Abstract
Background
Polymorphisms in the vitamin D receptor (VDR) play an effective role in the susceptibility of pulmonary tuberculosis (TB). Given the importance of this polymorphism and its association with pulmonary TB, this study aimed to investigate the prevalence of VDR polymorphisms in people with pulmonary TB.
Methods
The search process was performed from 2009 to 2023 according to PRISMA (Preferred reporting items for systematic reviews and meta-analyses). The strengthening of the reporting of observational studies in epidemiology (STROBE) checklist was used to qualify the articles. The data was entered into STATA version 14 software, then the fixed effects model and the random effects model, effect size (ES), and Q test (P < 0.10) were used for data analysis at a confidence interval level (CI) of 95%. Two-sided statistical tests were considered with α=0.05.
Results
In this research, 28 articles were analyzed. Polymorphisms showed a significant relationship with susceptibility to pulmonary TB (P = 0.000), and significant heterogeneity (P = 0.000) was seen between polymorphisms. FokI (95% CI: 0.39-0.46, P = 0.000, ES = 43%), ApaI (95% CI: 0.31-0.48, P = 0.000, ES = 39%) and BsmI (95% CI: 0.24-0.50, P = 0.000, ES = 37%) showed the most frequent gene polymorphisms after TaqI (95% CI: 0.34-0.77, P = 0.000, ES = 56%).
Conclusion
ApaI, BsmI, FokI, and TaqI polymorphisms were found in patients suffering from pulmonary TB. Polymorphisms related to the TaqI gene were the most frequent. Controlling and prescribing vitamin D may be needed in these patients.
Keywords: Vitamin D Receptor Genes, Polymorphisms, Pulmonary Tuberculosis.
↑What is “already known” in this topic:
Different results regarding the relationship of polymorphism of VDR genes with the pulmonary TB are performed at different time intervals, years, communities, but this relationship is still unknown.
→What this article adds:
Polymorphism of VDR genes in patients suffering from pulmonary TB in this study was shown. TaqI gene polymorphisms rate was higher than FokI, ApaI and BsmI.
Introduction
Mycobacterium tuberculosis (M. tuberculosis) infected approximately one-third of the world population (1). Tuberculosis is the second cause of death caused by an infectious disease in the world (after-acquired immunodeficiency syndrome (AIDS), and its incidence rate is 8.8 million cases, with the death of nearly 2 million people per year (2, 3).
The results of the evaluation of TB trends from 2010 to 2019 in Iran showed an annual increase of 0.84% in the incidence of TB. Between 2010 and 2013, the incidence of TB increased by 18.10% annually, and between 2013 and 2019, it increased by -5.42% annually (4). In another study conducted in Iran, 12% of 9717 TB cases were Afghans, and 72.3% of patients had pulmonary TB (5). Global research showed a 10% cumulative reduction in TB incidence from 2015 to 2021 (6). A person's lifetime risk of developing TB is between 5% and 15% (7). The risk of contracting TB for people is between 5 and 15 percent (8). The effects of some genes in the human body have been proven on the level of TB infection in different people (9, 10). Among the genes that affect TB are the human white blood cell antigen and white blood cell non-antigen genes. The human white blood cell antigen and the white blood cell non-antigen gene affect the emergence of TB. Vitamin D receptor (VDR) is in the group of non-antigenic genes of human white blood cells (11, 12). Monocytes have an active VDR (1,25(OH)2 Vitamin D) on their surface. Vitamin D (1, 25(OH)2D is the most important metabolite of vitamin D and it is produced from 25OHD by the enzyme 25OHD-1α hydroxylase (CYP27B1)). Vitamin D (1, 25(OH)2D enters the macrophage by the phagosome -lysosome integration. Vitamin D (1, 25(OH)2D by stimulating the release of nitric oxide radicals, increases the ability of this cell to destroy TB (12, 13).
Among the immune cells, alveolar macrophages (AM) are monocytes, which recognize bacteria through their membrane receptors, including Toll-like receptors, and in addition to ingesting it, they create the front line of defense against mycobacterium by creating an inflammatory response. Cytokines produced by macrophages cause other innate immune cells, including natural killer cells and Tγδ lymphocytes, to be called to the site of infection, which in turn increases the capacity to kill mycobacterium by releasing macrophage-activating cytokines (1). Polymorphism is the occurrence of two or more completely different forms or forms, also called alternative phenotypes, in a population of a species. Meanwhile, polymorphisms are the most common type of genetic variation that is presented in the human genome at a frequency higher than 1%. Polymorphisms differ from deoxyribonucleic acid (DNA) mutations, which are observed at extremely low frequencies in the population. Polymorphisms in the VDR gene may contribute to susceptibility to TB (12, 14, 15). The vitamin D receptor polymorphisms gene is on chromosome 12cen-q12 (including 14 exons and approximately 75 kilobases in genomic deoxyribonucleic acid (DNA)), including single nucleotide polymorphisms (SNPs) in FokI (F/f), BsmI (B/b), (ApaI (A/a) and TaqI (T/T). This polymorphism may or may not affect the activity of the VDR gene and the effects caused by vitamin D afterward (10).
Junaid and Rehman indicated that vitamin D deficiency is a risk factor for the development of active TB in patients (37.3±9.9 years old) with pulmonary TB. Mansy et al. reported that the frequency of AA, Aa, and aa polymorphism in the VDR gene was 58.5%, 17.9%, and 23.6%, respectively. Also, the frequency of TT, Tt, and tt polymorphism was 48%, 19%, and 33%, respectively. There was not any significant relationship between VDR gene polymorphism, ApaI and TaqI, and increased susceptibility to acute lower respiratory tract infection (16). In another study, Wang and Li showed that VDR gene polymorphisms including BsmI rs1544410 (odds ratio (OR); 0.79), FokI rs2228570 (OR; 0.89), and TaqI rs731236 (OR; 0.87) may act as genetic markers of TB in a certain population (17). Li et al. reported that the OR of TaqI gene polymorphism in the world population was 1.12 (9).
According to studies conducted in different populations at different time intervals in different years, there is still no convincing answer for a definitive relationship or lack of a definitive relationship regarding polymorphisms. Vitamin D receptor genes and their effect on pulmonary TB have not yet been found, and the results are contradictory. Their relationship is still the subject of discussion in the scientific communities related to this issue. In many studies, the polymorphism of all VDR genes has not been investigated, and many research studies may not have been included in these systematic review studies and meta-analyses in different periods. Therefore, an up-to-date meta-analysis is needed to obtain a more reliable assessment of the association between VDR gene polymorphisms and TB risk in global communities. Paying attention to the mentioned cases, investigating VDR gene polymorphisms and their effect on tuberculosis disease is a very effective step in the management of prevention, control, and care before and after the treatment of this disease, which in this research will be considered.
Methods
Information sources and search strategy
This research was performed according to the PRISMA (Preferred reporting items for systematic reviews and meta-analyses) guideline (http://www.prisma-statement.org/, https://meridian.cvm.iastate.edu/wp-content/uploads/2017/06/PRISMA-2009-checklist.pdf). The advanced search process was carried out from 2009 to 2023. Quotations were used to insert a specific phrase, and Google (https://www.google.co.uk/) was used to search a site. Databases include PubMed (Uniform Resource Locator (URL); https://pubmed.ncbi.nlm.nih.gov/), Scopus (URL; https://www.scopus.com/), Web of Sciences (URL; http://webofscience.com/), Google Scholar (URL; https://scholar.google.com/), and scientific information database (SID) (URL; https://www.sid.ir/journal/en) (18-21).
