Abstract
Objective
The study was aimed to determine the survivorship and functional outcomes of modular endoprosthetic reconstruction in the management of primary and metastatic bone tumors of the lower limbs and to investigate the rate and causes of implant failure.
Methods
A total of 84 limbs of 82 patients (49 male, 33 female; mean age=48 years, age range=13–78 years) with a minimum follow-up of 12 months in whom resection and modular endoprosthetic reconstructions were performed for primary or metastatic bone tumors of the lower extremity were retrospectively reviewed and included in the study. The mean follow-up was 43 (range=13–119) months. Functional status was assessed using the Musculoskeletal Tumor Society (MSTS) scoring system at the final follow-up. Implant survival was defined as the time from implantation until partial or complete exchange of the prosthesis secondary to mechanical or nonmechanical causes or amputation. The effects of the anatomical site on functional scores and implant survival were statistically analyzed. Additionally, the effects of diagnosis and adjuvant treatments on functional scores, implant survival, and failure rates were investigated.
Results
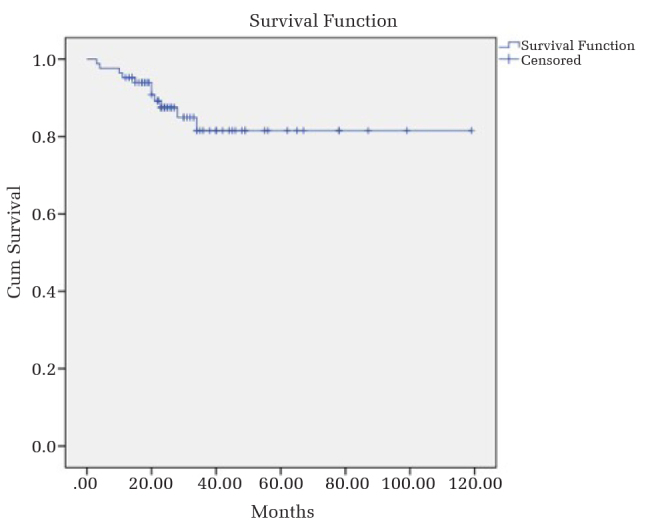
At the time of the study, 55 patients were still alive with a mean follow-up of 48 (range=15–119) months. The mean MSTS scores resulting from the final follow-up of all patients and of those surviving were 87.9% (range=16%–100%) and 86.8% (range=16%–100%), respectively. Overall implant survival was 95.2%, 89.2%, 87%, and 87% at 1, 2, 3, and 4 years, respectively. Statistically, both functional scores and implant survival analysis in different anatomical sites were found similar. In 15 of the patients (17.8%), endoprosthetic reconstructions had failed. The causes of failure were soft tissue failure (dislocation) in 5 patients, infection in 5, structural/mechanical failure in 2, local recurrence in two, and aseptic loosening in one. The diagnosis and receiving preoperative and/or postoperative adjuvant treatment did not affect functional scores, implant survival or failure rates.
Conclusion
The results of this study have shown that modular endoprosthetic replacement can provide satisfactory functional results and a durable mid-term limb salvage option in the management of patients with primary and metastatic bone tumors of the lower limbs.
Level of Evidence
Level IV, Therapeutic Study
Keywords: Limb salvage surgery, Modular endoprosthetic reconstruction, Lower extremity, Bone sarcomas, Metastatic bone tumors
Introduction
Better understanding of tumor biology, effective chemotherapy, improved imaging studies, new surgical techniques and prosthetic designs have enabled majority of bone cancers to be treated by limb salvage surgery. Lower extremity bone defects that mainly occur following removal of bone tumors can be managed by modular or custom-made endoprosthetic reconstruction or by biological reconstruction methods such as recycled tumor-bearing autografts with or without vascularized fibula autograft (VFA), VFA alone, bone transport methods using external fixators, massive bone allografts (MBA) combined with VFG, MBA alone and allograft-prosthesis composites (1–15).
In the past 30 years, modular endoprostheses have become the standard for tumor reconstructions about the hip and knee (11–15). These allow reconstruction of a wide variety of skeletal defects using off-the-shelf components without the expense or time required to manufacture a custom-made implant. Modular replacement prostheses can provide a stable and secure fixation which allows immediate weight-bearing and restoration of function in patients with primary or metastatic bone tumors. Besides their ability to provide a solid and functional limb, complications of endoprosthesis have also been reported in the literature including soft tissue failure, aseptic loosening, structural/mechanical failure, infection and tumor progression (16).
The overall survival and functional outcomes of megaprosthesis following limb-sparing surgery have been evaluated extensively in the literature (11–15, 17–22). However, there are few studies reported from the country in which the current study was conducted (23–25). We believe that the experience of a single musculoskeletal tumor referral center may also provide a contribution to the literature in terms of creating a standard approach for endoprosthetic replacements about the hip and knee. This study aimed to evaluate the overall survival of modular endoprosthetic reconstructions in the treatment of primary and metastatic bone tumors of the lower limbs and to assess the rate and causes of failure and ultimate functional outcome. The effect of the anatomical site on implant survival and functional scores was analyzed. In addition, the effect of diagnosis and adjuvant treatments on functional scores, implant survival and failure rates were investigated.
