Abstract
Development of disease is extremely rare in chimpanzees when inoculated with either T-cell-line-adapted neutralization-sensitive or primary human immunodeficiency virus type 1 (HIV-1), at first excluding a role for HIV-1 neutralization sensitivity in the clinical course of infection. Interestingly, we observed that short-term in vivo and in vitro passage of primary HIV-1 isolates through chimpanzee peripheral blood mononuclear cells (PBMC) resulted in a neutralization-sensitive phenotype. Furthermore, an HIV-1 variant reisolated from a chimpanzee 10 years after experimental infection was still sensitive to neutralization by soluble CD4, the CD4 binding site recognizing antibody IgG1b12 and autologous chimpanzee serum samples, but had become relatively resistant to neutralization by polyclonal human sera and neutralizing monoclonal antibodies. The initial adaptation of HIV-1 to replicate in chimpanzee PBMC seemed to coincide with a selection for viruses with low replicative kinetics. Neither coreceptor usage nor the expression level of CD4, CCR5, or CXCR4 on chimpanzee PBMC compared to human cells could explain the phenotypic changes observed in these chimpanzee-passaged viruses. Our data suggest that the increased neutralization sensitivity of HIV-1 after replication in chimpanzee cells may in part contribute to the long-term asymptomatic HIV-1 infection in experimentally infected chimpanzees.
Chimpanzees (Pan troglodytes) are the most commonly used nonhuman primates infected with human immunodeficiency virus type 1 (HIV-1). Upon inoculation of chimpanzees with HIV-1, virus isolation from peripheral blood mononuclear cells (PBMC) was repeatedly successful, and the development of HIV-1-specific antibodies together with an HIV-1-specific cytotoxic-T-lymphocyte response could be demonstrated (1, 2, 4, 17, 44, 45, 52). However, despite persistent HIV replication in vivo, clinical progression to AIDS-defining illnesses was generally not observed (17, 24, 44, 64), which reduced the relevance of this animal model for pathogenesis studies. A number of mechanisms have been postulated to explain the absence of disease progression. These include the inability of chimpanzee CD4+ cells to support HIV-1-induced syncytium formation (8), the inability of chimpanzee macrophages to support HIV-1 replication (18, 57, 64), and reduced HIV-induced apoptotic cell death in these animals (20, 23, 57). However, HIV-1 viruses highly adapted to replicate in chimpanzee PBMC through multiple in vivo or repetitious in vitro passages could form syncytia, replicated in macrophages, and induced apoptosis in CD4+ T cells (14, 18, 47, 59). Other proposed mechanisms for long-term survival of chimpanzees are the lack of impaired CD4+ T-cell renewal (24), major histocompatibility complex polymorphisms (2), differences in production of CD8+ T-cell factor (22), and the role of infiltration of CD8+ T cells in lymphoid tissue (29), which all remain to be clarified (25).
During HIV-1 infection in humans, increased replicative capacity of the virus is associated with an increase in viral load and subsequent disease progression. In 50% of the cases, a switch from non-syncytium- to syncytium-inducing variants (from CCR5 to CXCR4 usage) (12, 63) can be observed, which correlates with an accelerated CD4 T-cell decline and a more rapid disease progression (30). Although no correlation between disease progression and serum-neutralizing antibody responses were found (10, 21), a more broadly effective antibody response was found during the course of infection, which was most pronounced in long-term nonprogressors (37, 41, 48, 54, 67). This may indicate that normal regulation of the antibody response in HIV-1 infected individuals is impaired and therefore development of escape mutants cannot be prevented (5). It has been suggested that in long-term nonprogressors a more vigorous antibody response can develop, possibly because of reduced viral replication (54, 68).
Biological properties of HIV-1 such as replication rate and neutralization sensitivity, both considered relevant for AIDS pathogenesis, have not been extensively studied in chimpanzees. Here we studied primary HIV-1 variants after short-term in vivo and in vitro passage through chimpanzee cells and compared them with an HIV-LAI variant reisolated 10 years after experimental infection of a chimpanzee.
MATERIALS AND METHODS
Primary cells.
PBMC were isolated from buffy coats obtained from healthy blood donors or from heparinized vena punctures of healthy HIV-1-negative chimpanzees by Ficoll-Isopaque density gradient centrifugation. Cells (5 × 106/ml) were stimulated for 3 days in Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and phytohemagglutinin (PHA; 5 μg/ml). Subsequently, cells (106/ml) were grown in the absence of PHA, in medium supplemented with recombinant interleukin-2 (10 U/ml, a kind gift of R. Rombouts, Chiron Benelux). Prior to use of chimpanzee PBMC in 50% tissue culture infective dose (TCID50) or neutralization assays, CD8+ T cells were depleted using anti-CD8-coated magnetic beads (Dynal).
Viruses.
Virus isolation and virus stock preparation were performed on PHA-stimulated PBMC according to standard procedures (56). The HAN2 isolate was obtained from the European Programme for a Vaccine against AIDS (Programme EVA, Potters Bar, United Kingdom). Isolation and properties of the virus have previously been described (53). An inoculum of the HIV-LAI strain prepared on the H9 T-cell line was used to experimentally infect a chimpanzee. HIVAms37 was obtained from an AIDS patient visiting the Academic Medical Center in Amsterdam. The HIV-IIIB stock was also prepared on human PBMC. Each week, virus production in the supernatant was monitored in an in-house p24 antigen capture enzyme-linked immunosorbent assay (ELISA). If sufficient p24 antigen production could be demonstrated, the titer of the virus stock was quantified by determination of the TCID50 in PHA-stimulated healthy donor PBMC.
In vivo chimpanzee passage of a T-cell line-adapted (TCLA) and primary HIV-1 isolate.
All chimpanzees studied were housed in the Biomedical Primate Research Center in Rijswijk, The Netherlands. Ten years after the experimental infection with HIV-LAI (infected in 1984), biological virus clones were reisolated from chimpanzee Maya (ch-Ma). Experimental infection with the primary isolate HAN2 was performed as previously described (4). A female chimpanzee was inoculated with 100 TCID50 (as determined on chimpanzee PBMC) of a virus stock prepared on human PBMC. Blood samples were drawn every week and revealed a peak in HIV-1 RNA serum levels at 4.5 weeks postinoculation, and after 6 weeks antibodies could be detected (4). From a PBMC sample collected 4.5 weeks after inoculation, biological HIV-1 clones (HAN2/ch-in vivo) were obtained by cocultivation with human PHA-stimulated PBMC according to standard procedures (56).
Neutralizing agents.
