Abstract
Marijuana has been used by humans for thousands of years for both medicinal and recreational purposes. This included the treatment of pain, inflammation, seizures, and nausea. In the 1960s, the structure of the principal psychoactive ingredient Δ9-tetrahydrocannabinol was determined, and over the next few decades, two cannabinoid receptors were characterized along with the human endocannabinoid system and what it affects. This includes metabolism, the cardiovascular and reproductive systems, and it is involved in such conditions as inflammation, cancer, glaucoma, and liver and musculoskeletal disorders. In the central nervous system, the endocannabinoid system has been linked to appetite, learning, memory, and conditions such as depression, anxiety, schizophrenia, stroke, multiple sclerosis, neurodegeneration, addiction, and epilepsy. It was the profound effectiveness of cannabidiol, a non-psychoactive ingredient of marijuana, to relieve the symptoms of Dravet syndrome, a severe form of childhood epilepsy, that recently helped spur marijuana research. This has helped substantially to change society's attitude towards this potential source of useful drugs. However, research has also revealed that the actions of endocannabinoids, such as anandamide and 2-arachidonoylglycerol, and the phytocannabinoids, tetrahydrocannabinol and cannabidiol, were not just due to interactions with the two cannabinoid receptors but by acting directly on many other targets including various G-protein receptors and cation channels, such as the transient receptor potential channels for example. This mini-review attempts to survey the effects of these 4 important cannabinoids on these currently identified targets.
Keywords: Cannabidiol, Δ9-tetrahydrocannabinol, N-arachidonoylethanolamine, 2-arachidonoylglycerol, endocannabinoid system
1. Introduction
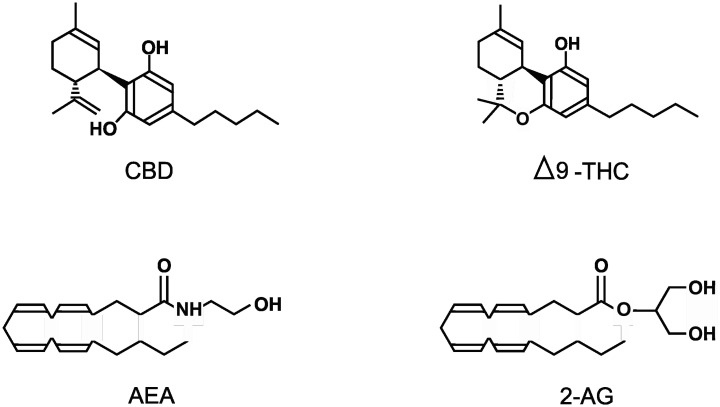
The cannabis plant, more commonly known as marijuana, has been used by humans for thousands of years but only recently has the cannabis genus (which includes the species Cannabis sativa, Cannabis indica, and Cannabis ruderalis) been investigated for its pharmacological potential. The first recorded medical use was in China over 5,000 years ago, where marijuana was used to treat pain, inflammation, seizures, and nausea. But it was also used recreationally and it was this use, especially in the West, that prevented its full medical potential from being explored [1],[2]. Nearly 60 years ago, Δ9-tetrahydrocannabinol (THC; see Figure 1) was isolated along with many other phytocannabinoids from marijuana [3]; this made possible the identification and characterization of the first endogenous cannabinoid receptor (CB1R) which is a G-protein coupled receptor (GPCR) [4],[5]. This was soon followed by the identification of a second endogenous cannabinoid receptor (CB2R; also a GPCR) [6],[7]. The expression of these 2 cannabinoid receptors, along with the presence of the principal endogenous endocannabinoids N-arachidonoylethanolamine (AEA: anandamide; see figure 1) and 2-arachidonoylglycerol (2-AG: see figure 1) helped define the endocannabinoid system [8]–[11]. The endocannabinoid system in the central nervous system (CNS) can affect appetite, learning, memory, and conditions such as depression, anxiety, schizophrenia, stroke, multiple sclerosis, neurodegeneration, addiction, and epilepsy [1],[10],[12],[13]. In the peripheral nervous system and other tissues this system can affect metabolism, nociception, the cardiovascular and reproductive systems, and conditions such as inflammation, cancer, glaucoma, and liver and musculoskeletal disorders [14],[15]. However, over the last couple of decades it became clear that the many actions of cannabinoids could not be just attributed to interactions with the two cannabinoid receptors. Now it is known that cannabinoids can interact with a wide variety of additional targets ranging from glycine receptors to opioid receptors to various transient receptor potential channels (TRPs). The purpose of this mini-review therefore is to summarize the current known direct targets of four important cannabinoids including cannabidiol (CBD; see Figure 1), THC, AEA, and 2-AG; the chemical structures of these cannabinoids are illustrated in Figure 1. In order to be included in this mini-review, the authors had to be able to state with a high degree of certainty that the cannabinoids were acting on the specific target and not via another pathway or receptor. This mini-review does not cover any transporter, metabolic, or indirect targets. No actions of metabolites or synthetic cannabinoids are discussed and the reader is referred to more specific reviews on these subjects.
Figure 1. Representations of the structures of the 4 cannabinoids discussed in this review including Cannabidiol (CBD), Δ9-Tetrahydrocannabinol (THC), N-Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG).
2. The agonists
CBD was first isolated from marijuana in 1940 by Adams et al. and from hashish resin by Jacob and Todd [16],[17], and its structure was later elucidated in the 1960's by Mechoulam and Shvo [18]. In 1964, Gaoni and Mechoulam described the structure of the psychoactive phytocannabinoid THC [3]. It is interesting to note that although CBD and THC are considered the major active phytochemicals in marijuana they are not thought to be normally synthesized in the plant but rather produced by heat-induced decarboxylation of other related phytocannabinoids such as cannabidiolic acid [19],[20]. The endogenous cannabinoids AEA and 2-AG are arachidonic acid derivatives formed from the cell membrane components N-arachidonoyl phosphatidylethanolamine and diacylglycerol [21]. AEA was the first endocannabinoid identified that acted on cannabinoid receptors and was described in 1992 [8] while 3 years later 2-AG was also identified [9].
