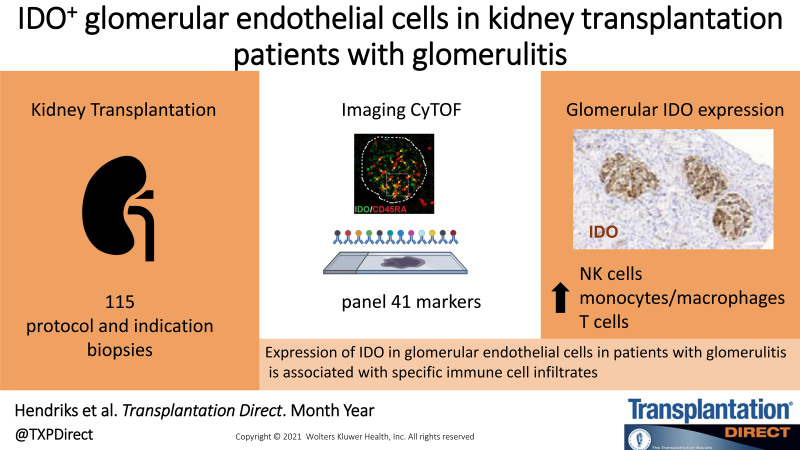
Abstract
Background.
Kidney transplantation is the preferred treatment option for patients with end-stage renal disease. However, long-term graft survival remains a challenge. The enzyme indoleamine 2,3 dioxygenase (IDO) has been reported to have immunomodulatory effects with IDO transcripts being elevated in both antibody-mediated rejection and T cell–mediated rejection.
Methods.
A metal-conjugated antibody panel for the staining of kidney biopsies was developed, allowing the visualization of 41 structural and immune markers on a single tissue slide to gain in-depth insight into the composition and localization of the immune cell compartment. Staining was applied to week 4 and 24 protocol biopsies of 49 patients as well as on 15 indication biopsies of the TRITON study and 4 additional transplantation biopsies with glomerulitis.
Results.
A highly distinctive and specific glomerular IDO expression was observed in biopsies from 3 of 49 patients in imaging mass cytometry. Immunohistochemistry confirmed IDO expression in glomeruli of 10 of 10 cases with glomerulitis. IDO was found to be expressed by CD31+ glomerular endothelial cells, accompanied by the presence of granzyme-B+Tbet+CD7+CD45RA+ natural killer cells and CD68+ macrophages. Furthermore, a proportion of both the immune cells and endothelial cells expressed Ki-67, indicative of cell proliferation, which was not observed in control glomeruli.
Conclusions.
Our results show glomerular IDO expression in transplanted kidneys with glomerulitis, which is accompanied by increased numbers of natural killer cells and macrophages and likely reflects local immune activation.
Kidney transplantation remains the preferred treatment for end-stage renal disease. However, despite significant improvements in short-term graft survival because of advances in immunosuppressive regimens, optimizing long-term kidney graft survival remains a challenge. In the past few years, several subtypes of kidney rejection have been defined.1 Designing and adjusting antirejection therapy specifically based on rejection type might help prolong graft survival.
In this study, we report on a distinct group of kidney transplantation patients who presented with indoleamine 2,3 dioxygenase (IDO) positivity specific to endothelial cells located in the glomeruli. IDO is an immunomodulatory enzyme produced by immune cells such as macrophages and dendritic cells in response to proinflammatory stimuli like interferon-gamma (IFN-γ).2 In addition, IDO can also be expressed by epithelial and endothelial cells, and local expression of IDO can contribute to the induction of regulatory T cell (Treg) and inhibition of NK and effector T cells.3,4
We used imaging mass cytometry (IMC) to understand the cell phenotypes in the environment of IDO glomerular endothelial cells. IMC has already been used to generate a detailed 2-dimensional map of the human kidney.5 Using IMC allowed us to maximize the amount of information that can be obtained from the limited quantity of tissue obtained from core-needle kidney biopsies. We developed a panel of 41 metal-conjugated antibodies, focusing on immune cell markers and structural and immune regulatory molecules. We performed IMC on all available biopsies of 49 patients, including 4 and 24-wk protocol biopsies and 15 indication biopsies.
In the current study, we show that IDO expression in biopsies from kidney transplantation recipients was prominent in glomerular endothelial cells in patients with glomerulitis, colocalized with NK cells and macrophages, and coinciding with local proliferation of both immune cells and endothelial cells.
