Abstract
BACKGROUND
Coagulopathy and thromboembolic events are associated with poor outcomes in coronavirus disease 2019 (COVID-19) patients. There is conflicting evidence on the effects of chronic anticoagulation on mortality and severity of COVID-19 disease.
AIM
To summarize the body of evidence on the effects of pre-hospital anticoagulation on outcomes in COVID-19 patients.
METHODS
A Literature search was performed on LitCovid PubMed, WHO, and Scopus databases from inception (December 2019) till June 2023 for original studies reporting an association between prior use of anticoagulants and patient outcomes in adults with COVID-19. The primary outcome was the risk of thromboembolic events in COVID-19 patients taking anticoagulants. Secondary outcomes included COVID-19 disease severity, in terms of intensive care unit admission or invasive mechanical ventilation/intubation requirement in patients hospitalized with COVID-19 infection, and mortality. The random effects models were used to calculate crude and adjusted odds ratios (aORs) with 95% confidence intervals (95%CIs).
RESULTS
Forty-six observational studies met our inclusion criteria. The unadjusted analysis found no association between prior anticoagulation and thromboembolic event risk [n = 43851, 9 studies, odds ratio (OR)= 0.67 (0.22, 2.07); P = 0.49; I2 = 95%]. The association between prior anticoagulation and disease severity was non-significant [n = 186782; 22 studies, OR = 1.08 (0.78, 1.49); P = 0.64; I2 = 89%]. However, pre-hospital anticoagulation significantly increased all-cause mortality risk [n = 207292; 35 studies, OR = 1.72 (1.37, 2.17); P < 0.00001; I2 = 93%]. Pooling adjusted estimates revealed a statistically non-significant association between pre-hospital anticoagulation and thromboembolic event risk [aOR = 0.87 (0.42, 1.80); P = 0.71], mortality [aOR = 0.94 (0.84, 1.05); P = 0.31], and disease severity [aOR = 0.96 (0.72, 1.26); P = 0.76].
CONCLUSION
Prehospital anticoagulation was not significantly associated with reduced risk of thromboembolic events, improved survival, and lower disease severity in COVID-19 patients.
Keywords: Prior anticoagulation, COVID-19, Prehospital anticoagulation, Chronic anticoagulation, Mortality, Severity, Thromboembolic events
Core Tip: Coronavirus disease 2019 (COVID-19) patients are at an increased risk of developing thromboembolic events and hypercoagulable disorders, which are poor prognostic indicators of COVID-19 disease. The results of different observational studies on the effects of chronic anticoagulation on the mortality and severity of COVID-19 disease in infected patients are inconsistent. This systematic review and meta-analysis provide a comprehensive assessment of the risk of thromboembolic events, mortality, and severity of COVID-19 disease in patients on prehospital anticoagulation treatment.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged at the end of 2019 and was later termed coronavirus disease 2019 (COVID-19)[1]. While Acute Respiratory Distress Syndrome (ARDS) was the predominant complication associated with a potentially fatal outcome, the infection progression could also be influenced by other risk factors like diabetes, coronary artery disease, chronic kidney disease, metabolic syndrome, psychiatric and neurological complications[2-12]. Due to the complexity of these complications, a standardized treatment approach for COVID-19 has yet to be universally agreed upon to guide healthcare professionals worldwide[10,13-15].
Studies have described that multi-organ involvement in COVID-19 is associated with endothelial dysfunction and thrombosis[16]. Moreover, COVID-19 has been observed to influence clotting system pathways and activate platelets, potentially resulting in vascular inflammation, hypercoagulability, and endothelial dysfunction[17]. As a result, thromboembolic events are found to be a common complication in these individuals, with an incidence rate of around 25% for Venous Thromboembolism (VTE)[16]. SARS-CoV-2-related coagulopathy is distinct from other causes of coagulopathy that result in VTE as it involves a significant element of inducing systemic inflammation and endothelitis, which might not be effectively addressed by conventional anticoagulant treatment methods[18]. Regardless, emerging research evidence suggests that anticoagulation therapy has shown effectiveness in COVID-19 patients, particularly in noncritically ill hospitalized cases[19].
Studies mostly emphasize using anticoagulation therapy during hospitalization; however, the relationship between pre-existing chronic anticoagulation due to non-COVID-19 indication and clinical outcomes in COVID-19-infected patients remains largely unknown. This meta-analysis aims to analyze the relationship between VTE risk in COVID-19 disease and prehospital anticoagulation and to study the impact of prehospital anticoagulation on COVID-19 disease severity and mortality.
MATERIALS AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed in this systematic review and meta-analysis[20]. The protocol for this meta-analysis was registered on PROSPERO (CRD42022306893).
Data sources and literature search strategy
Two independent investigators (Iqbal K and Rathore SS) utilized databases, including LitCovid, WHO, and Scopus, to conduct a systemic literature search for relevant articles from December 2019 to June 30, 2023 (Supplementary Table 1). A combination of keywords, such as “anticoagulant”, “direct oral anticoagulants”, “DOAC”, “vitamin K antagonist”, “VKA”, “dabigatran”, “rivaroxaban”, “apixaban”, “edoxaban”, “warfarin”, “heparin”, “prehospital”, “prehospital”, “preadmission”, “pre-admission”, “chronic”, “long-term”, “pre-hospital”, and “prior”, were searched electronically[21].
Study selection and inclusion criteria
Duplicates within the articles retrieved from our systemic search were identified and eliminated using Endnote (Clarivate Analytics, Thomson Reuters Corporation, Philadelphia, Pennsylvania). Two independent researchers (Iqbal K and Rathore SS) screened the articles based on their titles, abstracts, and keywords. The articles were subsequently assessed for relevance through full-text screening. Data review and collection were done by Bhurwal A, Iqbal A, Ahmed J, Iqbal K, Sharma N, Mehdi M, Kumar P, and Rathore SS. References to the shortlisted articles were also screened for additional studies. Any disagreement was resolved by discussing within the group or through an independent reviewer’s (Bansal V) inputs. Articles that failed to fulfill the inclusion criteria were removed. For the meta-analysis, the inclusion criteria were: (1) All retrospective or prospective observational studies enrolling patients ≥ 18 years; (2) Studies that compared outcomes of COVID-19-infected patients who had been on anticoagulation before their COVID-19 diagnosis for any indication vs those that did not receive any prehospital anticoagulation; and (3) Studies including data (raw or adjusted) for at least one of the outcomes of interest. Exclusion criteria consisted of: (1) Studies without a control group; (2) Studies with no outcome of interest (new incidence of thromboembolic events, all-cause mortality, severity of COVID-19 (defined as per WHO Ordinal scale 6-9); and (3) Literature reviews or narrative reviews or case reports.
Our primary outcome of interest was the incidence of VTE in COVID-19-infected adults on chronic anticoagulation prior to the infection. Mortality and disease severity were considered secondary outcomes. COVID-19 disease severity was based on the WHO ordinal scale[22]. A WHO clinical progression scale score of 6-9 indicated severe COVID-19, often necessitating intensive care unit (ICU) admission. In cases where this scale was not used, the highest WHO ordinal scale event was used as a substitute for severe infection. If ICU admission rates were unavailable, intubation or non-invasive mechanical ventilation rates were considered to assess COVID-19 severity. The cases included SARS-CoV-2 positive hospitalized patients with prehospital use of anticoagulants. The controls were COVID-19-positive hospitalized patients with no history of prehospital anticoagulation. Both raw data and their adjusted estimates were derived for the meta-analysis. Estimates from studies that performed propensity-matched scoring were also considered as adjusted estimates.
Statistical analysis
We used Review Manager v.5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014)[23] for unadjusted and adjusted analysis and Comprehensive Meta-Analysis software package 154 (Biostat, Englewood, NJ, United States)[24] for the meta-regression analysis. For the unadjusted analysis, we calculated the Mantel Haenszel crude odds ratios (ORs) using a random-effects model with a 95% confidence interval (95%CI). We also calculated the adjusted OR (aOR) with 95%CI to measure adjusted estimates. Adjusted estimates were used for the primary analysis to produce more reliable results as it considers the baseline differences between the two groups (prior anticoagulant users and non-users) that may influence the outcomes. In all cases, significance was defined by a P-value < 0.05, and all analyses utilized a random-effects model. Statistical heterogeneity was gauged among the included articles using the Higgins I2 statistics, and I2 > 50% was regarded as substantial heterogeneity. To reduce the inherent selection bias in observational studies, a subgroup analysis based on study design was performed[25].
We conducted univariate and multivariate meta-regression analyses with random effects (maximum likelihood approach) to examine potential study variations that could impact the effect magnitude. The age and gender distribution of the study sample, the study's country of origin, and the percentage of participants with comorbid conditions like obesity, diabetes, pulmonary illness, dyslipidemia, cardiovascular disease, and hypertension were all considered potential sources of variability. If covariates significantly (P < 0.05) altered the relationship between mortality or severity in COVID-19 hospitalized patients and prehospital anticoagulant usage, they were chosen for additional modeling. Two models were developed: one for mortality and the other for the severity of the condition. Then, using P = 0.05 as the cutoff threshold for elimination, preselected factors were included in a manual backward and stepwise multiple meta-regression analysis. P < 0.05 (P < 0.10 for heterogeneity) was used to define statistical significance. The meta-analysis and meta-regression tests were all two-tailed.
Risk of Bias and Quality assessment
Two investigators (Kumar P, Iqbal A) assessed the included studies’ methodological quality through the Newcastle-Ottawa Scale (Supplementary Table 2)[26]. The following categories were used to evaluate every study: (1) Study group selection; (2) Study group comparability; and (3) Determination of the desired outcomes (Supplementary Table 2). The publishing bias likelihood was tested using Egger regression methods, and the results were visually examined using funnel plots (Supplementary Figure 1). The degree of evidence certainty at the outcome level was evaluated using the GRADE pro profiler (GRADE working group, McMaster University, and Evidence Prime Inc; Supplementary Table 3)[27].
