Abstract
Introduction:
This study aimed to explore the clinical effects of blood purification therapy in patients with chronic renal disease, measured by renal function index and inflammation.
Methodology:
Data were collected from a tertiary care hospital in Pakistan between June 2022 and September 2023. Eighty-four patients undergoing maintenance hemodialysis for chronic renal failure were retrospectively included in this cohort.
Results:
Age, sex, BMI, course of disease, primary disease, and educational level were not related to the response to blood purification treatment. Blood purification therapy positively affected renal function, serological indices, and inflammatory factors (P<0.05).
Conclusion:
Blood purification therapy can improve toxin clearance and renal function and reduce inflammation. Therefore, the authors can conclude that this is an effective therapy for our population.
Keywords: blood purification, chronic renal failure, hemodialysis, hemoperfusion, toxin clearance
Introduction
Highlights
Hemoperfusion can save the residual renal function.
Blood purification therapy positively affected renal function, serological indices, and inflammatory factors.
Age, sex, BMI, course of disease, primary disease, and educational level were not related to the response to blood purification treatment.
Chronic renal failure (CRF) refers to the common manifestation of various primary or secondary chronic kidney diseases1,2. It can lead to renal dysfunction. Patients are unable to maintain the needs of the body, which is accompanied by increased serum creatinine, decreased glomerular filtration rate, and related metabolic products and toxin retention, water-electrolyte imbalance, and acid-base disturbances3,4. CRF can be divided into four stages according to the degree of renal damage: compensatory, decompensatory, failure, and uremic5. The compensatory stage is the first stage of renal insufficiency, with serum creatinine levels in the range of 133–177 μmol/l. The kidneys at this stage are normal or mildly abnormal, and the clinical symptoms are not obvious. If the patient is actively treated to remove the inducing factors, the condition may progress slowly or be relieved to some extent6. The decompensatory stage is the second stage of renal insufficiency, with serum creatinine levels in the range of 177–451 μmol/l. Currently, not only are there structural abnormalities in renal lesions, namely glomerulosclerosis, renal tubular atrophy, and interstitial fibrosis, but also abnormal renal function, which is seriously deranged7. The failure stage is the third stage of renal insufficiency, namely the early stage of uremia, with serum creatinine levels in the range of 451–707 μmol/l. The patient’s kidney reserve capacity gradually decreases, urine output gradually decreases, anemia aggravates, and volume load gradually increases, followed by heart failure, pulmonary edema, pericardial and pleural effusion, severe ion disorder, and metabolic acidosis8. Uremia is the fourth stage of renal insufficiency and the most serious stage of renal failure, with a serum creatinine level greater than 707 μmol/l. Patients have obvious clinical symptoms, such as nausea, vomiting, anorexia, stomach pain, severe edema, dyspnea, and other heart failure manifestations, most of which are accompanied by severe anemia, low calcium levels, and high phosphorus levels9.
Creatinine and blood urea nitrogen (BUN) levels are measured to assess kidney health10. Creatinine is a major uremic toxin, which when accumulated in the bloodstream, can lead to impair kidney function and hasten the ageing process of the kidneys11. Increased BUN and creatinine levels frequently indicate poor kidney function, thus it’s important to comprehend and treat these indicators in renal disease patients10. Elevated serum levels of β2- macroglobulin (β2-MG) were detected in patients with chronic kidney failure in 197312. Serum β2-MG has a strong correlation with kidney disease. High levels of β2-MG are linked to both mortality and morbidity in end-stage kidney disease (ESKD), including vascular calcification. Additionally, β2-MG is inversely related with glomerular filtration rate (GFR)13. Several inflammatory markers are associated with chronic kidney disease. These include interleukins (ILs), tumor necrosis factor (TNF), interferon (IFN), and transforming growth factor (TGF), chemokines, and cell adhesion molecules (CAMs)14 and C-reactive proteins (CRP)15. Measurement of them can give important information about kidney health14.
At present, early patients with CRF can be treated with drugs, but these are not suitable for advanced patients. If the disease progresses to a more serious stage, it needs to be treated with an alternative approach, such as peritoneal dialysis or hemodialysis and renal transplant, to help patients live a less painful life16. Kidney transplantation is the best therapeutic option, but limited blood supplies, high treatment costs, and other limitations prevent widespread use. As a result, blood purification therapy becomes an attractive substitute that is both practical and affordable, convenient, and highly effective. This therapy improves internal stability by quickly removing harmful compounds and retaining water in the patient’s body17. More than two million patients are treated with hemodialysis (HD) worldwide18. Clinically, patients with CRF are also treated with hemoperfusion, which can remove exogenous or endogenous toxins, drugs, or metabolic waste that cannot be removed by dialysis19. Studies20 also show that both high-flux hemodialysis and hemoperfusion can better protect patients’ residual renal function, effectively improve the therapeutic effect, and prolong their survival time. Therefore, this research was designed to study the outcomes of hemoperfusion in patients with CRF and analyze its clinical curative effect and influence on renal function indices. This finding may provide a reliable reference for the therapeutic efficacy of hemoperfusion in the Pakistani population. By focusing on a comprehensive set of outcomes, including kidney function indices, inflammatory markers, serologic indices and patient demographics, this research aims to contribute valuable insights into the optimization of blood purification therapies for CRF management in Pakistan.
