Abstract
INTRODUCTION
Mini-percutaneous nephrolithotomy (mPCNL ) has been described as an alternative to standard nephrolithotomy (sPCNL ) for select stones. Studies suggest that mPCNL has comparable stone-free rates, with potential for decreased complications and shorter hospital stay. Costs associated with both procedures present a challenge to Canadian institutions due to capital acquisitions of equipment and ongoing disposables. The objective of this study was to compare the cost-effectiveness of both procedures at our institution.
METHODS
A decision tree analytic model was developed to compare costs and outcomes of both procedures. Primary outcomes included assessment of total capital, operative, and hospitalization costs. Cost and outcome of peri- and postoperative parameters were obtained using a retrospective analysis of 20 mPCNL and 84 sPCNL procedures on 1–2.5 cm stones between January 2020 and June 2022, and supplemented with internal hospital expenditure records and literature outcome data. Descriptive statistics and regression models were performed.
RESULTS
The estimated total cost-per-patient was $7427.05 and $5036.29 for sPCNL and mPCNL, respectively, resulting in cost-savings of $2390.76 in favor of mPCNL, with a comparable stone-free rate. The savings were due to lower costs associated with complications and hospital stay. mPCNL had higher capital costs ($95 116.00) compared to sPCNL ($78 517.00), but per-procedure operative costs were lower for mPCNL ($2504.48) compared to sPCNL ($3335.72). Cost-per-case regression of total costs intersected at 5.51 cases when accounting for operative and hospitalization costs, and at 20 cases when only considering operative costs.
CONCLUSIONS
Despite higher upfront costs, mCPNL may represent a valid, cost-effective alternative to sPCNL for select stones due to clinical and economic benefits in Canadian institutions.
INTRODUCTION
Standard percutaneous nephrolithotomy (sPCNL ) is the established first-line treatment for nephrolithiasis >20 mm in several international guidelines given its high stone-free rate (SFR).1,2 The procedure has undergone several modifications since its inception to improve outcomes while reducing invasiveness. One such modification is the miniaturization of the access sheath. Minimally invasive PCNL (mPCNL ) was first developed for use in the pediatric population by Jackman et al, and subsequently adapted for adult patients by Lahme et al.3,4 The mPCNL technique makes use of smaller sheath sizes of 14–20 F as opposed to the sPCNL sheath sizes of 24–30 F.5 Several studies comparing mPCNL to sPCNL have since been published, exploring the complication profile and efficacy of both procedures.6–18 Recent metanalyses have suggested that mPCNL had comparable SFR to that of sPCNL, and may be associated with reductions in bleeding, perforation, and leakage, along with fewer transfusion, less pain, and shorter hospitalization.19–21
Containing Canadian healthcare costs has continued to be a major challenge. In 2021, total health spending in Canada was expected to reach $308 billion, representing 12.7% of Canada’s gross domestic product and equal to $8019 per Canadian.22 Healthcare has remained the largest expense in provincial spending, accounting for nearly one-third of total expenditure.23 Surgical procedures are one of the costliest interventions in healthcare. The Canadian Institute for Health Information (CIHI) describes the “Major Intervention on Upper Urinary Tract” category, which encompasses PCNL procedures, as having an average estimated hospital cost of $9535.24 With an estimated 1600 PCNL operations occurring annually in Canada, there is incentive to minimize costs associated with this procedure, particularly if newer technology can offer similar or improved outcomes.25 Costs associated with both sPCNL and mPCNL procedures currently present a challenge to Canadian institutions due to the capital acquisitions of required equipment and significant use of disposables.
In this study, we aimed to investigate and compare sPCNL and mPCNL procedures at our academic institution and conduct a cost-effectiveness analysis between both procedures. Our objective was to gain insight into the various systematic, operative, and postoperative factors driving costs associated with both procedures, while identifying opportunities for potential cost savings.
METHODS
Study design
A retrospective analysis was conducted of sPCNL and mPCNL procedures at our institution to establish baseline demographic and stone variables, along with major cost-defining peri- and postoperative parameters, including postoperative length of stay (LOS ) and operative time.
Data collection
Data for the study were obtained from a retrospective data review of 20 mPCNL and 84 sPCNL procedures performed on 1–2.5 cm stones between January 2020 and June 2022. Specific inclusion criteria included: age over >18 years, nephrolithiasis >15 mm, or >10 mm lower calyceal, non-contrast computed tomography (NCCT) available preoperatively, along with postoperative imaging of either plain kidney ureter bladder X-ray (KUB), ultrasound (US), or NCCT, and underwent mPCNL or sPCNL between January 2020 and June 2022. Specific exclusion criteria included patients with congenital renal abnormalities or solitary kidney and scheduled staged procedures for large stone burden.
The major cost-defining peri- and postoperative parameters, along with baseline demographic and stone variables, complications, emergency department encounters, and readmissions before the patient’s first postoperative outpatient visit (4–6 weeks postoperatively) were collected from patient records. A decision tree analytic model was subsequently developed to compare the costs and outcomes associated with each procedure, incorporating the parameters collected and supplemented with internal hospital expenditure records and literature outcome data.
