Abstract
Macrophages and dendritic cells are known to play an important role in the establishment and persistence of human immunodeficiency virus (HIV) infection. Besides antiretroviral therapy, several immune-based interventions are being evaluated with the aim of achieving better control of virus replication in reservoir cells. Murabutide is a safe synthetic immunomodulator presenting a capacity to enhance nonspecific resistance against viral infections and to target cells of the reticuloendothelial system. In this study, we have examined the ability of Murabutide to control HIV type 1 (HIV-1) replication in acutely infected monocyte-derived macrophages (MDMs) and dendritic cells (MDDCs). Highly significant suppression of viral replication was consistently observed in Murabutide-treated cultures of both cell types. Murabutide did not affect virus entry, reverse transcriptase activity, or early proviral DNA formation in the cytoplasm of infected cells. However, treated MDMs and MDDCs showed a dramatic reduction in nuclear viral two-long terminal repeat circular form and viral mRNA transcripts. This HIV-1-suppressive activity was not mediated by inhibiting cellular DNA synthesis or by activating p38 mitogen-activated protein kinase. Furthermore, Murabutide-stimulated cells expressed reduced CD4 and CCR5 receptors and secreted high levels of β-chemokines, although neutralization of the released chemokines did not alter the HIV-1-suppressive activity of Murabutide. These results provide evidence that a clinically acceptable immunomodulator can activate multiple effector pathways in macrophages and in dendritic cells, rendering them nonpermissive for HIV-1 replication.
Macrophages and dendritic cells are key antigen-presenting cells (APCs) which express surface CD4 molecules and are susceptible to human immunodeficiency virus type 1 (HIV-1) infection. These APCs are believed to be among the first cells to be infected by HIV-1 in patients and to act as reservoirs for virus dissemination (34, 40, 61). Unlike T cells, HIV-infected macrophages and dendritic cells show little or no virus-induced cytopathic effects in vitro (25, 34). While HIV-exposed dendritic cells are known to facilitate the lysis and loss of antigen-specific CD4+ T cells (12), infected macrophages have been shown to mediate apoptosis of CD4+ (3), as well as of CD8+ lymphocytes (23), and to act as the source of increasing viremia during opportunistic infections (45). Recently, the HIV-1 regulatory protein Nef was found to activate macrophages to release factors which allow productive infection and activation of resting T cells (62). These findings, together with the observation that macrophage-tropic (M-tropic) isolates of HIV-1 are much more readily transmitted than are lymphotropic (T-tropic) isolates (70), confirm the role of macrophage infection in HIV-1 pathogenesis. Currently used highly active antiretroviral therapy does not appear to be sufficiently efficient either in targeting the pool of cells that support low levels of virus replication (46) or in eliminating latently infected T cells (53). Additional strategies including immune-based interventions are now believed to be essential for the long-term control and eradication of HIV infection (46, 58).
HIV-1 infection of macrophages and dendritic cells has been shown to induce considerable immune dysfunction and to impair the participation of APCs in protective responses to a variety of pathogens (37, 51, 69). This has led to the evaluation of the capacity of several immunomodulators, either of exogenous or of endogenous origin, to control HIV-1 replication in APCs and to restore their functions (8, 10, 31, 65). Muramyl peptides are a family of synthetic immunomodulators that are endowed with numerous biological activities and target essentially cells of the reticuloendothelial system (4, 28, 60). One selected member of this family, Murabutide, has been found to enhance the host's nonspecific resistance to bacterial and viral infections, to induce colony-stimulating activity, and to be well tolerated by humans (5, 13, 14, 20). In contrast with most other exogenous immunomodulators, Murabutide is apyrogenic (13), does not induce inflammatory reactions (39, 71), and has the capacity to synergize with selected therapeutic cytokines to drive the release of T-helper 1 cytokines (6, 16). Moreover, the coadministration of Murabutide with alpha interferon (IFN-α) or with interleukin-2 (IL-2), was found to dramatically enhance the antitumor activity of either cytokine, as well as the antiviral and anti-inflammatory effects of IFN-α (6, 7, 52). Based on the highly interesting immunopharmacological profile of Murabutide and on its ability to regulate macrophage function (4, 55), we have studied the effects of this immunomodulator on HIV-1 replication in acutely infected monocyte-derived macrophages (MDMs). We have also extended our investigation to evaluate a potential effect of Murabutide on mature monocyte-derived dendritic cells (MDDCs) infected with M- or T-tropic HIV-1 strains. Stimulation of acutely infected cells with Murabutide suppressed viral replication through mechanisms that targeted proviral DNA integration and viral mRNA transcription. Reduced expression of virus receptors and elevated β-chemokine release were also observed in treated cultures. Our findings demonstrate a potent HIV-suppressive activity of a safe synthetic immunomodulator and a profile of APC activation that is associated with protective responses against infection.
MATERIALS AND METHODS
Preparation of human MDMs and MDDCs.
Monocytes were isolated from buffy coats prepared from normal volunteer donors. Peripheral blood mononuclear cells were prepared by Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia Biotech, Orsay, France) and were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum (Etablissement de Transfusion Sanguine, Lille, France). Monocytes were recovered, following overnight adherence to tissue culture flasks (Falcon, Le Pont de Claix, France), by gentle detachment with a cell scraper (ATGC, Noisy-Le-Grand, France). To generate MDMs, monocytes were cultured in 24-well plates (Falcon) at 5 × 105 cells/ml for a 7-day period in RPMI 1640 medium containing 10% AB serum. The same medium supplemented with recombinant human granulocyte-macrophage colony-stimulating factor–IL-4–tumor necrosis factor alpha (TNF-α) was used to differentiate monocytes into MDDCs (65). At the end of the differentiation period, >90% of the MDMs were CD14+ and MDDCs were found to represent mature dendritic cells as judged by morphologic (adherent cells with fine membrane projections) and phenotypic (CD14− CD3−, high levels of CD80 and CD86, >40% CD83+, and >60% CD4+) criteria.
Reagents and antibodies.
TNF-α, IL-4, and granulocyte-macrophage colony-stimulating factor were purchased from R&D Systems (Abingdon, United Kingdom). Lipopolysaccharide (LPS) from Salmonella enteritidis (gamma irradiated) was obtained from Sigma (St Quentin, Fallavier, France). Murabutide (N-acetyl-muramyl-l-alanyl-d-glutamine n-butyl ester), was provided by ISTAC S.A. (Lille, France) and was prepared as described elsewhere (13). The compound was dissolved in phosphate-buffered saline (PBS) at 5 mg/ml, and the absence of endotoxin contamination (<6 × 10−2 endotoxin unit/ml) was verified by the Limulus amebocyte lysate assay (BioWhittaker France, Fontenay-sous-bois, France). Anti-CD4–phycoerythrin (PE) (13B8.2), anti-CD14–PE (RMO52), anti-CD3–PE (UCHT1), anti-HLA-DR–PE (B8.12.2), anti-CD25–PE (B1.49.9), and anti-CD83–PE (HB15a) monoclonal antibodies (MAbs) and their isotype-matched controls were purchased from Immunotech (Beckman Coulter, Marseille, France). Anti-CD86–PE (B70/B7-2), anti-CCR5–PE (2D7), and anti-CXCR4–PE (12G5) MAbs and their isotype-matched controls were purchased from Pharmingen (Becton Dickinson, Rungis, France). Neutralizing goat immunoglobulin G (IgG) antibodies against macrophage inflammatory protein 1 alpha (MIP-1α), MIP-1β, and the protein regulating upon activation normal T expressed and secreted (RANTES) and normal goat IgG were obtained from R&D Systems. SB203580, a specific inhibitor of p38 mitogen-activated protein kinase (MAPK), was purchased from France Biochem (Meudon, France).