3. Cannabinoid targets
3.1. The cannabinoid receptors CB1R and CB2R
CB1R is a 472 amino acid GPCR in humans which has been characterized [4],[5],[22]–[24] together with 3 isoforms that exhibit high levels of expression [25] throughout the CNS in areas such as the olfactory bulb, hippocampus, basal ganglia, and cerebellum and peripherally in sympathetic terminals, trigeminal ganglion, dorsal root ganglia, and nociceptive terminals [26]–[29]. As a GPCR, this receptor acts via the heterotrimeric G-protein Gi/o (α-subunit) to inhibit adenylate cyclase (AC) and therefore lower the concentration of cyclic adenosine monophosphate (cAMP) in the cell [30]. It is thought that the β/γ-heterodimer can itself activate some AC isoforms [31],[32] and CB1R may also act via Gq/11 to increase intracellular calcium [33]. This receptor has also been reported to directly interact with certain calcium channels [34],[35] and potassium channels [36],[37] and may activate cell survival pathways [38],[39]. CB2R is a 360 amino acid GPCR in humans which is found as 2 differentially expressed isoforms [6],[7],[40]. This cannabinoid receptor is expressed primarily outside the CNS in peripheral tissues such as the immune, cardiovascular, reproductive, and gastrointestinal systems [6],[30]. This GPCR can also inhibit AC and therefore decrease intracellular cAMP, activate the mitogen-activated protein kinase (MAPK) pathway affecting gene expression [41],[42], and promote neuronal survival similarly to the CB1R [43]. Now, examining the actions of the 4 selected cannabinoids, CBD appeared to have little effect on receptor activation and may act as an antagonist on both receptors [44]–[46]. In fact, CBD can negatively modulate the responses to THC and 2-AG [47],[48]. In 2019, Tham et al. demonstrated CBD acting as a negative allosteric regulator on CB1R and as a partial agonist on CB2R in HEK293A cells [49]. THC can act as a partial agonist on both cannabinoid receptors [30],[50],[51]. AEA is a high affinity partial agonist of CB1R but appears virtually inactive on CB2R [52] whereas 2-AG only exhibits moderate affinity as a partial agonist for both cannabinoid receptors [7].
3.2. G-protein coupled receptors 3, 6, 12, 18, and 55
The current unnamed (orphan) GPCRs 3, 6, and 12 only appear to have been explored in their responses to CBD which acts as an inverse agonist for all 3 [53]–[55]. GPCR18 was isolated in 1997 by Gantz et al. and is typically coupled to Gi/o which inhibits cAMP production and is expressed at high levels in the brainstem, spleen, and testes [56],[57]. CBD appears to have little effect on this receptor at physiological concentrations [58] whereas THC is a potent agonist in HEC-1B cells expressing this receptor [59]. AEA and 2-AG seem to have little effect on GPCR18 although McHugh reported that AEA could act as an agonist in HEC-1B cells expressing this receptor [59]. GCPR55 was characterized by Ryberg et al. in 2007 and is highly expressed in the CNS, adrenal glands, and gastrointestinal tract, and it acts primarily through activation of G13 [60],[61]. Using HEK293 cells, this GPCR was also observed to couple to Gq [62]. CBD acts as an antagonist on this receptor [63] whereas Ryberg using HEK293 cells showed THC can be an agonist [61]. AEA may act as a partial agonist but 2-AG had little effect using an assay which observed increases in intracellular calcium in HEK293 cells and also in U2OS cells as evidence for GPCR55 activation [62],[64].
3.3. Opioid receptors µ, δ and κ
All opioid GPCRs couple principally to Gi/o and in most neurons reduce excitation by increasing potassium currents. In addition to inhibiting AC they activate MAPK [65]. It had been discovered in the 1970s that THC could reduce the symptoms of naloxone-induced opioid withdrawal due to interactions with the µ receptor [66],[67]. Labelled THC binding was observed at µ opioid receptors in rat brain [68],[69] and it was found both THC and CBD allosterically modulate both µ and δ opioid GPCRs negatively [70] while THC alone appears to interact with κ opioid receptors [71]. AEA and 2-AG had no apparent direct effect on opioid receptors, although there appears to be mounting evidence that cannabinoid receptors may directly interact with opioid receptors [72],[73].
3.4. Purine and adenosine receptors
Apart from being central in the energetic status of a cell, purines are involved in a wide range of physiological functions [74],[75]; adenosine receptors are part of the purine family. Adenosine receptors are GPCRs that typically couple to Gs or Gi/o to activate or inhibit AC, respectively. They can also interact with calcium and potassium channels via the activated G-proteins. These receptors used to be known as P1 receptors but now they have been renamed after their primary ligand adenosine receptors A1, A2A, A2B, and A3 [74],[76]. CBD allosterically enhances adenosine receptors A1 and A2A [77],[78] while 2-AG was found to allosterically inhibit A3 adenosine receptors [79]. THC and AEA appear not to have any actions on these receptors.
3.5. Peroxisome proliferator-activated receptors (PPARα and PPARγ)
PPARs are ligand-activated transcription factors that are members of the nuclear hormone receptor family and which are important in regulating energy and metabolic homeostasis; in particular they are involved in insulin sensitivity and enhancing both glucose and fatty acid metabolism [80]. It has been shown that CBD and THC can activate PPARγ [81]–[84] utilizing fatty acid binding proteins to transport them into the nucleus [85]. However, Alhamoruni saw little direct effect with either CBD or THC in Caco-2 cell cultures [86],[87]. Apparently, AEA and 2-AG can activate both PPARα and PPARγ [88]–[91].
3.6. Glycine receptor
The ionotropic glycine receptor is a pentameric ligand-gated chloride channel which mediates neuronal inhibition both in the brain stem and spinal cord [92]. It belongs to the Cys-loop ionotropic family of receptors which also includes nicotinic acetylcholine, serotonin type 3, and GABAA receptors [93],[94]. The effect of cannabinoids on these receptors appears to be subunit-dependent similarly to other such receptors in this family as discussed later. CBD and THC were found to potentiate glycine's actions in mice and this effect required certain alpha subunits (α-1, α-1-β, α–2 and α-3 subunits) in the receptor [95],[96]. Hejazi et al. found that THC and AEA also potentiated glycine currents allosterically in Xenopus oocytes expressing glycine receptors [97]. However, Lozovaya et al. found in isolated rat hippocampal neurons and cerebellar Purkinje neurons that AEA and 2-AG inhibited glycine receptors and accelerated desensitization [98]. In HEK293 cells expressing glycine receptors of varying subunit composition AEA potentiated the effects of these receptors if containing α-1 or α-1-β subunits but had no effect on receptors made up of α-2 or α-3 subunits [99]. Alvarez et al. recently published a paper using computer modelling, among other methods, to investigate the molecular mechanisms possibly involved in the positive allosteric modulation of glycine receptors by THC [100].
