Abstract
BACKGROUND
Emergency pancreaticoduodenectomy (EPD) is a rare event for complex periampullary etiology. Increased intraoperative blood loss is correlated with poor postoperative outcomes.
CASE SUMMARY
Two patients underwent EPD using a no-touch isolation technique, in which all arteries supplying the pancreatic head region were ligated and divided before manipulation of the pancreatic head and duodenum. The operative times were 220 and 239 min, and the blood loss was 70 and 270 g, respectively. The patients were discharged on the 14th and 10th postoperative day, respectively. Thirty-two patients underwent EPD for the treatment of neoplastic bleeding. The mean operative time was 361.6 min, and the mean blood loss was 747.3 g. The complication rate was 37.5%. The in-hospital mortality rate was 9.38%.
CONCLUSION
The no-touch isolation technique is feasible, safe, and effective for reducing intraoperative blood loss in EPD.
Keywords: No-touch isolation technique, Pancreaticoduodenectomy, Emergency pancreaticoduodenectomy, Neoplastic bleeding, Superior mesenteric artery first approach, Case report
Core Tip: Emergency pancreaticoduodenectomy (EPD) has been rarely reported as a life-saving procedure. Morbidity and mortality rates remain very high for EPD. Increased intraoperative blood loss has been linked to worse postoperative results. The non-touch isolation technique is feasible, safe, and effective in minimizing intraoperative blood loss in EPD.
INTRODUCTION
Emergency pancreaticoduodenectomy (EPD) is rarely performed in cases of trauma, endoscopic and/or postoperative complications, perforation, uncontrollable hemorrhage, or progressive multiple-organ failure in severe necrotizing pancreatitis. While the mortality rate of elective PD has shown a significant decrease during the last three decades, it remains higher for EPD[1]. Increased intraoperative blood loss can be correlated with poor postoperative outcomes in PD[2], and there is clearly a higher in-hospital mortality rate for patients with higher intraoperative blood loss in EPD[3]. Therefore, minimizing intraoperative blood loss during EPD is crucial. This report describes a no-touch isolation technique in which all arteries supplying the pancreatic head region are first ligated and divided before manipulation of the pancreatic head and duodenum in the early phase of resection during EPD.
CASE PRESENTATION
Chief complaints
A 67-year-old woman and an 88-year-old woman presented to emergency department with a complaint of melena and dyspnea, respectively.
History of present illness
Symptoms developed just before emergency transport in both patients.
History of past illness
Case 1: The first patient was scheduled to undergo pylorus-preserving PD (PPPD) because of a duodenal gastrointestinal stromal tumor (GIST).
Case 2: The second patient had painless mass that was gradually increasing in size over a period of six months.
Personal and family history
Both two patients denied any family history of malignant tumors.
Physical examination
Case 1: On physical examination, the vital signs were as follows: Body temperature (35.9 °C); blood pressure (75/34 mmHg); heart rate (124 beats per min); respiratory rate (28 breaths per min).
Case 2: On physical examination, the vital signs were as follows: Body temperature (36.4 °C); blood pressure (70/40 mmHg); heart rate (140 beats per min); respiratory rate (36 breaths per min).
Laboratory examinations
Case 1: Levels of serum hemoglobin were 3.7 g/dL.
Case 2: Levels of serum hemoglobin were 5.4 g/dL.
Imaging examinations
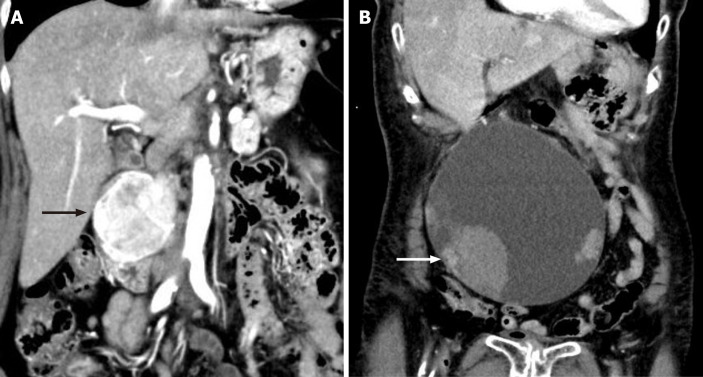
Case 1: Contrast-enhanced computed tomography demonstrated a duodenal hyper vascular mass, sized 5.0 cm × 6.0 cm (Figure 1A).