The search keywords according to MeSH database (VDR: https://www.ncbi.nlm.nih.gov/mesh/?term=VDR, Polymorphisms: https://www.ncbi.nlm.nih.gov/mesh/?term=Polymorphisms, Tuberculosis: https://www.ncbi.nlm.nih.gov/mesh/?term=Tuberculosis)was done. The search was also done with words that have the same meaning as the keywords, such as vitamin D receptor gene polymorphisms and TB. Boolean Operators are simple words (AND, OR, NOT, or AND NOT) that were used to limit the search results. Advanced search syntax included; ‘prevalence OR incidence OR frequency OR outbreaks OR occurrence OR epidemiology OR epidemiologic studies OR population-based’ AND ‘vitamin D receptor genes polymorphisms OR VDR’ AND ‘pulmonary tuberculosis’ AND ‘TB OR Mycobacterium tuberculosis’. The search syntax can be modified in databases (19, 22, 23).
The specific word was searched on the web page itself with Ctrl+F, and network search was used through a laptop or computer system. The desired format for searching was portable document format (PDF). All variables for data were sought bypopulation (patients suffering from pulmonary TB with/without VDR gene polymorphisms), intervention (VDR gene polymorphisms and susceptibility to TB), control (patients suffering from pulmonary TB without VDR gene polymorphisms), and outcomes (susceptibility to TB) (PICO) format. Also, assumptions were the following:
1. What is the prevalence of VDR polymorphisms in people with pulmonary TB in the world population?
2. What are the most common VDR gene polymorphisms (ApaI, BsmI, FokI, and TaqI)?
The search process was carried out by two researchers and was defined for each specific time independently.
In the screening process, each of the two researchers considered the articles resulting from their search to identify inclusion criteria. Next, the third researcher reviewed, considered, and selected the articles.
Then, the quality of the articles was evaluated by the relevant experts (three researchers) in this field. Disagreements between researchers regarding the inclusion criteria were resolved by discussion and consulting. Name of authors, year of study, country, continent, method, type of study, number of women and men, number of samples, age, and considered genes (ApaI (A/a), BsmI (B/b), FokI (F/f), and TaqI (T/T)) were selected for data extraction. All data was interested in an Excel file version 2010. In the end, information was analyzed by a statistics consultant.
For the quality control of the articles, accreditation of studies, as well as the ability to generalize the results in society, strengthening the reporting of observational studies in epidemiology (STROBE) checklist (https://www.strobe-statement.org/checklists/) was used (file:///C:/Users/Pascal/Downloads/STROBE-checklist-v4-combined.pdf). In the STROBE checklist, there are recommendations to clarify the design, implementation method, and findings of observational studies and to improve the reporting of observational studies (23, 24). PRISMA guideline is used for checking the risk of bias in each of the searched studies. Different stages in assessing the risk of bias in individual studies were performed according to the protocol (Table 1) (25-27).
Table 1. Stages used for checking the risk of bias in each of the searched studies (27).
| Stages in risk-of-bias assessment | Specific steps |
|---|---|
| 1. Develop a protocol | • Quality assessment, risk of bias, and concepts should be determined.
• The inclusion of specific risk-of-bias criteria and the choice of specific risk-of-bias rating tool(s) should be stated and justified, respectively. Introduce and specify the tools used to assess bias and the research-specific bias standards and operational definitions that justify the bias assessment criteria. The author should clarify how the risk of bias measures were summarized to obtain a low, moderate, high, or unclear risk of bias for individual outcomes. How to use the scales should be specified. For example, numerical scores lead to risk-of-bias categories. The author should determine how inconsistencies between pairs of risk of bias reviewers. The author should determine how the synthesis of the evidence will comprise an assessment of the risk of bias and how the studies with high or obscure risk of bias will or won't be used in the synthesis of the evidence. |
| 2. Pilot test and train | • The review team should be selected, and at least two reviewers are
required to figure out the risk of bias in each study. A third reviewer resolves
conflicts between reviewers. Train reviewers • Evaluation of a pilot scale of risk of bias instruments using a small subset of studies that represent the range of risk of bias in the evidence base should be considered. Identify problems and issues, then use the appropriate tools to solve the problems. |
| 3. Assess the risk of bias in individual studies | • The design of each study should be determined. Each risk of bias measure should be reviewed by preselected appropriate criteria for that study design and each prespecified outcome. Make a judgment about the overall risk of bias for each outcome, taking into account the conduct of the study. Classify the risk of bias in study design (low, moderate, high, or unknown), documentation of reasons for judgment, and process of finalizing judgment. The author should resolve disagreements in judging and record final ratings for each outcome. |
| 4. Use assessment of the risk of bias in the synthesis of evidence | • Conduct preplanned analyses • Consider additional required analyses • The author should include risk of bias assessment in quantitative/qualitative synthesis. Study design categories should be kept separate. |
| 5. Report assessment of risk of bias process and limitations | • Cite reports on validation of the selected tool(s), the assessment of
the risk of bias process (summarizing from the protocol), and limitations to the
process • Implement measures to improve the reliability assessment of the risk of bias |
Inclusion and exclusion criteria of primary studies
The inclusion criteria included studies that were in English or other languages with English abstracts with clear results (all necessary variables were checked, and the results are mentioned in the abstract). The types of studies were published in the form of an original article (observational studies (case-control, cross-sectional) and reported the prevalence of the desired cases (Patients with VDR gene polymorphisms suffering from pulmonary TB). The exclusion criteria also included the following studies: low quality that did not report the desired index, meta-analysis and review studies, letters to the editor, abstracts of articles presented in the congress, articles whose full text was not available, articles with unknown study time, and articles with insufficient sample size. The opinion was not specified in it (23, 24).
Data analysis method
The final information was summarized and entered into STATA 14 software. To check the heterogeneity more precisely, the I2 test was also used. If the heterogeneity of the studies was low after the qualitative examination, the Q test (P < 0.10) with the Chi-squared (χ2) heterogeneity statistical test was used, and finally, a meta-analysis was performed (9, 23).
According to the results of the heterogeneity of the studies, two statistical models, the fixed effects model and the random effects model, were used for data analysis at a confidence interval (CI) level of 95%, then the Meta and Metan commands, the combined values of the meta-analysis in the fixed model or random effects were estimated, which is the common model used in the combination of research and heterogeneous studies, in meta-analysis. Also, a weighted mean was used to determine the arithmetic mean of unequal and unbalanced data sets and the effect size (ES) (as evaluated index) of the effects along with the CI to measure the relationship of the data more effectively. The Meta command was used in both fixed and random models. The Metan command was also used for discrete and continuous quantitative data and is very similar to the Meta command. By executing this command, the results of the combined studies and the weighted value of the target index were estimated with the corresponding CI. The weight that was applied in this process for each research in calculating the weighted average in random effect meta-analysis was obtained in two steps: weighting with the inverse of the variance and weighting using the variance component of the random effect. A cumulative meta-analysis was also performed using the Metacum command, and the purpose of this analysis was to show the time course of the results of the studies. To show the effect of each study on the final result, a simple sensitivity analysis was also performed using the Metainf command. Meta-regression (The results of metaregression were not significant, so they were not reported in this paper) was also performed in subgroups to evaluate the heterogeneity (Heterogeneity was assessed using a random effects model, but the high level of heterogeneity among studies may be related to uncontrolled intervening factors) between studies such as the year of publication of the study and qualitative confounding factors that might affect the prevalence. Meta-regression also was done to find the actual source of heterogeneity, but the results were not significant. All two-sided statistical tests were considered with α=0.05 (18, 23, 24).
Results
Overview of search in studies
After the initial search in electronic databases, 116 articles were reviewed. Based on the exclusion and inclusion criteria, 44 articles were excluded, and 72 of them were included in this study (Figure 1).
Figure 1.
