Abstract
Xanthine oxidase inhibitors, including allopurinol and febuxostat, are the first-line treatment of hyperuricemia. This meta-analysis investigated the association between urate-lowering therapy and all-cause mortality in different chronic diseases to match its users and non-users in a real-world setting. Overall, 11 studies were included, which reported adjusted hazard ratios for all-cause mortality over at least 12 months. Meta-analysis of all included studies showed no effect of the therapy on all-cause mortality. However, subgroup analyses showed its beneficial effect in patients with chronic kidney disease (14% risk reduction) and hyperuricemia (14% risk reduction), but not in patients with heart failure (28% risk increase). Urate-lowering therapy reduces all-cause mortality among patients with hyperuricemia and chronic kidney disease, but it seems to increase mortality in patients with heart failure and should be avoided in this subgroup.
Keywords: all-cause mortality, allopurinol, febuxostat, hyperuricemia, xanthine oxidase inhibitors
Introduction
Hyperuricemia is defined as increased serum level of uric acid. When the serum level of uric acid exceeds the solubility threshold for monosodium urate, symptoms of gout may occur. The prevalence of hyperuricemia is estimated at 20% among the US population, whereas the prevalence of gout is estimated at 3.9% of US adults, including 5.2% of men and 2.7% of women [1]. The risk factors for hyperuricemia include genetic vulnerability, male sex, older age, lifestyle factors, chronic kidney disease, and use of numerous pharmaceuticals [2], including diuretics, low-dose aspirin, beta-blockers, angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers (except for losartan), and many others [3].
Xanthine oxidase inhibitors (XOI), including allopurinol and febuxostat, remain the first-line treatment of hyperuricemia [2]. Xanthine oxidase (XO) participates in conversion from hypoxanthine and guanine to uric acid, a part of purine catabolism pathway. Inhibition of XO leads, therefore, to a decrease in serum uric acid levels, with a concomitant increase in levels of hypoxanthine, xanthine, and guanine. These substances, however, exhibit significant toxicity [4, 5]. As a result, drugs lowering serum uric acid may lead to increased risk of cancer [6]. Additionally, a U-shaped association between serum uric acid, mortality [7], and cardiovascular risk [8] was reported. On the other hand, uric acid is known as an antioxidant, and it has been proposed to have neuroprotective properties [9]. It should also be noted that the relationship between serum uric acid level and symptoms of gout is not obvious; many patients with hyperuricemia remain asymptomatic, while in some patients with a relatively low serum uric acid level, a flare of gout may appear [2].
Therefore, we feel that it is reasonable to consider the most important endpoint, namely mortality, in decision making on the treatment of hyperuricemia. A reduction of all-cause mortality is a reliable measure of treatment efficacy in various populations. That is why all-cause mortality seems to be an adequate outcome to compare the benefits of urate-lowering therapy between patients with different diseases. Due to the shortage of randomized controlled trials on the subject, non-randomized studies were analyzed in the present study. Thus, the aim of our work was to analyze the association between urate-lowering therapy and all-cause mortality in different chronic diseases to match its users and non-users in a real-world setting.
Material and methods
Protocol and registration
The systematic review protocol was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA) guidance [10]. The study protocol was registered at PROSPERO (CRD42022346624).
Data sources and searches
This was a systematic review and meta-analysis. The PubMed, Scopus, and EMBASE electronic databases was searched for articles published in English from January 2000 to January 2023. Last search was performed on January 20, 2023. Relevant keywords were applied alone or in combination to identify data. The search strategy with the number of hits is presented in the study protocol. For abstracts potentially meeting the inclusion criteria, full-text publications were retrieved. Each study was assessed for eligibility by 2 independent reviewers, according to the criteria presented in the study protocol. Reasons for exclusion were briefly documented.
Study selection
Studies that reported adjusted hazard ratios (HR) for all-cause mortality over at least 12 months in febuxostat or allopurinol users vs. non-users in real-world matched cohorts were eligible. Only studies carried out among adults were included.
