Abstract
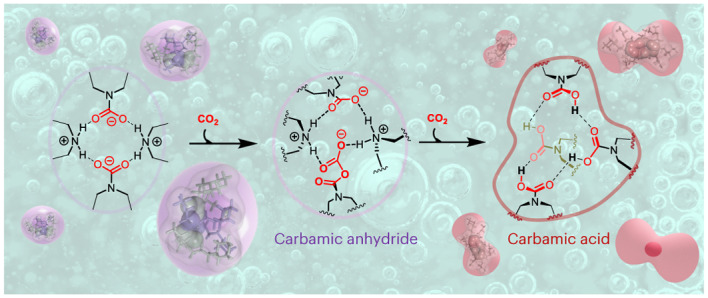
Carbon capture, utilization and storage is a key yet cost-intensive technology for the fight against climate change. Single-component water-lean solvents have emerged as promising materials for post-combustion CO2 capture, but little is known regarding their mechanism of action. Here we present a combined experimental and modelling study of single-component water-lean solvents, and we find that CO2 capture is accompanied by the self-assembly of reverse-micelle-like tetrameric clusters in solution. This spontaneous aggregation leads to stepwise cooperative capture phenomena with highly contrasting mechanistic and thermodynamic features. The emergence of well-defined supramolecular architectures displaying a hydrogen-bonded internal core, reminiscent of enzymatic active sites, enables the formation of CO2-containing molecular species such as carbamic acid, carbamic anhydride and alkoxy carbamic anhydrides. This system extends the scope of adducts and mechanisms observed during carbon capture. It opens the way to materials with a higher CO2 storage capacity and provides a means for carbamates to potentially act as initiators for future oligomerization or polymerization of CO2.
Subject terms: Self-assembly, Sustainability
Carbon capture, utilization and storage is key for climate change mitigation and developing more environmentally friendly technologies. Now it has been shown that CO2 capture in single-component water-lean solvents is accompanied by the self-assembly of reverse-micelle-like tetrameric clusters in solution that enable the formation of various CO2-containing compounds.
Main
In its latest report, the International Energy Agency confirmed that CO2 capture has a critical role in greenhouse gas mitigation and the clean energy transition1. Solvent-based technologies are the most mature option for point source CO2 capture, with many commercial offerings available2. Yet their substantial cost hampers deployment, and the thermal regeneration of solvent would consume energy at the gigatonne scale. This high energy penalty is inherent to the CO2 absorption thermodynamics of aqueous amines A. Despite decades of research and calls for change3,4, scrubbing is unlikely to advance with current half-loaded ammonium carbamate A(0)+A(1)1– and ammonium bicarbonate A(0)+W(1)– adduct pairs (Fig. 1a,e for notation).
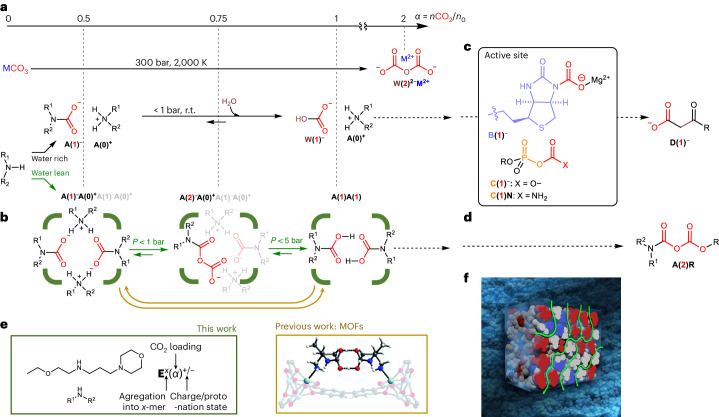
Fig. 1. Conventional versus unconventional CO2-binding adducts with increasing CO2/amine-A stoichiometry.
a,b, Conventional adducts from aqueous CO2 capture (ammonium carbamate A(0)+A(1), ammonium carbonate A(0)+W(1)– and metal percarbonate M2+W(2)2– (a)) versus adducts observed in a water-lean medium such as a MOF and in a water-lean solvent (green, this work) including anhydride A(2)– and carbamic acid A(1) (b). r.t., room temperature; nCO2, molar amount of CO2; n0 molar amount of absorbant. c,d, Biological (urea-based B(1)– and phosphate-based C(1)– and C(1)N) capture intermediates (c) and their final transformation products D(1)– versus synthetic alkoxy carbamic anhydride A(2)R (d). e,f, Structure of EEMPA, associated notation, X-ray structure of A(2) dimer within MOF9 (e; reproduced from ref. 4 with permission of the Royal Society of Chemistry) and aggregation domains within CO2-loaded water-lean solvents34 (f). MOF, metal organic framework.
Confining amines within nanoscopic sites in a solid material (either pre-synthesized5,6 or assembled during capture7) has been the only strategy to achieve cooperative and full loading8–10 (into carbamic acid A(1); Fig. 1b). Although carbamic acid was postulated to exist in moderately polar aprotic liquid media11–14, unambiguous evidence of its formation in solution is scarce10,15,16. The recent observation of pyrocarbonates M2+W(2)2– (M = Pb, Sr) proved that the theoretical limit of one CO2 per binding site can be overcome at extreme temperatures and pressures17.
Biological systems cooperatively manage oxygen uptake using haemoglobin18, though no equivalent molecule exists to absorb CO2. Instead, CO2 is transported as a water-bound species (bicarbonate) or a dissolved gas and converted into original adducts (Fig. 1c) such as N-carboxybiotin B(1)– (ref. 19) and carboxyphosphate C(1)– (ref. 20), or carbamoylphosphate C(1)N (ref. 21). These activated species, key intermediates for the biosynthesis of the building blocks of life D(1)–, are stabilized by a non-covalent hydrogen bonding network within the shielded active sites of enzymes. Through this confinement strategy, nature has extended the portfolio of capture reactions and products far beyond humanity’s current achievements.
Results and discussion
In this work, we explore the ability of a neat water-lean solvent to self-assemble into clusters with a shielded reactive site that enables atypical CO2 capture adduct (Fig. 1d) formation under mild conditions (temperature, T ≤ 313 K; CO2 equilibrium pressure, P < 15 bar; Fig. 1b). Single-component water-lean solvents, such as N-(2-ethoxyethyl)-3-morpholinopropan-1-amine (EEMPA, E), have emerged as promising for post-combustion CO2 capture. EEMPA has a higher solvent energy efficiency22 and lower capture costs23,24 than aqueous amines25 or two-component formulations in the peer-reviewed literature26–32. EEMPA and its diamine analogues were initially designed to form a stable intramolecularly hydrogen-bonded carbamic acid and adopt a folded hairpin structure33. Until now, experimental data could not support the formation of a carbamic acid under processing conditions (partial pressure p*(CO2) < 0.15 bar). Here we posit an alternative hypothesis, where the properties of EEMPA are directly related to its intermolecular self-assembly rather than intramolecular folding (Fig. 1b) after chemically fixing CO2. This is supported by the recent observation that water-lean alkoxyguanidines34–37 display a heterogeneous structure of aggregated ions upon loading (Fig. 1f). This spontaneous nanostructure formation may provide confined (re)active sites, likely explaining how E successfully performs the integrated capture and conversion of CO2 into fuels and chemicals including methanol38 and methane39.