RESULTS
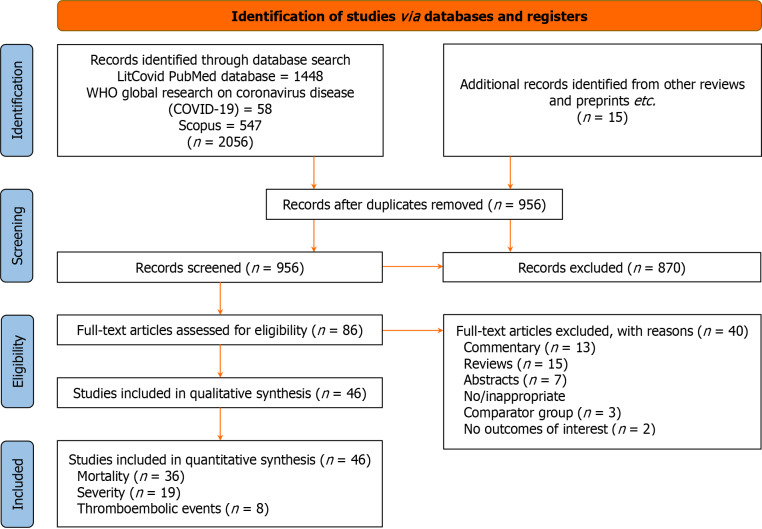
The initial database search produced 2056 articles of potential relevance. After eliminating the duplicates, 956 articles were filtered for appropriateness and relevancy using their titles, abstracts, and keywords. Of them, 86 full-text papers relevant to the manuscript's objectives were examined. The final analysis included 46 studies comprising 41 cohort studies (36 retrospective, 2 ambispective, and 3 prospective), three case-control studies, and one cross-sectional study. The PRISMA flowchart outlining the search process is shown in Figure 1. Table 1 provides an overview of the study characteristics of the included publications, and Table 2 shows the studies reporting adjusted estimates along with the covariates for which the estimates are adjusted.
Figure 1.
PRISMA flow diagram.
Table 1.
Study characteristic table for included studies
|
Ref.
|
Country of study
|
Study design
|
Setting
|
Total COVID-19 positive patients
|
Total patients with pre-admission anticoagulation
|
Type of anticoagulant
|
Duration of anticoagulant
|
Indication for anticoagulant Use
|
Definition Of Severity
|
Mean age ± SD (yr)
|
Female sex proportion (%)
|
Diabetes proportion (%)
|
Hypertension proportion (%)
|
Pulmonary disease proportion (%)
|
Arrhythmia proportion (%)
|
| Ageno et al[28], 2021 | Italy | Retrospective observational study | Inpatient hospitalized | 4396 | 43 | DOAC or VKA | - | AF | NA | - | - | - | - | - | 56.4 |
| Arachchillage et al[29], 2022 | United Kingdom | Ambispective cohort study | Inpatient hospitalized | 5883 | 963 | DOAC (rivaroxaban, apixaban, edoxaban, dabigatran, or VKA (warfarin) | - | VTE or heart disease or AF | ICU admission | - | 44.81 | 28.96 | 47.12 | 24.55 | - |
| Aslan et al[55], 2021 | Turkey | Retrospective cohort study | Inpatient hospitalized | 1710 | 79 | DOAC (dabigatran, rivaroxaban, apixaban, edoxaban) | - | AF, venous thrombosis | ICU admission | 62 (52-71) |
50.5 | 27 | 42 | 6 | 5 |
| Bauer et al[71], 2021 | United States | Case–control study | Hospitalized and non-hospitalized patients | 1449 | - | - | - | Admitted to the hospital/died | 54.7 ± 22.5 | 63 | 17 | 36 | 22 | - | |
| Boari et al[68], 2020 | Italy | Retrospective cohort study | Inpatient hospitalized | 258 | 29 | - | - | - | NA | 71.0 ± 13.8 | 32.9 | 26 | 58.5 | 14 | - |
| Brouns et al[53], 2020 | Netherlands | Retrospective case-series | Inpatient hospitalized | 101 | 18 | DOAC or VKA | - | - | - | - | - | - | - | - | - |
| Buenen et al[40], 2021 | Netherlands | Cohort study | Hospitalized and non-hospitalized patients | 497 | 110 | DOAC or VKA | - | - | NA | - | 36.2 | 20.5 | 52.1 | 26 | - |
| Chocron et al[41], 2021 | France | Retrospective cohort study | Inpatient hospitalized | 2878 | 382 | DOAC or VKA | - | AF, VTE | ICU admission | - | 40.3 | 30.3 | 75.3 | - | 24.8 |
| Corrochano et al[30], 2022 | Spain | Retrospective observational study | Inpatient hospitalized | 1598 | 155 | VKA (warfarin or acenocoumarol), DOAC (dabigatran, rivaroxaban, apixaban or edoxaban), or heparins | - | - | ICU admission | 66.5 (17.1) | 47.1 | 20 | 50.8 | - | - |
| Covino et al[42], 2021 | Italy | Retrospective observational study | Emergency department | 184 | 92 | DOAC or VKA | 1 month | AF | NA | 84 (81-87) |
50 | 18.5 | 41.8 | 17.4 | - |
| Denas et al[43], 2021 | Italy | Retrospective observational study | Hospitalized and non-hospitalized patients | 4697 | 651 | VKA, NOAC (dabigatran, rivaroxaban, apixaban) or edoxaban | 6 months | AF | ICU admission | - | 45.6 | 24.1 | 87.3 | - | - |
| Fauvel et al[54], 2020 | France | Retrospective observational study | Inpatient hospitalized | 1240 | 136 | VKA 47 (3.8), NOAC 78 (6.3), heparin 11 (0.9) | - | - | ICU admission and mechanical ventilation | - | - | - | - | - | - |
| Flam et al[59], 2021 | Sweden | Prospective cohort study | Inpatient hospitalized | 459402 | 103703 | DOAC (dabigatran, apixaban, rivaroxaban or edoxaban) | 0–6 months [7394 (7.1%)]; 7–24 months [29012 (28.0%)]; > 24 months [67245 (64.8%)] | AF and atrial flutter | Hospital admission and ICU admission | - | - | - | - | - | - |
| Fröhlich et al[31], 2021 | Germany | Retrospective cohort study | Inpatient hospitalized | 6637 | 731 | DOAC or VKA | 6 months | AF | NA | - | 50 | 25 | 93 | - | 76 |
| Fumagalli et al[44], 2022 | Italy | Retrospective cohort study | Inpatient hospitalized | 176 | 91 | DOAC or VKA | - | AF | NA | - | 48.3 | 33 | 71 | 21 | 11.9 |
| Gülcü et al[32], 2022 | Turkey | Retrospective cohort study | Inpatient hospitalized | 5575 | 451 | DOAC (rivaroxaban, apixaban, edoxaban, dabigatran), and VKA (warfarin) | - | - | NA | 64 (51–74) |
49.8 | 26.9 | 49.5 | 13.9 | 5.2 |
| Hanif et al[33], 2020 | United States | Retrospective cohort study | Inpatient hospitalized | 58 | 33 | DOAC (rivaroxaban, apixaban, edoxaban, and dabigatran), VKA (warfarin) | - | - | NA | - | 34.5 | - | - | - | - |
| Harrison et al[34], 2021 | United States | Retrospective cohort study | Inpatient hospitalized | 1027 | 132 | DOAC (rivaroxaban, apixaban, dabigatran), and VKA (warfarin) | - | - | NA | - | 52.5 | 38.7 | 74.3 | - | 21.1 |
| Ho et al[45], 2021 | United States | Retrospective cohort study | Hospitalized and non-hospitalized patients | 28076 | 304 | VKA (warfarin) or DOAC (dabigatran) | Within 3 months prior to SARS-Cov-2 diagnosis | - | ICU admission | - | 51.6 | 8.3 | 7.2 | - | - |
| Hozayen et al[35], 2021 | United States | Prospective cohort study | Outpatient and inpatient | 5597 | 160 | Enoxaparin, VKA (warfarin), DOAC | Within 3 months prior to SARS-Cov-2 diagnosis | - | NA | 51 ± 22 | 57.1 | 15.7 | 34.7 | 4.5 | 12 |
| Iaccarino et al[72], 2021 | Italy | Cross-sectional study | Inpatient hospitalized | 2377 | 125 | DOAC | 6 months | AF, mechanic valvularreplacement, pulmonary thromboembolism prophylaxis | ICU admission | 68.2 ± 0.38 | 37.3 | 18 | 59 | - | 4.7 |
| Klok et al[61], 2020 | Denmark | Retrospective cohort study | Intensive care | 184 | 17 | - | - | - | NA | - | - | - | - | - | - |
| Li et al[36], 2020 | China | Ambispective cohort study | Inpatient hospitalized | 547 | 16 | - | - | - | Severe COVID-19 infection | 60 (48-69) |
49.1 | 15.1 | 30.3 | 3.1 | - |
| Lodigiani et al[73], 2020 | Italy | Retrospective cohort study | Inpatient hospitalized | 388 | 33 | DOAC or VKA | - | - | ICU admission | 66 (55–75) |
32 | 22.7 | 47.2 | 9 | - |
| Ménager et al[60], 2020 | France | Retrospective cohort study | Inpatient hospitalized | 82 | 9 | VKA (warfarin or acenocoumarol or fluindione) | - | - | NA | 88 (85–92) | 47.6 | 23.2 | 63.4 | - | 36.6 |
| Middeldorp et al[62], 2020 | Netherlands | Retrospective cohort study | Inpatient hospitalized | 198 | 19 | - | - | AF | ICU admission | 61± 14 | 34 | - | - | - | - |
| Natali et al[63], 2020 | United States | Retrospective case control study | Inpatient hospitalized | 400 | 22 | - | - | - | NA | - | - | - | - | -- | - |
| Olcott et al[46], 2021 | England. | Retrospective cohort study | Inpatient hospitalized | 309 | 81 | DOAC or VKA | - | - | NA | - | 47.9 | - | - | - | - |
| Parker et al[47], 2021 | United Kingdom | Retrospective cohort study | Inpatient hospitalized | 1032 | 164 | DOAC, VKA, LMWH, fondaparinux | Taking the anticoagulant for > 1 month | AF, VTE, metallic heart valve, LV thrombus | ICU admission | 71 (56–83) |
44.9 | 29.3 | 44.3 | - | - |
| Philipose et al[66], 2020 | United Kingdom | Retrospective cohort study | Inpatient hospitalized | 466 | 68 | - | - | - | NA | - | - | - | 50.2 | 28.1 | - |
| Reilev et al[64], 2020 | Denmark | Cohort study | Community-managed and hospitalized | 11122 | 577 | - | At least one filled prescription within 6 months prior to the test date | - | ICU admission | 48 (33–62) |
62 | 6.4 | 24 | 12 | 4.6 |
| Rieder et al[37], 2022 | Multi-Country | Retrospective cohort study | Hospitalized and outpatient | 1433 | 334 | VKA or non-VKA DOAC (rivaroxaban, apixaban, edoxaban, dabigatran etexilate) | - | AF | NA | - | 39.8 | 31.2 | 86.5 | - | 25.4 |
| Rivera-Caravaca et al[58], 2021 | International, HOPE COVID-19 Registry | Retrospective cohort study | Inpatient hospitalized | 1002 | 110 | DOAC or VKA | - | AF, VTE, mechanical heart valves | NA | - | 40.8 | 31.2 | 82.1 | 18.3 | - |
| Rivera-Caravaca et al[38], 2021 | United States, Trinetx | Cohort study | Hospitalized and outpatient | 26006 | 13003 | DOAC (dabigatran, apixaban, rivaroxaban or edoxaban) | 1 yr | - | NA | - | 48.4 | 37.9 | 71.9 | 18.3 | 48.1 |
| Rodríguez-Molinero et al[67], 2020 | Spain | Retrospective cohort study | Inpatient hospitalized | 418 | 34 | - | - | - | Need for oxygen therapy through a nonrebreather mask or mechanical ventilation | 65.4 ± 16.6 | 43.1 | 23.7 | 52 | 9.8 | 10.8 |