Viruses were tested for their relative neutralization sensitivity against increasing concentrations of recombinant sCD4, HIV-1 immunoglobulin (HIVIG), pooled human sera (Amshps), and the human monoclonal antibodies (MAbs) gp13, gp68, IgG1b12, and 1577. HIVIG is a preparation of purified polyclonal immunoglobulin derived from plasma of multiple HIV-infected donors who had more than 400 CD4+ T cells/μl of blood (13). Amshps is a nonimmunoglobulin purified pooled plasma of 34 seropositive patients from the Amsterdam Cohort whose CD4+ T-cell counts ranged from 40 to 820 cells/μl. The gp13 and gp68 antibodies recognize epitopes surrounding the CD4bs of gp120 (58), and IgG1b12 recognizes the CD4bs of gp120 (7). MAb 1577 recognizes a highly conserved epitope of gp41 (residues 735 to 752) (16). Autologous serum neutralization of LAI/ch-Ma was studied with three serum samples that were obtained in March 1992, June 1994, and March 1996.
Neutralization sensitivity of HIV-1 variants.
From each virus isolate, a final inoculum of 10 TCID50 in a volume of 100 μl was incubated for 1 to 2 h at 37°C with increasing concentrations of the neutralizing agents. Subsequently, the mixtures of virus with sCD4, sera, or antibodies were added to 105 3-day PHA-stimulated PBMC of either human or chimpanzee origin in 96-well microtiter plates. The following day, plates incubated with HIVIG, Amshps, or chimpanzee serum were washed extensively. On days 7 and 14, virus production in culture supernatants was analyzed by an in-house p24 antigen capture ELISA. Means of quadruplicate experiments of each agent, tested at least twice, were plotted. The percent neutralization was calculated by determining the reduction in p24 production in the presence of the agent compared to the cultures with virus only. When possible 50% (IC50) and 90% (IC90) inhibitory concentrations were determined by linear regression. When sensitivity to neutralization was measured on chimpanzee PBMC, p24 production was monitored until day 10.
In vitro characterization of virus replication kinetics.
Analysis of replication kinetics was performed as described previously (63). Briefly, PHA-stimulated PBMC (5.0 × 106 cells/0.5 ml) were incubated with 102 TCID50 (1 ml) in a total volume of 1.5 ml for 2 h at 37°C. Virus supernatant was removed, and cells were incubated at a concentration of 1.0 × 106 cells/ml. Every day, 50 μl of supernatant was collected to measure p24 production. Fresh PHA-stimulated PBMC were added on days 5, 8, and 11.
125I radiolabeling of V3 peptides and HIV envelope protein.
In brief, 50-μl solutions of phosphate-buffered saline (PBS) containing either 20 μg of peptides or 5 μg of protein was used for labeling with Na-125I using chloramine T for 30 s (27). The radiolabeled preparations were purified from free iodine by dialysis and subsequently aliquoted in the presence of protease inhibitor phenylmethylsulfonyl fluoride and bovine serum albumin (BSA; fraction V; Sigma) in a final concentration of 0.1% and stored until use at −20°C. The peptide V3-IIIB (tip loop, SP104) was prepared as described earlier (35), and the circular V3 peptides were purchased from Zeneca (Cambridge Research Biochemicals) and are based on an isolate obtained from patient 168 from the Amsterdam Cohort (ACH.168). The recombinant gp160 from strain IIIB was expressed via a baculovirus expression system in insect cells and was kindly provided by Phage, La Jolla, Calif.
Binding of HIV envelope protein and V3 peptides to immobilized antibody (radioimmunoassay format).
The binding studies of antibodies to V3 peptides and gp160 protein were performed as follows: a serial dilution of serum was mixed with an excess of protein A-Sepharose beads suspended in PBS containing 0.1% BSA and 0.05% NP-40. After 2 h of incubation head over tail, the beads were washed four times with PBS containing 0.01% Tween 20. Antibodies bound to protein A-Sepharose beads were incubated head over tail with radiolabeled gp160 or V3 peptide (ca. 100,000 cpm) in PBS containing 0.1% BSA and 0.05% NP-40 for 16 h at room temperature. After washing the beads with PBS–0.01% Tween 20, radioactivity was measured in a gamma counter.
Determination of coreceptor use by HIV-1.
U87 cells stably expressing CD4 alone or in combination with CCR5 and CXCR4 (a kind gift of D. Littman) were seeded at 104 cells per well in 96-well plates in IMDM supplemented with 5 μg of Polybrene and 1 μg of puromycin per ml. Occasionally, 200 μg of G418 per ml was added to select for CD4-expressing cells. The next day, cells were washed in PBS, and 102 to 104 TCID50 of virus/ml were added in a 100-μl final volume. After 24 h, cells were washed twice with PBS, and 200 μl of fresh medium was added. Supernatants were harvested on days 7, 14, 21, and 28 and tested for the presence of p24 antigen by ELISA.
Cell surface expression of CD4, CCR5, and CXCR4.
Flow cytometry was used to analyze the expression of CD4, CCR5, and CXCR4. Human and chimpanzee PBMC (0.5 × 106) were incubated either with 5 μg of anti-CCR5 MAb 2D7 (kindly provided by C. Mackay) or with 5 μg of IgG2a isotype-matched control MAb (CLB, Amsterdam, The Netherlands) per ml. Cells were then washed and resuspended in 50 μl of fluorescein isothiocyanate-conjugated affinity-purified F(ab′)2 goat anti-mouse IgG (CLB). Subsequently, cells were incubated first with normal mouse serum (CLB) to diminish background staining, followed by an incubation with PerCP-labeled anti-CD4 MAbs (Becton Dickinson, San Jose, Calif.) and phycoerythrin-labeled anti-CXCR4 MAbs (Pharmingen). Cells were analyzed on the FACScan to determine the levels of cell surface expression. All incubation steps were performed at 4°C for 20 min. Between two incubation steps, two wash steps were performed with PBS supplemented with 0.5% BSA.
RESULTS
Phenotypic changes in HIV-1 variants induced by in vitro passage through chimpanzee cells.
We first studied the effect of in vitro passage of primary viruses through chimpanzee PBMC on neutralization sensitivity. PHA-stimulated chimpanzee PBMC were inoculated with virus stocks prepared on human PBMC of the primary isolates HIVAms37 and HAN2. When p24 production in the HAN2-infected chimpanzee PBMC culture could be demonstrated, supernatant (HAN2/ch-in vitro-p1) was harvested and in part used for cell-free inoculation of fresh PHA-stimulated chimpanzee PBMC. In this way, p2 and p3 isolates were also obtained. For further studies only the p1 and p3 HAN2 isolates were used. Virus production in HIVAms37-infected chimpanzee PBMC became evident after 3 weeks of culture, and this supernatant was used for further study. In parallel, parental viruses were passaged on human PBMC. Virus stocks from HAN2/ch-in vitro-p1 and -p3, HIVAms37/ch-in vitro, and all the parental viruses were prepared using a mixture of human PBMC derived from different donors. On the same cells, a TCID50 assay was performed for quantification of the virus titer. Original and passaged viruses were then analyzed for their neutralization sensitivity on human PBMC. For comparison the TCLA IIIB virus was included in the experiments.
