Abstract
Background
Microglial cell iron load and inflammatory activation are significant hallmarks of late-stage Alzheimer’s disease (AD). In vitro, microglia preferentially upregulate the iron importer, divalent metal transporter 1 (DMT1, gene name Slc11a2) in response to inflammatory stimuli, and excess iron can augment cellular inflammation, suggesting a feed-forward loop between iron import mechanisms and inflammatory signaling. However, it is not understood whether microglial iron import mechanisms directly contribute to inflammatory signaling and chronic disease in vivo. These studies determined the effects of microglial-specific knockdown of Slc11a2 on AD-related cognitive decline and microglial transcriptional phenotype.
Methods
In vitro experiments and RT-qPCR were used to assess a role for DMT1 in amyloid-β-associated inflammation. To determine the effects of microglial Slc11a2 knockdown on AD-related phenotypes in vivo, triple-transgenic Cx3cr1Cre − ERT2;Slc11a2flfl;APP/PS1+ or − mice were generated and administered corn oil or tamoxifen to induce knockdown at 5–6 months of age. Both sexes underwent behavioral analyses to assess cognition and memory (12–15 months of age). Hippocampal CD11b + microglia were magnetically isolated from female mice (15–17 months) and bulk RNA-sequencing analysis was conducted.
Results
DMT1 inhibition in vitro robustly decreased Aβ-induced inflammatory gene expression and cellular iron levels in conditions of excess iron. In vivo, Slc11a2KD APP/PS1 female, but not male, mice displayed a significant worsening of memory function in Morris water maze and a fear conditioning assay, along with significant hyperactivity compared to control WT and APP/PS1 mice. Hippocampal microglia from Slc11a2KD APP/PS1 females displayed significant increases in Enpp2, Ttr, and the iron-export gene, Slc40a1, compared to control APP/PS1 cells. Slc11a2KD cells from APP/PS1 females also exhibited decreased expression of markers associated with disease-associated microglia (DAMs), such as Apoe, Ctsb, Csf1, and Hif1α.
Conclusions
This work suggests a sex-specific role for microglial iron import gene Slc11a2 in propagating behavioral and cognitive phenotypes in the APP/PS1 model of AD. These data also highlight an association between loss of a DAM-like phenotype in microglia and cognitive deficits in Slc11a2KD APP/PS1 female mice. Overall, this work illuminates an iron-related pathway in microglia that may serve a protective role during disease and offers insight into mechanisms behind disease-related sex differences.
Keywords: microglia, iron, inflammation, DMT1, Slc11a2, Alzheimer’s disease, APP/PS1, neuroinflammation, sex differences, behavior
Background
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases and the most frequent cause of dementia. The disease is primarily characterized by the accumulation of extracellular amyloid-beta (Aβ) plaques and intraneuronal neurofibrillary tau tangles, which is thought to lead to neuronal loss and debilitating impairments in memory and cognition (1). In addition to Aβ and tau, other pathological features have been shown to contribute to AD development, including significant neuroinflammation, synaptic dysfunction, oxidative stress, and lysosomal dysfunction (2, 3). Additionally, mounting evidence demonstrates that excessive iron deposition in the brain is strongly associated with AD pathogenesis (4–6). Iron levels in the brain increase significantly with age (7, 8) and studies in patients with AD demonstrate that the degree of iron load in disease-associated brain regions (i.e., the hippocampus and frontal cortex) positively correlates with aberrant protein aggregation and severity of cognitive decline (9–11). Furthermore, iron has been found in dense core plaques and tau tangles in the brains of AD patients and mouse models (12–14), and directly binds to and exacerbates the toxicity of Aβ (15, 16). Although iron is critical for myelination, neurotransmitter synthesis, and mitochondrial metabolism in the healthy brain, excessive levels of iron can result in the harmful formation of toxic free radicals and production of reactive oxygen species (ROS), which can ultimately lead to lipid peroxidation, cellular damage, and ultimately cell death (17).
Microglial cells are the primary resident innate immune cell of the central nervous system (CNS) and play essential roles in brain development, maintenance of neural homeostasis, and response to injury and disease in the CNS. While it was been widely appreciated that microglial-mediated neuroinflammation is a key pathological hallmark of AD (18, 19), more recent work has also highlighted the prominent role microglia play in mediating brain iron dysregulation in disease (20–22). Microglia are equipped with the necessary machinery to import, store, and export and/or recycle iron (20, 23, 24). In fact, iron transport may occur preferentially in microglia compared to other cell types in the brain (25–27). Despite their high capacity to handle and store iron, microglia are particularly susceptible to iron-induced damage (28) and Ryan et al. recently demonstrated a predominant role for microglia in mediating the harmful effects of excess iron on other neural cells in a tri-culture system (29). Microglia are loaded with iron in AD and other neurodegenerative diseases, (30–34) and one of the key transcriptional changes in clusters of disease-associated microglia (DAMs) is an alteration in iron-storage genes such as Fth1 and Ftl in both humans and mice (35, 36). While microglial iron loading has been more widely recognized as a key component of AD pathology, it is still not understood how microglial iron-handling contributes to overall disease progression (5, 37, 38).
At the cellular level, an intimate relationship between microglial iron load and inflammatory signaling has been established. In a reciprocal manner, iron can enhance markers of inflammation and oxidative stress in some systems (21, 39, 40), and inflammatory signals induce the cellular uptake and storage of iron (24, 41). Specifically, microglia preferentially upregulate iron importer divalent metal transporter 1 (DMT1; gene name, Slc11a2) in response to acute inflammatory stimuli such as lipopolysaccharide (LPS) and Aβ (24, 41, 42). DMT1 is a widely-expressed proton-coupled ferrous iron (Fe2+) importer essential for life, found on both the cellular plasma membrane and endosomal membrane (43). This importer plays a role in both transferrin-bound and non-transferrin-bound iron uptake, as it mediates the immediate import of ferrous iron at the cell surface, and also transports iron reduced in the endosome into the cytosol so it can be utilized by the cell (44). Previous work has targeted DMT1 in cell culture systems resulting in a significant decrease in pro-inflammatory IL1β signaling in response to an acute stimulus of Aβ (45). Furthermore, our work showed that knocking down Slc11a2 in a short-term in vivo model of LPS-induced inflammation blunts the neural inflammatory response in male, but not female, mice (42). These results were observed in the absence of an additional iron load, suggesting a role for microglial DMT1 in helping to drive the baseline inflammatory response.
With these findings, it is intriguing to consider a role for microglial DMT1 in a disease of chronic cellular iron load and inflammation. However, to our knowledge, no studies have investigated whether targeting this microglial iron importer alters disease pathogenesis in vivo. In these studies, we generated an inducible, microglial-specific genetic knockdown of Slc11a2 in a model of AD in both male and female mice. We investigated whether microglial Slc11a2 knockdown alleviated markers of disease including microglial inflammatory and oxidative stress markers and changes in behavior and cognition.
Materials and methods
Experimental Animals
All mouse breeding, maintenance, and procedures were approved in advance and conducted in compliance with the Institutional Animal Care and Use Committee at Vanderbilt University. For the primary cell experiments from young and aged mice, young 9-week-old control C57BL/6J male mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) (#000664, JAX). C57BL/6J male mice between 27–30 months old were originally purchased from Jackson Laboratories and were aged and maintained in the Vanderbilt mouse facility. To determine the effect of decreased microglial DMT1 on disease, we generated a novel transgenic mouse model with inducible knockdown of Slc11a2 in microglial cells in the APP/PS1 model of AD. Cx3cr1Cre − ERT2 mice (B6.129P2(C)-Cx3cr1tm2.1(cre/ERT2)Jung/J; #020940) purchased from Jackson Laboratories (JAX, Bar Harbor, ME, USA) express a tamoxifen-inducible Cre-recombinase driven by the promoter for the microglial/macrophage Cx3cr1 chemokine receptor gene, allowing for conditional knockdown of loxP-containing genes in Cx3cr1-expressing cells (46). Slc11a2-’floxed’ mice (129S-Slc11a2tm2Nca/J; #017789, JAX) (47) mice were bred with Cx3cr1Cre − ERT2 homozygous mice to obtain Slc11a2flfl;Cx3cr1Cre++ homozygous animals. APP/PS1+ hemizygous animals were purchased from JAX and maintained in our facility (Tg(APPswe,PSEN1dE9)85Dbo; MMRRC_034832-JAX). These transgenic animals express a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant presenilin-1 (PS1-dE9), and have been widely used in AD research, particularly in relation to amyloid-β associated pathology (48–50). We chose this model of AD based on the well-characterized development of disease-associated symptoms (i.e., amyloid deposition, cognitive deficits) and the progressive nature of disease development over the course of several months. This slower onset compared to other models allows us to examine the early pathological changes that occur prior to the onset of symptoms later in the course of disease. Additionally, the APP/PS1 model has already been shown to exhibit significant microglial iron loading (21, 32), and an amyloid-driven model is relevant based on associations between iron and Aβ in the brain (15, 51). APP/PS1+ hemizygous animals were bred separately with Slc11a2flfl animals to yield Slc11a2flfl;APP/PS1+ mice. Resulting progeny from these crosses were then bred with Slc11a2flfl;Cx3cr1Cre − ERT2++ animals to yield triple-transgenic Slc11a2flfl;Cx3cr1Cre − ERT2+/−;APP/PS1+ or APP/PS1− (i.e., ‘WT’) mice (Additional File 1: Fig. S1A). All mice used in experiments were Slc11a2flfl;Cx3cr1Cre − ERT2+/− and either APP/PS1+ hemizygotes or WT as littermate controls. Experimental mice were on a mixed 129S/BL6 background, with > 80% BL/6J genetic makeup. All genotypes were confirmed with an ear snip via Transnetyx (Cordova, TN) using real-time PCR. Mice were weaned at 3 weeks of age and had ad libitum access to food (LabDiets, standard rodent chow 5001, 240 ppm iron) and water. Both male and female mice were used in our experiments and were group-housed (2–5 per cage) by sex in transparent cages at 22–25°C under a 12 h light/dark cycle in a specific pathogen-free facility. Control and experimental animals were randomly assigned across cages.
Tamoxifen Treatment
Tamoxifen (Sigma #T5648) was dissolved in corn oil (Sigma #C8267–2.5L, lot #MKCK6411, Saint Louis, MO) to generate a 20 mg/mL stock concentration by sonicating the mixture and stirring overnight in a glass vial at 37°C. Slc11a2flfl;Cx3cr1Cre − ERT2+/−;APP/PS1+ or − male and female mice at 5–6 months of age were administered a dose of 4 mg (maximum 200 μL volume) tamoxifen via oral gavage every day for five consecutive days (42, 52) (Additional File 1: Fig. S1B). All mice that received tamoxifen are denoted as ‘Slc11a2KD’, and are either APP/PS1 or WT. Littermate mice with the same genotypes (Slc11a2flfl;Cx3cr1Cre − ERT2+/−;APP/PS1+ or −) were administered gavage with corn oil as a control for the presence of Cre based on work showing effects of Cx3cr1-CreERT2 genotype alone on microglial function (53, 54). Corn-oil-treated animals are denoted as ‘Control,’ and are either APP/PS1 + or WT. The numbers of experimental animals used are shown in Supplemental Table 1. We chose to induce knockdown of Slc11a2 between 5–6 months of age in these mice, as it is a relatively early timepoint in this AD model when Aβ plaque deposition becomes visible and allowed us to assess the effect of early changes in microglial Slc11a2 on downstream disease development. Knockdown of Slc11a2 was confirmed in isolated microglia from all animals via RT-qPCR utilizing a primer targeting Slc11a2 exons 6–8 (Additional File 1: Fig. S1C).
Behavioral assays
All behavioral assays were conducted in the Vanderbilt Murine Neurobehavioral Core after mice were acclimated to the facility for at least one to two weeks. All mice underwent testing by two experimenters between 12–15 months of age (mouse numbers and weights shown in Additional File 2: Table S1, S2). The running order of assays was kept consistent for all animals in each study, and animals were run each day between 0630–1300 h with one task per day. For each task, mice were acclimated to the testing room for 30 min to 1 h prior to testing, and control and experimental groups were evenly and randomly distributed across cages, days, and time of each assay. Following completion of a trial, each apparatus was cleaned of feces, disinfected, and deodorized with an anti-bacterial spray (Peroxigard, Virox Technologies) in between animals. APP/PS1+ mice are known to be prone to spontaneous seizures (55) and any mouse that exhibited a seizure during an assay was excluded from that analysis (n = 3 male Slc11a2KD;APP/PS1+).
Nest building
As a measurement of general cognition and well-being, an overnight nest building assay was used. Nest building assessments were performed as the first behavioral task to minimize effects of stress on the mice from other behavioral assays. Mice were single-housed and given 5 g of cotton nestlet (Ancare, Bellmore, NY) in the afternoon the day prior. The next morning, amount shredded and quality of nests was scored by a blinded observer using a 0–5 scale adapted from previous work, in 0.5 increments (42, 56, 57). Following nest building assessment, mice were re-housed in groups of 4–5 before all other behavioral tasks.
Locomotor activity: Elevated Zero Maze and Open Field
For locomotor activity assessment, several assays were used. An elevated zero maze (white maze, width 5 cm; diameter 50 cm; wall height 15 cm, Stoelting Co. IL) was used first, where mice underwent a single 5 min trial of free exploration. Mice were video-recorded using a ceiling-mounted camera and movement was automatically tracked and scored using AnyMaze (Stoelting Co., Wood Dale, IL). Analysis parameters were set to ensure 80% of the mouse needed to be present in either the ‘open’ or ‘closed’ zone for an entry into that zone to be recorded. Total time in the open and closed zones and total distance traveled were measured. Sound-attenuating transparent open field chambers (27.5 × 27.5 cm) were used for a second measurement of baseline locomotor activity. Mice were placed in the center of the chamber and allowed to explore freely for 45 min. Distance traveled was recorded automatically via the breaking of infrared beams (MedAssociates ENV-510 software, Fairfax, VT). Additionally, time spent in the center area (19.05 × 19.05 cm) versus time in the ‘surround’ was calculated as a control measure of anxiety-like behavior.