| Rossi et al[57], 2020 | Italy | Retrospective observational study | Outpatient | 70 | 26 | DOAC (eivaroxaban, apixaban, edoxaban, dabigatran) | Regularly taken by the patient for at least 6 months | AF, pulmonary embolism, or DVT | NA | - | 50 | 25.7 | 61.4 | 15.7 | - |
| Russo et al[48], 2022 | Italy | Retrospective observational study | Inpatient hospitalized | 467 | 87 | DOAC (edoxaban, dabigatran, rivaroxaban, apixaban), VKA (warafrin) | - | AF, prosthetic heart valve, venous thromboembolism | NA | - | 33.3 | 25.3 | 74 | 18.8 | - |
| Ruzhentsova et al[56], 2021 | Italy | Retrospective cohort study | Outpatient | 76 | 26 | DOAC (rivaroxaban, apixaban, dabigatran) | - | - | NA | - | 56.6 | 26.3 | 77.6 | - | 36.8 |
| Schiavone et al[74], 2021 | Italy | Retrospective cohort study | Inpatient hospitalized | 844 | 65 | DOAC or VKA | - | - | ICU admission | 63.4 ± 16.1 | 38.3 | 16.6 | 45.1 | 7.4 | 9.2 |
| Sivaloganathan et al[49], 2020 | United Kingdom | Case-control study | Inpatient hospitalized | 180 | 31 | VKA (warfarin), DOAC (dabigatran, rivaroxaban, apixaban), or LMWH | - | - | ICU admission | - | - | - | - | - | - |
| Spiegelenberg et al[50], 2021 | Netherlands | Retrospective cohort study | Inpatient hospitalized | 1154 | 190 | DOAC or VKA | - | AF, VTE, mechanical valve replacement, cardiac arrest in history, or unknown (5%) | ICU admission | - | 32.2 | 27.3 | 53.8 | 26.1 | - |
| Tehrani et al[69], 2021 | Sweden | Retrospective cohort study | Inpatient hospitalized | 255 | 49 | - | - | - | NA | 66 ± 17 | 41 | 31 | 54 | 13 | - |
| Togano et al[39], 2021 | Japan | Retrospective cohort study | Inpatient hospitalized | 4026 | 105 | VKA (warfarin), DOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) | - | - | Mechanical ventilation/ supplemental oxygen/SPO2 ≤94% on room air/tachypnea | 52.0 (34–69) |
40.1 | 14.1 | 19 | 8.1 | - |
| Tremblay et al[70], 2020 | United States | Retrospective cohort study | Hospitalized and ambulatory patients | 3772 | 241 | - | - | - | Intubation-mechanical ventilation | 56.6 (18.2) | 45.2 | - | - | 14.9 | - |
| van Haaps et al[51], 2021 | Denmark | Retrospective cohort study | Inpatient hospitalized | 3006 | 445 | DOAC, VKA, LMWH | - | - | ICU admission | - | 35.1 | 37.8 | 59.5 | 8.3 | - |
| Wargny et al[65], 2021 | France | Retrospective cohort study | Inpatient hospitalized | 2796 | 501 | - | - | - | NA | 69.7 ± 13.2 | 36.3 | 100 | 76.8 | 9.6 | - |
Defined according to the 2019 clinical practice guideline from the Infectious Diseases Society of America and the American Thoracic Society for diagnosis and treatment of adults with community acquired pneumonia. DOAC: Direct-acting oral anticoagulants; VKA: Vitamin K antagonists; ARDS: Acute respiratory distress syndrome; NOAC: Non-Vitamin K oral anticoagulants; AF: Atrial fibrillation; VTE: Venous thromboembolism; DVT: Deep venous thrombosis; CAD: Coronary artery disease; NA: Not applicable; IQR: Interquartile range; OAC: Oral anticoagulant.
Table 2.
Studies reporting adjusted estimates and the factors for which they are adjusted
| Ref. | Outcome |
Adjusted estimates
|
| Ageno et al[28], 2021 | Mortality | Age, gender, and heparin use after admission, history of acute MI, T2D, HTN, cancer, COPD, renal function and CRP at hospital entry |
| Arachchillage et al[29], 2022 | 90-day mortality, thrombosis, and ICU admission | Age, gender, BMI, antiplatelet treatment prior to admission, autoimmune disease, malignancy, hypercholesterolaemia, heart disease, T2D, smoking status, liver disease, lung disease, existing renal failure and whether renal failure was dialysis dependent |
| Aslan et al[55], 2021 | In-hospital mortality | Age, male gender, T2D, ferritin, d-dimer, neutrophil, lymphocyte, creatinine, CRP, SaO2, procalcitonin, DOAC, HTN, HF, AF, CAD, COPD, systolic blood pressure and hematocrit in univariable logistic regression analysis |
| Buenen et al[40], 2021 | All-cause mortality within 30 d | Age, sex, symptom duration, home medication, and comorbidities |
| Covino et al[42], 2021 | All-cause in-hospital death. | Age, sex, comorbidity (categorized as CCI < 3 or CCI ≥ 3), and illness severity at admission (categorized as NEWS < 6 or NEWS ≥ 6) |
| Gülcü et al[32], 2022 | In-hospital all-cause mortality | Age, gender, HTN, DM, HF, CAD, eGFR, albumin, CRP, D-dimer, hemoglobin, platelet count, LDH, and oxygen saturation variables |
| Bauer et al[71], 2020 | Severity | Age and gender |
| Ménager et al[60], 2020 | 7-day mortality | Age, sex, severe undernutrition, T2D, HTN, prior MI, HF, prior stroke and/or TIA, CHA2DS2-VASc score, HAS-BLED score, and eGFR |
| Philipose et al[66], 2020 | Mortality | Age and gender |
| Rieder et al[37], 2022 | COVID-19 related mortality | Age, gender, BMI and smoking status, the phase of disease at diagnosis, solid tumor, AF, CAD, prior MI, peripheral artery disease, HTN, cerebrovascular disease, and T2D |
| Rivera-Caravaca et al[38], 2021 | Mortality and the composite of any thrombotic or thromboembolic event | Age, gender and ethnicity, all the included comorbidities |
| Rodríguez-Molinero et al[67], 2020 | Mortality | Age, sex, obesity, and corticosteroids |
| Russo et al[48], 2022 | Mortality | Age, arterial HTN, T2D, CAD, HF, previous stroke |
| Schiavone et al[74], 2021 | Mortality | Age > 65 years, male gender, CAD, CKD, COPD, HF, OAC, PaO2/FiO2, hydroxychloroquine, tocilizumab, antivirals, heparin |
| Spiegelenberg et al[50], 2021 | All-cause in hospital mortality and ICU admission | Age, sex, body mass index, active malignancy, COPD, T2D, HTN, CAD, MI, HF, non-ischaemic cardiomyopathy, previous heart surgery, electronic heart device, cerebrovascular accident, peripheral artery disease, immunosuppressive medication, no ICU policy |
| Togano et al[39], 2021 | Severity (mechanical ventilation/ supplemental oxygen/SpO2 ≤ 94% on room air/tachypnea) | Age, gender, BMI, smoking, alcohol consumption, myocardial infarction/congestive heart failure, peripheral artery disease, cerebrovascular disease, dementia, paralysis, COPD, liver dysfunction, hypertension, hyperlipidemia, diabetes, obesity, leukemia, lymphoma, immunosuppression |
| Tremblay et al[70], 2020 | All-cause mortality, mechanical ventilation | Age, gender, race, CCI and obesity |
| van Haaps et al[51], 2021 | 21-day all-cause mortality and ICU admission | Age, gender, T2D, HTN, CKD, asthma, obesity, time in pandemic, center, chronic cardiac disease, malignancy, liver disease, dementia, organ transplant, autoimmune disorder, and rheumatic disorder |
| Wargny et al[65], 2021 | Death within 28 d | Age |
| Harrison et al[34], 2021 | 21-day all-cause in hospital mortality | Age, gender, and confounding variables |
| Iaccarino et al[72], 2021 | Mortality, ICU admission | Age, multimorbidity (combined in the CCI score), and gender |
| Hozayen et al[35], 2021 | Mortality | Age, sex, self-identified race/ethnicity (as a proxy for social, not biological risk factors), Elixhauser comorbidity score, and the presence/absence of any cardiovascular, immunological or hematological comorbidities |
| Ho et al[45], 2021 | ICU admission, VTE, and mortality between date of SARS-CoV-2 diagnosis and 45 d after diagnosis | Age, sex, race/ethnicity, body mass index, CCI, HTN, T2D, and smoking history as well as the week of SARS-CoV-2 diagnosis |
| Denas et al[43], 2021 | ICU admission and all-cause mortality | Age, sex, HF, HTN, cancer, T2D, history of stroke/TIA, previous bleeding, history of MI, peripheral artery disease, abnormal renal function, abnormal hepatic function, use of antiplatelet drugs, NSAIDs and statin use |
| Corrochano et al[30], 2022 | All-cause mortality and ICU admission | Sex, age, CCI, and antithrombotic therapy |
| Chocron et al[41], 2021 | In-hospital mortality and ICU admission | Sex, age, cardiovascular comorbidities (history of HTN, dyslipidemia, BMI, T2D, and current smoking), plasma creatinine level (µmol/L), CRP (mg/L), fraction of inspired oxygen, the degree of pulmonary lesions with ground-glass opacities and areas of consolidation, and the use of in-hospital anticoagulation (preventive low or high dose and therapeutic dose) |
T2D: Type 2 diabetes mellitus; HTN: Hypertension; HF: Heart failure; CAD: Coronary artery disease; MI: Myocardial infarction; AF: Atrial fibrillation; TIA: Transient ischemic attack; CKD: Chronic kidney disease; COPD: Chronic obstructive pulmonary disease; VTE: Venous thromboembolism; OAC: Oral anticoagulant; DOAC: Direct oral anticoagulant; CRP: C-reactive protein; LDH: Lactate dehydrogenase; BMI: Body mass index; CCI: Charlson comorbidity index; NEWS: National Early Warning Score; ICU: Intensive care unit; NSAIDs: Non-steroidal anti-inflammatory drugs; eGFR: Estimated glomerular filtration rate; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019.