MATERIALS AND METHODS
Study Design and Tissue Material
Biopsies analyzed originate from the TRITON clinical trial, a randomized phase II, prospective, single-center, open-label study in living-donor kidney transplant recipients in which autologous bone marrow-derived mesenchymal stromal cell therapy with concomitant early tacrolimus withdrawal was compared with standard tacrolimus dosing. The trial design and protocol have been previously described and were approved by the local ethics committee, Leiden University Medical Center Leiden, and by the Central Committee on Research involving Human Subjects in the Netherlands.6,7 Written informed consent was obtained from all participants. During the trial, protocol kidney biopsies were obtained at 4 and 24 wk after transplantation. Among the 70 participants, 49 patients received their allocated treatment and biopsies were successfully taken. In addition, 15 indication biopsies were taken in case of suspected rejection. These tissues were analyzed by IMC. Additionally, biopsies of 4 patients with glomerulitis were added for immunohistochemistry (IHC) staining of IDO.
Hematoxylin and Eosin Stains, Banff, C4d, and IHC
Biopsies were processed by a standard light microscopy panel containing hematoxylin and eosin, periodic-acid Schiff, and Jones’ silver staining and scored according to the 2019 Banff classification by an expert renal pathologist (J.K.).8 C4d staining was performed on formalin-fixed paraffin-embedded tissues using the immuneperoxidase technique. IHC staining for IDO was performed with the same IDO antibody as in IMC, on both the biopsies of the TRITON trial and 4 additional glomerulitis cases.
Mass Cytometry Antibodies
Table S1 (SDC, http://links.lww.com/TXD/A678) lists all heavy metal isotope-tagged monoclonal antibodies used. All antibodies were conjugated with heavy metal isotopes using the MaxPar X8 Polymere Antibody Labeling Kit according to the manufacturer’s protocol (Fluidigm, CA) except for keratin, α-smoot muscle actin, CD4, Vimentin, and HLA-G. Conjugation of 2 keratin antibody clones to 106Pd was performed using a protocol adapted from Schulz et al.9 209Bi was conjugated to α-smoot muscle actin using a protocol adapted from Spitzer et al.10 Conjugations of Cisplatin 194 and 198 to Vimentin and HLA-G were performed using a protocol adapted from Mei et al.11 CD4 was stained using a secondary staining step with α-mouse-145Gd. Antibodies were titrated to determine the optimal labeling concentration. All antibodies were verified by IHC before use in IMC.
IMC Antibody Staining
IMC antibody staining was performed as previously described.12 In brief, 4-µm formalin-fixed paraffin-embedded sections were deparaffinized by a series of xylene and ethanol, after which citrate-microwave antigen retrieval was performed. After blocking with superblock solution, the slides were stained with anti-CD4 (mouse IgG1, dilution in Table S1, SDC, http://links.lww.com/TXD/A678) overnight at 4 °C. The sections were then washed and stained with a secondary anti-mouse-145Gd antibody for 1 h at room temperature. Next, the sections were washed and stained with the first metal-labeled antibody mix for 5 h at room temperature (Table S1, SDC, http://links.lww.com/TXD/A678). After washing, slides were stained with the second antibody mix and incubated overnight at 4 °C. Finally, the slides were stained with Iridium nuclear staining, washed with demineralized water, and dried under airflow.
IMC Data Acquisition and Analysis
The Hyperion was autotuned, using a 3-element tuning slide according to the manufacturer’s protocol (Fluidigm). Regions of interest (ROIs) on the IMC slides were selected by the renal pathologist (J.K.), making sure the renal cortex containing glomeruli and, where possible, larger arteries was ablated. This resulted in 2 ROIs per biopsy, each 800 × 1200 µm. Selected ROIs were ablated at 200 Hz. Pixel analysis was performed using in-house developed Cytosplore Imaging. First, glomeruli were selected, after which the total number of pixels and the number of IDO+ pixels were counted per glomerulus. In each glomerulus, the number of positive cells for a certain marker was counted manually in the IDO+ glomeruli of the IDO+ group (12/14 glomeruli), the IDO– glomeruli of the IDO– group (18 glomeruli), and the IDO–/antibody-mediated rejection+ group (15 glomeruli).
Statistical Analysis
Percentage of positive pixels and cell counts are presented as medians. All comparisons were performed with the Mann-Whitney U test and Bonferroni correction. P values of <0.05 were considered statistically significant. Mann-Whitney U tests were performed in GraphPad Prism version 8.4.2 (Dotmatics).
