Abstract
Background
Cardiovascular disease (CVD) is a significant cause of morbidity and mortality in men with prostate cancer; however, data on racial disparities in CVD outcomes are limited.
Objectives
We quantified the disparities in CVD according to self-identified race and the role of the structural social determinants of health in mediating disparities in prostate cancer patients.
Methods
A retrospective cohort study of 3,543 prostate cancer patients treated with systemic androgen deprivation therapy (ADT) between 2008 and 2021 at a quaternary, multisite health care system was performed. The multivariable adjusted association between self-reported race (Black vs White) and incident major adverse cardiovascular events (MACE) after ADT initiation was evaluated using cause-specific proportional hazards. Mediation analysis determined the role of theme-specific and overall social vulnerability index (SVI) in explaining the racial disparities in CVD outcomes.
Results
Black race was associated with an increased hazard of MACE (HR: 1.38; 95% CI: 1.16-1.65; P < 0.001). The association with Black race was strongest for incident heart failure (HR: 1.79; 95% CI: 1.32-2.43), cerebrovascular disease (HR: 1.98; 95% CI: 1.37-2.87), and peripheral artery disease (HR: 1.76; 95% CI: 1.26-2.45) (P < 0.001). SVI, specifically the socioeconomic status theme, mediated 98% of the disparity in MACE risk between Black and White patients.
Conclusions
Black patients are significantly more likely to experience adverse CVD outcomes after systemic ADT compared with their White counterparts. These disparities are mediated by socioeconomic status and other structural determinants of health as captured by census tract SVI. Our findings motivate multilevel interventions focused on addressing socioeconomic vulnerability.
Key Words: cardio-oncology, disparity, electronic health records, prostate cancer
Central Illustration
According to U.S. population data, death rates are higher in Black men with cancer compared with other racial groups, with several studies demonstrating worse overall survival in Black patients after a prostate cancer diagnosis.1, 2, 3, 4 A growing body of evidence suggests that the morbidity from nonprostate cancer–specific causes contributes considerably to overall survival disparities after prostate cancer diagnosis.4,5 Structural racism and the social determinants of health (SDOH) play critical roles in shaping these disparities, influencing access to health care, housing, socioeconomic status, and quality of care.6, 7, 8 However, limited data exist on the impact of SDOH on the noncancer causes that drive these differences in outcomes.
Cardiovascular disease (CVD) is a major cause of noncancer-specific morbidity and mortality in prostate cancer.9 In particular, systemic androgen deprivation therapy (ADT), commonly used in the treatment of men with prostate cancer both in the localized and metastatic disease settings, is associated with adverse metabolic and cardiovascular (CV) effects.10, 11, 12, 13 Observational studies indicate that ADT exposure increases the risk of adverse CV events, including myocardial infarction, stroke, heart failure, and CV death, particularly in patients with pre-existing CVD or multiple CV risk factors.14, 15, 16, 17, 18, 19
Thus, we sought to answer the following questions: What are the differences in major adverse cardiovascular events (MACE) in men with prostate cancer receiving ADT as a function of race (a social construct referencing diverse sets of people)? If differences exist, to what extent do the structural SDOH mediate and potentially explain those differences? In this study, we defined MACE as heart failure, coronary heart disease, cerebrovascular disease, peripheral artery disease, ventricular tachycardia/ventricular fibrillation, and CV mortality.
Methods
Study population
We performed a retrospective cohort study of prostate cancer patients treated with gonadotropin-releasing hormone (GnRH) agonist– or GnRH antagonist–based ADT at the University of Pennsylvania Health System (UPHS) between 2008 and 2021. UPHS is a 6-hospital, multisite, quaternary care academic medical center serving more than 1 million patients per year from urban and suburban areas mostly in the Greater Philadelphia region (Southeast Pennsylvania, Central and Southern New Jersey, and Delaware).20 The study was approved and informed consent requirement was waived by the Institutional Review Board of the University of Pennsylvania. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Patients with a prostate cancer diagnosis and at least 1 prescription of a GnRH agonist, GnRH antagonist, first-generation antiandrogen, abiraterone, or novel antiandrogen treated at UPHS-affiliated hospitals were identified (Supplemental Table 1). Detailed information including ADT and related medication orders, demographics, vital signs, laboratory measurements, CV diagnosis and procedure codes from inpatient and outpatient encounters, and CV medications (Supplemental Table 2) were extracted from Penn Data Store, the enterprise data warehouse for UPHS that contains aggregated longitudinal clinical data from the Epic electronic medical record (EMR) (Epic Systems Corporation) and other clinical source systems. Baseline was defined by the ADT start date. To ensure that the ADT start date was defined accurately, only nonhistorical, prescribed medication orders were considered; patients were required to have a prostate cancer–related encounter with oncology or urology providers before the earliest ADT medication order date, and manual patient chart review was performed by trained physicians if this was unclear or missing. Patients were excluded if: 1) there was no evidence of GnRH agonist or antagonist receipt; 2) ascertainment of the ADT start date was not possible; or 3) there was an observable window of <30 days between the last UPHS encounter and the ADT start date (Supplemental Figure 1).
CV risk factors and disease
Prevalent CV risk factors (hypertension, diabetes, and hyperlipidemia) and CVD (heart failure, coronary heart disease, cerebrovascular disease, and peripheral artery disease) were ascertained based on the presence of International Classification of Diseases-9th Revision-Clinical Modification and International Classification of Diseases-10th Revision-Clinical Modification diagnosis codes during 1 inpatient or 2 outpatient encounters before ADT start. Medications (for hypertension, diabetes, and hyperlipidemia), laboratory measurements (for diabetes), and revascularization procedures (for coronary heart disease and peripheral artery disease) were also used to define CV risk factors and CVD (Supplemental Table 3).21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Ventricular tachycardia/fibrillation was ascertained based on 1 inpatient or outpatient diagnosis code.34
Social determinants of health
The Centers for Disease Control and Prevention social vulnerability index (SVI), a composite structural SDOH measure, was used.35 The calculations are based on the relative rankings of census tracts in the United States on 15 social factors across socioeconomic status (below poverty, unemployed, income, and no high school diploma), household composition and disability (age 65 years or older, aged 17 years or younger, older than age 5 years with a disability, and single-parent households), minority status and language (minority and speak English “less than well”), and housing type and transportation (multiunit structures, mobile homes, crowding, no vehicle, and group quarters) themes using American Community Survey data. To obtain SVI data, the residential address at ADT start was geocoded using ArcGIS Pro version 3.0 (ESRI). Census tract SVI rankings were extracted from the Agency for Toxic Substances and Disease Registry by matching census tract geocodes (Supplemental Figure 2).
