Abstract
The V protein of Sendai virus (SeV) is nonessential to virus replication in cell culture but indispensable to viral pathogenicity in mice. The highly conserved cysteine-rich zinc finger-like domain in its carboxyl terminus is believed to be responsible for this viral pathogenicity. In the present study, we showed that the cysteine-rich domain of the SeV V protein could actually bind zinc by using glutathione-S-transferase fusion proteins. When the seven conserved cysteine residues at positions 337, 341, 353, 355, 358, 362, and 365 were replaced individually, the zinc-binding capacities of the mutant proteins were greatly impaired, ranging from 22 to 68% of that of the wild type. We then recovered two mutant SeVs from cDNA, which have V-C341S and V-C365R mutations and represent maximal and minimal zinc-binding capacities among the corresponding mutant fusion proteins, respectively. The mutant viruses showed viral protein synthesis and growth patterns similar to those of wild-type SeV in cultured cells. However, the mutant viruses were strongly attenuated in mice in a way similar to that of SeV VΔC, which has a truncated V protein lacking the cysteine-rich domain, by exhibiting earlier viral clearance from the mouse lung and less virulence to mice. We therefore conclude that the zinc-binding capacity of the V protein is involved in viral pathogenesis.
Sendai virus (SeV), which belongs to the genus Respirovirus in the family Paramyxoviridae, is a respiratory tract pathogen of rodents. Like other members within the order Mononegavirales, it is an enveloped virus with a single-stranded, negative-sense RNA genome of approximately 15.4 kb. The arrangement of the SeV genome starts with a short 3′ leader sequence, followed by six genes encoding the structural proteins, including N (nucleocapsid), P (phosphoprotein), M (matrix), F (fusion), HN (hemagglutinin-neuraminidase), and L (large) proteins and terminates with a 5′ trailer sequence (19).
Among the six genes, the P gene was found to be unique in that it encodes not a single but multiple proteins. The colinear transcript encodes the P protein as well as the C, C′, Y1, and Y2 proteins; these four proteins are translated in a shifted frame by alternative translational starts. The P gene also directs synthesis of an accessory mRNA for the V protein by inserting a pseudotemplated G residue at the specific editing site (30, 31). Consequently, the V and P proteins have the same 317 residues at the amino terminus (the P/V common region), while the V protein contains a 67-residue unique carboxyl terminus (the Vu region). This Vu region features a motif formed by seven cysteine residues (CX3CX11CXCX2CX3CX2C, where X refers to any amino acid residue), which are highly conserved in almost all the members of the subfamily Paramyxovirinae. The number and spacing of these cysteine residues resemble those of zinc finger structures and metalloproteins, and the V proteins of other members of the subfamily Paramyxovirinae, including simian virus 5 (SV5), measles virus, and Newcastle disease virus, have been shown to bind zinc (21, 25, 29). It is not known, however, whether the SeV V protein actually binds zinc ions. Furthermore, the biological significance of the zinc binding is unknown.
The proteins encoded by the P gene vary in their role in virus replication. By complexing with the L protein or forming a homotrimer, the P protein is involved in virus mRNA transcription and genome replication (4, 7, 13). It also binds soluble N protein, perhaps acting as a chaperone to mediate nucleocapsid formation (6, 13). In contrast to the well-characterized P protein, little is known about the functions of the C and V proteins. The C proteins have been shown to be indispensable for efficient virus multiplication and pathogenicity (18), and they have also been shown to block interferon-mediated antivirus responses (11, 12). The SeV V protein is reportedly able to suppress virus genome RNA replication in vitro with a defective interfering minigenome model (5). However, the exact function of the V protein in virus replication and pathogenicity is not clear so far. Recent studies using a reverse genetics system have provided insights into the role of the V protein in virus infection in mice (8, 9, 14, 15; reviewed in references 23 and 24). The V-deficient virus [V(−)] and the Vu-deficient virus (VΔC) both replicate as efficiently as the wild-type parent SeV in cell culture with no significant alterations in viral mRNA transcription, genome replication, or protein synthesis. In contrast, both the V(−) and VΔC viruses are remarkably attenuated in mice, indicating an imperative role for the V protein, predominantly the Vu domain, in SeV pathogenicity in vivo. It has been demonstrated that the V protein is necessary for a virus to maintain a high viral load in the mouse lung as well as to inhibit host innate immunity in the early stage of infection.
In the present study, we first proved that the Vu domain of the SeV V protein is capable of binding zinc atoms with its highly conserved cysteine residues. We further investigated the effect of cysteine-dependent zinc binding on viral pathogenicity in mice by generating V mutant SeVs.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
LLC-MK2 cells were grown in Eagle's minimal essential medium (MEM) supplemented with 10% fetal calf serum. The wild-type SeV (SeV WT) was derived from cDNA of the Z strain, and its mutants were propagated in embryonated chicken eggs. Infectivity was measured by an immunofluorescent infectious focus assay (17) and expressed as cell infectious units (CIU)/ml. Hemagglutinating units (HAU) were measured by the standard method using a microtiter plate. An anti-SeV antibody was prepared by immunizing rabbits with purified SeV virions, and antibodies against P, C, and Vu were prepared with histidine-tagged P, C, and Vu proteins, respectively, purified from Escherichia coli (14).
Gene cloning, expression, and purification of the SeV V, P/V common, and Vu proteins.