Methodology
Data were collected from a tertiary care hospital in Pakistan from June 2022 through September 2023 and analyzed retrospectively. Approval was obtained before data collection from the ethical committee of the hospital in May 2022 with IRB approval number # AIMS7575/2023. Due to its retrospective nature, consent was not taken directly from the patients. Tertiary care hospitals treat patients through hemodialysis on a regular basis, and some patients are treated with hemoperfusion. The choice of hemoperfusion is based on the patient’s choice, as this is not a common methodology. The present study aimed to analyze outcomes in patients in whom hemoperfusion was applied and evaluated if the method had better outcomes than regular hemodialysis. The evaluation was performed based on variables, such as inflammation, serological index, and renal function index. The statistical methods used mean and standard deviation, and ANOVA test for continuous variables.
Inclusion and exclusion criteria
Patients were included in the study if they were diagnosed with CRF for more than six months, were undergoing hemodialysis more than twice a month, and had complete data for clinical diagnosis and follow-up. Patients with incomplete data were excluded from the study. Patients who progressed to renal transplant, died during the 1-year period of study, had missing follow-up data, and laboratory investigations were also excluded. Pregnant women and patients with disorders that suppress the immune system or cause septicemia were not included. Patients with cerebrovascular and cardiovascular disorders and mental disorders such as chronic depression were also excluded. Patients with a history of parathyroidectomy within six months of hemodialysis were excluded (Fig. 1).
Figure 1.
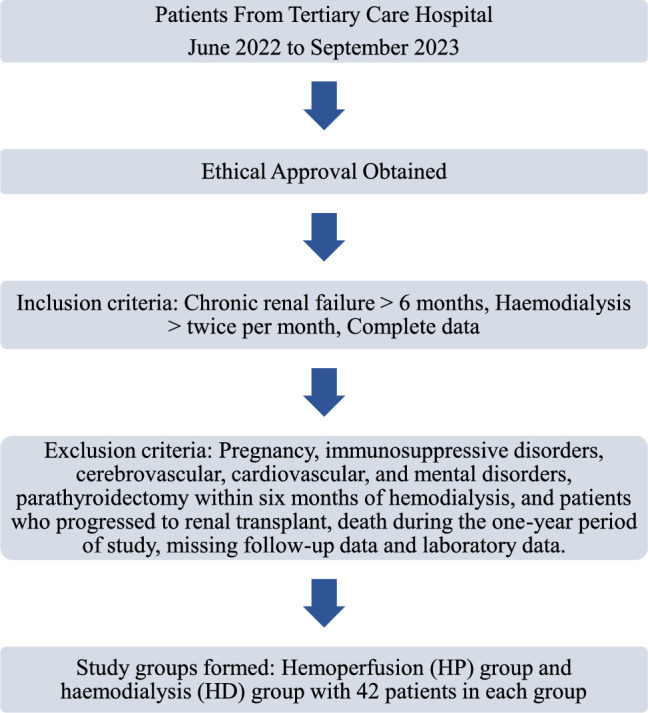
Patient recruitment chart.
Patients
Patients with chronic renal failure receiving renal replacement therapy and symptomatic treatment, including administration of folic acid, calcium carbonate, erythropoietin, and other treatments to reduce phosphorus, supplement calcium, and correct iron-deficiency anemia. Patients were monitored for glucose control and blood pressure regulation. The records of patients who had undergone hemoperfusion along with hemodialysis were searched, and a cohort of 42 patients was selected for whom complete data were available. To confirm the efficiency of hemoperfusion combined with dialysis, we needed patients for whom we could compare these patients. We selected a cohort of 42 patients that matched in clinical characteristics and were demographically matchable. These patients also underwent hemodialysis without hemoperfusion. The patients undergoing hemodialysis only were named “hemodialysis (HD) group” and those taking hemoperfusion and dialysis were names “hemodialysis- hemoperfusion (HP) group.”
Hemodialysis/Hemoperfusion procedure
The hemodialysis procedure at our tertiary care center was performed on a Fresenius 4008s hemodialysis machine and bicarbonate dialysate with a blood flow of up to 200 ml/min. Dialysis sessions were usually continued for 12 weeks, with three sessions per week, each lasting for almost 4 h. A disposable resin hemoperfusion apparatus was used in patients who underwent hemoperfusion along with hemodialysis. It was connected to a dialysis machine, which was continuously perfused for two hours, and the apparatus was removed after saturation.
Detection of kidney function indices
The renal function indices of patients were measured, including serum creatinine (S. Cr), BUN and β2-MG. Fasting venous blood (5 ml) was drawn before and after a treatment session in hemodialysis patients as part of regular observation. Only those patients’ data for which complete blood profiles were available were included in the study.
Detection of inflammatory factors
Inflammatory factors, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6 and CRP, were measured using enzyme-linked immunosorbent assay. These values were available for our cohorts.
Detection of serological indexes
Serological indices, including parathyroid hormone (PTH), homocysteine (Hcy), and blood phosphorus levels, were detected by automatic biochemical immunoassay.
