Abstract
Purpose
Although comprehensive and widespread guidelines on how to conduct systematic reviews of outcome measurement instruments (OMIs) exist, for example from the COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) initiative, key information is often missing in published reports. This article describes the development of an extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline: PRISMA-COSMIN for OMIs 2024.
Methods
The development process followed the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) guidelines and included a literature search, expert consultations, a Delphi study, a hybrid workgroup meeting, pilot testing, and an end-of-project meeting, with integrated patient/public involvement.
Results
From the literature and expert consultation, 49 potentially relevant reporting items were identified. Round 1 of the Delphi study was completed by 103 panelists, whereas round 2 and 3 were completed by 78 panelists. After 3 rounds, agreement (≥67%) on inclusion and wording was reached for 44 items. Eleven items without consensus for inclusion and/or wording were discussed at a workgroup meeting attended by 24 participants. Agreement was reached for the inclusion and wording of 10 items, and the deletion of 1 item. Pilot testing with 65 authors of OMI systematic reviews further improved the guideline through minor changes in wording and structure, finalized during the end-of-project meeting. The final checklist to facilitate the reporting of full systematic review reports contains 54 (sub)items addressing the review’s title, abstract, plain language summary, open science, introduction, methods, results, and discussion. Thirteen items pertaining to the title and abstract are also included in a separate abstract checklist, guiding authors in reporting for example conference abstracts.
Conclusion
PRISMA-COSMIN for OMIs 2024 consists of two checklists (full reports; abstracts), their corresponding explanation and elaboration documents detailing the rationale and examples for each item, and a data flow diagram. PRISMA-COSMIN for OMIs 2024 can improve the reporting of systematic reviews of OMIs, fostering their reproducibility and allowing end-users to appraise the quality of OMIs and select the most appropriate OMI for a specific application.
Note
In order to encourage its wide dissemination this article is freely accessible on the web sites of the journals: Health and Quality of Life Outcomes; Journal of Clinical Epidemiology; Journal of Patient-Reported Outcomes; Quality of Life Research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41687-024-00727-7.
Keywords: Systematic reviews, Outcome measurement instrument, Reporting guideline, Measurement properties, PRISMA, COSMIN
Plain language summary
Outcome measurement instruments are tools that measure certain aspects of health. When researchers want to know which tool is the best for their study, they do something called a systematic review. They gather all facts about these tools from the scientific literature, put them together, and then make a decision on which tool is the best for their research. The problem is that too many systematic review reports about these tools are missing important information. This makes it hard for readers of these reviews to understand them clearly and to pick the best tool. This study tried to solve this problem by creating a new guideline, called “PRISMA-COSMIN for Outcome Measurement Instruments”. This guideline helps researchers to report their reviews on tools in a clear and thorough way. The study identified 54 things that should be reported in any review of tools, covering everything from the report’s title to the discussion section. PRISMA-COSMIN for Outcome Measurement Instruments will make the reporting of these reviews of tools better, so people can understand them and choose the right tool for their needs.
Introduction
An outcome measurement instrument (OMI) refers to the tool used to measure a health outcome domain. Different types of OMIs exist, such as questionnaires or patient-reported outcome measures (PROMs) and its variations, clinical rating scales, performance-based tests, laboratory tests, scores obtained through a physical examination or observations of an image, or responses to single questions [1, 2]. OMIs are used to monitor patients’ health status and evaluate treatments in research and clinical practice [3, 4]. Systematic reviews of OMIs synthesize data from primary studies on the OMIs’ measurement properties, feasibility, and interpretability to provide insight into the suitability of an OMI for a particular use [2]. Systematic reviews of OMIs are an important tool in the evidence-based selection of an OMI for research and/or clinical practice.
Several organizations have developed methodology for conducting systematic reviews of OMIs, including Outcome Measures in Rheumatology (OMERACT) [5], JBI (formerly Joanna Briggs Institute) [6], and the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) initiative [2], the latter being the most widely used. Despite the availability of methodological guidance on the conduct of OMI systematic reviews, such reviews are often not reported completely [7–9]. For example, a recent study into the quality of 100 recent OMI systematic reviews shows that reporting is lacking on feasibility and interpretability aspects of OMIs, the process of data synthesis, raw data on measurement properties, and the number of independent reviewers involved in each of the steps of the review process (unpublished data). Incomplete reporting limits reproducibility and hinders the selection of the most suitable OMI for a specific application [10]. At present, a reporting guideline for systematic reviews of OMIs does not exist.
Reporting guidelines outline a minimum set of items to include in research reports, and their endorsement by journals has been shown to improve adherence, methodological transparency, and uptake of findings [11–13]. To improve the reporting of systematic reviews, the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline was developed, containing a checklist, an explanation and elaboration (E&E) document, and flow diagrams [14]. Endorsement of PRISMA has resulted in improved quality of reporting and methodological quality of systematic reviews [15]. PRISMA has been updated in 2020 and is primarily focused on systematic reviews of interventions [16]. Although systematic reviews of OMIs share common elements with systematic reviews of interventions, there are also several differences: for example, in a systematic review of OMIs, multiple reviews (i.e., one review per measurement property) are often included [17], and effect measures and evidence synthesis methods are different in systematic reviews of OMIs. As such, some PRISMA 2020 items are not appropriate for systematic reviews of OMIs, other items need to be adapted, and some items that are important are not included.
There is thus a need for reporting guidance specifically for systematic reviews of OMIs [18], which might also help to reduce the ongoing publication of poor-quality reviews in the literature [7, 8]. This study therefore aimed to develop the PRISMA-COSMIN for OMIs 2024 guideline as a stand-alone extension of PRISMA 2020 [16]. New in reporting guideline development, this study also aimed to integrate patient/public involvement in the development of PRISMA-COSMIN for OMIs 2024, as patients/members of the public are ultimately impacted by the results of these systematic reviews.
Methods
Details on integrating patient/public involvement in the development of PRISMA-COSMIN for OMIs 2024, our lessons learned and recommendations for future reporting guideline developers are outlined elsewhere [19]. Patient/public involvement has been reported according to the GRIPP2 short form reporting checklist in the current manuscript [20].
Project launch and preparation
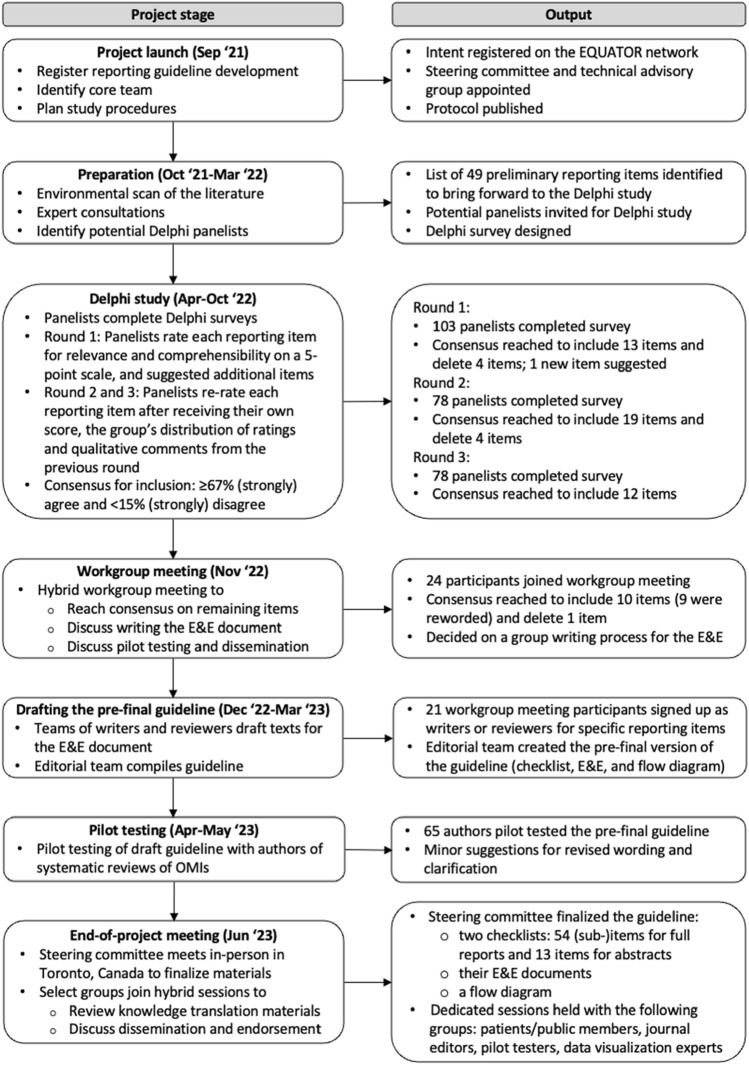
We registered the development of PRISMA-COSMIN for OMIs 2024 on the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) website [21] and the Open Science Framework [22]. Figure 1 shows the PRISMA-COSMIN for OMIs 2024 development process. A protocol was published previously [23] and Online Resource 1 states deviations from the protocol. The protocol details the project launch, preparation and PRISMA-COSMIN for OMIs 2024 item generation process. Briefly, a steering committee for project oversight, including a patient partner, and a technical advisory group for support and feedback were appointed (Online Resource 2 shows group membership). In the item generation process, we used PRISMA 2020 as the framework on which to modify, add, or delete items [16]. Potential items were identified by searching the literature for scientific articles and existing guidelines that describe potentially relevant reporting recommendations [2, 5, 6, 16, 24–51]. We applied this initial list of items to three different types of OMI systematic reviews: a systematic review of all available PROMs that measure a certain outcome domain in a certain population [52], a systematic review of one specific PROM [53], and a systematic review of a non-PROM (digital monitoring devices for oxygen saturation and respiratory rate) [54]. Application of the initial item list to these systematic reviews resulted in supporting, refuting, refining and supplementing the items. Findings were shared with the steering committee and technical advisory group, resulting in a list of preliminary items that were presented during the first round of the Delphi study [23].
