Abstract
The M2 ion channel of influenza A virus is a small integral membrane protein whose active form is a homotetramer with each polypeptide chain containing 96-amino-acid residues. To identify residues of the transmembrane (TM) domain that line the presumed central ion-conducting pore, a set of mutants was generated in which each residue of the TM domain (residues 25 to 44) was replaced by cysteine. The accessibility of the cysteine mutants to modification by the sulfhydryl-specific reagents methane thiosulfonate ethylammonium (MTSEA) and MTS tetraethylammonium (MTSET) was tested. Extracellular application of MTSEA evoked decreases in the conductances measured from two mutants, M2-A30C and M2-G34C. The changes observed were not reversible on washout, indicative of a covalent modification. Inhibition by MTSEA, or by the larger reagent MTSET, was not detected for residues closer to the extracellular end of the channel than Ala-30, indicating the pore may be wider near the extracellular opening. To investigate the accessibility of the cysteine mutants to reagents applied intracellularly, oocytes were microinjected directly with reagents during recordings. The conductance of the M2-W41C mutant was decreased by intracellular injection of a concentrated MTSET solution. However, intracellular application of MTSET caused no change in the conductance of the M2-G34C mutant, a result in contrast to that obtained when the reagent was applied extracellularly. These data suggest that a constriction in the pore exists between residues 34 and 41 which prevents passage of the MTS reagent. These findings are consistent with the proposed role for His-37 as the selectivity filter. Taken together, these data confirm our earlier model that Ala-30, Gly-34, His-37, and Trp-41 line the channel pore (L. H. Pinto, G. R. Dieckmann, C. S. Gandhi, C. G. Papworth, J. Braman, M. A. Shaughnessy, J. D. Lear, R. A. Lamb, and W. F. DeGrado, Proc. Natl. Acad. Sci. USA 94:11301–11306, 1997).
The M2 ion channel protein of influenza A virus has a high selectivity for protons (3, 8, 17, 21, 24). Even though the M2 protein is only a minor component of the viral envelope (30), the ion channel activity is nonetheless essential in the life cycle of the virus. Influenza virus enters cells via endocytosis, and in the low-pH environment found in the endosomal compartment, the M2 ion channel is activated (reviewed in reference 14). Protons flow into the virion, acidifying the virion interior, a pH change which is thought to weaken protein-protein interactions between the viral matrix protein and the ribonucleoprotein (RNP) complex (reviewed in references 10 and 14), a prerequisite in the uncoating process. A considerable body of evidence indicates the M2 ion channel is inhibited by the antiviral drug amantadine (3, 21, 29). In virus-infected cells treated with amantadine, the viral matrix protein fails to dissociate from the RNP, and import of the RNA into the nucleus does not occur. Hence, the RNPs cannot undergo replication in the nucleus and the viral life cycle is blocked (reviewed in references 10, 14, and 15).
The mature M2 protein is 96 amino acid residues (the N-terminal methionine is cleaved [27]), and it spans the membrane once: it has 23 extracellular residues, a transmembrane (TM) domain of 19 residues, and a 54-residue cytoplasmic tail (16). The M2 protein is disulfide linked (11, 25), and the ion channel active form is a homotetramer (22). Models of the structure of the M2 ion channel based on experimentally determined data (13, 20) and by molecular dynamics principles (1, 23, 31) indicate that the M2 TM domain forms a left-handed α-helical coiled-coil containing a central ion-conducting pore through the axis of symmetry. The majority of models predict occlusion of the ion-conducting pore by the inward facing histidine residue 37. The importance of histidine residue 37 in proton conduction is highlighted by the finding that (i) the curve of pH activation of the channel possesses a midpoint very near the pKa of histidine (28), (ii) substitution of His-37 for other residues causes altered ion channel properties (20, 28), (iii) coordination of Cu2+ ions with His-37 results in inhibition of channel activity (5), and (iv) the extent of the diminution of ion conduction in deuterium oxide solvent is consistent with protons binding to the channel as they traverse the pore (17).
To identify experimentally the residues which line the ion-conducting pore of the M2 channel, each residue in the presumed TM domain was changed individually to cysteine. The ion currents of the resulting mutant ion channels were tested for their susceptibility to modification by aqueous sulfhydryl-specific reagents. A change in current of a given mutant on addition of the sulfhydryl-specific reagent is interpreted as indicating that in the wild-type protein, the residue is exposed to the aqueous surface of the molecule and forms part of the ion-conducting pore. By application of reagents that do not readily cross the membrane to both the extracellular and intracellular sides of the channel, it was possible to investigate the point at which the channel is most narrow.
MATERIALS AND METHODS
Construction of M2 TM domain cysteine mutants.