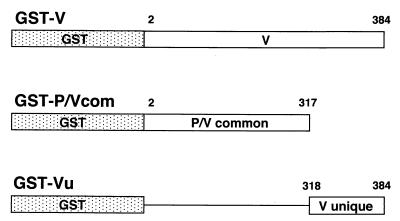
DNA manipulations were performed basically according to a manual (1). SeV V, P/V common, and Vu DNA fragments were prepared from the plasmid pIRES-V, harboring SeV V cDNA, with the Expand High Fidelity PCR system (Boehringer Mannheim, Indianapolis, Ind.). Primers used for V gene amplification were P/V-5 (5′-ATGGCGGATCCGAGCTCAGCATGGATCAAGATGC-3′) and Vstop (5′-ACCTTCTCGAGCCTTACGAGCGGAAGATTC-3′). For the P/V common gene, the primers used were P/V-5 and P/V stop (5′-ACCTTCTCGAGCCTTAGCCCTTTTTGTTGAGTC-3′). For the V unique gene, the primers used were Vu-5 (5′-ATGGCGGATCCGAGCTCACCATGCATAGGAGAGAACAC-3′) and Vstop. The PCR products were inserted into a glutathione-S-transferase (GST) fusion expression vector, pGEX-4T-1 (Amersham Pharmacia Biotech, Piscataway, N.J.), between the BamHI and XhoI sites. The constructs were then introduced into E. coli DH1 competent cells. The recombinant clones were confirmed by sequence analysis using a 310 genetic analyzer (PE Biosystems, Foster City, Calif.).
GST fusion proteins were expressed and purified according to the manufacturer's manual. Briefly, recombinant protein expression in BL21 cells was induced, and the cells were collected by centrifugation and disrupted by mild sonication. After centrifugation, supernatants were directly applied to a glutathione-Sepharose 4B affinity chromatography column and eluted with glutathione. Protein was quantified using the Bradford protein assay.
Protein analysis by Western blotting.
Western blotting was performed as described previously (28). Briefly, proteins were separated on sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS–15% PAGE) and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) using a semidry protein transfer apparatus (Sartoblot II-S; Sartorius, Edgewood, N.Y.). The blotted membranes were treated with rabbit anti-SeV serum or anti-Vu serum (diluted to 1:500) and subsequently with horseradish peroxidase-conjugated mouse anti-rabbit immunoglobulin G antibody (Organon Technika Cappel; 1:1,000). Reactive proteins were visualized in a visualization buffer (0.5 mg of 3,3′-diaminobenzidine per ml, 1.4% H2O2, 50 mM Tris-HCl [pH 7.5]).
Mutagenesis of the Vu gene.
A 0.8-kb SmaI fragment from SeV cDNA plasmid pSeV(+), corresponding to nucleotides 2764 to 3556 in the SeV antigenomic cDNA, was subcloned into plasmid pGEM-3 (Promega, Madison, Wis.). Site-directed mutagenesis was performed on double-stranded plasmid using the U.S.E. mutagenesis kit (Amersham Pharmacia Biotech). All of the mutants were screened by sequence analysis. The mutated Vu genes were further amplified by PCR and inserted into the expression vector pGEX-4T-1.
Zinc-binding assay.
Samples were separated by SDS–15% PAGE and electroblotted onto membranes as described above. The zinc-binding assay was performed on the blotted membrane using 65ZnCl2 (Amersham Pharmacia Biotech) as described previously (2). Membranes were equilibrated in renaturing buffer (100 mM Tris-HCl [pH 6.8], 50 mM NaCl, 10 mM dithiothreitol [DTT]) for 1 h with three changes of the buffer, then rinsed in labeling buffer (100 mM Tris-HCl [pH 6.8], 50 mM NaCl) twice, and incubated with 10 μM 65ZnCl2 in labeling buffer for 15 min. The labeling buffer was flushed with nitrogen gas to remove dissolved oxygen before use. The membranes were further rinsed in wash buffer (100 mM Tris-HCl [pH 6.8], 50 mM NaCl, 1 mM DTT) twice, then washed for 1 h with three changes of the buffer, and subjected to autoradiography with a Fujix BAS 2000 image analyzer (Fuji, Tokyo, Japan). Transferred proteins were further stained with amido black 10B, and they were quantified by densitometry using MacBAS software (Fuji).
Recovery of SeV from cDNA.
The 0.8-kb SmaI fragments (2764 to 3556) after mutagenesis were subcloned into a plasmid possessing the EcoRI2871-BamHI5011 fragment by using the two SmaI sites in the fragment and the vector, and the EcoRI-BamHI fragments were subsequently transferred into the ClaI fragment corresponding to nucleotides 2090 to 5335. The ClaI fragments were finally transferred to pSeV(+) plasmid to generate pSeV(+)-V-C341S and pSeV(+)-V-C365R. These steps were similar to those described before (26), and SeV was recovered from the recombinant plasmids as described previously (16).
Protein analysis by metabolic labeling and immunoprecipitation.
Confluent monolayers of LLC-MK2 cells in a 3.5-cm dish were infected with SeV at a multiplicity of infection (MOI) of 20. After 7 h, the cells were labeled with 35S-labeled cysteine-methionine ([35S]Pro-mix; 3.7 MBq/ml; Amersham Pharmacia Biotech) for 30 min in methionine- and cysteine-free Dulbecco's modified MEM. The cells were lysed in radioimmunoprecipitation assay buffer (10 mM Tris-HCl [pH 7.4], 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl). Polypeptides were immunoprecipitated with anti-SeV, anti-Vu, or anti-C serum and analyzed by SDS-PAGE as described previously (27). An autoradiogram was analyzed by using an image analyzer.