Data extraction and quality assessment
Two independent investigators (MMN and MN) extracted the following variables: adjusted HR, sample size, percentage of men, mean age, average follow-up (mean or median), and number of deaths. Disagreements were resolved by consensus. The total duration of follow-up was obtained from publications or calculated by multiplying the average follow-up by cohort size (patient-years). The number of deaths per 1000 patient-years was obtained from publications or calculated by dividing the number of deaths by the total follow-up duration.
Risk of bias assessment of the included studies was performed by 2 independent authors using the Newcastle-Ottawa scale (NOS). The NOS consists of 3 domains: (1) selection, (2) comparability, and (3) outcome [11]. Discrepancies were resolved by discussion. The certainty of evidence was assessed based on the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework.
Data synthesis and analysis
We conducted a meta-analysis of adjusted HRs for changes in all-cause mortality cardiovascular death in febuxostat or allopurinol users. Standard errors were calculated from 95% confidence intervals or from p-values [12]. The log-transformed values of point estimates and of standard errors were used in an inverse variance random-effects meta-analysis, with the restricted maximum-likelihood estimator for tau2 and the Q-profile method for the confidence interval of tau2 and tau. Heterogeneity was expressed with the I2 statistic and evaluated with Cochran’s Q test. Prediction intervals were calculated to aid the interpretation of the estimates, with consideration of heterogeneity [13]. Influential studies with the greatest impact on the estimate and heterogeneity were explored by a visual inspection of the Baujat plot [14]. Subgroup analyses were performed in the cohorts of patients with kidney disease, hyperuricemia, and heart failure. Also, we performed another subgroup analysis depending on the use of propensity score matching.
A restricted maximum-likelihood random-effects meta-regression analysis was used to explore heterogeneity, with the following covariates assessed: percentage of men, mean age, publication year, average follow-up, and number of deaths per 1000 person-years. A funnel plot and the Egger’s test were used to assess publication bias [15]. A p-value of less than 0.05 was considered statistically significant. R software (version 4.1.2) and the meta and dimeter packages were used for all analyses [16, 17]. The study did not receive any funding.
Results
Study characteristics
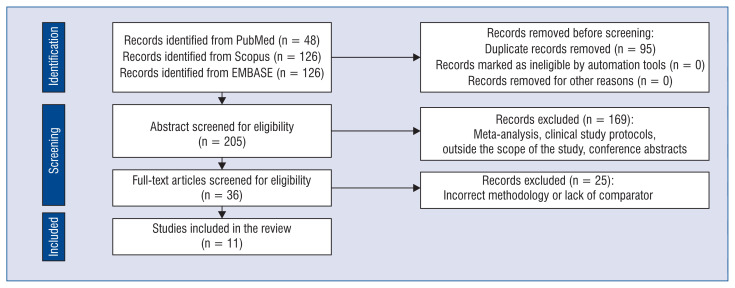
A total of 300 citations were identified, and 36 potentially eligible articles were retrieved in full text. Overall, 11 studies were included in the review (Fig. 1) [18–28]. Risk of bias assessment using NOS showed that the studies have low risk of bias. The characteristics of the included studies are summarized in Table 1. In the analysis, we included 2 cohorts from the study by Wei et al.
Figure 1.
PRISMA flow diagram.
Table 1.
Characteristics of included studies.