HIV-1 strains and in vitro infection.
The M-tropic HIV-1Ba-L and T-tropic HIV-1LAI strains were obtained from the Central Virology Laboratory in Lille, France. The primary strains used, HIV-1CHR-4 (M tropic) and HIV-1CHR-1 (dual tropic), were isolated in our laboratory and have been described elsewhere (27, 65). To infect differentiated cells, 104 cpm of virus reverse transcriptase (RT) activity was added to each well and incubated for 2 h at 37°C. Virus was then removed, cells were washed three times, and fresh medium was added. Cultures were fed twice a week by replacing half of the supernatants with an equal volume of fresh medium containing or not containing the same concentration of Murabutide. Viral replication was assessed by evaluating the content of viral RT or p24 protein in the supernatants as previously described (2, 27).
Detection of HIV-1 DNA and RNA.
Total cellular DNA was extracted from HIV-1Ba-L-infected cells and subjected to 25 or 40 repeated rounds of amplification with Amplitaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.). PCR amplification of β-actin sequences (5′-GGGTCAGAAGGATTCCTATG-3′ and 5′-GGTCTCAAACATGATCTGGG-3′) was performed to standardize for cell equivalence. HIV-1 proviral DNA in each sample was measured by using the GAG06 (5′-GCITTIAGCCCIGAAGTIATACCCATG-3′)-GAG04 (5′-CATICTATTTGTTCITGAAGGGTACTAG-3′) primer pair (49). In some experiments, low- and high-molecular-weight DNAs were extracted from HIV-1Ba-L-infected cells by the protocol described by Steinkasserer et al. (59). Low-molecular-weight DNA was subjected to 30 or 40 rounds of amplification using either the strong-stop viral DNA primer pair (5′-GGCTAACTAGGGAACCCACTG-3′ and 5′-CTGCTAGAGATTTTCCACACTGAC-3′), the γ-32P-labeled 2-LTR-sense (5′-GCCTCAATAAAGCTTGCCTTG-3′)–2-LTR-antisense (5′-TCCCAGGCTCAGATCTGGTCTAAC-3′) primer pair to detect the viral two-long terminal repeat (2-LTR) circle form, or the mitochondrial primer pair (5′-GAATGTCTGCACAGCCACTTT-3′ and 5′-ATAGAAAGGCTAGGACCAAAC-3′) as an internal PCR standard (59, 65). High-molecular-weight DNA was also subjected to PCR amplification using either the γ-32P-labeled M668 (5′-TTTCAGGTCCCTGTTCGGGCGCC-3′)-AluI (5′-GCCTCCCAAAGTGCTGGGATTA-3′) primer pair to analyze provirus integration (11) and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer pair (5′-CCACCCATGGCAAATTCCATGGCA-3′ and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′) as an internal PCR standard. To measure HIV-1 RNA levels, total cellular RNA was extracted using RNAZol (Bioprobe Systems, Montreuil, France) and was amplified using rTth polymerase (Perkin-Elmer) in the presence of the GAG06-GAG04 primer pair to detect the HIV-1 unspliced Gag-Pol mRNA and the BSS (5′-GGCTTGCTGAIGNGCICACIGCAAGAGG-3′)-KPNA (5′-AGAGTIGTGGTTGNTTCNTTCCACACAG-3′) primer pair to detect the singly spliced mRNA as previously described (2, 43, 56, 65). All PCR products were separated on acrylamide gels and visualized by ethidium bromide staining, except for the 2-LTR DNA circle product and the integrated provirus that were visualized after air drying by exposure to Kodak XAR-5 film (Eastman Kodak, Rochester, N. Y.). Using imaging systems (Image Master 1D prime; Amersham Pharmacia Biotech), the percentage of inhibition of HIV-1 DNA and RNA expression was deduced after normalization to the levels of the corresponding internal standards (β-actin, mitochondrial DNA, or GAPDH) as previously described (2, 65).
Flow cytometry analysis.
To assess surface receptor expression in MDMs, 2 × 105 cells were incubated for 30 min at 4°C with specific MAbs, or with isotype-matched controls, diluted 1:100 in PBS containing 2% heat-inactivated fetal calf serum (Sigma). Following two washes in PBS, cells were resuspended and fixed in 1% paraformaldehyde and analyzed with a FACSCalibur flow cytometer (Becton Dickinson). Live cells were gated on their forward and side light scatter characteristics, and the percentage of positive cells and the mean fluorescence intensity (MFI) were recorded. In some experiments, exclusion of dead cells was verified by propidium iodide staining.
Cytokine assays.
Cell supernatants were collected up to 6 days after infection and were filtered through 0.22-μm-pore-size membranes (Millipore, Eschborn, Germany), aliquoted, and stored at −70°C. The levels of constitutive or Murabutide-induced TNF-α, IFN-γ, IL-2, IL-6, IL-10, IL-12, IL-13, MIP-1α, MIP-1β, and RANTES were determined using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems. Specific ELISA kits for the detection of IFN-α (Endogen, Woburn, Mass.) and IL-16 (Biosource, Camarillo, Calif.) were also employed. All assays were performed in accordance with the manufacturer's instructions, and cytokine levels were calculated by comparison to standard curves using recombinant cytokines.
Detection of tritium-labeled thymidine uptake.
Monocytes seeded at 7.5 × 104 per well in 96-well plates (Falcon) were allowed to differentiate into MDMs or MDDCs over a 7-day culture period. Cells were then left unstimulated or were stimulated with Murabutide, in quadruplicate, for another 2 or 6 days. The level of DNA synthesis was measured after an 18-h pulse with 0.5 μCi of tritium-labeled thymidine (Amersham Pharmacia Biotech) per well. Cells were then lysed with 10 μl of DNAZol (Life Technologies, Cergy Pontoise, France) prior to harvesting on a filter mat for scintillation counting (Skatron, Lier, Norway). Radioactivity was read using a Tricard 1600LR liquid scintillation beta counter (Packard, Downers Grove, Ill.).
Statistical analyses.
The Wilcoxon matched-pair test was used to determine the statistical significance of all reported results unless otherwise mentioned. P values of <0.05 were considered statistically significant.
RESULTS
Murabutide suppresses HIV-1 replication in acutely infected MDMs.
