Abstract
Metformin is a common diabetes drug that may reduce lactate clearance by inhibiting mitochondrial oxidative phosphorylation, leading to metformin-associated lactic acidosis (MALA). As diabetes mellitus is a common chronic metabolic condition found in critically ill patients, pre-existing metformin use can often be found in critically ill patients admitted to the intensive care unit or the high dependency unit. The aim of this narrative mini review is therefore to update clinicians about MALA, and to provide a practical approach to its diagnosis and treatment. MALA in critically ill patients may be suspected in a patient who has received metformin and who has a high anion gap metabolic acidosis, and confirmed when lactate exceeds 5 mmol/L. Risk factors include those that reduce renal elimination of metformin (renal impairment from any cause, histamine-2 receptor antagonists, ribociclib) and excessive alcohol consumption (as ethanol oxidation consumes nicotinamide adenine dinucleotides that are also required for lactate metabolism). Treatment of MALA involves immediate cessation of metformin, supportive management, treating other concurrent causes of lactic acidosis like sepsis, and treating any coexisting diabetic ketoacidosis. Severe MALA requires extracorporeal removal of metformin with either intermittent hemodialysis or continuous kidney replacement therapy. The optimal time to restart metformin has not been well-studied. It is nonetheless reasonable to first ensure that lactic acidosis has resolved, and then recheck the kidney function post-recovery from critical illness, ensuring that the estimated glomerular filtration rate is 30 mL/min/1.73 m2 or better before restarting metformin.
Keywords: Acid-base equilibrium, Biguanides, Oxidative phosphorylation, Pyruvic acid, Renal replacement therapy
Core Tip: Metformin-associated lactic acidosis (MALA) in critically ill patients may be suspected in a patient who has received metformin and who has a high anion gap metabolic acidosis, and confirmed when lactate exceeds 5 mmol/L. Risk factors include those that reduce renal elimination of metformin and excessive alcohol consumption. Treatment of MALA involves immediate cessation of metformin, supportive management, treating other concurrent causes of lactic acidosis like sepsis, and treating any coexisting diabetic ketoacidosis. Severe MALA requires extracorporeal removal of metformin with either intermittent hemodialysis or continuous kidney replacement therapy.
INTRODUCTION
Diabetes mellitus is a common chronic metabolic condition found in critically ill patients[1], and is a poor prognostic factor for survival[2]. One of the first-line treatments for type 2 diabetes mellitus is the oral biguanide drug metformin, given its high efficacy, low cost, lack of hypoglycemia, potential of modest weight loss, and potential reduction of major adverse cardiovascular events[3] and mortality[4]. Therefore, pre-existing metformin use can often be found in critically ill patients admitted to the intensive care unit (ICU) or the high dependency unit.
Metformin is, however, a double-edged sword for critically ill patients. On the one hand, it helps to control hyper-glycemia via inhibition of hepatic gluconeogenesis[5]. On the other hand, during critical illness, metformin accumulation and severe toxicity in the form of metformin-associated lactic acidosis (MALA) can occur. MALA is not benign, with mortality exceeding 10% according to several studies. Twenty-five deaths (10.8% crude mortality) were recorded from 232 MALA cases reported to the United Kingdom National Poisons Information Service between 2010 and 2019[6]. A systematic review of 253 reported cases of MALA demonstrated a crude mortality rate of 16.2%, with higher lactate and metformin levels in non-survivors compared to survivors[7]. Among 82 adult patients with MALA admitted to a Taiwanese ICU, ICU mortality was 17.0%[8]. And among 105 patients with MALA hospitalized in Thailand, the 30-day mortality rate was 36.2%[9].
According to a population-based study conducted in the United Kingdom, metformin use, compared to no metformin use, has been associated with about 4.5-fold greater incidence of lactic acidosis in patients with type 2 diabetes mellitus (45.7 versus 11.8 cases per 100000 patient-years), with increasing risk with worsening kidney function[10]. The incidence of MALA ranges from 2.4 to 15 cases per 100000 patient-years in Sweden[11], 4.6 to 39 cases per 100000 patient-years in the United Kingdom[12], and 3 to 10 cases per 100000 patient-years based on a meta-analysis of 65 studies[13]. The incidence of lactic acidosis with metformin is several-fold lower than the 40-64 cases per 100000 patients-years with phenformin, another biguanide, which has been withdrawn from clinical use since the late 1970s for its strong association with lactic acidosis[14,15].
Given the high prevalence of diabetes mellitus and consequently of metformin use, clinicians should expect to encounter MALA in critically ill patients, including those admitted to the ICU[16-18]. To facilitate clinical management, this narrative mini review aims to provide updated information on the pathophysiology, diagnosis, and management of MALA in critically ill patients.
PATHOPHYSIOLOGY OF MALA
The mechanism leading to MALA involves lactate accumulation due to reduced clearance by oxidative phosphorylation in mitochondria[19], as illustrated in Figure 1. Metformin-induced inhibition of oxidative phosphorylation leads to mitochondrial dysfunction[20,21], which leads to lactic acidosis even in the presence of normal oxygen supply[22]. Such mitochondrial dysfunction resembles cyanide poisoning, and affects multiple sites such as the liver, skeletal muscle, heart, kidney, and platelets[23].
Figure 1.
