Abstract
An effective vaccine for AIDS may require development of novel vectors capable of eliciting long-lasting immune responses. Here we report the development and use of replication-competent and replication-defective strains of recombinant herpes simplex virus (HSV) that express envelope and Nef antigens of simian immunodeficiency virus (SIV). The HSV recombinants induced antienvelope antibody responses that persisted at relatively stable levels for months after the last administration. Two of seven rhesus monkeys vaccinated with recombinant HSV were solidly protected, and another showed a sustained reduction in viral load following rectal challenge with pathogenic SIVmac239 at 22 weeks following the last vaccine administration. HSV vectors thus show great promise for being able to elicit persistent immune responses and to provide durable protection against AIDS.
Preclinical vaccine testing for AIDS has relied principally on simian immunodeficiency virus (SIV) in macaque monkeys. Vaccine protection against pathogenic, difficult to neutralize strains of SIV, which could be considered representative of field strains of human immunodeficiency virus type 1 (HIV-1), has proven very difficult to achieve. Consequently, most studies have used a homologous strain of SIV for challenge 2 to 4 weeks after the last vaccine boost. Even with these optimized conditions, solid vaccine efficacies of even 50% have seldom been achieved (15, 26, 31, 33, 40, 43). The source of the problem seems to lie in the natural immune evasion strategies of SIV, HIV, and other lentiviruses (reviewed in reference 9). SIV and HIV are typically refractory to antibody-mediated neutralization and have evolved strategies that allow continuous viral replication in the face of apparently strong host immune responses. Vaccine protection against SIV and HIV may require approaches that yield immune responses that are persistently sustained and active at the time of live SIV or HIV exposure.
A hallmark of the herpesviruses is that they persist for the lifetime of the infected host in a latent state from which they can periodically reactivate. Strong humoral and cellular immune responses can be easily measured for decades after the time of initial infection (49). In animals infected experimentally with herpes simplex virus (HSV), cytokines remain at elevated levels for long periods of time in latently infected ganglionic tissue (4, 16, 25, 41), suggesting the persistence of activated T lymphocytes or other immune cells. Replication-deficient and replication-competent herpesvirus strains have been shown to induce durable antibody and protective immune responses (30). Thus, herpesviruses are attractive vaccine vectors for inducing long-lasting immune responses that could potentially be protective against AIDS.
Live HSV vaccines, which have the potential to serve as vaccine vectors, are of two general types: attenuated, replication-competent viruses (28, 42) and replication-defective viruses (12, 29, 32). As a first step in testing the potential of HSV recombinants to serve as vectors for AIDS vaccines, we have generated recombinant strains of both types: an attenuated, replication-competent HSV-1 recombinant expressing SIV envelope and Nef proteins and a replication-defective HSV-1 recombinant expressing SIV envelope and Nef proteins. We show here that these two recombinants are capable of inducing protection in rhesus macaques.
MATERIALS AND METHODS
Cells and viruses.
Vero (African green monkey kidney) cells were maintained in Dulbecco's modified minimal essential medium (Cellgrow, Atlanta, Ga.) supplemented with 5% fetal bovine serum (Gibco-BRL, Grand Island, N.Y.) and 5% newborn calf serum (HyClone, Provo, Utah) as described elsewhere (21). V827 cells (X. J. DaCosta and D. M. Knipe, unpublished results) were obtained by cotransformation of Vero cells with the neomycin resistance plasmid and the ICP8 and ICP27 genes as described elsewhere (14). KOS1.1 is a wild-type (WT) laboratory strain of HSV-1 (21). The HSV-1 d27 variant (37) contains a null deletion mutation in the ICP27 gene and is replication defective in normal cells but grows in ICP27-expressing cells such as V827 cells.
Bacterial strains and media.
Escherichia coli strains DH5α and JM109 were used in plasmid cloning procedures. E. coli strains were grown in Luria-Bertani medium for liquid culture or on Luria-Bertani agar plates supplemented with antibiotics as appropriate (ampicillin [200 μg/ml] or kanamycin [25 μg/ml]). Bacteria with plasmids containing SIV envelope sequences were grown at 30°C for increased stability of the DNA sequences.
Plasmids.
The low-copy-number plasmid pLG339-Sport (6) was obtained from Ron Montelaro (University of Pittsburgh). The mammalian eukaryotic expression vector plasmid pCI (Promega, Madison, Wis.), which contains the human cytomegalovirus immediate-early (CMV IE) promoter/enhancer and the simian virus 40 polyadenylation signal, was purchased from Promega. Plasmid p239SpE3′/nef-open, containing the 3′ half of the SIVmac239 genome, was the source of the SIV envelope nucleotide sequences. Plasmid p101086.7 BglII (5), which contains the HSV-1 thymidine kinase (TK) gene and flanking regions, was obtained from Don Coen (Harvard Medical School).
The expression cassette containing SIV sequences downstream from the CMV IE promoter/enhancer was constructed in several stages. First, the intron was removed from plasmid pCI by deletion of the 197-bp AflII fragment to generate plasmid pCIΔAflII. SIV sequences were isolated as a 3.8-kbp SphI-EcoRI restriction fragment from plasmid p239SpE3′/nef-open. These SIV sequences contain rev exon 1, the complete env reading frame, rev exon 2, and nef (34). The SIV DNA fragment was treated with T4 polymerase and ligated into the SmaI site of pCIΔAflII to generate the intermediate expression plasmid pCE4#7. The transfer vector was generated in several steps, commencing with isolation of the TK gene from plasmid p101086.7BglII by cleavage of the plasmid with XbaI and HindIII. The ends of the HSV-1 XbaI-HindIII fragment were filled in with Klenow enzyme and ligated into the HincII site of plasmid pLG339-Sport to generate plasmid pSTK. The DNA fragment containing the SIV env-nef expression cassette was excised from plasmid pCE4#7 by partial cleavage with BglII and complete cleavage with BamHI and ligated into the BglII site of pSTK to yield the final transfer vector plasmid, pSTCE. Nef-coding sequences overlap the 3′ end of the env reading frame in SIV and are believed to be expressed by splicing within this expression cassette (35).
Transfections and recombinant virus isolation.
HSV DNA was purified from infected cell lysates by sodium iodide gradient centrifugation (48). Cotransfection of infectious viral DNA and linearized plasmid DNA was performed using calcium phosphate precipitation (20). Plaque purification of recombinant viruses with agarose overlay medium and Southern blot hybridization analysis of viral DNA were performed as described previously (14). Selection of TK-negative viruses was performed with overlay medium containing 100 μM acycloguanosine (ACG; Sigma, St. Louis, Mo.).
Immunoprecipitation of Env protein from virus-infected cells in culture.
