Abstract
OBJECTIVES:
Interhospital transfer of patients with acute respiratory failure (ARF) is relevant in the current landscape of critical care delivery. However, current transfer practices for patients with ARF are highly variable, poorly formalized, and lack evidence. We aim to synthesize the existing evidence, identify knowledge gaps, and highlight persisting questions related to interhospital transfer of patients with ARF.
DATA SOURCES:
Ovid Medline, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Embase, CINAHL Plus, and American Psychological Association.
STUDY SELECTION:
We included studies that evaluated or described hospital transfers of adult (age > 18) patients with ARF between January 2020 and 2024 conducted in the United States. Using predetermined search terms and strategies, a total of 3369 articles were found across all databases. After deduplication, 1748 abstracts were screened by authors with 45 articles that advanced to full-text review. This yielded 16 studies that fit our inclusion criteria.
DATA EXTRACTION:
The studies were reviewed in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews by three authors.
DATA SYNTHESIS:
Included studies were mostly retrospective analyses of heterogeneous patients with various etiologies and severity of ARF. Overall, transferred patients were younger, had high severity of illness, and were more likely to have commercial insurance compared with nontransferred cohorts. There is a paucity of data examining why patients get transferred. Studies that retrospectively evaluated outcomes between transferred and nontransferred cohorts found no differences in mortality, although transferred patients have a longer length of stay. There is limited evidence to suggest that patients transferred early in their course have improved outcomes.
CONCLUSIONS:
Our scoping review highlights the sparse evidence and the urgent need for further research into understanding the complexity behind ARF transfers. Future studies should focus on defining best practices to inform clinical decision-making and improve downstream outcomes.
Keywords: acute respiratory failure, interhospital transfer, scoping review
KEY POINTS
Question: The aim of this scoping review study is to define and describe the “W” questions (who, why, when, where, and what) in the context of interhospital transfer of patients with acute respiratory failure (ARF).
Findings:
Who: Overall transferred patients were younger, commercially insured, with higher severity of illness compared with nontransferred patients.
Why: Most studies used administrative or electronic health records data, and reasons for transfer were not captured. Survey data suggests that stakeholders often disagree on why patients are transferred.
When: While few studies suggest that early transfer is associated with better outcomes, timing of transfer is not optimally answered by the literature.
Where: ARF patients were transferred to centers with higher volumes of mechanically ventilated patients, although transfer networks remain informal.
What: Studies that retrospectively evaluated outcomes between transferred and nontransferred cohorts found no differences in mortality, although transferred patients have a longer length of stay.
Meaning: Our findings highlight the sparse evidence and the urgent need for further research into understanding the complexity behind ARF transfers.
Acute respiratory failure (ARF) is one of the most common causes of critical illness and ICU admission across the United States and is associated with high mortality (1–3). Approximately one in 30 ARF patients admitted to an ICU undergoes a transfer to another hospital for multiple reasons, ranging from a need for highly specialized care or procedures to requests by patients or family members (4–7). Prior literature has showcased improved survival of ARF patients when cared for in hospitals with higher volume or resources, which has led to preliminary advocacy in building tiered ICU transfer networks for ARF patients (5, 8–10). Yet in contrast to other conditions like stroke, myocardial infarction, and trauma, current transfer practices for patients with ARF are poorly formalized and lack evidence.
The transfer of ARF patients as an intervention is not well studied. Most recently, the issue of ARF transfers took center stage due to the stress on the healthcare system related to the COVID-19 pandemic and worsening shortages of critical care clinicians. The considerable practice variability and fragmentation of the transfer decision-making process in the setting of severe resource strain led to long wait times for patients, considerable moral distress for triaging providers (11), and ultimately increased mortality for critically ill patients with COVID-19 (12). Thus, in our scoping review, we aimed to synthesize the existing evidence on who, why, when, where to, and what happens to patients before and after transfer. Our ultimate goal was to identify knowledge gaps and highlight enduring questions related to interhospital transfer of patients with ARF.
METHODS
Literature Search
We included articles that evaluated or described the process of interhospital transfer of adult (age > 18) patients with ARF between January 2020 and January 2024 conducted in the United States. Of note, our broad definition of ARF was inclusive of patients requiring mechanical ventilation, noninvasive ventilation (NIV), and high-flow nasal cannula for either hypoxia or hypercapnia.
We excluded articles not in English, editorials, case reports, commentaries, or literature from meetings or conferences. Additionally, transfer related studies primarily focused on stroke, myocardial infarction, trauma, emergency department setting, non-ICU/floor transfers, combat setting, aortic emergencies, neurosurgical patients, safety during transfer, and mode of transport were beyond the scope of this review and excluded. Search terms and strategies were developed closely with an experienced librarian; a full list of search strategies and terms used is provided in the search strategy appendix (Appendix, http://links.lww.com/CCX/B374). Approval from institutional research ethics board was not required as the study did not involve human subjects or de-identified data.