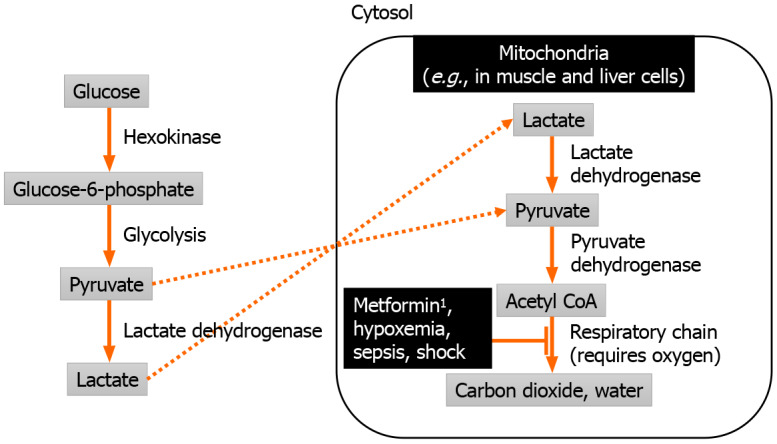
Pathophysiology of metformin-associated lactic acidosis. 1Metformin is eliminated unchanged by the kidney.
Metformin by itself usually does not lead to MALA, as evidenced by relatively safe use in most patients, unless acute ingestion of massive amounts metformin occurs[24,25], or when metformin accumulates in the setting of severe renal impairment[14]. Metformin is absorbed mainly through the small intestine[26], negligibly protein-bound in the blood, minimally metabolized, and eliminated unchanged by the kidneys[27]. Peak concentration of metformin during therapeutic dosing ranges from 1.5 to 3 mg/L[28]. Elevated metformin levels as defined by a plasma level exceeding 4 mg/L was associated with worse lactic acidosis[14,29].
Apart from metformin, oxidative phosphorylation can also be impaired by lack of oxygen supply from hypoxemic respiratory failure and circulatory failure (generally marked by a systolic blood pressure persistently lower than 90 mmHg[30] or a mean arterial pressure persistently lower than 65 mmHg[31]), sepsis-induced mitochondrial dysfunction, or hepatic failure from any viral, toxic, or other cause. In most cases, MALA is driven by a combination of metformin and one of the other causes, which may be present in critically ill patients.
DIAGNOSIS OF MALA
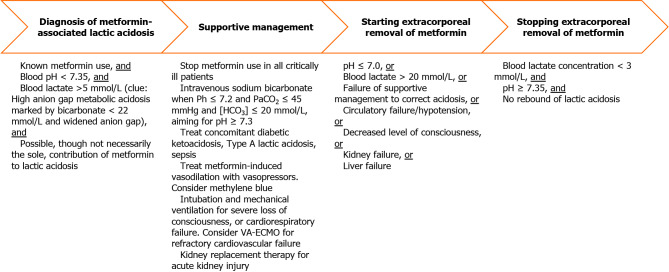
MALA may be suspected in a patient who has received metformin and who has a high anion gap metabolic acidosis (HAGMA)[32] on either arterial or venous blood gas analysis (Figure 2). Although high metformin levels support the diagnosis of MALA, low levels do not exclude it, with a sensitivity of only 67% at a threshold level of 9.9 mg/L[29]. HAGMA is the combination of low pH < 7.35[33], low bicarbonate < 22 mmol/L[17], and a widened anion gap (sodium – chloride – bicarbonate). The widened anion gap must be due to highly elevated lactic acid > 5 mmol/L[33,34] that cannot be completely explained by other causes such as inadequate tissue oxygenation[35]. Clinically, HAGMA leads to respiratory compensation of acidosis, which involves the patient breathing more quickly and deeply. The concomitant appearance of tachypnea and hyperpnea is a form of hyperventilation eponymously termed Kussmaul respiration[36,37]. For diabetic patients who are critically ill with HAGMA, one should be vigilant for diabetic ketoacidosis, which is another cause of HAGMA. Notably, diabetic ketoacidosis with normal blood glucose levels (i.e., euglycemic ketoacidosis) can occur in patients with combined use of metformin and sodium/glucose cotransporter 2 inhibitors (e.g., empagliflozin)[38].
Figure 2.
Diagnosis and management of metformin-associated lactic acidosis. HCO3: Serum bicarbonate concentration; PaCO2: Arterial partial pressure of carbon dioxide; VA-ECMO: Venoarterial extracorporeal membrane oxygenation.
Non-specific clinical features of MALA include fatigue, anorexia, nausea, hypothermia, hypotension, abdominal pain, altered consciousness[7,39]. Such non-specific features may lead to erroneous diagnoses like acute mesenteric ischemia[40], which may lead to unnecessary laparotomy[41]. Unusual clinical features include acute reversible blindness, which may be due to acidosis-related retinal cell impairment[42,43]. As cases of MALA with non-specific or unusual clinical features have been described, it may be prudent to screen any ill patient who has been taking metformin for the presence of metabolic acidosis with serum bicarbonate, as part of a basic electrolyte panel. Once metabolic acidosis is detected, further testing for lactic acidosis can then be done.