Measured parameters from data collection
Postoperative LOS
Patients were stratified based on their experience of a complication, ED presentation, and readmission in each of the sPCNL and mPCNL populations. Average LOS was subsequently calculated for each group and was used as a surrogate to quantify the cost of incurring a “complication.” Patients who had a prolonged LOS due to non-medical reasons (i.e., disposition) had an adjustment in their calculated LOS for analysis purposes by capturing the time between their postoperative status and the time their medical issues were deemed “resolved,” by standardized institutional criteria determined by the medial team. Due to a lack of patients who experienced a complication at our institution in the mPCNL arm, the mPCNL complication arm of the decision tree was assumed on a “worst case scenario” basis, where the LOS used was the LOS of the no-complication mPCNL arm multiplied by the ratio of sPCNL complication LOS arm/sPCNL no complication LOS arm.
Operative time
The average operative time was calculated from each of the sPCNL and mPCNL populations and used as a component of the operative cost in the analysis.
Assumed parameters
Although SFR and complication rates were calculated for our local population, to minimize risk of bias given the low sample size and as per other cost-effectiveness studies, we used literature-established parameters of the SFR and complication rate to calculate cost-effectiveness. SFR and complication rate parameters were extrapolated using literature outcome data from a recent meta-analysis comparing outcomes of sPCNL and mPCNL.20,21
Cost parameters
Internal hospital expenditure records were used to quantify the cost to the following parameters:
– LOS: calculated at a rate of $86.71/hour; and
– Operative time: calculated at a rate of $23.45/minute.
Internal hospital expenditure records combined with external Canadian Olympus™ (Tokyo, Japan) list prices (2022) were also used to quantify the cost of capital and operative equipment fees (Tables 1, 2).
Table 1.
Demographic and operative factors of sPCNL vs. mPCNL cases
| sPCNL | mPCNL | p | |
|---|---|---|---|
|
| |||
| Number of cases | 84 | 20 | |
|
| |||
| Age (years) | 59.61±15.51 | 58.05±10.61 | 0.3570 |
|
| |||
| Sex | >0.9999 | ||
| Male | 48 (57.1%) | 11 (55%) | |
| Female | 36 (42.9%) | 9 (45%) | |
|
| |||
| BMI | 29.63±6.39 | 30.37±5.88 | 0.6261 |
|
| |||
| Stone size (mm) | 18.03±3.96 | 15.95±3.62 | 0.1365 |
|
| |||
| Stone density (HU) | 837.7±333.8 | 878.4±254.2 | 0.6620 |
|
| |||
| Stone number | 1.44±0.68 | 1.60±0.88 | 0.5603 |
|
| |||
| Laterality | 0.7972 | ||
| Right | 32 (38.1%) | 7 (35%) | |
| Left | 52 (61.9%) | 13 (65%) | |
|
| |||
| Stone location | 0.0737 | ||
| Lower pole | 43/124 (34.1%) | 14/32 (43.8%) | |
| Mid pole | 3/124 (2.4%) | 2/32 (6.3%) | |
| Upper pole | 9/124 (7.3%) | 1/32 (3.1%) | |
| Renal pelvis | 34/124 (27.4%) | 8/32 (25%) | |
| Staghorn/multicalyx | 14/124 (11.3%) | 0 | |
| Proximal ureter | 9/124 (7.3%) | 0 | |
| UPJ | 12/124 (9.6%) | 7/32 (21.9%) | |
|
| |||
| Drainage type | <0.0001* | ||
| None | 1 | 4 | |
| Stent | 41 | 16 | |
| Nephrostomy tube | 39 | 0 | |
| Both | 3 | 0 | |
|
| |||
| Operative time | 87.37±23.33 | 93.75±24.00 | 0.2123 |
|
| |||
| Total length of hospitalization (including re-admissions/ED encounters due to complications) (hr) | 46.62±56.72 | 21.44±9.26 | <0.0001* |
|
| |||
| Total length of hospitalization (no complications) | 28.49±11.36 | 21.44±9.26 | 0.0069* |
|
| |||
| ED visits | 5 (5.95%) | 0 (0%) | 0.5804 |
|
| |||
| Readmissions | 7 (8.33%) | 0 (0%) | 0.3412 |
|
| |||
| Complication rate (any Clavien-Dindo) | 35 (41.67%) | 0 (0%) | 0.0001* |
|
| |||
| Stone-free rate | 64 (76.19%) | 17 (85.00%) | 0.5524 |
Values are presented as number of patients (%) or mean ± standard deviation (range) as appropriate. Mann-Whitney, Chi-squared (χ2), or Fisher’s exact tests were used as appropriate.
Significant.
BMI: body mass index; ED: emergency department; HU: Hounsfield unit; mPCNL: mini percutaneous nephrolithotomy; sPCNL: standard percutaneous nephrolithotomy; UPJ: ureteropelvic junction.