Materials and methods
Patients who underwent resection and modular endoprosthetic reconstruction for primary or metastatic bone tumors of the lower extremity between 2005 and 2013 were reviewed retrospectively. Patients lacking regular postoperative follow-up, having a follow-up period of less than 12 months or incomplete medical records were excluded from the cohort.
The study protocol was approved by the Local Ethics Committee of Marmara University School of Medicine (09.2013.153). Subsequently, the data were obtained from our extensive orthopedic oncology files, imaging studies, and operative and pathology reports. The age and sex of the patients, diagnosis and localization of the lesions, radiologic staging of primary tumors, previous oncological treatment and surgical treatment modalities were analyzed. Functional outcomes of the surgical procedure, implant survival, the rate and causes of failure were investigated. In addition, the overall survival of the patients was recorded.
All cases in this study were treated with a multidisciplinary approach based on the decision of the Bone and Soft Tissue Tumors Council of Marmara University Pendik Training and Research Hospital. Osteosarcoma and Ewing’s sarcoma patients had a standard treatment protocol including neoadjuvant and adjuvant chemotherapy, combined with wide resection and endoprosthetic reconstruction. In addition, 2 patients with Ewing’s sarcoma received postoperative radiotherapy for low tumor necrosis rate (one) or close surgical margin (one). Patients with chondrosarcoma and recurrent giant cell tumor of the bone (GCTB) underwent wide resection and endoprosthetic replacement, without any adjuvant treatment. Persistent pain and extensive bone destruction with actual or impending pathological fracture were the main indications for resection and endoprosthetic reconstruction in patients with bone metastasis in whom the Mirels score was at least 8 and in patients with multiple myeloma (MM) (26). The adjuvant treatments received by patients with metastatic bone disease and MM are summarized in Table 1.
Table 1.
The adjuvant treatments received by patients with metastatic bone disease and multiple myeloma
| Variable | Metastatic Bone Disease (%) | Multiple Myeloma (%) |
|---|---|---|
| Chemotherapy | ||
| Preoperative | 5 (13.1) | 2 (33.3) |
| Postoperative | 20 (52.6) | 1 (16.6) |
| Preoperative and postoperative | 9 (23.7) | - |
| Radiotherapy | ||
| Preoperative | 8 (21) | - |
| Postoperative | 13 (34.2) | 6 (100) |
| Preoperative and postoperative | 7 (18.4) | - |
We routinely performed magnetic resonance imaging showing entire length of the involved bone and the adjacent joint to determine intra- and extra-compartmental tumor extension. In addition, all patients had a whole-body bone scintigraphy (technetium-99 MDP whole-body bone scintigraphy) done as part of the radiological staging.
The surgical procedure included the resection of the tumor with wide margins followed by bone and soft tissue reconstruction. Surgical approaches for different anatomical sites were performed according to the general principles of limb salvage surgery (27, 28). The R classification was used to evaluate the surgical margin. According to the R classification, residual tumors are referred to as R0, R1, and R2 (R0: no residual tumor, margin ≥ 1mm; R1: no residual tumor [microscopic residual tumor], margin ≤1 mm; R2: macroscopic residual tumor) (29).
For thrombosis prophylaxis, 1×40 mg enoxaparin (low-molecular weight heparin derivative) was started 12–24 h before surgery and continued until the patients were able to ambulate. As antibiotic prophylaxis, cephalosporin with a total dose of 50 mg/kg was given twice daily at induction before surgery and administered until all drains were removed by the third or fourth postoperative day.
Patients with proximal and distal femoral reconstructions were allowed to bear full or partial weight immediately after surgery with walking aids. Those who underwent distal femoral reconstruction began isometric quadriceps exercises and mobilization of the knee on the second postoperative day. Following proximal tibial and extra-articular resections, the knee was kept in full extension for 3 weeks to allow healing of the extensor mechanism. Then extension exercises were started to prevent an extensor lag, followed by flexion of the knee.
A musculoskeletal oncology team consisting of 4 orthopedic surgeons did preoperative and postoperative clinical and radiological follow-up evaluations. Functional and radiological follow-up was performed at 3-month intervals in the first 2 years, at 6-month intervals in following 3 years, and then annually. Functional evaluation was done by Musculoskeletal Tumor Society (MSTS) scoring system (30).
Implant survival was defined as the time from implantation until partial or complete exchange (revision) of the prosthesis for mechanical (soft tissue failure, aseptic loosening, structural/mechanical failure) or nonmechanical (infection, tumor recurrence/progression) causes or amputation. Implant survival was not affected by interventions that did not consist of partial or complete exchange of the prosthesis. Complications resulting in reconstruction failure were also recorded. Henderson classification was used to define failure: Type 1, soft tissue failure; Type 2, aseptic loosening; Type 3, structural/mechanical failure; Type 4, infection; and Type 5, tumor recurrence (16).
