Abstract
Background
In the context of patients with hepatocellular carcinoma (HCC) treated with systemic therapy, the correlation between the appearance of adverse events (AEs) and reported efficacy outcomes is well-known and widely investigated. From other pathological settings, we are aware of the prognostic and predictive value of the occurrence of immune-related AEs in patients treated with immune-checkpoint inhibitors.
Objective
This retrospective multicenter real-world study aims to investigate the potential prognostic value of AEs in patients with HCC treated with atezolizumab plus bevacizumab in the first-line setting.
Patients and methods
The study population consisted of 823 patients from five countries (Italy, Germany, Portugal, Japan, and the Republic of Korea).
Results
Of the patients, 73.3% presented at least one AE during the study period. The most common AEs were proteinuria (29.6%), arterial hypertension (27.2%), and fatigue (26.0%). In all, 17.3% of the AEs were grade (G) 3. One death due to bleeding was reported. The multivariate analysis confirmed the appearance of decreased appetite G < 2 [versus G ≥ 2; hazard ratio (HR) 0.60; 95% confidence interval (CI) 0.13–0.90; p < 0.01] and immunotoxicity G < 2 (versus G ≥ 2; HR: 0.70; 95% CI 0.24–0.99; p = 0.04) as independent prognostic factors for overall survival, and the appearance of decreased appetite G < 2 (versus G ≥ 2; HR: 0.73; 95% CI 0.43–0.95; p = 0.01), diarrhea (yes versus no; HR: 0.57, 95% CI 0.38–0.85; p = 0.01), fatigue (yes versus no; HR: 0.82, 95% CI 0.65–0.95; p < 0.01), arterial hypertension G < 2 (versus G ≥ 2; HR: 0.68, 95% CI 0.52–0.87; p < 0.01), and proteinuria (yes versus no; HR: 0.79, 95% CI 0.64–0.98; p = 0.03) as independent prognostic factors for progression-free survival.
Conclusions
As demonstrated for other therapies, there is also a correlation between the occurrence of AEs and outcomes for patients with HCC for the combination of atezolizumab plus bevacizumab.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-024-01061-0.
Key Points
| There is a correlation between the occurrence of adverse events and outcomes for patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab. |
| This understanding is crucial for further enhancing our daily clinical practice and providing patients with hepatocellular carcinoma with the best possible management of the currently available therapeutic strategies. |
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and accounts for approximately 90% of cases [1]. Recently, the therapeutic armamentarium available for the systemic treatment of this disease has expanded. Sorafenib was approved in 2007 thanks to the results of two phase III trials [2, 3]. Lenvatinib became the new standard first-line therapy in 2017 due to the results of the REFLECT trial [4]. The combination of the monoclonal antibody, bevacizumab, which inhibits the vascular endothelial growth factor (VEGF), with atezolizumab, a monoclonal antibody inhibiting the programmed cell death ligand-1 (PD-L1), represents the first therapeutic doublet available for HCC treatment. The IMbrave150 trial showed that this combination can achieve a median overall survival (OS) of 19.2 months and a median progression-free survival (PFS) of 6.9 months [5]. Recently, an immunotherapeutic combination was approved by the Food and Drug Administration (FDA) and the European Medicines Agency in light of the final data from the HIMALAYA trial. This combination comprises a single dose of tremelimumab (anti-cytotoxic T-lymphocyte antigen 4) with another anti-PD-L1, durvalumab, and it achieved a median OS of 16.4 months [6]. Although this combination is indicated in international guidelines as the new standard of care for first-line HCC treatment, it is not yet reimbursed in all countries [7]. Currently, the most widely used systemic therapy, therefore, remains the combination of atezolizumab plus bevacizumab.
In the context of treating patients with HCC with systemic therapy, the correlation between the appearance of adverse events (AEs) and reported efficacy outcomes is well known and widely investigated. For sorafenib and lenvatinib, there is evidence regarding the prognostic and predictive value of the appearance of specific AEs during treatment, which has increased clinicians’ ability to better manage the use of these two tyrosine kinase inhibitors (TKIs) in daily clinical practice [8–29]. However, from other pathological settings (such as non-small-cell lung cancer and melanoma), we are aware of the prognostic and predictive value of the occurrence of immune-related AEs in patients treated with immune-checkpoint inhibitors [30–37]. However, studies investigating this aspect in the context of systemic treatment of patients with HCC are still very limited [38, 39].
This retrospective multicenter real-world study aims to investigate the potential prognostic and predictive value of AEs occurring during first-line treatment with the combination of atezolizumab plus bevacizumab in a large cohort of patients with HCC.
Methods
The study population consisted of patients with HCC from five countries (Italy, Germany, Portugal, Japan, and the Republic of Korea) being treated with atezolizumab plus bevacizumab between October 2018 and April 2022. Patients were treated with atezolizumab plus bevacizumab in the first-line setting for intermediate [Barcelona Clinic Liver Cancer (BCLC)-B] or advanced (BCLC-C) HCC, judged ineligible for loco-regional procedures. Patients were included in the study if they had HCC histological or imaging diagnosis following recent international guidelines and if they had not received any prior systemic treatments for this neoplasm. All patients were treated with 15 mg/kg of body weight of bevacizumab and 1200 mg of atezolizumab administered intravenously every 3 weeks as determined by the IMbrave150 trial [5]. Treatment interruptions and/or dose reductions were allowed to manage AEs. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 [40].
Statistical Analysis
This analysis aimed to examine the association between toxicity, graded using CTCAE 5.0, and clinical outcomes in patients with HCC treated with atezolizumab plus bevacizumab.
Categorical variables were compared with Fisher’s exact test. OS was defined as the time interval from the first day of treatment to the day of death. PFS was defined as the time interval from the first day of treatment to the progression of the disease or the day of death for any cause. OS and PFS were estimated by the Kaplan–Meier method, and curves were compared by the log-rank test. Hazard ratios (HRs) adjusted and not adjusted by baseline characteristics were calculated using the Cox proportional hazards model.
MedCalc package (MedCalc® version 16.8.4) was used for statistical analysis.