Out of the 46 studies included in our meta-analysis, 26 studies[28-54] described using both direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs). Five studies[55-59] had only DOAC as the prehospital anticoagulant, while a single study by Ménager et al[60] included patients only on VKAs. The anticoagulant type was unspecified in the remaining 13 studies[36,61-72]. In the 32 studies[28-35,37,39-51,53-60,73,74] studying DOACs Apixaban, Dabigatran, Edoxaban, and Rivaroxaban were the most commonly used, while on the other hand, VKAs were represented mostly by Warfarin. However, in two studies[30,60], Acenocoumarin was also taken into consideration. Supplemental prehospital use of other anticoagulants, including LMWH, Fondaparinux, and Enoxaparin, was observed in five studies[30,35,47,49,51].
Quality assessment and publication bias
The included studies’ methodological quality assessment demonstrated that 21 studies[25,28,30,34,36,38,39,43,44,50,54,56,58-60,63,66,68,69,71,72,73] had a high quality with very low risk of bias, while 24 studies[29,31-33,35,37,40-42,46-49,51,55,57,62,64,65,67,70,73,74] were of moderate quality (Supplementary Table 2). Out of the 24 studies with moderate quality, 11 studies[29,33,35,37,41,46-49,52,62] had an unclear risk of bias (not enough information to make a clear judgment), and the remaining 13 studies[31,32,40,46,55,57,64,65,67,70,73,74] had a low risk of bias, but some other potential flaws. Therefore, every study was approved for inclusion in the quantitative analysis. A single study was of poor quality and potentially high risk of bias[53]. Supplementary Figure 1 displays the publishing bias funnel plots. The results showed no discernible publication bias, and Supplementary Figure 1 displays the various P-values from Egger's regression test. We also evaluated the certainty of evidence at the outcome level using GRADE pro profiler (GRADE working group, McMaster University, and Evidence Prime Inc; Supplementary Table 3)[20].
Primary outcome
Thromboembolic event rate: A total of nine studies[29,30,37,40,45,50,54,60,70] evaluated the unadjusted risk, while six studies[29,45,50,54,58,61]calculated the adjusted risk between prehospital anticoagulation and new thromboembolic events in COVID-19 infected patients. Three studies[45,50,62] defined thromboembolic events as VTE, while three others[29,30,37] included both arterial and venous thromboembolic events, and the remaining three[40,70] did not clarify their definition of thromboembolic events.
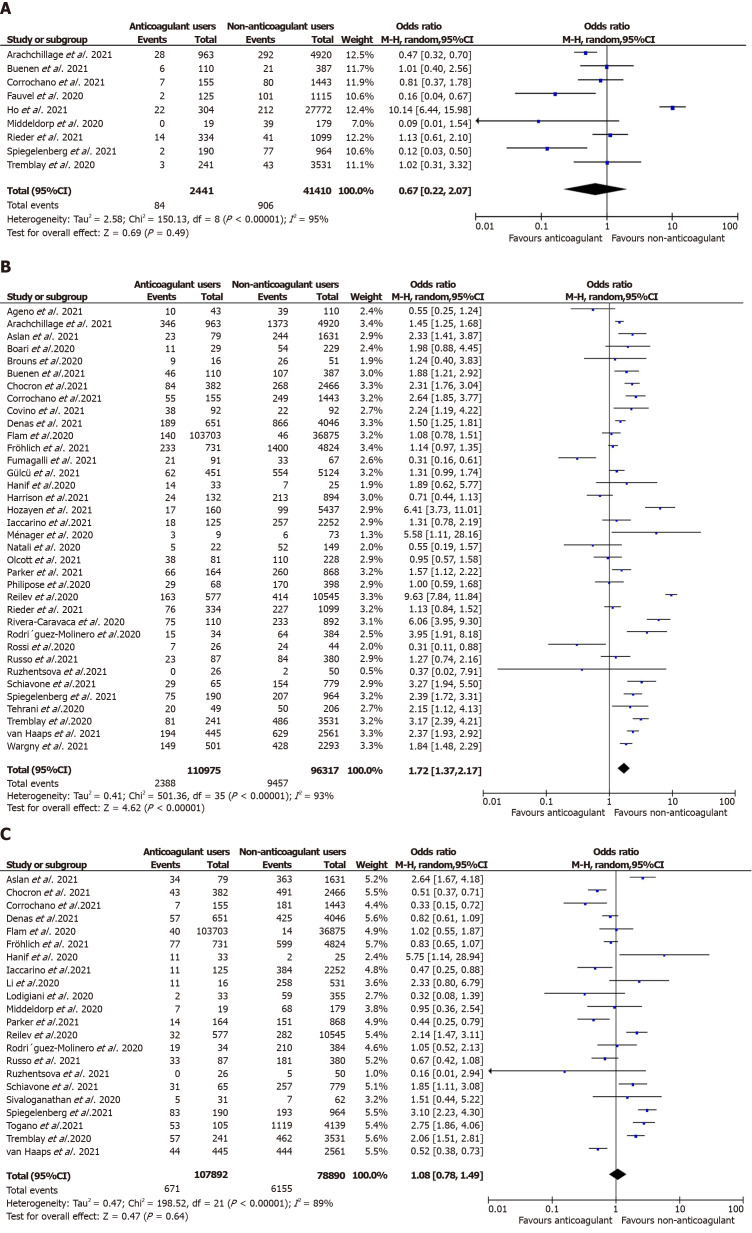
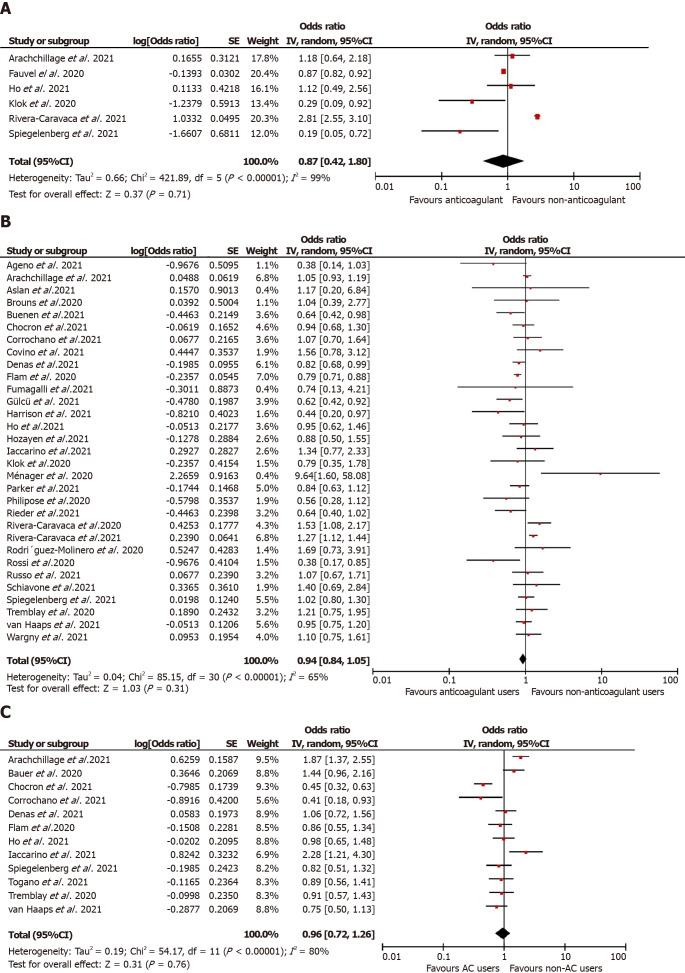
Overall, anticoagulation prior to COVID-19 diagnosis suggested a reduction in the unadjusted risk of new VTE in adult patients in these nine studies but did not attain statistical significance [n = 43851, OR = 0.67 (0.22, 2.07); P = 0.49; I2 = 95%; Figure 2A]. Similarly, in the predefined subgroup analysis, the use of VKA[40,50,54] [2658 participants, OR = 0.32 (0.05, 1.98); P = 0.22; I2 = 63%; Supplementary Figure 2A] or DOAC[40,50,54] [2699 participants, OR = 0.36 (0.10, 1.25); P = 0.11; I2 = 51%; Supplementary Figure 2B] or any anticoagulants [40960 participants, OR = 1.03 (0.26, 4.08); P = 0.97; I2 = 96%; Supplementary Figure 2C] did not show a statistical significant reduction of new VTE event risk associated with COVID-19 infection (P value for subgroup differences = 0.70, I2 = 0%). Our prespecified adjusted meta-analysis on six studies[29,45,50,54,61,74] did not achieve statistical significance in the lower odds of thromboembolic events in patients suffering from COVID-19 [aOR = 0.87 (0.42, 1.80); P = 0.71; I2 = 99%; Figure 3A].