We also grouped the patient cohort to see the effect of diagnosis and adjuvant treatments on MSTS scores, overall implant survival rates at 1, 2, 3 and 4 years, and the rate of complications resulting in reconstruction failure. Group 1 (metastatic bone disease and MM) and Group 2 (osteosarcoma and Ewing’s sarcoma) included patients who received neoadjuvant and/or adjuvant chemotherapy and radiotherapy. On the other hand, the patients in Group 3 (chondrosarcoma and GCTB) did not have any adjuvant treatment in the preoperative and postoperative period. First, we compared Group 1 and 2 to see if outcomes were changing or not with regard to the diagnosis. Then, Group 1+2 and Group 3 were compared to reveal if these parameters were affected by adjuvant treatments.
Descriptive analyses were presented using mean and standard deviation for normally distributed variables. The variables were investigated using visual (histogram, probability plots) and analytical methods (Kolmogorov-Simirnov\Shapiro-Wilk’s test) to determine whether or not they were normally distributed. Chi-square and t tests were used to compare categorical and continuous variables. Since MSTS scores were not normally distributed, nonparametric tests (Mann-Whitney U test) were used to compare these parameters. The Kaplan-Meier test was used for the endoprosthesis and patient survival analyses. The implantation of the prosthesis was the starting point of implant survival analysis. The end points were amputation or revision surgery for any cause, which was defined as partial or complete exchange of the endoprosthetic implant for any cause. The log-rank test was used to analyze different survival probabilities of factors. A p value of less than 0.05 was considered to be significant. Data obtained in the study were analyzed statistically using the Statistical Package for Social Sciences, version 22.0 software (IBM SPSS Corp.; Armonk, NY, USA).
Results
A total of 82 patients (49 male, 33 female) with primary or metastatic bone tumors of the lower extremity underwent 84 resection and endoprosthetic reconstructions. The mean age at surgery was 48±19.46 years (range, 13–78 years). The diagnosis of the tumors and localization of the lesions based on diagnosis are summarized in Tables 2 and 3 respectively. For primary tumors, radiological staging revealed stage IA (1), IB (1), IIA (2), IIB (28) and IIIB (2) malignant and, stage 3 benign (4) lesions according to the Enneking system (31). There were 4 patients with solitary metastasis, 7 with oligometastases [metastases only in a limited number (≤3) of sites] and 27 with multimetastases (metastases >3 sites).
Table 2.
The diagnosis of the tumors
| Diagnosis | n (%) |
|---|---|
| Metastatic bone disease | 38* (46.3) |
| Lung carcinoma | 16 (42.1) |
| Breast carcinoma | 12 (31.6) |
| Renal cell carcinoma | 4 (10.5) |
| Thyroid carcinoma | 2 (5.3) |
| Prostate carcinoma | 2 (5.3) |
| Bladder carcinoma | 1 (2.6) |
| Unknown origin | 1 (2.6) |
| Multiple myeloma | 6 (7.3) |
| Primary bone sarcoma | 34 (41.5) |
| Conventional osteosarcoma | 17 (50) |
| Ewing’s sarcoma | 9 (26.5) |
| High-grade chondrosarcoma | 6 (17.6) |
| Low-grade chondrosarcoma | 2 (5.9) |
| Recurrent giant cell tumor of bone | 4 (4.9) |
Two patients with metastatic bone disease had bilateral bone involvement
Table 3.
The anatomical localizations of the lesions based on diagnosis
| Diagnosis | Number of reconstructions | Proximal femur | Distal femur | Total femur | Proximal tibia |
|---|---|---|---|---|---|
| Metastatic bone disease or multiple myeloma, n (%) | 46 (54.7) | 41 (95.3) | 5 (17.2) | – | – |
| Primary bone sarcoma n (%) | 34 (40.5) | 2 (4.7) | 22 (75.9) | 4 (100) | 6 (75) |
| Giant cell tumor of bone, n (%) | 4 (4.8) | – | 2 (6.9) | – | 2 (25) |
| Total | 84 | 43 | 29 | 4 | 8 |
The mean follow-up was 43±18.96 months (range, 13–119 months); patients with metastatic disease/MM and primary bone tumors were followed a mean of 32±10.76 months (range, 13–56 months) and 52±11.74 months (range, 15–119 months) respectively. At the time of the study, 23 patients with metastatic disease or MM had died because of progression of the disease. In addition, 4 patients with primary bone sarcoma had died secondary to pulmonary metastases (3) or chemotherapy complications (one). 55 patients were still alive with a mean follow-up of 48±20.01 months (range, 15–119 months).
Surgical margins were reported as R0 (33) and R1 (one) following the resection of primary bone sarcomas. The case with R1 margin was an Ewing sarcoma, and underwent postoperative adjuvant radiotherapy. Except for one case with R1 margin, R0 margins were obtained in remaning 3 resections done for recurrent GCTB. We also achieved R0 margin in the majority of the patients (38/44; 86.4%) with metastatic bone disease or MM, even though clear margins do not affect patient survival, except for 4 patients with solitary renal cell (3) and thyroid (one) carcinoma metastasis. This approach was standardized r to prevent intra-operative tumor spillage and to minimize local tumor progression/recurrence in the postoperative period. The diagnoses of 6 patients with R1 and R2 margins were lung carcinoma (one), breast carcinoma (2), and MM (3), and all received postoperative radiotherapy.
