized molecule (Figure 4G). The RNA fragments were then incubated with whole-cell lysates from WI-38 fibroblasts (PDL22 or PDL40); pulldown of the proteins associated with the biotinylated RNA followed by western blot analysis revealed preferential binding of IGF2BP1 to the methylated form of CDS1792–2366, particularly in PDL40 (Figure 4H). The increased binding of IGF2BP1 in PDL40 relative to PDL22 was partly m6A-dependent, as unmethylated RNA also showed moderate binding. After consulting the ENCORI database, we included two control RBPs in this analysis; G3BP1 served as a negative control, as it does not interact with PTCHD4 mRNA, and YTHDF2 was tested as an m6A reader predicted to bind PTCHD4 mRNA, but showed high abundance in proliferating cells and low in senescent cells.
We further examined the binding of IGF2BP1 to specific regions of the CDS of the endogenous PTCHD4 mRNA, where the m6A peaks of interest were localized by RIP analysis (43). Endogenous RNA was fragmented into ∼200 nt segments and 2 μg of the fragmented RNA was incubated with 100 ng of human c-Myc-tagged IGF2BP1 recombinant protein. After pulldown using anti-c-Myc-magnetic beads and extraction of IGF2BP1-bound RNA fragments, RT-qPCR analysis using primer pairs spanning the entire PTCHD4 CDS in 100- to 140-nt amplicons was used to calculate the relative representation of each segment bound to IGF2BP1 relative to the input (Supplementary Figure S4E). Interestingly, IGF2BP1 preferentially bound the region identified by the m6A-eCLIP as being methylated (CDS1792–2366, Figure 4F). As controls of binding specificity, RIP analysis on total unfragmented RNA (after consulting the ENCORI database) confirmed strong binding of IGF2BP1 to PTCHD4 mRNA and MKI67 mRNA (the latter a positive control) but not to RPS29 mRNA (a negative control) (Supplementary Figure S4F). In sum, these data indicate that IGF2BP1 stabilizes PTCHD4 mRNA in senescent cells and binds preferentially to m6A-modified regions on PTCHD4 mRNA.
Our initial screening had uncovered other reader RBPs, HNRNPC and YTHDF2, capable of binding PTCHD4 mRNA, and whose silencing increased the levels of PTCHD4 mRNA (Figure 4A, B). Further analysis of HNRNPC revealed that its effect on PTCHD4 mRNA occurred transcriptionally and was dependent on p53 (Supplementary Figure S5A). For YTHDF2, however, we hypothesized that it might promote the decay of PTCHD4 mRNA in proliferating WI-38 cells, given that (i) YTHDF2 lowers the stability of many m6A-modified mRNAs (60); (ii) like other RBPs (61–63), YTHDF2 levels are high in proliferating cells but decline markedly in senescent cells (Figure 4H, Supplementary Figure S5B); and (iii) YTHDF2 was found to interact with methylated PTCHD4 mRNA in lysates from PDL22 but not PDL40 (Figure 4H). Supporting this hypothesis, we found that silencing YTHDF2 markedly increased PTCHD4 mRNA levels in PDL22 cells (∼2-fold) but only slightly increased them in PDL40 cells (∼1.2-fold) (Supplementary Figure S5C). Although further work is needed to confirm that YTHDF2 might play a destabilizing role on PTCHD4 mRNA, the increased presence of YTHDF2 in proliferating cells may contribute to the low levels of methylated PTCHD4 mRNA in this population, while the low YTHDF2 levels in senescence can enable the stabilization of PTCHD4 mRNA in senescent cells.
Silencing PTCHD4 accelerates growth arrest and DNA damage in pre-senescent cells
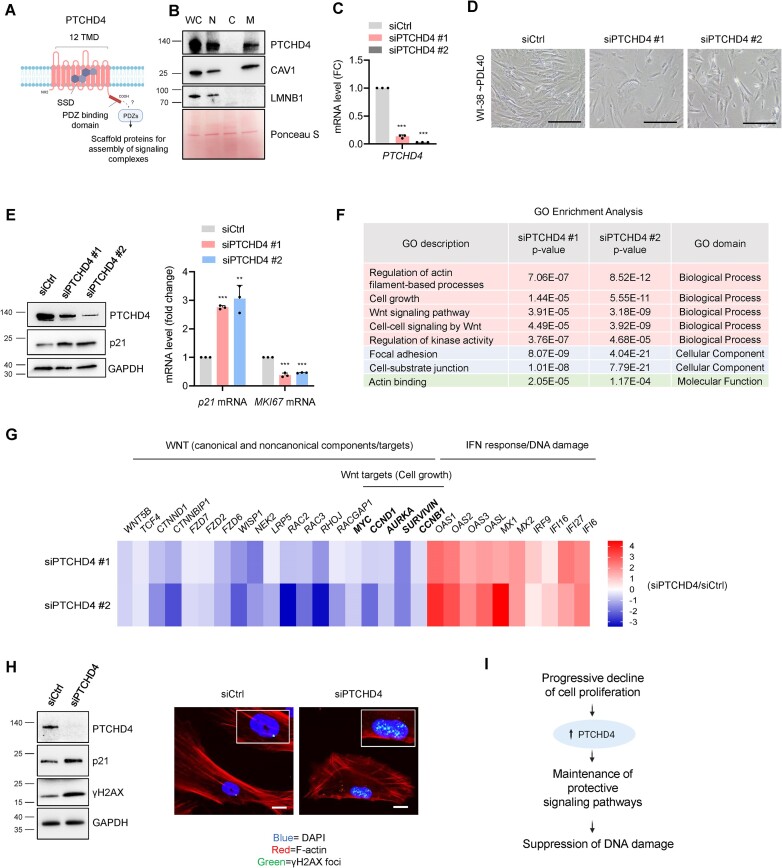
We then investigated the function of PTCHD4 protein in senescence. Although the specific function of PTCHD4 is poorly understood, it was described as an inhibitor of the Hedgehog signalling pathway in colorectal cancer (33), and PTCHD4 gene mutations were associated with cell membrane stiffness in prostate cancer (64). PTCHD4 belongs to the Patched protein family, characterized by 12 transmembrane domains and a sterol-sensing domain (SSD) (65) (Figure 5A). Within this family, only PTCHD4 and PTCHD1 have a C-terminal domain that can interact with cytoplasmic PDZ proteins, which function as scaffolding platforms, assembling signaling complexes and anchoring membrane proteins to the cytoskeleton [(66,67), Figure 5A]. In WI-38 cells (PDL40), cell fractionation confirmed that PTCHD4 is not a soluble cytosolic (C) protein, and is instead localized in membranous compartments of the cell, such as the nucleus (N) and insoluble rafts of plasma membrane and other organelles (M). Caveolin-1 and Lamin B1 served as markers of membrane rafts and the nuclear compartment, respectively (Figure 5B).
