Highlights
-
•
Mesenchymal stem cells (MSCs) play significant roles in driving cancer-induced bone disease.
-
•
New studies demonstrate MSC activities in cancer dormancy, metabolism, and immune-oncology.
-
•
Identifying novel MSC contributions to bone metastasis could improve current and future therapies.
Keywords: Bone metastasis, Mesenchymal stem cell, Stromal cell, MSC, Microenvironment, Bone
Abstract
The skeleton is a common site of cancer metastasis and malignancy with the resultant lesions often being incurable. Interactions between metastatic cancer cells and the bone microenvironment are critical for cancer cell survival, outgrowth, and progression. Mesenchymal Stem Cells (MSCs) are an essential stromal cell type in bone that are appreciated for their impacts on cancer-induced bone disease, however, newer evidence suggests that MSCs possess extensive roles in cancer-bone crosstalk, including cancer cell dormancy, metabolic demands, and immune-oncology. Emerging evidence has also identified the importance of MSC tissue source and the influence of ageing when studying MSC biology. Combining these considerations together with developing technologies such as spatial transcriptomics will contribute to defining the molecular mechanisms underlying complex stroma-cancer interactions in bone and assist with identification of therapeutically tractable targets.
1. Introduction
Solid malignancies such as breast and prostate cancer frequently metastasize to bone. The resultant lesions often become refractory to treatment leading to incurable disease that greatly contributes to patient morbidity and mortality [1]. The metastatic process to bone involves cancer cell seeding to favorable niches within the soil of the bone marrow. The surrounding bone marrow microenvironment is key for what happens next – either promoting cancer cell entry into dormancy for long periods of time or contributing to metastatic outgrowth. Defining the underlying molecular mechanisms that govern cancer-bone stroma interaction can reveal key biology that is therapeutically tractable. In this context, novel roles for stromal cells, in particular MSCs have recently been described.
MSCs are multipotent cells capable of differentiating toward chondroblast, osteoblast, and adipocyte lineages and can be defined by surface markers including but not limited to CD105, CD73, CD90, neural/glial 2(NG2), Nestin and leptin receptor (LepR) while negative for CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR (Table 1) [2], [3]. Although they can be derived from multiple tissues, bone is considered the natural reservoir for MSCs where they comprise 0.001 to 0.01 % of the total cell population [4]. MSCs can be localized in the bone marrow stem cell niche, perivascular niche, or endosteal niche. Functionally, MSCs are best characterized by their contribution to homeostatic bone turnover by differentiating into bone forming osteoblasts that in turn contribute to niche functions such as hematopoietic stem cell (HSC) maintenance and regulation of osteoclast bone resorbing activity [4]. Of note, the tissue origin of MSCs may affect their function [5]. The potential divergence and plasticity of MSCs is illustrated by a comparison of MSCs from adipose, umbilical cord blood, and bone marrow which found that all sources could differentiate to osteogenic and adipogenic lineages under specific culture conditions, and while there was a shared gene expression profile between MSCs from all three sources, many other genes were differentially expressed [6].
Table 1.
List of select surface markers used for MSC characterization.
| Positive | Negative |
|---|---|
| CD105 | CD45 |
| CD73 | CD34 |
| CD90 | CD14 or CD11b |
| Neural/Glial 2 (NG2)* | CD79a or CD19 |
| Nestin* | HLA-DR |
| Leptin receptor (LepR)* |
* Bone marrow-derived MSC specific.
