Abstract
Respiratory syncytial virus (RSV) infection is a major cause of morbidity in childhood worldwide. The first human RSV-specific cytotoxic T-lymphocyte epitope to be defined is described. This HLA B7-restricted epitope in nucleoprotein (NP) was detectable in four healthy, B7-positive adult subjects using B7-RSV-NP tetrameric complexes to stain CD8+ T cells.
Respiratory syncytial virus (RSV) infection is a major cause of morbidity in the first 2 years of life in both developed and developing countries (9, 21). By analogy with other acute virus infections, such as influenza (15, 22), antiviral cellular immunity might be expected to play a central part in controlling human RSV infection. There is compelling evidence that RSV-specific type 1 T-helper responses and cytotoxic T-lymphocyte (CTL) activity are indeed critical to the disease-free control of murine RSV infection (4, 11, 19). However, study of the cellular immune response to RSV in infected humans has been limited by technical difficulties in obtaining adequate information either from small-volume samples from acutely infected children or from the low frequency of RSV-specific memory CTL responses in adults.
Recent advances in the techniques which may be applied to the study of CTLs have enabled many of these obstacles to be overcome. The new methodologies of enzyme-linked immunospot (Elispot) and flow cytometry-based assays (1, 14), including the use of tetrameric complexes of viral peptide and major histocompatibility complex class I (tetramers), have greatly increased the sensitivity by which CTLs may be detected (7, 13, 14, 16, 20). In addition, data which previously would have required weeks or months to reach may now be obtained within hours.
Although precise definition of RSV-specific CTL epitopes in humans has not been achieved to date, studies of infected children have nonetheless hinted at the importance of the anti-RSV CTL response in control of the infection (6, 10, 12). Reports of RSV-specific memory CTL activity in adults have been directed at highlighting the RSV proteins which serve as targets for human CTL epitopes (2, 5). These have suggested significant differences between the proteins that are principally immunogenic for CTL responses in human RSV infection compared to those chiefly targeted by CTLs in murine RSV infection (17, 18).
This report describes the characterization of a novel HLA B7-restricted memory RSV-specific CTL epitope in humans. This is the first RSV-specific CTL epitope to be fully defined in human infection. This report also illustrates the application of the novel technologies of Elispot and flow cytometry-based assays to the study of human RSV infection, even when these cells are present at very low frequency, in order to identify CTL epitopes rapidly and ultimately to understand the role of virus-specific CTL responses in controlling RSV infection in humans.
Blood was collected from healthy adult volunteers, and peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Hypaque (Sigma, St. Louis, Mo.) density gradient centrifugation. HLA typing was performed by sequence-specific primer PCR (3). The class I HLA types of the six subjects studied were as follows: subject A, A2/3 B7/51 Cw5/7; subject B, A3/− B7/− Cw7/−; subject C, A3/26 B7/38 Cw7/8; subject D, 11/25 B7/18 Cw7/12; subject E, A34/68 B51/72 Cw2/16; subject F, A2/3 B8/44 Cw5/7. A panel of 48 overlapping peptides spanning the RSV nucleoprotein (NP) was generated. These studies initially focused on NP as a potential target for CTL epitopes based on earlier work (2, 5). These NP peptides were 16 to 18 amino acids in length and overlapped by 10 amino acids. Peptides were synthesized as free acids on a Synergy peptide synthesizer (model 432A; Applied Biosystems, Foster City, Calif.) using conventional 9-fluorenylmethoxy carbonyl chemistry. All peptides were analyzed for purity by reverse-phase high-pressure liquid chromatography. Peptides in all cases were >80% pure.
Elispot assays were performed as previously described (8). Briefly, 200,000 PBMC per well were incubated overnight with individual NP peptides, each at 10−5 M, in wells which had been precoated with anti-gamma interferon (IFN-γ) monoclonal antibody (MAb) 1-DIK (Mabtech, Stockholm, Sweden). The following day, cells were washed and incubated for 100 min with a second, biotinylated anti-IFN-γ MAb, 7-B6-1 biotin (Mabtech). Following washing, streptavidin-conjugated alkaline phosphatase (Mabtech) was added and the mixture was incubated at room temperature for 40 min. Spot-forming cells (sfc) were detectable as dark spots after a 20-min reaction with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium using an alkaline phosphatase-conjugated substrate (Bio-Rad, Richmond, Calif.). The number of specific T cells was calculated by subtracting the negative control values. The background was less than 20/106 PBMC (four spots per well at 200,000 PBMC per well). Cells were sorted in accordance with the manufacturer's instructions using magnetic MACS microbeads coated with anti-CD4 and anti-CD8 MAbs, respectively (Miltenyi Biotec, Auburn, Calif.). Briefly, PBMC were magnetically labeled by incubation together with the MACS microbeads for 10 min at room temperature, washed, and then run through a MACS column applied to a magnet. The cells collected initially thus represented the negatively sorted fraction. Following removal of the column from the magnet, positively sorted cells were eluted directly from the column.