Table 2.
sPCNL and mPCNL equipment cost per procedure
| Equipment | sPCNL | mPCNL | Number | Cost (1 unit) | Total cost |
|---|---|---|---|---|---|
| Urine drainage bag | X | X | 1 | 2.95 | 2.95 |
| Cystoscopy irrigation tubing | X | X | 1 | 21.62 | 21.62 |
| Sterile 50 cc syringe | X | X | 2 | 0.36 | 0.72 |
| Guidewire angiography package | X | X | 1 | 20.17 | 20.17 |
| Vertical isolation barrier drape | X | X | 1 | 18.28 | 18.28 |
| Contrast (iopamidol 41%) media 100 cc | X | X | 1 | 11.00 | 11.00 |
| Sponge gauze 4”x8” 12 pack | X | X | 4 | 0.24 | 0.96 |
| Sterile lubricating jelly | X | X | 4 | 0.71 | 2.84 |
| C-arm drape | X | X | 1 | 3.47 | 3.47 |
| Major pack all services * | X | X | 1 | 0.01 | 0.01 |
| Sterile surgical gown | X | X | 2 | 2.32 | 4.64 |
| 16 Fr 2-way Foley catheter | X | X | 1 | 0.45 | 0.45 |
| Amplatz guidewire | X | X | 1 | 27.00 | 27.00 |
| W-Bore tubing set | X | X | 1 | 9.78 | 9.78 |
| Solu-IV 4-pack sponge 0.05% | X | X | 1 | 4.35 | 4.35 |
| Sterile connecting tube | X | X | 1 | 0.93 | 0.93 |
| Olympus nephroscope instrument set * | X | X | 1 | 0.01 | 0.01 |
| Percutaneous instrument set * | X | X | 1 | 0.01 | 0.01 |
| 5 Fr flexible tip catheter | X | X | 1 | 11.50 | 11.50 |
| Camera drape | X | X | 1 | 9.79 | 9.79 |
| Sterile surgical glove | X | X | 2 | 0.68 | 1.36 |
| Minor plastic/ENT pack | X | X | 1 | 7.15 | 7.15 |
| Olympus shockpulse lithotriptor instrument set * | X | X | 1 | 0.01 | 0.01 |
| 0.9% NaCl irrigation solution 3000 cc | X | X | 1 | 8.21 | 8.21 |
| Angled glidewire | X | X | 1 | 45.39 | 45.39 |
| Trocar needle w/locking stylette | X | X | 1 | 22.59 | 22.59 |
| Tuohy-Borst adapter | X | X | 1 | 17.00 | 17.00 |
| 8/10 Dilator 8 Fr sheath stylet set, 10 Fr safety wire | X | X | 1 | 40.00 | 40.00 |
| #11 Scalpel blade | X | X | 1 | 0.69 | 0.69 |
| ysto-nephro flexible fiberscope instrument set * | X | X | 1 | 0.01 | 0.01 |
| Disposable shockpulse lithotripsy probe set | X | 1 | 750.00 | 750.00 | |
| Disposable 30 Fr renal clear sheath kit + balloon dilator | X | 1 | 244.00 | 244.00 | |
| 550 um Holmium laser fiber | X | 1 | 13.15 | 13.15 | |
| Total sPCNL equipment cost per procedure | 1286.89 | ||||
| Total mPCNL equipment cost per procedure | 306.04 |
Standard reusable institution-specific instrument sets without an identifiable cost, logged for sake of completeness at a cost of $0.01.
mPCNL: mini percutaneous nephrolithotomy; sPCNL: standard percutaneous nephrolithotomy.
Outcome measures
The primary outcomes assessed in this study included the total capital, operative, and hospitalization costs associated with each procedure.
Data analysis
Descriptive statistics were used to summarize the data obtained from the retrospective analysis. Regression models were performed to evaluate the relationship between costs and the number of cases for each procedure.
Cost analysis
The total cost per patient was estimated for each procedure. This included capital costs, operative costs, and hospitalization costs. Capital costs associated with acquiring the required equipment were assessed for both procedures. Operative costs included the expenses related to the procedure itself, such as equipment and operative time. Hospitalization costs were calculated based on the LOS and were used as a surrogate for quantifying the cost of a complication.
Cost-effectiveness analysis
The cost-effectiveness analysis was conducted by comparing the costs and outcomes of mPCNL and sPCNL procedures. The incremental cost-effectiveness ratio (ICER ) was calculated to determine the cost-effectiveness of mPCNL compared to sPCNL. The ICER was assessed based on the cost savings associated with complications and length of hospitalization, while assuming a comparable SFR from literature established parameters.
Statistical analysis
Appropriate statistical tests were performed to analyze the data and evaluate the significance of the findings, with demographic and outcome variables compared using Student-t, Mann-Whitney, Chi-squared (χ2), Fisher’s exact tests, and linear regression as appropriate. The statistical analysis aimed to support the conclusions drawn from the cost and outcome comparisons between mPCNL and sPCNL procedures.
Ethical considerations
This study was conducted in accordance with ethical guidelines and regulations. Institutional review board approval was obtained with the Ottawa Hospital Research Institute Research Ethics Board Protocol (number 20220444-01H), and patient data were anonymized and handled confidentially to protect patient privacy and confidentiality.
RESULTS
Baseline demographic data
Twenty mPCNL and 84 sPCNL procedures were included in the final retrospective data review. The patient characteristics are summarized in Table 1. Patients were homogenous in characteristics between both groups, with significant differences only apparent in drainage type. Notably, there was no difference in operative time between procedures (p=0.2123); however, there was a difference in length of hospitalization (46.62 vs. 21.44 days for sPCNL vs. mPCNL, respectively, p<0.0001). There was an 85% SFR in the mPCNL group compared to the sPCNL rate of 76.19%; this was not statistically significant. For complication rate, however, there was a statistically significant difference between the mPCNL rate of 0% compared to sPCNL rate of 41.67% (p=0.0001).