RESULTS
IDO+ Expression by HLA-DR+CD31+ Endothelial Cells in Glomeruli of Transplant Recipients
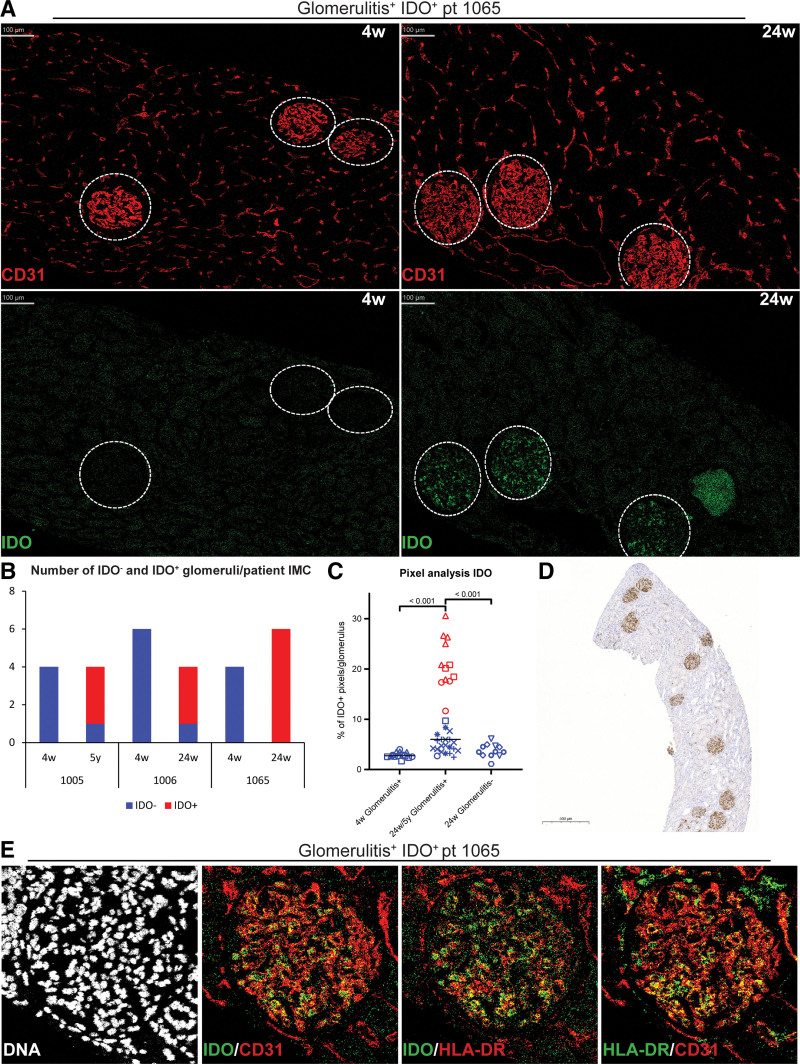
We applied IMC analysis on biopsies of a cohort of 49 kidney transplant recipient, including paired protocol biopsies obtained at 4 and 24 wk, and indication biopsies (n = 15) obtained at time points ranging from 4 wk till 5 y posttransplantation. Global analysis of glomeruli, characterized by CD31+ endothelial staining, and tubulointerstitial regions of all biopsies revealed in 3 patients the striking observation of IDO expression in glomeruli (Figure 1A). When analyzing biopsies of different time points from these 3 patients, IDO was observed in the majority of the glomeruli at week 24 (2/3) or after 5 y (1/3), with no IDO staining visible at 4 wk (Figure 1A and B). IDO expression was confirmed by a quantitative pixel analysis, where all positively scored glomeruli showed >10% positive pixels, ranging to 30% positivity (Figure 1C). We performed a similar pixel analysis on week 24 biopsies of 8 other patients from the trial, matched for demographics and clinical characteristics (Table S1, SDC, http://links.lww.com/TXD/A678). In all these cases, the glomeruli showed <10% positive pixels for IDO.
FIGURE 1.
IDO+ glomerular endothelial cells. A, Representative IMC images showing CD31 staining and IDO staining at week 4 and week 24. B, Bar graph showing the amount of IDO– and IDO+ glomeruli per patient in IMC for patient 1005, 1006, and 1065. C, Plot showing the percentage (%) of IDO+ pixels per glomeruli at 4 wk and 24 wk/5 y for the group that has glomerulitis at 24 wk/5 y and the glomerulitis– group at 24 wk. Significance was calculated between the blue (IDO–) and red (IDO+) glomeruli. Lines indicate medians (4 wk = 2.7%, 24 wk/5 y glomerulitis+ = 5.99, 24 wk/5 y glomerulitis+IDO+ = 20.5%, 24-wk glomerulitis– = 3.5). Each dot represents a glomeruli, the unique shape identifies the patient. D, Representative IHC stain for IDO in patient 1065. E, Representative IMC images showing DNA stain and merged images of IDO/CD31, IDO/HLA-DR, and HLA-DR/CD31 in a glomerulitis+IDO+ glomerulus. IDO, indoleamine 2,3 dioxygenase; IHC, immunohistochemistry; IMC, imaging mass cytometry.