Figure 1.
Imaging of the duodenal tumor and pancreatic tumor. A: Imaging of the duodenal tumor and pancreatic tumor. Contrast-enhanced computed tomography showed a well-defined, enhancing masse, sized 5.0 cm × 6.0 cm, with heterogeneous density at the second portion of the duodenum (arrow); B: Imaging of the pancreatic tumor. Contrast-enhanced computed tomography showed a huge cystic mass measuring 17 cm, with some solid components and adjacent hematoma (arrow).
Case 2: A complex, septate cystic mass in the pancreatic head measuring 17 cm, with solid components and adjacent hematoma (Figure 1B).
Further diagnostic work-up
A diagnosis of GIST was made in the case 1 by ultrasound-guided fine-needle aspiration.
FINAL DIAGNOSIS
The final diagnosis was a GIST and an intraductal papillary mucinous neoplasm (IPMN).
TREATMENT
Two patients underwent EPD using a no-touch isolation technique as a lifesaving procedure for massive pancreaticoduodenal neoplastic bleeding at our institution between May and June 2023 (Table 1). All procedures were performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and revised in Tokyo 2004. Appropriate written informed consent was obtained for the publication of these case reports and accompanying images.
Table 1.
Details of patients who underwent emergency pancreaticoduodenectomy using a no-touch isolation technique
|
Patients
|
1
|
2
|
| Age/sex | 67/female | 88/female |
| Pathology | Duodenal-GIST | IPMN |
| Hb (g/dL) transfusion, U | 3.7, 8 | 5.4, 10 |
| Tumor size (cm) | 5 | 17 |
| Procedure | PPPD | PPPD |
| Operative time (m) | 220 | 239 |
| Blood loss (g) | 70 | 270 |
| Complication | No | No |
| Length of stay (d) | 14 | 10 |
GIST: Gastrointestinal stromal tumor; PPPD: Pylorus-preserving pancreaticoduodenectomy; IPMN: Intraductal papillary mucinous neoplasm; Hb: Hemoglobin.
Case 1
Eight units of red blood cell concentrate were transfused, and emergency PPPD was performed using a no-touch isolation technique. The operative time was 220 min, and blood loss was 70 g.
Case 2
Ten units of red blood cell concentrate were transfused before surgery. Intraoperative findings showed that the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) and the transverse colon and mesentery firmly adhered to the huge cystic tumor over its entire length, which made dissection difficult, and intraoperative blood loss was increased. Finally, PPPD was performed using a no-touch isolation technique. The operative time was 239 min, and blood loss was 270 g.
Surgical procedure
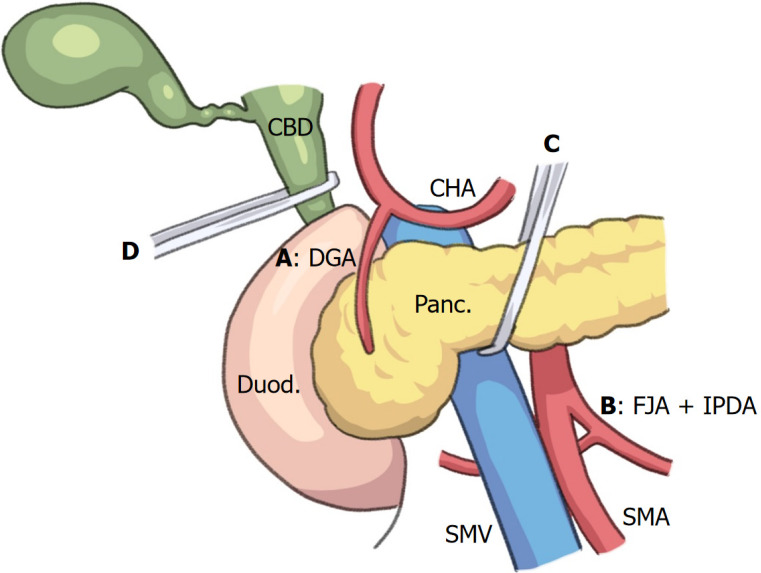
First, the gastrocolic ligament was divided and the omental bursa was opened to visualize the anterior surface of the pancreas. Dissection along the inferior surface of the pancreatic neck exposed the SMV. The SMA with nerve plexuses surrounding the SMA is taped just to the left side of the SMV. Dissection was performed along the superior surface of the pancreas and between the distal duodenum and the pancreas to isolate the common and proper hepatic arteries and the gastroduodenal artery (GDA), which was ligated and divided. After the pancreatic neck was separated from the SMV, it was taped. The hepatoduodenal ligament was dissected to isolate the portal vein and the common bile duct (CBD). Next, the ligament of Treitz was dissected and opened to visualize the aorta. The taped SMA was visualized immediately superior to the left renal vein. The inferior pancreaticoduodenal artery (IPDA) arises from the first jejunal artery (FJA) or the SMA. The IPDA usually branches from the left dorsal side of the SMA[4]. The proximal jejunum was pulled to the right and the SMA was rotated counterclockwise. The IPDA was visualized and then ligated and divided. The superior and inferior pancreaticoduodenal arteries were blocked (Figure 2). The inferior pancreatic artery (IPA) forms the arterial arcade between the GDA and the dorsal pancreatic artery (DPA)[5]. Therefore, ligation of the IPA is important. Early ligation of the IPA at the inferior border of the body of the pancreas or the arterial arcade of the DPA can be controlled by stay sutures on both the cranial and caudal sides of the remnant pancreas and amputation of the pancreas if the IPA cannot be isolated.