Figure 2.
Unadjusted meta-analysis for mortality, severity thromboembolic events in prehospital use of anticoagulants vs control cohort in COVID-19. A: Unadjusted thromboembolic events in prehospital use of anticoagulants vs control cohort; B: Unadjusted mortality in prehospital use of anticoagulants vs control cohort; C: Unadjusted severity in prehospital use of anticoagulants vs control cohort.
Figure 3.
Adjusted meta-analysis for mortality, severity thromboembolic events in prehospital use of anticoagulants vs control cohort in COVID-19. A: Adjusted thromboembolic events in prehospital use of anticoagulants vs control cohort; B: Adjusted mortality in prehospital use of Anticoagulants vs control cohort; C: Adjusted severity in prehospital use of Anticoagulants vs control cohort.
Secondary outcomes
Mortality: A total of 35[28-35,37,38,40,41,43,44,46-48,50,51,53,55-57,59,60,63-70,72,74] studies reported mortality in prior anticoagulant users (2388 deaths out of 110975 patients; 2.1%) vs non-users (9457 deaths out of 96317 patients; 9.8%). Prior anticoagulation was found to significantly increase the risk of mortality in COVID-19 patients in the unadjusted analysis [OR = 1.72 (1.37, 2.17); P < 0.00001; I2 = 93%; Figure 2B). Subgroup analysis by the type of anticoagulation medication showed that prior VKA use [19747 participants, OR = 1.91 (1.20, 3.06); P = 0.007; I2 = 83%; Supplementary Figure 3A] and any anticoagulant use [43643 participants, OR = 1.88 (1.40, 2.52); P < 0.00001; I2 = 94%; Supplementary Figure 3B] was associated with an increased mortality risk. However, prior use of DOAC [22374 participants, OR = 1.42 (0.95, 2.12); P = 0.08; I2 = 87%; Supplementary Figure 3C] and the adjusted estimates of 28 studies[28,30,32,34,35,37,38,40-45,48,50,51,53,55,57,60,61,65-67,70,72,74] [aOR = 0.94 (0.84, 1.05); P = 0.31; I2 = 65%; Figure 3B] revealed no statistically significant association between prehospital anticoagulation and mortality in patients hospitalized for COVID-19.
COVID-19 disease severity: Overall, 22 studies[30,31,33,36,39,41,43,47-51,55,56,59,62,64,67,70,72,73,74] documented the association between prehospital anticoagulation and COVID-19 infection severity. ICU admission was the surrogate marker of COVID-19 severity in 15 studies, while mechanical ventilation was used in five studies, and one study used ARDS development during hospitalization. Six hundred seventy-one patients out of 103,703 who received anticoagulants before COVID-19 diagnosis developed severe COVID-19 infection, while 6155 out of 78890 non-users progressed to severe COVID-19 illness. We derived no statistically significant association between prehospital anticoagulation and COVID-19 disease severity [OR = 1.08 (0.78, 1.49); P = 0.64; I2 = 89%; Figure 2C]. On carrying out a subgroup analysis based on the type of anticoagulants, VKAs [6947 participants, OR = 1.26 (0.57,2.77); P = 0.57; I2 = 82%; Supplementary Figure 4A], DOACs [149564 participants, OR = 1.12 (0.58, 2.15); P = 0.74; I2 = 88%; Supplementary Figure 4B], and any anticoagulant use [36854 participants, OR = 1.07 (0.72, 1.58); P = 0.75; I2 = 89%; Supplementary Figure 4C] all reported non-significant association with severe COVID-19 disease. Similarly, prehospital anticoagulation was not significantly associated with COVID-19 disease severity in the adjusted analysis [12 studies[29,30,39,41,43,45,50,51,59,70-72]; aOR = 0.96 (0.72, 1.26); P = 0.76; I2 = 80%; Figure 3C].
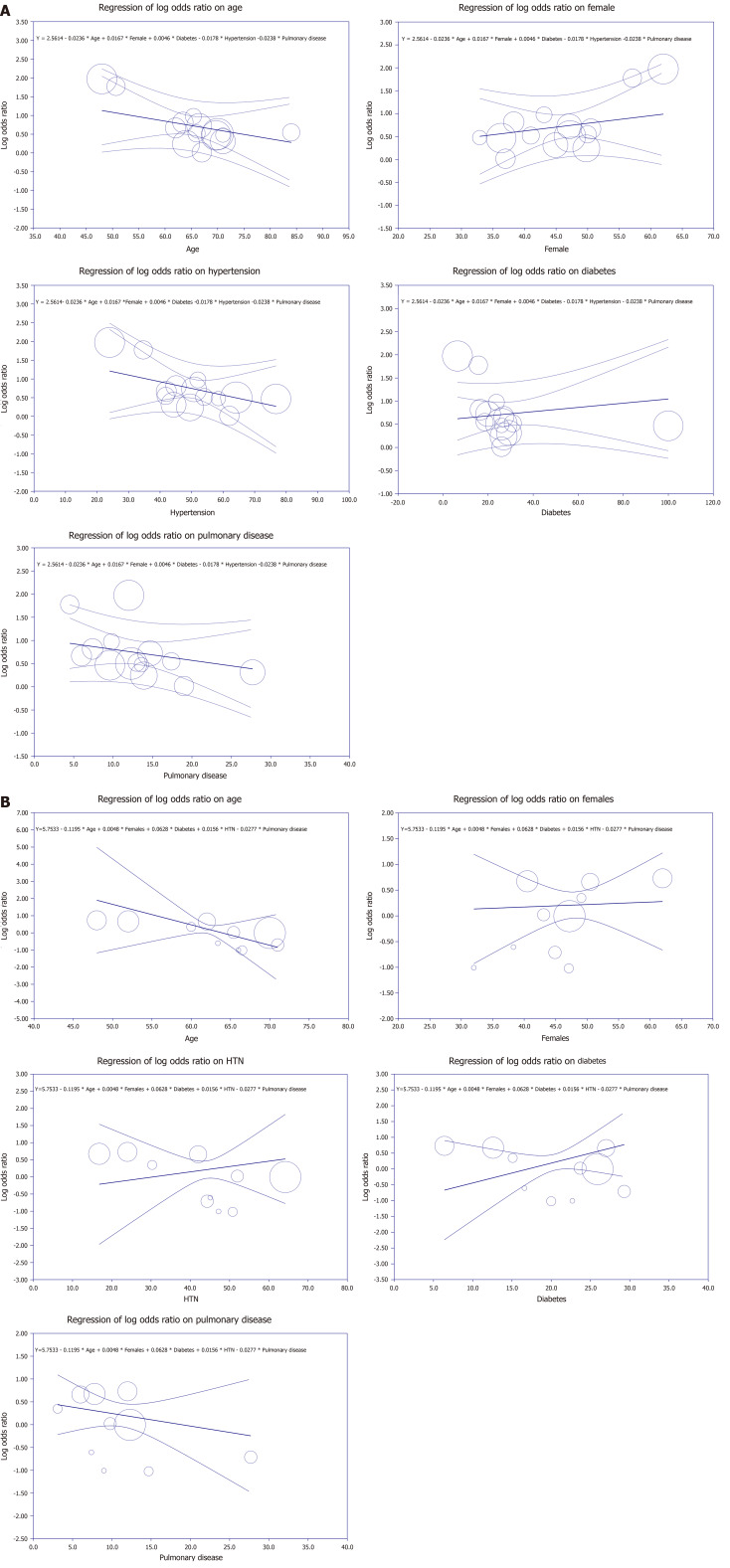
Multivariate meta-regression model for mortality outcome
To account for variations in the correlation between mortality and prehospital anticoagulant use, a multivariate meta-regression was performed. The findings demonstrated that when considered collectively, the proportion of age, diabetes, female gender, hypertension, and pulmonary diseases was significant. These variables accounted for R2 = 90% of the difference in mortality between studies (Figure 4A).
Figure 4.
Meta-regression analyses. A: Mortality meta-regression analysis; B: Severity meta-regression analysis.
Multivariate meta-regression model for severity outcome
Multivariate meta-regression was employed to take into consideration variations in the correlation between COVID-19 severity and prehospital anticoagulation. Together, age, female gender, and the proportion of hypertension, pulmonary disease, and diabetes were found to be significant covariates. Figure 4B demonstrates that these factors, all together, explained R2 = 100% for heterogeneity in severity among the included studies.
DISCUSSION
Our analysis offered a thorough examination of the risk of thromboembolic events, mortality, and severity of COVID-19 disease when anticoagulant medication was used prior to hospitalization. The meta-analysis identified no significant association between prehospital anticoagulation and decreased thromboembolic events risk and reduced COVID-19 disease severity. Though the unadjusted analysis exhibited a rise in mortality risk of COVID-19 patients with prior anticoagulation, adjusting the estimates revealed no significant difference in the odds of thromboembolic events, mortality, and severity between COVID-19 infected patients on prehospital anticoagulation and those without antecedent anticoagulation treatment.
Literature review and analysis of existing research
VTE: Coagulopathy and thromboembolic events are described as independent poor prognostic indicators in COVID-19[58,75]. Hospitalized COVID-19 patients tend to have a higher incidence of VTE than individuals with other illnesses[76], yet the exact mechanism of hypercoagulability remains unclear. COVID-19 infection can induce hyperinflammation[77], leading to endothelial dysfunction[78-80], platelet activation, blood stasis[58], and microvascular inflammation[81], all of which can influence consequent respiratory distress and other organ dysfunctioning[78-80]. This acute inflammatory state also increases the arterial and venous TE risk[61].