| Author, year | Population | Continent | Drug | Sample (total) | Sample (drug) | Mean age | Male % | Follow-up | Person-years | PSM | Adjustment covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Watanabe, 2020 | CKD | Asia | A | 2797 | 697 | 60 | 54 | 4.80 | 3.35 | − | Age, sex, smoking, BMI, comorbidities, eGFR, urinary protein, uric acid, hemoglobin, and albumin |
| Ju, 2019 | Gout | Asia | A or F | 7214 | 3607 | 72 | 71 | 1.97 | 7.11 | + | Age, sex, comorbidities, and medications |
| Hung, 2020 | ADPKD | Asia | A | 651 | 71 | 47 | 48 | 3.90 | 0.28 | − | Age, sex, residency, comorbidities, and medications |
| Kuo, 2015 | Gout | Europe | A | 7038 | 3519 | 64 | 72 | 3.00 | 10.56 | + | Age, sex, BMI, smoking, alcohol consumption, comorbidities, and medications |
| Larsen, 2015 | Hyperuricemia | Europe | A | 10338 | 5197 | 63 | 73 | 5.08 | 26.40 | + | Age, sex, comorbidities, medications, eGFR, glycated hemoglobin, total cholesterol, proteinuria, and uric acid |
| Weisman, 2021 | Gout | North America | A | 5937 | 1304 | 75 | 64 | 1.11 | 1.45 | + | Age, sex, residence, comorbidities, previous treatment burden, medications, eGFR, and uric acid |
| Luk, 2009 | Hyperuricemia | North America | A | 9924 | 2483 | 64 | 99 | 2.40 | 5.96 | − | Age, sex, race, BMI, comorbidities, health care utilization, medications, eGFR, cholesterol, and albumin |
| Wei J, 2022 | Gout and CKD | Europe | A | 10454 | 5227 | 74 | 61 | 5.00 | 26.14 | + | Sex, age, region, socioeconomic deprivation, BMI, smoking, alcohol, severity of kidney disease, and comorbidities |
| Dubreuil, 2014 | Hyperuricemia | Europe | A | 11854 | 5927 | 67 | 69 | 2.90 | 17.19 | + | Demographic, BMI, comorbidities, healthcare utilization, medications, and laboratory measurements |
| Wei L, 2009 (prevalent users) | Heart failure (prevalent users) | Europe | A | 4518 | 258 | 74 | 63 | 4.80 | 1.24 | − | Age, sex, Carstair’s deprivation code, comorbidities, and medications |
| Wei L, 2009 (incident users) | Heart failure (incident users) | Europe | A | 4527 | 267 | 69 | 59 | 4.80 | 1.28 | − | Age, sex, Carstair’s deprivation code, comorbidities, and medications |
| Tsuruta, 2014 | ESRD, Dialysis | Asia | A | 6252 | 1561 | 60 | 68 | 1.75 | 2.73 | + | Demographics, comorbidities, and laboratory measurements |
PSM — propensity score matching; A — allopurinol; F — febuxostat; CKD — chronic kidney disease; ADPKD — autosomal dominant polycystic kidney disease, ESRD — end-stage renal disease
Allopurinol and febuxostat use and all-cause mortality
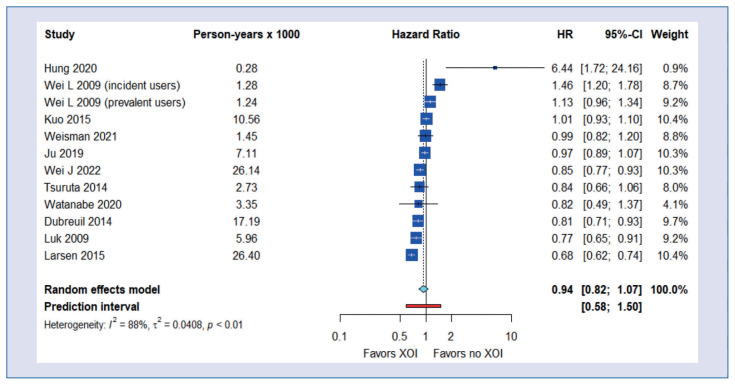
We found no significant correlation between allopurinol and febuxostat use and all-cause mortality (HR = 0.94; 95% CI, 0.82–1.07), but there was significant heterogeneity (I2 = 88%, tau2 = 0.0408, p < 0.01; prediction interval, 0.58–1.50; Central Illustration). Egger’s test showed no trend for a publication bias (p = 0.285).
Central Illustration.
Meta-analysis of all-cause mortality.