NMR-based identification of molecular and supramolecular speciation
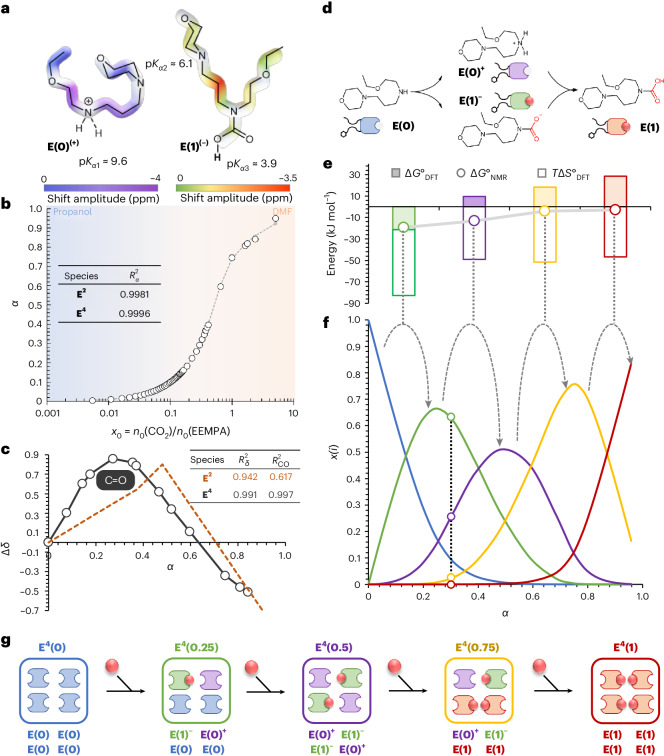
To elucidate the molecular features of CO2 capture by E and identify the supramolecular interactions during self-aggregation, quantitative 1H and 13C NMR spectra were recorded on a series of samples of neat E mixed with increasing molar ratios, x0, of CO2. Two sets of signals in slow exchange at the NMR timescale were observed. The signals correspond to free ammonium/amine and carbamic acid/carbamate pairs (respectively notated as E(0)(+) and E(1)(–); Supplementary Tables 1 and 2 for nomenclature), the former converting to the latter throughout CO2 absorption (Fig. 2a and Supplementary Figs. 12–17). Accurately quantifying both species produced the loading, α, that is, the molar fraction of CO2 effectively bound by E. Complemented at low x0 values by vapour–liquid equilibrium measurements (Supplementary Fig. 2)22, these data (Supplementary Fig. 5 and Supplementary Table 6) allowed us to plot the CO2 binding isotherm with its sigmoidal profile, typical of cooperative systems (Fig. 2b and Supplementary Figs. 10 and 11)7,8. Initial partial pressures of up to 70 bar CO2 (5 equiv. CO2 versus E) were required to reach a final α of almost 1, well above the 0.5 CO2 per EEMPA in unpressurized flue gas14. Under the studied experimental conditions, slow CO2 absorption by E, whose viscosity increases slightly, eventually leads to equilibrium pressure values no higher than 15 bar (Supplementary Figs. 6 and 47) and water content no higher than 700 ppm (an H2O:EEMPA molar ratio of <1:120). During loading, NMR peaks of both E(0)(+) and E(1)(–) underwent noticeable chemical shift perturbations (Supplementary Figs. 22–25) due to fast protonation and self-aggregation phenomena. Chemical shift perturbation mapping40 (Fig. 2a) and monitoring41 (Fig. 2c) confirmed that protonation occurred first on the most basic site (the secondary nitrogen atom of E(0); Supplementary Figs. 1 and 4), as expected, and then unexpectedly on the least basic site (the carbamic oxygen of E(1); Fig. 2c, inset). Together, these observations can be translated into a two-stage molecular scenario (Fig. 2d). Below partial pressures in CO2 of 1 bar, neat E(0) yields an equimolecular mixture of charged ammonium E(0)+ and carbamate E(1)–, which convert into pure carbamic acid E(1) upon gentle pressurization (equilibrium partial pressure p*f(CO2) < 20 bar; Supplementary Fig. 7). A convergent set of evidence (strong shielding of the carbonyl carbon42 and strong deshielding of the hydroxyl proton11 peaks in 13C and 1H NMR, respectively; Supplementary Figs. 15 and 17) supports the presence of this elusive adduct. The data indicate that carbamic acid formation begins at unexpectedly low loading values (around 0.3; Fig. 2c, inset). Experimental data, which encompass chemical shift perturbations Δδ(i), chemical loading values α and molar ratios x0, were fitted with a MATLAB in-house script (see Supplementary Fig. 3 for numbering i of each proton and carbon). Data processing provided the equilibrium binding constants of the two-stage covalent process and values of the chemical shifts δ(i) of the individual E(0), E(0)+, E(1)– and E(1) adducts (Supplementary Figs. 26 and 27). Interestingly, a conventional dimeric model (Fig. 2d) could not be reliably fitted to the experimental data (in particular the 13C chemical shift perturbation of the carbonyl group of E(1)(–); Fig. 2c, inset) in the absence or presence of an additional E(1)–E(1) dimerization equilibrium. Of all scenarios involving higher aggregates, the tetrameric model (Fig. 2g) provided the best match with the full set of experimental data (Fig. 2b,c, Supplementary Figs. 10, 11 and 22–25 and Supplementary Table 7).
Fig. 2. Tetrameric model based on EEMPA–CO2 constituents.
a, Chemical shift perturbation mapping from q13C NMR analyses on E(0)(+) and E(1)(−) (colour code reflects chemical shift perturbation amplitude; pKa1, pKa of the secondary amine; pKa2: pKa of the tertiary amine; pKa3: pKa of the carbamic acid). b,c, Fitting of the α and Δδ(i) values with increasing CO2/EEMPA initial molar ratio x0 by the dimeric E2 and tetrameric E4 models (dot, experimental; dashed line, model; R2α, R2δ and R2CO are root mean squared deviation values for α, δ(aliphatic) and δ(CO), respectively; DMF, N,N-dimethylformamide). d, The two-stage loading process of E(0) by CO2 yielding adducts E(0)+, E(1)– and E(1). e, CO2-binding Gibbs free energies (ΔG°DFT; filled bars), entropies (TΔS°DFT; empty bars) and enthalpies (sum) obtained from DFT and the thermodynamic model (ΔG°NMR; circles). f, The resulting speciation (x(i), the molar fraction versus α, the CO2 loading; vertical dotted line, loading selected for classical molecular dynamics modelling). g, Notation and schematic representation of oligomers and monomeric constituents.