RESULTS
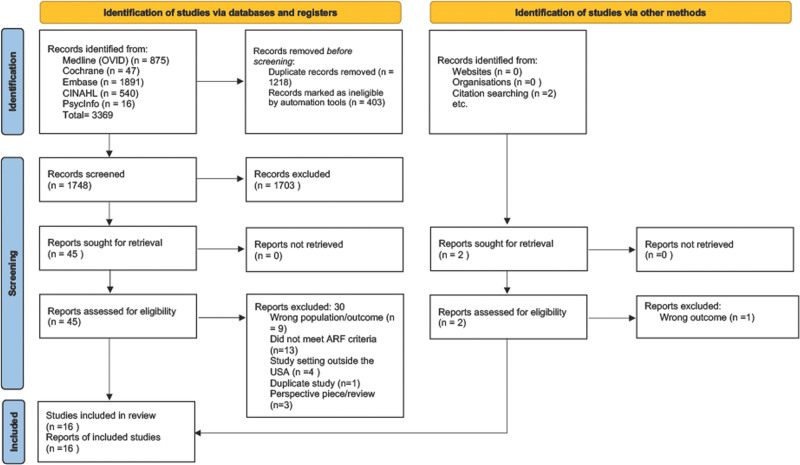
Our search strategy identified 3369 articles across all databases and relevant reviews. An additional two studies were identified via citation searching; 1748 remained after deduplication. A pilot screen of 100 random articles was initially performed by the reviewers (A.L., N.R.N.) to clarify inclusion and exclusion criteria. Two reviewers (A.L.) and (J.S.) screened the remaining results in Rayyan (13) using predetermined criteria. Conflicts were resolved via discussion between three authors (A.L., J.S., N.R.N.) before full-text screening. All full-text articles were screened separately by each reviewer, with conflicts resolved by discussion or a third reviewer. References of included articles were assessed for potential inclusion that led to identifying two additional articles. The abstract screening yielded 45 articles, which advanced to full-text reading. This yielded 16 articles that fit inclusion criteria and our definition of ARF (Fig. 1) (14).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 diagram flow for the identification of included studies. ARF = acute respiratory failure, USA = United States.
The 16 articles included in this review were observational in nature, with 14 retrospective analysis that used either administrative databases (15–20) or electronic health record data (21–28). One study was prospective in nature and examined the reasons behind patient transfer (29), while another evaluated the efficacy of an intervention on transfer wait times and outcomes (30). Eleven studies included patients that were predominantly mechanically ventilated, three of which included patients on extracorporeal membrane oxygenation (ECMO) (15, 24, 27) (Table 1). Three studies identified COVID-19 as the primary diagnosis (21, 27, 32) and two studies identified pulmonary embolism (PE) as the primary diagnosis (20, 26).
TABLE 1.
A Summary of Included Studies and Specified Level of Respiratory Support
| References | Study Year | Respiratory support |
|---|---|---|
| Aguayo et al (15) | 2010–2016 | All mechanically ventilated, on ECMO |
| Carroll et al (26) | 2012–2018 | 62% of transfers with intermediate/high risk pulmonary embolism on supplemental oxygen |
| Chen et al (21) | 2020 | All mechanically ventilated |
| Creel-Bulos et al (27) | 2023 | All mechanically ventilated |
| Hanane et al (22) | 2012–2014 | 44% mechanically ventilated or on high-flow oxygen |
| Hyder et al (30) | 2022 | 63% mechanically ventilated |
| Nadig et al (16) | 2012–2013 | All mechanically ventilated |
| Nadig et al (17) | 2015–2017 | All mechanically ventilated |
| Patel et al (23) | 2010–2014 | Median Fio2 on arrival 0.28 (interquartile range, 0.21–0.4) |
| Ranney et al (24) | 2009–2015 | All mechanically ventilated, on ECMO |
| Rush et al (18) | 2013 | All mechanically ventilated |
| Sedhom et al (20) | 2016–2019 | 56–65% of patients in three cohorts on mechanical ventilation |
| Siddiqui et al (28) | 2016–2018 | 58% mechanically ventilated |
| Tyler et al (19) | 2006–2012 | All mechanically ventilated |
| Usher et al (25) | 2020 | All with diagnosis of COVID-19 pneumonia, 59% mechanically ventilated |
| Wagner et al (29) | 2007–2008 | 47% mechanically ventilated |
| Wallace et al (31) | 2010 | All mechanically ventilated |
ECMO = extracorporeal membrane oxygenation.
