Abstract
Addressing the need for more equitable cardio-oncology care requires attention to existing disparities in cardio-oncologic disease prevention and outcomes. This is particularly important among those affected by adverse social determinants of health (SDOH). The intricate relationship of SDOH, cancer diagnosis, and outcomes from cardiotoxicities associated with oncologic therapies is influenced by sociopolitical, economic, and cultural factors. Furthermore, mechanisms in cell signaling and epigenetic effects on gene expression link adverse SDOH to cancer and the CVD-related complications of oncologic therapies. To mitigate these disparities, a multifaceted strategy is needed that includes attention to health care access, policy, and community engagement for improved disease screening and management. Interdisciplinary teams must also promote cultural humility and competency and leverage new health technology to foster collaboration in addressing the impact of adverse SDOH in cardio-oncologic outcomes.
Key Words: cardiology, cardio-oncology, care delivery model, disparities, epidemiology, lifestyle risk factors, signaling pathways, social determinants of health
Central Illustration
Highlights
-
•
The impact of SDOH on cardiovascular outcomes among patients with cancer remains understudied.
-
•
Common mechanistic pathways exist between SDOH and cancer therapy–related cardiotoxicities.
-
•
Integration of SDOH into the prevention and management of cardio-oncology outcomes is needed.
Introduction
State of the science
Social determinants of health (SDOH) are the conditions within which people are born, live, work, and age. They reflect systemic, structural (economic and environmental [ie, built, social, and food]), and psychosocial factors that play a critical role in cardiovascular disease (CVD) outcomes.1 For instance, prolonged exposure to adverse SDOH is directly associated with the development of CVD risk factors and poor cardiovascular health behaviors (ie, smoking, physical inactivity, low-quality dietary intake, and poor sleep quality). Moreover, chronic psychosocial and environmental stressors related to adverse SDOH trigger biologic pathways,2 such as activation of the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis, and the neural-hematopoietic axis, to create a proinflammatory milieu that promotes cancer,3 diabetes mellitus,4 and CVD.5
The relationship between SDOH and CVD extends across the life span, and this relationship is true among cancer survivors, particularly childhood cancer survivors. Well-known cardiovascular risk factors among childhood cancer survivors include hypertension, obesity, dyslipidemia, and prediabetes. Similarly, among both childhood and adult survivors of cancer, there is an increased risk of early-onset acquired heart disease, stroke, and arrhythmia.6,7 Childhood cancer survivors are more likely to have elevated blood pressure (38.4% vs control, 30.1%; P = 0.04).8 Moreover, 1 in 8 childhood adult survivors of childhood cancer will experience a life-threatening cardiovascular event within 30 years after oncologic therapy.7,9
Adverse CVD outcomes can either be associated with cancer therapy or occur independently of systemic cancer therapy or radiation therapy.9,10 Prior research suggests that when diagnosed with hypertension, adult survivors of childhood cancers who received chest-directed radiotherapy have a 28 times greater relative risk of coronary artery disease (relative excess risk because of interaction: 27.9; 95% CI: 14.6-51.0) and an 18 times greater relative risk of heart failure (relative excess risk because of interaction: 18.3; 95% CI: 7.6-37.4) compared to survivors without any cardiotoxic or radiation exposure.7 Pediatric and adult childhood cancer survivors are more likely to develop cardiomyopathy, heart failure, and valvular heart disease, whereas adult survivors are more likely to develop coronary artery disease decades before their age-matched peers.7,9 Furthermore, there is an increased CVD mortality risk among non-Hispanic Black (NHB) adults compared to non-Hispanic White (NHW) adults with cancer that mirrors the disproportionate adverse SDOH burden they face11 and this racial difference is attenuated by taking into consideration education and household income.16 This evidence demonstrates the complex interplay between cancer therapy, cardiovascular health, and the urgent need to further expand our understanding of the role of SDOH on CVD outcomes for cancer patients.
Available evidence supports a clear relationship between SDOH and CVD.1 In this review, we apply the health equity framework by Powell-Wiley et al1 to examine current evidence relating SDOH to CVD development in the setting of cancer and cancer therapeutics for both childhood and adult cancer survivors. We identify epidemiologic and translational studies of mechanistic pathways that connect oncologic treatments to CVD and examine the impact of SDOH on these pathways. We highlight SDOH domains salient to cardio-oncology for childhood and adult populations and provide future directions for integrating SDOH into interventions to reduce disparities and promote health equity in cardio-oncologic outcomes.
Disparities in cardio-oncology outcomes
Significant disparities in excess CVD risk related to cancer and specific cancer therapeutics continue to exist in groups traditionally under-represented in biomedical research (Table 1).12 Table 1 illustrates key examples of SDOH domains that have been demonstrated in prior research to impact disparities in cardio-oncology populations. As noted, NHB populations have the highest comorbid age-adjusted CVD- and cancer-related mortality compared to NHW populations in the United States.13 Although racial and ethnic differences in survival among childhood cancer patients are attenuated when adjusting for health insurance and area-level social deprivation index,14 current evidence suggests that patient populations who are exposed to clustered, adverse SDOH over their lifetime are disproportionately burdened by the cardiovascular side effects of cancer and cancer therapeutics. Thus, historically under-represented populations often experience the intersectional effects of several SDOH domains concurrently, which may influence their health-seeking behaviors and CVD outcomes.1,2
Table 1.
Specific SDOH and Impact on Cardio-Oncology Patient Survival and Cardiovascular Health
| SDOH Factor | Example SDOH Measures Used | Impact on CV Health of Oncology Patients |
|---|---|---|
| Structural racism and discrimination | Self-reported race | Among women diagnosed with noninvasive breast cancer who received therapies with potential cardiotoxicity, higher hazards of CVD mortality were observed among NHB women compared with NHW women.133 |
| Social Vulnerability index | Communities with high social vulnerability have higher age-adjusted mortality rates for comorbid cancer and CVD.13 | |
| Education and health care access | Highest level of education attained extracted from death certificates | Increased age-adjusted cancer mortality rates from 1989 to 2018 among individuals with <12 years of education but decreased among those with 12 or more years of education demonstrating an increased cancer mortality gap between the least educated and most educated for most malignancies.32 |
| Socioeconomic factors | County-level poverty | Increased CVD mortality with increased county-level poverty among women diagnosed with cancer from 1987 to 2015.30 |
| SEER definition of having medical coverage: private insurance, Medicare, or military coverage | Presence of non-Medicaid insurance is associated with lower CVD mortality in cancer patients compared to those without insurance or with Medicaid.123 | |
| Neighborhood and built environment | Area deprivation index | Residence in areas with higher area deprivation associated with 5- to 8-fold all-cause death in the St. Jude Lifetime cohort.17 |
| Community context and social risk: food access | County-level percentage of individuals experiencing food insecurity | Increased age-adjusted incidence and mortality with increased food insecurity.44,45 |
| Psychosocial/social environment | The Medical Outcomes Study 36-item Short Form Health Survey version 2 | Obesity, a CVD risk factor, is associated with health-related quality of life among adult survivors of childhood cancer.51 |
CVD = cardiovascular disease; NHB = non-Hispanic Black; NHW = non-Hispanic White; SDOH = social determinants of health; SEER = Surveillance, Epidemiology, and End Results.
Among childhood cancer survivors, NHB populations, Hispanic/Latinx populations, and individuals with lower household income and education are less likely to have health insurance and more likely to have obesity or diabetes.15 NHB childhood cancer survivors are also more likely to have hypertension and other CVD risk factors, whereas Hispanic/Latinx cancer survivors have higher rates of CVD-related deaths.16 Living in a census block group with a high area deprivation index (ADI) (ie, more resource deprived) is independently associated with increased mortality risk among childhood cancer survivors.17,18 The current evidence on disparities among cardio-oncology patients highlights the importance of a life course approach when examining the impact of SDOH on the cardiovascular outcomes related to cancer and its therapeutics.