Thermodynamic analysis of self-assembly and CO2 absorption
The full picture of the covalent adduct and non-covalent cluster populations, or chemical speciation, could be simulated after parameter adjustment through fitting the NMR data. Figure 2f,e shows the speciation and Gibbs free energies of CO2 absorption for each successive tetramer E4(α), respectively (ΔG°DFT, DFT-computed Gibbs free energy; ΔG°NMR, Gibbs free energy computed from NMR data; TΔS°NMR, entropic contribution from NMR data). This information, derived from a thermodynamic model, is fully consistent with the experimental observations. Simulations (Fig. 2e, yellow curve), in agreement with NMR data (Fig. 2c), show that carbamic acid is a key component of the cluster E4(0.75) that emerges as loading reaches 0.3. Although EEMPA displays clustering similarly to haemoglobin, its stepwise CO2 capture is negatively cooperative (Fig. 2e), with a drop in Gibbs free energy of ~7 kJ mol–1 between the first, second and third binding steps. The free energy reduction explains why pressurization is needed, despite the thermodynamic stabilization provided by tetrameric self-assembly. Energies and structures of each covalent species, E(0), E(0)+, E(1)– and E(1), as well as of their dimeric and tetrameric clusters were assessed by density functional theory (DFT) calculations with an implicit model of the EEMPA–CO2 medium43. This approach provides a good estimation of energies, which can be further refined by explicitly taking into account the molecules surrounding the computed structures. This preliminary campaign was complemented with classical molecular dynamics simulations. Thermodynamic data of the non-covalent (dimerization and tetramerization) and covalent processes (CO2 capture) were obtained from the DFT-computed energies and compared to experimental measurements to assess the modelling results. Although sizeable differences between computed and experimental enthalpies and entropies are expected, leading to large deviations between Gibbs free energies, good agreement was found for the covalent capture of CO2 by the tetramers (Fig. 2e and Supplementary Fig. 44).
Computed enthalpies of CO2 absorption by tetramers at low loading (α = 0.07–0.22) perfectly match values derived from prior vapour–liquid equilibrium measurements on EEMPA (–79 versus –75 kJ mol–1)22, further supporting our tetrameric model (Supplementary Figs. 32 and 41). The computed entropies of CO2 capture by the tetramers (Supplementary Figs. 33 and 42) match the order of magnitude of values of aqueous amines44,45. With the exception of the first capture step (–200 J mol–1 K–1), these entropies are relatively constant along the loading process (~150 J mol–1 K–1). This agrees with the first capture reaction of the gaseous reactant being accompanied by the tetramerization of E, while subsequent absorption steps involve only the loss of translational and rotational freedom of the gaseous reactant. We experienced the limits of the DFT method while assessing the entropy of all non-covalent pairing processes (2E → E2 and 2E2 → E4; Supplementary Figs. 33, 36 and 39). Though the calculations confirmed that higher aggregates are systematically enthalpically favoured (Supplementary Figs. 32, 35 and 38) regardless of loading, enthalpy values were compensated for by overestimating computed entropies, leading to low positive Gibbs free energies of tetramerization46. This predicted endergonicity for dimerization and tetramerization (Supplementary Figs. 34, 37 and 40) is imputed to the limitation inherent to the solvent model. In fact, classical molecular dynamics simulations (1 μS trajectory) of the E/CO2 system at 298 K and 0.25 loading showed the coexistence of the E4(0.25) and E4(0.50) tetramers (defined by dominant hydrogen bonds; Supplementary Fig. 46) in proportions (18% and 22% of the whole system, respectively) that qualitatively agree with the NMR-data-derived MATLAB model. Complementary evidence supporting the existence of CO2-rich tetramers such as E4(0.75) and E4(1) was provided by Fourier transform infrared and wide-angle X-ray scattering (WAXS) spectroscopies (vide supra). Static DFT calculations confirmed that non-covalent clustering acts as a genuine thermodynamic driving force, stabilizing covalent adducts enthalpically by around 15 kJ mol–1 compared to the isolated species (Supplementary Figs. 32 and 35). Calculations revealed that combining unloaded E(0), partially loaded E(0)+E(1)– and fully loaded E(1) into clusters opens the door to a broad range of absorption enthalpies during CO2 capture (with values decreasing between from –80 to –20 kJ mol–1 along the E4(0)–E4(0.75) series; Fig. 2e), far beyond those observed on solid absorbents9. Consequently, clustering empowered by water-lean solvents may allow chemists to choose the thermodynamic features of the capture and release cycle on demand by setting the loading range, and thus selecting the active tetrameric species.
Structural analysis of the E4 clusters
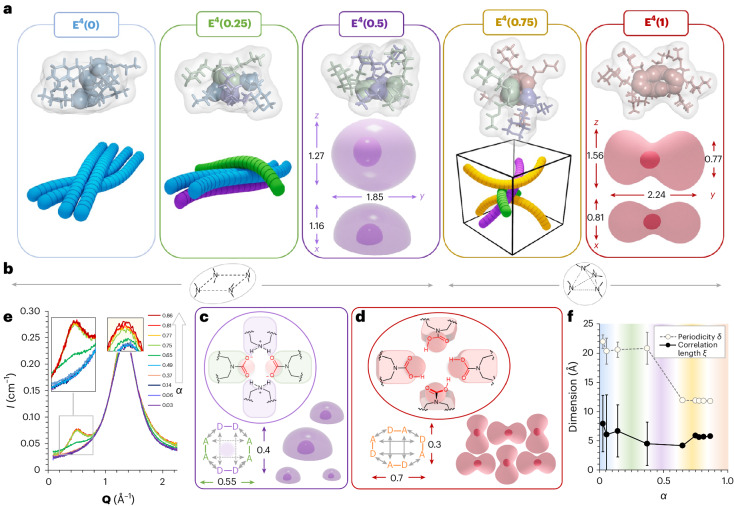
Given the level of agreement with experimental data, DFT modelling could be exploited to gain insights about the non-covalent interactions (Figs. 3c,d and 4c) that govern self-assembly and the structural features of the tetrameric clusters (Figs. 3a and 4a and Supplementary Fig. 45). The self-assembly of subunits E(0), E(0)+, E(1)– and E(1) is driven by a network of hydrogen bonds between the amine, ammonium, carbamate and carbamic acid polar head groups that form the tetrad at the core of the re(active) site. Low-loading tetramers E4(0) and E4(0.25) result from packing unfolded E(0) (E(0)+ and E(1)–) into cylindrical bundle-like tertiary structures, where the basic moieties of the (re)active site are buried and poorly accessible. In E4(0.5), the E(0)+ and E(1)– subunit chains individually fold into turns, with the polyether moieties gathered in one hemisphere (Fig. 3a). As a result, the tertiary structure of E4(0.5) is a half ovoid, exposing the square planar hydrogen-bonded (re)active site (Fig. 3b,c). Incorporation of an additional CO2 molecule leads to dramatic structural changes, both locally and globally (Fig. 3a,d). While carbamic acid formation is accompanied by hydrogen bonding reorganization, the polyether chains remain folded into turns as in E4(0.5). This induces a conformational change of the polar group tetrad from square planar to tetrahedral (Fig. 3b), affecting the orientation of the side chains. Consequently, E4(0.75) adopts a star-shaped tertiary structure with spaced side chains roughly pointing towards the vertices of a cube, shielding the active site from the solution. In E4(1), gathered pairs of ether chains (experimentally confirmed by NMR; Supplementary Fig. 17) yield a flattened figure-eight global structure (Fig. 3a,d).