Tetramers were prepared as previously described (1). The B7-expressing plasmid was kindly provided by G. Gillespie. Staining of PBMC by tetramers was done as follows. Cells were incubated for 30 min at 4°C with a tetramer at 0.5 mg/ml and then for a further 10 min with saturating amounts of a peridinin chlorophyll-α (PerCP)-conjugated anti-CD8 MAb and a fluorescein isothiocyanate-conjugated anti-CD4 MAb (Becton Dickinson). Stained samples were analyzed on a FACSCalibur flow cytometer using CellQuest software. Control samples for tetramer staining were PBMC from HLA-mismatched persons. Quadrant boundaries for tetramer staining were established by exclusion of >99.99% of control CD8+ T cells.
Peptide-specific CTL lines were generated as previously described (8). Briefly, pelleted PBMC were resuspended in 100 μl of 200 μM peptide NP-38 and incubated at 37°C for 1 h. These cells were then resuspended at 1.5 × 106/ml in wells of a 24-well plate (Costar) in RPMI 1640 medium (Sigma)–10% fetal calf serum (Sigma)–20 ng of interleukin-7 (IL-7; R&D Systems, Minneapolis, Minn.) per ml–10 mM HEPES buffer (Sigma) with antibiotics (2 mM l-glutamine, penicillin-streptomycin at 50 U/ml. Following a week in culture at 37°C and 5% CO2, medium exchanges were performed twice weekly with the same medium, except that 50 U of recombinant IL-2 (kindly provided by M. Gately, Hoffmann-La Roche [Nutley, N.J.]) per ml replaced the IL-7. Chromium release assays were performed following 2 to 4 weeks of culture.
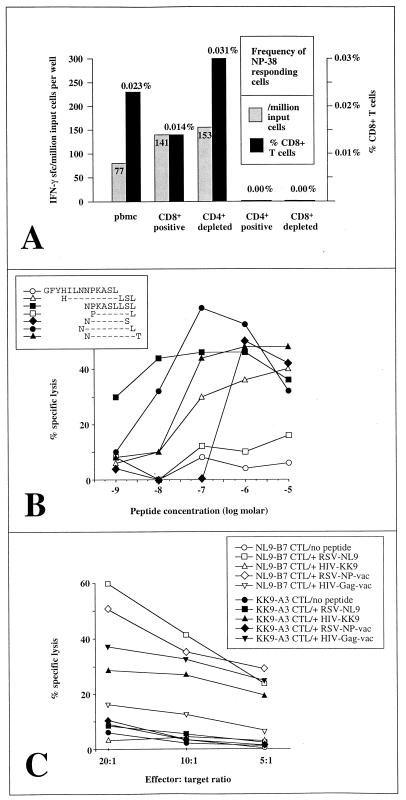
PBMC from subject A, a healthy adult volunteer, were screened in an Elispot assay for activity in response to each of the 48 overlapping NP peptides. Responses to the overlapping 16-mer peptide NP-38 (amino acid sequence, GFYHILNNPKASLLSL) were observed (Fig. 1A). Truncations of the 16-mer were synthesized and tested for recognition by a peptide-specific CTL line (Fig. 1B). The optimal epitope, defined as the peptide able to sensitize targets for 50% of maximal lysis by CTL clones at the lowest peptide concentration, was peptide NPKASLLSL (NL9). This response was HLA B7 restricted (Fig. 1C and data not shown). Only target cells presenting HLA B7 and pulsed with the NL9 peptide were recognized by the NL9-specific CTL line. Moreover, the specific binding of the B7-NL9 tetramer to these effector cells reconfirmed the HLA restriction of the response (see below).
FIG. 1.
(A) Elispot assay using unsorted and sorted PBMC from subject A (HLA type, A2/3 B7/51 Cw5/7). Responses to the 16-mer NP-38 (amino acid sequence, GFYHILNNPKASLLSL). The frequency of IFN-γ sfc per million input cells per well is shown in gray, and percent sfc among CD8+ T cells is shown in black. (B) Optimization of the epitope within the 16-mer NP-38. The optimal epitope is the 9-mer NL9. Effectors were a peptide-specific CTL line generated from subject A, and targets were HLA B7-matched Epstein-Barr virus-transformed B-lymphoblastoid cells from donor 004-TCH (HLA type, A∗0202/29 B7/B44 Cw7/7). (C) Recognition of processed NL9 peptide by RSV-specific effectors. Effectors were RSV NP-specific NL9-B7-restricted effectors (NL9-B7), as described for panel B, and HIV Gag-specific KK9-A3-restricted effectors (KK9-A3) from donor 019-TCH (HLA type, A3/30 B51/60 Cw 15/16). Targets were B-lymphoblastoid cells from subject B (HLA type, A3/− B7/− Cw7/−). Targets were either pulsed with no peptide, with the RSV NL9 peptide, or with the HIV KK9 peptide or infected with RSV NP-vaccinia virus (vac) or with HIV Gag-vaccinia virus. Thus, open circles represent targets pulsed with no peptide and incubated with NL9-B7-specific CTLs, open squares represent targets pulsed with the RSV NL9 peptide and incubated with NL9-B7-specific effectors, etc.