The cysteine substitution mutants in the M2 TM domain (influenza virus A/Udorn/72) were those described previously (20). In vitro synthesis of mRNA was performed using a mMessage mMachine T7 transcription kit (Ambion, Austin, Tex.).
Microinjection and culture of oocytes.
Oocytes from Xenopus laevis were prepared for mRNA injection and injected with M2 mRNA as described previously (5).
Electrophysiological recordings.
At 24 to 48 h after mRNA injection, whole cell currents were recorded with a two-electrode voltage-clamp apparatus consisting of a differential preamplifier (model MEZ-7101; Nihon Kohden, Tokyo, Japan) that recorded the voltage difference between a pipette (filled with 3 M KCl) located in the cell and another in the surrounding bath. A voltage-clamp amplifier (Nihon Kohden CEZ-1100) provided feedback current to the oocyte through a second intracellular pipette. Oocyte currents were recorded in standard Barth's solution (0.3 mM NaNO3, 0.71 mM CaCl2, 0.82 mM MgSO4, 1.0 mM KCl, 2.4 mM NaHCO3, 88 mM NaCl, 15.0 mM morpholineethanesulfonic acid [pH 6.2] or 15.0 mM HEPES [pH 7.5]). To check for nonspecific leaks, amantadine hydrochloride (100 mM stock in Barth's solution; Sigma Chemical Co., St. Louis, Mo.) was diluted and applied at a working concentration of 100 mM for 2 to 5 min at the end of measurements from an oocyte.
Reagents.
2-Aminoethyl methanethiosulfonate hydrobromide (MTSEA), [2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET), and 2-[(5-fluoresceinyl)aminocarbonyl]ethyl methanethiosulfonate (MTS-fluorescein) were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). Reagents were stored at −20°C in a desiccator. Individual aliquots of reagent powder were weighed out. For each experiment, an aliquot was freshly diluted in Barth's solution and immediately applied to the oocyte.
Polypeptide analysis.
Metabolic labeling of oocytes with [35S]cysteine was performed as described previously (12, 21). Oocytes were homogenized in radioimmunoprecipitation buffer and extracted with trifluoromethane as described previously (12, 30). Polypeptides were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions as described previously (11). Radioactivity was detected using a Fuji BAS 1000 Bioimager (Fuji Medical Instruments, Stanford, Conn.), and fluorescence was detected using a STORM imager (Molecular Dynamics, Perkin-Elmer, Norwalk, Conn.).
RESULTS
Effect of extracellular application of MTS reagents on the activity of M2 protein cysteine substitution mutants.
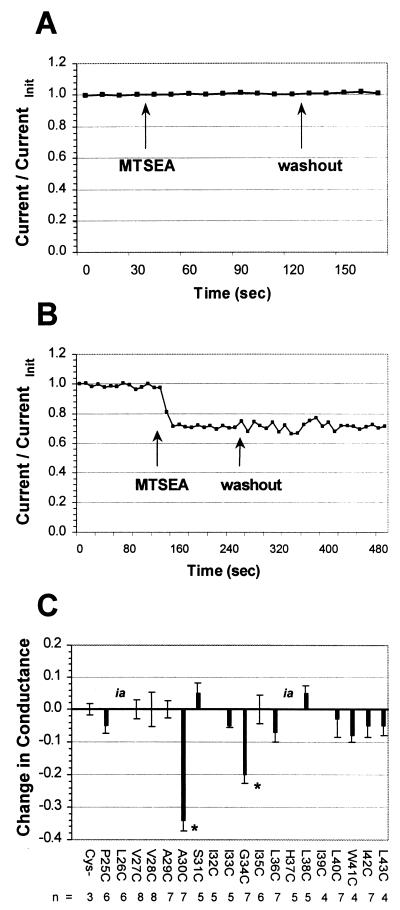
To determine which residues line the pore of the M2 ion channel, a set of mutants was generated in which each residue of the M2 TM domain (residues 25 to 44) was replaced by cysteine (20). The accessibility of the cysteine residues to aqueous sulfhydryl-specific reagents applied both extracellularly and intracellularly was tested. Oocytes were injected with mRNA for a given M2 mutant, and membrane currents were recorded by maintaining the oocyte at a holding voltage of −20 mV, with test pulses taken to −80 mV at 10-s intervals. The pH of the bath medium was lowered from 7.5 to 6.2 to activate the M2 ion channel, and the membrane current was allowed to reach a steady-state amplitude. A sulfhydryl-specific reagent was then added to the bath, and its effect on the current was recorded. After the membrane current reached a new steady-state amplitude, the reagent was washed out. Finally, amantadine (100 μM) was added to determine M2-specific currents. At the end of each step, the current-voltage relationship was determined by both a series of voltage clamp steps and a voltage ramp. As a control, the currents were recorded from an M2 ion channel that did not contain a cysteine residue. As shown in Fig. 1A, addition of MTSEA or its washout did not cause a perturbation to the recorded current for the nonsubstituted (cysteineless) protein. An example of the effect of MTSEA on a cysteine-containing M2 ion channel, M2-G34C, is shown in Fig. 1B. Immediately upon addition of MTSEA (2.5 mM) the current decreased, with maximum inhibition observed within 30 s. This decrease was not reversible upon washout, as expected for a covalent modification. To determine that this effect was specific to the MTS reagent and was not caused by a mechanical disturbance of the oocyte, the bath medium was changed between two solutions of identical composition. No change in current was observed when the buffer was exchanged.
