Abstract
The beneficial effects of Prosopis africana (PA) on human health have been demonstrated; however, its protective effects against heavy metals (HM) are not yet understood. This study evaluated the potential neuroprotective effects of PA in the cerebral cortex and cerebellum. To accomplish this, we divided 35 albino Sprague Dawley rats into five groups. Group I did not receive either heavy metal mixture (HMM) or PA. Group II received a HMM of PbCl2 (20 mg/kg), CdCl2 (1.61 mg/kg), HgCl2 (0.40 mg/kg), and NaAsO3 (10 mg/kg) orally for a period of two months. Groups III, IV, and V received HMM along with PA at doses of 500, 1000, and 1500 mg/kg, respectively. PA caused decreased levels of HM accumulation in the cerebral cortex and cerebellum and improved performance in the Barnes maze and rotarod tests. PA significantly reduced levels of IL-6 and TNF-α. PA increased concentrations of SOD, CAT, GSH, and Hmox-1 and decreased the activities of AChE and Nrf2. In addition, levels of MDA and NO decreased in groups III, IV, and V, along with an increase in the number of live neurons. In conclusion, PA demonstrates a complex neuroprotective effect with the potential to alleviate various aspects of HM-induced neurotoxicity.
Keywords: Prosopis africana, heavy metals, neuroprotective effects, cerebral cortex, cerebellum
1. Introduction
Motor and cognitive deficits, along with psychiatric and behavioral disturbances, are among neurophysiological disruptions caused by neurotoxicants [1],[2]. Humans have been exposed to various environmental toxicants, particularly metals and metalloids, since ancient times, and their toxic effects have been extensively investigated and documented [3]. Despite their unprecedented toxicity to the nervous system even at subliminal concentrations, many countries have implemented regulations to control their levels in different environmental matrices, including food, water, air, soil, etc. [4]. However, despite these measures, long-term exposure to neurotoxic metals through food and water consumption, as well as other routes such as occupational inhalation, tobacco smoking, and, more recently, electronic cigarette vaping [5]–[7], still presents a significant public health challenge.
Unlike other nutrients or essential metals, noxious elements have no known biological benefit to humans. These elements are outrightly noxious and pose risks to human health [8]. In humans, Pb, Hg, Cd, and As are absorbed with varying efficiencies, disrupt the blood-brain barrier, and preferentially accumulate in different regions of the brain where they can potentially cause adverse neurotoxic effects even at low concentrations [9].
Accumulating evidence suggests that there is no safe level of lead exposure [2],[10]. Pb exposure occurs through inhalation of lead-contaminated dust particles, consumption of food and water contaminated with lead, and exposure to lead-based paints commonly found in older homes. Chronic lead exposure leads to neurotoxicity, characterized by significant neurological deficits, including impairments in intelligence, memory, processing speed, reading and comprehension, visuospatial skills, and motor skills [8],[11]. Regarding Cd, its long half-life and poor elimination result in a tendency to bioaccumulate in peripheral organs such as the kidneys, lungs, and testes, also being able to reach the central nervous system (CNS). Cd-associated neurotoxicity is linked to neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, Huntington's disease, and multiple sclerosis [12]. Inspiration of contaminated dust, fumes, or mists, as well as ingestion of crops harvested from As-contaminated farmlands, are major sources of human exposure to As. In both humans and animals, As is readily absorbed and tends to bioaccumulate in the brain [13].
Heavy metals, particularly Hg, selectively cause degeneration of the nervous system and are toxic to the cerebral and cerebellar cortex [14]. Previous studies have reported toxicity and tissue bioaccumulation profiles of single heavy metals [15],[16], but human exposure to multiple heavy metals usually occurs concertedly, especially in the coastal regions of Niger Delta Nigeria, where we have reported a cocktail of these metals in vegetables, meat, fish, etc. [17],[18].
Nutraceuticals have demonstrated benefits in the management of non-communicable diseases. Recently, bioactive molecules isolated from plants have been extensively studied for therapeutic research. The benefit of nutraceuticals in preventing metal-induced neurotoxicity has garnered significant support, as various plant extracts have exhibited a range of pharmacological properties [19]. Reports suggest that phytoceuticals and nutraceuticals contain potent ingredients that are used in the management of metal-induced neurodegeneration [20],[21]. These agents have been shown to protect against neuronal injuries, attenuate neurotoxicity, and enhance neuronal survival [22].
The seed of Prosopis africana (PA) is rich in carbohydrates, fiber, protein [23], potassium, and magnesium, with significant amounts of essential amino acids [24],[25]. The fermentation of PA, known as Okpeye, enhances its nutritional value while reducing antinutritional and toxic factors. The PA is widely used in Africa, particularly in Nigeria, as a condiment, flavor enhancer, micronutrient, and protein supplement [26]. Previous studies from our lab demonstrated a significant antioxidant potential of PA [27]. Only recently, hormesis—a process in which small, nontoxic exposure to stress is used to induce adaptive responses that protect biological systems against subsequently large and potentially lethal stresses of the same, similar, or different nature—has generated a lot of interest. The field of hormetic/adaptive responses activated via natural ingredients (polyphenols, flavonoids, etc.) is emerging as a promising field for new nutritional antioxidant strategies in neurodegeneration in animal studies where the vast majority of observations have revealed biphasic dose–response hormetic effects [28],[29].
Oxidative stress plays a prominent role in the neuropathophysiology of various brain-related dysfunctions, particularly neurodegenerative diseases. The nuclear factor-erythroid 2 related factor 2/antioxidant-responsive element Nrf2-ARE system serves as the primary cellular defense against oxidative stress by regulating the expression of antioxidant molecules and enzymes. Dysregulation of this system and the excessive generation of reactive oxygen species (ROS) can damage crucial cellular components, leading to the loss of structural and functional integrity of neurons [30].
The current study aims to evaluate the influence of an aqueous extract of PA on the Nrf2-ARE system in the cerebral cortex and cerebellum of rats exposed to a mixture of heavy metals.
2. Methods
2.1. Materials
2.1.1. Prosopis africana
PA pods were harvested from Nsukka, Enugu State, Nigeria (latitude: 6.857816/N 60°51′28.138″; longitude: 7.411943/E 70°24′42.996″), identified by Mr. Ozioko, Department of Botany, University of Nigeria, Nsukka, washed, sun-dried for three days, and blended to a powdery form. Subsequently, 100 g of the powder was mixed with 1000 mL of deionized water and shaken for 48 h [31]. The slurry was sieved and filtered through a Whatman filter paper No.1 after vigorous shaking of the mixture. The extract was then separated and stored in a refrigerator at 4 °C. The crude extract was partially purified by sequential extraction using different solvents with different (increasing) polarity: petroleum ether, chloroform, ethyl acetate, and finally methanol.
Soxhlet apparatus (temperature 40 °C, time 2–3 h) was used for the fractionation. This was done by tying the dried methanol extract with a muslin cloth and then inserting into the thimble of the Soxhlet apparatus. Methanol was poured into the distillation flask below and allowed to heat at 40 °C. The vapor resulting from the distillation flask condensed in the thimble holder and dissolved the dried crude extract tied inside the thimble. When the condensed methanol in the extraction chamber reached the overflow level, the solution in the thimble holder was aspirated by a siphon and returned to the distillation flask. This was repeated until all soluble compounds in the methanol were extracted. The sample (dried crude extract) was then removed, untied, and air-dried. The methanol extract was concentrated with the rotary evaporator, and the dried residues were subjected to quantitative phytochemical screening. The methanol fraction was subjected to GC–MS analysis.
2.1.2. PA preparation for gas chromatography–mass spectrometry (GC–MS) analysis
Phytochemical analysis of methanol extracts of PA was carried out using Shimadzu GC–MS QP2010 Plus. At the start, the column oven temperature was set at 70 °C and kept for 5 min; thereafter, it was raised to 250 °C at 10 °C/min and held for 10 min. The temperature was subsequently increased to 300 °C at intervals of 10 °C per minute and held for another 10 min. The instrument specifications were as follows: pressure, 110.8 kPa; injection mode, splitless; total flow, 38.9 mL/min; column flow, 1.71 mL/min; solvent cut time, 3.5 min; detector gain mode, relative; injection temperature, 280 °C; purge flow, 3 mL/min; and sample injection volume, 2 µL. The detector operated at a temperature of 320 °C and helium was the carrier gas. Compounds were identified in the samples by comparing the mass spectra with the National Institute of Standards and Technology and Wiley 7 library.