In the 3 patients with IDO+ glomeruli, Banff scores indicated glomerulitis (3/3) combined with peritubular capillaritis in two-thirds of the patients (Table S2, SDC, http://links.lww.com/TXD/A678). With IMC, a limited ROI and a limited number of tissues can be measured. Therefore, to further investigate the link with glomerulitis, we performed IHC for IDO on the discussed cases and 4 additional glomerulitis cases (Figure 1D; Table S2, SDC, http://links.lww.com/TXD/A678). The percentage of IDO+ glomeruli was determined in patients with and without glomerulitis. In patients with glomerulitis, the percentage of IDO+ glomeruli was 22%–100% (median 89%, 10 cases), including the cases that were negative in IMC. In contrast, no glomerular IDO expression was observed in cases without glomerulitis 0%–11% (median 0%, 5 cases), confirming a significantly higher number of IDO+ glomeruli in glomerulitis (P < 0.001; Figure S2, SDC, http://links.lww.com/TXD/A678). Although IMC did not show any staining for IDO in the peritubular capillaries, in IHC 7 of 10 glomerulitis cases showed low to moderate IDO positivity in the peritubular capillaries (data not shown). Cases without glomerulitis did not show any IDO positivity in the peritubular capillaries.
To investigate which cells in the glomeruli expressed IDO, colocalization with specific markers was assessed in IMC. We observed colocalization of IDO with both CD31 and HLA-DR (Figure 1E), CD31+HLA-DR+ expression is indicative of glomerular endothelial cells.
Patients with glomerulitis showed both IDO+ and IDO– glomeruli. IMC was used to determine the inflammatory conditions and cellular composition of IDO+ glomeruli (3 patients) and compared this to IDO– glomeruli originating from both glomerulitis (5 patients) and nonglomerulitis patients (5 patients). Details of the 3 patients with IDO+ glomeruli are described in Table S3 (SDC, http://links.lww.com/TXD/A678), and biopsy characteristics with Banff scores of all patients and representative Jones’ silver stains are described in Table S2 and Figure S1 (SDC, http://links.lww.com/TXD/A678).
Glomeruli With IDO Expression Show Proliferation of Both Endothelial Cells and Leukocytes
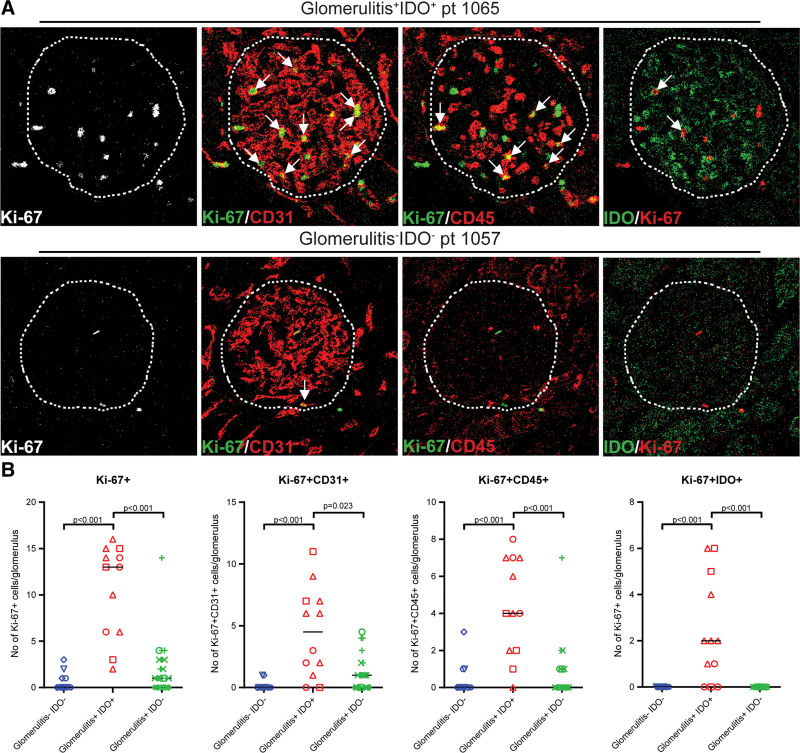
As a first step, we investigated the expression of Ki-67 as a sign of cell proliferation. This revealed that Ki-67 expression was increased in glomerulitis+IDO+ glomeruli but not in glomerulitis–IDO– nor glomerulitis+IDO– glomeruli (P < 0.001, P < 0.001; Figure 2A and B). The increase of Ki-67 expression was observed both for endothelial cells (Ki-67+CD31+) and for leukocytes (Ki-67+CD45+) in the glomerulitis+IDO+ glomeruli compared with the glomerulitis–IDO– glomeruli and glomerulitis+IDO– glomeruli (P < 0.001, P = 0.023, P < 0.001, P < 0.001; Figure 2A and B). There were also more Ki-67+IDO+ endothelial cells in the glomerulitis+IDO+ glomeruli compared with the glomerulitis–IDO– glomeruli and glomerulitis+IDO– glomeruli (P < 0.001, P < 0.001; Figure 2A and B). Thus, a subset of CD31+ endothelial cells and CD45+ immune cells are Ki-67+, indicative of cellular proliferation and part of these Ki-67+ endothelial cells are also IDO+.