Following infection of mammalian cells with virus for the indicated times, cells were pulse-labeled in methionine- and cysteine-free medium (supplemented with 10% dialyzed fetal bovine serum) containing 50 μCi of [35S]methionine-cysteine (Translabel; NEN) per ml for 1 h prior to harvesting into 1 ml of phosphate-buffered saline (PBS). Cells were lysed by freezing and thawing in IP (immunoprecipitation) buffer (300 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 50 mM HEPES-HCl [pH 7.5]) containing protease inhibitors phenylmethylsulfonyl fluoride (1 mM), Na-p-tosyl-l-lysine chloromethyl ketone (TLCK; 0.5 mM), leupeptin (5 μg/ml), bestatin (40 μg/ml), and aprotinin (1% [wt/vol]). Following centrifugation at 4°C at 13,000 rpm for 30 min, cleared cell lysates were incubated with serum from an SIV-infected rhesus monkey and protein A for 2 h. Immune complexes were washed three times with IP buffer prior to analysis by SDS-polyacrylamide gel electrophoresis (PAGE) in a 9.25% DATD-cross-linked polyacrylamide gel and exposure to film for autoradiography.
Detection of Nef protein in virus-infected cells in culture.
Vero cells were infected at a multiplicity of infection of 5 with recombinant viruses and harvested at 9 or 12 h postinfection (hpi). Infected cells were lysed in Laemmli sample buffer, and cell lysates were subjected to SDS-PAGE in a 12% DATD-cross-linked polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane by electroblotting (39). Nef protein was detectable on the blot following incubation with a 1:1,000 dilution of N27 anti-Nef ascites fluid and 1:5,000 dilution of a anti-mouse secondary antibody conjugated to horseradish peroxidase using an enhanced chemiluminescence (ECL) detection system (Amersham).
Rectal challenge.
Rectal challenge with cloned SIVmac239 used 103.5 tissue culture infectious doses (104.8 rhesus monkey infectious doses) by the intravenous route (virus containing 8.5 ng of p27). The preparation, titration, and use of this challenge stock have been described previously (23). Animals were tranquilized with Vetalar (15 to 20 mg/kg of body weight) given intramuscularly. The anal area was gently wiped clean with soap and water and rinsed well with water. Inoculum was loaded into a 1-ml syringe and connected to a metallic mouse watering tube, approximately 9 cm long, with a rounded end. The pelvic area of the animal was raised to a 45° angle with head tilted forward. The outside of the tube was lubricated with Lidocaine and K-Y jelly for easy insertion and slid gently into the rectum of the animal carefully, avoiding any trauma. Material was inoculated 0.1 to 0.2 ml at a time, making sure that there was no drainage from the anal area. Upon completion of the procedure, animals were kept in a slightly inverted position for 5 to 10 min.
Viral load measurements.
The number of infectious cells in peripheral blood mononuclear cells (PBMC), i.e., the cell-associated viral load, was quantitated as previously described (10, 19, 35, 50). Quantitation of viral RNA levels in plasma using real-time reverse transcriptase PCR has also been described (44).
Antibody detection by IP-WB.
SIVmac239 envelope protein contained within the supernatant of infected cells was used for the immunoprecipitation-Western blot (IP-WB) assay. Supernatant from clarified cultures was centrifuged at 1,300 rpm for 5 min to pellet residual cells and debris. The clarified supernatant was then filtered and centrifuged at 17,500 rpm for 3 h in a type 19 rotor to pellet whole virus. The supernatant, containing free gp120, was filtered again and aliquoted for storage at −80°C. For immunoprecipitations, 300 μl of this preparation was mixed with 30 μl of protein A/G-agarose beads (Santa Cruz), 620 μl of RPMI 1640 (Gibco), and 50 μl of test serum. This mixture was incubated at 4°C overnight with rocking. After incubation, the protein A/G-agarose was pelleted and the supernatant was removed. The beads were then washed three times with 1 ml of PBS–0.5% Tween 20. After the final wash, the beads were resuspended in 12 μl of Laemmli sample buffer and boiled for 3 min. The samples were then electrophoresed through a SDS–5% polyacrylamide gel and transferred onto an Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). Membranes were blocked with 5% skim milk in PBS–0.05% Tween 20 for 1 h at room temperature. The blot was then incubated with a mixture of monoclonal antibodies directed to SIVmac gp120, KK43, KK52, and KK54 and then a horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (IgG) monoclonal antibody (Pierce). The antibodies were detected using a Pico West chemiluminescence kit (Pierce), and the blot was either placed against film or visualized using a Fuji LAS-1000 charge-coupled device camera (Fuji, Inc., Tokyo, Japan).
Other measurements.
CD4 cell numbers were quantitated by flow cytometry with a whole blood lysis technique that we have used previously (19, 50). Procedures for amplifying across the nef gene by PCR for the analysis of WT DNA sequences have also been described (19, 50). SIV and HSV were purified with the use of column chromatography and used to coat enzyme-linked immunosorbent assay (ELISA) plates as described previously (19, 50). The presence of antibodies to both SIV and HSV was detected using alkaline phosphatase-conjugated goat anti-human IgG. The titer of anti-HSV antibodies was defined as the serum dilution that reduced the A410 in the ELISA determination to a value of 0.2. Neutralization of SIV was measured as previously described (8, 27).
Assay of virus-specific CTL activity. (i) HSV-specific CTL activity.
Techniques for detecting HSV-specific cytotoxic T lymphocytes (CTL) were adapted from those used in humans (46). Autologous cells (fibroblasts or B-lymphoblastoid cell lines [B-LCL]) infected with HSV KOS for 16 to 20 h and subsequently inactivated with long-wave UV irradiation in the presence of psoralen (10 μg/ml) were used for HSV-specific stimulation. Fresh PBMC were mixed with stimulators at a responder-to-stimulator ratio of 10:1 in RPMI 1640 containing 10% fetal calf serum (R-10 medium) and incubated at 37°C in a CO2 incubator. Interleukin-2 was added after 4 to 5 days, and cultures were tested for CTL activity 10 to 14 days after stimulation. In initial experiments, comparable CTL activity was obtained when either autologous HSV-infected fibroblasts or B-LCL were used for stimulation. In subsequent experiments (week 8 onwards for animal experiment group 602 [AE602] and from the onset for AE606), autologous B-LCL infected with HSV KOS were used for in vitro HSV-specific stimulation. CTL activity was measured by 51Cr release assays using standard methodology. Autologous B-LCL uninfected or infected with HSV were used as target cells. HSV-specific lysis was expressed as the difference in lysis between virus-infected and uninfected target cells, and a value of 10% or greater was considered significant.
(ii) SIV-specific CTL activity.