3.7. Nicotinic receptor
These ionotropic pentameric acetylcholine receptors that mediate excitation by sodium ion entry are found throughout the body specifically at autonomic ganglia and neuromuscular junctions (NMJ). In the CNS they are widespread and are typically found on presynaptic terminals where they can enhance or inhibit the release of other neurotransmitters. Additionally, their responses to cannabinoids are subunit dependent [101],[102] as seen with glycine receptors that are also members of the Cys-loop family. CBD has been shown to inhibit synaptic transmission at frog NMJs, human nicotinic receptors, and rat hippocampal neurons whereas THC appears to have little effect [103]–[105]. AEA and 2-AG antagonize nicotinic receptors in Xenopus oocytes [103],[106],[107]. Cannabinoids seem most effective on these receptors if homologous for α7 subunits or α4β2 combinations [108].
3.8. 5-Hydroxytryptamine (5-HT1A) receptor
This receptor is a GPCR expressed in the CNS which is typically coupled to Gi/o which inhibits AC resulting in a decrease in cAMP and neuronal inhibition [109]. It has been reported that CBD is an agonist of this receptor [110],[111]; the other 3 cannabinoids do not appear to have any effect on this receptor.
3.9. 5-Hydroxytryptamine (5-HT3) receptor
Of the 7 classes of 5-HT receptors, 5-HT3 is the only ionotropic cation channel Cys-loop member [112],[113]. Activation results in depolarization mediating fast synaptic transmission [114]. However, unlike the previously discussed nicotine and glycine receptors, 5-HT3 receptors are homopentameric so there are no subunit variations [113]. There are 2 alternate 5-HT3 transcripts (A and B) but they do not appear to offer any functional differences [115]. CBD, THC, and AEA all appear to directly inhibit 5-HT3 receptors when expressed in Xenopus oocytes or HEK-293 cultures [116]–[120]. Fan, as early as 1995, had observed AEA inhibition of these receptors in rat nodose ganglion neurons [121]. Interestingly, it was seen that the degree of inhibition of 5-HT3 receptors could vary with the expression system utilized and it has been suggested that this is due to different levels of receptor density at the plasma membrane [122]. 2-AG was not reported as having any effect on this receptor.
3.10. Dopamine D2 receptor
This is one of 5 GPCR dopamine receptors found throughout the CNS but especially in the cortex and limbic system; the D2 receptor is coupled to Gi/o and therefore inhibits AC resulting in a decrease in cAMP. Additionally, potassium channels are activated while calcium channels are inhibited resulting in a decrease in neuronal excitability [123]. Seeman has shown that CBD acts directly on D2 receptors where it acts as a partial agonist [124], but it appears there is no other data on the possible effects of the other 3 cannabinoids on this or other dopamine receptors.
3.11. Glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors
Glutamate is the major excitatory transmitter in the human cortex. The 2 principal ionotropic receptor subtypes are the AMPA and NMDA ligand-gated cation selective tetramers that depolarize and, specifically with regards to the NMDA receptor, have a high calcium permeability [125]. CBD negatively allosterically modulates the currents caused by activation of AMPA receptors [126] while AEA inhibits recombinant AMPA receptors expressed in Xenopus oocytes [127]. THC and 2-AG appear to have little direct effect on either type of glutamate receptor. AEA appears to enhance NMDA-induced currents expressed in Xenopus oocytes [128] and in the control of blood pressure in rats [129]. CBD appears not to have been tested on NMDA receptors.
3.12. γ-Aminobutyric Acid (GABAA) receptors
This pentameric GABA ionotropic chloride channel is the main inhibitory mediator in the CNS [130]. Like glycine, nicotinic, and 5-HT3 receptors it is a member of the Cys-loop family of ionotropic ligand-gated channels. So far, 19 GABAA receptor subunit genes have been found in humans which can be assigned to 8 different categories. However, functional channels are normally formed from 2 copies from the α and β categories plus one copy from the others to form a heteropentamer [130],[131]. Koe et al. used synthetic cannabinoid ligands to study GABAA binding, anticonvulsant, and analgesic effects in mice and proposed that cannabinoids might interact with this target [132],[133]. Indeed CBD appears to cause potentiation by acting allosterically with certain α-containing subunits in multiple systems including Xenopus [134]–[136]. In HEK-293 cells expressing this receptor, THC was also shown to potentiate the response [137] while in the same system AEA and 2-AG inhibited currents and increased desensitization of GABA receptors; this was also observed in isolated hippocampal neurons from rat brains [138].
3.13. Transient receptor potential (TRP) receptors
TRP receptors compose a large family of cation channels mediating excitation and often calcium entry typically responding to chemical or physical stimuli such as temperature, pressure and pain mediators. They are composed of 4 subunits and can be homo- or heterotetramers and are expressed primarily on sensory neurons [139],[140]. There is much evidence to suggest that many of these receptors are directly affected by cannabinoids to the extent that some in the field have given them the label of ionotropic cannabinoid receptors [7],[141],[142]. On TRPV1-4 receptors, all 4 cannabinoids (CBD, THC, AEA, and 2-AG) appear to act as agonists although the evidence for THC is slightly less compelling [143]–[145]. TRPA1 and TRPM8 are thought to be particularly associated with cold perception [146]. CBD, THC, and AEA all act as agonists on TRPA1 [143],[145],[147] and recently this was also confirmed for 2-AG by Heblinski et al. in HEK cells expressing these channels [148]. Interestingly, the TRPM8 receptor response to the agonists menthol and icilin is antagonized by CBD, THC, and AEA, although there appears to be no published data on the effects of 2-AG [145],[149].
3.14. Voltage-gated sodium channels
In most excitable cells the initial depolarizing phase of an action potential is mediated by voltage-gated sodium channels [150]–[152]. These channels typically consist of 3 subunits; a pore-forming α subunit plus 2 β subunits. Nine α subunits (resulting in the classifications of Nav1.1 to 1.9) and 4 β subunits have been identified and characterized in mammals [153]. Apart from the so-called gate that opens at the required membrane potential (threshold potential), these channels also inactivate using a second gate to limit excessive depolarization [154],[155]. Using various human and mouse Nav1.1 to 1.7 channels, it was found that CBD possibly inhibited these channels by stabilizing the inactivation state and preventing them from opening [156],[157]. THC, although possessing some anticonvulsant properties, does not appear to have similar actions to CBD and due to its psychotropic effects is difficult to study in animal models [158]. However, Turkanis did report inhibition of sodium currents in mouse neuroblastoma cells by THC [159]. Direct effects on these channels by AEA and 2-AG have not been reported.