FIGURE 2.
Ki-67+ expression in glomerulitis+IDO+ glomeruli compared with glomerulitis–IDO– and glomerulitis+IDO– glomeruli. A, Representative IMC images showing staining for Ki-67, merged images of Ki-67/CD31 and Ki-67/CD45 for both a glomerulitis+IDO+ glomerulus and a glomerulitis–IDO– glomerulus. Arrows indicate respectively Ki-67+CD31+ cells and Ki-67+CD45+ cells. B, Plots comparing the number of Ki-67+, Ki-67+CD31+, and Ki-67+CD45+ cells per glomerulus for groups glomerulitis+IDO+, glomerulitis–IDO– and glomerulitis+IDO–. Medians are indicated by lines, for Ki-67+, the medians are 0, 13, and 1; for Ki-67+CD45+, the medians are 0, 4, and 0; for Ki-67+CD31+, the medians are 0, 4.5, and 1; and for Ki-67+IDO+, the medians are 0, 2, and 0. Each dot represents a glomeruli, the unique shape identifies the patient. The group of glomerulitis–IDO– contains 14 glomeruli of 5 patients, glomerulitis+IDO+ contains 12 glomeruli of 3 patients, and glomerulitis+IDO– 21 glomeruli of 5 patients. IDO, indoleamine 2,3 dioxygenase; IMC, imaging mass cytometry.
Characterization of the Intragraft Immune Compartment in IDO+ and IDO– Glomeruli
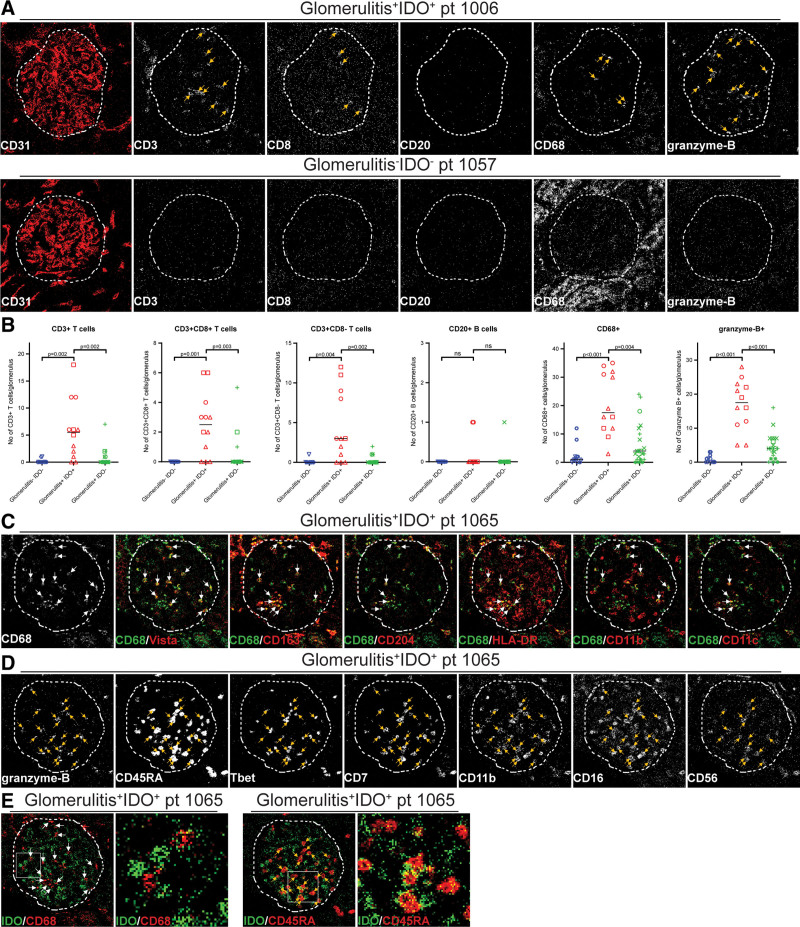
To further identify immune cells present within glomerulitis+IDO+ glomeruli, the following markers were used: CD3, CD8, CD20, CD68, and granzyme-B (Figure 3A). Because of low staining intensity, CD4 was not used. CD3+ T cells were increased in glomerulitis+IDO+ glomeruli when compared with glomerulitis–IDO– and glomerulitis+IDO– glomeruli (P = 0.002, P = 0.002). Both CD8+ and CD8− T cells were present in the glomeruli and their numbers were higher in the glomerulitis+IDO+ glomeruli compared with glomerulitis–IDO– (P = 0.001, P = 0.004) and glomerulitis+IDO glomeruli (P = 0.003, P = 0.002; Figure 3A–C). Both Treg (CD3+FoxP3+) and CD20+ B cells were largely absent in the glomeruli of all groups (Figure 3A and B; data not shown). In contrast, CD68+ macrophages and granzyme-B+ cells were present in large numbers in all glomerulitis+IDO+ glomeruli and significantly increased compared with glomerulitis–IDO– glomeruli (P < 0.001, P < 0.001) and glomerulitis+IDO– glomeruli (P = 0.004, P < 0.001; Figure 3B).