2.2. Experiments
A total of 35 male albino Sprague Dawley rats (n = 35; 90 days old) were sourced from the Department of Toxicology, University of Port Harcourt, Nigeria Animal House. The rats were placed in clean cages with two rats per cage and allowed to acclimate for 14 days under a 12-h reverse light/dark cycle (lights on at 7:00 a.m.). Throughout the acclimatization period and subsequent experimental phases, the rats had access to both feed and water. All experimental procedures were conducted in accordance with the guidelines outlined by the Animal Use and Care Committee at the University of Port Harcourt, Nigeria (UPH/CEREMAD/REC/MM73/014) and were consistent with the principles established in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
For a duration of 60 days, weight-matched Sprague Dawley male albino rats (7 rats per group) were assigned at random to five groups subjected to different treatments. The control group (group 1) received deionized water exclusively; group 2 was administered environmentally relevant doses of a mixture of heavy metals, namely PbCl2 (20 mg/kg), CdCl2 (1.61 mg/kg), HgCl2 (0.40 mg/kg), and NaAsO3 (10 mg/kg), as specified by [32] and [33]. Groups 3, 4, and 5 were also exposed to the metal mixture plus PA at doses of 500 mg/kg, 1000 mg/kg, and 1500 mg/kg, respectively [31]. There were no observable side effects of PA at the doses applied in this study. All treatments were administered through oral gavage for 60 days. The ARRIVE checklist was used to standardize the protocol and ensure transparency.
2.3. Behavioral tests
Each rat was brought from the holding room to the testing room immediately prior to testing. Rats were tested in both the morning (9:00 a.m. to 12:00 p.m.) and afternoon (1:00 p.m. to 4:00 p.m.) in the Barnes maze and rotarod tests. Rats in the rotarod test were tested a total of six times (three times a day over two days); rats in the Barnes maze test were not tested more than once [34]. Behavioral tests were carried out from day 50.
2.3.1. Barnes maze testing
The Barnes maze is a circular white Plexiglas platform (160 cm in diameter) with 18 circular holes and surrounded by a 45 cm high wall [35]. Holes were blocked with mesh but the target hole was left unblocked. A plastic transparent escape cage was placed under the escape hole, filled with bedding from the home cage. The start box was a white, open-ended cylinder, easily lifted from the platform to the roof, approximately 3 m above. The maze was positioned in a room with many extra-maze cues to allow the orientation of rats in the space.
The Barnes maze platform was evenly illuminated (300 lx) by fluorescent lights located on the ceiling. The procedure consisted of habituation trial, acquisition trials, and retention trial [35]. The habituation session started by putting the rats into the transparent escape box (filled with bedding from the home cage) for 120 s. Afterward, the rat was put near the escape hole surrounded by the start box and given 60 s to escape. If the rat did not enter the escape box within that time, it was carefully lifted and guided through the target hole into the escape box. The rat was left in the escape box for 120 s. Finally, the rat was put in the center of the maze and left undisturbed for the following 240 s for it to enter the escape box. If the rat failed to enter the escape box during the assigned time, it was placed into the escape box manually following the aforementioned procedure and kept there for 120 s.
Habituation sessions were separated by a 300-s resting period. In this resting period, the rat remained in its home cage [36]. After two days of habituation bout, rats were trained for five days and thereafter exposed to a retrieval stretch seven days later. Each training or retrieval bout comprised of four consecutive 270-s trials, separated by a 300-s resting period in the rat home cage. At the onset of each trial, each rat was kept in the start box in the center of the maze for 30 s until the trial was commenced by lifting the cylinder; subsequent free maze exploration occurred for the next 240 s. If the rat failed to enter the escape box within the assigned time, it was gently lifted and put in the escape box. The rat was left in the escape box for 120 s, after which it was returned to its home cage for 300 s. The escape hole remained at a constant position throughout all trials and sessions.
The time for locating the target hole was taken as the escape latency and recorded. All behavioral tests were blinded to the treatment groups to minimize bias.
2.3.2. Rotarod test
Rats were trained to walk on the accelerating rotarod (UgoBasile 47600, Milan, Italy). The rotarod comprised a cylinder with a diameter of 3 cm on which five rats could run simultaneously, separated by panels of sufficient size to prevent them from detecting each other visually. The speed of the rod was linearly increased from 4 rpm to 40 rpm for 300 s, after which the rats were returned to their cages. Once the rat was incapable of maintaining its balance and fell off the device, a sensor was triggered, and the time was recorded. The first week of the experiment was used to train the rats in the use of the device and to obtain baseline values. Rats were subjected to the rotarod on a daily basis [37],[38].
2.4. Necropsy, harvesting cerebral cortex and cerebellum, and ELISA assays
Rats were euthanized using pentobarbital anesthesia (IP 50 mg/kg). The brain from each rat was harvested with meticulous isolation of the cerebrum and cerebellar regions. Each of these regions was divided into two portions and stored at −80 °C. One portion was for metal analysis and the other half was used for biochemical analysis.
The cerebral cortex and cerebellum were homogenized separately in 9 volumes of cold phosphate buffer (0.1 M, pH 7.4). The homogenates were then subjected to centrifugation at 3000 rpm for 20 min at 4 °C to remove any nuclear debris. The cerebral cortex and cerebellum lysates were used for the assessments of acetylcholinesterase (AChE), MDA, NO, GSH, GPx, GST, and SOD, as well as ELISA assays targeting TNF-α, IL-6, NF-kB, Nrf2, hmox-1, and Casp-3 previously described in our lab according to manufacturer's instructions [39],[40].
2.5. Antioxidant and oxidative stress markers in the cerebral cortex and cerebellum
Glutathione peroxidase (GPx) activity was measured using the method described by Paglia and Valentine (1967) [41], with absorbance measured at 412 nm. Reduced glutathione (GSH) levels were determined following the procedure outlined by Jollow et al. (1974) [42], with absorbance measured at 412 nm. Glutathione S-transferase (GST) activity was determined using the method of Habig et al. [43], with absorbance measured at 310 nm. Superoxide dismutase (SOD) activity was assessed separately in the cerebral cortex and cerebellum [44]. Catalase (CAT) activity was determined by monitoring the breakdown of H2O2 at 240 nm, following Aebi's method [45]. Lipid peroxidation, evaluated as thiobarbituric acid reactive substances (TBARS), was determined by the adaptation of Esterbauer and Cheeseman's method (1990) [46]. Nitric oxide (NO) was assayed using the Griess reaction technique [47], with absorbance measured at 540 nm against the blank.
2.6. Determination of heavy metals
The harvested cerebral cortex and cerebellum were treated as previously described by Ikpeama et al. 2023 [48] and Pb, Cd, and As were determined with an atomic absorption spectrometer [49].
2.7. Histopathology
After the behavioral test, two rats per group were euthanized with 50 mg/kg pentobarbital IP and perfused with heparinized saline 0.9% solution followed by 4% paraformaldehyde in 0.2 M phosphate buffer. The histopathology of the cerebral cortex and cerebellum was done by a histopathologist who was blinded to the experimental groups. The number of live cells in the cerebral cortex and cerebellum of each group was also quantified with ImageJ version 1.48 software using standard Hounsfield unit ranges by delineating regions of interest (ROI) with a mouse computer interface by using the region selection tools in Image J Menu toolbar.
2.8. Molecular docking
Since the perceived protection from HMM is through a PA effect on acetylcholine esterase (AChE), AChE docking was included. The crystallized structure of AChE with ID: 3M3D was downloaded from the Protein Data Bank. The protein was prepared prior to molecular docking using AutoDock tools; this involved the removal of co-crystallized ligand, addition of charges, polar hydrogen, and grid setup. The 3D structure of resveratrol and galantamine (an FDA-approved AChE inhibitor) was downloaded from Pubchem (SDF file). These SDF files were then converted to PDB files using Pymol software [50]. However, the 3D structure of Model Oris (the modeled compound) was generated and optimized using Molecular Operating Environment (MOE) package (The MOE, 2015), and converted to PDB using Chem 3D software. Molecular docking of the ligand against AChE (target protein) was performed using AutoDock Vina software [51], and the binding affinities/energy were reported in kcal/mol. Resveratrol modeling by scalphold replacement was carried out using MOE package. This involved replacing the non-polar alkene groups linking the two polar ends of resvestarol, thereby optimizing this portion for further interaction within AChE, and hence improving its physiological roles. The model was further docked with the same grid option as in resveratrol and galantamine and the docking scores were reported.
2.9. Statistical analysis
Data were expressed as mean ± standard deviation (SD), with n = 5 except for behavioral studies, where n = 7. Microsoft Xlstat 2014 was used to perform analysis of variance and Tukey's multiple-comparison pairwise tests to check if the concentration of the biomarkers was significantly different between groups. The data analysis involved performing descriptive statistics of the metals and biomarkers concentration before ANOVA was used to establish if there was a significant difference in the concentration of the heavy metals and biomarkers among groups. All significant differences were at a p < 0.05.
3. Results
3.1. Phytoconstituents identified by GC–MS in aqueous extract PA
Table 1 presents the phytoconstituents detected in the water extract of PA; 40% of the constituents are flavonoids, 27% are alkaloids, 13% are polyphenols, 12% are phenolic acids, and 5% are amino acids.
