Abstract
Introduction:
Bronchopulmonary sequestration (BPS) is typically a rare congenital disorder characterized by the presence of non-functioning lung tissue. There are two types of BPS: intralobar and extralobar sequestration, where extralobar sequestration can either be intrathoracic or sub-diaphragmatic.
Case presentation:
In this case report, we present the case of a 70-year-old male with intralobar BPS who presented with recurrent chest infections, and a diagnosis of intralobar pulmonary sequestration was made based on a computed tomography (CT) scan.
Discussion:
The diagnosis of intralobar pulmonary sequestration can be delayed as the intralobar type can present with varying imaging findings. A diagnosis can be made based on CT or MRI findings. A CT scan or MRI can show mass or consolidation with or without a cyst. Both CT and MRI can be reliable modalities to identify the arterial supply of the sequestered lung tissue, which is commonly a branch of the descending aorta.
Conclusion:
Sequestration should be suspected when a posterobasal lung abnormality is supplied by an abnormal artery from the aorta or another systemic artery.
Keywords: bronchopulmonary sequestration, congenital disorder, recurrent pneumonia
Introduction
Highlights
Bronchopulmonary sequestration (BPS) is a rare congenital disorder that typically presents with recurrent chest infections.
Intralobar BPS can present with varying imaging findings, which can lead to a delay in diagnosis.
The diagnosis of BPS can be made with a computed tomography (CT) or an MRI.
A contrast-enhanced CT scan can help detect the aberrant blood supply to the congenital anomalous lung tissue.
Bronchopulmonary sequestration (BPS), also called pulmonary sequestration, is a rare congenital disorder of the lower airway occurring in approximately 1 in 10 000–35 000 live births1. It is made up of a non-functioning mass of lung tissue that obtains its arterial blood supply from the systemic circulation and lacks normal contact with the tracheobronchial tree2.
There are two types of BPS: intralobar and extralobar sequestration, where extralobar sequestration can either be intrathoracic or sub-diaphragmatic2. Among these, intralobar sequestration, which is the most common type, usually presents in late childhood or adolescence with features of recurrent chest infection2. Here, we present the case of a 70-year-old male with intralobar BPS. This case report has been reported in line with SCARE guidelines3.
Case presentation
A 70-year-old man presented to the emergency department of our hospital with shortness of breath and a cough that progressively increased over 2 months. He complained of two episodes of hemoptysis within 2 months. There was no chest pain, swelling of the legs, or fever. He was a known case of chronic obstructive pulmonary disease. He had received complete anti-tubercular treatment for sputum-positive pulmonary tuberculosis 45 years ago. He had recurrent episodes of pneumonia and lung abscesses. He had hernia surgery 5 years ago. He used to smoke a pack of cigarettes per day for 50 years and stopped smoking one and a half years ago. On examination, clubbing was present in bilateral hands. He had a pulse of 86 beats per minute and an oxygen saturation of 92% at 2 l/min with nasal prongs. On auscultation, he had decreased air entry on both sides, more on the right side, with wheeze present in the left inframammary area.
Laboratory tests, including a complete blood count, liver and renal function tests, and urinalysis, were all within the normal range. A chest radiograph showed a normal cardio-mediastinal contour with slightly hyperinflated bilateral lungs. Further investigation with computed tomography (CT) showed a multicystic lesion in the left lower lung lobe with a few intralesional cysts showing air-fluid level and supplied by an aberrant artery arising from the descending thoracic aorta-pulmonary sequestration (Figs 1, 2, and 3) (infected) with cystic degeneration.
Figure 1.