Flow diagram of the selection process of studies at present systematic review and meta-analysis
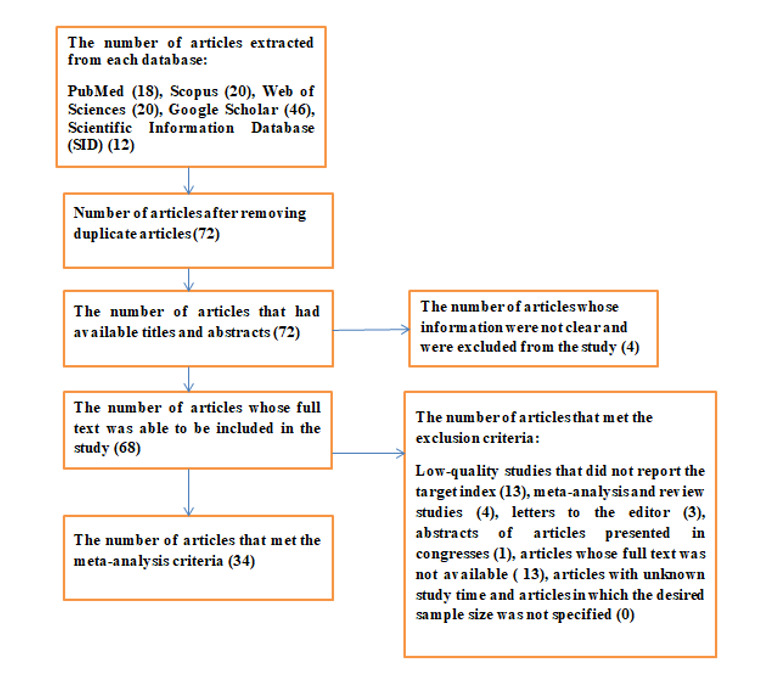
Data information
After completing the exclusion and inclusion procedures and data extraction, 34 articles were selected for final analysis (28-61). A higher rate of ApaI (50%), BsmI (68.40%), FokI (60%), and TaqI (91.40%) polymorphisms was observed in studies performed by Rashedi et al. 2014 in Iran (30), Sinaga et al. 2014 in Indonesia (48), Marashian et al. 2010 in Iran (29) and Wu et al. 2015 in China (50). Also, research performed by Rizvi et al. 2016 in India (32), Sari et al. 2022 in Indonesia (56), Acen et al. 2016 in Uganda (52), and Zhang et al. 2022 in Turkey (61) showed a lower rate of ApaI (19.20%), BsmI (0.56%), FokI (7.30%) and TaqI (0.27%), respectively (Table 2).
Table 2. Data extraction related to systematic review studies and meta-analysis related to polymorphism of VDR genes in people with pulmonary TB.
| Reference | Author | Year of Study | Country | Continent | Method | Type of Study | Women (%) | Men (%) | Number of samples | Age (M± SD) | ApaI (A/a) (%) | BsmI (B/b) (%) | FokI (F/f) (%) | TaqI (T / T) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | Tajik et al. | 2009 | Iran | Asia | PCR-SSP | Case-Control | ND | ND | 96 | ND | 44 | 44 | 52 | 40 |
| 29 | Marashian et al. | 2010 | Iran | Asia | PCR- RFLP | Case-Control | 48.20 | 58.80 | 164 | ND | 22 | 58 | 60 | 52 |
| 30 | Rashedi et al. | 2014 | Iran | Asia | PCR- RFLP | Case-Control | 40.47 | 59.25 | 84 | ND | 50 | 32.14 | 39.30 | 41.67 |
| 30 | Rashedi et al. | 2014 | Iran | Asia | PCR - RFLP | Case-Control | 40.47 | 59.25 | 84 | ND | 50 | ND | ND | ND |
| 31 | Fernández-Mestre et al. | 2015 | Venezuela | Americas | PCR - RFLP | Case-Control | 44 | 56 | 93 | ND | ND | ND | 50.50 | 59.30 |
| 32 | Rizvi et al. | 2016 | India | Asia | PCR-sequencing | Case-Control | ND | ND | 130 | 13.38 | 19.20 | ND | ND | 70.80 |
| 33 | Siregar et al. | 2017 | Indonesia | Asia | PCR - RFLP | Case-Control | 30.30 | 69.70 | 76 | ND | 47.20 | ND | 32 | ND |
| 34 | Zhang et al. | 2018 | China | Asia | Conventional PCR | Case-Control | 32.69 | 67.30 | 52 | 38.02±13.40 | 32.70 | 7.70 | 36.50 | 90.40 |
| 34 | Zhang et al. | 2018 | China | Asia | Conventional PCR | Case-Control | 23.43 | 76.56 | 128 | 38.02±16.37 | 39.10 | 11.70 | 47.70 | 88.30 |
| 35 | Silva-Ramírez et al. | 2019 | Mexico | Americas | Fast Real-Time PCR System | Case-Control | 35 | 65 | 257 | 4.26±18.45 | 48.60 | 37.30 | 45.30 | 51.30 |
| 36 | Kang et al. | 2011 | South Korea | Asia | PCR amplification | Case-Control | 42.58 | 57.41 | 155 | ND | ND | 8.67 | 56.31 | 89.93 |
| 37 | Ates et al. | 2011 | Turkey | Asia | PCR- RFLP | Case-Control | 38 | 62 | 128 | 6.84±26.47 | ND | 53 | 47 | 38 |
| 38 | Rathored et al. | 2012 | India | Asia | PCR- RFLP | Cross-sectional | 35.59 | 64.40 | 354 | 4.50±10.27 | ND | 40 | 51 | 42 |
| 38 | Rathored et al. | 2012 | India | Asia | PCR- RFLP | Cross-sectional | 28.80 | 71.89 | 338 | 37.40 ± 9.60 | ND | 61 | 34 | 42 |
| 39 | Salimi et al. | 2015 | Iran | Asia | PCR- RFLP | Case-Control | 62.50 | 37.50 | 120 | 7.50±19.51 | ND | 55 | 37 | 43 |
| 40 | Joshi et al. | 2014 | India | Asia | PCR- RFLP | Case-Control | 68 | 38 | 110 | ND | ND | 52.73 | 41.82 | ND |
| 41 | Singh et al. | 2011 | India | Asia | PCR- RFLP | Case-Control | 39.60 | 60.40 | 101 | 38.66 ± 2.69 | ND | 51.49 | 39.60 | 60.40 |
| 42 | Al-Tamim et al. | 2016 | Iraq | Asia | PCR- RFLP | Case-Control | 37.09 | 62.91 | 62 | 12.62± 8.82 | ND | 12.90 | 35.48 | 87.10 |
| 43 | Zhang et al. | 2010 | China | Asia | PCR - RFLP | Case-Control | 56.36 | 43.63 | 110 | 33.80 | ND | ND | 39.09 | ND |
| 44 | Wu et al. | 2013 | China | Asia | PCR- RFLP | Case-Control | ND | ND | 2013 | ND | ND | ND | 45.10 | 89.10 |
| 45 | Asgharzadeh et al. | 2014 | Iran | Asia | PCR- RFLP | Case-Control | 40.78 | 59.21 | 76 | ND | ND | ND | 39.30 | ND |
| 46 | Sulaja et al. | 2014 | India | Asia | PCR- RFLP | Case-Control | 54.47 | 44.77 | 134 | ND | ND | ND | 33.58 | ND |
| 47 | Mahmoud et al. | 2014 | Egypt | Africa | PCR and electrophoresis | Case-Control | 32.50 | 67.50 | 40 | 35.00±8.00 | ND | ND | 50 | ND |
| 48 | Sinaga et al. | 2014 | Indonesia | Asia | PCR - RFLP | Case-Control | 30.30 | 69.70 | 76 | ND | 43.40 | 68.40 | 55.30 | ND |