The role of SDOH domains in cardio-oncologic disparities
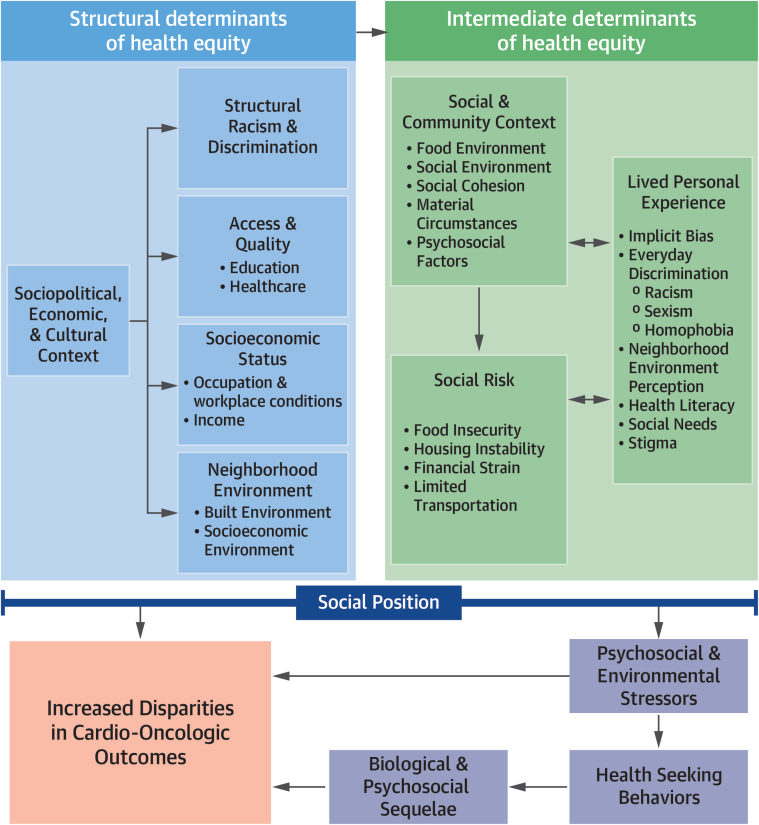
The social determinants of cardio-oncologic health disparities framework (Figure 1) integrates Powell-Wiley et al’s health equity–focused framework1 with the drivers of inequity in cardio-oncology12 to illustrate the increased burden and worsened outcomes relating to the intersection of adverse SDOH with cardiovascular outcomes in patients with cancer. Structural SDOH domains within the framework reflect the sociopolitical, economic, and cultural context (ie, structural racism and discrimination, access to quality education and health care, socioeconomic status [SES], and neighborhood environment) that shape the social risks and lived experience of an individual within a specific social and community context (intermediate SDOH domain).1,19 Overall, these structural and intermediate SDOH domains serve as antecedents that affect chronic stress burden and health-seeking behaviors of individuals. Health-seeking behaviors are goal-oriented actions in pursuit of symptom identification, interpretation, and management.20 These health-seeking behaviors can affect biologic pathways that determine CVD outcomes in patients with cancer. Guided by the proposed framework, we highlight specific examples of SDOH domains that affect health-seeking behaviors and cardio-oncologic outcomes for disparate population groups.
Figure 1.
The Social Determinants of Cardio-Oncologic Disparities
Disparities in cardio-oncologic outcomes are shaped by the trickle-down effects of systemic-, social-, and community-level factors at an individual’s social position. Marginalized individuals who experience the intersectional effects of multiple negative social determinants of health may also experience a double burden of poor cardiovascular disease outcomes and cancer mortality.
Structural racism and discrimination
Structural racism and discrimination represent systemic policies and ideologies that disadvantage under-represented populations, thereby promoting disparities in CVD risk and outcomes among those with cancer. It is perpetuated through the everyday experience of chronic microaggressions and discrimination. Effectively, it may hinder health-seeking behaviors, serving as a barrier to the detection and treatment of early cardiovascular toxicity and related CVD after cancer care in under-represented groups.21 Furthermore, structural racism has been established as a key component of persistent health disparities and adverse CVD outcomes (Table 1). For example, NHB and Hispanic/Latinx patients are more likely to delay preventive medical care, in part because of long-standing policies resulting from structural racism that limit access to education, employment, and health insurance.21 As such, this can potentially increase the burden of both cardiovascular and oncologic diseases. Compared to White individuals, more advanced staging at the time of diagnosis among NHB and Hispanic/Latinx individuals with adult-onset cancers is known to contribute to disparities in cancer-related mortality, although earlier detection may not fully eliminate those disparities.22
Furthermore, Hispanic/Latinx, Asian American, and Pacific Islander individuals residing in communities with high social vulnerability experience higher rates of age-adjusted mortality because of comorbid CVD and cancer compared to those of the same racial and ethnic groups residing in communities with low social vulnerability.13 The social vulnerability index is a composite measure of 4 main themes (ie, neighborhood-level SES,23 household composition and disability, transportation barriers, and minority status and language),23 some of which are markers of existing structural racism.24 Prior research illustrates that NHB women who experienced perceived racism or discrimination are at a significantly greater risk for low-grade chronic inflammation and subsequent CVD development,25,26 both of which could contribute to disparate cardio-oncology outcomes. Similarly, Indigenous Americans face a disproportionate burden of disease driven by long-standing racial and cultural divides, including the lack of accessible medical specialists (ie, cardio-oncologists).27 In parallel, despite limited data, disparities in cardio-oncology care and outcomes are inherent between sex and gender minorities along with immigrant populations within the United States. These are partially influenced by stigma faced in health care and hesitancy to pursue care because of limited trust in medical providers.21,27 More research using diverse populations is critically needed among cardio-oncology patients. Representative population samples will allow for the examination of both patient experiences of structural racism and discrimination as well as the impact of provider-level implicit bias on health care delivery and CVD outcomes. Additionally, clinical trials in oncology and cardiovascular medicine often use nonrepresentative samples, thus limiting trial access for and recruitment of diverse populations.27,28 Consequently, clinical trials may underestimate any effects of inherent structural racism and discrimination within cardio-oncology populations. Hence, special attention to equitable clinical trial enrollment is especially important to comprehensively understand and address structural factors in relation to cardio-oncologic outcomes.
Education and health care access
Lower educational attainment and lack of access to quality health care may further drive inequities within cardio-oncology populations. Several studies have shown that lower educational attainment is associated with a higher risk of CVD events, such as acute myocardial infarction, stroke, and heart failure,29 and others found no clear trends in the associations between educational attainment and cancer mortality.30 Prior research also shows that although the rates of attainment of an associate degree or higher increased from 2010 to 2022 across almost all racial and ethnic groups, the percentage of NHB or Hispanic/Latinx individuals with that degree remained significantly lower than NHW individuals (36% and 34% vs 56%).31 Evidently, although the effect that a patient’s educational attainment has on clinician bias, clinical care, and health outcomes requires further investigation, particularly in minoritized populations, educational level may shape the ability to recognize and describe symptoms or engage and advocate for quality medical care. This, in turn, may result in challenges navigating the health care system; delayed health screenings; limited access to medical care; and, overall, worse short- and long-term outcomes.32 Taken together, this evidence illustrates that marginalized and/or minoritized populations may be disproportionately affected by the adverse effects of a lower educational level.
Despite the exponential expansion of the cardio-oncology field globally, the availability of trained specialists remains limited. Within the United States, access to cardio-oncologists is mainly limited to the urban, coastal areas. These geographic limitations create a disparity in access to specialized cardio-oncology care for a large segment of the population. Rural populations are particularly disadvantaged by geographically inaccessible care, leading to suboptimal cardio-oncologic support. Several studies have established that increased travel time for health care and reduced geographic accessibility are linked to worse cancer and CVD outcomes.27 Conversely, in childhood cancer survivors, residence in high-income or high–physician density communities is associated with increased cardio-oncology referrals and echocardiographic screening.33 Additionally, patients within lower SES strata, those with transportation limitations, or those with medical insurance coverage barriers may not be able to access care even if geographically near specialists.34 Although there are few studies specifically examining these determinants within the cardio-oncology population, the current literature suggests that education and health care access have multilevel effects on quality of life and mortality.
Socioeconomic status
Both childhood and adult cancer patients living in poverty experience higher rates of relapse and death.35, 36, 37 Racial and ethnic inequalities in mortality are influenced by relative SES, including education and job attainment levels, as demonstrated by investigators who have found that racial and ethnic differences in outcomes are associated with the unequal distribution of resources and SES across racial and ethnic groups.21,38 In studies specifically evaluating childhood cancer survivors, NHB and Hispanic/Latinx individuals with lower household income and education were less likely to have health insurance and more likely to have obesity or diabetes,15 both risks for CVD. Among adolescent and young adult (AYA) cancer survivors, living in lower SES neighborhoods was associated with increased CVD events and mortality compared to living in higher SES neighborhoods.39 An even larger retrospective cohort study from the Surveillance, Epidemiology, and End Results registry data (242,940 AYA women and 158,347 AYA men with a broad spectrum of primary malignancies) found that after adjusting for ethnicity and race, increasingly severe degrees of poverty were linked to an increased risk of CVD death.30 Evidently, SES contributes to health inequities within cardio-oncology care and delivery, possibly through its associations with health literacy, resources, and accessibility.
Neighborhood built environment
Environmental SDOH are external factors in an individual’s physical surroundings that can significantly impact their health outcomes and overall well-being. These determinants include access to clean air and water, exposure to environmental toxins, and the built environment. Components of the built environment include access to pedestrian-friendly roads, green space, residential or recreation facility density, and public transportation that provide accessibility and proximity to health care and other social services. Increased rates of cardiovascular risk factors and pre-existing CVD among populations historically under-represented in biomedical research are influenced by these environmental determinants and contribute to increased cardiotoxicity incidence and mortality.34 Similarly, the St. Jude Lifetime Cohort Study, a cohort study of childhood cancer survivors, found that living in a disadvantaged (high ADI) U.S. Census–block group was associated with significant increases in all-cause and health-related late mortality as opposed to living in more advantaged (low ADI) areas.17 Additionally, individuals living in lower SES neighborhoods have increased levels of sedentary behavior and physical inactivity. Thus, neighborhood characteristics are important contributors to cardio-oncologic disparities.22 More work is needed to examine the effects of environmental toxins and natural environment features, particularly in the context of climate change, on CVD outcomes for patients with cancer.