Differentiating mitochondrial dysfunction from metformin versus inadequate tissue oxygenation from inadequate oxygen supply as the cause of lactic acidosis is crucial for appropriate therapy[35] (Table 1), and both can coexist in critically ill patients. Inadequate tissue oxygenation from insufficient oxygen supply to meet tissue oxygen demand to drive the respiratory chain causes Type A lactic acidosis, which is marked by a capillary refill time > 3 seconds[31]. If arterial blood gas and central venous blood gas analyses are available, a central venous oxygen saturation (ScvO2) < 70%, or a combination of ScvO2 ≥ 70% and a carbon dioxide gap > 6 mmHg (difference between the central venous partial pressure of carbon dioxide, as measured from a blood sample taken from a central line with its tip around the junction of the superior vena cava and right atrium, and the arterial partial pressure of carbon dioxide) indicates Type A lactic acidosis[35]. Treatment requires improving oxygen supply by increasing blood oxygenation, cardiac output, or hemoglobin. In addition, oxygen demand can be reduced through sedation and mechanical ventilation. Conversely, these treatments do not directly treat mitochondrial dysfunction, which leads to Type B lactic acidosis. Here, oxygen supply is adequate, but the respiratory chain is inhibited from utilizing the available oxygen. Elimination of the respiratory chain inhibitor – which is metformin in the case of MALA – will be necessary. Such elimination can be achieved naturally via the kidneys if renal function is adequate, or artificially via extracorporeal treatment (i.e., kidney replacement therapy).
Table 1.
Lactic acidosis due to inadequate tissue oxygenation versus mitochondrial dysfunction[35]
|
Clinically relevant considerations
|
Lactic acidosis due to inadequate tissue oxygen supply to meet demand (Type A lactic acidosis)
|
Lactic acidosis due to mitochondrial dysfunction (Type B lactic acidosis)
|
| Selected causes | Hypovolemia/dehydration | Metformin toxicity |
| Sepsis | Sepsis | |
| Heart failure | Cyanide poisoning | |
| Respiratory failure | ||
| Clinical and laboratory features | Capillary refill time > 3 s | Capillary refill time 0-3 s |
| ScvO2 < 70% | ScvO2 ≥ 70% and CO2 gap < 6 mmHg | |
| ScvO2 ≥ 70% and CO2 gap > 6 mmHg | ||
| Treatment | Fluid challenge, followed by fluid loading if capillary refill time or blood pressure improves | Stop further administration and consider extracorporeal removal of toxic agent (e.g., metformin) |
| Empirical broad-spectrum antimicrobials for suspected sepsis | Empirical broad-spectrum antimicrobials for suspected sepsis | |
| Vasopressors/inotropes to maintain a mean arterial pressure ≥ 65 mmHg | ||
| Supplemental oxygen and mechanical ventilation to maintain an arterial oxygen saturation of 94%-98% | ||
| Correct severe anemia and maintain hemoglobin 7-9 g/dL |
CO2 gap: Difference between the central venous partial pressure of carbon dioxide (measured from a blood sample taken from a central line with its tip around the junction of the superior vena cava and right atrium) and the arterial partial pressure of carbon dioxide (measured from an arterial blood sample); ScvO2: Central venous oxygen saturation, which is measured from a blood sample taken from a central line with its tip around the junction of the superior vena cava and right atrium.
In clinical practice, a definitive diagnosis of MALA may be challenging for the following reasons. Firstly, MALA must be diagnosed and treated in a timely manner. Secondly, lactic acidosis is commonly due to inadequate tissue oxygenation in critically ill patients, which may take several hours to resolve before a definitive diagnosis of MALA can be done. Thirdly, MALA may resolve concurrently with improvement of tissue oxygenation, and cannot be excluded if lactic acidosis and inadequate tissue oxygenation are both absent later in the course of critical illness. Overall, a working diagnosis of MALA will often anchor on a possible diagnosis rather than a definitive diagnosis, where metformin is a possible, though not necessarily the sole, contributor to lactic acidosis.
RISK FACTORS FOR MALA
As metformin is renally excreted by filtration and active tubular secretion[12], kidney disease with renal impairment, as indicated by a low estimated glomerular filtration rate (eGFR), is the main risk factor for metformin accumulation and toxicity (Table 2). Renal impairment can be due to a variety of pre-renal (e.g., post-operative gastrointestinal losses and dehydration[44]), renal, post-renal (i.e., obstructive) causes. In a large study in the United States involving 157430 patients, metformin use was associated with hospitalization with acidosis only when patients had eGFR < 30 ml/min/1.73 m2[45]. In a separate Taiwanese population-based cohort study, metformin was associated with increasing lactic acidosis risk when eGFR was below 30 ml/min/1.73 m2[46]. This eGFR threshold therefore marks the lower limit of kidney function for safe use of metformin in non-critically ill patients[47]. However, newer evidence might alter the safety threshold. For instance, among 320882 patients with type 2 diabetes mellitus and chronic kidney disease in the United States Veterans Administration, metformin use was associated with incident lactic acidosis when chronic kidney disease resulted in eGFR < 45 mL/min/1.73 m2[48].
Table 2.
Risk factors for metformin-associated lactic acidosis
|
Risk factors
|
Mechanism
|
| Kidney failure (any cause) | Reduced renal elimination of metformin[45] |
| Cimetidine | Reduced renal elimination of metformin[51] |
| Ribociclib | Reduced renal elimination of metformin[52] |
| Excessive alcohol consumption | Ethanol oxidation consumes nicotinamide adenine dinucleotides that are also required for lactate metabolism[50] |
Other risk factors for MALA include excessive alcohol consumption[49], as ethanol oxidation consumes nicotinamide adenine dinucleotides that are also required for lactate metabolism[50]. Drugs that reduce renal elimination of metformin, such as histamine-2 (H2) receptor antagonists[51] and ribociclib[52], predispose to metformin accumulation and MALA. A recent pharmacovigilance study using the United States Food and Drug Administration’s Adverse Event Reporting System demonstrated that metformin combined with cimetidine at conventional therapeutic doses was associated with increased risk of lactic acidosis[51]. Separately, a case report highlighted the development of MALA in a 62-year-old woman on metformin who also took ribociclib, a CDK4 and CDK6 inhibitors used in for the treatment of metastatic breast cancer[52]. Nevertheless, the absence of the above risk factors does not exclude MALA[39].