Figure 5.
PTCHD4 protein counteracts growth arrest and DNA damage in pre-senescent cells. (A) Schematic of PTCHD4 domains and cellular localization, created using BioRender; TMD, transmembrane domains; SSD, sterol sensing domain; PDZ proteins, cytoplasmic adapters that function as scaffolds for the assembly of signaling complexes. (B) Pre-senescent WI-38 cells (∼PDL40) were subjected to subcellular fractionation and western blot analysis to examine the distribution of PTCHD4 protein among crude nuclei (N), cytosol (C) and membrane rafts derived from the plasma membrane and intracellular organelles (M); WC, whole-cell extracts. Caveolin-1 (CAV1) and lamin B1 (LMNB1) were used as markers of membrane rafts and nuclei, respectively. Ponceau S was used to monitor equal loading and transfer. (C–E) WI-38 cells at ∼PDL40 were transfected for 6 h with siCtrl or individual PTCHD4-targeted siRNAs (siPTCHD4 #1 and siPTCHD4 #2); 48 h later, the level of silencing was evaluated by RT-qPCR analysis (C), and cell morphology and confluency were assessed by phase-contrast microscopy (scale bar: 100 μm) (D). The levels of PTCHD4, the growth arrest marker p21, and the loading control GAPDH were then assessed by western blot analysis (E, left), and the levels of p21 mRNA and MKI67 mRNA (normalized to GAPDH mRNA and 18S rRNA) were quantified by RT-qPCR analysis (E, right). (F) WI-38 cells at ∼PDL40 were transfected as in (C); 48 h later, RNA was collected and processed for RNA-seq analysis (GSE247621) and Gene Ontology (GO) enrichment analysis was performed. The GO terms over-represented and shared between the siPTCHD4 #1 and siPTCHD4 #2 transfection groups (relative to siCtrl) are indicated. The GO analysis included mRNAs that were differentially expressed at P< 0.05, |FC| > 1.5, and either upregulated or downregulated after PTCHD4 silencing. (G) WI-38 cells at ∼PDL40 were transfected with PTCHD4-targeted siRNAs and were subjected to RNA-seq analysis as described in (F); the heatmap shows representative mRNAs encoding protein components and/or targets of the Wnt pathway in cells transfected with siPTCHD4 #1 or siPTCHD4 #2, when compared to siCtrl (blue, lower than siCtrl; red, higher than siCtrl; unit:log2FC). The heatmap also includes representative mRNAs belonging to the interferon response pathway, often elevated following DNA damage. (H) WI-38 cells (∼PDL40) were transfected with either siCtrl or pooled PTCHD4-directed siRNAs (siPTCHD4) for 6 h, and harvested or fixed 48 h later. Western blot analysis (left) and immunofluorescence analysis (right) were performed to assess the levels of γH2AX, a marker of DNA damage and DNA foci. PTCHD4 levels were monitored to confirm the silencing efficiency; p21 levels were evaluated as a marker of growth arrest and DNA damage, and GAPDH as a loading control. In immunofluorescent micrographs, F-actin (red) was used to highlight the extensive cytoskeletal reorganization and DAPI (blue) to stain the nuclei. Scale bar: 20 μm. (I) Schematic of the proposed role of PTCHD4. As the senescent program advances, the rise in PTCHD4 helps to serve a protective role as a scaffold protein for the establishment of signal transduction pathways (including the mitotic Wnt pathway) to counteract growth arrest and DNA damage. Data in (C,E) represent the means and S.D. from at least three biological replicates; statistical significance (**P< 0.01; ***P< 0.001) was established using Student's t-test. Other data are representative of three or more biological replicates.