3.15. Voltage-gated calcium channels
For many excitable cells, voltage-gated calcium channels are the primary source of calcium entry, allowing it to act as a secondary messenger for such important functions as neurotransmitter release and muscle contraction [153]. There are 6 classes of voltage-gated calcium channels based on such factors as threshold, pharmacology and rate of inactivation. The classification has now been updated to align with that used for other voltage-gated channels such as L-type calcium channels which are found in many tissues including heart, skeletal muscle and the nervous system that are now called Cav1.1 to 1.4. They are particularly involved in regulating contraction in cardiac and smooth muscle. P and Q-channels are found in the nervous system, smooth muscle, and other tissues and are now classified as Cav2.1 while N and R-channels, also found in the nervous system and other tissues including heart and lung, are now called Cav2.2 and 2.3, respectively. P, Q, and N-channels are involved in neurotransmitter and hormone release. Finally, T-channels that are found in the nervous system, heart, smooth muscle, and other tissues are now classified as Cav3.1–3 and help regulate repolarization in neurons and cardiomyocytes among other functions [160]. These channels are heteropentamers with the main pore-forming subunit α1 typically associating with a β and a γ subunit and additionally α-related gene products such as α2δ [153]. CBD appears to only affect L-type channels/Cav1.1 to 1.4 inhibiting them as shown by Ali et al. in rat ventricular myocytes [161]. Additionally, 2-AG inhibited these channels while THC had no effect [162]. AEA was found to inhibit both T-type channels/Cav3.1–3.3 and L-type/Cav1.1–1.4 [163],[164].
3.16. Potassium channels
The potassium channel family is very large and diverse and serves a variety of functions throughout the body, especially in excitable tissues. Repolarization after the sodium-mediated depolarization of the action potential, setting the refractory period/action potential timing and generally opposing excitability when necessary are the main effects of these channels [165],[166]. Classification can generally be sub-divided into 4 smaller families including voltage-gated channels (Kv1 to 12.3/17 members), calcium or sodium-activated channels (KCa1.1 to 5.1 and KNA1.1 and 1.2/8 and 2 members respectively), the so-called two-pore domain or “leak” channels that help establish the resting membrane potential of excitable cells (K2p1.1 to 18.1/15 members) and inwardly rectifying channels (KIR1.1 to 7.1/16 members) and delayed rectifying channels(KDR) [166]. Several fairly recent studies have demonstrated that CBD can enhance the currents of certain voltage-gated potassium channels such as human Kv7.2 and 7.3 expressed in Chinese hamster ovary cells (CHO) cells, mouse superior cervical ganglion cells and cultured rat hippocampal neurons [167]. Isaev and Topal independently observed inhibition of various potassium delayed rectifier channels and others by CBD using rat, rabbit and dog ventricular myocyte systems [168],[169]. There appears to be little published data on the effects of THC on potassium channels, but back in 1996 Poling et al. published the observation that THC (and AEA) inhibited Kv1.2 channels in transfected fibroblasts [170]. AEA, apart from enhancing large-conductance KCa1.1 channels in various systems [171] seems to inhibit most potassium channels. Those affected include several cardiac types including human Kv4.3 and rat myocyte KATP channels) [172]–[174], Kv3.1 voltage-gated channels [175], several delayed rectifier channels [176],[177], KATP (cromakalin-induced) and K2p3.1 (TASK-1) channels [178]. Finally, 2-AG was also seen to inhibit several types of potassium channel including Kv4.3 myocyte channels [172] and IA current in mouse dopaminergic neurons [179], delayed rectifier channels [177],[180], and KATP channels in mouse insulinoma cells [180]. The direct actions of the 4 cannabinoids on these targets have been summarized in Table 1.
Table 1. A summary of the direct targets of the 4 cannabinoids cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), N-arachidonoylethanolamine (AEA), and 2-arachidonoylglycerol (2-AG) complete with principal references as discussed in this mini-review. When reporting the effects of these cannabinoids, terminology is used as published. Abbreviations used include (SD) for subunit dependent, N/E for no effect, and N/T for not tested.
| EFFECTOR | CBD | THC | AEA | 2-AG | References |
| CB1R | -Allosteric | Partial Agonist | Partial Agonist | Partial Agonist | [7],[30],[44]–[52] |
| CB2R | Partial Agonist | Partial Agonist | N/E | Partial Agonist | [7],[30],[44]–[52] |
| GPCRs 3,6&12 | Inverse Agonist | N/T | N/T | N/T | [53]–[55] |
| GPCR 18 | N/E | Agonist | Agonist | N/E | [58],[59] |
| GPCR 55 | Antagonist | Agonist | Partial Agonist | N/E | [60]–[64] |
| µ Opioid | -Allosteric | -Allosteric | N/E | N/E | [66]–[70] |
| δ Opioid | -Allosteric | -Allosteric | N/E | N/E | [70] |
| κ Opioid | N/E | Inhibit | N/E | N/E | [71] |
| Adenosine A1,2a | +Allosteric | N/E | N/E | N/E | [77],[78] |
| Adenosine A3 | N/E | N/E | N/E | -Allosteric | [79] |
| PPARs | Agonist | Agonist | Agonist | Agonist | [81]–[91] |
| Glycine (SD) | Potentiate | +Allosteric | +Allosteric | Inhibit | [95]–[99] |
| Nicotinic (SD) | Inhibit | N/E | Antagonist | Antagonist | [101]–[108] |
| 5-HT1A | Agonist | N/E | N/E | N/E | [110],[111] |
| 5-HT3 | Inhibit | Inhibit | Inhibit | N/E | [116]–[122] |
| Dopamine D2 | Partial Agonist | N/T | N/T | N/T | [124] |
| NMDA | N/E | N/E | Enhance | N/E | [128],[129] |
| AMPA | -Allosteric | N/E | Inhibit | N/E | [126],[127] |
| GABAA (SD) | +Allosteric | Potentiate | Inhibit | Inhibit | [132]–[138] |
| TRPV1-4 | Agonist | Agonist | Agonist | Agonist | [143]–[145] |
| TRPA1 | Agonist | Agonist | Agonist | Agonist | [143],[145],[147],[148] |
| TRPM8 | Antagonist | Antagonist | Antagonist | N/T | [145],[149] |
| Nav1.1-1.7 | Inhibit | Inhibit | N/T | N/T | [156]–[159] |
| Cav1.1-1.4 | Inhibit | N/E | Inhibit | Inhibit | [161]–[164] |
| Cav3.1-3.3 | N/T | N/T | Inhibit | N/T | [163],[164] |
| Kv7.2-7.3 | Enhance | N/T | N/T | N/T | [167] |
| KDR | Inhibit | N/T | Inhibit | Inhibit | [168],[169],[177]–[180] |
| Kv1.2 | N/T | Inhibit | Inhibit | N/T | [170] |
| KCa1.1 | N/T | N/T | Enhance | N/T | [171] |
| Kv4.3 | N/T | N/T | Inhibit | Inhibit | [172]–[174] |
| KATP | N/T | N/T | Inhibit | Inhibit | [172],[173],[178],[180] |
| Kv3.1 | N/T | N/T | Inhibit | N/T | [175] |
| K2P3.1 | N/T | N/T | Inhibit | N/T | [178] |
4. Future directions
Both the endocannabinoid system and the potential of marijuana phytocannabinoids are now becoming recognized as important areas requiring proper research and appropriate funding. This is finally becoming a reality now that the social stigma associated with marijuana's recreational use is receding. This plant has been used by humans for thousands of years and proper investigation of all its potential uses, contraindications, potential problems with long-term use, etc. need to be fully investigated as its use for medical purposes becomes more widespread. This summary of the actions of 4 principal cannabinoids illustrates the wide array of targets these compounds can affect and underscores why the phytocannabinoids produced by the marijuana plant can have the potential to affect many body systems and disease states. CBD and THC can act as allosteric modulators, both positive and negative, plus other yet to be defined effects on many of these targets. CBD can infact negatively allosterically modulate CB1R itself. AEA and 2-AG have less compelling evidence for allosteric modulation as yet but are still observed to affect many of these targets. Due to the high threshold for inclusion in this mini-review, many papers were not considered as the authors could not state with a high degree of certainty how the cannabinoids were acting. Many publications state cannabinoid-receptor independent but cannot conclude with certainty how the cannabinoid is acting. To overcome this problem, many of the papers included were utilizing fairly simple primary cell cultures expressing specific receptors so they could be certain. It would be nice to see these initial results confirmed in more complex and relevant systems, but this will require the production of very specific synthetic agonists and antagonists to both elucidate mechanisms of action and develop more useful pharmaceuticals. This will also enable further understanding of the function of the endocannabinoid system itself which may reveal a whole new level of subtle modulation of these targets and systems. Hopefully this summary has captured the majority of known direct targets and will help the reader to gain an overall perspective and insight on the functions of these selected cannabinoids and the endocannabinoid system and direct to more specific and detailed resources.
