Abstract
Latently infected resting CD4+ T cells provide a long-term reservoir for human immunodeficiency virus type 1 (HIV-1) and are likely to represent the major barrier to virus eradication in patients on combination antiretroviral therapy. The mechanisms by which viruses enter the latent reservoir and the nature of the chemokine receptors involved have not been determined. To evaluate the phenotype of the virus in this compartment with respect to chemokine receptor utilization, full-length HIV-1 env genes were cloned from latently infected cells and assayed functionally. We demonstrate that the majority of the viruses in the latent reservoir utilize CCR5 during entry, although utilization of several other receptors, including CXCR4, was observed. No alternative coreceptors were shown to be involved in a systematic fashion. Although R5 viruses are present in the latent reservoir, CCR5 was not expressed at high levels on resting CD4+ T cells. To understand the mechanism by which R5 viruses enter latent reservoir, the ability of an R5 virus, HIV-1 Ba-L, to infect highly purified resting CD4+ T lymphocytes from uninfected donors was evaluated. Entry of Ba-L could be observed when virus was applied at a multiplicity approaching 1. However, infection was limited to a subset of cells expressing low levels of CCR5 and markers of immunologic memory. Naive cells could not be infected by an R5 virus even when challenged with a large inoculum. Direct cell fractionation studies showed that latent virus is present predominantly in resting memory cells but also at lower levels in resting naive cells. Taken together, these findings provide support for the hypothesis that the direct infection of naive T cells is not the major mechanism by which the latent infection of resting T cells is established.
The demonstration of resting CD4+ T lymphocytes carrying replication-competent human immunodeficiency virus type 1 (HIV-1) genomes in patients successfully treated with highly active antiretroviral therapy (HAART) identifies the latent infection of these cells as an important mechanism of viral persistence (12, 20, 43). Although present at a low frequency, this latent reservoir persists for extremely long periods of time in the face of aggressive antiviral therapy (t1/2, 6 to 44 months) (8, 19, 51). Thus, these latently infected cells represent a serious obstacle to virus eradication.
While the clinical importance of the latent infection of resting CD4+ lymphocytes has been clearly demonstrated, the mechanisms that govern the formation of this latent reservoir are less clear. The existence of this reservoir can best be explained by considering the infection of CD4+ T cells in the context of the normal physiology of T-cell activation (reviewed in reference 33). Naive CD4+ T cells exit the thymus and enter the peripheral lymphoid tissues, where they persist in a resting state until they encounter cognate antigen. Following initial exposure to antigen, naive T cells undergo blast transformation and enter the cell cycle. Activation results in an increase in the size of nucleotide pools and increased expression of sets of molecules including transcription factors, effector molecules such as cytokines, and cell surface proteins such as cytokine receptors and adhesion molecules (15). After several rounds of division, some of the activated cells exit the cell cycle, lose expression of activation markers such as HLA-DR, and revert to a resting state in which they persist as memory T cells capable of responding to subsequent exposures to the initiating antigen. An appealing model for the formation of the latent reservoir involves the infection of activated CD4+ lymphocytes, which at some frequency survive both the cytopathic effects of the virus and antiviral immune responses. A fraction of these infected cells may exit the cell cycle into a quiescent state, carrying with them an integrated copy of the HIV-1 genome. The finding that the majority of integrated DNA in resting lymphocytes appears to be in the memory CD4+ T-lymphocyte subset provides evidence in support of this hypothesis (9).
The direct infection of quiescent lymphocytes may also play a role in the establishment of the latent compartment. Unlike the situation in activated CD4+ cells, the infection of resting lymphocytes is nonproductive due to a block upstream of the integration event. This block has been suggested to arise from an inability to complete the reverse transcription reaction (25, 46, 47) or a failure to import the preintegration complex into the nucleus (38). Previous work clearly demonstrates that this preintegration form of latent infection is labile, but if the infected cell is activated soon after infection, productive infection ensues (5, 47). Interestingly, recent experiments using pseudotyped HIV-1 vectors demonstrate that certain cytokines provide signals that make resting cells permissive for virus integration and gene expression (41). This is in agreement with previous work demonstrating virus propagation in purified resting cells treated with combinations of stimulatory cytokines (44). The proinflammatory environment of HIV-1 infection may provide a suitable milieu for cytokine-driven progression from the labile preintegration state of latency to stable latent infection. Thus, infected resting cells may transit from a labile preintegration state to a stable form of latency in both antigen-dependent and antigen-independent mechanisms and may play a role in the establishment of the stable latent reservoir of HIV-1 provirus in resting T cells.
One approach to understanding the establishment of the latent reservoir for HIV-1 involves an analysis of the chemokine receptors utilized by viruses that enter latency. The phenotype of the envelope (Env) protein of virus in the latent compartment may reflect the path by which infection in quiescent T cells was established. Latent infection of resting T cells occurs very soon after transmission and cannot be blocked by early treatment (10, 19, 51). This early establishment suggests an involvement of CCR5 simply because R5 viruses predominate during the acute phase of the disease and are clearly involved in transmission (reviewed in reference 1). However, the nature of the chemokine receptors utilized by viruses in the latent reservoir has not been previously determined and may involve additional coreceptor molecules. To identify additional coreceptors involved in establishing a latent infection, we cloned full-length functional env genes of virus in the latent reservoir and assayed their chemokine receptor utilization.
The ability of viruses utilizing CCR5 to enter highly purified resting cells has not been demonstrated. In fact, previous in vitro studies suggest that the infection of resting lymphocytes lacking CD25 by an R5 virus cannot occur (7). A consequence of this is that entry into the latent compartment by R5 viruses should occur only via an activated intermediate, as discussed above. If resting lymphocytes are indeed resistant to infection by R5 viruses, the presence of these viruses within the reservoir could provide support for the hypothesis that entry of the virus into the reservoir occurs via an activated T cell expressing both CCR5 and CXCR4. Furthermore, since the large majority of lymphocytes in the periphery are resting, the inability of R5 viruses to enter quiescent cells would have implications for pathogenesis and viral dynamics. To complement our analysis of coreceptor utilization, we have assessed the ability of viruses using CCR5 to directly infect highly purified populations of resting CD4+ lymphocytes. Together these studies provide new insights into the establishment of the latent reservoir.
MATERIALS AND METHODS
PCR amplification and cloning of full-length env genes.