Figure 2.
Illustration of no-touch isolation technique in emergency pancreaticoduodenectomy. All arteries (A-D) that supply the pancreatic head region are isolated before manipulation of the pancreatic head and duodenum in the early phase of resection during emergency pancreaticoduodenectomy. A: The gastroduodenal artery; B: First jejunal artery, inferior pancreaticoduodenal artery; C: Intrapancreatic arterial arcade, including the inferior pancreatic artery. D: The para-biliary arterial plexus, including the 3 or 9 o’clock arteries, anastomoses to the posterior superior pancreaticoduodenal artery. Panc: Pancreas; Duod: Duodenum; SMV: Superior mesenteric vein; SMA: Superior mesenteric artery; CHA: Common hepatic artery; CBD: Common bile duct; GDA: Gastroduodenal artery; IPDA: Inferior pancreaticoduodenal artery; FJA: First jejunal artery.
OUTCOME AND FOLLOW-UP
No patients required intra-or postoperative blood transfusions, and no complications were encountered. Patients were discharged on the 14th and 10th postoperative day, respectively. All lesions had clear surgical margins. The patients were still alive without recurrence.
DISCUSSION
EPD, which has been rarely reported mostly in complex pancreaticoduodenal trauma, perforations, life-threatening hemorrhage, and severe infection is still a very uncommon procedure[1]. Recent rapid developments in technological innovations, improvements in surgical skills, progress in perioperative management, and the extensive experience of surgeons have proven the feasibility and safety of PD[6]. While the mortality rate of elective PD has shown a significant decrease during the last three decades, it remains higher for EPD[7]. EPD has been reported mostly in trauma settings, while non-trauma cases have been also reported, although rarely, which include bleeding, complicated tumors of the pancreaticoduodenal complex, endoscopic complications, and caustic ingestion[1]. Emergent pancreatic resection for neoplastic disease also is associated with significantly higher mortality and morbidity rates compared to elective pancreatic resections[8]. In patients with upper gastrointestinal tract bleeding, including pancreaticoduodenal, endoscopic or radiological procedures can achieve hemostasis in most patients. However, conservative management with interventional radiologic coiling of feeding arteries may momentarily salvage the situation but does not treat the underlying disease, especially in cases of malignancies. Moreover, interventional angiography with embolization of the pancreaticoduodenal arcade might not solve the problem in the presence of erosive tumors. It could also be ineffective because of the notable collateral blood supply of the pancreaticoduodenal area from the celiac and superior mesenteric arterial circulation[5], and the rich extra-blood supply, especially in patients with huge extra-growing tumors[9]. Therefore, in cases of bleeding of neoplastic origin, emergency pancreatoduodenectomy can be a definite therapeutic option. The literature review was performed via MEDLINE and PubMed with the key words, “emergency/emergent” and “pancreaticoduodenectomy/pancreatoduodenectomy”. In addition, we manually searched the reference lists of all the identified articles to identify further relevant articles. We selected only literature with full texts published between January 2001 and December 2022. All recorded cases of EPD performed for neoplastic bleeding in an emergency setting were extracted. Thirty-two patients[1,10-32] were reported to undergo EPD for neoplastic bleeding causes (Table 2): Seven cases of pancreatic carcinomas, five GISTs, four IPMNs, three duodenal carcinomas, two