Table 1. Phytoconstituents detected in aqueous extract of Prosopis africana (PA) with chemical characteristics, concentration, and relevant activity.
| No | R. Time (minutes) | Molecular formula | Molecular weight g/mol | Compound name | Compound class | Concentration (ug/g) | Activity |
| 1 | 8.91 | C20H28O5 | 348.40 | Cuhumulone or Cohumulone | Phenolic acid | 3.355 | Antioxidant, anti-inflammatory |
| 2 | 10.65 | C21H30O5 | 362.50 | Humulone | Phenolic acid | 3.786 | Antioxidant, anti-inflammatory |
| 3 | 12.27 | C22H32O5 | 376.50 | Adhumulone | Phenolic acid | 4.470 | Antioxidant, anti-inflammatory |
| 4 | 13.74 | C15H12O2 | 224.25 | Flavonones or flavanones | Flavonoids | 4.143 | Antioxidant, anti-inflammatory |
| 5 | 14.69 | C6H13N3O3 | 175.19 | Citrulline | Amino acid | 4.572 | Improve SOD, CAT, GPx, and NO |
| 6 | 16.04 | C14H12O3 | 228.24 | Resveratrol | Polyphenol | 4.250 | Antioxidant, anti-inflammatory |
| 7 | 17.09 | C15H14O6 | 290.27 | Catechin | Polyphenol | 4.100 | Antioxidant, anti-inflammatory |
| 8 | 18.05 | C15H26N2 | 234.38 | Sparteine | Alkaloid | 7.723 | Bivalent chelator |
| 9 | 19.52 | C15H17NO4 | 275.30 | Ribalindine | Alkaloid | 11.149 | ROS scavenging |
| 10 | 20.59 | C15H14O2 | 226.27 | Flavan-3-ol | Flavonoids | 6.315 | Antioxidant |
| 11 | 21.67 | C15H14O6 | 290.27 | Epicatechin | Polyphenol | 1.372 | Antioxidant |
| 12 | 22.60 | C27H32O14 | 580.50 | Naringin | Flavonoid | 14.462 | Antioxidant, anti-inflammatory |
| 13 | 23.23 | C15H12O5 | 272.25 | Naringenin | Flavonoid | 5.156 | Antioxidant, anti-inflammatory |
| 14 | 23.97 | C31H28O12 | 592.50 | Proanthocyanin | Polyphenol | 2.887 | Antioxidant |
| 15 | 25.09 | C15H10O2 | 222.24 | Flavone | Flavonoids | 4.977 | Antioxidant |
| 16 | 25.62 | C12H20N2O | 208.30 | Ammodendrine | Alkaloid | 3.299 | - |
| 17 | 26.24 | C15H22N2O | 246.35 | Aphyllidine | Alkaloid | 1.037 | - |
| 18 | 26.99 | C11H16N2O | 192.26 | Dohydrocystisine | - | 1.286 | - |
The effect of PA on the body weight and absolute and relative weight of the cerebral cortex and the cerebellum of male albino rats treated with HMM is shown in Table 2. There were no significant differences in the absolute and relative weights of the cerebral cortex and cerebellum between the control and HMM-only exposed rats. Similarly, there were no significant differences in the absolute and relative weights of the cerebral cortex and cerebellum between the HMM-only exposed rats and the HMM-plus-PA co-treated groups. Rats in the different groups showed an increase in body weight, feed intake, and fluid intake.
Table 2. Effect of Prosopis africana (PA) on the body weight and absolute and relative weight of cerebral cortex and cerebellum of male albino rats treated with heavy metal mixture (HMM).
| Treatment |
Cerebral cortex
|
Cerebellum
|
|||||
| *Absolute (g) | *Relative (%) | *Absolute (g) | *Relative (%) | Body weight (g) | Feed intake | Fluid intake | |
| Deionized H2O (only) | 0.91±0.18a | 0.34±1.30 | 0.30±0.04a | 0.11±0.29 | I = 175±4.36a | 164.75±18.80a | 225.08±58.95a |
| F = 271.33±13.8a | |||||||
| %diff. = 55.05 | |||||||
| HMM (only) | 0.82±0.10a | 0.34±0.60 | 0.28±0.07a | 0.12±0.42 | I = 158±2b | 78.78±27.54c | 102.30±20.03d |
| F = 240±16.64ab | |||||||
| %diff. = 51.90 | |||||||
| HMM + 500 mg/kg PA | 0.83±0.03a | 0.36±0.21 | 0.30±0.01a | 0.13±0.07 | I = 151±1c | 88.53±20.90c | 148.10±27.13c |
| F = 230.33±14.57ab | |||||||
| %diff. = 52.54 | |||||||
| HMM + 1000 mg/kg PA | 0.82±0.05a | 0.38±0.18 | 0.27±0.06a | 0.13±0.22 | I = 146±1cd | 130.03±18.48b | 190.20±56.50b |
| F = 213.33±27.15b | |||||||
| %diff. = 46.12 | |||||||
| HMM + 1500 mg/kg PA | 0.78±0.04a | 0.37±0.19 | 0.24±0.02a | 0.12±0.10 | I = 141±1.73d | 158.55±18.4a | 218.31±58.98a |
| F = 208.33±20.82b | |||||||
| %diff. = 47.75 | |||||||
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture. *I = Initial weight, F = Final weight, %diff. = % difference.
Tables 3a and 3b illustrate the effect of PA on the bioaccumulation of As, Pb, and Cd (mg/kg) in the cerebral cortex and cerebellum, respectively, of rats exposed to a heavy metal mixture. There was a significant bioaccumulation of As, Pb, and Cd in the cerebral cortex and cerebellum of HMM-exposed animals. The aqueous extract of PA caused a significant (p < 0.05) decrease in the levels of As, Pb, and Cd in the cerebral cortex and cerebellum of rats.
Table 3a. Effect of Prosopis africana (PA) on the bioaccumulation of As, Pb, and Cd (mg/kg) in the cerebral cortex of rats exposed to heavy metal mixture (HMM).
| Treatment |
Arsenic
|
Lead
|
Cadmium
|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 0.01±0.01a | 0.02±0.01a | 0.01±0.01a |
| HMM | 1.47±0.14b | 3.24±0.16b | 2.75±0.05b |
| HMM + 500 mg/kg PA | 0.77±0.11c | 2.33±0.19c | 1.97±0.09c |
| HMM + 1000 mg/kg PA | 0.76±0.07c | 2.29±0.11c | 1.06±0.05c |
| HMM + 1500 mg/kg PA | 0.14±0.21a | 1.03±0.05a | 0.20±0.03a |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
Table 3b. Effect of Prosopis africana (PA) on the bioaccumulation of As, Pb, and Cd (mg/kg) in the cerebellum of rats exposed to heavy metal mixture (HMM).
| Treatment | Arsenic |
Lead |
Cadmium |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 0.03±0.01a | 0.03±0.01a | 0.03±0.01a |
| HMM | 1.11±0.15b | 4.30±0.16b | 2.75±0.053b |
| HMM + 500 mg/kg PA | 0.96±0.14b | 2.03±0.17c | 2.51±0.32b |
| HMM + 1000 mg/kg PA | 0.46±0.11c | 1.90±0.03c | 1.22±0.07c |
| HMM + 1500 mg/kg PA | 0.10±0.08a | 0.61±0.09a | 0.36±0.11a |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
The performance of rats treated with PA in the rotarod test is presented in Table 4. The control group exhibited a significantly higher latency of fall from the rotating wheel/rod of the rotarod than all the other groups (p < 0.05, df = 4, F = 2.4). The group treated with only the heavy metal mixture showed a significantly lower latency of fall, indicating neuromuscular coordination and balance abnormalities. The groups co-treated with PA had significantly higher latencies of fall compared to the HMM group.
Table 4. Effect of Prosopis africana (PA) on the rotarod and Barnes maze tests (time in seconds).
| Groups | Rotarod test | Barnes maze test |
| Deionized water | 11.00±3.53a | 20.39±2.95a |
| Heavy metals | 6.33±1.01b | 61.29±4.16b |
| HMM + 500 mg/kg PA | 7.50±0.33a | 49.44±6.73c |
| HMM + 1000 mg/kg PA | 8.22±0.63b | 30.11±8.25d |
| HMM + 1500 mg/kg PA | 10.78±3.40c | 25.95±4.01d |
Note: Values are expressed as mean ± SD, n = 7. Different superscripts (a, b, c, d) indicate a statistically significant difference between the means at p < 0.05, while similar superscript letters have no significant difference.
Table 4 also displays the performance of rats treated with PA in the Barnes maze test. The control group had a significantly lower escape latency in locating the hole compared to the group exposed only to the heavy metal mixture (p < 0.05, df = 4, F = 8.81). The groups treated with PA had significantly lower escape latencies compared to the HMM-only group.