While ample evidence supports anticoagulation benefits during hospitalization[82-84], the role of prehospital anticoagulation before COVID-19 diagnosis, particularly in thromboembolic event incidence, lacks sufficient data. Despite anticoagulation, people on long-term oral anticoagulation therapy that started prior to COVID-19 infection may be more prone to thrombosis because of the existing SARS-CoV-2 infection[45]. Initial research suggests potential negative effects of VKAs on COVID-19[50]. SARS-CoV-2 infected individuals have reduced extra-hepatic vitamin K stores, which could be further decreased with VKAs[50]. However, studies have produced conflicting results regarding prehospital anticoagulation's impact on COVID-19 patients' thromboembolic risk, with some studies showing increased thromboembolic event risk[58]. In contrast, others observed benefits from prehospital anticoagulation in reducing COVID-related TE risk[29,50]. However, as shown in both unadjusted and adjusted analyses, our study did not find a significant reduction in thromboembolic events among COVID-19 patients receiving prehospital anticoagulation.
Mortality: Diverse study outcomes exist on chronic anticoagulation's impact on COVID-19 mortality. Tremblay et al[70] noted higher mortality among prehospital anticoagulated COVID-19 patients, yet non-significance emerged after age, sex, race, obesity, and Charlson Comorbidity Index adjustment. Rivera-Caravaca et al[58] reported higher all-cause mortality rates in prehospital DOAC-treated patients, remaining significant post-adjustment. Conversely, studies like Fumagillin et al’s[44] observed lower mortality rates in pre-COVID-19 anticoagulated patients[41,43,44,68]. Our research aligns with studies[41-51,69] and an earlier meta-analysis published by Kamel et al[85], revealing no substantial prehospital anticoagulation-associated mortality impact. Unlike Kamel et al[85], our study comprehensively explores pre-admission anticoagulation effects on VTE risk, severity of disease, and mortality in COVID-19.
Severity: Divergent results have been observed regarding the effect of anticoagulation on the severity of COVID-19[47,48,52,64,66,74]. Aslan et al[55] revealed significantly higher ICU admission, ventilation, oxygen therapy, and mortality rates with DOAC use. Yet, Corrochano et al’s[30] study found ICU admission reduction with prehospital anticoagulation after age, sex, and CCI adjustment. Similarly, Iaccarino et al[72] linked oral anticoagulants to ICU admission risk reduction. However, their cross-sectional design for hypothesis generation might influence outcomes[33].
Assessment of potential biases
The existence of selection bias and confounding bias is one possible explanation for inconsistent results among studies[29,30,49,55,58], as some studies include patients with cardiovascular or coagulation issues (poorer premorbid state). Certain studies neglect the effects of other medications during hospitalization[84]. Another reason can be the nature of the study, as observational and retrospective studies limit the causal inference[27-30,55], even though propensity score matching efforts[58], as propensity scoring is dependent on covariates and confounders are not included in the scoring, resulting in a potential bias. Small sample size also introduces bias[28,55]. The focus on hospitalized patients can hamper the generalizability of milder and asymptomatic COVID-19 cases. Similarly, heterogeneous sample sizes impact data collection and yield inconsistent results. Socio-demographics, like age and gender, may also influence drug effects on severity and mortality.
Possible mechanisms underlying the findings
A hypercoagulative state is promoted by COVID-19-induced coagulative abnormalities and alterations in prothrombotic factors, such as enhanced factor VIII, plasma fibrinogen, microparticles, and increased platelet activation[86-89]. This hypercoagulative state, known as COVID-19-associated coagulopathy (CAC), differs from acute disseminated intravascular coagulation (DIC) in presentation, involving thrombosis instead of bleeding[90]. Although D-dimer levels are elevated in both CAC and DIC, additional coagulation factors differentiate CAC (higher levels of Von Willebrand factor (vWF) antigen, Factor VIII activity, and fibrinogen) from DIC (lower levels of fibrinogen, reduced Factor VIII activity, and decreased VWF)[88-91].
Evidence suggests coagulation factor Xa's role in virus entry by cleaving SARS Spike proteins, which certain anticoagulants inhibit, potentially affecting viral fusion with ACE-2 receptors[10]. Ageno et al[28] used this concept to show DOACs' potentially higher antiviral efficacy over VKAs, though with limitations. Meanwhile, in our meta-analysis, DOAC-treated cases showed more severe disease progression than VKA-treated ones.
The underlying pathophysiology of endothelial damage by SARS-COV-2 is thought to be related to the renin-angiotensin-aldosterone system (RAAS). Virus-host cell binding via ACE-2 receptors raises disease severity risk. Thus, ACEI and ARBs (RAAS inhibitors) could heighten disease severity by increasing ACE2 receptor expression[16]. Anticoagulants, not affecting ACE2 receptors, might explain the non-significant enhancement in disease severity, as seen in our meta-analysis with no prehospital anticoagulation-COVID-19 severity correlation.
Clinical implications and future directions
Our study has significant clinical implications. Monitoring coagulation function in ICU patients using repeated platelet count, prothrombin time, and D-dimer measurements is vital. Elevated serum D-dimer predicts VTE and is a prognostic tool for COVID-19 risk stratification[92,93]. High D-dimer or rapid respiratory decline may indicate suspected VTE. Middeldorp et al[62] noted significantly higher D-dimer levels in ICU-admitted COVID-19-infected patients, regardless of chronic prehospital anticoagulation. Our analysis found that chronic anticoagulation does not significantly reduce the risk of new thromboembolic events in COVID-19 patients. This suggests that prior anticoagulation does not protect against COVID-19-related thromboembolic events. Due to the controversial effects of anticoagulant therapy on COVID-19 severity, further studies are needed.
Strengths and limitations
This meta-analysis's strengths include a sizable sample size dispersed across several nations and moderate to high-quality studies included according to the New-Castle Ottawa Scale. However, there are notable limitations. First, the retrospective nature of most included research limits the generalizability of the conclusions, highlighting the need for expansive prospective investigations. Second, while adjusted estimates were prioritized in the original analysis, selection bias and the confounding impact typical of observational research cannot be completely eliminated. Furthermore, inconsistency existed in the definition of severity among the included articles. Lastly, data gaps regarding prehospital anticoagulation specifics—duration, indication, type, and dosage—in some studies impeded comprehensive analysis.
CONCLUSION
The current meta-analysis concludes that prehospital anticoagulation does not significantly correlate with reduced COVID-19-related thromboembolic events, enhanced survival, or lowered disease severity risk. This aligns with recent guidelines advocating prophylactic anticoagulant use in COVID-19 patients, irrespective of VTE risk. To gain deeper insights and robust evidence, we suggest well-designed prospective studies and randomized trials investigating the impact of prior anticoagulant usage on thromboembolic risk, mortality, and disease severity in COVID-19 cases. Furthermore, a thorough exploration of the reasons behind the limited efficacy of chronic anticoagulants in severe infections is warranted. Future research should focus on determining personalized VTE risks for COVID-19 patients, uncovering underlying pathogenic pathways, and identifying optimal anticoagulant interventions for VTE prevention.
ACKNOWLEDGEMENTS
Data from this study was presented as an abstract format for the SCCM 2023 Critical Care Congress in San Francisco, California, United States.
Footnotes
Conflict-of-interest statement: Dr. Bansal has nothing to disclose.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Adam CA, Romania S-Editor: Lin C L-Editor: A P-Editor: Zhang L
Contributor Information
Kinza Iqbal, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Akshat Banga, Department of Internal Medicine, Sawai Man Singh Medical College, Jaipur 302004, India.
Taha Bin Arif, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Sawai Singh Rathore, Department of Internal Medicine, Dr. Sampurnanand Medical College, Jodhpur 342003, Rajasthan, India.
Abhishek Bhurwal, Department of Gastroenterology and Hepatology, Rutgers Robert Wood Johnson School of Medicine, New Brunswick, NJ 08901, United States.
Syeda Kisa Batool Naqvi, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Muhammad Mehdi, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Pankaj Kumar, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Mitali Madhu Salklan, Department of Internal Medicine, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak 124001, Haryana, India.
Ayman Iqbal, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Jawad Ahmed, Department of Internal Medicine, Dow Medical College, Karachi 74200, Pakistan.
Nikhil Sharma, Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
Amos Lal, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN 55905, United States.
Rahul Kashyap, Department of Research, Wellspan Health, York, PA 17403, United States.
Vikas Bansal, Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States. bansal.vikas@mayo.edu.