ampullary carcinomas, two liposarcomas, two lymphomas, and seven others (four cases of metastases from renal cell carcinoma, adrenal cancer, colon cancer, or neuroendocrine tumor, one duodenal sarcoma, one gastric cancer, and one paraganglioma). There were 15 females (46.88%) and 17 males (53.12%), with a mean age of 61.2 years. Two of the 32 patients (6.25%) underwent transcatheter arterial embolization before EPD. Operative time and intraoperative blood loss were described in 11 and 13 patients, respectively. The mean operative time was 361.6 min, and mean blood loss was 747.3 g. The complication rate was 37.5% (n = 12). Pancreatic fistula was the most common complication, occurring in six patients (18.75%). The in-hospital mortality rate was 9.38% (two patients). The mean length of hospitalization was 25.5 d. The overall mortality rate of EPD for tumor bleeding was 9.38%. Blood loss during EPD was 20-1600 g (mean 747.3 g). Increased intraoperative blood loss can be correlated with poor postoperative outcomes in PD[2]. There was a higher in-hospital mortality rate in patients with higher intraoperative blood[3]. Therefore, minimizing intraoperative blood loss during EPD is crucial. A no-touch isolation technique was originally recommended for operations on periampullary cancer to prevent the scattering of cancer cells into the portal blood[33,34]. To the best of our knowledge, this is the first report of a no-touch isolation technique performed to reduce intraoperative blood loss in EPD. In this procedure, all arteries that supply the pancreatic head region are first ligated and divided before manipulation of the pancreatic head and duodenum[6,33,34]. In conventional PD using Kocher’s maneuver, the GDA is usually ligated and divided during surgery; however, IPDA ligation and division are performed in the final stage of resection, which might induce further tumor bleeding. Early ligation of the IPDA is an effective technique for minimizing intraoperative blood loss during PD[33]. Recently, the SMA first approach has been proposed[35]. Using this approach, we identified the origin of the IPDA arising from the posterior aspect of the SMA without mobilization of the duodenum or colon[4,35]. Rotating the SMA counterclockwise facilitates easy identification of the origin of the FJA or IPDA because these arteries arise from the left dorsal aspect of the SMA[4,35]. The arterial supply of the pancreas is marked by numerous anastomoses. Arterial arcades are formed by branches from different main supplying arteries, and between branches from each main artery. Major branches anastomose within the substance of the pancreas[5]. Moreover, the para-biliary arterial plexus, including the 3 or 9 o’clock arteries, anastomoses the posterior superior pancreaticoduodenal artery[36]. Therefore, both the pancreas and CBD should be divided before Kocher’s maneuver if bleeding cannot be controlled after ligation of the GDA and IPDA. Duo to our limited experiences, a larger cohort should be evaluated in a prospective, randomized study to elucidate appropriate indications and effects of this novel procedure.
Table 2.
Reported patients that underwent emergency pancreaticoduodenectomy for neoplastic hemorrhage
|
Characteristic
|
|
|
| Sex, n (female/male) | 15/17 | |
| Age, yr (mean) | 20-93 (61.2) | |
| Pre-operative TAE, n (%) | 2 (6.25) | |
| Operative time, min (mean) | 210-553 (361.6) | |
| Blood loss, g (mean) | 20-1600 (747.3) | |
| Morbidity, n (%) | 12 (37.5) | |
| Mortality, n (%) | 3 (9.38) | |
| Hospital stay, d (mean) | 7-63 (25.5) | |
| Underlying diagnosis, n (%) | Pancreatic carcinoma | 7 (21.8) |
| GIST | 5 (15.6) | |
| IPMN | 4 (12.5) | |
| Duodenal carcinoma | 3 (9.4) | |
| Ampullary carcinoma | 2 (6.3) | |
| Liposarcoma | 2 (6.3) | |
| Lymphoma | 2 (6.3) | |
| Others | 7 (21.8) |
GIST: Gastrointestinal tumor; IPMN: Intraductal papillary mucinous neoplasm; TAE: Transcatheter arterial embolization.