Operative equipment cost and fees
Itemized common and procedure-specific equipment costs were collected and are outlined in Table 2. Common equipment costs were $292.89. Total sPCNL equipment costs per-procedure were $1286.89 as compared to the total mPCNL equipment cost per-procedure of $306.04.
Micro-cost analysis
The total cost per patient was estimated to be $7378.14 for sPCNL and $4363.54 for mPCNL, resulting in a cost savings of $3014.60 in favor of mPCNL at our institution. mPCNL had higher capital costs ($95 116.00) compared to sPCNL ($78 517.00) due to the acquisition of required equipment; however, per-procedure operative costs were lower for mPCNL ($2504.48) compared to sPCNL ($3335.72). The detailed breakdown of capital, operative, and hospitalization costs is provided in Table 3.
Table 3.
Micro-cost analysis between both sPCNL and mPCNL procedures of capital, operative, and postoperative costs
| sPCNL | mPCNL | |
|---|---|---|
|
| ||
| Initial capital cost | ||
| Shockpulse lithotriptor system | $62 535.00 | |
| Standard nephroscope set | $15 982.00 | |
| Mini nephroscope set | $13 241.00 | |
| Holmium laser | $80 000.00 | |
| 550 um reusable laser fiber | $750.00 | |
| Total initial capital cost | $78 517.00 | $93 991.00 |
|
| ||
| Operative cost | ||
| Per-procedure equipment fee (Table 2) | $1286.89 | $306.04 |
| Operative time ($23.45/min) | $2048.83 | $2198.44 |
| Total operative cost | $3335.72 | $2504.48 |
|
| ||
| Postoperative cost | ||
| Total hospitalization cost (86.71$/hr) | $4042.42 | $1859.06 |
| Total postoperative cost | $4042.42 | $1859.06 |
|
| ||
| Total initial capital cost | $78 517.00 | $93 991.00 |
|
| ||
| Total per procedure cost | $7378.14 | $4363.54 |
Pricing as per Canadian Olympus list prices Nov 2022.
mPCNL: mini percutaneous nephrolithotomy; sPCNL: standard percutaneous nephrolithotomy.
Decision tree analysis
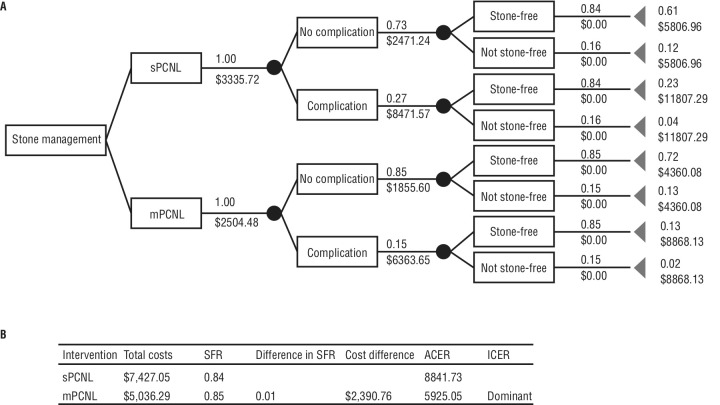
A decision tree analytic model was developed to compare the costs and outcomes mPCNL and sPCNL procedures while considering literature-established complication rate and SFR (Figure 1). Using a rollback method through summation of all weighted pathway costs and their associated probability, total procedural costs were $7427.05 and $5036.29 for sPCNL and mPCNL, respectively. This demonstrated a cost savings of $2390.76 in favor of mPCNL, with a dominant ICER due to lower costs associated with complications and length of hospitalization with a comparable SFR (Table 4).
Figure 1.
(A) Decision tree model comparing outcomes between percutaneous nephrolithotomy (PCNL) and mini (m)PCNL. The decision tree starts with the option of stone management with PCNL or mPCNL. Circles represent chance nodes with possible events. Top values are the probability of the event occurring, with the bottom value representing the cost of that event occurring. End points (triangles), have final cumulative probabilities and costs of occurring, represented by the top and bottom values respectively. Probability of a pathway=summated probability of all events in the pathway. Cost of a pathway=the sum of the cost of all events in the pathway. (B) Estimated total and incremental costs and outcomes for sPCNL and mPCNL. Total costs are calculated through the summation of all weighted pathway costs and their associated probability. ACER: average cost-effectiveness ratio; ICER: incremental cost-effectiveness ratio
Table 4.
Estimated total and incremental costs and outcomes for sPCNL and mPCNL
| Intervention | Total costs | SFR | Difference in SFR | Cost difference | ACER | ICER |
|---|---|---|---|---|---|---|
| sPCNL | $7427.50 | 0.84 | 8841.71 | |||
| mPCNL | $5036.29 | 0.85 | $0.01 | $2390.76 | 5925.05 | Dominant |
Total costs are calculated through the summation of all weighted pathway costs and their associated probability. ACER: average cost-effectiveness ratio; ICER: incremental cost-effectiveness ratio; mPCNL: mini percutaneous nephrolithotomy; sPCNL: standard percutaneous nephrolithotomy; SFR: stone-free rate.