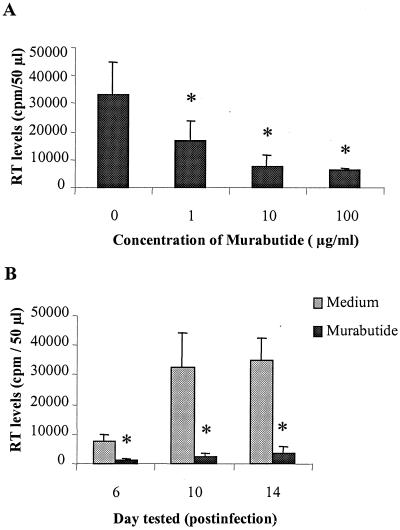
We examined the effects of different concentrations of Murabutide (0.01 to 100 μg/ml) on the level of viral replication in HIV-1Ba-L-infected MDMs. Viral RT activity was measured 12 days postinfection in supernatants of untreated or Murabutide-treated MDM cultures from six separate donors. Results shown in Fig. 1A demonstrate that the addition of Murabutide at 1, 10, or 100 μg/ml resulted, respectively, in 42, 84, or 85% mean inhibition of viral replication. Lower concentrations of Murabutide (0.01 and 0.1 μg/ml) had no significant inhibitory effect (<20%). We then evaluated, in six additional experiments, the kinetics of HIV-1Ba-L replication in acutely infected MDM cultures that were maintained for 2 weeks in the absence or presence of Murabutide at 10 μg/ml. Viral replication was detectable as of day 6 postinfection and was found to peak either on day 10 or on day 14. Treatment of infected cultures with Murabutide induced a dramatic inhibition of viral replication on day 6 (78% mean inhibition), and this effect was maintained at a similar level (90%) on days 10 and 14 (Fig. 1B). In two of the six experiments, viral replication was monitored over a period of 4 weeks and the inhibitory effect of Murabutide was found to be consistent (>85%) throughout the whole culture period. Microscopic examination of cultures at different time points and analysis by trypan blue dye revealed that the addition of Murabutide to infected MDMs did not alter the adherence characteristic or the viability of cells compared with unstimulated cultures. Two primary HIV-1 isolates, M-tropic HIV-1CHR-4 and dual-tropic HIV-1CHR-1, were also used to infect MDMs, and cultures were maintained for 2 weeks in the absence or presence of Murabutide (10 μg/ml). Peak levels of viral p24 protein in the supernatants of MDMs infected with the CHR4 and CHR1 strains were 8,067 and 19,490 pg/ml, respectively. The corresponding levels of p24 in cultures maintained with Murabutide were 1,185 and 2,377 pg/ml, representing 85 and 88% inhibition of viral replication. Moreover, to address a potential effect of Murabutide on baseline cellular DNA synthesis that could interfere with viral replication (30), [3H]thymidine uptake was evaluated in six independent experiments after 2- and 6-day culture periods. The levels of MDM proliferation (mean number of counts per minute ± the standard error of the mean [SEM]) on day 2 and on day 6 were 1,317 ± 279 and 1,058 ± 302, respectively. The proliferative activity of MDMs was not significantly modified (P > 0.05) by treatment with Murabutide (1,123 ± 212 on day 2 and 1,150 ± 207 on day 6). This is in contrast to the effects of other immunomodulators, including LPS, which have been reported to inhibit macrophage DNA synthesis (42).
FIG. 1.
Murabutide inhibits HIV-1Ba-L replication in acutely infected MDM cultures. Following the 2-h infection period, MDMs were washed and maintained in the absence or presence of Murabutide at 1 to 100 (A) or 10 (B) μg/ml. Viral RT levels were measured in culture supernatants 12 (A) or 6, 10, and 14 (B) days postinfection. The results presented are the means ± SEMs of six separate experiments in panel A and of another six independent experiments in panel B. ∗, RT levels significantly reduced compared with those of untreated cultures (P < 0.05).
Murabutide suppresses viral mRNA and proviral DNA levels in HIV-1-infected MDMs.
Analysis of total cellular RNA from HIV-1Ba-L-infected cultures was performed on samples maintained for 8 days postinfection in the absence or presence of Murabutide (10 μg/ml). Results from two representative experiments are shown in Fig. 2. The expression level of unspliced HIV-1 mRNA in both experiments was found to be inhibited by 98% in Murabutide-treated compared with untreated MDMs (Fig. 2A). On the other hand, the inhibitory effects of Murabutide on the expression level of singly spliced HIV-1 mRNA were 85 and 63% in experiments 1 and 2, respectively (Fig. 2B). No effect of Murabutide on the level of β-actin mRNA accumulation in MDMs could be detected (Fig. 2C).
FIG. 2.
Viral mRNA expression in HIV-1Ba-L-infected MDMs cultured for 8 days in the absence or presence of Murabutide (10 μg/ml). In two separate experiments (expt.), RNA samples (33, 100, and 300 ng) were subjected to RT-PCR amplifications with primer pair GAG06-GAG04 to detect unspliced Gag or Pol mRNA (A) and with primer pair BSS-KPNA to detect intermediate-size, singly spliced viral transcripts (B). These mRNAs were named as follows on the basis of the exons they contain and the proteins they produce (43): 1.4E Tat, exons 1 and 4E; 1.2.4BE Vpu/Env, exons 1, 2, and 4BE; 1.2.5E Vpu/Env, exons 1, 2, and 5E; 1.4BE Vpu/Env, exons 1 and 4BE; 1.5E Vpu/Env, exons 1 and 5E. Constitutively expressed β-actin mRNA in the same samples was also amplified (C). An equivalent amount of RNA from the 8E5 cell line was used as a positive control for RT-PCR amplification.
To determine whether Murabutide stimulation of HIV- 1Ba-L-infected MDMs may also affect proviral DNA formation, we evaluated the provirus content in total cell DNA extracts obtained 22 h postinfection. Representative results from two separate experiments are shown in Fig. 3A. It was noted that cultures maintained with Murabutide after the 2-h infection period presented dramatically lower levels of HIV-1 proviral DNA compared with untreated MDMs. The mean inhibition of proviral DNA corresponded to 74 and 78% in experiments 1 and 2, respectively. Since this approach cannot identify whether Murabutide targets the early step of reverse transcription or the nuclear transport of viral DNA and its integration, we decided to examine this issue by evaluating the levels of strong-stop DNA and the 2-LTR circular form in the low-molecular-weight DNA fraction and the level of integrated provirus in high-molecular-weight DNA. PCR analysis of the low-molecular-weight DNA, extracted at the 24-h time point, demonstrated the absence of a significant effect (<5% inhibition) of Murabutide on the early step of reverse transcription and the formation of strong-stop DNA (Fig. 3B). In contrast, the level of the viral 2-LTR DNA circle form, reflecting the transport of viral preintegration complexes to the nucleus, was greatly reduced (>92% inhibition) in Murabutide-treated, HIV-1Ba-L-infected MDMs (Fig. 3B). Identical inhibition was also observed in DNA samples extracted 48 h postinfection (data not shown). Furthermore, using the high-molecular-weight DNA fraction purified from total cellular DNA, the level of integrated provirus was analyzed in untreated and in Murabutide-treated MDMs. Results from two independent experiments (Fig. 3C) demonstrate that virus integration was reduced by greater than 87% following 48 h of treatment with Murabutide. Taken together, these results point to a dramatic inhibition by this synthetic immunomodulator of the transport of viral preintegration complexes to the nucleus and of virus integration, with no measurable effect on the early process of virus reverse transcription.