PREVENTION AND TREATMENT OF MALA
Since critically ill patients often have risk factors – such as hypoxemia, cardiac failure, and renal impairment – that combine with metformin to elevate the risk of MALA, it is prudent to stop metformin for these patients initially even in the absence of lactic acidosis. Glucose control in critically ill diabetic patients can be achieved with insulin rather than metformin, targeting 7.8-10 mmol/L for most patients[53]. As metformin achieves peak concentration only 1-3 h after ingestion of immediate release formulations and 6-8 h after ingestion of extended release formations[28], and has an elimination half-life of approximately 5 h even in patients with normal kidney function[26], the effects of metformin will continue to persist for hours after metformin has been discontinued on the patient’s medication list.
Once clinicians diagnose MALA, treatment needs to start immediately with the cessation of metformin. In the setting of severe metformin toxicity, supportive management of affected organ systems is necessary. Treatment of concomitant Type A lactic acidosis, and extracorporeal removal of metformin can facilitate resolution of MALA (Figure 2). In general, faster lactate clearance is associated with improved survival in critically ill patients[54]. Reversal of Type A lactic acidosis requires treatment of the underlying causes and improving cardiorespiratory parameters[35]. Empirical broad-spectrum antimicrobials should be promptly administered for suspected sepsis. To improve the oxygen supply, adequate volume expansion is required. Not all patients will require fluids, so fluid loading should only be done if a fluid challenge improves capillary refill time or blood pressure[55]. Vasopressors (e.g., noradrenaline, vasopressin[56]) and inotropes may be needed to maintain a mean arterial pressure ≥ 65 mmHg[31]. Intravenous methylene blue infusion has been reported in some case studies to reverse metformin-induced vasodilation, as it counters the activation of endothelial nitric oxide synthase by metformin[57]. Supplemental oxygen and mechanical ventilation may also be needed to maintain an arterial oxygen saturation of 94%-98%[58]. During mechanical ventilation, high minute ventilation is needed for respiratory compensation of acidemia, and higher tidal volumes (e.g., 8 mL/kg predicted body weight) while keeping plateau pressure under the safe upper limit of 30 cmH2O, together with high respiratory rates of up to 35 breaths per minute, may be used[59]. Furthermore, correction of severe anemia with blood transfusion, maintaining a hemoglobin level 7-9 g/dL[60], enhances the oxygen-carrying capacity of the blood. Rarely, refractory cardiovascular failure in MALA may require mechanical circulatory support in the form of venoarterial extracorporeal membrane oxygenation[61,62].
As part of supportive management, intravenous sodium bicarbonate infusion can mitigate profound acidemia, indicated by a pH ≤ 7.2. Mechanistically, such severe acidemia[63] and lactic acidosis[64] can impair cardiac function. Although direct improvement of cardiac function with bicarbonate has not been demonstrated, in patients with moderate/severe acute kidney injury (Acute Kidney Injury Network score of 2-3; serum creatinine > 2 times of the baseline or urine output < 0.5 mL/kg/h for > 12 h), with pH ≤ 7.2 and arterial partial pressure of carbon dioxide ≤ 45 mmHg and serum bicarbonate ≤ 20 mmol/L, giving intravenous sodium bicarbonate aiming for pH ≥ 7.3 reduces the composite outcome of all-cause mortality at day 28 and the presence of at least one organ failure at day 7[65]. These treatment thresholds are likely applicable to MALA since many patients would have concurrent acute kidney injury, and metformin is associated with elevated lactate level in critically ill patients when Acute Kidney Injury Network score is ≥ 2[66]. Theoretical concerns of bicarbonate generating excessive carbon dioxide and paradoxically worsening acidemia has led to the use of tris-hydroxymethyl aminomethane, a buffer that increases the intracellular pH of hepatocytes[67], though this has not been widely adopted.
Metformin is a small molecule of 129 Daltons that is water-soluble, minimally protein-bound, and easily dialyzable[68]. The Extracorporeal Treatments in Poisoning Workgroup has recommended the institution of extracorporeal removal when any one the following conditions of severity are met: Lactate > 20 mmol/L, pH ≤ 7.0, or failure of standard supportive measures (e.g., use of bicarbonate infusion)[28]. The clinical utility of measuring metformin levels before prescribing extracorporeal treatment is unclear as metformin toxicity cannot be excluded if levels are not high[28]. Extracorporeal removal of metformin can be considered when biochemical parameters are less severe in patients with circulatory failure/hypotension, decreased level of consciousness, kidney failure, or liver failure[28]. If the patient is hemodynamically stable, intermittent hemodialysis is the preferred modality as it is highly efficient in correcting acidemia and removing metformin. Alternatively, if intermittent hemodialysis is not available, or if the patient is hemodynamically unstable, then continuous kidney replacement therapy may be used. Despite concerns that metformin can inhibit citrate metabolism and lead to citrate accumulation, regional citrate anticoagulation to prolong filter life for continuous kidney replacement therapy can be safely used[69]. Extracorporeal treatment can be stopped when the lactate concentration falls below 3 mmol/L, and the pH corrects to at least 7.35, though repeated monitoring will be required to detect rebound of MALA due to redistribution of metformin from tissues to the intravascular space[28]. When intermittent hemodialysis is used, one study showed that a cumulative duration of 15 h was associated with normalization of metformin levels to the therapeutic range[70].