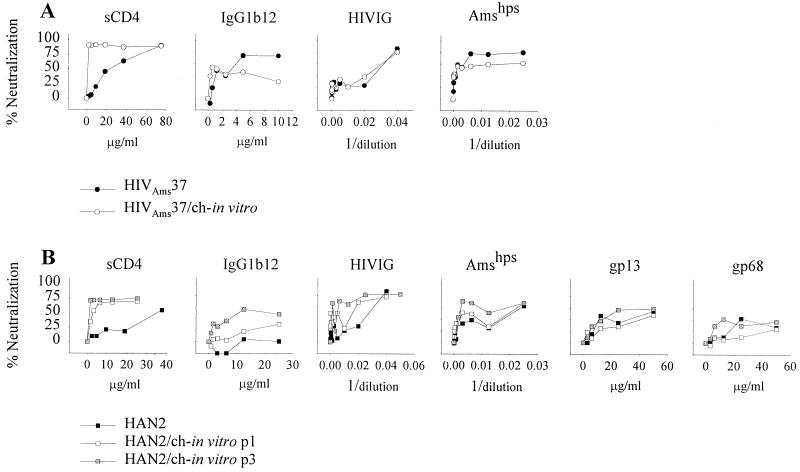
The sCD4 neutralization resistance of HIVAms37 decreased more than 20-fold from an IC50 of 35.0 μg/ml and an IC90 of 67.2 μg/ml for the primary virus to an sCD4 IC50 of 1.3 μg/ml and an IC90 of 3.9 μg/ml after in vitro passage through chimpanzee cells (Fig. 1A, Table 1). The same was observed for the HAN2 isolate, which originally showed an sCD4 IC50 of 51.9 μg/ml and an IC90 of 98.6 μg/ml but a 13-fold-decreased IC50 of 3.9 μg/ml and an IC90 of 29.0 μg/ml after a single passage through chimpanzee cells (Table 1, Fig. 1B).
FIG. 1.
Neutralization sensitivity as determined on human PHA-stimulated PBMC. (A) One hundred TCID50 of the primary isolate HIVAms37/ml, before (●) and after (○) in vitro passage through chimpanzee PBMC (HIVAms37/ch-in vitro). Viruses were incubated with increasing concentrations of sCD4, IgG1b12, gp68, and gp13 or increasing dilutions of HIVIG and Amshps as indicated. P24 production was measured, and mean OD values were calculated from quadruplicate cultures from at least duplicate experiments. The percent neutralization was calculated by determining the reduction in supernatant p24 production in the presence of the neutralizing agents relative to control cultures lacking these agents. (B) Neutralization sensitivity of the primary isolates HAN2 (■) and HAN2 after one (HAN2/ch-in vitro-p1; □) or three (HAN2/ch-in vitro-p3; ▨) in vitro passages through chimpanzee PBMC.
TABLE 1.
ICs of HIV-1 isolates passaged through chimpanzee PBMC
| Virus | Neutralizing MAb, human sera, or sCD4 titer of the indicated virus isolate to:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sCD4 (μg/ml)a
|
MAbsa
|
Pooled anti-HIV-1-positive serab
|
||||||||||||
| IgG1b12 (μg/ml)
|
gp13 (μg/ml)
|
gp68 (μg/ml)
|
1577 (μg/ml)
|
HIVIG
|
Amshps
|
|||||||||
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| HAN2 | 51.9 | 98.6 | 41.8 | 69.2 | 40 | 78.3 | >50d | >50 | >65 | >65 | 41 | 22 | 39 | 19 |
| HAN2/ch-in vitro-p1 | 3.9 | 29.0 | 53.5 | 96.81 | 51.3 | >50 | >50 | >50 | NDc | ND | 63 | 22 | 64 | 17 |
| HAN2/ch-in vitro-p3 | 1.1 | 6.7 | 10.2 | 44.8 | 20.1 | >50 | >50 | >50 | ND | ND | 247 | 38 | 480 | 21 |
| HIVAms37 | 35.0 | 67.2 | 3.1 | 5.9 | ND | ND | ND | ND | ND | ND | 42 | 20 | 882 | 44 |
| HIVAms37/ch-in vitro | 1.27 | 3.9 | 0.54 | 15.0 | ND | ND | ND | ND | ND | ND | 43 | 20 | 904 | 24 |
| IIIB | 3.5 | 8.7 | 0.5 | 1.4 | 34 | 53 | 5.9 | 34.9 | 5.9 | 50.6 | 941 | 177 | 511 | 42 |
| LAI/ch-Ma | 2.3 | 7.2 | 0.9 | 3.5 | 50.4 | >50 | >50 | >50 | >65 | >65 | 182 | 21 | 32 | 14 |
| HAN2/ch-in vivo | 2.5 | 23.3 | 9.4 | 32.5 | >50 | >50 | >50 | >50 | >65 | >65 | 187 | 43 | 291 | 45 |
The 50 and 90% neutralization titers were determined by assaying p24 inhibition.
Reciprocal 50 and 90% neutralization titers.
ND, not done.
Instances in which no neutralization was observed with the highest concentration of MAb used are indicated with a “>” sign.
Differences in neutralization of HAN2 by antibody and pooled sera following one passage through chimpanzee PBMC was not observed. However, the HAN2/ch-in vitro-p3 isolate had become even more sensitive for sCD4 and also an increased sensitivity for IgG1b12, Amshps, HIVIG, and gp13 MAb could be measured (Table 1, Fig. 1B).
For HIVAms37/ch-in vitro there was no change in neutralization sensitivity for IgG1b12, HIVIG, and Amshps, a finding which may be related to the fact that the primary HIVAms37 isolate was already sensitive for neutralization by IgG1b12 and Amshps (Fig. 1A).
Neutralization sensitivity of a primary HIV-1 variant after short-term in vivo passage through a chimpanzee.