Community context and social risk
Multiple factors contribute to an individual’s social and community context including the food environment, social cohesion, and material circumstances (ie, food security, housing stability, and financial strain).40 In regard to the food environment, access to nutritious food and overall dietary intake have established implications in biological and psychological mechanisms related to an individual’s cardiovascular health, including immune and stress responses.41 Previous literature using a comparative risk assessment model of nationally representative data found that poor dietary factors contributed to about one-half of cardiometabolic deaths.42 Unfortunately, food insecurity disproportionally impacts individuals from socioeconomically, ethnically, and racially disadvantaged backgrounds, which further widens the divide in health outcomes.41 Recent research indicates that NHB and Hispanic/Latinx patients experience a greater health care burden because of a lack of access to nutritious food.43 Particularly, U.S. counties with the greatest amount of food insecurity were found to have not only increased cancer and CVD incidences but also increased mortality rates for both.44,45 Sedentary behavior, which can be partially attributed to the lack of availability and accessibility of recreational facilities, also markedly increases CVD risk in patients with and without cancer.29 In essence, limited access to high-quality food, community-tailored health care resources, and their intersection with other adverse SDOH can lead to delayed diagnoses, inadequate treatment, and poorer outcomes while further widening disparities within cardio-oncology care.
Psychosocial stressors
Factors that impact individuals psychologically may also influence cardiovascular health outcomes in patients with cancer. Longitudinal studies have revealed psychosocial determinants that increase the likelihood of poor cardiovascular health such as adverse childhood experiences,46 chronic psychological stress,47, 48, 49 and depression.50 Numerous studies have also shown that interpersonal connections and a sense of community play a protective role in medical conditions including CVD and cancer.29 Survivors of childhood cancer also report better health-related quality of life scores when there is greater lifetime adherence to cardiovascular health behaviors (lifestyle factors), such as being physically active and choosing a healthy diet.51 Similarly, factors such as cultural values and beliefs, social norms, and faith also influence the self-care, monitoring, and maintenance behaviors of cancer survivors.52 Evidently, several SDOH domains influence not only baseline cardiovascular health factors (ie, body mass index, blood pressure, and cholesterol) but also a cancer survivor’s adherence to and adaptation of healthy lifestyle behaviors (ie, diet, exercise, avoiding smoking or vaping, and quality sleep).53 Although this evidence indicates that the cardio-oncology population experiences the intersectional effects of multiple adverse SDOH, it also illustrates that psychosocial stressors may be a pathway linking SDOH to disparities in cardio-oncologic outcomes.
SDOH and Biological Pathways in Cardio-Oncology
Chronic exposure to adverse SDOH and associated stressors promote the development of CVD by known signaling mechanisms. Few studies have examined the biologic effects of adverse SDOH in cardio-oncology patients; however, there are common signaling pathways related to chronic stress responses relevant in CVD outcomes for patients treated for oncologic conditions.
Signaling mechanisms involved in chronic stress responses, CVD, and cardio-oncology
Chronic psychosocial and environmental stressors (PSES) related to adverse SDOH lead to activation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system as well as the sympatho-adrenomedullary axis.2,54 As summarized in prior reviews, the consequences of activation of the body’s stress response results in an increased release of cortisol and catecholamines such as epinephrine, norepinephrine, and dopamine.2
Although increased circulating levels of cortisol could initially be an anti-inflammatory mechanism, a proinflammatory response can also be observed in the setting of chronic stress.55 This response can occur because of glucocorticoid resistance, which is present in individuals experiencing chronic PSES.2,56 Glucocorticoid resistance leads to a decreased anti-inflammatory function of cortisol and a subsequent increase in proinflammatory cytokine release by immune cells.57 When increasing catecholamine levels activate their respective receptors, intracellular signaling can switch to a noncanonical proinflammatory response, further accelerating inflammation.58,59 A common feature involved in pathways that further accelerates proinflammatory cytokine production is the activation of nuclear factor kappa B. This pathway has been linked to chronic stress,60 CVD,61 and various cancers.62
Proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha are upregulated in individuals with adverse SDOH and chronic PSES. These cytokines are also upregulated in individuals who develop cancer therapy–related cardiovascular toxicities (CTR-CVTs) from select cancer therapeutics including anthracyclines, T-cell therapies, and immune checkpoint inhibitors.1,2,63, 64, 65 Similarly, chronic inflammation plays a role in both CVD and cancer risk.66 In the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) trial, a randomized, double-blinded, placebo-controlled trial of the IL-1β inhibitor canakinumab, treatment significantly reduced recurrent cardiovascular events in patients with high CVD risk and lung cancer incidence and mortality.67
Additionally, mitochondrial dysfunction, related DNA alterations, and cellular apoptosis are most commonly associated with anthracycline-induced cardiomyopathies, along with other CTR-CVTs, such as vascular and cardiac dysfunction from vascular epidermal growth factor receptor tyrosine kinase inhibitors.64,68,69 Mitochondrial DNA (mtDNA)70 is a biomarker of mitochondrial dysfunction measurable in peripheral blood; it has been altered in a variety of diseases71 including CVD72 and cancer.73 In a recent cross-sectional study of 391 adults (183 NHW, 110 Black, and 98 Hispanic), the associations between mitochondrial DNA copy numbers (mtDNAcns) with behavioral factors and depression were investigated; moderation of these associations by sex, race and ethnicity, and age was also studied.74 Although no associations were found between mtDNAcns and depression, a significant relationship between mtDNAcns and smoking was evident, which was more apparent in men and Black study participants.74 mtDNAcn levels in leukocytes have been associated with increasing household density and markers of the built environment in a cohort of Mexican American adults.75 A potential pathway impacting mtDNA release because of increased inflammation involves the activation of nuclear factor kappa B, which was found to actively bind to mtDNA itself and may regulate mitochondrial dynamics.76,77
Clonal hematopoiesis as a common pathway in cancer, adverse cardiovascular outcomes, and chronic PSES
Clonal hematopoiesis (CH) (ie, hematopoietic stem cell clonal expansion) can be the consequence of chronic inflammation, subsequently creating a proinflammatory life cycle for all immune cells. CH may affect immune cells intergenerationally.78 CH is a risk factor for acute myeloid leukemia, chronic lymphocytic leukemia, breast cancer, and myelodysplastic syndromes, among others,79, 80, 81 as well as coronary artery disease and heart failure.81, 82, 83, 84, 85
Somatic sequence variations in 3 epigenetic regulator genes (DNMT3A, TET2, and ASXL1), also known as DTA genes, are frequent genetic sequence variation associated with hematologic cancers and CVD.81,86 Although much of CH can be related to aging and independent of cancer therapies, therapy-related clonal hematopoiesis (t-CH) is commonly found in cancer survivors, highlighting potential mechanisms for CTR-CVTs.87 In an analysis of the DTA genes, as well as JAK2, TP53, SRSF2, and SF3B1, among 393 patients who underwent anthracycline-based acute myeloid leukemia treatment, those with t-CH sequence variants had a 74% increased risk of an adverse cardiovascular event compared to those without t-CH alterations.88 Furthermore, 1 study assessing >30,000 patients diagnosed with solid tumor malignancies found associations between t-CH sequence variants with the use of pyrimidine analogs, tubulin binding agents, and immunotherapy, whereas sequence variants in genes associated with DNA damage response and repair were associated with the use of poly (adenosine phosphatase–ribose) polymerase inhibitor, platinum agents, and anthracyclines.89 TP53, PPM1D (a gene within the damage response and repair pathway), and TET2 have been implicated as possible t-CH sequence variant sites important in the development of cancer therapy–related cardiomyopathy after anthracycline-based therapies (Figure 2).90, 91, 92
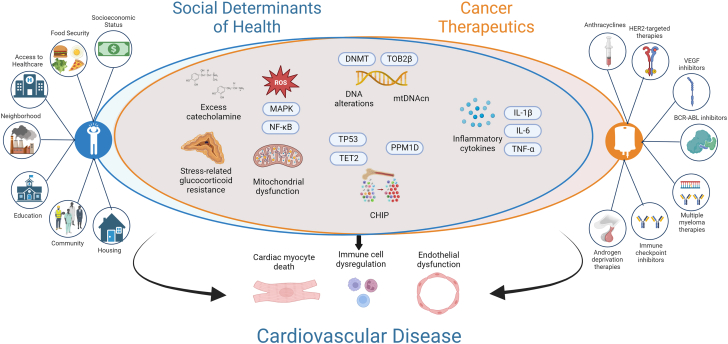
Figure 2.
Commonalities in Signaling Pathways Between SDOH and Cancer Therapeutics in CVD
All pathways mentioned in this figure, but especially the ones common for social determinants of health (SDOH) and cancer therapeutics, have been shown to be crucial for cardiovascular disease (CVD) development along with progression. BCR-ABL1 = breakpoint cluster region-Abelson leukemia gene 1; CHIP = clonal hematopoiesis of indeterminate potential; DNMT = DNA methyltransferase; HER2 = human epidermal growth factor receptor 2; IL = interleukin; MAPK = mitogen-activated protein kinase; mtDNAcn = mitochondrial DNA copy number; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; PPM1D = protein phosphatase, Mg2+/Mn2+ dependent 1D; ROS = reactive oxygen species; TET2 = ten-eleven translocation 2; TOP2β = topoisomerase; TNF = tumor necrosis factor; TP53 = tumor protein 53; VEGF = vascular endothelial growth factor.