FIGURE 3.
Immune cells in the IDO+ glomeruli compared with IDO– and IDO–/AMR+ glomeruli. A, Representative IMC images showing staining of glomerulitis+IDO+ and glomerulitis–IDO– glomeruli for immune markers CD3, CD8, CD20, CD68, and granzyme-B. Arrows indicated positive cells. B, Plots comparing the number of positive cells for each marker per glomerulus for groups glomerulitis–IDO–, glomerulitis+IDO+ and glomerulitis+IDO– with lines indicating medians. The medians are 0, 5.5, and 0 for CD3+ T cells; 0, 2.5, and 0 for CD3+CD8+ T cells; 0, 3, and 0 for CD3+CD8− T cells; 0, 0 and 0 for CD20+ B cells; 1, 17.5, and 4 for CD68+ macrophages; and 0, 17.5, and 4 for granzyme-B+ cells. Each dot represents a glomerulus, the unique shape identifies the patient. The group of glomerulitis–IDO– contains 14 glomeruli of 5 patients, glomerulitis+IDO+ contains 12 glomeruli of 3 patients, and glomerulitis+IDO– 21 glomeruli of 5 patients. C, Representative IMC images of a glomerulitis+IDO+ glomerulus with merged images for IDO/CD68, CD68/Vista, CD68/CD163, CD68/CD204, CD68/HLA-DR, CD68/CD11b, and CD68/CD11c. Arrows indicated double positive cells. D, Representative IMC images of a glomerulitis+IDO+ glomeruli showing staining for granzyme-B, CD45RA, Tbet, CD7, CD11b, CD16, and CD56. Arrows indicate granzyme-B+ NK cells. E, Zoomed in merged IMC images of IDO/CD68 and IDO/CD45RA showing the location of IDO+ endothelial cells with respect to CD68+ macrophages and CD45RA+ NK cells. AMR, antibody-mediated rejection; IDO, indoleamine 2,3 dioxygenase; IMC, imaging mass cytometry; NK, natural killer.
We further investigated the phenotypes of the CD68+ and granzyme-B+ cells in glomerulitis+IDO+ glomeruli. The CD68+ cells were found to be HLA-DR+ and to display variable expressions of Vista, CD163, CD204, CD11b, and CD11c, consistent with a macrophage phenotype (Figure 3C). The granzyme-B+ cells were Tbet+CD7+CD45RA+CD11b±CD16±CD56±, in the absence of CD3, consistent with NK cells (Figure 3D).
Finally, we investigated the possible cellular interaction between infiltrating cells and the endothelial cells expressing IDO. CD68+ macrophages were not consistently located next to IDO+ endothelial cells (Figure 3E). In contrast, close interactions between NK cells and IDO+ endothelial cells were observed. For this colocalization, we had to use surface-expressed CD45RA, resulting in yellow pixels at the interface of 2 cells (Figure 3E), because granzyme-B, used for the initial characterization, is an intracellular protein.
DISCUSSION
In this study, we applied in-depth immune profiling to tissue sections of biopsies obtained from kidney transplant recipients. We show that biopsies with glomerulitis were accompanied by IDO expression in the glomerular endothelial cells and a local increase in the number of granzyme-B+Tbet+CD7+CD45RA+ NK cells and CD68+ macrophages. Additionally, we observed that under these conditions, several immune and endothelial cells expressed Ki-67, indicative of cell proliferation, whereas this was not observed in control IDO– glomeruli.
