Abstract
Background
The rising prevalence of familial multiple sclerosis (MS) in Iran has spurred interest in the potential impact of parental consanguinity on the risk of developing the disease. This study aims to aggregate current knowledge on parental consanguinity and its possible effect on MS risk, particularly among familial MS patients from various regions and ethnicities in Iran. The objective is to enhance the understanding of MS genetics and encourage further research in this field.
Materials and methods
A cross-sectional study was conducted on clinically definite familial MS (FMS) patients registered in the nationwide MS registry of Iran (NMSRI). Data were extracted and supplemented with structured telephone follow-ups to gather detailed histories of MS in relatives and the familial relationships of the patients' parents. A family penetration score was proposed. Descriptive statistics and inferential statistical tests were used to analyze the data at a significance level of 0.05, adhering to ethical guidelines.
Results
Out of 19,911 individuals registered in the NMSRI, 2307 FMS patients across 13 provinces were included in the final analysis. Among these, 385 (19.3 %) reported parental consanguinity, with 283 (14.2 %) having parents who were cousins and 102 (5.1 %) having parents who were distant relatives. The data showed no significant association between parental kinship and variables such as MS phenotype, number of affected relatives with MS, hospitalization rates, and expanded disability status scale score. Similarly, MS severity did not differ based on parental consanguinity (P-value >0.05). While the rate of consanguineous marriage was higher among patients with an onset age less than 18 years, there was no statistically significant difference in disease onset age based on parental consanguinity status.
Conclusion
Our study highlights the complexity of factors influencing MS development, including genetic and environmental components. These results highlight the need for further research to achieve a more comprehensive understanding of MS etiology.
Keywords: Multiple sclerosis, Familial multiple sclerosis, Parental consanguinity, Iran
Highlights
-
•
There is no association between parental consanguinity and risk of multiple sclerosis occurrence.
-
•
There is no association between parental consanguinity and age at MS onset.
-
•
The number of affected familial MS is not associated with increasing risk of primary progressive MS.
-
•
The age at onset among subjects with more than one familial MS is significantly lower than those with one familial MS cases.
1. Introduction
Multiple sclerosis (MS) is a complex and multifactorial autoimmune disorder that affects the central nervous system (CNS) [1]. While the precise etiology of MS remains elusive, it is widely accepted that both genetic and environmental factors play significant roles in disease susceptibility [2]. Iran, a country with a high prevalence of MS, has been experiencing an upward trend in the prevalence of familial MS (FMS) [3,4]. Recently, there has been growing interest in the impact of parental consanguinity—the practice of individuals with shared ancestry marrying and having children—on the risk of developing hereditary and autoimmune diseases [5]. The relationship between consanguineous marriage and autoimmune diseases, such as primary immunodeficiency (PID) and type 1 diabetes mellitus (T1DM), has been demonstrated in various studies [6,7]. This unique aspect of human genetics offers an intriguing avenue for exploring the genetic landscape of MS and shedding light on the underlying mechanisms of disease development [8].
The prevalence of consanguineous marriages varies globally, with higher rates observed in certain regions due to cultural, religious, and social factors. In Iran, estimated consanguinity ratios range from 30 to 85 % across different ethnic and religious populations. Patterns of consanguinity among several Iranian populations have been previously reported, with a significant percentage of first cousin marriages [[9], [10], [11], [12]]. In Saudi Arabia, parental consanguineous marriage was observed in 32 % of patients with MS (PwMS), with 56 % in familial MS and 28 % in sporadic MS [13]. Another study in Saudi Arabia found that consanguineous marriage is more common in Middle and Far Eastern countries, where the incidence of MS is low, and did not increase the risk of familial MS [14].
The relationship between consanguinity, genetic diversity, and the expression of protective alleles is complex and requires further research to establish clear connections and mechanisms [[15], [16], [17], [18], [19]]. A case-control study conducted in Iran found that offspring of consanguineous unions had a lower risk of MS compared to offspring of unrelated parents [5]. Based on these studies, it appears that consanguinity can lead to decreased genetic diversity, but the potential impact on the prevalence of protective alleles is not well understood.
Some studies have reported both recessive and dominant patterns of inheritance among MS patients with a family history of the disease. Consanguineous unions increase the likelihood of offspring inheriting recessive genetic variations [20,21]. In the context of MS, investigating the association between parental consanguinity and disease risk has the potential to elucidate the role of recessive genetic factors that may contribute to either susceptibility or protection against multiple sclerosis.
Overall, the search results suggest that the relationship between consanguineous marriage and MS is complex and may depend on various factors, such as the population studied and the type of consanguineous relationship. Considering that only a few small-scale studies have addressed this topic, this article aims to comprehensively analyze the current state of knowledge regarding parental consanguinity in the context of MS and its potential clinical impact on familial MS patients across different geographical regions of Iran with various ethnicities. By examining existing studies and genetic investigations, we seek to explore the potential impact of consanguineous unions on MS risk. Through this comprehensive study, we aim to contribute to the growing body of knowledge surrounding MS epidemiology and genetics and to foster further research in this intriguing field. Therefore, the goal of this study is to investigate the potential impact of consanguineous unions on the risk of MS occurrence in a large-scale study.
2. Materials and Methods
Study Design: This registry-based cross-sectional study was conducted on clinically definite MS patients registered in the Nationwide MS Registry of Iran (NMSRI), the official leading MS registry in Iran [22].
Study Population: Out of 19,911 patients with MS (PwMS) registered in the NMSRI as of October 8th, 2022, 2847 individuals were classified as having familial MS across 13 provinces, including East Azerbaijan, Isfahan, Tehran, Chaharmahal and Bakhtiari, Razavi Khorasan, Khuzestan, Fars, Qazvin, Kerman, Kermanshah, Lorestan, Mazandaran, and Markazi. Of these, 2307 patients were included in the final analysis, resulting in a response rate of 81 %. The inclusion criteria comprised all definite MS patients with a history of familial MS in any first, second, or third-degree relatives, while the exclusion criteria were the lack of consent for participation in follow-up interviews.
Data collection: Registered data were primarily extracted from the NMSRI datasets. Additionally, supplementary unregistered data were obtained through structured telephone follow-ups. A team of 18 trained researchers conducted these follow-ups, ensuring precise quality checks over a four-month period from June 6th to October 8th, 2022. The questions covered various aspects of MS, including baseline characteristics, MS history in relatives, and parental consanguinity [[22], [23], [24]].
Demographic characteristics of the subjects were recorded, including sex, age at the time of the study, date of birth, place of residence in the previous year, parental consanguinity, family history of MS, and date of visit. Additionally, disease characteristics and progression were documented, including the date and age of diagnosis, age at onset of first symptoms, type of MS, expanded disability status scale (EDSS) score, and hospitalization history for MS [25].
Nationwide MS registry of Iran (NMSRI): As a population-based registry, the NMSRI has been collecting epidemiological and clinical information on Iranian patients with MS (PwMS) since April 17th, 2018, with the assistance of experienced neurologists and trained registrars across 18 provinces. All cases are registered after being confirmed as MS patients according to the 2017 McDonald's criteria by neurologists [26]. The registry data are available through the District Health Information Software (DHIS2), and universal coverage and completeness of patient information are the most important features of the NMSRI [23,24]. Duplicate cases were systematically excluded based on the unique national identification code.