Evidence Synthesis
Who: Epidemiology of Patients With ARF Who Undergo Interhospital Transfer
In multiple retrospective analyses, younger patients were more likely to be transferred than older patients (15, 19, 20, 33). As an example, an analysis using administrative data from Florida’s Healthcare Cost and Utilization Project (HCUP) state inpatient database (n = 2091) during 2012–2013 found that mechanical ventilated transferred patients were likely to be younger than 65 (mean age 61 vs. 65; p < 0.001) (16). More recently, a cohort study from a Midwestern hospital of mechanically ventilated COVID-19 patients (n = 117) also found that transferred patients were younger compared with those that were not transferred (median age 57 vs. 61; p < 0.001) (21). Similarly, three studies found that older age (> 65) was associated with lower probabilities of undergoing transfer (19, 20, 32).
Race and ethnicity appeared to be a recurring factor in these retrospective studies. Notably, in a large nationwide inpatient sample from 2006 to 2012 (n = 3095), there were no differences in illness severity between transferred groups compared with those who were not; however, Black and Hispanic patients (odds ratio [OR], 0.79; 95% CI, 0.70–0.89; p < 0.001 and OR, 0.79; 95% CI, 0.69–0.90; p < 0.01, respectively) and patients in the Southern states (OR, 0.79; 95% CI, 072–0.88; p < 0.01) were less likely to be transferred (19). Conversely, a recent study of transferred mechanically ventilated COVID-19 patients (n = 117) found no differences in race or ethnicity in comparison to directly admitted ICU patients, but transferred patients were more likely to state English as their preferred language (21).
Studies have also evaluated patient’s insurance, or lack thereof as a variable associated with interhospital transfer. In a large cohort of ARF patients (n = 2091), the authors found that patients insured via Medicaid had a lower adjusted odds of transfer in comparison to commercially insured patients (adjusted OR, 0.65; 95% CI, 0.56–0.75; p < 0.001) (16). Conversely, in another cohort (n = 3095) authors found that patients with insurance coverage were associated with lower probability of transfer compared with uninsured patients in univariate analysis (94% vs. 92%; p < 0.01) (19).
Transferred patients with ARF have also been found to be more severely ill and resource intensive as evidenced by an increasing percentage of transferred patients requiring ECMO support (15, 21, 24). In a large nationwide analysis of patients with PE (n = 11,341), transferred patients were more likely to have high risk features like acute cor pulmonale, saddle PE, or concomitant deep vein thrombosis (20). Some studies have used acute illness severity indicators like the Acute Physiology and Chronic Health Evaluation scoring system (22) and Sequential Organ Failure Assessment (SOFA) scores (23) to describe patient characteristics, which are both predictive scoring systems to assess the magnitude of a patient’s illness as well as prognosticate risk of mortality. However, a majority have used administrative data sources and report use of Charlson Comorbidity Index (16, 17), total Elixhauser comorbidity score (15, 18, 19, 25), or other models (21) that largely capture comorbidities and not acuity at time of disease presentation. Some have excluded severity of illness altogether (24, 29, 31), leading to the inability to accurately risk stratify patients either during their initial hospital course or events that occur after transfer.
Why: Reasons for Interhospital Transfer of Patients With ARF
Given the use of administrative data and/or retrospective cohort studies, information on why patients get transferred is sparse. Among the limited pool of studies, reasons for ARF patient transfers include patient complexity with high oxygenation needs, need for ECMO capabilities, or advanced interventions not available at the initial hospital (20, 26, 27) and for cohorting patients during the COVID-19 pandemic (25). Only one prospective study has evaluated reasons for interhospital transfer from 62 hospitals to a large academic medical center by surveying the referring physician, the accepting physician, and/or the patient/ their surrogate (29). Of 138 patients transferred within the study period, 124 surveys were received from accepting physicians (90%), 27 from referring physicians (20%), and 46 from patients and/or their surrogates (33%). The most stated reasons for transfer included access to higher quality of care, availability of specialized tests or procedures not available at the referring hospital, and to increase the patient’s likelihood of survival (29). Additionally, the study found that physician and patient/surrogate pairs had little to no agreement on the specific reasons for transfer.