Fig. 1.
Development process of PRISMA-COSMIN for OMIs 2024. E&E explanation and elaboration; EQUATOR Enhancing the QUAlity and Transparency Of health Research; OMI outcome measurement instrument
Delphi study
We conducted a 3-round international Delphi study between April and September 2022 using Research Electronic Data Capture (REDCap) [55]. The aim of the Delphi study was to obtain consensus on the inclusion and wording of items for PRISMA-COSMIN for OMIs 2024. We invited persons involved in the design, conduct, publication, and/or application of systematic reviews of OMIs as panelists. They were identified by the steering committee (researchers in the steering committee were not able to participate) and from other relevant Delphi studies [1, 40, 56–58]. Persons who co-authored at least three systematic reviews of OMIs, identified through the COSMIN database for systematic reviews [59], were also invited. Invitees could forward the invitation to other qualified colleagues. Besides the patient partner, we selected five patients/members of the public to join through newsletters and contact persons of relevant organizations [60–63]. Patients/members of the public attended a 90-min virtual onboarding session led by the patient partner and project lead with information about the purpose of the study, OMIs, systematic reviews, reporting guidelines, and the Delphi method. Support was offered throughout the process, if needed.
Registered panelists were invited for each round, irrespective of their responses to previous rounds. Each round was open for approximately four weeks, and weekly reminders were sent two weeks after the initial invitation. For each proposed item, panelists indicated whether it should be reported in a systematic review of OMIs, and whether the wording was clear. Both questions were scored on a five-point Likert scale: strongly disagree, disagree, neutral, agree, strongly agree. Panelists could also opt to select ‘not my expertise’; these responses were not included for calculating consensus. As decided a priori, consensus for inclusion was achieved when at least 67% of the panelists agreed or strongly agreed with a proposal [24, 56, 57, 64] and less than 15% disagreed or strongly disagreed [1, 58]. Panelists were encouraged to provide a rationale for their ratings and suggestions for improved wording.
In round 1, panelists also voted on original PRISMA 2020 items that were thought to have limited relevance for systematic reviews of OMIs. For these items, panelists indicated whether they were indeed not applicable, using the five-point Likert scale described above. In addition, panelists were asked to suggest new items not included in the list. Round 2 of the Delphi study included all round 1 items (except original PRISMA 2020 items that achieved consensus for inclusion and wording), as well as any new items that were suggested during round 1. If panelists made compelling arguments for the deletion of an item in round 1, this was brought forward in round 2, where panelists indicated whether they agreed with the deletion. Round 3 included items that did not reach consensus during rounds 1 or 2, or items with modified wording.
Following each round, frequencies of responses across all panelists and for each group (academia, patients/members of the public, other) were calculated. The project lead (EE) reviewed and summarized qualitative arguments to identify arguments against the overall trend in frequencies. The steering committee checked the summaries of qualitative arguments. A feedback report detailing frequencies and all anonymized qualitative comments was created and shared with panelists in each subsequent round. Each subsequent round also included the summary of qualitative arguments, the percentage consensus for inclusion and wording, and panelists’ own rating from the previous Delphi round.
Workgroup meeting
We held a 3-h hybrid workgroup meeting in Toronto, Canada, and through Zoom in November 2022. This meeting was held to reach agreement on the inclusion and wording of items that had no consensus for inclusion after round 3 of the Delphi study, or for items for which the wording was revised. The steering committee selected participants with a variety of backgrounds from diverse geographic locations from the Delphi panelists who completed all three rounds; however, we did not use the specific responses of panelists in the Delphi study as a criterion for their selection to participate in the workgroup meeting. Additionally, certain members of the technical advisory group, knowledge users, and a limited number of editors were invited, irrespective of their participation in the Delphi study.
Ten days before the meeting, all attendees received an information package via email, including 1) an agenda, meeting details, and practical preparation steps for the meeting, 2) a full list of items detailing their changes over the Delphi rounds, specifying the items that needed discussion at the meeting, 3) the feedback report from Delphi round 3, and 4) short bio statements and photos from participants in the workgroup meeting. Attendees were asked to review the information prior to the meeting. A pre-meeting was held with patients/members of the public to go over the aims and materials for the workgroup meeting.
A facilitator presented each item selected to be discussed, providing a summary of Delphi round 3 results orally and visually on slides. For items that needed agreement on wording, the chair of the meeting summarized main points, and final wording was decided. Where consensus for inclusion was required, attendees voted on each item via a poll. Voting options were “include”, “exclude”, or “abstain”, and ≥70% include/exclude was needed for consensus [65], not taking the abstainers into account. The meeting was audio recorded and a notetaker documented the results of each poll, as well as the final wording of the items agreed upon.
Developing the guideline
Drafting the pre-final guideline
After the workgroup meeting, we drafted the pre-final guideline, consisting of 1) the PRISMA-COSMIN for OMIs 2024 checklists (a checklist for full reports and a checklist for abstracts) with a glossary explaining technical terms used; 2) their respective explanation and elaboration (E&E) documents, including a rationale and detailed guidance for the reporting of each item; and 3) the PRISMA-COSMIN for OMIs 2024 flow diagram. We invited workgroup participants to contribute to drafting the E&E document by signing up for specific items in teams of two writers and two reviewers. We made explicit effort to align the wording and structure with PRISMA 2020 [16], as this is expected to facilitate the usability and uptake of PRISMA-COSMIN for OMIs 2024.
Pilot testing
Authors in the process of drafting or publishing their systematic review of OMIs, or who recently (2022/2023) published their review were eligible for pilot testing the pre-final guideline. Pilot testers were recruited through the network of the steering committee, by emailing corresponding authors of systematic reviews published in 2022/2023 included in the COSMIN database [59], and by emailing contact persons of ongoing or completed (but not yet published) systematic reviews of OMIs registered in PROSPERO between January 1, 2020, and January 1, 2023 [66]. Pilot testers received the pre-final guideline and were asked to apply it to their drafted, submitted or recently published systematic review of OMIs. Pilot testers provided feedback on the relevance and understandability of each item and its E&E text using a structured survey in REDCap [55]. Responses from pilot testers were reviewed and used to improve the guideline.
End-of-project meeting
We held a hybrid two-day end-of-project meeting in Toronto, Canada, and over Zoom in June 2023, with most members of the steering committee attending in-person. The main goals of the meeting were to finalize the guideline based on the feedback from the pilot testers and discuss its implementation, dissemination, and endorsement. We held hybrid sessions ranging from 60–90 min on Zoom with the following groups: patients/members of the public, journal editors, pilot testers, and data visualization/OMI systematic review experts. Two weeks before the meeting, attendees received an information package via email, including 1) the agenda, session aims, meeting details, and practical information, 2) the bios and photos from participants relevant to their session, and 3) any session-specific documents, if applicable. Attendees were asked to review the information ahead of the meeting.
Results
Delphi study
In total, 252 potential panelists were invited for the Delphi study, of which 81 registered (response rate 32%). Additionally, 38 persons registered through referral. One person withdrew before the start of the first Delphi round, resulting in 118 invited panelists for each round. Of these, 109 panelists responded to at least one round (Online Resource 3a); their characteristics are presented in Table 1. Round 1 was completed by 103 panelists, whereas rounds 2 and 3 were completed by 78 panelists.
Table 1.