Fresh PBMC infected for 90 min with a recombinant vaccinia virus that expresses the Gag and Pol proteins of SIVmac251 and the Env protein of SIVmac239 were mixed with autologous uninfected PBMC at a ratio of 1:10 in R-10 medium and incubated for 10 days, interleukin-2 being added to the culture after 4 to 5 days. We have found that this method of stimulation is comparable to that using autologous B-LCL and has the added advantage of decreasing background levels of lysis. CTL activity was measured in a standard 51Cr release assay as previously described (18). Autologous B-LCL infected overnight with recombinant vaccinia virus expressing SIVmac239 Env protein (rVV-239; provided by M. Mulligan) or WT vaccinia virus NYCBH were used as test and control target cells. Effectors and targets were incubated in duplicate for 4 h on a 96-well plate, and percent lysis was calculated according to standard formula. Cold targets infected with WT vaccinia virus NYCBH were used at a cold/hot target ratio of 15:1 to reduce background lysis.
RESULTS
Construction of vaccine vectors and SIV Env expression.
HSV-1 recombinants capable of expressing SIV Env and Nef proteins were generated by introduction of a single SIV env-nef expression cassette into the TK gene of the HSV-1 genome using homologous recombination. For the isolation of a replication-competent recombinant virus expressing SIV proteins, infectious viral DNA from the KOS1.1 WT viral strain was cotransfected with linearized plasmid pSTCE into Vero cells to allow recombination between the viral genome and the transfer vector sequences. Recombination of the expression cassette into the TK locus would result in a recombinant virus which was TK negative and therefore resistant to the antiviral drug ACG. Thus, we harvested the progeny from transfection and plated the viruses in 100 μM ACG. ACG-resistant viruses were subjected to three rounds of plaque purification. One of the recombinants, K81, was shown to contain the SIV sequences in the TK locus by Southern blot hybridization. This recombinant virus expressed SIV Env and Nef proteins as described below.
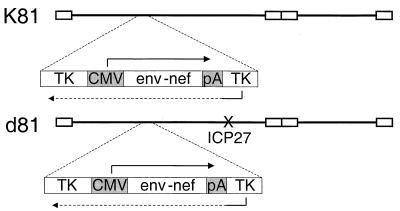
In addition to the K81 replication-competent recombinant virus, we isolated a replication-defective recombinant virus that was capable of expressing SIV Env and Nef proteins. For this construction we transfected viral DNA from the d27 mutant virus, which contains a deletion in the essential ICP27 gene (37), with linearized pSTCE plasmid DNA into the ICP27-complementing cell line V827. Viral progeny from the transfected cells were harvested and plated on V827 cells in the presence of ACG as above. One recombinant virus, d81, was isolated following three cycles of plaque purification in ACG. Southern blot hybridization showed that the d81 genome contained the SIV expression cassette inserted into the TK gene, and this recombinant virus expressed SIV Env and Nef as described below. Thus, the two recombinant viruses both contain the SIV env and nef genes inserted into the TK locus in a manner that caused its disruption (Fig. 1). K81 is replication competent, while d81 has a deletion in the essential ICP27 gene in addition to the deletion in TK, rendering it replication defective in Vero cells.
FIG. 1.
Genomic maps of K81 and d81 HSV recombinants. SIV sequences were inserted into the TK gene in each recombinant by the methods described in the text. CMV, promoter/enhancer sequences of the CMV IE gene; PA, signal sequences for poly(A) addition. The SIV sequences are from the SphI site (nucleotide 6450) rightward in SIVmac239 (34). These include rev exon 1, the entire env reading frame, rev exon 2, and the nef open reading frame.
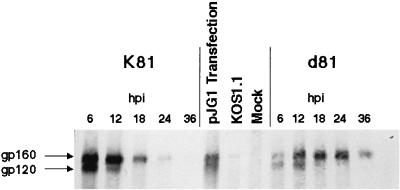
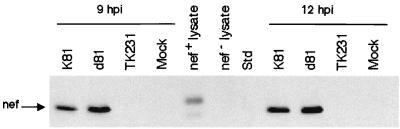
To determine the kinetics of SIV Env protein expression by K81 and d81 in monkey cells, we infected Vero cells with K81 or d81 and at various times postinfection (p.i.) labeled the cultures with [35S]methionine-cysteine for 60 min. The labeled cells were harvested, and SIV Env protein was immunoprecipitated from the resulting cell lysates (Fig. 2). Env expression by K81 peaked at early times, approximately 6 hpi (Fig. 2 and results not shown). Both gp160 and gp120 were observed on these gels, indicating that proteolytic processing of at least some Env protein occurred in the HSV-infected cells during the brief labeling time, although the amount of gp120 was always greatly reduced at late times p.i. The kinetics of Env protein expression were somewhat delayed in d81-infected cells in that peak levels of expression were not apparent until 18 hpi (Fig. 2, lanes 11 and 12). Processing of gp160 was also reduced after 12 hpi in d81-infected cells. Immunofluorescence staining of K81- and d81-infected Vero cells demonstrated cytoplasmic and cell surface staining of SIV env (E. McNamee, C. Murphy, W. Lucas, and D. Knipe, unpublished results). Thus, Env expression was readily detected in cells infected with K81 and d81, although the kinetics of expression were somewhat delayed in d81-infected cells. Nef protein expression were also specifically detected in extracts from K81- and d81-infected cells, but not from uninfected or HSV TK−-infected control cells, by Western blot reactivity to the N27 anti-Nef monoclonal antibody (Fig. 3). Nef protein detected in lysates from transfected cells migrated slightly more slowly due to the presence of an epitope tag (Fig. 3).
FIG. 2.
Kinetics of Env protein expression by recombinant viruses K81 and d81 in cell cultures. Vero cells were infected with the indicated viruses or mock infected. The infected cells were labeled with [35S]methionine-cysteine for 1 h prior to harvest at the times indicated. Env proteins were immunoprecipitated with anti-SIV sera and resolved by SDS-PAGE. The resulting autoradiogram is shown. pJG1 transfection lane shows Env protein expressed in cells transfected with plasmid pJG1. KOS1.1 is WT HSV-1.
FIG. 3.
Nef protein expression as detected by Western blots. Vero cells were infected with the indicated viruses or mock infected. Cells were harvested at 9 or 12 hpi, and lysates were resolved by SDS-PAGE. Nef protein was detected following incubation with the N27 anti-Nef monoclonal antibody in K81- and d81-infected cell lysates as well as in the Nef-positive control lysate, which migrated slower due to an epitope tag. Nef protein was not detected in cells infected with TK− HSV, in mock-infected cells, or in nef− lysates.
Vaccine phase.
Five rhesus monkeys were immunized with the replication-competent HSV recombinant K81, and two were immunized with the replication-deficient HSV recombinant d81. Three groups of rhesus monkeys received their first immunizations on different dates. Monkeys received booster immunizations according to the schedules shown in Table 1. One control monkey (Mm 285-95 [referred to hereafter by number alone]) received replication-competent TK− vector without insert. All monkeys were derived from our specific pathogen-free rhesus monkey breeding colony and were free of herpes B and HSV upon enrollment in the study. Each animal received 9 × 107 PFU of HSV recombinant subcutaneously and 1.8 × 108 PFU of HSV recombinant intramuscularly at each immunization.