When: Timing of Interhospital Transfer for Patients With ARF
Only a few studies included have evaluated timing of transfer as a variable. One retrospective study using HCUP state inpatient database (2015–2017) from five states (n = 6718) found that 65% of the transferred cohort were transferred within 2 days of initial ICU admission characterized as early transfers; these early transfers were found to have a lower relative risk of in patient mortality (0.442; 95% CI, 0.403–0.497; p < 0.0001) and shorter length of hospital stay (20.7 fewer days [13.0 vs. 33.7; p < 0.0001]) compared with a propensity matched cohort transferred later (> 3 d). However, this study was limited and did not have information on clinically derived illness severity scores, reason(s) for transfer, ICU capacity strain, and staffing models (17). A similar retrospective study of ARF transfers (n = 1269) at a single academic center showcased a median length of stay (LOS) of 4 days (interquartile range [IQR], 2–8 d) at the initial hospital before transfer and found that a longer LOS at the initial hospital was independently associated with increased mortality (OR, 1.029; 95% CI, 1.008–1.050; p < 0.005) (22). This suggests that the benefit of transfer is derived from interventions that are most effective when implemented early in a patient’s illness course similar to early antibiotic initiation in sepsis (34) or proning in acute respiratory distress syndrome (35). However, this effect was not observed consistently in other studies. A single-center study (n = 773) evaluating predictors of mortality after interhospital transfer found that the median LOS for the transferred patients at the initial hospital was 1 day (IQR, 0–2 d). However, the timing did not affect their primary outcome of 24-hour mortality after transfer (23). Similarly, a small study (n = 76) evaluating the effectiveness of a quality improvement intervention—a daily transfer huddle of key stakeholders—to shorten wait times for transferred patients did not show a statistically significant decrease in mortality or LOS despite decreasing wait times (30).
Where: Destinations for Patients With ARF Who Undergo Interhospital Transfer
Two retrospective studies using administrative data and evaluating transfer patterns in multiple states report that transfers occur typically from smaller hospitals (bed number < 100) to larger hospitals (bed number > 300); larger hospitals in these studies were further characterized by including information on annual ARF volume, annual ICU volume, and availability of expertise with ECMO and transplants (16, 17). Given the demonstrated safety of transport while on ECMO support, access to high-volume regional centers has increased due to expansion of their catchment area (27). In fact, one study of ARF transfers in a single academic center reports receiving transfers from 350 referral centers within the 4-year time period of the study (23). Studies have also reported receiving interhospital transfers across state lines ranging from 11% to 45%, although characterization of receiving hospitals remains scant (22, 24, 29). One study of mechanically ventilated ICU patients from the Nationwide Inpatient Sample (n = 3095) found that compared with nonteaching hospitals, patients admitted to urban teaching hospitals had lower odds of transfer (OR, 0.31; 95% CI, 0.28–0.33; p < 0.001) (19).
What Happens to ARF Patients After Transfer?
A small set of studies have evaluated clinical outcomes associated with transferred ARF patients (Table 2). Of note, these patients have predominantly been compared with nontransferred cohorts directly admitted to tertiary or academic ICUs. The majority of studies showcase no difference in mortality rates between transferred and nontransferred cohorts (15, 18, 21). One study evaluated risk factors for early mortality within 24 hours of transfer and identified a cardiac arrest before transfer, high SOFA score on the day of transfer, and high Fio2 needs (0.8–1.0) on arrival to the transferring unit as risk factors for an increased odds of mortality (23). In disease specific cohorts, a study of ARF patients with PE suffered higher rates of PE-related mortality when transferred compared with those that did not (2.3% vs. 0.6%; p = 0.004) despite receiving more advanced therapy beyond anticoagulation (12.5 vs. 3.2%; p < 0.001) (26). Studies examining the centralization of care of patients with COVID-19 requiring mechanical ventilation did not find any difference in mortality between transferred and nontransferred cohorts (21, 25). The only study to show a mortality benefit was in a study evaluating outcomes in transferred patients with ARF requiring ECMO support, which showed lower mortality (48.7% vs. 51.6%; p < 0.001) when patients were transferred to high-volume centers compared with low-volume centers (15).
TABLE 2.
