Abstract
Cytotoxic T cells react to cardiac peptides in patients with cancer that receive immunotherapy, resulting in myocarditis. Identifying the targets for T cells in patients who are immunosuppressed will enable a better understanding of individual risk before treatment is initiated
In the past decade, the discovery of immune checkpoint inhibitors (ICIs) ushered in a new age of cancer treatment, prolonging life for patients with previously incurable metastatic disease. What has become apparent during therapy is the development of immune-related adverse events (irAEs) in upwards of around 40% of patients in a variety of tissue locations, including the heart. Typically, irAEs are minor. However, rare but life-threatening reactions do occur that necessitate not only discontinuation of the cancer therapy itself, but also initiation of aggressive immunosuppression1. Mechanistically, irAEs are thought to be due to the release of inhibitory ‘brakes’ that are present in T cells, allowing the T cells to target normal tissues inappropriately rather than targeting the malignancy. Myocarditis, although an uncommon irAE, has a fatality rate of up to 50% and is frequently accompanied by myositis (skeletal muscle inflammation) in a process that is poorly understood2. Thus, there is impetus to gain a better understanding of the immune-based mechanisms through which irAEs can emerge and, by extension, a need for prognostic and therapeutic strategies to detect and mitigate the irAEs in patients with cancer that receive ICI treatment. In a recent issue of Nature, Axelrod et al.3 explore these urgent questions using a mouse model of ICI-induced myocarditis and report the identification of important correlations in human patients.
Immune checkpoints refer to a group of receptors and the associated signaling pathways that positively or negatively regulate immune homeostasis, particularly by modulating T cell activity. For example, programmed cell death 1 (PD-1) is expressed by T cells. When bound to programmed death-ligand 1 (PD-L1), PD-1 provides an inhibitory signal that suppresses T cell activation. Tumor cells in many cancers express high levels of PD-L1, evading destruction by cytotoxic CD8+ T cells. Similarly, cytotoxic T lymphocyte antigen-4 (CTLA-4) negatively regulates T cell proliferation and activation through a non-redundant mechanism by outcompeting CD28 for binding with CD80 and/or CD86 on dendritic cells, which would otherwise provide crucial costimulatory signals4. Removing these brakes on T cell activation using combinatorial drugs that block these pathways can restore antitumor activity and promote survival in patients with cancer.
Axelrod et al. utilize a mouse model5 that lacks the gene encoding PD-1 (Pdcd1) and possesses only one copy of the gene encoding CTLA-4 (Ctla4). Pdcd1−/−Ctla4+/− mice experience premature death and myocarditis that is characterized predominantly by CD8+ T cell infiltration into the myocardium, as in patients with cancer that receive combination ICI therapy and experience fulminant myocarditis6. Using single-cell RNA sequencing (scRNA-seq) and T cell receptor sequencing (TCR-seq), the authors identify specific TCR clonotypes among T cell infiltrates in murine ICI-associated myocarditis (ICI-MC), providing a granular look into the antigen-specificity of clonal T cell populations underlying the disease. In a series of elegant experiments, CD8+ T cells are confirmed as necessary for myocarditis to occur in this model, as myocarditis and survival are ameliorated following treatment with CD8-depleting antibody or reproduced in recipient mice that received splenocytes containing CD8+ T cells from Pdcd1−/−Ctla4+/− mice. The question is, why are CD8+ T cells able to recognize cardiac tissue antigens?
α-Myosin (MYH6) is a protein that facilitates myocyte contraction and relaxation, and peptides of this protein were revealed as autoantigens that are recognized by the dominant CD8+ T cell clonotypes in murine ICI-MC. Autoreactive CD8+ T cells are typically deleted in the thymus, where virtually all self-proteins are expressed to eliminate selfrecognition. Notably, Myh6 was among a group of four genes (the other three being Nppb, Nppa and Sbk2) that were enriched in the heart yet not expressed in the thymus, creating an opportunity for autoreactive T cells specific for cardiac antigen to escape into circulation. Observations in the mouse extended into human patients – MYH6 peptides expanded autoreactive T cells from peripheral blood mononuclear cells (PBMCs) of ICI-MC patients and healthy controls. Moreover, TCR-seq of cardiac and skeletal muscle tissue from ICI-MC patients showed similar TCR clonotypes as seen in MYH6-expanded cultures. Previously, myosin peptides were linked to human myocarditis through antibody-mediated7 or CD4+ T cell-mediated responses8, and MYH6 was identified as an autoantigen in a murine model of spontaneous myocarditis9. In the current study, Axelrod et al.3 show that CD8+ T cells underlie both mouse and human ICI-MC, presenting strong correlative evidence that the presence of MYH6-derived peptides provides a biomarker for myocarditis risk as well as prospective candidates for targeted approaches that seek to attenuate myocarditis in patients receiving ICI treatment.