FIG. 1.
Effect of extracellular MTSEA on the conductances of M2 cysteine substitution mutants. (A) Application of MTSEA had no effect on the currents of a cysteineless M2 ion channel. (B) Time course of inhibition of the M2-G34C ion channel by MTSEA. Current was immediately decreased upon addition of MTSEA (2.5 mM) to the bathing medium and did not recover upon washout. (C) The conductances of only two mutants (M2-A30C and M2-G34C) were decreased by extracellular application of 2.5 mM MTSEA (∗, P < 0.05). Two of the mutants (labeled “ia”) had activity that was too low to measure inhibition accurately in this assay.
The effect of extracellular MTSEA application on mutant channels with cysteines substituted at every position in the putative TM domain was tested (Fig. 1C). The conductances of only two mutants, M2-A30C and M2-G34C, were decreased (34% ± 3% [n = 7] and 20% ± 3% [n = 7], respectively; P < 0.05 by one-way analysis of variance using the Student-Newman-Keuls test). The currents of the M2-G34C ion channel were also inhibited by the sulfhydryl-specific reagents MTSET (2 mM) and N-ethylmaleimide (1 mM), although the inhibition observed was less (14% ± 5% [n = 3] and 7% ± 2% [n = 5], respectively). No other mutant ion channels were inhibited in this way, although two (M2-L26C and M2-H37C) could not be tested because they had very low currents, even when measured at very low pH. These results are consistent with Ala-30 and Gly-34 lining the channel pore.
Activity of the M2-L26C ion channel.
Extracellular application of MTS reagents did not identify any residues that were exposed to the aqueous pore that were situated closer to the extracellular mouth of the channel than Ala-30. However, if the channel forms a helical coiled-coil bundle, then at least one additional N-terminal residue should line the pore between the extracellular opening and Ala-30. One residue in this region that could not be tested was Leu-26, because when substituted by cysteine the activity of the resulting mutant ion channel was too low to measure inhibition accurately. One possible explanation for the low activity of M2-L26C is that introduced cysteine residues form an intersubunit disulfide bond (2), leading to decreased channel activity. Therefore, we tested whether treatment with the reducing agent dithiothreitol (DTT) would reduce such bonds, thereby increasing the currents observed.
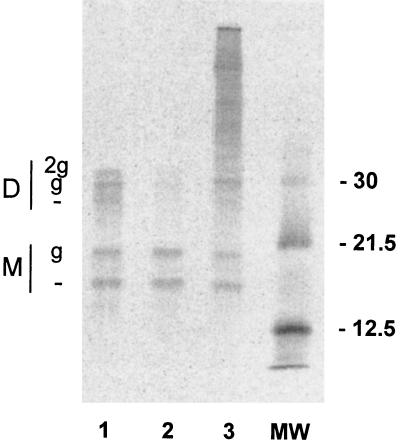
Application of DTT (2 min, 5 mM) evoked an increase in currents measured from the M2-L26C ion channel at low pH (Table 1). For the wild-type M2 ion channel, the currents measured at pH 6.2 were approximately eightfold greater than those measured at pH 7.5. In contrast, the ratio of current measured in Barth's solution at pH 6.2 to that at pH 7.5 for the M2-L26C ion channel was near 1, indicating that it was not appreciably activated by low pH. After application of DTT, this ratio increased somewhat (usually over 40% [Table 1]). As the increased current was both activated by low pH and sensitive to the M2-specific inhibitor amantadine, the data indicate that the increase in current was not due to nonspecific effects of the DTT treatment. Furthermore, the increase in current evoked by DTT could be partially reversed by subsequent treatment with the oxidizing reagent H2O2 (2 min, 0.1% [Table 1]). These results are consistent with the spontaneous formation of reversible intersubunit disulfide bonds. To confirm directly that M2-L26C when expressed in oocytes formed interchain disulfide bonds, M2-L26C was examined by SDS-PAGE under nonreducing conditions. As shown in Fig. 2, M2-L26C formed disulfide-linked dimers that were in the large part reduced by DTT treatment (lane 2) and were reformed on oxidation after H2O2 treatment (lane 3). The multiple M2 species are due to the fact that a population of the M2-L26C species are modified by the addition of N-linked carbohydrate chains to Asn residue 22 (21).