IDO is an immunomodulatory enzyme, rate limiting for tryptophan catabolism through the kynurenine pathway.2 IDO is considered to have immune suppressive properties and has been shown to be abundant in the placenta, where it is essential for a successful pregnancy.13,14 In view of the immunoregulatory properties, the expression and activity of IDO have been previously studied in the context of kidney transplantation.15,16 Plasma kynurenine levels were measured as a substitute for IDO activity with the rationale that IDO might be protective for the allograft because of its immunosuppressive properties.16 However, these studies revealed that higher levels of kynurenine, and thus IDO activity, did not protect against rejection but were even elevated in patients with acute rejection.15,16 Whereas elevated numbers of IDO transcripts have been described in both antibody-mediated rejection and T cell–mediated rejection, these studies did not allow for investigating IDO in the context of spatial orientation and do, therefore, not pinpoint where in the kidney biopsy this expression is located.17 Furthermore, these studies did not relate IDO expression to glomerulitis. On a cellular level, IDO can be produced by immune cells, such as macrophages and dendritic cells, in response to proinflammatory stimuli like IFN-γ. Although the expression of IDO has been associated with immunoregulation, we did not observe FoxP3+ Treg in regions of glomerular IDO expression. Although IDO expression in the context of kidney disease and transplantation has been previously observed in human tubular epithelial cells and podocytes, these studies did not relate IDO expression with the presence of CD68+ macrophages nor NK cells in the glomeruli.15,18,19
In the present study, we observed that IDO is expressed in the glomerular endothelial cells of 10 patients in combination with glomerulitis. In 7 of these patients, IDO was also present in the peritubular capillaries. Our study demonstrates heterogeneity within renal allografts with glomerulitis, as evidenced by the presence of both IDO+ and IDO– glomeruli within the same patient. The fact that IDO expression is accompanied by specific immune cell infiltrates highlights the importance of local interactions and communications to regulate immune-activating or regulatory mechanisms. We observed that glomerular IDO expression was especially associated with elevated numbers of granzyme-B+Tbet+CD7+CD45RA+ NK cells. We showed that these NK cells are in close proximity to the endothelial cells, implying interaction between the 2 cell types. NK cells are known to be able to harm endothelial cells by releasing granzyme-B and by producing IFN-γ, attracting monocytes and macrophages.1 We therefore envision an effector role of the NK cells in the IDO+ glomeruli, leading to endothelial damage. This is supported by our finding that the endothelium is expressing Ki-67 and thus proliferating, possibly to replace the damaged endothelial cells and repair vascular damage. In response to the damage, the endothelium might produce IDO with the aim to induce negative feedback on the NK cells because IDO can suppress NK cell activity.20 Therefore, we hypothesize that the endothelium produces IDO in response to NK cell activity. No specific features, including HLA-A/B/DR or killer-cell immunoglobulin-like receptor ligand mismatch, viral status, donor-specific antibodypositivity in serum, or C4d deposition in the biopsy, could explain the infiltration of NK cells (data not shown).
In the glomerulitis+IDO+ glomeruli, an increase of CD68+HLA-DR+ monocytes/macrophages displaying variable expression of Vista, CD163, CD204, CD11b, and CD11c was observed. Macrophages are associated with chronic glomerular injury and inferior graft function.21 However, in mice, kidney-resident macrophages expressing Vista were reported to prevent IFN-γ/interleukin-9 axis-mediated tubulointerstitial fibrosis after acute glomerular injury and accelerate repair from ischemic injury.22,23 We observed both Vista+ and Vista– macrophages in the IDO+ glomeruli, but their role in either injury or repair remains unknown.
In this study, the use of a 41-marker panel allowed us to identify IDO as a unique identifier in glomerulitis patients. Although the use of this extensive panel guided us to the discovery of IDO expression in the glomeruli of glomerulitis patients, the use of IMC limits the area that can be ablated and therefore visualized. Additionally, IHC is more sensitive than IMC, increasing the visualization of IDO in the glomeruli. Therefore, some patients with IDO+ glomeruli in IHC did not show IDO+ glomeruli in IMC.
In conclusion, we identified glomerular IDO expression in patients with glomerulitis after kidney transplantation. It will be important to extend our findings to a larger cohort of patients with glomerulitis and glomerular IDO expression, both to elucidate the cause of IDO expression and the presence of granzyme-B+ NK cells and CD68+ macrophages as well as to assess IDO expression in relation to clinical outcome.
ACKNOWLEDGMENTS
The authors thank Kyra Dijkstra, BSc, for cutting the slides; Boudewijn P.F. Lelieveldt, PhD, for developing Cytosplore Imaging and the Flow cytometry Core Facility of the Leiden University Medical Center for their help with IMC experiments.
Supplementary Material
Footnotes
ZonMW-TAS provided funding for this study (grant/award number: 116004104).
J.K. declares consultancy fees from Novartis AG and Alentis Theurapeutics AG, and speaker fees from Chiesi Pharmaceuticals BV, all outside the scope of the current submission.