Highly purified resting CD4+ T lymphocytes were isolated from the peripheral blood mononuclear cells (PBMC) of eight patients on HAART as previously reported (20). One additional patient successfully treated with HAART was acquired separately for study because of documented history of X4 virus in the circulation. Latent virus was cultured from this patient as described (20). To detect and clone full-length envelope genes from the genomic DNA of these resting T cells, a novel nested PCR strategy employing a thermostable polymerase cocktail with proofreading activity was performed. A mass of 500 ng of genomic DNA was amplified in a reaction containing 1 μM each of outer primer (sense, 5′-ATGGCAGGAAGAAGCGGAGACAG-3′; antisense, 5′-TGTGTAGTTCTGCCAATCAGGGAAGTAGCCTTGTG-3′) 200 μM each of the four deoxynucleoside triphosphates, buffer containing 1.5 mM MgCl2, and 3.5 U of Expand High Fidelity polymerase cocktail (Boehringer Mannheim). A 3-min hot start at 94°C was performed, followed by 25 cycles of 94°C for 30 s, 65°C for 30 s, and 68°C for 3 min. An additional 5 s were added to the extension step of the last 15 cycles of this reaction. A final extension at 68°C was performed for 7 min. An aliquot of the reaction product was diluted 1:4 in distilled water for use in a second reaction with inner primers (sense, 5′-GATAGACGCGTAGAAAGAGCAGAAGACAGTGGCAATG-3′; antisense, 5′-CCTTGTCCGGCGGCCGCCTTAAAGGTACCTGAGGTCTGACTGG-3′) of 20 cycles of 94°C for 30 s, annealing at 68°C for 30 s, and 68°C for 3 min. Again, an additional 5 s of extension were added during the last 10 cycles of the reaction. A 7-min final extension was also performed. PCR products were gel purified (Qiagen), digested with NotI and MluI, and cloned into the pCI-PRE vector as described (C. Buck and R. F. Siliciano, submitted for publication). The resulting constructs were transformed into Escherichia coli JM109 competent bacteria and grown at 30°C to minimize recombination and bacterially induced mutagenesis within env. Because our strategy did not distinguish defective genomes from those competent to replicate, env genes from replication-competent virus were also cloned. Viruses isolated from resting CD4+ T cells of three patients on HAART were used to infect phytohemagglutinin-stimulated, CD8-depleted primary lymphoblasts. DNA was then isolated and used as a template for the PCR and cloning strategy described above.
Functional activity of env clones from the latent reservoir.
env clones were assayed for functional activity as measured by the ability to utilize a panel of chemokine receptors in a previously described luciferase reporter virus infection assay (17). Briefly, Env and NL-luc E−R− plasmids were transfected into 293T cells using CaPO4. Viral supernatants were harvested 2 days posttransfection, cleared of cell debris by low-speed centrifugation, and used to infect feline CCC cells transfected with CD4 and various HIV-1 coreceptors. Infection of target cells by pseudotyped virus leads to expression of luciferase, which was quantified in cell lysates 2 to 3 days following addition of virus. To maximize our ability to sample viruses in the latent compartment, analysis was performed on multiple clones representing multiple independent PCR amplifications.
Isolation and analysis of highly purified resting T cells.
Resting CD4+ lymphocytes were prepared using a modification of a previously published purification strategy (9, 11, 20). PBMC from seronegative donors were prepared by centrifugation on Ficoll-Hypaque gradients. Monocytes were removed by adherence during overnight culture in RPMI 1640 supplemented with 10% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM glutamine (minimal medium [MM]). Initial enrichment for resting CD4+ T cells was achieved by a bead depletion strategy. Cells were incubated for 30 min on ice with a cocktail of monoclonal antibodies against cell surface markers expressed on other cell lineages (CD8, CD19, CD16, and CD14) or activated CD4+ T cells (CD69, CD25, and HLA-DR). Excess antibody was removed by washing in phosphate-buffered saline (pH 7.2) supplemented with 2% fetal calf serum, 0.1% glucose, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 12 mM HEPES (pH 7.2). Cells carrying bound antibody were removed by incubation with magnetic beads conjugated to sheep anti-mouse immunoglobulin G (Dynal) at 4°C for 25 min, followed by magnetic separation. The resulting fractionated cells were stained with phycoerythrin (PE)-conjugated OKT4 (Ortho-immune Diagnostics) and Tri-Color (TC)-labeled anti-HLA-DR antibody (Caltag) for 30 min on ice in preparation for sorting. OKT4 does not block infection by HIV-1. Cells staining CD4+ and DR− were collected using an Elite cell sorter (Coulter). Isolation of quiescent populations depleted of either CD45 RA+ or CD45 RO+ cells was performed as described above by including the relevant fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies in both the depletion and sorting steps. Sorting was then performed by collecting cells positive for CD4+ and negative for HLA-DR− and the relevant CD45 isoform. This strategy allowed the exclusion of cells expressing both RO and RA isoforms from either population.
The fraction of highly purified resting cells expressing CCR5 was enumerated using three-color fluorescence-activated cell sorting (FACS) analysis. Two hundred thousand cells were stained with the FITC-conjugated monoclonal antibody against CCR5 (2D7; Pharminagen) in conjunction with the PE- and Tri-Color-labeled antibodies against CD4 and HLA-DR (respectively) described above. The fraction of resting cells expressing CCR5 was determined by gating on CD4+ lymphocytes lacking HLA-DR.
Infection of highly purified resting T lymphocytes.
High-titer HIV-1 stocks were obtained commercially (ABI) and treated with DNase (Boehringer Mannheim) in the presence of 1 mM MgCl2 for 30 min at 37°C. DNase treatment was required to remove contaminating DNA in the stock arising from cell death and lysis during virus production. Between 5 × 105 and 1 × 106 purified resting lymphocytes were resuspended in 100 μl of MM and infected at a multiplicity of infection (MOI) of either 0.1 or 1. To account for intravirion reverse transcription products (27, 40, 48–50) and contaminating DNA in the viral stocks, an aliquot of virus was heat inactivated at 70°C for 30 min, and an equivalent amount was applied to resting cells. Three hours following application of the virus, the cells were washed in 1 ml of trypsin-EDTA. A second 1-ml volume of 1× trypsin was added and incubated at 37°C for 10 min. A final wash with 1 ml of MM was performed. Cells were cultured in MM where indicated, followed by lysis. Cell lysis was performed in 200 μl of PCR lysis buffer containing 100 mM KCl, 20 mM Tris (pH 8.3), 0.1% NP-40, and 500 μg of proteinase K per ml (17).