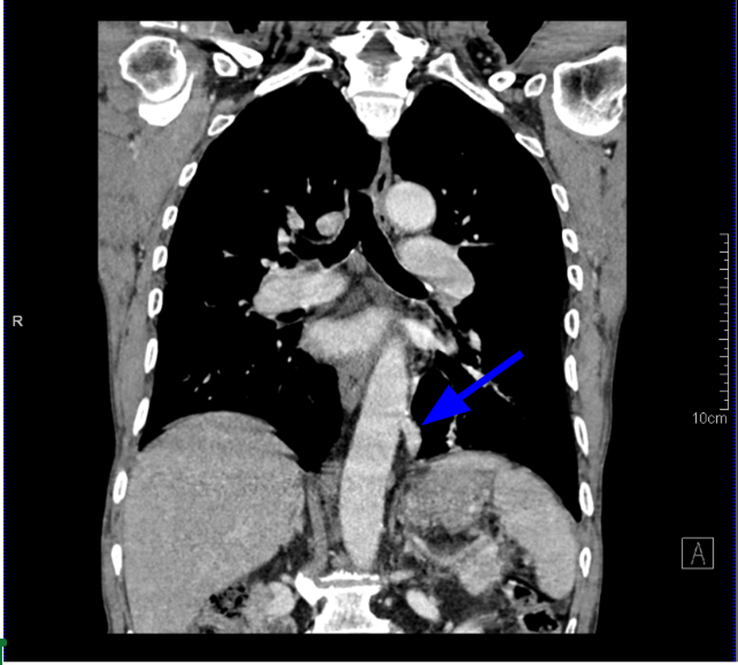
Coronal CT scan imaging in mediastinal algorithm showing aberrant artery arising from descending aorta and supplying the pulmonary sequestration area separately (blue arrow).
Figure 2.
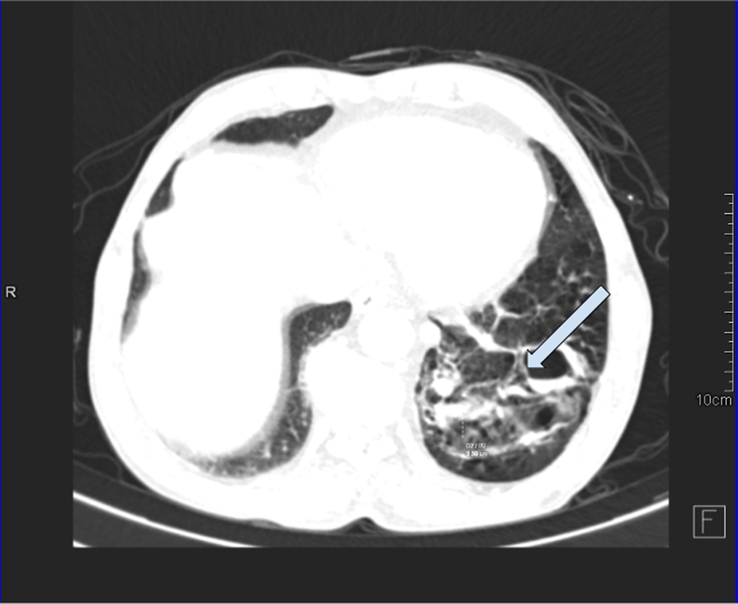
Axial CT scan imaging in the lung algorithm showing multicystic lesion in the left lower lung lobe with a few intralesional cysts showing an air-fluid level and supplied by an aberrant artery arising from the descending thoracic aorta-pulmonary sequestration (infected) with cystic degeneration. COPD (chronic obstructive pulmonary disease) changes with centrilobular emphysema and cardiomegaly were noted in bilateral lung fields.
Figure 3.
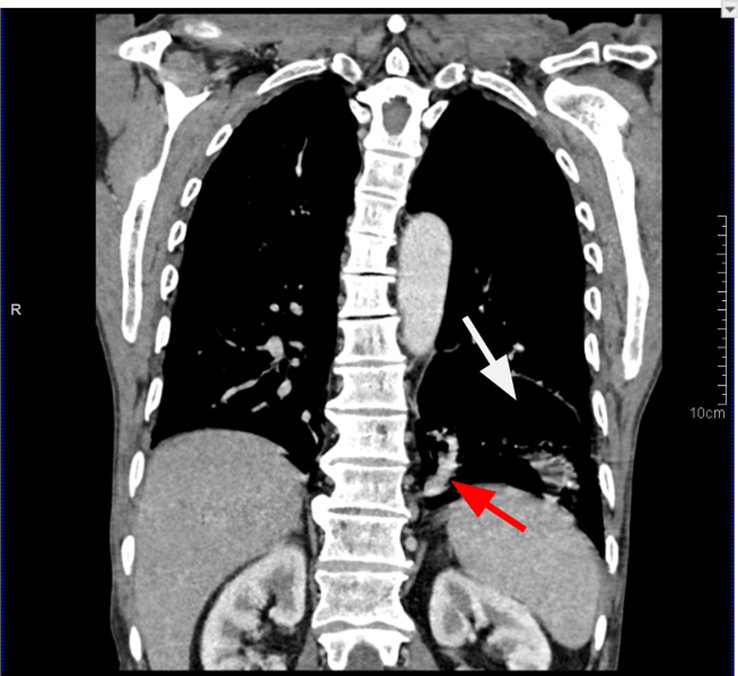
Chest coronal section contrast-enhanced computed tomography in mediastinal algorithm showing a section of the pulmonary sequestration as a cystic lesion (white arrow) with the systemic feeder aberrant artery from the aorta (red arrow).