Vital status
The National Death Index was used to ascertain vital status and obtain the underlying cause of death (Supplemental Methods).36 Diagnosis codes between I00 and I99 defined CV-related deaths; C61 defined death from prostate cancer.37
Study outcomes
The primary outcome was time to first MACE including heart failure, coronary heart disease, cerebrovascular disease, peripheral artery disease, ventricular tachycardia/ventricular fibrillation, and CV mortality. Individual components of the MACE outcome were evaluated as secondary outcomes. To define incident CV events in patients without pre-existing CVD, International Classification of Diseases-Ninth Revision-Clinical Modification and International Classification of Diseases-10th Revision-Clinical Modification diagnosis codes during 1 inpatient or 2 outpatient encounters were used. In those with pre-existing CVD, to account for the possibility that diagnosis codes could represent instances of follow-up encounters rather than true events, only primary inpatient diagnosis codes were used (Supplemental Table 4).38 Prostate cancer mortality was analyzed as an exploratory outcome (Supplemental Methods).
Statistical analysis
Baseline characteristics were summarized using count (proportions) for categoric variables and median (Q1-Q3) for continuous variables. Our comparative analysis focused on Black and White patients; the number of patients who self-identified as other races was small, altogether <7%. Differences in prevalent CV risk factors and disease were tested using the chi-square test. Overall and theme-specific census tract SVI percentile rankings were compared using the Wilcoxon rank sum test.
The cause-specific hazards method was used for the primary analysis of time-to-event outcomes given the etiologic nature of our study questions.39 Unadjusted rates of incident CV outcomes were estimated and compared using the Kaplan-Meier estimator and the log-rank test. Adjusted associations between self-identified race and outcomes were evaluated using Cox proportional hazards models with results presented as HRs with 95% CIs. Schoenfeld residuals analysis was conducted to validate the proportional hazards assumption. An approach based on the modified disjunctive cause criterion was implemented to select possible confounders.40 These were selected a priori based on their known associations with CV outcomes, including age, systolic blood pressure, body mass index, hypertension, diabetes, dyslipidemia, self-reported smoking status, pre-existing CVD (atherosclerotic CVD and heart failure), CV medications (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, and statins), abiraterone, and novel antiandrogen therapy.41 Because abiraterone and novel antiandrogens could be prescribed at different time points during the course of ADT (ie, for the initial management of castration-sensitive disease or at the time of castration resistance), these were treated as time-varying covariates with ascertainment of start/stop dates from the EMR based on prescription data and manual chart review. To address possible confounding related to changing practice patterns over the study period, the ADT start year was additionally included in the models as a proxy variable. Sensitivity analysis was performed for unadjusted differences in CV outcomes based on Gray’s test for competing risks, and adjusted associations were modeled using the Fine and Gray method.39 In this sensitivity analysis, abiraterone and novel antiandrogen use were treated as fixed covariates.
In secondary analysis, we explored through an interaction analysis whether race-based differences in CV outcomes differed according to prevalent CVD and cancer stage. Prevalent CVD was defined based on prevalent atherosclerotic CVD or heart failure at ADT start. Cancer stage at diagnosis was assessed by the American Joint Committee on Cancer system and defined as localized (stages 1-3) or metastatic (stage 4) disease.
Mediation analysis was performed based on the method of Valeri and Vanderweele42 to determine whether differences in MACE according to race were mediated by structural SDOH-related mechanisms, specifically census tract SVI. This analysis decomposed the total effect of race into a direct effect (non–SVI-mediated effect) and an indirect effect mediated by SVI (SVI-mediated effect) and determined the proportion of the overall effect mediated by SVI using the following formula: (direct effect · [indirect effect − 1])/(direct effect · indirect effect − 1).43 Details on the mediation analysis methods are provided in the Supplemental Methods.
A 2-sided significance level of 0.05 was used to assess statistical significance. All analyses were performed using R 4.4.2 (R Foundation for Statistical Computing).
Results
Missing data
A detailed manual chart review was conducted to reduce the missingness in the data. After the review, the proportion of missing values was low at <5%.
Baseline characteristics
Among the 3,543 included patients, 1,079 (30.4%) were Black, 2,227 (62.9%) were White, 63 (1.8%) were Asian, and 96 (2.7%) self-identified as other (Table 1). The demographics of the entire patient population at UPHS during that time frame was as follows: 62% White, 21% Black, 3% Hispanic, and 3% Asian.20 In comparison, the demographics of Philadelphia consist of 37% White, 40% Black, 16% Hispanic, and 7% Asian.44 In summary, our study population exhibited a higher proportion of Black patients.
Table 1.