Results
The overall cohort included 852 patients treated with atezolizumab plus bevacizumab. A total of 29 patients were excluded for follow-up (FU), since, at less than 3 months, the first re-evaluation computed tomography scan for the assessment of the response to treatment had not yet been performed; hence, 823 were available for the analysis. Median FU was 10.4 months (95% CI 9.6–11.0). Clinical and laboratory baseline characteristics are summarized in Supplementary Table 1. The most frequent etiology of cirrhosis was viral (53.7%). Most of the patients were asymptomatic [performance status (PS) 0: 74.7%] and in the advanced stage (BCLC-C: 59.3%). A total of 93.4% were Child–Pugh A class.
Survival Outcomes
Median OS was 15.9 months (95% CI 14.7–23.9), and median PFS was 7.6 months (95% CI 6.7–8.6).
A proportion of 73.3% of patients presented at least one AE during the study period (Table 1). The most common AEs were proteinuria (29.6%), arterial hypertension (27.2%), and fatigue (26.0%). Of the AEs, 17.3% were grade (G) 3. One death due to bleeding was reported.
Table 1.
Adverse events
| GRADE 0 | GRADE 1 | GRADE 2 | GRADE 3 | GRADE 4 | GRADE 5 | |
|---|---|---|---|---|---|---|
| Arterial hypertension | 599 (72.8%) | 65 (7.9%) | 107 (13.0%) | 52 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| Fatigue | 609 (74.0%) | 145 (17.6%) | 55 (6.7%) | 14 (1.7%) | 0 (0.0%) | 0 (0.0%) |
| Decreased appetite | 647 (78.6%) | 112 (13.6%) | 48 (5.8%) | 16 (1.9%) | 0 (0.0%) | 0 (0.0%) |
| Proteinuria | 579 (70.3%) | 102 (12.4%) | 85 (10.3%) | 57 (6.9%) | 0 (0.0%) | 0 (0.0%) |
| Diarrhea | 756 (91.9%) | 48 (5.8%) | 15 (1.8%) | 4 (0.5%) | 0 (0.0%) | 0 (0.0%) |
| Hypothyroidism | 779 (94.6%) | 14 (1.7%) | 27 (3.3%) | 3 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| Immunotoxicity | 717 (87.1%) | 54 (6.6%) | 27 (3.3%) | 21 (2.5%) | 4 (0.5%) | 0 (0.0%) |
| Other | 659 (80.1%) | 49 (5.9%) | 57 (6.9%) | 48 (5.8%) | 9 (1.1%) | 1 (0.1%) |
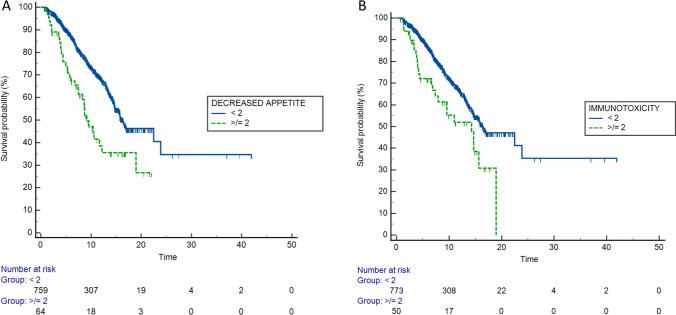
At univariate analysis, the occurrence of several AEs was related to OS, as shown in Table 2. After adjusting for positive clinical covariates at univariate analysis, the multivariate analysis confirmed the appearance of decreased appetite G < 2 (versus G ≥ 2; HR: 0.60; 95% CI 0.13–0.90; p < 0.01) and immunotoxicity G < 2 (versus G ≥ 2; HR: 0.70; 95% CI 0.24–0.99; p = 0.04) as independent prognostic factors for OS (Table 2; Fig. 1).
Table 2.
Univariate and multivariate analyses for OS
| Characteristics | Univariate analysis OS | Multivariate analysis OS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex | 0.16 | |||
| Female | 1 | |||
| Male | 0.80 (0.59–1.09) | |||
| Age | 0.18 | |||
| ≤ 70 years | 0.84 (0.66–1.08) | |||
| > 70 years | 1 | |||
| Etiology | 0.75 | |||
| Viral | 0.96 (0.75–1.23) | |||
| Non-viral | 1 | |||
| BCLC | 0.03 | 0.79 | ||
| B | 0.76 (0.59–0.98) | 0.96 (0.72–1.28) | ||
| C | 1 | 1 | ||
| AFP | < 0.01 | < 0.01 | ||
| < 400 ng/mL | 0.47 (0.36–0.62) | 0.65 (0.38–0.85) | ||
| ≥ 400 ng/mL | 1 | 1 | ||
| Child–Pugh | < 0.01 | |||
| A | 0.10 (0.05–0.20) | |||
| B | 1 | |||
| NLR | < 0.01 | < 0.01 | ||
| < 3 | 0.56 (0.43–0.73) | 0.67 (0.42–0.87) | ||
| ≥ 3 | 1 | 1 | ||
| EHD | 0.77 | |||
| Yes | 1 | |||
| No | 0.96 (0.75–1.24) | |||
| ALBI | < 0.01 | < 0.01 | ||
| 1 | 0.11 (0.06–0.19) | 0.13 (0.07–0.22) | ||
| 2 | 1 | 1 | ||
| PS | 0.01 | |||
| ≤ 1 | 0.24 (0.07–0.76) | |||
| > 1 | 1 | |||
| PVT | < 0.01 | |||
| Yes | 1 | |||
| No | 0.53 (0.39–0.71) | |||
| Decreased appetite | < 0.01 | |||
| Yes | 1 | |||
| No | 0.59 (0.43–0.80) | |||
| Decreased appetite | < 0.01 | < 0.01 | ||
| G < 2 | 0.37 (0.23–0.60) | 1 | ||
| G ≥ 2 | 1 | 0.60 (0.13–0.90) | ||
| Fatigue | < 0.01 | |||
| Yes | 1 | |||
| No | 0.57 (0.43–0.77) | |||
| Fatigue | < 0.01 | 0.21 | ||
| G < 2 | 0.50 (0.31–0.80) | 0.84 (0.47–1.07) | ||
| G ≥ 2 | 1 | 1 | ||
| Proteinuria | 0.25 | |||
| Yes | 0.86 (0.66–1.11) | |||
| No | 1 | |||
| Proteinuria | 0.23 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.83 (0.61–1.13) | |||
| Hypertension | < 0.01 | 0.10 | ||
| Yes | 0.69 (0.53–0.89) | 0.77 (0.57–1.05) | ||
| No | 1 | 1 | ||
| Hypertension | 0.03 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.72 (0.54–0.97) | |||
| Immunotoxicity | 0.02 | |||
| Yes | 1 | |||
| No | 0.66 (0.46–0.94) | |||
| Immunotoxicity | < 0.01 | 0.04 | ||
| G < 2 | 0.45 (0.27–0.77) | 0.70 (0.24–0.99) | ||
| G ≥ 2 | 1 | 1 | ||
| Diarrhea | 0.46 | |||
| Yes | 0.85 (0.56–1.30) | |||
| No | 1 | |||
| Diarrhea | 0.12 | |||
| G < 2 | 0.50 (0.21–1.18) | |||
| G ≥ 2 | 1 | |||
| Hypothyroidism | 0.47 | |||
| Yes | 0.83 (0.51–1.37) | |||
| No | 1 | |||
| Hypothyroidism | 0.91 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.97 (0.52–1.80) | |||
| Other toxicity | 0.56 | |||
| Yes | 1 | |||
| No | 0.92 (0.68–1.23) | |||
| Other toxicity | 0.10 | |||
| G < 2 | 0.75 (0.53–1.06) | |||
| G ≥ 2 | 1 | |||
p < 0.05 are reported in bold
AFP alpha-feto-protein, ALBI albumin-bilirubin, BCLC Barcelona Clinic Liver Cancer, EHD extrahepatic disease, G grade, NLR neutrophil-lymphocyte ratio, OS overall survival, PS performance status, PVT portal vein thrombosis
Fig. 1.