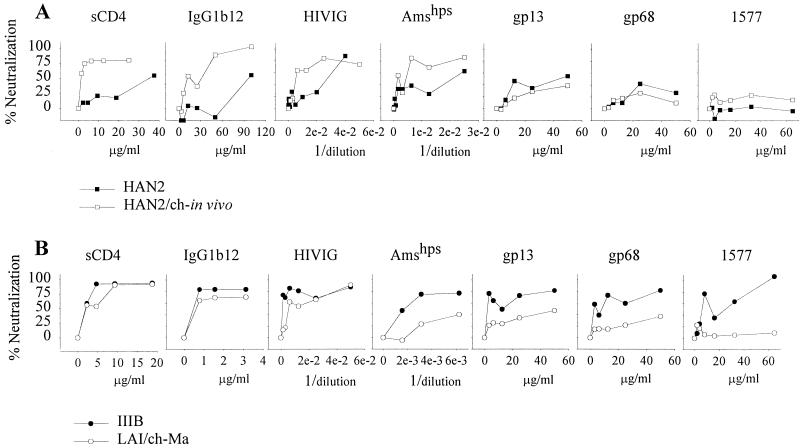
The increased neutralization-sensitive phenotype of the primary isolates after in vitro passage through chimpanzee PBMC suggest that adaptation to replicate in these cells selects for a neutralization-sensitive envelope configuration. We next compared the neutralization sensitivity of the primary neutralization-resistant HAN2 virus and the HIV-1 biological clone reisolated from PBMC from a chimpanzee that had been experimentally infected with this HAN2 virus for 4.5 weeks (4). The neutralization sensitivity of the reisolated HAN2 variant was increased 20- and 5-fold, respectively, for sCD4 and IgG1b12 (Fig. 2A). IC50 values for HIVIG and Amshps were decreased respectively by 5- and 7.5-fold (Table 1). There were no differences for gp13 and gp68 neutralization sensitivity between the primary and in vivo-passaged HAN2 virus.
FIG. 2.
Neutralization sensitivity of the HAN2 isolate (■) and HAN2 reisolated 4.5 weeks after experimental infection of a chimpanzee (HAN2/ch-in vivo; □) (A) and the TCLA IIIB virus (●) and the virus reisolated 10 years after experimental infection of a chimpanzee (LAI/ch-Ma; ○) (B). The assay was performed as described in the legend to Fig. 1 except that, along with the other agents, MAb 1577 neutralization sensitivity was also determined.
Neutralization sensitivity of an HIV-1 variant reisolated 10 years after experimental infection of a chimpanzee.
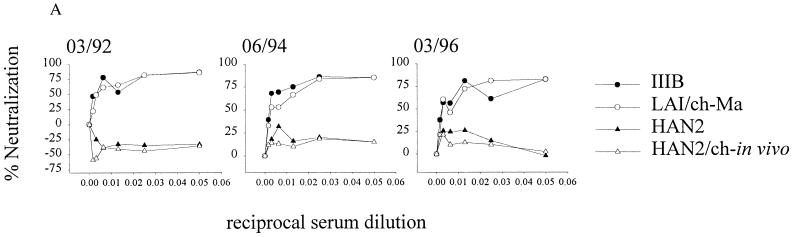
The effect on HIV-1 neutralization sensitivity upon long-term in vivo passage in a chimpanzee was then studied. The LAI variant (LAI/ch-Ma) that was reisolated from chimpanzee Maya 10 years after experimental infection was analyzed for its sCD4 and antibody neutralization sensitivity. Soluble CD4 concentrations that resulted in a 50% (IC50) or 90% (IC90) reduction in titer of the LAI/ch-Ma isolate were 2.3 and 7.2 μg/ml, respectively. This was in the same range as the concentrations required for neutralization of the TCLA IIIB isolate (IC50 = 3.5 μg/ml and IC90 = 8.7 μg/ml). The MAb IgG1b12, known to recognize an epitope within the gp120 CD4 binding domain, could also neutralize the LAI/ch-Ma variant at an IC50 concentration of 1.4 μg/ml and an IC90 concentration of 3.5 μg/ml (Fig. 2B, Table 1). The LAI/ch-Ma virus had become relatively resistant for neutralization by HIVIG, Amshps, the anti-gp41 MAb 1577, and the two MAbs gp13 and gp68. Resistance for HIVIG and Amshps increased 5- and 16-fold, respectively, while 15 and 8 times more gp13 and gp68 antibodies, respectively, were required for 50% inhibition of the LAI/ch-Ma variant. The LAI/ch-Ma variant was very well neutralized by autologous serum obtained at time points before, at, and after the moment the LAI/ch-Ma variant was isolated (Fig. 3A). The same efficient neutralization was observed for IIIB but not for HAN2 and HAN2/ch-in vivo, indicating the specificity of the humoral immune response.
FIG. 3.
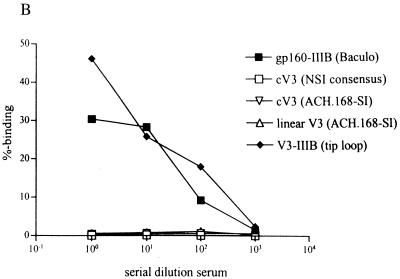
Analysis of the neutralizing capacity of diluted autologous chimpanzee serum. (A) A standard neutralization assay was performed with serum obtained at different time points (March 1992, June 1994, and March 1996) of chimpanzee Maya and used to test the viruses HIV-IIIB (●), LAI/ch-Ma (reisolated from chimpanzee Maya in 1994) (○), HAN2 (▴), and HAN2/ch-in vivo (▵). Serum and viruses were washed away 24 h after incubation on human PHA-stimulated PBMC. The percent neutralization compared with that of the control is shown. (B) Serum of chimpanzee Maya obtained in 1991 was tested for binding to different HIV-envelope-related antigens using a radioimmunoassay (see Materials and Methods).
The specificity was confirmed in a radioimmunoassay with radiolabeled env-derived antigens and the serum of chimpanzee Maya (Fig. 3B). Serum antibodies, present in a 1991 sample recognized unprocessed gp160, monomeric gp120 (also from MN, data not shown) or the V3 loop of IIIB in a dose-dependent manner. A consensus V3 loop of a non-syncytium-inducing (CCR5 coreceptor-using) virus was not recognized. Furthermore, the linear and folded V3 peptide of a virus isolate from patient ACH.168, a dual-tropic primary isolate, were also not recognized.
The presence of a relatively neutralization-sensitive virus variant 10 years after infection in the presence of specific antibodies may imply that full escape from humoral immunity is not necessary for HIV persistence in the chimpanzee.
Sensitivity to neutralization as measured on chimpanzee PBMC.
