Abstract
Selected members of the adenovirus family have been shown to interact with the coxsackie adenovirus receptor, αv integrins, and sialic acid on target cells. Initial interactions of subgenus D adenoviruses with target cells have until now been poorly characterized. Here, we demonstrate that adenovirus type 8 (Ad8), Ad19a, and Ad37 use sialic acid as a functional cellular receptor, whereas the Ad9 and Ad19 prototypes do not.
The 51 human adenovirus (Ad) serotypes fall into six subgenera, A through F (8, 20). Several adenoviruses of subgenus D exhibit tropism for the human eye (4, 5, 10, 13), but Ad8, Ad19a, and Ad37 are the only types which are frequently associated with epidemic keratoconjunctivitis (10, 13). Adenovirus binding to target cells is mediated by its fiber (7, 9, 12, 18), and the cellular receptor for most adenoviruses, including Ad9 and Ad19p of subgenus D, has been identified recently as the coxsackie adenovirus receptor (CAR) (6, 17, 19). Ad37 was found recently to use α(2→3)-linked sialic acid as a receptor (2) instead of CAR. Ad9 has been shown to use its penton base to interact with αv integrins not only during internalization, which is the proposed mechanism for most adenoviruses (14), but also during attachment to target cells (18). Here, we investigated the role of sialic acid and αv integrins during the early steps of infection for five distinct subgenus D adenoviruses.
Sialic acid is used as a primary receptor by Ad8, Ad19a, and Ad37.
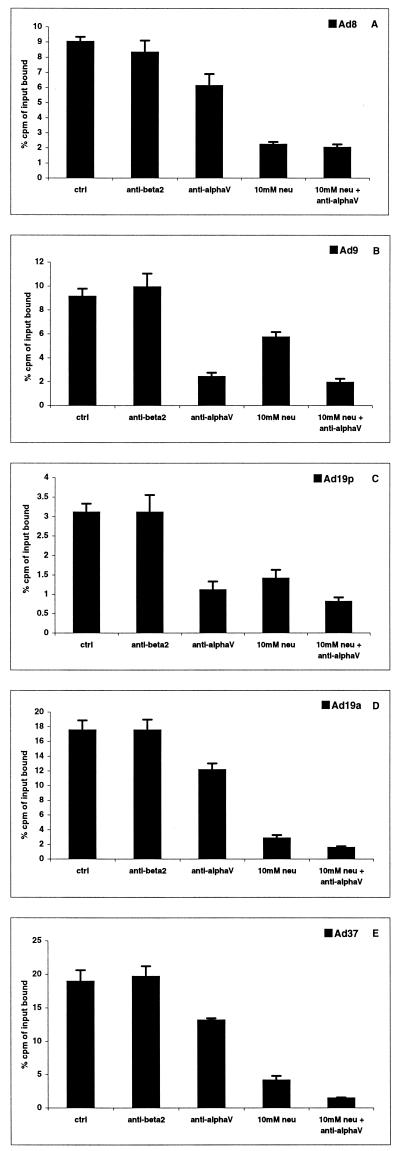
Using 35S-labeled adenoviruses (7) in a virus-cell binding assay (2), we found that the binding of Ad8, Ad19a, and Ad37 to A549 cells was highly sensitive to neuraminidase treatment of the cells (Fig. 1). We also found that Ad9 and Ad19p binding was sensitive to neuraminidase treatment of cells, but to a lesser extent. These results suggested that the adenoviruses Ad8, Ad19a, and Ad37 all use sialic acid as cellular receptors. At this point, we could not exclude the possibility that sialic acid was also used as a receptor by Ad9 and Ad19p.
FIG. 1.
Interaction of subgenus D adenoviruses with A549 cells. A total of 2 × 105 A549 cells in suspension were treated with (i) anti-β2 integrin MAb P4H9 on ice (negative control), (ii) anti-αv integrin MAbs P1F6 and LM609 on ice, (iii) neuraminidase (Vibrio cholerae) at 37°C, or (iv) first with P1F6 and LM609 and then with neuraminidase. The binding of 35S-labeled virions to the cells was measured in a Wallac scintillation counter. Data are the means of three independent experiments.
The penton base proteins of most, but not all, adenoviruses contain αv-integrin-recognizing RGD (Arg-Gly-Asp) motifs (1). The αvβ5 integrins have been shown to interact with most adenoviruses, supporting their internalization into the host cell (15). Nucleotide sequencing and comparison of the respective penton base proteins revealed that they all contained the RGD motif (Fig. 2). Full-length penton base genes of Ad19p, Ad19a, and Ad37 were generated by PCR (3) and sequenced as described earlier (3). An incomplete Ad9 penton base gene was generated by PCR (3) and sequenced by Cybergene (Huddinge, Sweden). The RGD-flanking region of Ad8 has been published (1). Other than for the regions flanking the RGD motifs, the Ad9, Ad19a, Ad19p, and Ad37 sequences were highly homologous. Function-blocking monoclonal antibodies (MAbs) against αv integrins inhibited Ad9 and Ad19p binding to A549 cells significantly more than the binding of Ad8, Ad37, and Ad19a to A549 cells (Fig. 1). Cotreatment of the cells with neuraminidase and αv MAbs had little additional effect beyond what was achieved with neuraminidase alone (Ad8, Ad19a, and Ad37) or with αv MAbs alone (Ad9 and Ad19p). Taken together, these experiments indicated that sialic acid is a functional attachment molecule for Ad8, Ad19a, and Ad37, and that Ad9 and Ad19p are more dependent on αv integrins for attachment to target cells.