Acknowledgments
Thanks to my long-suffering wife Vanessa for creating the figure.
Footnotes
Conflicts of interest: Nicholas J. D. Wright is an editorial board member for AIMS Neuroscience and was not involved in the editorial review or the decision to publish this article. The author declares that there are no competing interests.
References
- 1.Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R. Cannabinoids As Therapeutic Agents. (1st Edition) Chapman and Hall/CRC; 1986. The Pharmacohistory of Cannabis Sativa; pp. pp. 1–20. [DOI] [Google Scholar]
- 3.Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 4.Devane WA, Dysarz FA, 3rd, Johnson MR, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 5.Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 6.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 7.Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devane WA, Hanuš L, Breuer A, et al. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 10.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 12.Kano M, Ohno-Shosaku T, Hashimotodani Y, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16:30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller LK, Devi LA. The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications. Pharmacol Rev. 2011;63:461–470. doi: 10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams R, Hunt M, Clark JH. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J Am Chem Soc. 1940;62:196–200. doi: 10.1021/ja01858a058. [DOI] [Google Scholar]
- 17.Jacob A, Todd AR. Cannabidiol and Cannabol, Constituents of Cannabis indica Resin. Nature. 1940;145:350–350. doi: 10.1038/145350a0. [DOI] [Google Scholar]
- 18.Mechoulam R, Shvo Y. Hashish. I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–2078. doi: 10.1016/0040-4020(63)85022-X. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Okamoto K. Distribution of tetrahydrocannabinolic acid in fresh wild cannabis. Experientia. 1970;26:819–820. doi: 10.1007/BF02114192. [DOI] [PubMed] [Google Scholar]
- 20.Taura F, Sirikantaramas S, Shoyama Y, et al. Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chem Biodivers. 2007;4:1649–1663. doi: 10.1002/cbdv.200790145. [DOI] [PubMed] [Google Scholar]
- 21.Araque A, Castillo PE, Manzoni OJ, et al. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB(1) Nature. 2017;547:468–471. doi: 10.1038/nature23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua T, Vemuri K, Pu M, et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167:750–762.e714. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Z, Yin J, Chapman K, et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540:602–606. doi: 10.1038/nature20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Mariscal I, Krzysik-Walker SM, Doyle ME, et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapper JR, Moreno-Sanz G, Russo R, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price TJ, Helesic G, Parghi D, et al. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam J, Trembovler V, Di Marzo V, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. Faseb J. 2008;22:285–294. doi: 10.1096/fj.06-7957com. [DOI] [PubMed] [Google Scholar]
- 29.Veress G, Meszar Z, Muszil D, et al. Characterisation of cannabinoid 1 receptor expression in the perikarya, and peripheral and spinal processes of primary sensory neurons. Brain Struct Funct. 2013;218:733–750. doi: 10.1007/s00429-012-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 31.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 32.Rhee M-H, Bayewitch M, Avidor-Reiss T, et al. Cannabinoid Receptor Activation Differentially Regulates the Various Adenylyl Cyclase Isozymes. J Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- 33.Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabó GG, Lenkey N, Holderith N, et al. Presynaptic calcium channel inhibition underlies CB1 cannabinoid receptor-mediated suppression of GABA release. J Neurosci. 2014;34:7958–7963. doi: 10.1523/JNEUROSCI.0247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- 37.Mackie K, Lai Y, Westenbroek R, et al. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galve-Roperh I, Rueda D, Gómez del Pulgar T, et al. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol. 2002;62:1385–1392. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- 39.Gómez del Pulgar T, Velasco G, Guzmán M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/bj3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu QR, Pan CH, Hishimoto A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–530. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouaboula M, Poinot-Chazel C, Bourrié B, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouaboula M, Poinot-Chazel C, Marchand J, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 43.Viscomi MT, Oddi S, Latini L, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertwee RG, Ross RA, Craib SJ, et al. (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/S0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- 46.Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morales P, Goya P, Jagerovic N, et al. Allosteric Modulators of the CB(1) Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016;1:22–30. doi: 10.1089/can.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176:1455–1469. doi: 10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. doi: 10.2174/0929867306666220401124036. [DOI] [PubMed] [Google Scholar]
- 51.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 52.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown KJ, Laun AS, Song ZH. Cannabidiol, a novel inverse agonist for GPR12. Biochem Biophys Res Commun. 2017;493:451–454. doi: 10.1016/j.bbrc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laun AS, Shrader SH, Brown KJ, et al. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40:300–308. doi: 10.1038/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laun AS, Shrader SH, Song Z-H. Novel inverse agonists for the orphan G protein-coupled receptor 6. Heliyon. 2018;4:e00933. doi: 10.1016/j.heliyon.2018.e00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gantz I, Muraoka A, Yang Y-K, et al. Cloning and Chromosomal Localization of a Gene (GPR18) Encoding a Novel Seven Transmembrane Receptor Highly Expressed in Spleen and Testis. Genomics. 1997;42:462–466. doi: 10.1006/geno.1997.4752. [DOI] [PubMed] [Google Scholar]