Short-term spatial working memory
A single-trial Y-maze was used as another measurement of baseline locomotor and exploratory behavior, as well as an assay to measure short-term working memory function. A clear plexiglass three-arm Y-maze (each arm 5 cm in width, 34.5 cm long) with differentiated arms (different colors of paper with or without patterns placed underneath the maze) was used. All mice were placed in the same point of the same arm and allowed to freely explore for 6 min. A ceiling-mounted camera recorded video of the mice and AnyMaze automatically measured total distance traveled and order of arm entries. Entry into another arm was predicated on having at least 80% of the mouse cross into at least 1 cm of the arm. Spontaneous alternation as a measure of intact working memory was calculated by hand using arm entry order data from AnyMaze. A ‘correct’ alternation is defined by three consecutive entries into three different arms (e.g., ABC, BCA, CAB). Percent alternation was calculated using: ((Number of spontaneous alternations) / (Number of total arm entries – 2)) * 100.
Morris water maze
Mice underwent testing in the Morris water maze (MWM) to assess the effect of Slc11a2 knockdown on learning and memory (58). Briefly, a circular pool approximately 1 m in diameter filled approximately 30 cm deep with 22–27°C water was used for this task. A white round platform (10 cm in diameter) was used to provide animals an escape from the water. Mice first underwent two visual training days, where the platform jutted above the water with a pole attached to allow mice to see the target platform. This platform was moved around to each of the four quadrants on each session during training days to allow the opportunity for each animal to swim and survey the room, which contained multiple visual spatial cues kept constant throughout. Each training day comprised four trials per mouse, and each mouse was given 60 sec to find the platform. If a mouse did not reach the platform in 60 sec, it was guided to and placed on the platform for at least 5 sec. On subsequent days following the two visual training days, the water was made opaque with non-toxic tempura white paint, and the platform was submerged approximately 0.5 cm under the water. The platform was kept in the same location for each trial and day, and mice were randomly placed in different locations in the pool so that the use of spatial cues for navigation was necessitated. Mice underwent four trials per day for five days, with each trial lasting 60 sec to assess learning and short-term memory. If mice did not find the platform within 60 sec, they were guided to the platform and escape latency was recorded as 60 sec. Following the final day of testing, the platform was removed and mice were allowed to swim freely for 60 sec. Total time spent in the target quadrant where the platform used to be, time spent around the location of the platform, swim speed, total distance traveled, and time spent in perimeter were recorded as measurements of platform location memory.
Fear conditioning assay
Following completion of all other behavioral tasks, a fear conditioning assay was conducted to assess differences in fear-associated memory. Mice were placed in sound-attenuating chambers with a wire grid floor. On the first day (training trial), mice were placed in the chambers for 8 min and allowed to explore freely. Every 2 min, a 30 sec tone was played, followed immediately by a small shock administered through the wire floor (1 sec, 0.5 mA). This tone-shock pairing occurred 3 times during the training trial. To assess contextual fear conditioning, mice were placed back into the same chamber the next day and allowed to run around freely for 4 min with no tone or shock presented. Total time freezing – indicative of fear memory – was recorded automatically (VideoFreeze, MedAssociates). To assess cued fear conditioning (memory of the tone), mice underwent a second testing trial. This trial included a different experimenter handling the mice, significant alterations to the chamber with white walls, white floor inserts, and red light, and the scent of vanilla placed in an open tube outside the chamber. Mice freely explored the chamber for 2 min before the tone was administered for the final 2 min (without a shock pairing). Total time spent freezing during the no-tone and tone segments were recorded as a measurement of cued fear memory.
Mouse euthanasia and tissue collection
At the time of euthanasia, mice were deeply anesthetized with isoflurane and 500–700 μL of blood was collected via cardiac puncture. Immediately following blood collection, mice were trans-cardially perfused with 20 mL of cold 1x Dulbecco’s phosphate-buffered saline (DPBS) to remove circulating blood and decapitated for rapid brain removal. Whole brains were either placed on ice for mincing and processing for cellular isolation, or bilateral hippocampus was isolated first before proceeding to cellular isolation.
Tissue digestion and single-cell suspension preparation
Brains were rapidly removed and Briefly placed in 3 mL cold, sterile 1x Hank’s buffered saline solution (HBSS, Gibco, #14175095) containing 1% fetal bovine serum (FBS, heat-inactivated; Gibco, #10082147) to remove any residual blood. Microglia isolation was performed following published protocols, with slight modifications (59–61). Briefly, whole brains were transferred and finely minced with scissors in cold, sterile “IMG media” [Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4.5 g/L) and L-glutamine media (Gibco, #11965092) containing 10% FBS and 1% penicillin-streptomycin (Gibco, #15140122). Minced tissue was transferred into 50 mL conical tubes and 5 mL of digestion media (IMG media + 100 units Papain, #LK003176; 500 Kunitz units DNase, #LK003170, Worthington Biochemicals, Lakewood, NJ) was added to each tube. Whole-brain samples were enzymatically-dissociated by placing in an orbital shaker for 1 h at 37°C, diluted with 10 mL IMG media, and strained through 70 μm sterile filters (Corning, #431751). Bilateral hippocampus samples in the RNA-sequencing studies were treated in the same manner described above, with slight modification. Bilateral hippocampus samples were isolated and immediately placed in 15 mL conical tubes on ice and 2.5 mL digestion media was added. Samples were incubated for 30 min in an orbital shaker at 37°C. Every 10 min, samples were triturated up and down with a serological pipette of decreasing size before straining samples through filters and proceeding with subsequent steps. All samples were further processed at 4°C unless otherwise indicated.
Percoll gradient
Cells were centrifuged for 5 min at 500 × g, re-suspended in a solution of 30% isotonic Percoll and IMG media (Cytiva, #17-0891-01), and slowly layered onto a 70% Percoll gradient with HBSS + 1% FBS. HBSS + 1% FBS (without Percoll) was layered on top, and samples were centrifuged for 15 min at room temperature at 600 × g with the brake set to the lowest setting to allow for density separation. The supernatant containing myelin and neuronal debris was removed, and cells at the interface between the 30–70% gradients were carefully collected and placed on ice into 8 mL HBSS + 1% FBS in a fresh tube to wash residual Percoll. Cells were centrifuged at 500 × g for 5 min at 4°C, and pelleted cells were re-suspended in appropriate media for downstream assays.
Plating and treatment for primary cell experiments
For experiments conducted in isolated primary glial cells from young and aged mice, all steps above were performed under sterile conditions in a cell culture hood with autoclaved tools and sterile-filtered reagents. Glial cells isolated and pelleted from the Percoll gradient were re-suspended in 1 mL IMG media for counting and plating. Cells were counted using the Nexcelom Cellometer Auto T4 Cell Counter (Nexcelom Biosciences) and plated at a density of 100,000 cells per well in poly-L-lysine-coated 48-well plates in pre-warmed sterile IMG media containing 5 ng/mL GM-CSF (R&D Systems, #415-ML-010). Media was changed the next day, and then every other day for five days before stimulation with Aβ as described below.
CD11b immunomagnetic microglial isolation
For experiments analyzing gene expression (RNA sequencing and RT-qPCR) in microglia, Percoll-isolated glial samples were further processed for enrichment of CD11b+ microglia. Following Percoll gradient separation, centrifugation, and pelleting, cells were re-suspended in 400 μL cold “MACS” buffer (1x PBS containing 0.5% FBS and 2 mM EDTA) and transferred to 5 mL tubes. Cells were centrifuged at 4°C for 5 min at 500 × g, pelleted, and re-suspended in 90 μL MACS buffer for magnetic labeling and separation according to manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, samples were incubated with magnetic anti-CD11b MicroBeads (Miltenyi Biotec, #130-093-634; 10 μl per 90 μl buffer/brain) for 15 min at 4°C. Magnetic separation was performed utilizing MS columns, and CD11b+ cells and the effluent non-magnetic fractions (CD11b− cells) were obtained. Following a final centrifugation for 5 min at 500 × g, cells were immediately re-suspended in RLT lysis buffer (Qiagen, #74004) supplemented with 1% beta-mercaptoethanol, Briefly vortexed, and flash-frozen in liquid nitrogen. Samples were stored at −80°C until RNA isolation.
In vitro cells and experimental treatments
The immortalized microglial cell line, “IMG” (62), was used for in vitro experiments to assess the effect of pharmacological inhibition of DMT1 on Aβ-induced inflammation. IMG cells were purchased from Millipore (Cat. #SCC134, RRID:CVCL_HC49), and cultured as described using Accutase for dissociation and passaging (45). Briefly, cells were cultured up to a maximum of 10 passages in sterile Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4.5 g/L) + 2.5 mM glutamine (Gibco, #11965092) supplemented with 10% fetal bovine serum (FBS, heat-inactivated, Gibco, #16140071) and 1% penicillin/streptomycin (“IMG media”).
Ebselen treatments
The drug ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one] was chosen as a robust inhibitor of DMT1 (63). Ebselen was purchased from Focus Biomolecules (#10–2288) and re-suspended in sterile dimethyl sulfoxide (DMSO; Sigma, #276855). IMG cells were plated and grown overnight in six-well-plates (150,000–200,000 cells/well) in IMG media. The next day, cells were treated for 24 h with either 25 μM ebselen or control DMSO. This concentration of ebselen was chosen as the treatment dose following preliminary experiments indicating this dose decreased cellular iron content and following similar reported doses from previous work (64). Following 24 h of ebselen/DMSO treatment, cells were further treated as described below.
Amyloid-β and iron treatments
In both IMG cells and primary isolated glia in the young and aged mice experiments, amyloid-β1–42 was used as an acute AD-associated inflammatory stimulus. Aβ (HFIP-treated, rPeptide #A-1163–2) and scrambled Aβ (rPeptide #A-1004–2) were purchased from rPeptide and 5 mM stock solutions were prepared with sterile, anhydrous DMSO (Sigma #276855) and sonicated for 15 min before storing aliquots at −20°C. The day before cell stimulation, oligomeric Aβ1–42 was prepared as previously described (39) using cold, sterile phenol-free Ham’s F-12 media (R&D Systems, #M25350) and allowed to rest at 4°C for 24 h. The next day, cells were treated with 1 μM Aβ1–42 or scrambled Aβ for 24 h before lysis and collection for RNA isolation. For in vitro experiments in IMG cells, ferric ammonium citrate (FAC, Sigma, #F5879) was used as a non-transferrin-bound form of iron. FAC was re-suspended fresh in sterile RNase-free water immediately before each experiment, and cells were treated for 24 h with 50 μM FAC based on literature recommendations (39, 65) or water (control), with or without Aβ prior to lysis and collection for RNA isolation or ICP-MS, as described below.
Inductively-coupled Plasma Mass Spectrometry (ICP-MS)
Following 24 h of treatment with scrambled Aβ or 1 μm Aβ1–42 ± FAC, IMG cells were collected for ICP-MS analysis of intracellular iron content. After washing twice with ice-cold 1x PBS, cells were collected into metal-free tubes using Accutase, and total cell counts were measured for data normalization. After centrifugation at 600×g for 5 min and removal of supernatant, cells were acid-digested in 150 μL trace-metal grade nitric acid (70%, OPTIMA Grade HNO3, Fisher-Sci, #A467–250), and 30% ultra trace-grade hydrogen peroxide (Thermofisher) was added at a 1:4 dilution (37.5 μL H2O2). Samples were vortexed, incubated at 65°C overnight, and diluted the next day with Ultrapure Milli-Q water (Ω18.2) at 10 times the volume of nitric acid (1.5 mL water). ICP-MS was performed at the Vanderbilt Mass Spectrometry Research Center using an Agilent 7700 ICP-MS (Agilent) attached to a Teledyne autosampler (CETAC Technologies, Omaha, NE). The following settings were used: cell entrance = −40V, cell exit = −60V, plate bias = −60V, OctP bias = −18V, and collision and cell helium flow = 4.5 mL/min. Samples were introduced by peristaltic pump and taken up at 0.5 rps for 30 s, followed by 30 s at 0.1 rps for signal stabilization. A calibration curve for each isotope was made at 0, 1, 10, 100, 1000, 5000, and 10,000 ppb, and blanks were run following standard calibration to wash out signal from the 10,000 ppb standard. Data were acquired and analyzed using the Agilent Mass Hunter Workstation Software version A.01.02.
RNA isolation, cDNA synthesis, and RT-qPCR
Lysed cell samples from all experiments (i.e., CD11b + microglia and primary isolated glia) were processed for total mRNA using an RNeasy Micro Kit with DNase treatment according to manufacturer’s instructions (Qiagen, Hilden, Germany, #74004), with the exception of the IMG experiments, which used the RNeasy Mini Kit (Qiagen, # 74104). Following on-column RNA purification and elution, cellular RNA was reverse transcribed into cDNA at equal concentrations across samples using iScript Reverse Transcriptase (BioRad, Hercules, CA). RT-qPCR was conducted to assess the expression of several genes and confirm Slc11a2 knockdown using FAM-conjugated TaqMan Gene Expression Assay primers (Thermofisher, shown in Additional File 3: Table S3) and iQ Supermix (BioRad). PCR reactions were performed in duplicate under thermal conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 45 s. The expression of each gene measured was normalized to a housekeeping gene (either 18S or ActinB where indicated), and relative expression values were analyzed utilizing the comparative cycle threshold 2−ΔΔCT method (66).