Variables and Measurements: According to the current literature and our expert panel consensus, the variables measured in this study are described in detail as follows:
-
•
Familial MS: having MS with at least one affected relative with MS [4].
-
•
Pediatric-onset MS (POMS): PwMS under 18 years of age [27].
-
•
Adult-onset MS (AOMS): PwMS with the onset between 18 and 49.9 years old
-
•
Late-onset MS (LOMS): PwMS with the onset after 50 years of age [28].
-
•
First-degree relatives: parents, offspring, and siblings
-
•
Second-degree relatives: grandparents, aunts, and uncles
-
•
Third-degree relatives: cousins and other relatives [29].
-
•
Latency period: the time between disease onset and diagnosis of MS
- •
Our team also proposed a novel score to differentiate between different levels of family involvement among PwMS, termed the “family penetration score.” The scoring formula assigns coefficients of 1, 0.5, and 0.25 to first-degree, second-degree, and more distant relatives, respectively. The final score is determined by multiplying the number of individuals in each category by their corresponding coefficient.
Data Analysis and Statistical Considerations: Data were presented as mean (standard deviation) for quantitative variables and frequency (percentage) for qualitative variables. Comparisons of quantitative variables between study groups were performed using independent t-tests and analysis of variance (ANOVA). Qualitative variables were compared using chi-square and Fisher exact tests. Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 26 (IBM Co., Armonk, NY). A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics characteristics and sex differences
Table 1 displays the demographic characteristics of our studied population. The sex analysis revealed a female to male ratio of 3.07:1, with 1502 (75.4 %) females and 489 (24.6 %) males. The mean (SD) age of the patients was 36.97 (9.54) years, the age at onset was 28.71 (8.74) years, and the age at diagnosis was 29.84 (9.00) years. More than 91 % of the individuals had an age at onset between 18 and 50 years; 119 (6.1 %) had pediatric-onset MS, and 46 (2.3 %) had late-onset MS.
Table 1.
Comparison of demographic and clinical manifestations between females and males.
| Qualitative Variables | Total | Female (n = 1502) | Male (n = 482) | P-value1 | |
|---|---|---|---|---|---|
| Age at Onset | <18 18–50 ≥50 |
119 (6.1 %) | 92(6.2 %) | 27(5.6 %) | 0.241 |
| 1800 (91.6 %) | 1361(91.8 %) | 439(91.1 %) | |||
| 46 (2.3 %) | 30(2.0 %) | 16(3.3 %) | |||
| Consanguinity of Parents | Yes No |
385 (19.3 %) | 289(19.2 %) | 96(19.6 %) | 0.849 |
| 1606 (80.7 %) | 1213(80.8 %) | 393(80.4 %) | |||
| MS Phenotype | RR SP PP RP |
1597 (80.8 %) | 1238(83.3 %) | 359(73.4 %) | <0.001 |
| 269 (13.6 %) | 185(12.4 %) | 84(17.2 %) | |||
| 98 (5.0 %) | 56(3.8 %) | 42(8.6 %) | |||
| 12 (0.6 %) | 8(0.5 %) | 4(0.8 %) | |||
| Number of Affected Relatives | 1 >1 |
1540 (77.3 %) | 1156(77.0 %) | 384(78.5 %) | 0.473 |
| 451 (22.7 %) | 346(23.0 %) | 105(21.5 %) | |||
| Hospitalization due to MS | 0 ≥1 |
568 (27.7 %) | 433(29.0 %) | 135(27.8 %) | 0.610 |
| 1480 (72.3 %) | 1058(71.0 %) | 350(72.2 %) | |||
| Conversion to SPMS | Yes No |
278 (14.5 %) | 195(13.5 %) | 83(17.6 %) | 0.028 |
| 1641 (85.5 %) | 1252(86.5 %) | 389(82.4 %) | |||
| Quantitative Variables | Mean (SD) | P-value2 | ||
|---|---|---|---|---|
| Age | 36.97 (9.54) | 36.94(9.45) | 37.07(9.85) | 0.787 |
| Age at Onset | 28.71 (8.74) | 28.55(8.62) | 29.20(9.11) | 0.152 |
| Age at Diagnosis | 29.84 (9.00) | 29.68(8.90) | 30.33(9.27) | 0.172 |
| Latency Period | 1.12 (2.79) | 1.13(2.79) | 1.09(2.80) | 0.827 |
| Number of Affected Relatives | 1.30 (0.65) | 1.30(0.67) | 1.29(0.64) | 0.761 |
| EDSS Score | 2.20 (1.92) | 2.07(1.87) | 2.58(2.02) | <0.001 |
N: Number, SD: Standard Deviation, RR: Relapsing Remitting, SP: Secondary Progressive, PP: Primary Progressive, RP: Relapsing Progressive, EDSS: Expanded Disability Status Scale.
P-value1: P-value computed by Chi-square test, P-value2: P-value computed by independent t-test.
Relapsing-remitting MS (RRMS) was the most common type, with a prevalence of over 80 %. In contrast, 13.6 % of patients (269 PwMS) later developed secondary progressive MS (SPMS), the most prevalent progressive MS type. The mean (SD) Expanded Disability Status Scale (EDSS) score was 2.20 (1.92). Significant differences in MS phenotypes were observed between males and females (P-value <0.001). There was a high prevalence of RRMS in women (84 %), whereas the SPMS, primary-progressive MS (PPMS), and progressive relapsing MS (PRMS) forms were more common in men (17.2 %, 8.6 %, and 0.8 %, respectively). Additionally, the disease progressed to SPMS more frequently in men (P-value = 0.028). The mean (SD) EDSS score was significantly higher in males at 2.58 (2.2) compared to 2.07 (1.87) in females (P-value <0.001). There were no significant sex differences among other qualitative and quantitative variables, including consanguinity. The geographical distribution of our studied population is depicted in Supplementary Table 1.
3.2. Familial aspects
As shown in Table 2, regarding the familial aspects of MS, 1540 participants (77 %) had just one relative with MS, while 451 (22.7 %) had more than one relative with the disease; among these, 26 (1.3 %) had four or more affected relatives, including one patient with 10 affected relatives. Overall, the mean (SD) number of affected relatives was 1.30 (0.66). Additionally, 385 patients (19.3 %) reported parental consanguinity.
Table 2.
Familial aspects of MS.
|
Number of Affected Relatives (N, %) |
1 | 1540 | (77.3 %) |
| ≥1 |
451 |
(22.7 %) |
|
|
Number of Affected Relatives (N, %) |
1 | 1540 | (77.3 %) |
| 2 | 348 | (17.5 %) | |
| 3 | 77 | (3.9 %) | |
| ≥4 |
26 |
(1.3 %) |
|
|
Number of Affected Relatives (Mean, SD) |
1.30 |
(0.66) |
|
| Consanguinity of Parents (N, %) | Yes | 385 | (19.3) |
| No | 1606 | (80.7) |
N: Number, SD: Standard Deviation.