Animal experiments.
Specific-pathogen-free, 3-week-old male mice of the ICR/Crj (CD-1) strain, purchased from Charles River Japan, Inc. (Atsugi, Japan), were intranasally inoculated with 106, 107, or 108 CIU of virus inoculum per mouse under mild anesthesia with ether, and their body weights and clinical symptoms were checked daily. In an experiment for virus replication, the mice were sacrificed and virus infectivity in the lung was measured at certain time intervals. All of the mice were kept in a bioclean condition in the facility for animal experiments of the Hiroshima University School of Medicine.
RESULTS
Expression of SeV V, P/V common, and Vu proteins in E. coli.
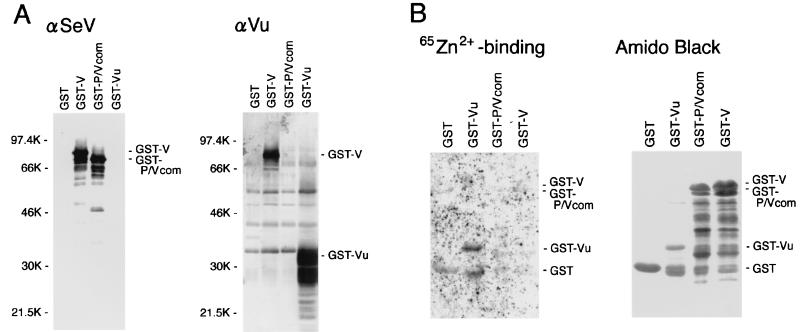
To determine whether the SeV V protein can bind zinc ion, the V-related polypeptides were expressed in E. coli. The V, P/V common, and V unique genes were cloned into the pGEX-4T-1 expression vector to generate GST fusion proteins (Fig. 1). SDS-PAGE and Western blot assay results (Fig. 2A) confirmed the expression of GST-V, GST-P/V common (P/Vcom), and GST-Vu proteins. GST-V and GST-P/Vcom were reactive to rabbit anti-SeV serum raised against purified virions, while GST-Vu was not. This observation is consistent with the observation that the V protein is not a structural protein in SeV virions (5). Anti-Vu serum, raised against the purified Vu peptide expressed in E. coli, reacted with both GST-V and GST-Vu proteins but not with the GST-P/Vcom protein. These results show the authenticity of the fusion proteins.
FIG. 1.
Schematic representation of GST fusion proteins. SeV V, P/Vcom, and Vu peptides were fused to the carboxyl terminus of GST. The amino acid residue numbers of the V protein are indicated.
FIG. 2.
(A) Western blotting to detect GST fusion proteins. Expression of GST-V, GST-P/Vcom, and GST-Vu proteins as well as GST was induced in E. coli, and the proteins were purified by glutathione-Sepharose 4B affinity chromatography. The proteins were analyzed by SDS-PAGE and transferred to a membrane. The blots were stained with anti-SeV antibody (αSeV) or anti-Vu antibody (αVu) as a primary antibody, as described in Materials and Methods. Sizes are shown in kilodaltons. (B) Zinc-binding assay of GST fusion proteins. The blot was probed with 65ZnCl2 in reducing conditions to detect zinc binding and processed for autoradiography (65Zn2+ binding). The same membrane was then stained with amido black 10B to detect blotted proteins.
Zinc-binding assay for the GST-V, GST-P/Vcom, and GST-Vu proteins.
The GST fusion proteins were processed for the zinc-binding assay. Partially purified GST-V, GST-P/Vcom, and GST-Vu proteins were resolved by SDS-PAGE and subsequently transferred to a membrane. The membrane was then incubated with 65ZnCl2 (Fig. 2B). After autoradiography, the blotted membrane was further stained with amido black 10B (Fig. 2B), and protein density was measured. Zinc-binding activity determined by this assay was corrected for the amounts of blotted protein and its molecular size.
It was found that although GST itself bound 65Zn2+ to some extent, GST-Vu bound 7.2-fold more zinc than did GST, exhibiting its potential zinc-binding capacity. As predicted, GST-P/Vcom did not bind zinc, but unexpectedly, neither did GST-V (Fig. 2B). This is probably because GST-V might be in a denatured form unfavorable to zinc binding after purification from E. coli or because GST coupling at the N terminus of the V protein might interfere with the binding of zinc ions. However, it is evident that the highly conserved, cysteine-rich unique domain at the carboxyl terminus of the V protein is capable of coupling zinc ions.
Mutagenesis of a Vu gene segment.