RNA sequencing and library preparation
Following on-column purification and DNase treatment with the Qiagen RNeasy Micro Kit, total mRNA extracted from hippocampal CD11b+ samples was submitted to the Vanderbilt Technologies for Advanced Genomics (VANTAGE) Core facility for sample quality control assessment and RNA sequencing (RNA-seq). Only hippocampal CD11b+ microglia isolated from female animals were used for RNA-seq, following earlier findings of significant behavioral differences primarily in Slc11a2KD female APP/PS1 animals. The concentration of RNA samples was determined by NanoDrop (ThermoScientific). Sample Quality Control analysis was assessed using fluorometry Qubit and integrity by BioAnalyzer, and a RIN value of > 7 was confirmed for all samples before proceeding to library preparation and sequencing. Paired-end sequencing libraries were constructed using a standard mRNA NEBNext Poly(A) selection Library Prep Kit (Illumina). Library Quality Control analysis was performed by using Qubit and BioAnalyzer to determine the concentration and size bp. Samples were then sequenced at multiplex Paired-End 150 bp using the Illumina NovaSeq 6000 sequencing platform. To confirm sequencing quality, Illumina Quality Scores were calculated utilizing the following equation: Q = −10log10I. All samples sequenced reached sequencing quality of at least Q(30).
Sequencing analysis: alignment, mapping, quantification, differential expression
Gene alignment, read mapping, gene counts quantification, and differential gene expression analyses were conducted at the Creative Data Solutions (CDS) Core at Vanderbilt. RNA-seq reads were adapter-trimmed and quality-filtered using Trimgalore v0.6.7 (67) and Cutadapt 1.18 (68) to remove adapter sequences and pairs that were either shorter than 20 bp or that had Phred scores less than 20. An alignment reference was generated from the mm39 mouse genome and GENCODE comprehensive gene annotations (M31), to which trimmed reads were aligned and counted using Spliced Transcripts Alignment to a Reference (STAR) v2.7.9a (69) with the –quantMode GeneCounts parameter. About 30–50 million uniquely mapped reads were acquired per sample. DESeq2 package v1.36.0 (70) was used to perform sample-level quality control, low count filtering, normalization and downstream differential gene expression analysis. Genomic features counted fewer than five times across at least three samples were removed. The measure of standard deviation (sd) and quantiles on principal component 1 (PC1) among samples was used to assess whether any samples were a statistical outlier. One sample in the Control APP/PS1 group was removed from analyses after exhibiting a deviation of > 2 standard deviations and an interquartile range of > 1.5 in PC1 compared to its respective group (sample shown in Additional File 4: Fig. S2B and C). Five to six biological replicates per condition were included for the differential expression analysis. Differentially expressed genes were identified using a false discovery rate (FDR) adjusted p-value threshold of 0.05, calculated using the Benjamini-Hochberg (BH) procedure for multiple hypothesis testing correction, and a log2 fold change threshold of greater than 1. Gene set enrichment analysis (GSEA) (71) was performed using the R package Clusterprofiler (72) with gene sets from the Mouse MSigDB database (73). Coverage of reads across annotated exons in the Slc11a2 gene analysis was done using the R package ggcoverage 1.3.0 (74). All data processing was performed at the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University.
Data and statistical analyses
Data are presented as mean ± S.E.M. All experiments were analyzed using analysis of variance (ANOVA) for multiple comparisons followed by appropriate post-hoc analyses unless otherwise noted. Male and female data were first compared using ANOVA (2(Sex) × 2(Genotype) × 2(Treatment), followed by Sidak’s corrections for multiple comparisons and analysis of interaction effects. Based on our previous work showing sex differences in Slc11a2 expression between males and females, most primary analyses were conducted within each sex separately to assess the effect of Slc11a2 knockdown in each sex. To do this, a 2(Genotype) × 2(Treatment) ANOVA followed by Sidak’s corrections was used. In analyzing MWM data, repeated measures ANOVA (2(Knockdown) × 2(APP/PS1 Genotype) × 5(Day)) was used to analyze latency data from multiple training days and Tukey’s post-hoc analysis was used following significant F values to establish differences among all groups. Data from primary cell and IMG cell experiments were analyzed using either 2(Treatment) × 2(Age) ANOVA or 3(Treatment) × 2(ebselen/DMSO) ANOVA, respectively. Sidak’s post-hoc analysis was used for interaction effects and corrections for multiple comparisons. Statistical outliers within each group for all studies were identified using either the ROUT or Grubb’s method for outliers and excluded from statistical analyses. GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses outside of RNA-seq analyses conducted in R. Differences among groups were considered significant at values of p < 0.05.
Results
Age and Aβ stimulation synergize to increase microglial Slc11a2 and iron loading markers in primary microglia.
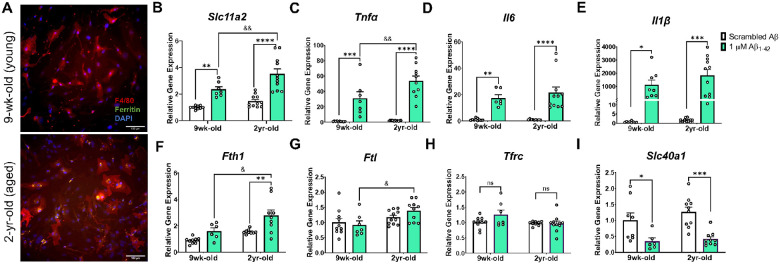
To assess a potential role for microglial iron and Slc11a2 in aging and amyloid-related pathology, we isolated glia from young and aged mice for primary cell in vitro experiments. We first observed significant ferritin (FtL) protein deposits in cells isolated from aged compared to young mice (Fig. 1A), demonstrating, as others have shown, a key iron-loading microglial phenotype in aging (38, 75). To determine whether Slc11a2 contributes to this age-associated increase in iron and whether the transporter gene plays a role in amyloid-related disease conditions, isolated microglia from young and aged mice were treated in vitro with an acute stimulus of 1 μM oligomeric Aβ for 24 h and gene expression of Slc11a2 was measured. As others have also shown (24, 45), there was a significant increase in microglial Slc11a2 in response to acute Aβ exposure (Fig. 1B, Treatment, F(1, 35) = 48.91, p < 0.0001). Additionally, glia from the two-year-old aged mice exhibited an augmented Aβ-induced Slc11a2 response, which was significantly greater than the response observed in the cells from young mice (Age, F(1, 35) = 11.21, p = 0.002; young vs. old Aβ, p = 0.005). In addition, there was a robust increase in pro-inflammatory cytokines Tnfα, Il1β, and Il6 in response to Aβ (Fig. 1C–E, Il6: Treatment, F(1, 32) = 41.20, p < 0.0001; Il1β: F(1, 34) = 24.23, p < 0.0001; Tnfα: F(1, 34) = 77.83, p < 0.0001), which was even greater in the glia from the aged mice compared to those isolated from the young mice (significant for Tnfα: Age, F(1, 34) = 6.52, p = 0.015, Interaction F(1, 34) = 5.57, p = 0.024; young vs. aged Aβ p = 0.005). Along with differences in Aβ-induced Slc11a2 gene levels in the glia from the aged mice, there was a significant increase in iron-storage genes Ftl and Fth1 in response to Aβ only in the cells from the aged animals (Fig. 1F–G). Specifically, Aβ induced an increase in Fth1 in the aged glia (Age, F(1, 29) = 12.46, p = 0.001, Treatment, F(1, 29) = 13.67, p = 0.0009), and Fth1 and Ftl were significantly higher in response to Aβ in the aged cells when compared to the young cells (Fth1, young vs. aged, p = 0.01; Ftl: Age, F(1, 34) = 7.92, p = 0.008, young vs. aged, p = 0.02). There were no differences in Tfrc gene expression – another main iron importer gene – due to age or Aβ treatment (Fig. 1H, p > 0.05), suggesting that a specific gene expression increase in Slc11a2 may accompany age- and Aβ-related changes in cellular iron and inflammatory status. Aβ also decreased Slc40a1 levels (gene for ferroportin, main iron exporter) to a similar degree in the cells from the young and aged animals (Fig. 1I, Treatment, F(1, 30) = 23.40, p < 0.0001), further suggesting that a specific alteration in Slc11a2 in response to age and amyloid may be involved in the progression of disease.
Figure 1.
Age and Aβ stimulation synergize to increase microglial Slc11a2 and iron-loading markers in primary microglia.
A) Representative images of Percoll-isolated glia from young (top image, 9-week-old) and aged (bottom image, 2-year-old) mouse showing ferritin deposits in microglia from the aged mouse. Isolated glia were stained with antibodies raised against ferritin-L and F4/80, along with DAPI to visualize ferritin, microglia, and nuclei, respectively. Images shown at 20x, scale bar = 100 μm. B-I) Relative gene expression (compared to control scrambled Aβ) of B Slc11a2, C Tnfα, D Il6, E Il1β, F Fth1, G Ftl, H Tfrc, and I Slc40a1 via RT-qPCR. Isolated cells from young and aged mice were plated and treated with scrambled Aβ or 1μM Aβ1–42 for 24 h before collection for RNA isolation and RT-qPCR analysis. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 effect of treatment. &p<0.05, &&p<0.01 effect of age × treatment. ns = not significant. Data represent the mean ± S.E.M of 7–11 mice per group. Statistical outliers were removed using the Grubb’s test.
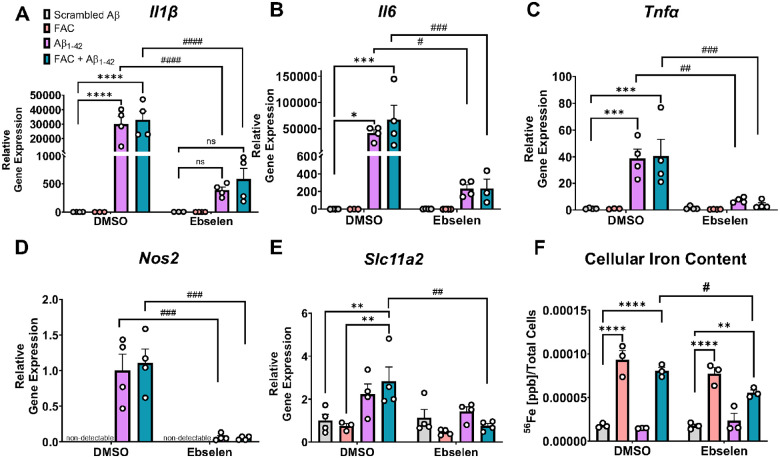
DMT1 inhibition in vitro significantly blunts Aβ-induced inflammation and decreases cellular iron levels in immortalized microglia.
Based on the purported roles for DMT1 in Aβ stimulation and iron load, we characterized the effect of inhibiting DMT1 on Aβ and iron-induced inflammation in an in vitro system. Cells from the murine immortalized microglial cell line, “IMG” cells (62), were treated with ebselen, a pharmacological inhibitor of DMT1 (63), before subsequent treatment with scrambled Aβ, oligomeric Aβ1–42 alone, or iron (50 μM FAC) + Aβ1–42. Aβ stimulation leads to a robust increase in microglial pro-inflammatory Il1β, Il6, Tnfα, and Nos2 transcription, as expected (Fig. 2A–D, Il1β: Treatment, F(3, 22) = 16.78, p < 0.0001; Il6: Treatment, F(3, 23) = 5.28, p = 0.006; Tnfα: Treatment, F(3, 23) = 10.89, p = 0.0001; Nos2: Treatment, F(3, 23) = 16.31, p < 0.0001). Addition of 50 μM FAC did not have a significant effect on Aβ-induced inflammatory markers. Ebselen profoundly decreased the Aβ-induced pro-inflammatory cytokine response for all three cytokines assayed along with Nos2, even in the absence of excess iron added to the media (Aβ alone condition) (Fig. 2A–D, Il1β: Interaction, F(3, 22) = 15.76, p < 0.0001; Il6: Interaction, F(3, 23) = 4.81, p = 0.0096; Tnfα: Interaction, F(3, 23) = 6.89, p = 0.0018; Nos2: Interaction, F(3, 23) = 13.18, p < 0.0001). Aβ induced a significant upregulation in Slc11a2 and ebselen inhibited this increase when a bolus of FAC was added as well (Fig. 2E, Treatment, F(3, 23) = 5.07, p = 0.008, Interaction, F(3, 23) = 3.49, p = 0.032). This was paralleled by a change in total intracellular iron levels as measured via ICP-MS, where ebselen significantly decreased cellular iron levels in the FAC + Aβ1–42 condition (Fig. 2F, Treatment, F(3, 16) = 72.53, p < 0.0001, Ebselen, F(1, 16) = 4.15, p = 0.058, Interaction, F(3, 16) = 3.52, p < 0.05). These data demonstrate associations between DMT1 inhibition, decreases in cellular iron levels, and blunted Aβ-induced pro-inflammatory responses in IMG cells.
Figure 2.
DMT1 inhibition in vitro significantly blunts Aβ-induced inflammation and decreases cellular iron levels in immortalized microglia.