Characteristics of Delphi panelists and participants in the workgroup meeting
| Self-reported characteristic | Delphi study (total n = 109); n (%) | Workgroup meeting (total n = 24); n (%) |
|---|---|---|
| Primary perspective | ||
| Academia | 94 (86) | 18 (75) |
| Hospital | 4 (4) | 1 (4) |
| Industry | 2 (2) | 1 (4) |
| Government | 1 (1) | 0 (0) |
| Editor | 1 (1) | 0 (0) |
| Non-profit | 1 (1) | 0 (0) |
| Patient | 4 (4) | 3 (13) |
| Patient representative | 1 (1) | 1 (4) |
| Public member | 1 (1) | 0 (0) |
| Job titlea | ||
| PhD student | 6 (6) | 2 (10) |
| Research assistant | 2 (2) | 1 (5) |
| Postdoctoral research fellow | 15 (15) | 2 (10) |
| (Senior) researcher/research associate | 16 (16) | 7 (35) |
| (Senior) lecturer | 7 (7) | 0 (0) |
| (Assistant/associate) professor | 35 (34) | 4 (20) |
| Clinician/therapist (various) | 12 (12) | 1 (5) |
| Editor/reader/information specialist | 5 (5) | 1 (5) |
| Director/dean/chair | 5 (5) | 2 (10) |
| Country of workplaceb | ||
| UK | 28 (26) | 3 (13) |
| Canada | 27 (25) | 16 (67) |
| USA | 15 (14) | 2 (8) |
| Australia | 12 (11) | 0 (0) |
| Spain | 9 (8) | 0 (0) |
| Netherlands | 5 (5) | 2 (8) |
| Italy | 3 (3) | 1 (4) |
| Japan | 2 (2) | 0 (0) |
| Otherc | 8 (7) | 0 (0) |
| Relevant groupa, d | ||
| OMI systematic review author | 78 (76) | 12 (60) |
| Systematic review author | 66 (64) | 13 (65) |
| OMI developer | 62 (60) | 11 (55) |
| Clinician | 42 (41) | 4 (20) |
| Core outcome set developer | 32 (31) | 11 (55) |
| Epidemiologist | 21 (20) | 11 (55) |
| Psychometrician/clinimetrician | 18 (17) | 9 (45) |
| Journal editor | 17 (17) | 4 (20) |
| Reporting guideline developer | 12 (12) | 9 (45) |
| Biostatistician | 5 (5) | 0 (0) |
| Highest level of educationa | ||
| Master’s degree | 11 (11) | 3 (15) |
| MD | 10 (10) | 1 (5) |
| PhD | 56 (54) | 12 (60) |
| MD/PhD | 26 (25) | 4 (20) |
| Expertise on systematic reviews of OMIsa | ||
| High | 57 (55) | 11 (55) |
| Average | 41 (40) | 8 (40) |
| Low | 5 (5) | 1 (5) |
| Expertise on PRISMAa | ||
| High | 60 (58) | 12 (60) |
| Average | 36 (35) | 4 (20) |
| Low | 7 (7) | 4 (20) |
| Expertise on COSMINa | ||
| High | 37 (36) | 9 (45) |
| Average | 53 (51) | 8 (40) |
| Low | 13 (13) | 3 (15) |
| Previously involved in researche | ||
| As participant | 5 (58) | 4 (100) |
| As patient/public research partner | 6 (100) | 4 (100) |
| Previously involved in methodological researche | ||
| As participant | 2 (33) | 2 (50) |
| As patient/public research partner | 4 (67) | 3 (75) |
| Previously involved in a Delphi studye | 4 (67) | 3 (75) |
aNot asked to patients, patient representatives and public members; b Patients, patient representatives and public members were asked for their country of residence; c Other countries (all n = 1): Belgium, China, South Korea, Singapore, France, South Africa, New Zealand, Switzerland; d Panelists could select multiple responses; e Only asked to patients, patient representatives and public members
COSMIN COnsensus-based Standards for the selection of health Measurement Instruments; OMI outcome measurement instrument; PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses
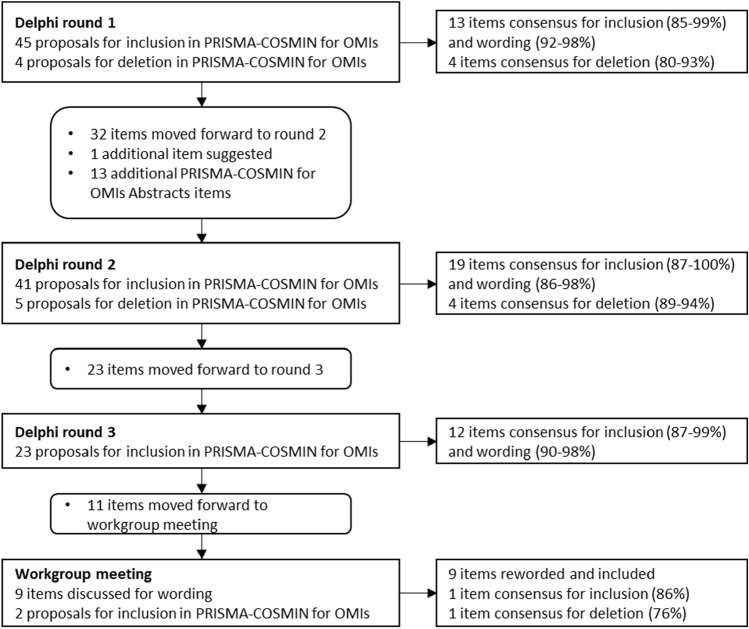
In round 1, 49 potentially relevant items were proposed. Thirteen original PRISMA 2020 items reached consensus for inclusion and wording, whereas 4 original PRISMA 2020 items with limited relevance to systematic reviews of OMIs (related to data items, effect measures, and reporting biases) reached consensus for deletion (Fig. 2, Online Resource 4). Panelists made many qualitative arguments and suggestions for rewording. Wording was revised for all other items based on suggestions from panelists, and these items moved forward to round 2. For two items, related to the name and description of the OMI of interest and citing studies that appear to meet inclusion criteria but were excluded, panelists made compelling arguments for deletion in round 1. Panelists were asked to confirm deletion of these items in round 2, despite the high percentage of consensus for inclusion obtained in round 1.
Fig. 2.
Proposals and consensus for items in each Delphi round and the workgroup meeting. COSMIN COnsensus-based Standards for the selection of health Measurement Instruments; OMI outcome measurement instrument; PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses
While analyzing responses from round 1, we observed misunderstanding among panelists for the item pertaining to the abstract and for the items pertaining to the syntheses. Therefore, we extensively revised these items for round 2. Instead of one abstract item covering all elements, we added thirteen more specific abstract items, based on the PRISMA for Abstracts checklist [16, 67]. Three syntheses items were thought to be of limited relevance for systematic reviews of OMIs, and panelists were asked to confirm the deletion of these items in round 2. Based on suggestions for additional items, we drafted 1 new item pertaining to author contributions for consideration in round 2.
In round 2, 19 additional items reached consensus for inclusion and wording, whereas 4 items reached consensus for deletion (Fig. 2, Online Resource 4). Wording was revised for the other items based on suggestions of the panelists, and moved forward to round 3, despite having mostly high percentages of consensus for inclusion and wording.
In round 3, 12 additional items reached consensus for inclusion and wording (Fig. 2, Online Resource 4). Wording was slightly revised for 9 remaining items, although most of these items had high percentages of consensus for inclusion and wording. Consensus for inclusion was not reached for 2 items. These 11 items moved forward for discussion during the workgroup meeting.
Besides the confusion on the abstract item and syntheses items in round 1, panelists’ comments revolved around terminology for ‘studies’ and ‘reports’ as unit of analysis within these types of reviews. Within the context of measurement property evaluation, there is ongoing confusion about what constitutes a ‘study’. To avoid such confusion among review authors, we suggested to replace the PRISMA 2020 items that ask to report “the number of studies included in the review” by “the number of reports included in the review”. Ultimately, consensus on terminology was reached (see Box 1 for definitions of ‘study’, ‘report’, and ‘study report’) and the term “study reports” was used in those items.
Box 1.