Axelrod et al.3 established a comprehensive map of the immune landscape using scRNA-seq and showed that activated T cells comprised a larger proportion of the overall immune cell population in the hearts of Pdcd1−/−Ctla4+/− mice with myocarditis (34%) relative to healthy controls (2%). Of the T cells, TCR-seq identified a high degree of clonality in mice with myocarditis, in which a large proportion expressed Cd8a (which encodes the α-chain of the CD8 receptor) and cytotoxicityrelated genes. By contrast, T cells that did not undergo clonal expansion included a larger proportion of Cd4+ cells and naive populations. This suggests that clonally expanded CD8+ T cells are the primary drivers of disease while, surprisingly, CD4+ T cells may not be required to provide ‘help’ – a function that they typically serve. This was confirmed in vivo when anti-CD8 but not anti-CD4 antibody treatment improved survival in Pdcd1−/−Ctla4+/− mice. Similarly, adoptive transfer of whole splenocytes from Pdcd1−/−Ctla4+/− mice, but not splenocytes that lacked CD8+ T cells, induced fatal myocarditis in recipient Rag1−/− mice, which lack mature T and B cells. The immune mechanisms through which this could occur will be an interesting area for future investigation. TCR screening revealed the dominant clonotypes from three independent CD8+ T cell repertoires as having high recognition for MYH6 epitopes, highlighting MYH6 as an important major histocompatibility complex class I (MHC-I)-restricted autoantigen in murine ICI-MC.
Next, the authors3 found that the total number of T cells from human PBMCs of both ICI-MC and healthy donors could be expanded through stimulation with MYH6 peptides, suggesting that autoreactive MYH6-specific T cells pre-existed in peripheral circulation. This is not surprising given that MYH6 is not expressed by thymic epithelial cells or antigen-presenting cells in the mouse or human thymus10. However, TCR-seq of T cells from ICI-MC patients showed overlapping anti-MYH6 clonal repertoires across inflamed cardiac and skeletal muscle tissues, similar to those in expanded PBMC cultures – supporting MYH6 as an important autoantigen in the pathology of human myocarditis and myositis, as in the mouse. Furthermore, anti-MYH6 clonal populations that were enriched in the diseased heart had high expression of CD8A and cytotoxicity genes (for example, NKG7 and GZMB), supporting CD8+ T cells as mediators of pathology. Defining the full TCR repertoire is costly and challenging, making translatability to large numbers of patients difficult. To this end, Axelrod et al.3 designed MHC-I tetramers loaded with MYH6 peptides to identify circulating anti-MYH6 CD8+ T cells in mice and humans. Tetramers are soluble peptide-MHC complexes that bind TCRs specific for a given antigen and can be used to survey patient blood samples for T cell populations of interest. This work thus provides a useful diagnostic tool for the identification of autoreactive CD8+ T cells that are specific for MYH6 using standard flow cytometry approaches. Together, these human studies show evidence for a predominantly CD8+ T cell-mediated mechanism of myocarditis, specifically in the context of ICI treatment, and provide the basis for argument against a direct pathogenic role for CD4+ T cells.
