Abstract
Introduction:
Positive effects of vitamin D (vitD) supplementation on comorbidities of pregnancy (COP) have been explored; however, few studies have elucidated the pathophysiology behind the development of these COP and the potential relationship with derangements in placental development and morphology. Additionally, it is known that placentas weighing 10th-90th % for gestational age are associated with better outcomes. Therefore, the objective of this study was to assess the impact of resulting circulating serum 25(OH)D concentrations associated with intake of high or low doses of supplementary vitD on placental development and morphology in women who participated in a randomized double blind, placebo-controlled trial of vitD supplementation. We hypothesized that if maternal serum 25(OH)D concentration (vitD status marker) is insufficient/deficient, then placental weight and % for gestational age (GA) will be smaller and will correlate with increased vascular and inflammatory placental pathologic findings.
Methods:
The findings of the present study are a secondary analysis of data generated from a previously reported randomized controlled trial (RCT), the Kellogg Vitamin D Pregnancy Study. Pregnant women (n=297) in this RCT (January 2013 - April 2018) were randomly assigned to 400 IU vs. 4400 IU vitD/day (10–14 weeks’ gestational age) and followed to delivery. 132 placentas were analyzed by pathologists blinded to treatment, and the 2016 Amsterdam Consensus Criteria were used to categorize grouping/grading of placental pathology and weight. Total [25(OH)D] was measured using radioimmunoassay (ng/mL). Chi-square and Student’s t-test were used to show the difference in maternal characteristics by treatment group and by placental weight. Chi-square analysis was used to determine differences between the percent pathology findings by treatment group. Students t-test was used to determine the differences in vitD status and the frequency of placental lesions. Association between [25(OH)D] area under the curve (AUC) and placental morphology were determined in a regression model that included maternal BMI ≥30kg/m2, race/ethnicity, and vitD treatment group allocation. Data were analyzed using SAS v9.4 (Cary, NC) and statistical significance was indicated by p<0.05.
Results:
The percent pathology findings by treatment group were not significantly different for each of the placental pathology categories as defined by the 2016 Amsterdam Consensus Criteria including placental weight. However, when using 25(OH)D as a biomarker for vitD status, linear regression model showed maternal serum [25(OH)D] AUC was significantly associated with greater placental weight (p=0.023). Logistic regression models showed mothers with BMI ≥30kg/m2 had larger placental weight (p=0.046), and Hispanic and white/Caucasian mothers had greater placental weights than Black American mothers (p=0.025). When placentas ≥90th % for GA, n=7, were removed from the placental pool, Pearson correlation still showed a positive association between maternal serum 25(OH)D AUC and placental weight (p=0.011). In a second linear regression model of placentas ≥90th % for GA (n=7) vs. placentas <90th % (n=108), maternal serum 25(OH)D AUC was significantly greater in those placentas ≥90th % (p=0.03); however, this was not associated with increased perinatal mortality.
Conclusion:
Findings suggest increasing maternal serum [25(OH)D] via vitamin D supplementation during pregnancy did not adversely affect placental morphology; trends showed those in the treatment group had fewer placental lesions. Placental weight was found to be significantly associated with [25(OH)D] AUC, which represents maternal vitamin D status over the course of pregnancy; 7 placentas ≥90th % for GA were not associated with perinatal mortality.
Keywords: vitamin D, 25-hydroxyvitamin D, placenta, placental weight, pregnancy, inflammation
1. Introduction
The placenta offers the fetus both immunologic and physical protection and provides a fetal-maternal interface essential for appropriate nutrient and waste exchange, and cell signaling. As the fetus develops, the amniotic fluid in its surroundings affords it movement, maintenance of temperature, a protective buffer, and proper lung and gastrointestinal development. Combined, the placenta, umbilical cord, and amniotic fluid work in synergy to make sure that the nutritional needs of the fetus are met to allow for appropriate growth.
Many of the effects of micronutrients, such as vitamins, folate, and iron on placental function have been explored, including vitamin D.2 Adequate vitamin D supplementation during pregnancy has been associated with the reduced risk of numerous comorbidities of pregnancy (COP), such as preeclampsia,3–6 fetal growth restriction (FGR),6 preterm birth,5,7,8 small for gestational age infants,5,9 gestational diabetes,5 and even inflammatory processes such as amniotic fluid infection.4,5,10 Many of these pathologies are thought to have a root cause stemming from inadequate placental development and morphology, which may be assessed by observing gross and microscopic placental findings.
During pregnancy, maternal 1,25(OH)2D significantly increases due to hydroxylation of 25-hydroxyvitamin D (25(OH)D) by CYP27B1 in the maternal kidney and placenta.2,4 It has been found recently that instead of passive diffusion of 25(OH)D across the maternal-fetal interface, the syncytiotrophoblast cells that make up the placental villi are responsible for taking up maternal 25(OH)D via endocytosis.11 Once in the cell, 25(OH)D is enzymatically converted to the active form 1,25(OH)2D and either stored or utilized by the placenta or transferred to the fetal or maternal circulation.11 The associated elevation in maternal 1,25(OH)2D concentration causes greater calcium absorption by the maternal gut to help meet the demands of the developing fetus.10 Downstream effects of 25(OH)D on the placenta also have been studied, including activation of degrading enzyme CYP24A111 and transcription of the vitamin D receptor itself.12
Because of the role that the placenta has in the production of 1,25(OH)2D and its use of this metabolite, it has been hypothesized that in the event of decreased maternal concentrations of 25(OH)D, there might be changes to placental morphometry. In studies on mice, dietary restriction of vitamin D has been shown to cause smaller placental weight, decreased villi labyrinth vascularization, and decreased angiogenic VEGF-α expression.2 In a large human cohort, it was found that higher maternal circulating 25(OH)D reduced the risk of placental vascular pathology.13 In addition, associations have been made between placental pathology and clinical outcomes. For example, the shape and size of the placenta has been associated with fetal growth restriction, reduced fetal movement, and long term health complications.12 Chronic villitis is believed to impair fetoplacental circulation and later manifest itself as adverse neurological outcomes such as cerebral palsy.12,14 Placental inflammation, by means of amniotic fluid infection, has also been associated with premature delivery.15,16 Furthermore, the fetal inflammatory response has been associated with poor fetal clinical outcomes such as intraventricular hemorrhage, neurologic disease, and chronic lung disease such as asthma.12,15,17 Vitamin D also has been recognized for its role in immune function.4 Adequate maternal blood vitamin D concentration plays an important role on the maternal-fetal interface and immunity.4,18 Thus, assessment of placental pathology, especially that which might be associated with impaired immune function such as amniotic fluid infection or inflammation, might also show a correlation with total maternal 25(OH)D concentration during the first, second, and third trimesters.19
There are many previously described associations between placental pathologic findings, comorbidities of pregnancy, and poor fetal outcomes. Few studies, however, demonstrate the effects of adequate vitamin D supplementation during pregnancy on placental pathologic findings alone.13 The studies that have assessed the effects of vitamin D on the placenta have examined different subgroups of placental pathology depending on their hypotheses (i.e. vascular,13 inflammatory components, or both17,20). Most present their placental pathologic findings in addition to their assessment of gene regulation in the placenta,7 or expression of inflammatory markers in maternal10 or fetal cord blood, or both.20 Some of these studies are limited by their use of small placental sample size,7,17 maternal concentrations of 25(OH)D measured only at one interval (first or third trimesters, baseline or delivery)17 or a single measure of neonatal cord blood 25(OH)D,7 and the use of only term13 or preterm7 placentas.