We then silenced PTCHD4 with two individual siRNAs (siPTCHD4 #1 and siPTCHD4 #2) (Figure 5C) in pre-senescent WI-38 cells (PDL40), which proliferate much more slowly than WI-38 cells at PDL22. After PTCHD4 silencing, cell morphology changed markedly, and cell density was reduced (Figure 5D); these changes were also seen after pooling both siRNAs, each at one-half the concentration (Supplementary Figure S6A). By 48 h after transfecting the siRNAs, PTCHD4 silencing in pre-senescent cells strikingly reduced cell numbers compared to control populations, whereas proliferating cells (PDL22), which express low levels of PTCHD4, only showed a slight reduction in cell numbers (Supplementary Figure S6B). These changes were accompanied by an increase in p21 mRNA, encoding a growth arrest protein, and a reduction in MKI67 mRNA, encoding a proliferative protein (Figure 5E), suggesting that PTCHD4 silencing inhibited growth. Pooling the siRNAs similarly increased p21 mRNA levels and reduced the levels of MYC and CCND1 mRNAs, encoding proliferative proteins MYC and Cyclin D1 (Supplementary Figure S6C).
To study the pathways that might be modulated by PTCHD4, RNA-seq analysis was performed 48 h after PTCHD4 silencing using each siRNA (GSE247621 and Supplementary Table S4). Gene Ontology (GO) and pathway enrichment analysis of the transcriptomes shared between both siRNAs revealed the terms ‘cell growth’, ‘actin-filament based process’, ‘Wnt signaling pathway’, and ‘cell-cell signaling by Wnt’ (Figure 5F). Reactome pathway enrichment analysis indicated changes in ‘signal transduction’ and ‘RHO GTPase cycle’, linked to the noncanonical Wnt pathway and cytoskeleton rearrangement (Supplementary Figure S6D) (68). Accordingly, the levels of components and targets of the Wnt pathway were reduced, including the proteins encoded by MYC, CCND1, CCNB1 and AURKA mRNAs (Figure 5G, Supplementary Figure S6E), which promote cell division (69–71). Among the transcripts upregulated by PTCHD4 silencing, several mRNAs encode proteins in the interferon pathway often associated with DNA damage [(72), Figure 5G, Supplementary Figure S6E]. Analysis of the presence of DNA damage revealed that silencing PTCHD4 increased the levels of γH2AX, and the nuclear γH2AX foci compared to control cells (Figure 5H). Finally, in PTCHD4-silenced cells we observed gross rearrangements in the actin cytoskeleton and in cell morphology (as visualized by staining with rhodamine phalloidin), likely linked to the changes in Wnt/Rho signaling pathways and cell adhesion and polarity (68) (Figure 5H). Overexpressing PTCHD4 in proliferating WI-38 cells (PDL22) did not increase the levels of senescence markers like p21 or SA-β-Gal (Supplementary Figure S6F). In conclusion, PTCHD4 does not trigger cellular senescence, but rather suppresses DNA damage and growth arrest in pre-senescent cells (Figure 5I).
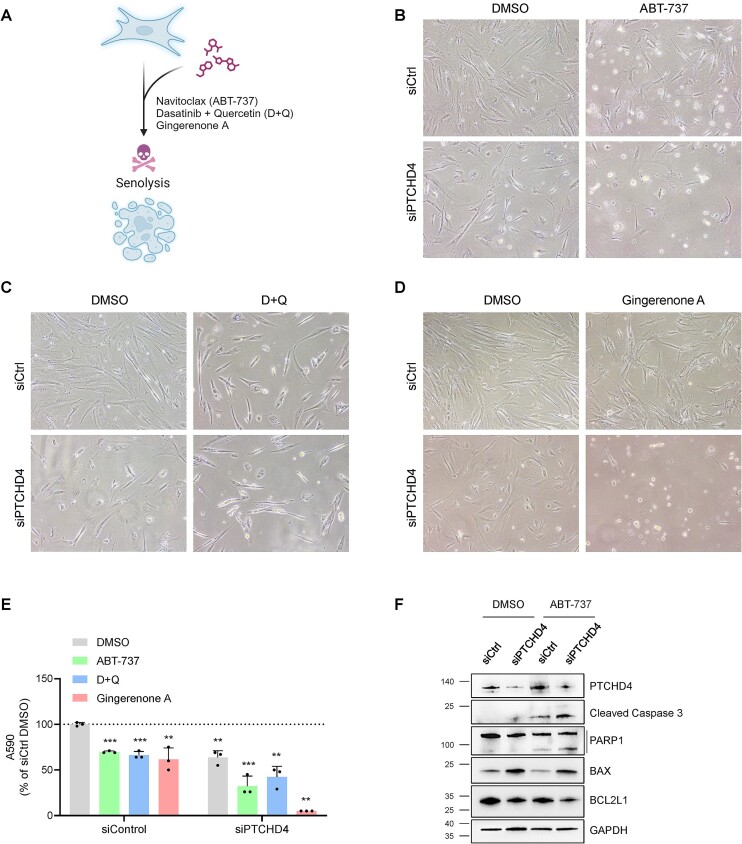
PTCHD4 promotes senescent cell survival and resistance to senolysis
Given that PTCHD4 silencing elevated markers of DNA damage and cellular stress in cells progressing towards replicative exhaustion (Figure 5G, H), we hypothesized that PTCHD4 might play a role in the survival of senescent and pre-senescent cells. Therefore, in keeping with the notion that once cells become senescent, they are more resistant to apoptotic stimuli (73), we tested whether silencing PTCHD4 sensitized WI-38 cells (PDL40) to apoptosis with specific treatments. Senolytic drugs [compounds that are preferentially toxic to senescent cells (9,10)] routinely studied in cell culture include ABT-737, Dasatinib plus Quercetin (D + Q), and Gingerenone A (10,74) (Figure 6A). We thus transfected WI-38 cells at PDL40 with either siCtrl or a pool of PTCHD4-targeting siRNAs (siPTCHD4); 48 h later, we further treated them for 24 h with individual senolytic compounds (10 μM ABT-737, 20 μM Gingerenone A, or 20 μM D + 8 μM Q) or with vehicle alone (DMSO). As shown, cell numbers declined in PTCHD4-silenced relative to control cultures, and this preferential reduction was accentuated after 24 h of treatment with senolytic drugs (Figure 6B–E). Western blot analysis revealed that the senolytic treatment induced the cleavage of PARP1 and Caspase-3 in WI-38 cells, with a moderate increase of these apoptotic markers in PTCHD4-silenced cells over control cells (Figure 6F). Western blot analysis also showed increased levels of the pro-apoptotic protein BAX and reduced levels of the anti-apoptotic protein BCL2L1 (Bcl-xL) in PTCHD4-silenced cells, before and after senolytic treatment (ABT-737) (Figure 6F). Collectively, these data indicate that suppressing PTCHD4 production sensitizes pre-senescent cells to apoptosis.