To our knowledge, very limited research has examined CH among populations chronically exposed to adverse SDOH. Studies have evaluated the association of healthy weight,93 nutritious diet and eating habits,94 and smoking95 with CH. However, individuals residing in more deprived neighborhoods or individuals of lower SES disproportionally experience obesity,96 exposure to food deserts,97 and nicotine.98 Therefore, it is critical to understand the existence and association of CH in individuals chronically exposed to adverse SDOH. It is important to investigate the underlying mechanisms of CH in adults, particularly in the context of intergenerational trauma, which ultimately affects children and their response to childhood cancer and its therapy.
Gene expression and DNA modification-related mechanisms in chronic stress and cardio-oncology
Whole genome sequencing for genetic variants provides a potential role for predicting cardiac dysfunction postchemo/radiotherapy. Although associations exist between single-nucleotide variation (SNV) and an increased risk of CVD postchemo/radiotherapy, there are no conclusive findings to distinguish low- vs high-risk patients. Specifically, retinoic acid receptor-y SNVs rs2229774 and rs17863833 have been associated with an increased risk of heart failure in childhood cancer survivors who were treated with anthracycline-based therapies.99 It is hypothesized that retinoic acid receptor-y variants influence cardiotoxicity by the suppression of cardioprotective enzymes and mechanism activation post–anthracycline therapy.100 Additionally, studies have shown that truncating variants in the titin gene are highly expressed in patients with cancer therapy–associated cardiomyopathy.101 Individuals with truncating variants in the titin gene and cancer therapy–associated cardiomyopathy were found to be more likely to experience atrial fibrillation, heart failure, and diminished recovery of cardiac function.102 Neither of these studies describe if any differences existed by ancestry, a marker of genetic origin of a population, rather than the social constructs of race or ethnicity.103 Few studies have described differences in European vs African ancestry with chemotherapy-induced cardiomyopathy. Specifically, patients with European ancestry with SNV rs1786814 on the CELF4 gene (a gene regulating messenger ribonucleic acid expression) have a genotype distribution of GG (66%), GA (29%), and AA (4%). GG genotype confers a 10-fold risk for anthracycline-induced cardiomyopathy compared with GA or AA.104 These observed effects are speculated to at least in part be related to the impact of CELF4 loss on the neuronal sodium channel Nav1.6,105 which has been shown to impact heart function.106 In a study using genotype determination of ancestry, survivors of childhood cancer with African ancestry demonstrate a 2.5-fold higher rate of chemotherapy-induced cardiomyopathy compared with those of European descent. This is postulated to be secondary to hypomethylation of the putative homeodomain transcription factor 1. Additionally, the SNV rs6689879∗C variant conferred a 4.2% ejection fraction reduction in survivors of African ancestry and a 5.4-fold cardiomyopathy risk vs those with European ancestry (ejection fraction reduction = 0.4%, 1.31-fold risk).107
Epigenetic mechanisms, which are crucial in transcriptional control of gene expression and implicated in adverse cardio-oncology outcomes, are largely understudied in the context of chronic PSES among cardio-oncology patients.108 At present, most evidence is available on the epigenetic effects of doxorubicin in relation to cardiovascular outcomes.108,109 Recently, a study suggested epigenetic memory after anthracycline therapy may lead to CVD.110 Therefore, it is important to establish overlapping epigenetic pathways related to the effects of cancer therapy, experiences caused by adverse SDOH, and CVD outcomes to identify specific predictors and therapeutic targets for populations at highest risk for cardiotoxicity after cancer treatment. This is particularly critical because therapies targeting epigenetic alterations are under investigation in both cardiovascular and oncologic medicine. Collectively, these findings support the need for the enrollment of diverse patient populations into cancer therapeutic clinical trials that involve sequencing to identify SNVs, genetic variations, and epigenetic markers predictive of CVD outcomes after cancer treatment. Ultimately, one might speculate that common signaling pathways related to both chronic exposure to adverse SDOH and poor CVD outcomes in oncologic patients promote inflammation or immune cell dysfunction; however, data from diverse cohorts of patients with cancer are needed to test these hypotheses.
Integration of SDOH Into Cardio-Oncology Intervention Development
Integrating SDOH into the prevention and management of cardio-oncology outcomes is crucial for comprehensive patient care. The integration of SDOH into cardio-oncology care acknowledges that individuals with cancer and those who experience the cardiac side effects of cancer therapeutics are affected by a complex interplay of factors beyond medical conditions. Multilevel interventions are needed to mitigate the effects of adverse SDOH, improve health outcomes, and enhance equity. An effective approach often involves a combination of individual, community, and health care system–level interventions tailored to the specific social needs and challenges of each patient and community.1 Emphasis should be placed on implementing interventions that promote and facilitate sustainable community engagement. Multilevel approaches must be grounded in cultural humility and patient advocacy to facilitate access to health care, quality health insurance, and improved patient-provider ratios in minoritized and underserved populations (Central Illustration).
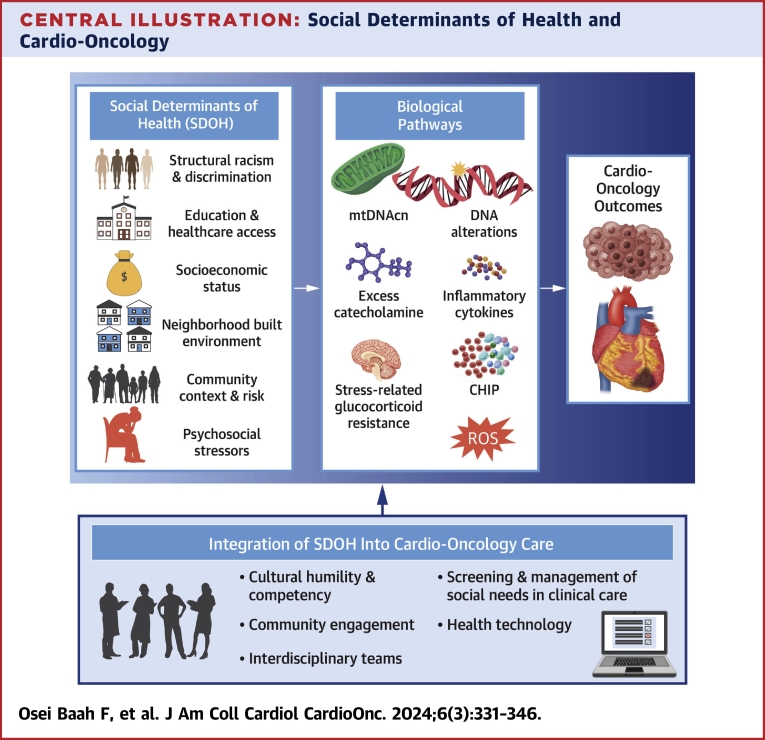
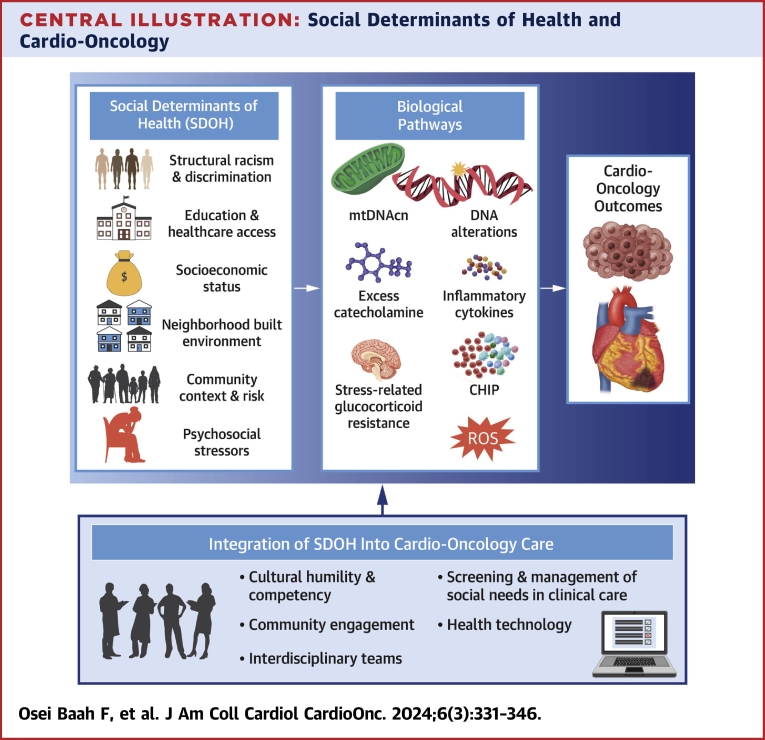
Central Illustration.