HIV-1 entry was detected using a modification of the PCR strategy previously described by Chen and colleagues (46). Since the entry of viruses into resting cells is complicated by the protracted kinetics of reverse transcription in quiescent cells, the generation of both early (long terminal repeat [LTR]) and late (LTR-Gag) products of the reaction was monitored using the previously published primer pairs M667-AA55 and M667-M661, respectively. These reactions were performed using chemistry similar to that described above and cycling the reaction between 94 and 65°C. Products of the reaction were subjected to 2% agarose electrophoresis followed by alkaline transfer to nitrocellulose and hybridization with an end-labeled oligonucleotide probe. Products of reactions employing M667 and AA55 were detected with a specific end-labeled oligonucleotide probe (5′-GTG CTT CAA GTA GTG TGT GCC C-3′), while those amplified by M667 and M661 were detected with AA55. To control for the relative number of cells in each lysate, β-globin was amplified and detected by Southern hybridization as described (9).
Quantitation of viruses in the latent reservoir.
The frequency of cells harboring latent HIV-1 among purified resting naive and resting memory subsets of CD4+ T cells was evaluated using a previously described limiting-dilution culture assay (19). These studies were carried out on blood samples from previously characterized patients on combination therapy who had undetectable plasma virus levels. This ensures that the latent viruses detected are derived from the stable latent reservoir (19). Results are expressed as infectious units per million (IUPM) resting CD4+ T cells.
RESULTS
Cloning and characterization of functional envelope genes from quiescent lymphocytes of patients on HAART.
To identify the phenotype of env from viruses resident in latently infected resting T lymphocytes of patients on HAART, viral envelopes were cloned from resting T cells from selected patients and assayed for their ability to utilize a panel of chemokine receptors in a functional assay. Patients who were being treated with combinations of three to five antiretroviral drugs, including a protease inhibitor, and who had undetectable plasma HIV-1 RNA (<200 copies/ml) were selected for study. The characteristics of the patient population are summarized in Table 1. In patients with effective suppression of viral replication, viruses isolated from resting CD4+ T cells are likely to be derived from cells with stably integrated HIV-1 DNA (20). DNA was isolated from purified resting CD4+ cells and used as the template in a novel nested PCR strategy for the amplification and cloning of the full-length env gene. This assay could detect as few as 50 copies of env in a background of 500 ng of HIV-1− genomic DNA (data not shown). Approximately 50% of the clones studied were functional in cell fusion (not shown) or pseudotyping experiments.
TABLE 1.
Characteristics of patients studieda
| Patient no. | CD4 nadir (cells/μl) | Drug regimen | Time on HAART (mo) | Purity of sorted resting CD4+ T cells (%) | No. of functional env genes cloned/total no. cloned |
|---|---|---|---|---|---|
| 1 | 612 | d4T, 3TC, RTV, SQV | 5.0 | 95.3 | 3/6 |
| 6 | 440 | AZT, 3TC, RTV | 1.7 | 97.0 | 5/5 |
| 10 | 151 | d4T, 3TC, IDV | 8.5 | 99.4 | 1/5 |
| 11 | 981 | d4T, (RTV, SQV)→(3TC, NFV) | 1.5 | 96.1 | 6/9 |
| 12 | 133 | d4T, 3TC, RTV→SQV | 17.0 | 97.8 | 2/8 |
| 13 | 477 | d4T, 3TC, RTV | 8.0 | 97.3 | 1/6 |
| 14 | 253 | d4T, 3TC, IDV | 10.5 | 96.3 | 7/9 |
| 16 | 472 | d4T, 3TC, RTV | 30.0 | 99.1 | 4/9 |
| 56 | 717 | AZT, 3TC, IDV | 30.0 | 5/8 |
Patients were adult volunteers who gave informed consent before giving blood for this study. Drugs used in HAART regimens included the nucleoside analogues zidovudine (AZT), stavudine (d4T), and lamivudine (3TC), the nonnucleoside reverse transcriptase inhibitor nevirapine (NVP), and the protease inhibitors ritonavir (RTV), saquinavir (SQV), indinavir (IDV), and nelfinavir (NFV). In most cases, three drugs for which the patient had no prior experience were started simultaneously. In two cases, one protease inhibitor was substituted for another (i.e., RTV→SQV) because of toxicity, but in all cases treatment with three or four drugs was maintained. Several of the patients had had prior monotherapy, generally with nucleoside analogues (20).
The chemokine receptor utilization of 34 clones from nine patients was determined using previously described pseudotyping technology (13). The vast majority of clones assayed could infect CD4+ target cells expressing CCR5 but not cells expressing CXCR4 (Table 2). However, clones representing both the X4 and R5/X4 viral phenotypes were identified. To decrease the chance that the low frequency of X4 virus identified during our studies was a consequence of inadequate sampling, envelopes derived from independent cloning experiments were analyzed. Only envelopes from patients 12 and 56 had the capacity to utilize CXCR4 in the absence of CCR5. Interestingly, studies performed prior to the administration of HAART revealed that the phenotype of viruses in the circulation of patient 56 included those capable of utilizing CXCR4. The env genes cloned from the resting T cells of this patient utilized either CCR5 or CXCR4 in luciferase reporter virus infections. That R5 virus was detected in the reservoir years after the detection of X4 virus in the peripheral blood highlights the capacity of the reservoir to preserve viruses representing the complete life history of the infection within an individual (Table 2). Envelopes from patient 10 were dual tropic, capable of utilizing both CCR5 and CXCR4. Other coreceptors, including V28, GPR15, STRL33, and APJ, were able to support reporter virus entry in a few instances, but the reduced efficiency and low frequency of usage of alternate receptors suggest that they do not play a major role in establishment of the latent virus reservoir. The phenotype of clones from virus cultured from the latent reservoir of patients 14, 16, and 56 could not be distinguished from the env genes of viruses cloned directly from the DNA of resting cells of these patients, indicating that direct sampling of proviral DNA in purified resting CD4+ T cells provides a representative picture of the replication-competent viruses harbored in the latent reservoir.
TABLE 2.