RESTARTING METFORMIN AFTER CRITICAL ILLNESS OR MALA
The optimal time to restart metformin has not been well-studied. It is nonetheless reasonable to first ensure that lactic acidosis has resolved, and then recheck the kidney function post-recovery from critical illness, ensuring that the eGFR is 30 mL/min/1.73 m2 or better before restarting metformin. Metformin should be avoided in patients with advanced chronic kidney disease with creatinine > 530 µmol/L, given 35% increased odds of mortality compared to non-use of metformin[71]. Dose adjustment of metformin when eGFR is between 30-60 mL/min/1.73 m2 may help avoid metformin accumulation above the safe upper limit of 5 mg/L: Maximum total daily doses should be kept at 1500 mg when eGFR is between 45-59 mL/min/1.73 m2, and at 1000 mg when eGFR is between 30-44 mL/min/1.73 m2[72]. Additionally, for stable patients with chronic liver disease, who are not in liver failure and who do not excessively consume alcohol, hepatic elimination of lactate remains sufficient for safe use of metformin[73].
CONCLUSION
MALA in critically ill patients is associated with a mortality rate above 10% and occurs with 2.4-39 incident cases per 100000 patient-years. It may be suspected in a patient who has received metformin and who has a HAGMA, and confirmed when lactate exceeds 5 mmol/L. Risk factors include those that reduce renal elimination of metformin (renal impairment from any cause, H2 receptor antagonists, ribociclib) and excessive alcohol consumption (as ethanol oxidation consumes nicotinamide adenine dinucleotides that are also required for lactate metabolism). Treatment of MALA involves immediate cessation of metformin, supportive management, treating other concurrent causes of lactic acidosis like sepsis, and treating any coexisting diabetic ketoacidosis. Severe MALA requires extracorporeal removal of metformin with either intermittent hemodialysis or continuous kidney replacement therapy.
Footnotes
Conflict-of-interest statement: Dr. See reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from Novartis, outside the submitted work.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: Singapore
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Berkovic MC, Croatia; McGinnis CB, United States S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
References
- 1.Carpenter DL, Gregg SR, Xu K, Buchman TG, Coopersmith CM. Prevalence and Impact of Unknown Diabetes in the ICU. Crit Care Med. 2015;43:e541–e550. doi: 10.1097/CCM.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen CF, Johansen MB, Christensen S, O'Brien JM, Tønnesen E, Sørensen HT. Type 2 diabetes and 1-year mortality in intensive care unit patients. Eur J Clin Invest. 2013;43:238–247. doi: 10.1111/eci.12036. [DOI] [PubMed] [Google Scholar]
- 3.Roumie CL, Chipman J, Min JY, Hackstadt AJ, Hung AM, Greevy RA Jr, Grijalva CG, Elasy T, Griffin MR. Association of Treatment With Metformin vs Sulfonylurea With Major Adverse Cardiovascular Events Among Patients With Diabetes and Reduced Kidney Function. JAMA. 2019;322:1167–1177. doi: 10.1001/jama.2019.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viollet B, Foretz M. Revisiting the mechanisms of metformin action in the liver. Ann Endocrinol (Paris) 2013;74:123–129. doi: 10.1016/j.ando.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Hughes BW, Gray LA, Bradberry SM, Sandilands EA, Thanacoody RH, Coulson JM. Metformin-associated lactic acidosis reported to the United Kingdom National Poisons Information Service (NPIS) between 2010 and 2019: a ten-year retrospective analysis. Clin Toxicol (Phila) 2023;61:445–452. doi: 10.1080/15563650.2023.2198667. [DOI] [PubMed] [Google Scholar]
- 7.Yeh HC, Ting IW, Tsai CW, Wu JY, Kuo CC. Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy: a systematic review of case reports and case series. BMC Nephrol. 2017;18:229. doi: 10.1186/s12882-017-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CC, Weng SF, Tseng KL, Ho CH. Clinical presentations and prognosis of metformin-associated lactic acidosis patients in the intensive care unit: A 20-year survey. Medicine (Baltimore) 2022;101:e29918. doi: 10.1097/MD.0000000000029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thammavaranucupt K, Phonyangnok B, Parapiboon W, Wongluechai L, Pichitporn W, Sumrittivanicha J, Sungkanuparph S, Nongnuch A, Jayanama K. Metformin-associated lactic acidosis and factors associated with 30-day mortality. PLoS One. 2022;17:e0273678. doi: 10.1371/journal.pone.0273678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly PJ, Lonergan M, Soto-Pedre E, Donnelly L, Zhou K, Pearson ER. Acute kidney injury, plasma lactate concentrations and lactic acidosis in metformin users: A GoDarts study. Diabetes Obes Metab. 2017;19:1579–1586. doi: 10.1111/dom.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiholm BE, Myrhed M. Metformin-associated lactic acidosis in Sweden 1977-1991. Eur J Clin Pharmacol. 1993;44:589–591. doi: 10.1007/BF02440866. [DOI] [PubMed] [Google Scholar]
- 12.Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care. 2014;37:2291–2295. doi: 10.2337/dc14-0464. [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vecchio S, Giampreti A, Petrolini VM, Lonati D, Protti A, Papa P, Rognoni C, Valli A, Rocchi L, Rolandi L, Manzo L, Locatelli CA. Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol (Phila) 2014;52:129–135. doi: 10.3109/15563650.2013.860985. [DOI] [PubMed] [Google Scholar]