Outcome measures
Therapeutic effects were compared between the two groups. Total effective rate=(markedly effective + effective)/total number of patients × 100. The therapy was considered markedly effective when the symptoms were resolved to the maximum, effective if the symptom resolution was partial, and ineffective if the symptoms were not resolved. In the two groups, the changes in renal function indices (SCr, BUN, and β2-MG), inflammatory factors (TNF-α, IL-1, IL-6, and CRP), and serological indices (PTH, Hcy, and blood phosphorus levels) were compared before and after treatment. Comparisons were performed using a paired-sample t-test. The work has been reported in line with the STROCSS criteria21 under UIN: researchregistry9859, https://www.researchregistry.com/browse-the-registry#home/registrationdetails/658b80b2d81db40027714562/.
Results
The mean and standard deviation of continuous variables including age, BMI, and disease duration for both groups (hemodialysis, HD; hemoperfusion, HP) were determined (Table 1). Years in the HD group was 47.64±9.28 years and 53.07±11.21 years in the HP group. The basic characteristics of the groups (Table 2), such as sex and clinical characteristics of patients, including primary diagnosis and effectiveness of the treatment, are categorical variables. There were 52.4% males in the HD group and 59.5% males in the HP group, while diabetic nephropathy was 31.7% and 31.0%, respectively. Comparing the basic clinical data, we found that there was no statistical difference between the groups in terms of sex (P=0.519) and primary disease (P=0.599). Variables, including age, body mass index (BMI), and gender for both groups (hemodialysis, HD; hemoperfusion, HP), were determined using SPSS version 23.0 ANOVA Test (Table 3). Age in the HD group is 480.033 between the group and 4.924 within the group. Age in the HP group is 36.790 between the group and 144.096 within the group. BMI in HD group is 4.855 between the group and 3.831 within the group, whereas and BMI in HP group is 8.531 between the group and 3.929 within the group. Gender in HD group was 0.209 between the group and 0.265 within the group and the gender in HP group was 0.340 between the group and 0.228 within the group. All variables have (P<0.05). S. Cr, blood urea nitrogen (BUN) and β2-MG, TNF-a, IL-1, IL -6 and CRP, PTH, Hcy, and blood phosphorus (BP) are also continuous variables expressed as mean ± standard deviation (Tables 4–6 and Fig. 2). These variables were compared between the two groups.
Table 1.
Mean and standard deviation for continuous variables.
| Variables | Mean ± standard deviation | Median |
|---|---|---|
| Age | ||
| HD | 47.64±9.28 | 48.00 |
| HP | 53.07±11.21 | 54.50 |
| BMI | ||
| HD | 22.13±2.0 | 22.250 |
| HP | 23.304±2.17 | 23.250 |
| Duration of disease time in years | ||
| HD | 6.50±1.64 | 7.00 |
| HP | 7.42±1.68 | 8.00 |
HD, hemodialysis group; HP, hemodialysis-hemoperfusion group.
Table 2.
Basic characteristics of both cohorts.
| Variable | Frequency | Percentage |
|---|---|---|
| Sex | ||
| HD | ||
| Male | 22 | 52.4 |
| Female | 20 | 47.6 |
| HP | ||
| Male | 25 | 59.5 |
| Female | 17 | 40.5 |
| Primary diagnosis | ||
| HD | ||
| Chronic glomerulonephritis | 14 | 34.1 |
| Chronic pyelonephritis | 2 | 4.9 |
| Diabetic nephropathy | 13 | 31.7 |
| Hypertensive nephropathy | 8 | 19.5 |
| Other | 4 | 9.8 |
| HP | ||
| Chronic glomerulonephritis | 16 | 38.1 |
| Chronic pyelonephritis | 2 | 4.8 |
| Diabetic nephropathy | 13 | 31.0 |
| Hypertensive nephropathy | 9 | 21.4 |
| Other | 2 | 4.8 |
| Effectiveness | ||
| HD | ||
| Ineffective | 6 | 14.3 |
| Effective | 25 | 59.5 |
| Markedly effective | 11 | 26.2 |
| HP | ||
| Ineffective | 1 | 2.4 |
| Effective | 23 | 54.8 |
| Markedly effective | 18 | 42.9 |
| Adverse effects | ||
| HD | ||
| No adverse effects | 32 | 76.2 |
| Muscle spasm | 4 | 9.5 |
| Itchy skin | 3 | 7.1 |
| Cardiovascular event | 2 | 4.8 |
| Dysarteriotony | 1 | 2.4 |
| HP | ||
| No adverse effects | 38 | 90.5 |
| Muscle spasm | 3 | 7.1 |
| Itchy skin | 1 | 2.4 |
HD, hemodialysis group; HP, hemodialysis-hemoperfusion group.
Table 3.