Glossary of terms used in PRISMA-COSMIN for OMIs 2024
|
Systematic review A study design that uses explicit, systematic methods to collect data from primary studies, critically appraises the data, and synthesizes the findings descriptively or quantitatively in order to address a clearly formulated research question [65, 68, 69]. Typically, a systematic review includes a clearly stated objective, pre-defined eligibility criteria for primary studies, a systematic search that attempts to identify all studies that meet the eligibility criteria, risk of bias assessments of the included primary studies, and a systematic presentation and synthesis of findings of the included studies [65]. Systematic reviews can provide high quality evidence to guide decision making in healthcare, owing to the trustworthiness of the findings derived through systematic approaches that minimize bias [70] Outcome domain Refers to what is being measured (e.g., fatigue, physical function, blood glucose, pain) [1, 2]. Other terms include construct, concept, latent trait, factor, attribute Outcome measurement instrument (OMI) Refers to how the outcome is being measured, i.e., the OMI used to measure the outcome domain. Different types of OMIs exist such as questionnaires or patient-reported outcome measures (PROMs) and their variations, clinical rating scales, performance-based tests, laboratory tests, scores obtained through a physical examination or observations of an image, or responses to single questions [1, 2]. An OMI consists of a set of components and phases, i.e., ‘equipment’, ‘preparatory actions’, ‘collection of raw data’, ‘data processing and storage’, and ‘assignment of the score’ [57]. A specific type of OMIs is clinical outcome assessments (COAs) [71], which specifically focus on outcomes related to clinical conditions, often emphasizing the patient’s experience and perspective Report A document with information about a particular study or a particular OMI. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information such as a manual for an OMI or the PROM itself [68]. A study report is a document with information about a particular study like a journal article or a preprint Record The title and/or abstract of a report indexed in a database or website. Records that refer to the same report (such as the same journal article) are “duplicates” [68] Study The empirical investigation of a measurement property in a specific population, with a specific aim, design and analysis Quality The technical concept ‘quality’ is used to address three different aspects defined by COSMIN, OMERACT, and GRADE: 1) quality of the OMI refers to the measurement properties; 2) quality of the study refers to the risk of bias; and 3) quality of the evidence refers to the certainty assessment [2, 5, 72] Measurement properties The quality aspects of an OMI, referring to the validity, reliability, and responsiveness of the instrument’s score [64]. Each measurement property requires its own study design and statistical methods for evaluation. Different definitions for measurement properties are being used. COSMIN has a taxonomy with consensus-based definitions for measurement properties [64]. Another term for measurement properties is psychometric properties Feasibility The ease of application and the availability of an OMI, e.g., completion time, costs, licensing, length of an OMI, ease of administration, etc. [5, 26]. Feasibility is not a measurement property, but is important when selecting an OMI [2] Interpretability The degree to which one can assign meaning to scores or change in scores of an OMI in particular contexts (e.g., if a patient has a score of 80, what does this mean?) [64]. Norm scores, minimal important change and minimal important difference are also relevant concepts related to interpretability. Like feasibility, interpretability is not a measurement property, but is important to interpret the scores of an OMI and when selecting an OMI [2] Measurement properties’ results The findings of a study on a measurement property. Measurement properties’ results have different formats, depending on the measurement property. For example, reliability results might be the estimate of the intraclass correlation coefficient (ICC), or structural validity results might be the factor loadings of items to their respective scales and the percentage of variance explained Measurement properties’ ratings The comparison of measurement properties’ results against quality criteria, to give a judgement (i.e., rating) about the results. For example, the ICC of an OMI might be 0.75; this is the result. A quality criterion might prescribe that the ICC should be >0.7. In this case the result (0.75) is thus rated to be sufficient Risk of bias Risk of bias refers to the potential that measurement properties’ results in primary studies systematically deviate from the truth due to methodological flaws in the design, conduct or analysis [68, 73]. Many tools have been developed to assess the risk of bias in primary studies. The COSMIN Risk of Bias checklist for PROMs was specifically developed to evaluate the risk of bias of primary studies on measurement properties [44]. It contains standards referring to design requirements and preferred statistical methods of primary studies on measurement properties, and is specifically intended for PROMs. The COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of OMIs can be used for any type of OMI [57] Synthesis Combining quantitative or qualitative results of two or more studies on the same measurement property and the same OMI. Results can be synthesized quantitatively or qualitatively. Meta-analysis is a statistical method to synthesize results. Although this can be done for some measurement properties (i.e., internal consistency, reliability, measurement error, construct validity, criterion validity, and responsiveness), it is not very common in systematic reviews of OMIs because the point estimates of the results are not used. Instead, the score obtained with an OMI is used. End-users therefore only need to know whether the result of a measurement property is sufficient or not. For some measurement properties it is not even possible to statistically synthesize the results by meta-analysis or pooling (i.e., content validity, structural validity, and cross-cultural validity/measurement invariance). In general, most often the robustness of the results is described (e.g., the found factor structure, the number of confirmed and unconfirmed hypotheses), or a range of the results is provided (e.g., the range of Cronbach’s alphas or ICCs) Certainty (or confidence) assessment Together with the synthesis, often an assessment of the certainty (or confidence) in the body of evidence is provided. Authors conduct such an assessment to reflect how certain (or confident) they are that the synthesized result is trustworthy. These assessments are often based on established criteria, which include the risk of bias, consistency of findings across studies, sample size, and directness of the result to the research question [2]. A common framework for the assessment of certainty (or confidence) is GRADE (Grading of Recommendations Assessment, Development, and Evaluation) [72]. A modified GRADE approach has been developed for communicating the certainty (or confidence) in systematic reviews of OMIs [2] OMI recommendations Systematic reviews of OMIs provide a comprehensive overview of the measurement properties of OMIs and support evidence-based recommendations for the selection of suitable OMIs for a particular use. Unlike systematic reviews of interventions, systematic reviews of OMIs often make recommendations about the suitability of OMIs for a particular use, although in some cases this might not be appropriate (e.g., if restricted by the funder). Making recommendations also facilitates much needed standardization in use of OMIs, although their quality and score interpretation might be context dependent. Making recommendations essentially involves conducting a synthesis at the level of the OMI, across different measurement properties, taking feasibility and interpretability into account as well. Various methods and tools for OMI recommendation exist (e.g., from COSMIN, OMERACT and others) [2, 74, 75] |
Notably, patient/public involvement impacted the inclusion of reporting items pertaining to 1) feasibility and interpretability of the OMI, 2) recommendations on which OMI (not) to use, and 3) the plain language summary. Although other Delphi panelists saw little relevance for these items in the first Delphi round, patients/members of the public felt strongly about including these items. Their arguments ultimately persuaded other Delphi panelists to vote for inclusion of these items.
Workgroup meeting
In total, 33 persons were invited to the hybrid workgroup meeting, of which 24 (72%) attended the meeting (16 through Zoom, 8 in-person). Attendants included nine steering committee members (one member was unable to attend), four members of the technical advisory group (all Delphi panelists), three knowledge users (two Delphi panelists), three patients/members of the public (all Delphi panelists), and five Delphi panelists (Online Resource 2). Their characteristics are presented in Table 1.
Through discussions, we reached agreement for wording for the 9 items that had their wording revised based on comments of panelists in Delphi round 3 (Fig. 2, Online Resource 4). Two items required voting on inclusion/deletion (one on citing reports that were excluded, one on author contributions). The first item reached 86% agreement for inclusion; the second item reached 76% agreement for deletion.
Developing the guideline
All but three workgroup meeting participants contributed to drafting the E&E document for specific items. E&E text for each item was drafted by at least two writers and checked by at least 2 reviewers. Patients/members of the public signed up to be reviewers for reporting items that would benefit greatly from their input (e.g., items pertaining to the plain language summary, feasibility and interpretability of the OMI, and recommendations on which OMI (not) to use), as well as some other items, resulting in a clearer guideline. Select members of the steering committee made editorial edits for accuracy and consistency across items. A PRISMA-COSMIN for OMIs 2024 flow diagram was created.
We approached 515 potential pilot testers, of which 65 registered (response rate 13%). Additionally, 27 persons registered through referral, resulting in 92 registered pilot testers. These pilot testers were all in the process of drafting or publishing their systematic review, or recently published their review. Of these, 65 contributed to pilot testing by applying the guideline to their systematic review (Online Resource 3b); their characteristics are presented in Table 2. Pilot testers commented on the usability of the guideline and E&E document and made suggestions to improve clarity of the items and the E&E document.
Table 2.