The present study is unique because it builds upon prior work using a larger sample size of placentas (n=115) to assess unique parameters and analyze preterm and term placentas as a function of maternal blood 25(OH)D concentrations at first, second, and third trimesters and over the course of pregnancy as well as 25(OH)D of neonatal cord blood at delivery. In the present study, we assess the impact of circulating serum 25(OH)D concentrations associated with intake of supplementary vitamin D on placental development and morphology in participants of a randomized double blind, placebo-controlled trial of vitamin D supplementation. We explore the effects of maternal serum and neonatal cord blood 25(OH)D concentrations on placental findings grouped using the 2016 Amsterdam Consensus Criteria: 1) maternal and fetal vascular pathology, 2) placental weight, 3) inflammatory patterns in the placental histopathology, such as amniotic fluid infection (AFI) and villitis of unknown etiology (VUE), and 4) other placental lesions (subgroups: nonspecific (NS), and delayed villous maturation (DVM)) and their associated clinical relevance. We hypothesized that if maternal serum [25(OH)D] and/or neonatal cord blood [25(OH)D] was insufficient or deficient, then placental weight and percentile for gestational age would be smaller and would correlate with increased vascular and inflammatory placental pathologic findings.
2. Materials and Methods
2.1. Study Overview and Design
The present study is a secondary goal of the Kellogg Vitamin D Pregnancy Study; a double blind, randomized placebo-controlled clinical trial that focused on understanding the underlying mechanisms of vitamin D deficiency during pregnancy and associated adverse maternal and fetal outcomes, possibly contributing to health disparities in pregnancy outcomes. The RCT took place between January 2013 and April 2018 at the Medical University of South Carolina (MUSC), an urban medical center in the southeastern United States, that manages both normal and high-risk pregnancies, and performs approximately 3000 deliveries per year. All women enrolled in the study took a standard prenatal vitamin (containing 400 IU vitamin D3) and were randomized to receive either daily placebo or 4000 IU vitamin D3 in the form of gummies (Church & Dwight, Ewing, NJ). This RCT was conducted in accordance with the declaration of Helsinki and approved by the Institutional Review Board at MUSC, study protocol (Pro00020570), and was registered via clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT01932788). The primary trial results have previously been reported.19,21,22
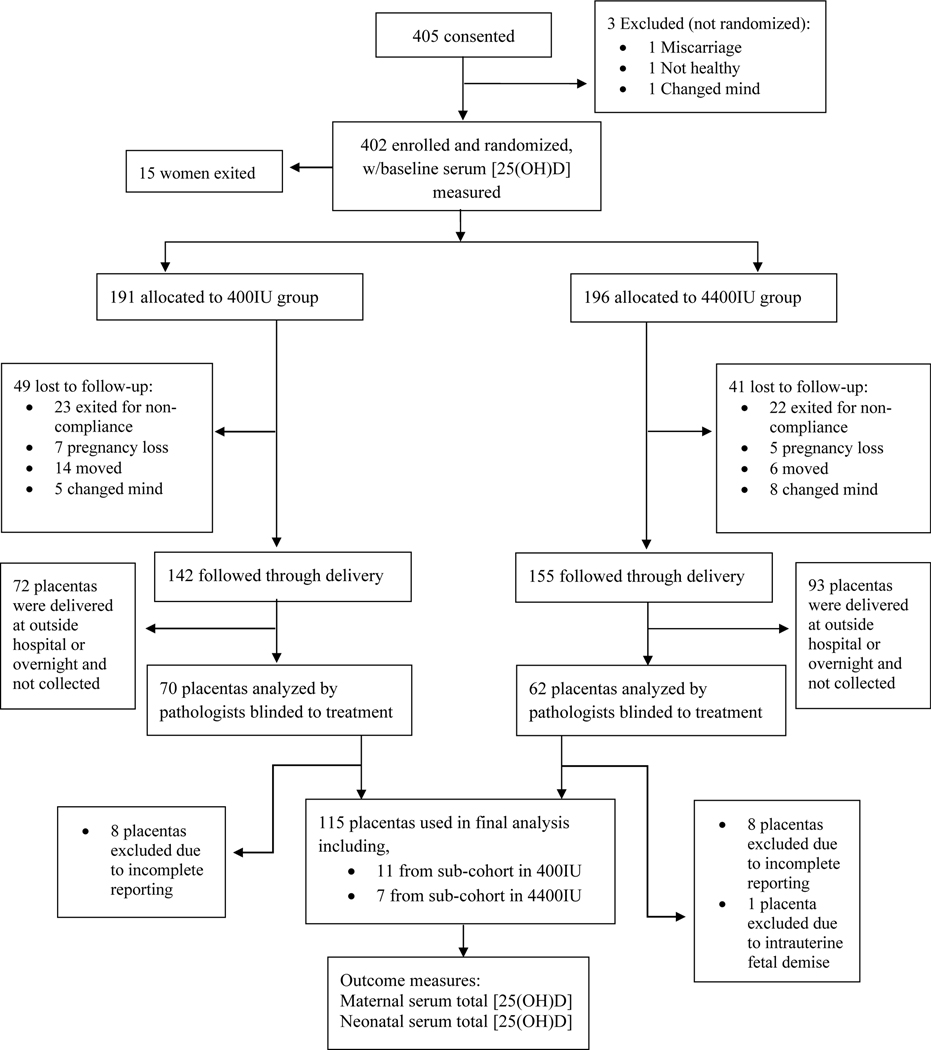
As part of this RCT, 132 placentas that were delivered at MUSC were collected as a convenience sample between the hours of 7 am and 9 pm and analyzed by attending pathologists or residents trained in placental examination. Their associated pathologic findings were reported in the Redcap database. 115 placentas were used in the final statistical analysis. Placentas that were delivered at a hospital other than MUSC were not included in the convenience sample. All evaluators and study team members were blinded to treatment assignment. The CONSORT Flow diagram can be seen in Figure 1.
Figure 1.
CONSORT Flow Diagram
2.2. Participants
Women ages 18–45, in good overall health, who presented to their obstetrician or midwife at MUSC weeks 10–14 after their last menstrual period and had a confirmed singleton pregnancy were eligible for enrollment (n=405). Women with pre-existing parathyroid disease or uncontrolled thyroid disease, multiple fetuses, sickle cell disease, sarcoidosis, Crohn’s disease, or ulcerative colitis were not eligible for enrollment. A sub-cohort of eighteen participants with known diabetes, hypertension, human deficiency virus (HIV), or morbid obesity (BMI>49kg/m2) in the original RCT delivered placentas and were included in this secondary goal of the RCT. In total, the placentas of 132 of the 405 originally consented participants were analyzed.
Maternal sociodemographic information was collected via questionnaire at visit 1 and included marital, insurance, employment status, level of education, alcohol use, and smoking status. Maternal age, body mass index (BMI; based on height and weight taken at the first outpatient visit), and self-reported race (Black American, white/Caucasian, Hispanic) were documented. Comorbidities of pregnancy (such as hypertensive disorders of pregnancy [pregnancy-induced hypertension, preeclampsia, eclampsia, or HELLP Syndrome], intrauterine growth restriction, clinically diagnosed chorioamnionitis, bacterial vaginosis, sexually transmitted infection (trichomoniasis, gonorrhea, chlamydia), urinary tract infection (UTI), and gestational diabetes) and birth characteristics (such as gestational age, neonatal birth weight, and mode of delivery) also were reported. No mothers in the present study experienced eclampsia. Neonatal cord blood 25(OH)D concentrations were used in analysis. All neonates in the present study were born living.