CONCLUSION
Despite our limited experience, and pending future studies to establish appropriate indications, we believe that a non-touch isolation technique is feasible, safe, and effective in minimizing intraoperative blood loss in EPD. The benefits of this approach, however, require further validation, understanding the importance of careful patient selection for successful treatment.
Footnotes
Informed consent statement: Informed written consent was obtained from the patients for publication of this report and any accompanying images.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Tang H, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
Contributor Information
Akihiro Cho, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan. acho5184@gmail.com.
Satoshi Katagiri, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Masao Ota, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Shunsuke Onizawa, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Ryota Higuchi, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Toshiya Sugishita, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Yukiko Niwa, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Takeshi Ishita, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Toshihiko Mouri, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Akita Kato, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
Moe Iwata, Division of Gastroenterological Surgery, Tokyo Women’s Medical University, Yachiyo Medical Center, Chiba 276-8524, Japan.
References
- 1.Standop J, Glowka T, Schmitz V, Schaefer N, Hirner A, Kalff JC. Emergency Kausch-Whipple procedure: indications and experiences. Pancreas. 2010;39:156–159. doi: 10.1097/MPA.0b013e3181bb98d2. [DOI] [PubMed] [Google Scholar]
- 2.Seykora TF, Ecker BL, McMillan MT, Maggino L, Beane JD, Fong ZV, Hollis RH, Jamieson NB, Javed AA, Kowalsky SJ, Kunstman JW, Malleo G, Poruk KE, Soares K, Valero V 3rd, Velu LKP, Watkins AA, Vollmer CM Jr Pancreas Fistula Study Group. The Beneficial Effects of Minimizing Blood Loss in Pancreatoduodenectomy. Ann Surg. 2019;270:147–157. doi: 10.1097/SLA.0000000000002714. [DOI] [PubMed] [Google Scholar]
- 3.Popa C, Schlanger D, Chirică M, Zaharie F, Al Hajjar N. Emergency pancreaticoduodenectomy for non-traumatic indications-a systematic review. Langenbecks Arch Surg. 2022;407:3169–3192. doi: 10.1007/s00423-022-02702-6. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki I, Minagawa M, Takano K, Takizawa K, Hatakeyama K. Left posterior approach to the superior mesenteric vascular pedicle in pancreaticoduodenectomy for cancer of the pancreatic head. JOP. 2011;12:220–229. [PubMed] [Google Scholar]
- 5.Bertelli E, Di Gregorio F, Bertelli L, Mosca S. The arterial blood supply of the pancreas: a review. I. The superior pancreaticoduodenal and the anterior superior pancreaticoduodenal arteries. An anatomical and radiological study. Surg Radiol Anat. 1995;17:97–106, 1. doi: 10.1007/BF01627566. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Liu Y, Dong J, Chai Y, Tang H. Laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy for carcinoma of the ampulla of Vater in a medium-volume center: a propensity score matching analysis. J Int Med Res. 2023;51:3000605231219061. doi: 10.1177/03000605231219061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krige JE, Nicol AJ, Navsaria PH. Emergency pancreatoduodenectomy for complex injuries of the pancreas and duodenum. HPB (Oxford) 2014;16:1043–1049. doi: 10.1111/hpb.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driedger MR, Puig CA, Thiels CA, Bergquist JR, Ubl DS, Habermann EB, Grotz TE, Smoot RL, Nagorney DM, Cleary SP, Kendrick ML, Truty MJ. Emergent pancreatectomy for neoplastic disease: outcomes analysis of 534 ACS-NSQIP patients. BMC Surg. 2020;20:169. doi: 10.1186/s12893-020-00822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho A, Ryu M, Ochiai T. Successful resection, using pancreas-sparing duodenectomy, of extrahepatically growing hepatocellular carcinoma associated with direct duodenal invasion. J Hepatobiliary Pancreat Surg. 2002;9:393–396. doi: 10.1007/s005340200048. [DOI] [PubMed] [Google Scholar]