Fig. 3. Structural analysis of the E4 tetrameric clusters and of their packing in solution.
a, DFT-derived van der Waals surface of dominant tetramers and schematic tertiary structure (distances in nm). b–d, Simplified developed representation of the internal nitrogen-based tetrads (b) and of their hydrogen-bond network and tetramer packing modes (c and d; distances in nm; A, hydrogen-bond acceptor; D, hydrogen-bond donor). e, Stack of WAXS spectra recorded at increasing loading. Q, scattering vector; I, differential scattering cross-section per unit volume. Insets show zoomed-in views. f, Evolution of periodicity δ and correlation length ξ with loading. Data are presented as mean values. Details on error bar calculation are in the Supplementary Methods.
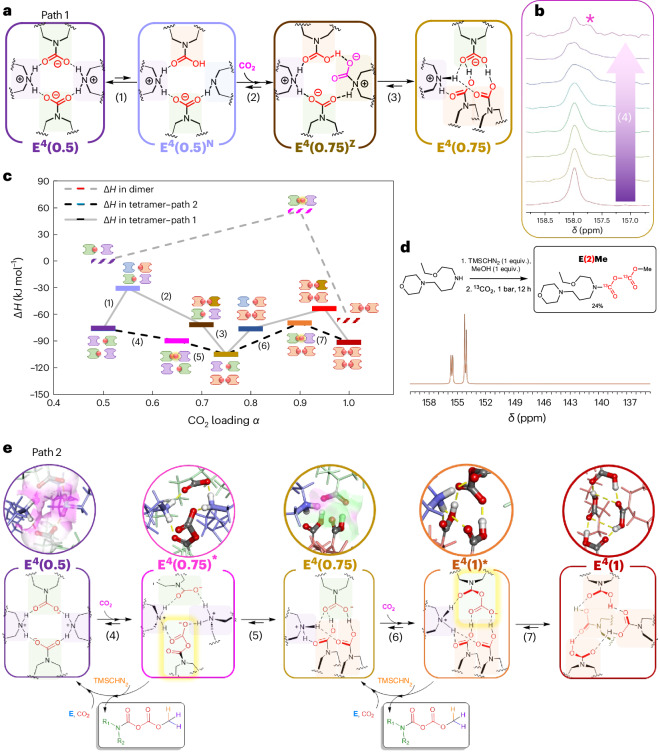
Fig. 4. Mechanism of carbamic acid formation involving anhydride intermediate within E4 clusters.
a, Zwitterion-mediated hypothetical carbamate to carbamic acid conversion pathway (path 1, steps 1–3; E4(0.5)N and E4(0.75)Z are neutral and zwitterionic intermediates, respectively). b, The q13C NMR carbonyl signal evolution upon CO2 loading above ambient pressure (step 4, bottom to top; * marks the additional CO signal from the anhydride). c, DFT-derived enthalpies (ΔH) of E2 and E4 clusters involved in the zwitterion-mediated (path 1, steps 1–3) and anhydride-mediated (path 2, steps 4–5 and 6–7) pathways. d, TMSCHN2 derivatization of 13C-labelled E(2)– into E(2)Me (schematic on top and snapshot of the carbonyl region of the 13C NMR spectrum on the bottom). e, Proposed mechanistic pathway. The asterisk marks a carbamic anhydride-containing intermediate.
Fourier transform infrared spectroscopy experimentally confirmed the presence of EEMPA clusters at high loading. While the conversion of ammonium carbamates to carbamic acid dimers is generally accompanied by a marked increase in the stretching frequency of the carbonyl signal47,48, the observed decrease (Supplementary Fig. 28) agrees with reports about higher carboxylic aggregates (Supplementary Fig. 29)13. WAXS analysis provided a second set of experimental evidence for the tetrameric clusters and information about their morphological features in pressurized and unpressurized conditions (Fig. 3e). The Q region (where Q is the scattering vector) exhibits a CO2 loading-dependent structure factor fit using the Teubner–Strey model49–51. The model qualitatively describes the segregation between the polar reactive moieties and ethoxyethyl and morpholinopropane arms encountered in the tetramers particularly well. The presence of this polar core provides the required electron density contrast and enables the observation of molecular-scale phase segregation at a CO2 loading above 0.6. The lack of phase segregation at lower CO2 loading values may be ascribed to the solution composition, which includes several tetramers (E4(0.25) to E4(0.75)) with differing morphologies poorly adapted to regular and dense packing. WAXS was used to probe the dimensions of these micelle-like clusters and their polar cores. The measured cluster dimensions, including a tetrad correlation length (ξ) of 5.8 Å and periodicity between adjacent tetrads (δ) of ~11.8 Å, quantitatively match with the DFT results (Fig. 3f and Supplementary Figs. 30, 31 and 45). The WAXS analysis also shows a cubic bicontinuous phase based on the head-to-hip packing of CO2-saturated tetramers (Fig. 3c,d). The WAXS data provide additional experimental evidence for the formation of tetramers, agreeing with the NMR-based speciation and DFT modelling.
Anhydride-based mechanism of CO2 capture
The molecular mechanism leading to the formation of carbamic acid-containing clusters was explored by a coupled experimental (NMR of neat pressurized samples) and theoretical (DFT calculations) approach. In the classical zwitterion model, the first CO2 addition proceeds via a carbamic acid intermediate, which converts into ammonium carbamate upon deprotonation by a second amine52. In this framework, carbamic acid can be produced only from a neutral amine precursor. In our system, carbamic acid-containing species E4(0.75) and E4(1) arise from E4(0.5) via a stepwise CO2 absorption. We expected that forming carbamic acid from the E4(0.5) ammonium carbamate tetrad would first require an energetically uphill proton transfer from the nitrogen atom of one ammonium group to a neighbouring carbamate oxygen (Fig. 4a, step 1). The free amine centre of the unstable intermediate E4(0.5)* would then bind to a third CO2 molecule (Fig. 4a, step 2), generating the zwitterion E4(0.75)z that would relax into E4(0.75) (Fig. 4a, step 3).
