Abstract
INTRODUCTION
Most robot-assisted surgery (RAS) systems in Canada are donor-funded, with constraints on implementation and access due to significant costs, among other factors. Herein, we evaluated the impact of the growing multispecialty use of RAS on urologic RAS access and outcomes in the past decade.
METHODS
We conducted a retrospective review of all RAS performed by different surgical specialties in two high-volume academic hospitals between 2010 and 2019 (prior to the COVID pandemic). The assessed outcomes included the effect of increased robot access over the years on annual robotic-assisted radical prostatectomy (RARP) volumes, surgical waiting times (SWT), and pathologically positive surgical margins (PSM). Data were collected and analyzed from the robotic system and hospital databases.
RESULTS
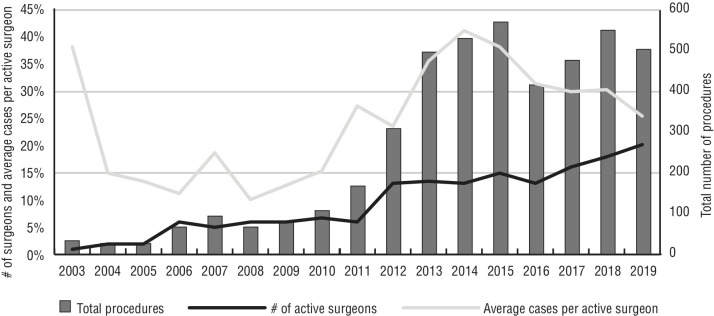
In total, six specialties (urology, gynecology, general, cardiac, thoracic, and otorhinolaryngologic surgery) were included over the study period. RAS access by specialty doubled since 2010 (from three to six). The number of active robotic surgeons tripled from seven surgeons in 2010 to 20 surgeons in 2019. Moreover, there was a significant drop in average case volume, from a peak of 40 cases in 2014 to 25 cases in 2019 (p=0.02). RARP annual case volume followed a similar pattern, reaching a maximum of 166 cases in 2014, then declining to 137 cases in 2019. The mean SWT was substantially increased from 52 days in 2014 to 73 days in 2019; however, PSM rates were not affected by the reduction in surgical volumes (p<0.05).
CONCLUSIONS
Over the last decade, RAS access by specialty has increased at two Canadian academic centers due to growing multispecialty use. As there was a fixed, single-robotic system at each of the hospital centers, there was a substantial reduction in the number of RAS performed per surgeon over time, as well as a gradual increase in the SWT. The current low number of available robots and unsustainable funding resources may hinder universal patient access to RAS.
INTRODUCTION
Surgery has become substantially technology-driven, with robot-assisted surgery (RAS) being a notable innovation in several surgical fields.1 RAS overcomes the ergonomic limitations of conventional laparoscopy and offers various advantages.1,2 With the emergence of new technologies, the global market for surgical robots was valued at $4.4 billion USD in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 18.0% from 2023–2030.3
According to Intuitive Surgical 2010 Annual Report, the da Vinci® surgical (DVS) system was predominantly used for prostatectomies and hysterectomies, accounting for over 70% of procedures robotic-assisted radical prostatectomy (RARP). Several studies have demonstrated the safety and effectiveness of RARP, highlighting its benefits compared to open radical prostatectomy (ORP) and conventional laparoscopy, such as reduced blood loss, transfusion rate, complications, and hospital stay.1–3 Furthermore, compared to conventional laparoscopy, RARP enables larger degrees of wrist movement, increased work efficiency, and precision with three-dimensional visualization of the operative field.1,3
While urology has achieved significant growth in RAS use, other specialties, such as general surgery, gastrointestinal, cardiothoracic, gynecologic, and otorhinolaryngologic, have also witnessed increasing adoption of robotic surgery in recent years.2
In Canada, most RAS systems (30 DaVinci robots) have been funded by hospital foundations through philanthropy donations, thereby limiting their availability and use. This expanded use of RAS across specialties, especially in urology, poses significant challenges, given the limited resources in the Canadian socialized, single-payer, healthcare system; however, the impact of the increased use of RAS on urologic access, surgical volumes, and outcomes remains unknown. Therefore, our study aimed to assess the effects of the growing multispecialty use of RAS coupled with a limited number of robotic systems, specifically on urologic access and oncologic outcomes over the past decade.