Baseline Characteristics
| Overall (N = 3,543) | Black (n = 1,079) | White (n = 2,227) | P Valueb | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 70 (64-76) | 68 (62-75) | 70 (65-77) | <0.001 |
| Race | ||||
| Asian | 63 (1.8) | |||
| Black | 1,079 (30.4) | |||
| White | 2,227 (62.9) | |||
| Other | 96 (2.7) | |||
| Unknown | 78 (2.2) | |||
| Ethnicity, non-Hispanic non-LatinX | 3,435 (98.5) | 1,055 (98.6) | 2,159 (98.5) | 0.94 |
| Prostate cancer | ||||
| AJCC stage at diagnosisa | <0.001 | |||
| 1 | 173 (5.9) | 53 (5.9) | 107 (5.8) | |
| 2 | 1,107 (37.7) | 357 (39.5) | 679 (36.9) | |
| 3 | 728 (24.8) | 196 (21.7) | 491 (26.7) | |
| 4 | 622 (21.2) | 177 (19.6) | 401 (21.8) | |
| Unknown | 306 (10.4) | 121 (13.4) | 160 (8.7) | |
| ADT start year | <0.001 | |||
| 2008-2009 | 207 (5.8) | 65 (6.0) | 131 (5.9) | |
| 2010-2011 | 565 (15.9) | 224 (20.8) | 306 (13.7) | |
| 2012-2013 | 375 (10.6) | 119 (11.0) | 221 (9.9) | |
| 2014-2015 | 409 (11.5) | 113 (10.5) | 271 (12.2) | |
| 2016-2017 | 504 (14.2) | 130 (12.0) | 332 (14.9) | |
| 2018-2019 | 786 (22.2) | 230 (21.3) | 504 (22.6) | |
| 2020-2021 | 697 (19.7) | 198 (18.4) | 462 (20.7) | |
| Abiraterone use | 619 (17.5) | 145 (13.4) | 433 (19.4) | <0.001 |
| Novel antiandrogen use | 507 (14.4) | 141 (13.1) | 331 (14.9) | 0.19 |
| CV risk factors | ||||
| SBP, mm Hg | 135 (124-148) | 136 (124-149) | 134 (124-147) | 0.14 |
| DBP, mm Hg | 76 (70-83) | 77 (70-84) | 76 (70-82) | 0.048 |
| BMI, kg/m2 | 28 (25-31) | 28 (25-31) | 28 (25-31) | 0.12 |
| Current or past smoking | 1,741 (51.2) | 628 (60.4) | 1,008 (47.1) | <0.001 |
| Obesity | 1,136 (33.4) | 363 (34.8) | 717 (33.7) | 0.57 |
| Hypertension | 2568 (72.5) | 856 (79.3) | 1,546 (69.4) | <0.001 |
| Diabetes mellitus | 824 (23.3) | 363 (33.6) | 398 (17.9) | <0.001 |
| Hyperlipidemia | 2,186 (61.7) | 606 (56.2) | 1,439 (64.6) | <0.001 |
| CV disease | ||||
| Heart failure | 231 (6.5) | 110 (10.2) | 111 (5.0) | <0.001 |
| Coronary heart disease | 642 (18.1) | 158 (14.6) | 455 (20.4) | <0.001 |
| Cerebrovascular disease | 309 (8.7) | 117 (10.8) | 176 (7.9) | 0.006 |
| Peripheral artery disease | 285 (8.0) | 97 (9.0) | 174 (7.8) | 0.28 |
| History of CV medication use | ||||
| ACEI or ARB | 1,644 (46.4) | 532 (49.3) | 1,000 (44.9) | 0.019 |
| Sacubitril/valsartan | 5 (0.1) | 1 (0.1) | 4 (0.2) | 0.90 |
| Beta-blocker | 1,281 (36.2) | 411 (38.1) | 810 (36.4) | 0.36 |
| MRA | 77 (2.2) | 40 (3.7) | 36 (1.6) | <0.001 |
| Calcium-channel blocker | 1,134 (32.0) | 501 (46.4) | 568 (25.5) | <0.001 |
| Diuretics | 1,051 (29.7) | 445 (41.2) | 556 (25.0) | <0.001 |
| Statins | 1,933 (54.6) | 546 (50.6) | 1,272 (57.1) | <0.001 |
| Aspirin | 1,706 (48.2) | 478 (44.3) | 1,134 (50.9) | <0.001 |
| P2Y12 inhibitors | 282 (8.0) | 86 (8.0) | 183 (8.2) | 0.86 |
| Warfarin | 242 (6.8) | 59 (5.5) | 170 (7.6) | 0.026 |
| Direct-acting oral anticoagulants | 205 (5.8) | 46 (4.3) | 151 (6.8) | 0.005 |
Values are median (Q1-Q3) or n (%).
ACEI = angiotensin-converting enzyme inhibitor; ADT = androgen deprivation therapy; AJCC = American Joint Committee on Cancer; ARB = angiotensin receptor blocker; BMI = body mass index; CV = cardiovascular; DBP = diastolic blood pressure; MRA = mineralocorticoid receptor antagonist; SBP = systolic blood pressure.
Prostate cancer–related variables were obtained from the Penn Cancer Registry; 2,936 of the 3,543 in the analytic cohort have data available in the Penn Cancer Registry.
Differences were tested using the Wilcoxon rank sum test for continuous variables or chi-square test for categorical variables.
The median (Q1-Q3) age was 70 years (Q1-Q3: 64-76 years). The majority received a GnRH agonist (37.6%) or GnRH agonist with first-generation antiandrogen (52.9%) as their initial ADT regimen, whereas only 0.8% received a GnRH antagonist. The remaining 8.7% received abiraterone or novel antiandrogen with a GnRH agonist or antagonist as part of their initial ADT regimen. Abiraterone and/or novel antiandrogen use at any time during the course of ADT was documented in 24.2%.
Prevalent CV risk factors and disease according to self-identified race
The prevalence of smoking (60.4% vs 47.1%), hypertension (79.3% vs 69.4%), and diabetes (33.6% vs 17.9%) was greater in Black compared with White patients (P < 0.001). Similarly, heart failure (10.2% vs 5.0%; P < 0.001) and cerebrovascular disease (10.8% vs 7.9%; P = 0.006) were more prevalent in Black patients. However, the prevalence of hyperlipidemia (56.2% vs 64.6%) and coronary heart disease (14.6% vs 20.4%) was greater in White patients (P < 0.001) (Table 1).