To determine whether this epitope was presented by target cells processing the RSV NP, HLA B7-matched target cells were infected with an RSV NP-vaccinia virus recombinant (17). The targets were recognized as efficiently as the same targets pulsed with the RSV NL9 peptide. RSV-specific CTLs did not recognize the same targets pulsed with a control human immunodeficiency virus (HIV) peptide or targets that had been infected with an HIV Gag-vaccinia virus recombinant. Control HIV Gag-specific CTLs from an HIV-infected donor, which recognized an HLA A3-restricted epitope in p17 Gag, KIRLRPGGK (KK9; p17 Gag 18-26 [http://hiv-web.lanl.gov/immunology/index.html]), only lysed the same targets (also A3 matched) either when expressing an HIV Gag-vaccinia virus recombinant or when targets were pulsed with the HIV KK9 peptide (Fig. 1C).
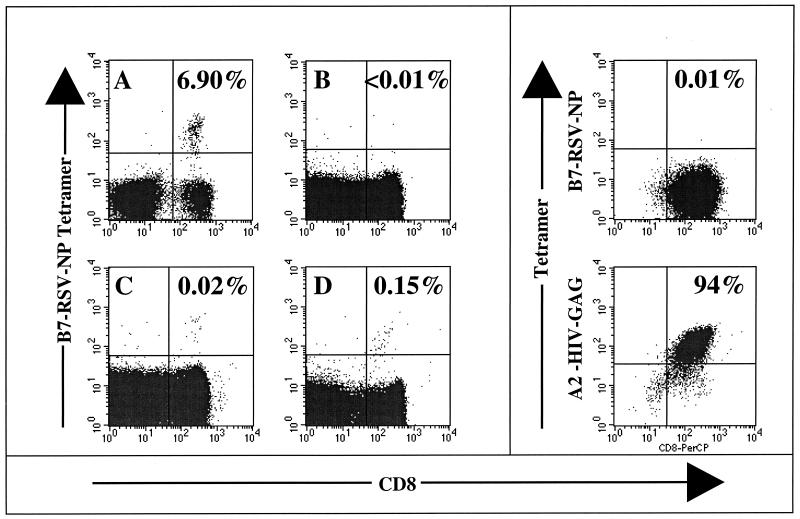
In order to characterize this response further, peptide NL9-HLA B7 tetramers were synthesized and used to stain PBMC from three further persons with B7 (subjects B to D) and two B7-negative persons (subjects E and F) (Fig. 2). In the B7-negative control subjects, the frequency of NP9-B7-specific CD8+ T cell binding to the tetramer was <0.01% of CD8+ T cells. NL9-B7-specific CD8+ T cells were detectable in all four donors with B7 who were studied, and the frequency of CD8+ T-cell binding to the NL9-B7 tetramer varied from 0.02 to 0.15% of the CD8+ T cells (Fig. 2). As a further negative control, CTL clones from a donor with HLA B7 (subject 161j [HLA type, A2/3 B7/60 Cw3/7]) that were specific for A2-HIV Gag epitope SLYNTVATL (http://hiv-web.lanl.gov/immunology/index.html) were stained with the B7-RSV NP tetramer (Fig. 2, top right), as well as with the A2-Gag tetramer (Fig. 2, bottom right). Despite the low frequency of PBMC from subjects A and C (both 0.02% of CD8+ T cells), NL9-specific CTL lines were successfully generated from both PBMC samples, in one case staining 6.9% of the CD8+ T cells (Fig. 2A) and in the other staining 40% of the CD8+ T cells (not shown). Additional support for the tetramer data shown is the finding of recognition of peptide NP-38 (frequency range, 1 of 13,883 to 1 of 25,000 PBMC) in Elispot assays using PBMC even in subjects A to C, whose PBMC showed a low frequency of tetramer staining, whereas no recognition of NP-38 was observed in Elispot assays of PBMC from the B7-negative subjects studied.
FIG. 2.