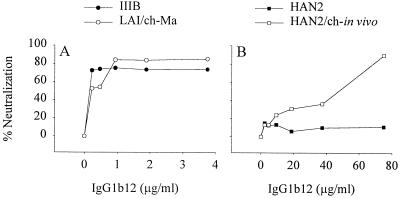
Next, we wanted to exclude that the increased neutralization sensitivity after passage through chimpanzee PBMC in vivo and in vitro is due to the type of target cell used for the neutralization assay. We compared the sensitivities of IIIB, LAI/ch-Ma, HAN2, and HAN2/ch-in vivo to IgG1b12 neutralization on chimpanzee PBMC. First, the TCID50 was determined on CD8 T-cell-depleted chimpanzee PHA-stimulated PBMC before 100 TCID50 was used in the neutralization assay. We found the same neutralization sensitivity irrespective of the use of chimpanzee or human PBMC. Both on chimpanzee PBMC and on human PBMC, HIV-IIIB and LAI/ch-Ma were highly sensitive, and the primary HAN2 isolate was resistant, whereas relative to the parental HAN2, the HAN2/ch-in vivo exhibited an increased sensitivity for neutralization by IgG1b12 (Fig. 4).
FIG. 4.
Sensitivity to neutralization by the IgG1b12 MAb of parental and chimpanzee passaged isolates as measured on CD8 T-cell-depleted PHA-stimulated chimpanzee PBMC. (A) HIV-IIIB (●) and LAI/ch-Ma (○). (B) HAN2 (■) and HAN2/ch-in vivo (□). The TCID50 of the on human PBMC-grown virus stocks was first determined on the same CD8 T-cell-depleted chimpanzee PBMC. Depicted in the graph is the percent neutralization compared to that of untreated controls.
Influence of in vivo and in vitro passage of HIV-1 through chimpanzee PBMC on replication kinetics.
It has been hypothesized that viral adaptation to a new environment would select for virus variants who most efficiently enter and duplicate in these new target cells (28, 46, 60). This process could influence replication kinetics, which in this case coincides with a neutralization-sensitive HIV-1 envelope glycoprotein. We therefore analyzed how this neutralization-sensitive phenotype associated with the viral replicative capacity.
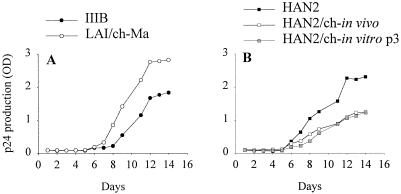
As demonstrated in Fig. 5, serial passage of the HAN2 isolate through chimpanzee cells reduced its replicative capacity on human PBMC. The same was observed for the virus reisolated after 4.5 weeks of in vivo replication in a chimpanzee. The LAI/ch-Ma isolate, which was still sCD4 and IgG1b12 sensitive but resistant to pooled sera, showed relatively high replication kinetics compared to the IIIB isolate and the HAN2 isolates. The reduced replicative capacity of chimpanzee-passaged HIV-1 was also evident when chimpanzee PBMC were used as target cells (data not shown).
FIG. 5.
Replication kinetics of progeny viruses before and after passage through chimpanzee PBMC. (A) HIV-IIIB (●) and LAI/ch-Ma (○). (B) HAN2 (■), HAN2/ch-in vivo (□), and HAN2/ch-in vitro-p3 ( ).
).
Coreceptor usage of HIV-1 variants before and after passage through chimpanzee cells.
The increased neutralization sensitivity of HIV-1 upon passage through chimpanzee PBMC closely resembles the phenotypic changes associated with adaptation to grow in T-cell lines. Passage of primary isolates through T-cell lines or cell lines expressing one of the different coreceptors (55) does not normally induce a change in coreceptor usage during transition from neutralization resistance to neutralization sensitivity (33, 40, 62). To exclude a role for coreceptor use during chimpanzee PBMC passage in vitro and in vivo, we tested whether passage was associated with changes in coreceptor use.
The neutralization-resistant isolates HIVAms37 and HAN2 both used CCR5 and CXCR4. In vitro passage of these isolates through chimpanzee cells did increase the neutralization sensitivity and reduced the replicative capacity but did not change the capacity to use both CCR5 and CXCR4 (Table 2). In vivo passage of the HAN2 isolate through chimpanzee cells, which also resulted in a highly neutralization-sensitive progeny virus, also did not change the capacity to use both CCR5 and CXCR4. The LAI/ch-Ma isolate could only use CXCR4 as a coreceptor.
TABLE 2.
Coreceptor usage of primary and chimpanzee-passaged HIV-1 variants
| Virus | CD4 expressing U87 cells cotransfected with:
|
|
|---|---|---|
| CXCR4 | CCR5 | |
| IIIB | + | − |
| LAI/ch-MA | + | − |
| HAN2 | + | + |
| HAN2/ch-in vivo | + | + |
| HAN2/ch-in-vitro-p1 | + | + |
| HIVAms37 | + | + |
| HIVAms37/ch-in vitro | + | + |
Expression of CD4, CCR5, and CXCR4 on human and chimpanzee PBMC.
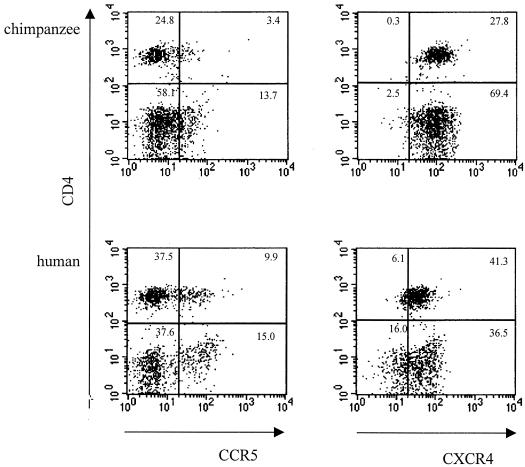
It has been suggested that the low expression of CD4 on cell lines compared to the expression of CD4 on primary T cells would select for viruses with the highest affinity for CD4 (31, 49), thus explaining why TCLA viruses are highly sensitive to sCD4 neutralization. Since passage through chimpanzee cells exerted the same effect as T-cell-line adaptation, we analyzed the expression of CD4 on PBMC of both human and chimpanzee origin. In addition, we examined the expression of the HIV-1 coreceptors CCR5 and CXCR4. Analysis of the membrane expression of the different molecules did not reveal a difference between PBMC originating from either humans or chimpanzees (Fig. 6).
FIG. 6.
CCR5 and CXCR4 expression on CD4-positive lymphocyte populations of chimpanzee (top panels) or human (bottom panels) PBMC. A three-color staining protocol was used to assess CCR5 expression (x axis of left panels) and CXCR4 expression (x axis of right panels) on the total number of CD4-positive cells (y axis in all plots).