Chemokine receptor utilization of viruses in latently infected CD4+ resting T cellsa
| Patient no. | Clone | Signal strength with coreceptor:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCR2b | CCR3 | CCR5 | CXCR4 | STRL33 | GPR1 | GPR15 | APJ | V28 | CCR8 | ChemR23 | CD4 alone | ||
| HXB2 | − | + | − | ++++ | − | − | − | + | − | − | − | − | |
| SIVmac251 | − | − | ++++ | − | ++ | ++ | ++ | − | − | − | − | − | |
| Ba-L | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| 1 | A1.2 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − |
| A1.3 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| A2.3 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| 6 | C1.1 | + | + | ++++ | + | + | ++ | + | + | + | + | + | + |
| C1.2 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| C2.2 | + | ++ | ++++ | + | − | − | − | − | − | − | − | − | |
| C2.4 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| C2.5 | − | ++ | ++++ | − | − | − | + | − | − | − | − | − | |
| 10 | D1.4b | ++ | ++ | ++++ | +++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ |
| 11 | E1.4 | − | + | ++++ | − | ++ | − | − | − | − | − | − | − |
| E2.4 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| E11.A1 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| E11.A6 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| E11.A7 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| E11.A8 | − | ++ | ++++ | − | − | − | − | − | ++ | − | − | − | |
| 12 | F1.20 | − | − | − | ++++ | − | − | − | + | − | − | − | − |
| F1.23 | − | − | +++ | ++++ | − | − | − | + | − | − | − | − | |
| 13 | G1.2 | − | − | ++++ | − | − | − | − | − | − | − | − | − |
| 14 | H1.1 | − | + | ++++ | − | − | − | − | − | − | − | − | − |
| H1.2 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| H2.3 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| H2.4 | − | + | ++++ | − | + | − | − | − | − | − | − | − | |
| H2.5 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| V14-4Bc | − | + | ++++ | − | − | + | − | − | − | + | − | + | |
| V14-5Bc | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| 16 | I1.4 | − | + | ++++ | − | − | − | − | − | − | − | − | − |
| I1.5 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| I2.1 | − | + | ++++ | − | − | − | − | − | − | − | − | − | |
| V16-3Bc | − | ++ | ++++ | − | + | − | − | − | − | − | − | − | |
| 56 | X4 | − | + | ++++ | − | − | − | − | − | − | − | − | − |
| X7 | + | ++ | ++ | ++++ | − | − | + | ++ | + | − | − | − | |
| X8 | − | ++ | ++++ | − | − | − | − | − | − | − | − | − | |
| VX2c | − | ++ | ++++ | + | − | − | ++ | − | − | − | − | − | |
| VX2c | ++ | ++ | − | ++++ | − | − | + | ++ | − | − | − | − | |
env genes cloned by PCR directly from highly purified resting T cells of patients on HAART were tested for coreceptor specificity using a luciferase reporter virus assay (17). Pseudotyped virions for each env containing the luciferase gene capable of a single round of infection were used to infect feline CCC cells transiently expressing CD4 and various HIV coreceptors. Infection was quantified by measurement of luciferase in target cell lysates 2 to 3 days postinfection. Results for each env were normalized to the coreceptor (CCR5 or CXCR4 in all cases) that gave the highest signal, which is indicated as ++++. Signals that were 50 to 99%, 11 to 49%, 5 to 10%, and <5% of the activity of the principal coreceptor are indicated as +++, ++, +, and −, respectively. The X4 HIV-1 Env HXBc2 and R5 SIVmac251 Env BK28 were used as positive controls. Coreceptor use of the R5 HIV-1 Env Ba-L is also shown and was calculated from previously published work (13, 17).
High background on CCC cells likely indicates use of endogenous feline coreceptor.
env genes cloned from biological isolates.
Expression of CCR5 on resting lymphocytes.
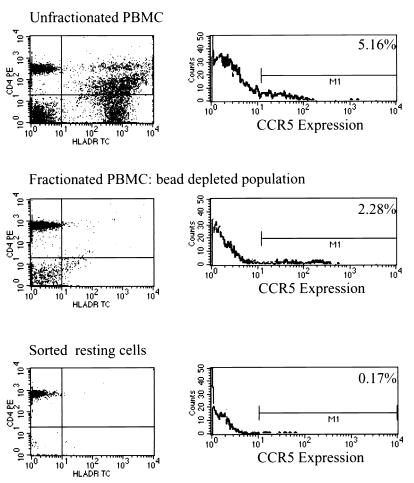
The results presented above suggest that R5 viruses predominate in the latent reservoir for HIV-1 in resting CD4+ T cells. To understand this finding with respect to the capacity of R5 viruses to directly infect resting T cells, we studied the expression of CCR5 on resting T-cell populations of uninfected donors. Earlier studies of PBMC demonstrated CCR5 expression on a subset of cells expressing markers of both activation and memory (CD26+ and CD45RO+), while CXCR4 was shown to have a reciprocal pattern, being expressed predominantly on naive cell populations (CD45 RA+) (3, 9). Recent four-color FACS analysis of PBMC demonstrated the expression of CXCR4 on both naive and memory T-cell subsets, while CCR5 was found to be absent on naive T cells (35). Interpretation of the published data is complicated by the failure of some studies to distinguish clearly between resting CD4+ T cells, which can harbor latent virus, and activated T cells, which are fully permissive for viral replication. Making use of the same rigorous cell purification procedure used in the delineation of the latent reservoir, we isolated resting CD4+ T cells that were negative for expression of the activation markers HLA-DR, CD25, and CD69. The level of CCR5 on these highly purified resting CD4+ T lymphocytes was then measured. Considering what is now known about the factors regulating its promoter, high levels of expression of CCR5 on quiescent lymphocytes would not be predicted (22, 31). In agreement with these observations, highly purified resting cells consistently demonstrated lower levels of CCR5 than partially purified or unpurified PBMC from the same donor (Fig. 1). The percentage of cells expressing CCR5 was a function of the fraction of cells expressing HLA-DR, suggesting that among PBMC, most of the cells expressing CCR5 coexpress markers of activation. This finding highlights the association between CCR5 expression and markers of activation. For all individuals studied, less than 6.3% of highly purified resting CD4+ T cells (containing <1% CD4+ DR+ cells) had detectable expression of CCR5. Thus, CCR5 expression can be detected at low levels on only a small fraction of the resting CD4+ T-lymphocyte population known to harbor latent HIV-1.
FIG. 1.