Subgroup analysis
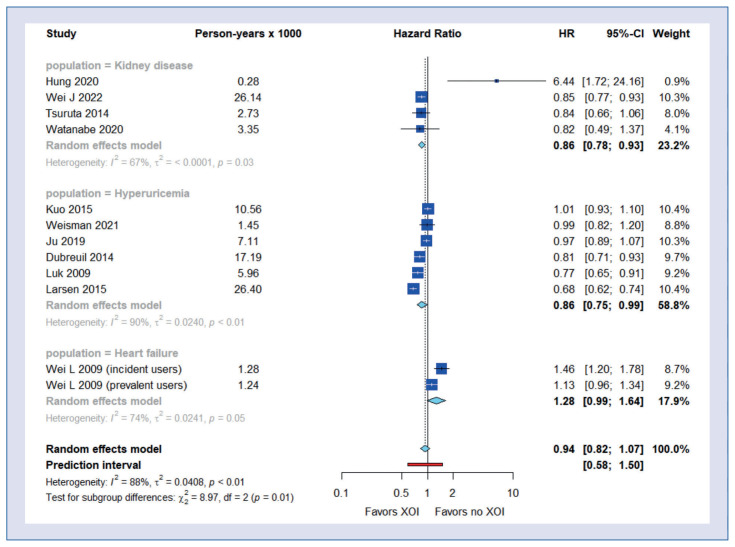
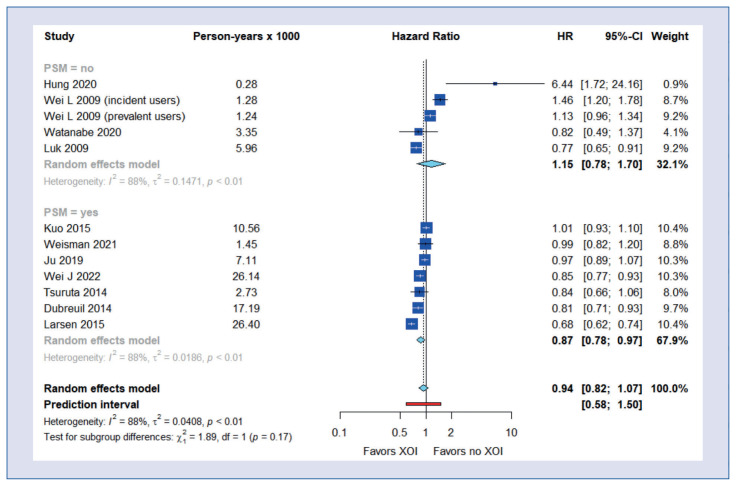
Subgroup analyses showed a beneficial effect of XOI use on all-cause mortality in patients with chronic kidney disease (HR = 0.86; 95% CI, 0.78–0.93) and hyperuricemia (HR = 0.86; 95% CI, 0.75–0.99) but not with heart failure (HR = 1.28; 95% CI, 0.99–1.64) (Fig. 2). The beneficial effect of XOI use on all-cause mortality was also observed in the group of studies using propensity score matching (HR = 0.87; 95% CI, 0.78–0.97) (Fig. 3).
Figure 2.
Subgroup analysis of all-cause mortality – by disease type.
Figure 3.
Subgroup analysis of all-cause mortality – by propensity score matching.
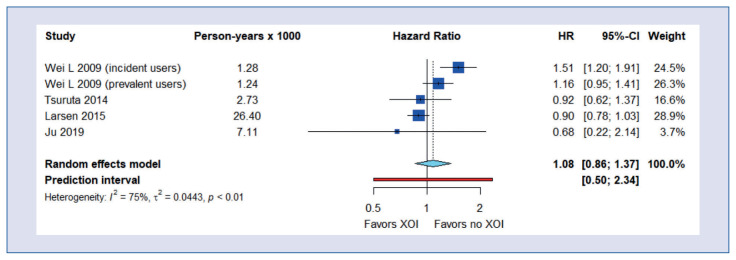
Allopurinol and febuxostat use and cardiovascular mortality
There was no beneficial effect of allopurinol and febuxostat therapy on cardiovascular death (HR = 1.08; 95% CI, 0.86–1.37) (Fig. 4).
Figure 4.
Meta-analysis of cardiovascular death.
