Abstract
The ongoing shortage of human donor organs for transplantation has catalyzed new interest in the application of pig organs (xenotransplantation). One of the biggest concerns about the transplantation of porcine grafts into humans is the transmission of pig endogenous retroviruses (PERV) to the recipients or even to other members of the community. Although nonhuman primate models are excellently suited to mimic clinical xenotransplantation settings, their value for risk assessment of PERV transmission at xenotransplantation is questionable since all of the primate cell lines tested so far have been found to be nonpermissive for PERV infection. Here we demonstrate that human, gorilla, and Papio hamadryas primary skin fibroblasts and also baboon B-cell lines are permissive for PERV infection. This suggests that a reevaluation of the suitability of the baboon model for risk assessment in xenotransplantation is critical at this point.
The increasing shortage of human organs for transplantation has led to growing efforts in experimental xenotransplantation. However, reports of pig endogenous retroviruses (PERV), which are able to infect human cell lines in vitro (6, 12, 18), have raised significant objections against the clinical use of porcine donor organs. Therefore, research and evaluation of the infection risk is considered to be essential.
Recent investigation of patients after limited contact with porcine cells or tissues did not provide any evidence of PERV infection (5, 10, 11). However, although those patient samples are the most suitable ones currently available for assessment of PERV transmission, these retrospective studies have several shortcomings (5, 10, 11). (i) Patients had not undergone whole-organ xenotransplantation. (ii) Less than 10% of the subjects analyzed had undergone pharmacologic immunosuppression. (iii) No cells or tissues from pigs transgenic for human immunoregulators were used. (iv) Reduction of serum complement levels, which could support survival of the virus and enhance the risk of potential PERV infection, is unlikely in most cases (barring the patients with acute liver failure) and was not analyzed. (v) Reduction of the natural anti-Gal antibodies by immunoadsorption (Gal columns or whole-organ perfusion), which would increase PERV survival in serum (12), was only analyzed in two patients after extracorporeal kidney perfusion (10, 11).
In theory, these issues could be addressed by the use of suitable nonhuman primate models. Unfortunately, all nine of the cell lines derived from five primate species that have been tested to date have been found to be nonpermissive for PERV infection (7, 12, 16, 18). Therefore, nonhuman primates are currently believed not to be suitable for assessment of the potential risk of PERV infection of xenograft recipients (2). To challenge this hypothesis, we investigated a panel of primate primary fibroblasts and several baboon lymphocytic cell lines for permissivity to PERV infection by assaying for PERV-specific cell surface receptors and transmission of PERV sequences.
Primary fibroblasts obtained from the European Cell Bank of Primates, Munich, Germany, were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, and baboon B-cell lines isolated from Papio hamadryas individuals of the primate colony at the Institute of Medical Primatology, Sukumi, Russia, were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. Application of replication-incompetent PERV-specific pseudotypes transferring the retroviral MFGnlslacZ vector and detection of target cells expressing PERV subtype-specific cell surface receptors by β-galactosidase staining were performed as described recently (16). To ensure constant titers of the applied pseudotypes, supernatants of the producer cell lines were stored frozen at −70°C until use. Infection experiments with replication-competent PERV released by the PK15 (17) and PAE (9) cell lines were performed as previously described (12), with the exception that no Polybrene was used. For infection of primate primary fibroblasts, about 5 × 104 cells were either cocultured with 2 × 105 lethally X-irradiated (100 Gy) PK15 or PAE cells or exposed for 24 h to 0.45-μm-filtered overnight supernatant of these cell lines (12). Detection of transmitted PERV by PCR was performed essentially as previously described (6; P. Le Tissier, J. P. Stoye, Y. Takeuchi, C. Patience, and R. A. Weiss, Letter, Nature 389:681–682, 1997). Contaminating porcine cells or DNAs were detected by PCR specific for pig mitochondrial DNA (3, 15). The sensitivities of the applied primer pairs were 1 PK15 or PAE cell in a background of 105 human cells for PERV pol and envA and 1 PK15 or PAE cell in 104 human cells for PERV envB and envC. Pig mitochondrion-specific primers (cytochrome oxidase II and cytochrome b) allowed the detection of 1 PK15 or PAE cell in a background of 106 to 107 human cells. PERV-specific reverse transcription (RT)-PCR and an RT-PCR-based assay for reverse transcriptase activity in cell culture supernatant were performed as previously described (6, 13).