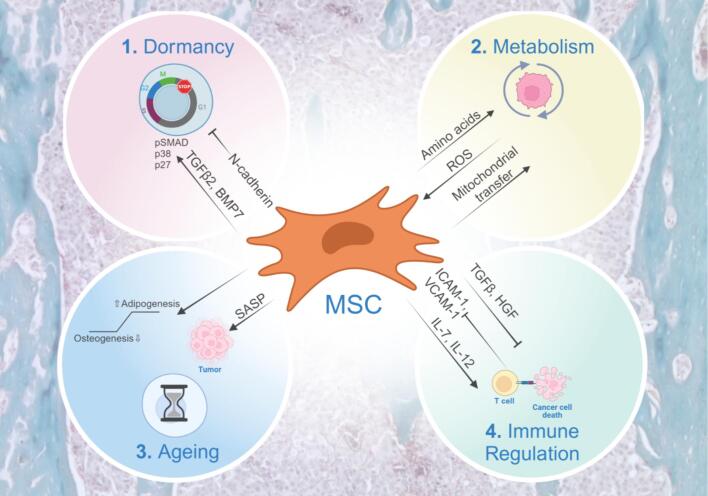
In the context of primary cancer, MSCs contribute to tumorigenesis and metastasis, but their engagement of cancer cells that have disseminated to the bone marrow and their subsequent roles in the establishment of bone metastases is less clear. However, new studies have begun to address this gap in knowledge [3], [7]. For example, MSCs can promote the chemoattraction and migration of cancer cells via the production of chemokines such as C-X-C chemokine motif 12 (CXCL12) [8]. Further, MSCs can protect against the establishment of bone metastatic prostate cancer by inducing apoptosis via Fas ligand (FasL) and Interleukin-28 (IL-28). In doing so, MSCs drive the selection of apoptotic resistant cancer cells that are also cross-resistant to conventional chemotherapies such as docetaxel [9]. Following cues from cancer cells, MSCs contribute to aberrant bone formation in diseases like bone metastatic prostate cancer or can suppress bone formation in skeletal malignancies such as multiple myeloma [10]. Emerging areas of cancer research, such as dormancy, metabolism, ageing, and immune modulation have allowed us to delve deeper into how MSCs contribute to the fate of bone metastatic cancer cells (Fig. 1).
Fig. 1.
Emerging areas for mesenchymal stem cells (MSCs) in bone metastasis. 1) MSCs can maintain dormancy in breast cancer cells via TGFβ2 and BMP7, but also induce proliferation of dormant cancer cells via N-cadherin. 2) Cancer cells rewire metabolism of MSCs and other stromal cells, impairing normal hematopoiesis while facilitating cancer cell survival, proliferation, and chemoresistance via transfer of reactive oxygen species (ROS), organelles, and amino acids such as glutamine. 3) Age-related changes in MSCs have profound effects on cancer progression by increased senescence, secretion of senescence associated secretory phenotypes (SASP) factors, and increased bone marrow adipocytes. 4) Immunomodulatory roles of MSCs include inhibitory effects via adhesion molecules and release of TGFβ and HGF, but engineering MSCs to deliver cargo, such as IL-7 and IL-12, offers improved efficacy of CAR-T cells. Figure created with BioRender.com.
2. Dormancy
Upon arriving in the metastatic site, disseminated tumor cells (DTCs) may enter a period of dormancy until specific signals initiate awakening and outgrowth [11]. Formative studies have shown that stromal cells influence this process. For example, contact between DTCs and endothelial cells induces dormancy until transforming growth factor-beta-1 (TGFβ1) and periostin from sprouting vasculature promote awakening. Interestingly, interrupting integrin-mediated interactions with the perivascular niche offers a strategy to sensitize dormant breast cancer cells to chemotherapy [12], [13]. Further, osteoblasts have known implications in prostate cancer dormancy via a growth arrest-specific 6 (GAS6)/Axl and transforming growth factor-beta-2 (TGFβ2) dependent mechanisms [14]. Multipotent MSCs also impact dormancy, by secretion of factors such as TGFβ2, CXCL12, GAS6, and bone morphogenetic proteins (BMPs) [15], [16], [17]. Specifically, NG2/Nestin positive MSCs in the perivascular niche provide TGFβ2 and BMP7 to maintain dormancy of E0771 cells via TGFβR3 and BMPRII and subsequent signaling through SMAD, p38, and p27 signaling [16]. Interestingly, although osteoblasts are also a source of TGFβ2, they did not compensate for loss of TGFβ2 when it was ablated in the MSC compartment. Additional studies utilizing hanging drop co-cultures of bone marrow derived MSC and MDA-MB-231 breast cancer cells led to MSCs surrounding the breast cancer cells and the formation of cancer cell spheroids. MSCs were subsequently internalized and degraded which resulted in the breast cancer cells acquiring a unique molecular signature indicative of dormancy [18]. Bone fracture and repair have been hypothesized to contribute to skeletal metastasis and dormancy reawakening. Recent studies show that pathologic bone remodeling can provoke outgrowth of disseminated cancer cells from primary tumors via interactions mediated through N-cadherin and that Cre-mediated depletion of NG2 positive MSCs abrogated this effect, suggesting that MSC control of dormancy entry/awakening acts in concert with additional microenvironmental cues [3]. Nevertheless, understanding MSC-derived dormancy mechanisms may aid in developing strategies to sustain dormancy or awaken the cancer cells so that they are susceptible to systemic therapy and are eradicated, thus preventing metastatic relapse.