- 57.Penumarti A, Abdel-Rahman AA. The Novel Endocannabinoid Receptor GPR18 Is Expressed in the Rostral Ventrolateral Medulla and Exerts Tonic Restraining Influence on Blood Pressure. J Pharmacol Exp Ther. 2014;349:29–38. doi: 10.1124/jpet.113.209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McHugh D, Roskowski D, Xie S, et al. Δ(9)-THC and N-arachidonoyl glycine regulate BV-2 microglial morphology and cytokine release plasticity: implications for signaling at GPR18. Front Pharmacol. 2014;4:162. doi: 10.3389/fphar.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McHugh D, Page J, Dunn E, et al. Δ(9)-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol. 2012;165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obara Y, Ueno S, Yanagihata Y, et al. Lysophosphatidylinositol Causes Neurite Retraction via GPR55, G13 and RhoA in PC12 Cells. PLOS ONE. 2011;6:e24284. doi: 10.1371/journal.pone.0024284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Brit J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauckner JE, Jensen JB, Chen H-Y, et al. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whyte LS, Ryberg E, Sims NA, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharir H, Console-Bram L, Mundy C, et al. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J Neuroimmune Pharmacol. 2012;7:856–865. doi: 10.1007/s11481-012-9351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbett AD, Henderson G, McKnight AT, et al. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006;147 Suppl 1:S153–162. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hine B, Friedman E, Torrelio M, et al. Morphine-Dependent Rats: Blockade of Precipitated Abstinence by Tetrahydrocannabinol. Science. 1975;187:443–445. doi: 10.1126/science.1167428. [DOI] [PubMed] [Google Scholar]
- 67.Cox BM, Christie MJ, Devi L, et al. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Brit J Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tulunay FC, Ayhan IH, Portoghese PS, et al. Antagonism by chlornaltrexamine of some effects of Δ9-tetrahydrocannabinol in rats. Eur J Pharmacol. 1981;70:219–224. doi: 10.1016/0014-2999(81)90217-X. [DOI] [PubMed] [Google Scholar]
- 69.Vaysse PJ, Gardner EL, Zukin RS. Modulation of rat brain opioid receptors by cannabinoids. J Pharmacol Exp Ther. 1987;241:534–539. [PubMed] [Google Scholar]
- 70.Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 71.Smith PB, Welch SP, Martin BR. Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther. 1994;268:1381–1387. [PubMed] [Google Scholar]
- 72.Reis GML, Pacheco D, Perez AC, et al. Opioid receptor and NO/cGMP pathway as a mechanism of peripheral antinociceptive action of the cannabinoid receptor agonist anandamide. Life Sci. 2009;85:351–356. doi: 10.1016/j.lfs.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Haller VL, Stevens DL, Welch SP. Modulation of opioids via protection of anandamide degradation by fatty acid amide hydrolase. Eur J Pharmacol. 2008;600:50–58. doi: 10.1016/j.ejphar.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Burnstock G. Purinergic Signalling: Therapeutic Developments. Front Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 76.Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 77.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonca E, Darıcı F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: the role of adenosine A1 receptors. J Cardiovasc Pharmacol Ther. 2015;20:76–83. doi: 10.1177/1074248414532013. [DOI] [PubMed] [Google Scholar]
- 79.Lane JR, Beukers MW, Mulder-Krieger T, et al. The endocannabinoid 2-arachidonylglycerol is a negative allosteric modulator of the human A3 adenosine receptor. Biochem Pharmacol. 2010;79:48–56. doi: 10.1016/j.bcp.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Tyagi S, Gupta P, Saini AS, et al. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burstein S. PPAR-γ: A nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77:1674–1684. doi: 10.1016/j.lfs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 82.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Brit J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173:1899–1910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khosropoor S, Alavi MS, Etemad L, et al. Cannabidiol goes nuclear: The role of PPARγ. Phytomedicine. 2023;114:154771. doi: 10.1016/j.phymed.2023.154771. [DOI] [PubMed] [Google Scholar]
- 85.Hughes ML, Liu B, Halls ML, et al. Fatty Acid-binding Proteins 1 and 2 Differentially Modulate the Activation of Peroxisome Proliferator-activated Receptor α in a Ligand-selective Manner. J Biol Chem. 2015;290:13895–13906. doi: 10.1074/jbc.M114.605998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alhamoruni A, Lee AC, Wright KL, et al. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 87.Alhamoruni A, Wright KL, Larvin M, et al. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol. 2012;165:2598–2610. doi: 10.1111/j.1476-5381.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouaboula M, Hilairet S, Marchand J, et al. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 89.Kozak KR, Gupta RA, Moody JS, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 90.Rockwell CE, Snider NT, Thompson JT, et al. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 91.Sun Y, Alexander SP, Garle MJ, et al. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J Biol Chem. 2012;287:40216–40223. doi: 10.1074/jbc.R112.408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- 94.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 95.Ahrens J, Demir R, Leuwer M, et al. The Nonpsychotropic Cannabinoid Cannabidiol Modulates and Directly Activates Alpha-1 and Alpha-1-Beta Glycine Receptor Function. Pharmacology. 2009;83:217–222. doi: 10.1159/000201556. [DOI] [PubMed] [Google Scholar]
- 96.Xiong W, Cheng K, Cui T, et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol. 2011;7:296–303. doi: 10.1038/nchembio.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hejazi N, Zhou C, Oz M, et al. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- 98.Lozovaya N, Yatsenko N, Beketov A, et al. Glycine Receptors in CNS Neurons as a Target for Nonretrograde Action of Cannabinoids. J Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Z, Aubrey KR, Alroy I, et al. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem Pharmacol. 2008;76:1014–1023. doi: 10.1016/j.bcp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez LD, Alves NRC. Molecular determinants of tetrahydrocannabinol binding to the glycine receptor. Proteins. 2023;91:400–411. doi: 10.1002/prot.26438. [DOI] [PubMed] [Google Scholar]
- 101.Albuquerque EX, Pereira EF, Alkondon M, et al. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96:302–311. doi: 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Mahgoub M, Keun-Hang SY, Sydorenko V, et al. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;720:310–319. doi: 10.1016/j.ejphar.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 104.Oz M, Al Kury L, Keun-Hang SY, et al. Cellular approaches to the interaction between cannabinoid receptor ligands and nicotinic acetylcholine receptors. Eur J Pharmacol. 2014;731:100––105. doi: 10.1016/j.ejphar.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Turkanis SA, Karler R. Effects of delta-9-tetrahydrocannabinol, 11 -hydroxy-delta-9-tetrahydrocannabinol and cannabidiol on neuromuscular transmission in the frog. Neuropharmacology. 1986;25:1273–1278. doi: 10.1016/0028-3908(86)90147-4. [DOI] [PubMed] [Google Scholar]