Cost-per-case regression
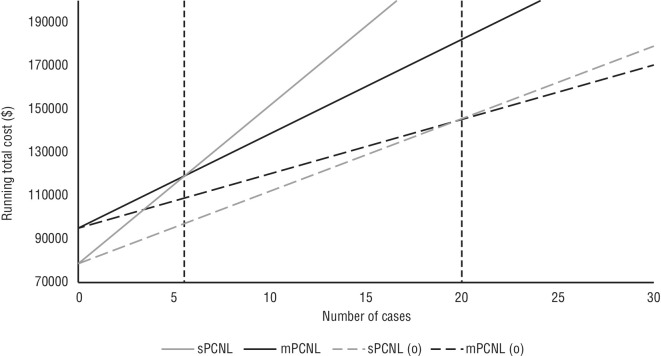
A regression analysis of the total costs between mPCNL and sPCNL procedures was performed (Figure 2). When considering both operative and hospitalization costs, the cost-per-case intersection point occurred at 5.51 cases. This suggests that mPCNL becomes more cost-effective than sPCNL after this threshold. When considering operative costs alone, the cost-per-case intersection point was at 20 cases, further supporting the cost-effectiveness of mPCNL.
Figure 2.
Cost-per-case regression of total running costs comparison between standard percutaneous nephrolithotomy (sPCNL) and mini (m)PCNL intersects at 5.51 cases, and at 20 cases when eliminating hospitalization costs (only operative costs).
DISCUSSION
In this study, we aimed to investigate and compare the sPCNL and mPCNL procedures in terms of their cost-effectiveness at our academic institution. Our findings shed light on the various factors driving costs associated with both procedures and identified opportunities for potential cost savings from a Canadian perspective.
The total cost per patient showed a significant cost saving in favor of mPCNL at our institution, primarily driven by the reduced operative costs and shorter hospitalization associated with mPCNL.
The operative costs, comprising both operative time and equipment fees, demonstrate that it is the latter that contributes to the significant cost difference. The total per-procedure equipment fee for sPCNL is more than three times that of mPCNL, approaching $1286.89. Further analysis demonstrates that it is the sPCNL procedure-specific equipment that primarily contributes to that figure, most notably the cost of the disposable equipment (i.e., shock pulse lithotripsy probes, balloon dilators, and sheaths). These procedure- specific disposable equipment costs alone add up to $994 — almost 77% of the total equipment cost and almost 30% of the total operative cost. This is consistent with previous studies that have demonstrated the cost of disposable balloon dilatation to be high relative to other dilatation techniques in the PCNL procedure.26,27
Other studies have evaluated the use of alternative, more cost-efficient dilatation techniques in sPCNL. Specifically, Frattini et al introduced a novel “one-shot” dilatation with a 25 Fr or 30 Fr Amplatz dilator and compared it to previously established dilatation techniques, including Alken metal telescoping dilators and balloon dilatation.26 The authors demonstrated the technique compared favorably with both of the other dilation techniques without an increase in morbidity and with significant reductions in X-ray exposure and costs, with the one-shot technique costing approximately $60 USD. A systematic review and meta-analysis of all dilatation techniques further supported this finding, with one-shot dilatation significantly decreasing fluoroscopy time and resulting in less hemoglobin decrease compared to other dilatation techniques. One-shot dilatation should be considered for most patients who undergo PCNL procedures.28
The use of balloon dilation at our institution is consistent with contemporary dilation techniques that most urologists in Canada currently use for PCNL procedures; however, there is clearly room for cost savings in using alternative techniques, as outlined previously. The operative costs of mPCNL are relatively lower due to more reprocessed materials (dilators, sheaths, and laser fibers). While these fees may vary across institutions, they are unlikely to reach that of sPCNL in centers that already have established holmium or thulium laser infrastructure and contracts. There were no significant differences in operative time between both procedures at our institution, with costs associated with operative time approaching $2048.83 and $2198.44. Operative time and its cost calculation is subject to surgeon experience, practice variation, and institutional practices. Furthermore, while some studies have shown mPCNL may take longer, the mean difference in operative time was shown to be 12 minutes in one meta-analysis, which is unlikely to contribute to a major difference in operative costs.21
Based on our institutional data, there was a significant difference in length of hospitalization — 28.49 hours for sPCNL compared with 21.44 hours for mPCNL — even among those procedures that had no complications. When looking to include procedures that had complications, the sPCNL length of hospitalization significantly increases to almost double at 46.62 hours, which accounts for a difference of $2183.36 between procedures. This difference is present even with both groups showing homogenous preoperative factors, including stone burden and location, among other variables. This highlights that the difference is not due to more complicated cases in the sPCNL population. The postoperative cost of hospitalization alone comprises 54% of the total per-patient procedural cost of sPCNL, as compared to 42% in mPCNL. The increased length of hospitalization of sPCNL compared to mPCNL is supported in other studies and is likely attributed to the increased complications and morbidity associated with the procedure, further highlighting the economic advantages of mPCNL compared to sPCNL.19,21
Our analysis also revealed that mPCNL had higher capital costs compared to sPCNL due primarily to the acquisition of a holmium laser and mPCNL set, resulting in an initial capital difference of almost $15 474. Our regression analysis, however, revealed that this difference is quickly made up after approximately 20 mPCNL procedures or even at five procedures when accounting for the postoperative costs of both procedures. Furthermore, the capital costs of acquisition of the holmium laser can be negligible in institutions that already have established laser capabilities and may be even less than stated when compared to costs from other vendors that this study did not explore. This shows the significant impact cost-savings this initial investment can have on healthcare institutions, particularly in resourceconstrained environments.