Social Determinants of Health and Cardio-Oncology
These social determinants of health (SDOH) domains have been shown to be integral to the development and progression of cardio-oncologic outcomes. Moreover, clonal hematopoiesis, as well as epigenetic (related to mitochondrial DNA copy number and DNA alterations) and cell signaling mechanistic pathways, which influence catecholamines and inflammatory cytokines, further link SDOH to cardio-oncology. It is imperative to consider the integration of SDOH into targeted interventions that encourage health equity within the field of cardio-oncology. The rightward arrow indicates the influence of adverse SDOH on biological pathways. The upward arrow indicates the integration of SDOH into cardio-oncologic care to impact cardio-oncology outcomes. CHIP = clonal hematopoiesis of indeterminate potential; ROS = reactive oxygen species.
Cultural humility and competency
Cultural humility and competence reflect congruent behaviors, attitudes, beliefs, and policies that facilitate effective cross-cultural collaboration.111 Cultural competency includes 4 tenets: 1) awareness (insight into ones’ personal biases, prejudice, and stereotypes toward others); 2) knowledge (of personal cultural norms and beliefs and that of others); 3) attitudes (of respect and acceptance of difference); and 4) skills (that promote interaction with diverse cultures).112 These 4 tenets must permeate all levels of interventions aimed at mitigating the adverse effects of SDOH. Training health care providers to be culturally sensitive and aware of diverse backgrounds can facilitate patient-provider communication and promote trust.113,114 Cultural competency can also include making SDOH training a core competency within the cardio-oncology field.115
Additionally, being aware of the impact of structural racism within communities and how it contributes to health disparities is crucial.12 Structural racism leads to “differential access to the goods, services, and opportunities of society by race,”116 determining societal values and power hierarchies, all of which underlie persistent health disparities in the United States. The presidential advisory statement for the American Heart Association acknowledged and emphasized that a society free from structural racism results in considerable social, economic, and health benefits for all those living in the United States, including those who are direct beneficiaries of the status quo and historically marginalized populations.117 Structural racism contributes to higher rates of CVD and risk factors, under-representation in research, socioeconomic and cultural barriers, and lack of access to specialty care. Efforts to dismantle structural racism include but are not limited to increasing diversity in the health care workforce and leadership, incorporating patient cultural values into treatment plans, and increasing antiracism and cultural competency training through well-developed cultural immersion programs.21
Community engagement
With the increasing awareness of the importance of incorporating SDOH into patient care frameworks, emerging models must identify and address barriers to care at the individual, population, and policy levels. To accomplish this, it is imperative that frameworks be designed leveraging feedback from those with greatest insight into the specific multilevel needs (ie, the cardio-oncology patients and communities most impacted by health inequities driven by adverse SDOH). By recognizing the voices of these communities, medical care recommendations and advocacy policies can be systematically tailored to design impactful, standardized interventions. Furthermore, through active and continuous engagement of community stakeholders, medical providers will not only be able to actively foster trust within the diverse populations they serve but also gain a more nuanced perspective into the difficulties their patients face in obtaining and receiving quality care. Involvement of community advisory boards has been shown to increase the participation of a more diverse group of participants in clinical trials. A study investigating the health and needs related to cardiovascular health and obesity within a cohort of patients in Washington, DC, effectively used a community advisory board to better inform their comprehensive understanding of this community’s needs.118 Thus, community engagement is needed to provide more equitable cardio-oncology care.
Interdisciplinary care teams
Bringing together health care professionals from different disciplines such as clinicians, social workers, psychologists, and nutritionists can provide holistic care addressing both medical and social needs. Clinicians can make patients aware of within-system resources (ie, clinic food pantry and produce prescriptions) and refer to social workers or case managers for other local resources along with eligibility for government food programs (ie, the Special Supplemental Nutrition Program for Women, Infants, and Children). Ideally, each cardio-oncology patient should have access to a multidisciplinary team invested in their well-being. Moreover, “compassionate surveillance” is the “process of working to identify individuals who have been negatively impacted by trauma and other SDOH in order to connect families to necessary resources and supports.” In doing so, providers can identify resource-related barriers to health promotion among cancer survivors and connect patients along with their families to community- based resources.119 Addressing psychosocial trauma related to poverty and community-level stressors is vital.
Addressing social needs and access to health care through policy and advocacy
Collaborating with and investing in community organizations to address social needs such as quality housing, food security and access to high-produce supply stores (stores with healthy food options), insurance options, accessible primary care, employment opportunities, and raising awareness of disparities will contribute to overall well-being and improved treatment outcomes.120 Similar multilevel systems and supports have been proposed for patients with cardiovascular-kidney-metabolic syndrome, underscoring the need to provide consistent and equitable care across all variety of medical specialties.121,122 Moreover, implementing standardized education platforms aimed at not only increasing awareness of cardio-oncology but also improving outcomes within the field is crucial.115
Ensuring equitable access to medical care, including screenings, treatment, and timely follow-up, is critical across the life course. This may include improving transportation options, reducing financial barriers, and offering language assistance services. Financial assistance programs to access insurance or medical expense supplements can alleviate financial stressors that might hinder cardiovascular care for oncology patients. Insurance status was identified as a key determinant of subsequent cardiovascular risk in long-term outcomes after cancer treatment.123 The quality of health insurance has also been found to influence self-care behaviors of community-dwelling individuals with heart failure,124 a possible cardiac complication of cancer therapeutics. This indicates that policy interventions aimed at addressing access to quality health care must also target both insurance type and the quality of insurance to adequately address the health care needs of cardio-oncology patients from historically under-represented groups. Overall, policy interventions that consider and mitigate social needs and health care access may enhance equitable care and improve health outcomes for all cardio-oncology patients.
Screening and management of social needs
Expansion of SDOH to children’s hospitals in the U.S. News and World Report Rankings (USNWR) may encourage hospitals to implement screening of SDOH among childhood cancer survivors. USNWR publishes annual rankings of hospitals and specialties.125,126 For instance, pediatric oncology programs are ranked based on a variety of factors that include survival rates, patient volume, prevention of specific adverse events, quality improvement efforts, and patient supports. In 2022, USNWR published information on the plan to include health equity in rankings. Three new domains were created: access, outcomes, and SDOH. Per Binger et al,127 “the social determinants of health domain examine ways in which hospitals address social conditions that create and exacerbate health inequalities.” These domains were included in the 2022 to 2023 best hospital rankings, although this was only applied to adult hospitals. A reported goal of ranking in these domains was to “incentivize hospitals to compete on measures of equity.”128 An expansion of these health equity ratings to children’s hospitals could incentivize research and spending to address SDOH not only for childhood cancer survivors but for all children. By improving identification of these SDOH, interventions could be designed that would hopefully mitigate disparate cardiac outcomes for childhood cancer survivors experiencing these SDOH.
A second broad approach is to encourage increased adoption of value-based care or alternative payment models for reimbursement. In traditional fee-for-service payment models, both measurement of and interventions to address SDOH are not traditionally reimbursed. However, value-based care and alternative payment models typically have built-in reimbursement related to quality or health outcome.129 A recent cross-sectional study of over 2,800 U.S. hospitals found that hospitals using value-based care models were more likely to implement additional SDOH screening strategies and potentially more efforts to address SDOH.130 Although no payment strategy is perfect, continued advocacy for new reimbursement models that reward those addressing SDOH could reduce barriers to care faced by both childhood and adult cancer survivors.
Health technology for expanding access to cardio-oncologic care
Implementing telehealth services can improve access to care, especially for those in remote or underserved areas, as well as enhance patient monitoring. Incorporating screening tools into electronic health records (EHRs) and using resource navigators are also effective approaches in using health technology to bridge the gaps in care. Furthermore, machine learning can be used to create mortality risk assessments using a composite of risk factors through the EHR. As an example, the classification and regression tree has been used to assess the predictors of mortality among cancer survivors using 56 sociodemographic variables. Analyzing data from 987,009 patients within 2717 U.S. counties, factors such as teen birth, pre-1960 housing (lead paint indicator), ADI, medical factors, household income, number of hospitals, and exposure to particulate matter were determined to be the most important contributors to cardio-oncology mortality.131 With the expanding landscape of technological advancements, integration of such tools into clinical care may increase the efficiency and effectiveness of CVD surveillance and management among cancer survivors.
Future directions in integrating SDOH into cardio-oncology
Moving forward there are many ways to further incorporate SDOH into the field of cardio-oncology (Table 2). Given the well-known limitations in the existing evidence on SDOH-related epidemiologic research (nonrepresentative samples and low-powered studies), the development and validation of SDOH-informed cardio-oncology tools that reflect a diverse population is vital in tailoring interventions that ensure all patients and populations receive equitable care. Subsequently, these standardized measures can be included in artificial intelligence medical tools along with EHRs. In doing so, it may potentially increase awareness of both the direct and indirect impact of SDOH at the individual, community, and policy levels. Health care systems should also allocate resources toward training practitioners and researchers in identifying and addressing barriers to quality care. By expanding knowledge on SDOH, health care providers can better recognize and prioritize specific needs of their patients. In a similar vein, institutions should continue to engage in community partnerships not only to encourage increased participation in research activities but also to gain insight and amplify respective needs. Finally, well-curated data also provide evidence to advocate for policy changes and resource allocation at local, regional, or national levels as well as demonstrate the value for systemic improvements to support population health. Thus, emphasis on SDOH in future patient and community-centered interventions is key in addressing cardio-oncologic health disparities.