Two different types of modular prostheses (in 56 patients; TMTS/HBRS, CoCrMo alloy, Hipokrat Inc, İzmir, Turkey and in 28 patients; MUTARS, TiA16V4 alloy, Implantcast, Buxtehude, Germany), which were available in the market at the time of the study, were used for reconstruction. Details of the surgical procedures are given in Table 4.
Table 4.
The details of the surgical procedures
|
Postoperative histopathological examination approved intra-articular tumor contamination in all.
Attachment tube: polyethylene terephthalate ‘Trivera tube,’ MUTARS, Implantcast, Buxtehude, Germany).
GC: gastrocnemius; GM: gluteus medius; VL: vastus lateralis muscles
The mean MSTS scores at the last follow-up of all patients and of those surviving were 87.9%±13.24% (range, 16%–100%) and 86.8%±12.72% (range, 16%–100%) respectively. Table 5 summarizes functional scores of endoprosthetic reconstructions in different anatomical sites, demonstrating a statistically insignificant difference (p=0.572). The last follow-up MSTS scores of metastatic/MM patients (Group 1) and osteosarcoma/Ewing’s sarcoma patients (Group 2) did not show a statistically significant difference (p=0.421). Additionally, final MSTS scores were similar when comparing Groups 1 and 2 with Group 3, which included patients with chondrosarcoma and GCTB, demonstrating that adjuvant treatments did not affect functional improvement (p=0.096).
Table 5.
The MSTS scores with regard to anatomical localizations of all patients and of those surviving at the time of study
| Anatomical localizations of reconstructions | MSTS scores due to the latest follow-up; mean% (SD) | |
|---|---|---|
|
| ||
| All patients (%) | Patients surviving at the time of study (%) | |
| Proximal femur | 87.2±9.99 | 85.7±14.3 |
| Distal femur | 88.4±17.24 | 87.7±13.7 |
| Proximal tibia | 86.2±15.56 | 86.2±15.56 |
| Total femur | 89.9±13.4 | 89.9±13.4 |
| Overall | 87.9±13.24 | 86.8±12.37 |
| p values (Mann-Whitney U test) | p=0.071 | p=0.092 |
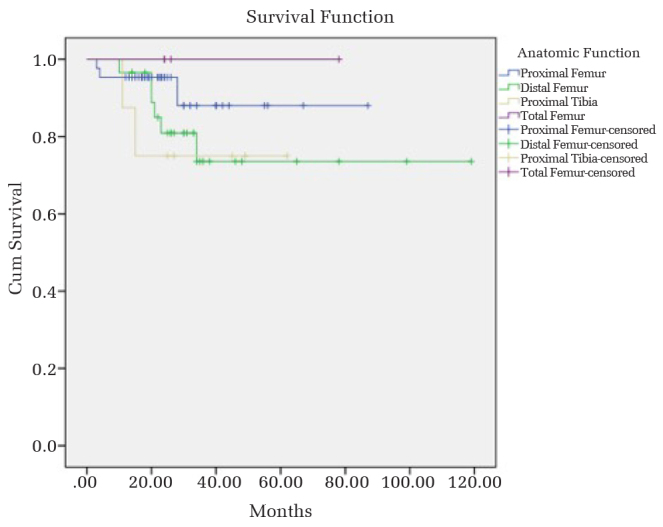
Table 6 shows the overall implant survival and survivorship of endoprostheses with respect to specific anatomic localizations. Based on the Kaplan-Meier analysis, the overall survival of endoprosthetic reconstructions and endoprosthetic survivorship in terms of anatomical localization are shown in Figures 1 and 2, respectively. The log-rank test showed no statistical difference of implant survival probabilities among anatomical localizations (p=0.452). The implant survival rates of Groups 1, 2 and 3 at 1, 2, 3 and 4 years are shown on Table 7. Even though a statistical analysis was not performed, implant survival rates were similar in all groups, giving an impression that diagnosis and adjuvant treatments did not affect this parameter.
Table 6.
The overall implant survival and survivorship of the implants with respect to specific anatomical localizations
| Anatomical localizations of endoprosthesis | 1-year survival | 2-year survival | 3-year survival | 4-year survival |
|---|---|---|---|---|
| Overall survival | 95.2% (80/84) | 89.2% (75/84) | 87% (73/84) | 87% (73/84) |
| Proximal femur | 95.3% (41/43) | 95.3% (41/43) | 93% (40/43) | 93% (40/43) |
| Distal femur | 96.5% (28/29) | 82.7% (24/29) | 79.3% (23/29) | 79.3% (23/29) |
| Total femur | 100% (4/4) | 100% (4/4) | 100% (4/4) | 100% (4/4) |
| Proximal tibia | 87.5% (7/8) | 75% (6/8) | 75% (6/8) | 75% (6/8) |
| p values (log-rank test) | p=0.165 | p=0.091 | p=0.432 | p=0.302 |
Figure 1.
Overall survival of endoprosthetic reconstructions
Figure 2.
Endoprosthetic survival chart in terms of anatomical localizations
Table 7.