Incident CV outcomes according to self-identified race
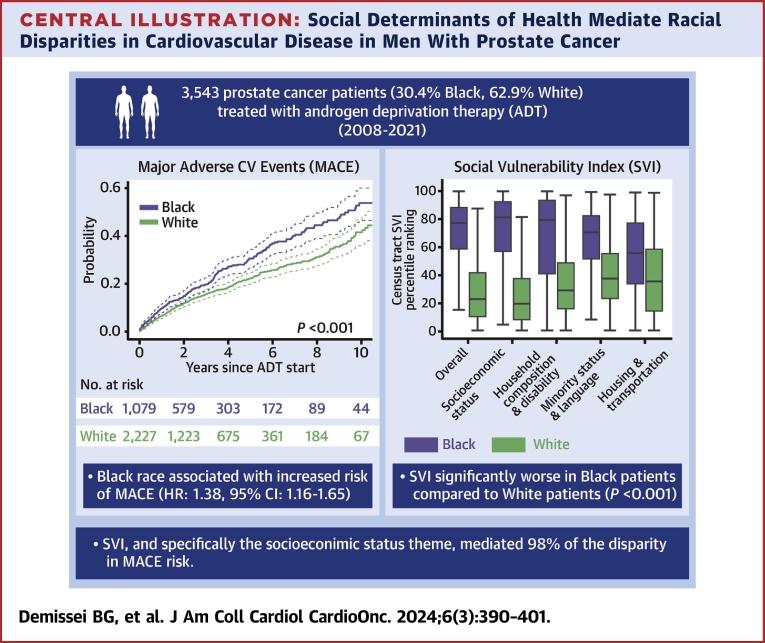
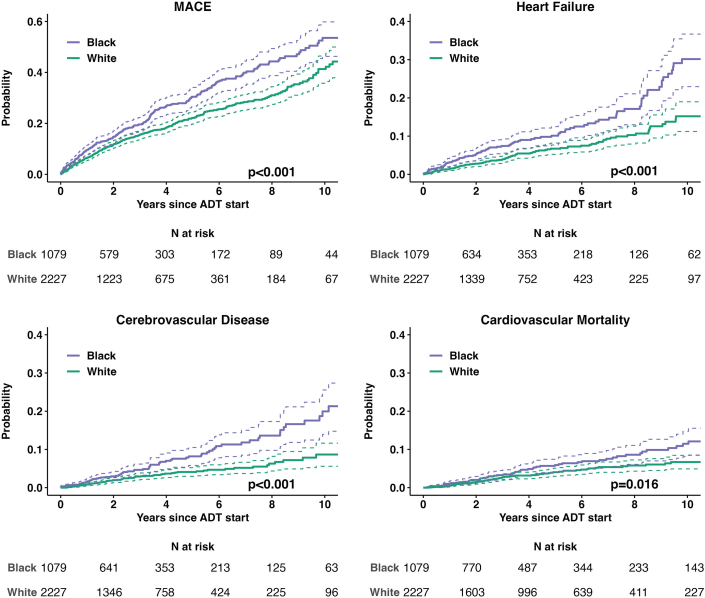
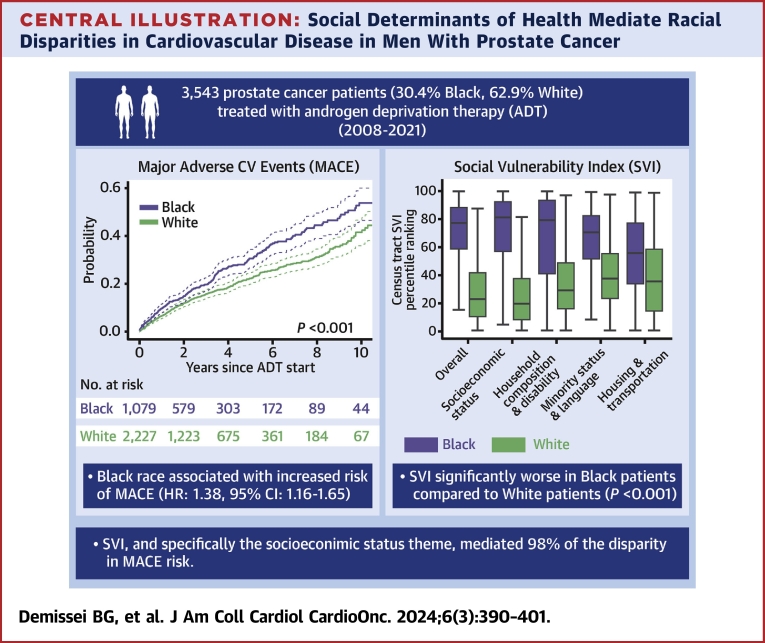
The unadjusted rate of incident MACE was significantly greater in Black compared with White patients over a median (Q1-Q3) follow-up time of 2.8 years (Q1-Q3: 1.3-5.6 years) (Figure 1, Supplemental Figures 3 to 5). In multivariable analysis, Black patients had 38% greater hazard of MACE compared to White patients (HR: 1.38; 95% CI: 1.16-1.65; P < 0.001). Black race was independently associated with increased hazard of heart failure (HR: 1.79; 95% CI: 1.32-2.43; P < 0.001), cerebrovascular disease (HR: 1.98; 95% CI: 1.37-2.87; P < 0.001), and peripheral artery disease (HR: 1.76; 95% CI: 1.26-2.45; P < 0.001) events (Figure 1, Table 2). Moreover, Black race was associated with CV mortality in unadjusted analysis (HR: 1.51; 95% CI: 1.08-2.12; P = 0.017), although this was not statistically significant after adjustment for pre-existing CV risk factors and disease. A sensitivity analysis based on the Fine and Gray competing risks method showed similar findings (Supplemental Figures 4 and 5, Supplemental Table 5). Differences in MACE risk between Black and White patients were consistent in subgroup analyses based on pre-existing CVD or cancer stage (Supplemental Figures 6 and 7, Supplemental Table 6).
Figure 1.
MACE and Cardiovascular Outcomes by Self-Identified Race
The probability of incident major adverse cardiovascular events (MACE) and individual cardiovascular outcomes following systemic androgen deprivation therapy (ADT) in Black vs White prostate cancer patients estimated based on the Kaplan-Meier estimator; the difference according to self-identified race was tested using the log-rank test.
Table 2.