Figure 6.
PTCHD4 promotes senescent-cell survival and resistance to senolysis. (A) Schematic representation of senolytic compounds analyzed; created using BioRender. (B–D) WI-38 cells at ∼PDL40 were transfected for 6 h with siCtrl or with pooled PTCHD4 siRNAs (siPTCHD4). Forty-eight h later, cells were treated with ABT-737 (10 μM) (B), with D + Q (20 μM + 8 μM) (C), with Gingerenone A (20 μM) (D), or with vehicle alone (DMSO) for 24 h, whereupon micrographs were taken. (E) For each treatment described in (B–D), the number of adherent cells was quantified by crystal violet staining and OD reading at 590 nm; values are shown as percentages relative to siCtrl-transfected cells treated with vehicle (DMSO). (F) WI-38 cells were treated as in (B) and the whole-cell lysates were subjected to western blot analysis to assess the level of PTCHD4 protein, pro-apoptotic markers (BAX, cleaved PARP1, and cleaved Caspase-3), anti-apoptotic markers (BCL2L1), and loading control GAPDH. Data in (E) represent the means and S.D. from at least three biological replicates; statistical significance (**P< 0.01; ***P< 0.001) was established using Student's t-test. Other data are representative of three or more biological replicates.
Discussion
Over the lifetime, the accumulation of senescent cells in a tissue can give rise to a pro-inflammatory environment which in turn may alter normal organ architecture. Accordingly, cellular senescence has been described as a hallmark of aging, and the low-grade inflammation characteristic of increasing age (‘inflammaging’) has been associated with several age-related pathologies like cancer, diabetes, and cardiovascular and neurodegenerative diseases (2,7,8). Here, we report that in senescent fibroblasts, PTCHD4 mRNA is transcriptionally induced by p53, co-transcriptionally m6A-methylated by METTL3, and post-transcriptionally stabilized by IGF2BP1, which has specific affinity for the m6A-modified PTCHD4 mRNA. In turn, the rise in PTCHD4 levels promoted the survival of senescent fibroblasts.
We used microarrays to identify m6A-methylated mRNAs in senescent cells and integrated these data with the transcriptomic signature of cell senescence (29), identifying PTCHD4 mRNA as being highly abundant and highly methylated in senescent cells (Figure 1). While p53 transcriptionally induced PTCHD4 mRNA in senescence (Figure 2), PTCHD4 mRNA stability increased in senescent cells relative to proliferating cells, and METTL3 was important for maintaining the m6A content and stability of PTCHD4 mRNA (Figure 3). We further identified IGF2BP1 as an m6A reader and stabilizing RBP for PTCHD4 mRNA; m6A-eCLIP analysis followed by MeRIP validation revealed a high density of m6A peaks in the last exon of the PTCHD4 coding region that was preferentially bound by IGF2BP1 (Figure 4).
Although in colorectal cancer cells PTCHD4 inhibited the Hedgehog pathway and functioned as a tumor suppressor (33), a role for PTCHD4 in cell senescence was not studied. Silencing PTCHD4 in pre-senescent WI-38 cells reduced several components of the canonical and noncanonical Wnt pathway, triggering growth inhibition and increasing expression of stress and DNA damage proteins (Figure 5). These findings prompted us to investigate the role of PTCHD4 in senolysis, joining widespread efforts in the field to identify senolytic therapies to eliminate senescent cells to improve age-related conditions (9,10). Interestingly, PTCHD4-silenced cells were sensitized to the effects of senolytic drugs ABT-737, Gingerenone A, and D + Q (Figure 6). A schematic summarizing these findings and a proposed model for PTCHD4 regulation and function in senescence is offered (Graphical Abstract).