Table 2.
Recommendations for Patient and Community-Centered Interventions to Address Cardio-Oncologic Health Disparities
|
|
|
|
|
Abbreviations as in Table 1.
Conclusions
Providing more equitable access to cardio-oncology care in the United States is needed.132 Disparities exist in cardio-oncology prevention and outcomes, especially among minoritized populations and those with adverse SDOH. Complex SDOH domains intersect to influence CVD among patients with cancer and cardiotoxicity. Additionally, mechanisms of adverse SDOH, cancer, and CVD are similar in cell signaling and genetic expression. A comprehensive approach encompassing cultural humility, community engagement, SDOH screening and management, emerging health technology, and interdisciplinary team-based care is needed to decrease disparities in cardio-oncology outcomes.
Funding Support and Author Disclosures
The Powell-Wiley Lab is funded by the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health and the Intramural Research Program of the National Institute on Minority Health and Health Disparities. This research was made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the National Institutes of Health and generous contributions to the Foundation for the National Institutes of Health from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank our study participants; the community advisory board; the Washington, DC, Cardiovascular Health and Obesity Collaborative; and our community for their participation and support. The authors also thank the National Institutes of Health Office of Intramural Training and Education and the Graduate Summer Opportunity to Advance Research program for their trainee support for this project.
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institute on Minority Health and Health Disparities; the National Institutes of Health; or the U.S. Department of Health and Human Services.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Powell-Wiley T.M., Baumer Y., Baah F.O., et al. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumer Y., Pita M.A., Baez A.S., et al. By what molecular mechanisms do social determinants impact cardiometabolic risk? Clin Sci (Lond) 2023;137:469–494. doi: 10.1042/CS20220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad M.H., Rizvi M.A., Fatima M., Mondal A.C. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol Cell Endocrinol. 2021;520 doi: 10.1016/j.mce.2020.111093. [DOI] [PubMed] [Google Scholar]
- 4.Gianotti L., Belcastro S., D’Agnano S., Tassone F. The stress axis in obesity and diabetes mellitus: an update. Endocrines. 2021;2:334–347. [Google Scholar]
- 5.Stiekema L.C.A., Schnitzler J.G., Nahrendorf M., Stroes E.S.G. The maturation of a 'neural- hematopoietic' inflammatory axis in cardiovascular disease. Curr Opin Lipidol. 2017;28:507–512. doi: 10.1097/MOL.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 6.Chow E.J., Chen Y., Armstrong G.T., et al. Underdiagnosis and undertreatment of modifiable cardiovascular risk factors among survivors of childhood cancer. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipshultz E.R., Chow E.J., Doody D.R., et al. Cardiometabolic risk in childhood cancer survivors: a report from the Children’s Oncology Group. Cancer Epidemiol Biomarkers Prev. 2022;31:536–542. doi: 10.1158/1055-9965.EPI-21-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong G.T., Liu Q., Yasui Y., et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer R.A., Aboumsallem J.P., Bracun V., et al. A new classification of cardio-oncology syndromes. Cardiooncology. 2021;7:24. doi: 10.1186/s40959-021-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu C., Shi T., Jiang C., Liu B., Baldassarre L.A., Zarich S. Racial and ethnic disparities in all-cause and cardiovascular mortality among cancer patients in the U.S. J Am Coll Cardiol CardioOnc. 2023;5:55–66. doi: 10.1016/j.jaccao.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Addison D., Branch M., Baik A.H., et al. Equity in cardio-oncology care and research: a scientific statement from the American Heart Association. Circulation. 2023;148:297–308. doi: 10.1161/CIR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 13.Ganatra S., Dani S.S., Kumar A., et al. Impact of social vulnerability on comorbid cancer and cardiovascular disease mortality in the United States. J Am Coll Cardiol CardioOnc. 2022;4:326–337. doi: 10.1016/j.jaccao.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Han X., Zheng Z., et al. Racial/ethnic disparities in childhood cancer survival in the United States. Cancer Epidemiol Biomarkers Prev. 2021;30:2010–2017. doi: 10.1158/1055-9965.EPI-21-0117. [DOI] [PubMed] [Google Scholar]
- 15.Noyd D.H., Liu Q., Yasui Y., et al. Cardiovascular risk factor disparities in adult survivors of childhood cancer compared with the general population. J Am Coll Cardiol CardioOnc. 2023;5:489–500. doi: 10.1016/j.jaccao.2023.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q., Leisenring W.M., Ness K.K., et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34 doi: 10.1200/JCO.2015.66.3567. 1634-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrhardt M.J., Liu Q., Dixon S.B., et al. Association of modifiable health conditions and social determinants of health with late mortality in survivors of childhood cancer. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.55395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schraw J.M., Peckham-Gregory E.C., Rabin K.R., Scheurer M.E., Lupo P.J., Oluyomi A. Area deprivation is associated with poorer overall survival in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28525. [DOI] [PubMed] [Google Scholar]
- 19.Baah F.O., Teitelman A.M., Riegel B. Marginalization: conceptualizing patient vulnerabilities in the framework of social determinants of health-an integrative review. Nurs Inq. 2019;26 doi: 10.1111/nin.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momeni M., Rafii F. Help-seeking behaviour for cancer symptoms: an evolutionary concept analysis. Scand J Caring Sci. 2020;34:807–817. doi: 10.1111/scs.12788. [DOI] [PubMed] [Google Scholar]
- 21.Fazal M., Malisa J., Rhee J.W., Witteles R.M., Rodriguez F. Racial and ethnic disparities in cardio-oncology: a call to action. J Am Coll Cardiol CardioOnc. 2021;3:201–204. doi: 10.1016/j.jaccao.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis L., Canchola A.J., Spiegel D., Ladabaum U., Haile R., Gomez S.L. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency for Toxic Substances and Disease Registry CDC SVI documentation 2018. 2022. https://www.atsdr.cdc.gov/placeandhealth/svi/documentation/SVI_documentation_2018.html
- 24.Pérez-Stable EJ. NIMHD Director Statement in Support of NIH Efforts to Address Structural Racism. 2024. Accessed December 5, 2023. https://www.nimhd.nih.gov/resources/understanding-health-disparities/srd.html
- 25.Simons R.L., Lei M.K., Klopack E., Zhang Y., Gibbons F.X., Beach S.R.H. Racial discrimination, inflammation, and chronic illness among African American women at midlife: support for the weathering perspective. J Racial Ethn Health Disparities. 2021;8:339–349. doi: 10.1007/s40615-020-00786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conway-Phillips R., Dagadu H., Motley D., et al. Qualitative evidence for Resilience, Stress, and Ethnicity (RiSE): a program to address race-based stress among Black women at risk for cardiovascular disease. Complement Ther Med. 2020;48 doi: 10.1016/j.ctim.2019.102277. [DOI] [PubMed] [Google Scholar]
- 27.Patel S.R., Suero-Abreu G.A., Ai A., Ramachandran M.K., Meza K., Florez N. Inequity in care delivery in cardio-oncology: dissecting disparities in underrepresented populations. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1124447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner B.E., Steinberg J.R., Weeks B.T., Rodriguez F., Cullen M.R. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am. 2022;11 doi: 10.1016/j.lana.2022.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obeidat O., Charles K.R., Akhter N., Tong A. Social risk factors that increase cardiovascular and breast cancer risk. Curr Cardiol Rep. 2023;25(10):1269–1280. doi: 10.1007/s11886-023-01957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson C., Lund J.L., Weaver M.A., Wood W.A., Olshan A.F., Nichols H.B. Disparities in mortality from noncancer causes among adolescents and young adults with cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1417–1426. doi: 10.1158/1055-9965.EPI-18-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Statistics NCES Educational Attainment of Young Adults. Condition of Education. 2023 [Google Scholar]
- 32.Barcelo A., Duffett-Leger L., Pastor-Valero M., Pereira J., Colugnati F.A.B., Trapido E. The role of education on Cancer amenable mortality among non-Hispanic blacks and non- Hispanic whites in the United States (1989-2018) BMC Cancer. 2021;21:907. doi: 10.1186/s12885-021-08633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caplin D.A., Smith K.R., Ness K.K., et al. Effect of population socioeconomic and health system factors on medical care of childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Adolesc Young Adult Oncol. 2017;6:74–82. doi: 10.1089/jayao.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohman R.E., Yang E.H., Abel M.L. Inequity in cardio-oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sangaramoorthy M., Shariff-Marco S., Conroy S.M., et al. Joint associations of race, ethnicity, and socioeconomic status with mortality in the multiethnic cohort study. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afshar N., English D.R., Blakely T., et al. Differences in cancer survival by area-level socio- economic disadvantage: a population-based study using cancer registry data. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang R.J., Shah S.C., Camargo M.C., Palaniappan L., Hwang J.H. County rurality and socioeconomic deprivation is associated with reduced survival from gastric cancer in the United States. Gastroenterology. 2020;159:1555–1557.e2. doi: 10.1053/j.gastro.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rod N.H., Bengtsson J., Budtz-Jørgensen E., et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. Lancet. 2020;396:489–497. doi: 10.1016/S0140-6736(20)30621-8. [DOI] [PubMed] [Google Scholar]