3.3. Parental consanguinity
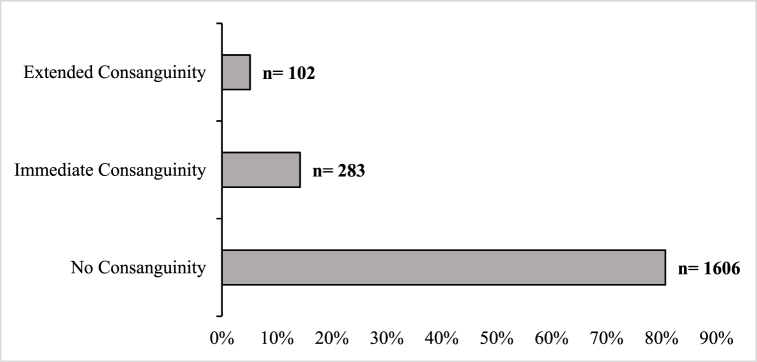
The relationship between parents was divided into three categories: (1) parents with no consanguinity, (2) parents with immediate consanguinity, and (3) parents with extended consanguinity. The FMS registry showed that the parents of 1606 patients (80.7 %) were not related, while 385 patients (19.3 %) reported some degree of parental relationship. As shown in Fig. 1, 283 parents (14.2 %) were cousins, and 102 (5.1 %) were distant relatives.
Fig. 1.
Consanguinity of parents among FMS cases.
Table 3 compares quantitative and qualitative variables based on different types of parental consanguinity. The table indicates no significant association between parental kinship and variables such as MS phenotype, number of affected relatives with MS, and hospitalization rates. Additionally, the mean EDSS score was not significantly different between the “All Consanguinity” group (2.19 [1.80]) and the “No Consanguinity” group (2.20 [1.95]) (P-value = 0.897); hence, MS severity did not differ by parental consanguinity.
Table 3.
Comparison of demographic and clinical manifestations based on parental consanguinity.
| Qualitative Variables | Parental Consanguinity |
||||||
|---|---|---|---|---|---|---|---|
| No Consanguinity |
Consanguineous Marriage |
||||||
| All Consanguinity Types |
P-value1 | Immediate Consanguinity |
Extended Consanguinity |
P-value1 | |||
| N (%) | N (%) | ||||||
| Age at Onset |
<18 | 88(5.6 %) | 31(8.2 %) | 0.095 |
19(6.8 %) | 12(11.9 %) | 0.085 |
| 18–50 | 1457(91.9 %) | 343(90.3 %) | 256(91.8 %) | 87(86.1 %) | |||
| ≥50 |
40(2.5 %) |
6(1.6 %) |
4(1.4 %) |
2(2.0 %) |
|||
| Sex |
Female | 1213(75.5 %) | 289(75.1 %) | 0.894 |
216(76.3 %) | 73(71.6 %) | 0.621 |
| Male |
393(24.5 %) |
96(24.9 %) |
67(23.7 %) |
29(28.4 %) |
|||
| MS Phenotype |
RR | 1280(80.3 %) | 317(83.0 %) | 0.585 |
237(84.3 %) | 80(79.2 %) | 0.184 |
| SP | 224(14.1 %) | 45(11.8 %) | 34(12.1 %) | 11(10.9 %) | |||
| PP | 81(5.1 %) | 17(4.5 %) | 9(3.2 %) | 8(7.9 %) | |||
| RP |
9(0.6 %) |
3(0.8 %) |
1(0.4 %) |
2(2.0 %) |
|||
| Number of Affected Relatives |
1 | 1249(77.8 %) | 291(75.6 %) | 0.357 |
217(76.7 %) | 74(72.5 %) | 0.455 |
| >1 |
357(22.2 %) |
94(24.4 %) |
66(23.3 %) |
28(27.5 %) |
|||
| Hospitalization due to MS |
0 | 465(29.1 %) | 103(27.3 %) | 0.497 |
74(26.7 %) | 29(29.0 %) | 0.723 |
| ≥1 |
1134(70.9 %) |
274(72.7 %) |
203(73.3 %) |
71(71.0 %) |
|||
| Conversion to SPMS | Yes | 228(14.7 %) | 50(13.5 %) | 0.559 | 36(13.3 %) | 14(14.0 %) | 0.828 |
| No | 1321(85.3 %) | 320(86.5 %) | 234(86.7 %) | 86(86.0 %) | |||
| Quantitative Variables | Mean (SD) | P-value2 | Mean (SD) | P-value2 | ||
|---|---|---|---|---|---|---|
| Age | 37.08(9.69) | 36.49(8.92) | 0.279 | 36.71(8.75) | 35.88(9.39) | 0.420 |
| Age at Onset | 28.85(8.82) | 28.14(8.40) | 0.154 | 28.28(8.52) | 27.74(8.07) | 0.315 |
| Age at Diagnosis | 29.99(9.07) | 29.23(8.68) | 0.141 | 29.37(8.63) | 28.84(8.86) | 0.298 |
| Latency Period | 1.13(2.77) | 1.07(2.87) | 0.733 | 1.06(2.86) | 1.10(2.92) | 0.938 |
| Number of Affected Relatives | 1.30(0.68) | 1.31(0.60) | 0.774 | |||
| EDSS Score | 2.20(1.95) | 2.19(1.80) | 0.897 | 2.23(1.83) | 2.07(1.71) | 0.758 |
N: Number, SD: Standard Deviation, RR: Relapsing Remitting, SP: Secondary Progressive, PP: Primary Progressive, RP: Relapsing Progressive, EDSS: Expanded Disability Status Scale.
P-value1: P-value computed by Chi-square test, P-value2: P-value computed by independent t-test.
3.4. Age at disease onset
Clinical presentations of MS varied significantly depending on the age of disease onset (P-value = 0.001). While 84 % of pediatric-onset MS (POMS) cases experienced RRMS, 21 % of late-onset MS (LOMS) cases had PPMS from the beginning. Among the different age groups, hospitalization rates were significantly lower for patients diagnosed after 50 years of age. Although the rate of consanguineous marriage was higher among patients with an onset age of less than 18 years, this difference was not statistically significant, and there was no significant difference in disease onset age based on parental consanguinity status.
Most quantitative variables showed significant differences when categorized by disease onset age groups. For POMS patients, the latency period between disease onset and diagnosis was less than a year. This period was 1.08 years for adult-onset MS (AOMS) and 4.70 years for LOMS (P-value <0.001). Notably, the EDSS score for patients with symptom onset over 50 was 3.08, significantly higher than that in other age groups (P-value = 0.002) (Table 4).
Table 4.