3. Metabolism
The bone marrow niche harbors a highly dynamic metabolic microenvironment exemplified by energy-intensive processes such as bone resorption and formation. These processes are exploited by cancer cells, causing metabolic reprogramming that facilitates survival and progression [19], [20]. Reciprocally, proliferating cancer cells can rewire the metabolism of MSCs and other cells in the stroma which in turn impairs normal hematopoiesis and bone formation. For example, breast cancer cells exhibit elevated enzymes for serine synthesis and release lactate which promotes osteoclast differentiation and osteolysis [21] while multiple myeloma cells deplete amino acids such as glutamine leading to activation of glutamine synthetase in bone marrow-MSCs [22]. This has been linked to decreased bone mass in MM patients, as glutamine is key for osteoblast mediated bone formation [19], [23]. In the stroma, metabolic reprogramming can also influence cancer cell behavior via transfer of reactive oxygen species (ROS), dysfunctional mitochondria, and amino acids. In acute myeloid leukemia (AML), increased glycolysis in the cancer cell promotes the transfer of ROS to the MSC cell line MS5 via gap junctions, triggering the conversion of pyruvate to acetate that can then be utilized by the cancer cells to fuel the TCA cycle and lipid biosynthesis [24]. MSCs can also transfer mitochondrial content to cancer cells to support tumor growth and chemotherapy resistance via the genesis of tunneling nanotubules (TnTs), gap junctions or partial cell fusions [25], [26]. The resultant transfer leads to increased oxidative metabolism, ATP levels and lowered ROS as well as chemoresistance [22], [27]. Further, MSCs can protect leukemic cancer cells from cell death by maintaining low ROS levels and by supplying cysteine and asparagine [28], [29]. In bone metastatic breast cancer, malignant cells have an increased dependency on extracellular glutamine for survival which is linked with serine biosynthesis, allowing cells to overcome the low glucose levels in the metastatic niche [30]. Given the rapidly emerging data regarding metabolic demands of cancer cells and their dependance on a supporting stromal microenvironment, it is clear that these vulnerabilities can be exploited therapeutically for the treatment of skeletal malignancies.
4. Ageing
Cellular damage and genomic changes at both transcriptional and epigenetic levels increase with ageing and consequently the risk of developing cancer becomes greater. Studies also show that age-related changes in the stroma, including MSCs, can have profound effects on cancer progression. These changes include decreased extracellular matrix integrity, inflamm-ageing, immune suppression, induction of senescence, and senescence associated secretory phenotype (SASP) [31]. In senescent osteoblasts, IL-6 driven SASP secretion promotes osteoclast mediated bone resorption. This, in turn, fosters the formation of premetastatic niches, increasing the likelihood of successful breast cancer seeding in the bone marrow [32]. However, MSC SASP can have opposing effects on cancer cells and may be cancer-stage dependent. For example, SASP from bone marrow MSCs impairs the growth of immortalized primary prostate cancer cells in vitro but not that of metastatic prostate cancer cells [33]. Further senescence-related abnormalities in MSCs from multiple myeloma patients have been reported. When comparing myeloma patients MSC secretome to healthy donors, there was increased interleukin-1 beta (IL-1β), IL-6 and Tumor Necrosis Factor alpha (TNF-α). These can function as growth factors for MM cells and induce migration, adhesion, and osteoclastogenesis [34]. The presented evidence suggests that stromal, senescent, and age-related alterations are sufficient to instigate changes favoring tumor cell-seeding and growth.