Kaplan–Meier curves for OS according to the occurrence of decreased appetite (A) and immunotoxicity (B)
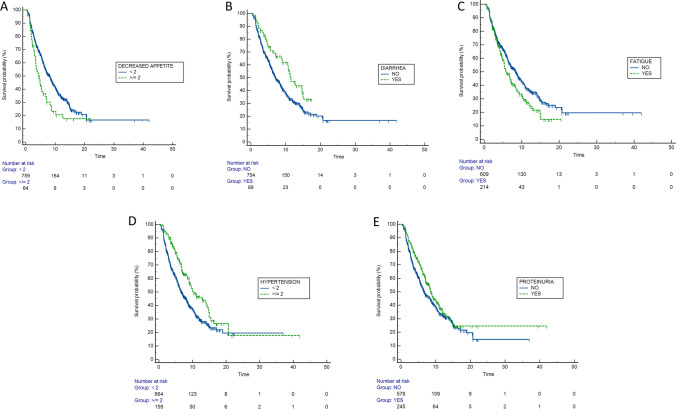
At univariate analysis, the occurrence of several AEs was related to PFS, as shown in Table 3. After adjusting for positive clinical covariates at univariate analysis, the multivariate analysis confirmed the appearance of decreased appetite G < 2 (versus G ≥ 2; HR: 0.73; 95% CI 0.43–0.95; p = 0.01), diarrhea (yes versus no; HR: 0.57; 95% CI 0.38–0.85; p = 0.01), fatigue (yes versus no; HR: 0.82; 95% CI 0.65–0.95; p < 0.01), arterial hypertension G < 2 (versus G ≥ 2; HR: 0.68; 95% CI 0.52–0.87; p < 0.01), and proteinuria (yes versus no; HR: 0.79; 95% CI 0.64–0.98; p = 0.03) as independent prognostic factors for PFS (Table 3; Fig. 2).
Table 3.
Univariate and multivariate analyses for PFS
| Characteristics | Univariate analysis PFS | Multivariate analysis PFS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex | 0.65 | |||
| Female | 0.95 (0.75–1.19) | |||
| Male | 1 | |||
| Age | 0.80 | |||
| ≤ 70 years | 1 | |||
| > 70 years | 0.98 (0.81–1.17) | |||
| Etiology | 0.39 | |||
| Viral | 0.92 (0.77–1.11) | |||
| Non–viral | 1 | |||
| BCLC | 0.03 | 0.86 | ||
| B | 0.82 (0.68–0.98) | 0.98 (0.80–1.21) | ||
| C | 1 | 1 | ||
| AFP | < 0.01 | < 0.01 | ||
| < 400 ng/mL | 0.60 (0.49–0.74) | 0.74 (0.57–0.89) | ||
| ≥ 400 ng/mL | 1 | 1 | ||
| Child–Pugh | < 0.01 | |||
| A | 0.48 (0.31–0.75) | |||
| B | 1 | |||
| NLR | < 0.01 | < 0.01 | ||
| < 3 | 0.69 (0.57–0.84) | 0.81 (0.66–0.94) | ||
| ≥ 3 | 1 | 1 | ||
| EHD | 0.85 | |||
| Yes | 1 | |||
| No | 0.98 (0.81–1.19) | |||
| ALBI | < 0.01 | 0.01 | ||
| 1 | 0.53 (0.36–0.78) | 0.74 (0.45–0.95) | ||
| 2 | 1 | 1 | ||
| PS | < 0.01 | |||
| ≤ 1 | 0.28 (0.12–0.65) | |||
| > 1 | 1 | |||
| PVT | < 0.01 | |||
| Yes | 1 | |||
| No | 0.71 (0.56–0.89) | |||
| Decreased appetite | < 0.01 | |||
| Yes | 1 | |||
| No | 0.67 (0.53–0.84) | |||
| Decreased appetite | < 0.01 | 0.01 | ||
| G < 2 | 0.55 (0.38–0.80) | 0.73 (0.43–0.95) | ||
| G ≥ 2 | 1 | 1 | ||
| Fatigue | < 0.01 | < 0.01 | ||
| Yes | 1 | 1 | ||
| No | 0.74 (0.60–0.91) | 0.82 (0.65–0.95) | ||
| Fatigue | 0.11 | |||
| G < 2 | 0.75 (0.53–1.07) | |||
| G ≥ 2 | 1 | |||
| Proteinuria | 0.03 | 0.03 | ||
| Yes | 0.80 (0.66–0.98) | 0.79 (0.64–0.98) | ||
| No | 1 | 1 | ||
| Proteinuria | 0.07 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.81 (0.64–1.02) | |||
| Hypertension | < 0.01 | |||
| Yes | 0.72 (0.59–0.87) | |||
| No | 1 | |||
| Hypertension | < 0.01 | < 0.01 | ||
| G < 2 | 1 | 1 | ||
| G ≥ 2 | 0.67 (0.54–0.83) | 0.68 (0.52–0.87) | ||
| Immunotoxicity | 0.14 | |||
| Yes | 1 | |||
| No | 0.81 (0.61–1.07) | |||
| Immunotoxicity | 0.01 | 0.07 | ||
| G < 2 | 0.55 (0.36–0.86) | 0.77 (0.42–1.06) | ||
| G ≥ 2 | 1 | 1 | ||
| Diarrhea | 0.01 | 0.01 | ||
| Yes | 0.67 (0.49–0.91) | 0.57 (0.38–0.85) | ||
| No | 1 | 1 | ||
| Diarrhea | 0.81 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.93 (0.51–1.70) | |||
| Hypothyroidism | 0.07 | |||
| Yes | 0.71 (0.49–1.03) | |||
| No | 1 | |||
| Hypothyroidism | 0.17 | |||
| G < 2 | 1 | |||
| G ≥ 2 | 0.72 (0.45–1.15) | |||
| Other toxicity | 0.21 | |||
| Yes | 0.87 (0.70–1.08) | |||
| No | 1 | |||
| Other toxicity | 0.98 | |||
| < 2 | 1 | |||
| ≥ 2 | 1.00 (0.77–1.29) | |||
p < 0.05 are reported in bold
AFP alpha-feto-protein, ALBI albumin-bilirubin, BCLC Barcelona Clinic Liver Cancer, EHD extrahepatic disease, G grade, NLR neutrophil-lymphocyte ratio, PFS progression-free survival, PS performance status, PVT portal vein thrombosis
Fig. 2.