FIG. 3.
Effect of Murabutide on HIV-1Ba-L-proviral DNA levels in MDMs. (A) Total DNA was extracted 22 h postinfection from untreated or Murabutide-treated MDMs, and various concentrations (6, 30, and 150 ng) were subjected to PCR amplification with primer pair GAG06-GAG04 to detect the HIV-1 gag gene. Cell equivalence was determined by amplification of the β-actin housekeeping gene, and DNA extracts from 8E5 cells were used as PCR standards. (B) Different dilutions (6, 30, and 150 ng) of low-molecular-weight DNA were amplified using strong-stop DNA or 2-LTR circular DNA primer sets. Cell equivalence was determined by mitochondrial gene (Mito) amplification. (C) High-molecular-weight DNA extracts (6, 30, and 150 ng) made 48 h postinfection were amplified using the M668-AluI primer pair to detect the cellular–HIV-1 DNA junction sequences (fragments of >659 bp). Cell equivalence was determined by amplification of the GAPDH gene. Extracts from HIV-1-infected peripheral blood mononuclear cells (PBL) were used as PCR standards.
Further characterization of the Murabutide-suppressive activity on HIV-1 replication in MDMs.
To address the question of whether the mechanism of HIV-1 suppression by Murabutide is exclusively linked to the inhibition of nuclear localization of proviral DNA, experiments were performed in which treatment with Murabutide only started 48 h postinfection. This time period is known to be sufficient for complete reverse transcription and nuclear import of viral preintegration complexes into MDMs (15). Results from five independent experiments have demonstrated that the percentages of inhibition of HIV-1Ba-L replication measured 10 days postinfection were similar among cultures that were stimulated with Murabutide either immediately after infection (77% ± 7%) or 48 h postinfection (62% ± 8%; P = 0.1172, Mann-Whitney U test). This effect, which was confirmed at the level of viral transcripts, also points to the ability of Murabutide to control viral replication at a step that follows the full integration of proviral DNA. Moreover, to analyze whether Murabutide could directly interfere with virus binding and entry, experiments were performed in which the compound was mixed with HIV-1Ba-L and added to cultures during the 2-h infection period. In three separate experiments, identical levels of proviral DNA were detected 24 and 48 h postinfection in MDM cultures exposed to HIV-1Ba-L in the absence or presence of Murabutide (10 μg/5 × 105 cells). Similarly, the presence of Murabutide with the virus during the infection period had no effect whatsoever on the viral RT levels measured 10 days postinfection (data not shown). Thus, Murabutide does not seem to compete with HIV-1 binding to its receptors or to exert a direct inhibitory activity on viral enzymes. This latter point was further demonstrated by the inability of Murabutide, when mixed with HIV-1 particles purified by ultracentrifugation, to reduce the enzymatic activity of RT in vitro (data not shown).
Activation by LPS of p38 MAPK in MDMs has been recently suggested as a major mechanism mediating the HIV-suppressive activity of endotoxin (72). To determine whether this mechanism is implicated in Murabutide activity, we have evaluated the effect of blocking p38 MAPK activation, using the specific inhibitor SB203580, on the inhibition of viral replication induced either by Murabutide or by LPS. Results of one out of three identical experiments shown in Fig. 4 demonstrate that the HIV-suppressive activity of Murabutide was not modified by the presence of the inhibitor SB203580. In contrast, blocking of p38 MAPK activation was found to abrogate the LPS-induced suppression of viral replication. This further differentiates the activity of Murabutide from that of LPS at the level of intracellular signaling events.
FIG. 4.
Inhibition of p38 MAPK activation with SB203580 does not abrogate the HIV-suppressive activity of Murabutide. MDM cultures were maintained during and continuously after infection in the absence or presence of 1 μM SB203580. Wells from each condition were left untreated (Medium) or were stimulated with Murabutide or with LPS following the 2-h infection period. Viral RT levels were assessed 10 days postinfection, and the data presented are means ± SDs of a single representative experiment done in triplicate out of three experiments performed.
Murabutide inhibits HIV-1 replication in MDDCs.
Cultures of differentiated MDDCs were infected with HIV-1Ba-L and then maintained for 3 weeks in the absence or presence of Murabutide (10 μg/ml). Detectable viral replication in untreated cultures was consistently observed 8 to 10 days postinfection, and RT levels in the supernatants continued to increase until the end of the culture period. The addition of Murabutide to infected cultures from six separate donors, greatly reduced the RT levels on days 15 and 19 postinfection (Fig. 5), and the mean percentages of inhibition of viral replication were 69 and 77%, respectively. Dose-response studies indicated that even at a concentration of 0.1 μg/ml, Murabutide exerted significant HIV-suppressive activity (>50%) in MDDC cultures from three out of four tested donors. Similar to the effects on MDMs, Murabutide was also found to inhibit proviral DNA and viral mRNA levels in infected MDDCs but not to interfere with cell proliferative activity (data not shown).
FIG. 5.
Murabutide inhibits HIV-1Ba-L replication in acutely infected MDDCs. Following the 2-h infection period, MDDCs were maintained in the absence or presence of Murabutide (10 μg/ml). Supernatants collected on days 15 and 19 postinfection were evaluated for viral RT levels. The data presented are means ± SEMs of six independent experiments. ∗, RT levels significantly reduced compared with those of unstimulated cultures (P < 0.05).
Regulation by Murabutide of the expression of HIV-1 receptors.
We then questioned the ability of Murabutide to regulate, in MDMs and MDDCs, the level of expression of different cell surface receptors, including CD14, CD4, HLA-DR, CCR5, and CXCR4. Stimulation with Murabutide for 6, 24, and 48 h had no measurable effect on CD14, HLA-DR, and CXCR4 expression in both cell populations. MDDCs did not express detectable CD14 either before or after Murabutide stimulation (data not shown). However, both MDMs and MDDCs cultured with Murabutide for 24 or 48 h, but not those cultured for 6 h, presented reduced expression of CD4 and CCR5 and this effect was maximal at the 24-h time point (Fig. 6). Histograms demonstrating the staining profile of cells from one representative donor are shown in Fig. 6A. Furthermore, analysis of results from five independent experiments revealed that both the percentage of positive cells (Fig. 6B) and the MFI of the positive populations (Fig. 6C) were significantly reduced following stimulation with Murabutide (P < 0.05). Nevertheless, this effect was quite limited in magnitude and is unlikely to be a major pathway mediating the HIV-suppressive activity of the immunomodulator.
FIG. 6.