| 49 | Karmila et al. | 2015 | Indonesia | Asia | PCR- RFLP | Case-Control | 46.66 | 53.33 | 30 | ND | ND | 43.33 | ND | |
| 50 | Wu et al. | 2015 | China | Asia | PCR- RFLP and SSCP | Case-Control | ND | ND | 151 | ND | ND | ND | 46.40 | 91.40 |
| 51 | Škodrić-Trifunović et al. | 2015 | Serbia | Europe | PCR - RFLP | Case-Control | 35.45 | 64.54 | 110 | 60.33± 17.82 | ND | ND | 47 | ND |
| 52 | Acen et al. | 2016 | Uganda | Africa | PCR–direct sequencing | Case-Control | 26.80 | 73.20 | 41 | 34.20±12.00 | ND | ND | 7.30 | ND |
| 53 | Sinaga et al. | 2018 | Indonesia | Asia | PCR- RFLP | Case-Control | 41.90 | 58.10 | 43 | ND | ND | ND | 53.50 | ND |
| 53 | Sinaga et al. | 2018 | Indonesia | Asia | PCR- RFLP | Case-Control | 26.80 | 73.20 | 56 | ND | ND | ND | 46.40 | ND |
| 54 | Panda et al. | 2019 | India | Asia | PCR- RFLP | Cross-sectional | 32 | 68 | 150 | 39.30 ±8.10 | ND | ND | 38.60 | ND |
| 55 | Harishankar et al. | 2017 | India | Asia | PCR- RFLP | Case-Control | ND | ND | 50 | 39.70±10.10 | ND | ND | ND | 42 |
| 56 | Sari et al. | 2022 | Indonesia | Asia | Real-Time PCR | Case-Control | 96 | 80 | 176 | 18-60 | ND | 0.56 | 57.95 | 5.68 |
| 57 | Yu et al. | 2023 | China | Asia | Multiplex PCR | Case-Control | 109 | 112 | 221 | ND | ND | ND | ND | 16.28 |
| 58 | Marpaung et al. | 2021 | Indonesia | Asia | PCR-RFLP | Case-Control | 18 | 47 | 65 | 18-65 | ND | ND | 44.61 | ND |
| 59 | Hussein et al. | 2023 | Egypt | Africa | PCR-RFLP | Case-Control | 64 | 136 | 200 | 0-14 | ND | ND | ND | 94 |
| 60 | Sibuea et al. | 2023 | Indonesia | Asia | PCR-RFLP | Case-Control | ND | ND | 32 | ND | ND | ND | ND | ND |
| 61 | Zhang et al. | 2022 | China | Asia | PCR-RFLP | Case-Control | 298 | 433 | 731 | ND | ND | ND | ND | 0.27 |
Results of studied polymorphisms
Considering that the CI was not considered as 1 (This is while the more this CI number is away from 0.5, the less likely it is to be true), other polymorphisms showed a significant relationship with susceptibility to pulmonary TB (P = 0.000). Study design, subjects, and outcomes affect the heterogeneity of studies in a meta-analysis. The results of significant heterogeneity were considered as I2<50%. In this study, significant heterogeneity (P = 0.000) was seen between studies in polymorphisms. According to Egger and Begg tests, there was no publication bias to gene polymorphisms. Due to the existence of more than 50% heterogeneity in all studied polymorphisms, the random effects model was used. Sensitivity analysis was performed by sequentially removing each study. The combined OR after removing each study did not significantly deviate from the sequential removal; these materials indicated strong results in terms of statistics. TaqI gene polymorphisms (95% CI: 0.34-0.77, P = 0.000, ES = 56%) were the most frequent among all studies. ApaI (95% CI: 0.31-0.48, P = 0.000, ES = 39%) and BsmI (95% CI: 0.24-0.50, P = 0.000, ES = 37%) gene polymorphisms rates were lower than the TaqI andFokI (95% CI: 0.39-0.46, P = 0.000, ES = 43%) (Table 3).
Table 3. The results of a meta-analysis of studied polymorphisms, degree of heterogeneity between studies, and publication bias.
| Gene Polymorphisms | Number of Cases in all Articles | Pooled Mean Effect Size (ES) | Confidence Interval (CI) 95% for ES (Lower –Upper) | Z-Statistic for ES | P-Value for ES | I2 (Degree of Heterogeneity) | Publication Bias-Test Begg, Egger P-Value Test | Model |
|---|---|---|---|---|---|---|---|---|
| ApaI (A/a) | 10 | 39% | (0.31-0.48) | 9.30 | 0.000 | 88.8% | 0.221 | Random Effect |
| BsmI (B/b) | 14 | 37% | (0.24- 0.50) | 5.52 | 0.000 | 98.9% | 0.392 | Random Effect |
| FokI (F/f) | 28 | 43% | (0.39- 0.46) | 23.48 | 0.000 | 83.23% | 0.537 | Random Effect |
| TaqI (T / T) | 17 | 56% | (0.34- 0.77) | 5.17 | 0.000 | 99.8% | 0.211 | Random Effect |
* Z-Statistic for ES =0
Results of TaqI gene polymorphisms
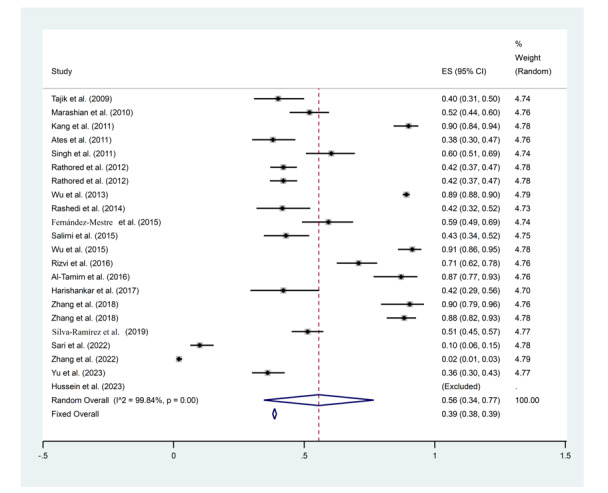
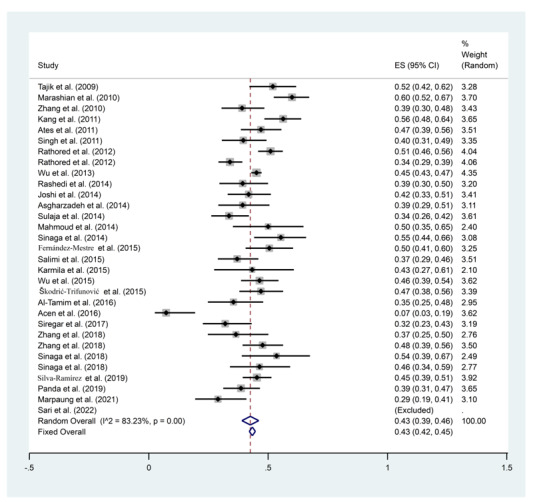
Wu et al. 2015 (95% CI: 0.86-0.95, ES = 91%, Weight: 4.78) (50) and Zhang et al. 2022 (95% CI: 0.01-0.03, ES = 0.02%, Weight: 4.79) (61) showed the highest and the lowest rate of TaqI gene polymorphisms among all studies, respectively (Figure 2).
Figure 2.