Implant survival rates at 1-, 2-, 3-, and 4-year based on diagnosis
| Diagnosis | 1-year (%) | 2-year (%) | 3-year (%) | 4-year (%) |
|---|---|---|---|---|
| Metastatic bone disease/ MM (Group 1) | 97 | 86 | 86 | 79 |
| OS/ EWS | ||||
| (Group 2) | 95 | 82 | 80 | 76 |
| CS/ GCTB | ||||
| (Group 3) | 100 | 84 | 84 | 84 |
MM: Multiple myeloma; OS: Osteosarcoma; EWS; Ewing sarcoma; CS: Chondro sarcoma, GCTB: Giant cell tumor of bone
Fifteen (17.8%) of 84 reconstructions failed, requiring partial (3 patients; 3.6%) or complete (3 patients; 3.6%) exchange of the prosthesis, soft tissue reconstruction to restore joint stability (5 patients; 5.9%) and amputation (4 patient; 4.7%). Table 8 demonstrates the causes of reconstruction failures and how they were managed during follow-up. Soft tissue failure (Henderson Type 1 failure) leading to the dislocation of the hip joint was seen in 4 THAs (4/13; 30.7%) and one bipolar prosthesis was seen (1/34; 2.9%), indicating the type of articulation was an important prognostic factor (p=0.024). Two late infections were managed by amputation as the definitive treatment at initial presentation, because of the insufficient soft tissue coverage. Statistically, the rate of complications resulting in reconstruction failure was similar between Groups 1 and 2 (p=0.054), and Group 1 + 2 and Group 3 (p=0.636).
Table 8.
The causes of reconstruction failures and their management during follow-up
| Patient | Diagnosis | Localization | Complication (Type of failure*) | Time of complication | Treatment | Final status at F-U |
|---|---|---|---|---|---|---|
| 1 | Lung Ca | Proximal femur | Dislocation (Type 1 failure) | p.o 1 mo | OR + ST Reconstruction | Event free |
| 2 | Breast Ca | Proximal femur | Dislocation (Type 1 failure) | p.o 2 mo(s) | OR + ST Reconstruction | Event free |
| 3 | OS | Proximal femur | Dislocation (Type 1 failure) | p.o 1 mo | OR + ST Reconstruction | Event free |
| 4 | Breast Ca | Proximal femur | Dislocation (Type 1 failure) | p.o 4 mo(s) | OR + ST Reconstruction | Event free |
| 5 | RCC | Proximal femur | Dislocation (Type 1 failure) | p.o 2 mo(s) | OR + ST Reconstruction | Event free |
| 6 | Recurrent GCTB | Distal femur | Aseptic loosening (Type 2 failure) | p.o 10 mo(s) | One-stage revision (partial exchange of EPR) | Event free |
| 7 | OS | Proximal femur | Stem fracture (Type 3 failure) | p.o 30 mo(s) | One-stage revision (Complete exchange of EPR) | Event free |
| 8 | Lung Ca | Distal femur | Polyethylene wear (Type 3 failure) | p.o 18 mo(s) | One-stage revision (partial exchange of EPR) | Event free |
| 9 | EWS | Proximal femur | Infection (Type 4 failure) | p.o 1 mo | Debridement + One-stage revision (partial exchange of EPR) | Event free |
| 10 | OS | Distal femur | Infection (Type 4 failure) | p.o 10 mo(s) | Two-stage revision (complete exchange of EPR) | Event free |
| 11 | H-G CS | Proximal tibia | Infection (Type 4 failure) | p.o 12 mo(s) | Two-stage revision (complete exchange of EPR) | Recurrent infection → Amputation |
| 12 | OS | Distal femur | Infection (Type 4 failure) | p.o 16 mo(s) | Amputation | Event free |
| 13 | H-G CS | Proximal tibia | Infection (Type 4 failure) | p.o 18 mo(s) | Amputation | Event free |
| 14 | OS | Distal femur | Local recurrence (Type 5 failure) | p.o 14 mo(s) | Amputation + Systemic CT | Event free |
| 15 | OS | Distal femur | Local recurrence (Type 5 failure) | p.o 26 mo(s) | Amputation+ Systemic CT | Event free |
Failures due to Henderson classification.
Ca: carcinoma; OS: osteosarcoma; RCC: renal cell carcinoma; GCTB: giant cell tumor of bone; EWS: Ewing’s sarcoma; H-G CS: high-grade chondrosarcoma; p.o: postoperative; mo(s): month(s); OR: open reduction; ST: soft tissue; CT: chemotherapy; F-U: follow-up; EPR: endoprosthetic reconstruction
Discussion
The current study demonstrated that modular endoprosthetic reconstruction of the lower extremities could provide good functional outcomes and high implant survival rates with a relatively low rate of complications resulting in reconstruction failure in mid-term follow-up. The functional scores and implant survival analysis of endoprosthetic reconstructions in different anatomical sites did not show statistically significant differences. Soft tissue failure/dislocation (Henderson Type 1 failure) and infection (Henderson Type 4 failure) were the leading causes of failure following reconstructions around the hip and knee respectively. Bipolar articulation in the hip was indicated as an important prognostic factor to prevent dislocation. Even though it was not significant statistically, reconstructions around the knee were more prone to partial or complete exchange of the prosthesis in our study. The diagnosis and preoperative and/or postoperative adjuvant treatment did not affect functional scores, implant survival and implant failure rates.