Regulation by Murabutide of CD4 and CCR5 membrane expression in MDMs. Following 24 h of culture in the absence or presence of Murabutide, MDMs were stained with CD4 or CCR5 MAbs and with isotype-matched control antibodies. Histograms from one representative experiment showing the percentage of positive cells and the MFI for CD4 and CCR5 (shaded area), as well as the staining profile obtained with isotype-matched antibodies (open area) are presented in panel A. The data shown in panels B and C are means ± SEMs of five separate experiments reflecting the percentage of positive cells (B) and the MFI (C) in untreated (Medium) or Murabutide-treated cultures. ∗, expression of CD4 and CCR5 significantly reduced compared with that in unstimulated cells (P < 0.05).
Profile of cytokines and chemokines induced by Murabutide in HIV-1Ba-L-infected MDM and MDDC cultures.
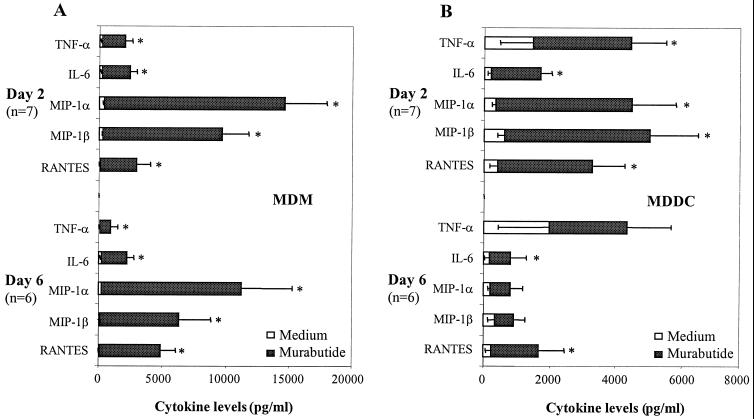
The question of whether Murabutide modulates the endogenous cytokine network in MDM and MDDC cultures was addressed by evaluating the levels of secreted cytokines and chemokines 2 and 6 days postinfection. Supernatants from untreated or Murabutide-treated cultures in seven independent experiments were found to lack detectable IFN-α, IFN-γ, IL-2, IL-12, IL-13, and IL-16. Low levels of IL-10 (<200 pg/ml) were consistently present in MDM and MDDC supernatants, although these levels were not significantly modified upon stimulation with Murabutide (data not shown). It is of interest that the absence of IL-2 and IFN-γ in supernatants from all of the cultures reflects the high purity and the absence of lymphocytes in the MDM and MDDC preparations. On the other hand, treatment of MDMs with Murabutide for either 2 or 6 days induced a significant release of TNF-α, IL-6, MIP-1α, MIP-1β, and RANTES (Fig. 7A). However, the balance was much more tipped toward the induction of β-chemokines than toward the induction of proinflammatory cytokines. A similar profile of Murabutide-induced cytokines, although weaker in magnitude, was also observed in MDDCs treated for 2 days (Fig. 7B). Contrary to the effect in MDMs, longer stimulation of MDDC with Murabutide (6 days) did not maintain significantly induced levels of MIP-1α, MIP-1β, or TNF-α (Fig. 7B).
FIG. 7.
Profiles of released cytokines in HIV-1Ba-L-infected MDM and MDDC cultures that were maintained in the absence (Medium) or in the presence of Murabutide (10 μg/ml). Culture supernatants were collected 2 and 6 days postinfection, and the levels of different cytokines and chemokines were analyzed with ELISA kits. The values shown are means ± SEMs of six or seven separate experiments with MDM (A) and MDDC (B) cultures. ∗, values significantly different from those of unstimulated cultures (P < 0.05).
The HIV-suppressive activity of Murabutide is not blocked by antichemokine antibodies.
To evaluate the role of induced β-chemokines in the HIV-suppressive activity of Murabutide, the effect of neutralizing antibodies against MIP-1α, MIP-1β, and RANTES was examined. In three separate experiments, stimulation of HIV-1Ba-L-infected MDMs with Murabutide for 8 to 10 days postinfection resulted in 79% mean inhibition (range, 64 to 90%) of RT levels compared with those of untreated controls. The level of Murabutide-induced inhibition of HIV-1 replication was equally evident in MDM cultures that were continuously maintained either with normal goat IgG at 150 μg/ml (mean inhibition: 78%; range, 66 to 91%) or with a mixture of each of the three antichemokine antibodies at 50 μg/ml (mean inhibition: 78%; range, 56 to 93%). Evaluation of the levels of β-chemokines in the same supernatants using ELISA kits revealed that the addition of antichemokine antibodies, but not of normal goat IgG, reduced the detectability of the released chemokines by >90%. This suggests that the neutralized chemokines were no longer accessible for binding and strongly argues for chemokine-independent inhibition of HIV-1 replication by Murabutide. To further elucidate this point, we tested the effect of Murabutide on HIV-1LAI replication in MDMs and MDDCs. This T-tropic strain had no capacity to replicate in MDMs, a finding that is consistent with its phenotype, and cultures maintained in the presence of Murabutide continued to be negative for p24 (<5 pg/ml). On the other hand, productive infection with HIV-1LAI in MDDCs was observed in cultures from two out of four tested donors. Peak p24 levels occurred on day 20 postinfection and corresponded to 35,700 and 31,424 pg/ml. The addition of Murabutide to infected cultures inhibited the release of p24 protein into the supernatants by 76 and 58%, respectively. These results clearly demonstrate that the HIV-suppressive activity of Murabutide is not restricted to M-tropic viruses and that the observed effect of the immunomodulator is not necessarily dependent on its capacity to induce β-chemokine release.
DISCUSSION
Macrophages and dendritic cells are major targets for HIV-1 and have been found to contribute to several immune defects observed in infected subjects (29, 40, 68). Agents capable of controlling HIV-1 infection and/or replication in APCs, as well as of stimulating cellular immune functions, may have an important place in the management of HIV disease. Synthetic muramyl dipeptide and several of its hydrophilic structural derivatives have been found to activate cells of the myeloid lineage, to regulate cytokine release, and to enhance the host's nonspecific resistance to infections and tumors (16, 36, 47, 48). However, many of these molecules were found to induce prohibiting toxicities and only a few analogues presented a real potential for clinical development (5, 64). Studies with experimental animals and with humans revealed that Murabutide, selected by screening of over 200 analogues, presented unique immunopharmacologic activities that were associated with a safe clinical profile (5–7, 13, 52). Based on these findings, the potential use of Murabutide among the nonspecific immune-based strategies for the immunotherapy of chronic viral infections has been envisaged (5).