We exploited the viscosity increase that accompanies CO2 uptake by E4(0.5) to slow the decay of the elusive intermediates involved in the formation of E4(0.75) and E4(1). In practice, an EEMPA sample was overpressurized with CO2 (α = 0.8–1 range) until equilibrium was attained and then transferred into an NMR tube with a headspace under 1 bar of CO2. The quantitative 13C (q13C) NMR spectra were immediately recorded over time once the transfer was complete. The monitored phenomenon is governed by slow mass transfer within the viscous medium. It corresponds to the stripping of E4(0.75) back into E4(0.5) and gaseous CO2 through the intermediate(s) species. Examining the stack of spectra recorded over time in reverse order (from the end to the beginning of the experiment; Fig. 4b, bottom to top) provides a sequence of snapshots of the intermediate states encountered during the E4(0.5) + CO2 → E4(0.75) absorption step (step 4; Fig. 4c,e). While the NMR signals of the aliphatic backbone for both E(0)(+) and E(1)(–) match those recorded at the same loadings at equilibrium (Supplementary Figs. 47 and 48 versus Supplementary Figs. 12–15), the carbamate signal displays a shouldered peak at high loadings (Fig. 4b and Supplementary Fig. 49). Relative integration of the signals corresponding to CO2-bearing versus CO2-free species in this loading range indicated that the CO2-bearing species is bound to more than one CO2 molecule on average.
To identify this carbamate-like intermediate species, we attempted in situ trapping via alkylation with trimethylsilyldiazomethane (TMSCHN2), previously employed for carbamate to urethane conversions53. To our surprise, traces of the methyl carbamic anhydride of EEMPA, notated as E(2)Me, could be directly detected by 13C NMR in the crude mixture (with 0.5 equiv. TMSCHN2; Supplementary Figs. 52 and 53). To confirm this observation and isolate the intermediate, CO2-free EEMPA was premixed with 1 equiv. TMSCHN2 and pressurized with CO2. Remarkably, E(2)Me formed at up to 37% conversion from the crude mixture. An analytically pure (99.5%) sample was recovered in a 24% isolated yield after column chromatography and characterized by mass spectrometry (Supplementary Figs. 63–65) and NMR spectroscopy (Fig. 4c and Supplementary Figs. 54–57). To further confirm the structure of this intermediate, the analogue E(2)tBu (tBu, tert-butyl) was synthesized ex situ from E with di-tert-butyldicarbonate54–57. Thus, we could compare the 13C NMR pattern of the intermediate trapped in situ with the spectra of these derivatives and unambiguously confirm the identity of E(2)Me. The most characteristic feature is the set of two doublets around 150 ppm in 13C NMR observed for both E(2)Me and E(2)tBu (Supplementary Figs. 55, 58, 61 and 66). This pattern is indicative of a non-symmetrical bis(carbonyl) system split given the bulkiness of the capping end group (tBu versus Me), explaining the slight difference in splitting patterns observed between the adducts (Fig. 4d).
DFT calculations confirmed that the anhydride intermediate is strongly stabilized within the reactive site of the tetrameric reverse-micelle-like clusters and favoured over the conventional zwitterion intermediate (Fig. 4a,c,e; Supplementary Figs. 50 and 51 for more detail). The enthalpic cost of proton transfer from the ammonium carbamate to the amine carbamic acid is rather high (Fig. 4a,c, step 1; more than 45 kJ mol–1), whereas the anhydride pathway follows a downhill energetic trajectory (Fig. 4c,e, step 4; –14 kJ mol–1) from E4(0.5). The unique stereoelectronic features of the tetrameric cluster obviously favour this alternative reaction pathway, as carbamic anhydride formation from a traditional ammonium carbamate dimer such a E2(0.5) is thermodynamically strongly disfavoured (by +56 kJ mol–1). Electrostatic potential surface mapping (Fig. 4e) of the internal cavity of E4(0.5) additionally reveals an electron-poor cleft reminiscent of hydrolases’s oxyanion hole58,59. In these confined sites, poorly reactive moieties (alcohols and amides) are activated by a network of hydrogen bonds, which also stabilizes the electron-rich intermediates formed. The same phenomenon is believed to be at work here, to both activate the nucleophilicity of the carbamate and the electrophilicity of CO2 and stabilize the carbamic anhydride. Experimentally, this intermediate species instantaneously forms at room temperature. The rate-limiting step is its conversion into the carbamic acid-containing tetrad E4(0.75). Hydrogen-bond pairing between the members of the E(1)E(1)–E(1)E(0)+ tetrad of E4(0.75) seems particularly efficient, as the resulting electrostatic potential surface is much less electrodeficient than in E4(0.5). This complementarity is perturbed in the carbamic anhydride-containing intermediate tetrad E4(1)*. As a result, the fourth CO2 uptake is both kinetically and thermodynamically less favourable than the third (–14 versus –9 kJ mol–1). Remarkably, TMSCHN2 can selectively methylate and abstract the anhydride from this highly complex system as well as displace the cascade of reversible CO2 absorption events (Fig. 4c,e). This assertion is supported by the fact that premixing TMSCHN2 and unloaded EEMPA leads to the formation of substantial amounts of E(2)Me upon exposure to CO2 in standard temperature and pressure conditions. With this rather simple reactive system, two CO2 molecules can be linearly bound to a single nitrogen centre, doubling the amount of greenhouse gas that may be captured or sequestered into a carbon-based material. In agreement with recent reports60,61, the two urethane analogues E(2)Me and E(2)tBu display moderate stability (conversion into E(1)R within days at room temperature and pressure). Continued studies of structure–reactivity relationships and stability are underway, where we anticipate making the next generation of CO2-rich urethanes with increased robustness. Ultimately, the reactive system identified here may have unlocked the principles needed to oligomerize and store several carbon dioxide molecules on the same backbone. If the recently discovered pyrocarbonate is the strict carbon analogue of pyrophosphate, anhydride E(2)– can be viewed as an analogue of ADP, one of the universal molecules used to transport and store energy by nature. Capture within a clustered water-lean solvent may provide a conceptual framework to build a sustainable carbon value chain inspired by cellular metabolism, an intriguing approach to effectively mitigating CO2 emissions.
Conclusion
The quest for an ideal CO2 capture solvent has spanned almost a century, beginning with patenting the amine scrubbing process and intensifying with the threat of global warming62. Single-component water-lean solvents have recently emerged as promising, displaying high energy efficiencies and low operational costs. However, it has been challenging to determine what molecular and mechanistic features give rise to the advantageous properties and performance of EEMPA and its analogues. It appears that combining a central basic secondary amine site with two flexible, mildly polar side chains enables EEMPA to behave as a proto-surfactant, forming micelle-like ionic clusters after CO2 binding. These stable supramolecular aggregates not only explain EEMPA’s unusual physical properties (low viscosity and high conductivity) but also enable a different covalent capture chemistry to take place within shielded and well-structured nano-environments. These cavities are highly reminiscent of enzymatic active sites, with structural and physical features that stabilize intermediates and catalyse numerous reactions in exceptionally mild conditions. A ‘double-tailed surfactant’-like secondary amine backbone seems to be a general prerequisite for self-assembly into reverse micelle clusters among water-lean solvents and opens the door to exciting reactivity, such as carbamic anhydride formation. We have gathered preliminary evidence of self-assembly and anhydride formation for a series of secondary–tertiary diamine analogues of EEMPA. The reactivity of EEMPA and CO2 can be conceptually depicted using a dynamic combinatorial framework63. Simple constituents such as E, CO2 and protons reversibly and covalently connect to yield a collection of capture products (E(0), E(0)+, E(1), E(1)–), themselves the components of higher tetrameric aggregates. These original proto-protein architectures may enable a shift beyond the canonical mono- and bimolecular capture adducts, in terms of both thermodynamics and kinetics. Tetramerization opens the door for stepwise CO2 capture, with each step displaying highly contrasting binding constants and absorption enthalpies beyond the values obtained with aqueous and metal–organic framework-appended absorbents. While EEMPA displays negative cooperativity, we believe it is just an example in a series towards positively cooperative liquid absorbents. Kinetically speaking, the shielded and hydrogen-bonded reactive core enables the formation of intriguing intermediates such as the CO2-enriched anhydride E(2)–. The way these pseudo-active sites modulate reactivity is illustrated by the alkylation process, which selectively proceeds on the unusual anhydride with respect to the carbamate. The spontaneous clustering of water-lean solvents dramatically expands the scope of thermodynamic and kinetic capture features but may serve as an original medium for the integrated transformation of unexpected capture intermediates such as E(2)– into different end products.