Comparison between and within groups using ANOVA test.
| ANOVA | |||||
|---|---|---|---|---|---|
| Sum of Squares | df | Mean square | F | Sig. | |
| Age HD | |||||
| Between groups | 3360.234 | 7 | 480.033 | 97.493 | 0.000 |
| Within groups | 167.409 | 35 | 4.924 | ||
| Total | 3527.643 | 42 | |||
| BMI HD | |||||
| Between groups | 33.987 | 7 | 4.855 | 1.267 | 0.0255 |
| Within groups | 130.246 | 35 | 3.831 | ||
| Total | 164.233 | 42 | |||
| Age HP | |||||
| Between groups | 257.529 | 7 | 36.790 | 0.255 | 0.007 |
| Within groups | 4899.256 | 35 | 144.096 | ||
| Total | 5156.786 | 42 | |||
| BMI HP | |||||
| Between groups | 59.718 | 7 | 8.531 | 2.171 | 0.032 |
| Within groups | 133.584 | 35 | 3.929 | ||
| Total | 193.302 | 42 | |||
| Gender HD | |||||
| Between groups | 1.462 | 7 | 0.209 | 0.788 | 0.012 |
| Within groups | 9.014 | 35 | 0.265 | ||
| Total | 10.476 | 42 | |||
| Sex HP | |||||
| Between groups | 2.383 | 7 | 0.340 | 1.496 | 0.002 |
| Within groups | 7.736 | 35 | 0.228 | ||
| Total | 10.119 | 42 | |||
HD, hemodialysis group; HP, hemodialysis-hemoperfusion group; Sig., significance.
Table 4.
Change of renal function indexes before and after treatment.
| Pre_HD_SCr | 750.4±15.90 | 0.538 |
| Pre_HP_SCr | 752.38±15.9 | |
| Post_HD_SCr | 503.4±10.7 | 0.000 |
| Post_HP_SCr | 400.1±78.7 | |
| Pre_HD_BUN | 24.8±2.9 | 0.001 |
| Pre_HP_BUN | 26.5±0.9 | |
| Post_HD_BUN | 15.04±1.2 | 0.000 |
| Post_HP_BUN | 8.1±1.1 | |
| Pre_HD_β2MG | 7.85±1.2 | 0.000 |
| Pre_HP_β2MG | 6.01±1.85 | |
| Post_HD_β2MG | 4.05±1.4 | 0.000 |
| Post_HP_β2MG | 1.84±0.44 |
P<0.05 shows significance.
β2-MG, β2- macroglobulin; BUN, blood urea nitrogen; HD, hemodialysis group; HP, hemodialysis-hemoperfusion group; S. Cr, Serum creatinine.
Table 6.
Changes of serological indexes before and after treatment.
| Pre_HD_PTH | 59.45±6.97 | 0.107 |
| Pre_HP_PTH | 61.32±4.82 | |
| Post_HD_PTH | 39.12±6.71 | 0.000 |
| Post_HP_PTH | 21.86±2.42 | |
| Pre_HD_Hcy | 37.55±1.88 | 0.159 |
| Pre_HP_Hcy | 39.1±6.8 | |
| Post_HD_Hcy | 21.81±2.47 | 0.015 |
| Post_HP_Hcy | 20.60±1.91 | |
| Pre_HD_BP | 3.08±0.93 | 0.001 |
| Pre_HP_BP | 4.00±1.43 | |
| Post_HD_BP | 2.02±0.66 | 0.125 |
| Post_HP_BP | 1.85±0.44 |
P<0.05 is significant.
BP, blood phosphorus; Hcy, homocysteine; HD, hemodialysis group; HP, hemodialysis-hemoperfusion group; PTH, parathyroid hormone.
Figure 2.
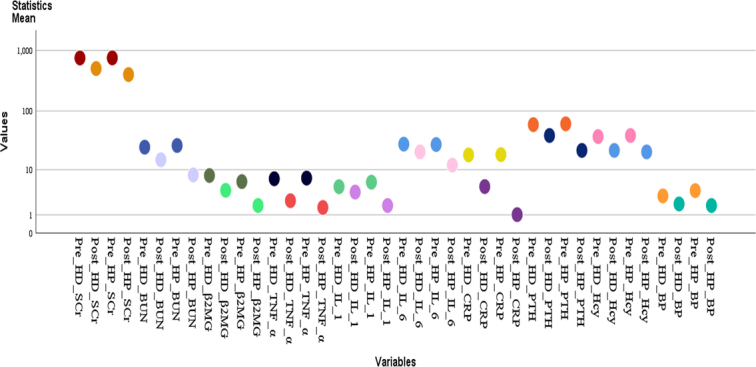
Comparison of means for renal function and serologic index and inflammatory markers.
Comparison of renal function indices
By comparing the renal function indices before and after treatment, it was found that there was no significant difference in serum creatinine between the two groups before treatment (P>0.05), but BUN and β2-MG were different. After treatment, the renal function indices of the HP group were lower than those of the HD group (P<0.05). In addition, further intra-group comparisons showed that the levels of S. Cr, BUN, and β2-MG in both groups before treatment were significantly higher than those after treatment (P<0.05). (Fig. 3) (Table 4).
Figure 3.