Several aspects of our study need to be addressed in the future, such as identifying the precise m6A sites on PTCHD4 mRNA at a single-nucleotide resolution. The m6A-eCLIP analysis revealed a high density of m6A peaks on the last exon of the PTCHD4 CDS; unfortunately, it did not offer precision greater than 70 nt, and for each peak there were multiple DRACH motifs that could be potential sites for m6A methylation. Interestingly, most m6A modifications occur in terminal exons (in the CDS or 3′UTR), likely influencing alternative polyadenylation and stability (58,59). We did not see prominent peaks in the long (>20-kb) PTCHD4 3′UTR, and thus further analysis of the impact of m6A modifications was not pursued.
Although we identified IGF2BP1 as an m6A reader that promotes PTCHD4 mRNA stability, other RBPs might influence PTCHD4 mRNA stability through mechanisms that might be m6A-dependent or -independent. For example, other RBPs like HNRNPC and YTHDF2 might reduce the levels of PTCHD4 mRNA in proliferating and/or senescent cells (Supplementary Figure S5A–C), transcriptionally or post-transcriptionally. We also cannot exclude the possibility that the m6A modification might regulate other aspects of PTCHD4 mRNA metabolism besides stability, even though our preliminary data comparing proliferating and senescent cells did not show significant changes in the cellular localization or the translation rate of PTCHD4 mRNA (not shown). Finally, even though we mainly focused on the regulation of PTCHD4 mRNA, more studies are necessary to assess the possible roles of PTCHD4 protein modification and turnover.
In the future, it will also be crucial to determine whether PTCHD4 mRNA is upregulated in models of senescence in vivo. Unlike humans, the promoter of the mouse Ptchd4 gene does not appear to possess a p53-binding site, and therefore we do not anticipate a high increase in the levels of Ptchd4 mRNA in murine senescent cells. While the mouse may not be amenable to study the function of PTCHD4 in senescence, other model organisms ought to be examined.
It will also be important to study the expression of PTCHD4 in senescent cells accumulating in human organs using approaches such as single-cell RNA sequencing (sc-RNA-seq) or spatial omics (transcriptomic or proteomic) analyses. Although little is known at present about the expression and function of PTCHD4 across organs as well as in physiological and disease processes, PTCHD4 appears to be expressed widely in multiple cell types. It will be interesting to study if PTCHD4 expression levels increase similarly in response to damaging stimuli in other developmental paradigms.
To guide future strategies directed at PTCHD4, it will be important to delineate the signaling cascade associated with PTCHD4. PTCHD1 and PTCHD4 are the only members of the Patched family bearing a C-terminal PDZ-binding domain (75), which serves as a platform to assemble signaling complexes, and a scaffold for anchoring membrane proteins to the cytoskeleton (66,67). Therefore, it is crucial to identify the interacting partners of this domain on PTCHD4 in order to uncover its specific role in the activation of the Wnt pathway and other pathways. The identification of PTCHD4-interacting proteins is underway in our laboratory, although we are aware that the highly hydrophobic feature of PTCHD4 poses challenges to solubilize and immunoprecipitate endogenous PTCHD4 protein.
In closing, the finding that reducing PTCHD4 mRNA sensitized pre-senescent cells to senolysis points to the therapeutic value of this mRNA. Interventions directed at m6A modifications could thus be exploited in senolytic treatment regimens to eliminate senescent cells, although further work is needed to develop strategies to target specific methylated sites. Given that modulating p53, METTL3 or IGF2BP1 can influence a multitude of transcripts, we propose that strategies to specifically target PTCHD4 mRNA m6A sites will permit a more precise clearance of senescent cells.
Supplementary Material
Acknowledgements
Author contributions: M.R., K.A., and M.G. conceived the study. M.R., D.T., K.A., K.M.M. designed experiments. M.R., N.B., C.H.S., C.A., D.T., J.H.Y., R.M., J.L.M., Y.P., and K.W.G.L. performed and analyzed experiments. N.B., C.A., D.T., R.M., J.L.M., X.Y., K.M.M., J.F., E.L., K.W.G.L., S.D. and K.A. contributed intellectually and provided technical support. M.R., K.A. and M.G. wrote the manuscript. All authors commented on the manuscript.
Contributor Information
Martina Rossi, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Nirad Banskota, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Chang Hoon Shin, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Carlos Anerillas, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Dimitrios Tsitsipatis, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Jen-Hao Yang, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA; Institute of Biomedical Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan.
Rachel Munk, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Jennifer L Martindale, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Xiaoling Yang, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Yulan Piao, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Krystyna Mazan-Mamczarz, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Jinshui Fan, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Elin Lehrmann, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Kwan-Wood Gabriel Lam, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Supriyo De, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Kotb Abdelmohsen, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Myriam Gorospe, Laboratory of Genetics and Genomics, National Institute on Aging (NIA) Intramural Research Program (IRP), National Institutes of Health (NIH), Baltimore, MD, USA.