He was treated with IV antibiotics. The patient was advised for surgery; however, the patient refused. The patient was referred to another center for further treatment.
Discussion
BPS is a bronchopulmonary foregut malformation that can be intralobar, extralobar, or hybrid. Intralobar type makes up 75% of BPS, which lies inside the lobe and does not have a separate visceral pleura. It is usually discovered in adolescence and early adulthood as a result of recurrent infections2. Patients can also present with chest pain or hemoptysis4. Intralobar sequestration is more common in the left lower lobe2.
Most intralobar pulmonary sequestration occurs before the age of 20 and occurs rarely after 50 years of age. In a study by the Mayo Clinic, the median age of diagnosis of pulmonary sequestration among adults was 42 years5. In our case, intralobar pulmonary sequestration was diagnosed at 70 years of age. Our patient had recurrent episodes of lung abscess and two episodes of hemoptysis. The extralobar type accounts for about 25% of BPS, lies outside the normal lung, has its own visceral pleura, and presents early in life2. This type is usually associated with congenital anomalies like pulmonary hypoplasia, foregut duplication cysts, heart defects, or a diaphragmatic hernia or eventration. It can be diagnosed with prenatal or neonatal ultrasound, or magnetic resonance imaging (MRI)6. Most extralobar pulmonary sequestration also occurs in the left hemithorax.
BPS arises from an aberrant pulmonary lung bud and obtains its blood supply from foregut vessels7. If the accessory lung bud develops before the formation of the visceral pleura, sequestered lung tissue along with the normal pulmonary parenchyma is covered by the same visceral pleura, forming intralobar sequestration7. But if the accessory lung bud only forms after the formation of the visceral pleura, the sequestered lung tissue will then form its own pleura, forming extralobar pulmonary sequestration7. Blood supply to pulmonary sequestration is through an anomalous vessel from the systemic circulation, often from the descending thoracic aorta4. Many cases are incidentally diagnosed in CT chest scans. Hence, we should be aware of the clinical features suggestive of intralobar sequestration, which include persistent cough, back pain, hemoptysis, and dyspnea, with the most common being recurrent pneumonia8.
The diagnosis of intralobar pulmonary sequestration can be delayed as the intralobar type can present with varying imaging findings. The plain radiograph of BPS may show a solitary nodule or mass, a triangular opacity, consolidation, or a cystic or multicystic lesion if infected4. A CT scan or MRI can show mass or consolidation with or without a cyst, and both CT and MRI can be reliable modalities to identify the arterial supply of the sequestered lung tissue, which is commonly a branch of the descending aorta4. The supplying artery may also arise from the splenic artery, celiac artery, abdominal aorta, or even the coronary artery4,9. Sometimes, the supplying artery may not be small or thin and may not be detected9. The imaging finding of extralobar type differs from intralobar type in that it occurs medially to the lung and is well defined, usually airless, and homogenous4.
The treatment for both intralobar and extralobar pulmonary sequestration is surgical excision10. Even when the patient is asymptomatic, intralobar type is treated with surgical excision due to the risk of future recurrent infections, abscesses, or hemoptysis11. For the asymptomatic extralobar type detected incidentally in adulthood, treatment has been debated, with some recommending radiological monitoring every 5–10 years12. Regardless of the type, when surgery has been planned, radiological evaluation of the anatomy of the blood supply of the lesion is recommended to avoid the transection of any unsuspected blood vessel4.