A-E) Relative gene expression (compared to scrambled Aβ DMSO) via RT-qPCR of A Il1β, B Il6, C Tnfα, D Nos2, and E Slc11a2 in IMG cells. IMG cells were treated for 24 h with DMSO or 25 μM ebselen, followed by 24 h treatment with scrambled Aβ or 1 μM Aβ1–42 ± 50 μM ferric ammonium citrate (FAC). F) ICP-MS analysis of intracellular 56Fe content from IMG cells following 24 h treatment with DMSO or ebselen, and 24 h scrambled Aβ ± FAC or Aβ1–42 ± FAC treatment. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 effect of Aβ or FAC treatment. #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 effect of treatment × ebselen. ns = not significant. Data show a representative experiment with the mean ± S.E.M of 3–4 technical replicates, and experiment was repeated three times. Statistical outliers were removed using the Grubb’s test.
Microglial Slc11a2 knockdown results in a hyperactive phenotype in female APP/PS1 mice and worsens hyperactivity in male APP/PS1 mice at 12–15 months.
To determine the effects of knocking down Slc11a2 in vivo, we generated a transgenic mouse line allowing for inducible knockdown of Slc11a2 in microglia between 5–6 months of age. Between 7–9 months after tamoxifen treatment, when mice were 12–15 months of age, male and female control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 mice were run through a series of behavioral assays to assess the effect of microglial Slc11a2 knockdown on aspects of behavior and cognition.
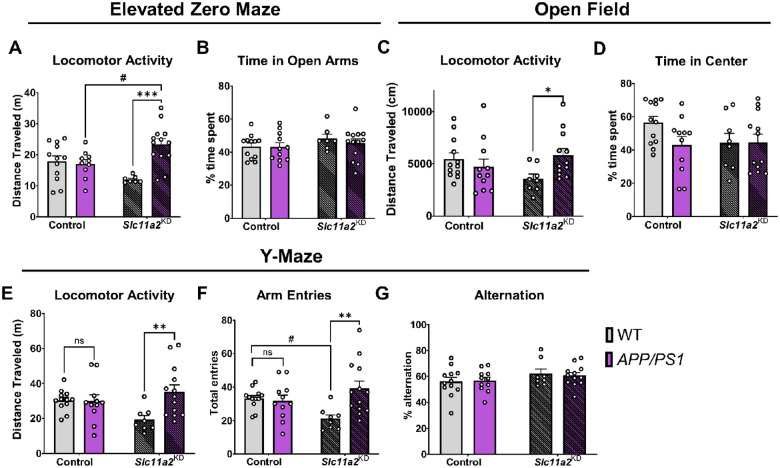
First, to assess locomotor activity, mice were tested in elevated zero maze (EZ maze, 5 min), open field chambers (45 min), and one-trial spontaneous alternation Y-maze tests (6 min) and total distance traveled was measured in each. In females, control APP/PS1 mice did not exhibit differences in baseline locomotor activity compared to control WT female mice in any of the assays tested (Fig. 3A–F; p > 0.05). However, microglial Slc11a2KD female APP/PS1 animals exhibited a significant increase in distance traveled in all three activity measurement assays compared to their non-APP/PS1 counterparts (Fig. 3A, C, E, F; activity measurements, EZ maze: APP/PS1, F(1, 38) = 9.28, p = 0.004, Interaction effect, F(1, 38) = 12.29, p = 0.001; open field: Interaction, F(1, 39) = 5.36, p = 0.03; Y-maze activity: APP/PS1, F(1, 40) = 5.23, p = 0.03, Interaction, F(1, 40) = 5.92, p = 0.02; arm entries in Y-maze: APP/PS1, F(1, 40) = 5.76, p = 0.02, Interaction, F(1, 40) = 7.93, p = 0.008). As control measurements to assess for anxiety-like behavior, the amount of time spent in the open arms of the EZ maze (Fig. 3B, p > 0.05) or in the center area of the open field chambers were not significantly different (Fig. 3D, p > 0.05). Additionally, there were no significant differences in Y-maze spontaneous alternation capacity between any groups (Fig. 3G, p > 0.05).
Figure 3.
Microglial Slc11a2 knockdown results in a hyperactive phenotype in female APP/PS1 mice at 12–15 months.
A-B) Elevated zero maze. A Total distance traveled (m) in control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 female mice. B Total percent time spent in open arms. C-D) Open field locomotor activity assay. C Total distance traveled (cm). D Total percent time spent in the center. E-G) Exploratory Y-maze. E Total distance traveled (m). F Total number of different arm entries. G Total percent alternation. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001 effect of APP/PS1 genotype, #p<0.05 Slc11a2KD vs. Control. ns = not significant. Data represent the mean ± S.E.M of 8–13 female mice per group. Statistical outliers were removed using the Grubb’s test.
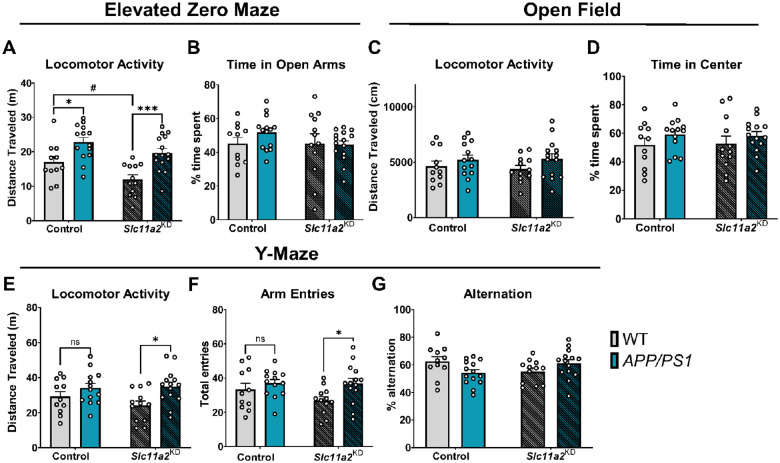
Male APP/PS1 mice exhibited a significant increase in activity in the EZ maze compared to WT controls (Fig. 4A; EZ Maze: APP/PS1 effect, F(1, 48) = 22.61, p < 0.0001). There was a significant main effect of Slc11a2 knockdown on activity in the EZ maze in males (Knockdown effect, F(1, 48) = 8.18, p = 0.0063), and post-hoc analyses revealed that Slc11a2 knockdown had a greater effect on the hyperactive phenotypes observed in the APP/PS1 males compared to corresponding controls (Fig. 4A, EZ maze: Control vs. APP/PS1, p = 0.013, Slc11a2KD Control vs. Slc11a2KD APP/PS1, p = 0.0006). This was observed in the absence of a significant anxiety-like phenotype, with no difference in time spent in the open arms of the EZ maze (Fig. 4B). Male APP/PS1 mice did not show any significant differences in total distance traveled or anxiety-like behavior in the open field chambers over 45 min (Fig. 4C–D, p > 0.05).
Figure 4.
Microglial Slc11a2 knockdown worsens hyperactivity in a novel environment in male APP/PS1 mice at 12–15 months.
A-B) Elevated zero maze. A Total distance traveled (m) in control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 male mice. B Total percent time spent in open arms. C-D) Open field locomotor activity assay. C Total distance traveled (cm). D Total percent time spent in the center. E-G) Exploratory Y-maze. E Total distance traveled (m). F Total number of different arm entries. G Total percent alternation. Two-way ANOVA, *p<0.05, ***p<0.001 effect of APP/PS1 genotype. #p<0.05 Slc11a2KD vs. Control. ns = not significant. Data represent the mean ± S.E.M of 11–15 male mice per group. Statistical outliers were removed using the Grubb’s test.
However, there was a significant APP/PS1-associated increase in activity in a 6 min Y-maze in the males (Fig. 4E; Y-maze: APP/PS1 effect, F(1, 46) = 8.40, p = 0.006), which was exacerbated in the Slc11a2 knockdown animals (Fig. 4E, Y-maze activity post-hoc comparisons: Control vs. APP/PS1, p = 0.39, Slc11a2KD Control vs. Slc11a2KD APP/PS1, p = 0.012; Fig. 4F, Y-maze arm entries: F(1, 46) = 5.65, p = 0.02; post-hoc comparisons: Control WT vs. APP/PS1, p = 0.61, Slc11a2KD WT vs. Slc11a2KD APP/PS1, p = 0.03). There were no significant differences in Y-maze spontaneous alternation capacity as a measure of working memory (Fig. 4G). Overall, these data suggest that microglial Slc11a2 knockdown is associated with an exaggerated hyperactive phenotype in the APP/PS1 animals, particularly in female mice.
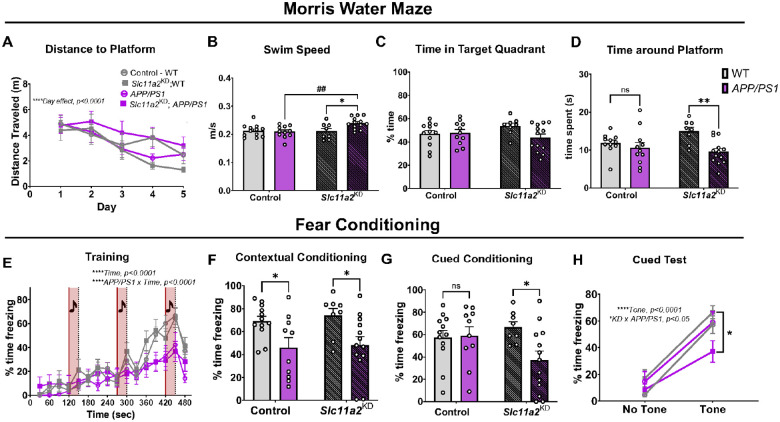
Slc11a2 knockdown worsens memory performance in Morris water maze and cued fear conditioning assay in APP/PS1 females.
To determine whether Slc11a2 knockdown affected measurements of well-being, cognition, and longer-term learning and memory, several behavioral tasks were utilized. An overnight nest building assay revealed a robust APP/PS1-associated deficit in nestlet amount shredded in the females; however, there was no additional effect of Slc11a2 knockdown on this measurement of cognition and well-being (Control WT mean, 4.3 g ± 0.28; Control APP/PS1 mean, 1.73 g ± 0.30; Slc11a2KD WT mean, 3.5 g ± 0.47; Slc11a2KD APP/PS1 mean, 1.48 g ± 0.35; APP/PS1 effect, F(1, 38) = 43.54, p < 0.0001). To assess learning and spatial memory, mice underwent five days of trials to find a hidden platform in Morris water maze (MWM), a widely used test for hippocampal-dependent spatial navigation and memory. Over the course of five days, all female mice (regardless of APP/PS1 genotype or Slc11a2 knockdown) effectively learned the location of the platform compared to their baseline on day one, exhibiting significantly shorter path lengths to find the platform by day five (Fig. 5A; Day effect, F(2.75, 110.1) = 11.38, p < 0.0001). Female APP/PS1 mice were not different than control WT females at finding the hidden platform during training days. However, microglial Slc11a2KD female APP/PS1 animals exhibited slightly longer path lengths to find the hidden platform, although this was not statistically significant (p = 0.1). Furthermore, in accordance with data from earlier tasks assessing locomotor activity, female Slc11a2KD APP/PS1 mice were significantly more hyperactive in the water maze (i.e., greater average swim speed) compared to all other groups (Fig. 5B; Knockdown × APP/PS1 Interaction, F(1, 40) = 5.45, p = 0.025). Mice underwent one 60 sec probe trial for memory of platform location 24 h after the last set of training trials, in which the platform was removed from the pool and mice were allowed to swim freely. There were no significant differences in time spent in the target quadrant where the platform location was previously (Fig. 5C, p > 0.05); however, female APP/PS1 mice overall exhibited a decrease in time spent around the exact platform location (exact platform location, plus 1.5 cm surrounding radius) compared to WT littermate controls (Fig. 5D; Females: APP/PS1 effect, F(1, 39) = 8.90, p = 0.005). Female Slc11a2KD APP/PS1 mice exhibited a significant further reduction in time spent around the platform location, suggesting an exacerbated loss of memory function in these animals (Females: post hoc analysis: Control WT vs. Control APP/PS1, p = 0.68; Slc11a2KD WT vs. Slc11a2KD APP/PS1, p = 0.004). To further assess the effects of Slc11a2 knockdown on memory function, we utilized a fear conditioning assay in which a tone was succeeded by a mild foot shock. During the initial training session, all groups significantly increased freezing by the third tone presentation, albeit APP/PS1 females overall froze less over the course of the 8 min training session (Fig. 5E, Time effect, F(6.9, 279.3) = 41.1, p < 0.0001; Interaction of Time × APP/PS1, F(15,600) = 4.91, p < 0.0001). In the contextual fear conditioning assay, female APP/PS1 mice exhibited a disease model-associated deficit in fear memory (Fig. 5F, APP/PS1 effect, F(1, 39) = 12.26, p = 0.0012); although, there was no additional effect of Slc11a2 knockdown. However, in the cued fear conditioning memory task, female Slc11a2KD APP/PS1 mice displayed a significant worsening in fear memory associated with presentation of a tone (Fig. 5G, Knockdown × APP/PS1 Interaction, F(1, 39) = 4.19, p = 0.047). Indeed, although all females exhibited an increase in freezing in response to the presentation of the tone (Tone, F(1, 39) = 145.2, p < 0.0001), female Slc11a2KD APP/PS1 mice were significantly less responsive compared to all other groups (Fig. 5H; Interaction of Knockdown × APP/PS1, F(1, 39) = 5.39, p = 0.026).
Figure 5.
Microglial Slc11a2 knockdown worsens memory performance in Morris Water Maze and cued fear conditioning assay in APP/PS1 female mice.