Characteristics of pilot testers
| Self-reported characteristic | Pilot testers (total n = 65); n (%) |
|---|---|
| Job title | |
| (Post)graduate student | 3 (5) |
| PhD student | 19 (29) |
| Research assistant | 6 (9) |
| Postdoctoral research fellow | 4 (6) |
| (Senior) researcher/research associate | 7 (11) |
| (Senior) lecturer | 8 (12) |
| (Assistant) professor | 11 (17) |
| Clinician/therapist (various) | 7 (11) |
| Country of workplace | |
| Brazil | 8 (12) |
| UK | 7 (11) |
| Canada | 6 (9) |
| Germany | 4 (6) |
| Belgium | 4 (6) |
| Mexico | 4 (6) |
| Australia | 3 (5) |
| The Netherlands | 3 (5) |
| Italy | 3 (5) |
| Spain | 3 (5) |
| China | 2 (3) |
| Iran | 2 (3) |
| Switzerland | 2 (3) |
| Turkey | 2 (3) |
| United states | 2 (3) |
| Othera | 10 (15) |
| Highest level of education | |
| Bachelor’s degree | 3 (5) |
| Master’s degree | 21 (32) |
| MD | 21 (32) |
| PhD | 5 (8) |
| MD/PhD | 9 (14) |
| Other | 6 (9) |
| Participated in the Delphi study | |
| Yes | 6 (9) |
| No | 59 (91) |
| Use of guidelineb | |
| As a checklist after drafting the review | 55 (93) |
| As a writing tool during drafting the rewiew | 38 (65) |
| As a peer-review tool for someone else’s review | 14 (24) |
| As a teaching tool | 19 (32) |
| Review used for pilot testingc | |
| Published | 13 (22) |
| Not yet published | 46 (78) |
| Role in review used for pilot testingc | |
| First author | 45 (76) |
| Co-author | 8 (14) |
| PI/senior author | 6 (10) |
aOther countries (all n = 1): Greece, Ireland, Malaysia, Malta, Norway, Portugal, Saudi Arabia, Singapore, France, Sri Lanka; bOnly asked to participants who completed pilot testing (n = 59); participants could select multiple responses; cOnly asked to participants who completed pilot testing (n = 59)
Seven members of the steering committee met in-person for the two-day end-of-project meeting to finalize the guideline and E&E document based on the feedback of pilot testing, whereas two joined through Zoom (one was unable to attend). In addition, the following groups attended hybrid sessions: patients/members of the public (n = 5), journal editors (n = 7), pilot testers (n = 4), and data visualization/OMI systematic review experts (n = 6).
Feedback from pilot testing resulted in minor changes in wording and restructuring of the items, but not to changes in the content of the checklist. Most importantly, we changed the title of the section ‘other information’ to ‘open science’ and moved this section before the items on the introduction, consistent with the recently published CONSORT 2023 statement.
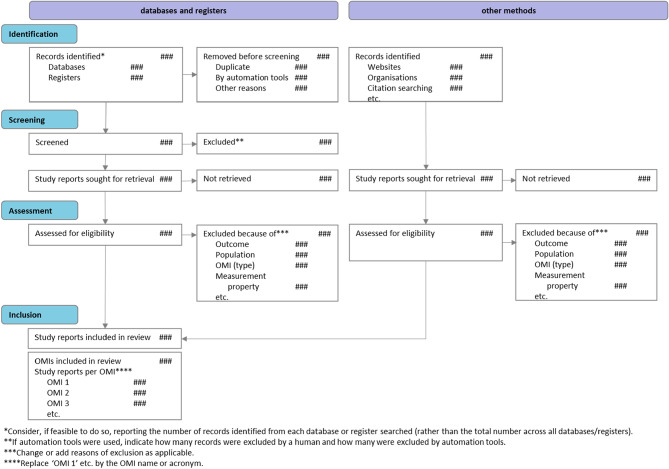
The PRISMA-COSMIN for OMIs 2024 guideline consists of a checklist for full systematic review reports with 54 (sub)items (Table 3), and a glossary of technical terms used (Box 1). The 13 items pertaining to the title and abstract are also included in a separate checklist that authors drafting e.g., conference abstracts could use. Their respective E&E documents (Online Resource 5 shows the E&E for full reports) contain a rationale for each item, essential and additional elements, and quoted examples from a published systematic review of OMIs. The PRISMA-COSMIN for OMIs 2024 flow diagram is shown in Fig. 3.
Table 3.
PRISMA-COSMIN for OMIs 2024 checklist with Abstract items featured
| Section and Topic | # | Checklist itema | Location |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review and include as applicable the following (in any order): outcome domain of interest, population of interest, name/type of OMIs of interest, and measurement properties of interest | |
| Abstract | |||
| Open Science | |||
| Fundingb | 2.2 | Specify the primary source of funding for the review | |
| Registration | 2.3 | Provide the register name and registration number | |
| Background | |||
| Objectives | 2.4 | Provide an explicit statement of the main objective(s) or question(s) the review addresses | |
| Methods | |||
| Eligibility criteria | 2.5 | Specify the inclusion and exclusion criteria for the review | |
| Information sources | 2.6 | Specify the information sources (e.g., databases, registers) used to identify studies and the date when each was last searched | |
| Risk of bias | 2.7 | Specify the methods used to assess risk of bias in the included studies | |
| Measurement properties | 2.8 | Specify the methods used to rate the results of a measurement property | |
| Synthesis methods | 2.9 | Specify the methods used to present and synthesize results | |
| Results | |||
| Included studies | 2.10 | Give the total number of included OMIs and study reports | |
| Synthesis of results | 2.11 | Present the syntheses of results of OMIs, indicating the certainty of the evidence | |
| Discussion | |||
| Limitations of evidence | 2.12 | Provide a brief summary of the limitations of the evidence included in the review (e.g., study risk of bias, inconsistency, and imprecision) | |
| Interpretation | 2.13 | Provide a general interpretation of the results and important implications | |
| Plain Language Summary | |||
| Plain language summary | 3 | If allowed by the journal, provide a plain language summary with background information and key findings | |
| Open Science | |||
| Registration and protocol | 4a | Provide registration information for the review, including register name and registration number, or state that the review was not registered | |
| 4b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | ||
| 4c | Describe and explain any amendments to information provided at registration or in the protocol | ||
| Support | 5 | Describe sources of financial or non-financial support for the review, and the role of the funders in the review | |
| Competing interests | 6 | Declare any competing interests of review authors | |
| Availability of data, code, and other materials | 7 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review | |
| Introduction | |||
| Rationale | 8 | Describe the rationale for the review in the context of existing knowledge | |
| Objectives | 9 | Provide an explicit statement of the objective(s) or question(s) the review addresses and include as applicable the following (in any order): outcome domain of interest, population of interest, name/type of OMIs of interest, and measurement properties of interest | |
| Methods | |||
| Followed guidelines | 10 | Specify, with references, the methodology and/or guidelines used to conduct the systematic review | |
| Eligibility criteria | 11 | Specify the inclusion and exclusion criteria for the review | |
| Information sources | 12 | Specify all databases, registers, preprint servers, websites, organizations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted | |
| Search strategy | 13 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used | |
| Selection process | 14 | Specify the methods used to decide whether a study met the inclusion criteria of the review, e.g., including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools/AI used in the process | |
| Data collection process | 15 | Specify the methods used to collect data from reports, e.g., including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools/AI used in the process | |
| Data items | 16 | List and define which data were extracted (e.g., characteristics of study populations and OMIs, measurement properties’ results, and aspects of feasibility and interpretability). Describe methods used to deal with any missing or unclear information | |
| Study risk of bias assessment | 17 | Specify the methods used to assess risk of bias in the included studies, e.g., including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools/AI used in the process | |
| Measurement properties | 18 | Specify the methods used to rate the results of a measurement property for each individual study and for the summarized or pooled results, e.g., including how many reviewers rated each study and whether they worked independently | |
| Synthesis methods | 19a | Describe the processes used to decide which studies were eligible for each synthesis | |
| 19b | Describe any methods used to synthesize results | ||
| 19c | If applicable, describe any methods used to explore possible causes of inconsistency among study results (e.g., subgroup analysis) | ||
| 19d | If applicable, describe any sensitivity analyses conducted to assess robustness of the synthesized results | ||
| Certainty assessment | 20 | Describe any methods used to assess certainty (or confidence) in the body of evidence | |
| Formulating recommendations | 21 | If appropriate, describe any methods used to formulate recommendations regarding the suitability of OMIs for a particular use | |
| Results | |||
| Study selection | 22a | Describe the results of the search and selection process, from the number of records identified in the search to the number of study reports included in the review, ideally using a flow diagram. If applicable, also report the final number of OMIs included and the number of study reports relevant to each OMI. [T] | |
| 22b | Cite study reports that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded | ||
| OMI characteristics | 23a | Present characteristics of each included OMI, with appropriate references. [T] | |
| 23b | If applicable, present interpretability aspects for each included OMI. [T] | ||
| 23c | If applicable, present feasibility aspects for each included OMI. [T] | ||
| Study characteristics | 24 | Cite each included study report evaluating one or more measurement properties and present its characteristics. [T] | |
| Risk of bias in studies | 25 | Present assessments of risk of bias for each included study. [T] | |
| Results of individual studies | 26 | For all measurement properties, present for each study: (a) the reported result and (b) the rating against quality criteria, ideally using structured tables or plots. [T] | |
| Results of syntheses | 27a | Present results of all syntheses conducted. For each measurement property of an OMI, present: (a) the summarized or pooled result and (b) the overall rating against quality criteria. [T] | |
| 27b | If applicable, present results of all investigations of possible causes of inconsistency among study results | ||
| 27c | If applicable, present results of all sensitivity analyses conducted to assess the robustness of the synthesized results | ||
| Certainty of evidence | 28 | Present assessments of certainty (or confidence) in the body of evidence for each measurement property of an OMI assessed. [T] | |
| Recommendations | 29 | If appropriate, make recommendations for suitable OMIs for a particular use | |
| Discussion | |||
| Discussion | 30a | Provide a general interpretation of the results in the context of other evidence | |
| 30b | Discuss any limitations of the evidence included in the review | ||
| 30c | Discuss any limitations of the review processes used | ||
| 30d | Discuss implications of the results for practice, policy, and future research | ||
aIf an item is marked with [T], a template for data visualization is available. These templates can be downloaded from www.prisma-cosmin.ca. bItem #2.1 in the PRISMA-COSMIN for OMIs 2024 Abstracts checklist refers to the title. Item #2.1 in the Abstracts checklist is identical to item #1 in the Full Report checklist
Fig. 3.