The effect of PA on the levels of MDA (µmol/mL) and NO (µM/L) in the cerebral cortex and cerebellum of male albino rats treated with HMM is shown in Table 5. Treatment with the HMM significantly increased both MDA (µmol/mL) and NO (µM/L) levels compared with the control group that received deionized water (p < 0.05). Administration of aqueous PA significantly reduced the concentrations of both MDA (µmol/mL) and NO (µM/L) compared with the rats exposed to HMM alone.
Table 5. Effect of Prosopis Africana (PA) on the MDA (µmol/mL) and NO (µM/L) levels of heavy metal mixtures (HMM)-treated cerebral cortex and cerebellum of male albino rats.
| Treatment |
MDA
|
NO
|
||
| CC | CE | CC | CE | |
| Control | 0.51±0.10a | 0.41±0.13a | 6.55±0.65a | 4.85±1.46a |
| HMM | 0.78±0.03b | 0.75±0.06b | 13.53±3.12b | 14.33±6.93b |
| HMM + 500 mg/kg PA | 0.39±0.10c | 0.46±0.13a | 4.53±1.60c | 3.22±1.00a |
| HMM + 1000 mg/kg PA | 0.50±0.16a | 0.48±0.13a | 7.00±3.30a | 4.49±1.13a |
| HMM + 1500 mg/kg PA | 0.32±0.22c | 0.46±0.01a | 9.87±3.93d | 6.23±0.83c |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
There was a significant decrease (p < 0.05) in the antioxidants SOD, CAT, and GSH in the cerebral cortex and cerebellum of rats exposed only to HMM compared with the control group that received deionized water (Table 6). However, co-treatment with the aqueous extract of PA and HMM caused a significant increase (p < 0.05) in SOD, CAT, and GSH levels in the cerebral cortex and cerebellum. Co-treatment with the aqueous extract of PA and HMM did not result in a significant decrease in GPx levels in the cerebral cortex and cerebellum (Table 6).
Table 6. Effect of Prosopis africana (PA) on antioxidants [SOD (U/mg), GPx (IU), CAT (mM), GSH (µmol/mL)] in the cerebral cortex and cerebellum of HMM-treated male albino rats.
| Treatment |
SOD
|
GPx
|
CAT
|
GSH
|
||||
| CC | CE | CC | CE | CC | CE | CC | CE | |
| Control | 0.30±0.10a | 0.28±0.02a | 0.11±0.01a | 0.09±0.01a | 2.32±0.24a | 1.24±0.99a | 1.17±0.23a | 1.43±0.48a |
| HMM | 0.12±0.01b | 0.13±0.03b | 0.08±0.01a | 0.07±0.01a | 0.68±0.21b | 0.76±0.24b | 0.91±0.12b | 0.64±0.10b |
| HMM + 500 mg/kg PA | 0.42±0.14c | 0.33±0.12a | 0.08±0.01a | 0.07±0.01a | 3.42±0.15c | 4.13±1.06c | 1.15±0.07a | 1.05±0.09a |
| HMM + 1000 mg/kg PA | 0.35±0.11a | 0.35±0.05a | 0.09±0.02a | 0.09±0.01a | 3.99±0.17c | 3.95±0.86c | 1.23±0.22a | 1.36±0.26a |
| HMM + 1500 mg/kg PA | 0.50±0.15b | 0.37±0.12c | 0.09±0.01a | 0.08±0.01a | 3.96±0.76c | 3.41±1.23c | 1.40±0.18c | 1.15±0.08a |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
Table 7 illustrates the effect of PA on the pro-inflammatory cytokines (pg/mL) in the cerebral cortex and cerebellum of male albino rats treated with a heavy metal mixture. Treatment with the heavy metal mixture alone caused a significant increase (p < 0.05) in IL-6 and TNF-α levels compared with the control group. However, administration of the aqueous extract of PA resulted in a dose-dependent and significant decrease (p < 0.05) in IL-6 and TNF-α levels compared with rats exposed to the HMM alone.
Table 7. Effect of Prosopis africana (PA) on the pro-inflammatory cytokines (pg/mL) in the cerebral cortex and cerebellum of HMM-treated male albino rats.
| Treatment |
IL-6
|
TNF-α | ||
| CC | CE | CC | CE | |
| Control | 41.90±8.48a | 23.63±6.30a | 34.53±7.4a | 32.40±5.82a |
| HMM | 68.03±8.72b | 50.70±7.83b | 68.03±6.56b | 56.53±8.89b |
| HMM + 500 mg/kg PA | 67.67±8.82b | 57.43±5.91c | 34.80±6.13a | 48.13±5.62c |
| HMM + 1000 mg/kg PA | 54.23±4.67c | 53.33±7.42c | 44.10±4.10c | 42.00±2.29c |
| HMM + 1500 mg/kg PA | 31.53±4.24d | 44.73±8.10d | 30.93±7.5d | 17.40±6.02d |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c, d) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
The effect of aqueous PA on the levels of Nrf2 (pg/mL), Nf-kb (pg/mL), and Hmox-1 (pg/mL) in the cerebral cortex and cerebellum of male albino rats treated with the heavy metal mixture is presented in Table 8. Treatment with the heavy metal mixture alone caused a significant increase (p < 0.05) in Nrf2 levels, a significant decrease (p < 0.05) in Hmox-1 levels, and a non-significant increase in Nf-kb levels. Co-treatment with HMM + PA at doses of 500, 1000, and 1500 mg/kg significantly decreased Nrf2 levels in the cerebral cortex, whereas co-treatment with HMM + PA at doses of 1000 and 1500 mg/kg significantly decreased Nrf2 levels in the cerebellum compared to the HMM-only group. There was a significant increase (p < 0.05) in Hmox-1 levels after co-administration of HMM + PA.
Table 8. Effect of Prosopis africana (PA) on the Nrf2 (pg/mL), Nfkb (pg/mL), and Hmox-1 (pg/mL) in the cerebral cortex and cerebellum of HMM-treated male albino rats.
| Treatment |
Nrf2
|
NF-Kb
|
Hmoxl
|
|||
| CC | CE | CC | CE | CC | CE | |
| Control | 7.27±0.29a | 6.53±0.99a | 0.07±0.01a | 0.08±0.00a | 1.24±0.13c | 0.93±0.64a |
| HMM | 12.03±1.63b | 11.93±1.06b | 0.09±0.01a | 0.09±0.02a | 0.57±0.37b | 0.65±0.05b |
| HMM + 500 mg/kg PA | 10.77±0.59b | 11.27±1.32b | 0.12±0.01a | 0.12±0.03a | 0.85±0.30c | 0.67±0.03a |
| HMM + 1000 mg/kg PA | 8.90±0.17c | 7.50±0.95c | 0.09±0.01a | 0.09±0.03a | 0.99±0.36c | 0.70±0.18a |
| HMM + 1500 mg/kg PA | 7.83±0.58c | 7.80±0.36c | 0.11±0.03a | 0.10±0.00a | 1.07±0.20c | 0.70±0.18a |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
The effect of the aqueous extract of PA on caspase 3 levels in the cerebral cortex and cerebellum of male albino rats treated with a heavy metal mixture is shown in Table 9. Treatment with the heavy metal mixture alone caused a significant increase (p < 0.05) in the level of caspase 3 in the cerebellum compared to the control group. Co-treatment of HMM with the aqueous extract of PA resulted in a significant decrease (p < 0.05) in the level of caspase 3 compared to the HMM-only treated group. However, the administration of the aqueous extract of PA did not show a significant (p > 0.05) reduction in the level of caspase 3 in the cerebral cortex of HMM-exposed rats.
Table 9. Effect of Prosopis africana (PA) on Caspase 3 (µmol/mL) in the cerebral cortex and cerebellum of HMM-treated male albino rats.
| Treatment |
Caspase 3
|
|
| CC | CE | |
| Control | 0.52±0.16a | 0.21±0.07a |
| HMM | 0.58±0.17a | 0.64±0.16b |
| HMM + 500 mg/kg PA | 0.59±0.21a | 0.50±0.02c |
| HMM + 1000 mg/kg PA | 0.55±0.18a | 0.52±0.12c |
| HMM + 1500 mg/kg PA | 0.50±0.27a | 0.44±0.08c |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
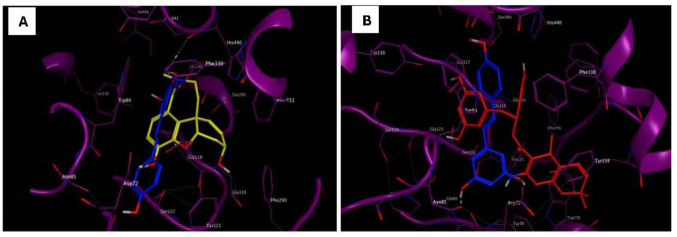
3.1.1. Molecular docking
Several inhibitors of AChE are composed mainly of two pharmacophores joined via an appropriate group or chain. During catalysis, these pharmacophores/polar groups bind simultaneously to the catalytic and peripheral sites of AChE, which are approximately 14 Å from each other, found at the top and the bottom of the AChE gorge (Figure 1). Resveratrol presented, in the structure displays, various forms of interactions (hydrophobic) with a binding affinity of −9.0 kcal/mol, which is responsible for its AChE inhibitory activity. The molecular docking study showed that the hydroxyl group attached to the benzene ring of resveratrol present at both ends formed a hydrogen bond with the carboxylic group of Glu199 in the peripheral pocket and the OH group at the backbone of Tyr70 at the other end, enabling the strengthening of its binding affinity; the rest of resveratrol formed hydrophobic interactions with Ser122, Tyr121, Phe330, and Trp84. Galantamine (standard drug) was also found to be interacting with similar residues at the active site, though with a lesser binding affinity of −8.9 kcal/mol, implying the therapeutic potential of resveratrol as a AChE inhibitor.