A-D) Morris water maze (MWM). A Total distance traveled (m) before reaching hidden platform over course of five training days in control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 female mice. Four trials of 60 s each were conducted each day and averaged per animal. Three-way ANOVA, ****p<0.0001 effect of day. B Average speed (m/s) measured during probe trial. Two-way ANOVA, *p<0.05 effect of APP/PS1 genotype. ##p<0.01 Slc11a2KD vs. Control. C Total percent time spent in the target quadrant in probe trial for memory. D Total time (s) spent around where the platform previously was (exact platform location + 1.5 cm radius) during probe trial for memory. E-H) Fear conditioning assay. E Percent component time freezing during the 8 min training protocol. Every 2 min, a 30 s tone was played, followed by a mild foot-shock. Increased freezing behavior over the course of the assay is shown. ****p<0.0001 effect of time, ****p<0.0001 effect of APP/PS1 × time. F Percent time freezing during 4 min contextual fear conditioning test. G Total percent time spent freezing during the 4 min of cued fear conditioning testing. H Percent component time spent freezing during 2 min of no-tone versus 2 min of tone presentation in cued fear conditioning test. ****p<0.0001 effect of tone, *p<0.05 Slc11a2KD APP/PS1 vs. Control APP/PS1. Data represent the mean ± S.E.M. of 8–13 mice per group. Statistical outliers were removed using the Grubb’s test.
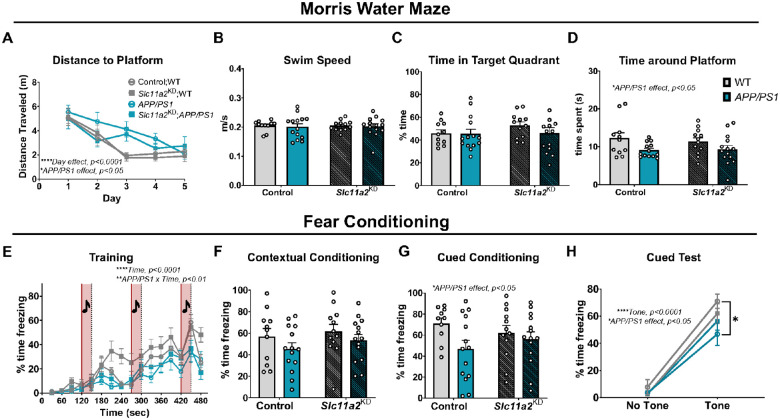
Male APP/PS1 animals displayed a significant deficit in nest building capacity compared to littermate WT control mice, with no additional effect due to Slc11a2KD (Control WT mean, 3.17 g ± 0.52; Control APP/PS1 mean, 2.03 g ± 0.41; Slc11a2KD WT mean, 3.82 g ± 0.34; Slc11a2KD APP/PS1 mean, 2.35 g ± 0.44; APP/PS1 effect, F(1, 47) = 9.15, p = 0.004). In the MWM, all males regardless of experimental group learned the location of the platform by the end of five training days, albeit APP/PS1 males exhibited longer path lengths over the course of the training compared to WT controls (Fig. 6A; Males: Day effect, F(2.891, 135.9) = 20.45, p < 0.0001; APP/PS1 effect, F(1, 47) = 5.99, p = 0.018). This behavioral phenotype was observed in the absence of differences in swim speeds between groups (Fig. 6B, p > 0.05), demonstrating a disease model-associated learning deficit in the males. In the MWM probe trial, there were no significant differences between groups in time spent in the target quadrant of the previous platform location (Fig. 6C, p > 0.05); however, male APP/PS1 mice overall spent significantly less time around the remembered platform location (platform location, including 1.5 cm surrounding radius) compared to WT controls (Fig. 6D; Males: APP/PS1 effect, F(1, 46) = 6.55, p = 0.01). There were no differences in male Slc11a2KD animals compared to Slc11a2-intact control animals in MWM. In the fear conditioning task, male APP/PS1 animals exhibited decreased freezing during the training session (Fig. 6E, Interaction of Time × APP/PS1, F(15, 705) = 2.25, p = 0.004). There were no significant differences between any groups of the males in the contextual fear conditioning assay (Fig. 6F, p > 0.05), although male APP/PS1 mice overall performed worse on the cued fear conditioning task for memory compared to WT controls (Fig. 6G–H, APP/PS1 effect, F(1, 46) = 4.15, p = 0.047). Slc11a2 knockdown had no effect on performance in these assays in the males. Overall, these data suggest that microglial Slc11a2 knockdown is associated with significant worsening of cognitive dysfunction in several tasks in a sex-specific manner, particularly in female APP/PS1 animals.
Figure 6.
Microglial Slc11a2 knockdown has no effect on memory performance in male mice.
A-D) Morris water maze (MWM). A Total distance traveled (m) before reaching hidden platform over course of five training days in control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 male mice. Four trials of 60 s each were conducted each day and averaged per animal. Three-way ANOVA, ****p<0.0001 effect of day, *p<0.05 effect of APP/PS1. B Average speed (m/s) measured during probe trial. Two-way ANOVA, *p<0.05 effect of APP/PS1 genotype. ##p<0.01 Slc11a2KD vs. Control. C Total percent time spent in the target quadrant in probe trial for memory. D Total time (s) spent around where the platform previously was (exact platform location + 1.5 cm radius) during probe trial for memory. E-H) Fear conditioning assay. E Percent component time freezing during the 8 min training protocol. Every 2 min, a 30 s tone was played, followed by a mild foot-shock. Increased freezing behavior over the course of the assay is shown. ****p<0.0001 effect of time, **p<0.0001 effect of APP/PS1 × time. F Percent time freezing during 4 min contextual fear conditioning test. G Total percent time spent freezing during the 4 min of cued fear conditioning testing. H Percent component time spent freezing during 2 min of no-tone versus 2 min of tone presentation in cued fear conditioning test. *p<0.05 effect of APP/PS1 genotype, ****p<0.0001 effect of tone. Data represent the mean ± S.E.M. of 11–14 mice per group. Statistical outliers were removed using the Grubb’s test.
Hippocampal microglia from female Slc11a2KD APP/PS1 animals exhibit significant alterations in DAM-like inflammatory and oxidative gene markers.
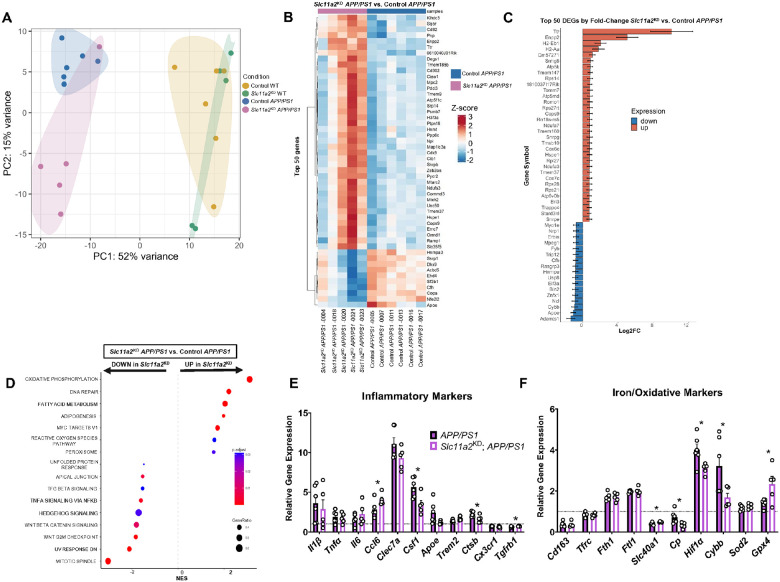
Significant alterations in gene expression from isolated microglia have been shown in AD models and human patients (35, 36). Thus, to examine transcriptomic changes in microglia in our studies, we magnetically isolated CD11b + microglia from the bilateral hippocampus from female mice for bulk RNA-sequencing. Primarily, we sought to determine changes in hippocampal microglia that may underlie the behavioral and memory-associated deficits observed primarily in the female Slc11a2KD APP/PS1 animals. In the Slc11a2 knockdown microglia, we first confirmed abrogation of expression in the Slc11a2 gene between exons 6–8 (Additional File 4: Fig. S2A), similar to what has been shown by others in this mouse model used to knockdown Slc11a2 (76, 77). Principal component analysis revealed a primary effect of APP/PS1 genotype on overall gene expression in isolated cells (Fig. 7A). As expected, hippocampal microglia isolated from APP/PS1 control animals exhibited a significant and robust pattern of differential gene expression compared to microglia isolated from WT controls. We found 1,301 differentially expressed genes (DEG) that were elevated in microglia from APP/PS1 control animals and 1,236 genes that were significantly downregulated in APP/PS1 controls compared to WT controls (adjusted p-value < 0.05). In examining the top 50 DEG (by fold-change and adj. p-value) in hippocampal microglia isolated from APP/PS1 compared to WT control females, we observed changes in similar gene markers previously reported in AD-associated microglia. Specifically, there were robust increases in microglial phagocytic marker Cd68 (78), hypoxia-related gene Hif1α (79), aging-associated marker Clec7a (80), lipid-droplet-associated marker Plin2 (81), as well as Type I IFN-signaling gene, Mamdc2 (82) (Additional File 5: Fig. S3A). DEGs that were downregulated in APP/PS1 hippocampal microglia compared to WT controls included homeostatic microglial marker Tmem119, as well as iron export gene, Slc40a1 (ferroportin) (Additional File 5: Fig. S3B). Gene-set enrichment analysis (GSEA) revealed significant upregulations in genes involved in cholesterol homeostasis, cellular metabolism, and inflammatory activation in APP/PS1 microglia (Additional File 5: Fig. S3C), similar to what others have shown previously in AD models (83).
Figure 7.
Slc11a2 knockdown shifts transcriptional profile and alters DAM-related gene markers in hippocampal microglia from female APP/PS1 mice.
A) Principal component analysis (PCA) of bulk RNA-seq gene expression in sorted CD11b+ microglia from control WT, control APP/PS1, Slc11a2KD WT, and Slc11a2KD APP/PS1 mice. Primary differences in overall gene expression are a result of APP/PS1 genotype. B) Heat map of top 50 DEGs (by adjusted p-value in RNA-seq dataset) between Slc11a2KD APP/PS1 versus control APP/PS1 microglia. Blue = downregulated in Slc11a2KD cells, lighter blue and/or red = upregulated in Slc11a2KD cells. C) Top 50 DEGS by fold-change in RNA-seq analysis between Slc11a2KD APP/PS1 and control APP/PS1 microglia. Red = upregulated in Slc11a2KD, blue = downregulated in Slc11a2KD cells. D) GSEA analysis of hallmark gene pathways significantly altered between Slc11a2KD APP/PS1 and control APP/PS1 microglia. E-F) Relative gene expression of targeted E inflammatory markers and F iron and oxidative stress markers from Slc11a2KD APP/PS1 versus control APP/PS1 microglia in the RNA-seq dataset. Gene expression is relative to control WT average (black dotted line set to 1). *p<0.05, student’s t-test comparing Slc11a2KD APP/PS1 vs. control APP/PS1. Data represent the mean ± S.E.M. of 5–6 mice per group.
To determine the effect of Slc11a2 knockdown on hippocampal microglia, we first compared microglial gene expression between Slc11a2KD and Control WT females. As a result of knockdown alone, we only found 7 DEGs (Additional File 6: Fig. S4A). Top genes altered included Ccr6 and Cd5 (Additional File 6: Fig. S4B-C). We then aimed to determine how Slc11a2 knockdown affects microglial gene expression in the APP/PS1 female animals. There were 150 genes significantly upregulated and 484 downregulated genes in microglia isolated from Slc11a2KD APP/PS1 animals compared to microglia from control APP/PS1 mice. Of these DEGs, Enpp2 and Ttr were robustly upregulated in knockdown cells compared to controls (Fig. 7B). Of the top 50 identified DEGs between Slc11a2KD APP/PS1 and control APP/PS1 females, Apoe (encoding apolipoprotein E), Cybb (gene for NOX2), and homeostatic marker Bin2 were also significantly downregulated in the knockdown cells compared to the control APP/PS1 cells (Fig. 7B–C). GSEA in the Slc11a2KD and control APP/PS1 microglia revealed significant increases in genes associated with cellular metabolism – in particular, oxidative phosphorylation and fatty acid metabolism – and reactive oxygen species (ROS) pathways (Fig. 7D). Slc11a2 knockdown cells also exhibited significant decreases in genes associated with TNF and NFκB inflammatory signaling and Wnt signaling. When comparing relative expression of specific genes in the sequencing dataset, we observed significant alterations in several genes involved in inflammatory and iron-related pathways in Slc11a2KD versus control cells from APP/PS1 females. Specifically, we observed a significant decrease in DAM markers Ctsb and Csf1 (84) in the knockdown cells, as well as a significant increase in Tgfbr1 (p < 0.05) and increase in Trem2 compared to control cells (although not statistically significant, p = 0.068) (Fig. 7E). In examining genes related to iron handling and redox status, we observed a significant increase in iron exporter gene Slc40a1 and antioxidant gene Gpx4 in Slc11a2KD APP/PS1 cells compared to control APP/PS1 microglia (Fig. 7F). Additionally, Slc11a2 knockdown cells exhibited decreases in pro-oxidant genes, such as Hif1α and Cybb, and a robust decrease in the iron-related gene encoding ceruloplasmin (Cp) (Fig. 7F). Although Slc11a2KD cells isolated from APP/PS1 mice exhibited significant differences in the expression of several DAM markers compared to control APP/PS1 microglia, Slc11a2KD APP/PS1 microglia displayed a transcriptional profile still distinct from control, non-APP/PS1 WT cells (black dotted line, Fig. 7E, F). In comparison to control WT cells, Slc11a2KD APP/PS1 microglia upregulated DAM and aging-related markers Csf1, Hif1α, Cybb, and Ctsb – albeit, to a lesser degree than control APP/PS1 microglia.