TABLE 1.
Timing of booster inoculations and challenge in vaccinated monkeysa
| Animal expt | Animal no. | HSV strain | Time(s) (wk) of:
|
|
|---|---|---|---|---|
| Booster inoculations | Challenge | |||
| 602 | 137-96 | K81 | 6, 40 | 62 |
| 285-95 | TK− | 6, 40 | 62 | |
| 606 | 342-96 | K81 | 6, 24 | 46 |
| 1-97 | K81 | 6, 24 | 46 | |
| 159-96 | d81 | 6, 24 | 46 | |
| 70-97 | d81 | 6, 24 | 46 | |
| 608 | 178-97 | K81 | 6, 10 | 32 |
| 198-97 | K81 | 6, 10 | 32 | |
Vaccinations were started at different times in the different animal experiments, but all vaccinated animals were challenged together on the same day.
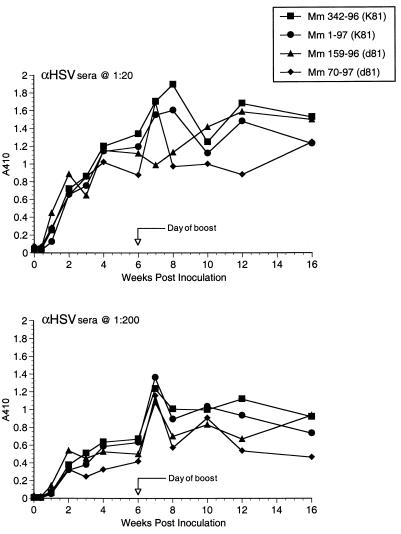
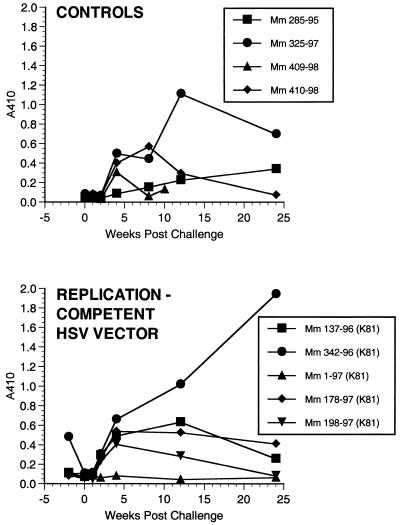
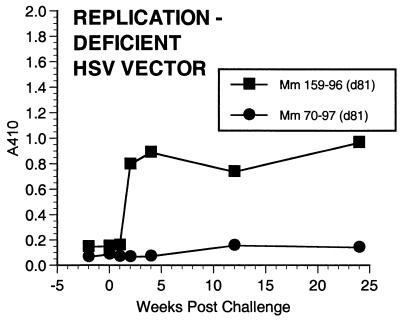
Each animal responded with a strong, stable antibody response to HSV after the initial immunization (Fig. 4 and other data not shown). Anti-HSV antibody responses were not significantly different between the animals that received K81 and those that received d81 (Fig. 4). ELISA-based tests for the detection of antibody responses to SIV envelope protein produced only weak signals. We thus developed a more sensitive, more specific assay for detection of antibodies to SIV gp120. Using this new method, we had no difficulty demonstrating the appearance of anti-gp120 antibody responses. The new assay relies on immunoprecipitation of gp120 from culture supernatants and detection of immunoprecipitated gp120 by Western blotting using monoclonal anti-gp120 antibody and ECL detection. The assay is able to detect 10 pg or less of immunoprecipitated gp120. Anti-SIV antibodies were specifically detected in all immunized animals except animal 285-95, which received the replication-competent, control TK− HSV without insert (Fig. 5A and B and Table 2). Anti-SIV antibodies were in general slow to develop but once present were continually detected at similar levels over the course of months up until the time of challenge (Fig. 5A and B and other data not shown). Dilutions of sera from rhesus monkeys infected with WT or mutant strains of SIV showed that levels of anti-gp120 antibodies could be roughly quantitated over at least a 10,000-fold range (Fig. 5C). Levels of anti-gp120 antibodies induced by the recombinant herpesviruses were roughly 1,000- to 10,000-fold lower than the levels observed in monkeys infected with WT SIVmac239 (Fig. 5). Also, there seemed to be considerable animal-to-animal variation in the strength of anti-SIV antibody responses (Fig. 5A and C). Sera taken immediately prior to challenge had no neutralizing activity against the difficult to neutralize challenge virus SIVmac239, consistent with the low level of anti-SIV antibodies in the IP-WB assay. These same sera also had no neutralizing activity against lab-adapted SIVmac251, a virus which is typically quite sensitive to antibody-mediated neutralization (22, 27).
FIG. 4.
Anti-HSV antibody responses. Week 0 is the time of initial immunization. Antibodies were determined by ELISA using plates coated with purified, lysed HSV.
FIG. 5.
Anti-SIV antibody responses. Antibodies to SIV gp120 were detected by IP-WB and ECL detection on film. The time line of samples is shown above each gel. (A) Three of the immunized animals from AE606; (B) the two immunized animals from AE608; (C) comparisons of week 26 sera from vaccinated monkeys to more highly diluted sera from a monkey infected with WT SIV in the right three lanes. As described previously (10), very weak anti-SIV antibody responses were elicited by SIVΔVif in monkey 151-93 (lane 1, week 0; lane 2, week 16; lane 3, week 28) but not by heat-inactivated (h.i.) SIVΔvif in monkey 180-93.
TABLE 2.