2.3. Laboratory Measurements
Whole blood was obtained from participants at each month of gestation and at delivery. The samples used for this study were obtained at visits 1, 4, and 1 month prior to delivery, which roughly corresponded to 2, 5, and 8 months of gestation or first, second, and third trimesters, respectively. One hundred and nine participants had their blood drawn 1 month prior to delivery. Whole blood also was drawn from the umbilical vein of the placenta at the time of delivery, representing neonatal blood; of which, 111 neonatal blood samples were collected. All whole blood samples were separated by centrifugation producing plasma which was stored at −80℃ until processed and analyzed in the Hollis/Wagner lab (MUSC). Total circulating maternal and neonatal 25(OH)D concentration was measured using a commercially available radioimmunoassay (Diasorin, Stillwater, MN). 25(OH)D deficiency was defined according to the Endocrine Society criteria as total circulating 25(OH)D <50nmol/L (<20ng/mL), insufficiency as ≥50 to <80nmol/L (≥20 to <32ng/mL), and sufficiency as ≥80nmol/L (≥32ng/mL).23
2.4. Placental Sampling, Examination, and Histological Analysis
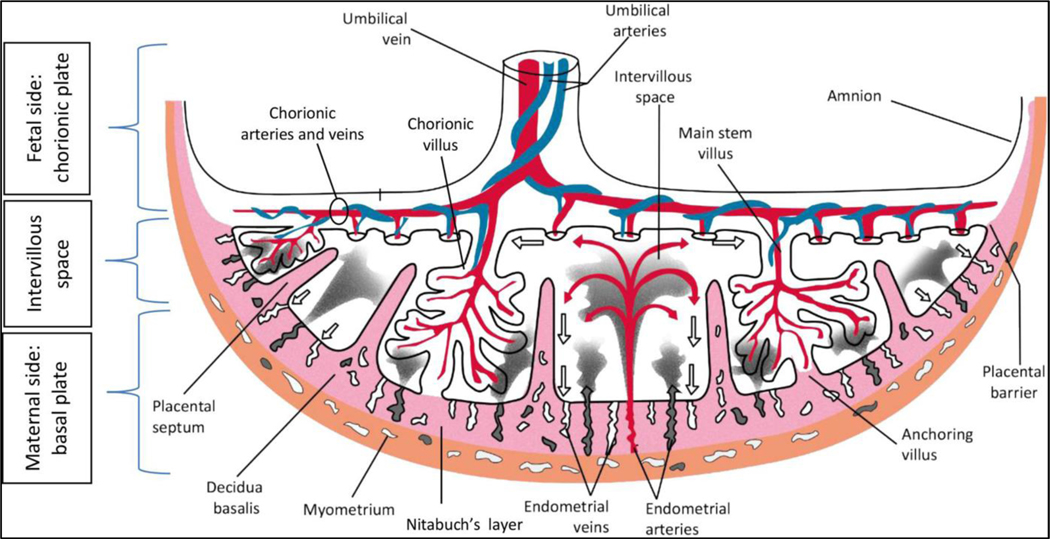
After delivery, 132 singleton placentas were submitted in the fresh state and refrigerated to minimize the effects of autolysis. After removing the umbilical cord and trimming of the extraplacental membranes, the placentas were each fixed into a specimen container of 10% neutral buffered formalin, which increased their weight by approximately 5%. The corrected weight for each placenta was determined by multiplying the fixed weight by 0.95. The term ‘placental weight’ is used in lieu of ‘corrected placental weight’ in this paper. Gross pathologic examination and histological analyses of the placentas were performed by attending pathologists or residents specifically trained in placental analysis who were blinded to the vitamin D status of the pregnancies. All findings were originally reported on individual forms, then entered into a secure Redcap database. An illustration of placental anatomy is included for reference (Figure 2).1
Figure 2.
Gross placental anatomy 1
Placental findings were grouped by association into maternal vascular pathology, fetal vascular pathology, infectious pathology including inflammatory/immune mediated pathology such as chorioamnionitis and villitis of unknown etiology, and miscellaneous groups (nonspecific findings and additional groupings) as described by the Amsterdam group consensus published in 201612 and further refined by Dr. Redline.24,25 More information on gross and microscopic examination of the placentas and organization of placental findings according to the 2016 Amsterdam Consensus Criteria can be found in Supplementary Material (Table 1).
2.5. Statistical Analysis
The primary outcome measure was maternal serum [25(OH)D] at first, second, third trimester, and over time from month 3 to the month prior to delivery (steady state after 2 months of supplementation expressed as area under the curve; AUC) and placental pathology. Data were analyzed using SAS v9.4, Cary, NC. Chi-square was used to associate participant sociodemographic information, preexisting chronic conditions, baseline maternal serum [25(OH)D], and birth characteristics with treatment groups. Student’s t-test was used to associate both participant education and mean maternal serum [25(OH)D] AUC by treatment groups. Chi-square, Fisher’s exact test, Student’s t-test, and Wilcoxon two-sample test were used to compare participant characteristics of those in the original RCT whose placentas were analyzed versus those who were not. Chi-square analysis was used to determine the potential difference in percent placental pathology findings by treatment group. Student’s t-test was used to test for the difference in mean serum [25(OH)D] in mothers with—versus—without placental lesions in each category during the first, second, third trimester, and AUC.
Pearson correlation was used to associate placental weight and maternal serum [25(OH)D] during the first, second, third trimester, and AUC, and with maternal baseline BMI, neonatal cord blood [25(OH)D], and neonatal birth weight. Pearson correlation was also used to associate neonatal cord blood [25(OH)D] with maternal serum [25(OH)D] during the first, second, third trimester and AUC. Student’s t-test was used to test for the difference in mean placental weight and maternal BMI at baseline. Analysis of variance (ANOVA) was used to show difference in mean placental weight among Black American, white/Caucasian, and Hispanic mothers.
ANOVA also was used to determine differences in placental weight in participants who developed COP. Chi-square and Fisher’s exact test were used to determine the difference between the frequency of placental pathology findings by category and those who developed COP. Chi-square and Fisher’s exact test were used to determine the difference in the number of participants who developed COP by treatment group. Student’s t-test was used to show the difference in mean maternal serum [25(OH)D] during the first, second, third trimester, AUC, and neonatal cord blood [25(OH)D] in participants who developed COP.
Linear and logistic regressions were used to associate placental pathologies by category and patient characteristics including maternal serum [25(OH)D] (first, second, third trimester, and AUC) or neonatal cord blood [25(OH)D], race/ethnicity, maternal baseline BMI≥30kg/m2, and vitamin D treatment group allocation. Statistical significance was indicated by p<0.05.
3. Results
3.1. Participant Characteristics
The characteristics of participants whose placentas were used in the final analysis are displayed in Table 1 and organized by treatment allocation (n=115). No significant differences were noted between the treatment groups regarding maternal sociodemographic information, preexisting chronic conditions (sub-cohort), maternal serum [25(OH)D] at baseline and throughout the course of pregnancy (AUC), and birth characteristics. As expected, maternal serum [25(OH)D] AUC and neonatal cord blood [25(OH)D] at delivery were significantly greater in the treatment group (Table 1). There were few significant differences in participant characteristics between those enrolled in the original RCT whose placentas were analyzed in the present study (n=115) versus those that were not. The characteristics that were different included prior/current alcohol use (fewer analyzed in placental group), cesarean delivery (more), GA at delivery (smaller), preterm delivery (fewer), neonatal birth length (shorter), APGAR scores at 1 and 5 minutes (lower), and neonatal cord blood [25(OH)D] (lower; all p<0.05).