Kaplan–Meier curves for PFS according to the occurrence of decreased appetite (A), diarrhea (B), fatigue (C), arterial hypertension (D), and proteinuria (E)
Response Evaluation
The objective response rate (ORR) was 27.3%: 35 (4.2%) had a complete response (CR), 190 (23.1%) had a partial response, 428 (52.0%) had stable disease, and 170 (20.7%) had progressive disease. We analyzed the correlation between the appearance of AEs and the response obtained. The appearance of hypothyroidism of any G (odds ratio: 0.52; 95% CI 0.28–0.97; p = 0.04) and immunotoxicity of any G (odds ratio: 0.54; 95% CI 0.36–0.82; p < 0.01) was correlated with higher ORR, while the absence of fatigue of any G (odds ratio: 3.90; 95% CI 1.18–12.87; p = 0.02) and decreased appetite of any G (odds ratio: 9.71; 95% CI 1.31–71.41; p = 0.02) were correlated with more CR (Table 4). There were no differences in baseline characteristics between patients presenting and not presenting with immunotoxicity and hypothyroidism during treatment with atezolizumab plus bevacizumab (Supplementary Table 2). Patients presenting with decreased appetite more frequently had non-viral etiology (p < 0.01), an advanced stage (BCLC-C; p = 0.03), and PS ≥ 1 (p < 0.01) compared with patients not presenting with decreased appetite, while patients with presenting fatigue more frequently had macrovascular invasion compared with patients not presenting with fatigue (p = 0.03; Supplementary Table 3).
Table 4.
ORR and CR in patients treated with atezolizumab plus bevacizumab
| N (%) | p value | Odds ratio | N (%) | p value | Odds ratio | ||
|---|---|---|---|---|---|---|---|
|
Decreased appetite ORR No ORR |
49 (27.8%) 127 (72.1%) |
0.87 | 0.97 |
Decreased appetite CR No CR |
1 (0.6%) 175 (99.4%) |
0.02 | 9.71 |
|
No decreased appetite ORR No ORR |
176 (27.2%) 471 (72.8%) |
(0.67–1.40) |
No decreased appetite CR No CR |
34 (5.2%) 613 (94.7%) |
(1.31–71.41) | ||
|
Decreased appetite G < 2 ORR No ORR |
210 (27.7%) 549 (72.3%) |
0.47 | 0.80 |
Decreased appetite G < 2 CR No CR |
35 (4.6%) 724 (95.4%) |
0.20 | 0.16 |
|
Decreased appetite G ≥ 2 ORR No ORR |
15 (23.4%) 49 (76.6%) |
(0.44–1.46) |
Decreased appetite G ≥ 2 CR No CR |
0 (0.0%) 64 (100.0%) |
(0.01–2.61) | ||
|
Fatigue ORR No ORR |
49 (22.9%) 165 (77.1%) |
0.09 | 1.37 |
Fatigue CR No CR |
3 (1.4%) 211 (98.6%) |
0.02 | 3.90 |
|
No fatigue ORR No ORR |
176 (28.9%) 433 (71.1%) |
(0.95–1.97) |
No fatigue CR No CR |
32 (5.2%) 577 (94.7%) |
(1.18–12.87) | ||
|
Fatigue G < 2 ORR No ORR |
212 (28.1%) 542 (71.9%) |
0.10 | 0.59 |
Fatigue G < 2 CR No CR |
35 (4.6%) 719 (95.3%) |
0.18 | 0.14 |
|
Fatigue G ≥ 2 ORR No ORR |
13 (18.8%) 56 (81.1%) |
(0.32–1.11) |
Fatigue G ≥ 2 CR No CR |
0 (0.0%) 69 (100.0%) |
(0.01–2.40) | ||
|
Proteinuria ORR No ORR |
78 (32.0%) 166 (68.0%) |
0.05 | 0.72 |
Proteinuria CR No CR |
13 (5.3%) 231 (94.7%) |
0.32 | 0.70 |
|
No proteinuria ORR No ORR |
147 (25.4%) 432 (74.6%) |
(0.52–1.00) |
No proteinuria CR No CR |
22 (3.8%) 557 (96.2%) |
(0.35–1.42) | ||
|
Proteinuria G < 2 ORR No ORR |
183 (26.9%) 498 (73.1%) |
0.51 | 1.14 |
Proteinuria G < 2 CR No CR |
31 (4.5%) 650 (95.4%) |
0.36 | 0.61 |
|
Proteinuria G ≥ 2 ORR No ORR |
42 (29.6%) 100 (70.4%) |
(0.77–1.70) |
Proteinuria G ≥ 2 CR No CR |
4 (2.8%) 138 (97.2%) |
(0.21–1.75) | ||
|
Hypertension ORR No ORR |
71 (31.7%) 153 (68.3%) |
0.09 | 0.74 |
Hypertension CR No CR |
12 (5.3%) 212 (94.6%) |
0.34 | 0.70 |
|
No hypertension ORR No ORR |
154 (25.7%) 445 (74.3%) |
(0.53–1.04) |
No hypertension CR No CR |
23 (3.8%) 576 (96.2%) |
(0.34–1.44) | ||
|
Hypertension G < 2 ORR No ORR |
179 (26.9%) 485 (73.0%) |
0.61 | 1.10 |
Hypertension G < 2 CR No CR |
27 (4.1%) 637 (95.9%) |
0.59 | 1.25 |
|
Hypertension G ≥ 2 ORR No ORR |
46 (28.9%) 113 (71.1%) |