CCR5 expression on resting CD4+ T cells at various stages of purification. Highly purified resting CD4+ T cells were isolated from the peripheral blood of HIV-negative donors by removing unwanted cell lineages and activated CD4+ T cells through sequential bead depletion and cell sorting procedures. HLA-DR was used as a marker of activation. The purity of resting CD4+ T cells was invariably greater than 99%. Cells at multiple points of the procedure were stained for CCR5 expression using a FITC conjugate of the anti-CCR5 monoclonal 2D7.
Infection of quiescent CD4+ T lymphocyte populations is inefficient when mediated by CCR5 due to low surface expression of the coreceptor.
A small number of resting CD4+ T cells express CCR5 at levels that can be detected by flow cytometry. To determine whether this level of coreceptor is sufficient to mediate infection, the ability of the R5 isolate Ba-L to enter resting T lymphocytes was measured and compared to the ability of the X4 virus HIV-1 IIIb to infect these cells. Previous studies demonstrate a relationship between the amounts of CCR5 and CD4 present on the surface of a cell required for infection by an R5 virus, concluding that in the presence of high levels of CD4, low-level expression of CCR5 could mediate infection (34, 45). The level of CXCR4 on highly purified resting T cells is sufficient for entry by X4 viruses (9, 25). Since the infection of resting lymphocytes is nonproductive due to either a block in nuclear import (4, 38) or incomplete reverse transcription (25, 46, 47), entry of HIV-1 was detected by a modification of a previously published PCR-based entry assay which detects the products of reverse transcription (46).
Several control experiments were performed to ensure that the signals detected represented infection rather than the products of intravirion reverse transcription (27, 40, 48–50) or contaminating DNA in the virus stock. The generation of HIV-1 DNA-specific signals following infection could be blocked by inclusion of monoclonal antibodies to CD4 or the gp41 peptide fusion inhibitor DP372 (data not shown) (42). Furthermore, the reaction detecting complete reverse transcription products could be blocked by the addition of zidovudine prior to infection (data not shown). In addition, as is discussed below, the time-dependent increase in the strength of the signals demonstrated that the assay was detecting products of reverse transcription being generated in infected cells following virus entry.
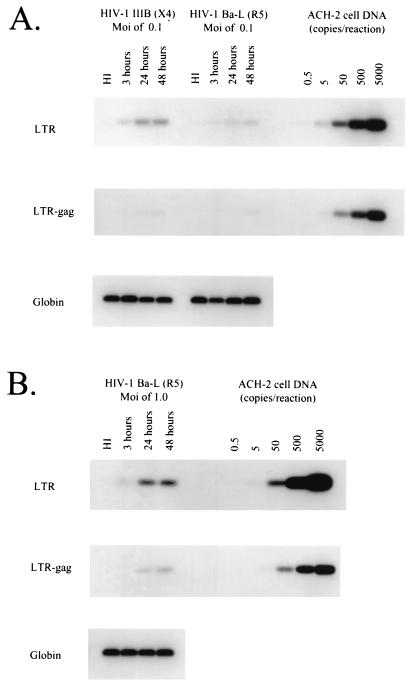
Using this assay, entry of HIV-1 Ba-L and HIV-1 IIIb into quiescent CD4+ T lymphocytes purified from the blood of seronegative donors was measured. Purified cells were universally greater than 99% pure, as judged by the absence of contaminating activated cells expressing HLA-DR. When infections were performed at a multiplicity of 0.1, a significant and reproducible difference in the ability of these two viruses to enter resting cells was observed. Entry of IIIb could be demonstrated at a lower multiplicity than that of the R5 virus Ba-L (Fig. 2A). This result likely reflects the large difference in expression of CXCR4 and CCR5 coreceptors on resting cells. In some donors, infection with Ba-L at an MOI of 0.1 did not yield significant entry by our assay, consistent with the reported variability of expression of CCR5 from donor to donor. However, the resting lymphocytes of all donors studied could be infected by R5 viruses at an MOI of 1, ruling out an absolute block to infection of resting lymphocytes by a virus utilizing CCR5 (Fig. 2B). These experiments were performed in duplicate on samples from four different donors. The titers of the Ba-L and IIIb stocks used were equivalent, as evidenced by the fact that both viruses could infect activated CD4+ T cells with similar efficiencies (data not shown).
FIG. 2.
Levels of CCR5 on highly purified resting T lymphocytes are sufficient to mediate infection by the R5 virus Ba-L. The ability of highly purified resting cells to be infected with the R5 virus HIV-1 Ba-L and the X4 virus HIV-1 IIIb was determined. Cells were infected at an MOI of 0.1 (A) or 1.0 (B). After incubation for the indicated time at 37°C, cells were lysed, and entry was monitored by PCR assay for early (LTR) and late (LTR-Gag) products of reverse transcription as described (46). In control lanes, virus was heat-inactivated (HI) before infection. The sensitivity of the PCR in each experiment was assayed using a series of ACH-2 dilutions as a target for the PCR described above. A fragment of the β-globin gene was amplified to normalize for the number of cells used in each PCR experiment. Infection experiments were performed at least twice on cells from four different donors.
The coreceptor utilization of Ba-L has been characterized extensively and has been shown to be almost exclusively CCR5 (Table 2) (16, 32). To rule out the possibility that the infection of resting cells by HIV-1 Ba-L shown in our previous experiments was mediated by an alternative or undiscovered coreceptor, we attempted to block infection by preincubating the cells with monoclonal antibodies to CCR5. A cocktail of anti-CCR5 monoclonals (R&D Technologies) blocked infection with an efficiency that roughly equaled that of anti-CD4. Neither an irrelevant antibody nor a monoclonal antibody against CXCR4 was able to diminish the entry of Ba-L (data not shown).
Infection of quiescent lymphocytes by an R5 virus can be eliminated by removing cells expressing CD45RO.