The present study was aimed at evaluating whether macrophage activation by Murabutide could trigger cellular effector mechanisms that are able to control HIV-1 replication. Moreover, in the absence of knowledge of the outcome of interactions between muramyl peptides and dendritic cells, we also sought to analyze this issue using Murabutide-treated, HIV-infected dendritic cells. The addition of Murabutide to MDM or MDDC cultures, either immediately or 2 days after infection with HIV-1, resulted in highly significant inhibition of viral replication. This activity was demonstrated to be independent of a direct effect on the virus or on virus entry but to involve activation of cellular pathways capable of interfering with proviral DNA integration or/and viral transcription. Although the effects of Murabutide seem to have certain features in common with those reported for LPS or IFN-α (31), it is apparent that the mechanisms involved are not identical. Activation of p38 MAPK by LPS in acutely infected MDMs has been reported to be essential for inhibition of the viral 2-LTR circular DNA form (72). Our results obtained by using a specific inhibitor of p38 MAPK activation indicate that the Murabutide-induced inhibition of nuclear proviral DNA is not dependent on the activation of this signaling molecule. In addition, whereas the presence of Murabutide during the 2-h infection period had no effect on virus entry and replication, the use of LPS under similar conditions has been found to enhance virus entry but to inhibit nuclear import of viral preintegration complexes (72). On the other hand, suppression of HIV-1 replication by LPS is known to depend on the capacity of endotoxin to induce IFN-α release and the subsequent upregulation of CCAAT enhancer binding protein beta (26). Although no measurable IFN-α could be detected in Murabutide-stimulated MDM cultures, our results cannot rule out the possibility of upregulation by Murabutide of CCAAT enhancer binding protein beta or of other HIV-1 LTR-repressing transcription factors (38, 41), an issue that needs to be addressed in future studies.
Macrophages are permissive for M-tropic HIV-1 isolates that use CCR5 for entry but are resistant to CXCR4-dependent T-tropic strains (9). This has been recently explained by the expression of a low-molecular-weight monomeric form of CXCR4 in MDMs (35). However, primary dual-tropic HIV-1 isolates are known to be capable of infecting macrophages via a CXCR4-dependent process (67). Our results obtained by using the CHR4 and CHR1 primary isolates demonstrate the capacity of Murabutide to control the replication of both M-tropic and dual-tropic HIV-1 strains in MDMs. On the other hand, mature dendritic cells have been found to support productive infection by M- and T-tropic strains which use CCR5 and CXCR4, respectively (22, 34). We were able to get productive infection of MDDCs with the T-tropic strain LAI in cultures from only two of the four tested donors. Nevertheless, in both cases, stimulation of infected cells with Murabutide led to significant suppression of T-tropic HIV-1 replication. This clearly indicates that the HIV-suppressive activity of Murabutide in APCs is not restricted to M-tropic virus isolates. Furthermore, in Murabutide-treated MDM and MDDC cultures, a selective profile of high β-chemokine induction and low proinflammatory cytokine release has been observed. These results suggest that the HIV-suppressive activity of Murabutide depends, at least in part, on its capacity to induce MIP-1α, MIP-1β, and RANTES. However, a role for the Murabutide-induced β-chemokines in mediating the inhibition of HIV replication in MDMs could be neither observed nor deduced. This is based on (i) the failure of the maintenance of cultures in the presence of neutralizing antibodies to the three β-chemokines to modify the HIV-suppressive activity of Murabutide, (ii) the ability of the immunomodulator to inhibit the replication of β-chemokine-insensitive dual-tropic and T-tropic strains, and (iii) restriction of the activity of β-chemokines on HIV replication to processes that do not seem to be affected by Murabutide, including virus entry (1) or a postbinding fusion step (44). Nevertheless, the ability of Murabutide to induce the secretion of β-chemokines in MDM and MDDC cultures may be responsible for the observed downregulation of CCR5 expression, possibly through receptor internalization following ligand binding. Indeed, the downregulation of CCR5 expression by Murabutide was evident in a period corresponding to the presence of maximum levels of induced β-chemokines in treated cultures. On the other hand, the mechanism involved in the Murabutide-induced downregulation of CD4 expression has not been elucidated in the present study. However, based on the absence of detectable IL-16 in Murabutide-treated cultures, we can rule out a potential role for the natural ligand of CD4 in mediating the observed downregulation of its receptor (24). Similarly, the absence of CD3+ lymphocytes in our culture system (<1%) and the absence of detectable IL-2 levels in supernatant of Murabutide-treated APCs exclude the possibility of IL-2-mediated downregulation of CD4 in MDMs (33). Alternative explanations, including Murabutide-induced downregulation of CD4 gene expression, cannot be disregarded and will be addressed in future studies.
Biologically active muramyl peptides are known to interact directly with macrophages and B cells, although the receptors mediating this interaction are still controversial. Recently, the monocyte surface antigen CD14, a major LPS receptor, has been suggested to serve as a binding site for MDP (17, 66). Nevertheless, MDP and other derivatives were found to exert direct effects on purified CD14− B lymphocytes (57) and MDDCs (present work), arguing for a multiplicity of muramyl peptide receptors. Moreover, the possibility that muramyl peptides and LPS share the same signaling receptors is highly unlikely in view of the finding that LPS-hyporesponsive C3H/HeJ mice, having a missense point mutation in the toll-like receptor 4 (TLR4) gene (50), are highly responsive to muramyl peptides (19). This is further substantiated by a recent report implicating TLR2, and not TLR4, in the mediation of peptidoglycan signaling in macrophages (63). In addition, certain effects of muramyl peptides have been shown to be dependent on the interaction of the compounds with intracellular receptors (18), which may include nuclear histones (21). In this context, it is relevant to point out that whereas several muramyl peptides were found to bind to the brain centers responsible for regulating temperature and sleep, the safe analogue Murabutide presented no such binding activity (32, 54). Taken together, these reports argue in favor of the presence of multiple cellular receptors for muramyl peptides and suggest that a limited structural modification could dictate the receptor usage, the immunopharmacologic effects, and the clinical acceptability of a defined derivative.
Finally, the ability of certain immunomodulators to potentiate the host's resistance to viral infections has been frequently associated with their capacity to regulate cytokine release (4, 47, 54). However, it may well be that immunomodulators also regulate the expression of other cellular genes that are needed for the completion of different steps in the virus life cycle. Our data strongly suggest the regulation by Murabutide of cellular factors necessary for HIV integration and transcription. Although these factors have not been identified in the present study, the observed abilities of Murabutide to suppress HIV-1 replication, to reduce the expression of virus receptors, and to enhance the release of HIV-suppressive chemokines could have important implications for the nonspecific immunotherapy of HIV infection. Ongoing studies in our laboratory have also revealed the ability of Murabutide to control HIV replication in endogenously infected T cells and in the humanized severe combined immunodeficient mouse model. Confirmation of these results and the good clinical tolerance of Murabutide by HIV-infected patients may rapidly contribute to the serious evaluation of a new immunotherapeutic approach, as an adjunct to antiretroviral agents, in the management of HIV disease.
ACKNOWLEDGMENTS
We are grateful to V. Vidal for valuable comments and to N. Bethencourt for preparation of the manuscript.
This work was supported by grants from the Agence Nationale pour la Valorisation et l'Avancement de la Recherche (ANVAR) and from the Association Stop SIDA (Lille, France).