Discussion
Our systematic review evaluated evidence from real-world studies assessing the impact of treatment of hyperuricemia on all-cause mortality [29]. Meta-analysis of all included studies showed no correlation between the treatment and all-cause mortality. However, subgroup analyses showed its beneficial effect on all-cause mortality in patients with chronic kidney disease, and hyperuricemia, with risk reduction in both clinical conditions by 14%, but not in these with heart failure, with risk increase of 28%. Substantial heterogeneity was noted both in the main analysis, and in subgroup analyses. Additionally, only in 7 included studies was propensity score matching used. Therefore, the overall evidence about the association of urate-lowering therapy with all-cause mortality in real-world clinical settings is of suboptimal quality. However, the funnel plot suggested a low possibility of publication bias towards studies reporting favorable effects of XOI.
In patients with heart failure, cellular damage leads to increased amounts of hypoxanthine and xanthine, which are converted to uric acid. Therefore, increased serum uric acid levels in patients with heart failure are frequently observed. Moreover, elevated serum uric acid levels in this population are considered a risk factor for poor outcome. XOI were proven to improve cardiac function in early studies. However, a series of clinical trials on urate-lowering therapy in heart failure failed to show clinical benefits [30]. XO, mediating conversion from hypoxanthine and xanthine to uric acid, forms reactive oxygen species, resulting in oxidative stress. However, other metabolic pathways are also involved in the generation of reactive oxygen species in heart failure [31]. Most probably, this is the reason why XOI are not effective in limitation of oxidative stress in heart failure, which results in lack of clinical benefits, as shown in our meta-analysis. In other words, overproduction of uric acid is a compensatory mechanism, eliminating toxic products of purine catabolism, and serum uric acid level is a marker of severity of cell death and oxidative stress in heart failure. The treatment with uric acid-lowering drugs may increase mortality and should be avoided in this subgroup of patients.
It should be mentioned that the impact of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease was assessed in the ALL-HEART study, which included more than 5700 participants aged 60 years or older, with ischemic heart disease, but without gout, and without heart failure in most cases. No benefits of allopurinol therapy were observed; however, harmful effects were also absent [32]. Possibly, increased mortality on allopurinol is limited to patients with developed heart failure.
We feel that our results should lead to modification of existing guidelines on urate-lowering therapy in patients with high cardiovascular risk. For example, according to the Polish-Italian expert consensus [33], XOI are first-line urate-lowering medicines, and they are recommended for achievement of targeted serum uric acid < 6 mg/dL, or < 5 mg/dL in those with high cardiovascular risk. Based on the above arguments, we think that indications for XOI should be narrowed, and this group of medicines should be used much more cautiously in patients with cardiovascular diseases, especially heart failure.
Chronic kidney disease is often accompanied by the retention of serum uric acid [34]. In patients with chronic kidney disease, increased serum levels of uric acid accelerate the progression of renal failure. On the other hand, XOI use does not slow the disease progression [9, 34]. However, there is evidence for the reduction of cardiovascular risk in chronic kidney disease patients with the reduction of serum uric acid levels, most probably via inhibition of atherogenesis. While chronic kidney disease is associated with extremely high cardiovascular risk [34], this may be the explanation for the positive correlation between urate-lowering therapy and mortality in this group of patients.
Of note, in one study [19] that included patients with autosomal dominant polycystic kidney disease (ADPKD), the effect of XOI on all-cause mortality was unfavorable. ADPKD is one of the common reasons for chronic kidney disease. However, it differs in many aspects from other conditions leading to chronic kidney disease. Therefore, even though the cited study [19] included a relatively low number of patients, and the number of person-years was the lowest among studies included in our meta-analysis, these results cannot be marginalized. Mechanisms of cell death participate in the pathogenesis of ADPKD [35], and, possibly, serum uric acid levels reflect the intensity of these processes, but they should not be considered a target for therapy. Thus, before further studies on mortality of ADPKD patients on urate-lowering therapy are published, this group of pharmaceuticals should be used cautiously in this subgroup, especially because, contrary to other causes of chronic kidney disease, hyperuricemia was not reported to be a risk factor for the progression of chronic kidney disease in ADPKD [36].