Figure 1. (a) Superimposed view of the active-site interaction of resveratrol (blue color) and galantamine (in yellow). (b) Superimposed view of the active-site interaction of resveratrol (blue color) and Model Oris (in red).
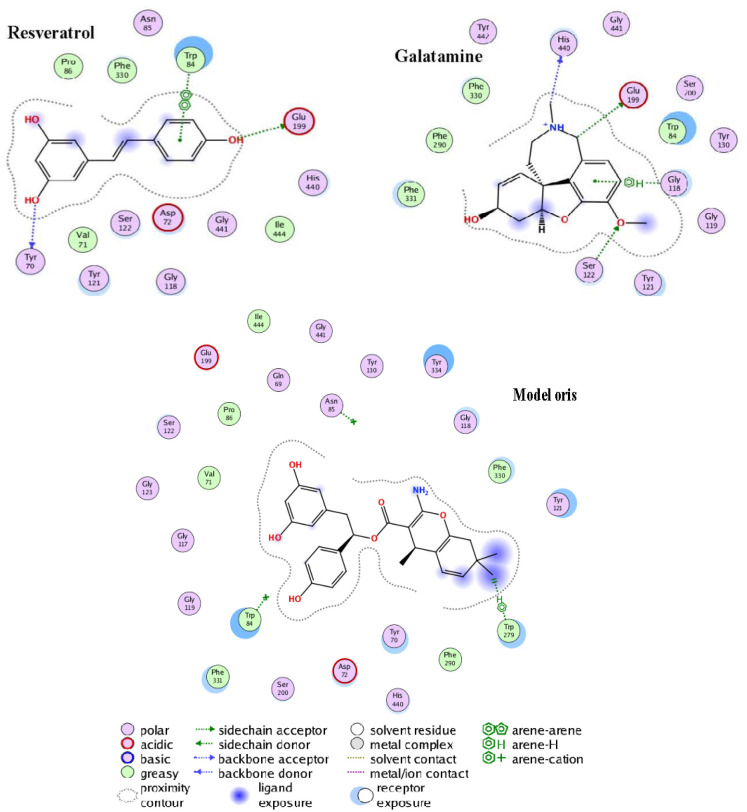
However, an active-site view of the resveratrol–AChE complex revealed possible points of optimization in resveratrol in order to boost its therapeutic benefits (Figure 2). Due to the non-polar alkene group joining both reactive ends of resveratrol, there was no interaction at this region. A fragment modeling through scaffold replacement of these groups was performed using MOE, 2015 while placing a pharmacophore filter to keep key interactions prior to scalphold replacement search. Over 22,856 fragments of replaceable linkers (scaffolds) were generated, from which the best was picked (Model Oris). Molecular docking study of Model Oris against AChE gave a binding affinity of −10.6 kcal/mol, much higher than resveratrol and galantamine. This could be because of more interactions initiated at AChE active sites (Try334, Gln69, His440, Try84, and Ser200) following resveratrol modeling.
Figure 2. Acetylcholine esterase (AChE) active site interactions (2D diagram) docked compounds showing amino acid residues forming major interactions. Hydrogen-bonding interactions of ligands and residues are shown in a green line. More details of the interactions are shown in the attached legend.
Table 10 presents the effect of the aqueous extract of PA on AChE activity in the cerebral cortex and cerebellum of male albino rats treated with a heavy metal mixture. Administration of the heavy metal mixture alone showed a significant increase (p < 0.05) in AChE activity in the cerebral cortex (237.40 ± 159.35) and cerebellum (294.30 ± 135.71) compared to the control group (32.40 ± 3.24 and 66.67 ± 14.44 in the cerebral cortex and cerebellum, respectively). Co-treatment of HMM with the aqueous extract of PA caused a significant reduction in AChE activity in both the cerebral cortex and cerebellum compared to the HMM-only treated group.
Table 10. Effect of Prosopis africana (PA) on the AChE (µmol/mL) activity in the cerebral cortex and cerebellum of HMM-treated male albino rats.
| Treatment |
AChE
|
|
| CC | CE | |
| Control | 32.40±3.24a | 66.67±14.44a |
| HMM | 237.40±159.35b | 294.30±135.71b |
| HMM + 500 mg/kg PA | 130.23±175.89c | 85.90±8.00d |
| HMM + 1000 mg/kg PA | 119.50±104.55c | 101.13±17.75c |
| HMM + 1500 mg/kg PA | 82.53±47.91d | 104.27±40.64c |
*Values = mean ± SD, n = 5, data with different superscripts (a, b, c) are significantly different from each other (p < 0.05), data with the same superscripts are not significantly different; HMM: heavy metal mixture.
3.2. Histopathology
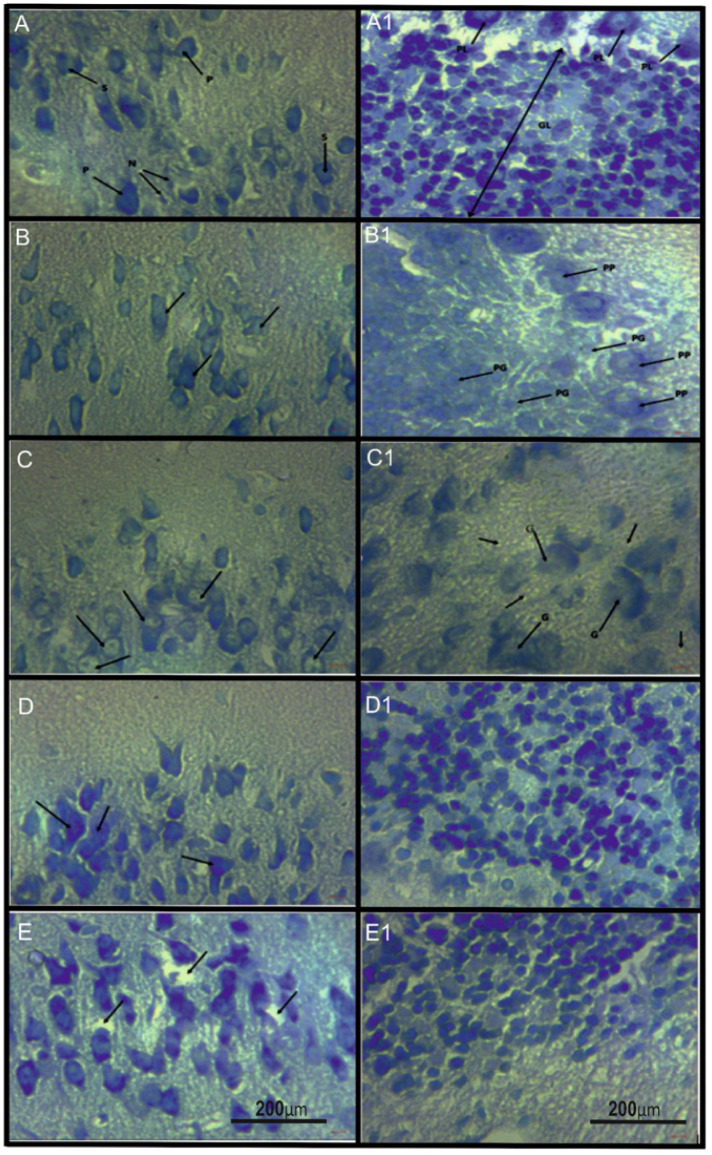
Photomicrographs (×400, toluidine blue) (Figure 3A–E) of the cerebral cortex are shown. The first image displays normal neuronal cells, indicating their healthy state. In the second image, cell loss is observed in the outer granular layer, accompanied by abnormal neuronal cell morphology (arrow). The third image reveals cell loss and diffuse neuronal cell cytoplasmic vacuolation. The fourth image displays the outer granular layer containing numerous normal neuronal cells (arrows). Finally, the fifth image (GRP 5 HMM + 1500 mg/kg PA CC ×400) exhibits restored neurons with minimal neutrophil vacuolation (arrows) in the outer granular layer.