- 15.Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999;22:925–927. doi: 10.2337/diacare.22.6.925. [DOI] [PubMed] [Google Scholar]
- 16.Mariano F, Pozzato M, Inguaggiato P, Guarena C, Turello E, Manes M, David P, Berutti S, Consiglio V, Amore A, Campo A, Marino A, Berto M, Carpani P, Calabrese G, Gherzi M, Stramignoni E, Martina G, Serra A, Comune L, Roscini E, Marciello A, Todini V, Vio P, Filiberti O, Boero R, Cantaluppi V. Metformin-Associated Lactic Acidosis Undergoing Renal Replacement Therapy in Intensive Care Units: A Five-Million Population-Based Study in the North-West of Italy. Blood Purif. 2017;44:198–205. doi: 10.1159/000471917. [DOI] [PubMed] [Google Scholar]
- 17.Peters N, Jay N, Barraud D, Cravoisy A, Nace L, Bollaert PE, Gibot S. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12:R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biradar V, Moran JL, Peake SL, Peter JV. Metformin-associated lactic acidosis (MALA): clinical profile and outcomes in patients admitted to the intensive care unit. Crit Care Resusc. 2010;12:191–195. [PubMed] [Google Scholar]
- 19.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protti A, Lecchi A, Fortunato F, Artoni A, Greppi N, Vecchio S, Fagiolari G, Moggio M, Comi GP, Mistraletti G, Lanticina B, Faraldi L, Gattinoni L. Metformin overdose causes platelet mitochondrial dysfunction in humans. Crit Care. 2012;16:R180. doi: 10.1186/cc11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A, Foti G, Rossi A, Mistraletti G, Gattinoni L. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14:R22. doi: 10.1186/cc8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreis DT, Mallat J, Tettamanti M, Chiarla C, Giovannini I, Gatti S, Protti A. Increased ratio of P[v-a]CO(2) to C[a-v]O(2) without global hypoxia: the case of metformin-induced lactic acidosis. Respir Physiol Neurobiol. 2021;285:103586. doi: 10.1016/j.resp.2020.103586. [DOI] [PubMed] [Google Scholar]
- 23.Protti A, Fortunato F, Monti M, Vecchio S, Gatti S, Comi GP, De Giuseppe R, Gattinoni L. Metformin overdose, but not lactic acidosis per se, inhibits oxygen consumption in pigs. Crit Care. 2012;16:R75. doi: 10.1186/cc11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiew AL, Wright DFB, Dobos NM, McArdle K, Mostafa AA, Newth A, Roberts MS, Isbister GK. 'Massive' metformin overdose. Br J Clin Pharmacol. 2018;84:2923–2927. doi: 10.1111/bcp.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manouchehri A, Rashidian H, Zakariaei Z. Severe metabolic acidosis due to massive metformin overdose in a man: a case report. Oxf Med Case Reports. 2023;2023:omad049. doi: 10.1093/omcr/omad049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Sirtori CR, Franceschini G, Galli-Kienle M, Cighetti G, Galli G, Bondioli A, Conti F. Disposition of metformin (N,N-dimethylbiguanide) in man. Clin Pharmacol Ther. 1978;24:683–693. doi: 10.1002/cpt1978246683. [DOI] [PubMed] [Google Scholar]
- 28.Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, Gosselin S, Hoffman RS, Nolin TD, Ghannoum M Extracorporeal Treatments in Poisoning Workgroup. Extracorporeal Treatment for Metformin Poisoning: Systematic Review and Recommendations From the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med. 2015;43:1716–1730. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 29.Bennis Y, Bodeau S, Batteux B, Gras-Champel V, Masmoudi K, Maizel J, De Broe ME, Lalau JD, Lemaire-Hurtel AS. A Study of Associations Between Plasma Metformin Concentration, Lactic Acidosis, and Mortality in an Emergency Hospitalization Context. Crit Care Med. 2020;48:e1194–e1202. doi: 10.1097/CCM.0000000000004589. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 31.Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN), Hernández G, Ospina-Tascón G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Cavalcanti AB, Bakker J, Hernández G, Alegría L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavéz N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, González H, Arancibia JM, Muñoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Muñoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpán B, Fasce F, Luengo C, Medel N, Cortés C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, González MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudín A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, García F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garcés P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Peréz V, Delgado G, López A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderón A, Paredes G, Barberán JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernán Portilla A, Dávila H, Mora JA, Calderón LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elshafei MN, Alamin M, Mohamed MFH. Osmolar-gap in the setting of metformin-associated lactic acidosis: Case report and a literature review highlighting an apparently unusual association. Medicine (Baltimore) 2020;99:e22492. doi: 10.1097/MD.0000000000022492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;2010:CD002967. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luft D, Deichsel G, Schmülling RM, Stein W, Eggstein M. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol. 1983;80:484–489. doi: 10.1093/ajcp/80.4.484. [DOI] [PubMed] [Google Scholar]