Comparison of demographic and clinical manifestations by age at onset.
| Qualitative Variables | Age at Onset |
||||
|---|---|---|---|---|---|
| <18 |
18–50 |
≥50 |
P-value1 | ||
| N (%) | |||||
| Sex |
Female | 92(77.3 %) | 1361(75.6 %) | 30(65.2 %) | 0.241 |
| Male |
27(22.7 %) |
439(24.4 %) |
16(34.8 %) |
||
| Parental Consanguinity |
Yes | 31(26.1 %) | 343(19.1 %) | 6(13.0 %) | 0.095 |
| No |
88(73.9 %) |
1457(80.9 %) |
40(87.0 %) |
||
| MS Phenotype |
RR | 101(84.9 %) | 1451(81.2 %) | 30(65.2 %) |
<0.001 |
| SP | 16(13.4 %) | 240(13.4 %) | 6(13.0 %) | ||
| PP | 2(1.7 %) | 84(4.7 %) | 10(21.7 %) | ||
| PR |
0(0.0 %) |
12(0.7 %) |
0(0.0 %) |
||
| Number of Affected Relatives | 1 | 84(70.6 %) | 1400(77.8 %) | 37(80.4 %) | 0.170 |
| >1 | 35(29.4 %) | 400(22.2 %) | 9(19.6 %) | ||
| Hospitalization due to MS |
0 | 32(26.9 %) | 507(28.4 %) | 24(52.2 %) |
0.002 |
| ≥1 |
87(73.1 %) |
1280(71.6 %) |
22(47.8 %) |
||
| Conversion to SPMS | Yes | 16(13.9 %) | 254(14.6 %) | 4(8.7 %) | 0.527 |
| No | 99(86.1 %) | 1488(85.4 %) | 42(91.3 %) | ||
| Quantitative Variables | Mean (SD) | P-value2 | ||
|---|---|---|---|---|
| Age | 25.59(7.23) | 37.16(8.70) | 57.46(4.61) | <0.001 |
| Age at Onset | 14.76(2.33) | 29.11(7.57) | 49.85(7.35) | <0.001 |
| Age at Diagnosis | 15.10(2.21) | 30.18(7.59) | 54.54(3.96) | <0.001 |
| Latency Period | 0.34(0.95) | 1.08(2.62) | 4.70(6.98) | <0.001 |
| Number of Affected Relatives | 1.36(0.65) | 1.30(0.67) | 1.20(0.40) | 0.340 |
| EDSS Score | 1.94(1.76) | 2.19(1.92) | 3.08(1.73) | 0.002 |
N: Number, SD: Standard Deviation, FMS: Familial MS, RR: Relapsing Remitting, SP: Secondary Progressive, PP: Primary Progressive, RP: Relapsing Progressive, EDSS: Expanded Disability Status Scale.
P-value1: P-value computed by Chi-square test, P-value2: P-value computed by independent t-test.
3.5. Extent of individual and family involvement
Table 5 shows the association between the number of affected relatives and the number of hospitalizations with clinical or demographic factors. The number of PwMS in the family was not associated with sex, age at onset, or MS phenotype. There was no difference between those with or without parental consanguinity regarding the number of affected relatives or the number of hospitalizations.
Table 5.
Comparison of demographic and clinical factors by the number of hospitalizations and affected relatives.
| Number of Affected Relatives |
Number of Hospitalizations |
||||||
|---|---|---|---|---|---|---|---|
| 1 |
>1 |
P-value1 | 0 |
≥1 |
P-value1 | ||
| Qualitative Variables | N (%) | N (%) | |||||
| Sex |
Female | 1156 (75.1 %) | 346 (76.7 %) | 0.473 |
433 (76.2 %) | 1058 (75.1 %) | 0.610 |
| Male |
384 (24.9 %) |
105 (23.3 %) |
135 (23.8 %) |
350 (24.9 %) |
|||
| Age at Onset |
<18 | 84 (5.5 %) | 35 (7.9 %) | 0.170 |
32 (5.7 %) | 87 (6.3 %) | 0.002 |
| 18–50 | 1400 (92.0 %) | 400 (90.1 %) | 507 (90.1 %) | 1280 (92.2 %) | |||
| ≥50 |
37 (2.4 %) |
9 (2.0 %) |
24 (4.3 %) |
22 (1.6 %) |
|||
| Parental Consanguinity |
Yes | 291 (18.9 %) | 94 (20.8 %) | 0.357 |
103 (18.1 %) | 274 (19.5 %) | 0.497 |
| No |
1249 (81.1 %) |
357 (79.2 %) |
465 (81.9 %) |
1134 (80.5 %) |
|||
| MS Phenotype |
RR | 1245 (81.5 %) | 352 (78.6 %) | 0.101 |
495 (87.5 %) | 1092 (78.3 %) |
<0.001 |
| SP | 194 (12.7 %) | 75 (16.7 %) | 41 (7.2 %) | 225 (16.1 %) | |||
| PP | 78 (5.1 %) | 20 (4.5 %) | 28 (4.9 %) | 68 (4.9 %) | |||
| RP |
11 (0.7 %) |
1 (0.2 %) |
2 (0.4 %) |
10 (0.7 %) |
|||
| Hospitalization due to MS |
0 | 453 (29.7 %) | 115 (25.6 %) | 0.089 |
– | – | |
| ≥1 |
1073 (70.3 %) |
335 (74.4 %) |
– |
– |
|||
| Conversion to SPMS | Yes | 202 (13.6 %) | 76 (17.4 %) | 42 (7.7 %) | 232 (17.0 %) | ||
| No | 1281 (86.4 %) | 360 (82.6 %) | 0.047 | 504 (92.3 %) | 1133 (83.0 %) | <0.001 | |
| Quantitative Variables | Mean (SD) | P-value2 | Mean (SD) | P-value2 | |||
|---|---|---|---|---|---|---|---|
| Age | 37.01 (9.45) | 36.82 (9.86) | 0.708 | 36.16 (9.67) | 37.25 (9.48) | 0.022 | |
| Age at Onset | 28.95 (8.68) | 27.87 (8.90) | 0.022 | 29.55 (9.16) | 28.33 (8.55) | 0.005 | |
| Age at Diagnosis | 30.07 (8.98) | 29.04 (9.02) | 0.034 | 30.89 (9.31) | 29.39 (8.84) | <0.001 | |
| Latency Period | 1.12 (2.82) | 1.12 (2.71) | 0.972 | 1.31 (2.90) | 1.05 (2.76) | 0.057 | |
| Number of Affected Relatives | – | – | 1.29 (0.76) | 1.30 (0.62) | 0.747 | ||
| EDSS Score | 2.17 (1.88) | 2.31 (2.04) | 0.164 | 1.68 (1.67) | 2.40 (1.97) | <0.001 | |
N: Number, SD: Standard Deviation, FMS: Familial MS, RR: Relapsing Remitting, SP: Secondary Progressive, PP: Primary Progressive, RP: Relapsing Progressive, EDSS: Expanded Disability Status Scale.
P-value1: P-value computed by Chi-square test, P-value2: P-value computed by independent t-test.
The age at symptom onset and disease diagnosis in patients with more than one family member with MS was significantly lower than in those with only one family member with MS (P-value = 0.022 and P-value = 0.034, respectively). The number of hospitalizations in patients with secondary progressive multiple sclerosis (SPMS) was significantly higher than in other types of MS (P-value <0.001). Additionally, among cases with conversion to SPMS, 17 % had at least one hospitalization, while 7.7 % had no history of hospitalization (P-value <0.001).