- 39.Keegan T.H.M., Kushi L.H., Li Q., et al. Cardiovascular disease incidence in adolescent and young adult cancer survivors: a retrospective cohort study. J Cancer Surviv. 2018;12:388–397. doi: 10.1007/s11764-018-0678-8. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell A.M., Heitz H.K., Leach S.M., Berghuis K.J. Material circumstances, health care access, and self-reported health: a latent class analysis. J Health Psychol. 2023;28:675–689. doi: 10.1177/13591053221132899. [DOI] [PubMed] [Google Scholar]
- 41.Chang R., Javed Z., Taha M., et al. Food insecurity and cardiovascular disease: current trends and future directions. Am J Prev Cardiol. 2022;9 doi: 10.1016/j.ajpc.2021.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Micha R., Peñalvo J.L., Cudhea F., Imamura F., Rehm C.D., Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317:912–924. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt E.J., Mozaffarian D., Leung C.W., Berkowitz S.A., Murthy V.L. Diet and food and nutrition insecurity and cardiometabolic disease. Circ Res. 2023;132:1692–1706. doi: 10.1161/CIRCRESAHA.123.322065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen K.Y., Blackford A.L., Hussaini S.M.Q. County-level food insecurity to predict cancer incidence and mortality in the United States, 2015-2020. J Clin Oncol. 2023;41 10539-10539. [Google Scholar]
- 45.Alexander G.K., Bashore L., Brooks V. Improving food literacy and access among young adult cancer survivors: a cross-sectional descriptive study. Cancer Nurs. 2022;45:161–166. doi: 10.1097/NCC.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 46.Deschênes S.S., Graham E., Kivimäki M., Schmitz N. Adverse Childhood experiences and the risk of diabetes: examining the roles of depressive symptoms and cardiometabolic dysregulations in the Whitehall II cohort study. Diabetes Care. 2018;41:2120–2126. doi: 10.2337/dc18-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Everson-Rose S.A., Roetker N.S., Lutsey P.L., et al. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2318–2323. doi: 10.1161/STROKEAHA.114.004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohlin B., Nilsson P.M., Nilsson J.A., Berglund G. Chronic psychosocial stress predicts long- term cardiovascular morbidity and mortality in middle-aged men. Eur Heart J. 2004;25:867–873. doi: 10.1016/j.ehj.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Stewart R.A.H., Colquhoun D.M., Marschner S.L., et al. Persistent psychological distress and mortality in patients with stable coronary artery disease. Heart. 2017;103:1860–1866. doi: 10.1136/heartjnl-2016-311097. [DOI] [PubMed] [Google Scholar]
- 50.Wu Q., Kling J.M. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang F.F., Hudson M.M., Huang I.C., et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2018;124:3918–3923. doi: 10.1002/cncr.31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeom J.W., Yeom I.S., Park H.Y., Lim S.H. Cultural factors affecting the self-care of cancer survivors: an integrative review. Eur J Oncol Nurs. 2022;59 doi: 10.1016/j.ejon.2022.102165. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life’s Essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell G., Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15:525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 55.Barnes P.J., Adcock I.M. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 56.Cohen S., Janicki-Deverts D., Doyle W.J., et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh C.P., Ewing L.J., Cleary J.L., et al. Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain Behav Immun. 2018;69:364–373. doi: 10.1016/j.bbi.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh C.P., Bovbjerg D.H., Marsland A.L. Glucocorticoid resistance and beta2-adrenergic receptor signaling pathways promote peripheral pro-inflammatory conditions associated with chronic psychological stress: a systematic review across species. Neurosci Biobehav Rev. 2021;128:117–135. doi: 10.1016/j.neubiorev.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole S.W. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole S.W., Hawkley L.C., Arevalo J.M., Sung C.Y., Rose R.M., Cacioppo J.T. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumori A. Nuclear factor-κB is a prime candidate for the diagnosis and control of inflammatory cardiovascular disease. Eur Cardiol Rev. 2023;18 doi: 10.15420/ecr.2023.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiordelisi A., Iaccarino G., Morisco C., Coscioni E., Sorriento D. NFkappaB is a key player in the crosstalk between inflammation and cardiovascular diseases. Int J Mol Sci. 2019;20(7):1599. doi: 10.3390/ijms20071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganatra S., Dani S.S., Yang E.H., Zaha V.G., Nohria A. Cardiotoxicity of T-cell antineoplastic therapies: JACC: CardioOncology primer. J Am Coll Cardiol CardioOnc. 2022;4:616–623. doi: 10.1016/j.jaccao.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clayton Z.S., Brunt V.E., Hutton D.A., et al. Tumor necrosis factor alpha-mediated inflammation and remodeling of the extracellular matrix underlies aortic stiffening induced by the common chemotherapeutic agent doxorubicin. Hypertension. 2021;77:1581–1590. doi: 10.1161/HYPERTENSIONAHA.120.16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leiva O., AbdelHameid D., Connors J.M., Cannon C.P., Bhatt D.L. Common pathophysiology in cancer, atrial fibrillation, atherosclerosis, and thrombosis: JACC: CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2021;3:619–634. doi: 10.1016/j.jaccao.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 68.Eaton H., Timm K.N. Mechanisms of trastuzumab induced cardiotoxicity - is exercise a potential treatment? Cardiooncology. 2023;9:22. doi: 10.1186/s40959-023-00172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stuhlmiller T.J., Zawistowski J.S., Chen X., et al. Kinome and transcriptome profiling reveal broad and distinct activities of erlotinib, sunitinib, and sorafenib in the mouse heart and suggest cardiotoxicity from combined signal transducer and activator of transcription and epidermal growth factor receptor inhibition. J Am Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClintock C.R., Mulholland N., Krasnodembskaya A.D. Biomarkers of mitochondrial dysfunction in acute respiratory distress syndrome: a systematic review and meta-analysis. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.1011819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castellani C.A., Longchamps R.J., Sun J., Guallar E., Arking D.E. Thinking outside the nucleus: mitochondrial DNA copy number in health and disease. Mitochondrion. 2020;53:214–223. doi: 10.1016/j.mito.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L., Zhang Q., Yuan K., Yuan J. mtDNA in the pathogenesis of cardiovascular diseases. Dis Markers. 2021;2021 doi: 10.1155/2021/7157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X., Zhan L., Chen Y., et al. Increased mtDNA copy number promotes cancer progression by enhancing mitochondrial oxidative phosphorylation in microsatellite-stable colorectal cancer. Signal Transduct Target Ther. 2018;3:8. doi: 10.1038/s41392-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vyas C.M., Ogata S., Reynolds C.F., III, et al. Lifestyle and behavioral factors and mitochondrial DNA copy number in a diverse cohort of mid-life and older adults. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao H., Shen J., Leung E., Zhang X., Chow W.H., Zhang K. Leukocyte mitochondrial DNA copy number and built environment in Mexican Americans: a cross-sectional study. Sci Rep. 2020;10 doi: 10.1038/s41598-020-72083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laforge M., Rodrigues V., Silvestre R., et al. NF-κB pathway controls mitochondrial dynamics. Cell Death Differ. 2016;23:89–98. doi: 10.1038/cdd.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kracht M., Müller-Ladner U., Schmitz M.L. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling. J Allergy Clin Immunol. 2020;146:694–705. doi: 10.1016/j.jaci.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 78.von Bonin M., Jambor H.K., Teipel R., et al. Clonal hematopoiesis and its emerging effects on cellular therapies. Leukemia. 2021;35:2752–2758. doi: 10.1038/s41375-021-01337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S.J., Bejar R. Clonal hematopoiesis in cancer. Exp Hematol. 2020;83:105–112. doi: 10.1016/j.exphem.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gibson C.J., Fell G., Sella T., et al. Clonal hematopoiesis in young women treated for breast cancer. Clin Cancer Res. 2023;29:2551–2558. doi: 10.1158/1078-0432.CCR-23-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Genovese G., Kähler A.K., Handsaker R.E., et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Natarajan P., Jaiswal S., Kathiresan S. Clonal hematopoiesis: somatic mutations in blood cells and atherosclerosis. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rauch P.J., Silver A.J., Gopakumar J., et al. Loss-of-function mutations in Dnmt3a and Tet2 lead to accelerated atherosclerosis and convergent macrophage phenotypes in mice. Blood. 2018;132:745. [Google Scholar]
- 85.Sikking MA, Stroeks S, Henkens M, et al. Clonal hematopoiesis has prognostic value in dilated cardiomyopathy independent of age and clone size. J Am Coll Cardiol HF. 2024;12(5):905-914. https://doi.org/10.1016/j.jchf.2023.06.037 [DOI] [PubMed]
- 86.Fuster J.J., Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018;122:523–532. doi: 10.1161/CIRCRESAHA.117.312115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Natarajan P. Genomic Aging, clonal hematopoiesis, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2023;43:3–14. doi: 10.1161/ATVBAHA.122.318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calvillo-Argüelles O., Schoffel A., Capo-Chichi J.M., et al. Cardiovascular disease among patients with AML and CHIP-related mutations. J Am Coll Cardiol CardioOnc. 2022;4:38–49. doi: 10.1016/j.jaccao.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sonnenschein A., Moore E., Lo C., et al. Novel associations between clonal hematopoiesis and therapeutic exposures revealed in patients with solid tumors using real world evidence. Blood. 2022;140:2879–2880. [Google Scholar]