PRISMA-COSMIN for OMIs 2024 flow diagram
Discussion
This paper outlines the development of PRISMA-COSMIN for OMIs 2024, including a Delphi study, workgroup meeting, pilot testing and an end-of-project meeting, and contains the checklist and E&E document for full reports. PRISMA-COSMIN for OMIs 2024 is intended to guide the reporting of systematic reviews of OMIs, in which at least one measurement property of at least one OMI is evaluated. These systematic reviews support decision making on the suitability of an OMI for a specific application. PRISMA-COSMIN for OMIs 2024 is not intended for reviews that only provide an overview (characteristics) of OMIs used, as these reviews are more scoping in nature. Systematic reviews of OMIs conducted with any methodology can use PRISMA-COSMIN for OMIs 2024; it does not apply specifically to systematic reviews conducted with the methodology or tools from the COSMIN initiative, although it is consistent with COSMIN guidance [2].
Similar to PRISMA 2020 [16], PRISMA-COSMIN for OMIs 2024 consists of two checklists (one for full reports and one for abstracts), their respective E&E documents, and a flow diagram. To develop PRISMA-COSMIN for OMIs 2024, we adapted PRISMA 2020 and made the following revisions to the checklist for full reports: 9 new items were added, 8 items were deleted because they were deemed not relevant for systematic reviews of OMIs, 24 items were modified, and 22 items kept as original. This checklist thus contains 54 (sub)items addressing the title, abstract, plain language summary, open science, introduction, methods, results, and discussion sections of a systematic review report. The 13 items pertaining to the title and abstract are also included in a separate Abstract checklist, accompanied by a separate E&E document that authors could use when drafting abstracts (e.g., conference abstracts).
The rigorous development process ensured that PRISMA-COSMIN for OMIs 2024 was informed by the knowledge of those who have expertise in OMIs and OMI systematic review methods, and patients/members of the public with lived experience. We were fortunate to include a good cross-section of stakeholders. Pilot testing with a large sample of authors of various OMI systematic reviews further improved PRISMA-COSMIN for OMIs 2024, confirming its broad applicability to different types and fields of OMI systematic reviews. We included patients/members of the public in the development process, as they are ultimately impacted by the results of systematic reviews of OMIs and the OMIs that are selected based on these reviews. Impact of patient/public involvement was evident, as four items were included that might have been disregarded, and their suggestions for rewording made the guideline clearer. As patient/public involvement in reporting guideline development is still in its infancy [76], we extensively evaluated this part of the process, reflected on lessons learned and provide recommendations for future reporting guideline developers elsewhere [19].
The field of evaluating OMIs is continuously evolving. For the development of PRISMA-COSMIN for OMIs 2024, we took PRISMA 2020 [16] as a guiding framework and used consensus methodology to modify, add, and delete reporting items based on the OMI literature and existing guidelines. The COSMIN guideline for systematic reviews of OMIs [2] was particularly important, as this currently is the most comprehensive and widely used guideline. Novel developments to evaluate OMIs, such as modern validity theory [77, 78] and qualitative research methods to investigate the impact of response processes and consequences of measurement [79, 80], might become increasingly important. Review authors who apply these methods are also able to use PRISMA-COSMIN for OMIs 2024 to guide their reporting. We will monitor the need for adaptations to the guideline should these methods be applied more frequently in OMI systematic reviews and require specific additional reporting items.
Despite the rigorous development process, we cannot be certain that we would have obtained exactly the same results if we would have done the process again, either with the same or with different participants. For example, in the Delphi study and workgroup meeting, we had relatively low representation of people from lower- and middle-income countries. This might have impacted our results, although representation in the pilot study was better. Another potential limitation is that we did not systematically search the literature to identify potential items in the preparation phase of the process. This was largely for pragmatic reasons, as we assumed that not much information on reporting recommendations for systematic reviews of OMIs would exist, as opposed to reporting guidance for primary studies on measurement properties [40]. Instead, we took PRISMA 2020 [16] as an evidence-informed and consensus-based framework and, based on our experiences with conducting, authoring, and reviewing systematic reviews of OMIs, we modified, added or deleted items. By applying the initial item list to three high-quality OMI systematic reviews we were able to confirm the relevance of items. The Delphi study and pilot testing with large and diverse samples validated these decisions. Moreover, our definition of consensus (67%) is somewhat arbitrary, although it has been used in other Delphi studies [24, 57, 64]. However, we ultimately reached at least 80% agreement on inclusion and wording in the Delphi study, so even if we had used a higher cut-off, this would not have changed our results.
Complete and transparent reporting of systematic reviews of OMIs is essential to foster reproducibility of systematic reviews and allow end-users to select the most appropriate OMI for a specific application. We hope that PRISMA-COSMIN for OMIs 2024 will improve the reporting of systematic reviews of OMIs as well as the quality of such reviews [7, 8]. PRISMA-COSMIN for OMIs 2024 will be published on the websites of the EQUATOR network, PRISMA, COSMIN, and www.prisma-cosmin.ca. To promote its uptake, a social media campaign to increase awareness, a short video (2–3 min) explaining the resources available to guide reporting systematic reviews of OMIs, and 1-page tip sheets outlining how to report each item will be created, in addition to patient-targeted materials. Furthermore, we are considering an automated e-mail system, whereby authors who register their OMI systematic review in PROSPERO [66] receive PRISMA-COSMIN for OMIs 2024. We will monitor the need for updating PRISMA-COSMIN for OMIs 2024, to reflect changes in best practice health research reporting and to stay consistent with PRISMA terminology.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all Delphi panelists for their participation: Pervez Sultan, Sunita Vohra, Achilles Thoma, Chris Gale, Eun-Hyun Lee, Reinie Cordier, Montse Ferrer, James O’Carroll, Karla Gough, Claire Snyder, Grace Turner, Kathleen Wyrwich, Michelle Hyczy de Siqueira Tosin, Lex Bouter, Estelle Jobson, James Webbe, Carolina Barnett-Tapia, Dr Himal Kandel, Jill Carlton, Beverley Shea, Frank Gavin, Jean-Sébastien Roy, Martin Howell, Shona Kirtley, Nadir Sharawi, Christine Imms, Jose Castro-Piñero, Pete Tugwell, Vibeke Strand, Ana Belen Ortega-Avila, Alexa Meara, Susan Darzins, Brooke Adair, Daniel Gutiérrez Sánchez, Tim Pickles, Krishnan PS Nair, Désirée van der Heijde, Marco Tofani, Nadine Smith, Sarah Wigham, Hayley Hutchings, Mirkamal Tolend, Giovanni Galeoto, Maarten Boers, Philip Powell, Pablo Cervera-Garvi, Philip van der Wees, Edith Poku, Loreto Carmona, Jan Boehnke, Martin Heine, Hideki Sato, Yuexian Shi, and Lisa McGarrigle, as well as the panelists that wished to be anonymous. We thank all pilot testers for their participation: Erin Macri, Tjerk Sleeswijk Visser, Sanaa Alsubheen, Karla Danielle Xavier Do Bomfim, Sorina Andrei, Patrick Lavoie, Tiziana Mifsud, Emma Stallwood, Luciana Sayuri Sanada, Maribel Salas, Sandrine Herbelet, Lena Sauerzopf, Li Rui, Elizabeth Greene, Samuel Miranda Mattos, Angela Contri, Nusrat Iqbal, Tanya Palmer, Liesbeth Delbaere, Elissa Dabkowski, Ben Rimmer, Mareike Löbberding, Cheng Ling Jie, Vikram Mohan, Vanessa Monserrat Vázquez Vázquez, Giovanni Galeoto, Zhao Hui Koh, Ana Isabel de Almeida Costa, Maite Barrios, Çağdaş Isiklar, Walid Mohamed, Connie Cole, Leila Kamalzadeh, Arianna Magon, Beatrice Manduchi, Natalia Hernández-Segura, Olga Antsygina, Yang Panpan, Vahid Saeedi, Shenhao Dai, Sabien van Exter, Miguel Angel Jorquera Ruzzi, Tonje L Husum, Karina Franco-Paredes, Carole Délétroz, Lauren Shelley, Tayomara Ferreira Nascimento, Anuji De Za, Denis de Jesus Batista, Ayse Ozdede, Maria Misevic, Jolien Duponselle, Sinaa Al-aqeel, Germain Honvo, Leonardo Mauad, Andrew Dyer and Lilián Elizabeth Bosques-Brugada, as well as the pilot testers that wished to be anonymous. We thank Prof. Maarten Boers for his improvements to the PRISMA-COSMIN for OMIs 2024 flow diagram. We thank Prof. Galeoto for assisting Anna Berardi in drafting texts for the E&E document.