Frequency results of TaqI gene polymorphisms with a combined ES of 56%
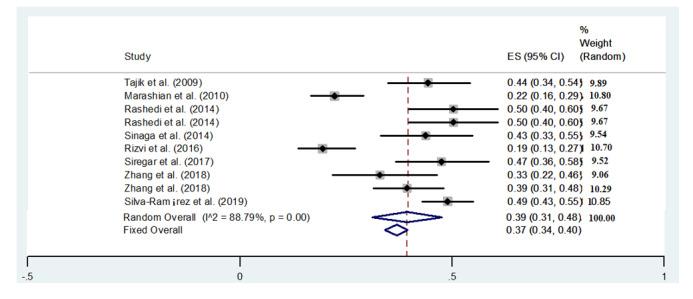
Results of ApaI gene polymorphisms
Rashedi et al. 2014 (95% CI: 0.40-0.60, ES = 50%, Weight: 9.67) (30) and Silva-Ramيrez et al. 2019 (95% CI: 0.43-0.55, ES = 49%, Weight: 10.58) (35) showed the highest rate of ApaI gene polymorphisms among all studies, and Rizvi et al. 2016 (95% CI: 0.19-0.13, ES = 19%,
Weight: 10.70) (32) showed the lowest rate (Figure 3).
Figure 3.
Frequency results of ApaI gene polymorphisms with a combined ES of 39%
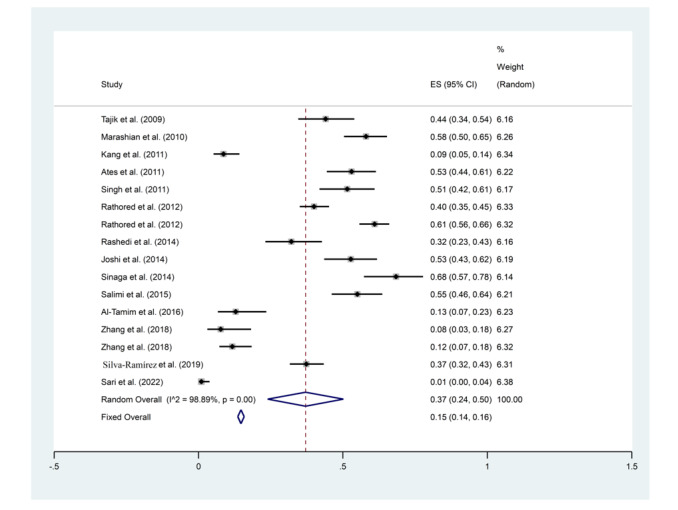
Results of BsmI gene polymorphisms
The random model about BsmI in the studied population showed that the higher rate of gene polymorphisms was related to Sinaga et al. 2014 (95% CI: 0.57-0.78, ES = 68%, Weight: 6.14) (48) and the lowest rate of it was related to Sari et al. 2022 (95% CI: 0.00-0.04, ES = 0.01%, Weight: 6.38) (56) (Figure 4).
Figure 4.
Frequency results of BsmI gene polymorphisms with a combined ES of 37%
Results of FokI gene polymorphisms
The highest and lowest rate of FokI polymorphisms were observed in Marashian et al. 2010 (95% CI: 0.52-0.67, ES=60%, Weight: 3.70) (29) and Acen et al. 2016 (95% CI: 0.03-0.19, ES=0.07%, Weight: 3.62) (52), respectively (Figure 5).
Figure 5.
Frequency results of FokI gene polymorphisms with a combined ES of 43%
Discussion
Different polymorphisms have been determined in the sequences of the VDR gene, but the practical effect of these changes on the human body is still unclear. These genes play many important roles, such as synthesis, activation, delivery, and binding of the activated vitamin D. Vitamin D produces cathelicidin, which destroys the microbial membrane and has an antimicrobial effect. Vitamin D deficiency is very common in patients with TB, and low serum vitamin D levels are more common in these patients (62-64). Research has shown that genetic factors are involved in 10% of patients who suffer from TB. Apart from environmental factors (such as bad economic conditions, malnutrition, stress, and bad health conditions), the pathogenesis of TB can depend on biological factors such as the active metabolite of 1, 25-dihydroxy vitamin D. Vitamin D can increase immunity against M. tuberculosis infection by increasing phagocytosis through activation of macrophages and regulation of the innate and acquired immune system (65, 66). Results of determining the polymorphism frequency of VDR genes in our study
found that TaqI gene polymorphism was the most frequent (ES of 56%). A higher rate of ApaI (50%) also was observed in Rashedi et al. 2014 in Iran (30). In 2011, Su et al. showed that the VDR gene is an early prospect gene for TB vulnerability, but the results of several studies are somewhat contradictory. Therefore, further investigation into the relationship between gene polymorphisms and the risk of pulmonary TB seems necessary. In the study performed by Su et al.,it was found that Fok I polymorphism (OR=1.12) had a definite relationship with susceptibility to pulmonary TB. Fok I polymorphism of the VDR gene may have a high-risk role in pulmonary TB (65). Sadykov et al. 2020 showed that TB infection has been able to cause one of the most important health problems in society and also created a significant economic burden in Asian countries. Recent findings show that the amount of processes related to immunity and host defense against M. tuberculosis is influenced by various genes of the human genome, including genes that play a role in vitamin D metabolism. Similar to our results, Sadykov et al. showed a significant relationship between polymorphism of Bsm I (OR = 0.425, 39%) and Taq I (OR = 0.443, 62%) in patients suffering from pulmonary TB. In our study, TaqI and BsmI gene polymorphisms were found at 56% and 37%, respectively, and a significant relationship between TaqI and BsmI gene polymorphisms with pulmonary TB was found, too. So, genetic variation in VDR genes can affect susceptibility to pulmonary TB. These findings can provide researchers with initial clues to understand individual genetic differences in pulmonary TB susceptibility (67). Sari et al., in 2022, found a significant relationship between Vitamin D levels and patients suffering from TB.
Vitamin D deficiency was observed in 85.1% of TB patients with VDR gene polymorphisms (56). In a research conducted in 2013 by Wu et al., the study of genetic changes in the VDR gene and its effect on the risk of TB was investigated. In Wu et al. study, contrary to the results of other studies, the association between BsmI VDR gene polymorphisms according to allele model (b versus (vs). B: OR = 0.78), homozygote model (bb vs. BB: OR = 0.61), recessive model (bb vs. Bb+BB: OR = 0.70) and dominant model (bb+Bb vs. BB: OR = 0.77) was associated with a reduced risk of pulmonary TB in the Asian population. The frequency of VDR polymorphisms, the presence and/or absence of the relationship between this type of polymorphism or susceptibility to pulmonary TB is different based on the race and genotype of the studied population, methods, and also sampling. Also, gene polymorphisms are complex and fluctuating, mostly attributed to different ethnic groups. In addition, the burden of pulmonary TB is geographically highest in Asia and Africa (68). In our study, a higher rate of ApaI (50%), BsmI (68.40%), FokI (60%), and TaqI (91.40%) polymorphisms was observed in Iran (30), Indonesia (48), Iran (29), and China (50). In the study of Protas et al. in 2023, it was shown that the VDR polymorphism frequency may depend on ethnicity (69). The TB report of the World Health Organization (WHO) has shown that the yellow-skinned race is more susceptible to TB than the black and white-skinned race. Also, several environmental factors can affect the VDR gene expression levels, including food habits, intensity, and hours of sunlight are also effective on this factor (68). Mohammadi et al. in 2020 showed that FokI and ApaI polymorphisms did not show any significant effect on the development of pulmonary TB in Iranian populations, but TaqI (Allele comparison (OR: 1.57), additive model of tt/TT (OR: 1.57), recessive model (tt/Tt + TT) (OR: 1.99) and dominant model (tt + Tt/TT) (OR: 1.98)) and BsmI (Dominant genotype (bb + bB/BB) (OR: 1.44)) had a significant effect on the risk of pulmonary TB (70).