Initial assessment of overall gene expression via PCA and DEGs in these samples revealed significant variance in gene expression in one sample in the Slc11a2KD APP/PS1 group compared to the rest of the Slc11a2KD APP/PS1 biological replicates (sample labeled as −0004 in PCA plot shown in Additional File 4: Fig. S2B and in heat map Fig. 7B). Although this sample was not considered to be a statistical outlier, further RNA-seq analyses conducted following the removal of this sample are shown in Additional File 7: Fig. S5. In this analysis, there were 2,210 genes significantly upregulated and 2,230 significantly downregulated in the Slc11a2KD versus control APP/PS1 females (Additional File 7: Fig. S5B). The top DEGs revealed upregulations in genes including phagocytic-associated Igkc, along with Ttr and Enpp2 (Additional File 7: Fig. S5C and D). Slc11a2KD cells also exhibited significant downregulations in heat-shock-related genes Hspa1a and Hspa1b, as well as in inflammatory-related genes Lag3, Inpp5d, Erap1, and H2-Aa, and in LDAM (lipid-droplet-accumulating microglia) marker Ly9 (Additional File 7: Fig. S5C and D). Overall, these data suggest that microglial Slc11a2 knockdown in females decreases the DAM-like and aging-associated transcriptional signatures in the APP/PS1 model.
Discussion
Iron-loaded microglia are a hallmark of several neurodegenerative diseases, including AD (85–87). Reactive microglia surrounding Aβ plaques exhibit a significant upregulation in ferritin-L (Ftl1) across AD mouse models and human patients and is thus a defining feature of DAMs across multiple disease models (31, 35, 36, 88). Furthermore, recent in vitro work showed that iron loading specifically in microglia underlies subsequent neurotoxicity and cell death in a tri-culture system, positioning microglial iron load as a central mediator of neurodegeneration (29). At the in vitro level in microglia, inflammatory signals and iron import mechanisms are intimately connected [(20, 24), our data also in IMG cells]. Increased iron levels have been shown to enhance pro-inflammatory cytokine secretion (45), toxic ROS production (40), and cellular senescence and dysfunction (75, 89). Reciprocally, AD-associated pro-inflammatory signals such as Aβ and bacterial lipopolysaccharide (LPS) upregulate microglial iron importer DMT1 (gene, Slc11a2).
In our studies in primary microglia from aged and young mice treated in vitro with pro-inflammatory oligomeric Aβ1–42, we observed that the Aβ-induced increase in Slc11a2 was exacerbated in microglia from aged compared to young mice. This age-associated increase in Slc11a2 expression was accompanied by a significant upregulation in iron storage genes Ftl1 and Fth1 and augmented Aβ-induced inflammatory markers. An exacerbated Aβ-provoked inflammatory response in aged glia has been similarly observed by others and suggests a primed cellular state (90, 91). Our findings demonstrate an association between augmented Aβ-induced inflammation and iron loading markers in aged cells and suggest a synergy between age and Aβ leading to increased microglial Slc11a2 expression. Considering the purported role of iron in promoting cellular senescence and inflammation, it may be that DMT1/Slc11a2 plays a role in mediating the concomitant cellular iron and inflammatory load observed in neurodegenerative disease. Indeed, a role for DMT1 in Parkinson’s disease is well-appreciated (92, 93). However, no studies to our knowledge have directly examined whether altering microglial DMT1/Slc11a2 in vivo affects the development of chronic inflammation and disease-associated hallmarks in AD.
To investigate the effects of cell-specific alteration of Slc11a2 in AD, we generated a novel model of tamoxifen-inducible, microglial-specific knockdown of Slc11a2 in the APP/PS1 mouse model of AD. In female Slc11a2KD APP/PS1 mice, we observed a significant worsening of behavioral phenotypes and cognitive performance at 12–15 months of age following Slc11a2 knockdown at 5–6 months of age. Specifically, female Slc11a2KD APP/PS1 animals were significantly more hyperactive than all other female groups in multiple assays conducted. Previous studies have demonstrated significant hyperactivity in mouse models of AD (94–97), and AD human patients often exhibit disruptions in psychiatric behaviors such as hyperactivity, impulsivity, and disinhibition (98). Thus, microglial Slc11a2 knockdown exacerbates disruptions in these neuropsychiatric-like symptoms in our model, particularly in female APP/PS1 mice.
To assay for changes in memory function, we utilized the MWM test – a gold standard for testing hippocampal-dependent spatial memory acquisition and retention in rodents (58, 99). Slc11a2 knockdown resulted in a significant worsening of memory function in APP/PS1 females in the MWM memory probe trial. To further probe this memory phenotype, we used a fear conditioning assay consisting of both a contextual conditioning and cued conditioning task. We observed a significant deficit in learned cued fear memory in female Slc11a2KD APP/PS1 mice compared to control WT and APP/PS1 mice. The cued fear conditioning task utilizing the re-presentation of a cue (tone previously paired with a shock) requires the use of separate, parallel neural processing systems from the contextual fear memory task, involving inputs from the amygdala, insular cortex, regions in the parietal and temporal lobes, sensory cortices, and thalamus (100, 101). These complex networks likely converge with hippocampal circuits to acquire and express fear memory associated with a conditioned stimulus (102). Interestingly, dysfunction and neurodegeneration in the amygdala (103–105) and insular cortex (106, 107) have been implicated in AD models and patients as an early indicator of disease, and may also underlie many of the neuropsychiatric symptoms observed in AD patients, such as hyperactivity and agitation (108). The deficits we observed in cued fear memory in the female Slc11a2KD APP/PS1 mice, paired with their significant hyperactivity, suggest that Slc11a2 knockdown may worsen AD-associated behavior mediated by both hippocampal and non-hippocampal-dependent circuits in female mice. These data thus reflect a sex-specific, disease-modifying cognitive effect of Slc11a2 knockdown in female, but not male, APP/PS1 mice.
The sex-specific effects of microglial Slc11a2 knockdown found in our work are of particular interest in relation to AD development. In humans, females are significantly more likely to develop AD than males (109, 110), and female mice display enhanced pathological hallmarks compared to males in AD models (111–114). In the studies reported here, we observed effects of microglial Slc11a2 knockdown in female, but not male, APP/PS1 mice, suggesting a potential pathway involved in worsening disease parameters in female mice. Sex differences in brain iron-handling and changes in iron-associated markers related to disease development are not well understood. In humans, brain ferritin levels are generally higher in older men than women in several regions (115), which is thought to contribute to the risk for males developing neurodegenerative disease at earlier ages than females (116). In females, but not males, prior iron-deficiency anemia is associated with the development of dementia in females (117). On the other hand, there is a significant rise in serum ferritin levels associated with menopause in aging females (118), and this rise in iron status during female mid-life has been directly correlated with declining cognitive performance (119). In mice, males have higher brain iron levels than females (120), and recent work showed that adult male and female mice differentially alter brain iron stores in iron-deficient conditions (121). Additionally, research has illuminated significant sex differences in microglial morphology, inflammatory markers, and activity in age and disease, which may also contribute to sex differences in AD development (122, 123). Although the exact associations between brain iron status, sex, and microglial function are still being elucidated, our work suggests that a particular microglial inflammatory-iron-related pathway may be relevant to sex-dependent differences in inflammation and disease progression.
Although we were technically limited to gene expression analyses in these studies, future work aimed at characterizing iron load in the Slc11a2KD cells would be needed to expand upon these findings. While we cannot make definitive conclusions related to iron levels and/or protein-level changes in DMT1 and inflammatory makers per se, others have demonstrated an important role for transcription-level changes in inflammatory and iron-related genes (124, 125). Furthermore, many studies have characterized the microglial transcriptional landscape during AD (80, 126, 127). We conducted RNA-seq on isolated hippocampal microglia from the female mice in these studies to assess transcriptional changes that may underlie the cognitive differences observed. We found robust increases in Enpp2 and Ttr in Slc11a2KD APP/PS1 microglia compared to controls. Although there is a possibility these genes are associated with choroid plexus contamination in the hippocampal samples (128, 129), previous work in models of neuroinflammation and AD has identified downregulations in the expression of Enpp2, or ectonucleotide pyrophosphatase 2, and Ttr, the gene encoding for transthyretin, in specific DAM subsets (130–132). These two genes play roles in protein folding, Aβ binding, and lipid signaling, and have been suggested to play significant deleterious roles in microglia during aging and disease, when they are increased (133, 134).
Slc11a2 KD cells from APP/PS1 females also exhibited decreases in DAM-related and age-associated markers, such as Apoe, Csf1, Cybb, and Ctsb. Upregulations in these genes in AD-associated microglia are thought to be representative of a ‘primed’ microglial expression state during disease pathogenesis (132). While much work remains to elucidate the specific functions of heterogeneous microglial populations during AD, this DAM-like phenotype is posited to be a protective cell state initiated in response to growing AD pathology (35, 135, 136). Thus, decreases in these markers in the Slc11a2KD cells may reflect the loss of a protective transcriptional state. Slc11a2KD cells from APP/PS1 females also displayed a significant decrease in Bin2, a marker related to cell migration and phagocytosis, and decreases in oxidative stress regulators Hif1α and Nfe2l2. A decrease in Bin2 expression has been shown to mark a disease-promoting transition in microglia during AD progression (137, 138), and increases in Hif1α and Nfe2l2 in AD are thought to be a response to prevent excessive oxidative damage and microgliosis in disease (36, 139, 140). Our data thus suggest that Slc11a2 knockdown in microglia during AD progression leads to a lessening of the appearance of ‘protective’ DAM-related markers associated with limiting cellular damage (i.e., Apoe, Hif1α, Csf1, Nfe2l2), and instead an exacerbation of deleterious transcriptional changes observed in aged and AD-associated microglia (i.e., Bin2, Enpp2). Interestingly, although microglial Slc11a2 knockdown improved inflammatory markers and sickness in our acute LPS model (42), it may be that a shift in these inflammatory pathways in microglia in the APP/PS1 model is an important defensive measure in the context of chronic disease and long-term cognitive protection.
With the exception of differences in iron export gene, Slc40a1, and ceruloplasmin gene, Cp, there were few significant changes in iron-associated genes in Slc11a2KD cells in our RNA-seq data. This could suggest that the effect of Slc11a2 knockdown is primarily on inflammatory markers and not on iron markers per se, or it could reflect a time-dependent transcriptional change in those markers during AD progression that was not captured at the time point tested. Our in vitro work utilizing ebselen to inhibit DMT1 demonstrated that at the cellular level, ebselen robustly decreases Aβ-induced inflammatory markers and alters iron load. Ebselen functions as both a potent DMT1 inhibitor (63) and is also a peroxidase mimetic, and thus holds potential as a therapeutic to simultaneously limit cellular iron uptake, ROS production, and inflammatory signaling (141–143). Indeed, ebselen can improve phenotypes in AD models, and this may be due in part to its effects exerted on DMT1 and iron-handling (144, 145). While our in vivo work cannot definitively demonstrate that the effects of DMT1 knockdown are due to differences in iron load per se, it is intriguing to note that the directional changes in inflammatory and oxidative markers in microglia from the Slc11a2KD females mimic the anti-inflammatory and antioxidant effects of ebselen. While ebselen targets several pathways in the cell, it may be that some of the anti-inflammatory and antioxidant effects of ebselen are due in part to DMT1 inhibition (146, 147). Future work is needed to measure levels of iron in knockdown cells to conclusively determine the cellular effects of knockdown. However, even if total cellular iron levels do not change due to Slc11a2 knockdown, it could be that alterations in DMT1 affect the localization or distribution of iron in the cell that then alters inflammatory signaling.
Lastly, it is intriguing to consider a different time point for manipulation of microglial Slc11a2 during the disease process or for microglial collection for analysis. It may be that microglial increases in iron-related markers are initially a neuroprotective measure, whereas a late transition to an iron-import transcriptional phenotype results in exceeding the cell’s capacity for non-toxic iron-handling and leads to neurodegenerative consequences (29, 148).
Conclusions
In conclusion, this work highlights a sex-specific effect of microglial knockdown of iron import gene Slc11a2 on behavior and cognitive function in the APP/PS1 mouse model of AD. Female Slc11a2KD APP/PS1 mice are significantly more hyperactive and display worsened memory phenotypes compared to control animals. Associated with these behavioral changes, microglia from Slc11a2KD APP/PS1 females display a transcriptional shift demonstrating decreased DAM markers purported to be protective. These data suggest that knockdown of iron import gene, Slc11a2, leads to a progressive worsening of disease parameters in female AD mice and illuminate a microglial inflammatory-iron-associated pathway that holds relevance to our understanding of the complex roles of iron and microglia in neurodegenerative disease.
Acknowledgements
The authors would like to acknowledge Nathan Winn, PhD, and Teri Doss for assistance with tamoxifen gavage. The authors thank Dr. Rich Breyer for providing the aged mice in the primary cell experiments. Behavioral experiments were conducted in the Murine Neurobehavior Core lab (MNL) at the Vanderbilt University Medical Center, and the authors would like to thank Dr. John Allison and Krista Paffenroth for technical training. The MNL receives support from the Vanderbilt Kennedy Center P50 HD103537. Bulk RNA-seq preparation, sequencing, and initial quality control analysis was performed in the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core laboratory. VANTAGE is supported in part by Clinical and Translational Science Award Grant 5UL1 RR024975-03, Vanderbilt Ingram Cancer Center Grant P30 CA68485, Vanderbilt Vision Center Grant P30 EY08126, and National Institutes of Health/National Center for Research Resources Grant G20 RR030956. Additionally, RNA-seq data analysis in this work was performed by Creative Data Solutions, part of the Vanderbilt Center for Stem Cell Biology. RNA-seq processing leveraged the resources provided by the Vanderbilt Advanced Computing Center for Research and Education (ACCRE), a collaboratory operated by and for Vanderbilt faculty.