TABLE 1.
Ratios of currents at pH 6.2 to pH 7.5 of oocytes expressing M2-L26C under four different treatmentsa
| Treatment | Ratio, current at pH 6.2/current at pH 7.5
|
|||
|---|---|---|---|---|
| Assay 1 (n = 5) | Assay 2 (n = 5) | Assay 3 (n = 4) | Assay 4 (n = 1) | |
| No DDT | 1.04 ± 0.02 | 1.23 ± 0.13 | 0.78 ± 0.06 | 1.31 |
| 5 mM DTT | 1.55 ± 0.17 | 1.68 ± 0.14 | 1.31 ± 0.05 | 1.85 |
| % Change | 48 | 37 | 67 | 41 |
| Amantadine | H2O2 | MTSEA | MTSET | |
| Reagent | 0.84 ± 0.06 | 1.33 ± 0.14 | 1.29 ± 0.05 | 1.83 |
| % change | −46 | −21 | −2 | −1 |
DTT increases activity of the M2-L26C ion channel. As shown in the first two rows, currents from oocytes expressing the M2-L26C mutant protein showed little pH activation before DTT treatment, whereas after incubation in DTT (2 min, 5 mM), activation in response to low pH was markedly increased. This increased activity was partly inhibited by amantadine (assay 1), consistent with previous observations that changes at residue 26 can lead to amantadine resistance (6). In a separate batch of oocytes, the increased current evoked by DTT treatment was partially reversed by H2O2 (2 min, 0.1%) (assay 2). In other separate batches of oocytes, the activity of the M2-L26C ion channel after DTT treatment was not affected by extracellular application of either 2.5 mM MTSEA (assay 3) or 2 mM MTSET (assay 4).
FIG. 2.
The M2-L26C mutant protein forms reversible intersubunit disulfide bonds. Radiolabeled oocyte lysates were immunoprecipitated with the anti-M2 antibody 14C2 (30). Oocytes were either untreated controls (lane 1), DTT treated (lane 2), or DTT treated and then H2O2 treated (lane 3). Note that the ratio of M2 dimers (D) to monomers (M) decreases upon DTT treatment and is restored upon H2O2 treatment. —, nonglycosylated species; g and 2g, mono- and diglycosylated species, respectively. Positions of molecular weight (MW) markers are shown at the right in kilodaltons.
As a means to obtain measurable activity from the M2-L26C ion channel had been established, it was possible to determine the accessibility of M2-L26C to sulfhydryl-specific reagents. Neither MTSEA (2.5 mM) nor MTSET (2 mM) had a significant effect on the conductance of the M2-L26C channel after pretreatment with DTT (Table 1, assays 3 and 4). To confirm that residual reducing reagent was not inactivating the MTS reagents, we confirmed that the M2-G34C ion channel could still be inhibited by MTSEA after treatment with DTT (data not shown).
Fluorescent labeling of M2 cysteine substitution mutants.
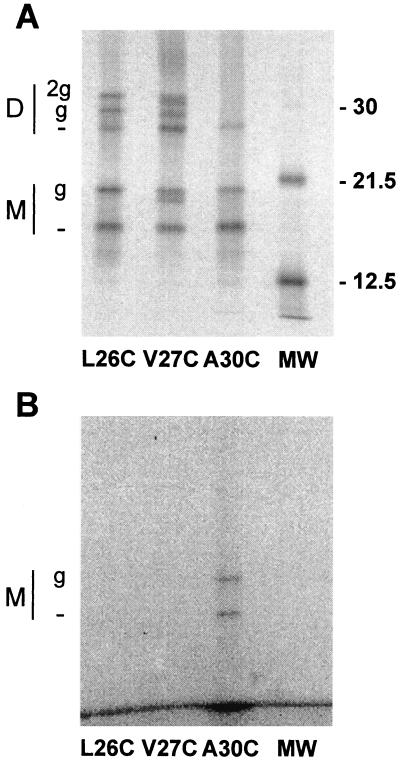
One possibility for the lack of effect of MTSEA on cysteine residues substituted at positions N-terminal to Ala-30 is that the channel pore widens near its extracellular mouth, such that even if a MTS reagent reacts with a cysteine in this region, the modifying moiety is too small to occlude the pore. To test whether such biochemical modification was occurring, fluorescently conjugated MTS derivatives were used. Oocytes expressing the M2-L26C, M2-V27C, and M2-A30C ion channels were metabolically labeled with [35S]cysteine and then incubated in Barth's solution (pH 7.5) to which MTS-fluorescein (ca. 500 μM) was added. Excess unreacted reagent was thoroughly washed out before the oocytes were lysed. The M2 proteins were immunoprecipitated, the peptides were analyzed by SDS-PAGE, and the gels were analyzed for both radioactivity, to visualize total protein expression, and fluorescence, to detect the covalently linked fluorescent MTS reagent. By superimposing the two gel images, it could be determined which M2 proteins were labeled with the fluorescent reagent.