METHODS
Study design and data collection
After obtaining the ethics approval for this retrospective, multi-institutional study, a review with patients involved in all RAS procedures performed in different surgical specialties (urology, gynecology, general, cardiac, thoracic, and otorhinolaryngologic surgery) between 2010 and 2019 at the Centre Hospitalier de l’Université de Montréal (CHUM) and the Hôpital du Sacré-Coeur-de-Montréal (HSCM) was conducted. Both academic centers are located in the large metropolitan city, Montreal, QC, Canada. The decision for these years included the initiation of RAS in 2010 and 2019 year to avoid clinical impact from the COVID pandemic.
Over the same period, another retrospective review of a prospectively maintained RARP institutional review board-approved database was conducted in the same academic centers. The data was collected from the institutions’ electronic medical records (EMR) platform OACIS. The platform allowed access to the electronic surgical scheduling system (OPERA) and the resource management systems. Procedural data was also collected from each of the DVS robotic systems.
The assessed outcomes included the effect of increased robot access over the years on annual RARP volumes, surgical wait times (SWT), and pathological positive surgical margins (PSM ). The measured variables included the total number of RAS procedures, active RAS surgeons, and average number of cases per active surgeon across different surgical specialties, total RARP volume, SWT in days, and PSM (including the total percentage of positive margins, percentage of positive pT2 margins, and percentage of positive pT3 margins).
Statistical analysis
Data were collected and analyzed from the robotic system and hospital databases. All analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, U.S.). Descriptive statistics, such as means and standard deviations, were calculated to summarize the baseline characteristics. The statistical significance level was set at p<0.05.
RESULTS
In total, six specialties (urology, gynecology, general, cardiac, thoracic, and otorhinolaryngologic surgery) developed RAS programs over the studied period, and RAS access by specialty doubled from three (2010) to six (2019) (Figure 1). In terms of procedural volume, urology remained at the top among surgical specialties, with more than 42% of the total procedure volume (Figure 1). The number of active robotic surgeons tripled from seven surgeons in 2010 to 20 surgeons in 2019. Urologic and gynecologic surgeons together represented half of the total number of active surgeons. There was a gradual decrease in the number of RAS procedures performed per surgeon over time. Over the years, there was a significant decrease in the average RAS volume per active robotic surgeon (all surgical specialties included), from a high of 40 cases in 2014 to 25 cases in 2019 (Figure 2).
Figure 1.
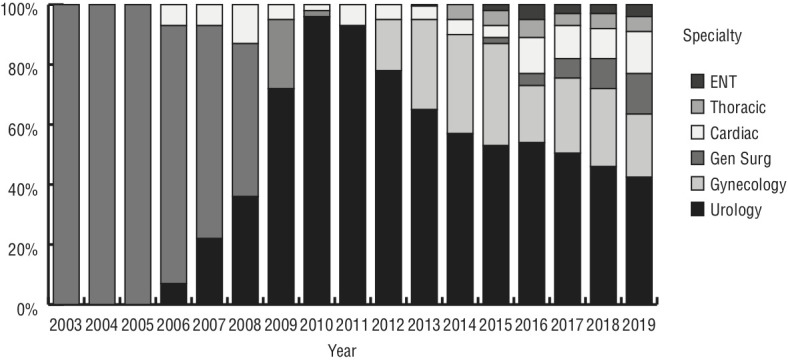
Total procedure volume accessed by speciality over time.
Figure 2.
Total procedures vs. number of active surgeons vs. average cases per active surgeon.
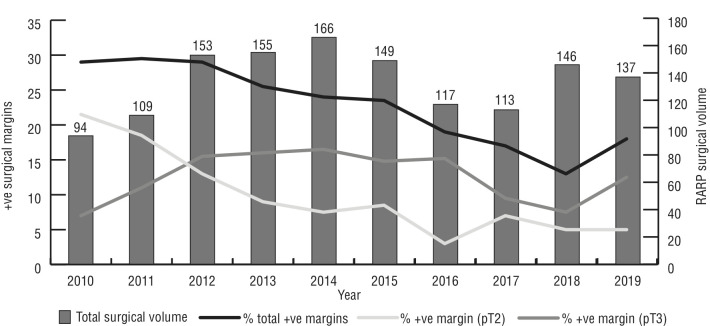
The total RARP surgical volume between 2010 and 2019 was 1339 procedures across the two centers, ranging from a low of 94 procedures in 2010 to a high of 166 procedures in 2014. There were 802 procedures performed at the HSCM, while the CHUM performed 537 procedures throughout the same period.