A Summary of Studies Evaluating Outcomes in Patients Who Undergo Interhospital Transfer
| References | Duration of Study | Population and Sample Size | Database | Primary Outcome | Results |
|---|---|---|---|---|---|
| Aguayo et al (15) | 2010–2016 | n = 29,298 ECMO patients, 36.8% transferred (n = 10,807) | Nationwide inpatient sample | Survival to hospital discharge | No difference between transfer and nontransferred |
| 47.9% vs. 47.9% (p = 0.97) | |||||
| Carroll et al (26) | August 2012–2018 | n = 2050 patients with PE, 21% transferred (n = 423) | Single-center study (Boston, MA) | PE-related mortality | Worse mortality in transferred group |
| 2.3% transferred vs. 0.6% in nontransferred (p = 0.004) | |||||
| Chen et al (21) | March 17, 2020, to October 14, 2020 | n = 298 patients with COVID-19 pneumonia, 39% transferred (n = 117) | Single-center study (Chicago, IL) | In-hospital mortality | No difference |
| 43% vs. 43% (p = 0.95) | |||||
| Patel et al (23) | January 2010, 2014, to April 15, 2014 | n = 773 patients | Single-center study (Madison, WI) | Predictors of 24-hr mortality | High Sequential Organ Failure Assessment scores the day of transfer (OR, 7.7; 95% CI, 1.21–66.26; p = 0.04), high Fio2 on arrival, and cardiac arrest before transfer (OR, 4.94; 95% CI, 1.43–15.96; p = 0.0009) |
| Ranney et al (24) | June 2009–December 2015 | n = 387 total ECMO patients, 34.4% transferred (n = 133) | Single-center study (Durham, NC) | Survival to decannulation, hospital discharge | Venoarterial ECMO: 66.2% survived to decannulation, 48.1% to hospital discharge |
| Venovenous ECMO: 76.8% survived to decannulation, 69.5% to hospital discharge | |||||
| Rush et al (18) | 2013 | n = 1,630 patients transferred for sepsis requiring mechanical ventilation compared with n = 1,630 propensity matched cohort who did not undergo transfer | U.S. hospitals nationwide readmission database | Hospital mortality, LOS | No difference in mortality between two cohorts (12.3% vs. 12.7%; p = 0.74) |
| Longer LOS in transferred cohort (12.8 vs. 9.1 d; p < 0.01) |
ECMO = extracorporeal membrane oxygenation, LOS = length of stay, OR = odds ratio, PE = pulmonary embolism.
Few studies evaluating LOS showcase a trend toward higher LOS among the transferred cohort (18), as well as higher cumulative cost of care (15). Palliative care consultation is often underutilized for transferred patients and is positively correlated with higher LOS (28). Additionally, transferred cohorts have shown higher frequency of being discharged to long-term acute care hospitals or rehabilitation facilities (21).
SUMMARY AND DISCUSSION
In summary, the investigations related to interhospital transfer of ARF patients in the United States are scant, with only 16 studies identified over 20 years of literature. Only a few contemporary studies reflect the changing landscape of ARF transfers in the aftermath of the COVID-19 pandemic, and while presumably prevalence has increased over time this has not been reevaluated in the literature. Furthermore, the evidence reviewed here is predominantly retrospective with a heterogeneous ARF patient population. While the majority of studies used diagnosis or procedural coding to specifically identify ARF patients who required mechanical ventilation (15–19, 21, 24, 31), other studies included patient cohorts with a broader range of respiratory support, from supplemental oxygen to ECMO with varying clinical characteristics provided (20, 22, 23, 26–29). Additionally, there was a paucity of studies specifically addressing the utilization of NIV and high-flow oxygen as modes of support for the transferred ARF population despite a large increase in prevalence of NIV in recent years (36). Future research can address these limitations by designing studies that specifically evaluate patients with ARF and include the broad range of respiratory support provided to patients. Additionally, we need future work to take into account the etiology of ARF, which may be due to pulmonary or extrapulmonary organ involvement.
Overall, among the included studies we found that patients who undergo interhospital transfer for ARF are likely to be young, White, have higher severity of illness, and be commercially insured. This highlights the fact that current transfer practices may provide more aggressive care to patients who are younger, have more severe illness, and for those with higher socioeconomic status, including offering transfer as a medical intervention. This raises concern that inequities exist during transfer, but current observational studies fail to provide adequate insights and reasons for such variation.
Additionally, there are only a few studies objectively evaluating reasons for transfer. Any transfer involves a stakeholder triad including the referring physician, accepting physician, and patient/family. The primary driver motivating transfer decisions may vary from stakeholder to stakeholder and is subject to a myriad of implicit or explicit biases. Current evidence showcases gaps in decision-making and reasoning regarding transfer, highlighting the need for future studies to develop a standardized framework for patient selection and for devising a shared nomenclature for the various reasons for transfer.
Further, the question of optimal timing of transfer is not answered by the current literature. There is a robust and growing body of literature demonstrating that earlier access to aggressive and appropriate care for ARF improves outcomes (37). Accordingly, the benefit from transfer may not be realized if patients are transferred late in their illness trajectory. Until we have a better understanding of how to identify patients with ARF who will benefit the most from interhospital transfer, determining the optimal timing of transfer will remain difficult.
ARF transfers seem to traverse an informal network from a sizable catchment area generally directing patients toward higher resourced, larger hospitals in urban areas. However, there are several factors that dispute the practicality and generalizability of this transfer pattern. For example, capacity strain at high resourced hospitals may limit their ability to accept all patients in a timely manner. Clinicians may have preexisting referral relationships with certain hospitals whereas families may choose closer, more prestigious, or better advertised hospitals. Presumably, an underlying principle behind the transfer of most ARF patients is improving access to highly capable centers that are best equipped at caring for these patients. One commonly used surrogate of expertise is hospital case volume and ECMO capability, which has shown to be associated with lower mortality in patients with ARF (38–40). Thus, we advocate for formalizing transfer networks for patients with ARF to improve access to highly capable centers similar to other conditions with high mortality like stroke (41, 42), myocardial infarction (43, 44), and more recently sepsis (45).