Strength of anti-SIV antibody responses
| Animal | Response at indicated time (wk) after initial vaccination
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 8 | 12 | 16 | 22 | 24 | 26 | 36 | 40 | 42 | 52 | |
| AE608 | |||||||||||||
| 178-97 | − | − | +/− | +++ | +++ | +++ | |||||||
| 198-97 | − | − | − | +++ | +++ | +++ | |||||||
| AE606 | |||||||||||||
| 342-96 | − | − | − | + | ++ | ++ | +++ | ++ | |||||
| 1-97 | − | − | − | − | − | − | ++ | + | |||||
| 159-96 | − | − | +/− | +/− | + | + | + | + | |||||
| 70-97 | − | − | − | +/− | + | + | ++ | + | |||||
| AE602 | |||||||||||||
| 137-96 | − | − | − | − | − | − | +/− | ++ | ++ | ||||
| 285-95 | − | − | − | − | − | − | − | − | − | ||||
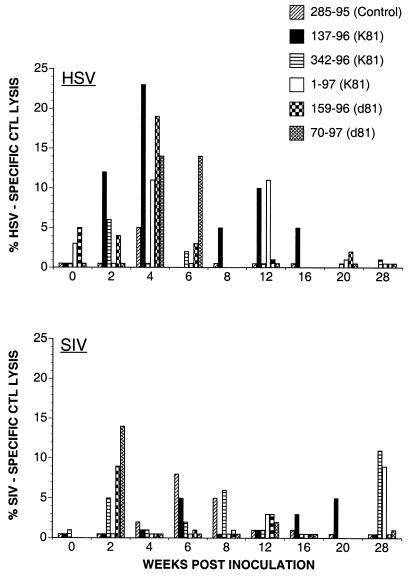
CTL responses to HSV and SIV envelope protein were evaluated in six animals (AE602 and AE606). CTL activity directed toward HSV was detected 4 weeks after initial immunization in the two animals immunized with the replication-deficient HSV vector d81 and in two animals (137-96 and 1-97) immunized with the replication-competent HSV vector. Levels of specific lysis after in vitro antigen-specific stimulation with autologous cells infected with HSV KOS ranged from 10 to 23% at effector-to-target (E/T) ratios of 14:1 to 43:1 (Fig. 6). The primary HSV-specific CTL response did not differ in strength or kinetics between animals immunized with the replication-deficient or replication-competent HSV vectors.
FIG. 6.
HSV-specific and SIV-specific CTL activity after vaccination and before SIV challenge. Percent HSV-specific CTL lysis denotes the difference in lysis between uninfected target cells and target cells infected with HSV-KOS. Percent SIV-specific CTL lysis is the difference in lysis between autologous B-LCL infected with WT vaccinia virus NYCBH and recombinant vaccinia virus expressing the SIVmac239 envelope. Cold targets infected with WT vaccinia virus NYCBH were used to reduce background lysis in SIV-specific CTL assays. Background lysis was generally <8% and did not exceed 12% at time points with positive Env-specific CTL activity.
CTL responses to SIV envelope protein were measured after in vitro SIV-specific stimulation. Lysis of Env-expressing target cells ≥10% above control target cells was considered to be significant. Weak SIV Env-specific CTL activity (10 to 15% specific lysis at E/T ratios of 40:1 to 60:1) was detected at single time points in two animals (Fig. 6). It was detected 2 weeks after immunization with the replication-deficient HSV vector d81 in animal 70-97 and 8 weeks after the second booster inoculation in animal 342-96 immunized with the replication-competent HSV vector K81. In addition, CTL activity just below threshold level (8 to 9% at E/T ratios of 40:1 to 45:1) was detected in animal 159-96 2 weeks after immunization with the replication-deficient HSV vector d81 and in animal 1-97 8 weeks after the second booster inoculation with the replication-competent HSV vector K81. In all of these animals, Env-specific CTL activity of ≥8% lysis was observed at two or more E/T ratios. Since Env-specific CTL activity was detected at single time points in individual animals, we were not able to determine if it was mediated by CD8+ lymphocytes or restricted by major histocompatibility complex class I.
Challenge phase.
The seven rhesus monkeys vaccinated with HSV-SIV recombinants and four controls were challenged rectally with SIVmac239. The four controls included the one monkey (285-95) that received replication-competent TK− HSV with no insert of SIV sequences. Challenge was performed 22 weeks after the last vaccine boost, which represented 32 to 62 weeks after the primary immunization (Table 1). Challenge was with 0.5 ml of a 1:2.5 dilution of a stock of SIVmac239 which had been titered previously by the intravenous route (23). This dilution of SIVmac239 contained 8.5 ng of p27, 4.7 × 103 tissue culture infectious doses, and 6 × 104 animal infectious doses by the intravenous route.
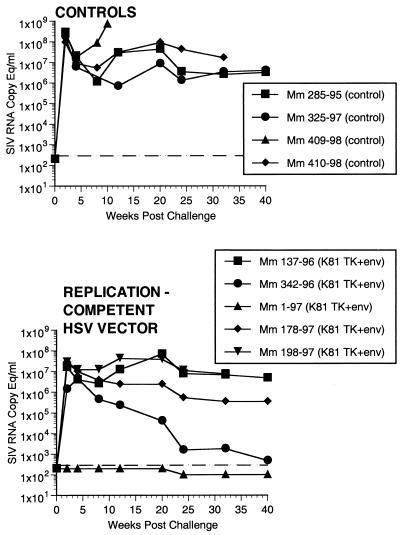
All four of the control monkeys became infected by all criteria following the SIV exposure. All four showed peaks in plasma antigenemia, in levels of SIV RNA in plasma, and in numbers of infectious cells in PBMC by 2 weeks after exposure (Fig. 7 to 9). At least three of the four controls had anti-SIV antibody responses after challenge (Fig. 10). The one control monkey with weak or no anti-SIV antibody responses was a rapid progressor that died at 10 weeks p.i. Set points of viral RNA in the other three controls were approximately 106 to 107 copies per ml of plasma (Fig. 8), as we have seen previously (10). We and others (A. Aldovini and D. Watkins, personal communication) have exposed 11 additional naive monkeys with the same dose of the same stock of SIVmac239 by the rectal route, and all 11 have similarly become infected. The challenge dose used for these experiments contained approximately 10 animal infectious doses by the rectal route because one of two macaques became systemically infected following rectal exposure to 0.5 ml of a 1:25 dilution of the same stock.
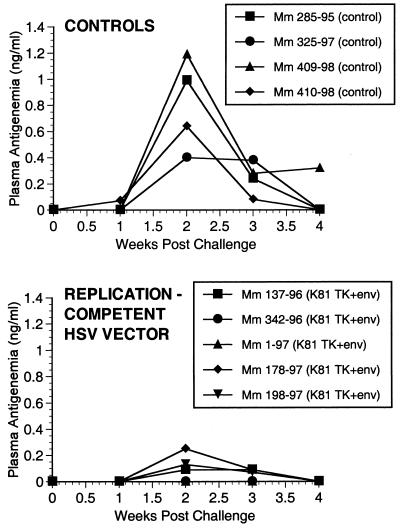
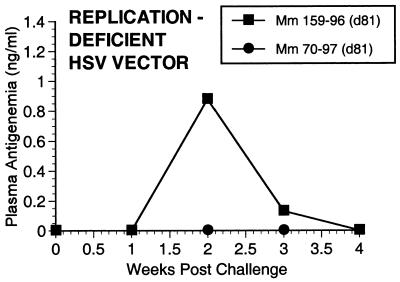
FIG. 7.
SIV p27 antigen in plasma postchallenge.