DISCUSSION
We observed here that short-term passage through chimpanzee cells of primary neutralization-resistant viruses resulted in HIV-1 progeny with increased neutralization sensitivity for soluble CD4 (sCD4), pooled human sera, and the CD4 binding site (CD4bs) recognizing antibody IgG1b12. This neutralization-sensitive phenotype appeared to be relatively stable since an HIV-1 variant reisolated from a long-term asymptomatic chimpanzee that had been infected with HIV for 10 years was still sensitive to sCD4, IgG1b12, and autologous serum even in a retrospective fashion, suggesting that within a timeframe of 2 years no neutralization-resistant escape variants developed. By using radiolabeled HIV antigens, we showed that this autologous serum had IIIB-envelope-specific reactivity which most likely occurred in the context of the highly antigenic -QR-GPGR motif in the V3 loop of HIV-IIIB (26). This QR motif is absent in the NSI consensus and in the ACH.168 V3 loop. The increased neutralization sensitivity after short-term passage through chimpanzee PBMC coincided with a reduced replicative capacity. The efficient replication of the long term in vivo-passaged isolate may suggest a process of ongoing adaptation to growth in chimpanzee PBMC, although neutralization resistance to CD4bs-directed agents was not regained. The immediate increase in neutralization sensitivity for CD4bs-recognizing agents of primary HIV-1 variants upon passage through chimpanzee PBMC may indicate that the loss of sCD4 and IgG1b12 resistance was due to selection for or rapid development of viruses with a gp120-gp41 configuration that could efficiently support entry into chimpanzee CD4+ T cells. It can be stated that this phenotypic change both in vivo and in vitro occurred in the absence of neutralizing antibodies since in vivo the first antibodies were detected 6 weeks after infection. It therefore cannot be excluded that in the absence of neutralizing antibodies viruses are selected that are sensitive for neutralization. However, this is different from observations in humans, where the development of neutralization-sensitive viruses during primary infection does not occur (36). The adaptation to grow in chimpanzee cells may result in reduced levels of protection against a chimpanzee CD4bs-directed neutralization. Long-term passage in a chimpanzee did not fully revert the neutralization sensitivity of LAI/ch-Ma. It thus seems that not merely the presence of HIV-1-neutralizing antibodies in vivo (3, 6, 15, 19) but also the sensitivity of circulating HIV-1 variants to CD4bs-recognizing antibodies may correlate with the benign clinical course of infection in HIV-1-infected chimpanzees.
It could be that the affinity of chimpanzee CD4 for HIV-1 gp120 is different. Although 5-amino-acid differences have been observed between human and chimpanzee CD4, there was no difference in affinity and association rate for TCLA monomeric gp120 between human or chimpanzee CD4 (8). Also, no differences in infection of HIV-IIIB on human or chimpanzee CD4-transfected cells were found. Our observation that neutralization sensitivity was not dependent on the use of chimpanzee or human PBMC confirmed these results. Furthermore, no differences between human and chimpanzee CD4 glycosylation have been found, and CD4 epitopes recognized by a panel of 19 different anti-CD4 MAbs showed that epitopes were equally expressed on human and chimpanzee cells (38), which seems to exclude major conformational differences between human and chimpanzee CD4. Therefore, higher CD4 affinity as a general mechanism for increased neutralization sensitivity seems unlikely. Another possibility could be a differential expression or functioning of coreceptors CCR5 and CXCR4 on human and chimpanzee CD4+ T cells. We showed here that the expression of coreceptors is comparable in cells of human and chimpanzee origin. Moreover, human and chimpanzee CXCR4 show complete sequence homology, while in CCR5 2-amino-acid substitutions have been reported that do not disturb coreceptor functioning (50, 61). Altogether, these results do not suggest that differences in expression or function of coreceptors influence the neutralization sensitivity of progeny virus.
The phenotypic changes as observed after adaptation of HIV-1 variants to chimpanzee PBMC resemble the alterations observed after T-cell-line passage. The increased neutralization sensitivity during T-cell-line adaptation may be related to shielding of relevant epitopes on primary viruses (32, 66), resulting in a less-than-optimal binding efficiency to CD4 cells but creating an optimal adaptation for replication in the presence of neutralizing antibodies in vivo (43). TCLA HIV-1 isolates would then be optimally adapted to replicate in vitro, by trading of protection from neutralizing antibodies for an envelope configuration that allows more efficient CD4 binding and subsequent cell fusion (31, 42, 62). Since the neutralization-sensitive phenotype of the LAI/ch-Ma persisted in the presence of high neutralizing antibody titers (1, 19, 45, 46), the phenotypic similarities between HIV-1 adaptation to chimpanzee PBMC and T-cell lines may be based on different mechanisms; otherwise, the hypothesis needs to be adjusted.
With respect to HIV-1 infection in humans, we reisolated a slowly replicating IIIB variant that was sensitive to neutralization by CD4bs-recognizing agents, 3 years after accidental infection of a laboratory worker with the IIIB virus (65). An isolate obtained 4 years later had become neutralization resistant and possessed an increased replicative capacity (unpublished data). It cannot be excluded that in chimpanzees, the same evolution of biological properties is ongoing, but with delayed kinetics due to low-level replication and consequently slow accumulation of required mutations (11). In favor of this hypothesis is the observation that serial passage of HIV-1 through chimpanzees, and thus adaptation to this host, indeed seems to result in a virus that is more pathogenic for chimpanzees (14, 47, 59). In these studies, changes in the neutralization sensitivity of the virus were not considered. However, based on our observations in the accidentally infected laboratory worker and on recent observations in macaques (9, 28), it is tempting to speculate that HIV-1 variants that cause AIDS in chimpanzees are neutralization resistant for CD4bs-recognizing agents, with a fully restored replicative capacity (9, 39).
The increased neutralization sensitivity of primary HIV-1 isolates upon passage through chimpanzee cells, as demonstrated here, argues for caution in the interpretation of HIV-1 vaccine studies in this animal. The ability of experimental vaccines to protect from infection may be overestimated in chimpanzees since at least the HIV-1 isolates tested here, immediately became neutralization sensitive in this species. A vaccine that is considered to induce protective humoral immunity based on experiments in chimpanzees could possibly fail in humans since the level of neutralizing antibodies and the difference in neutralization sensitivity of the viruses that provide protection in chimpanzees might be insufficient against primary neutralization-resistant HIV-1 variants present in humans. Finally, a correlation between neutralization sensitivity and the clinical course of an HIV-1 infection may indeed exist. Elucidation of the molecular basis for neutralization resistance of primary HIV-1 variants may reveal strategies to prevent infection (32, 34, 51, 66).