Endoprosthetic reconstruction of the lower extremities following bone tumor resection usually provides immediate weight-bearing and a well-functioning, durable reconstruct in the long term. High MSTS scores, ranging from 60% to 80%, have usually been achieved with long-term follow-up of endoproshetic replacements of the hip and knee regions (15, 20, 24). In a long-term analysis of 32 patients with primary bone sarcomas of the femur and proximal tibia, Ham et al. obtained the highest functional scores in patients with distal femoral endoprosthesis, and the lowest functional scores in patients with total femoral replacements (17). In the current series, a mean MSTS score of 87.9% was achieved at mid-term follow-up of patients who underwent limb salvage with endoprosthetic reconstruction for lower extremity bone neoplasia. Functional scores did not differ statistically with anatomical sites. We can conclude that a standard surgical approach in all locations and individualized postoperative rehabilitation allow early mobilization and clinical recovery, which contribute to relatively high MSTS scores at mid-term follow-up.
Survivorship and complications of endoprosthetic reconstructions have been studied in large series including primary and/or metastatic bone tumors (11–15, 17, 21, 22). A literature search reveals that high, long-term implant survival rates can be achieved with modular or custom-made endoprostheses in the lower extremity (15, 17, 32–34). Ahlmann et al. achieved an overall implant survival of 78%, 60% and 60% at 5, 10 and 15 years of follow-up in patients who had undergone limb salvage with endoprostheses for lower extremity bone neoplasia (15). In a series of 250 patients with malignant bone and soft-tissue tumors of the upper and lower extremities, Gosheger et al. reported 89.7% and 68.5% 5-year prosthetic survival rates for the upper and lower extremities respectively (18). Prosthetic survival without any re-operation was 73.4% at 3 years and 60.4% at 5 years postoperatively. The results of the current study were comparable to the literature data. Even though, the survivorship of endoprostheses with respect to anatomical localization revealed a higher rate in the proximal femur compared to distal femur and proximal tibia, this was not significant statistically. Total femoral endoprosthetic replacements achieved 100% implant survival rate at 4 years, supporting the high overall survival of total femoral endoprosthetic replacement (35, 36).
Instability remains a frequent cause of failure following endoprosthetic reconstruction of the proximal femur (15, 18, 21, 37). Type of articulation (bipolar versus THA), preservation of the joint capsule and technique of abductor repair are the factors affecting hip instability. In the current series, soft tissue failure (Henderson Type 1 failure) leading to dislocation of the hip joint was seen in 5.9% of the reconstructions within 4 months of the initial procedure. We agree that acetabular preservation and a meticulous soft tissue reconstruction including capsulorrhaphy and abductor mechanism repair recreate hip stability and avoid dislocation (18, 36, 38). In the current series, in patients with metastatic bone disease or MM of the proximal femur, we preserved the continuity of the abductor mechanism by a split trochanteric osteotomy if the trochanter major had not been invaded by the tumor. On the other hand, in the remaining metastatic/ myeloma lesions or primary tumors of this region, the trochanter major was removed and abductor repair was achieved by primary suturing of the gluteus medius and vastus lateralis muscles, and the use of Trevira tube/prolene mesh, when there was soft tissue defect.
In the current series, the rates of aseptic loosening and mechanical failure were 1.1% and 2.3% respectively. These rates were lower than that reported in the literature, ranging from 2.4% to 19% for aseptic loosening and 4.3% to 48% for mechanical complications (15, 18, 21, 39). Aseptic loosening was observed in one patient with a distal femoral metadiaphyseal resection reconstructed by an uncemented endoprosthesis. As mentioned by Gosheger et al. cemented stems should be preferred in reconstructions of distal femoral metadiaphyseal defects (18). The lower incidence of mechanical complications was probably related to the relatively short follow-up.
Infection is a leading cause of failure following endoprosthetic reconstruction of the lower extremities. In a retrospective analysis of 50 consecutive resections and endoprosthetic reconstructions for tumors around the knee, Sim et al. reported 4 superficial wound infections (8%) and 6 deep infections (12%) (19). Gosheger et al. emphasized deep prosthetic infection as the most common complication following endoprosthetic reconstruction in extremities (18). Predilection sites were proximal femur (19.5%), proximal tibia (16.7%), and distal femur (11.7%). In the current series, the infection rate was 5.9%. When compared to similar series in the literature with 3 to 25% infection rates (15, 17, 21, 34), our overall infection rate was acceptable. However, we experienced a high rate of infection following proximal tibial reconstructions, even though a transpositional medial gastrocnemius flap was routinely used for soft tissue coverage. This can be explained by the low number of proximal tibial reconstructions in the current series.
Even though one patient with an early infection was managed by mechanical debridement and change of mobile components, the remaining 4 patients with late infections required a two-stage revision or amputation in our series. One of the 2 patients who underwent two-stage revision had a recurrent infection, and an amputation was performed to overcome this complication. Insufficient soft tissue envelope was the main indication for an amputation at initial presentation in the remaining 2 patients. We think that if a healthy, soft tissue coverage cannot be obtained by local flaps, which might already be used in the first surgery in some patients, or if local conditions are not suitable for free flaps, then an amputation is a reasonable option at initial treatment of an infected megaprosthesis.