Patients with a history of at least one hospitalization had higher mean age 37.25 (9.48) years (P-value = 0.022), lower age at onset 28.33 (8.55) years (P-value = 0.005), lower age at diagnosis 29.39 (8.84) years (P-value <0.001), and higher EDSS scores 2.40 (1.97) (P-value <0.001) compared to those with no history of hospitalization.
After scoring the patients according to the family penetration coefficient, sex was the only variable associated with the final scores. Men reported significantly higher family penetration scores than women (P-value = 0.005). There was no association between the family penetration score and variables such as age at onset, MS phenotype, number of hospitalizations due to MS, or disease progression to SPMS. The results are summarized in Table 6.
Table 6.
Comparison of demographic and clinical factors by family penetration score.
| Variables | Family Penetration Score |
P- value | |
|---|---|---|---|
| Mean (SD) | |||
| Sex |
Female | 0.62 (0.52) | 0.005a |
| Male |
0.70 (0.56) |
||
| Age at Onset |
<18 | 0.67 (0.53) | 0.470b |
| 18–50 | 0.63 (0.53) | ||
| ≥50 |
0.71 (0.56) |
||
| MS Phenotype |
RR | 0.63 (0.52) | 0.108b |
| SP | 0.71 (0.60) | ||
| PP | 0.64 (0.53) | ||
| RP |
0.45 (0.42) |
||
| Hospitalization due to MS |
0 | 0.62 (0.51) | 0.327a |
| ≥1 |
0.65 (0.54) |
||
| Number of Hospitalizations due to MS |
0 | 0.62 (0.51) | 0.089b |
| 1 | 0.61 (0.53) | ||
| 2 | 0.69 (0.54) | ||
| 3 | 0.71 (0.58) | ||
| 4 |
0.64 (0.53) |
||
| Conversion to SPMS | Yes | 0.69 (0.59) | 0.069a |
| No | 0.63 (0.52) | ||
SD: Standard Deviation, RR: Relapsing Remitting, SP: Secondary Progressive, PP: Primary Progressive, RP: Relapsing Progressive.
: P-value computed by independent t-test.
: P-value computed by ANOVA.
4. Discussion
In this study, we investigated the possible role of consanguinity in the occurrence and course of MS among a multi-ethnic Iranian population of FMS cases from NMSRI. The majority of our sample comprised young individuals (average age ∼37 years) who had experienced MS for less than 10 years, which may account for the relatively low mean EDSS score. The study revealed a lower prevalence of parental consanguinity (PC) among patients (19.3 %) compared to rates reported in other studies in the region, such as 31 % in Saudi Arabia and 40 % in Palestine [13,32]. However, some other studies in Iran, like that of Maghzi et al. [5], reported lower rates, with 26.1 % of consanguinity among their cases. It is important to note that our data is derived from a national-level population-based registry, which provides a more accurate reflection of the actual population.
According to our results, MS severity factors were significantly higher in males, as evidenced by clinical presentations, progression to SPMS, and EDSS scores. Additionally, MS phenotypes differed significantly between males and females. Relapsing-remitting MS (RRMS) forms were more prevalent in women, consistent with the well-established fact that females predominate in RRMS, with a female-to-male ratio significantly higher (∼3.3:1) among RRMS patients who experience at least four relapses within five years of MS onset [33,34]. Furthermore, primary-progressive MS (PPMS) was more prevalent in males, aligning with the known association between male sex and PPMS characteristics, as well as the role of male sex in the progression from RIS to PPMS [33]. Males also exhibited a higher frequency of progression to SPMS and significantly higher EDSS scores compared to females. Previous research indicated that males experienced a shorter time and younger age for conversion to SPMS, as well as a considerably faster progression in EDSS scores compared to females [33,35,36].
No sex differences were observed in variables such as the number of family members affected by MS, the prevalence of primary care (PC) utilization, hospitalization rates, and the latency period. Our findings on the number of family members affected by MS are consistent with those reported by Salehi et al. [4]. Moreover, there was no significant difference in the sex ratios observed in pediatric-onset MS (POMS), adult-onset MS (AOMS), or late-onset MS (LOMS) patients, which is consistent with previous studies [4,28,37,38].
Overall, our results demonstrated that parental consanguinity (PC) did not affect the manifestation of MS, a topic that has been controversial for some time. A previous study conducted in Saudi Arabia by Al Jumah et al. indicated that MS patients with any degree of PC were more likely to have another MS-affected family member, although this was not statistically significant [14]. Another study in the Aseer region of Saudi Arabia suggested that the presence of PC doubles the risk of developing MS compared to individuals without PC [39]. Additionally, a study of the Caucasian Canadian population suggested that the offspring of consanguineous couples have a higher recurrence rate of familial MS [40]. Conversely, multiple studies have shown no statistically significant association between PC and MS. A case-control study by Maghzi et al. indicated that the offspring of consanguineous unions exhibit a reduced risk of MS compared to the offspring of unrelated parents, possibly due to the recessive inheritance of protective alleles for MS [5]. Furthermore, another study in Iran found no association between PC and disease severity [41]. Despite the high prevalence of consanguinity among Emirati individuals, no significant differences in clinical and demographic characteristics were observed between those with familial and sporadic MS [42]. Previous studies have identified certain rare genetic risk variants that contribute to the heritability of MS, possibly explaining its occurrence in specific families [20,43], along with some hormonal contributors [44]. In contrast, additional studies have demonstrated a significant link between environmental factors and the development of MS, such as sunlight exposure, cigarette smoking, measles infection, Epstein-Barr virus (EBV) infection, and stressful events [[45], [46], [47]].
There was no apparent association between parental consanguinity (PC) and age at MS onset, which may diminish the perceived role of genetic factors in the onset of MS. Conversely, an Italian cohort study examined the correlation between weighted genetic risk scores and MS clinical features and found a reverse correlation between genetic burden and age at symptom onset. After dividing the patients into groups based on MS familiarity, the study validated the link between genetic burden and age at onset in sporadic MS (SMS) but not in those with familial MS (FMS). This suggests that common genetic variants associated with MS do not significantly contribute to familial MS [48].
The initial presentation of MS was found to vary significantly between late-onset MS (LOMS) and other patients. For LOMS, 60 % had a relapsing-remitting MS (RRMS) phenotype and 21 % had a primary-progressive MS (PPMS) phenotype. In contrast, for those with pediatric-onset MS (POMS) and adult-onset MS (AOMS), around 80 % exhibited an RRMS phenotype. As demonstrated by previous studies, the proportion of patients with PPMS and secondary-progressive MS (SPMS) increased dramatically with age. Additionally, two (1.7 %) POMS patients had a PPMS phenotype, similar to findings in other studies indicating that approximately two percent of POMS patients have a PPMS phenotype [28,38,49].
Hospitalization rates were significantly higher in POMS and AOMS cases compared to LOMS cases, which aligns with other studies showing higher relapse rates leading to more hospitalizations for these patients [28,38,50]. Another explanation is that patients diagnosed earlier experience longer years of morbidity, and there is heightened concern for younger patients compared to the elderly.