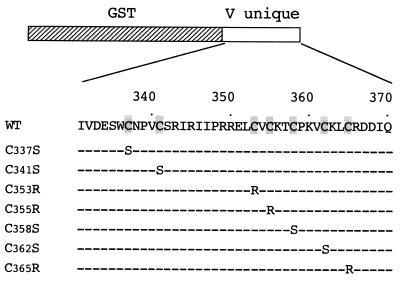
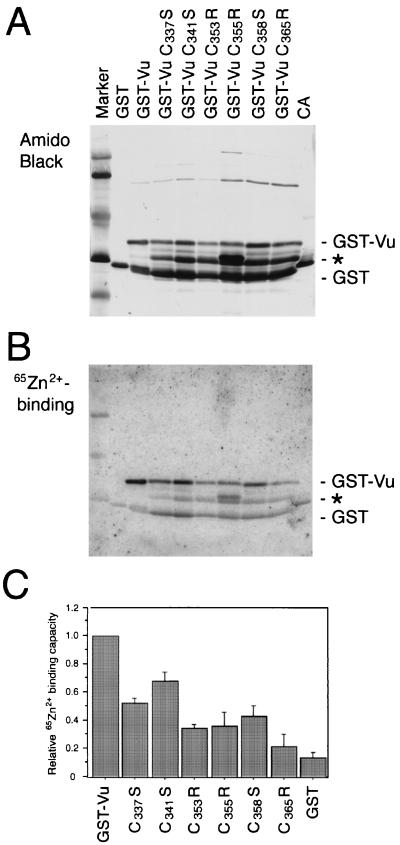
To investigate involvement of the conserved cysteine residues in zinc binding, the seven cysteine residues in the zinc finger-like motif were individually mutated by site-specific mutagenesis. Mutations were designed so as not to introduce a mutation into the overlapping P coding frame, because the same mutations were to be introduced into virus in a later experiment. The mutations were Cys337→Ser (C337S), C341S, C353R, C355R, C358S, C362S, and C365R (Fig. 3). Mutated Vu genes were then inserted in frame into pGEX-4T-1 to construct a recombinant expression vector. Purified mutant GST-Vu fusion proteins were analyzed by SDS-PAGE and transferred to a membrane (Fig. 4A). It was observed the C362S mutant GST-Vu protein was not expressed in E. coli, probably due to degradation by a host protease (data not shown). For the other GST-Vu proteins, an intermediate band between the full-sized GST-Vu protein and the GST protein appeared (∗ in Fig. 4A). This was thought to be because the mutant proteins acquired partial susceptibility to a protease in E. coli, probably due to a conformational change by a single point mutation. If this is in fact the case, since mutations at different positions generated a similar band, changes in each cysteine residue may lead to a common conformation. Another intermediate band appeared in the C355R mutant (Fig. 4A), suggesting that the mutation may have given rise to another protein conformation.
FIG. 3.
Schematic representation of mutant GST-Vu proteins. The seven cysteine residues conserved among the subfamily Paramyxovirinae (shaded) were individually replaced with serine or arginine. Mutations were designed to change a cysteine and, at the same time, not to change an amino acid of the overlapping P coding frame for later reverse genetics experiments.
FIG. 4.
Western blotting of the mutant GST-Vu proteins and their zinc-binding capacities. Expression of the mutant GST-Vu proteins as well as GST was induced in E. coli, and the proteins were purified by glutathione-Sepharose 4B affinity chromatography. The purified proteins and carbonic anhydrase (CA) were analyzed by SDS-PAGE and transferred to a membrane. The blot was probed with 65ZnCl2 in reducing conditions to detect zinc binding and processed for autoradiography (B, 65Zn2+-binding). The same membrane was then stained with amido black 10B to detect blotted proteins (A). An asterisk indicates partially degraded GST-Vu proteins. The relative ratio of zinc binding by the GST-Vu mutants to that of the wild type was then calculated from data from three independent experiments (C). Bars indicate standard deviations.
Zinc-binding assay of mutant Vu proteins.
Purified mutant GST-Vu proteins were tested for their zinc-binding activity (Fig. 4B). We quantified zinc binding to full-sized GST-Vu bands with correction for the amounts of blotted proteins. GST-Vu did not bind zinc ions when DTT was not included in the binding assay buffer (data not shown). Thus, zinc-binding experiments must be performed in reducing conditions for GST-Vu to maintain zinc-binding ability, suggesting that this coordination is cysteine-mediated in nature. This is also consistent with the case for papillomavirus E6 and E7 proteins (2). We included carbonic anhydrase, which binds zinc through three histidine residues, in this analysis (Fig. 4B). In reducing conditions, however, carbonic anhydrase did not bind zinc ions strongly.
The results from three independent experiments (Fig. 4C) clearly showed that the zinc-binding capacity was substantially impaired by substitution of the conserved cysteine residues. Cys365 appeared to be most essential to the zinc-binding capacity of Vu protein, since the C365R mutation caused a remarkable loss of binding capacity, down to only 22% of that of the wild type. Mutations C337S and C341S led to a relatively mild reduction in binding capacity, 52 and 68% of wild type, respectively. For mutations C353R, C355R, and C358S, the zinc-binding capacities were 34, 36, and 43% of wild type, respectively. These results indicate that the zinc-binding capacity of the V protein is a function of the unique domain in its carboxyl terminus and clearly cysteine dependent.
Generation of SeV with mutations at the cysteine residues of the Vu motif.
To investigate the effect of the cysteine mutations on viral pathogenicity, we created two viruses possessing the mutations C341S and C365R, which correspond to the highest and lowest zinc-binding capacities, respectively, among the GST-Vu mutants examined. Mutated DNA fragments were introduced into the whole SeV genomic cDNA, and virus was recovered from the constructs. The viruses were designated SeV V-C341S and SeV V-C365R. Virus recovery rate and infectivity in embryonated eggs were equivalent to those of the wild-type virus (data not shown). These results of reverse transcription-PCR and nucleotide sequencing confirmed the introduced mutations in the recovered viruses.