The calculated total per-procedure cost at our institution was found to be $7378.14 and $4363.54 for sPCNL and mPCNL, respectively, with a raw cost savings of $3014.6. This finding is with consideration of our local complication rates for mPCNL and sPCNL, which were 0% and 41.67%, respectively. To account for our institution’s low patient population and lack of complications in our mPCNL population, which may skew cost differences, a decision tree analytic model was developed considering the rates of complications and SFR obtained from literature outcome data, while using our local, stratified, postoperative hospitalization data to obtain a more accurate analysis. This analysis supported our institutional findings, with the total per procedure costs approaching $7427.05 and 5036.29 for sPCNL and mPCNL, respectively. This further supports the external validity of our results and highlights the economic advantage of mPCNL over sPCNL, even when incorporating literature-supported complication data.
“mPCNL is associated with lower procedural costs compared to sPCNL, as well as a comparable SFR, indicating that mPCNL is a cost-effective alternative to sPCNL.”
Ultimately, the decision tree analysis demonstrated that mPCNL was associated with lower procedural costs compared to sPCNL, resulting in a cost savings of $2390.76. This finding, along with a comparable SFR, indicates that mPCNL is a cost-effective alternative to sPCNL with a dominant ICER. Notably, the ICER would be more dominant and continue to support our findings when using our local complication rates and SFR, which show a mPCNL SFR of 85% (similar to that of the literature-extrapolated SFR parameter) and a complication rate of 0% (a much more favorable rate than that of the literature-extrapolated complication rate of 15%).
Limitations
The study has several important limitations that should be taken into consideration when interpreting the findings. Firstly, the study was conducted at a single academic institution in Canada, which may limit the generalizability of the findings to other healthcare settings or countries with different healthcare systems. Variations in patient populations, surgical techniques, and resource availability across different regions could significantly affect the cost-effectiveness outcomes observed.
Furthermore, the sample size in the study, particularly in the mPCNL group, was relatively small. A sample size calculation was also not performed, as we were limited by the relatively small number of mPCNL procedures performed at our institution. This may affect the statistical power and precision of the results, limiting the ability to draw definitive conclusions. The small sample size also increases the risk of selection bias and reduces the representativeness of the study population. This is especially apparent when addressing our calculated local SFR and complication rates, which are significantly affected by the small population.
While our local SFR was reasonably similar to that of literature-established SFR of mPCNL and sPCNL, our compilation rates were much more heterogenous. Although the decision tree analysis was performed using literature parameters, the postoperative LOS data could be subject to overestimation given the differences in complication rates. In addition, although preoperative stone factors were statistically homogenous between both groups, it is important to acknowledge the 11.3% staghorn population in the sPCNL group.
Stone characteristics in this study were based on preoperative CT reports and operative notes, which introduce an element of subjectivity in defining a “true” staghorn calculus. Given the homogeneity in other stone characteristics and all stones assessed in this study being under 2.5 cm, it is also unlikely these stones were true staghorn calculi. In any case, it is worth noting that inclusion of this population in the cost-analysis may overestimate postoperative costs and results should be interpreted accordingly.
Furthermore, only patients with stone sizes of 1–2.5 cm were chosen for this study, with the complication and SFR parameters from the literature data that was used not being restricted to those stone sizes. Although this may affect the true cost-effectiveness of the procedures, a recent meta-analysis comparing sPCNL and mPCNL specifically in stone sizes >2 cm has demonstrated no significant difference in SFR between both procedures.29 Given this, the cost-effectiveness of mPCNL found in this study likely would not significantly change with varying stone size.
Additionally, the analysis in this study considered only the direct costs associated with the procedures, such as surgical equipment, operative time, and hospital stay. The study used internal hospital expenditure records and external list prices to estimate the costs associated with the procedures; however, the cost of equipment and supplies can vary among healthcare institutions and may be subject to negotiations and discounts, especially with varying vendors. This study also used postoperative length of hospitalization as a surrogate for complications and did not explore specific complication-related costs (imaging, bloodwork, medications, etc.) that may affect the final cost.
Nevertheless, LOS is known to be one of the most significant costs associated with a patient’s admission and so was used as a reasonable surrogate for the purposes of this study. Furthermore, given the relatively short time period that was chosen for this study and the heterogeneity in choice of urinary drainage between surgeons, indirect costs related to repeat procedures, including costs related to productivity loss or long-term followup expenses (nephrostomy tube vs. stent, cystoscopy appointments to remove stents, repeat procedures, etc.), were not included in the economic evaluation. These indirect costs can have an impact on the overall cost-effectiveness of a procedure and should be considered when assessing the value of different treatment options. Inclusion of indirect costs would provide a more comprehensive picture of the economic implications of mPCNL and sPCNL.