Juan Pablo Domecq, Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry: Erratum. Crit Care Med. 2021;49:e562. doi: 10.1097/CCM.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon T, Sharma R, Earthineni G, Iftikhar H, Sondhi M, Shams S, Khurshid Ahmed N, Khan H, Rathore SS, Singh R. Association of Gastrointestinal System With Severity and Mortality of COVID-19: A Systematic Review and Meta-Analysis. Cureus. 2021;13:e13317. doi: 10.7759/cureus.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon T, Sharma R, Kataria S, Sardar S, Adhikari R, Tousif S, Khan H, Rathore SS, Singh R, Ahmed Z. The Association of Acute Kidney Injury With Disease Severity and Mortality in COVID-19: A Systematic Review and Meta-Analysis. Cureus. 2021;13:e13894. doi: 10.7759/cureus.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathore SS, Rojas GA, Sondhi M, Pothuru S, Pydi R, Kancherla N, Singh R, Ahmed NK, Shah J, Tousif S, Baloch UT, Wen Q. Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int J Clin Pract. 2021;75:e14470. doi: 10.1111/ijcp.14470. [DOI] [PubMed] [Google Scholar]
- 6.Shah K, Bedi S, Onyeaka H, Singh R, Chaudhari G. The Role of Psychological First Aid to Support Public Mental Health in the COVID-19 Pandemic. Cureus. 2020;12:e8821. doi: 10.7759/cureus.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah K, Mann S, Singh R, Bangar R, Kulkarni R. Impact of COVID-19 on the Mental Health of Children and Adolescents. Cureus. 2020;12:e10051. doi: 10.7759/cureus.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheraton M, Deo N, Kashyap R, Surani S. A Review of Neurological Complications of COVID-19. Cureus. 2020;12:e8192. doi: 10.7759/cureus.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Kashyap R, Hutton A, Sharma M, Surani S. A Review of Cardiac Complications in Coronavirus Disease 2019. Cureus. 2020;12:e8034. doi: 10.7759/cureus.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Rathore SS, Khan H, Bhurwal A, Sheraton M, Ghosh P, Anand S, Makadia J, Ayesha F, Mahapure KS, Mehra I, Tekin A, Kashyap R, Bansal V. Mortality and Severity in COVID-19 Patients on ACEIs and ARBs-A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Front Med (Lausanne) 2021;8:703661. doi: 10.3389/fmed.2021.703661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Zu Y, Alkhatib A, Pham TT, Gill F, Jang A, Radosta S, Chaaya G, Myers L, Zifodya JS, Bojanowski CM, Marrouche NF, Mauvais-Jarvis F, Denson JL. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans. Diabetes Care. 2020;44:188–193. doi: 10.2337/dc20-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denson JL, Gillet AS, Zu Y, Brown M, Pham T, Yoshida Y, Mauvais-Jarvis F, Douglas IS, Moore M, Tea K, Wetherbie A, Stevens R, Lefante J, Shaffer JG, Armaignac DL, Belden KA, Kaufman M, Heavner SF, Danesh VC, Cheruku SR, St Hill CA, Boman K, Deo N, Bansal V, Kumar VK, Walkey AJ, Kashyap R Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw Open. 2021;4:e2140568. doi: 10.1001/jamanetworkopen.2021.40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal V, Mahapure KS, Bhurwal A, Gupta I, Hassanain S, Makadia J, Madas N, Armaly P, Singh R, Mehra I, O'Horo JC, Kashyap R. Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2020;7:606429. doi: 10.3389/fmed.2020.606429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal V, Mahapure KS, Mehra I, Bhurwal A, Tekin A, Singh R, Gupta I, Rathore SS, Khan H, Deshpande S, Gulati S, Armaly P, Sheraton M, Kashyap R. Mortality Benefit of Convalescent Plasma in COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2021;8:624924. doi: 10.3389/fmed.2021.624924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R, Shaik L, Mehra I, Kashyap R, Surani S. Novel and Controversial Therapies in COVID-19. Open Respir Med J. 2020;14:79–86. doi: 10.2174/1874306402014010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb Haemost. 2020;120:949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 19.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhari PB, Banga A. Writing strategies for improving the access of medical literature. World J Exp Med. 2023;13:50–58. doi: 10.5493/wjem.v13.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrance Training. Review Manager (RevMan). [cited 24 April 2022]. Available from: https://training.cochrane.org/online-learning/core-software/revman .
- 24.Brüggemann P, Rajguru K. Comprehensive Meta-Analysis (CMA) 3.0: a software review. J Market Anal. 2022:10: 425–429. [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. [cited 24 April 2022]. Available from: https://cmr.cochrane.org/?CRGReportID=2972 .
- 27.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ageno W, De Candia E, Iacoviello L, Di Castelnuovo A CORIST investigators. Protective effect of oral anticoagulant drugs in atrial fibrillation patients admitted for COVID-19: Results from the CORIST study. Thromb Res. 2021;203:138–141. doi: 10.1016/j.thromres.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arachchillage DJ, Rajakaruna I, Odho Z, Crossette-Thambiah C, Nicolson PLR, Roberts LN, Allan C, Lewis S, Riat R, Mounter P, Lynch C, Langridge A, Oakes R, Aung N, Drebes A, Dutt T, Raheja P, Delaney A, Essex S, Lowe G, Sutton D, Lentaigne C, Sayar Z, Kilner M, Everington T, Shapiro S, Alikhan R, Szydlo R, Makris M, Laffan M. Clinical outcomes and the impact of prior oral anticoagulant use in patients with coronavirus disease 2019 admitted to hospitals in the UK - a multicentre observational study. Br J Haematol. 2022;196:79–94. doi: 10.1111/bjh.17787. [DOI] [PubMed] [Google Scholar]
- 30.Corrochano M, Acosta-Isaac R, Mojal S, Miqueleiz S, Rodriguez D, Quijada-Manuitt MÁ, Fraga E, Castillo-Ocaña M, Amaro-Hosey K, Albiol N, Soria JM, Antonijoan RM, Souto JC. Impact of pre-admission antithrombotic therapy on disease severity and mortality in patients hospitalized for COVID-19. J Thromb Thrombolysis. 2022;53:96–102. doi: 10.1007/s11239-021-02507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fröhlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, Kastrati A, Leistner DM, Skurk C, Landmesser U, Günster C. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021;110:1041–1050. doi: 10.1007/s00392-020-01783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gülcü O, Aksakal E, Aydemir S, Doğan R, Saraç İ, Aydın SŞ, Öztürk M, Aksu U, Kalkan K, Tanboğa İH. Association between previous anticoagulant use and mortality among hospitalized patients with COVID-19. J Thromb Thrombolysis. 2022;53:88–95. doi: 10.1007/s11239-021-02489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanif A, Khan S, Mantri N, Hanif S, Saleh M, Alla Y, Chinta S, Shrestha N, Ji W, Attwood K, Adrish M, Jain KR. Thrombotic complications and anticoagulation in COVID-19 pneumonia: a New York City hospital experience. Ann Hematol. 2020;99:2323–2328. doi: 10.1007/s00277-020-04216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison RF, Forte K, Buscher MG Jr, Chess A, Patel A, Moylan T, Mize CH, Werdmann M, Ferrigno R. The Association of Preinfection Daily Oral Anticoagulation Use and All-Cause in Hospital Mortality From Novel Coronavirus 2019 at 21 Days: A Retrospective Cohort Study. Crit Care Explor. 2021;3:e0324. doi: 10.1097/CCE.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hozayen SM, Zychowski D, Benson S, Lutsey PL, Haslbauer J, Tzankov A, Kaltenborn Z, Usher M, Shah S, Tignanelli CJ, Demmer RT. Outpatient and inpatient anticoagulation therapy and the risk for hospital admission and death among COVID-19 patients. EClinicalMedicine. 2021;41:101139. doi: 10.1016/j.eclinm.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieder M, Gauchel N, Kaier K, Jakob C, Borgmann S, Classen AY, Schneider J, Eberwein L, Lablans M, Rüthrich M, Dolff S, Wille K, Haselberger M, Heuzeroth H, Bode C, von Zur Mühlen C, Rieg S, Duerschmied D. Pre-medication with oral anticoagulants is associated with better outcomes in a large multinational COVID-19 cohort with cardiovascular comorbidities. Clin Res Cardiol. 2022;111:322–332. doi: 10.1007/s00392-021-01939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera-Caravaca JM, Núñez-Gil IJ, Vivas D, Viana-Llamas MC, Uribarri A, Becerra-Muñoz VM, Trabattoni D, Fernández Rozas I, Feltes G, López-Pais J, El-Battrawy I, Macaya C, Fernandez-Ortiz A, Estrada V, Marín F HOPE COVID-19 Investigators. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. Eur J Clin Invest. 2021;51:e13436. doi: 10.1111/eci.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togano T, Uemura Y, Asai Y, Hayakawa K, Matsunaga N, Terada M, Ohtsu H, Suzuki S, Toyoda A, Hara H, Sato R, Ishikane M, Kinoshita-Iwamoto N, Hangaishi A, Ohmagari N. The influence of pre-admission antiplatelet and anticoagulation therapy on the illness severity in hospitalized patients with COVID-19 in Japan. J Infect Chemother. 2021;27:1498–1503. doi: 10.1016/j.jiac.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buenen AG, Sinkeldam M, Maas ML, Verdonschot M, Wever PC. Prior use of anticoagulation is associated with a better survival in COVID-19. J Thromb Thrombolysis. 2021;52:1207–1211. doi: 10.1007/s11239-021-02486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chocron R, Galand V, Cellier J, Gendron N, Pommier T, Bory O, Khider L, Trimaille A, Goudot G, Weizman O, Alsac JM, Geneste L, Schmeltz A, Panagides V, Philippe A, Marsou W, Ben Abdallah I, Deney A, El Batti S, Attou S, Juvin P, Delmotte T, Messas E, Pezel T, Planquette B, Duceau B, Gaussem P, Sutter W, Sanchez O, Waldman V, Diehl JL, Mirault T, Bonnet G, Cohen A, Smadja DM Critical COVID‐19 France Investigators. Anticoagulation Before Hospitalization Is a Potential Protective Factor for COVID-19: Insight From a French Multicenter Cohort Study. J Am Heart Assoc. 2021;10:e018624. doi: 10.1161/JAHA.120.018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Covino M, De Matteis G, Della Polla D, Burzo ML, Pascale MM, Santoro M, De Cristofaro R, Gasbarrini A, De Candia E, Franceschi F. Does chronic oral anticoagulation reduce in-hospital mortality among COVID-19 older patients? Aging Clin Exp Res. 2021;33:2335–2343. doi: 10.1007/s40520-021-01924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denas G, Gennaro N, Ferroni E, Fedeli U, Lorenzoni G, Gregori D, Iliceto S, Pengo V. Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: A population-based propensity score matched study. Int J Cardiol. 2021;329:266–269. doi: 10.1016/j.ijcard.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fumagalli S, Trevisan C, Del Signore S, Pelagalli G, Volpato S, Gareri P, Mossello E, Malara A, Monzani F, Coin A, Bellelli G, Zia G, Antonelli Incalzi R GeroCovid Working Group. COVID-19 and Atrial Fibrillation in Older Patients: Does Oral Anticoagulant Therapy Provide a Survival Benefit?-An Insight from the GeroCovid Registry. Thromb Haemost. 2022;122:105–112. doi: 10.1055/a-1503-3875. [DOI] [PubMed] [Google Scholar]