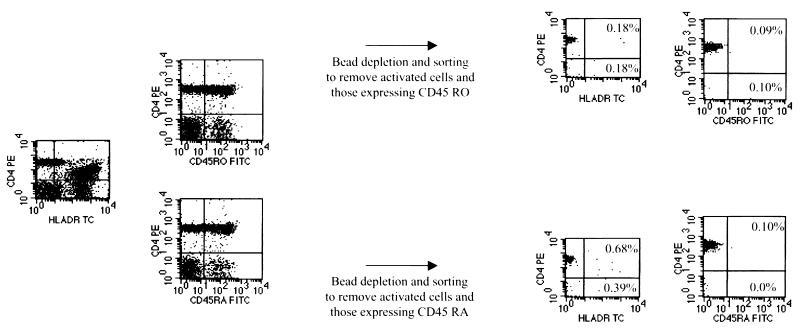
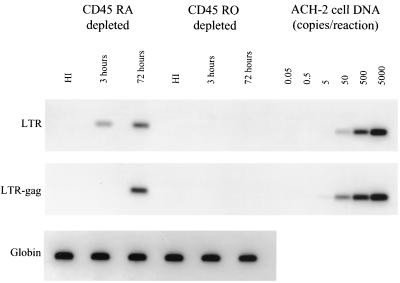
To further characterize the subset of resting cells permissive for infection by Ba-L, highly purified quiescent cells were further depleted of markers associated with a naive or memory phenotype. Cells obtained using a modification of a previously described depletion and sorting strategy were greater than 99% free of contaminating cells expressing HLA-DR and the unwanted CD45 isoform (Fig. 3). The resulting cells were infected at a multiplicity of 1 with Ba-L. PCR analysis clearly demonstrated that the infection of quiescent lymphocytes is restricted to subsets containing cells expressing CD45RO (Fig. 4). Thus, CCR5-utilizing isolates can enter a subset of resting memory T cells but not naive cells.
FIG. 3.
Purification of naive and memory resting CD4+ T cell. PBMC from HIV-1-negative donors were subjected to magnetic depletion using monoclonal antibodies to CD8, CD19, CD16, CD14, CD69, CD25, HLA-DR, and either CD45 RA or RO. Cells were sorted for those expressing CD4 while lacking expression of either HLA-DR or the relevant CD45 isoform. Sorted populations contained less than 1.0% contaminants expressing HLA-DR or the inappropriate CD45 isoform.
FIG. 4.
Infection of resting cells by R5 viruses is restricted to those expressing markers of immunologic memory. Resting CD4+ lymphocytes depleted of either naive or memory subsets were obtained and infected with HIV-1 Ba-L at a multiplicity of 1.0. Infection was monitored over the course of 3 days. Infection was monitored through the detection of the products of reverse transcription as described using M661 and M667 (46). The sensitivity of the PCR in each experiment was assayed using a series of ACH-2 dilutions as a target for the PCR against the LTR-Gag junction. Amplification of β-globin was performed to demonstrate the presence of an equal mass of DNA in each sample. This experiment was performed on samples from two donors with identical results.
Latent HIV-1 can be found in resting memory CD4+ T cells in vivo and to a lesser extent in naive cells.
Given that the R5 viruses that comprise the latent reservoir in most patients cannot directly infect naive resting CD4+ T cells, it was of interest to determine whether latent virus could be isolated from the naive subset of resting CD4+ T cells obtained from infected individuals. Using a previously described purification scheme that yields naive and memory CD4+ T cell subsets that have <1% contamination with the other subset or with activated cells, the frequency of cells harboring latent, replication-competent HIV-1 was measured by limiting-dilution cultures (Table 3). In most patients, latent virus was found predominantly in the memory subset, consistent with the idea that latently infected cells arise through infection of activated cells, which then revert to a resting memory state. However, for some patients, latent virus could also be isolated from naive cells at a lower frequency. When considered in the context of the above studies demonstrating the inability of R5 viruses to enter naive cells, these results suggest that latent infection is established in naive cells through a mechanism other than direct infection of the cells while they are in a resting, naive state.
TABLE 3.
Quantitation of latent virus from both naive and memory resting T lymphocytes in patients on HAARTa
| Patient no. | Time on HAART (mo) | Plasma HIV-1 RNA (copies/ml) | Frequency of latently infected CD4+ T cells (IUPM)
|
Purityb (%)
|
||
|---|---|---|---|---|---|---|
| CD45RA+ | CD45RO+ | CD45RA+ | CD45RO+ | |||
| 9 | 29.8 | N.D.c | 1.1 | <1.6 | 99.8 | 99.4 |
| 35.9 | <50 | 0.09 | 0.5 | N.D. | N.D. | |
| 10 | 41.0 | 129 | <0.4 | 5.0 | 98.6 | 98.3 |
| 11 | 29.2 | <50 | 0.1 | 3.0 | 99.7 | 99.2 |
| 21 | 34.0 | 141 | 3.0 | 8.0 | 99.7 | 99.5 |
| 50 | 24.8 | <50 | <0.2 | 1.1 | 99.4 | 98.8 |
Patients studied had been on long-term HAART with suppression of plasma HIV-1 RNA to below 200 copies/ml.
Purity refers to the percentage of CD4+ HLA-DR− cells in the sorted population and was calculated as (1−[number of CD4+ HLA-DR+ cells/number of CD4+ HLA-DR− cells]) × 100.
N.D., not done.
DISCUSSION
The establishment of replication-competent HIV-1 genomes within the resting CD4+ T lymphocytes of infected individuals is an important mechanism of viral persistence and represents a major barrier to HIV-1 eradication with antiretroviral drugs (12, 20, 43). The manner in which this stable infection is established can only be inferred. HIV-1 replicates most efficiently in activated CD4+ T lymphocytes which express high levels of CD4, CXCR4, and CCR5. One model suggests that at some frequency, an infected activated T lymphocyte may exit the cell cycle, enter a quiescent state, and circulate as a latently infected resting memory CD4+ T cell. This model is supported by the finding that the majority of stable latent virus in resting cells can be found in memory cells. In this study, the mechanisms by which viruses enter the latent reservoir in resting CD4+ T cells has been evaluated through an analysis of the patterns of chemokine receptor utilization by viruses in the reservoir, of the distribution of these viruses among naive and memory subsets of resting CD4+ T cells, and of the direct entry of X4 and R5 viruses into these subsets.
The contribution of the direct infection of resting cells to the establishment of the latent reservoir is not clear. Since, at any given time, the majority of lymphocytes are in a quiescent state, resting lymphocytes likely represent a major target for infection by HIV-1. Previous studies characterizing the ability of HIV-1 to enter unstimulated CD4+ T-cell populations involved the use of laboratory-adapted strains of HIV-1 that utilize CXCR4 (21, 23, 36, 38, 39, 47). Subsequent studies using highly purified resting T cells confirmed these initial findings (9, 25). Although several reports include experiments demonstrating the capacity of R5 isolates to infect unpurified, unstimulated peripheral blood populations (39, 46, 47), rigorous analysis of the capacity of R5 viruses to enter highly purified resting T cells is lacking in the literature. In fact, resting cells (CD25−) have been reported to be refractory to entry by R5 viruses, presumably due to low or absent coreceptor expression (7). Therefore, the presence of R5 viruses within resting T cells that are themselves refractory to direct infection would support a model in which the reservoir is established through the infection of an activated T cell, which then exits the cell cycle into a quiescent state. To test this hypothesis, the chemokine receptor utilization of viruses derived from latently infected CD4+ T lymphocytes was determined and compared to the capacity of these viruses to directly infect resting CD4+ T lymphocytes.