CO2 anhydrides represent an emerging class of CO2 storage products, with higher mass content and similarities to the phosphate-rich biomolecules that control cellular energy storage. A next step is to extend the proof of feasibility to higher CO2 content and obtain an adenosine triphosphate analogue. This would pave the way towards a CO2-only oligomerization process, with solvent acting as an initiator. Ultimately, these structures provide a wealth of valuable information on CO2 reactivity in an unconventional, yet simple, medium. This may be the base for the next generation of impactful carbon capture utilization and storage technologies.
Methods
All reagents were purchased from commercial sources and used as received. Regular liquid-state NMR spectra were recorded on either a 500 MHz Varian iNOVA, 500 MHz Bruker Avance NEO or 600 MHz Bruker NEO equipped with the Cryo Probe using standard pulse sequences. High-pressure liquid-state NMR spectra were recorded on a 500 MHz Varian iNOVA spectrometer, using in-house manufactured PEEK NMR tubes connected to a commercial Parr reactor and Teledyne ISCO pump. The 13C magic angle spinning NMR spectra were collected on a 600 MHz Bruker Avance III spectrometer using 5 mm zirconia rotors spinning at 3–5 kHz, a home-built custom HX probe and an in-house-developed WHiMS rotor system. Mass spectrometry analysis was performed using a Q Exactive Plus mass spectrometer (Thermo Scientific) outfitted with a heated electrospray ionization source and on a time-of-flight secondary ion mass spectrometer (ToF-SIMS 5, IONTOF). High- and low-pressure infrared spectra were collected on Nicolet iS10 Thermo Scientific instrument using a high-pressure demountable transmission liquid Harrick cell and OMNIC 9 software. WAXS experiments were carried out on a Xenocs Xeuss 2.0 small-angle X-ray scattering (SAXS)/WAXS system employing a monochromated Cu Kα (average wavelength λavg = 1.54189 Å) source and an effective Q range of 0.1–2.3 Å–1. Electron paramagnetic resonance measurements were performed on a Bruker ELEXSYS E580 spectrometer equipped with an SHQE resonator.
The Supplementary Information contains details of syntheses, spectroscopic analyses and computational studies.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-024-01495-z.
Supplementary information
Supplementary Figs. 1–71, Tables 1–11 and Methods.
MATLAB code for dimeric model.
MATLAB code for tetrameric model.
Raw data for MATLAB fitting.
DFT optimized structures of all compounds.
Computed energies of all compounds studied, used as source data for Fig. 4c.
Animation showing the 1 μs classical molecular dynamics trajectory of E4(0.25) and E4(0.5) clusters in a 25 mol% CO2 loading system.
Source data
Source data for Fig. 2.
Source data for Fig. 3.
Acknowledgements
French authors were supported by the LABEX iMUST of the University of Lyon (ANR-10-LABX-0064), created within the ‘Plan France 2030’ set up by the French government and managed by the French National Research Agency (ANR) and by the Region Auvergne-Rhone Alpes (Pack Ambition Recherche 2019). US authors acknowledge the US Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Understanding and Control of Reactive Separations (FWP 75428). Data in this publication were obtained using the Pacific Northwest National Laboratory (PNNL) Catalysis Science NMR Facility. This research used resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science User Facility located at Lawrence Berkeley National Laboratory, operated under contract no. DE-AC02-05CH11231. PNNL is operated by Battelle for the US Department of Energy under contract no. DE-AC05-76RL01830. We thank the Centre Commun de RMN of the Université Claude Bernard Lyon 1 (CCRMN UCBL) for assistance with NMR analyses, B. Mundy for help with technical editing and P. Koech for fruitful discussions.
Author contributions
J.L. and D.J.H. jointly conceived the study, secured funding and administered the project. J.L., J.S., D.J.H., K.G., J.L.B., D.Z., M.T.N. and E.W. designed the experimental and theoretical methodologies. M.H., J.S., K.G., D.Z., J.L.B., E.W., M.T.N., D.R., S.I.A., D.M., W.J. and J.K. performed experiments and interpreted data. Visualization and construction of figures were performed by J.L., D.J.H., D.Z., M.T.N. and J.L.B. Writing was led by J.L. and D.J.H. with review and editing by all other authors.
Peer review
Peer review information
Nature Chemistry thanks Ivan Gladich, Thomas Moore, Fabio Pietrucci, Jesse Thompson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information and data files. Should any raw data files be needed in another format, they are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The MATLAB script as well as the dataset used for the parametric fitting are available as supplementary data files. They have been uploaded to and are accessible from Zenodo at 10.5281/zenodo.10649310 (ref. 64).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julien Leclaire, Email: julien.leclaire@univ-lyon1.fr.
David J. Heldebrant, Email: david.heldebrant@pnnl.gov
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-024-01495-z.