FIG. 2.
Amino acid sequences of the variable RGD-flanking regions in the penton base of five subgenus D adenoviruses. The conserved RGD motifs are shown in boldface, and conserved amino acids are indicated by asterisks. The GenBank accession numbers are AF217407 (Ad9), AF217408 (Ad37), AF217409 (Ad19p), and AF217410 (Ad19a). The sequence of Ad8 is from Albinsson and Kidd (1).
Sialic acid is a functional receptor for Ad8, Ad19a, and Ad37 but not for Ad9 or Ad19p.
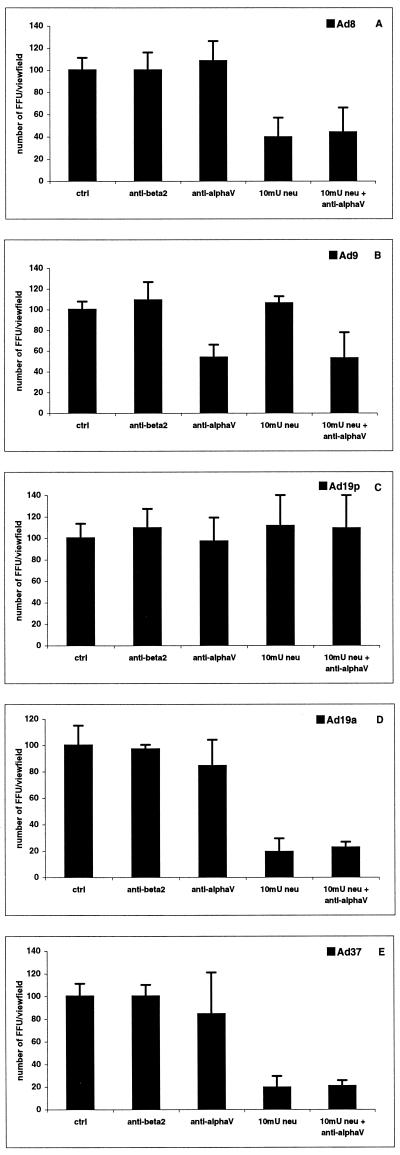
To determine the role of sialic acid and αv integrins as functional receptors for subgenus D adenoviruses, we treated A549 cells with neuraminidase and/or αv MAbs and infected the cells with the different adenovirus types as described earlier (2), with the exception that the neuraminidase treatment of the cells was followed by incubation of virus-infected cells on ice for 1 h, washing, and warming of the cells thereafter to 37°C. Our immunofluorescence assays indicated that infection of A549 cells with Ad8, Ad19a, and Ad37 was strongly inhibited by pretreatment of the cells with neuraminidase (Fig. 3). The efficacy of Ad8 infection decreased by 60% and that of Ad19a and Ad37 infection decreased by 80%, suggesting that sialic acid is a functional receptor for these viruses. These data agree with an earlier observation that Ad8 partially competes for Ad37 binding to conjunctival cells (12).
FIG. 3.
Uptake and replication of subgenus D adenoviruses in A549 cells. A total of 2 × 105 adherent A549 cells were treated with (i) anti-β2 integrin MAb P4H9 on ice (negative control), (ii) anti-αv integrin MAbs P1F6 and LM609 on ice, (iii) neuraminidase (V. cholerae) at 37°C, or (iv) first with P1F6 and LM609 and then with neuraminidase. Added virions were diluted to 5,000 fluorescent focus units (FFU) per virus type and well, resulting in approximately 100 FFU per view field in control wells. Data are the means of three independent experiments.
We then assayed the ability of subgenus D adenoviruses to infect A549 cells preblocked with αv MAbs (Fig. 3). αv MAbs did not appreciably decrease the infectivity of Ad8, Ad19a, and Ad37, and the combination of neuraminidase and αv MAbs had no additional effect compared to neuraminidase alone. This supports the suggestion that sialic acid is used as a functional receptor for Ad8, Ad19a, and Ad37.