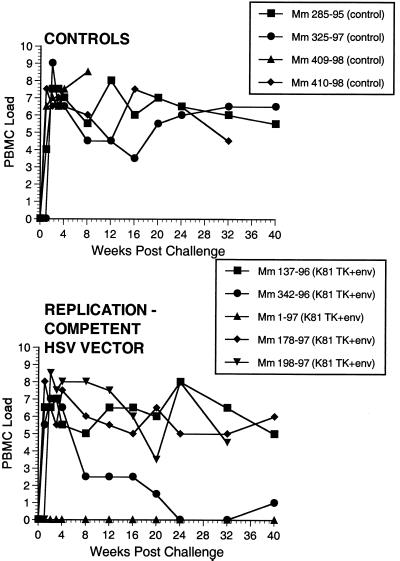
FIG. 9.
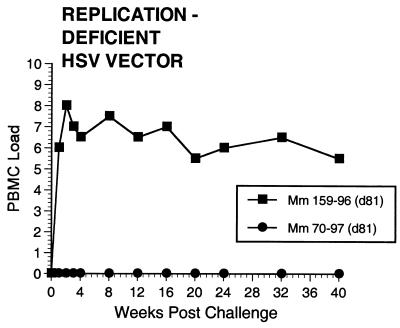
Numbers of infectious cells in PBMC. Numbers on y axis are code: 0, no SIV recovery even with 106 PBMC; 1, 50% recovery with 106 PBMC; 2 to 8, 50% recovery with 333,333, 111,111, 37,037, 12,345, 4,115, 1,372, and 454 PBMC, respectively.
FIG. 10.
Anti-SIV antibody responses following challenge. Week 0 is the time of rectal challenge with SIVmac239. Antibodies were determined by ELISA using plates coated with purified, lysed SIV. Antibodies were determined using a 1:20 dilution of plasma from the indicated animals and conjugate at 1:100.
FIG. 8.
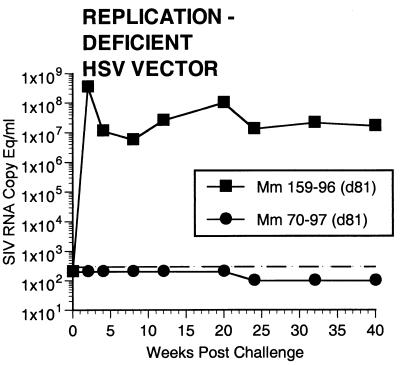
SIV RNA in plasma postchallenge. A shift in the baseline corresponds to use of assay with a lower detection limit.
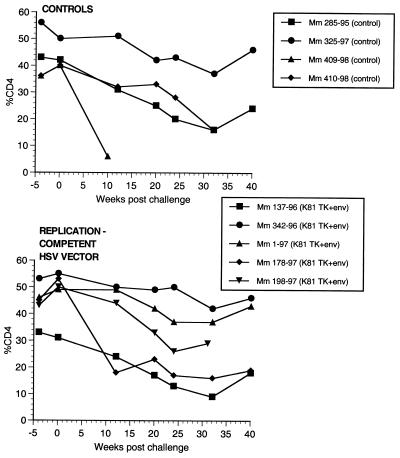
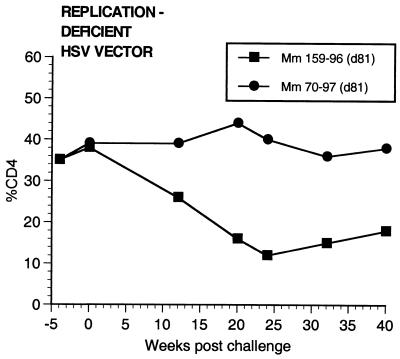
Two of the seven vaccinated monkeys (1-97 and 70-97) were strongly protected by all criteria. These two animals had no p27 antigen detectable in plasma at any time point (Fig. 7), no SIV RNA detectable in plasma at any time point (Fig. 8), and no SIV recoverable from PBMC (Fig. 9). In contrast, the other five vaccinated animals did have detectable SIV RNA in plasma (Fig. 8) and recoverable SIV in PBMC (Fig. 9). Four of these five also had detectable SIV p27 antigen in plasma; only 342-96 did not (Fig. 7). SIV DNA sequences were not detected in PBMC taken from 1-97 and 70-97 at 2 and 4 weeks after challenge using a sensitive nested PCR. SIV DNA sequences were detected at both 2 and 4 weeks in 342-96, 159-96, 198-97, 409-98, and 410-98 (data not shown). Changes in anti-SIV antibody levels postchallenge were also consistent with these observations. The five unprotected monkeys had clear, rapid increases in anti-SIV antibody levels by ELISA following challenge; the two protected monkeys did not (Fig. 10). These antibody results were confirmed in the IP-WB assay (data not shown). Despite the lack of sterilizing immunity, SIV RNA loads in plasma in the SIV-infected monkeys previously vaccinated with K81 were 1.2 logs lower at peak than those observed in the four control monkeys (Fig. 8). This difference was statistically significant (P = 0.021) in a Mann-Whitney test. These observations agree with the consistent differences in plasma antigenemia in control versus vaccinated animals (Fig. 7). Four of the five unprotected monkeys appeared to have plasma RNA loads and cell-associated viral loads at set point similar to those seen in control animals (Fig. 8 and 9). However, 342-96 exhibited decreasing viral loads in both assays that were significantly lower than in the controls in this study (Fig. 8 and 9), lower than those observed in all additional control macaques that received the same dose of this same stock rectally, and lower than what we have ever observed previously with SIVmac239 (10, 17, 36). This same animal (342-96) had no detectable SIV p27 antigen in plasma (Fig. 7) and had the lowest viral RNA levels of the infected animals at peak (Fig. 8). Vigorous and sustained Gag- and Env-directed CTL activity was detected 1 to 3 weeks after SIV challenge in the four control animals and in three of the five unprotected animals (data not shown). There were no apparent differences in the strength or kinetics of CTL activity between control and vaccinated animals that became SIV infected. Declines in CD4+ lymphocyte concentrations were observed in control and unprotected animals. However, CD4+ lymphocyte concentrations were relatively stable in the solidly protected animals 1-97 and 70-97 and in the partially protected animal 342-96 (Fig. 11).
FIG. 11.
Percentages of CD4+ T lymphocytes in monkeys following challenge. Week 0 is the time of rectal challenge with SIVmac239. The results are expressed as the percentage of cells in PBMC that were CD4+ T lymphocytes.
Correlations with protection.
Examination of anti-SIV binding antibodies through the course of the vaccine phase and on the day of challenge revealed no obvious correlations with protection. In fact, anti-gp120 antibody levels in protected monkeys 1-97 and 70-97 assessed by the IP-WB assay were at the lower end of the spectrum among the seven vaccinated monkeys. Protections were achieved in the absence of detectable neutralizing activity in serum at the time of challenge. Monkey 1-97 was vaccinated with the replication-competent vector, and 70-97 was vaccinated with the replication-deficient vector.