In the study conducted by Marpaung et al. in 2021, FokI polymorphisms, including FF (41.5%), ff (44.6%), and ff (13.8%) were found in patients with pulmonary TB. However, there was no significant relationship between FokI polymorphism and pulmonary TB (58). Yu et al. in 2023 reported that allele and genotype frequencies of Fok I, Taq I, Apa I, and Bsm I, in VDR were not related to TB susceptibility (57). Also, in our study, ApaI, BsmI, TaqI, and FokI gene polymorphisms were found in patients suffering from pulmonary TB. However, the effects of these genes should be assessed in a larger sample size. In the meantime, the relationship between polymorphism of VDR and susceptibility to pulmonary TB may be moderated by host vitamin D status. Vitamin D deficiency in the body may increase the risk of developing pulmonary TB. Also, in some studies, TaqI has not played any role in the development of TB and it is not present in the population with a high frequency of TB, so more research is needed to be considered (68). On the other hand, the contradictory results presented in different studies and the individual high-risk genotypes proposed in each population may be due to the selection of patients from different populations, different races, types of nutrition, and living environments or geographic regions (71-73). Types of vitamin D also may play a different role in each population. In each study, the diversity of the genetic background among each ethnicity and the genotypes and allelic frequencies of vitamin D polymorphisms are other effective factors in the amount and relationship of vitamin D polymorphisms and the risk of TB (68). This study has some limitations. Articles used in the present study were limited to ten years and some countries. Some other VDR genes should be considered, and the results should be compared together. To check keywords according to Emtree, a formal university account was needed. This account was not available to us. On the other hand, this analysis is done using statistical methods to summarize independent studies to find the exact form of the relationship between the investigated variables. Meta-analysis results are one of the most reliable research methods in the field of evidence-based medicine. It is a synthesis and combination of different studies that exist on a specific topic and provides the possibility of strengthening and clarifying the results of different studies. This is an essential method for the synthesis of studies and it allows us to provide an accurate and universal answer according to all current knowledge.
Conclusion
A higher rate of ApaI, BsmI, FokI, and TaqI polymorphisms was observed in Iran, Indonesia, Iran and China, respectively. Significant heterogeneity was observed between studies in terms of polymorphisms. TaqI gene polymorphisms were the most frequent in the studies. The highest rate of TaqI, ApaI, BsmI, and FokI gene polymorphisms among all studies was shown by studies in 2015, 2014, 2014, and 2010, respectively. According to the results, it seems that investigations of patients suffering from TB in terms of polymorphism of these genes are needed. Also, to control this disease, it is necessary to prescribe vitamin D and take it under the supervision of a related specialist.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgment
We would like to express our gratitude to the Research and Technology Vice-Chancellor of Qazvin University of Medical Sciences for the material and spiritual assistance in advancing the present project.
Ethical Approval
The present study was approved by the Ethics Committee of Qazvin University of Medical Sciences, Qazvin, Iran, with code IR.QUMS.REC.1399.430.
Authors Contribution
Study conception and design: RR and SR; Acquisition of data: AHP and RZ; Analysis and interpretation of data: SAG and SR; Drafting of the manuscript: SR and AP; Critical revision: SR, SAG, and NR; All the authors read and approved the final article. All authors contributed to the study design.
Cite this article as : Samimi R, Hosseinpanahi A, Zaboli R, Peymani A, Rouhi S, Ahmadi Gooraji S, Rajaei N. Prevalence of Vitamin D Receptor Gees Polymorphisms in People with Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Med J Islam Repub Iran. 2024 (25 Mar);38:32. https://doi.org/10.47176/mjiri.38.32
References
- 1.Shen H, Liu Q, Huang P, Fan H, Zang F, Liu M. et al. Vitamin D receptor genetic polymorphisms are associated with oral lichen planus susceptibility in a Chinese Han population. BMC Oral Health. 2020;20(1):1–7. doi: 10.1186/s12903-020-1002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol. 2016;14(11):677–691. doi: 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]
- 3.Cain KP, Anekthananon T, Burapat C, Akksilp S, Mankhatitham W, Srinak C. et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis. 2009;15(2):258. doi: 10.3201/eid1502.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alavi SM, Enayatrad M, Cheraghian B, Amoori N. Incidence trend analysis of tuberculosis in Khuzestan Province, southwest of Iran: 2010–2019. Glob Epidemiol. 2023;6:100118. doi: 10.1016/j.gloepi.2023.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doosti A, Nasehi M, Moradi G, Roshani D, Sharafi S. et al. The pattern of tuberculosis in Iran: a national cross-sectional study. Iran J Public Health. 2023;52(1):193–200. doi: 10.18502/ijph.v52i1.11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagcchi S. WHO's global tuberculosis report 2022. Lancet Microbe. 2023;4(1):e20. doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 7.Owolabi KM, Pindza E. A nonlinear epidemic model for tuberculosis with caputo operator and fixed point theory. Healthcare Analytics. 2022;2:100111. doi: 10.1016/j.health.2022.100111. [DOI] [Google Scholar]
- 8.Reichler MR, Khan A, Sterling TR, Zhao H, Moran J, McAuley J. et al. Tuberculosis epidemiologic studies consortium task order 2 team. risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. J Infect Dis. 2018;218(6):1000–1008. doi: 10.1093/infdis/jiy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Wen F, Wang Z. Correlation between polymorphism of vitamin D receptor TaqI and susceptibility to tuberculosis: An update meta-analysis. Medicine (Baltimore) 2022;101(16):e29127. doi: 10.1097/MD.0000000000029127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wimalawansa SJ. Vitamin D adequacy and improvements of comorbidities in persons with intellectual developmental disabilities. J Child Dev Disord. 2016;2:22–33. doi: 10.4172/2472-1786.100030. [DOI] [Google Scholar]
- 11.Cândido FG, Bressan J. Vitamin D: link between osteoporosis, obesity, and diabetes. Int J Mol Sci. 2014;15(4):6569. doi: 10.3390/ijms15046569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Cao WC, Zhang CY, Tian L, Wu XM, Habbema JD. et al. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case-control study. Int J Tuberc Lung Dis. 2004;8(4):428. [PubMed] [Google Scholar]
- 13.Annweiler C, Annweiler T, Montero-Odasso M, Bartha R, Beauchet O. Vitamin D and brain volumetric changes: Systematic review and meta-analysis. Maturitas. 2014;78(1):30. doi: 10.1016/j.maturitas.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Chiarella P, Capone P, Sisto R. Contribution of genetic polymorphisms in human health. Int J Environ Res Public Health. 2023;20(2):912. doi: 10.3390/ijerph20020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junaid K, Rehman A. Impact of vitamin D on infectious disease-tuberculosis-a review. Clin Nutr Exp. 2019;25:1–10. [Google Scholar]