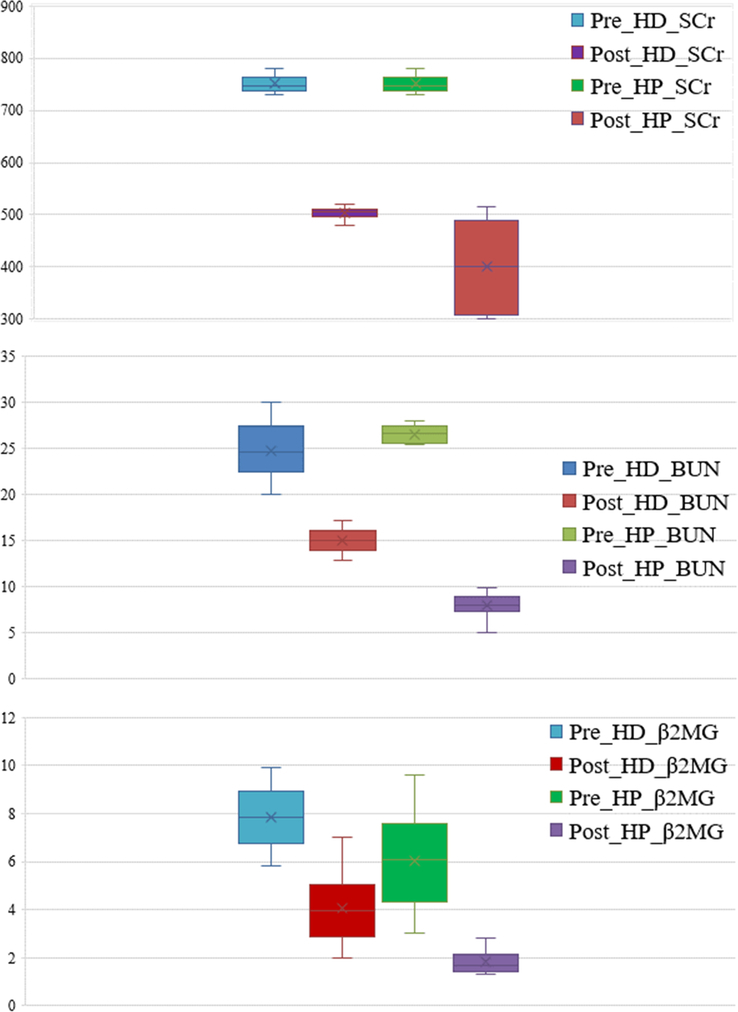
Comparison of renal function indexes before and after the hemodialysis (HD) and hemodialysis-hemoperfusion (HP) treatment. β2MG, β2- macroglobulin; BUN, blood urea nitrogen; SCr, serum creatinine.
Comparison of inflammatory factors before and after treatment
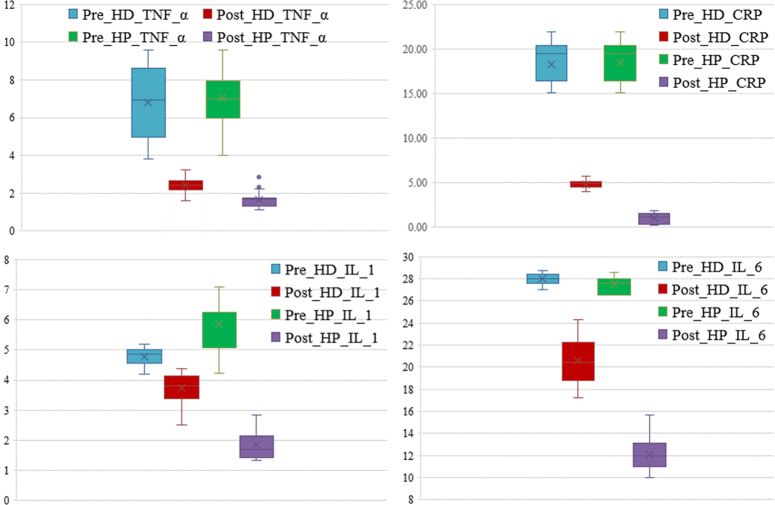
By comparing the inflammatory factors before and after treatment, it was found that there was no significant difference in TNF-α and CRP levels between the two groups before treatment (P>0.05), but IL-1 and IL-6 levels were different. After treatment, the TNF-α, IL-1, IL-6, and CRP levels of the HP group were obviously lower than those of the HD group (P<0.05). In addition, further intra-group comparisons showed that the levels of TNF-α, IL-1, IL-6, and CRP in both groups before treatment were significantly higher than those after treatment (P<0.05). (Fig. 4) (Table 5).
Figure 4.
Comparison of inflammatory factors before and after the hemodialysis (HD) and hemodialysis-hemoperfusion (HP) treatment. CRP, C-reactive protein; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Table 5.
Changes of inflammatory factors before and after treatment.
| Pre_HD_TNF_α | 6.81±1.82 | 0.484 |
| Pre_HP_TNF_α | 7.04±1.21 | |
| Post_HD_TNF_α | 2.41±0.33 | 0.000 |
| Post_HP_TNF_α | 1.64±0.37 | |
| Pre_HD_IL_1 | 4.78±0.28 | 0.000 |
| Pre_HP_IL_1 | 5.86±0.81 | |
| Post_HD_IL_1 | 3.73±0.48 | 0.000 |
| Post_HP_IL_1 | 1.84±0.44 | |
| Pre_HD_IL_6 | 27.98±0.46 | 0.000 |
| Pre_HP_IL_6 | 27.53±0.7 | |
| Post_HD_IL_6 | 20.59±2.1 | 0.000 |
| Post_HP_IL_6 | 12.09±1.45 | |
| Pre_HD_CRP | 18.26±2.16 | 0.052 |
| Pre_HP_CRP | 18.5±1.99 | |
| Post_HD_CRP | 4.81±0.41 | 0.000 |
| Post_HP_CRP | 1.02±0.61 |
value of P<0.05 shows significance.