With regards to urologic RAS, there was a general decreasing trend in RARP surgical volume over time. There were two active RARP surgeons performing these procedures. Surgeon 1 performed 925 procedures between 2010 and 2019, with a low of 35 procedures in 2010 and a high of 127 procedures in 2014. Surgeon 2 performed 414 procedures in total, with a low of 25 procedures in 2017 and a high of 59 procedures in 2010. The mean SWT for RARP procedures ranged from a low of 52 days in 2014 to a high of 118 days in 2011. Throughout the study period, there was a gradual increase in the mean SWT.
The total percentage of positive margins on pathology ranged from 13.1% in 2018 to 29.4% in 2011. The percentage of positive pT2 margins ranged from 5.1% in 2019 and 21.2% in 2010. The percentage of positive pT3 margins ranged from 7.4% in 2010 to 16.2% in 2014. A decreasing trend was observed in PSM between 2010 and 2019, and PSM rates were not affected by the increase in mean SWT or the decrease in surgical volumes (Figure 3).
Figure 3.
Total surgical volume and positive surgical margins over time.
DISCUSSION
Minimally invasive robotic surgery offers numerous benefits across various surgical fields, including urology. The confined and intricate nature of urologic pelvic procedures makes it challenging to perform using conventional laparoscopic or open surgery. Robotic assistance, however, addresses these challenges by providing improved access, maneuverability, and visualization. The introduction of RARP in 2000 by Binder et al revolutionized the field of urology.4 RARP has demonstrated superior outcomes compared to ORP, including reduced blood loss, shorter hospital stays, and reduced postoperative complications.5–8 Additionally, the adoption of robotic surgical systems has expanded beyond urology to include procedures such as nephrectomy (partial and radical), cystectomy, and adrenalectomy.
In Canada, the publicly funded healthcare system and limited market size pose inherent challenges for implementing robotic surgery. Access to medical devices is often delayed, resulting in longer SWT and limited availability of robotic technology. Unlike in the U.S., where hospitals purchase robots, all DVS systems in Canada have been acquired through donor-funded initiatives, restricting their widespread implementation. Consequently, Canadian patients may face longer travel distances for surgery and extended SWT. The use of robotic surgery has experienced exponential growth worldwide, particularly in urology, but its expansion into other specialties has led to a plateau in the growth of robotic prostatectomies.9,10 Therefore, our study aimed to assess the effects of the growing multispecialty use of RAS on urologic access and outcomes over the past decade.
In the present study, we observed that RAS access by specialty has increased in the past decade (2010–2019) due to growing multispecialty use. Additionally, there was a significant reduction in number of RAS performed per surgeon over time, with a corresponding increase SWT. Fortunately, however, the drop in annual urology surgical volumes was not associated with worse oncologic outcomes. This is likely related to the fact that all urologic RAS surgeons had extensive experience, fellowship training, and had overcome their learning curves.
Moreover, our findings indicate a notable increase in specialty-specific access to RAS, with the number of specialties offering RAS doubling from 2010 to 2019. Additionally, we observed a consistent decrease in the number of RAS procedures performed by individual surgeons over time. The average case volume per active robotic surgeon, encompassing all specialties, showed a significant decline from a peak of 40 cases in 2014 to 25 cases in 2019. With regards to a minimum number of cases per year per surgeon to maintain proficiency, this may be of concern, with the addition of other surgeons in upcoming years with a fixed number of robotic systems.
In line with this trend, the annual case volume for RARP exhibited a similar pattern. It reached its highest point of 166 cases in 2014, but subsequently decreased to 113 cases in 2017 and 137 cases in 2019. Furthermore, our analysis revealed a significant increase in SWT throughout the study period. The average SWT rose from 52 days in 2014 to 73 days in 2019. While the precise impact of prolonged waiting on disease recurrence remains uncertain, there is a growing concern about the potential negative influence of extended waiting periods on patient outcomes, particularly in terms of psychological well-being and oncologic progression, as well as secondary treatment for disease recurrence. Despite the surgical delays and reduction in surgical volumes, overall PSM from RARP remained relatively unaffected in the present study, decreasing from 29.6% in 2010 to 17.5% in 2019.