Associations Between Self-Identified Black Race (Compared With White Race) and CV Events After ADT
| CV Outcome | Unadjusted HR (95% CI) | P Value | Adjusted HRa (95% CI) | P Value |
|---|---|---|---|---|
| MACE | 1.43 (1.22-1.67) | <0.001 | 1.38 (1.16-1.65) | <0.001 |
| Heart failure | 1.91 (1.46-2.49) | <0.001 | 1.79 (1.32-2.43) | <0.001 |
| Coronary heart disease | 0.99 (0.74-1.32) | 0.95 | 0.95 (0.69-1.30) | 0.76 |
| Cerebrovascular disease | 2.16 (1.56-3.01) | <0.001 | 1.98 (1.37-2.87) | <0.001 |
| Peripheral artery disease | 1.93 (1.43-2.61) | <0.001 | 1.76 (1.26-2.45) | <0.001 |
| Ventricular tachycardia/fibrillation | 1.13 (0.68-1.86) | 0.64 | 1.11 (0.64-1.93) | 0.70 |
| CV mortality | 1.51 (1.08-2.12) | 0.017 | 1.20 (0.81-1.78) | 0.37 |
Associations were modeled using cause-specific proportional hazards models.
MACE = major adverse cardiovascular event; other abbreviations as in Table 1.
Models adjusted for age, year of ADT start, SBP, current or past smoking, BMI, hypertension, diabetes mellitus, hyperlipidemia, heart failure, pre-existing atherosclerotic CV disease and CV medication use at ADT start (ACEI/ARB, beta-blockers, MRA, and statins), abiraterone, and novel antiandrogen therapy. Abiraterone and novel antiandrogen therapy were modeled as time-varying covariates.
Prostate cancer mortality according to self-identified race
Supplemental Figure 8 presents the unadjusted rate of prostate cancer mortality according to race. After adjustment for age and cancer stage, no significant association was observed between Black race and prostate cancer mortality (HR: 1.13; 95% CI: 0.91-1.42; P = 0.27).
Structural SDOH as mediator of racial disparities in CV outcomes
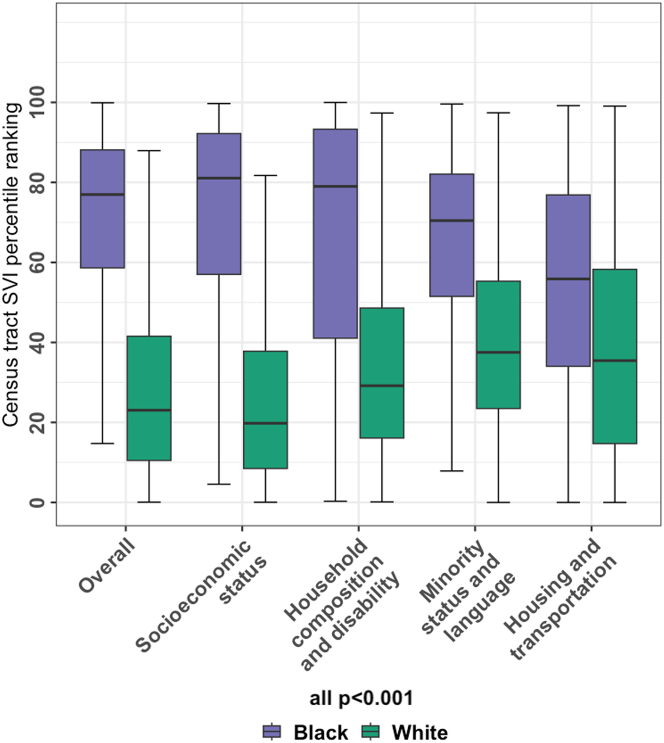
Census tract SVI was collected in 3,458 patients (1,063 Black and 2,163 White and the remainder other races) with geocodable address information. Patients (n = 85) with missing or nongeocodable address (PO Box instead of physical address) were excluded. A total of 1,419 discrete geocodes were represented in our study. The median (Q1-Q3) census tract overall SVI ranking was significantly higher in Black at 77% (Q1-Q3: 58%-88%) compared to White patients at 23% (Q1-Q3: 10%-42%) (P < 0.001). These differences, indicative of greater social vulnerability among Black patients, were observed consistently across all SVI themes (Figure 2).
Figure 2.
Social Vulnerability According to Self-Identified Race
Distribution of census tract social vulnerability index (SVI) percentile rankings in Black vs White prostate cancer patients at androgen deprivation therapy start. Higher SVI rankings correlate with greater social vulnerability.
Our analysis examining the census tract SVI as a potential mediator of the observed racial disparities in MACE indicated that the overall SVI rankings mediated 81% of the association between Black race and MACE (Table 3). The indirect SVI-mediated effect was significantly different with an HR of 1.28 (95% CI: 1.07-1.72), whereas the non–SVI-mediated effect was not (HR: 1.07; 95% CI: 0.76-1.38). In additional analyses exploring each theme-specific SVI measure as a mediator of the association between race and MACE in individual models, the socioeconomic status theme had the strongest effect; the indirect effect mediated by this theme accounted for 98% of the association between race and MACE (Table 3). In contrast, the effects of the other themes evaluated were less substantial.
Table 3.
Mediation Analysis Using Overall and Theme-Specific Census Tract SVI Rankings
| SVI Measure | Natural Indirect Effect HR (95% CI)a | Natural Direct Effect HR (95% CI)b | Proportion Mediated |
|---|---|---|---|
| Overall SVI rankings | 1.28 (1.07-1.72) | 1.07 (0.76-1.38) | 0.81 |
| Theme-specific rankings | |||
| Socioeconomic status | 1.35 (1.10-1.74) | 1.01 (0.75-1.25) | 0.98 |
| Household composition and disability | 1.13 (0.99-1.29) | 1.21 (0.97-1.50) | 0.42 |
| Minority status and language | 1.03 (0.85-1.25) | 1.33 (1.05-1.77) | 0.11 |
| Housing type and Transportation | 1.02 (0.94-1.14) | 1.32 (1.07-1.63) | 0.09 |
Details of the specific methods used in the mediation analysis are provided in the Supplemental Methods.