Last, studies evaluating outcomes among transferred patients have not consistently used a comparison cohort. Some studies have included nontransferred patients who either continue to stay at the initial hospital, while some studies have used patients directly admitted to the tertiary hospital, which one can argue are inherently different from the transferred cohort. The gold standard for evaluating this question would be to design a randomized controlled interventional trial (transfer vs. no transfer), which is ethically challenging. Alternatively, future researchers can employ target trial emulation methodologies and specify the protocol for a hypothetical randomized trial of ARF transfers and then emulate this using the available observational data to get at causal inference and outcomes related to interhospital transfers (39, 46).
LIMITATIONS
Our scoping review has several limitations. Our review was limited to studies conducted within the United States, and as a result, we may have excluded key international studies that would address some of our questions. The ARF patient population included in this review are heterogeneous with limited clinical generalizability. Furthermore, our review on interhospital transfers does not take into account the progress that has occurred in the treatment of ARF over the last 15 years. Additionally, our review fails to capture the evolving healthcare infrastructure changes impacting transfer patterns, including the ongoing reorganization into hospital networks utilizing hub and spoke models, the growing adoption of ICU telemedicine with improved access to real-time expert consultation (47, 48), and increased strain on the critical care delivery system related to shortages of critical care-trained providers (49, 50).
CONCLUSIONS
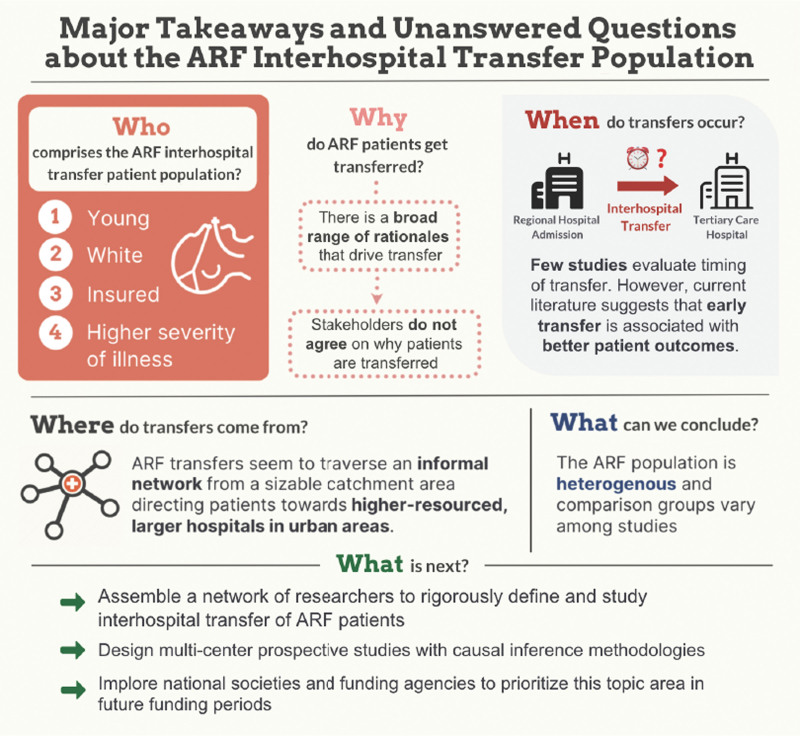
In conclusion, our scoping review highlights the sparse evidence and the urgent need for further research into understanding the complexity behind ARF transfers (Fig. 2). The recent COVID-19 pandemic presented a missed opportunity to deploy a nuanced structure for transfers; instead, we relied on variable and institution-specific mechanisms, which led to significant patient mortality, morbidity, and capacity constraints (51). Thus, on the heels of the pandemic, there is both an urgent need and a unique opportunity to compile a network of researchers, clinicians, and health system administrators engaged in interhospital transfers to rigorously define and study interhospital transfer of ARF patients. Our scoping review serves as preliminary data to formulate the next set of research questions and subsequently design multicenter prospective studies with causal inference methodologies. We implore national societies and funding agencies to prioritize this topic area in the coming years.
Figure 2.
A summary of the major takeaways of the acute respiratory failure (ARF) literature highlights many unanswered questions.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Jennifer Slota, Email: jennifer.slota@northwestern.edu.
Denise A. Nunes, Email: denise.nunes@northwestern.edu.
Kelly C. Vranas, Email: vranas@ohsu.edu.
Jacqueline M. Kruser, Email: jkruser@wisc.edu.