Abbreviations
- COSMIN
COnsensus-based Standards for the selection of health Measurement Instruments
- E&E
Explanation and elaboration
- EQUATOR
Enhancing the QUAlity and Transparency Of health Research
- JBI
Joanna Briggs Institute
- OMERACT
Outcome Measures in Rheumatology (OMERACT)
- OMI
Outcome measurement instrument
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM
Patient-Reported Outcome Measure
- REDCap
Research Electronic Data Capture
Funding
The research is funded by the Canadian Institutes of Health Research (grant number 452145). The funder had no role in the design or conduct of this research. Dr. Tricco holds a Tier 2 Canada Research Chair in Knowledge Synthesis.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Individual, anonymized responses from panelists or pilot testers are available from the corresponding author upon reasonable request.
Declarations
Ethical approval
This is a methodological study. The Research Ethics Board of the Hospital for Sick Children has confirmed that no ethical approval is required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
Drs. Terwee and Mokkink are the founders of COSMIN; Dr. Moher is a member of the PRISMA executive; Dr. Tricco is the co-editor in chief of the Journal of Clinical Epidemiology. The other authors have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juanna Ricketts has been a valued patient contributor who recently passed away but has given her explicit permission to be a co-author.
References
- 1.Butcher NJ, Monsour A, Mew EJ, Chan A-W, Moher D, Mayo-Wilson E, Terwee CB, Chee-A-Tow A, Baba A, Gavin F. Guidelines for reporting outcomes in trial reports: The CONSORT-outcomes 2022 extension. JAMA. 2022;328(22):2252–2264. doi: 10.1001/jama.2022.21022. [DOI] [PubMed] [Google Scholar]
- 2.Prinsen CA, Mokkink LB, Bouter LM, Alonso J, Patrick DL, De Vet HC, Terwee CB. COSMIN guideline for systematic reviews of patient-reported outcome measures. Quality of Life Research. 2018;27(5):1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter ME. What is value in health care. New England Journal of Medicine. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 4.Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015 doi: 10.1136/bmj.g7818. [DOI] [PubMed] [Google Scholar]
- 5.OMERACT . The OMERACT handbook for establishing and implementing core outcomes in clinical trials across the spectrum of rheumatologic conditions. OMERACT; 2021. [Google Scholar]
- 6.Stephenson M, Riitano D, Wilson S, Leonardi-Bee J, Mabire C, Cooper K, Monteiro da Cruz D, Moreno-Casbas M, Lapkin S. Chapter 12: Systematic reviews of measurement properties. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 7.Mokkink LB, Terwee CB, Stratford PW, Alonso J, Patrick DL, Riphagen I, Knol DL, Bouter LM, de Vet HC. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Quality of Life Research. 2009;18(3):313–333. doi: 10.1007/s11136-009-9451-9. [DOI] [PubMed] [Google Scholar]
- 8.Terwee CB, Prinsen C, Garotti MR, Suman A, De Vet H, Mokkink LB. The quality of systematic reviews of health-related outcome measurement instruments. Quality of Life Research. 2016;25(4):767–779. doi: 10.1007/s11136-015-1122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorente S, Viladrich C, Vives J, Losilla J-M. Tools to assess the measurement properties of quality of life instruments: A meta-review. British Medical Journal Open. 2020;10(8):e036038. doi: 10.1136/bmjopen-2019-036038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna SP, Heaney A. Setting and maintaining standards for patient-reported outcome measures: Can we rely on the COSMIN checklists? Journal of Medical Economics. 2021;24(1):502–511. doi: 10.1080/13696998.2021.1907092. [DOI] [PubMed] [Google Scholar]
- 11.Altman DG, Simera I. Using reporting guidelines effectively to ensure good reporting of health research. Guidelines for reporting health research: A User’s Manual. 2014;25:32–40. [Google Scholar]
- 12.Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Medicine. 2010;7(2):e1000217. doi: 10.1371/journal.pmed.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Sanger N, Shams I, Luo C, Shahid H, Li G, Bhatt M, Zielinski L, Bantoto B, Wang M. Does the medical literature remain inadequately described despite having reporting guidelines for 21 years? A systematic review of reviews: an update. Journal of Multidisciplinary Healthcare. 2018;27:495–510. doi: 10.2147/JMDH.S155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*, t Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12):e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. International journal of surgery. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 17.COSMIN (2023) Guideline for systematic reviews of outcome measurement instruments. Available via https://www.cosmin.nl/tools/guideline-conducting-systematic-review-outcome-measures/. Accessed Apr 2023
- 18.Butcher NJ, Monsour A, Mokkink LB, Terwee CB, Tricco AC, Gagnier J, Offringa M (2021) Needed: guidance for reporting knowledge synthesis studies on measurement properties of outcome measurement instruments in health research
- 19.Elsman EB, Smith M, Hofstetter C, Gavin F, Jobson E, Markham S, Ricketts J, Baba A, Butcher NJ, Offringa M (2024) A blueprint for patient and public involvement in the development of a reporting guideline for systematic reviews of outcome measurement instruments: PRISMA-COSMIN for OMIs 2024. Res Involv Engagem 10(33). 10.1186/s40900-024-00563-5 [DOI] [PMC free article] [PubMed]
- 20.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, Altman D, Moher D, Barber R, Denegri S. GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. BMJ. 2017 doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EQUATOR (2023) PRISMA-COSMIN? Recommendations for reporting systematic reviews of outcome measurement instruments. Available via https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/#PRISMACOSMIN. Accessed 14 Sept 2023
- 22.Elsman EB, Baba A, Butcher NJ, Offringa M, Moher D, Terwee CB, Mokkink LB, Smith M, Tricco, A (2023) PRISMA-COSMIN. OSF
- 23.Elsman EB, Butcher NJ, Mokkink LB, Terwee CB, Tricco A, Gagnier JJ, Aiyegbusi OL, Barnett C, Smith M, Moher D. Study protocol for developing, piloting and disseminating the PRISMA-COSMIN guideline: A new reporting guideline for systematic reviews of outcome measurement instruments. Systematic Reviews. 2022;11(1):121. doi: 10.1186/s13643-022-01994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terwee CB, Prinsen CA, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, Bouter LM, De Vet HC, Mokkink LB. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A delphi study. Quality of Life Research. 2018;27(5):1159–1170. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, Williamson PR, Terwee CB. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”–a practical guideline. Trials. 2016;17(1):1–10. doi: 10.1186/s13063-016-1555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8. doi: 10.1186/1745-6215-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bulletin of the World Health Organization. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, De Vet HC. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Clinical Chemistry. 2015;61(12):1446–1452. doi: 10.1373/clinchem.2015.246280. [DOI] [PubMed] [Google Scholar]
- 30.Kottner J, Audige L, Brorson S, Donner A, Gajewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. International Journal of Nursing Studies. 2011;48(6):661–671. doi: 10.1016/j.ijnurstu.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA (2009) Guidance for Industry - Patient-Reported Outcome Measures: use in Medical Product Development to Support Labeling Claims. U.S. Department of Health and Human Services Food and Drug Administration
- 33.Revicki DA, Erickson PA, Sloan JA, Dueck A, Guess H, Santanello NC, Group M.F.P.-R.O.C.M Interpreting and reporting results based on patient-reported outcomes. Value in Health. 2007;10:S116–S124. doi: 10.1111/j.1524-4733.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 34.Staquet M, Berzon R, Osoba D, Machin D. Guidelines for reporting results of quality of life assessments in clinical trials. Quality of Life Research. 1996;5(5):496–502. doi: 10.1007/BF00540022. [DOI] [PubMed] [Google Scholar]
- 35.AHRQ . Methods guide for effectiveness and comparative effectiveness reviews. Agency for Healthcare Research and Quality; 2019. [PubMed] [Google Scholar]
- 36.Aromataris E, Munn Z. JBI Manual for evidence synthesis. JBI; 2020. [Google Scholar]