We observed that LOMS patients had significantly longer latency periods than others, delaying treatment and resulting in more severe MS courses. While a comprehensive cohort study estimated a longer diagnostic delay for POMS [38], Ghadiri et al. reported extended latent periods in LOMS and PPMS types [37]. Furthermore, significant differences were observed in the EDSS scores among the three onset age groups. Despite having MS for a longer duration, individuals in the POMS group had the lowest EDSS scores, while those in the LOMS group had the highest. In simpler terms, individuals in the POMS group had a significantly lower probability of developing disabilities and progressing in the EDSS score. This may be attributed to the more remarkable ability for recovery in the developing CNS, as suggested by Simone et al. [50].
The findings of our study revealed that the number of PwMS in family members was not associated with sex, age at onset, PC, or MS phenotype. Conversely, some studies reported that the presence of PC in PwMS increased the probability of having another family member with MS [14,40]. Our study showed that the age at symptom onset and disease diagnosis in patients with more than one family member with MS was significantly lower than in those with only one family member with MS. Previous studies on the age of disease onset have reported no difference between familial MS and sporadic MS [4,13]. Our results might be due to familial aggregation of the disease or increased awareness among the affected individuals' families regarding the nature of the disease. Nevertheless, further investigation is necessary to provide a more definitive statement.
It was reported that sex was not significantly different in familial and sporadic MS patients [42]. However, the results of our current study indicate that the family penetration score was higher among male patients with familial MS. The observed increase in the family penetration score among male patients suggests that physicians should be more cautious about the diagnosis of MS in close relatives of male PwMS.
5. Limitations
To our knowledge this is the first study to investigate the role of consanguinity in FMS patients in a large national scale. However, it's important to note that our study had certain limitations. First, the cross-sectional nature of the study makes it difficult to build a causation relationship between involved factors. Despite these limitations, our findings provide valuable insights into the genetic features of MS and is enlightening a pathway for future investigations.
To build upon our findings, here are some suggestions for further research regarding the impact of parental consanguinity on MS: (1) future studies could be conducted as longitudinal studies to track the progression of the disease in patients with a history of parental consanguinity and establish causation relationship between parental consanguinity and the risk of multiple sclerosis; (2) genetic studies could investigate the impact of parental consanguinity on the specific genetic variations associated with familial MS. These are just a few recommendations for further research based on the search results. Further research in these areas could help to improve forward our understanding of familial MS and its genetic and environmental features, as well as improve the management and treatment of the disease.
6. Conclusion
In conclusion, our study found no association between consanguinity and MS risk as well as a lower prevalence of parental consanguinity among FMS cases compared to other studies in the region. These results suggest that MS is a multifactorial disease influenced by genetic and environmental factors. Further research is needed to explore these findings and provide more conclusive statements. By addressing these gaps, we can advance our understanding of the MS etiology and pave the way for potential applications or interventions.
Funding
Tehran University of Medical Sciences and Health Services.
Ethical considerations
This study was overseen by the Ethics Committee of Tehran University of Medical Sciences and the study bears the registration code IR. TUMS.NI.REC.1401.026.
Consent for publication
Not applicable.
Data availability statement
The data sets used and analyzed during the study are available from the corresponding author on reasonable request. Moreover, the entire data of the study has been sent to the journal in Excel file format and if needed, it will be provided by contacting the website https://nmsri.ir/or by email to corresponding Author (sh_eskandaeieh@yahoo.com).
Name of the repository: Sharareh Eskandarieh.
CRediT authorship contribution statement
Zahra Salehi: Writing – original draft, Formal analysis. Mohammad Mehdi Naghizadeh: Writing – review & editing, Investigation. Sajjad Ghane Ezabadi: Writing – review & editing, Investigation. Azadeh Ebrahimitirtashi: Writing – review & editing, Investigation. Naghmeh Abbasi Kasbi: Writing – review & editing, Data curation. Faezeh Khodaie: Writing – review & editing, Investigation. Shahram Aliyari: Writing – review & editing, Methodology, Formal analysis. Fereshteh Ashtari: Writing – review & editing, Investigation. Seyed Mohammad Baghbanian: Writing – review & editing, Investigation. Seyed Massood Nabavi: Writing – review & editing, Investigation. Samaneh Hosseini: Writing – review & editing, Investigation. Nazanin Razazian: Writing – review & editing, Investigation. Vahid Shaygannejad: Writing – review & editing, Investigation. Nastaran Majdi-Nasab: Writing – review & editing, Investigation. Mohammad Hossein Harirchian: Writing – review & editing, Investigation. Asghar Bayati: Writing – review & editing, Investigation. Hoda Kamali: Writing – review & editing, Investigation. Nahid Hosseni Nejad Mir: Writing – review & editing, Investigation. Nahid Beladi Moghadam: Writing – review & editing, Investigation. Maryam Poursadeghfard: Writing – review & editing, Investigation. Hossein Mozhdehipanah: Writing – review & editing, Investigation. Nazanin Jalali: Writing – review & editing, Investigation. Mohammad Ali Nahayati: Writing – review & editing, Investigation. Fardin Faraji: Writing – review & editing, Investigation. Naser Kamyari: Writing – review & editing, Investigation. Mohammad Ali Sahraian: Writing – review & editing, Supervision, Methodology, Investigation. Zhila Maghbooli: Writing – review & editing, Data curation. Sharareh Eskandarieh: Writing – review & editing, Project administration, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Sharareh Eskandarieh reports financial support was provided by Tehran University of Medical Sciences. Sharareh Eskandarieh reports a relationship with Tehran University of Medical Sciences that includes: consulting or advisory, employment, equity or stocks, and funding grants. Sharareh Eskandarieh has patent licensed to NMSRI. None If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Support for the Reported Work: Tehran University of Medical Sciences.
Relevant support: advisory positions, consulting fees.
Intellectual Property: National multiple sclerosis registry system of Iran (NMSRI).
Other Activities: None.
The entire data of the study has been sent to the journal in Excel file format.
If needed, it will be provided by contacting the website https://nmsri.ir/or by email.
Acknowledgements
We would like to express our sincere gratitude to all the diligent individuals working in the NMSRI. The authors are extremely grateful to Ms. Saeideh Ayoubi and Mr. Mohammad Reza Zabihi who were of great help during this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32946.