ACKNOWLEDGMENTS
We thank R. Sweet (SmithKline Beecham) for providing recombinant sCD4 and A. Prince for HIVIG. Amshps was obtained from J. Goudsmit, gp13 and gp68 were a kind gift of M. Schutten, and the IgG1b12 MAb was kindly provided by P. W. H. I. Parren and D. R. Burton. The 1577 gp41 MAb was obtained through the AIDS Research and Reference Reagent Program, NIH, and was contributed by M. Ferguson. We thank Aran Labrijn, Rene van Lier, and Frank Miedema for critically reading the manuscript.
This work was supported by The Dutch AIDS Foundation (grant number 1304).
REFERENCES
- 1.Alter H J, Eichberg J W, Masur H, Saxinger W C, Gallo R C, Macher A M, Lane H C, Fauci A S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984;226:549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- 2.Balla-Jhagjhoorsingh S S, Koopman G, Mooij P, Haaksma T G M, Teeuwsen V J P, Bontrop R E, Heeney J L. Conserved CTL epitopes shared between HIV-infected human long-term survivors and chimpanzees. J Immunol. 1999;162:2308–2314. [PubMed] [Google Scholar]
- 3.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 4.Bogers W M J M, Koornstra W, Dubbes R, ten Haaft P J F, Verstrepen B E, Jhagjhoorsingh S, Haaksma T, Niphuis H, Laman J D, Norley S, Schuitemaker H, Goudsmit J, Hunsmann G, Heeney J L, Wigzell H. Characteristics of primary infection of a European human immunodeficiency virus type 1 clade B isolate in chimpanzee. J Gen Virol. 1998;79:2895–2903. doi: 10.1099/0022-1317-79-12-2895. [DOI] [PubMed] [Google Scholar]
- 5.Bradney A P, Scheer S, Crawford J M, Buchbinder S P, Montefiori D C. Neutralization escape in human immunodeficiency virus type 1-infected long-term nonprogressors. J Infect Dis. 1999;179:1264–1267. doi: 10.1086/314711. [DOI] [PubMed] [Google Scholar]
- 6.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van Opstal O, Culp J, Rosenberg M, De Wilde M, Heidt P. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzee. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 7.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E M, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 8.Camerini D, Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990;60:747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- 9.Cayabyab M, Karlsson G B, Etemad-Moghadam B A, Hofmann W, Steenbeke T, Halloran M, Fanton J W, Axthelm M K, Letvin N L, Sodroski J G. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiple passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–984. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecilia D, Kleeberger C A, Munoz A, Giorgi J V, Zolla-Pazner S. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J Infect Dis. 1999;176:1365–1374. doi: 10.1086/314773. [DOI] [PubMed] [Google Scholar]
- 11.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins L M, Weinhold K J, Matthews T J, Langlois A J, Perno C F, Condie R M, Allain J P. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood. 1991;77:1111–1117. [PubMed] [Google Scholar]
- 14.Davis I C, Girard M, Fultz P N. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J Virol. 1998;72:4623–4632. doi: 10.1128/jvi.72.6.4623-4632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 16.Evans D J, McKeating J, Meredith J M, Burke K L, Katrak K, John A, Ferguson M, Minor P D, Weiss R A, Almond J W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989;339:385–388. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- 17.Fultz P N, McClure H M, Swenson B, McGrath C R, Brodie A, Getchell J P, Jensen F C, Anderson D C, Broderson J R, Francis D P. Persistent infection of chimpanzees with human T-lymphotropic virus type III/lymphadenopathy associated virus: a potential model for acquired immunodeficiency syndrome. J Virol. 1986;58:116–124. doi: 10.1128/jvi.58.1.116-124.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gendelman H E, Ehrlich G D, Baca L M, Conley S, Ribas J, Kalter D C, Meltzer M S, Poiesz B J, Nara P. The inability of human immunodeficiency virus to infect chimpanzee monocytes can be overcome by serial viral passage in vivo. J Virol. 1991;65:3853–3863. doi: 10.1128/jvi.65.7.3853-3863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard M, Kieny M-P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kaczorek M, Gomard E, Gluckman J-C, Fultz P N. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gougeon M, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 21.Hay C M, Ruhl D J, Basgoz N O, Wilson C C, Billingsley J M, DePasquale M P, D'Aquila R T, Wolinsky S M, Crawford J M, Montefiori D C, Walker B D. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–5519. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeney J, Bogers W, Buijs L, Dubbes R, ten Haaft P, Koornstra W, Niphuis H, Nara P, Teeuwsen V. Immune strategies utilized by lentivirus infected chimpanzees to resist progression to AIDS. Immunol Lett. 1996;51:45–52. doi: 10.1016/0165-2478(96)02554-0. [DOI] [PubMed] [Google Scholar]
- 23.Heeney J, Jonker R, Koornstra W, Dubbes R, Niphuis H, Di Rienzo A M, Gougeon M L, Montagnier L. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 24.Heeney J L. AIDS: a disease of impaired Th-cell renewal? Immunol Today. 1995;16:515–519. doi: 10.1016/0167-5699(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 25.Heeney J L, Bruck C, Goudsmit J, Montagnier L, Schultz A, Tyrrell D, Zolla-Pazner S. Immune correlates of protection from HIV infection and AIDS. Immunol Today. 1997;18:4–8. doi: 10.1016/s0167-5699(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 26.Huisman, J. G., A. Carotenuto, A. F. Labrijn, C. H. M. Papavoine, J. D. Laman, M. M. Schellekens, M. G. H. M. Koppelman, and C. W. Hilbers. Recognition properties of V3-specific antibodies to V3-loop peptides derived from HIV-1 gp120 presented in multiple conformations. Biochemistry, in press. [DOI] [PubMed]
- 27.Huisman J G, Winkel I N, Lelie P N, Tersmette M, Goudsmit J, Miedema F. Detection of early anti-p24 HIV responses in EIA- and immunoblot-negative individuals. Implications for confirmatory testing. Vox Sang. 1987;53:31–36. doi: 10.1111/j.1423-0410.1987.tb04910.x. [DOI] [PubMed] [Google Scholar]
- 28.Kimata J T, Kuller L, Anderson D B, Dailey P, Overbaugh J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat Med. 1999;5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]
- 29.Koopman G, Haaksma A G, ten Velden J, Hack C E, Heeney J L. The relative resistance of HIV type 1-infected chimpanzees to AIDS correlates with maintenance of follicular architecture and the absence of infiltration by CD8+ cytotoxic lymphocytes. AIDS Res Hum Retrovir. 1999;15:365–373. doi: 10.1089/088922299311330. [DOI] [PubMed] [Google Scholar]