Conclusion
In an adult, BPS might manifest as a consolidation, a mass, a cystic or multicystic lesion, or any combination of these. An abnormal artery from the aorta or another systemic artery supplying a posterobasal lung abnormality should raise suspicion for sequestration. It is best detected by CT or MRI. As opposed to intralobar sequestration, extralobar sequestration is medial to the lung, nearly invariably airless, clearly defined, and homogeneous.
Ethical approval
Ethical approval was not required for case reports as per our Institutional review committee of our center. Patient anonymity is maintained throughout this manuscript, and consent was obtained for publication from the patient.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of of the written consent is available for review by the Editor-in-Chief of this journal on request. We also ensured, none of the identifying characteristics are included in the case report.
Sources of funding
No funding was obtained.
Author contribution
A.P.: manuscript preparation, editing, and review; A.P.: data collection, obtaining consent from the patient, and manuscript review; S.K.: manuscript preparation, editing, and review; A.D.: concept, manuscript preparation, editing, and review; S.A.: manuscript editing and review; A.C.: concept, manuscript editing, and review; A.C.: concept, manuscript editing, and review; P.P.: manuscript editing and review; S.B.: manuscript preparation, editing, and review.
Conflicts of interest disclosure
All authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Since this is a case report, research registration has not been done.
Guarantor
Dr Suraj Kesari, Department of Radiology, Kathmandu University School of Medical Sciences, Dhulikhel, Kavre 45210, Nepal.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 18 March 2024
Contributor Information
Abhishek Pandey, Email: aabhishek152@gmail.com.
Archana Pandey, Email: dr.pandeyarchana@gmail.com.
Suraj Keshari, Email: cheerfulsrj.sk@gmail.com.
Aliza Dulal, Email: alizadulal17@gmail.com;alizadulal@gmail.com.
Suyash Acharya, Email: acharyasuyash555@gmail.com.
Aashutosh Chaudhary, Email: aashutoshc007@gmail.com.
Ashlesha Chaudhary, Email: ashlesha04.ac@gmail.com.
Prasamsa Pande, Email: prasamsa.pande07@gmail.com.
Sushant Bhardwaj, Email: bhardwajsushant30@gmail.com.
References
- 1.Durell J, Thakkar H, Gould S, et al. Pathology of asymptomatic, prenatally diagnosed cystic lung malformations. J Pediatr Surg 2016;51:231–235. [DOI] [PubMed] [Google Scholar]
- 2.Landing BH, Dixon LG. Congenital malformations and genetic disorders of the respiratory tract (larynx, trachea, bronchi, and lungs). Am Rev Respir Dis 1979;120:151–185. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabi C, Mathew G, Maria N, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2023;109:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker CM, Wu CC, Gilman MD, et al. The imaging spectrum of bronchopulmonary sequestration. Curr Probl Diagn Radiol 2014;43:100–114. [DOI] [PubMed] [Google Scholar]
- 5.Alsumrain M, Ryu JH. Pulmonary sequestration in adults: a retrospective review of resected and unresected cases. BMC Pulm Med 2018;18:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter R. Pulmonary sequestration. Ann Thorac Surg 1969;7:68–88. [DOI] [PubMed] [Google Scholar]
- 7.Biyyam DR, Chapman T, Ferguson MR, et al. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010;30:1721–1738. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty RK, Modi P, Sharma S. Pulmonary Sequestration. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 9.Ikezoe J, Murayama S, Godwin JD, et al. Bronchopulmonary sequestration: CT assessment. Radiology 1990;176:375–379. [DOI] [PubMed] [Google Scholar]
- 10.Savic B, Birtel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabalza Marinucci B, Maurizi G, Vanni C, et al. Surgical treatment of pulmonary sequestration in adults and children: long-term results. Interact Cardiovasc Thorac Surg 2020;31:71–77. [DOI] [PubMed] [Google Scholar]
- 12.Laberge J-M, Puligandla P, Flageole H. Asymptomatic congenital lung malformations. Semin Pediatr Surg 2005;14:16–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