This study is also subject to possible selection bias. Although patients had similar “stone burden” between both procedures, theoretically, to equally undergo either procedure, we acknowledge that there may be hidden, unaccounted-for patient factors (comorbidities, body mass index, patient preference, etc.) or possible surgeon factors (available operative resources, surgeon preference/experience, relative novelty of the mPCNL procedure in our institution, etc.) that may lead to selection bias of one procedure over the other. The study also did not account for variations in surgeon experience and practice patterns, which can influence operative time and outcomes. Surgeons with different skill levels or preferences may have different outcomes and associated costs. Therefore, the generalizability of the findings to other surgical teams should be interpreted with caution. Further research with larger sample sizes, multicenter studies, and a broader range of outcome measures is warranted to validate and expand upon these findings.
Despite these limitations, the study contributes valuable information regarding the cost-effectiveness of mPCNL compared to sPCNL. There has been an effort in recent years to explore the idea of ambulatory PCNL in Canada, with studies demonstrating success in select patients.25,30–32 One study estimated a possible cost savings of over $3000 per PCNL in Canada by shifting PCNL from an inpatient to an outpatient procedure. 25 In our study, we show that similar cost savings can be achieved by simply shifting from the sPCNL to mPCNL, with comparable outcomes in select cases.
CONCLUSIONS
Our study provides evidence supporting the cost-effectiveness of mPCNL compared to sPCNL in the Canadian healthcare system. The cost savings associated with mPCNL are driven by lower operative costs, shorter hospital stays, and reduced equipment fees. Despite higher initial capital costs, mPCNL becomes more cost-effective after a certain number of procedures. These findings have implications for healthcare institutions aiming to minimize costs while maintaining favorable outcomes for patients with nephrolithiasis. Future research with larger sample sizes and multicenter studies would strengthen the evidence base and provide more robust guidance on the cost-effectiveness of mPCNL in different healthcare contexts.
KEY MESSAGES.
■ Despite higher upfront costs (that are negated if the institution already has laser capabilities), mPCNL may represent a valid, cost-effective alternative to sPCNL for select stones.
■ sPCNL disposable equipment costs drive the significant difference in per-procedure operative costs at more than 3 times that of mPCNL ’s equipment costs.
■ Cost-per-case regression of total costs between both procedures demonstrate mPCNL is more effective after ~20 cases, and even less when considering differences in hospitalization costs.
Footnotes
See related commentary on page 179
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Skolarikos A, Petřík A, Somani B, et al. EAU guidelines on urolithiasis. European Association of Urology. 2022. [Accessed Feb. 15, 2024]. Available at: https://uroweb.org/guidelines/urolithiasis.
- 2.Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART II. J Urol. 2016;196:1161–9. doi: 10.1016/j.juro.2016.05.091. [DOI] [PubMed] [Google Scholar]
- 3.Jackman SV, Hedican SP, Peters CA, et al. Percutaneous nephrolithotomy in infants and preschool age children: Experience with a new technique. Urology. 1998;52:697–701. doi: 10.1016/S0090-4295(98)00315-X. [DOI] [PubMed] [Google Scholar]
- 4.Lahme S, Bichler KH, Strohmaier WL, et al. Minimally invasive PCNL in patients with renal pelvic and calyceal stones. Eur Urol. 2001;40:619–24. doi: 10.1159/000049847. [DOI] [PubMed] [Google Scholar]
- 5.Wright A, Rukin N, Smith D, et al. ‘Mini, ultra, micro’ - nomenclature and cost of these new minimally invasive percutaneous nephrolithotomy (PCNL) techniques. Ther Adv Urol. 2016;8:142–6. doi: 10.1177/1756287215617674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giusti G, Piccinelli A, Taverna G, et al. Miniperc? No, thank you! Eur Urol. 2007;51:810–5. doi: 10.1016/j.eururo.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Knoll T, Wezel F, Michel MS, et al. Do patients benefit from miniaturized tubeless percutaneous nephrolithotomy? A comparative prospective study. J Endurol. 2010;24:1075–9. doi: 10.1089/end.2010.0111. [DOI] [PubMed] [Google Scholar]
- 8.Li LY, Gao X, Yang M, et al. Does a smaller tract in percutaneous nephrolithotomy contribute to less invasiveness? A prospective comparative study. Urology. 2010;75:56–61. doi: 10.1016/j.urology.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Cheng F, Yu W, Zhang X, et al. Minimally invasive tract in percutaneous nephrolithotomy for renal stones. J Endurol. 2010;24:1579–82. doi: 10.1089/end.2009.0581. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Zeng G, Wu W, et al. Minimally invasive percutaneous nephrolithotomy with multiple mini tracts in a single session in treating staghorn calculi. Urol Res. 2011;39:117–22. doi: 10.1007/s00240-010-0308-z. [DOI] [PubMed] [Google Scholar]