Funding
This project was funded by DK121520-02S1, a Pilot and Feasibility Award from the Vanderbilt Diabetes and Research Training Center (DK020593) and a Pilot and Feasibility Award from the Vanderbilt Memory and Alzheimer’s Center (P20-AG068082) to AHH. AHH is also supported by a Career Scientist Award from the Veterans Affairs (IK6 BX005649). KVR was supported by the Molecular Endocrinology Training Program (T32 DK007563-31), the Interdisciplinary Alzheimer’s Disease Training Program (T32-AG058524) and an individual Ruth L. Kirschstein National Research Service Award (1F31AG081025). FEH is supported by R01ES031401-05.
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- Apoe
apolipoprotein E
- APP/PS1
APPswe,PSEN1dE9
- CNS
central nervous system
- DAM
disease-associated microglia
- DEG
differentially expressed genes
- DMSO
dimethyl sulfoxide
- DMT1
divalent metal transporter 1
- EZM
elevated zero maze
- FAC
ferric ammonium citrate
- Fth
ferritin heavy chain
- Ftl
ferritin light chain
- GSEA
gene set enrichment analysis
- Hif1α
hypoxia-inducible factor 1 α
- ICP-MS
inductively-coupled plasma mass spectrometry
- IL
interleukin
- IMG
immortalized microglial cell line
- KD
knockdown
- MWM
Morris water maze
- PCA
principal component analysis
- ROS
reactive oxygen species
- Slc11a2
solute carrier family 11 member 2
- Tnfα
tumor necrosis factor α
- WT
wild-type
Funding Statement
This project was funded by DK121520-02S1, a Pilot and Feasibility Award from the Vanderbilt Diabetes and Research Training Center (DK020593) and a Pilot and Feasibility Award from the Vanderbilt Memory and Alzheimer’s Center (P20-AG068082) to AHH. AHH is also supported by a Career Scientist Award from the Veterans Affairs (IK6 BX005649). KVR was supported by the Molecular Endocrinology Training Program (T32 DK007563-31), the Interdisciplinary Alzheimer’s Disease Training Program (T32-AG058524) and an individual Ruth L. Kirschstein National Research Service Award (1F31AG081025). FEH is supported by R01ES031401-05.
Footnotes
Ethics approval and consent to participate
All studies using animals were approved by and conducted in compliance with the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University, protocol number #M2000113–00. No research was done with human participants, material, or data (Not applicable).
Competing Interests
The authors declare that they have no competing interests.
Supplementary Files
Contributor Information
Katrina Volk Robertson, Vanderbilt University.
Alec S. Rodriguez, Vanderbilt University
Jean-Philippe Cartailler, Vanderbilt University.
Shristi Shrestha, Vanderbilt University.
Kyle R. Schroeder, Vanderbilt University
Arianna M. Valenti, Vanderbilt University
Fiona E. Harrison, Vanderbilt University Medical Center
Alyssa H. Hasty, Vanderbilt University
Availability of data and materials
The datasets supporting the conclusions of this article are available in the NCBI Gene Expression Omnibus (GEO) repository with accession ID GSE269314 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269314). Other datasets used and/or analyzed are available from the corresponding authors on reasonable request. Additionally, sperm from Slc11a2flfl;Cx3cr1Cre-ERT2;APP/PS1+ triple-transgenic male mice (129S;C57BL/6J) were cryopreserved at the Vanderbilt Genome Editing Resource to preserve the genetic line used in these studies, and are available for sharing upon request.
References
- 1.Association As. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598 – 695. [DOI] [PubMed] [Google Scholar]
- 2.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings JL, Osse AML, Kinney JW. Alzheimer’s Disease: Novel Targets and Investigational Drugs for Disease Modification. Drugs. 2023;83(15):1387–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem. 2016;139(Suppl 1):179–97. [DOI] [PubMed] [Google Scholar]
- 5.Kenkhuis B, Bush AI, Ayton S. How iron can drive neurodegeneration. Trends Neurosci. 2023;46(5):333–5. [DOI] [PubMed] [Google Scholar]
- 6.Liu JL, Fan YG, Yang ZS, Wang ZY, Guo C. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front Neurosci. 2018;12:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Shapiro JS, Chang HC, Miller RA, Ardehali H. Aging is associated with increased brain iron through cortex-derived hepcidin expression. Elife. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ficiara E, Stura I, Guiot C. Iron Deposition in Brain: Does Aging Matter? Int J Mol Sci. 2022;23(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Y, Zhu W, Zhan C, Zhao L, Wang J, Tian Q, et al. Investigation on positive correlation of increased brain iron deposition with cognitive impairment in Alzheimer disease by using quantitative MR R2’ mapping. J Huazhong Univ Sci Technolog Med Sci. 2011;31(4):578. [DOI] [PubMed] [Google Scholar]
- 10.van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, et al. Cortical Iron Reflects Severity of Alzheimer’s Disease. J Alzheimers Dis. 2017;60(4):1533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spotorno N, Acosta-Cabronero J, Stomrud E, Lampinen B, Strandberg OT, van Westen D, et al. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain. 2020;143(5):1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res. 1992;31(1):75–83. [DOI] [PubMed] [Google Scholar]
- 13.Meadowcroft MD, Connor JR, Smith MB, Yang QX. MRI and histological analysis of beta-amyloid plaques in both human Alzheimer’s disease and APP/PS1 transgenic mice. J Magn Reson Imaging. 2009;29(5):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94(18):9866–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Moloney A, Meehan S, Morris K, Thomas SE, Serpell LC, et al. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J Biol Chem. 2011;286(6):4248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30(4):447–50. [DOI] [PubMed] [Google Scholar]
- 17.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345(1–2):91–104. [DOI] [PubMed] [Google Scholar]
- 18.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217(2):459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemonnot AL, Hua J, Ulmann L, Hirbec H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front Aging Neurosci. 2019;11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nnah IC, Wessling-Resnick M. Brain Iron Homeostasis: A Focus on Microglial Iron. Pharmaceuticals (Basel). 2018;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh A, Mela V, Harty C, Minogue AM, Costello DA, Kerskens C, et al. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 2019;29(5):606–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Gao X, Zhou S. New Target for Prevention and Treatment of Neuroinflammation: Microglia Iron Accumulation and Ferroptosis. ASN Neuro. 2022;14:17590914221133236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winn NC, Volk KM, Hasty AH. Regulation of tissue iron homeostasis: the macrophage ferrostat. JCI Insight. 2020;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy RC, Sosa JC, Gardeck AM, Baez AS, Lee CH, Wessling-Resnick M. Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem. 2018;293(20):7853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop GM, Dang TN, Dringen R, Robinson SR. Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox Res. 2011;19(3):443–51. [DOI] [PubMed] [Google Scholar]
- 26.Healy S, McMahon J, Owens P, FitzGerald U. Significant glial alterations in response to iron loading in a novel organotypic hippocampal slice culture model. Sci Rep. 2016;6:36410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinert A, Morawski M, Seeger J, Arendt T, Reinert T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao L, Li X, Luo Y, Wei J, Ding X, Xiong H, et al. Iron metabolism mediates microglia susceptibility in ferroptosis. Front Cell Neurosci. 2022;16:995084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan SK, Zelic M, Han Y, Teeple E, Chen L, Sadeghi M, et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat Neurosci. 2023;26(1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeineh MM, Chen Y, Kitzler HH, Hammond R, Vogel H, Rutt BK. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol Aging. 2015;36(9):2483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenkhuis B, Somarakis A, de Haan L, Dzyubachyk O, ME IJ, de Miranda N, et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol Commun. 2021;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland R, McIntosh AL, Finucane OM, Mela V, Rubio-Araiz A, Timmons G, et al. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun. 2018;68:183–96. [DOI] [PubMed] [Google Scholar]
- 33.Gillen KM, Mubarak M, Nguyen TD, Pitt D. Significance and In Vivo Detection of Iron-Laden Microglia in White Matter Multiple Sclerosis Lesions. Front Immunol. 2018;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adeniyi PA, Gong X, MacGregor E, Degener-O’Brien K, McClendon E, Garcia M et al. Ferroptosis of microglia in aging human white matter injury. Ann Neurol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169(7):1276–e9017. [DOI] [PubMed] [Google Scholar]
- 36.Prater KE, Green KJ, Mamde S, Sun W, Cochoit A, Smith CL, et al. Human microglia show unique transcriptional changes in Alzheimer’s disease. Nat Aging. 2023;3(7):894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in Neurodegeneration - Cause or Consequence? Front Neurosci. 2019;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angelova DM, Brown DR. Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J Neurochem. 2019;151(6):676–88. [DOI] [PubMed] [Google Scholar]
- 39.Nnah IC, Lee CH, Wessling-Resnick M. Iron potentiates microglial interleukin-1beta secretion induced by amyloid-beta. J Neurochem. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yauger YJ, Bermudez S, Moritz KE, Glaser E, Stoica B, Byrnes KR. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J Neuroinflammation. 2019;16(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126(4):541–9. [DOI] [PubMed] [Google Scholar]
- 42.Volk Robertson K, Schleh MW, Harrison FE, Hasty AH. Microglial-specific knockdown of iron import gene, Slc11a2, blunts LPS-induced neuroinflammatory responses in a sex-specific manner. Brain Behav Immun. 2023;116:370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mims MP, Prchal JT. Divalent metal transporter 1. Hematology. 2005;10(4):339–45. [DOI] [PubMed] [Google Scholar]
- 44.Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr. 2012;70:169–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nnah IC, Lee CH, Wessling-Resnick M. Iron potentiates microglial interleukin-1beta secretion induced by amyloid-beta. J Neurochem. 2020;154(2):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheli VT, Santiago Gonzalez DA, Marziali LN, Zamora NN, Guitart ME, Spreuer V, et al. The Divalent Metal Transporter 1 (DMT1) Is Required for Iron Uptake and Normal Development of Oligodendrocyte Progenitor Cells. J Neurosci. 2018;38(43):9142–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–70. [DOI] [PubMed] [Google Scholar]
- 49.Izco M, Martinez P, Corrales A, Fandos N, Garcia S, Insua D, et al. Changes in the brain and plasma Abeta peptide levels with age and its relationship with cognitive impairment in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuroscience. 2014;263:269–79. [DOI] [PubMed] [Google Scholar]
- 50.Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009;29(34):10706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: Two sides of the same coin. Neurobiol Dis. 2015;81:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willis EF, Vukovic J. Protocol for brain-wide or region-specific microglia depletion and repopulation in adult mice. STAR Protoc. 2020;1(3):100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahasrabuddhe V, Ghosh HS. Cx3cr1-Cre induction leads to microglial activation and IFN-1 signaling caused by DNA damage in early postnatal brain. Cell Rep. 2022;38(3):110252. [DOI] [PubMed] [Google Scholar]
- 54.Faust TE, Feinberg PA, O’Connor C, Kawaguchi R, Chan A, Strasburger H, et al. A comparative analysis of microglial inducible Cre lines. Cell Rep. 2023;42(9):113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes-Marin KE, Nunez A. Seizure susceptibility in the APP/PS1 mouse model of Alzheimer’s disease and relationship with amyloid beta plaques. Brain Res. 2017;1677:93–100. [DOI] [PubMed] [Google Scholar]
- 56.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1(3):1117–9. [DOI] [PubMed] [Google Scholar]
- 57.Consoli DC, Spitznagel BD, Owen BM, Kang H, Williams Roberson S, Pandharipande P, et al. Altered EEG, disrupted hippocampal long-term potentiation and neurobehavioral deficits implicate a delirium-like state in a mouse model of sepsis. Brain Behav Immun. 2023;107:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nunez J. Morris Water Maze Experiment. J Vis Exp. 2008(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grabert K, McColl BW. Isolation and Phenotyping of Adult Mouse Microglial Cells. Methods Mol Biol. 2018;1784:77–86. [DOI] [PubMed] [Google Scholar]
- 60.Lee JK, Tansey MG. Microglia isolation from adult mouse brain. Methods Mol Biol. 2013;1041:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bordt EA, Block CL, Petrozziello T, Sadri-Vakili G, Smith CJ, Edlow AG et al. Isolation of Microglia from Mouse or Human Tissue. STAR Protoc. 2020;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy RC, Lu DY, Alkhateeb A, Gardeck AM, Lee CH, Wessling-Resnick M. Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-beta. J Neuroinflammation. 2016;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol. 2006;13(9):965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhee JW, Yi H, Thomas D, Lam CK, Belbachir N, Tian L, et al. Modeling Secondary Iron Overload Cardiomyopathy with Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Rep. 2020;32(2):107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenkhuis B, van Eekeren M, Parfitt DA, Ariyurek Y, Banerjee P, Priller J, et al. Iron accumulation induces oxidative stress, while depressing inflammatory polarization in human iPSC-derived microglia. Stem Cell Rep. 2022;17(6):1351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 67.Felix Krueger F, James P, Ewels E, Afyounian M, Weinstein B, Schuster-Boeckler. Gert Hulselmans, & scalmons. FelixKrueger/TrimGalore: v0.6.10 - add default decompression path (0.6.10) Zenodo. 2023.