Data availability
The data in this article (m6A microarray, m6A-eCLIP-seq, and RNA-seq) are available at Gene Expression Omnibus (GEO) Superseries GSE247621. The individual submissions can be accessed within the Superseries: m6A microarray analysis (GSE246934), m6A-eCLIP-seq analysis (GSE247595), and PTCHD4 silencing RNA-seq analysis (GSE247596).
Supplementary data
Supplementary Data are available at NAR Online.
Funding
National Institute on Aging Intramural Research Program, National Institutes of Health [Z01-AG000394]. The open access publication charge for this paper has been waived by Oxford University Press -– NAR Editorial Board members are entitled to one free paper per year in recognition of their work on behalf of the journal.
Conflict of interest statement. None declared.
References
- 1. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell. Res. 1965; 37:614–636. [DOI] [PubMed] [Google Scholar]
- 2. Muñoz-Espín D., Serrano M.. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014; 15:482–496. [DOI] [PubMed] [Google Scholar]
- 3. Hernandez-Segura A., Nehme J., Demaria M.. Hallmarks of cellular senescence. Trends Cell Biol. 2018; 28:436–453. [DOI] [PubMed] [Google Scholar]
- 4. González-Gualda E., Baker A.G., Fruk L., Muñoz-Espín D.. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021; 288:56–80. [DOI] [PubMed] [Google Scholar]
- 5. Huang W., Hickson L.J., Eirin A., Kirkland J.L., Lerman L.O.. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 2022; 18:611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker D.J., Narita M., Muñoz-Cánoves P.. Cellular senescence: beneficial, harmful, and highly complex. FEBS J. 2023; 290:1156–1160. [DOI] [PubMed] [Google Scholar]
- 7. Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M.P., Invidia L., Celani L., Scurti M.et al.. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007; 128:92–105. [DOI] [PubMed] [Google Scholar]
- 8. McHugh D., Gil J.. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 2018; 217:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gasek N.S., Kuchel G.A., Kirkland J.L., Xu M.. Strategies for targeting senescent cells in human disease. Nat. Aging. 2021; 1:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L., Pitcher L.E., Prahalad V., Niedernhofer L.J., Robbins P.D.. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 2023; 290:1362–1383. [DOI] [PubMed] [Google Scholar]
- 11. Mullani N., Porozhan Y., Mangelinck A., Rachez C., Costallat M., Batsché E., Goodhardt M., Cenci G., Mann C., Muchardt C.. Reduced RNA turnover as a driver of cellular senescence. Life Sci. Alliance. 2021; 4:e202000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harries L.W. Dysregulated RNA processing and metabolism: A new hallmark of ageing and provocation for cellular senescence. FEBS J. 2023; 290:1221–1234. [DOI] [PubMed] [Google Scholar]
- 13. Fang X., Li M., Yu T., Liu G., Wang J.. Reversible N6-methyladenosine of RNA: The regulatory mechanisms on gene expression and implications in physiology and pathology. Genes Dis. 2020; 7:585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murakami S., Jaffrey S.R.. Hidden codes in mRNA: control of gene expression by m6A. Mol. Cell. 2022; 82:2236–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang S., Lv W., Li T., Zhang S., Wang H., Li X., Wang L., Ma D., Zang Y., Shen J.et al.. Dynamic regulation and functions of mRNA m6A modification. Cancer Cell Int. 2022; 22:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang C., Hu Y., Zhou B., Bao Y., Li Z., Gong C., Yang H., Wang S., Xiao Y.. The role of m6A modification in physiology and disease. Cell Death. Dis. 2020; 11:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang X., Liu B., Nie Z., Duan L., Xiong Q., Jin Z., Yang C., Chen Y.. The role of m6A modification in the biological functions and diseases. Sig. Transduct Target Ther. 2021; 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Śledź P., Jinek M.. Structural insights into the molecular mechanism of the m6A writer complex. eLife. 2016; 5:e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satterwhite E.R., Mansfield K.D.. RNA methyltransferase METTL16: Targets and function. Wiley Interdiscip. Rev. RNA. 2022; 13:e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Y., Hsu P.J., Chen Y.-S., Yang Y.-G.. Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018; 28:616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., Zhao B.S., Mesquita A., Liu C., Yuan C.L.et al.. Recognition of RNA N6-methyladenosine by IGF2BP Proteins Enhances mRNA Stability and Translation. Nat. Cell Biol. 2018; 20:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casella G., Tsitsipatis D., Abdelmohsen K., Gorospe M.. mRNA methylation in cell senescence. Wiley Interdiscip Rev. RNA. 2019; 10:e1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J., Cheng B., Su Y., Li M., Ma S., Zhang Y., Zhang A., Cai S., Bao Q., Wang S.et al.. The potential role of m6A RNA methylation in the aging process and aging-associated diseases. Front. Genet. 2022; 13:869950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu F., Zhang L., Lai C., Peng X., Yu S., Zhou C., Zhang B., Zhang W.. Dynamic alteration profile and new role of RNA m6A methylation in replicative and H2O2-induced premature senescence of human embryonic lung fibroblasts. Int. J. Mol. Sci. 2022; 23:9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Z., Shi Y., Lu M., Song M., Yu Z., Wang J., Wang S., Ren J., Yang Y.-G., Liu G.