Figure 3. Histopathological findings in five examined groups of animals (A–E: cerebral cortex; A1–E1: cerebellum).
In Figures 3A1–E1, photomicrographs (×400, toluidine blue) of the cerebellum are presented. The first image depicts the normal cerebellar architecture, showcasing the Purkinje cell (PL) and granule cells in the granular layer. In the second image, multiple pyknotic Purkinje cells (PP) and granule cells (PG) are observed. The third image reveals moderate cell loss with hypertrophy of scanty cells (G) and diffuse neutrophil vacuolation (arrows). The fourth image displays a restored density of granular cells in the cerebellum. Lastly, the fifth image exhibits restored cells in the cerebellum.
4. Discussion and conclusion
This study evaluated the behavioral changes induced by exposure to a quaternary metal mixture in rats, as well as the potential protective effects of the aqueous extract from Prosopis africana (PA) seeds. The findings confirm that chronic exposure to the quaternary metal mixture has a negative impact on memory retention and balance, as assessed by the Barnes maze test and the rotarod test, respectively. These effects may be attributed to the ability of the metal mixture to interfere with downstream effector molecules that are essential for long-term potentiation. Impairment of these molecules could lead to memory loss and neurobehavioral changes [52],[53]. The parasympathetic neurotransmitter acetylcholine is crucial for learning and memory [54]. The neurotransmitter acetylcholine, which plays a crucial role in learning and memory [54], is affected by the metal mixture. The primary degradation enzyme of acetylcholine, acetylcholinesterase (AChE), is widely recognized as an indicator of damage to cholinergic neurons in the brain cleft [55]. Furthermore, lead (Pb) interferes with neurotransmitter release and disrupts the functions of cholinergic systems and gamma-aminobutyric acid (GABA) signaling. It also inhibits N-methyl D-aspartate (NMDA) ion channels [2]. Although no significant differences were observed in the absolute and relative weights of the cerebral cortex and cerebellum between the control group exposed to deionized water and the group exposed only to the quaternary metal mixture, as well as between the group exposed only to the metal mixture and the group co-treated with the metal mixture and PA extract, significant memory and motor impairments were detected in the rats exposed to the metal mixture in the present study. This was evident from the results of the Barnes maze and rotarod performance tests, respectively. Both impairments were significantly reversed by the administration of PA. Moreover, the considerable bioaccumulation of As, Pb, and Cd in the cerebral cortex and cerebellum of the rats exposed to the metal mixture was significantly reduced in a dose-dependent manner by the co-administration of PA extract. Therefore, it is reasonable to suggest that these observations can be attributed, at least in part, to the beneficial effects of PA in reversing the memory and motor impairments caused by the metal mixture.
Oxidative stress plays a significant role in the process of tissue damage. It occurs when an imbalance arises between the production of reactive oxygen species (ROS) and the body's ability to neutralize or repair their harmful effects [56]. Over time, persistent oxidative stress can contribute to different organs toxicity. Managing oxidative stress and bolstering antioxidant defenses are essential strategies in preventing and mitigating tissue damage and its associated health consequences [57].
Some bioactive compounds of PA have been reported to have antioxidant and anti-inflammatory properties [58],[59]. Resveratrol, one of the active ingredients of PA has been demonstrated to inhibit MDA and IL-1β levels, while improving the levels of SOD, CAT, GSH, and GPx [60]. Catechin, a major component of tea, exists in various forms such as epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate. These forms possess potent antioxidant properties that can effectively neutralize ROS and RNS, thereby contributing to their antioxidant and anti-inflammatory effects [61]. Epicatechins, polyphenolics found in PA, possess both antioxidant and anti-inflammatory properties [62],[63].
Our study demonstrated that exposure to a quaternary metal mixture significantly increased the activity of both cortical and cerebellar acetylcholinesterase (AChE) in rats. This increased AChE activity might be attributed to allosteric interactions between metallic cations and the anionic sites of AChE in the brain [64]. Antioxidant and anti-inflammatory flavonoids and other polyphenols found in PA have shown efficacy in the treatment of Alzheimer's disease by targeting specific enzymes like AChE [65],[66]. The flavonoid quercetin 3-(20,60-diacetylglucoside) has been reported to inhibit AChE activity [67]. Additionally, in another study, the significant reduction in AChE activity following treatment with the methanolic extract of H. abyssinica was attributed to its phenolic and flavonoid content [68]. From the molecular docking study, Model Oris could be further investigated through molecular dynamics simulation and clinical trials as an inhibitor of AChE in the treatment of AChE-related disease conditions.
Furthermore, metals and metalloids can induce oxidative stress in neurons, acting as pro-oxidants that increase amounts of lipid peroxide in the brain [69]. The present study revealed significantly elevated levels of MDA and NO in animals exposed to the quaternary mixture, which were effectively reversed by co-administration of the aqueous extract of PA. This counteractive effect on oxidative stress can be ascribed to the extract's rich contents of antioxidant phenolics and flavonoids [70]. Polyphenols have demonstrated the ability to protect rats from metal-induced brain neuroinflammation and cognitive impairments [71],[72].
Metals induce expression of tumor necrosis factor alpha (TNFα), a marker of glial inflammation, in the cerebellum following sub-chronic exposure to mercury [8]. Pro-inflammatory cytokines like TNF-α, IL-1, and apoptotic factor caspase-3 are crucial in neurodegeneration and apoptosis [73],[74]. In vitro and in vivo studies have shown that inorganic arsenic (As) causes oxidative stress, leading to decreased neurite outgrowth and, at higher doses, neuronal cell death [75]. However, co-exposure to antioxidants can alleviate As-mediated oxidative stress [76]–[78]. In this study, treatment with the aqueous extract of PA demonstrated a decrease in pro-inflammatory cytokines, decreased cortical apoptotic factor caspase-3, and the restoration of normal histological appearance in the cerebral cortex and cerebellum compared to rats treated with the quaternary metal mixture alone. These observations are like the restoration of normal expression of the neuronal pro-survival protein Bcl2 and downregulation of the pro-apoptotic protein Bax elicited by H. abyssinica of animals treated with AlCl3 may be mediated by the neuroprotective flavonoid and phenolics of Prosopis Africana PA [50],[79].
Nutraceuticals are known to scavenge ROS and ultimately alleviate cellular injury caused by oxidative stress [80],[81]. Additionally, many nutraceuticals possess a pharmacological modulatory potential that can co-activate the Nrf2-ARE antioxidant defense and neurotrophin signaling-mediated cell survival systems [82]–[85]. Therefore, bioactive compounds in nutraceuticals may hold therapeutic potential in the management of neurodegenerative diseases.
In the current study, exposure to the quaternary metal mixture resulted in a significant increase in Nrf2 levels and a significant decrease in hmox-1 levels in the cerebral cortex and cerebellum. However, co-administration of HMM with 1000 mg/kg and 1500 mg/kg of PA extract led to a significant decrease in Nrf2 levels in these brain regions. The activation of the Nrf2-ARE pathway upregulates a network of cooperating enzymes that constitute an antioxidant defense system [86],[87], which maintains redox homeostasis by involving antioxidant synthesis and metabolism and iron homeostasis, including Hmox-1. Hmox-1 overexpression has been observed in neurodegenerative diseases, potentially serving as a compensatory mechanism against oxidative damage [88],[89]. This study has demonstrated remarkable changes in SOD, CAT, and GSH levels following exposure to the metal mixture, which were subsequently reversed by co-treatment with aqueous extract of PA. Natural plants such as Prosopis africana are known to display hormetic biphasic dose–response effects, with low-dose aqueous extracts of PA activating or stimulating intracellular Nrf2 antioxidant pathway for neuroprotection to environmental toxicants (heavy metals); at the same time, high doses of polyphenols can be toxic and inhibit these protective pathways. This is in line with the results of the study, indicating that a dose of 500 mg/kg of PA is protective against heavy metals by a significant increase in the Nrf2 level in the cortex and cerebellum of rats, while doses of 1000 or 1500 mg/kg induce a downregulation of Nrf2 levels.
The histopathological findings supported these biochemical parameters, further confirming the potential benefits of PA extract in mitigating neurotoxicity induced by the metal mixture in rats. Collectively, PA extract may exert neuroprotective activity against memory impairment induced by the quaternary metal mixture through the modulation of the oxido-inflammatory response through the Nrf2 signaling pathway. To demonstrate the increased trafficking of Nrf2 from the cytosol to the nucleus, immunoblotting will be done in future studies. Furthermore, the relevant mechanisms behind the decrease in heavy metal content in target organs following treatment with PA should be explored in future studies. Perhaps a weakness of the present study is the lack of mercury data, which will also be addressed in future studies.