The evolution of coreceptor utilization by HIV-1 throughout the course of infection has been widely documented (1, 14) Viruses involved in transmission are almost universally dependent upon CCR5, as demonstrated by the profound resistance of individuals null at the CCR5 locus to infection by HIV-1 (16, 26, 37). In some patients, disease progression correlates with a broadening of the repertoire of chemokine receptors utilized by HIV-1 during infection (14). The coreceptor utilization of viruses in the latent compartment has not been systematically analyzed. At least some viruses cultured from the resting CD4+ T-cell compartment in previous studies were cytopathic or capable of forming syncytia in the MT-2 cell line (12, 20). To determine the nature of the coreceptors utilized by viruses in the latent reservoir, we cloned and analyzed the phenotype of env genes amplified from viruses resident in highly purified resting CD4+ T lymphocytes from nine patients on HAART. We demonstrate here for the first time that viral envelopes from the latent reservoir show predominant usage of the coreceptor CCR5. This finding is consistent with the fact that the reservoir is formed soon after infection, when virtually all of the viruses have the R5 phenotype. The maintenance of R5 genomes in resting cells likely ensures that a broadening of coreceptor utilization occurs with disease progression, rather than a replacement of R5 viruses with those of the X4 phenotype, since R5 viruses present early in infection will be stored in the latent reservoir thereafter. This may account for the identification of R5 viruses in a patient with documented X4 virus in the blood prior to study.
Interpreting the low frequency of viruses utilizing CXCR4 in the latent reservoir is complicated by an incomplete understanding of the nature of the viruses present in each individual studied prior to the initiation of therapy and of the mechanism by which the viruses entered the resting compartment. An undetectable level of circulating virus was a requirement for inclusion in this study, prohibiting a comparison of the virus in the periphery to that in the reservoir. Furthermore, not all patients acquire virus of the X4 phenotype. Only patient 56 had documented utilization of CXCR4 prior to HAART. The analysis of several independently derived clones suggested that the bias against virus utilizing CXCR4 was unlikely a result of inadequate sampling of the reservoir. It is interesting to speculate that the cytopathology associated with X4 viruses provides strong selection against entrance into the reservoir, although such viruses can clearly enter the reservoir. Utilization of additional “minor” coreceptors, such as CCR3, STRL33, GPR15, and APJ, was also observed in envelopes from several patients. While the biological relevance of this finding is difficult to interpret without a better understanding of the role in vivo of these receptors, our study suggests that these receptors do not play a universal role in the establishment of the latent reservoir.
Longitudinal analysis of the frequency of latently infected cells in treated patients reveals a very slow decay of the virus in this compartment (19, 51). While several mechanisms have been discussed to explain the persistence of virus in this compartment, the long life span of memory T cells in vivo likely plays an important role. As a result, an interesting feature of this reservoir is that it appears to act as an archive of viral quasi species, containing viruses from many points during the life history of infection. The analysis of antiviral drug resistance conferring mutations from virus cultured from the resting T lymphocytes of individuals on HAART provides support for the archival nature of this compartment (20). Should therapy fail or be discontinued, virus may reemerge from the latent compartment and rekindle infection. In this setting the virus reestablishing infection may not reflect the phenotype of the virus circulating immediately prior to the initiation of therapy due to the fact that the breakthrough infection may have been the result of archival virus emerging from the latent reservoir. Thus, the phenotype of viruses in the latent compartment may have clinical significance as a repository representing all stages of the disease course even in the presence of successful therapy.
To better understand the significance of R5 viruses in the latent compartment, we next determined whether these same viruses had the capacity to infect resting T lymphocytes directly. Highly purified resting lymphocytes were assayed for levels of CCR5 sufficient to mediate infection by the R5 virus Ba-L. We demonstrate that only a small fraction of resting T cells express CCR5. Thus, HIV-1 can enter only a subset of quiescent T cells, all of which had a memory phenotype. The PCR analysis performed in this study suggests that infection of resting cells by R5 virus is inefficient and likely limited to the subset expressing detectable levels of CCR5. This is supported by the concordance of an absence of CCR5 on naive T cells and our inability to demonstrate infection of these populations by an R5 virus (35). Additionally, our ability to demonstrate infection of resting cells only during infection at high multiplicity is consistent with the infection's being restricted to a small fraction of resting cells expressing CCR5. This is a likely explanation for the discrepancy between our results and those of Chou et al. (7), who were unable to detect infection of resting CD4+ T cells by R5 viruses. That a larger inoculum of Ba-L was required to achieve detectable levels of infection compared to an X4 virus supports a quantitative role of coreceptor expression during entry (34, 45).
While infection of resting cells is nonproductive, the molecular processes preventing the production of progeny virions are less clear and the subject of extensive study. The inability to complete the process of reverse transcription (25, 46, 47), a block preventing the import of the preintegration complex into the nucleus (38), and the absence of a required cellular factor(s) (24) have all been proposed to explain the fate of the virus in an infected quiescent T lymphocyte. Our approach to detecting entry into resting cells relied on two different PCRs detecting either the early or late products of reverse transcription. This analysis was useful in demonstrating the absence of activated cells in our resting-cell population. Our ability to detect reverse transcription products with primers that span the junction of the LTR and gag suggests that reverse transcription in resting T cells proceeds largely to completion over the course of several days. The kinetics of this reaction are much slower than what has been reported and observed by us for the infection of activated cells (11, 18, 30, 47). These results differ from studies in the literature that conclude that the reverse transcription process does not proceed to completion in resting T cells (25, 46, 47). We believe the basis of this difference is the length of time over which infection was monitored following the application of the virus. In our hands, the reverse transcription process is largely incomplete at early time points and proceeds to completion over the course of a few days. Additionally, previous studies of the status of reverse transcription reaction in resting cells from HIV-1-infected individuals clearly demonstrate both the absence of a build-up of incompletely reverse-transcribed genomes and the presence of full-length linear double-stranded viral cDNA (5, 8).