As shown in Fig. 3, the M2-A30C ion channel was labeled by MTS-fluorescein. Note that only the monomeric species (both monoglycosylated and nonglycosylated) were labeled, because only the monomer protein possesses a free sulfhydryl group. Thus, these data confirm those obtained using extracellular MTSEA application and indicate that Ala-30 lines the channel pore. However, neither the M2-L26C nor the M2-V27C ion channels were labeled by MTS-fluorescein, although it should be noted that we were not able to perform the fluorescent labeling after a DTT pretreatment. Since a large proportion of monomers in these particular mutant channels are known to form disulfide-linked dimers (Fig. 2 and reference 2), reduction of these bonds may be necessary for there to be sufficient free sulfhydryl groups available with which a detectable number of MTS-fluorescein molecules may react. We were therefore unable to identify the pore-lining residue(s) near the N-terminal mouth of the channel.
FIG. 3.
MTS-fluorescein labeling of M2 cysteine substitution mutants. [35S]cysteine-labeled oocytes were incubated in a solution containing MTS-fluorescein reagent (two 5-min applications of ca. 500 μM), and immunoprecipitations were then performed. The resulting polyacrylamide gel was analyzed both for radioactivity (A) and for fluorescence (B). By superimposing the two gel images, it was determined that only the M2-A30C nonglycosylated and monoglycosylated monomeric species were fluorescently labeled. Other details are as for Fig. 2.
Effect of intracellular application of MTS reagents.
The currents of the M2 ion channel are thought to be in the order of 1 to 10 fA (17), too small to examine the effect of MTS reagents in an outside-out patch. Instead, we employed the technique of direct microinjection of a concentrated reagent solution while the oocyte was under two-electrode voltage clamp (26).
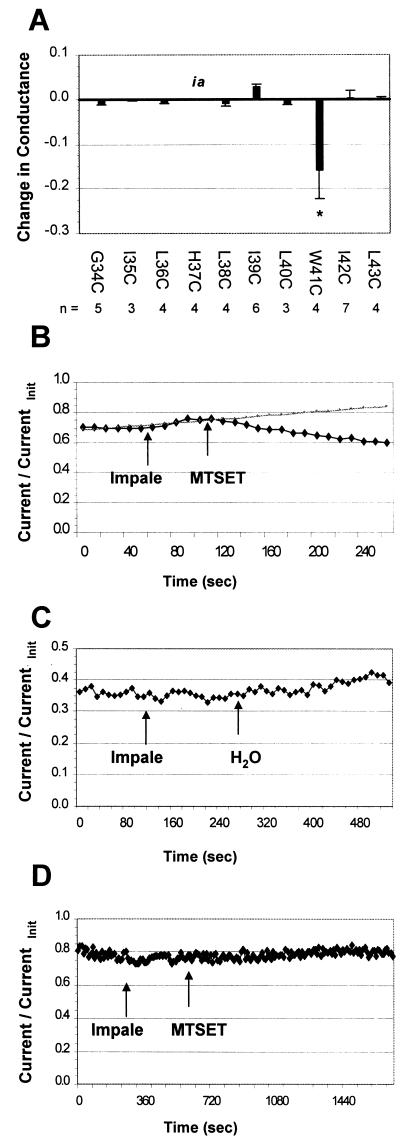
Oocytes expressing 10 individual M2 mutant proteins (M2-G34C to M2-L43C) were injected with a concentrated MTSET solution (40 mM, yielding a final cytoplasmic concentration of ca. 2 mM), and the effects on currents were measured (Fig. 4A). Only the currents of oocytes expressing the M2-W41C mutant were decreased (Fig. 4A and B), although again we were not able to test His-37 because of low activity when it was substituted with cysteine. Injection with water had no effect on observed currents (Fig. 4C), indicating that the decreased current observed with the M2-W41C mutant was a specific effect of the reagent and was not due to diminution of the leakage current, increase of oocyte volume, or other nonspecific mechanical effects. Also, note that impalement of the oocyte with the injection pipette had no effect on the stability of the recording.
FIG. 4.