SVI = social vulnerability index.
Represents the association between Black race and the time to major adverse cardiovascular event that is mediated by the SVI variable under consideration.
Represents the association between Black race and the time to major adverse cardiovascular event through other pathways not related to the SVI theme under consideration.
Discussion
In this large retrospective cohort study of prostate cancer patients, we examined racial disparities in CV outcomes after ADT. Our analysis demonstrates 3 main findings (Central Illustration). First, Black patients had a greater prevalence of pre-existing CV risk factors and disease at ADT initiation. Second, although there were no differences in prostate cancer mortality in adjusted analyses, Black patients were significantly more likely to experience incident MACE following ADT compared with White patients. Third, these disparities in CV outcomes were largely explained by differences in structural SDOH as defined by census tract SVI, specifically the socioeconomic status theme. To our knowledge, this is the first study demonstrating the marked racial disparities in CV outcomes in patients with prostate cancer that also serves to identify census tract–based structural SDOH as the main mediators of these inequities.
Central Illustration.
Social Determinants of Health Mediate Racial Disparities in Cardiovascular Disease in Men With Prostate Cancer
In a cohort of prostate cancer patients treated with androgen deprivation therapy (ADT) at a quaternary, multisite health care system, Black race was associated with a 1.38-fold increased hazard of major adverse cardiovascular event (MACE) as defined by cause-specific proportional hazards. Mediation analysis determined that the social vulnerability index (SVI), specifically the socioeconomic status theme, mediated 98% of this disparity in MACE risk. CV = cardiovascular.
The care for this cohort of patients was driven by oncology and genitourinary providers within our multisite health care system, and we hypothesize that this oncology-focused, equal-access cancer care may explain why there were no differences in prostate cancer mortality according to self-identified race.45 However, not all patients received CV care46, 47, 48, 49 despite over 90% of the patients in our cohort having at least 1 CV risk factor, and 29% had CVD before ADT initiation. MACE occurred in a substantial proportion of patients (ie, 22% at 5 years after ADT). Moreover, the burden of CVD in our cohort was greater in Black patients. Black race was associated with up to 38% increased hazard of incident MACE even after adjustment for CV risk factors, CVD, and confounders. Altogether, our findings demonstrate that CVD is an important driver of racial disparities in noncancer morbidity in prostate cancer, and this was consistent across patients with localized or metastatic cancer and those with or without pre-existing CVD. Our work identifies an urgent, unmet need for greater attention to CVD management in men with prostate cancer. As the most common cancer in men in the United States, this represents a critically important public health priority.
Social vulnerability has emerged as an important determinant of health outcomes across multiple disease conditions.50, 51, 52, 53, 54 Residence in neighborhoods with greater social vulnerability correlates with disadvantages in individual SDOH (eg, income, education, employment, health insurance coverage, health literacy, and access to health care).55 Neighborhoods with greater social vulnerability are also likely to have limited access to physical activity–promoting resources, healthy foods, safety, transportation, economic opportunities, and social cohesion. All of these factors have been associated with adverse CV outcomes; social vulnerability is a proxy for a multitude of downstream processes and barriers that negatively impact CV risk factor control and subsequent risk of CV events.55, 56, 57 Within our health system, the majority of Black patients resided in census tracts with the highest vulnerability, whereas the majority of White patients resided in census tracts in the lower 2 quartiles (less vulnerability). Importantly, mediation analysis indicated that these differences in census tract overall SVI rankings accounted for 81% of the association between Black race and MACE, with socioeconomic status demonstrating the strongest effect (98%) in theme-specific analysis. Our results suggest that census tract–based SDOH, socioeconomic status specifically, explains the marked racial disparities in CV outcomes after ADT.
Our findings have important implications in the clinical care of prostate cancer patients receiving ADT. The substantial burden of CVD in this population highlights a critical need for the implementation of more effective, accessible, and inclusive cardiology and cardio-oncology care.13,48,49 Our findings, derived from a quaternary, multisite health care system with specialized CV and cardio-oncology care, are sobering and raise concerns that systems with less resources may have even greater disparities in CV outcomes. However, recent interventions in cardiology that provide access to care equal to Black men, such as blood pressure medication management in Black barbershops, have resulted in statistically and clinically significant blood pressure reductions.58 Moreover, retrospective analyses in oncology that account for access to care and standardized treatment suggest that Black men have similar or better prostate cancer outcomes.4,45 We also emphasize the need for greater consideration of SDOH in the care of prostate cancer patients. Addressing SDOH is complex and requires further actions at the provider, health care system, community, and government level.56,57 Comprehensive assessment of SDOH can play a crucial role in identifying adverse SDOH in individual patients, which is key to informing multitargeted, tailored interventions.
Our findings related to disparate socioeconomic status, and rising costs of medical care also place greater emphasis on financial toxicity. Financial toxicity has been noted to be greatest among patients suffering from both CVD and cancer, and this may help explain our findings.59 Strategies focused on addressing the multidimensional financial toxicity experienced by cancer patients on the individual, community, and organizational level need to be developed.60 We believe that discussions regarding the burden of CVD and MACE in men with prostate cancer should be revisited in the context of these important contributions of SDOH and the need for additional race-conscious research, redirecting public health efforts at the community level to address the unmet needs of patients from vulnerable neighborhoods.55
Study limitations
This was a retrospective cohort study, and limitations inherent to such study design, including unmeasured confounding and misclassification, exist. The study was conducted at a quaternary academic health care system, which might affect the generalizability of our findings. The study period spans a relatively long time frame, during which substantial changes have occurred in prostate cancer management. Importantly, there has been increasing and earlier use of ADT in combination with abiraterone and/or novel antiandrogens, which carry increased CV risk. Although we adjusted for these therapies and additionally attempted to account for possible confounding related to changing clinical practice patterns by including ADT start year as a proxy, there is a possibility of residual confounding. In addition, there are inherent limitations to an EMR-based study. Although our study captures valuable insights, it is important to acknowledge that EMR data may not fully capture all relevant information, posing challenges to ascertain the factors driving the observed health outcomes.