Abbreviations
- AOMS
Adult-Onset MS
- CNS
Central nervous system
- EDSS
Expanded disability status scale
- FMS
Familial MS
- LOMS
Late-Onset MS
- MS
Multiple Sclerosis
- NMSRI
Nationwide MS registry of Iran
- PC
Parental consanguinity
- POMS
Pediatric-Onset MS
- PPMS
Primary-Progressive MS
- PRMS
Progressive-Relapsing MS
- RRMS
Relapsing-Remitting MS
- SMS
Sporadic MS
- SPMS
Secondary-Progressive MS
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Dobson R., Giovannoni G. Multiple sclerosis–a review. Eur. J. Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 2.Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 3.Ezabadi S.G., Ayoubi S., Sahraian M.A., Omrani M.A., Eskandarieh S. The incidence and prevalence of crude and familial multiple sclerosis in Tehran, Iran in 2021. Neurol. Sci. 2023 doi: 10.1007/s10072-023-07043-w. [DOI] [PubMed] [Google Scholar]
- 4.Salehi Z., Almasi-Hashiani A., Sahraian M.A., Ashtari F., Baghbanian S.M., Razazian N., Moghadasi A.N., Bayati A., Azimi A.R., Beladimoghadam N. Epidemiology of familial multiple sclerosis in Iran: a national registry-based study. BMC Neurol. 2022;22:1–8. doi: 10.1186/s12883-022-02609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maghzi H., Shaygannejad V., Minagar A., Hassanzadeh A., Maghzi A.-H. Consanguinity and multiple sclerosis susceptibility: a case control study. Mult Scler Relat Disord. 2016;10:179–180. doi: 10.1016/j.msard.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Al-Herz W., Aldhekri H., Barbouche M.-R., Rezaei N. Consanguinity and primary immunodeficiencies. Hum. Hered. 2014;77:138–143. doi: 10.1159/000357710. [DOI] [PubMed] [Google Scholar]
- 7.Albishi L.A., AlAmri E., Mahmoud A.A. Relationships among consanguinity, family history, and the onset of type 1 diabetes in children from Saudi Arabia. Prim Care Diabetes. 2022;16:102–106. doi: 10.1016/j.pcd.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sawcer S., Franklin R.J.M., Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi: 10.1016/S1474-4422(14)70041-9. [DOI] [PubMed] [Google Scholar]
- 9.Tayebi N., Yazdani K., Naghshin N. The prevalence of congenital malformations and its correlation with consanguineous marriages. Oman Med. J. 2010;25:37. doi: 10.5001/omj.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadat M., Zarghami M. Consanguineous marriages among Iranian Mandaeans living in south-west Iran. J. Biosoc. Sci. 2018;50:451–456. doi: 10.1017/S0021932017000207. [DOI] [PubMed] [Google Scholar]
- 11.Saadat M., Ansari-Lari M., Farhud D.D. Short report consanguineous marriage in Iran. Ann. Hum. Biol. 2004;31:263–269. doi: 10.1080/03014460310001652211. [DOI] [PubMed] [Google Scholar]
- 12.Rafiee L., Saadat M. Prevalence of consanguineous marriages among Iranian Georgians. J. Biosoc. Sci. 2011;43:47–50. doi: 10.1017/S0021932010000295. [DOI] [PubMed] [Google Scholar]
- 13.AlJumah M., Al Otaibi H., Al Towaijri G., Hassan A., Kareem A., Kalakatawi M., Alrajeh S., Al Mejally M., Algahtani H., Almubarak A. Familial aggregation of multiple sclerosis: results from the national registry of the disease in Saudi Arabia, Multiple Sclerosis. Journal–Experimental, Translational and Clinical. 2020;6 doi: 10.1177/2055217320960499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Jumah M., Kojan S., Al Khathaami A., Al Abdulkaream I., Al Blawi M., Jawhary A. Familial multiple sclerosis: does consanguinity have a role? Multiple Sclerosis Journal. 2011;17:487–489. doi: 10.1177/1352458510390406. [DOI] [PubMed] [Google Scholar]
- 15.Erzurumluoglu A.M., Shihab H.A., Rodriguez S., Gaunt T.R., Day I.N.M. Importance of genetic studies in consanguineous populations for the characterization of novel human gene functions. Ann. Hum. Genet. 2016;80:187–196. doi: 10.1111/ahg.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temaj G., Nuhii N., Sayer J.A. The impact of consanguinity on human health and disease with an emphasis on rare diseases. Journal of Rare Diseases. 2022;1:2. [Google Scholar]
- 17.Khayat A.M., Alshareef B.G., Alharbi S.F., AlZahrani M.M., Alshangity B.A., Tashkandi N.F. Consanguineous marriage and its association with genetic disorders in Saudi Arabia: a review. Cureus. 2024;16 doi: 10.7759/cureus.53888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Al-Bustan S., Feng Q., Guo W., Ma Z., Marafie M., Jacob S., Al-Mulla F., Xu S. The influence of admixture and consanguinity on population genetic diversity in Middle East. J. Hum. Genet. 2014;59:615–622. doi: 10.1038/jhg.2014.81. [DOI] [PubMed] [Google Scholar]
- 19.Bittles A.H. Vogel and Motulsky's Human Genetics. Springer; 2010. Consanguinity, genetic drift, and genetic diseases in populations with reduced numbers of founders; pp. 507–528. [Google Scholar]
- 20.Salehi Z., Keramatipour M., Talebi S., Arab S.S., Moghadasi A.N., Sahraian M.A., Izad M. Exome sequencing reveals novel rare variants in Iranian familial multiple sclerosis: the importance of POLD2 in the disease pathogenesis. Genomics. 2021;113:2645–2655. doi: 10.1016/j.ygeno.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Shams H., Shao X., Santaniello A., Kirkish G., Harroud A., Ma Q., Isobe N., Schaefer C.A., McCauley J.L. Polygenic risk score association with multiple sclerosis susceptibility and phenotype in Europeans. Brain. 2023;146:645–656. doi: 10.1093/brain/awac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahin S., Eskandarieh S., Moghadasi A.N., Razazian N., Baghbanian S.M., Ashtari F., bayati A., Manouchehrinia A., Beiki O., Mohebi F., Dezfuli M.M., Sahraian M.A. Multiple sclerosis national registry system in Iran: Validity and reliability of a minimum data set. Mult Scler Relat Disord. 2019;33:158–161. doi: 10.1016/j.msard.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Ayoubi S., Asadigandomani H., Bafrani M.A., Shirkoohi A., Nasiri M., Sahraian M.A., Eskandarieh S. The national multiple sclerosis registry system of Iran (NMSRI): aspects and methodological dimensions. Mult Scler Relat Disord. 2023;72 doi: 10.1016/j.msard.2023.104610. [DOI] [PubMed] [Google Scholar]
- 24.Ezabadi S.G., Sahraian M.A., Maroufi H., Shahrbaf M.A., Eskandarieh S. Global assessment of characteristics of multiple sclerosis registries; A systematic review. Mult Scler Relat Disord. 2022;63 doi: 10.1016/j.msard.2022.103928. [DOI] [PubMed] [Google Scholar]
- 25.Ghadiri F., Sahraian M.A., Baghbanian S.M., Ashtari F., Razazian N., Majdinasab N., Poursadeghfard M., Hatamian H., Harirchian M.H., Beladimoghadam N. Prescription trends of disease-modifying treatments for multiple sclerosis in Iran over the past 30 years. Mult Scler Relat Disord. 2022;61 doi: 10.1016/j.msard.2022.103777. [DOI] [PubMed] [Google Scholar]
- 26.Zipp F., Oh J., Fragoso Y.D., Waubant E. Implementing the 2017 McDonald criteria for the diagnosis of multiple sclerosis. Nat. Rev. Neurol. 2019;15:441–445. doi: 10.1038/s41582-019-0194-0. [DOI] [PubMed] [Google Scholar]