Even though the binding assay indicated that sialic acid may support Ad9 and Ad19p attachment to some extent (Fig. 1B and C, respectively), the infectivity assay clearly demonstrated that a productive infection by Ad9 or Ad19p does not require sialic acid (Fig. 3B and C). Rather, we found that Ad9 infectivity for A549 cells was significantly inhibited by αv MAbs (Fig. 3), while Ad19p infectivity was not inhibited. The Ad9 result agrees with the previous finding that preincubation of A549 cells with soluble penton base decreased Ad9 virion binding (18), presumably by blocking access to αv integrins acting as receptors. The combined treatment of neuraminidase and αv MAbs did not further inhibit Ad9 binding or infection compared to treatment with αv MAbs alone. Uniquely, the variable region of the Ad9 penton base contains three threonines immediately upstream of the RGD motif (Fig. 2). This is also a feature of vitronectin and fibronectin, proteins which interact efficiently with αv integrins. One possible explanation for the unusual ability of Ad9 to interact with αv integrins could be the presence of these threonines. Most adenoviruses have been shown to interact with the αvβ5 integrin heterodimer, and the most efficient interaction is exhibited by Ad37 (15). The relative efficiency by which Ad9 interacts with this integrin has not been determined, but from the data presented here and from the data of others (18), we would expect this interaction to be among the most efficient.
It has been shown previously that Ad19p virions interact with αvβ5 integrins (15). In the binding assay we found that αv integrins were used by Ad19p, but only to a limited extent. In the infectivity assay we found that Ad19p infection was independent of αv integrins, and a combination of neuraminidase treatment and αv MAb preincubation had little or no effect on the entry of Ad19p. This was unexpected, since the binding assay indicated that sialic acid and αv integrins together accounted for 74% of the total binding. In part, this may be explained by the known interaction between Ad19p and CAR (17). It may also be that sialic acid and/or αv integrins are used by Ad19p for binding and internalization but Ad19p is less competent in replication. The high particle/PFU ratio (1,600:1) found for Ad19p (11) supports this suggestion.
Sialic acid appears to be a functional cellular receptor for the adenovirus types that cause epidemic keratoconjunctivitis (i.e., Ad8, Ad19a, and Ad37), and it is found on the surface of many cell types. Consequently, the reason for the limited tropism of these viruses may lie at a postattachment level (i.e., during early replication), or at an even later point, during cell-cell spread. The latter consideration has been neglected somewhat in discussions of virus tropism.
Adenoviruses have been used as vectors for gene therapy, but with limited success (16, 21). Here, we demonstrated that a selected group of adenoviruses uses sialic acid as a primary, functional receptor. Since sialic acid is expressed on most cells, including erythrocytes, these findings should be useful for the application of adenovirus-based vectors in human gene therapy.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (K99-06X-0568820C and GT), the Swedish Cancer Foundation (1547-B99-10X and 1547-B99-08XAC), Lions Foundation (AMP-97-128), and the Foundation for Advanced Research at Umeå University Hospital.
REFERENCES
- 1.Albinsson B, Kidd A H. Adenovirus type 41 lacks an RGD αv-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999;64:125–136. doi: 10.1016/s0168-1702(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 2.Arnberg N, Edlund K, Kidd A H, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnberg N, Mei Y, Wadell G. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology. 1997;227:239–244. doi: 10.1006/viro.1996.8269. [DOI] [PubMed] [Google Scholar]
- 4.Bell S D, Rondon Rota T, McComb D E. Adenoviruses isolated from Saudi Arabia. Six new serotypes. Am J Trop Med Hyg. 1960;9:523–526. [Google Scholar]
- 5.Bennett F M, Law B B, Hamilton W, MacDonald A. Adenovirus eye infection in Aberdeen. Lancet. 1957;ii:670–673. doi: 10.1016/s0140-6736(57)92109-8. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 7.Defer C, Belin M-T, Caillet-Boudin M-L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong J C, Wermenbol A G, Verweij-Uijterwaal M W, Slaterus K W, Wertheim-Van Dillen P, Van Doornum G J J, Khoo S H, Hierholzer J C. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbiol. 1999;37:3940–3945. doi: 10.1128/jcm.37.12.3940-3945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon Y J, Aoki K, Kinchington P R. Adenovirus keratoconjunctivitis. In: Pepose J S, editor. Ocular infections and immunity. St. Louis, Mo: Mosby; 1996. pp. 877–894. [Google Scholar]
- 11.Green M, Pina M, Kimes R C. Biochemical purification of adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967;31:562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Reddy V, Dasgupta N, Nemerow G R. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73:2798–2802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp M C, Hierholzer J C, Cabradilla C P, Obijeski J F. The changing etiology of epidemic keratoconjunctivitis. Antigenic and restriction enzyme analysis of types 19 and 37 isolated over a 10-year period. J Infect Dis. 1983;148:24–33. doi: 10.1093/infdis/148.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathias P, Galleno M, Nemerow G R. Interactions of soluble recombinant integrin αvβ5 with human adenoviruses. J Virol. 1998;72:8669–8675. doi: 10.1128/jvi.72.11.8669-8675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackie-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadell G, Hammarskjöld M-L, Winberg G, Varsanyi T M, Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- 21.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]