- 106.Oz M, Zhang L, Ravindran A, et al. Differential Effects of Endogenous and Synthetic Cannabinoids on α<sub>7</sub>-Nicotinic Acetylcholine Receptor-Mediated Responses in <em>Xenopus</em> Oocytes. J Pharmacol Exp Ther. 2004;310:1152–1160. doi: 10.1124/jpet.104.067751. [DOI] [PubMed] [Google Scholar]
- 107.Spivak CE, Lupica CR, Oz M. The Endocannabinoid Anandamide Inhibits the Function of α4β2 Nicotinic Acetylcholine Receptors. Mol Pharmacol. 2007;72:1024–1032. doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- 108.Oz M, Yang KS, Mahgoub MO. Effects of cannabinoids on ligand-gated ion channels. Front Physiol. 2022;13:1041833. doi: 10.3389/fphys.2022.1041833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 110.Espejo-Porras F, Fernández-Ruiz J, Pertwee RG, et al. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology. 2013;75:155–163. doi: 10.1016/j.neuropharm.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 111.Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 112.Barnes NM, Hales TG, Lummis SCR, et al. The 5-HT3 receptor – the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lummis SCR. 5-HT3 Receptors*. J Biol Chem. 2012;287:40239–40245. doi: 10.1074/jbc.R112.406496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Hooft JA, Yakel JL. 5-HT3 receptors in the CNS: 3B or not 3B? Trends Pharmacol Sci. 2003;24:157–160. doi: 10.1016/S0165-6147(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 116.Barann M, Molderings G, Brüss M, et al. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Brit J Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oz M, Zhang L, Morales M. Endogenous cannabinoid, anandamide, acts as a noncompetitive inhibitor on 5-HT3 receptor-mediated responses in Xenopus oocytes. Synapse. 2002;46:150–156. doi: 10.1002/syn.10121. [DOI] [PubMed] [Google Scholar]
- 118.Xiong W, Koo BN, Morton R, et al. Psychotropic and nonpsychotropic cannabis derivatives inhibit human 5-HT3A receptors through a receptor desensitization-dependent mechanism. Neuroscience. 2011;184:28–37. doi: 10.1016/j.neuroscience.2011.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang KH, Galadari S, Isaev D, et al. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther. 2010;333:547–554. doi: 10.1124/jpet.109.162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang KHS, Isaev D, Morales M, et al. The effect of Δ9-tetrahydrocannabinol on 5-HT3 receptors depends on the current density. Neuroscience. 2010;171:40–49. doi: 10.1016/j.neuroscience.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 121.Fan P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- 122.Xiong W, Hosoi M, Koo B-N, et al. Anandamide Inhibition of 5-HT<sub>3A</sub> Receptors Varies with Receptor Density and Desensitization. Mol Pharmacol. 2008;73:314–322. doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]
- 123.Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors - IUPHAR Review 13. Br J Pharmacol. 2015;172:1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6:e920. doi: 10.1038/tp.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hansen KB, Wollmuth LP, Bowie D, et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol Rev. 2021;73:1469–1658. doi: 10.1124/pharmrev.120.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu Y, Yang Z, Jin B, et al. Cannabidiol inhibits febrile seizure by modulating AMPA receptor kinetics through its interaction with the N-terminal domain of GluA1/GluA2. Pharmacol Res. 2020;161:105128. doi: 10.1016/j.phrs.2020.105128. [DOI] [PubMed] [Google Scholar]
- 127.Akinshola BE, Taylor RE, Ogunseitan AB, et al. Anandamide inhibition of recombinant AMPA receptor subunits in Xenopus oocytes is increased by forskolin and 8-bromo-cyclic AMP. N-S Arch Pharmacol. 1999;360:242–248. doi: 10.1007/s002109900078. [DOI] [PubMed] [Google Scholar]
- 128.Hampson AJ, Bornheim LM, Scanziani M, et al. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- 129.Malinowska B, Zakrzeska A, Kurz CM, et al. Involvement of central β2-adrenergic, NMDA and thromboxane A2 receptors in the pressor effect of anandamide in rats. N-S Arch Pharmacol. 2010;381:349–360. doi: 10.1007/s00210-010-0497-6. [DOI] [PubMed] [Google Scholar]
- 130.Sigel E, Steinmann ME. Structure, Function, and Modulation of GABAA Receptors*. J Biol Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olsen RW. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018;136:10–22. doi: 10.1016/j.neuropharm.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koe BK, Milne GM, Weissman A, et al. Enhancement of brain [3H]flunitrazepam binding and analgesic activity of synthetic cannabimimetics. Eur J Pharmacol. 1985;109:201–212. doi: 10.1016/0014-2999(85)90421-2. [DOI] [PubMed] [Google Scholar]
- 133.KOE BK, WEISSMAN A. Facilitation of Benzodiazepine Binding by Levonantradol. J Clin Pharmacol. 1981;21:397S–405S. doi: 10.1002/j.1552-4604.1981.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 134.Bakas T, van Nieuwenhuijzen PS, Devenish SO, et al. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABA(A) receptors. Pharmacol Res. 2017;119:358–370. doi: 10.1016/j.phrs.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 135.Anderson LL, Absalom NL, Abelev SV, et al. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019;60:2224–2234. doi: 10.1111/epi.16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruffolo G, Cifelli P, Roseti C, et al. A novel GABAergic dysfunction in human Dravet syndrome. Epilepsia. 2018;59:2106–2117. doi: 10.1111/epi.14574. [DOI] [PubMed] [Google Scholar]
- 137.Yao L, Wells M, Wu X, et al. Membrane cholesterol dependence of cannabinoid modulation of glycine receptor. FASEB J. 2020;34:10920–10930. doi: 10.1096/fj.201903093R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Golovko T, Min R, Lozovaya N, et al. Control of Inhibition by the Direct Action of Cannabinoids on GABAA Receptors. Cereb Cortex. 2015;25:2440–2455. doi: 10.1093/cercor/bhu045. [DOI] [PubMed] [Google Scholar]