Studies of quiescent lymphocytes can be complicated by the presence of contaminating activated cells. It is unlikely that the infection demonstrated in this study reflects infection of contaminating activated cells for several reasons. The sorted populations used in this study were obtained using technology that allows for the isolation of cells from uninfected donors that are invariably greater than 99% free of cells bearing the HLA-DR marker of activation. These cells have been previously shown to be truly quiescent, as measured by the fact they do not incorporate thymidine in culture or expresses thymidine kinase (11). Finally, as discussed above, the design of our experiments allowed us to distinguish the infection of activated and resting cells on the basis of the kinetics of the reverse transcription reaction. As shown in Fig. 2, the synthesis of strong-stop DNA is largely complete at 12 h postinfection, while the product representing completion of reverse transcription is largely undetectable. In contrast, the viral genome can undergo both reverse transcription and integration during this time frame in activated T cells. Thus, the infection of activated cells cannot account for a significant amount of the signal produced during our experiments.
An understanding of the capacity of R5 viruses to enter quiescent T lymphocytes and the chemokine receptor utilization of viruses that comprise the latent reservoir together provide some insight into the mechanisms by which the latent reservoir may arise. The experiments presented here demonstrate that CCR5 exists at levels sufficient to mediate infection of only a subset of resting memory T cells. The discovery that the majority of viruses in resting T cells utilize CCR5 during infection was surprising due to the refractory nature of most resting cells to infection by viruses of this phenotype. It is interesting to speculate that the R5 viruses cloned from the resting cells of patients only had the capacity to enter the latent compartment through an activated T lymphocyte or a memory cell with continued expression of CCR5. We demonstrate here that the R5 viruses that comprise the latent reservoir in most patients cannot directly enter the naive subset of resting CD4+ T cells. However, some of the latent virus present in resting CD4+ T cells in vivo is in naive cells. Taken together, these results suggest that latently infected naive CD4+ T cells arise through a mechanism that does not involve direct infection of resting naive cells. One possibility is that some resting memory cells with integrated HIV-1 DNA might revert to the naive phenotype. Such phenotypic reversion has been observed in normal uninfected humans and in rodent systems (6, 28, 29). Infection of thymocytes is another possibility. Our results are consistent with other studies demonstrating the presence of R5 viruses in naive CD4+ T lymphocytes of HIV-1-infected individuals (2, 32). The overall conclusion of this work is that the latent reservoir for HIV-1 comprises mainly R5 viruses, viruses that do not enter most resting CD4+ T cells. The generation of the latent reservoir therefore likely proceeds through infection of other cell populations such as activated CD4+ T cells that can convert to resting cells carrying integrated HIV-1 genomes that become latent when cells are in a resting state.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 43222 to R.F.S. and AI 40880 to R.W.D.
We acknowledge Stuart Ray, Raj Gandi, Lucy Carruth, Monika Hermankova, and remaining members of the Siliciano lab for helpful discussions. We are also grateful to the individuals who donated the blood used in these experiments.
REFERENCES
- 1.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 2.Blaak H, van't Wout A B, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc Natl Acad Sci USA. 2000;97:1269–1274. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes [see comments] Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunce C, Bell E B. CD45RC isoforms define two types of CD4 memory T cells, one of which depends on persisting antigen. J Exp Med. 1997;185:767–776. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou C S, Ramilo O, Vitetta E S. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch C P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 9.Chun T W, Chadwick K, Margolick J, Siliciano R F. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun T W, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano R F. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 12.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 14.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree G R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 16.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, OBrien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. . (Erratum, 274:1069.) [DOI] [PubMed] [Google Scholar]
- 17.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 18.Farnet C M, Haseltine W A. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc Natl Acad Sci USA. 1990;87:4164–4168. doi: 10.1073/pnas.87.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 20.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 21.Gowda S D, Stein B S, Mohagheghpour N, Benike C J, Engleman E G. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989;142:773–780. [PubMed] [Google Scholar]
- 22.Guignard F, Combadiere C, Tiffany H L, Murphy P M. Gene organization and promoter function for CC chemokine receptor 5 (CCR5) J Immunol. 1998;160:985–992. [PubMed] [Google Scholar]
- 23.Helbert M R, Walter J, LAge J, Beverley P C. HIV infection of CD45RA+ and CD45RO+ CD4+ T cells. Clin Exp Immunol. 1997;107:300–305. doi: 10.1111/j.1365-2249.1997.280-ce1170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita S, Chen B K, Kaneshima H, Nolan G P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 25.Korin Y D, Zack J A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 27.Lori F, di Marzo Veronese F, De Vico A L, Lusso P, Reitz M S, Jr, Gallo R C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992;66:5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mclean A R, Michie C A. In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michie C A, McLean A, Alcock C, Beverley P C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 30.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriuchi H, Moriuchi M, Fauci A S. Cloning and analysis of the promoter region of CCR5, a coreceptor for HIV-1 entry. J Immunol. 1997;159:5441–5449. [PubMed] [Google Scholar]
- 32.Ostrowski M A, Chun T W, Justement S J, Motola I, Spinelli M A, Adelsberger J, Ehler L A, Mizell S B, Hallahan C W, Fauci A S. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierson T, McArthur J, Siliciano R F. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 34.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabin R L, Park M K, Liao F, Swofford R, Stephany D, Farber J M. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–3850. [PubMed] [Google Scholar]
- 36.Roederer M, Raju P A, Mitra D K, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang S, Patterson B, Levy J A. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unutmaz D, KewalRamani V N, Marmon S, Littman D R. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 44.Woods T C, Roberts B D, Butera S T, Folks T M. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 45.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 47.Zack J A, Haislip A M, Krogstad P, Chen I S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Bagasra O, Niikura M, Poiesz B J, Pomerantz R J. Intravirion reverse transcripts in the peripheral blood plasma of human immunodeficiency virus type 1-infected individuals. J Virol. 1994;68:7591–7597. doi: 10.1128/jvi.68.11.7591-7597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Zhang Y, Spicer T, Henrard D, Poiesz B J. Nascent human immunodeficiency virus type 1 reverse transcription occurs within an enveloped particle. J Virol. 1995;69:3675–3682. doi: 10.1128/jvi.69.6.3675-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Zhang Y, Spicer T P, Abbott L Z, Abbott M, Poiesz B J. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993;9:1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Ramratnam B, Tenner R K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]