(0.75–1.62) |
Hypertension G ≥ 2 CR No CR |
8 (5.0%) 151 (95.0%) |
(0.56–2.81) | ||
|
Immunotoxicity ORR No ORR |
43 (38.7%) 68 (61.3%) |
< 0.01 | 0.54 |
Immunotoxicity CR No CR |
6 (5.7%) 105 (94.6%) |
0.52 | 0.74 |
|
No immunotoxicity ORR No ORR |
182 (25.6%) 530 (74.4%) |
(0.36–0.82) |
No immunotoxicity CR No CR |
29 (26.1%) 683 (95.9%) |
(0.30–1.83) | ||
|
Immunotoxicity G < 2 ORR No ORR |
210 (27.2%) 563 (72.8%) |
0.66 | 1.15 |
Immunotoxicity G < 2 CR No CR |
34 (4.4%) 739 (95.6%) |
0.43 | 0.44 |
|
Immunotoxicity G ≥ 2 ORR No ORR |
15 (30.0%) 35 (70.0%) |
(0.62–2.15) |
Immunotoxicity G ≥ 2 CR No CR |
1 (2.0%) 49 (98.0%) |
(0.06–3.31) | ||
|
Diarrhea ORR No ORR |
15 (22.4%) 52 (77.6%) |
0.34 | 1.33 |
Diarrhea CR No CR |
2 (3.0%) 65 (97.0%) |
0.59 | 1.48 |
|
No diarrhea ORR No ORR |
210 (27.8%) 546 (72.2%) |
(0.73–2.42) |
No diarrhea CR No CR |
33 (4.4%) 723 (95.6%) |
(0.35–6.32) | ||
|
Diarrhea G < 2 ORR No ORR |
222 (27.6%) 582 (72.4%) |
0.26 | 0.49 |
Diarrhea G < 2 CR No CR |
35 (4.3%) 769 (95.6%) |
0.68 | 0.55 |
|
Diarrhea G ≥ 2 ORR No ORR |
3 (15.8%) 16 (84.2%) |
(0.14–1.70) |
Diarrhea G ≥ 2 CR No CR |
0 (0.0%) 19 (100.0%) |
(0.03–9.39) | ||
|
Hypothyroidism ORR No ORR |
18 (40.9%) 26 (59.1%) |
0.04 | 0.52 |
Hypothyroidism CR No CR |
2 (4.5%) 42 (95.4%) |
0.92 | 0.93 |
|
No hypothyroidism ORR No ORR |
207 (26.6%) 572 (73.4%) |
(0.28–0.97) |
No hypothyroidism CR No CR |
33 (4.2%) 746 (95.8%) |
(0.21–4.00) | ||
|
Hypothyroidism G < 2 ORR No ORR |
212 (26.7%) 581 (73.3%) |
0.05 | 2.09 |
Hypothyroidism G < 2 CR No CR |
33 (4.2%) 760 (95.8%) |
0.51 | 1.64 |
|
Hypothyroidism G ≥ 2 ORR No ORR |
13 (43.3%) 17 (56.7%) |
(1.00–4.39) |
Hypothyroidism G ≥ 2 CR No CR |
2 (6.7%) 28 (93.3%) |
(0.37–7.20) | ||
|
Other toxicity ORR No ORR |
50 (30.5%) 114 (69.5%) |
0.31 | 0.82 |
Other toxicity CR No CR |
8 (4.9%) 156 (95.1%) |
0.66 | 0.83 |
|
No other toxicity ORR No ORR |
175 (26.5%) 484 (73.4%) |
(0.57–1.19) |
No other toxicity CR No CR |
27 (4.1%) 632 (95.9%) |
(0.37–1.87) | ||
|
Other toxicity G < 2 ORR No ORR |
191 (27.0%) 517 (73.0%) |
0.56 | 1.14 |
Other toxicity G < 2 CR No CR |
30 (4.2%) 678 (95.8%) |
0.96 | 1.03 |
|
Other toxicity G ≥ 2 ORR No ORR |
34 (29.6%) 81 (70.4%) |
(0.74–1.75) |
Other toxicity G ≥ 2 CR No CR |
5 (4.3%) 110 (95.6%) |
(0.39–2.70) |
The reference groups are indicated in bold
CR complete response, G grade, ORR objective response rate
Discussion
Ours is the first international real-world investigation to explore the correlation between AEs and outcomes in a large cohort of patients with HCC treated with the combination of atezolizumab plus bevacizumab. In our analysis, at least one AE was observed in 73.3% of patients, with G 3 AEs occurring at a frequency of 17.3%. The most common AEs were proteinuria (29.6%), arterial hypertension (27.2%), and fatigue (26.0%). These data are consistent with those from a retrospective study conducted by Tada and colleagues, who reported a frequency ≥ 20% for proteinuria, decreased appetite, and fatigue in a cohort of 263 patients with HCC treated with atezolizumab plus bevacizumab [39]. These real-world results confirmed findings from prospective studies. Similarly, the same AEs were the most frequent in the pivotal phase III IMbrave 150 trial, in which 86.0% of patients receiving the combination experienced at least one AE, with 43.0% of these being G 3 or 4 [5]. Very similar data were also observed in another prospective study, the phase II–III ORIENT-32 trial, which investigated the efficacy of combining a PD-1 inhibitor, sintilimab, with a biosimilar bevacizumab in the first-line setting for 595 Chinese patients with HCC. This trial reported that 88.7% of patients experienced at least one AE, of which 33.7% were G 3/4 [41].