-H.et al.. METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Res. 2020; 48:11083–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z., Xue M., Chen J., Wang Z., Ju F., Ni J., Sun J., Wu H., Zheng H., Lou Z.et al.. METTL14 regulates intestine cellular senescence through m6A modification of lamin B receptor. Oxid Med. Cell Longev. 2022; 2022:9096436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu P., Li F., Lin J., Fukumoto T., Nacarelli T., Hao X., Kossenkov A.V., Simon M.C., Zhang R.. m6A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype. Nat. Cell Biol. 2021; 23:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernandez-Segura A., Brandenburg S., Demaria M.. Induction and validation of cellular senescence in primary human cells. J. Vis. Exp. 2018; 136:57782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casella G., Munk R., Kim K.M., Piao Y., De S., Abdelmohsen K., Gorospe M.. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019; 47:7294–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian Y., Chen X.. Senescence regulation by the p53 protein family. Methods Mol. Biol. 2013; 965:37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rufini A., Tucci P., Celardo I., Melino G.. Senescence and aging: the critical roles of p53. Oncogene. 2013; 32:5129–5143. [DOI] [PubMed] [Google Scholar]
- 32. Mowla S.N., Lam E.W.-F., Jat P.S.. Cellular senescence and aging: the role of B-MYB. Aging Cell. 2014; 13:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung J.H., Larsen A.R., Chen E., Bunz F.. A PTCH1 homolog transcriptionally activated by p53 suppresses Hedgehog signaling. J. Biol. Chem. 2014; 289:33020–33031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivkov R., Bunz F.. Pathways to chromothripsis. Cell Cycle. 2015; 14:2886–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ye X., Zerlanko B., Kennedy A., Banumathy G., Zhang R., Adams P.D.. Downregulation of Wnt signaling is an early signal for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol. Cell. 2007; 27:183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X., Ortiz M.A., Kotula L.. The physiological role of Wnt pathway in normal development and cancer. Exp. Biol. Med. (Maywood). 2020; 245:411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith T., Heger A., Sudbery I.. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 2017; 27:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 17:10–12. [Google Scholar]
- 39. Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R.. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopez-Delisle L., Rabbani L., Wolff J., Bhardwaj V., Backofen R., Grüning B., Ramírez F., Manke T.. pyGenomeTracks: reproducible plots for multivariate genomic datasets. Bioinformatics. 2021; 37:422–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panda A.C., Martindale J.L., Gorospe M.. Affinity pulldown of biotinylated RNA for detection of protein-RNA complexes. Bio Protoc. 2016; 6:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng Y., Wang S., Gao S., Soares F., Ahmed M., Guo H., Wang M., Hua J.T., Guan J., Moran M.F.et al.. Refined RIP-seq protocol for epitranscriptome analysis with low input materials. PLoS Biol. 2018; 16:e2006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Müller M., Schauer T., Becker P.B.. Identification of intrinsic RNA binding specificity of purified proteins by in vitro RNA immunoprecipitation (vitRIP). Bio Protoc. 2021; 11:e3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J., Li K., Cai J., Zhang M., Zhang X., Xiong X., Meng H., Xu X., Huang Z., Peng J.et al.. Landscape and regulation of m6A and m6Am methylome across human and mouse tissues. Mol. Cell. 2020; 77:426–440. [DOI] [PubMed] [Google Scholar]
- 45. Gillespie M., Jassal B., Stephan R., Milacic M., Rothfels K., Senff-Ribeiro A., Griss J., Sevilla C., Matthews L., Gong C.et al.. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022; 50:D687–D692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ge S.X., Jung D., Yao R.. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020; 36:2628–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hernandez-Segura A., de Jong T.V., Melov S., Guryev V., Campisi J., Demaria M.. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 2017; 27:2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwiatkowska K.M., Mavrogonatou E., Papadopoulou A., Sala C., Calzari L., Gentilini D., Bacalini M.G., Dall’Olio D., Castellani G., Ravaioli F.et al.. Heterogeneity of cellular senescence: cell type-specific and senescence stimulus-dependent epigenetic Alterations. Cells. 2023; 12:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wechter N., Rossi M., Anerillas C., Tsitsipatis D., Piao Y., Fan J., Martindale J.L., De S., Mazan-Mamczarz K., Gorospe M.. Single-cell transcriptomic analysis uncovers diverse and dynamic senescent cell populations. Aging (Albany NY). 2023; 15:2824–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shen H., Maki C.G.. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr. Pharm. Des. 2011; 17:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menendez D., Nguyen T.-A., Freudenberg J.M., Mathew V.J., Anderson C.W., Jothi R., Resnick M.A.. Diverse stresses dramatically alter genome-wide p53 binding and transactivation landscape in human cancer cells. Nucleic Acids Res. 2013; 41:7286–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee Y., Choe J., Park O.H., Kim Y.K.. Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 2020; 36:177–188. [DOI] [PubMed] [Google Scholar]