- 10.Iede K, Nakao A, Oshima K, Suzuki R, Yamada H, Tashiro M, Oshima Y, Kobayashi H. Controlling the arterial supply into the pancreatic head region as a whole peripancreatic arterial arcade via a mesenteric approach during isolated pancreatoduodenectomy. Surg Today. 2021;51:1819–1827. doi: 10.1007/s00595-021-02298-2. [DOI] [PubMed] [Google Scholar]
- 11.Parray FQ, Lone IM, Chowdri NA, Wani I, Wani MA, Gulzar GM, Thakur N. Emergency pancreaticoduodenectomy in duodenal paraganglioma: case report. ISRN Surg. 2011;2011:268674. doi: 10.5402/2011/268674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakakima Y, Inoue S, Fujii T, Hatsuno T, Takeda S, Kaneko T, Nagasaka T, Nakao A. Emergency pylorus-preserving pancreatoduodenectomy followed by second-stage pancreatojejunostomy for a gastrointestinal stromal tumor of the duodenum with an intratumoral gas figure: report of a case. Surg Today. 2004;34:701–705. doi: 10.1007/s00595-004-2771-z. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Okabayashi T, Kobayashi M, Araki K, Kohsaki T, Nishimori I, Onishi S, Ito S, Ogawa Y, Okuda H, Shuin T. Emergency pancreatoduodenectomy for pancreatic metastasis from renal cell carcinoma in a patient with von Hippel-Lindau disease: a case report. Dig Dis Sci. 2006;51:1383–1387. doi: 10.1007/s10620-005-9032-x. [DOI] [PubMed] [Google Scholar]
- 14.Stratigos P, Kouskos E, Kouroglou M, Chrisafis I, Fois L, Mavrogiorgis A, Axiotis E, Zamtrakis S. Emergency pancreatoduodenectomy (whipple procedure) for massive upper gastrointestinal bleeding caused by a diffuse B-cell lymphoma of the duodenum: report of a case. Surg Today. 2007;37:680–684. doi: 10.1007/s00595-007-3465-0. [DOI] [PubMed] [Google Scholar]
- 15.Hecker A, Hecker B, Bassaly B, Hirschburger M, Schwandner T, Janssen H, Padberg W. Dramatic regression and bleeding of a duodenal GIST during preoperative imatinib therapy: case report and review. World J Surg Oncol. 2010;8:47. doi: 10.1186/1477-7819-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okabayashi T, Shima Y, Iwata J, Sumiyoshi T, Kozuki A, Tokumaru T, Hata Y, Noda Y, Inagaki T, Morishita S, Morita M. Primary liposarcoma of the duodenum: a first case presentation. Intern Med. 2013;52:2753–2757. doi: 10.2169/internalmedicine.52.1230. [DOI] [PubMed] [Google Scholar]
- 17.Gulla A, Tan WP, Pucci MJ, Dambrauskas Z, Rosato EL, Kaulback KR, Pundzius J, Barauskas G, Yeo CJ, Lavu H. Emergent pancreaticoduodenectomy: a dual institution experience and review of the literature. J Surg Res. 2014;186:1–6. doi: 10.1016/j.jss.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 18.Strobel O, Schneider L, Philipp S, Fritz S, Büchler MW, Hackert T. Emergency pancreatic surgery--demanding and dangerous. Langenbecks Arch Surg. 2015;400:837–841. doi: 10.1007/s00423-015-1321-z. [DOI] [PubMed] [Google Scholar]
- 19.Nentwich MF, Reeh M, Uzunoglu FG, Bachmann K, Bockhorn M, Izbicki JR, Vashist YK. Non-trauma Emergency Pancreatoduodenectomies: A Single-Center Retrospective Analysis. World J Surg. 2016;40:2261–2266. doi: 10.1007/s00268-016-3525-y. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CY, Lai BR, Wang SY, Liao CH, Liu YY, Kang SC, Yeh CN, Jan YY, Yeh TS. The impact of preoperative etiology on emergent pancreaticoduodenectomy for non-traumatic patients. World J Emerg Surg. 2017;12:21. doi: 10.1186/s13017-017-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupascu C, Trofin A, Zabara M, Vornicu A, Cadar R, Vlad N, Apopei O, Grigorean V, Lupascu-Ursulescu C. Emergency Backwards Whipple for Bleeding: Formidable and Definitive Surgery. Gastroenterol Res Pract. 2017;2017:2036951. doi: 10.1155/2017/2036951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirea CS, Ciorbagiu MC, Obleagă CV, Moraru E, Mogoantă SŞ, Ciurea RN, Foarfă MC, Vîlcea AM, Vîlcea ID. Stage IV duodenal GIST requiring emergency pancreaticoduodenectomy - diagnosis difficulties and therapeutic options. Rom J Morphol Embryol. 2018;59:543–548. [PubMed] [Google Scholar]