- 16.Mansy W, Ibrahim NH, Al-Gawhary S, Alsubaie SS, Abouelkheir MM, Fatani A. et al. Vitamin D status and vitamin D receptor gene polymorphism in Saudi children with acute lower respiratory tract infection. Mol Biol Rep. 2019;46(2):1955–1962. doi: 10.1007/s11033-019-04645-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li HJ. Meta-analysis on associations between vitamin D receptor genetic variants and tuberculosis. Microb Pathog. 2019;130:59–64. doi: 10.1016/j.micpath.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadpour B, Rouhi S, Moradi M, Ramazanzadeh R, Saniyi E, Zandi S. et al. Prevalence of metallo-β-lactamases in Acinetobacter baumannii in Iran: a review and meta-analysis. Infect Disord Drug Targets. 2019;19(4):350–361. doi: 10.2174/1871526518666181016101430. [DOI] [PubMed] [Google Scholar]
- 19.Aslam S, Emmanuel P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31:47–50. doi: 10.4103/0253-7184.69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev. 2014;3:54. doi: 10.1186/2046-4053-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkis-Onofre R, Catalá-López F, Aromataris E, Lockwood C. How to properly use the PRISMA statement. Syst Rev. 2021;10:117. doi: 10.1186/s13643-021-01671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekhuis T, Demner-Fushman D, Crowley RS. Comparative effectiveness research designs: an analysis of terms and coverage in Medical Subject Headings (MeSH) and Emtree. J Med Libr Assoc. 2013;101:92–100. doi: 10.3163/1536-5050.101.2.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammadi B, Ramazanzadeh R, Nouri B, Rouhi S. Frequency of codon 306 mutations in embB gene of Mycobacterium tuberculosis resistant to ethambutol: a systematic review and meta-analysis. Int J Prev Med. 2020;11:112. doi: 10.4103/ijpvm.IJPVM_114_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadpour B, Rouhi S, Khodabandehloo M, Moradi M. Prevalence and association of human papillomavirus with esophageal squamous cell carcinoma in Iran: a systematic re-view and meta-analysis. Iran J Public Health. 2019;48(7):1215–1226. [PMC free article] [PubMed] [Google Scholar]
- 25.Erfani Y, Rasti A, Janani L. Prevalence of Gram-negative bacteria in ventilator-associated pneumonia in neonatal intensive care units: a systematic review and meta-analysis protocol. BMJ Open. 2016;6(10):e012298. doi: 10.1136/bmjopen-2016-012298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JSK, Harky A. The importance of risk of bias assessment in meta-analyses: does controlling heterogeneity suffice?. Eur J Cardiothorac Surg. 2020;58(5):1102. doi: 10.1093/ejcts/ezaa174. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M. et al. Introduction on 'assessing the risk of bias of individual studies' in systematic review of health-care intervention programs revised by the Agency for Healthcare Research and Quality. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Mar 8; North Carolina, United States of America. 20085
- 28.Tajik N, Jafari M, Nasiri M, Mousavi T, Farnia P, Salekmogaddam A. The study of the association between Vitamin D receptor common genetic polymorphisms and susceptibility to pulmonary tuberculosis. RJMS. 2009;16:14–21. [Google Scholar]
- 29.Marashian SM, Farnia P, Seyf S, Anoosheh S, Velayati AA. Evaluating the role of vitamin D receptor polymorphisms on susceptibility to tuberculosis among Iranian patients: a case-control study. Tuberk Toraks. 2010;58(2):147. [PubMed] [Google Scholar]
- 30.Rashedi J, Asgharzadeh M, Moaddab S, Amani M, Mazani M. ApaI polymorphism of Vitamin D receptor gene and association with susceptibility to tuberculosis. J Ardabil Univ Med Sci. 2013;13:379–387. [Google Scholar]
- 31.Fernández-Mestre M, Villasmil Á, Takiff H, Fuentes Alcala. NRAMP1 and VDR gene polymorphisms in susceptibility to tuberculosis in Venezuelan population. Dis Markers. 2015;2015:860628. doi: 10.1155/2015/860628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizvi I, Garg RK, Jain A, Malhotra HS, Singh AK, Prakash S. et al. Vitamin D status, vitamin D receptor and toll like receptor-2 polymorphisms in tuberculous meningitis: a case–control study. Infection. 2016;44(5):633. doi: 10.1007/s15010-016-0907-x. [DOI] [PubMed] [Google Scholar]
- 33.Siregar Y, Sinaga BY. Is there any impact of VDR gene polymorphism in Bataks Ethnic to have tuberculosis. J Health Sci. 2017;5:1–8. [Google Scholar]
- 34.Zhang Y, Zhu H, Yang X, Guo S, Liang Q, Lu Y. et al. Serum vitamin D level and vitamin D receptor genotypes may be associated with tuberculosis clinical characteristics: a case-control study. Medicine (Baltimore) 2018;97(30):e11732. doi: 10.1097/MD.0000000000011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Ramírez B, Saenz-Saenz CA, Bracho-Vela LA, Peñuelas-Urquides K, Mata-Tijerina V, Escobedo-Guajardo BL. et al. Association between vitamin D receptor gene polymorphisms and pulmonary tuberculosis in a Mexican population. Indian J Tuberc. 2019;66(1):70–75. doi: 10.1016/j.ijtb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Kang TJ, Jin SH, Yeum CE, Lee SB, Kim CH, Lee SH. et al. Vitamin D receptor gene TaqI, BsmI and FokI polymorphisms in Korean patients with tuberculosis. Immune Netw. 2011;11(5):253–257. doi: 10.4110/in.2011.11.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ates O, Dolek B, Dalyan L, Musellim B, Ongen G, Topal-Sarikaya A. The association between BsmI variant of vitamin D receptor gene and susceptibility to tuberculosis. Mol Biol Rep. 2011;38(4):2633. doi: 10.1007/s11033-010-0404-8. [DOI] [PubMed] [Google Scholar]
- 38.Rathored J, Sharma SK, Singh B, Banavaliker JN, Sreenivas V, Srivastava AK. et al. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25 (OH) D. Int J Tuberc Lung Dis. 2012;16(11):1522. doi: 10.5588/ijtld.12.0122. [DOI] [PubMed] [Google Scholar]
- 39.Salimi S, Farajian-Mashhadi F, Alavi Naini, Talebian G, Narooie-Nejad M. Association between vitamin D receptor polymorphisms and haplotypes with pulmonary tuberculosis. Biomed Rep. 2015;3(2):189–194. doi: 10.3892/br.2014.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi L, Ponnana M, Penmetsa SR, Nallari P, Valluri V, Gaddam S. Serum Vitamin D Levels and VDR Polymorphisms (B sm I and F ok I) in Patients and their Household Contacts Susceptible to T uberculosis. Scand J Immunol. 2014;79(2):113. doi: 10.1111/sji.12127. [DOI] [PubMed] [Google Scholar]
- 41.Singh A, Gaughan JP, Kashyap VK. SLC11A1 and VDR gene variants and susceptibility to tuberculosis and disease progression in East India. Int J Tuberc Lung Dis. 2011;15(11):1468. doi: 10.5588/ijtld.11.0089. [DOI] [PubMed] [Google Scholar]
- 42.Al-Tamimi B, Al-Harbi S, Al-Janabi IA, Al-Taee RA, Al-Hamawandi JA. Impact of Vitamin D receptor gene polymorphisms on the susceptibility to tuberculosis among Iraqi patients. Int J Pharmtech Res. 2016;9:376–384. [Google Scholar]
- 43.Zhang HQ, Deng A, Guo CF, Wang YX, Chen LQ, Wang YF. et al. Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Int J Tuberc Lung Dis. 2010;41(1):46. doi: 10.1016/j.arcmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Wu F, Zhang W, Zhang L, Wu J, Li C, Meng X. et al. NRAMP1, VDR, HLA-DRB1, and HLA-DQB1 gene polymorphisms in susceptibility to tuberculosis among the Chinese Kazakh population: a case-control study. Biomed Res Int. 2013;2013:484535. doi: 10.1155/2013/484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asgharzadeh M, Rashedi J, Moaddab SR, Ansarin K, Mirgasemi Z, Shirazi S. Foki polymorphism, vitamin D receptor gene start codon and its association with tuberculosis. Stud Med Sci. 2014;2:778–783. [Google Scholar]