The results of subgroup analysis in which studies on patients with hyperuricemia, including gout, were analyzed show a beneficial effect of urate-lowering therapy in this group. Inflammation during flare causes not only pain and debilitation [2] but may also lead to further consequences. It is believed that hyperuricemia is associated with increased cardiovascular risk in people without chronic kidney disease [32]. It may explain the positive impact of XOI in this population, as shown by our analysis.
Some limitations of our study should also be mentioned. First, as noted above, the quality of available data is far from optimal. Second, our main question was whether urate-lowering therapy impacts all-cause mortality. As we mentioned, a U-shaped relationship between serum uric acid levels and clinical outcomes has been reported [7, 8]. Therefore, it cannot be excluded that not the therapy itself, but rather the serum level of uric acid achieved with it, is what impacts the mortality. Third, the diversity of effects achieved in different subpopulations limits the clinical utility of our results; in clinical settings different clinical conditions frequently coexist. Therefore, our results do not elucidate whether to treat or not, for instance, the patients with heart failure and co-existing chronic kidney disease. Thus, further investigation is needed to facilitate clinical decision-making in the future.
Conclusions
According to available data, the effect of urate-lowering therapy on all-cause mortality depends on the indication for initiation of therapy. It reduces all-cause mortality among patients with hyperuricemia and chronic kidney disease, except for ADPKD. On the other hand, in patients with heart failure it may increase mortality and should be avoided.
Due to the suboptimal quality of literature data, further research is needed in this field.
Acknowledgments
Editorial assistance was provided by Proper Medical Writing, Warsaw, Poland.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Contribution statement: Conceptualization: MMN and LP; methodology: MMN; validation: MMN, MN, and LP; formal analysis: SG and LP; investigation: MMN and MN; data curation: MN; writing — original draft preparation: MMN and MN; writing — review and editing: SG and LP; visualization: MN; supervision: LP; project administration: SG and LP. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
References
- 1.Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The national health and nutrition examination survey, 2007–2016. Arthritis Rheumatol. 2019;71(6):991–999. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikuls T. Gout. New England journal of medicine. 2022;387(20):1877–1887. doi: 10.1056/nejmcp2203385. [DOI] [PubMed] [Google Scholar]
- 3.Salem CB, Slim R, Fathallah N, et al. Drug-induced hyperuricaemia and gout. Rheumatology. 2016:kew293. doi: 10.1093/rheumatology/kew293. [DOI] [PubMed] [Google Scholar]
- 4.Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19(4):491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 5.Pang Bo, McFaline JL, Burgis NE, et al. Defects in purine nucleotide metabolism lead to substantial incorporation of xanthine and hypoxanthine into DNA and RNA. Proc Natl Acad Sci USA. 2012;109(7):2319–2324. doi: 10.1073/pnas.1118455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang HC, Nguyen PA, Islam M, et al. Gout drugs use and risk of cancer: A case-control study. Joint Bone Spine. 2018;85(6):747–753. doi: 10.1016/j.jbspin.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Cho SK, Chang Y, Kim I, et al. U-Shaped association between serum uric acid level and risk of mortality: A cohort study. arthritis rheumatol. 2018;70(7):1122–1132. doi: 10.1002/art.40472. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Park W, Suh YJu, et al. Association of serum uric acid with cardiovascular disease risk scores in Koreans. Int J Environ Res Public Health. 2019;16(23):4632. doi: 10.3390/ijer-ph16234632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos GK, Goldfarb DS. Update on uricaAcid and the kidney. Curr Rheumatol Rep. 2022;24(5):132–138. doi: 10.1007/s11926-022-01069-3. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. doi: 10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- 13.IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baujat B, Mahé C, Pignon JP, et al. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21(18):2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrer M, Cuijpers P, Furukawa T, Ebert DD. Doing meta-analysis with R, A hands-on guide. chapman and Hall/CRC; New York: 2021. [Google Scholar]