In PERV subtype-specific pseudotype infection assays, PERV A-specific, but not PERV B- or C-specific, cell surface receptors have been detected on human, ape, and baboon primary fibroblasts. No evidence of any PERV-specific receptors on cells of other Old or New World primates has been obtained (Table 1).
TABLE 1.
Infection of primary primate fibroblasts by cell-free murine leukemia virus core-PERV env pseudotype particles
| Cell type | PERV pseudotypea:
|
Control
|
||
|---|---|---|---|---|
| envA | envB | envC | MLVb A | |
| Human foreskin fibroblast | + | − | − | + |
| Human MRC5 fetal fibroblast | + | − | − | + |
| Pan troglodytes PTR | + | − | − | + |
| Gorilla gorilla GGO205 | + | − | − | + |
| Papio hamadryas 312 | + | − | − | + |
| Papio hamadryas 419 | + | − | − | + |
| Papio hamadryas 420 | + | − | − | + |
| Papio hamadryas 421 | + | − | − | + |
| Papio hamadryas 423 | + | − | − | + |
| Papio papio 373 | (+) | ? | − | + |
| Macacca nemestrina | − | − | − | + |
| Macacca fascicularis 383 | − | − | − | + |
| Macacca nigra 381 | − | − | − | + |
| Macacca nigra 382 | − | − | − | + |
| Cercopithecus aethiops CAE27 | − | − | − | + |
| Cercopithecus aethiops CAEB | − | − | − | + |
| Pygathrix nemaeus PNE | − | − | − | + |
| Colobus guerezza CGU | − | − | − | + |
| Saimiri boliviensis 374 | − | − | − | + |
| Alouatta seniculus ASE | − | − | − | + |
| Controls | ||||
| HEK293 | + | + | − | + |
| ST-IOWA | + | + | + | − |
Results of pseudotype assays are depicted. The symbol (+) means that the results could not be reproduced in all of the experiments. envB infection in P. papio could not be clearly interpreted due to very high β-galactosidase background staining. Murine leukemia virus A was the amphotropic positive control. Infection experiments were reproduced at least three times.
MLV, murine leukemia virus.
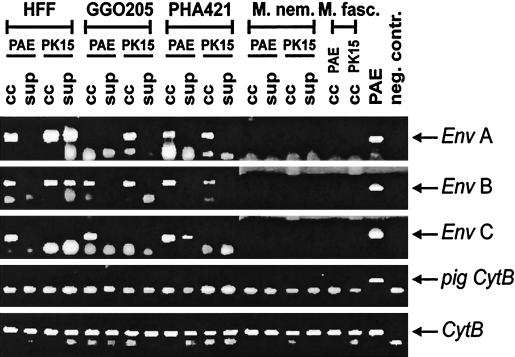
Moreover, we exposed human, gorilla, baboon (P. hamadryas), and macaque (Macaca fascicularis and M. nemestrina) primary fibroblasts to replication-competent PERV released by PK15 and PAE cells, which produce different sets of PERV subtypes (A/B versus A/B/C) (16). Transfer of all three PERV subtypes to human, gorilla, and baboon fibroblasts was observed by PCR (Fig. 1). Since receptors for PERV B and C could not be demonstrated on these cells, their genomes are probably present due to phenotypic mixing (16). However, gorilla fibroblasts cocultivated with PAE cells and baboon cells exposed to PAE supernatant are clearly positive for subtypes B/C and C, respectively, while PERV A transmission in these cultures is hardly detectable. Since PERV changes during adaptation to new host cells (19; our unpublished observations), these findings might indicate counterselection against PERV A in a PAE-PERV context, dependent on the target cell species and route of infection.
FIG. 1.