Staining of CD8+ T cells with the HLA B7-NL9 (RSV-NP) tetramer. (A) Peptide-specific line used in assays shown in Fig. 1 from subject A (HLA type, A2/3 B7/51 Cw5/7). (B) PBMC from B7-negative subject E (HLA type, A34/68 B51/72 Cw2/16). (C) PBMC from B7-positive subject C (HLA type, A3/26 B7/38 Cw7/8). (D) PBMC from subject D (HLA type, 11/25 B7/18 Cw7/12). (Top right) A2-HIV Gag CTL clones from subject 161j (HLA type, A2/3 B7/60 Cw3/7). (Bottom right) Staining of the same CTL clone as above but using the A2-HIV Gag tetramer.
In summary, RSV-specific CTL epitopes may be rapidly defined from memory CTLs in adults in spite of the low frequency (0.02 to 0.15% of CD8+ T cells in the case of the NL9-B7 epitope) with which these cells are present. Although the epitope described above lies within NP, previous studies (5) have shown that strong responses are also frequently directed toward epitopes within SH, F, M, and NS1b in human RSV-specific CTL activity. Thus, this definition of a single epitope is but a first step toward the characterization of the total RSV-specific CTL response. Once the dominant RSV-specific CTL epitopes have been identified, it will be possible to address the role of RSV-specific CTLs in controlling disease in pediatric infection. This would initially involve analysis of the timing of the appearance of RSV-specific CTLs with virus clearance and correlation of the magnitude of the CTL response with measures of disease severity and with viral load in age-matched children.
Acknowledgments
This work was supported by grants to P.J.R.G. from the Elizabeth Glaser Pediatric AIDS Foundation and the Medical Research Foundation (United Kingdom) (grant G108/274) and to B.D.W. through the National Institutes of Health (AI28568 and AI30914) and the Doris Duke Charitable Foundation. P.J.R.G. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. B.D.W. is a Doris Duke Distinguished Clinical Science Professor.
The gift of the B7-expressing plasmid from G. Gillespie is gratefully acknowledged. The RSV NP-vaccinia virus recombinant was one of the RSV-vaccinia virus recombinants made and donated by Peter Collins to the World Health Organization repository. The recombinant viruses from the World Health Organization Reagent Bank are maintained by Judy Beeler, Center for Biologics Evaluation and Research, Food and Drug Administration.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Bangham C R M, Openshaw P J M, Ball L A. Human and murine CTL specific to RSV recognize the viral nucleoprotein (NP) but not the major glycoprotein (G) expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 3.Bunce M, O'Neill C M, Barnardo M C, Krausa P, Browning M, Morris P, Welsh K. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 4.Cannon M J, Openshaw P J M, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with RSV. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherrie A H, Anderson K, Wertz G W, Openshaw P J M. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1981;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 7.Goulder, P. J. R., Y. Tang, C. Brander, M. Betts, M. A. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, Jr., K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 8.Goulder P J R, Brander C, Annamalai K, Mngqundaniso N, Govender U, Tang Y, He S, Hartman K E, O'Callaghan C A, Ogg G S, Altfeld M A, Rosenberg E S, Cao H, Kalams S A, Hammond M G, Bunce M, Pelton S I, Burchett S A, McIntosh K, Coovadia H M, Walker B D. Differential narrow focusing of immunodominant human immunodeficiency virus Gag-specific cytotoxic T-lymphocyte responses in infected African and Caucasoid adults and children. J Virol. 2000;74:5679–5690. doi: 10.1128/jvi.74.12.5679-5690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall C B. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 10.Hall C B, Powell K R, MacDonald N E. RSV infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 11.Hussell T, Baldwin C J, O'Garra A, Openshaw P J M. CD4+ T cells control Th2-driven pathology during pulmonary RSV infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs D, Bangham C R M, McMichael A J. Cell-mediated cytotoxic response to RSV in infants with bronchiolitis. Lancet. 1987;ii:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys by cell staining with a tetrameric MHC class I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMichael A J, Gotch F M, Noble G R, Beare P A. Cytotoxic T cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 16.Murali Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a revealuation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 17.Nicholas J A, Rubino K L, Levely M E, Adams E G, Collins P L. Cytolytic T-lymphocyte responses to respiratory syncytial virus effector cell phenotype and target proteins. J Virol. 1990;64:4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Openshaw P J M, Anderson K, Wertz G M, Askonas B A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64:1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Melero J A, Openshaw P J M. Eliminating a region of RSV attachment protein allows induction of protective immunity without vaccine-induced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A J, Callan M F. A reevaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 21.Weber M W, Mulholland E K, Greenwood B M. RSV infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–290. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 22.Yap K L, Ada G L, McKenzie I F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]