CRP, C-reactive protein; HD, hemodialysis group; HP, hemodialysis-hemoperfusion group; IL, interleukin; TNF-α, tumor necrosis factor-α.
Comparison of serological indices
Comparison of the serological indices before and after treatment showed that there was no significant difference in PTH and Hcy levels between the two groups before treatment (P>0.05), but after treatment, the PTH and Hcy levels of the HP group were obviously lower than those of the HD group (P<0.05). BP levels showed a different trend; the values were significantly different between the groups before treatment. In addition, further intra-group comparisons showed that the PTH, Hcy, and blood phosphorus levels in both groups before treatment were significantly higher than those after treatment (P<0.05) (Fig. 5) (Table 6). For example, the mean value for BP was 4.0 in HP group before and 1.85 after treatments which shows great change of values.
Figure 5.
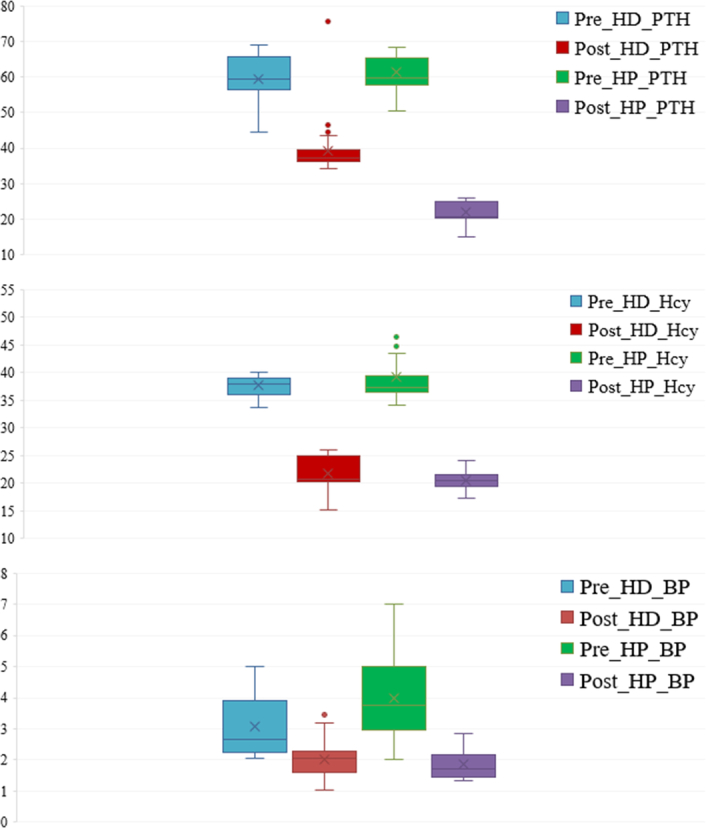
Comparison of serological indexes before and after the hemodialysis (HD) and hemodialysis-hemoperfusion (HP) treatment. BP, blood phosphorus; Hcy, homocysteine; PTH, parathyroid hormone.
Adverse events
In the HD group, adverse outcomes were observed in 23.81% (n=10) patients and in 9.55 (n=4) of patients in HP group. The HP group experienced fewer adverse outcomes than the HD group.
Effectiveness
The effectiveness in the HP group was 41/42 × 100=97.6%, and in the HD group, it was observed to be 36/42 × 100=85.7%, which was lower than that of the HP group (Table 2).
Discussion
CRF is a chronic disease that seriously threatens human health22,23. Clinically, some patients with early acute renal failure can recover within one month after treatment, but some patients can also delay for more than half a year24. The possibility of curing early renal failure is related to its pathological type, degree of severe organ involvement, complications, infection, and other factors, and there are significant individual differences25. Chronic kidney disease-related mortality has increased by 41.5% and the rate is increasing. Morbidity and mortality associated with renal failure and cardiovascular risk pose a serious burden on health systems globally26. In Pakistan, the prevalence of chronic renal failure ranges from 12.5 to 29.9% and the major cause is diabetic nephropathy27. According to statistics, nearly 90% of patients with CRF require hemodialysis. However, although dialysis techniques and drug therapy have made great progress, the dialysis mortality rate is still very high, ~18–20% per year28. At present, it is believed that the high mortality rate of patients is caused by failure to replace the endocrine function of the kidney and insufficient toxin clearance during dialysis, which leads to various complications and poor prognosis. Therefore, it is necessary to develop a better plan to treat patients with CRF and improve its clinical efficacy.
Currently, dialysis is a common clinical kidney replacement therapy, which can be generally divided into hemodialysis and peritoneal dialysis, among which hemodialysis is frequently used29. Hemodialysis is designed to treat patients by draining blood from the patient’s body to the outside of the body to remove metabolic wastes, toxins, and other useless substances in the blood, reducing the content of poisons in the blood, and then returning it to the body. Zhai et al. 30. showed that hemodialysis can significantly improve prognosis and enhance therapeutic effect in patients with chronic renal insufficiency complicated by coronary heart disease. However, hemodialysis has some disadvantages. Long-term hemodialysis may produce side effects including nausea, vomiting, convulsions, coma, and various complications (heart failure, coronary heart disease, severe anemia, and hypoxemia)31,32.