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC-CKR-5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Amiel C, Darcissac E, Truong M J, Dewulf J, Loyens M, Mouton Y, Capron A, Bahr G M. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179:83–91. doi: 10.1086/314550. [DOI] [PubMed] [Google Scholar]
- 3.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahr G M, Chedid L. Immunological activities of muramyl peptides. Fed Proc. 1986;45:2541–2544. [PubMed] [Google Scholar]
- 5.Bahr G M, Darcissac E, Bevec D, Dukor P, Chedid L. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on Murabutide. Int J Immunopharmacol. 1995;17:117–131. doi: 10.1016/0192-0561(94)00094-5. [DOI] [PubMed] [Google Scholar]
- 6.Bahr G M, Darcissac E, Pouillart P R, Chedid L A. Synergistic effects between recombinant interleukin-2 and the synthetic immunomodulator Murabutide: selective enhancement of cytokine release and potentiation of antitumor activity. J Interferon Cytokine Res. 1996;16:169–178. doi: 10.1089/jir.1996.16.169. [DOI] [PubMed] [Google Scholar]
- 7.Bahr G M, Pouillart P R, Chedid L A. Enhancement in vivo of the antiinflammatory and antitumor activities of type I interferon by association with the synthetic immunomodulator Murabutide. J Interferon Cytokine Res. 1996;16:297–306. doi: 10.1089/jir.1996.16.297. [DOI] [PubMed] [Google Scholar]
- 8.Barker E, Bossart K N, Levy J A. Primary CD8+ cells from HIV-infected individuals can suppress productive infection of macrophages independent of β-chemokines. Proc Natl Acad Sci USA. 1998;95:1725–1729. doi: 10.1073/pnas.95.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 10.Bernstein M S, Tong-Starksen S E, Locksley R M. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Investig. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briant L, Coudronnière N, Robert-Hebmann V, Benkirane M, Devaux C. Binding of HIV-1 virions or gp120–anti-gp120 immune complexes to HIV-1-infected quiescent peripheral blood mononuclear cells reveals latent infection. J Immunol. 1996;156:3994–4004. [PubMed] [Google Scholar]
- 12.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 13.Chedid L A, Parant M A, Audibert F M, Riveau G J, Parant F J, Lederer E, Choay J P, Lefrancier P L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35:417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomel J J, Simon-Lavoine N, Thouvenot D, Valette M, Choay J, Chedid L, Aymard M. Prophylactic and therapeutic effects of Murabutide in OF1 mice, infected with influenza A/H3N2 (A/Texas/1/77) virus. J Biol Response Mod. 1988;7:581–586. [PubMed] [Google Scholar]
- 15.Collins M, Gordon S. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology. 1994;200:114–120. doi: 10.1006/viro.1994.1169. [DOI] [PubMed] [Google Scholar]
- 16.Darcissac E C, Bahr G M, Pouillart P R, Riveau G J, Parant M A. Selective potentiation of cytokine expression in human whole blood by Murabutide, a muramyl peptide analogue. Cytokine. 1996;8:658–666. doi: 10.1006/cyto.1996.0088. [DOI] [PubMed] [Google Scholar]
- 17.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 18.Fogler W E, Fidler I J. The activation of tumoricidal properties in human blood monocytes by muramyl dipeptide requires specific intracellular interaction. J Immunol. 1986;136:2311–2317. [PubMed] [Google Scholar]
- 19.Galelli A, Chedid L. Induction of colony-stimulating activity (CSA) by a synthetic muramyl peptide (MDP): synergism with LPS and activity in C3H/HeJ mice and in endotoxin-tolerized mice. J Immunol. 1986;137:3211–3215. [PubMed] [Google Scholar]
- 20.Galelli A, Lefrancier P, Chedid L. Colony-stimulating activity induced by synthetic muramyl peptides: variation with chemical structure and association with anti-infectious activity. Infect Immun. 1984;46:495–500. doi: 10.1128/iai.46.2.495-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golovina T, Fattakhova G, Swiderek K, Makarov E, Bovin N, Shively J, Nesmeyanov V. Specific binding of glucosaminylmuramyl peptides to histones. FEBS Lett. 1999;454:152–156. doi: 10.1016/s0014-5793(99)00689-4. [DOI] [PubMed] [Google Scholar]
- 22.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 24.Hermann E, Darcissac E, Idziorek T, Capron A, Bahr G M. Recombinant interleukin-16 selectively modulates surface receptor expression and cytokine release in macrophages and dendritic cells. Immunology. 1999;97:241–248. doi: 10.1046/j.1365-2567.1999.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho D D, Rota T R, Hirsch M S. Infection of monocytes/macrophages by human T lymphotropic virus type III. J Clin Investig. 1986;77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda Y, Rogers L, Nakata K, Zhao B-Y, Pine R, Nakai Y, Kurosu K, Rom W N, Weiden M. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)β, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idziorek T, Khalife J, Billaut-Mulot O, Hermann E, Aumercier M, Mouton Y, Capron A, Bahr G M. Recombinant IL-16 inhibits HIV-1 replication and protects against activation-induced cell death (AICD) Clin Exp Immunol. 1998;112:84–91. doi: 10.1046/j.1365-2249.1998.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johannsen L. Biological properties of bacterial peptidoglycan. APMIS. 1993;101:337–344. doi: 10.1111/j.1699-0463.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 29.Knight S C. AIDS. A problem of antigen presentation? Curr Biol. 1994;4:1131–1134. doi: 10.1016/s0960-9822(00)00255-4. [DOI] [PubMed] [Google Scholar]
- 30.Koostra N A, Schuitemaker H. Proliferation-dependent replication in primary macrophages of macrophage-tropic HIV type 1 variants. AIDS Res Hum Retrovir. 1998;14:339–345. doi: 10.1089/aid.1998.14.339. [DOI] [PubMed] [Google Scholar]
- 31.Kornbluth R S, Oh P S, Munis J R, Cleveland P H, Richman D D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger J M, Walter J, Karnovsky M L, Chedid L, Choay J P, Lefrancier P, Lederer E. Muramyl peptides. Variation of somnogenic activity with structure. J Exp Med. 1984;159:68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutza J, Hayes M P, Clouse K A. Interleukin-2 inhibits HIV-1 replication in human macrophages by modulating expression of CD4 and CC-chemokine receptor-5. AIDS. 1998;12:F59–F64. doi: 10.1097/00002030-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Langhoff E, Terwilliger E F, Bos H J, Kalland K H, Poznansky M C, Bacon O M, Haseltine W A. Replication of human immunodeficiency virus type 1 in primary dendritic cell culture. Proc Natl Acad Sci USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapham C K, Zaitseva M B, Lee S, Romanstseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. [DOI] [PubMed] [Google Scholar]