The length of time from the initial immunization or the last boost to the challenge also did not correlate with protection. The two solidly protected monkeys and the partially protected monkey were all part of a group (AE606) whose vaccine phase was 46 weeks (Table 1). Thus, one monkey that had a longer vaccine phase and two that had a shorter vaccine phase were not protected. It is interesting that the three protected monkeys (1-97, 70-97, and 342-96) had an SIV envelope-specific CTL activity more consistently observed during the vaccine phase than unprotected animals. However, the low level of the Env-specific lysis observed precludes any definite conclusions regarding the correlation of CTL activity with protection.
DISCUSSION
Our current level of knowledge suggests that simple immunological memory in the absence of activated effector cells may not be sufficient to provide vaccine protection against field strains of SIV and HIV. Once SIV and HIV establish infection in a susceptible host, they have a remarkable ability to replicate unrelentingly in the face of apparently strong host immune responses. A multiplicity of mechanisms are used by these lentiviruses to achieve continuous replication (9). They include selection of antigenic variants that escape immune recognition within a single infected individual, destruction of CD4+ helper cell activity, and an envelope coat structure that is not easily accessible to antibodies. The failure of vaccines in animal models may be explained by a failure to blunt early, high-level replication by the virus, thus allowing the natural immune evasion strategies of the virus to take effect. Conversely, the success of live attenuated vaccine approaches in animal models may depend on the persistent, controlled nature of the infection with the attenuated strains. For an AIDS vaccine to be practically effective, we may need to develop strategies that result in persistently active immune responses that can be poised to quickly suppress the early replication of the virus.
Most vaccine approaches currently being forwarded for AIDS do not have persistence of antigen or persistent antigen expression. These include envelope subunit approaches, poxvirus recombinants, inactivated whole virus, DNA, and prime and boost regimens with combinations of these approaches. While it is theoretically possible that immunizations by such approaches could provide durable protection if the response time following live virus exposure was quick enough, they typically have not done so in animal models when challenge used pathogenic, difficult to neutralize strains of virus (15, 26, 31, 33, 40, 43). For influenza virus, memory CTL take at least 4 days to expand and home to the site of infection (13). Postexposure drug studies also suggest that 4 days may be too late to block establishment of a persistent infection (24, 38). Herpesviruses, which persist for the lifetime of the infected host, induce humoral and cellular immune responses that also persist for life. Persisting cellular responses have been postulated to keep HSV in a latent state because decreases in cellular responses are associated with reactivation of latent HSV (45). Thus, herpesvirus recombinant vectors may provide an alternative to live, attenuated lentivirus strains as AIDS vaccines that induce host immune responses which are persistently activated or can be rapidly induced into an active state.
What is impressive about the current results is that protection was achieved against difficult to neutralize, pathogenic SIV 5 months after the last vaccine administration. The vast majority of SIV and SHIV vaccine experiments that have used nonpersisting immunogens have scheduled the challenge at 2 to 4 weeks after the last vaccine boost, when immune responses are at or near their peak. The one exception is the study of Benson et al. (2), in which intravenous challenge at 6 months showed no protection but rectal challenge at 9 months surprisingly showed protection in 5 of 11 monkeys despite the fact that antiviral immune responses had declined to very low or undetectable levels by that time. Further studies will be needed to determine whether a longer time interval between the last boost with a nonpersisting immunogen and challenge actually favors protection despite dramatic declines in the levels of measurable antiviral immune responses. Our results nonetheless illustrate the potential of herpesviruses as vaccine vectors for AIDS and indicate the need for development of alternate vaccine strategies that can achieve persistently active immune responses.
What is the basis for optimism if only two of seven monkeys in this study were solidly protected against challenge? Previous studies with live attenuated SIV have suggested an important role for cellular responses to core Gag-Pol antigens in protection against challenge (19, 33, 50), and other studies have indicated a protective role for CTL to Tat and Rev (3, 47). The present study used SIV env and nef genes in the recombinant vaccines, but in the future it will be easily feasible to incorporate genes that express core, Tat, Rev, and other antigens. There is also reason to believe that other herpesviruses, such as other HSV strains that replicate better in rhesus monkey fibroblasts in culture (W. T. Lucas, C. G. Murphy, and D. M. Knipe, unpublished results) or the rhesus monkey rhadinovirus (11), may elicit stronger immune responses in rhesus monkeys. We also now know that HSV strains which express much higher levels of Env protein can be constructed (Lucas et al., unpublished). Once conditions can be defined for achieving solid protection in most or all animals, herpesvirus recombinants should be an ideal means for learning the types of immune responses most important for achieving vaccine protection in the SIV/rhesus monkey system.
The basis for the comparable strength of responses to replication-competent versus replication-defective HSV is currently not understood. However, there is precedent for this type of phenomenon in poxvirus systems. Replication-defective poxviruses can elicit antiviral antibody responses comparable in strength to replication-competent counterparts (1). There are several possible explanations for the equivalent responses to replication-competent and replication-defective strains. First, some of the primary infected cells may be the major antigen-presenting cells, and both types of strains may infect these cells. Second, even replication-competent HSV, or at least the HSV strain used in this study, may replicate so poorly in rhesus monkeys that the response to it is not detectably different from that to replication-defective HSV. Finally, there may be cells in the monkey that support replication of the mutant strain such that it is not replication defective in vivo.
Despite the potential advantages of durable immunity induced by a herpesvirus recombinant vector, preexisting immunity in the population and concern for safety could potentially limit practical application of this approach. Preexisting immunity to HSV might limit infection by the recombinant vaccine and the immune response to a new antigen expressed by the HSV vector. However, initial results in mouse models indicate that preexisting immunity to HSV does not necessarily restrict the immune response to a new antigen expressed by an HSV vector (M. Brockman and D. M. Knipe, unpublished results). With respect to safety concerns, vector replication could conceivably result in disease in immunocompromised individuals or under certain circumstances. Such safety concerns could potentially be minimized with improved vector designs (7).
ACKNOWLEDGMENTS
The first three authors contributed equally to this work and should be considered co-first authors.
We thank Ron Montelaro for the gift of plasmid pLG339Sport, Don Coen for the gift of plasmid p101086.7BglII, and Jim Hoxie for the N27 anti-Nef monoclonal antibody. We also thank Keith Mansfield, Prabhat Sehgal, and their staff for veterinary care and blood sampling and M. Piatak and L. Li for assistance with plasma viral load analyses.
This work was supported by National Cooperative Vaccine Discovery Group grant AI38131, HIV Vaccine Research and Development grant AI46006 from the Division of AIDS of the National Institute of Allergy and Infectious Diseases, and grant RR00168 from NCRR. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000.