Hemoperfusion is one of the most used blood purification therapies in hemodialysis clinics. It also draws the patient’s blood from the body and removes toxins, drugs, and metabolic wastes that cannot be removed during dialysis through adsorbents in the perfusion device, thus achieving blood purification and disease treatment33. Therefore, the combination of hemoperfusion and hemodialysis in the treatment of patients with CRF can not only remove macromolecular toxins from the body but also effectively treat uremic complications, including peripheral neuropathy and stubborn hypertension, which is a potentially effective treatment scheme. In this study, patients with CRF undergoing hemodialysis combined with hemoperfusion were studied, and the renal function indices (S. Cr, BUN, B2-MG), inflammatory factors (TNF-α, IL-1, IL-6, CRP), and serological indices (PTH, Hcy, and blood phosphorus levels) were analyzed and compared before and after treatment. This cohort was compared with a parallel cohort using hemodialysis only.
The results showed that there were no significant differences in serum Cr, TNF-α, CRP, PTH, and Hcy levels before treatment between the two groups, but after treatment, the levels in the HP group were significantly lower than those in the HD group. Further intra-group comparisons showed that the indices of the two groups were significantly higher before treatment than after treatment, indicating that hemodialysis combined with hemoperfusion can effectively improve the renal function of patients, reduce inflammatory reactions, and improve the toxin clearance effect, thus helping to reduce tissue damage and improve the treatment effect. The research of Wang et al. 34. shows that the combined treatment of hemodialysis and hemoperfusion can improve renal function, significantly reduce pruritus and anemia in patients with acute renal failure, and effectively reduce the incidence of adverse events, which is similar to our research.
In addition, we analyzed and compared the therapeutic effects on patients between the two groups. The results showed that the total effective rate of the patients in the HD group was significantly lower than that of the patients in the HP group, indicating that compared with hemodialysis alone, the combined treatment of hemodialysis and hemoperfusion is more conducive to improving the renal function of patients with CRF and can effectively improve clinical symptoms and abnormal signs.
At the end, we analyzed and compared the incidence of adverse reactions between the two groups. The results showed that the total incidence of adverse reactions in the HP group was obviously lower than that in the HD group, indicating that hemoperfusion can compensate for the inefficacy of hemodialysis in removing some macromolecular substances and that the combination of the two can effectively reduce the complications and incidence of adverse reactions in patients with CRF.
In this study, we revealed the clinical curative effect of hemodialysis combined with hemoperfusion in patients with CRF and its influence on renal function indices and inflammatory factors. However, this study had some limitations. First, this was a retrospective study with a limited sample size, which may have led to inaccurate experimental results. Second, patients could not be followed up in this study; therefore, the long-term efficacy and prognosis of patients could not be compared and observed. Therefore, we hope to conduct more experiments and future studies to improve our research.
Conclusion
Hemodialysis combined with hemoperfusion is effective in the treatment of patients with CRF. It can effectively improve the renal function of patients, reduce inflammatory reactions and complications, improve the toxin clearance effect and quality of life, and has high clinical application value.
Ethical approval
Ethical committee of Abbas institute of Medical Sciences ERB number AIMS7575/2023.
Consent
No written/verbal consent was availed from individual patients as the data was collected from the hospital record and not directly from the patient.
Source of funding
The authors declare that they have no competing interest.
Author contribution
Concepts: M.S.R.A. Resources: A.A., S.K.J. Data curation: M.T., A.A. formal analysis: S.T., A.Z. Methodology: A.A., F.A.H. Writing—original draft: B.B., A.A. Writing—review and editing: S.K.J., M.S.R.A., A.Z. Software: S.A. Supervision: M.A.
Conflicts of interest disclosure
The authors have declared that no competing interests exist.
Research registration unique identifying number (UIN)
Researchregistry9859. https://researchregistry.knack.com/research-registry#userresearchregistry/registerresearchdetails/658b80b2d81db40027714562/
Guarantor
None.
Data availability statement
Data analysis files present, can be provided on reasonable demand.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
The authors acknowledge Dr Waheeda Khan Bhettani, Pathologist at National Institute of Health Islamabad, Pakistan for revising their manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 May 2024
Contributor Information
Batool Butt, Email: batoolbutt7@gmail.com.
Adnan Mushtaq, Email: charlie181220@gmail.com.
Fatima Abdul Hameed, Email: fatimaahameed27@gmail.com.
Muhammad Sajid Rafique Abbasi, Email: abbasisajid2023@gmail.com.
Maham Tariq, Email: dr.mahamtariq2000@gmail.com.
Amna Akbar, Email: amna.akbar1324@gmail.com.
Sarosh Khan Jadoon, Email: saroshkhanjadoon@outlook.com.
Mumtaz Ahmad, Email: mumtazahmad8166@gmail.com.
Anam Zeb, Email: zebanam90@gmail.com.
Sarosh Alvi, Email: alvisarosh500@gmail.com.