Acknowledgments
None.
Footnotes
Ethical approval: All procedures involving animals and the experimental protocol followed guidelines for the safe use of animals in research and were approved by the University of Port Harcourt animal research committee (UPH/CEREMAD/REC/MM73/014).
Consent to Participate: Not applicable.
Consent to Publish: All authors have given their consent for publication.
Funding: None.
Conflicts of interests: Authors confirm that there was no conflict of interest.
Availability of data and materials: All data have been provided.
References
- 1.Bjørklund G, Chartrand MS, Aaseth J. Manganese exposure and neurotoxic effects in children. Environ Res. 2017;155:380–384. doi: 10.1016/j.envres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014;2014:840547. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florea AM, Büsselberg D. Occurrence, use and potential toxic effects of metals and metal compounds. Biometals. 2006;19:419–427. doi: 10.1007/s10534-005-4451-x. [DOI] [PubMed] [Google Scholar]
- 4.Jaishankar M, Tseten T, Anbalagan N, et al. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aherrera A, Olmedo P, Grau-Perez M, et al. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ Res. 2017;159:313–320. doi: 10.1016/j.envres.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Olmedo P, Goessler W, Tanda S, et al. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ Health Perspect. 2018;126:027010. doi: 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D, Navas-Acien A, Ilievski V, et al. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ Res. 2019;174:125–134. doi: 10.1016/j.envres.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gade M, Comfort N, Re DB. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res. 2021;201:111558. doi: 10.1016/j.envres.2021.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/S0041-008X(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh G, Singh V, Sobolewski M, et al. Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front Genet. 2018;9:89. doi: 10.3389/fgene.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 12.Aliomrani M, Sahraian MA, Shirkhanloo H, et al. Correlation between heavy metal exposure and GSTM1 polymorphism in Iranian multiple sclerosis patients. Neurol Sci. 2017;38:1271–1278. doi: 10.1007/s10072-017-2934-5. [DOI] [PubMed] [Google Scholar]
- 13.Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin-Zaki L, Elhassani S, Majeed MA, et al. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974;54:587–595. doi: 10.1542/peds.54.5.587. [DOI] [PubMed] [Google Scholar]
- 15.Fløtre CH, Varsi K, Helm T, et al. Predictors of mercury, lead, cadmium and antimony status in Norwegian never-pregnant women of fertile age. 2017;12:e0189169. doi: 10.1371/journal.pone.0189169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Zhong H, Guo Z, et al. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res. 2014;160:437–444. doi: 10.1007/s12011-014-0057-x. [DOI] [PubMed] [Google Scholar]
- 17.Okoye EA, Bocca B, Ruggieri F, et al. Metal pollution of soil, plants, feed and food in the Niger Delta, Nigeria: Health risk assessment through meat and fish consumption. Environ Res. 2021;198:111273. doi: 10.1016/j.envres.2021.111273. [DOI] [PubMed] [Google Scholar]
- 18.Okoye EA, Bocca B, Ruggieri F, et al. Arsenic and toxic metals in meat and fish consumed in Niger delta, Nigeria: Employing the margin of exposure approach in human health risk assessment. Food Chem Toxicol. 2022;159:112767. doi: 10.1016/j.fct.2021.112767. [DOI] [PubMed] [Google Scholar]
- 19.Abbaoui A, Chatoui H, El Hiba O, et al. Neuroprotective effect of curcumin-I in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson's disease. Neurosci Lett. 2017;660:103–108. doi: 10.1016/j.neulet.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15:1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 21.Moosmann B, Behl C. Dietary phenols: antioxidants for the brain? Nutr Neurosci. 2000;3:1–10. doi: 10.1080/1028415X.2000.11747298. [DOI] [PubMed] [Google Scholar]
- 22.Li H-w, Lan T-j, Yun C-x, et al. Mangiferin exerts neuroprotective activity against lead-induced toxicity and oxidative stress via Nrf2 pathway. Chin Herb Med. 2020;12:36–46. doi: 10.1016/j.chmed.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falade KO, Akeem SA. Physicochemical properties, protein digestibility and thermal stability of processed African mesquite bean (Prosopis africana) flours and protein isolates. J Food Meas Charact. 2020;14:1481–1496. doi: 10.1007/s11694-020-00398-0. [DOI] [Google Scholar]
- 24.Aremu M, Awala E, Opaluwa O, et al. Effect of processing on nutritional composition of African locust bean (Parkia biglobosa) and mesquite bean (Prosopis africana) seeds. Commun Appl Sci. 2015;3 [Google Scholar]
- 25.Aremu M, Olonisakin A, Atolaye B, et al. Some nutritional composition and functional properties of Prosopis africana. Bangladesh J Sci Ind Res. 2007;42:269–280. doi: 10.3329/bjsir.v42i3.665. [DOI] [Google Scholar]
- 26.Keay R. Clarendon. Oxford: Oxford University) Press; 1989. Trees of Nigeria. [Google Scholar]
- 27.Ozoani H, Ezejiofor AN, Okolo KO, et al. Ameliorative Effects of Zn and Se Supplementation on Heavy Metal Mixture Burden via Increased Renal Metal Excretion and Restoration of Redoxo-Inflammatory Alterations. Biol Trace Elem Res. 2024;202:643–658. doi: 10.1007/s12011-023-03709-w. [DOI] [PubMed] [Google Scholar]
- 28.Murakami A. Novel mechanisms underlying bioactivities of polyphenols via hormesis. Curr Opinion Toxicol. 2022;30:100337. doi: 10.1016/j.cotox.2022.02.010. [DOI] [Google Scholar]
- 29.Murakami A. Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Curr Opinion Biotechnol. 2024;86:103074. doi: 10.1016/j.copbio.2024.103074. [DOI] [PubMed] [Google Scholar]
- 30.Hannan MA, Dash R, Sohag AAM, et al. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front Mol Neurosci. 2020;13:116. doi: 10.3389/fnmol.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezike AC, Akah PA, Okoli CO, et al. Medicinal Plants Used in Wound Care: A Study of Prosopis africana (Fabaceae) Stem Bark. Indian J Pharm Sci. 2010;72:334–339. doi: 10.4103/0250-474X.70479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anyanwu BO, Orish CN, Ezejiofor AN, et al. Neuroprotective effect of Costus afer on low dose heavy metal mixture (lead, cadmium and mercury) induced neurotoxicity via antioxidant, anti-inflammatory activities. Toxicol Rep. 2020;7:1032–1038. doi: 10.1016/j.toxrep.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messarah M, Klibet F, Boumendjel A, et al. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol. 2012;64(3):167–174. doi: 10.1016/j.etp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Tarantino LM, Gould TJ, Druhan JP, et al. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome. 2000;11:555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- 35.Popović N, Madrid JA, Rol MÁ, et al. Barnes maze performance of Octodon degus is gender dependent. Behav Brain Res. 2010;212:159–167. doi: 10.1016/j.bbr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Popović N, Baño-Otalora B, Rol MÁ, et al. Effects of long-term individual housing of middle-aged female Octodon degus on spatial learning and memory in the Barnes maze task. Front Behav Neurosci. 2023;17:1221090. doi: 10.3389/fnbeh.2023.1221090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg R, Laman JD, van Meurs M, et al. Rotarod motor performance and advanced spinal cord lesion image analysis refine assessment of neurodegeneration in experimental autoimmune encephalomyelitis. J Neurosci Meth. 2016;262:66–76. doi: 10.1016/j.jneumeth.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Pritchett K, Mulder GB. The rotarod. J Am Assoc Lab Anim. 2003;42:49–49. [PubMed] [Google Scholar]
- 39.Eddie-Amadi BF, Ezejiofor AN, Orish CN, et al. Zn and Se abrogate heavy metal mixture induced ovarian and thyroid oxido-inflammatory effects mediated by activation of NRF2-HMOX-1 in female albino rats. Curr Res Toxicol. 2023;4:100098. doi: 10.1016/j.crtox.2022.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eddie-Amadi BF, Ezejiofor AN, Orish CN, et al. Zinc and selenium mitigated heavy metals mixture (Pb, Al, Hg and Mn) mediated hepatic-nephropathy via modulation of oxido-inflammatory status and NF‑kB signaling in female albino rats. Toxicology. 2022;481:153350. doi: 10.1016/j.tox.2022.153350. [DOI] [PubMed] [Google Scholar]
- 41.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 42.Jollow D, Mitchell J, Zampaglione Na, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 43.Wh H. Glutathione S-transferase. J Biol Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 44.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 45.Bergmeyer HU, Bernt E. Methods of enzymatic analysis. Elsevier; 1974. UV-assay with pyruvate and NADH; pp. pp. 574–579. [DOI] [Google Scholar]