Reiping Huang, Email: rhuang@facs.org.
Julie K. Johnson, Email: julie.k.johnson@northwestern.edu.
Tara C. Lagu, Email: tara.lagu@nm.org.
Nandita R. Nadig, Email: nandita.nadig@northwestern.edu.
REFERENCES
- 1.Esteban A, Anzueto A, Frutos F, et al. ; Mechanical Ventilation International Study Group: Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 2002; 287:345–355 [DOI] [PubMed] [Google Scholar]
- 2.Barrett ML, Smith MW, Elixhauser A, et al. : Utilization of intensive care services, 2011: Statistical Brief #185. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, Agency for Healthcare Research and Quality (US), 2006;114. [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 4.Glance LG, Li Y, Osler TM, et al. : Impact of patient volume on the mortality rate of adult intensive care unit patients. Crit Care Med 2006; 34:1925–1934 [DOI] [PubMed] [Google Scholar]
- 5.Kahn JM, Goss CH, Heagerty PJ, et al. : Hospital volume and the outcomes of mechanical ventilation. N Engl J Med 2006; 355:41–50 [DOI] [PubMed] [Google Scholar]
- 6.Needham DM, Bronskill SE, Rothwell DM, et al. : Hospital volume and mortality for mechanical ventilation of medical and surgical patients: A population-based analysis using administrative data. Crit Care Med 2006; 34:2349–2354 [DOI] [PubMed] [Google Scholar]
- 7.Pronovost PJ, Angus DC, Dorman T, et al. : Physician staffing patterns and clinical outcomes in critically ill patients: A systematic review. JAMA 2002; 288:2151–2162 [DOI] [PubMed] [Google Scholar]
- 8.Barnato AE, Kahn JM, Rubenfeld GD, et al. : Prioritizing the organization and management of intensive care services in the United States: The PrOMIS Conference. Crit Care Med 2007; 35:1003–1011 [DOI] [PubMed] [Google Scholar]
- 9.Nates JL, Nunnally M, Kleinpell R, et al. : ICU admission, discharge, and triage guidelines: A framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med 2016; 44:1553–1602 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen YL, Wallace DJ, Yordanov Y, et al. : The volume-outcome relationship in critical care: A systematic review and meta-analysis. Chest 2015; 148:79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlan EA, Mubarak E, Firn J, et al. : Inter-hospital transfer decision-making during the COVID-19 pandemic: A qualitative study. J Gen Intern Med 2023; 38:2568–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators: Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouzzani M, Hammady H, Fedorowicz Z, et al. : Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, Moher D, Bossuyt PM, et al. : PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguayo E, Kwon OJ, Dobaria V, et al. : Impact of interhospital transfer on clinical outcomes and costs of extracorporeal life support. Surgery 2020; 168:193–197 [DOI] [PubMed] [Google Scholar]
- 16.Nadig NR, Goodwin AJ, Simpson AN, et al. : Patient and hospital characteristics associated with interhospital transfer for adults with ventilator-dependent respiratory failure. Ann Am Thorac Soc 2017; 14:730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadig NR, Brinton DL, Simpson KN, et al. : The impact of timing on clinical and economic outcomes during inter-ICU transfer of acute respiratory failure patients: Time and tide wait for no one. Crit Care Explor 2022; 4:e0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rush B, Tyler PD, Stone DJ, et al. : Outcomes of ventilated patients with sepsis who undergo interhospital transfer: A nationwide linked analysis. Crit Care Med 2018; 46:e81–e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyler PD, Stone DJ, Geisler BP, et al. : Racial and geographic disparities in interhospital ICU transfers. Crit Care Med 2018; 46:e76–e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedhom R, Beshai R, Elkaryoni A, et al. : Trends and outcomes of interhospital transfer for high-risk acute pulmonary embolism: A nationwide analysis. Am J Med Open 2023; 10:100053 [Google Scholar]
- 21.Chen E, Longcoy J, McGowan SK, et al. : Interhospital transfer outcomes for critically ill patients with coronavirus disease 2019 requiring mechanical ventilation. Crit Care Explor 2021; 3:e0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanane T, Wiles S, Senussi MH, et al. : Interhospital transfers of the critically ill: Time spent at referring institutions influences survival. J Crit Care 2017; 39:1–5 [DOI] [PubMed] [Google Scholar]
- 23.Patel JJ, Kurman J, Al-Ghandour E, et al. : Predictors of 24-h mortality after inter-hospital transfer to a tertiary medical intensive care unit. J Intensive Care Soc 2018; 19:319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranney DN, Bonadonna D, Yerokun BA, et al. : Extracorporeal membrane oxygenation and interfacility transfer: A regional referral experience. Ann Thorac Surg 2017; 104:1471–1478 [DOI] [PubMed] [Google Scholar]