References
- 1.Borisov VV, Shilov EM. Chronic Renal Failure. Urologiia 2017;1(supplement):11–18. [DOI] [PubMed] [Google Scholar]
- 2.Li QM, Chena HR, Zha XQ, et al. Renoprotective effect of Chinese chive polysaccharides in adenine-induced chronic renal failure. Int J Biol Macromol 2018;106:988–993. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Z, Zheng K, Zhang H, et al. Physical exercise and patients with chronic renal failure: a meta-analysis. Biomed Res Int 2017;2017:7191826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Zheng H, Liu Z. Effect of calcitriol combined with sevelamer carbonate on serum parathyroid hormone in patients with chronic renal failure. Cell Mol Biol (Noisy-le-grand) 2020;66:31–35. [PubMed] [Google Scholar]
- 5.Wanner C, Ketteler M. Chronic renal failure. Dtsch Med Wochenschr 2011;136:1591–1593. [DOI] [PubMed] [Google Scholar]
- 6.Padmanabhan A, Gohil S, Gadgil NM, et al. Chronic renal failure: an autopsy study. Saudi J Kidney Dis Transpl 2017;28:545–551. [DOI] [PubMed] [Google Scholar]
- 7.Olsen E, van Galen G. Chronic renal failure-causes, clinical findings, treatments and prognosis. Vet Clin North Am Equine Pract 2022;38:25–46. [DOI] [PubMed] [Google Scholar]
- 8.Koushik NS, McArthur SF, Baird AD. Adult chronic kidney disease: neurocognition in chronic renal failure. Neuropsychol Rev 2010;20:33–51. [DOI] [PubMed] [Google Scholar]
- 9.Dhondup T, Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif 2017;43:179–188. [DOI] [PubMed] [Google Scholar]
- 10.Felxa J. Understanding creatinine and BUN levels in kidney patients: maintenance and management. Int J Urol Nephrol 2023;11:1–2. [Google Scholar]
- 11.Gao C, Zhang Q, Yang Y, et al. Recent trends in therapeutic application of engineered blood purification materials for kidney disease. Biomater Res 2022;26:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall PW, Vasiljevic M. Beta 2 -microglobulin excretion as an index of renal tubular disorders with special reference to endemic B alkan nephropathy. J Lab Clin Med 1973;81:897–904. [PubMed] [Google Scholar]
- 13.Assounga AG1,2. Beta 2 microglobulin in kidney failure: a review and an algorithm for renal replacement therapy. Saudi J Kidney Dis Transplant 2021;32:1214–1220. [DOI] [PubMed] [Google Scholar]
- 14.Petreski T, Piko N, Ekart R, et al. Review on inflammation markers in chronic kidney disease. Biomedicines 2021;9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Chen J, Lan HY, et al. Role of C-reactive protein in kidney diseases. Kidney Dis (Basel) 2022;9:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson BM, Akizawa T, Jager KJ, et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016;388:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Tao R, Gao J, et al. Vascular access blood purification treatments in chronic renal failure: impact on quality of life. Cardiovasc Re 2023;1:12–17. [Google Scholar]
- 18.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–1270. [DOI] [PubMed] [Google Scholar]
- 19.Cheng W, Luo Y, Wang H, et al. Survival outcomes of hemoperfusion and hemodialysis versus hemodialysis in patients with end-stage renal disease: a systematic review and meta-analysis. Blood Purif 2022;51:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Ren C, Han X, et al. The protective effect of different dialysis types on residual renal function in patients with maintenance hemodialysis: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew G, Agha R, for the STROCSS Group . STROCSS 2021: Strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 22.Zadrazil J. Aetiology and a clinical picture of chronic renal failure. Vnitr Lek 2011;57:607–613. [PubMed] [Google Scholar]
- 23.Long M, Li QM, Fang Q, et al. Renoprotective effect of laminaria japonica polysaccharide in adenine-induced chronic renal failure. Molecules 2019;24:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabst D, Sanchez-Cueva PA, Soleimani B, et al. Predictors for acute and chronic renal failure and survival in patients supported with veno-arterial extracorporeal membrane oxygenation. Perfusion 2020;35:402–408. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann JN, Schwartz K, Chow WH, et al. The association between chronic renal failure and renal cell carcinoma may differ between black and white Americans. Cancer Causes Control 2013;24:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imtiaz S, Ashar A. Epidemiology and Demography of Chronic Kidney Disease in Pakistan- a Review of Pakistani Literature. Pak J Kidney Dis 2023;7:2–7. [Google Scholar]
- 28.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2018;71:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller D, Goldstein SL. Hemodialysis in children with end-stage renal disease. Nat Rev Nephrol 2011;7:650–658. [DOI] [PubMed] [Google Scholar]
- 30.Zhai H, Li L, Yin Y, et al. The efficacy of hemodialysis in interventional therapy in coronary artery disease patients with chronic renal insufficiency. Ren Fail 2016;38:437–441. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadmehrabi S, Tang WHW. Hemodialysis-induced cardiovascular disease. Semin Dial 2018;31:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morfin JA, Fluck RJ, Weinhandl ED, et al. Intensive hemodialysis and treatment complications and tolerability. Am J Kidney Dis 2016;68:S43–S50. [DOI] [PubMed] [Google Scholar]
- 33.Ricci Z, Romagnoli S, Reis T, et al. Hemoperfusion in the intensive care unit. Intensive Care Med 2022;48:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Li Z, Zhang Y, et al. Comparison of combined hemodialysis and hemoperfusion with hemoperfusion alone in 106 patients with diabetic ketoacidosis and acute renal failure: a retrospective study from a single center in China. Med Sci Monit 2021;27:e922753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analysis files present, can be provided on reasonable demand.