References
- 1.CCUS in Clean Energy Transitions (IEA, 2020); https://www.iea.org/reports/ccus-in-clean-energy-transitions
- 2.Rochelle GT. Amine scrubbing for CO2 capture. Science. 2009;325:1652–1654. doi: 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- 3.Leclaire J, Heldebrant DJ. A call to (green) arms: a rallying cry for green chemistry and engineering for CO2 capture, utilisation and storage. Green Chem. 2018;20:5058–5081. [Google Scholar]
- 4.Forse AC, Milner PJ. New chemistry for enhanced carbon capture: beyond ammonium carbamates. Chem. Sci. 2021;12:508–516. doi: 10.1039/d0sc06059c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclaire J, et al. Structure elucidation of a complex CO2-based organic framework material by NMR crystallography. Chem. Sci. 2016;7:4379–4390. doi: 10.1039/c5sc03810c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leclaire J, et al. CO2 binding by dynamic combinatorial chemistry: an environmental selection. J. Am. Chem. Soc. 2010;132:3582–3593. doi: 10.1021/ja909975q. [DOI] [PubMed] [Google Scholar]
- 7.McDonald TM, et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature. 2015;519:303–308. doi: 10.1038/nature14327. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CA, Anderson HL. What is cooperativity? Angew. Chem. Int. Ed. 2009;48:7488–7499. doi: 10.1002/anie.200902490. [DOI] [PubMed] [Google Scholar]
- 9.Forse AC, et al. Elucidating CO2 chemisorption in diamine-appended metal-organic frameworks. J. Am. Chem. Soc. 2018;140:18016–18031. doi: 10.1021/jacs.8b10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhardt R, et al. Cooperative CO2 absorption isotherms from a bifunctional guanidine and bifunctional alcohol. ACS Cent. Sci. 2017;3:1271–1275. doi: 10.1021/acscentsci.7b00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampe EM, Rudkevich DM. Exploring reversible reactions between CO2 and amines. Tetrahedron. 2003;59:9619–9625. [Google Scholar]
- 12.Rudkevich, D. M. & Xu, H. Carbon dioxide and supramolecular chemistry. Chem. Commun.10.1039/b500318k (2005). [DOI] [PubMed]
- 13.Masuda K, Ito Y, Horiguchi M, Fujita H. Studies on the solvent dependence of the carbamic acid formation from ω-(1-naphthyl)alkylamines and carbon dioxide. Tetrahedron. 2005;61:213–229. [Google Scholar]
- 14.Dijkstra ZJ, et al. Formation of carbamic acid in organic solvents and in supercritical carbon dioxide. J. Supercrit. Fluids. 2007;41:109–114. [Google Scholar]
- 15.Kikkawa S, et al. Direct air capture of CO2 using a liquid amine−solid carbamic acid phase-separation system using diamines bearing an aminocyclohexyl group. ACS Environ. Au. 2022;2:354–362. doi: 10.1021/acsenvironau.1c00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki F, Matsumoto C, Iwata T, Mukai C. CO2-selective absorbents in air: reverse lipid bilayer structure forming neutral carbamic acid in water without hydration. J. Am. Chem. Soc. 2017;139:4639–4642. doi: 10.1021/jacs.7b01049. [DOI] [PubMed] [Google Scholar]
- 17.Spahr D, et al. Sr[C2O5] is an inorganic pyrocarbonate salt with [C2O5]2– complex anions. J. Am. Chem. Soc. 2022;144:2899–2904. doi: 10.1021/jacs.2c00351. [DOI] [PubMed] [Google Scholar]
- 18.Ogata RT, McConnell HM. Mechanism of cooperative oxygen binding to hemoglobin. Proc. Natl Acad. Sci. USA. 1972;69:335–339. doi: 10.1073/pnas.69.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowles, J. R. The mechanism of biotin-dependent enzymes. Ann. Rev. Biochem.58, 195–221 (1989). [DOI] [PubMed]
- 20.Phillips NFB, Snoswell MA, Chapman-Smith A, Bruce Keech D, Wallace JC. Isolation of a carboxyphosphate intermediate and the locus of acetyl-CoA action in the pyruvate carboxylase reaction. Biochemistry. 1992;31:9445–9450. doi: 10.1021/bi00154a017. [DOI] [PubMed] [Google Scholar]
- 21.Holden, H. M., Thoden, J. B. & Raushel, F. M. Carbamoyl phosphate synthetase: an amazing biochemical odyssey from substrate to product. Cell. Mol. Life Sci.56, 507–522 (1999). [DOI] [PMC free article] [PubMed]
- 22.Zheng RF, et al. A single-component water-lean post-combustion CO2 capture solvent with exceptionally low operational heat and total costs of capture-comprehensive experimental and theoretical evaluation. Energy Environ. Sci. 2020;13:4106–4113. [Google Scholar]
- 23.Jiang Y, et al. Techno-economic comparison of various process configurations for post-combustion carbon capture using a single-component water-lean solvent. Int. J. Greenh. Gas Control. 2021;106:103279. [Google Scholar]
- 24.Jiang Y, et al. Energy-effective and low-cost carbon capture from point-sources enabled by water-lean solvents. J. Clean. Prod. 2023;388:135696. [Google Scholar]
- 25.Heldebrant DJ, et al. Water-lean solvents for post-combustion CO2 capture: fundamentals, uncertainties, opportunities, and outlook. Chem. Rev. 2017;117:9584–9824. doi: 10.1021/acs.chemrev.6b00768. [DOI] [PubMed] [Google Scholar]
- 26.Lail M, Tanthana J, Coleman L. Non-aqueous solvent (NAS) CO2 capture process. Energy Procedia. 2014;63:580–594. [Google Scholar]
- 27.Zhou, S. J. et al. Pilot Testing of a Non-Aqueous Solvent (NAS) CO2Capture Process (United States Department of Energy’s Office of Fossil Energy Carbon Management, 2018).
- 28.Brown, A. et al. ION Engineering Final Project Report National Carbon Capture Center Pilot Testing (United States Department of Energy’s Office of Fossil Energy Carbon Management, 2017).
- 29.Xiao M, et al. CO2 absorption intensification using three-dimensional printed dynamic polarity packing in a bench-scale integrated CO2 capture system. AlChE J. 2022;68:e17570. [Google Scholar]
- 30.Sarmah M, et al. Matching CO2 capture solvents with 3D-printed polymeric packing to enhance absorber performance. SSRN Electron. J. 2021 doi: 10.2139/ssrn.3814402. [DOI] [Google Scholar]
- 31.Ye, Y. & Rochelle, G. T. Water-lean solvents for CO2 capture will not use less energy than aqueous amines. In 14thGreenhouse Gas Control Technologies Conference Melbourne (SSRN, 2018); 10.2139/ssrn.3366219
- 32.Yuan Y, Rochelle GT. Lost work: a comparison of water-lean solvent to a second generation aqueous amine process for CO2 capture. Int. J. Greenh. Gas Control. 2019;84:82–90. [Google Scholar]
- 33.Malhotra D, et al. Directed hydrogen bond placement: low viscosity amine solvents for CO2 capture. ACS Sustain. Chem. Eng. 2019;7:7535–7542. [Google Scholar]
- 34.Gao J, et al. The interfacial compatibility between a potential CO2 separation membrane and capture solvents. Carbon Capture Sci. Technol. 2022;2:100037. [Google Scholar]
- 35.Cantu DC, et al. Molecular-level overhaul of γ-aminopropyl aminosilicone/triethylene glycol post-combustion CO2-capture solvents. ChemSusChem. 2020;13:3429–3438. doi: 10.1002/cssc.202000724. [DOI] [PubMed] [Google Scholar]