Effect of intracellular MTS reagent on the conductances of M2 cysteine substitution mutants. (A) The conductance of only one mutant, M2-W41C, was decreased by intracellular application of 2 mM MTSET. The M2-H37C mutant (labeled “ia”) had activity that was too low to measure inhibition accurately in this assay. (B) M2-W41C currents were immediately decreased upon microinjection of a concentrated solution of MTSET (40 mM) into the oocyte. The trend line shown in gray is the extrapolated current in the absence of MTSET. Note that impaling the oocyte with the injection pipette while under two-electrode voltage clamp had no significant effect on the stability of the recording. (C) Mock injection with water had no effect on the observed current. (D) M2-G34C currents were not affected by microinjection of MTSET, in contrast to the inhibition observed when sulfhydryl-specific reagent was applied extracellularly (Fig. 1).
Importantly, M2-G34C was not affected by internal MTSEA, in contrast to the results obtained when the reagent was applied extracellularly (Fig. 4D; compare with Fig. 1A). Thus, these data for M2-G34C indicate that the reagent is not able to permeate through to the outer membrane region of the pore. Therefore, we did not test susceptibility of residues nearer the extracellular end of the TM domain than Gly-34 to MTS reagent applied from the cytoplasmic side of the M2 ion channel.
DISCUSSION
Extracellular application of MTS reagents inhibited currents observed from the M2-A30C and M2-G34C ion channels, whereas intracellular application inhibited currents from the M2-W41C ion channel. These data indicate that residues Ala-30, Gly-34, and Trp-41 line the aqueous pore of the M2 ion channel, results which are consistent with the M2 TM domain forming a coiled coil (Fig. 5). These results agree with conclusions drawn from circular dichroism spectroscopy studies of the M2 TM domain (4) and molecular dynamics simulations (23, 31), and in particular they confirm the identity of the residues that we predicted in a previous study to be pore lining (20).
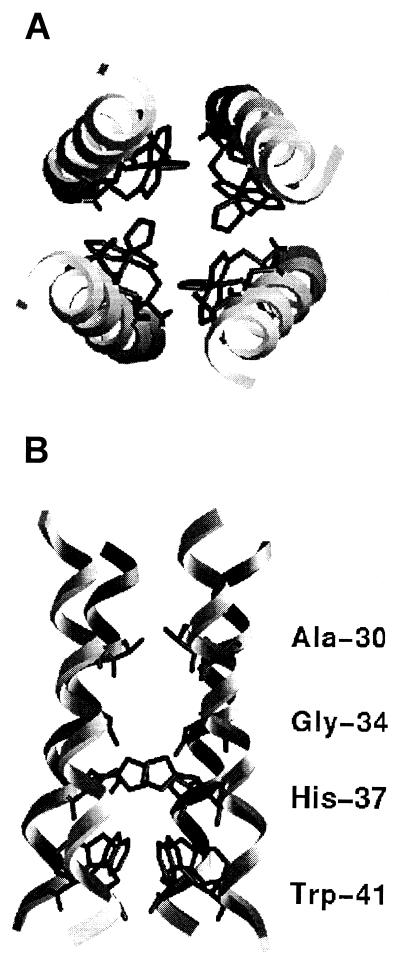
FIG. 5.
Model of the proposed TM domain of the M2 protein, showing top view as seen from the extracellular side (A) and a cross-section in the plane of the lipid bilayer (B). Residues which were identified as facing the ion-conducting aqueous pore are indicated. The model of the M2 channel is taken from that calculated in reference 20. The figure was generated using the program GRASP (18).
The finding that the M2-G34C ion channel could be inhibited only by extracellular addition of sulfhydryl reagent while the M2-W41C could be inhibited only by intracellular addition of sulfhydryl reagent indicates that the narrowest part of the pore lies between positions 34 and 41. This is consistent with our hypothesis that His-37 serves as the selectivity filter for the channel via a proton-relay mechanism (17, 20). We were not able to test the M2-H37C ion channel directly in this assay, however, as mutating this functionally critical residue to cysteine abolished channel activity. Nonetheless, a body of evidence suggests that the His-37 residue forms part of the ion-conducting pathway. This evidence includes inhibition of M2 ion channel activity by transition metals (5), mutagenesis studies, and pH titration data (28).
Influenza viruses grown in the presence of the M2-specific inhibitor amantadine acquire mutations in the TM domain of the M2 protein. These mutations occur predominantly at Leu-26, Val-27, Ala-30, Ser-31, and Gly-34 (6, 8, 9, 25). As we show here that two of these residues, Ala-30 and Gly-34, form part of the interior surface of the channel pore, the data are consistent with amantadine binding to a site within the pore of the M2 channel, in the fashion of an open pore blocker.