- 27.Krupp L.B., Banwell B., Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 28.Mirmosayyeb O., Brand S., Barzegar M., Afshari-Safavi A., Nehzat N., Shaygannejad V., Sadeghi Bahmani D. Clinical characteristics and disability progression of early-and late-onset multiple sclerosis compared to adult-onset multiple sclerosis. J. Clin. Med. 2020;9:1326. doi: 10.3390/jcm9051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerlind H., Ramanujam R., Uvehag D., Kuja-Halkola R., Boman M., Bottai M., Lichtenstein P., Hillert J. Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain. 2014;137:770–778. doi: 10.1093/brain/awt356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefranc M.-P., Lefranc G. In: Consanguinity. Maloy S., of G K.B.T.-B.E., Hughes Second E., editors. Academic Press; San Diego: 2013. pp. 158–162. [DOI] [Google Scholar]
- 31.Hart P.S., Markello T.C., Gahl W.A. In: F.P.B.T.-P. And P. of C.R. (Third E. Ognibene. Gallin J.I., editor. Academic Press; Boston: 2012. Chapter 50 - harnessing information using genomic platforms; pp. 727–744. [DOI] [Google Scholar]
- 32.Jbara A., Saidi I., Ishtayah M., Ghanim M., Al-Othman N., Rabayaa M. Familial versus sporadic multiple sclerosis in Palestine: a retrospective cross-sectional pilot study, Palestinian Medical and Pharmaceutical. Journal (Pal. Med. Pharm. J.) 2021;8(1):2. [Google Scholar]
- 33.Golden L.C., Voskuhl R. The importance of studying sex differences in disease: the example of multiple sclerosis. J. Neurosci. Res. 2017;95:633–643. doi: 10.1002/jnr.23955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalincik T., Vivek V., Jokubaitis V., Lechner-Scott J., Trojano M., Izquierdo G., Lugaresi A., Grand'Maison F., Hupperts R., Oreja-Guevara C. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136:3609–3617. doi: 10.1093/brain/awt281. [DOI] [PubMed] [Google Scholar]
- 35.Confavreux C., Vukusic S., Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 36.Koch M., Kingwell E., Rieckmann P., Tremlett H. The natural history of secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2010;81:1039–1043. doi: 10.1136/jnnp.2010.208173. [DOI] [PubMed] [Google Scholar]
- 37.Ghadiri F., Sahraian M.A., Razazian N., Ashtari F., Poursadeghfard M., Nabavi S.M., Navardi S., Baghbanian S.M., Shaygannejad V., Harirchian M.H., Beladimoghadam N., Majdinasab N., Hosseini S., Azimi A., Kamali H., Sharifipour E., Hosseini Nejad Mir N., Bayati A., Nahayati M.A., Heidari H., Mozhdehipanah H., Ghalyanchi Langroodi H., Jalali N., Ayoubi S., Asadollahzadeh E., Ebadi Z., Eskandarieh S., Naser Moghadasi A. Late-onset multiple sclerosis in Iran: a report on demographic and disease characteristics. Mult Scler Relat Disord. 2023;70 doi: 10.1016/j.msard.2022.104493. [DOI] [PubMed] [Google Scholar]
- 38.McKay K.A., Hillert J., Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. 2019;92:e2764–e2773. doi: 10.1212/WNL.0000000000007647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui A.F., Alsabaani A.A., Abouelyazid A.Y., Wassel Y.I. Risk factors of multiple sclerosis in Aseer region, Kingdom of Saudi Arabia A case-control study. Neurosciences Journal. 2021;26:69–76. doi: 10.17712/nsj.2021.1.20200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadovnick A.D., Yee I.M.L., Ebers G.C., Group C.C.S. Recurrence risks to sibs of MS index cases: impact of consanguineous matings. Neurology. 2001;56:784–785. doi: 10.1212/wnl.56.6.784. [DOI] [PubMed] [Google Scholar]
- 41.Baghizadeh S., Sahraian M.A., Beladimoghadam N. Clinical and demographic factors affecting disease severity in patients with multiple sclerosis, Iran. J. Neurol. 2013;12(1):1. [PMC free article] [PubMed] [Google Scholar]
- 42.Ceccarelli A., Mifsud V.A., Dogar A. Demographic and clinical characteristics of familial and sporadic multiple sclerosis: a single center exploratory study from Abu Dhabi. J. Clin. Neurosci. 2020;76:145–147. doi: 10.1016/j.jocn.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Everest E., Ahangari M., Uygunoglu U., Tutuncu M., Bulbul A., Saip S., Duman T., Sezerman U., Reich D.S., Riley B.P. Investigating the role of common and rare variants in multiplex multiple sclerosis families reveals an increased burden of common risk variation. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-21484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soleimani A., Ezabadi S.G., Möhn N., Esfandabadi Z.M., Khosravizadeh Z., Skripuletz T., Azimzadeh M. Influence of hormones in multiple sclerosis: focus on the most important hormones. Metab. Brain Dis. 2023;38:739–747. doi: 10.1007/s11011-022-01138-7. [DOI] [PubMed] [Google Scholar]
- 45.Maroufi H., Mortazavi S.H., Sahraian M.A., Eskandarieh S. Environmental risk factors of multiple sclerosis in the Middle East and North Africa region: a systematic review. Curr J Neurol. 2021;20:166. doi: 10.18502/cjn.v20i3.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortazavi S.H., Moghadasi A.N., Almasi-Hashiani A., Sahraian M.A., Goudarzi H., Eskandarieh S. Waterpipe and cigarette smoking and drug and alcohol consumption, and the risk of primary progressive multiple sclerosis: a population-based case-control study. Curr J Neurol. 2023;22:72. doi: 10.18502/cjn.v22i2.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alsharie A.M., Rafiee F., Rezaeimanesh N., Moghadasi A.N., Sahraian M.A., Eskandarieh S. Stressful life events and the risk of primary progressive multiple sclerosis: a population-based case-control study. Mult Scler Relat Disord. 2021;51 doi: 10.1016/j.msard.2021.102937. [DOI] [PubMed] [Google Scholar]
- 48.Esposito F., Guaschino C., Sorosina M., Clarelli F., Mascia E., Santoro S., Pagnesi M., Radaelli M., Colombo B., Moiola L. Impact of MS genetic loci on familial aggregation, clinical phenotype, and disease prediction. Neurology-Neuroimmunology Neuroinflammation. 2015;2 doi: 10.1212/NXI.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boiko A., Vorobeychik G., Paty D., Devonshire V., Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59:1006–1010. doi: 10.1212/wnl.59.7.1006. [DOI] [PubMed] [Google Scholar]
- 50.Simone I.L., Carrara D., Tortorella C., Liguori M., Lepore V., Pellegrini F., Bellacosa A., Ceccarelli A., Pavone I., Livrea P. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology. 2002;59:1922–1928. doi: 10.1212/01.wnl.0000036907.37650.8e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and analyzed during the study are available from the corresponding author on reasonable request. Moreover, the entire data of the study has been sent to the journal in Excel file format and if needed, it will be provided by contacting the website https://nmsri.ir/or by email to corresponding Author (sh_eskandaeieh@yahoo.com).
Name of the repository: Sharareh Eskandarieh.