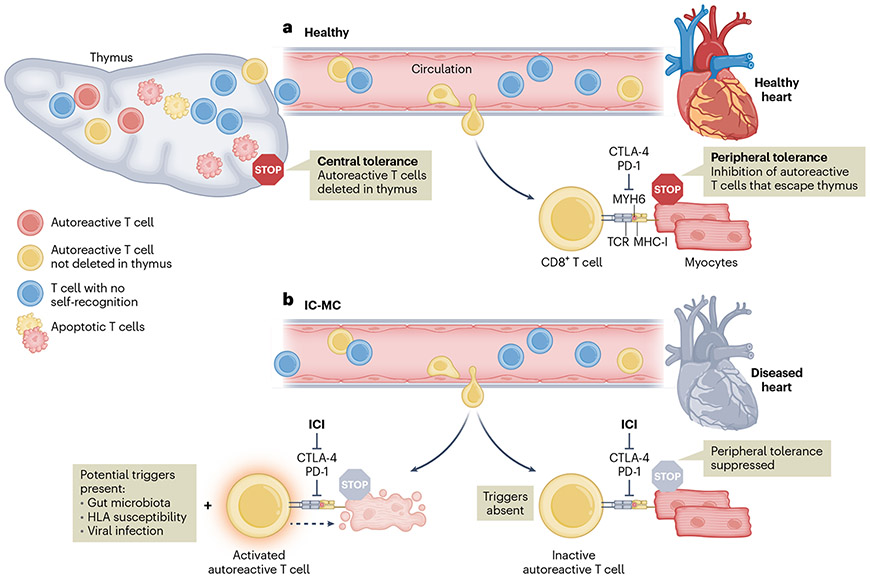
In summary, this study provides an important example of autoimmune toxicities that result from disrupted immune homeostasis. As MYH6 is not expressed in the thymus, anti-MYH6 T cells may bypass central tolerance mechanisms and escape into the periphery, even in the steady state. In healthy individuals, the activity of autoreactive T cells is suppressed through peripheral tolerance mechanisms such as CTLA-4- or PD-1-mediated inhibition. However, when these checkpoints are removed via ICI treatment, autoreactive T cells, if present, would be allowed to encounter antigens unabated (Fig. 1). It is crucial that this work is contextualized with insights from recent investigations of non-ischemic cardiomyopathy. Gil-Cruz et al.9 found that a gut-microbiota-derived peptide primed cross-reactive CD4+ T cells in the intestine, which ultimately caused lethal myocarditis in animal models and demonstrated potential causation in human patients with heart failure. A commensal bacterial species typically found in the human gut produced a protein with sequence homology to MYH6, leading to activation of CD4+ T cells with self-recognition of MYH6 in the myocardium. Only individuals with a specific variant of the human leukocyte antigen (HLA) allele HLA-DQB1 were susceptible, indicating that the combination of a specific peptide and HLA variant could predispose or exacerbate myocarditis in immunocompromised patients. If autoreactive anti-MYH6 CD8+ T cells are found in circulation, why do only some patients develop myocarditis? Perhaps these additional factors (for example, the gut microbiome and HLA haplotype) are involved in modulating inflammation. It is likely there is also heterogeneity in mechanisms of autoreactivity, with CD4+ T cells having a more important role in patients with heart failure, but CD8+ T cells being a central player in ICI-MC.
Fig. 1 ∣. CD8+ T cells mature in the thymus and those that recognize selfantigen are deleted via central tolerance mechanisms.
MYH6 is not expressed in the thymus, and thus anti-MYH6 CD8+ T cells can escape into peripheral circulation. a, Peripheral tolerance mechanisms include immune checkpoint receptors, such as CTLA-4 and PD-1, that inhibit T cell activity in healthy individuals and prevent potential tissue damage to the heart from anti-MYH6 CD8+ T cells. b, Treatment with ICIs suppresses negative regulation of autoreactive T cells from CTLA-4 and PD-1, which may manifest in immune-related adverse events such as myocarditis. Some individuals may be predisposed due to additional triggers that can modulate the inflammatory response, such as the gut microbiota, HLA genetic susceptibility or viral infection.
Moving forward, it will be interesting to utilize tetramer- and gut-microbiome-based studies in larger patient cohorts to better understand potential predisposition to future ICI-MC before therapy is initiated, so that certain subsets of patients can be followed more closely to mitigate or prevent myocarditis.
References
- 1.Johnson DB et al. Nat. Rev. Clin. Oncol 19, 254–267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang DY et al. JAMA Oncol. 4, 1721–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelrod ML et al. Nature 611, 818–826 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A & Wolchok DJ Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei SC et al. Cancer Discov. 11, 614–625 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DB et al. New Engl. J. Med 375, 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caforio ALP et al. Int. J. Cardiol 179, 166–177 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Myers JM et al. JCI Insight 1, e85851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil-Cruz C. et al. Science 366, 881–886 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Lv H. et al. J. Clin. Invest 121, 1561–1573 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]