Fatigue and decreased appetite are well-known symptoms characterizing patients with impaired liver function and advanced disease; both are often present in the neoplastic cachexia scenarios that can arise in these cases [42–45]. In our population, patients who experienced these symptoms more frequently had advanced-stage disease (p = 0.03), along with macrovascular invasion (p = 0.03), and worse PS (p < 0.01). It is not surprising, then, that G ≥ 2 decreased appetite was associated with both worse OS (HR: 0.60 95% CI 0.13–0.90; p < 0.01) and worse PFS (HR: 0.73; 95% CI 0.43–0.95; p = 0.01). The onset of fatigue of any G was identified as a negative prognostic factor for PFS (HR: 0.82; 95% CI 0.65–0.95; p < 0.01), and the absence of these symptoms correlated with higher CR rates (Table 4). Our findings are consistent with the multivariate analyses conducted by Japanese colleagues, who highlighted how the appearance of fatigue of any G is independently associated with worse OS (HR: 2.35; 95% CI 1.30–4.51; p = 0.01) [39]. Despite having completely different mechanisms of action, decreased appetite and fatigue have been linked to worse outcomes even in patients treated with lenvatinib as a first-line therapy [9–11]. Therefore, these negative prognostic factors need to be carefully considered in daily clinical practice for the proper management of patients with HCC.
A crucial point of our discussion is certainly the correlation between immune-related AEs and the efficacy of immunological therapies, a topic that has been widely discussed for several years in various disease contexts. Indeed, we are aware that, in patients with non-small-cell lung cancer, melanoma, urothelial carcinoma, and other conditions, the occurrence of immunotoxicity correlates with improved efficacy outcomes [30–37]. This aspect remains a field to be explored in HCC treatment. The most significant data available to us stem from an FDA pooled analysis conducted by Pinato and colleagues on a cohort of 406 patients with HCC treated with immunotherapy within international clinical trials. This analysis highlighted how the development of immune-related G ≥ 2 AEs was linked to longer OS (HR: 0.49; 95% CI 0.34–0.70) and PFS (HR: 0.43; 95% CI 0.32–0.59). The authors further confirmed these correlations in a cohort of 357 patients from an international consortium comprising 10 centers [38]. The other significant data we have available are from the Japanese study mentioned above. However, this study did not identify any significant correlation between immune-related AEs and the efficacy outcomes of the combination of atezolizumab plus bevacizumab [39]. These data appear to be inconsistent, but it is important to consider that the results of the Japanese study might have been influenced by the small sample size. Additionally, in the FDA pooled analysis, the population cohorts were very heterogeneous from a standpoint, including patients treated with both immunological combinations and monotherapies, as well as different molecules [38]. Amidst this complex landscape, our data demonstrate that the emergence of G ≥ 2 immunotoxicity is associated with worse OS (HR: 0.70; 95% CI 0.24–0.99; p = 0.04). Moreover, the appearance of immunotoxicity of any G is linked to a higher response rate (p < 0.01). Furthermore, our analysis suggests that the onset of diarrhea of any G is associated with a longer PFS (HR: 0.57; 95% CI 0.38–0.85; p = 0.01), while the development of hypothyroidism of any G relates to a higher ORR (p = 0.04). These data may appear contradictory, as the onset of a response is a well-known positive prognostic factor for patients with HCC [46, 47]. Some careful reflections are therefore necessary. The first point to consider is that we did not observe any differences in baseline characteristics between patients who experienced immunotoxicity and those who did not, or between patients who experienced hypothyroidism and those who did not. This could lead us to the conclusion that no other factors related to patients’ baseline characteristics might have influenced response rates or median OS and PFS. The fact that the increase in response rates in those with immunotoxicity does not necessarily translate into an increase in survival could be explained by considering that, in clinical practice, higher G AEs often result in long-term or permanent therapy interruption and in the use of steroid-based therapeutic regimens, with potentially negative repercussions on efficacy and the overall trend of median survival. There are still incompletely clarified aspects underlying the biological mechanisms that cause immune-related AEs and the use of high-dose steroids for their treatment. We are, in fact, aware of the immunosuppressive effect of steroid therapy, but numerous studies have shown how its use not only does not involve a reduction in the effectiveness of immunotherapy but also, if used to treat immune-related AEs, translates into better outcomes given by the possibility for patients to continue antineoplastic treatment [48, 49]. The second reflection pertains to the results regarding diarrhea and hypothyroidism, two AEs typically linked to atezolizumab-induced activation of the immune system. HCC clinicians have been familiar with managing these AEs for years, as they are quite common even in patients treated in first-line with sorafenib or lenvatinib, even though they are caused by entirely different mechanisms of action [2–4, 8–29]. Given their early and timely management in daily clinical practice, these AEs rarely escalate into higher-G immune-related toxicities, which our analysis identifies as factors independently correlated with worse survival. Certainly, further studies are required to definitively clarify these aspects and solidify our understanding of the biological phenomena underpinning the efficacy and toxicity induced by immunotherapy in the treatment of patients with HCC, just as has been done for other pathologies [50–54].
Another important aspect of our discussion concerns two AEs typically related to bevacizumab administration: arterial hypertension and proteinuria. Our data demonstrate that the occurrence of G ≥ 2 hypertension or any G proteinuria is associated with longer PFS. These findings are consistent with the multivariate analysis from the Japanese study, which also indicates a positive correlation between PFS and patients with any G of arterial hypertension [39]. Proteinuria and arterial hypertension are the result of the nephrotoxic action and damage to the vascular endothelium induced by VEGF inhibitory drugs, such as bevacizumab, lenvatinib, and sorafenib [55, 56]. Even in patients treated in the first-line setting with these TKIs, there is ample evidence of a positive correlation between survival outcomes and the occurrence of these AEs [10, 11, 20–22]. In the case of the combination of atezolizumab plus bevacizumab, it was highlighted that proteinuria was the AE that most frequently (29.7%) caused early bevacizumab interruption, resulting in a negative prognostic factor for both OS and PFS, as demonstrated in an interesting Japanese study involving 239 patients with HCC [57]. All this further reinforces the awareness we already had about the key role of the VEGF pathway in HCC pathogenesis and how its inhibition is central to the mechanisms underlying the demonstrated efficacy of the combination of atezolizumab plus bevacizumab [58].