It is important to note that the cost-effectiveness of RARP in Canada’s healthcare system continues to remain a topic of debate. While RARP offers several benefits, including improved surgical outcomes, its higher equipment cost ($2000–3000 CAD more per case in disposables) compared to laparoscopic or open surgeries needs to be considered. Previous economic assessments have shown favorable cost-effectiveness for RARP when the annual case volume per system exceeds 150 cases; however, more research is needed to provide conclusive evidence on the cost-effectiveness of RARP.11–15
Limitations
It is crucial to acknowledge the limitations of our study. First, our research was conducted in only two academic hospitals within Canada and in a single province, limiting the generalizability of the findings. National multispecialty studies are needed to validate these results and inform future policies on the coverage and accessibility of RAS.
Additionally, such studies can contribute to the development of sustainable strategies that enhance efficiency and accessibility of RAS. One such strategy to consider is to assign a maximum number of surgeons or specialities per robotic platform to protect against significant dilutional effects and access limitations we have observed.
Furthermore, our study was conducted by a limited number of highly trained robotic surgeons. Additionally, the retrospective design of our study restricts the analysis of other variables, such as the complexity of specific procedures in non-RARP cases. Therefore, ongoing studies with regression analyses to examine trends over time are still required.
CONCLUSIONS
Over the last decade, RAS access by specialty has significantly increased in Canada; however, our study demonstrates a gradual decline in the number of RAS performed per surgeon over time, as well as a gradual increase in the mean SWT with a fixed and limited number of robotic platforms this past decade. While significant or comprehensive conclusions regarding robotic surgery programs cannot be drawn from this two-institution retrospective study alone, the lack of growth and acquisition of additional surgical robots, the significant costs related to the use of surgical robots, the constant increase in RAS demand, and the increase in RAS application across different surgical specialties in Canada will eventually affect patient access to RAS.
Footnotes
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Labban M, Dasgupta P, Song C, et al. Cost-effectiveness of robotic-assisted radical prostatectomy for localized prostate cancer in the UK. JAMA Netw Open. 2022;5:e225740. doi: 10.1001/jamanetworkopen.2022.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giri S, Sarkar DK. Current status of robotic surgery. Indian J Surg. 2012;74:242–7. doi: 10.1007/s12262-012-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu HY, Hevelone ND, Lipsitz SR, et al. Use, costs and comparative effectiveness of robotic assisted, laparoscopic, and open urological surgery. J Urol. 2012;187:1392–8. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 4.Binder J, Kramer W. Robotically assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–10. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted vs. open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology. 2004;63:819–22. doi: 10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: The Vattikuti Urology Institute experience. Urology. 2002;60:864–8. doi: 10.1016/S0090-4295(02)01881-2. [DOI] [PubMed] [Google Scholar]
- 7.Rocco B, Matei DV, Melegari S, et al. Robotic vs open prostatectomy in a laparoscopically naive center: A matched-pair analysis. BJU Int. 2009;104:991–5. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V, Novara G, Fracalanza S, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–9. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 9.Zorn KC, Zanaty M, El-Hakim A. Robotic prostatectomy and access to care: Canadian vs. U.S. experience. Can Urol Assoc J. 2016;10:202–3. doi: 10.5489/cuaj.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad F, Finelli A, Dranitsaris G, et al. Does prolonging the time to prostate cancer surgery impact long-term cancer control: A systematic review of the literature. Can J Urol. 2006;13:16–24. [PubMed] [Google Scholar]
- 11.Annual report 2010–2011. Intuitive Surgical Inc; [Google Scholar]
- 12.Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16:1–313. doi: 10.3310/hta16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: A comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–50. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parackal A, Tarride JE, Xie F, et al. Economic evaluation of robot-assisted radical prostatectomy compared to open radical prostatectomy for prostate cancer treatment in Ontario, Canada. Can Urol Assoc J. 2020;14:E350–7. doi: 10.5489/cuaj.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Close A, Robertson C, Rushton S, et al. Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: A health technology assessment from the perspective of the UK National Health Service. Eur Urol. 2013;64:361–369. doi: 10.1016/j.eururo.2013.02.040. [DOI] [PubMed] [Google Scholar]