Of note, ageing also impacts MSC function with evidence supporting a shift towards adipogenic rather than osteogenic differentiation and resultant adipocytes contributing to cancer progression in bone. For example, peroxisome proliferators-activated receptor γ (PPARγ) is upregulated in MSCs with advancing age, leading to increased adipogenesis while the co-factors core-binding factor subunit beta (CBFβ) and minor allele frequency (MAF) that promote Runt-related transcription factor 2 (RUNX2) expression, a key driver of the osteogenic program, are downregulated [35]. Furthermore, ageing has been shown to alter the miRNA content of MSCs and extracellular vesicles [36]. miR-31a-5p levels are increased in aged MSCs and this again can promote a shift in differentiation towards the adipogenic lineage [37]. This is reflected in elderly individuals where expanded populations of bone marrow adipocytes (BMAs) are seen [35]. BMAs store energy in the form of lipids and release triglycerides and fatty acids to respond to energy demands and in doing so promote cancer cell proliferation, migration and invasion. For example, adipocytes in proximity to breast cancer cells contribute to growth and drug resistance which is in part mediated by adipokines such as leptin and adiponectin [38]. Additionally, IL-6, TNF-⍺, and CXCL12 secreted from BMA are shown to promote cell proliferation, inhibit apoptosis and activate autophagy as a form of chemoresistance in multiple myeloma [39]. In summary, considering that cancer is primarily a disease in older patients, understanding how ageing impacts MSC differentiation/function could reveal novel therapeutic opportunities to treat skeletal malignancies.
5. Immunomodulation
Bone marrow is home to many subsets of immune cells which is unsurprising given it is the cradle of hematopoiesis. MSCs have long been described as having immunomodulatory roles that are often suppressive. For example, studies have shown that MSCs can restrict CD4 + and CD8 + cytotoxic T cell proliferation in a TGFβ1 and HGF dependent manner with other key players such as Fas/FasL, Jagged-1/Notch-1, and TNFα signaling also having described roles [40], [41]. Recent studies reveal that MSCs can also express ICAM-1 and VCAM-1 much like endothelial cells, thereby facilitating T cell adhesion that in turn can exert immunosuppressive effects on the T cell compartment [42]. In the setting of skeletal malignancy, MSCs could therefore have profound effects on the efficacy of T-cell based therapies like TIL and CAR-T that are currently being trialed (NCT05732948, NCT05022849, NCT01140373, NCT06094842, NCT05805371, NCT03089203). New in vitro evidence suggests that bone marrow derived MSCs potently diminished the efficacy of lower affinity, moderately lytic BCMA, CD38, and CD138 targeting CAR-T cells. But surprisingly, the suppressive effect of MSCs was not noted in CAR-T cells that had a higher affinity for these myeloma presenting antigens suggesting that CAR design and choice of T-cell vehicle will be critical for the efficacy of these approaches in treating bone metastatic and skeletal malignancies[43].
While these studies imply that MSCs may negatively impact CAR-T cell activity overall, it is possible that the therapeutic potential of MSCs may be exploited to improve their efficacy. Case in point, MSCs engineered to release IL-7 and IL-12 were shown to improve CAR-T cell activation leading to elimination of colorectal adenocarcinoma cells when co-injected in a xenograft mouse model [44]. In addition to significantly enhanced tumor regression, MSC derived IL-7 and IL-12 also increased CAR-T cell amplification and decreased activation induced cell death (AICD). Clearly, we are at the beginning of our understanding of how MSCs may influence the efficacy of molecular and cellular based immunotherapies that are geared toward the treatment of bone metastatic disease and other skeletal malignancies, especially in the context of ageing where MSC biology is notably altered as described. However, further studies may reveal how to overcome the potentially immunosuppressive MSC effects and either facilitate or enhance the cytotoxic effect of these game-changing therapies.