The local recurrence rate of 5.8% (2 in 34 sarcomas) seen in this series compares favorably with previous reports for primary bone sarcomas (15, 17, 22). Achieving a good response to chemotherapy and tumor free margins can explain the acceptable rates of local recurrence. Even though, an aggressive approach including amputation and systemic chemotherapy was applied, tumor relapse was associated with poor prognosis.
When 3 groups of the patient cohort were compared with regard to MSTS scores, implant survival rates and failure, the outcomes were found to be similar, indicating that diagnosis and preoperative and/or postoperative adjuvant treatment did not have a significant effect on functional improvement, implant survival, and implant failure rates, following endoprosthetic reconstruction of the lower extremities.
This study has some limitations. First, it is a retrospective study of a mixed group of patients. This might be a disadvantage, but bone tumors requiring endoprosthetic reconstruction are rare, limiting the overall number of procedures performed at any single institution. The second limitation is the lack of a control group. Third, the duration of follow-up is short. Fourth, even though frequently used in clinical studies, the MSTS score is not a Turkish-translated and validated score. Finally, the fifth limitation is the lack of a preoperative functional outcome score and a quality of life measure. On the other hand, all procedures were performed in a standard approach by the same surgeon in the same institution. In addition, the patients were followed up extensively with detailed clinical, functional and oncological records.
In conclusion, this study demonstrates that modular endoprosthetic reconstruction of the lower extremities can provide good functional outcomes and high implant survival rates with acceptable failure rates at mid-term follow-up. The functional scores of endoprosthetic reconstructions in different anatomical sites did not show a statistically significant difference. Even though, the survivorship of endoprostheses with respect to anatomical localization revealed a higher rate in the proximal femur compared to distal femur and proximal tibia, this was not significant statistically. Soft tissue failure/dislocation and infection were the most common causes of failure. Bipolar hip articulation was defined as an important prognostic factor to decrease the rate of dislocation.
HIGHLIGHTS.
Modular endoprosthetic reconstructions can provide satisfactory functional results in the management of patients with primary and metastatic diseases in lower extremity.
According to different anatomical sites, functional scores of endoprosthetic reconstructions did not show a statistically significant difference.
Bipolar hip articulation was found to be an important prognostic factor to decrease the rate of dislocation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Local Ethics Committee of Marmara University (06.09.2013, decision no: 09.2013.153).
Informed Consent: N/A.
Author Contributions: Concept - O.M.T., B.E.; Design - Ö.S., O.M.T.; Supervision - O.M.T., B.E.; Resources - O.M.T., Ö.S.; Materials - O.M.T., E.Ş., Ö.S.; Data Collection and/or Processing - Ö.S., E.Ş.; Analysis and/or Interpretation - O.M.T., E.Ş.; Literature Search - Ö.S., E.Ş.; Writing Manuscript - O.M.T., E.Ş., Ö.S.; Critical Review - E.Ş., B.E.
Conflict of Interests: The authors have no conflict of interests to declare.
Financial Disclosure: The authors declare that this study has received no financial support.
References
- 1. Mankin HJ, Gebhardt MC, Jenning LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 2. Ortiz-Cruz E, Gebhardt MC, Jennings LC, Springfield DS, Mankin HJ. The results of transplantation of intercalary allografts after resection of tumors. a long-term follow-up study. J Bone Joint Surg Am. 1997;79:97–106. doi: 10.2106/00004623-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 3. Manabe J, Ahmed AR, Kawaguchi N, Matsumoto S, Kuroda H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:258–66. doi: 10.1097/00003086-200402000-00042. [DOI] [PubMed] [Google Scholar]
- 4. Uyttendaele D, De Schryver A, Claessens H, Roels H, Berkvens P, Mondelaers W. Limb conservation in primary bone tumors by resection, extracorporeal irradiation and re-implantation. J Bone Joint Surg Br. 1988;70:348–53. doi: 10.1302/0301-620X.70B3.3163694. [DOI] [PubMed] [Google Scholar]
- 5. Tsuchiya H, Wan SL, Sakayama K, Yamamoto N, Nishida H, Tomita K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J Bone Joint Surg Br. 2005;87:218–25. doi: 10.1302/0301-620X.87B2.15325. [DOI] [PubMed] [Google Scholar]
- 6. Hsu RW, Wood MB, Sim FH, Chao EY. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br. 1997;79:36–42. doi: 10.1302/0301-620X.79B1.0790036. [DOI] [PubMed] [Google Scholar]
- 7. Zaretski A, Amir A, Meller I, et al. Free fibula long bone reconstruction in orthopedic oncology: a surgical algorithm for reconstructive options. Plast Reconstr Surg. 2004;113:1989–2000. doi: 10.1097/01.PRS.0000122213.82011.C5. [DOI] [PubMed] [Google Scholar]
- 8. Tsuchiya H, Tomita K, Minematsu K, Mori Y, Asada N, Kitano S. Limb salvage using distraction osteogenesis. a classification of the technique. J Bone Joint Surg Br. 1997;79:403–11. doi: 10.1302/0301-620X.79B3.0790403. [DOI] [PubMed] [Google Scholar]
- 9. Anacak Y, Sabah D, Demirci S, Kamer S. Intraoperative extracorporeal irradiation and re-implantation of involved bone for the treatment of musculoskeletal tumors. J Exp Clin Cancer Res. 2007;26:571–4. [PubMed] [Google Scholar]