Table 1.
Participant Characteristics. Maternal sociodemographic information was collected via questionnaire at visit 1.
| Participants | Control (400 IU/d) N=62 | Treatment (4400 IU/d) N=53 | P |
|---|---|---|---|
| Maternal age at delivery in years (Mean, range) | 29 (20 – 40) | 28 (19 – 38) | 0.11 |
| BMI at baseline, visit 1 (kg/m2) (Mean, range) | 30.11 (17.80 – 52.37) | 28.08 (19.51 – 58.06) | 0.16 |
| Race/Ethnicity (n, %) | |||
| Black American | 21 (33.87) | 17 (32.08) | 0.89 |
| White/Caucasian | 23 (37.10) | 22 (41.51) | |
| Hispanic | 18 (29.03) | 14 (26.42) | |
| Insurance (n, %) | |||
| None/Medicaid | 40 (64.52) | 27 (50.94) | 0.14 |
| Private | 22 (35.48) | 26 (49.06) | |
| Maternal Education (n, %) | |||
| < High school or high school | 32/52 (61.54) | 25/49 (51.02) | 0.29 |
| Some college + ** | 20/52 (38.46) | 24/49 (48.98) | |
| Marital Status (n, %) | |||
| Married | 26 (41.94) | 29 (54.72) | 0.17 |
| Single | 36 (58.06) | 24 (45.28) | |
| Other Demographics | |||
| Gravidity (median, IQR) | 3.0 (2.0 – 4.0) | 2.0 (2.0 – 3.0) | 0.12 |
| Parity (median, IQR) | 1.0 (0.0 – 2.0) | 1.0 (0.0 – 2.0) | 0.40 |
| Once smoker (n, %) | 10 (16.13) | 7 (13.21) | 0.66 |
| Alcohol use prior to pregnancy (n, %) | 18/61 (29.50) | 15/53 (28.30) | 0.89 |
| Preexisting Chronic Conditions (n, %) | |||
| Cohort | 51 (82.26) | 46 (86.79) | 0.50 |
| Sub-cohort | 11 (17.74) | 7 (13.21) | |
| Maternal [25(OH)D] (ng/mL) (mean, range) | |||
| Baseline (visit 1/first trimester) | 25.63 (7.3 – 47.7) | 24.83 (10.9 – 50.0) | 0.65 |
| AUC (mean [25(OH)D] over the course of pregnancy) | 86.94 (27.1 – 164.0) | 137.4 (41.4 – 210.2) | <0.0001* |
| Mode of Delivery (n, %) | |||
| Cesarean | 32 (51.61) | 19 (35.85) | 0.09 |
| Vaginal delivery | 30 (48.38) | 34 (64.15) | |
| Birth Characteristics | |||
| Mean GA at delivery ± SD (weeks) (mean ± SD, range) | 38.11 ± 3.39 (24.3 – 41.0) |
38.19 ± 2.01 (33.4 – 41.2) |
0.88 |
| Term birth (>37 weeks) (n, %) | 55 (88.71) | 40 (75.47) | 0.06 |
| Preterm birth (<37 weeks) (n, %) | 7 (11.29) | 13 (24.53) | |
| Neonatal birth weight (g) (mean ± SD, range) | 3158.1 ± 841.0 (Range 595.0 – 4635.0) |
3249.5 ± 536.7 (Range 1955.0 – 4900.0) |
0.49 |
| Neonatal birth length (cm) (mean ± SD, range) | 46.03 ± 5.68 (Range: 30.0 – 50.0) n=57 |
48.29 ± 2.93 (Range: 42.0 – 50.0) n=48 |
0.15 |
| Neonatal birth head circumference (cm) (mean ± SD, range) | 33.33 ± 3.33 (Range: 22.0 – 39.50) n=54 |
33.81 ± 1.73 (Range: 29.50 – 36.50) n=47 |
0.38 |
| Neonatal Sex | |||
| Female (n, %) | 30/61 (49.18) | 23/53 (43.4) | 0.54 |
| Male (n, %) | 31/61 (50.82) | 30/53 (56.6) | |
| APGAR Scores (median, IQR) | |||
| 1 minute | 8.0 (6.0–9.0) | 8.0 (8.0–9.0) | 0.33 |
| 5 minute | 9.0 (9.0–9.0) | 9.0 (9.0–9.0) | 0.86 |
| Neonatal Cord Blood [25(OH)D] (ng/mL) (mean, range) | |||
| At delivery | 21.35 (6.70 – 53.90) n=58 | 33.00 (6.70 – 56.70) n=63 | <0.001* |
indicates significance
indicates mothers with some college or any degree greater than high school
3.2. Findings within each Placental Pathology Category by Treatment Group
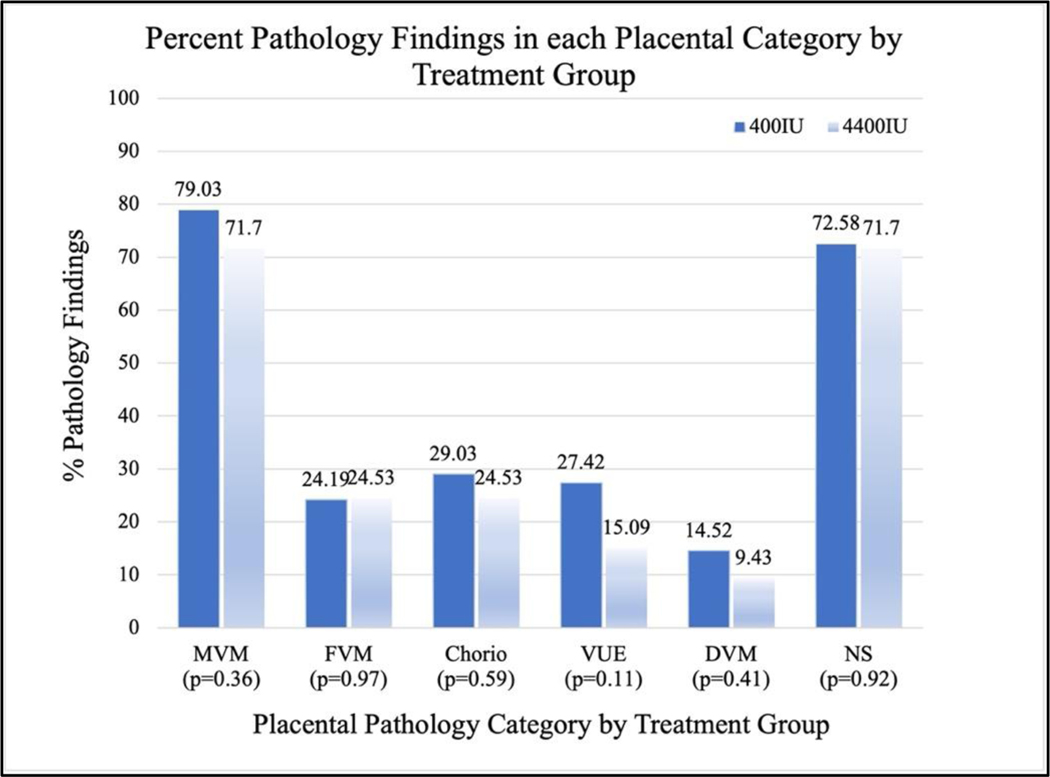
There was no significant difference in the percent of pathology findings between treatment and control groups within their respective placental pathology categories (Figure 3). However, trends in several of the categories showed that placentas in the treatment group had fewer lesions. Additionally, placentas in the treatment group weighed more on average (448.4g) than those in the control group (418.6g) but this finding was not significant (p=0.1183).