- 18.Dubreuil M, Zhu Y, Zhang Y, et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2015;74(7):1368–1372. doi: 10.1136/annrheumdis-2014-205269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung PH, Lin CH, Hung KY, et al. Clinical burden of autosomal dominant polycystic kidney disease. Aging (Albany NY) 2020;12(4):3899–3910. doi: 10.18632/aging.102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju C, Lai RW, Li KaH, et al. Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology (Oxford) 2020;59(9):2340–2349. doi: 10.1093/rheumatology/kez576. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CF, Grainge MJ, Mallen C, et al. Effect of allopurinol on all-cause mortality in adults with incident gout: propensity score-matched landmark analysis. Rheumatology (Oxford) 2015;54(12):2145–2150. doi: 10.1093/rheumatology/kev246. [DOI] [PubMed] [Google Scholar]
- 22.Larsen KS, Pottegård A, Lindegaard HM, et al. Effect of allopurinol on cardiovascular outcomes in hyperuricemic patients: A cohort study. Am J Med. 2016;129(3):299–306.e2. doi: 10.1016/j.amjmed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Luk AJ, Levin GP, Moore EE, et al. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford) 2009;48(7):804–806. doi: 10.1093/rheumatology/kep069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuruta Y, Nitta K, Akizawa T, et al. Association between allopurinol and mortality among Japanese hemodialysis patients: results from the DOPPS. Int Urol Nephrol. 2014;46(9):1833–1841. doi: 10.1007/s11255-014-0731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe K, Nakayama M, Yamamoto T, et al. Different clinical impact of hyperuricemia according to etiologies of chronic kidney disease: Gonryo Study. PLoS One. 2021;16(3):e0249240. doi: 10.1371/journal.pone.0249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Choi HK, Neogi T, et al. Allopurinol Initiation and All-cause mortality among patients with gout and concurrent chronic kidney disease: A population-based cohort study. Ann Intern Med. 2022;175(4):461–470. doi: 10.7326/M21-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei L, Fahey T, Struthers AD, et al. Association between allopurinol and mortality in heart failure patients: a long-term follow-up study. Int J Clin Pract. 2009;63(9):1327–1333. doi: 10.1111/j.1742-1241.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 28.Weisman A, Tomlinson GA, Lipscombe LL, et al. Allopurinol and renal outcomes in adults with and without type 2 diabetes: A retrospective, population-based cohort study and propensity score analysis. Can J Diabetes. 2021;45(7):641–649.e4. doi: 10.1016/j.jcjd.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie I, Hawkey C, Ford I, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. The Lancet. 2022;400(10359):1195–1205. doi: 10.1016/s0140-6736(22)01657-9. [DOI] [PubMed] [Google Scholar]
- 30.Kanbay M, Afsar B, Siriopol D, et al. Effect of uric acid-lowering agents on cardiovascular outcome in patients with heart failure: A systematic review and meta-analysis of clinical studies. angiology. 2020;71(4):315–323. doi: 10.1177/0003319719897509. [DOI] [PubMed] [Google Scholar]
- 31.Tamariz L, Hare JM. Xanthine oxidase inhibitors in heart failure: where do we go from here? Circulation. 2015;131(20):1741–1744. doi: 10.1161/CIRCULATIONAHA.115.016379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie IS, Hawkey CJ, Ford I, et al. ALL-HEART study group. allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet. 2022;400(10359):1195–1205. doi: 10.1016/S0140-6736(22)01657-9. [DOI] [PubMed] [Google Scholar]
- 33.Borghi C, Domienik-Karłowicz J, Tykarski A, et al. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol J. 2021;28(1):1–14. doi: 10.5603/CJ.a2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejaz AA, Nakagawa T, Kanbay M, et al. Hyperuricemia in kidney disease: A major risk factor for cardiovascular events, vascular calcification, and renal damage. semin nephrol. 2020;40(6):574–585. doi: 10.1016/j.semnephrol.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agborbesong E, Li LX, Li Lu, et al. Molecular mechanisms of epigenetic regulation, Inflammation, and cell death in ADPKD. Front Mol Biosci. 2022;9:922428. doi: 10.3389/fmolb.2022.922428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park HC, Kang AY, Jang JY, et al. Chronic asymptomatic pyuria precedes overt urinary tract infection and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol. 2013;14:1. doi: 10.1186/1471-2369-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]