6. Conclusion and future directions
The establishment and outgrowth of metastatic cancers or skeletal malignancies in bone is critically dependent on surrounding stromal cells, many of which are MSC derived. While MSCs were traditionally studied as a component of cancer induced bone disease, either through their osteogenic suppression or enhanced osteoblast differentiation in lytic or blastic lesions respectively, newer evidence suggests that they are far more involved in cancer-bone crosstalk. These new roles include, but are by no means limited to, dormancy, facilitating cancer cell metabolic demand and potentially protecting cancer cells from immune eradication given the reported immunosuppressive roles of MSCs. Further, emerging evidence is beginning to reveal the importance of studying MSC-cancer interactions in an age-appropriate manner as MSCs derived from older adults (cancer naïve or cancer bearing) are profoundly different (epigenetically/genetically) to those derived from their younger counterparts. Emerging technologies such as spatial transcriptomics may allow us to unravel some of these complex issues but there is no doubt that further resolution of MSC roles in cancer progression in bone can identify new therapeutic targets or strategies to enhance the efficacy of applied therapies such as those being employed by the immuno-oncology field.
7. Outstanding questions
-
1)
How do bone stromal MSCs contribute to dormant and active cancer cell metabolism?
-
2)
Can we define how the ageing bone stroma shapes the progression and drug response of skeletal metastases and malignancies?
-
3)
What is the impact of bone stromal MSCs on the efficacy of immune-oncology based therapies for the treatment of skeletal metastases and malignancies?
Funding
This work was supported by NCI 1U01CA244101-01A1-CCL.
CRediT authorship contribution statement
Karl J. Nyman: Writing – review & editing, Writing – original draft, Conceptualization. Jeremy S. Frieling: Writing – review & editing, Writing – original draft, Conceptualization. Conor C. Lynch: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W., et al. Bone Metastasis Initiation Is Coupled with Bone Remodeling through Osteogenic Differentiation of NG2+ Cells. Cancer Discov. 2023;13(2):474–495. doi: 10.1158/2159-8290.CD-22-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Li C., Zhao H., Wang B. Mesenchymal stem/stromal cells: Developmental origin, tumorigenesis and translational cancer therapeutics. Transl. Oncol. 2021;14(1) doi: 10.1016/j.tranon.2020.100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner W., et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Galland S., Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 2020;250(5):555–572. doi: 10.1002/path.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X.H., et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154(5):1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuire J.J., et al. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat. Commun. 2021;12(1):723. doi: 10.1038/s41467-021-20962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz V., et al. Bone-metastatic prostate carcinoma favors mesenchymal stem cell differentiation toward osteoblasts and reduces their osteoclastogenic potential. J. Cell. Biochem. 2011;112(11):3234–3245. doi: 10.1002/jcb.23258. [DOI] [PubMed] [Google Scholar]
- 11.E.N., The Spread of Tumours in the Human Body. Postgraduate Medical Journal, 1953. 29(329): p. 160-160.