S.H.H. conceived the study, performed experiments, and analyzed the data. J.K. and M.E.IJ. provided imaging mass cytometry input. M.E.IJ. performed the immunohistochemistry experiments. M.E.J.R. designed and coordinated the clinical trial and provided samples for the study. J.E. developed Imaging Cytosplore, J.K. provided Banff scores and pathology advise. S.H., C.v.K., and F.K. supervised the study. S.H.H., S.H., C.v.K., and F.K. wrote the article in collaboration with all coauthors.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Clinical trial information: ClinicalTrials.gov NCT00734396
Contributor Information
Sanne H. Hendriks, Email: s.h.hendriks@lumc.nl.
Sebastiaan Heidt, Email: s.heidt@lumc.nl.
Juliette Krop, Email: j.krop@lumc.nl.
Marieke E. IJsselsteijn, Email: m.e.ijsselsteijn@lumc.nl.
Jeroen Eggermont, Email: j.eggermont@lumc.nl.
Jesper Kers, Email: j.kers@lumc.nl.
Marlies E.J. Reinders, Email: m.e.j.reinders@erasmusmc.nl.
Frits Koning, Email: f.koning@lumc.nl.
REFERENCES
- 1.Callemeyn J, Lamarthee B, Koenig A, et al. Allorecognition and the spectrum of kidney transplant rejection. Kidney Int. 2022;101:692–710. [DOI] [PubMed] [Google Scholar]
- 2.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. 2005;338:12–9. [DOI] [PubMed] [Google Scholar]
- 3.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frumento G, Rotondo R, Tonetti M, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Avigan ZM, Kliegel JA, et al. Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight. 2019;4:e129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinders ME, Bank JR, Dreyer GJ, et al. Autologous bone marrow derived mesenchymal stromal cell therapy in combination with everolimus to preserve renal structure and function in renal transplant recipients. J Transl Med. 2014;12:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinders MEJ, Groeneweg KE, Hendriks SH, et al. Autologous bone marrow-derived mesenchymal stromal cell therapy with early tacrolimus withdrawal: the randomized prospective, single-center, open-label TRITON study. Am J Transplant. 2021;21:3055–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz AR, Mei HE. Surface barcoding of live PBMC for multiplexed mass cytometry. In: McGuire HM, Ashhurst TM, eds. Mass Cytometry: Methods And Protocols. Springer; 2019:93–108. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei HE, Leipold MD, Maecker HT. Platinum-conjugated antibodies for application in mass cytometry. Cytometry A. 2016;89:292–300. [DOI] [PubMed] [Google Scholar]
- 12.Ijsselsteijn ME, van der Breggen R, Farina Sarasqueta A, et al. A 40-marker panel for high dimensional characterization of cancer immune microenvironments by imaging mass cytometry. Front Immunol. 2019;10:2534–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curti A, Trabanelli S, Salvestrini V, et al. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113:2394–2401. [DOI] [PubMed] [Google Scholar]
- 14.Ban Y, Chang Y, Dong B, et al. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. J Int Med Res. 2013;41:1135–1149. [DOI] [PubMed] [Google Scholar]
- 15.Brandacher G, Cakar F, Winkler C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007;71:60–67. [DOI] [PubMed] [Google Scholar]
- 16.Lahdou I, Sadeghi M, Daniel V, et al. Increased pretransplantation plasma kynurenine levels do not protect from but predict acute kidney allograft rejection. Hum Immunol. 2010;71:1067–1072. [DOI] [PubMed] [Google Scholar]
- 17.Halloran PF, Venner JM, Famulski KS. Comprehensive analysis of transcript changes associated with allograft rejection: combining universal and selective features. Am J Transplant. 2017;17:1754–1769. [DOI] [PubMed] [Google Scholar]
- 18.Vavrincova-Yaghi D, Seelen MA, Kema IP, et al. Early posttransplant tryptophan metabolism predicts long-term outcome of human kidney transplantation. Transplantation. 2015;99:e97–104. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary K, Shinde R, Liu H, et al. Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy. J Immunol. 2015;194:5713–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park A, Yang Y, Lee Y, et al. Indoleamine-2,3-dioxygenase in thyroid cancer cells suppresses natural killer cell function by inhibiting NKG2D and NKp46 expression via STAT signaling pathways. J Clin Med. 2019;8:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Choi SE, Lim BJ, et al. Clinical significance of macrophage polarization in antibody-mediated rejection of renal allograft. Transplant Proc. 2018;50:1005–1008. [DOI] [PubMed] [Google Scholar]
- 22.Kim MG, Yun D, Kang CL, et al. Kidney VISTA prevents IFN-gamma/IL-9 axis-mediated tubulointerstitial fibrosis after acute glomerular injury. J Clin Invest. 2022;132:e151189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JG, Lee CR, Kim MG, et al. Kidney residency of VISTA-positive macrophages accelerates repair from ischemic injury. Kidney Int. 2020;97:980–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.