Our study has some limitations. The primary limitation is its retrospective nature, which means we cannot rule out biases and gaps in the collected data, especially those related to minor AEs that may not have been reported correctly. Moreover, the available data did not allow the analysis of all types of immune-related AEs, except for diarrhea and hypothyroidism, and the potential correlation between steroid treatment of immune-related AEs and patients’ outcomes. Another important limitation is the lack of a centralized review of reevaluation exams, which would have ensured more robust data regarding response rates and median survival. Nonetheless, our study provides valuable insight into our real-world daily clinical practice, encompassing a large population of both Eastern and Western patients from five different countries. However, further research is needed to confirm our findings and to address the limitations of our study.
In conclusion, similar to what has been demonstrated for other therapies used in HCC treatment, there is a correlation between the occurrence of AEs and patient outcomes for the combination of atezolizumab plus bevacizumab as well. The presence of G < 2 decreased appetite or G < 2 immunotoxicity was found to be a positive prognostic factor for OS, while the occurrence of any grade proteinuria or diarrhea or G ≥ 2 hypertension was associated with improved PFS. This understanding is crucial for further enhancing our daily clinical practice and providing patients with HCC with the best possible management of the currently available therapeutic strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement.
Conflict of interest
Andrea Casadei-Gardini is an advisor for AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, GSK, and MSD and received grants and personal fees from Bayer, Eisai, and MSD. Mario Scartozzi is an advisor for AMGEN, Eisai, MERCK, MSD, and SERVIER. Masatoshi Kudo received research grants from AbbVie, Astellas Pharma, Bayer, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Medico’s Hirata, MSD, Otsuka, Sumitomo Dainippon, Takeda, and Taiho; received advisory consulting fees from BMS, Chugai, Eisai, MSD, Ono pharmaceutical, and Taiho; and received lecture fees from Bayer, Chugai, EA Pharma, Eisai, and MSD. Mara Persano, Margherita Rimini, Toshifumi Tada, Goki Suda, Shigeo Shimose, Federico Rossari, Changhoon Yoo, Jaekyung Cheon, Fabian Finkelmeier, Ho Yeong Lim, José Presa, Gianluca Masi, Francesca Bergamo, Elisabeth Amadeo, Francesco Vitiello, Takashi Kumada, Naoya Sakamoto, Hideki Iwamoto, Tomoko Aoki, Hong Jae Chon, Vera Himmelsbach, Massimo Alberto Iavarone, Giuseppe Cabibbo, Margarida Montes, Francesco Giuseppe Foschi, Caterina Vivaldi, Caterina Soldà, Takuya Sho, Takashi Niizeki, Naoshi Nishida, Christoph Steup, Mariangela Bruccoleri, Masashi Hirooka, Kazuya Kariyama, Joji Tani, Masanori Atsukawa, Koichi Takaguchi, Ei Itobayashi, Shinya Fukunishi, Kunihiko Tsuji, Toru Ishikawa, Kazuto Tajiri, Hironori Ochi, Satoshi Yasuda, Hidenori Toyoda, Chikara Ogawa, Takashi Nishimura, Takeshi Hatanaka, Satoru Kakizaki, Noritomo Shimada, Kazuhito Kawata, Atsushi Hiraoka, Fujimasa Tada, Hideko Ohama, Kazuhiro Nouso, Asahiro Morishita, Akemi Tsutsui, Takuya Nagano, Norio Itokawa, Tomomi Okubo, Michitaka Imai, Hisashi Kosaka, Atsushi Naganuma, Yohei Koizumi, Shinichiro Nakamura, Masaki Kaibori, Hiroko Iijima, Yoichi Hiasa, Silvia Foti, Silvia Camera, Fabio Piscaglia, and Stefano Cascinu declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
The ethics committee of each involved center approved this trial, in agreement with the provisions of the Good Clinical Practice guidelines, the Declaration of Helsinki, the local laws, and the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 regarding the protection of natural persons and the processing of personal data.
Consent to participate
All patients provided written informed consent before enrollment in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
Conception and design: Andrea Casadei-Gardini, Mara Persano, and Margherita Rimini. Acquisition of data (acquired and managed patients): All authors. Analysis and interpretation of data: Andrea Casadei-Gardini, Mara Persano, and Margherita Rimini. Writing, review, and/or revision of the manuscript: Andrea Casadei-Gardini, Mara Persano, and Margherita Rimini. Final approval of manuscript: All authors.
Footnotes
Mara Persano and Margherita Rimini are co-first authors.
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomized phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Ghassan KAA, George L, Cheng AL, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM. 2022;1(8):EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 7.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung MW, Finn RS, Qin S, et al. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT) J Clin Oncol. 2019;37(4):317. [Google Scholar]
- 9.Ohki T, Sato K, Kondo M, et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs Real World Outcomes. 2020;7:141–149. doi: 10.1007/s40801-020-00179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimose S, Iwamoto H, Niizeki T, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel). 2020;12(7):1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapposelli IG, Tada T, Shimose S, et al. Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int. 2021;41:2997–3008. doi: 10.1111/liv.15014. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512–519. doi: 10.1007/s00535-016-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koizumi Y, Hirooka M, Hiraoka A, et al. Lenvatinib-induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr J. 2019;66(9):787–792. doi: 10.1507/endocrj.EJ19-0140. [DOI] [PubMed] [Google Scholar]
- 14.Shomura M, Okabe H, Sato E, et al. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers (Basel). 2020;12(11):3078. doi: 10.3390/cancers12113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persano M, Casadei-Gardini A, Burgio V, Scartozzi M, Cascinu S, Rimini M. Five years of lenvatinib in hepatocellular carcinoma: are there any predictive and/or prognostic factors? Expert Rev Anticancer Ther. 2023;23(1):19–27. doi: 10.1080/14737140.2023.2156340. [DOI] [PubMed] [Google Scholar]
- 16.Marisi G, Cucchetti A, Ulivi P, et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J Gastroenterol. 2018;24(36):4152–4163. doi: 10.3748/wjg.v24.i36.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85–92. doi: 10.1634/theoncologist.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Tan G, Zhu M, Li W, Zhai B, Sun X. Hand-foot skin reaction is a beneficial indicator of sorafenib therapy for patients with hepatocellular carcinoma: a systemic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:1–8. doi: 10.1080/17474124.2017.1373018. [DOI] [PubMed] [Google Scholar]