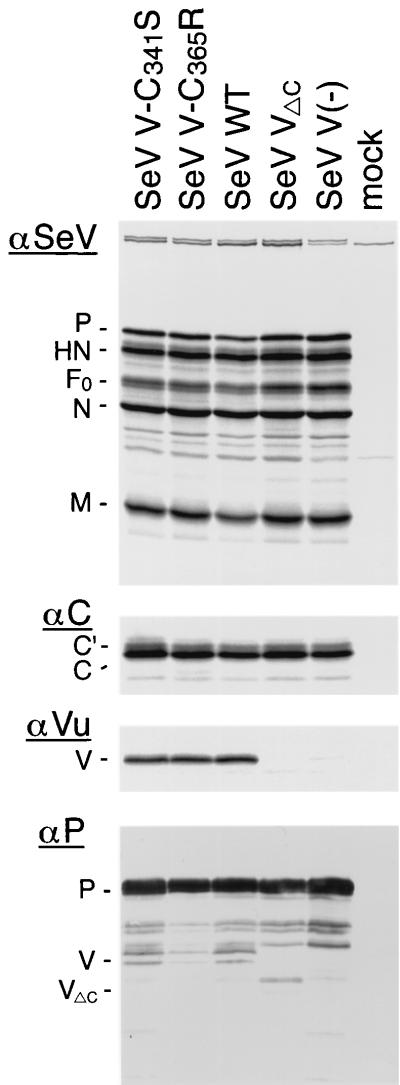
Viral protein synthesis was then investigated. LLC-MK2 cells were infected with the SeV mutants and metabolically labeled with 35S-labeled cysteine-methionine. We included the V-deficient virus, which has a mutation at the V editing site [SeV V(−)] (14), and a virus with a truncated V protein lacking the Vu domain because of a stop codon introduced just after the V editing site (SeV VΔC) (15). Immunoprecipitation with antiserum against SeV virions revealed that major viral proteins were similarly synthesized among all the viruses investigated (Fig. 5). Anti-C antibody precipitated equivalent amounts of C-related polypeptides C′ and C and a faster-migrating band probably corresponding to Y1 or Y2 (Fig. 5).
FIG. 5.
Protein synthesis of SeV V mutants in cultured cells. SeV V-Cys mutants V-C341S and V-C365R as well as SeV WT, V(−), and VΔC were used to infect LLC-MK2 cells at an MOI of 20. Proteins were labeled with [35S]Cys-Met for 30 min at 7 h postinfection and immunoprecipitated with anti-SeV serum (αSeV), anti-C serum (αC), or anti-Vu serum (αVu). The proteins were analyzed by SDS-PAGE and processed for autoradiography. In a separate panel, infected cell lysates were analyzed by Western blotting. The blotted membrane was probed with anti-P serum as a primary antibody (αP). Mock, uninfected cell lysates were used for immunoprecipitation; F0, precursor of the F protein.
The anti-Vu antibody precipitated the V protein of SeV V-C341S, SeV V-C365R, and SeV WT, but not that of SeV VΔC or SeV V(−), as expected (Fig. 5). The amounts of V protein were equivalent between SeV V-Cys mutants and SeV WT, suggesting that the mutated V proteins were synthesized as efficiently as the wild-type V protein. VΔC was identified in Western blotting using infected cell lysates and the anti-P antibody (Fig. 5). In this Western blot, SeV V-C365R appears to have a smaller amount of V protein. However, this was probably due to the different amounts loaded on the gel, as judged by the different amounts of P protein (Fig. 5).
The stability of the mutant V proteins was estimated by a 15-min pulse label and 3-h chase, followed by immunoprecipitation and SDS-PAGE. There was no difference in the amounts of V protein after a 3-h chase among SeV V-C341S, SeV V-C365R, and SeV WT (data not shown), suggesting that the mutant V proteins were stable in mammalian cells in spite of the point mutations.
Replication of SeV V-C341S and SeV V-C365R in cultured cells.
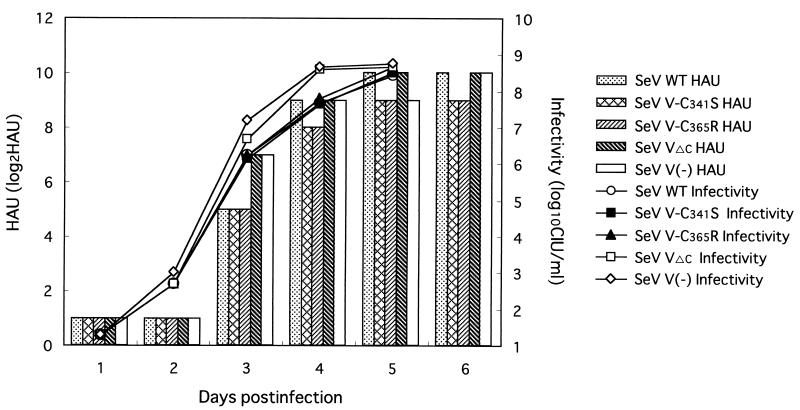
In order to examine whether the introduced mutations caused any change in virus replication in cell culture, LLC-MK2 cells were infected with SeV V-C341S or SeV V-C365R at an MOI of 0.01. SeV V(−) and SeV VΔC initially replicated a little faster than did the SeV V-Cys mutants and SeV WT, but finally all of the viruses reached a comparable level on day 5 after infection (Fig. 6). The relatively rapid replication of SeV V(−) and SeV VΔC in CV1 cells was previously reported (14, 15), and the present study indicates that this is also true for LLC-MK2 cells. SeV V-C341S and SeV V-C365R, however, grew as well as SeV WT (Fig. 6); the new mutants are closer to the wild-type virus in this respect. SeV V-C341S and SeV V-C365R were similar to SeV WT in plaque size and shape in CV1 cells (data not shown). Furthermore, all of the viruses displayed an identical replication pattern in LLC-MK2 cells at an input MOI of 20 (data not shown). These results demonstrate that SeV V-C341S and SeV V-C365R replicate similarly to the parent wild-type SeV in cultured cells. Thus, mutations in cysteine residues did not apparently have any detrimental effect on virus replication in cell culture.