- 36.Lederer E. Natural and synthetic immunomodulators derived from the mycobacterial cell wall. In: Bizzini B, Bonmassan E, editors. Advances in immunomodulation. Rome, Italy: Pythogora; 1988. pp. 9–36. [Google Scholar]
- 37.Macatonia S E, Lau R, Patterson S, Pinching A J, Knight S C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 38.Maciaszek J W, Parada N A, Cruikshank W W, Center D M, Kornfeld H, Viglianti G A. IL-16 represses HIV-1 promoter activity. J Immunol. 1997;158:5–8. [PubMed] [Google Scholar]
- 39.McAdam K P W J, Foss N T, Garcia C, DeLellis R, Chedid L, Rees R J W, Wolff S M. Amyloidosis and the serum amyloid A protein response to muramyl dipeptide analogs and different mycobacterial species. Infect Immun. 1983;39:1147–1154. doi: 10.1128/iai.39.3.1147-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meltzer M S, Skillman D R, Gomatos P J, Kalter D C, Gendelham H E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 41.Musso T, Bosco M C, Longo D L, Espinoza-Delgado I, Sica A, Cox G W, Gusella G L, Forni G, Varesio L. LPS-inducible nuclear factor in human monocytes that binds the negative regulatory element of the HIV LTR. J Leukoc Biol. 1994;56:21–26. doi: 10.1002/jlb.56.1.21. [DOI] [PubMed] [Google Scholar]
- 42.Nagao S, Ikegami S, Tanaka A. Inhibition of macrophage DNA synthesis by immunomodulators. Cell Immunol. 1984;89:427–438. doi: 10.1016/0008-8749(84)90344-7. [DOI] [PubMed] [Google Scholar]
- 43.Neumann M, Harrison J, Sartarelli M, Hadziyannis E, Erfle V, Felber B K, Pavlakis G N. Splicing variability in HIV type 1 revealed by quantitative RNA polymerase chain reaction. AIDS Res Hum Retrovir. 1994;10:1531–1542. doi: 10.1089/aid.1994.10.1531. [DOI] [PubMed] [Google Scholar]
- 44.Oravecz T, Pall M, Norcross M A. β-Chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 45.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 46.Pantaleo G. How immune-based interventions can change HIV therapy. Nat Med. 1997;3:483–486. doi: 10.1038/nm0597-483. [DOI] [PubMed] [Google Scholar]
- 47.Parant M A. Muramyl peptides as enhancers of host resistance to bacterial infections. In: Majde J A, editor. Immunopharmacology of infectious diseases: vaccine adjuvants and modulators of non-specific resistance. New York, N.Y: Alan R. Liss, Inc.; 1987. pp. 235–244. [Google Scholar]
- 48.Parant M A, Pouillart P, Le Contel C, Parant F J, Chedid L A, Bahr G M. Selective modulation of lipopolysaccharide-induced death and cytokine production by various muramyl peptides. Infect Immun. 1995;63:110–115. doi: 10.1128/iai.63.1.110-115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piatak M, Jr, Luk K C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–81. [PubMed] [Google Scholar]
- 50.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 51.Polyak S, Chen H, Hirsch D, George I, Hershberg R, Sperber K. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J Immunol. 1997;159:2177–2188. [PubMed] [Google Scholar]
- 52.Pouillart P R, Audibert F M, Chedid L A, Lefrancier P L, Bahr G M. Enhancement by muramyl peptides of the protective response of interferon-α/β against encephalomyocarditis virus infection. Int J Immunopharmacol. 1996;18:183–192. doi: 10.1016/0192-0561(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 53.Ramratnam B, Mittler J E, Zhang L, Boden D, Hurley A, Fang F, Macken C M, Perelson A S, Markowitz M, Ho D D. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 54.Riveau G, Chedid L. Comparison of the immuno- and neuropharmacological activities of MDP and Murabutide. In: Majde J A, editor. Immunopharmacology of infectious diseases: vaccine adjuvants and modulators of non-specific resistance. New York, N.Y: Alan R. Liss, Inc.; 1987. pp. 213–222. [Google Scholar]
- 55.Riveau G J, Brunel-Riveau B G, Audibert F M, Chedid L A. Influence of a muramyl dipeptide on human blood leukocyte functions and their membrane antigens. Cell Immunol. 1991;134:147–156. doi: 10.1016/0008-8749(91)90338-c. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz S, Felber B K, Benko D M, Fenyo E M, Pavlakis G N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souvannavong V, Adam A. Increased expression of alkaline phosphatase activity in stimulated B lymphocytes by muramyl dipeptide. Immunol Lett. 1990;29:247–251. doi: 10.1016/0165-2478(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 58.Starr S E. Immune reconstitution in HIV-infected individuals—what will it take? Immunologist. 1998;6:19–22. [Google Scholar]
- 59.Steinkasserer A, Harrison R, Billich A, Hammerschmid F, Werner G, Wolff B, Peichl P, Palfi G, Schnitzel W, Mlynar E, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus type 1 (HIV-1): interference with early and late events in HIV-1 replication. J Virol. 1995;69:814–824. doi: 10.1128/jvi.69.2.814-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St Georgiev V. Immunomodulatory activity of small peptides. Trends Pharmacol Sci. 1990;11:373–378. doi: 10.1016/0165-6147(90)90183-9. [DOI] [PubMed] [Google Scholar]
- 61.Stoler M H, Eskin T A, Benn S, Angerer R C, Angerer L M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986;256:2360–2364. [PubMed] [Google Scholar]
- 62.Swingler S, Mann A, Jacqué J, Brichacek B, Sasseville V G, Williams K, Lackner A A, Janoff E N, Wang R, Fisher D, Stevenson M. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 64.Telzak E, Wolff S M, Dinarello C A, Conlon T, El Kholy A, Bahr G M, Choay J P, Morin A, Chedid L. Clinical evaluation of the immunoadjuvant Murabutide, a derivative of MDP, administered with a tetanus toxoid vaccine. J Infect Dis. 1986;153:628–633. doi: 10.1093/infdis/153.3.628. [DOI] [PubMed] [Google Scholar]
- 65.Truong M J, Darcissac E C A, Hermann E, Dewulf J, Capron A, Bahr G M. Interleukin-16 inhibits human immunodeficiency virus type 1 entry and replication in macrophages and in dendritic cells. J Virol. 1999;73:7008–7013. doi: 10.1128/jvi.73.8.7008-7013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel E T, Flad H-D, Ulmer A J. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 69.Zerlauth G, Maier E, Chehadeh H, Zimmermann K, Eibl M M, Mannhalter J W. Interaction with human immunodeficiency virus type 1 modulates innate effector functions of human monocytes. J Infect Dis. 1995;172:1598–1601. doi: 10.1093/infdis/172.6.1598. [DOI] [PubMed] [Google Scholar]
- 70.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 71.Zidek Z. Differences in proinflammatory activity of several immunomodulatory derivatives of muramyl dipeptide (MDP) with special reference to the mechanism of the MDP effects. Agents Actions. 1992;36:136–145. doi: 10.1007/BF01991241. [DOI] [PubMed] [Google Scholar]
- 72.Zybarth G, Reiling N, Schmidtmayerova H, Sherry B, Bukrinsky M. Activation-induced resistance of human macrophage to HIV-1 infection in vitro. J Immunol. 1999;162:400–406. [PubMed] [Google Scholar]