- 68.May Martin M.. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal. 2011;S.1(v. 17, n. 1):pp. 10 – 2. [Google Scholar]
- 69.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castanza AS, Recla JM, Eby D, Thorvaldsdottir H, Bult CJ, Mesirov JP. Extending support for mouse data in the Molecular Signatures Database (MSigDB). Nat Methods. 2023;20(11):1619–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song Y, Wang J. ggcoverage: an R package to visualize and annotate genome coverage for various NGS data. BMC Bioinformatics. 2023;24(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia. 2008;56(10):1048–60. [DOI] [PubMed] [Google Scholar]
- 76.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115(5):1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlson ES, Tkac I, Magid R, O’Connor MB, Andrews NC, Schallert T, et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139(4):672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopperton KE, Mohammad D, Trepanier MO, Giuliano V, Bazinet RP. Markers of microglia in postmortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry. 2018;23(2):177–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.March-Diaz R, Lara-Urena N, Romero-Molina C, Heras-Garvin A, Ortega-de San Luis C, Alvarez-Vergara MI, et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat Aging. 2021;1(4):385–99. [DOI] [PubMed] [Google Scholar]
- 80.Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Commun. 2015;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23(2):194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Luo W, Wang X, Ma Y, Huang L, Wang Y. MAMDC2, a gene highly expressed in microglia in experimental models of Alzheimers Disease, positively regulates the innate antiviral response during neurotropic virus infection. J Infect. 2022;84(2):187–204. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Colonna M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J Exp Med. 2021;218(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu YJ, Au NPB, Ma CHE. Functional and Phenotypic Diversity of Microglia: Implication for Microglia-Based Therapies for Alzheimer’s Disease. Front Aging Neurosci. 2022;14:896852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwiatek-Majkusiak J, Dickson DW, Tacik P, Aoki N, Tomasiuk R, Koziorowski D, et al. Relationships between typical histopathological hallmarks and the ferritin in the hippocampus from patients with Alzheimer’s disease. Acta Neurobiol Exp (Wars). 2015;75(4):391–8. [PubMed] [Google Scholar]
- 86.Levi S, Ripamonti M, Moro AS, Cozzi A. Iron imbalance in neurodegeneration. Mol Psychiatry. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia. 2007;55(10):1074–84. [DOI] [PubMed] [Google Scholar]
- 88.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570(7761):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Angelova DM, Brown DR. Model Senescent Microglia Induce Disease Related Changes in alpha-Synuclein Expression and Activity. Biomolecules. 2018;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niraula A, Sheridan JF, Godbout JP. Microglia Priming with Aging and Stress. Neuropsychopharmacology. 2017;42(1):318–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105(47):18578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee HP, Zhu X, Liu G, Chen SG, Perry G, Smith MA, et al. Divalent metal transporter, iron, and Parkinson’s disease: a pathological relationship. Cell Res. 2010;20(4):397–9. [DOI] [PubMed] [Google Scholar]
- 94.Wang T, Chen Y, Zou Y, Pang Y, He X, Chen Y, et al. Locomotor Hyperactivity in the Early-Stage Alzheimer’s Disease-like Pathology of APP/PS1 Mice: Associated with Impaired Polarization of Astrocyte Aquaporin 4. Aging Dis. 2022;13(5):1504–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tag SH, Kim B, Bae J, Chang KA, Im HI. Neuropathological and behavioral features of an APP/PS1/MAPT (6xTg) transgenic model of Alzheimer’s disease. Mol Brain. 2022;15(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jul P, Volbracht C, de Jong IE, Helboe L, Elvang AB, Pedersen JT. Hyperactivity with Agitative-Like Behavior in a Mouse Tauopathy Model. J Alzheimers Dis. 2016;49(3):783–95. [DOI] [PubMed] [Google Scholar]
- 97.Oblak AL, Lin PB, Kotredes KP, Pandey RS, Garceau D, Williams HM, et al. Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Front Aging Neurosci. 2021;13:713726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandez M, Gobartt AL, Balana M, Group CS. Behavioural symptoms in patients with Alzheimer’s disease and their association with cognitive impairment. BMC Neurol. 2010;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48(1–2):29–69. [DOI] [PubMed] [Google Scholar]
- 100.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knafo S, Venero C, Merino-Serrais P, Fernaud-Espinosa I, Gonzalez-Soriano J, Ferrer I, et al. Morphological alterations to neurons of the amygdala and impaired fear conditioning in a transgenic mouse model of Alzheimer’s disease. J Pathol. 2009;219(1):41–51. [DOI] [PubMed] [Google Scholar]
- 102.Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317–31. [DOI] [PubMed] [Google Scholar]
- 103.Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Alzheimer’s Disease Neuroimaging I. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res. 2011;194(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Del Prieto L, Cantero JL, Atienza M. Atrophy of amygdala and abnormal memory-related alpha oscillations over posterior cingulate predict conversion to Alzheimer’s disease. Sci Rep. 2016;6:31859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin TW, Liu YF, Shih YH, Chen SJ, Huang TY, Chang CY, et al. Neurodegeneration in Amygdala Precedes Hippocampus in the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Curr Alzheimer Res. 2015;12(10):951–63. [DOI] [PubMed] [Google Scholar]
- 106.Kitamura J, Nagai M, Ueno H, Ohshita T, Kikumoto M, Toko M, et al. The Insular Cortex, Alzheimer Disease Pathology, and Their Effects on Blood Pressure Variability. Alzheimer Dis Assoc Disord. 2020;34(3):282–91. [DOI] [PubMed] [Google Scholar]
- 107.Bonthius DJ, Solodkin A, Van Hoesen GW. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J Neuropathol Exp Neurol. 2005;64(10):910–22. [DOI] [PubMed] [Google Scholar]
- 108.Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Aspects Med. 2015;43–44:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J Alzheimers Dis. 2018;64(4):1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Feng Y, Wu W, Zhao J, Fu C, Li Y, et al. Sex differences between APPswePS1dE9 mice in A-beta accumulation and pancreatic islet function during the development of Alzheimer’s disease. Lab Anim. 2016;50(4):275–85. [DOI] [PubMed] [Google Scholar]
- 112.Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14(3):318–27. [DOI] [PubMed] [Google Scholar]
- 113.Yang JT, Wang ZJ, Cai HY, Yuan L, Hu MM, Wu MN, et al. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci Bull. 2018;34(5):736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiao SS, Bu XL, Liu YH, Zhu C, Wang QH, Shen LL, et al. Sex Dimorphism Profile of Alzheimer’s Disease-Type Pathologies in an APP/PS1 Mouse Model. Neurotox Res. 2016;29(2):256–66. [DOI] [PubMed] [Google Scholar]
- 115.Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, et al. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28(3):414–23. [DOI] [PubMed] [Google Scholar]
- 116.Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci. 2004;1012:224–36. [DOI] [PubMed] [Google Scholar]
- 117.Chung SD, Sheu JJ, Kao LT, Lin HC, Kang JH. Dementia is associated with iron-deficiency anemia in females: a population-based study. J Neurol Sci. 2014;346(1–2):90–3. [DOI] [PubMed] [Google Scholar]
- 118.Whit field JB, Treloar S, Zhu G, Powell LW, Martin NG. Relative importance of female-specific and non-female-specific effects on variation in iron stores between women. Br J Haematol. 2003;120(5):860–6. [DOI] [PubMed] [Google Scholar]
- 119.Andreeva VA, Galan P, Arnaud J, Julia C, Hercberg S, Kesse-Guyot E. Midlife iron status is inversely associated with subsequent cognitive performance, particularly in perimenopausal women. J Nutr. 2013;143(12):1974–81. [DOI] [PubMed] [Google Scholar]
- 120.Hahn P, Song Y, Ying GS, He X, Beard J, Dunaief JL. Age-dependent and gender-specific changes in mouse tissue iron by strain. Exp Gerontol. 2009;44(9):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palsa K, Neely EB, Baringer SL, Helmuth TB, Simpson IA, Connor JR. Brain iron acquisition depends on age and sex in iron-deficient mice. FASEB J. 2024;38(1):e23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lynch MA. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front Aging Neurosci. 2022;14:868448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guillot-Sestier MV, Araiz AR, Mela V, Gaban AS, O’Neill E, Joshi L, et al. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun Biol. 2021;4(1):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang E, Ong WY, Go ML, Connor JR. Upregulation of iron regulatory proteins and divalent metal transporter-1 isoforms in the rat hippocampus after kainate induced neuronal injury. Exp Brain Res. 2006;170(3):376–86. [DOI] [PubMed] [Google Scholar]
- 125.Ingrassia R, Lanzillotta A, Sarnico I, Benarese M, Blasi F, Borgese L, et al. 1B/(−)IRE DMT1 expression during brain ischemia contributes to cell death mediated by NF-kappaB/RelA acetylation at Lys310. PLoS ONE. 2012;7(5):e38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018;22(3):832–47. [DOI] [PubMed] [Google Scholar]
- 127.Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell. 2018;173(5):1073–81. [DOI] [PubMed] [Google Scholar]
- 128.Stankiewicz AM, Goscik J, Majewska A, Swiergiel AH, Juszczak GR. The Effect of Acute and Chronic Social Stress on the Hippocampal Transcriptome in Mice. PLoS ONE. 2015;10(11):e0142195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sousa JC, Cardoso I, Marques F, Saraiva MJ, Palha JA. Transthyretin and Alzheimer’s disease: where in the brain? Neurobiol Aging. 2007;28(5):713–8. [DOI] [PubMed] [Google Scholar]
- 130.Shippy DC, Watters JJ, Ulland TK. Transcriptional response of murine microglia in Alzheimer’s disease and inflammation. BMC Genomics. 2022;23(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X, Buxbaum JN. Transthyretin and the brain re-visited: is neuronal synthesis of transthyretin protective in Alzheimer’s disease? Mol Neurodegener. 2011;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sobue A, Komine O, Hara Y, Endo F, Mizoguchi H, Watanabe S, et al. Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang L, Mu H, Xu F, Xie D, Su W, Xu J, et al. Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery. J Cereb Blood Flow Metab. 2020;40(1suppl):S81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amaral RF, Geraldo LHM, Einicker-Lamas M, TCLS ES, Mendes F, Lima FRS. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA(1) receptor. J Neurochem. 2021;156(4):499–512. [DOI] [PubMed] [Google Scholar]
- 135.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity. 2017;47(3):566–81. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen AT, Wang K, Hu G, Wang X, Miao Z, Azevedo JA, et al. APOE and TREM2 regulate amyloid-responsive microglia in Alzheimer’s disease. Acta Neuropathol. 2020;140(4):477–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sebastian Monasor L, Muller SA, Colombo AV, Tanrioever G, Konig J, Roth S et al. Fibrillar Abeta triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boche D, Gordon MN. Diversity of transcriptomic microglial phenotypes in aging and Alzheimer’s disease. Alzheimers Dement. 2022;18(2):360–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lastres-Becker I, Innamorato NG, Jaworski T, Rabano A, Kugler S, Van Leuven F, et al. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain. 2014;137(Pt 1):78–91. [DOI] [PubMed] [Google Scholar]
- 140.Ollonen T, Kurkela M, Laitakari A, Sakko S, Koivisto H, Myllyharju J, et al. Activation of the hypoxia response protects mice from amyloid-beta accumulation. Cell Mol Life Sci. 2022;79(8):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghazaiean M, Aliasgharian A, Karami H, Darvishi-Khezri H, Ebselen. A promising therapy protecting cardiomyocytes from excess iron in iron-overloaded thalassemia patients. Open Med (Wars). 2023;18(1):20230733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Landgraf AD, Alsegiani AS, Alaqel S, Thanna S, Shah ZA, Sucheck SJ. Neuroprotective and Anti-neuroinflammatory Properties of Ebselen Derivatives and Their Potential to Inhibit Neurodegeneration. ACS Chem Neurosci. 2020;11(19):3008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren X, Zou L, Lu J, Holmgren A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic Biol Med. 2018;127:238–47. [DOI] [PubMed] [Google Scholar]
- 144.Martini F, Rosa SG, Klann IP, Fulco BCW, Carvalho FB, Rahmeier FL, et al. A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J Psychiatr Res. 2019;109:107–17. [DOI] [PubMed] [Google Scholar]
- 145.Xie L, Zheng W, Xin N, Xie JW, Wang T, Wang ZY. Ebselen inhibits iron-induced tau phosphorylation by attenuating DMT1 up-regulation and cellular iron uptake. Neurochem Int. 2012;61(3):334–40. [DOI] [PubMed] [Google Scholar]
- 146.Davis MT, Bartfay WJ. Ebselen decreases oxygen free radical production and iron concentrations in the hearts of chronically iron-overloaded mice. Biol Res Nurs. 2004;6(1):37–45. [DOI] [PubMed] [Google Scholar]
- 147.Mukem S, Sayoh I, Maungchanburi S, Thongbuakaew T, Ebselen. Iron Uptake Inhibitor, Alleviates Iron Overload-Induced Senescence-Like Neuronal Cells SH-SY5Y via Suppressing the mTORC1 Signaling Pathway. Adv Pharmacol Pharm Sci. 2023;2023:6641347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Swanson MEV, Mrkela M, Murray HC, Cao MC, Turner C, Curtis MA, et al. Microglial CD68 and L-ferritin upregulation in response to phosphorylated-TDP-43 pathology in the amyotrophic lateral sclerosis brain. Acta Neuropathol Commun. 2023;11(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the NCBI Gene Expression Omnibus (GEO) repository with accession ID GSE269314 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269314). Other datasets used and/or analyzed are available from the corresponding authors on reasonable request. Additionally, sperm from Slc11a2flfl;Cx3cr1Cre-ERT2;APP/PS1+ triple-transgenic male mice (129S;C57BL/6J) were cryopreserved at the Vanderbilt Genome Editing Resource to preserve the genetic line used in these studies, and are available for sharing upon request.