- 20.Casadei Gardini A, Scarpi E, Marisi G, et al. Early onset of hypertension and serum electrolyte changes as potential predictive factors of activity in advanced HCC patients treated with sorafenib: results from a retrospective analysis of the HCC-AVR group. Oncotarget. 2016;7:15243–15251. doi: 10.18632/oncotarget.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong BY, Ni CF, Chen L, Zhu HD, Teng GJ. Early sorafenib-related biomarkers for combination treatment with transarterial chemoembolization and sorafenib in patients with hepatocellular carcinoma. Radiology. 2017;284:583–592. doi: 10.1148/radiol.2017161975. [DOI] [PubMed] [Google Scholar]
- 22.Howell J, Pinato DJ, Ramaswami R, et al. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-center, prospective study. Aliment Pharmacol Ther. 2017;45:1146–1155. doi: 10.1111/apt.13977. [DOI] [PubMed] [Google Scholar]
- 23.Shin SY, Lee YJ. Correlation of skin toxicity and hypertension with clinical benefit in advanced hepatocellular carcinoma patients treated with sorafenib. Int J Clin Pharmacol Ther. 2013;51:837–846. doi: 10.5414/CP201907. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka T, Eguchi Y, Kawazoe S, et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2012;42:879–886. doi: 10.1111/j.1872-034X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 25.Bettinger D, Schultheiss M, Knüppel E, Thimme R, Blum HE, Spangenberg HC. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2012;56:789–790. doi: 10.1002/hep.25637. [DOI] [PubMed] [Google Scholar]
- 26.Koschny R, Gotthardt D, Koehler C, Jaeger D, Stremmel W, Ganten TM. Diarrhea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology. 2013;84:6–13. doi: 10.1159/000342425. [DOI] [PubMed] [Google Scholar]
- 27.Di Costanzo GG, De Stefano G, Tortora R, et al. Sorafenib off-target effects predict outcomes in patients treated for hepatocellular carcinoma. Future Oncol. 2015;11:943–951. doi: 10.2217/fon.14.291. [DOI] [PubMed] [Google Scholar]
- 28.Di Costanzo GG, Casadei Gardini A, Marisi G, et al. Validation of a simple scoring system to predict sorafenib effectiveness in patients with hepatocellular carcinoma. Target Oncol. 2017;12:795–803. doi: 10.1007/s11523-017-0522-5. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Rahman O, Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic review and metaanalysis of the impact on survival. Expert Rev Gastroenterol Hepatol. 2017;11:75–83. doi: 10.1080/17474124.2017.1264874. [DOI] [PubMed] [Google Scholar]
- 30.Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37:2730e7. doi: 10.1200/JCO.19.00318. [DOI] [PubMed] [Google Scholar]
- 31.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374e8. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479e85. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
- 33.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71e4. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Lisberg A, Tucker DA, Goldman JW, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6:288e94. doi: 10.1158/2326-6066.CIR-17-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886e94. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773e81. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 37.Cortellini A, Buti S, Agostinelli V, et al. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;46:362e71. doi: 10.1053/j.seminoncol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Pinato DJ, Marron TU, Mishra-Kalyani PS, et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. 2021;157:140–152. doi: 10.1016/j.ejca.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Tada T, Kumada T, Hiraoka A, et al. Adverse events as potential predictive factors of therapeutic activity in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancer Med. 2022;00:1–12. doi: 10.1002/cam4.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Ren Z, Fan J, Xu J, et al. LBA2: Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32) Ann Oncol. 2020;31:S1287. [Google Scholar]
- 42.Rimini M, Persano M, Tada T, et al. Survival outcomes from atezolizumab plus bevacizumab versus lenvatinib in Child Pugh B unresectable hepatocellular carcinoma patients. J Cancer Res Clin Oncol. 2023;149(10):7565–7577. doi: 10.1007/s00432-023-04678-2. [DOI] [PubMed] [Google Scholar]
- 43.Persano M, Rimini M, Tada T, et al. Role of the Prognostic nutritional index in predicting survival in advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Oncology. 2023;101(5):283–291. doi: 10.1159/000528818. [DOI] [PubMed] [Google Scholar]
- 44.Rimini M, Kang W, Burgio V, et al. Validation of the easy-to-use lenvatinib prognostic index to predict prognosis in advanced hepatocellular carcinoma patients treated with lenvatinib. Hepatol Res. 2022;52(12):1050–1059. doi: 10.1111/hepr.13824. [DOI] [PubMed] [Google Scholar]
- 45.Rapposelli IG, Shimose S, Kumada T, et al. Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open. 2021;6(4):100190. doi: 10.1016/j.esmoop.2021.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudo M, Finn RS, Qin S, et al. Analysis of survival and objective response (OR) in patients with hepatocellular carcinoma in a phase III study of lenvatinib (REFLECT) J Clin Oncol. 2019;37(4):186. [Google Scholar]
- 47.Persano M, Rimini M, Tada T, et al. Clinical outcomes with atezolizumab plus bevacizumab or lenvatinib in patients with hepatocellular carcinoma: a multicenter real-world study. J Cancer Res Clin Oncol. 2023;149(9):5591–5602. doi: 10.1007/s00432-022-04512-1. [DOI] [PubMed] [Google Scholar]
- 48.Fessas P, Possamai LA, Clark J, et al. Immunotoxicity from checkpoint inhibitor therapy: clinical features and underlying mechanisms. Immunology. 2020;159(2):167–177. doi: 10.1111/imm.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson DB. Toxicities and outcomes: do steroids matter? Cancer. 2018;124(18):3638–3640. doi: 10.1002/cncr.31627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo JA, Fisher DE, Flaherty KT. Prognostic significance of cutaneous adverse events associated with pembrolizumab therapy. JAMA Oncol. 2015;1:1340–1341. doi: 10.1001/jamaoncol.2015.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 55.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200. doi: 10.1681/ASN.2018080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu C, Rimassa L, Sun HC, Vogel A, Kaseb AO. Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther Adv Med Oncol. 2021;13:17588359211031141. doi: 10.1177/17588359211031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatanaka T, Hiraoka A, Tada T, et al. Association of early bevacizumab interruption with efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma: a landmark analysis. Hepatol Res. 2022;52(5):462–470. doi: 10.1111/hepr.13748. [DOI] [PubMed] [Google Scholar]
- 58.Llovet JM, Pinyol R, Kelley RK, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022;3(4):386–401. doi: 10.1038/s43018-022-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.