Figure 3.
Chi-square analysis was used to determine the potential difference in percent placental pathology findings by treatment group. Trends did not reach statistical significance.
MVM: maternal vascular malperfusion FVM: fetal vascular malperfusion Chorio: acute chorioamnionitis VUE: villitis of unknown etiology DVM: delayed villous maturation NS: nonspecific
3.3. Maternal [25(OH)D] and Associated Findings within each Placental Pathology Category
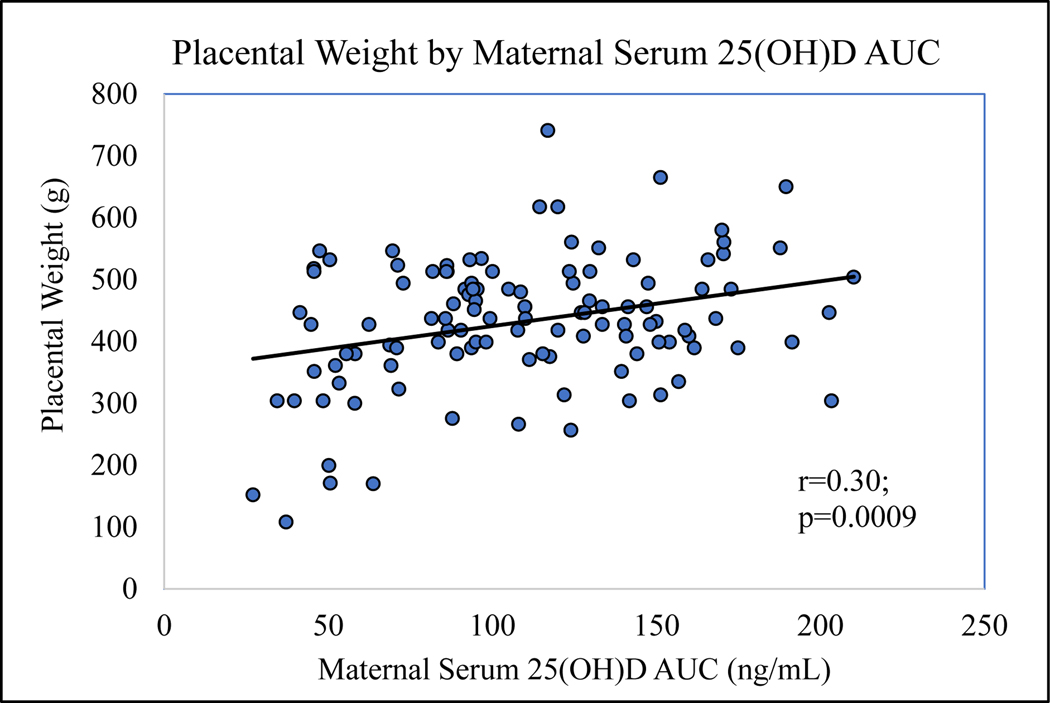
No significant differences were found by t-test between mean serum [25(OH)D] concentration in mothers with versus without placental lesions in each of the placental pathology categories during their first, second, third trimesters, or for AUC. However, placental weight was found to be positively correlated with maternal serum [25(OH)D] during the first, second, third trimesters, and AUC. These correlations were significant for all time points during pregnancy except during the first trimester (p=0.1188, p=0.0078, p=0.0320, and p=0.0009, respectively). The positive and significant correlation with AUC can be seen in Figure 4 (r=0.3, p=0.0009).
Figure 4.
Pearson correlation was used to show that maternal serum [25(OH)D] AUC (representing mean [25(OH)D] over the course of pregnancy) was positively correlated with placental weight (r=0.30; p=0.0009).
3.4. Maternal Characteristics and their Influence on Placental Weight
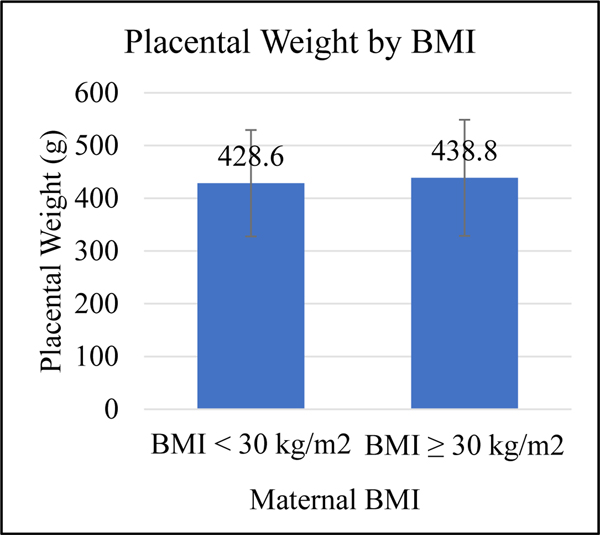
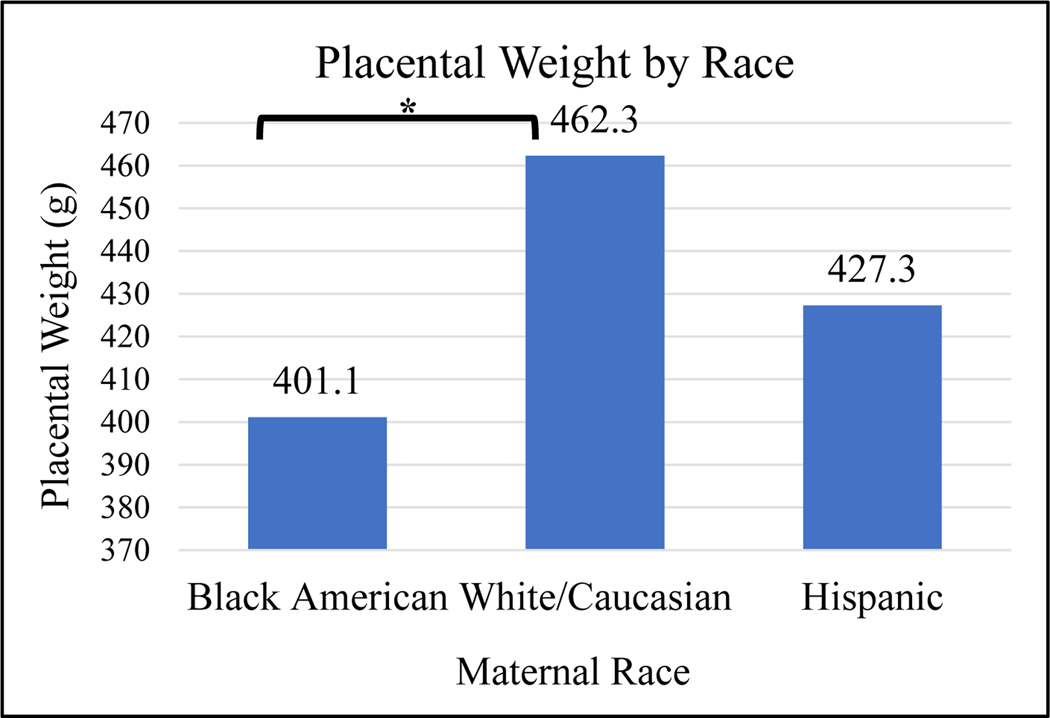
Body mass index (BMI) and race are known influencers of total serum [25(OH)D].26–28 Therefore, these variables were assessed independently with regards to placental weight. When mean placental weight was compared between mothers with baseline BMI less than 30kg/m2 and those mothers with BMI greater than or equal to 30kg/m2, no significant difference was found in a bivariate analysis (p=0.61; Figure 5A). However, mean placental weight was found to be different between the three maternal races, Black American, white/Caucasian, and Hispanic. Placental weights were significantly greater in white/Caucasian mothers than Black American mothers (p=0.03; Figure 5B). Baseline maternal BMI was positively correlated with placental weight but not significantly associated (r=0.45; p=0.7132).