In our experiments, inhibition of the M2 cysteine substitution proteins by MTS reagents was measured at pH 6.2, conditions at which the M2 ion channel is activated. Therefore, the results obtained here most likely reflect the conformation of the channel in the ion-conducting state. From studies of disulfide bond formation in M2 cysteine substitution proteins, it was concluded that conformational changes may occur near the cytoplasmic end of the channel pore when the medium is changed from pH 7.5 to pH 6.2 (2). However, we could not test this region of the protein at neutral pH due to technical limitations of our assay in applying MTS reagents intracellularly.
Labeling experiments using a fluorescently conjugated MTS derivative applied extracellularly confirmed that Ala-30 is a pore-lining residue. The fact that a reagent as large as MTS-fluorescein (molecular weight of 513.54) could penetrate so deeply into the pore suggests that the pore diameter may be surprisingly large near the extracellular opening. Results concerning pore-lining residues in the external region of the protein were inconclusive, however.
One mutant ion channel with a cysteine substituted near the extracellular mouth of the pore, M2-L26C, had very low activity which could be increased upon incubation in DTT. Subsequent treatment with H2O2 partially reversed this effect. Immunoprecipitation experiments confirmed that this was due to the reduction and subsequent re-formation of intersubunit disulfide bonds between the introduced cysteines on neighboring monomers. The fact that cysteines introduced at this position can form reversible disulfide bonds very readily might suggest that Leu-26 is also a pore-lining residue, as discussed previously (2). However, alternative explanations are possible. The monomer α-helices may rotate with respect to one another, briefly exposing minor conformations in which introduced cysteines are located favorably for disulfide bond formation. When such bonds are formed, they may lock the protein in a twisted conformation that does not conduct current. It is only upon the reduction of these bonds that the protein may return to its native structure, in which case it can conduct at least a small amount of current.
Attempts to test directly the accessibility of the reduced cysteine side chain of M2-L26C to modification by sulfhydryl-specific reagents did not yield measurable changes in current, nor was this residue labeled by a fluorescently conjugated reagent. The cysteine side chain in this mutant protein may therefore be inaccessible to reagents applied in the aqueous medium. However, it is always a concern that a mutated residue may not occupy the analogous position in the structure of the mutant protein as the corresponding wild-type residue. The low activity observed from the M2-L26C ion channel could also be due to the amino acid change causing an alteration to the protein structure. Similarly, the activity observed from the M2-V27C ion channel is markedly different from that of the wild-type channel, in that although increased current is observed upon lowered pH, the channel conductance does not change, nor is the current sensitive to amantadine. Given these limitations, it may not be possible to identify either Leu-26 or Val-27 as pore lining by the substituted-cysteine accessibility method.
The results presented here confirm the current model of the M2 ion channel, in which the TM domains of each subunit adopt an α-helical conformation. Our results also agree with the details of other studies (2, 20), which locate residues Ala-30, Gly-34, His-37, and Trp-41 on the surface of the ion-conducting pore. Furthermore, our observation that an obstruction in the vicinity of His-37 prevents passage of MTS reagents introduced from either side of the membrane is consistent with the hypothesis that His-37 serves as the selectivity filter for the channel (20). These results therefore indicate that detailed biophysical measurements of the structure and activity of the M2 ion channel with methods such as site-directed spin labeling (7), solid-state nuclear magnetic resonance spectroscopy (13), and unnatural amino acid substitution (19) will yield a more detailed understanding of the function of this model ion channel.
ACKNOWLEDGMENTS
We thank Kent Baker for help in producing the model of the M2 ion channel.