- 46.Esterbauer H, Cheeseman KH. Methods in enzymology. Elsevier; 1990. [42] Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal; pp. pp. 407–421. [DOI] [PubMed] [Google Scholar]
- 47.Sosroseno W, Sugiatno E, Samsudin AR, et al. The role of nitric oxide on the proliferation of a human osteoblast cell line stimulated with hydroxyapatite. Jb Oral Implantol. 2008;34:196–202. doi: 10.1563/0.910.1. [DOI] [PubMed] [Google Scholar]
- 48.Ikpeama EU, Orish CN, Ezejiofor AN, et al. Essential Trace Elements Prevent the Impairment in the Retention Memory, Cerebral Cortex, and Cerebellum Damage in Male Rats Exposed to Quaternary Metal Mixture by Up-regulation, of Heme Oxygynase-1 and Down-regulation of Nuclear Factor Erythroid 2-related Factor 2-NOs Signaling Pathways. Neuroscience. 2023;512:70–84. doi: 10.1016/j.neuroscience.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Okoye EA, Ezejiofor AN, Nwaogazie IL, et al. Heavy metals and arsenic in soil and vegetation of Niger Delta, Nigeria: Ecological risk assessment. Case Studies in Chemical and Environmental Engineering. 2022;6:100222. doi: 10.1016/j.cscee.2022.100222. [DOI] [Google Scholar]
- 50.Doungue HT, Kengne APN, Kuate D. Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract, and juice in aluminum chloride-induced Alzheimer's disease rats. Nutrire. 2018;43:1–12. doi: 10.1186/s41110-018-0082-1. [DOI] [Google Scholar]
- 51.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed MQ, Alenazi FS, Fazaludeen MF, et al. Pathology and Management of Alzheimer's disease: A review. Int J Pharm Res Alli. 2018;7 [Google Scholar]
- 53.Colizzi C. The protective effects of polyphenols on Alzheimer's disease: a systematic review. Alzh Dement-TRCI. 2019;5:184–196. doi: 10.1016/j.trci.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu Z, Zhang J, Yang H, et al. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiol Behav. 2016;154:114–125. doi: 10.1016/j.physbeh.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afzal S, Abdul Manap AS, Attiq A, et al. From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol. 2023;14:1269581. doi: 10.3389/fphar.2023.1269581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu B, Ouyang Y, Zhao T, et al. Antioxidant Hydrogels: Antioxidant Mechanisms, Design Strategies, and Applications in the Treatment of Oxidative Stress-Related Diseases. Adv Healthc Mater. 2024;2303817 doi: 10.1002/adhm.202303817. [DOI] [PubMed] [Google Scholar]
- 58.Oluwafemi R, Agubosi O, Alagbe J. Proximate, minerals, vitamins and amino acid composition of Prosopis africana (African mesquite) seed oil. Asian J Adv Res. 2021;4:1011–1017. [Google Scholar]
- 59.Alagbe JO, Agubosi OC, Oluwafemi RA. Histopathology of broiler chickens fed diets supplemented with Prosopis africana (African mesquite) essential oil. Brazilian J Sci. 2023;2:49–59. doi: 10.14295/bjs.v2i9.385. [DOI] [Google Scholar]
- 60.Hu H-C, Lei Y-H, Zhang W-H, et al. Antioxidant and Anti-inflammatory Properties of Resveratrol in Diabetic Nephropathy: A Systematic Review and Meta-analysis of Animal Studies. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.841818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial Properties of Green Tea Catechins. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tvrda E, Straka P, Galbavy D, et al. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules. 2019;24 doi: 10.3390/molecules24183226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu Z, Liu A, Li P, et al. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit Rev Food Sci. 2021;61:211–233. doi: 10.1080/10408398.2020.1723057. [DOI] [PubMed] [Google Scholar]
- 64.Auti ST, Kulkarni YA. Neuroprotective Effect of Cardamom Oil Against Aluminum Induced Neurotoxicity in Rats. Front Neurol. 2019;10:399. doi: 10.3389/fneur.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Hawary SS, Sobeh M, Badr WK, et al. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J Biol Sci. 2020;27:2829–2838. doi: 10.1016/j.sjbs.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elsawi SA, Aly HF, Elbatanony MM, et al. Phytochemical evaluation of Lagerstroemia indica (L.) Pers leaves as anti-Alzheimer's. J Mater Environ Sci. 2018;9:2575–2586. [Google Scholar]
- 67.Olennikov DN, Kashchenko NI, Chirikova NK, et al. Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int J Mol Sci. 2017;18:1685. doi: 10.3390/ijms18081685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anwar HM, Georgy GS, Hamad SR, et al. A leaf extract of harrisonia abyssinica ameliorates neurobehavioral, histological and biochemical changes in the hippocampus of rats with aluminum chloride-induced alzheimer's disease. Antioxidants. 2021;10:947. doi: 10.3390/antiox10060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taïr K, Kharoubi O, Taïr OA, et al. Aluminium-induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammada scoparia) J Acute Dis. 2016;5:470–482. doi: 10.1016/j.joad.2016.08.028. [DOI] [Google Scholar]
- 70.El-Hawary S, Abd El-Kader E, Rabeh M, et al. Eliciting callus culture for production of hepatoprotective flavonoids and phenolics from Sequoia sempervirens (D. Don Endl) Nat Prod Res. 2020;34:3125–3129. doi: 10.1080/14786419.2019.1607334. [DOI] [PubMed] [Google Scholar]
- 71.Amri Z, Ghorbel A, Turki M, et al. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement Altern Med. 2017;17:339. doi: 10.1186/s12906-017-1842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kujawska M, Jourdes M. Neuroprotective Effects of Pomegranate Juice against Parkinson's Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int J Mol Sci. 2020;21(1):202. doi: 10.3390/ijms21010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Wei M, Lu X, et al. Aluminum trichloride caused hippocampal neural cells death and subsequent depression-like behavior in rats via the activation of IL-1β/JNK signaling pathway. Sci Total Environ. 2020;715:136942. doi: 10.1016/j.scitotenv.2020.136942. [DOI] [PubMed] [Google Scholar]
- 74.Yaseen AA, Al-Okbi SY, Hussein AM, et al. Potential protection from Alzheimer's disease by wheat germ and rice bran nano-form in rat model. J Appl Pharm Sci. 2019;9:067–076. doi: 10.7324/JAPS.2019.S108. [DOI] [Google Scholar]
- 75.Lu T-H, Tseng T-J, Su C-C, et al. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett. 2014;224:130–140. doi: 10.1016/j.toxlet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Chakraborti D, Singh SK, Rahman MM, et al. Groundwater arsenic contamination in the Ganga River Basin: a future health danger. Int J Env Res Pub He. 2018;15:180. doi: 10.3390/ijerph15020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Firdaus F, Zafeer MF, Anis E, et al. Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol Rep. 2018;5:411–417. doi: 10.1016/j.toxrep.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jahan-Abad AJ, Morteza-Zadeh P, Negah SS, et al. Curcumin attenuates harmful effects of arsenic on neural stem/progenitor cells. Avicenna J Phytomedi. 2017;7:376. [PMC free article] [PubMed] [Google Scholar]
- 79.Essa AF, Teleb M, El-Kersh DM, et al. Natural acylated flavonoids: Their chemistry and biological merits in context to molecular docking studies. Phytochem Rev. 2023;22:1469–1508. doi: 10.1007/s11101-022-09840-1. [DOI] [Google Scholar]
- 80.Naoi M, Inaba-Hasegawa K, Shamoto-Nagai M, et al. Neurotrophic function of phytochemicals for neuroprotection in aging and neurodegenerative disorders: modulation of intracellular signaling and gene expression. J Neural Transm. 2017;124:1515–1527. doi: 10.1007/s00702-017-1797-5. [DOI] [PubMed] [Google Scholar]
- 81.Hannan MA, Sohag AAM, Dash R, et al. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine. 2020;69:153201. doi: 10.1016/j.phymed.2020.153201. [DOI] [PubMed] [Google Scholar]
- 82.Gao Y, Xu X, Chang S, et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol Appl Pharmacol. 2015;289:142–154. doi: 10.1016/j.taap.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Fang J, Wang H, Zhou J, et al. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des Devel Ther. 2018;12:2497–2508. doi: 10.2147/DDDT.S163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui HY, Zhang XJ, Yang Y, et al. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen Res. 2018;13:2119–2128. doi: 10.4103/1673-5374.241463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hui Y, Chengyong T, Cheng L, et al. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β(1-42) in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem Res. 2018;43:297–305. doi: 10.1007/s11064-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 86.Dinkova-Kostova AT, Kostov RV, Kazantsev AG. The role of Nrf2 signaling in counteracting neurodegenerative diseases. Febs J. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuadrado A, Rojo AI. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des. 2008;14:429–442. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- 89.Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radical Bio Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]