Figure 5A.
Student’s t-test was used to show difference s in mean placental weight and maternal baseline BMI ≥30kg/m2. No significance was found by t-test (p=0.61); however, in regression analysis, the difference is significant (see Table 2).
Figure 5B.
ANOVA was used to show difference in mean placental weight among the races.
*Indicates that Black American mothers have significantly smaller placental weights than white/Caucasian mothers (p=0.03).
3.5. Regression Models and Placental Pathology Categories
Potentially influencing variables such as race/ethnicity, maternal baseline BMI, treatment allocation, and maternal serum [25(OH)D] at each time point were included in logistic regression models. In these regression analyses, parameters included each of the placental pathology categories listed in Table 1 (Supplementary Material). Maternal serum [25(OH)D] AUC remained significantly associated with greater placental weight (p=0.023). Mothers with BMI greater than or equal to 30kg/m2 had significantly larger placental weights (p=0.046; Table 2). Hispanic and white/Caucasian mothers had significantly greater placental weights than Black American mothers (p=0.025; Table 2). Regression models with the parameters for the other placental pathology categories did not show any significant findings with variables race/ethnicity, maternal baseline BMI≥30kg/m2, treatment allocation, and maternal serum [25(OH)D] during first, second, third trimester, or AUC.
Table 2.
The association between [25(OH)D] AUC and placental morphology (placental weight) was determined in a regression model that included maternal race/ethnicity, BMI≥30kg/m2, and treatment group allocation.
| Variable | Beta Estimate | Standard Error | p-value |
|---|---|---|---|
| Black American mothers | −54.5 | 24 | 0.025* |
| Hispanic mothers | −31.5 | 23 | 0.17 |
| Maternal baseline BMI ≥30kg/m2 | 42.1 | 20.9 | 0.046* |
| Maternal serum [25(OH)D] AUC | 0.6 | 0.3 | 0.023* |
| Treatment group | 8.4 | 23.5 | 0.72 |
indicates significant findings.
3.6. Analyses including Placental Weights ≤10th and/or ≥90th Percentile for GA
Placentas less than the 10th or greater than the 90th percentile for gestational age are not always considered healthy.29,30 Given the significant positive correlation found between placental weight and maternal serum [25(OH)D] AUC, we investigated further by examining placentas that were less than the 10th or greater than the 90th percentile for gestational age. When placentas greater than or equal to (≥) the 90th percentile for gestational age, n=7, were removed from the placental pool, a positive correlation was still associated between maternal serum [25(OH)D] AUC and placental weight (p=0.011). In a second logistic regression model of placentas ≥90th percentile for gestational age (n=7) versus placentas <90th percentile (n=108), maternal 25(OH)D AUC was significantly greater in those placentas ≥90th percentile (p=0.03); however, this was not associated with increased perinatal mortality (Table 3, Supplementary Material). No other variables (BMI, race, treatment group allocation) showed a significant difference in their outcomes with regards to the parameter placental weight in these regression models. Mothers with placentas in the ≤10th percentile for gestational age had significantly lower serum [25(OH)D] during the second trimester and AUC than those with placentas in ≥90th percentile when comparing the three groups: ≤10th, 11–89th, and ≥90th, in analysis of variance (p=0.0148; p=0.0198).
For additional analyses regarding the development of COP as well as neonatal associations as they relate to 25(OH)D concentration and placental pathology, please refer to Supplementary Materials.
4. Discussion
A secondary goal of a RCT of participants supplemented with 400IU versus 4400IU vitamin D during pregnancy was determining whether or not maternal vitamin D status during pregnancy was significantly correlated with placental morphology and weight. The biomarker, 25(OH)D, is considered a better proxy for maternal vitamin D status than participant treatment group allocation because treatment allocation is affected more substantially by nonadherence in the high dose treatment group.31–33 The finding that elevated total serum [25(OH)D] during pregnancy is associated with increased placental weight is significant clinically because achieving appropriate placental weight for gestational age is critical for providing the developing fetus with adequate nutrition and waste exchange, and cell signaling. Even when accounting for confounding variables in the regression models, maternal race and BMI also were associated with placental weight. The association between skin color and BMI with serum [25(OH)D] is well known,26,34 but our findings regarding the association between placental weight and 25(OH)D concentration during pregnancy (AUC) are unique and important.
There are risks associated with placental insufficiency (weight <10th percentile for gestational age) and with oversized placentas (weight >90th percentile for gestational age).29 For example, low placental weight (<10th percentile for GA) has been associated with fetal growth restriction (FGR) and hypertensive disorders of pregnancy such as preeclampsia.35 Interestingly, low 25(OH)D concentration has also been associated with development of hypertensive disorders of pregnancy and small for gestational age infants.5,36 In addition, small placentas have been associated with increased risk for stillbirth 29 and may have smaller villous surface areas limiting the potential for nutrient and waste exchange.30 On the contrary, another study determined that after adjusting for birth weight, very large placentas were significantly associated with adverse neonatal outcomes including an APGAR score at five minutes of less than 7, neonatal seizures, and 3 minutes or greater of required ventilation.29
Our study showed that mean maternal serum [25(OH)D] AUC, representing vitamin D status during the course of pregnancy, was significantly correlated with placental weight; however, this included placentas that were greater than the 90th percentile for gestational age. Despite this finding, only 7 placentas out of 115 that were analyzed fell within this group. Maternal and neonatal outcomes can be found in Table 3 (Supplementary Materials). Notably, several of these participants had chronic maternal conditions and/or comorbidities of pregnancy, some of which are known to influence placental (and in some cases neonatal) weights. Four in this group delivered prematurely, but none were associated with neonatal mortality. Additionally, it has been documented that large placentas may be attributed to maternal conditions such as gestational diabetes or anemias.29 Development of a large placenta may also be the result of a response to maternal environmental conditions, such as high altitude or to hypoxic conditions as with cigarette smoking.29 Therefore, it is difficult to attribute a strong association directly with the effects of vitamin D and the outcomes found in participants with placentas weighing ≥90th percentile for gestational age in the present study.
In addition, no differences were found between the treatment and control groups and the frequency of COP development (Table 2, Supplementary Materials). Other studies have found that mothers supplemented with vitamin D, or those found to have greater concentrations of serum 25(OH)D measured during pregnancy, developed fewer COP.3–6,10 Interestingly, elevated maternal serum 25(OH)D concentrations at several time points throughout pregnancy resulted in fewer accounts of urinary tract infection (UTI). This is consistent with the role that vitamin D plays in both innate and adaptive immunity.4,18,19 This was not a primary outcome measure of the present study but an important finding relevant to the care of pregnant women and their fetuses with similar findings represented elsewhere.37 Elevated neonatal cord blood 25(OH)D concentrations also were associated with decreased maternal UTI likely because their serum 25(OH)D was found to be positively correlated with maternal 25(OH)D concentrations. Though no significant difference was determined between the frequency of UTI in each treatment group, a substantial number of women developed UTI during their pregnancies bolstering the power of the aforementioned statistical analyses (n=25; Table 2, Supplementary Materials). This contrasts with the five mothers who developed gestational diabetes and the association with increased neonatal cord blood 25(O)D concentration. Arguably, this finding does not hold much power. Few participants developed COP, except for UTI and bacterial vaginosis (Table 2, Supplementary Materials).