- 37.Brundage M, Blazeby J, Revicki D, Bass B, De Vet H, Duffy H, Efficace F, King M, Lam CL, Moher D. Patient-reported outcomes in randomized clinical trials: Development of ISOQOL reporting standards. Quality of Life Research. 2013;22(6):1161–1175. doi: 10.1007/s11136-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, CONSORT PRO Group, f.t Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013;309(8):814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 39.CRD . CRD’s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination; 2009. [Google Scholar]
- 40.Gagnier JJ, Lai J, Mokkink LB, Terwee CB. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Quality of Life Research. 2021;30:1–22. doi: 10.1007/s11136-021-02822-4. [DOI] [PubMed] [Google Scholar]
- 41.Higgins J, Lasserson T, Chandles J, Tovey D, Flemyng E, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane; 2021. [Google Scholar]
- 42.Lohr KN. Assessing health status and quality-of-life instruments: Attributes and review criteria. Quality of life Research. 2002;11(3):193–205. doi: 10.1023/A:1015291021312. [DOI] [PubMed] [Google Scholar]
- 43.McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 44.Mokkink LB, De Vet HC, Prinsen CA, Patrick DL, Alonso J, Bouter LM, Terwee CB. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Quality of Life Research. 2018;27(5):1171–1179. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton S, Berg A, Levit L, Eden J. Finding what works in health care standards for systematic reviews. Institute of Medicine; 2011. Standards for Reporting Systematic Reviews. [PubMed] [Google Scholar]
- 46.NQF . Guidance for Measure Testing and Evaluating Scientific Acceptability of Measure Properties. National Quality Forum; 2011. [Google Scholar]
- 47.PCORI (2021) Draft final research report: instructions for awardee. Patient-Centered Outcomes Research Institute
- 48.Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, Schwartz C, Revicki DA, Moinpour CM, McLeod LD. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Quality of Life Research. 2013;22(8):1889–1905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 49.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015 doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 50.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 51.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 52.Elsman EB, Mokkink LB, Langendoen-Gort M, Rutters F, Beulens J, Elders PJ, Terwee CB. Systematic review on the measurement properties of diabetes-specific patient-reported outcome measures (PROMs) for measuring physical functioning in people with type 2 diabetes. BMJ Open Diabetes Research and Care. 2022;10(3):e002729. doi: 10.1136/bmjdrc-2021-002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abma IL, Butje BJ, Peter M, van der Wees PJ. Measurement properties of the dutch-flemish patient-reported outcomes measurement information system (PROMIS) physical function item bank and instruments: A systematic review. Health and Quality of Life Outcomes. 2021;19(1):1–22. doi: 10.1186/s12955-020-01647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehdipour A, Wiley E, Richardson J, Beauchamp M, Kuspinar A. The performance of digital monitoring devices for oxygen saturation and respiratory rate in COPD: A systematic review. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2021;18(4):469–475. doi: 10.1080/15412555.2021.1945021. [DOI] [PubMed] [Google Scholar]
- 55.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, De Vet HC. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international delphi study. Quality of Life Research. 2010;19(4):539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mokkink LB, Boers M, van der Vleuten C, Bouter LM, Alonso J, Patrick DL, De Vet HC, Terwee CB. COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: A delphi study. BMC Medical Research Methodology. 2020;20(1):1–13. doi: 10.1186/s12874-020-01179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butcher NJ, Monsour A, Mew EJ, Chan A-W, Moher D, Mayo-Wilson E, Terwee CB, Chee-A-Tow A, Baba A, Gavin F. Guidelines for reporting outcomes in trial protocols: The SPIRIT-outcomes 2022 extension. JAMA. 2022;328(23):2345–2356. doi: 10.1001/jama.2022.21243. [DOI] [PubMed] [Google Scholar]
- 59.COSMIN. COSMIN database of systematic reviews. Available via www.cosmin.nl/tools/database-systematic-reviews/. Accessed 30 Jan 2022
- 60.Cochrane (2023) Cochrane consumer network: a network for patients and carers within Cochrane. Available via https://consumers.cochrane.org/healthcare-users-cochrane. Accessed 6 Apr 2023
- 61.COMET (2023) COMET POPPIE Working Group. Available via https://www.comet-initiative.org/Patients/POPPIE. Accessed 6 Apr 2023
- 62.OMERACT (2023) OMERACT Patient Research Partners. Available via https://omeractprpnetwork.org/. Accessed 6 Apr 2023
- 63.SPOR (2023) Strategy for patient-oriented research. Available via https://sporevidencealliance.ca/. Accessed 6 Apr 2023
- 64.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. Journal of Clinical Epidemiology. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Gates M, Gates A, Pieper D, Fernandes RM, Tricco AC, Moher D, Brennan SE, Li T, Pollock M, Lunny C. Reporting guideline for overviews of reviews of healthcare interventions: Development of the PRIOR statement. BMJ. 2022 doi: 10.1136/bmj-2022-070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.PROSPERO (2023) PROSPERO: international prospective register of systematic reviews. Centre for Reviews and Dissemination
- 67.Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, Gotzsche PC, Lasserson T, Tovey D, Group, P.f.A PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Medicine. 2013;10(4):e1001419. doi: 10.1371/journal.pmed.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2022) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane [DOI] [PMC free article] [PubMed]
- 70.Salameh J-P, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, Deeks JJ, Leeflang M, Korevaar DA, Whiting P. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ. 2020 doi: 10.1136/bmj.m2632. [DOI] [PubMed] [Google Scholar]
- 71.Walton MK, Powers JH, III, Hobart J, Patrick D, Marquis P, Vamvakas S, Isaac M, Molsen E, Cano S, Burke LB. Clinical outcome assessments: Conceptual foundation—report of the ISPOR clinical outcomes assessment–emerging good practices for outcomes research task force. Value in Health. 2015;18(6):741–752. doi: 10.1016/j.jval.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schünemann H, Brożek J, Guyatt G, Oxman A (2013) Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October, 2013
- 73.Boutron I, Page M, Higgins J, Altman D, Lundh A, Hrobjartsson A, et al. Chapter 7: considering bias and conflicts of interest among the included studies. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane 2020. In: Higgins J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3? Cochrane; 2022. [Google Scholar]
- 74.Beaton DE, Maxwell LJ, Shea BJ, Wells GA, Boers M, Grosskleg S, Bingham CO, Conaghan PG, D’Agostino MA, De Wit MP. Instrument selection using the OMERACT filter 21: The OMERACT methodology. The Journal of Rheumatology. 2019;46(8):1028–1035. doi: 10.3899/jrheum.181218. [DOI] [PubMed] [Google Scholar]
- 75.Lewis CC, Mettert KD, Stanick CF, Halko HM, Nolen EA, Powell BJ, Weiner BJ. The psychometric and pragmatic evidence rating scale (PAPERS) for measure development and evaluation. Implementation Research and Practice. 2021;2:26334895211037391. doi: 10.1177/26334895211037391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlussel MM, Sharp MK, de Beyer JA, Kirtley S, Logullo P, Dhiman P, MacCarthy A, Koroleva A, Speich B, Bullock GS. Reporting guidelines used varying methodology to develop recommendations. Journal of Clinical Epidemiology. 2023 doi: 10.1016/j.jclinepi.2023.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Kane M. The argument-based approach to validation. School Psychology Review. 2013;42(4):448–457. doi: 10.1080/02796015.2013.12087465. [DOI] [Google Scholar]
- 78.Weinfurt KP. Constructing arguments for the interpretation and use of patient-reported outcome measures in research: An application of modern validity theory. Quality of Life Research. 2021;30(6):1715–1722. doi: 10.1007/s11136-021-02776-7. [DOI] [PubMed] [Google Scholar]
- 79.AERA . The Standards for Educational and Psychological Testing. The American Educational Research Association (AERA); 2014. [Google Scholar]
- 80.Zumbo B, Chan E. Validity and validation in social, behavioral, and health sciences (Social Indicators Research Series. Springer International Publishing; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information. Individual, anonymized responses from panelists or pilot testers are available from the corresponding author upon reasonable request.