Furthermore, our study centered on Black and White patients. Although the greatest health disparities in cancer and CVD have been reported in Black patients, further investigation on the disparities in prostate cancer among other diverse race/ethnic groups should also be pursued. Finally, prior studies have determined that racial disparities in cancer and CV outcomes are not driven by socioeconomic status alone. Further investigation should be conducted to assess the role of other structural and SDOH, including but not limited to individual SDOH measures and structural measures including those that represent environmental burden.
Of note, we also highlight the strengths of this study. These include the following: original research that answers a clinically relevant question in a population with prevalent CVD and of great need, rigorous curation of a comprehensive data set through detailed manual chart review with minimal missing data, innovative methodologic approach to understanding inequities in care through mediation analysis, and observational findings that provide important data to support multilevel strategies to overcome health care disparities.
Conclusions
Black men with prostate cancer have a greater burden of adverse CVD outcomes after ADT compared with their White counterparts. These disparities are mediated by socioeconomic status and other structural determinants of health as captured by census tract SVI. Multilevel targeted interventions tailored to the needs of patients from socioeconomically vulnerable communities are necessary to reduce the disparities in CVD in patients with prostate cancer.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with prostate cancer treated with androgen deprivation therapy, Black patients have worse SVI and are significantly more likely to experience MACE compared to White patients. SVI, and particularly socioeconomic status, largely mediated the association between race and MACE.
TRANSLATIONAL OUTLOOK: Future research needs to focus on further understanding and mitigating the needs of patients from socioeconomically vulnerable communities. Interventions that effectively address the SDOH are needed to overcome disparities in cardiovascular outcomes in prostate cancer.
Funding Support and Author Disclosures
Supported by the American Heart Association Strategically Focused Research Network Cardio-Oncology Disparities award (Drs Demissei, Yancy, Narayan, and Ky [PI]) and the National Institutes of Health grant K24 HL167127-01A1 (Dr Ky [PI] and Ms. Smith and Ko). Dr Ky is PI of a Pfizer investigator-initiated study in cardio-oncology disparities unrelated to this work. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Husam Abdel-Qadir, MD, PhD, served as Guest Associate Editor for this paper. Paaladinesh Thavendiranathan, MD, MSc, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Cronin K.A., Scott S., Firth A.U., et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. Cancer. 2022;128:4251–4284. doi: 10.1002/cncr.34479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Siegel R.L., Sauer A.G., et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 3.Wen W., Luckenbaugh A.N., Bayley C.E., Penson D.F., Shu X.O. Racial disparities in mortality for patients with prostate cancer after radical prostatectomy. Cancer. 2021;127:1517–1528. doi: 10.1002/cncr.33152. [DOI] [PubMed] [Google Scholar]
- 4.Dess R.T., Hartman H.E., Mahal B.A., et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5:975–983. doi: 10.1001/jamaoncol.2019.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovtun K.A., Chen M.H., Braccioforte M.H., Moran B.J., D’mico A.V. Race and mortality risk after radiation therapy in men treated with or without androgen-suppression therapy for favorable-risk prostate cancer. Cancer. 2016;122:3608–3614. doi: 10.1002/cncr.30224. [DOI] [PubMed] [Google Scholar]
- 6.Bailey Z.D., Feldman J.M., Bassett M.T. How structural racism works — racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2020;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchwell K., Elkind M.S.V., Benjamin R.M., et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 8.Vince R.A. Jr, Jiang R., Bank M., et al. Evaluation of social determinants of health and prostate cancer outcomes among Black and White patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.50416. e2250416-e2250416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmehrath A.O., Afifi A.M., Al-Husseini M.J., et al. Causes of death among patients with metastatic prostate cancer in the US from 2000 to 2016. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.19568. e2119568-e2119568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer E.M., Srinivas S., Adra N., et al. NCCN Guidelines® insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20:1288–1298. doi: 10.6004/jnccn.2022.0063. [DOI] [PubMed] [Google Scholar]
- 11.Hu J.R., Duncan M.S., Morgans A.K., et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer: contemporary meta-analyses. Arterioscler Thromb Vasc Biol. 2020;40:e55–e64. doi: 10.1161/ATVBAHA.119.313046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challa A.A., Calaway A.C., Cullen J., et al. Cardiovascular toxicities of androgen deprivation therapy. Curr Treat Options Oncol. 2021;22:47. doi: 10.1007/s11864-021-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayan V., Ross A.E., Parikh R.B., Nohria A., Morgans A.K. How to treat prostate cancer with androgen deprivation and minimize cardiovascular risk: a therapeutic tightrope. J Am Coll Cardiol CardioOnc. 2021;3:737–741. doi: 10.1016/j.jaccao.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating N.L., O’Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 15.Haque R., UlcickasYood M., Xu X., et al. Cardiovascular disease risk and androgen deprivation therapy in patients with localised prostate cancer: a prospective cohort study. Br J Cancer. 2017;117:1233–1240. doi: 10.1038/bjc.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico A.V., Chen M.H., Renshaw A., Loffredo M., Kantoff P.W. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314:1291–1293. doi: 10.1001/jama.2015.8577. [DOI] [PubMed] [Google Scholar]
- 17.Ziehr D.R., Chen M.H., Zhang D., et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015;116:358–365. doi: 10.1111/bju.12905. [DOI] [PubMed] [Google Scholar]