PERV transmission to primary fibroblasts of different primate species. Primary human foreskin fibroblasts (HFF) and gorilla (GGO205), baboon (P. hamadryas, PHA421), M. nemestrina (M.nem.), and M. fascicularis (M.fasc.) skin fibroblasts were infected by cocultivation (cc) with lethally irradiated PK15 or PAE cells and by cell-free supernatant (sup), respectively, and analyzed for PERV transmission by PERV env-specific PCR. False-positive results due to amplification of PERV sequences from contaminating porcine DNA were excluded by pig cytochrome b-specific PCR (pig CytB). Mammalian cytochrome b-specific PCR (CytB) was the positive control.
In any case, cocultivation experiments with lethally irradiated porcine cells and even exposure to cell-free supernatants of PK15 and PAE cells resulted in PERV transmission to all of the species tested, with the exception of macaque cells, which again could not be infected (16, 18).
Since apes have been ruled out as animal models for ethical and practical reasons, we further concentrated on the susceptibility of baboon cells to PERV infection.
Since fibroblasts may not be the primary target for type C retroviral replication and do not represent the main interface for PERV particles released by a xenotransplant, we attempted to infect baboon lymphocytic cells. Cocultivation experiments were performed with irradiated PK15 cells and two P. hamadryas B-lymphocyte cell lines, C42 and KM93 (1). After several passages (>20 days), PERV transmission could be demonstrated in both cell lines (Fig. 2a). In contrast to the cocultivation experiments, cell-free infection of both B-cell lines using PK15 culture supernatant, as well as cocultivation with another P. hamadryas cell line (PTLV-L) of T-lymphocytic origin (4), did not result in virus transmission (data not shown).
FIG. 2.
(a) In vitro transmission of PERV to baboon lymphocytic cells. The results of PERV pol-specific PCRs of the cell types tested are shown. False-positive results due to contaminating porcine DNA were excluded by pig cytochrome oxidase II (pig CyOII)-specific PCR. β-Actin was used as the positive control. (b) PERV mRNA expression in infected baboon B cells. RT-PCRs were performed at time points when porcine DNA sequences were no longer detectable in the cell cultures. Internal control reactions without reverse transcriptase (RT) excluded contamination with genomic DNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the positive control.
To investigate whether the infection of the baboon cell lines was productive, we analyzed PERV mRNA expression in the infected baboon cells. KM93 and C42 showed strong PERV pol mRNA expression and PERV A and B envelope mRNA (Fig. 2b).
Our data provide clear evidence that baboon cells, similar to human cells, are permissive for PERV infection. In this context, we emphasize that no agents which might enable unspecific or non-receptor-mediated entrance of the virus into the cell were used in our infection experiments. Of special importance is the fact that two baboon B-lymphocytic cell lines could be infected by PERV. This cell type represents one of the cell types which will, during xenotransplantation, come in close contact with porcine endothelial cells, which have been shown to release infectious PERV in vitro (6). Although, we could not demonstrate PERV release either by the infected baboon cell lines or by primary fibroblasts (data not shown), these results do not exclude the possibility of PERV production due to adaptation or activation at later time points. It is noteworthy that the majority of infected human cell types which show PERV mRNA expression also do not release viral particles (8, 12, 18, 19). Nevertheless, our results indicate that the baboon probably represents the best animal model currently available for preclinical risk assessment.
Our observations suggest that the shortcomings of the retrospective studies discussed (5, 10, 11) could be readily addressed in infection experiments in the baboon model. Even extended survival of transgenic whole-organ xenografts for up to 100 days (F. N. K. Bhatti et al., abstr. 138, p. 36, in XVII World Congr. Transplant. Soc. 1998) and long-term survival of the recipients (after removal of orthotopic transplanted grafts) can be achieved. If such experiments result in no evidence of PERV infection, carefully monitored clinical trials with pig organs modified to abrogate complement-mediated virolysis and/or a cytotoxic T-cell response are necessary and justified.
Acknowledgments
We thank A. Haverich and B. Reichart for their continuous strong support. Moreover, we are thankful to V. Z. Agrba and M. Chikobava for supplying the baboon B-lymphocytic cell lines and E. Borst for human primary foreskin fibroblasts. PTLV-L cells were a kind gift of A. M. Vandamme, Leuven, Belgium.
The present study was supported by grants from the Bayerische Forschungsstiftung, the Hannover Medical School (HILF program), and the Medical Research Council, United Kingdom.