- 46.Sulaja BD, UK C. rs10735810 of vitamin D receptor (VDR) gene: association with pulmonary tuberculosis in children. J Mol Biol. 2014;2:32. [Google Scholar]
- 47.Mahmoud AA, Ali AH. Vitamin D receptor gene polymorphism and 25 hydroxy vitamin D levels in Egyptian patients with pulmonary tuberculosis. Egypt J Chest Dis Tuberc. 2014;63(3):651–655. [Google Scholar]
- 48.Sinaga BY, Amin M, Siregar Y, Sarumpaet SM. Correlation between Vitamin D receptor gene FOKI and BSMI polymorphisms and the susceptibility to pulmonary tuberculosis in an Indonesian Batak-ethnic population. Acta Med Indones. 2014;46(4):275. [PubMed] [Google Scholar]
- 49.Karmila A, Nazir M, Yangtjik K, Yuwono Y. Serum vitamin D and vitamin D receptor gene FokI polymorphisms in children with tuberculosis. Paediatr Indones. 2015;55(5):263. doi: 10.14238/pi55.5.2015.263-7. [DOI] [Google Scholar]
- 50.Wu L, Deng H, Zheng Y, Mansjö M, Zheng X, Hu Y. et al. An association study of NRAMP1, VDR, MBL and their interaction with the susceptibility to tuberculosis in a Chinese population. Int J Infect Dis. 2015;38:129. doi: 10.1016/j.ijid.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Škodrić-Trifunović V, Buha I, Jovanović D, Vučinić V, Stjepanović M, Spasovski V. et al. Variants in VDR and NRAMP1 genes as susceptibility factors for tuberculosis in the population of Serbia. Genetika. 2015;47:1021. [Google Scholar]
- 52.Acen EL, Worodria W, Mulamba P, Kambugu A, Erume J. The frequency distribution of vitamin D Receptor fok I gene polymorphism among Ugandan pulmonary TB patients. F1000Research. 2016;5:ISCB Comm J-1890. doi: 10.12688/f1000research.9109.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinaga BY, Amir Z, Siagian P. The role of FokI polymorphism of vitamin D receptor gene and vitamin D level in multidrug-resistant tuberculosis occurrence in Medan city, Indonesia. Arch Med Sci Civiliz Dis. 2018;3:e153. [Google Scholar]
- 54.Panda S, Tiwari A, Luthra K, Sharma SK, Singh A. Association of Fok1 VDR polymorphism with vitamin D and its associated molecules in pulmonary tuberculosis patients and their household contacts. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-51803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harishankar M, Selvaraj P. Influence of Cdx2 and TaqI gene variants on vitamin D3 modulated intracellular chemokine positive T-cell subsets in pulmonary tuberculosis. Clin Ther. 2017;39(5):946–957. doi: 10.1016/j.clinthera.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Sari DK, Dharmajaya R, Sari MI, Masyithah D. Vitamin D Receptor Gene Polymorphism Affecting Vitamin D and Beta Carotene Deficiency in Tuberculosis Patients. Open Access Maced J Med Sci. 2022;10:30. doi: 10.3889/oamjms.2022.9284. [DOI] [Google Scholar]
- 57.Yu J, Liu M, Mijiti X, Liu H, Wang Q, Yin C. et al. Association of single-nucleotide polymorphisms in the VDR gene with tuberculosis and infection of Beijing genotype Mycobacterium tuberculosis. Infect Drug Resist. 2023;16:3157–3169. doi: 10.2147/IDR.S407595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marpaung DO, Sinaga BY, Siagian P, Mutiara E. The association between vitamin D receptor gene FokI polymorphism and pulmonary tuberculosis. Res Sci. 2021;2(1):1–7. [Google Scholar]
- 59.Hussein MM, Mohamed EM, Kamal TM, Deraz TE. Increased susceptibility to complicated pneumonia among egyptian children with FokI (rs2228570), not TaqI (rs731236), vitamin D receptor gene polymorphism in association with vitamin D deficiency: a case-control study. BMC Pediatr. 2023;23(1):394. doi: 10.1186/s12887-023-04192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sibuea CV, Pardosi M, Simbolon I, Tampubolon PA. Polimorfisme gen vitamin D receptor (VDR) BsmI pada multi drug resistant tuberculosis (MDR TB) NJM. 2023;9:49–52. [Google Scholar]
- 61.Zhang X, Zhang Y, Yin Z, Xia W, Mao H, Bao L. et al. Relationship between vitamin D receptor gene polymorphisms and second acid-fast bacilli smear-positive during treatment for tuberculosis patients. Infect Genet Evol. 2022;103:105324. doi: 10.1016/j.meegid.2022.105324. [DOI] [PubMed] [Google Scholar]
- 62.Azimzadeh P, Montazerhaghighi M, Mohebbi SR, Romani S, Fatemi SR, Pourhoseingholi MA. et al. Evaluation of the association between FokI and BsmI polymorphisms of vitamin D receptor gene and risk of colorectal cancer in Iranian patients referred to Taleghani hospital, Tehran. Medical Sciences. 2011;21:55–60. [Google Scholar]
- 63.Shah Mohamadi, Ahmadizadeh C. A study of Polymorphism of Vitamin D Receptor and miR-378 on Vaccination non-responding in Pregnant Women suffering from Chronic Hepatitis B Infection. Yafte. 2019;20(4):116–126. [Google Scholar]
- 64.Wang Y, Li HJ. A meta-analysis on associations between vitamin D receptor genetic variants and tuberculosis. Microb Pathog. 2019;130:59–64. doi: 10.1016/j.micpath.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 65.Su Q, Ma X, Lin H, Li Y, Hu D, Xiong H. et al. Association between gene polymorphisms of vitamin D receptor and pulmonary tuberculosis susceptibility: a meta-analysis. Journal of Medical Colleges of PLA. 2011;26:63–75. [Google Scholar]
- 66.Cervantes JL, Oak E, Garcia J, Liu H, Lorenzini PA, Batra D, Chhabra A, Salazar JC, Roca X. Vitamin D modulates human macrophage response to Mycobacterium tuberculosis DNA. Tuberculosis. 2019;116(2019):S131. doi: 10.1016/j.tube.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadykov M, Azizan A, Kozhamkulov U, Akilzhanova A, Yerezhepov D, Salfinger M. et al. Association of genetic variations in the vitamin D pathway with susceptibility to tuberculosis in Kazakhstan. Mol Biol Rep. 2020;47(3):1659–1666. doi: 10.1007/s11033-020-05255-3. [DOI] [PubMed] [Google Scholar]
- 68.Wu YJ, Yang X, Wang XX, Qiu MT, You YZ, Zhang ZX. et al. Association of vitamin D receptor BsmI gene polymorphism with risk of tuberculosis: a meta-analysis of 15 studies. PloS One. 2013;8:e66944. doi: 10.1371/journal.pone.0066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Protas VV, Pogossyan GP, Li KG, Danilenko MP. Frequency of rs2228570 single nucleotide polymorphism of Vitamin-D Receptor (VDR) gene among the Kazakh ethnic group. Series Biology Medicine Geography. 2023;1:117. [Google Scholar]
- 70.Mohammadi A, Khanbabaei H, Nasiri-Kalmarzi R, Khademi F, Jafari M, Tajik N. Vitamin D receptor ApaI (rs7975232), BsmI (rs1544410), Fok1 (rs2228570), and TaqI (rs731236) gene polymorphisms and susceptibility to pulmonary tuberculosis in an Iranian population: a systematic review and meta-analysis. J Microbiol Immunol Infect. 2020;53:827. doi: 10.1016/j.jmii.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Roshani D, Ramazanzadeh R, Farhadifar F, Ahmadi A, Derakhshan S, Rouhi S. et al. A PRISMA systematic review and meta-analysis on Chlamydia trachomatis infections in Iranian women (1986-2015) Medicine (Baltimore) 2018;97(16):e0335. doi: 10.1097/MD.0000000000010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rouhi S, Roshani D, Shakib P, Ahangarkani F, Ramazanzadeh R. A 10-year survey on prevalence and occurrence rate of multi-drug resistant Mycobacterium tuberculosis in Latin American and Mediterranean families: a systematic review and meta-analysis. JBRMS. 2018;5:51–61. [Google Scholar]
- 73.Ramazanzadeh R, Roshani D, Shakib P, Rouhi S. Prevalence and occurrence rate of Mycobacterium tuberculosis Haarlem family multi-drug resistant in the worldwide population: A systematic review and meta-analysis. J Res Med Sci. 2015;20:78–88. [PMC free article] [PubMed] [Google Scholar]