This research was supported by Public Health Service research grants R37 AI-20201 (R.A.L.) and AI-31882 (L.H.P.) from the National Institute of Allergy and Infectious Diseases. K.S. was supported by National Institutes of Health Training Program in Cellular and Molecular Basis of Disease grant GM-08061. R.A.L. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arkin I T, Brunger A T. Statistical analysis of predicted transmembrane alpha-helices. Biochim Biophys Acta. 1998;1429:113–128. doi: 10.1016/s0167-4838(98)00225-8. [DOI] [PubMed] [Google Scholar]
- 2.Bauer C M, Pinto L H, Cross T A, Lamb R A. The influenza virus M2 ion channel protein: probing the structure of the transmembrane domain in intact cells by using engineered disulfide cross-linking. Virology. 1999;254:196–209. doi: 10.1006/viro.1998.9552. [DOI] [PubMed] [Google Scholar]
- 3.Chizhmakov I V, Geraghty F M, Ogden D C, Hayhurst A, Antoniou M, Hay A J. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J Physiol (London) 1996;494:329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duff K C, Kelly S M, Price N C, Bradshaw J P. The secondary structure of influenza A M2 transmembrane domain. A circular dichroism study. FEBS Lett. 1992;311:256–258. doi: 10.1016/0014-5793(92)81114-2. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi C S, Shuck K, Lear J D, Dieckmann G R, DeGrado W F, Lamb R A, Pinto L H. Cu(II) inhibition of the proton translocation machinery of the influenza A virus M2 protein. J Biol Chem. 1999;274:5474–5482. doi: 10.1074/jbc.274.9.5474. [DOI] [PubMed] [Google Scholar]
- 6.Grambas S, Bennett M S, Hay A J. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology. 1992;191:541–549. doi: 10.1016/0042-6822(92)90229-i. [DOI] [PubMed] [Google Scholar]
- 7.Gross A, Columbus L, Hideg K, Altenbach C, Hubbell W L. Structure of the KcsA potassium channel from Streptomyces lividans: a site-directed spin labeling study of the second transmembrane segment. Biochemistry. 1999;38:10324–10335. doi: 10.1021/bi990856k. [DOI] [PubMed] [Google Scholar]
- 8.Hay A J. The action of adamantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Semin Virol. 1992;3:21–30. [Google Scholar]
- 9.Hay A J, Wolstenholme A J, Skehel J J, Smith M H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helenius A. Unpacking the incoming influenza virus. Cell. 1992;69:577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- 11.Holsinger L J, Lamb R A. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991;183:32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- 12.Holsinger L J, Shaughnessy M A, Micko A, Pinto L H, Lamb R A. Analysis of the posttranslational modifications of the influenza virus M2 protein. J Virol. 1995;69:1219–1225. doi: 10.1128/jvi.69.2.1219-1225.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs F A, Cross T A. Transmembrane four-helix bundle of influenza A M2 protein channel: structural implications from helix tilt and orientation. Biophys J. 1997;73:2511–2517. doi: 10.1016/S0006-3495(97)78279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb R A, Holsinger L J, Pinto L H. The influenza A virus M2 ion channel protein and its role in the influenza virus life cycle. In: Wimmer E, editor. Receptor-mediated virus entry into cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1994. pp. 303–321. [Google Scholar]
- 15.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Press; 1996. pp. 1353–1395. [Google Scholar]
- 16.Lamb R A, Zebedee S L, Richardson C D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 17.Mould J A, Li H-C, Dudlak C S, Lear J D, Pekosz A, Lamb R A, Pinto L H. Mechanism for proton conduction of the M2 ion channel of influenza A virus. J Biol Chem. 2000;275:8592–8599. doi: 10.1074/jbc.275.12.8592. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls A, Sharp K A, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 19.Nowak M W, Gallivan J P, Silverman S K, Labarca C G, Dougherty D A, Lester H A. In vivo incorporation of unnatural amino acids into ion channels in Xenopus oocyte expression system. Methods Enzymol. 1998;293:504–529. doi: 10.1016/s0076-6879(98)93031-2. [DOI] [PubMed] [Google Scholar]
- 20.Pinto L H, Dieckmann G R, Gandhi C S, Papworth C G, Braman J, Shaughnessy M A, Lear J D, Lamb R A, DeGrado W F. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci USA. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi T, Tu Q, Pinto L H, Lamb R A. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc Natl Acad Sci USA. 1997;94:5000–5005. doi: 10.1073/pnas.94.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sansom M S, Kerr I D, Smith G R, Son H S. The influenza A virus M2 channel: a molecular modeling and simulation study. Virology. 1997;233:163–173. doi: 10.1006/viro.1997.8578. [DOI] [PubMed] [Google Scholar]
- 24.Shimbo K, Brassard D L, Lamb R A, Pinto L H. Ion selectivity and activation of the M2 ion channel of influenza virus. Biophys J. 1996;70:1335–1346. doi: 10.1016/S0006-3495(96)79690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugrue R J, Hay A J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai K K, Wang K W, Goldstein S A. MinK potassium channels are heteromultimeric complexes. J Biol Chem. 1997;272:1654–1658. doi: 10.1074/jbc.272.3.1654. [DOI] [PubMed] [Google Scholar]
- 27.Tobler K, Kelly M L, Pinto L H, Lamb R A. Effect of cytoplasmic tail truncations on the activity of the M2 ion channel of influenza A virus. J Virol. 1999;73:9695–9701. doi: 10.1128/jvi.73.12.9695-9701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Lamb R A, Pinto L H. Activation of the M2 ion channel of influenza virus: a role for the transmembrane domain histidine residue. Biophys J. 1995;69:1363–1371. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Takeuchi K, Pinto L H, Lamb R A. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zebedee S L, Lamb R A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong Q, Husslein T, Moore P B, Newns D M, Pattnaik P, Klein M L. The M2 channel of influenza A virus: a molecular dynamics study. FEBS Lett. 1998;434:265–271. doi: 10.1016/s0014-5793(98)00988-0. [DOI] [PubMed] [Google Scholar]