- 10. Erol B, Bascı O, Topkar MO, Caypinar B, Basar H, Tetik C. Mid-term radiological and functional results of biological reconstructions of extremity-located bone sarcomas in children and young adults. J Pediatr Orthop B. 2015;24:469–78. doi: 10.1097/BPB.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 11. Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89:1632–7. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 12. Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–6. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 13. Plötz W, Rechl H, Burgkart R, et al. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin Orthop Relat Res. 2002;405:207–15. doi: 10.1097/00003086-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 14. Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–93. doi: 10.2106/00004623-200606000-00016. [DOI] [PubMed] [Google Scholar]
- 15. Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–5. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 16. Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–29. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 17. Ham SJ, Schraffordt Koops H, Veth RPH, van Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Annals of Surg Oncol. 1998;5:423–36. doi: 10.1007/BF02303861. [DOI] [PubMed] [Google Scholar]
- 18. Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–71. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 19. Sim IW, Tse LF, Ek ET, Powell GJ, Choong PFM. Salvaging the limb salvage: management of complications following endoprosthetic reconstruction for tumors around the knee. Eur J Surg Oncol. 2007;33:796–802. doi: 10.1016/j.ejso.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20. Malo M, Davis AM, Wunder J, et al. Functional evaluation in distal femoral endoprosthetic replacement for bone sarcoma. Clin Orthop Relat Res. 2001;389:173–80. doi: 10.1097/00003086-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 21. Shehadeh A, Noveau J, Malawer M, Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010;468:2885–95. doi: 10.1007/s11999-010-1454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30:458–64. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selek H, Basarır K, Yıldız Y, Saglık Y. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty. 2008;23:112–7. doi: 10.1016/j.arth.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 24. Yalnız E, Çiftdemir M, Memisoglu S. Functional results of patients treated with modular prosthetic replacement for bone tumors of the extremities. Acta Orthop Traumatol Turc. 2008;42:238–45. doi: 10.3944/AOTT.2008.238. [DOI] [PubMed] [Google Scholar]
- 25. Bekmez S, Ayvaz M, Yücekul A, Tokgozoglu M. Modular cementless prosthetic reconstruction after resection of lower extremity malignant tumor. Acta Orthop Traumatol Turc. 2016;50:674–80. doi: 10.1016/j.aott.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirels H. Metastatic disease in long bones. a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–64. doi: 10.1097/00003086-198912000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Malawer MM, Sugarbaker PH, editors. Musculoskeletal cancer surgery: treatment of sarcomas and allied diseases. Dordrecht: Kluwert: Academic Publishers; 2001. [Google Scholar]
- 28.Simon MA, Springfield D, editors. Surgery for bone and soft-tissue tumors. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 29. Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115:3483–8. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 30. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–6. doi: 10.1097/00003086-199301000-00035. [DOI] [PubMed] [Google Scholar]
- 31. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop Relat Res. 2003;415:4–18. doi: 10.1097/01.blo.0000093891.12372.0f. [DOI] [PubMed] [Google Scholar]
- 32. Smolle MA, Andreou D, Tunn PU, Leithner A. Advances in tumour endoprostheses: a systematic review. EFORT Open Rev. 2019;4:445–59. doi: 10.1302/2058-5241.4.180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wedin R, Bauer HCF. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–7. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]
- 34. Quill G, Gitelis S, Morton T, Piasecki P. Complications associated with limb salvage for extremity sarcomas and their management. Clin Orthop Relat Res. 1990;260:242–50. doi: 10.1097/00003086-199011000-00040. [DOI] [PubMed] [Google Scholar]
- 35. Mankin HJ, Hornicek FJ, Harris M. Total femur replacement procedures in tumor treatment. Clin Orthop Relat Res. 2005;438:60–4. doi: 10.1097/00003086-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 36. Ruggieri P, Bosco G, Pala E, Errani C, Mercuri M. Local recurrence, survival and function after total femur resection and megaprosthetic reconstruction for bone sarcomas. Clin Orthop Relat Res. 2010;468:2860–6. doi: 10.1007/s11999-010-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Potter BK, Chow VE, Adams SC, Letson GD, Temple HT. Endoprosthetic proximal femur replacement: metastatic versus primary tumors. Surg Oncol. 2009;18:343–9. doi: 10.1016/j.suronc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 38. Gosheger G, Hillmann A, Lindner N, et al. Soft tissue reconstruction of megaprostheses using a Trevira tube. Clin Orthop Relat Res. 2001;393:264–71. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 39. Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. doi: 10.1302/0301-620X.78B1.0780005. [DOI] [PubMed] [Google Scholar]