FIG. 6.
Replication of SeV V mutants in cultured cells. Three dishes of LLC-MK2 cells were infected with SeV V-C341S, SeV V-C365R, SeV WT, SeV V(−), and SeV VΔC at an MOI of 0.01, and a part of the culture medium was taken daily. Mean HAU values (bars) and virus titers (symbols and lines) are shown in the graph.
Pathogenicity and replication of SeV V-C341S and SeV V-C365R in mice.
We next investigated the pathogenicity of the mutant viruses to mice. In the infection experiment, three to five 3-week-old ICR mice were infected in each group. When 106 CIU of virus was inoculated into a mouse, SeV WT killed four of the five mice, whereas SeV V-C341S killed no mice and SeV V-C365R killed only one of the five mice (Fig. 7). At 107 CIU/mouse, SeV WT killed all of the mice, whereas SeV V-C341S killed only two of the five mice. SeV V-C365R killed four of the five mice, but they survived longer than with SeV WT (Fig. 7). Comparison of the 50% mouse lethal dose (MLD50) demonstrated that SeV V-C341S pathogenicity was only 3% of that of the wild-type virus and that SeV V-C365R pathogenicity was 10% of the wild-type level (Table 1), indicating that the virulence of these mutants is attenuated in mice compared with the parent wild-type virus. It is noteworthy that the MLD50 values for SeV V-C341S and SeV V-C365R are close to that of SeV VΔC (Table 1). This shows that a single point mutation at the conserved cysteine residues in the Vu domain abolished the function of the entire Vu domain.
FIG. 7.
Body weight changes of mice infected with SeV V-Cys mutants. Three to five mice were infected with 106 or 107 CIU of SeV V-C341S, SeV V-C365R, or SeV WT per mouse, and the body weight of each mouse was checked daily. Each dot indicates an individual mouse, and an X marks the death of a mouse.
TABLE 1.
MLD50 of SeV V mutantsa
| Virus | MLD50, CIU (relative pathogenicity)
|
|
|---|---|---|
| Expt. 1 | Expt. 2 | |
| SeV WT | 3.2 × 105 (1.00) | 7.9 × 105 (1.00) |
| SeV V-C341S | 1.3 × 107 (0.03) | ND |
| SeV V-C365R | 3.2 × 106 (0.10) | ND |
| SeV VΔC | ND | 2.0 × 107 (0.04) |
| SeV V(−) | ND | 1.3 × 108 (0.01) |
Values are MLD50 of SeV V mutants in CIU in two experiments. Relative pathogenicity (reciprocal relative ratios of MLD50) compared with wild-type SeV, which is set at 1.0, is shown in parentheses. ND, not done.
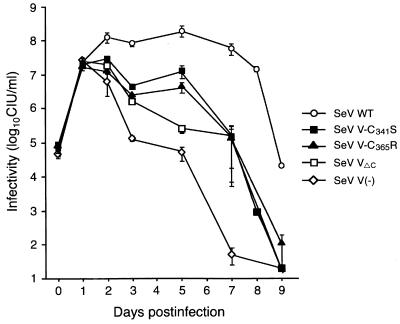
Virus replication in the mouse lung was then investigated in detail (Fig. 8). When three mice in each group were infected with 107 CIU of wild-type or mutant virus per mouse, all of the viruses replicated equally to about 107 CIU per mouse lung on day 1. Virus replication then diverged: wild-type virus replicated further to reach a maximal level, which was maintained from days 2 to 5, while in contrast, the virus titer of SeV V(−) rapidly decreased from day 2 (Fig. 8) (14). Replication of SeV VΔC was very similar to that of SeV V(−), but SeV VΔC grew more efficiently than SeV V(−) (Fig. 8) (15). The difference between SeV WT and SeV VΔC was far more obvious than that between SeV VΔC and SeV V(−). This suggests that the Vu domain is mainly involved in virus replication in mice, although the P/V common region also has some involvement. In SeV V-C341S and SeV V-C365R infection, virus replication was almost the same as that of SeV VΔC, and this is consistent with the MLD50 values of these viruses. An exception was that, unlike SeV VΔC, the virus titers of SeV V-C341S and SeV V-C365R slightly increased rather than decreased around day 5, which was somewhat similar to SeV WT (Fig. 8).
FIG. 8.
Replication of SeV V mutants in the mouse lung. Mice were infected with 107 CIU of SeV V-C341S, SeV V-C365R, SeV V(−), SeV VΔC, and SeV WT per mouse, and three mice for each virus were sacrificed on days 0, 1, 2, 3, 5, 7, and 9. The lungs were removed from each mouse, homogenized in 1 ml of MEM, and infectivity was measured. Each point represents the mean infectivity in three mice, and bars indicate the standard deviation. One mouse each infected with SeV WT and SeV V-C365R died on day 8, and the lungs were removed on that day.