- 35.See KC. Management of circulatory shock and hypotension. Singapore Med J. 2022;63:239–244. doi: 10.11622/smedj.2022075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryant SM, Cumpston K, Lipsky MS, Patel N, Leikin JB. Metformin-associated respiratory alkalosis. Am J Ther. 2004;11:236–237. doi: 10.1097/00045391-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Pepper GM, Schwartz M. Lactic acidosis associated with Glucophage use in a man with normal renal and hepatic function. Diabetes Care. 1997;20:232–233. doi: 10.2337/diacare.20.2.232b. [DOI] [PubMed] [Google Scholar]
- 38.Nzomessi D, Massie E, Gariani K, Giraud R, Meyer P. Combined lactic acidosis and ketoacidosis in a female diabetic patient with severe heart failure. Cardiovasc Endocrinol Metab. 2023;12:e0287. doi: 10.1097/XCE.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuda M, Hirayu N, Nabeta M, Goto M, Takasu O. Metformin-Associated Lactic Acidosis in Individuals Without Chronic Kidney Disease on Therapeutic Dose: A Case Report. Cureus. 2023;15:e48683. doi: 10.7759/cureus.48683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang QC, Hastings C, Johnson K, Slaven E. Metformin-Associated Lactic Acidosis Presenting Like Acute Mesenteric Ischemia. J Emerg Med. 2019;57:720–722. doi: 10.1016/j.jemermed.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Ali S, Labuschagne H, Azarov N, Hindi Z, Oud L. Metformin-associated lactic acidosis mimicking ischaemic bowel. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-221686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang R, Sun W. Reversible acute blindness in suspected metformin-associated lactic acidosis: a case report. J Med Case Rep. 2023;17:487. doi: 10.1186/s13256-023-04219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rueda Prada L, Knopps L, Dumic I, Barusya C, Subramanian A, Charokopos A, Zurob AS. Transient Complete Blindness Due to Metformin-Associated Lactic Acidosis (MALA) Reversed with Hemodialysis. Am J Case Rep. 2022;23:e935730. doi: 10.12659/AJCR.935730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angeletti C, Vergani C, Troili S, Carrocci C, De Martinis G, Venturoni F, Marinangeli F, Gentili L. Two cases of metformin-associated lactic acidosis in post-operative period in emergency department: time to be aware-case reports. AME Case Rep. 2023;7:39. doi: 10.21037/acr-22-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarus B, Wu A, Shin JI, Sang Y, Alexander GC, Secora A, Inker LA, Coresh J, Chang AR, Grams ME. Association of Metformin Use With Risk of Lactic Acidosis Across the Range of Kidney Function: A Community-Based Cohort Study. JAMA Intern Med. 2018;178:903–910. doi: 10.1001/jamainternmed.2018.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CC, Ko Y, Chen CH, Hung YJ, Wei TE, Chang TH, Ke SS, Kuo KN, Chen C. Relationship between metformin use and lactic acidosis in advanced chronic kidney disease: The REMIND-TMU study. Am J Med Sci. 2022;364:575–582. doi: 10.1016/j.amjms.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 47.Orloff J, Min JY, Mushlin A, Flory J. Safety and effectiveness of metformin in patients with reduced renal function: A systematic review. Diabetes Obes Metab. 2021;23:2035–2047. doi: 10.1111/dom.14440. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez CA, Halm EA, Pugh MJV, McGuire DK, Hennessy S, Miller RT, Lingvay I, Vouri SM, Zullo AR, Yang H, Chansard M, Mortensen EM. Lactic acidosis incidence with metformin in patients with type 2 diabetes and chronic kidney disease: A retrospective nested case-control study. Endocrinol Diabetes Metab. 2021;4:e00170. doi: 10.1002/edm2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubas TC, Johnson WJ. Metformin-induced lactic acidosis: potentiation by ethanol. Res Commun Chem Pathol Pharmacol. 1981;33:21–31. [PubMed] [Google Scholar]
- 50.Yamagishi H, Sekiguchi N, Hirano A, Oshima A, Imai T. Metformin-associated Lactic Acidosis Induced by Excessive Alcohol Consumption. Intern Med. 2023 doi: 10.2169/internalmedicine.2707-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie W, Li J, Kong C, Luo W, Zheng J, Zhou Y. Metformin-Cimetidine Drug Interaction and Risk of Lactic Acidosis in Renal Failure: A Pharmacovigilance-Pharmacokinetic Appraisal. Diabetes Care. 2024;47:144–150. doi: 10.2337/dc23-1344. [DOI] [PubMed] [Google Scholar]