- 23.Emiloju O, Candelario N, Dourado C. Metastatic clear cell endometrial carcinoma: an unusual cause of a common clinical presentation. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-235051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbarayan R, Anand S, Selvaraj S. Emergency Pancreaticoduodenectomy for Exsanguinating Ampullary Malignancy. Indian J Crit Care Med. 2020;24:1279–1280. doi: 10.5005/jp-journals-10071-23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyama A, Chikaishi Y, Kobayashi D, Matsuo K, Ochi T, Nakamura K, Endo T, Kikuchi K, Katsuno H, Nishijima A, Morise Z. A case of non-ampullary duodenal adenosquamous carcinoma with successful emergency pancreaticoduodenectomy for gastrointestinal hemorrhage. Surg Case Rep. 2023;9:161. doi: 10.1186/s40792-023-01749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye N, Bao X, Zhao X, Wang B. Signet-ring cell carcinoma of the duodenal bulb presenting with gastrointestinal hemorrhage: a case report and literature review. BMC Gastroenterol. 2022;22:226. doi: 10.1186/s12876-022-02267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y, Oshiro H, Yoshida A, Morishima K, Miki A, Sasanuma H, Sakuma Y, Lefor AK, Sata N. Pancreaticoduodenectomy for a primary duodenal capicua transcriptional repressor (CIC) -rearranged sarcoma with severe bleeding: a case report. BMC Gastroenterol. 2020;20:105. doi: 10.1186/s12876-020-01266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun M, Wei Y, Li S, Liu Z, Han X. Emergency laparoscopic pancreatoduodenectomy as treatment for pancreatic head cancer-induced acute gastrointestinal hemorrhage, safe or not? Hepatobiliary Surg Nutr. 2021;10:146–148. doi: 10.21037/hbsn-20-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokue H, Morita H, Tokue A, Tsushima Y. Successful management of life-threatening bleeding of intraductal papillary mucinous neoplasms in the pancreatic head. SAGE Open Med Case Rep. 2017;5:2050313X17741014. doi: 10.1177/2050313X17741014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha DE, Horn C, Passeri M. Triple threat: pancreatic cystic lesion presenting with spontaneous hemorrhage is found to harbor three distinct neoplasms. World J Surg Oncol. 2021;19:15. doi: 10.1186/s12957-021-02119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long TB, Binh NT, Dung LV, Linh LT, Luu DT, My TT, Duc NM. Diagnosis and treatment of hemosuccus pancreaticus induced by intraductal papillary mucinous neoplasm: a case report and review of the literature. Radiol Case Rep. 2021;16:3099–3103. doi: 10.1016/j.radcr.2021.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azizan N, Hayati F, Zakaria AD, Shukor NA. Intraductal papillary mucinous neoplasm presenting as bleeding duodenal mass: A surgical rarity. IIUM Med J Malaysia. 2019;18:123–126. [Google Scholar]
- 33.Nakao A, Takagi H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepatogastroenterology. 1993;40:426–429. [PubMed] [Google Scholar]
- 34.Hirota M, Shimada S, Yamamoto K, Tanaka E, Sugita H, Egami H, Ogawa M. Pancreatectomy using the no-touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: a pilot study. JOP. 2005;6:143–151. [PubMed] [Google Scholar]
- 35.Cho A, Yamamoto H, Kainuma O. Tips of laparoscopic pancreaticoduodenectomy: superior mesenteric artery first approach (with video) J Hepatobiliary Pancreat Sci. 2014;21:E19–E21. doi: 10.1002/jhbp.54. [DOI] [PubMed] [Google Scholar]
- 36.Cho A, Ryu M. New Liver Anatomy. 1st ed. New York: Springer, 2009. [Google Scholar]