REFERENCES
- 1.Agrba V Z, Yakoleva L A, Lapin B A, Sangulija I A, Timanovskaya V V, Markajan D S, Chuvirov G N, Salmanova E A. The establishment of continuous lymphoblastoid suspension cell cultures from hematopoietic organs of baboon (Papio hamadryas) with malignant lymphoma. Exp Pathol. 1975;10:318–332. doi: 10.1016/s0014-4908(75)80040-8. [DOI] [PubMed] [Google Scholar]
- 2.Birmingham K. FDA subcommittee finds no evidence of PERV transmission. Nat Med. 1999;5:855. doi: 10.1038/11275. [DOI] [PubMed] [Google Scholar]
- 3.Blusch, J. H., C. Roos, and H. Nitschko. A PCR based protocol for the detection of transmission of pig endogenous retroviruses in pig to human xenotransplantation. Transplantation, in press. [DOI] [PubMed]
- 4.Goubau P, Van Brussel M, Vandamme A M, Liu H F, Desmyter J. A primate T-lymphotropic virus, PTLV-L, different from human T-lymphotropic viruses types I and II, in a wild-caught baboon (Papio hamadryas) Proc Natl Acad Sci USA. 1994;91:2848–2852. doi: 10.1073/pnas.91.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneine W, Tibell A, Switzer W M, Sandstrom P, Rosales G V, Mathews A, Korsgren O, Chapman L E, Folks T M, Groth C G. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998;352:695–699. doi: 10.1016/S0140-6736(98)07145-1. [DOI] [PubMed] [Google Scholar]
- 6.Martin U, Kiessig V, Blusch J H, Haverich A, von der Helm K, Herden T, Steinhoff G. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998;352:692–694. doi: 10.1016/S0140-6736(98)07144-X. [DOI] [PubMed] [Google Scholar]
- 7.Martin U, Steinhoff G, Kiessig V, Chikobava M, Anssar M, Morschheuser T, Lapin B, Haverich A. Porcine endogenous retrovirus is transmitted neither in vivo nor in vitro from porcine endothelial cells to baboons. Transplant Proc. 1999;31:913–914. doi: 10.1016/s0041-1345(98)01832-6. [DOI] [PubMed] [Google Scholar]
- 8.Martin, U., M. E. Winkler, M. Id, H. Radeke, L. Arseniev, Y. Takeuchi, A. R. Simon, C. Patience, A. Haverich, and G. Steinhoff. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation, in press. [DOI] [PubMed]
- 9.Miyazono K, Okabe T, Urabe A, Takaku F, Heldin C H. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. 1987;262:4098–4103. [PubMed] [Google Scholar]
- 10.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer W M, Chapman L E, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 11.Patience C, Patton G S, Takeuchi Y, Weiss R A, McClure M O, Rydberg L, Breimer M E. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 12.Patience C, Takeuchi Y, Weiss R A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 13.Silver J, Maudru T, Fujita K, Repaske R. An RT-PCR assay for the enzyme activity of reverse transcriptase capable of detecting single virions. Nucleic Acids Res. 1993;21:3593–3594. doi: 10.1093/nar/21.15.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoye J. No clear answers on safety of pigs as tissue donor source. Lancet. 1998;352:666–667. doi: 10.1016/S0140-6736(98)22035-6. [DOI] [PubMed] [Google Scholar]
- 15.Switzer W M, Shanmugam V, Chapman L, Heneine W. Polymerase chain reaction assays for the diagnosis of infection with the porcine endogenous retrovirus and the detection of pig cells in human and nonhuman recipients of pig xenografts. Transplantation. 1999;68:183–188. doi: 10.1097/00007890-199907270-00003. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi Y, Patience C, Magre S, Weiss R A, Banerjee P T, Le Tissier P, Stoye J P. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998;72:9986–9991. doi: 10.1128/jvi.72.12.9986-9991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todaro G J, Benveniste R E, Lieber M M, Sherr C J. Characterization of a type C virus released from the porcine cell line PK(15) Virology. 1974;58:65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson C A, Wong S, Muller J, Davidson C E, Rose T M, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson C A, Wong S, VanBrocklin M, Federspiel M J. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol. 2000;74:49–56. doi: 10.1128/jvi.74.1.49-56.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]