In prior studies, placental weight has been shown to influence neonatal birth weight.38,39 Our study also found that placental weight was significantly positively associated with neonatal birth weight and there were trends that showed neonatal cord blood 25(OH)D concentration was positively associated with greater placental weight. In addition, other studies have explored the influence of maternal parity, fetal sex, and maternal age on fetoplacental weight.40 The long-term implications of hypovitaminosis D during pregnancy and derangements in placental pathology have both been shown to manifest in infantile disease including increased risk of developing chronic lung disease, such as asthma,17 schizophrenia and other neurologic disease.12,15
Hypovitaminosis D has also been associated with numerous pathologies pertaining to pregnancy including preeclampsia associated with impaired extravillous trophoblast invasion,3,4 and decreased immune function.4 The effects of hypovitaminosis D on pregnancy have been explored more fully, but the effects on placental health and function are still being assessed and must continue to be assessed. Trends in the present study showed that the treatment group had fewer placental pathology findings compared to the control group. These trends might lead to significance with a larger sample size, which is important because negative clinical associations can be attributed to placental pathology findings in each category as defined by the 2016 Amsterdam Consensus Criteria.12,14,15,24,25 Some placental pathology findings in the nonspecific category were associated with some COP; pregnancy-induced hypertension, preeclampsia, and urinary tract infection. These findings cannot accurately be associated with clinical outcomes as they are nonspecific and can be attributed to many different pathologies.24
Additionally, in the present study, the average baseline (visit 1) maternal total serum 25(OH)D concentration in the treatment group was 24.83 ng/mL and in the control group was 25.63 ng/mL, both considered insufficient levels (≥20 to <32ng/mL).23 In a population of women with deficient baseline levels of 25(OH)D (<20ng/mL), such as in regions with cooler climate or less sunlight, there is an increased possibility that placental pathology will be more pronounced. Further research is warranted to determine if racial differences influence other aspects of placental pathology as well as placental weight. Addressing factors associated with social determinants of health such as maternal support, insurance status, education, and parity might also reveal trends with placental weight and other aspects of placental pathology.
4.1. Strengths and Limitations
Our study is unique because maternal serum 25(OH)D concentration was measured during each trimester and monthly starting at 10–14 weeks of gestation until delivery (represented by AUC). Neonatal cord blood 25(OH)D concentration also was measured at delivery and used in analyses. A strength of our study is that a significant number of placentas were used in this analysis (n=115), including both term (≥37weeks; n=95) and preterm samples (n=20), but a limitation is that only 115 placentas were analyzed from a possible 402 participants who were enrolled in the RCT, the Kellogg Pregnancy Study. Therefore, significant differences were found between study participant characteristics in those who had their placentas analyzed versus those who did not. Further investigation with a larger sample size and an aim to analyze the placental pathology of all RCT participants should be conducted to ensure validity and generalization. A study with such a cohort is currently underway in Denmark.41 Another strength involves the inclusion of 18 participants with preexisting chronic conditions such as diabetes, hypertension, human immunodeficiency virus, or morbid obesity. These participants did not meet exclusion criteria and were thus, included as part of the main analyses of this secondary study, which also helped with generalizability of the study.
The placental pathology findings of the present study were grouped according to the 2016 Amsterdam Consensus Criteria. Pathologists have been working to reduce the amount of interobserver variability by creating more reproducible diagnostic schema for decades.12,15 In 2015, the Amsterdam Placental Workshop formed a group census to elucidate sampling and diagnostic criteria, and pathologic terminologies and descriptions of the placenta.12 Placental findings were grouped based on vascular pathology (maternal and fetal vascular malperfusion), inflammatory/immune pathology, and delayed villous maturation.12 Studies assessing interobserver reliability are still underway.42 Redline et al. recently published a study that showed reliability was better for inflammatory lesions than vascular lesions.42 Placental weight is an objective finding. Future work might explore differences in pathology findings in placentas delivered at different gestational ages because, as placentas get older, the number of lesions may increase. This study did not take into consideration multiple placental pathology findings simultaneously or COP that developed at the same time and the associated potential adversities found
If maternal nutrition is below sufficient thresholds, development of a healthy placenta and fetus is jeopardized.43 Because the desired result of pregnancy is a healthy placenta and newborn, additional studies could explore neonatal outcomes as they relate to vitamin D status, placental weight, and other placental pathology findings. Prior studies have demonstrated the importance of vitamin D sufficiency during the conception period and its effects on placentation.43,44 Therefore, future studies might determine the effects of vitamin D sufficiency prior to conception and placental pathology outcomes. Moreover, ongoing work needs to be done to fully understand why the extremes of placental weight for gestational age might be associated with adverse perinatal/neonatal outcomes.29
5. Conclusion
The results of the present study, a secondary goal of a RCT, revealed that elevated maternal serum 25(OH)D concentration via vitamin D supplementation during pregnancy did not adversely affect placental morphology as trends showed those in the treatment group had fewer placental lesions. In addition, placental weight was found to be significantly associated with [25(OH)D] AUC, which represents maternal vitamin D status over the course of pregnancy, and neonatal birth weight. Therefore, the importance of adequate vitamin D supplementation and nutrition during pregnancy is supported. The present study emphasized that sustained, increased maternal serum 25(OH)D concentrations over the course of pregnancy are critically important for appropriate placental growth, and elevating maternal serum [25(OH)D] via supplementation during pregnancy is safe. Sociodemographic factors and their effects on placental outcomes warrant further investigation.
Supplementary Material
Highlights:
There are positive effects of vitamin D supplementation on pregnancy outcomes.
Maternal 25(OH)D AUC was significantly associated with greater placental weight.
Mothers with BMI≥30kg/m2 had larger placental weight.
7 placentas ≥90th % for GA were not associated with perinatal mortality.
Increasing maternal 25(OH)D in pregnancy did not adversely affect placenta.
Funding:
Funded in part by W. K. Kellogg Foundation and the SCTR Institute, NIH/NCAT (Grant number UL1 TR000062).
Footnotes
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The Medical University of South Carolina.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The data are available upon request.