REFERENCES
- 1.Belyakov I M, Wyatt L S, Ahlers J D, Earl P, Pendleton C D, Kelsall B L, Strober W, Moss B, Berzofsky J A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, Borsetti A, Belli R, Akerblom L, Corrias F, Butto S, Heeney J, Verani P, Titti F, Ensoli B. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 4.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S H, Cook W J, Grove K L, Coen D M. Human thymidine kinase can functionally replace herpes simplex virus type 1 thymidine kinase for viral replication in mouse sensory ganglia and reactivation from latency upon explant. J Virol. 1998;72:6710–6715. doi: 10.1128/jvi.72.8.6710-6715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham T P, Montelaro R C, Rushlow K E. Lentivirus envelope sequences and proviral genomes are stabilized in Escherichia coli when cloned in low-copy-number plasmid vectors. Gene. 1993;124:93–98. doi: 10.1016/0378-1119(93)90766-v. [DOI] [PubMed] [Google Scholar]
- 7.DaCosta X J, Jones C A, Knipe D M. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci USA. 1999;96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel M D, Letvin N L, Sehgal P K, Hunsmann G, Schmidt D K, King N W, Desrosiers R C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C. Strategies used by human immunodeficiency virus that allow persistent viral replication. Nat Med. 1999;5:723–725. doi: 10.1038/10439. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desrosiers R C, Sasseville V G, Czajak S C, Zhang X, Mansfield K G, Kaur A, Lackner A A, Jung J U. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell H E, McLean C S, Harley C, Efstathiou S, Inglis S, Minson A C. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol. 1994;68:927–932. doi: 10.1128/jvi.68.2.927-932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 14.Gao M, Knipe D M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giavedoni L D, Planelles V, Haigwood N L, Ahmad S, Kluge J D, Marthas M L, Gardner M B, Luciw P A, Yilma T D. Immune response of rhesus macaques to recombinant simian immunodeficiency virus gp130 does not protect from challenge infection. J Virol. 1993;67:577–583. doi: 10.1128/jvi.67.1.577-583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford W P, Gebhardt B M, Carr D J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 17.Ilyinskii P O, Simon M A, Czajak S C, Lackner A A, Desrosiers R C. Induction of AIDS by simian immunodeficiency virus lacking NF-κB and SP1 binding elements. J Virol. 1997;71:1880–1887. doi: 10.1128/jvi.71.3.1880-1887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson R P, Lifson J D, Czajak S C, Cole K S, Manson K H, Glickman R, Yang J, Montefiori D C, Montelaro R, Wyand M, Desrosiers R C. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relation of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knipe D M, Ruyechan W T, Roizman B. Molecular genetics of herpes simplex virus. III. Fine mapping of a genetic locus determining resistance to phosphonoacetate by two methods of marker transfer. J Virol. 1979;29:698–704. doi: 10.1128/jvi.29.2.698-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knipe D M, Spang A E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982;43:314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langlois A J, Desrosiers R C, Lewis M G, KewalRamani V N, Littman D R, Zhou J Y, Manson K, Wyand M S, Bolognesi D P, Montefiori D C. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis M G, Bellah S, McKinnon K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G E. Titration and characterization of two rhesus derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 24.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D M, Kiser R F, Coalter V J, Walsh G, Imming R J, Fisher B, Flynn B M, Bischofberger N, Piatak M J, Hirsch V M, Nowak M A, Wodarz D. Containment of simian immunodeficiency virus: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Tang Q, Hendricks R L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meignier B, Martin B, Whitley R J, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162:313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- 29.Morrison L A, Knipe D M. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison L A, Knipe D M. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology. 1996;220:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- 31.Myagkikh M, Alipanah S, Markham P D, Tartaglia J, Paoletti E, Gallo R C, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:985–992. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen L H, Knipe D M, Finberg R W. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–7072. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polacino P S, Stallard V, Klaniecki J E, Pennathur S, Montefiori D C, Langlois A J, Richardson B A, Morton W R, Benveniste R E, Hu S L. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in macaques. J Virol. 1999;73:8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 35.Reitter J N, Desrosiers R C. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J Virol. 1998;72:5399–5407. doi: 10.1128/jvi.72.7.5399-5407.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 37.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenwirth B, ten Haaft P, Bogers W M J M, Nieuwenhuis I G, Niphuis H, Kuhn E-M, Bischofberger N, Heeney J L, Uberla K. Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques. J Virol. 2000;74:1704–1711. doi: 10.1128/jvi.74.4.1704-1711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Schlienger K, Montefiori D C, Mancini M, Riviere Y, Tiollais P, Michel M. Vaccine-induced neutralizing antibodies directed in part to the simian immunodeficiency virus (SIV) V2 domain were unable to protect rhesus monkeys from SIV experimental challenge. J Virol. 1994;68:6578–6588. doi: 10.1128/jvi.68.10.6578-6588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimeld C, Whiteland J L, Nicholls S M, Grinfeld E, Easty D L, Gao H, Hill T J. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 42.Spector F C, Kern E R, Palmer J, Kaiwar R, Cha T A, Brown P, Spaete R R. Evaluation of a live attenuated recombinant virus RAV 9395 as a herpes simplex virus type 2 vaccine in guinea pigs. J Infect Dis. 1998;177:1143–1154. doi: 10.1086/515278. [DOI] [PubMed] [Google Scholar]
- 43.Stott E J, Almond N, Kent K, Walker B, Hull R, Rose J, Silvera P, Sangster R, Corcoran T, Lines J, Silvera K, Luciw P, Murphy-Corb M, Momin P, Bruck C. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effect of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus- HIV-1 chimeric virus. J Gen Virol. 1998;79:423–432. doi: 10.1099/0022-1317-79-3-423. [DOI] [PubMed] [Google Scholar]
- 44.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load by determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 45.Thong Y H, Vincent M M, Hensen S A, Fuccillo D A, Rola-Pleszczynski M, Bellanti J A. Depressed specific cell-mediated immunity to herpes simplex virus type 1 in patients with recurrent herpes labialis. Infect Immun. 1975;12:76–80. doi: 10.1128/iai.12.1.76-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tigges M A, Koelle D, Hartog K, Sekulovich R E, Corey L, Burke R L. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J Virol. 1992;66:1622–1634. doi: 10.1128/jvi.66.3.1622-1634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Baalen C A, Pontesilli O, Huisman R C, Geretti A M, Klein M R, de Wolf F, Miedema F, Gruters R A, Osterhaus A D. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J Gen Virol. 1997;78:1913–1918. doi: 10.1099/0022-1317-78-8-1913. [DOI] [PubMed] [Google Scholar]
- 48.Walboomers J M, Schegget J T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976;74:256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- 49.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. Philadelphia, Pa: Raven Publishers; 1996. pp. 2297–2342. [Google Scholar]
- 50.Wyand M S, Manson K, Montefiori D C, Lifson J D, Johnson R P, Desrosiers R C. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]