- 36.Yu XY, et al. Mesoscopic structure facilitates rapid CO2 transport and reactivity in CO2 capture solvents. J. Phys. Chem. Lett. 2018;9:5765–5771. doi: 10.1021/acs.jpclett.8b02231. [DOI] [PubMed] [Google Scholar]
- 37.Bañuelos JL, et al. Subtle changes in hydrogen bond orientation result in glassification of carbon capture solvents. Phys. Chem. Chem. Phys. 2020;22:19009–19021. doi: 10.1039/d0cp03503c. [DOI] [PubMed] [Google Scholar]
- 38.Kothandaraman J, et al. Integrated capture and conversion of CO2 to methanol in a post-combustion capture solvent: heterogeneous catalysts for selective C—N bond cleavage. Adv. Energy Mater. 2022;12:2202369. [Google Scholar]
- 39.Kothandaraman J, et al. Integrated capture and conversion of CO2 to methane using a water-lean, post-combustion CO2 capture solvent. ChemSusChem. 2021;14:4812–4819. doi: 10.1002/cssc.202101590. [DOI] [PubMed] [Google Scholar]
- 40.Williamson MP. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 2013;73:1–16. doi: 10.1016/j.pnmrs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Septavaux J, et al. Simultaneous CO2 capture and metal purification from waste streams using triple-level dynamic combinatorial chemistry. Nat. Chem. 2020;12:202–212. doi: 10.1038/s41557-019-0388-5. [DOI] [PubMed] [Google Scholar]
- 42.Kortunov PV, Siskin M, Baugh LS, Calabro DC. In situ nuclear magnetic resonance mechanistic studies of carbon dioxide reactions with liquid amines in non-aqueous systems: evidence for the formation of carbamic acids and zwitterionic species. Energy Fuels. 2015;29:5940–5966. [Google Scholar]
- 43.Cantu DC, et al. Molecular-level overhauling of GAP/TEG post-combustion CO2 capture solvents. ChemSusChem. 2020;13:3429–3438. doi: 10.1002/cssc.202000724. [DOI] [PubMed] [Google Scholar]
- 44.Xiao M, et al. Thermodynamic analysis of carbamate formation and carbon dioxide absorption in N-methylaminoethanol solution. Appl. Energy. 2021;281:116021. [Google Scholar]
- 45.Septavaux, J., Germain, G. & Leclaire, J. Dynamic covalent chemistry of carbon dioxide: opportunities to address environmental issues. Acc. Chem. Res.50, 1692–1701 (2017). [DOI] [PubMed]
- 46.Jeamet, E. et al. Wetting the lock and key enthalpically favours polyelectrolyte binding. Chem. Sci. 10, 277–283 (2019). [DOI] [PMC free article] [PubMed]
- 47.Switzer JR, et al. Reversible ionic liquid stabilized carbamic acids: a pathway toward enhanced CO2 capture. Ind. Eng. Chem. Res. 2013;52:13159–13163. [Google Scholar]
- 48.Didas SA, Sakwa-Novak MA, Foo GS, Sievers C, Jones CW. Effect of amine surface coverage on the co-adsorption of CO2 and water: spectral deconvolution of adsorbed species. J. Phys. Chem. Lett. 2014;5:4194–4200. doi: 10.1021/jz502032c. [DOI] [PubMed] [Google Scholar]
- 49.Teubner M, Strey R. Origin of the scattering peak in microemulsions. J. Chem. Phys. 1987;87:3195–3200. [Google Scholar]
- 50.Harris MA, Kinsey T, Wagle DV, Baker GA, Sangoro J. Evidence of a liquid–liquid transition in a glass-forming ionic liquid. Proc. Natl Acad. Sci. USA. 2021;118:e2020878118. doi: 10.1073/pnas.2020878118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morkved TL, Stepanek P, Krishnan K, Bates FS, Lodge TP. Static and dynamic scattering from ternary polymer blends: bicontinuous microemulsions, Lifshitz lines, and amphiphilicity. J. Chem. Phys. 2001;114:7247–7259. [Google Scholar]
- 52.Yoon B, Hwang GS. Facile carbamic acid intermediate formation in aqueous monoethanolamine and its vital role in CO2 capture processes. Ind. Eng. Chem. Res. 2022;61:4475–4479. [Google Scholar]
- 53.Ito Y, Ushitora H. Trapping of carbamic acid species with (trimethylsilyl)diazomethane. Tetrahedron. 2006;62:226–235. [Google Scholar]
- 54.Aresta M, Dibenedetto A. Mixed anhydrides: key intermediates in carbamates forming processes of industrial interest. Chem. Eur. J. 2002;8:685–690. doi: 10.1002/1521-3765(20020201)8:3<685::AID-CHEM685>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 55.Aresta M, Quaranta E. Mechanistic studies on the role of carbon dioxide in the synthesis of methylcarbamates from amines and dimethylcarbonate in the presence of CO2. Tetrahedron. 1991;47:9489–9502. [Google Scholar]
- 56.Ram S, Ehrenkaufer RE. Rapid reductive-carboxylation of secondary amines. One pot synthesis of tertiary N-methylated amines. Tetrahedron Lett. 1985;26:5367–5370. [Google Scholar]
- 57.Basel Y, Hassner A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols. J. Org. Chem. 2000;65:6368–6380. doi: 10.1021/jo000257f. [DOI] [PubMed] [Google Scholar]
- 58.Simón L, Goodman JM. Enzyme catalysis by hydrogen bonds: the balance between transition state binding and substrate binding in oxyanion holes. J. Org. Chem. 2010;75:1831–1840. doi: 10.1021/jo901503d. [DOI] [PubMed] [Google Scholar]
- 59.Ménard R, Storer AC. Oxyanion hole interactions in serine and cysteine proteases. Biol. Chem. 1992;373:393–400. doi: 10.1515/bchm3.1992.373.2.393. [DOI] [PubMed] [Google Scholar]
- 60.Sampaio-Dias, I. E. et al. Isolation and structural characterization of stable carbamic-carbonic anhydrides: an experimental and computational study. Org. Chem. Front.9, 2154–2163 (2022).
- 61.Kemp, D. S. & Curran, T. P. Base-catalyzed epimerization behavior and unusual reactivity of N-substituted derivatives of 2,5-dicarbalkoxypyrrolidine. Preparation of a novel mixed carbamic carbonic anhydride by a 4-(dimethylamino)pyridine-catalyzed acylation. J. Org. Chem.53, 5729–5731 (1988).
- 62.Bottoms, R. R. Process for separating acidic gases. US patent US1783901A (1930).
- 63.Lehn JM. Dynamic combinatorial chemistry and virtual combinatorial libraries. Chem. Eur. J. 1999;5:2455–2463. [Google Scholar]
- 64.Leclaire, J. et al. Nano-clustering in water-lean solvents establishes novel CO2 chemistry. Zenodo10.5281/zenodo.10649309 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–71, Tables 1–11 and Methods.
MATLAB code for dimeric model.
MATLAB code for tetrameric model.
Raw data for MATLAB fitting.
DFT optimized structures of all compounds.
Computed energies of all compounds studied, used as source data for Fig. 4c.
Animation showing the 1 μs classical molecular dynamics trajectory of E4(0.25) and E4(0.5) clusters in a 25 mol% CO2 loading system.
Source data for Fig. 2.
Source data for Fig. 3.
Data Availability Statement
The data supporting the findings of this study are available within the paper and its Supplementary Information and data files. Should any raw data files be needed in another format, they are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
The MATLAB script as well as the dataset used for the parametric fitting are available as supplementary data files. They have been uploaded to and are accessible from Zenodo at 10.5281/zenodo.10649310 (ref. 64).