- 53. Poh H.X., Mirza A.H., Pickering B.F., Jaffrey S.R.. Alternative splicing of METTL3 explains apparently METTL3-independent m6A modifications in mRNA. PLoS Biol. 2022; 20:e3001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Jonge H.J.M., Fehrmann R.S.N., de Bont E.S.J.M., Hofstra R.M.W., Gerbens F., Kamps W.A., de Vries E.G.E., van der Zee A.G.J., te Meerman G.J., ter Elst A.. Evidence based selection of housekeeping genes. PLoS One. 2007; 2:e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caracausi M., Piovesan A., Antonaros F., Strippoli P., Vitale L., Pelleri M.C.. Systematic identification of human housekeeping genes possibly useful as references in gene expression studies. Mol Med Rep. 2017; 16:2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang Y., Wu J., Liu F., He J., Wu F., Chen J., Jiang Z.. IGF2BP1 promotes the liver cancer stem cell phenotype by regulating MGAT5 mRNA stability by m6A RNA methylation. Stem Cells Dev. 2021; 30:1115–1125. [DOI] [PubMed] [Google Scholar]
- 57. Zhang L., Wan Y., Zhang Z., Jiang Y., Gu Z., Ma X., Nie S., Yang J., Lang J., Cheng W.et al.. IGF2BP1 overexpression stabilizes PEG10 mRNA in an m6A-dependent manner and promotes endometrial cancer progression. Theranostics. 2021; 11:1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ke S., Alemu E.A., Mertens C., Gantman E.C., Fak J.J., Mele A., Haripal B., Zucker-Scharff I., Moore M.J., Park C.Y.et al.. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015; 29:2037–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R.. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012; 149:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M., Ma J., Wu L.. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016; 7:12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dong Q., Wei L., Zhang M.Q., Wang X.. Regulatory RNA binding proteins contribute to the transcriptome-wide splicing alterations in human cellular senescence. Aging (Albany NY). 2018; 10:1489–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryu S., Jung M., Kim C., Kang H., Han S., Cha S., Jeong S.M., Lee E.K.. Loss of RNA binding protein HuD facilitates the production of the senescence-associated secretory phenotype. Cell Death. Dis. 2022; 13:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Masuda K., Kuwano Y., Nishida K., Rokutan K.. General RBP expression in human tissues as a function of age. Ageing Res. Rev. 2012; 11:423–431. [DOI] [PubMed] [Google Scholar]
- 64. Feng D., Wang J., Shi X., Li D., Wei W., Han P.. Membrane tension-mediated stiff and soft tumor subtypes closely associated with prognosis for prostate cancer patients. Eur. J. Med. Res. 2023; 28:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kowatsch C., Woolley R.E., Kinnebrew M., Rohatgi R., Siebold C.. Structures of vertebrate patched and smoothened reveal intimate links between cholesterol and Hedgehog signalling. Curr. Opin. Struct. Biol. 2019; 57:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee H.-J., Zheng J.J.. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun. Signal. 2010; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Romero G., von Zastrow M., Friedman P.A.. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity. Adv. Pharmacol. 2011; 62:279–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schlessinger K., Hall A., Tolwinski N.. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009; 23:265–277. [DOI] [PubMed] [Google Scholar]
- 69. Dutta-Simmons J., Zhang Y., Gorgun G., Gatt M., Mani M., Hideshima T., Takada K., Carlson N.E., Carrasco D.E., Tai Y.-T.et al.. Aurora kinase A is a target of Wnt/β-catenin involved in multiple myeloma disease progression. Blood. 2009; 114:2699–2708. [DOI] [PubMed] [Google Scholar]
- 70. Bornschein J., Nielitz J., Drozdov I., Selgrad M., Wex T., Jechorek D., Link A., Vieth M., Malfertheiner P.. Expression of aurora kinase A correlates with the Wnt-modulator RACGAP1 in gastric cancer. Cancer Med. 2016; 5:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lecarpentier Y., Schussler O., Hébert J.-L., Vallée A.. Multiple targets of the canonical WNT/β-catenin signaling in cancers. Front. Oncol. 2019; 9:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brzostek-Racine S., Gordon C., Van Scoy S., Reich N.C.. The DNA damage response induces interferon. J. Immunol. 2011; 187:5336–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hu L., Li H., Zi M., Li W., Liu J., Yang Y., Zhou D., Kong Q.-P., Zhang Y., He Y.. Why senescent cells are resistant to apoptosis: an insight for senolytic development. Front. Cell Dev. Biol. 2022; 10:822816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moaddel R., Rossi M., Rodriguez S., Munk R., Khadeer M., Abdelmohsen K., Gorospe M., Ferrucci L.. Identification of gingerenone A as a novel senolytic compound. PLoS One. 2022; 17:e0266135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ung D.C., Iacono G., Méziane H., Blanchard E., Papon M.-A., Selten M., van Rhijn J.-R., Montjean R., Rucci J., Martin S.et al.. Ptchd1 deficiency induces excitatory synaptic and cognitive dysfunctions in mouse. Mol. Psychiatry. 2018; 23:1356–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nguyen T.-A.T., Grimm S.A., Bushel P.R., Li J., Li Y., Bennett B.D., Lavender C.A., Ward J.M., Fargo D.C., Anderson C.W.et al.. Revealing a human p53 universe. Nucleic Acids Res. 2018; 46:8153–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this article (m6A microarray, m6A-eCLIP-seq, and RNA-seq) are available at Gene Expression Omnibus (GEO) Superseries GSE247621. The individual submissions can be accessed within the Superseries: m6A microarray analysis (GSE246934), m6A-eCLIP-seq analysis (GSE247595), and PTCHD4 silencing RNA-seq analysis (GSE247596).