- 139.Voets T, Talavera K, Owsianik G, et al. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- 140.Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107–1116. doi: 10.1021/cn5000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Akopian AN, Ruparel NB, Jeske NA, et al. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- 143.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Brit J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Petrocellis L, Orlando P, Moriello AS, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 2012;204:255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 145.De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 146.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Petrocellis L, Schiano Moriello A, Imperatore R, et al. A re-evaluation of 9-HODE activity at TRPV1 channels in comparison with anandamide: enantioselectivity and effects at other TRP channels and in sensory neurons. Br J Pharmacol. 2012;167:1643–1651. doi: 10.1111/j.1476-5381.2012.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heblinski M, Santiago M, Fletcher C, et al. Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels. Cannabis Cannabinoid Res. 2020;5:305–317. doi: 10.1089/can.2019.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Petrocellis L, Starowicz K, Moriello AS, et al. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 150.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ghovanloo MR, Aimar K, Ghadiry-Tavi R, et al. Physiology and Pathophysiology of Sodium Channel Inactivation. Curr Top Membr. 2016;78:479–509. doi: 10.1016/bs.ctm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 152.Ghovanloo MR, Ruben PC. Cannabidiol and Sodium Channel Pharmacology: General Overview, Mechanism, and Clinical Implications. Neuroscientist. 2022;28:318–334. doi: 10.1177/10738584211017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Catterall WA, Swanson TM. Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Mol Pharmacol. 2015;88:141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jiang D, Shi H, Tonggu L, et al. Structure of the Cardiac Sodium Channel. Cell. 2020;180:122–134.e110. doi: 10.1016/j.cell.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.West JW, Patton DE, Scheuer T, et al. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ghovanloo MR, Shuart NG, Mezeyova J, et al. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 2018;293:16546–16558. doi: 10.1074/jbc.RA118.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sait LG, Sula A, Ghovanloo M-R, et al. Cannabidiol interactions with voltage-gated sodium channels. eLife. 2020;9:e58593. doi: 10.7554/eLife.58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 159.Turkanis SA, Partlow LM, Karler R. Delta-9-tetrahydrocannabinol depresses inward sodium current in mouse neuroblastoma cells. Neuropharmacology. 1991;30:73–77. doi: 10.1016/0028-3908(91)90045-D. [DOI] [PubMed] [Google Scholar]
- 160.Kushner J, Ferrer X, Marx SO. Roles and Regulation of Voltage-gated Calcium Channels in Arrhythmias. J Innov Card Rhythm Manag. 2019;10:3874–3880. doi: 10.19102/icrm.2019.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ali RM, Al Kury LT, Yang KH, et al. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium. 2015;57:290–299. doi: 10.1016/j.ceca.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 162.Oz M, Tchugunova Y, Dinc M. Differential effects of endogenous and synthetic cannabinoids on voltage-dependent calcium fluxes in rabbit T-tubule membranes: comparison with fatty acids. Eur J Pharmacol. 2004;502:47–58. doi: 10.1016/j.ejphar.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 163.Al Kury LT, Voitychuk OI, Yang KH, et al. Effects of the endogenous cannabinoid anandamide on voltage-dependent sodium and calcium channels in rat ventricular myocytes. Br J Pharmacol. 2014;171:3485–3498. doi: 10.1111/bph.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chemin J, Monteil A, Perez-Reyes E, et al. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. Embo J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hille B. Ion channels of excitable membranes. Sunderland, Mass.: Sinauer Sunderland, Mass; 2001. [Google Scholar]
- 166.McCoy MT, Jayanthi S, Cadet JL. Potassium Channels and Their Potential Roles in Substance Use Disorders. Int J Mol Sci. 2021;22:1249. doi: 10.3390/ijms22031249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang HB, Heckman L, Niday Z, et al. Cannabidiol activates neuronal Kv7 channels. Elife. 2022;11 doi: 10.7554/eLife.73246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Isaev D, Shabbir W, Dinc EY, et al. Cannabidiol Inhibits Multiple Ion Channels in Rabbit Ventricular Cardiomyocytes. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.821758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Topal L, Naveed M, Orvos P, et al. The electrophysiological effects of cannabidiol on action potentials and transmembrane potassium currents in rabbit and dog cardiac ventricular preparations. Arch Toxicol. 2021;95:2497–2505. doi: 10.1007/s00204-021-03086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Poling JS, Rogawski MA, Salem N, Jr., et al. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35:983–991. doi: 10.1016/0028-3908(96)00130-X. [DOI] [PubMed] [Google Scholar]
- 171.Bondarenko AI, Panasiuk O, Okhai I, et al. Direct activation of Ca(2+) and voltage-gated potassium channels of large conductance by anandamide in endothelial cells does not support the presence of endothelial atypical cannabinoid receptor. Eur J Pharmacol. 2017;805:14–24. doi: 10.1016/j.ejphar.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Amorós I, Barana A, Caballero R, et al. Endocannabinoids and cannabinoid analogues block human cardiac Kv4.3 channels in a receptor-independent manner. J Mol Cell Cardiol. 2010;48:201–210. doi: 10.1016/j.yjmcc.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 173.Li Q, Ma HJ, Song SL, et al. Effects of anandamide on potassium channels in rat ventricular myocytes: a suppression of I(to) and augmentation of K(ATP) channels. Am J Physiol Cell Physiol. 2012;302:C924–930. doi: 10.1152/ajpcell.00228.2011. [DOI] [PubMed] [Google Scholar]
- 174.Oz M, Yang KH, Dinc M, et al. The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J Pharmacol Exp Ther. 2007;323:547–554. doi: 10.1124/jpet.107.125336. [DOI] [PubMed] [Google Scholar]
- 175.Oliver D, Lien CC, Soom M, et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 176.Van den Bossche I, Vanheel B. Influence of cannabinoids on the delayed rectifier in freshly dissociated smooth muscle cells of the rat aorta. Br J Pharmacol. 2000;131:85–93. doi: 10.1038/sj.bjp.0703521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vignali M, Benfenati V, Caprini M, et al. The endocannabinoid anandamide inhibits potassium conductance in rat cortical astrocytes. Glia. 2009;57:791–806. doi: 10.1002/glia.20807. [DOI] [PubMed] [Google Scholar]
- 178.Maingret F, Patel AJ, Lazdunski M, et al. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. Embo J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gantz SC, Bean BP. Cell-Autonomous Excitation of Midbrain Dopamine Neurons by Endocannabinoid-Dependent Lipid Signaling. Neuron. 2017;93:1375–1387.e1372. doi: 10.1016/j.neuron.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spivak CE, Kim W, Liu Q-R, et al. Blockade of β-cell KATP channels by the endocannabinoid, 2-arachidonoylglycerol. Biochem Bioph Res Co. 2012;423:13–18. doi: 10.1016/j.bbrc.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]