Citations:
- 1.Jansen C, Kastelein AW, Kleinrouweler CE, et al. Development of placental abnormalities in location and anatomy. Acta Obstet Gynecol Scand 2020;99(8):983–993. DOI: 10.1111/aogs.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker BC, Hayes DJ, Jones RL. Effects of micronutrients on placental function: evidence from clinical studies to animal models. Reproduction 2018;156(3):R69–R82. DOI: 10.1530/REP-18-0130. [DOI] [PubMed] [Google Scholar]
- 3.Chan SY, Susarla R, Canovas D, et al. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015;36(4):403–9. DOI: 10.1016/j.placenta.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Karras SN, Wagner CL, Castracane VD. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018;86:112–123. DOI: 10.1016/j.metabol.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2013;26(9):889–99. DOI: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 6.Vestergaard AL, Justesen S, Volqvartz T, et al. Vitamin D insufficiency among Danish pregnant women-Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet Gynecol Scand 2021;100(3):480–488. DOI: 10.1111/aogs.14019. [DOI] [PubMed] [Google Scholar]
- 7.Budhwar S, Verma P, Verma R, et al. Altered cord serum 25-hydroxyvitamin D signaling and placental inflammation is associated with pre-term birth. Am J Reprod Immunol 2020;83(2):e13201. DOI: 10.1111/aji.13201. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CL, Baggerly C, McDonnell SL, et al. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. J Steroid Biochem Mol Biol 2015;148:256–60. DOI: 10.1016/j.jsbmb.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol 2011;204(6):556 e1–4. DOI: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta 2010;31(12):1027–34. DOI: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley B, Simner C, Manousopoulou A, et al. Placental uptake and metabolism of 25(OH)vitamin D determine its activity within the fetoplacental unit. Elife 2022;11. DOI: 10.7554/eLife.71094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khong TY, Mooney EE, Ariel I, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016;140(7):698–713. DOI: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 13.Gernand AD, Bodnar LM, Klebanoff MA, Parks WT, Simhan HN. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am J Clin Nutr 2013;98(2):383–8. DOI: 10.3945/ajcn.112.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol 2005;192(2):452–7. DOI: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6(5):435–48. DOI: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood CJ. Recent advances in elucidating the pathogenesis of preterm delivery, the detection of patients at risk, and preventative therapies. Curr Opin Obstet Gynecol 1994;6(1):7–18. (https://www.ncbi.nlm.nih.gov/pubmed/8180354). [PubMed] [Google Scholar]
- 17.He M, Mirzakhani H, Chen L, et al. Vitamin D Sufficiency Has a Limited Effect on Placental Structure and Pathology: Placental Phenotypes in the VDAART Trial. Endocrinology 2020;161(6). DOI: 10.1210/endocr/bqaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD. Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol 2015;224(3):R107–21. DOI: 10.1530/JOE-14-0642. [DOI] [PubMed] [Google Scholar]
- 19.Khatiwada A, Wolf BJ, Mulligan JK, et al. Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr Res 2021;89(3):554–562. DOI: 10.1038/s41390-020-0885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotiros A, Thornhill D, Post MD, Winn VD, Armstrong J. Inflammatory cytokines, placental pathology, and neurological outcomes in infants born to preterm preeclamptic mothers. PLoS One 2021;16(11):e0260094. DOI: 10.1371/journal.pone.0260094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson KK, Parikh HI, Garcia EM, et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J Perinatol 2019;39(6):824–836. DOI: 10.1038/s41372-019-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell AM, Shary JR, Louden C, Ramakrishnan V, Eckard AR, Wagner CL. Association of Bacterial Vaginosis with Vitamin D in Pregnancy: Secondary Analysis from the Kellogg Pregnancy Study. AJP Rep 2019;9(3):e226–e234. DOI: 10.1055/s-0039-1693163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26(10):2341–57. DOI: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015;213(4 Suppl):S21–8. DOI: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 25.Redline RW, Ravishankar S, Bagby CM, Saab ST, Zarei S. Four major patterns of placental injury: a stepwise guide for understanding and implementing the 2016 Amsterdam consensus. Mod Pathol 2021;34(6):1074–1092. DOI: 10.1038/s41379-021-00747-4. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JS, Bowles S, Evans AL. Vitamin D in obesity. Curr Opin Endocrinol Diabetes Obes 2017;24(6):389–394. DOI: 10.1097/MED.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. American journal of perinatology 2010;204(Jul 16):6:556.e1–556.e4. (In Eng). DOI: 10.1016/j.ajog.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton SA, McNeil R, Hollis BW, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. Int J Endocrinol 2010;2010:917428. DOI: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutcheon JA, McNamara H, Platt RW, Benjamin A, Kramer MS. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol 2012;119(6):1251–8. DOI: 10.1097/AOG.0b013e318253d3df. [DOI] [PubMed] [Google Scholar]
- 30.McNamara H, Hutcheon JA, Platt RW, Benjamin A, Kramer MS. Risk factors for high and low placental weight. Paediatr Perinat Epidemiol 2014;28(2):97–105. DOI: 10.1111/ppe.12104. [DOI] [PubMed] [Google Scholar]
- 31.Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up? Int J Endocrinol 2010;2010:631971. DOI: 10.1155/2010/631971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abercrombie M, Shary J, Ebeling M, Hollis B, Wagner C. Analysis of the NICHD Vitamin D Pregnancy Cohort on a Per-Protocol vs. Intent-to-Treat Basis: The Effect of Adherence on Trial Results. Journal of Nutrition & Food Sciences 2018;8(3). DOI: 10.4172/2155-9600.1000696. [DOI] [Google Scholar]
- 33.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014;72(1):48–54. DOI: 10.1111/nure.12090. [DOI] [PubMed] [Google Scholar]
- 34.Karras S, Paschou SA, Kandaraki E, et al. Hypovitaminosis D in pregnancy in the Mediterranean region: a systematic review. Eur J Clin Nutr 2016;70(9):979–86. DOI: 10.1038/ejcn.2016.12. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa J, Arakawa K, Nakamura M, et al. Analysis of placental weight centiles is useful to estimate cause of fetal growth restriction. J Obstet Gynaecol Res 2011;37(11):1658–65. DOI: 10.1111/j.1447-0756.2011.01600.x. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol 2011. (In Eng). DOI: S0002–9378(11)00344–9 [pii] 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haghdoost S, Pazandeh F, Darvish S, Khabazkhoob M, Huss R, Lak TB. Association of serum vitamin D levels and urinary tract infection in pregnant women: A case control study. Eur J Obstet Gynecol Reprod Biol 2019;243:51–56. DOI: 10.1016/j.ejogrb.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Panti AA, Ekele BA, Nwobodo EI, Yakubu A. The relationship between the weight of the placenta and birth weight of the neonate in a Nigerian Hospital. Niger Med J 2012;53(2):80–4. DOI: 10.4103/0300-1652.103547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams LA, Evans SF, Newnham JP. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 1997;314(7098):1864–8. DOI: 10.1136/bmj.314.7098.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flatley C, Sole-Navais P, Vaudel M, et al. Placental weight centiles adjusted for age, parity and fetal sex. Placenta 2022;117:87–94. DOI: 10.1016/j.placenta.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Vestergaard AL, Christensen M, Andreasen MF, Larsen A, Bor P. Vitamin D in pregnancy (GRAVITD) - a randomised controlled trial identifying associations and mechanisms linking maternal Vitamin D deficiency to placental dysfunction and adverse pregnancy outcomes - study protocol. BMC Pregnancy Childbirth 2023;23(1):177. DOI: 10.1186/s12884-023-05484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redline RW, Vik T, Heerema-McKenney A, et al. Interobserver Reliability for Identifying Specific Patterns of Placental Injury as Defined by the Amsterdam Classification. Arch Pathol Lab Med 2022;146(3):372–378. DOI: 10.5858/arpa.2020-0753-OA. [DOI] [PubMed] [Google Scholar]
- 43.Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol 2008;199(6 Suppl 2):S345–56. DOI: 10.1016/j.ajog.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 44.Ganguly A, Tamblyn JA, Finn-Sell S, et al. Vitamin D, the placenta and early pregnancy: effects on trophoblast function. J Endocrinol 2018;236(2):R93–R103. DOI: 10.1530/JOE-17-0491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request.