- 18.Nanda A., Chen M.H., Moran B.J., et al. Neoadjuvant hormonal therapy use and the risk of death in men with prostate cancer treated with brachytherapy who have no or at least a single risk factor for coronary artery disease. Eur Urol. 2014;65:177–185. doi: 10.1016/j.eururo.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 19.Bosco C., Bosnyak Z., Malmberg A., Adolfsson J., Keating N.L., Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68:386–396. doi: 10.1016/j.eururo.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Chen Y., Landis J.R., Mahoney K.B. Difference between users and nonusers of a patient portal in health behaviors and outcomes: retrospective cohort study. J Med Internet Res. 2019;21 doi: 10.2196/13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace R., Peters T., Rahme E., Dasgupta K. Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol. 2017;33:1052–1059. doi: 10.1016/j.cjca.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad F.S., Chan C., Rosenman M.B., et al. Validity of cardiovascular data from electronic sources: the Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation. 2017;136:1207–1216. doi: 10.1161/CIRCULATIONAHA.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt S.E., Pereira K., Granger B.B., et al. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus. J Am Med Inform Assoc. 2017;24:e121–e128. doi: 10.1093/jamia/ocw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulme O.L., Khurshid S., Weng L.C., et al. Development and validation of a prediction model for atrial fibrillation using electronic health records. J Am Coll Cardiol EP. 2019;5:1331–1341. doi: 10.1016/j.jacep.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tison G.H., Chamberlain A.M., Pletcher M.J., et al. Identifying heart failure using EMR-based algorithms. Int J Med Inform. 2018;120:1–7. doi: 10.1016/j.ijmedinf.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saczynski J.S., Andrade S.E., Harrold L.R., et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick N., Lacaille D., Bhole V., Avina-Zubieta J.A. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick N., Lacaille D., Bhole V., Avina-Zubieta J.A. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu K., Mitiku T., Lee D.S., Guo H., Tu J.V. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD) Can J Cardiol. 2010;26:e225–e228. doi: 10.1016/s0828-282x(10)70412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mentz R.J., Newby L.K., Neely B., et al. Assessment of administrative data to identify acute myocardial infarction in electronic health records. J Am Coll Cardiol. 2016;67:2441–2442. doi: 10.1016/j.jacc.2016.03.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu K., Wang M., Young J., et al. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;29:1388–1394. doi: 10.1016/j.cjca.2013.07.676. [DOI] [PubMed] [Google Scholar]
- 32.Fan J., Arruda-Olson A.M., Leibson C.L., et al. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Inform Assoc. 2013;20:e349–e354. doi: 10.1136/amiajnl-2013-001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalbaugh C.A., Kucharska-Newton A., Wruck L., et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among Medicare fee-for-service beneficiaries in the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamariz L., Harkins T., Nair V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):148–153. doi: 10.1002/pds.2340. [DOI] [PubMed] [Google Scholar]
- 35.Agency for Toxic Substances and Disease Registry CDC/ATSDR social vulnerability index. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 36.National Center for Health Statistics . National Center for Health Statistics; 2013. National Death Index User’s Guide. [Google Scholar]
- 37.Olubowale O.T., Safford M.M., Brown T.M., et al. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the National Death Index: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan V., Liu T., Song Y., et al. Early increases in blood pressure and major adverse cardiovascular events in patients with renal cell carcinoma and thyroid cancer treated with VEGFR TKIs. J Natl Compr Canc Netw. 2023;21:1039–1049.e10. doi: 10.6004/jnccn.2023.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanderWeele T.J. Principles of confounder selection. Eur J Epidemiol. 2019;34:211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacovelli R., Ciccarese C., Bria E., et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16:e645–e653. doi: 10.1016/j.clgc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderweele T.J., Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Census Bureau. Population estimates, July 1, 2023, (V2023) - Philadelphia City, Pennsylvania. 2023. Accessed on March 6th, 2024. https://www.census.gov/quickfacts/fact/table/philadelphiacitypennsylvania/SBO001217
- 45.Rasmussen K.M., Patil V., Li C., et al. Survival outcomes by race and ethnicity in veterans with nonmetastatic castration-resistant prostate cancer. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.37272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shore N.D., Saad F., Cookson M.S., et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 47.Leong D.P., Fradet V., Shayegan B., et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203:1109–1116. doi: 10.1097/JU.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 48.Sun L., Parikh R.B., Hubbard R.A., et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klimis H., Pinthus J.H., Aghel N., et al. The burden of uncontrolled cardiovascular risk factors in men with prostate cancer: a RADICAL-PC Analysis. J Am Coll Cardiol CardioOnc. 2023;5:70–81. doi: 10.1016/j.jaccao.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am J Prev Med. 2020;59:317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauer C., Zhang K., Xiao Q., Lu J., Hong Y.R., Suk R. County-level social vulnerability and breast, cervical, and colorectal cancer screening rates in the US, 2018. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.33429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aris I.M., Perng W., Dabelea D., et al. Associations of neighborhood opportunity and social vulnerability with trajectories of childhood body mass index and obesity among US children. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.47957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan S.U., Javed Z., Lone A.N., et al. Social vulnerability and premature cardiovascular mortality among US counties, 2014 to 2018. Circulation. 2021;144:1272–1279. doi: 10.1161/CIRCULATIONAHA.121.054516. [DOI] [PubMed] [Google Scholar]
- 54.Ganatra S., Dani S.S., Kumar A., et al. Impact of social vulnerability on comorbid cancer and cardiovascular disease mortality in the United States. J Am Coll Cardiol CardioOnc. 2022;4:326–337. doi: 10.1016/j.jaccao.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barber S., Hickson D.A., Wang X., Sims M., Nelson C., Diez-Roux A.V. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health. 2016;106:2219–2226. doi: 10.2105/AJPH.2016.303471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz W.M., Kelli H.M., Lisko J.C., et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandt E.J., Tobb K., Cambron J.C., et al. Assessing and addressing social determinants of cardiovascular health: JACC state-of-the-art review. J Am Coll Cardiol. 2023;81:1368–1385. doi: 10.1016/j.jacc.2023.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Victor R.G., Lynch K., Li N., et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med. 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valero-Elizondo J., Chouairi F., Khera R., et al. Atherosclerotic cardiovascular disease, cancer, and financial toxicity among adults in the United States. J Am Coll Cardiol CardioOnc. 2021;3:236–246. doi: 10.1016/j.jaccao.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamel L.M., Dougherty D.W., Hastert T.A., et al. The DISCO App: A pilot test of a multi-level intervention to reduce the financial burden of cancer through improved cost communication. PEC Innov. 2022;1 doi: 10.1016/j.pecinn.2021.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.