- 25.Usher MG, Tignanelli CJ, Hilliard B, et al. : Responding to COVID-19 through interhospital resource coordination: A mixed-methods evaluation. J Patient Saf 2022; 18:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll BJ, Beyer SE, Shanafelt C, et al. : Interhospital transfer for the management of acute pulmonary embolism. Am J Med 2022; 135:531–535 [DOI] [PubMed] [Google Scholar]
- 27.Creel-Bulos C, Miller C, Hassani B, et al. : “Pushing geographic boundaries: Interfacility transport and remote extracorporeal membrane oxygenation cannulation of patients during COVID-19 pandemic.” Perfusion 2023; 38:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui ST, Xiao E, Patel S, et al. : Impact of palliative care on interhospital transfers to the intensive care unit. J Crit Care Med (Targu Mures) 2022; 8:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner J, Iwashyna TJ, Kahn JM: Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care 2013; 28:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyder S, Tang R, Huang R, et al. : Implementation of an interdisciplinary transfer huddle intervention for prolonged wait times during inter-ICU transfer. Jt Comm J Qual Patient Saf 2024; 50:371–376 [DOI] [PubMed] [Google Scholar]
- 31.Wallace DJ, Angus DC, Seymour CW, et al. : Geographic access to high capability severe acute respiratory failure centers in the United States. PLoS One 2014; 9:e94057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usher MG, Fanning C, Fang VW, et al. : Insurance coverage predicts mortality in patients transferred between hospitals: A cross-sectional study. J Gen Intern Med 2018; 33:2078–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durairaj L, Will JG, Torner JC, et al. : Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med 2003; 31:1981–1986 [DOI] [PubMed] [Google Scholar]
- 34.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e1143 [DOI] [PubMed] [Google Scholar]
- 35.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 36.Kempker JA, Abril MK, Chen Y, et al. : The epidemiology of respiratory failure in the United States 2002-2017: A serial cross-sectional study. Crit Care Explor 2020; 2:e0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peek GJ, Clemens F, Elbourne D, et al. : CESAR: Conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006; 6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 39.Hajage D, Combes A, Guervilly C, et al. ; COVID-ICU Investigators: Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: An emulated target trial analysis. Am J Respir Crit Care Med 2022; 206:281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ike JD, Kempker JA, Kramer MR, et al. : The association between acute respiratory distress syndrome hospital case volume and mortality in a U.S. cohort, 2002-2011. Crit Care Med 2018; 46:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinaldo L, Brinjikji W, Rabinstein AA: Transfer to high-volume centers associated with reduced mortality after endovascular treatment of acute stroke. Stroke 2017; 48:1316–1321 [DOI] [PubMed] [Google Scholar]
- 42.Alberts MJ, Wechsler LR, Jensen ME, et al. : Formation and function of acute stroke-ready hospitals within a stroke system of care recommendations from the brain attack coalition. Stroke 2013; 44:3382–3393 [DOI] [PubMed] [Google Scholar]
- 43.Saito Y, Tateishi K, Kanda M, et al. : Volume-outcome relationships for percutaneous coronary intervention in acute myocardial infarction. J Am Heart Assoc 2022; 11:e023805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harold JG, Bass TA, Bashore TM, et al. ; Presidents and Staff: ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (writing committee to revise the 2007 clinical competence statement on cardiac interventional procedures). Circulation 2013; 128:436–472 [DOI] [PubMed] [Google Scholar]
- 45.Ofoma UR, Deych E, Mohr NM, et al. : The relationship between hospital capability and mortality in sepsis: Development of a sepsis-related hospital capability index. Crit Care Med 2023; 51:1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Admon AJ, Donnelly JP, Casey JD, et al. : Emulating a novel clinical trial using existing observational data. Predicting results of the PreVent study. Ann Am Thorac Soc 2019; 16:998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortis S, Sarrazin MV, Beck BF, et al. : ICU telemedicine reduces interhospital ICU transfers in the Veterans Health Administration. Chest 2018; 154:69–76 [DOI] [PubMed] [Google Scholar]
- 48.Kahn JM, Cicero BD, Wallace DJ, et al. : Adoption of ICU telemedicine in the United States. Crit Care Med 2014; 42:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halpern NA, Pastores SM, Oropello JM, et al. : Critical care medicine in the United States: Addressing the intensivist shortage and image of the specialty. Crit Care Med 2013; 41:2754–2761 [DOI] [PubMed] [Google Scholar]
- 50.Angus DC, Kelley MA, Schmitz RJ, et al. ; Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS): Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA 2000; 284:2762–2770 [DOI] [PubMed] [Google Scholar]
- 51.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]