DISCUSSION
In this work, we demonstrated that the Vu domain of SeV was capable of binding zinc ions. Similar findings have also been reported for SV5, measles virus, and Newcastle disease virus (21, 25, 29). In the case of SV5, it was found, by using inductively coupled argon plasma atomic emission spectroscopy, that each molecule of the V protein binds two atoms of zinc (25). The zinc-binding assay employed in our study did not allow a quantitative analysis of the molar ratio of zinc ions per molecule of V protein. However, GST-Vu showed a strong zinc-binding signal, 7.2-fold greater than that of GST, indicating its potential zinc-binding capacity. Generally, both cysteine and histidine residues can mediate zinc binding. For example, two histidine residues and two cysteine residues can bind one zinc atom in the C2H2 zinc finger structure. In order to test whether the zinc-binding activity of the Vu domain is cysteine dependent, we performed the assay under reducing conditions that specifically allowed the detection of zinc binding through cysteine residues (2). It was observed that the GST-Vu fusion protein definitely required reducing conditions to bind zinc (data not shown), indicating that coordination of the metal atoms could be mediated by cysteine residues. In contrast, carbonic anhydrase, a well-characterized zinc-binding metalloprotein which binds one zinc atom through three histidine residues, was used as a control protein and bound only a trace amount of zinc ions (Fig. 4B). Furthermore, mutations in cysteine residues considerably reduced zinc-binding activity, suggesting that zinc binding of the Vu domain is dependent on these conserved cysteine residues. The impairment of zinc binding may be related to the distinct conformation, as indicated by partial protease susceptibilities of the mutant GST-Vu proteins in E. coli. Given the reducing environment in the cytosol of eukaryotic cells and the presence of zinc, we predict that the V protein would bind zinc in vivo.
Although the V proteins of SeV and other paramyxoviruses were shown to bind zinc, the biological significance of the zinc binding was still an enigma. The present study using cysteine mutant viruses provided evidence supporting the notion that the zinc-binding capacity is involved in viral pathogenesis in vivo. Two mutant viruses, SeV V-C341S and SeV V-C365R, which represent the maximal and minimal zinc-binding capacity of corresponding fusion proteins, respectively, were constructed with a reverse genetics system. Like SeV V(−) and SeV VΔC, these cysteine mutant viruses exhibited a change in neither major viral protein synthesis nor replication in cultured cells. There was also no observable difference in stability of the mutant V proteins compared with that of the wild-type V protein in cultured cells. On the contrary, the virulence of these two mutants was attenuated remarkably in mice. The MLD50 values of SeV V-C341S and SeV V-C365R indicated that the mutants were only 3 and 10% as pathogenic as the wild-type parent SeV, respectively, and nearly as pathogenic as SeV VΔC. This attenuation was also observed in studies on viral replication in the mouse lung and mouse body weight changes. These results clearly indicate that even a single mutation in the highly conserved cysteine residues in the Vu domain could disrupt the function of the entire Vu domain.
The growth patterns of two cysteine mutant viruses in the mouse lung were very similar but not identical to that of SeV VΔC. On day 5 postinfection, the titers of V cysteine mutants appeared to increase slightly, as with wild-type SeV (Fig. 8). This is possible because SeV V-C341S and SeV V-C365R are single-amino-acid-substituted viruses, whereas SeV V(−) and SeV VΔC have deletions in the Vu domain. As a result, the V protein function of the cysteine mutants might not be entirely abolished, making the viruses somewhat resistant to host antiviral response. After day 5, specific cellular immunity participated in the antivirus response, and the viruses were cleared thereafter (Fig. 8).
One unexpected result was that although GST-Vu-C341S could bind more zinc ions (68% of the wild-type level) than GST-V-C365R (22% of the wild-type level), SeV V-C341S appeared to be less virulent than SeV V-C365R in vivo. This discrepancy implies that zinc-binding capacity and virulence in mice are not strongly correlated. It can be considered that zinc binding is a correct but incomplete marker of the proper conformation and function of the V protein. In such a scenario, although Cys341 is less important for zinc binding than the other cysteine residues, it may be critical for structure formation or function of the V protein by an as yet unknown mechanism.
It seems that the highly conserved cysteine residues are involved both in the zinc-binding capacity of the Vu domain and in viral pathogenesis in vivo. Although some enzymes utilize zinc in their active centers, most zinc-binding proteins can form a stable structural element within the protein itself through zinc binding. This structure could participate in protein-protein and protein-nucleic acid interactions. The results of early studies suggest that zinc fingers mediate nucleic acid binding in transcription factors (3) and homodimerization of proteins (10). More recent studies have revealed that many zinc fingers can mediate protein-protein interaction (22). It is possible that the V protein can also adopt a particular conformation in its unique carboxyl terminus through zinc binding and subsequently interacted with host cell protein(s) in vivo. A recent study showed that the V protein of SV5 interacts via the conserved cysteine residues with a 127-kDa subunit (DDB1) of the damage-specific DNA-binding protein (DDB), a host factor controlling cell cycle progression (20). However, it was also demonstrated in this study that the SeV V protein does not interact with DDB1. We assume that the SeV V protein may instead interact with another host factor(s) and may play an important role in facilitating virus multiplication by modifying early host immunity through interaction. The validity of this hypothesis remains to be clarified in a future study.
In conclusion, our study showed that the conserved cysteine residues in the SeV V protein are important for its zinc-binding capacity and for virus pathogenicity in mice. A function of the V protein, which is probably related to circumvention of innate host immunity, is thought to be mediated through its cysteine cluster in the carboxyl terminus. A possible host partner of the SeV V protein should be investigated to understand the biological activity of the V protein.
ACKNOWLEDGMENTS
We thank the Research Center for Molecular Medicine, Hiroshima University School of Medicine, for the use of their facilities.