- 11.Mishra S, Sharma R, Garg C, et al. Prospective comparative study of miniPERC and standard PNL for treatment of 1 to 2 cm size renal stone. BJU Int. 2011;108:896–900. doi: 10.1111/j.1464-410X.2010.09936.x. [DOI] [PubMed] [Google Scholar]
- 12.Song L, Chen Z, Liu T, et al. The application of a patented system to minimally invasive percutaneous nephrolithotomy. J Endourol. 2011;25:1281–6. doi: 10.1089/end.2011.0032. [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Shi H, Zhu J, et al. A prospective comparative study of haemodynamic, electrolyte, and metabolic changes during percutaneous nephrolithotomy and minimally invasive percutaneous nephrolithotomy. World J Urol. 2014;32:1275–80. doi: 10.1007/s00345-013-1204-2. [DOI] [PubMed] [Google Scholar]
- 14.Abdelhafez MF, Wendt-Nordahl G, Kruck SM, et al. Minimally invasive versus conventional large-bore percutaneous nephrolithotomy in the treatment of large-sized renal calculi: Surgeon’s preference? Scand J Urol. 2016;50:212–5. doi: 10.3109/21681805.2016.1155078. [DOI] [PubMed] [Google Scholar]
- 15.Sakr A, Salem E, Kamel M, et al. Minimally invasive percutaneous nephrolithotomy vs. standard PCNL for management of renal stones in the flank-free modified supine position: Single-center experience. Urolithiasis. 2017;45:585–9. doi: 10.1007/s00240-017-0966-1. [DOI] [PubMed] [Google Scholar]
- 16.Thapa BB, Niranjan V. Mini PCNL over standard PCNL: What makes it better? Surg J. 2020;6:e19–23. doi: 10.1055/s-0040-1701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ElSheemy MS, Elmarakbi AA, Hytham M, et al. Mini vs standard percutaneous nephrolithotomy for renal stones: A comparative study. Urolithiasis. 2019;47:207–14. doi: 10.1007/s00240-018-1055-9. [DOI] [PubMed] [Google Scholar]
- 18.Zeng G, Cai C, Duan X, et al. mini percutaneous nephrolithotomy is a noninferior modality to standard percutaneous nephrolithotomy for the management of 20–40 mm renal calculi: A multicenter, randomized controlled trial. Eur Urol. 2021;79:114–21. doi: 10.1016/j.eururo.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Liu Y, Liu L, et al. Minimally invasive versus standard percutaneous nephrolithotomy: A meta-analysis. Urolithiasis. 2015;43:563–570. doi: 10.1007/s00240-015-0808-y. [DOI] [PubMed] [Google Scholar]
- 20.Deng J, Li J, Wang L, et al. Standard versus mini-percutaneous nephrolithotomy for renal stones: A meta-analysis. Scand J Surg. 2021;110:301–11. doi: 10.1177/1457496920920474. [DOI] [PubMed] [Google Scholar]
- 21.Wan C, Wang D, Xiang J, et al. Comparison of postoperative outcomes of mini percutaneous nephrolithotomy and standard percutaneous nephrolithotomy: A meta-analysis. Urolithiasis. 2022;50:523–33. doi: 10.1007/s00240-022-01349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Expenditure Trends. Snapshot | CIHI; 2021. [Accessed Feb 15, 2024]. [cited 2022 Mar 25]. Available at: https://www.cihi.ca/en/national-health-expenditure-trends-2021-snapshot. [Google Scholar]
- 23.The Daily - Three-fifths of total federal, provincial, territorial and local spending went to social protection, health care and education in 2019. [Accessed Feb 15, 2024]. [cited 2022 Mar 25]. Available at: https://www150.statcan.gc.ca/n1/daily-quotidien/201127/dq201127a-eng.htm.
- 24.Patient Cost Estimator. CIHI; [Accessed Feb 15, 2024]. [cited 2022 Mar 25]. Available at: https://www.cihi.ca/en/patient-cost-estimator. [Google Scholar]
- 25.Kroczak T, Pace KT, Andonian S, et al. Ambulatory percutaneous nephrolithotomy in Canada: A cost-reducing innovation. Can Urol Assoc J. 2018;12:427–9. doi: 10.5489/cuaj.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frattini A, Barbieri A, Salsi P, et al. One shot: A novel method to dilate the nephrostomy access for percutaneous lithotripsy. J Endourol. 2001;15:919–23. doi: 10.1089/089277901753284143. [DOI] [PubMed] [Google Scholar]
- 27.Penbegul N, Dede O, Daggulli M, et al. A novel percutaneous nephrolithotomy (PCNL) set: The ‘Economical One-shot PCNL Set’ (Ecoset) Arab J Urol. 2017;15:199–203. doi: 10.1016/j.aju.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao D, Liu L, Liu H, et al. A comparison among four tract dilation methods of percutaneous nephrolithotomy: A systematic review and meta-analysis. Urolithiasis. 2013;41:523–30. doi: 10.1007/s00240-013-0598-z. [DOI] [PubMed] [Google Scholar]
- 29.Qin P, Zhang D, Huang T, et al. Comparison of mini percutaneous nephrolithotomy and standard percutaneous nephrolithotomy for renal stones >2 cm: A systematic review and meta-analysis. Int Braz J Urol. 2022;48:637–48. doi: 10.1590/s1677-5538.ibju.2021.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosier GW, Visram K, McGregor T, et al. Ambulatory percutaneous nephrolithotomy is safe and effective in patients with extended selection criteria. Can Urol Assoc J. 2022;16:89–95. doi: 10.5489/cuaj.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beiko D, Lee L. Outpatient tubeless percutaneous nephrolithotomy: The initial case series. Can Urol Assoc J. 2013;4:E86–90. doi: 10.5489/cuaj.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrour W, Andonian S. Ambulatory percutaneous nephrolithotomy: Initial series. Urology. 2010;76:1288–92. doi: 10.1016/j.urology.2010.08.001. [DOI] [PubMed] [Google Scholar]