- 90.Sano S., Wang Y., Ogawa H., et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight. 2021;6(13) doi: 10.1172/jci.insight.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yura Y., Miura-Yura E., Katanasaka Y., et al. The cancer therapy-related clonal hematopoiesis driver gene ppm1d promotes inflammation and non-ischemic heart failure in mice. Circ Res. 2021;129:684–698. doi: 10.1161/CIRCRESAHA.121.319314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hatakeyama K., Hieda M., Semba Y., et al. TET2 clonal hematopoiesis is associated with anthracycline-induced cardiotoxicity in patients with lymphoma. J Am Coll Cardiol CardioOnc. 2022;4:141–143. doi: 10.1016/j.jaccao.2022.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haring B., Reiner A.P., Liu J., et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the Women’s Health Initiative. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharya R., Zekavat S.M., Uddin M.M., et al. Association of diet quality with prevalence of clonal hematopoiesis and adverse cardiovascular events. JAMA Cardiol. 2021;6:1069–1077. doi: 10.1001/jamacardio.2021.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levin M.G., Nakao T., Zekavat S.M., et al. Genetics of smoking and risk of clonal hematopoiesis. Sci Rep. 2022;12:7248. doi: 10.1038/s41598-022-09604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powell-Wiley T.M., Cooper-McCann R., Ayers C., et al. Change in neighborhood socioeconomic status and weight gain: Dallas Heart Study. Am J Prev Med. 2015;49:72–79. doi: 10.1016/j.amepre.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honório O.S., Pessoa M.C., Gratão L.H.A., et al. Social inequalities in the surrounding areas of food deserts and food swamps in a Brazilian metropolis. Int J Equity Health. 2021;20:168. doi: 10.1186/s12939-021-01501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen A., Machiorlatti M., Krebs N.M., Muscat J.E. Socioeconomic differences in nicotine exposure and dependence in adult daily smokers. BMC Public Health. 2019;19:375. doi: 10.1186/s12889-019-6694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ehrhardt M.J., Leerink J.M., Mulder R.L., et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2023;24:e108–e120. doi: 10.1016/S1470-2045(23)00012-8. [DOI] [PubMed] [Google Scholar]
- 100.Magdy T., Jiang Z., Jouni M., et al. RARG variant predictive of doxorubicin-induced cardiotoxicity identifies a cardioprotective therapy. Cell Stem Cell. 2021;28:2076–2089.e7. doi: 10.1016/j.stem.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y., Wang Y., Han X., et al. Cardio-oncology: a myriad of relationships between cardiovascular disease and cancer. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.727487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia-Pavia P., Kim Y., Restrepo-Cordoba M.A., et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. 2019;140:31–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu C., Ahmed R., Lamri A., Anand S.S. Use of race, ethnicity, and ancestry data in health research. PLOS Glob Public Health. 2022;2 doi: 10.1371/journal.pgph.0001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X., Sun C.L., Quiñones-Lombraña A., et al. CELF4 variant and anthracycline-related cardiomyopathy: a Children’s Oncology Group genome-wide association study. J Clin Oncol. 2016;34:863–870. doi: 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagnon J.L., Briese M., Sun W., et al. CELF4 regulates translation and local abundance of a vast set of mRNAs, including genes associated with regulation of synaptic function. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noujaim S.F., Kaur K., Milstein M., et al. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J. 2012;26:63–72. doi: 10.1096/fj.10-179770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sapkota Y., Qin N., Ehrhardt M.J., et al. Genetic variants associated with therapy-related cardiomyopathy among childhood cancer survivors of African ancestry. Cancer Res. 2021;81:2556–2565. doi: 10.1158/0008-5472.CAN-20-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papazoglou P., Peng L., Sachinidis A. Epigenetic mechanisms involved in the cardiovascular toxicity of anticancer drugs. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.658900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tantawy M., Pamittan F.G., Singh S., Gong Y. Epigenetic changes associated with anthracycline-induced cardiotoxicity. Clin Transl Sci. 2021;14:36–46. doi: 10.1111/cts.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robinson E.L., Ameri P., Delrue L., et al. Differential expression of epigenetic modifiers in early and late cardiotoxic heart failure reveals DNA methylation as a key regulator of cardiotoxicity. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.884174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pokharel B., Yelland J., Hooker L., Taft A. A systematic review of culturally competent family violence responses to women in primary care. Trauma Violence Abuse. 2023;24:928–945. doi: 10.1177/15248380211046968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brottman M.R., Char D.M., Hattori R.A., Heeb R., Taff S.D. Toward cultural competency in health care: a scoping review of the diversity and inclusion education literature. Acad Med. 2020;95:803–813. doi: 10.1097/ACM.0000000000002995. [DOI] [PubMed] [Google Scholar]
- 113.Beach M.C., Price E.G., Gary T.L., et al. Cultural competence: a systematic review of health care provider educational interventions. Med Care. 2005;43:356–373. doi: 10.1097/01.mlr.0000156861.58905.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paez K.A., Allen J.K., Beach M.C., Carson K.A., Cooper L.A. Physician cultural competence and patient ratings of the patient-physician relationship. J Gen Intern Med. 2009;24:495–498. doi: 10.1007/s11606-009-0919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alvarez-Cardona J.A., Ray J., Carver J., et al. Cardio-oncology education and training: JACC council perspectives. J Am Coll Cardiol. 2020;76:2267–2281. doi: 10.1016/j.jacc.2020.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones C.P. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Churchwell K., Elkind M.S.V., Benjamin R.M., et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 118.Powell-Wiley T.M. Centering patient voices through community engagement in cardiovascular research. Circulation. 2023;147:105–107. doi: 10.1161/CIRCULATIONAHA.122.061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapke T.L., Karst J., LiaBraaten B., et al. Family caregiver acceptability of assessing caregiver adverse childhood experiences (ACEs) and distress in pediatric specialty care. Children (Basel) 2023;10(2):382. doi: 10.3390/children10020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mensah G.A., Cooper R.S., Siega-Riz A.M., et al. Reducing cardiovascular disparities through community-engaged implementation research: a National Heart, Lung, and Blood Institute workshop report. Circ Res. 2018;122:213–230. doi: 10.1161/CIRCRESAHA.117.312243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ndumele C.E., Neeland I.J., Tuttle K.R., et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. 2023;148(20):1636–1664. doi: 10.1161/CIR.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 122.Ndumele C.E., Rangaswami J., Chow S.L., et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606–1635. doi: 10.1161/CIR.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 123.Shi T., Jiang C., Zhu C., Wu F., Fotjhadi I., Zarich S. Insurance disparity in cardiovascular mortality among non-elderly cancer survivors. Cardiooncology. 2021;7:11. doi: 10.1186/s40959-021-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osei Baah F., Brawner B.M., Teitelman A.M., Ruger J.P., Riegel B. A mixed-methods study of social determinants and self-care in adults with heart failure. J Cardiovasc Nurs. 2023;38:555–567. doi: 10.1097/JCN.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.U.S. News & World Report Best children’s hospitals for cancer. https://health.usnews.com/best-hospitals/pediatric-rankings/cancer
- 126.U.S. News & World Report U.S. News best hospitals. 2023. https://health.usnews.com/best-hospitals
- 127.Binger T., Chen H., Harder B. Hospital rankings and health equity. JAMA. 2022;328:1805–1806. doi: 10.1001/jama.2022.19001. [DOI] [PubMed] [Google Scholar]
- 128.Binger T., Chen H., Adams Z., et al. U.S. News & World Report 2022-2023 Best Hospitals Health Equity Measures. https://health.usnews.com/media/best-hospitals/Best-Hospitals-Health-Equity-2022-23
- 129.Melzer S.M. Addressing social determinants of health in pediatric health systems: balancing mission and financial sustainability. Curr Opin Pediatr. 2022;34:8–13. doi: 10.1097/MOP.0000000000001083. [DOI] [PubMed] [Google Scholar]
- 130.Ashe J.J., Baker M.C., Alvarado C.S., Alberti P.M. Screening for health-related social needs and collaboration with external partners among US hospitals. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.30228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Motairek I., Dong W., Salerno P.R., et al. Geographical patterns and risk factor association of cardio-oncology mortality in the United States. Am J Cardiol. 2023;201:150–157. doi: 10.1016/j.amjcard.2023.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X., Fluchel M.N., Kirchhoff A.C., Zhu H., Onega T. Geographic access to pediatric cancer care in the US. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.51524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Collin L.J., Troeschel A.N., Liu Y., et al. A balancing act: racial disparities in cardiovascular disease mortality among women diagnosed with breast cancer. Ann Cancer Epidemiol. 2020;4:4. doi: 10.21037/ace.2020.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]