This study was supported in part by the Japan-China Sasakawa Medical Fellowship awarded to C. Huang.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1998. [Google Scholar]
- 2.Barbosa M S, Lowy D R, Schiller J T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989;63:1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg J M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986;232:485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- 4.Curran J. A role for the Sendai virus P protein trimer in RNA synthesis. J Virol. 1998;72:4274–4280. doi: 10.1128/jvi.72.5.4274-4280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran J, de Melo M, Moyer S, Kolakofsky D. Characterization of the Sendai virus V protein with an anti-peptide antiserum. Virology. 1991;184:108–116. doi: 10.1016/0042-6822(91)90827-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran J, Marq J B, Kolakofsky D. An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during the nascent chain assembly step of genome replication. J Virol. 1995;69:849–855. doi: 10.1128/jvi.69.2.849-855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran J, Marq J B, Kolakofsky D. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology. 1992;189:647–656. doi: 10.1016/0042-6822(92)90588-g. [DOI] [PubMed] [Google Scholar]
- 8.Delenda C, Hausmann S, Garcin D, Kolakofsky D. Normal cellular replication of Sendai virus without the trans-frame, nonstructural V protein. Virology. 1997;228:55–62. doi: 10.1006/viro.1996.8354. [DOI] [PubMed] [Google Scholar]
- 9.Delenda C, Taylor G, Hausmann S, Garcin D, Kolakofsky D. Sendai viruses with altered P, V, and W protein expression. Virology. 1998;242:327–337. doi: 10.1006/viro.1998.9027. [DOI] [PubMed] [Google Scholar]
- 10.Frankel A D, Bredt D S, Pabo C O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988;240:70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- 11.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotoh B, Takeuchi K, Komatsu T, Yokoo J, Kimura Y, Kurotani A, Kato A, Nagai Y. Knockout of the Sendai virus C gene eliminates the viral ability to prevent the interferon-alpha/beta-mediated responses. FEBS Lett. 1999;459:205–210. doi: 10.1016/s0014-5793(99)01241-7. [DOI] [PubMed] [Google Scholar]
- 13.Horikami S M, Curran J, Kolakofsky D, Moyer S A. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J Virol. 1992;66:4901–4908. doi: 10.1128/jvi.66.8.4901-4908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato A, Kiyotani K, Sakai Y, Yoshida T, Shioda T, Nagai Y. Importance of the cysteine-rich carboxyl-terminal half of V protein for Sendai virus pathogenesis. J Virol. 1997;71:7266–7272. doi: 10.1128/jvi.71.10.7266-7272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Nagai Y. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1:569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- 17.Kiyotani K, Takao S, Sakaguchi T, Yoshida T. Immediate protection of mice from lethal wild-type Sendai virus (HVJ) infections by a temperature-sensitive mutant, HVJpi, possessing homologous interfering capacity. Virology. 1990;177:65–74. doi: 10.1016/0042-6822(90)90460-9. [DOI] [PubMed] [Google Scholar]
- 18.Kurotani A, Kiyotani K, Kato A, Shioda T, Sakai Y, Mizumoto K, Yoshida T, Nagai Y. Sendai virus C proteins are categorically nonessential gene products but silencing their expression severely impairs viral replication and pathogenesis. Genes Cells. 1998;3:111–124. doi: 10.1046/j.1365-2443.1998.00170.x. [DOI] [PubMed] [Google Scholar]
- 19.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 1177–1204. [Google Scholar]
- 20.Lin G Y, Paterson R G, Richardson C D, Lamb R A. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology. 1998;249:189–200. doi: 10.1006/viro.1998.9317. [DOI] [PubMed] [Google Scholar]
- 21.Liston P, Briedis D J. Measles virus V protein binds zinc. Virology. 1994;198:399–404. doi: 10.1006/viro.1994.1050. [DOI] [PubMed] [Google Scholar]
- 22.Mackay J P, Crossley M. Zinc fingers are sticking together. Trends Biochem Sci. 1998;23:1–4. doi: 10.1016/s0968-0004(97)01168-7. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y. Paramyxovirus replication and pathogenesis: reverse genetics transforms understanding. Rev Med Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Nagai Y, Kato A. Paramyxovirus reverse genetics is coming of age. Microbiol Immunol. 1999;43:613–624. doi: 10.1111/j.1348-0421.1999.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 25.Paterson R G, Leser G P, Shaughnessy M A, Lamb R A. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology. 1995;208:121–131. doi: 10.1006/viro.1995.1135. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi T, Kiyotani K, Kato A, Asakawa M, Fujii Y, Nagai Y, Yoshida T. Phosphorylation of the Sendai virus M protein is not essential for virus replication either in vitro or in vivo. Virology. 1997;235:360–366. doi: 10.1006/viro.1997.8701. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi T, Leser G P, Lamb R A. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol. 1996;133:733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi T, Uchiyama T, Fujii Y, Kiyotani K, Kato A, Nagai Y, Kawai A, Yoshida T. Double-layered membrane vesicles released from mammalian cells infected with Sendai virus expressing the matrix protein of vesicular stomatitis virus. Virology. 1999;263:230–243. doi: 10.1006/viro.1999.9960. [DOI] [PubMed] [Google Scholar]
- 29.Steward M, Samson A C, Errington W, Emmerson P T. The Newcastle disease virus V protein binds zinc. Arch Virol. 1995;140:1321–1328. doi: 10.1007/BF01322759. [DOI] [PubMed] [Google Scholar]
- 30.Thomas S M, Lamb R A, Paterson R G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988;54:891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal S, Curran J, Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990;64:239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]