- 12.Carlson P., et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 2019;21(2):238–250. doi: 10.1038/s41556-018-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghajar C.M., et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yumoto K., et al. Axl is required for TGF-beta2-induced dormancy of prostate cancer cells in the bone marrow. Sci. Rep. 2016;6:36520. doi: 10.1038/srep36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker A.M., et al. Sympathetic Signaling Reactivates Quiescent Disseminated Prostate Cancer Cells in the Bone Marrow. Mol. Cancer Res. 2017;15(12):1644–1655. doi: 10.1158/1541-7786.MCR-17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobre A.R., et al. Bone marrow NG2(+)/Nestin(+) mesenchymal stem cells drive DTC dormancy via TGFbeta2. Nat. Cancer. 2021;2(3):327–339. doi: 10.1038/s43018-021-00179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu P.F., et al. Downregulation of CXCL12 in mesenchymal stromal cells by TGFbeta promotes breast cancer metastasis. Oncogene. 2017;36(6):840–849. doi: 10.1038/onc.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartosh T.J., et al. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs) PNAS. 2016;113(42):E6447–E6456. doi: 10.1073/pnas.1612290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitburn J., Edwards C.M. Metabolism in the Tumour-Bone Microenvironment. Curr. Osteoporos. Rep. 2021;19(5):494–499. doi: 10.1007/s11914-021-00695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirado H.A., et al. Metabolic crosstalk between stromal and malignant cells in the bone marrow niche. Bone Rep. 2023;18 doi: 10.1016/j.bonr.2023.101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollari S., et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 2011;125(2):421–430. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- 22.Marlein C.R., et al. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res. 2019;79(9):2285–2297. doi: 10.1158/0008-5472.CAN-18-0773. [DOI] [PubMed] [Google Scholar]
- 23.Stegen S., et al. Glutamine Metabolism in Osteoprogenitors Is Required for Bone Mass Accrual and PTH-Induced Bone Anabolism in Male Mice. J. Bone Miner. Res. 2021;36(3):604–616. doi: 10.1002/jbmr.4219. [DOI] [PubMed] [Google Scholar]
- 24.Vilaplana-Lopera N., et al. Crosstalk between AML and stromal cells triggers acetate secretion through the metabolic rewiring of stromal cells. Elife. 2022;11 doi: 10.7554/eLife.75908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Rooij B., et al. Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia. 2017;31(7):1651–1654. doi: 10.1038/leu.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burt R., et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matula Z., et al. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers (basel) 2021;13(14) doi: 10.3390/cancers13143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W., et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012;14(3):276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu M., et al. ALL blasts drive primary mesenchymal stromal cells to increase asparagine availability during asparaginase treatment. Blood Adv. 2021;5(23):5164–5178. doi: 10.1182/bloodadvances.2020004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandon M., et al. Bone metastatic breast cancer cells display downregulation of PKC-zeta with enhanced glutamine metabolism. Gene. 2021;775 doi: 10.1016/j.gene.2021.145419. [DOI] [PubMed] [Google Scholar]
- 31.Fane M., Weeraratna A.T. Normal Aging and Its Role in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020;10(9) doi: 10.1101/cshperspect.a037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo X., et al. Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development. Cell Rep. 2016;14(1):82–92. doi: 10.1016/j.celrep.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessio N., et al. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging (Albany NY) 2019;11(15):5817–5828. doi: 10.18632/aging.102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zdzisinska B., et al. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch. Immunol. Ther. Exp. (Warsz) 2008;56(3):207–221. doi: 10.1007/s00005-008-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massaro F., et al. Aging of Bone Marrow Mesenchymal Stromal Cells: Hematopoiesis Disturbances and Potential Role in the Development of Hematologic Cancers. Cancers (basel) 2020;13(1) doi: 10.3390/cancers13010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis C., et al. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng. A. 2017;23(21–22):1231–1240. doi: 10.1089/ten.tea.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu R., et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17(4):e12794. doi: 10.1111/acel.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris E.V., Edwards C.M. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front Endocrinol (lausanne) 2016;7:90. doi: 10.3389/fendo.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6(33):34329–34341. doi: 10.18632/oncotarget.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Nicola M., et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 41.Yan L., Li J., Zhang C. The role of MSCs and CAR-MSCs in cellular immunotherapy. Cell Commun. Signal. 2023;21(1):187. doi: 10.1186/s12964-023-01191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma S., et al. Repairing effects of ICAM-1-expressing mesenchymal stem cells in mice with autoimmune thyroiditis. Exp. Ther. Med. 2017;13(4):1295–1302. doi: 10.3892/etm.2017.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holthof L.C., et al. Bone Marrow Mesenchymal Stromal Cells Can Render Multiple Myeloma Cells Resistant to Cytotoxic Machinery of CAR T Cells through Inhibition of Apoptosis. Clin. Cancer Res. 2021;27(13):3793–3803. doi: 10.1158/1078-0432.CCR-20-2188. [DOI] [PubMed] [Google Scholar]
- 44.Hombach A.A., et al. IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells. 2020;9(4) doi: 10.3390/cells9040873. [DOI] [PMC free article] [PubMed] [Google Scholar]