- 45.Ho G, Dusendang JR, Schmittdiel J, Kavecansky J, Tavakoli J, Pai A. Association of chronic anticoagulant and antiplatelet use on disease severity in SARS-COV-2 infected patients. J Thromb Thrombolysis. 2021;52:476–481. doi: 10.1007/s11239-021-02383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olcott F, Hunt C, Chan T, Williams G. Pre-admission anti-coagulation does not improve all-cause mortality in geriatric COVID-19 patients. Clin Med (Lond) 2021;21:7–8. doi: 10.7861/clinmed.21-2-s7. [DOI] [PubMed] [Google Scholar]
- 47.Parker K, Hamilton P, Hanumapura P, Castelino L, Murphy M, Challiner R, Thachil J, Ebah L. Chronic anticoagulation is not associated with a reduced risk of acute kidney injury in hospitalised Covid-19 patients. BMC Nephrol. 2021;22:224. doi: 10.1186/s12882-021-02436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russo V, Bottino R, D'Andrea A, Silverio A, Di Maio M, Golino P, Nigro G, Valsecchi O, Attena E, Canonico ME, Galasso G, Parodi G, Scudiero F. Chronic Oral Anticoagulation and Clinical Outcome in Hospitalized COVID-19 Patients. Cardiovasc Drugs Ther. 2022;36:705–712. doi: 10.1007/s10557-021-07194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivaloganathan H, Ladikou EE, Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. 2020;190:e192–e195. doi: 10.1111/bjh.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiegelenberg JP, van Gelder MMHJ, Maas ML, Hovens MMC, Esselink A, Dofferhoff ASM, Janssen R, van de Maat J, Janssen N, Blaauw M, Hassing RJ, van Apeldoorn M, Kerckhoffs A, Veerman K, Hoogerwerf J, Kramers C, Leentjens J. Prior use of therapeutic anticoagulation does not protect against COVID-19 related clinical outcomes in hospitalized patients: A propensity score-matched cohort study. Br J Clin Pharmacol. 2021;87:4839–4847. doi: 10.1111/bcp.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Haaps TF, Collard D, van Osch FHM, Middeldorp S, Coppens M, de Kruif MD, Vlot EA, Douma RA, Ten Cate H, Juffermans NP, Gritters N, Vlaar AP, Reidinga AC, Heuvelmans MA, Oudkerk M, Büller HR, van den Bergh JPW, Maas A, Ten Wolde M, Simsek S Corona Research Fund Amsterdam UMC, Beudel M, van Es N; Dutch COVID & Thrombosis Coalition. Pre-admission anticoagulant therapy and mortality in hospitalized COVID-19 patients: A retrospective cohort study. Thromb Res. 2021;208:35–38. doi: 10.1016/j.thromres.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brouns SH, Brüggemann R, Linkens AEMJH, Magdelijns FJ, Joosten H, Heijnen R, Ten Cate-Hoek AJ, Schols JMGA, Ten Cate H, Spaetgens B. Mortality and the Use of Antithrombotic Therapies Among Nursing Home Residents with COVID-19. J Am Geriatr Soc. 2020;68:1647–1652. doi: 10.1111/jgs.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, Benmansour O, Godeau G, Mecheri Y, Lebourdon R, Yvorel C, Massin M, Leblon T, Chabbi C, Cugney E, Benabou L, Aubry M, Chan C, Boufoula I, Barnaud C, Bothorel L, Duceau B, Sutter W, Waldmann V, Bonnet G, Cohen A, Pezel T Critical Covid-19 France Investigators. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aslan B, Akyüz A, Işık F, Çap M, İnci Ü, Kaya İ, Karahan MZ, Aktan A, Bilge Ö, Özbek M, Altıntaş B, Boyraz B. The effect of chronic DOAC treatment on clinical outcomes of hospitalized patients with COVID-19. Int J Clin Pract. 2021;75:e14467. doi: 10.1111/ijcp.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruzhentsova TA, Khavkina DA, Chukhliaev PV, Garbuzov AA, Ploskireva AA. [Effect of anticoagulant therapy on the course of COVID-19 in comorbid patients] Vopr Virusol. 2021;66:40–46. doi: 10.36233/0507-4088-14. [DOI] [PubMed] [Google Scholar]
- 57.Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID-19 era. Eur J Intern Med. 2020;77:158–160. doi: 10.1016/j.ejim.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera-Caravaca JM, Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Marín F, Lip GYH. Direct-acting oral anticoagulants use prior to COVID-19 diagnosis and associations with 30-day clinical outcomes. Thromb Res. 2021;205:1–7. doi: 10.1016/j.thromres.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flam B, Wintzell V, Ludvigsson JF, Mårtensson J, Pasternak B. Direct oral anticoagulant use and risk of severe COVID-19. J Intern Med. 2021;289:411–419. doi: 10.1111/joim.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ménager P, Brière O, Gautier J, Riou J, Sacco G, Brangier A, Annweiler C Geria-Covid Study Group OBOT. Regular Use of VKA Prior to COVID-19 Associated with Lower 7-Day Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Cohort Study. Nutrients. 2020;13 doi: 10.3390/nu13010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natali KM, Chan KH, Atallah L, Nagarakanti S, Slim J. 552. Could Anticoagulant Use Prior to Infection with COVID-19 Decrease Mortality? Open Forum Infect Di. 2020;7:S342–S342. [Google Scholar]
- 64.Reilev M, Kristensen KB, Pottegård A, Lund LC, Hallas J, Ernst MT, Christiansen CF, Sørensen HT, Johansen NB, Brun NC, Voldstedlund M, Støvring H, Thomsen MK, Christensen S, Gubbels S, Krause TG, Mølbak K, Thomsen RW. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wargny M, Potier L, Gourdy P, Pichelin M, Amadou C, Benhamou PY, Bonnet JB, Bordier L, Bourron O, Chaumeil C, Chevalier N, Darmon P, Delenne B, Demarsy D, Dumas M, Dupuy O, Flaus-Furmaniuk A, Gautier JF, Guedj AM, Jeandidier N, Larger E, Le Berre JP, Lungo M, Montanier N, Moulin P, Plat F, Rigalleau V, Robert R, Seret-Bégué D, Sérusclat P, Smati S, Thébaut JF, Tramunt B, Vatier C, Velayoudom FL, Vergès B, Winiszewski P, Zabulon A, Gourraud PA, Roussel R, Cariou B, Hadjadj S CORONADO investigators. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64:778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Philipose Z, Smati N, Wong CSJ, Aspey K, Mendall MA. Obesity, old age and frailty are the true risk factors for COVID-19 mortality and not chronic disease or ethnicity in Croydon. 2020 Preprint. Available from: https://www.medrxiv.org/content/10.1101/2020.08.12.20156257v3 .
- 67.Rodríguez-Molinero A, Gálvez-Barrón C, Miñarro A, Macho O, López GF, Robles MT, Dapena MD, Martínez S, Milà Ràfols N, Monaco EE, Hidalgo García A COVID-19 Research Group of CSAPG. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLoS One. 2020;15:e0239571. doi: 10.1371/journal.pone.0239571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boari GEM, Chiarini G, Bonetti S, Malerba P, Bianco G, Faustini C, Braglia-Orlandini F, Turini D, Guarinoni V, Saottini M, Viola S, Ferrari-Toninelli G, Pasini G, Mascadri C, Bonzi B, Desenzani P, Tusi C, Zanotti E, Nardin M, Rizzoni D. Prognostic factors and predictors of outcome in patients with COVID-19 and related pneumonia: a retrospective cohort study. Biosci Rep. 2020;40 doi: 10.1042/BSR20203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tehrani S, Killander A, Åstrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tremblay D, van Gerwen M, Alsen M, Thibaud S, Kessler A, Venugopal S, Makki I, Qin Q, Dharmapuri S, Jun T, Bhalla S, Berwick S, Feld J, Mascarenhas J, Troy K, Cromwell C, Dunn A, Oh WK, Naymagon L. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood. 2020;136:144–147. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauer AZ, Gore R, Sama SR, Rosiello R, Garber L, Sundaresan D, McDonald A, Arruda P, Kriebel D. Hypertension, medications, and risk of severe COVID-19: A Massachusetts community-based observational study. J Clin Hypertens (Greenwich) 2021;23:21–27. doi: 10.1111/jch.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iaccarino G, Grassi G, Borghi C, Grassi D, Mancusi C, Muiesan ML, Salvetti M, Volpe M, Ferri C. Preexisting Oral Anticoagulant Therapy Ameliorates Prognosis in Hospitalized COVID-19 Patients. Front Cardiovasc Med. 2021;8:633878. doi: 10.3389/fcvm.2021.633878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiavone M, Gasperetti A, Mancone M, Curnis A, Mascioli G, Mitacchione G, Busana M, Sabato F, Gobbi C, Antinori S, Galli M, Forleo GB. Oral anticoagulation and clinical outcomes in COVID-19: An Italian multicenter experience. Int J Cardiol. 2021;323:276–280. doi: 10.1016/j.ijcard.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Shen L, Le KJ, Pan MM, Kong LC, Gu ZC, Xu H, Zhang Z, Ge WH, Lin HW. Incidence of Venous Thromboembolism in Hospitalized Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2020;7:151. doi: 10.3389/fcvm.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowenstein CJ, Solomon SD. Severe COVID-19 Is a Microvascular Disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. Author Correction: COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:448. doi: 10.1038/s41577-020-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talaei F, Banga A, Pursell A, Gage A, Pallipamu N, Seri AR, Adhikari R, Kashyap R, Surani S. New-onset atrial fibrillation among COVID-19 patients: A narrative review. World J Crit Care Med. 2023;12:236–247. doi: 10.5492/wjccm.v12.i5.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, Kahn SR, Klok FA, Lee AI, Neumann I, Pai A, Pai M, Righini M, Sanfilippo KM, Siegal D, Skara M, Touri K, Akl EA, Bou Akl I, Boulos M, Brignardello-Petersen R, Charide R, Chan M, Dearness K, Darzi AJ, Kolb P, Colunga-Lozano LE, Mansour R, Morgano GP, Morsi RZ, Noori A, Piggott T, Qiu Y, Roldan Y, Schünemann F, Stevens A, Solo K, Ventresca M, Wiercioch W, Mustafa RA, Schünemann HJ. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, Skanderson M, Brittain E, King JT Jr, Ho YL, Eden S, Kundu S, Lann MF, Greevy RA Jr, Ho PM, Heidenreich PA, Jacobson DA, Douglas IJ, Tate JP, Evans SJW, Atkins D, Justice AC, Freiberg MS. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamel AM, Sobhy M, Magdy N, Sabry N, Farid S. Anticoagulation outcomes in hospitalized Covid-19 patients: A systematic review and meta-analysis of case-control and cohort studies. Rev Med Virol. 2021;31:e2180. doi: 10.1002/rmv.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395:1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, Bacon CL, Gaule R, Gillett A, Byrne M, Ryan K, O'Connell N, O'Sullivan JM, Conlon N, O'Donnell JS. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]