- 52.Lagampan C, Poovorawan N, Parinyanitikul N. Lactic acidosis, a potential toxicity from drug-drug interaction related to concomitant ribociclib and metformin in preexisting renal insufficiency: A case report. Cancer Rep (Hoboken) 2022;5:e1575. doi: 10.1002/cnr2.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.See KC. Glycemic targets in critically ill adults: A mini-review. World J Diabetes. 2021;12:1719–1730. doi: 10.4239/wjd.v12.i10.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis*. Crit Care Med. 2014;42:2118–2125. doi: 10.1097/CCM.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 55.Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, Rhodes A, Cecconi M. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. 2017;21:207. doi: 10.1186/s13054-017-1796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galiero F, Consani G, Biancofiore G, Ruschi S, Forfori F. Metformin intoxication: Vasopressin's key role in the management of severe lactic acidosis. Am J Emerg Med. 2018;36:341.e5–341.e6. doi: 10.1016/j.ajem.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 57.Workum JD, Keyany A, Jaspers TCC. Methylene blue as treatment for vasoplegic shock in severe metformin overdose: A case report. Toxicol Rep. 2023;11:141–144. doi: 10.1016/j.toxrep.2023.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Driscoll BR, Howard LS, Earis J, Mak V British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:ii1–ii90. doi: 10.1136/thoraxjnl-2016-209729. [DOI] [PubMed] [Google Scholar]
- 59.Frakes MA, McWade J, Ender V, Cohen JE, Wilcox SR. Ventilator Management in Metformin-Associated Lactic Acidosis: A Case Report. Air Med J. 2023;42:300–302. doi: 10.1016/j.amj.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Carson JL, Stanworth SJ, Guyatt G, Valentine S, Dennis J, Bakhtary S, Cohn CS, Dubon A, Grossman BJ, Gupta GK, Hess AS, Jacobson JL, Kaplan LJ, Lin Y, Metcalf RA, Murphy CH, Pavenski K, Prochaska MT, Raval JS, Salazar E, Saifee NH, Tobian AAR, So-Osman C, Waters J, Wood EM, Zantek ND, Pagano MB. Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA. 2023;330:1892–1902. doi: 10.1001/jama.2023.12914. [DOI] [PubMed] [Google Scholar]
- 61.Akkaoui KK, Andersen LV, Nørgaard MA, Andreasen JB. Surviving cardiac arrest from severe metformin-associated lactic acidosis using extracorporeal membrane oxygenation and double continuous venovenous haemodialysis. BMJ Case Rep. 2023;16 doi: 10.1136/bcr-2023-254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling SK, Chung KW, Ma HY. A case of metformin-associated lactic acidosis with cardiogenic and vasoplegic shock supported by ECPella. Perfusion. 2023:2676591231181851. doi: 10.1177/02676591231181851. [DOI] [PubMed] [Google Scholar]
- 63.Rodríguez-Villar S, Kraut JA, Arévalo-Serrano J, Sakka SG, Harris C, Awad I, Toolan M, Vanapalli S, Collins A, Spataru A, Eiben P, Recea V, Brathwaite-Shirley C, Thompson L, Gurung B, Reece-Anthony R Acid-Base Working Group. Systemic acidemia impairs cardiac function in critically Ill patients. EClinicalMedicine. 2021;37:100956. doi: 10.1016/j.eclinm.2021.100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teplinsky K, O'Toole M, Olman M, Walley KR, Wood LD. Effect of lactic acidosis on canine hemodynamics and left ventricular function. Am J Physiol. 1990;258:H1193–H1199. doi: 10.1152/ajpheart.1990.258.4.H1193. [DOI] [PubMed] [Google Scholar]
- 65.Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, Pottecher J, Demoule A, Ferrandière M, Asehnoune K, Dellamonica J, Velly L, Abback PS, de Jong A, Brunot V, Belafia F, Roquilly A, Chanques G, Muller L, Constantin JM, Bertet H, Klouche K, Molinari N, Jung B BICAR-ICU Study Group. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392:31–40. doi: 10.1016/S0140-6736(18)31080-8. [DOI] [PubMed] [Google Scholar]
- 66.Posma RA, Hulman A, Thomsen RW, Jespersen B, Nijsten MW, Christiansen CF. Metformin use and early lactate levels in critically ill patients according to chronic and acute renal impairment. Crit Care. 2020;24:585. doi: 10.1186/s13054-020-03300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yusim D, Tiru B, Abdullin M, Landry DL, Hodgins S, Braden GL. Treatment of severe metformin-associated lactic acidosis with renal replacement therapy and tris-hydroxymethyl aminomethane: a case report. J Med Case Rep. 2023;17:462. doi: 10.1186/s13256-023-04201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correia MS, Horowitz BZ. Continuous extracorporeal clearance in metformin-associated lactic acidosis and metformin-induced lactic acidosis: a systematic review. Clin Toxicol (Phila) 2022;60:1266–1276. doi: 10.1080/15563650.2022.2127363. [DOI] [PubMed] [Google Scholar]
- 69.Brunoni B, Zadek F, Mulazzani F, Verza G, Marrazzo F, Spina S, Protti A, Fumagalli R, Langer T. Calcium-Citrate Anticoagulation during Continuous Renal Replacement Therapy in Patients with Metformin Intoxication: A Case Series, Mathematical Estimation of Citrate Accumulation, and Literature Review. Blood Purif. 2023;52:802–811. doi: 10.1159/000531953. [DOI] [PubMed] [Google Scholar]
- 70.Seidowsky A, Nseir S, Houdret N, Fourrier F. Metformin-associated lactic acidosis: a prognostic and therapeutic study. Crit Care Med. 2009;37:2191–2196. doi: 10.1097/CCM.0b013e3181a02490. [DOI] [PubMed] [Google Scholar]
- 71.Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, Hsu CC, Tarng DC. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015;3:605–614. doi: 10.1016/S2213-8587(15)00123-0. [DOI] [PubMed] [Google Scholar]
- 72.Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease Stages 3A, 3B, or 4. Diabetes Care. 2018;41:547–553. doi: 10.2337/dc17-2231. [DOI] [PubMed] [Google Scholar]
- 73.Smith FC, Stocker SL, Danta M, Carland JE, Kumar SS, Liu Z, Greenfield JR, Braithwaite HE, Cheng TS, Graham GG, Williams KM, Day RO. The safety and pharmacokinetics of metformin in patients with chronic liver disease. Aliment Pharmacol Ther. 2020;51:565–575. doi: 10.1111/apt.15635. [DOI] [PubMed] [Google Scholar]