- 30.Koot M, Keet I P M, Vos A H V, De Goede R E Y, Th M, Roos L, Coutinho R A, Miedema F, Th P, Schellekens A, Tersmette M. Prognostic value of human immunodeficiency virus type 1 biological phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kozak S L, Platt E J, Madani N, Ferro F E, Peden K, Kabat D. CD4, CXCR-4, and CCR5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Numberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 35.Laman J D, Schellekens M M, Abacioglu Y H, Lewis G K, Tersmette M, Fouchier R A M, Langedijk J P M, Claassen E, Boersma W J A. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J Virol. 1992;66:1823–1831. doi: 10.1128/jvi.66.3.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis J, Balfe P, Arnold C, Kaye S, Tedder R S, McKeating J A. Development of a neutralizing antibody response during acute primary human immunodeficiency virus type 1 infection and the emergence of antigenic variants. J Virol. 1998;72:8943–8951. doi: 10.1128/jvi.72.11.8943-8951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomis-Price L D, Cox J H, Mascola J R, VanCott T C, Michael N L, Fouts T R, Redfield R R, Robb M L, Wahren B, Sheppard H W, Birx D L. Correlation between humoral responses to human immunodeficiency virus type 1 envelope and disease progression in early-stage infection. J Infect Dis. 1998;178:1306–1316. doi: 10.1086/314436. [DOI] [PubMed] [Google Scholar]
- 38.McClure M O, Sattentau Q, Beverley P C L, Hearns J P, Fitzgerald A K, Zuckerman A J, Weiss R A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987;330:487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- 39.Mo H, Stamatatos L, Ip J E, Barbas C F, Parren P W H I, Burton D R, Moore J P, Ho D D. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–6874. doi: 10.1128/jvi.71.9.6869-6874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9(Suppl. A):S117–S136. [PubMed] [Google Scholar]
- 43.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nara P, Hatch W, Kessler J, Kelliher J, Carter S. The biology of human immunodeficiency virus-1 IIIB infection in the chimpanzee: in vivo and in vitro correlations. J Med Primatol. 1989;18:343–355. [PubMed] [Google Scholar]
- 45.Nara P L, Robey W G, Arthur L O, Asher D M, Wolff A V, Gibbs C J, Gajdusek D C, Fischinger P J. Persistent infection of chimpanzees with human immunodeficiency virus: serological responses and properties of reisolated viruses. J Virol. 1987;61:3173–3180. doi: 10.1128/jvi.61.10.3173-3180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nara P L, Smit L, Dunlop N, Hatch W, Merges M, Waters D, Kelliher J, Gallo R C, Fischinger P J, Goudsmit J. Emergence of virus resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990;64:3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novembre F J, Saucier M, Anderson D C, Klumpp S A, O'Neal S P, Brown II C R, Hart C E, Guenthner P C, Swenson R B, McClure H M. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 49.Platt E J, Madani N, Kozak S L, Kabat D. Infectious properties of human immunodeficiency virus type 1 mutants with distinct affinities for the CD4 receptor. J Virol. 1997;71:883–890. doi: 10.1128/jvi.71.2.883-890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pretet J L, Zerbib A C, Girard M, Guillet J G, Butor C. Chimpanzee CXCR4 and CCR5 act as coreceptors for HIV type 1. AIDS Res Hum Retrovir. 1997;13:1583–1587. doi: 10.1089/aid.1997.13.1583. [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto C D, Wyatt R, Hernández-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 52.Santra S, Fultz P N, Letvin N L. Virus-specific cytotoxic T lymphocytes in human immunodeficiency virus type 1-infected chimpanzees. J Virol. 1999;73:7065–7069. doi: 10.1128/jvi.73.8.7065-7069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauermann U, Schneider J, Mous J, Brunckhorst U, Schedel I, Jentsch K D, Hunsmann G. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res Hum Retrovir. 1990;6:813–823. doi: 10.1089/aid.1990.6.813. [DOI] [PubMed] [Google Scholar]
- 54.Scarlatti G, Leitner T, Hodara V, Jansson M, Karlsson A, Wahlberg J, Rossi P, Uhlén M, Fenyö E M, Albert J. Interplay of HIV-1 phenotype and neutralizing antibody response in pathogenesis of AIDS. Immunol Lett. 1996;51:23–28. doi: 10.1016/0165-2478(96)02550-3. [DOI] [PubMed] [Google Scholar]
- 55.Scarlatti G, Tresoldi E, Björndal Å, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyö E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 56.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, De Goede R E Y, Van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuitemaker H, Meyaard L, Kootstra N A, Otto S A, Dubbes R, Tersmette M, Heeney J L, Miedema F. Lack of T-cell dysfunction and programmed cell death in human immunodeficiency virus type-1 infected chimpanzees correlates with absence of monocytotropic variants. J Infect Dis. 1993;168:1140–1147. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- 58.Schutten M, McKnight A, Huisman R C, Thali M, McKeating J A, Sodroski J, Goudsmit J, Osterhaus A D. Further characterization of an antigenic site of HIV-1 gp120 recognized by virus neutralizing human monoclonal antibodies. AIDS. 1993;7:919–923. doi: 10.1097/00002030-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane C H, Martin M A. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan N, Sun Y, Li J, Hofman W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ten Haaft P J F, Murthy K K, Verstrepen B E, Eichberg J W, Heeney J L. Intact CCR-5 coreceptors in HIV-1 infected chimpanzees. AIDS. 1997;11:1291–1293. doi: 10.1097/00002030-199710001-00001. [DOI] [PubMed] [Google Scholar]
- 62.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium inducing and non-syncytium inducing biological virus clones in relation to replication kinetics during the course of HIV-1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe M, Ringler D J, Fultz P N, MacKey J J, Boyson J E, Levine C G, Letvin N L. A chimpanzee-passaged human immunodeficiency virus isolate is cytopathic for chimpanzee cells but does not induce disease. J Virol. 1991;65:3344–3348. doi: 10.1128/jvi.65.6.3344-3348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss S H, Goedert J J, Gartner S, Popovic M, Waters D, Markham P, Di Marzo Veronese F, Gail M H, Barkley W E, Gibbons J, Gill F A, Leuther M, Shaw G M, Gallo R C, Blattner W A. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988;239:68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J. The antigenic structure of the HIV gp120 envelope protein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 67.Zhang P F, Chen X, Fu D W, Margolick J B, Quinnan G V J. Primary virus envelope cross-reactivity of the broadening neutralizing antibody response during early chronic human immunodeficiency virus type 1 infection. J Virol. 1999;73:5225–5230. doi: 10.1128/jvi.73.6.5225-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y J, Fracasso C, Fiore J R, Björndal A, Angarano G, Gringeri A, Fenyö E M. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J Infect Dis. 1997;176:1180–1187. doi: 10.1086/514111. [DOI] [PubMed] [Google Scholar]