Summary
Oyster reefs are hotspots of denitrification mediated removal of dissolved nitrogen (N), however, information on their denitrifier microbiota is scarce. Furthermore, in oyster aquaculture, triploids are often preferred over diploids, yet again, microbiome differences between oyster ploidies are unknown. To address these knowledge gaps, farmed diploid and triploid oysters were collected over an annual growth cycle and analyzed using shotgun metagenomics and quantitative microbial elemental cycling (QMEC) techniques. Regardless of ploidy, Psychrobacter genus was abundant, with positive correlations found for genes of central metabolism, DNA metabolism, and carbohydrate metabolism. MAGs (metagenome-assembled genomes) yielded multiple Psychrobacter genomes harboring norB, narH, narI, and nirK denitrification genes, indicating their functional relevance within the eastern oysters. QMEC analysis indicated the predominance of carbon (C) and nitrogen (N) cycling genes, with no discernable patterns between ploidies. Among the N-cycling genes, the nosZII clade was overrepresented, suggesting its role in the eastern oyster’s N removal processes.
Subject areas: aquatic biology, aquatic science, genomics, invertebrate aquaculture, microbiology, microbiome, zoology
Graphical abstract

Highlights
-
•
Psychrobacter genus predominated in farmed diploid and triploid eastern oysters
-
•
Reconstructed MAGs of Psychrobacter spp. contained several denitrification genes
-
•
QMEC analysis indicated predominance of carbon (C) and nitrogen (N) cycling genes
-
•
The nosZII denitrifier clade was overrepresented regardless of ploidy and seasons
Aquatic biology; Aquatic science; Genomics; Invertebrate aquaculture; Microbiology; Microbiome; Zoology.
Introduction
Oysters are consumed widely on a global scale due to their unique taste as well as nutritional value, which is high in proteins, polyunsaturated fatty acids (PUFA), vitamins, and minerals.1,2 According to estimates from 2020, U.S. oyster landings totaled 9 million kg,2,3,4 with an annual value of almost $187.2 million USD.3,4,5 Crassostrea virginica, the eastern oyster or the American oyster, accounts for as much as 75% of all commercialized oysters in the United States, with the coastal regions of Florida, Texas, Louisiana, Mississippi, and Alabama, contributing to more than 60% of the total production.5
Oysters also provide ecosystem services, including maintaining water quality and sustaining estuarine habitats6 and thus, are recognized as keystone species across North American coasts from Florida to Maine. In fact, a single adult oyster can filter as much as 50 gallons of estuarine water per day,7 which is a significant bioextractive mechanism resulting in a net positive impact on water quality by way of removing particulate material and nutrients, especially the microbially mediated removal of nitrogen (N), thus mitigating algal blooms and fish kills. In this regard, oyster aquaculture has been recognized as a long-term tool to mitigate coastal eutrophication problems.8,9,10
Despite the significance of oysters as stated above, their populations continue to decline with up to 85% of oyster habitats decimated globally, as a function of overharvesting, habitat fragmentation, decreased water quality, shifts in estuarine water salinity, and diseases.11,12 According to Kirby,13 oyster fisheries expanded and collapsed in a linear sequence along easter North America, western North America, and eastern Australia. To address this, oyster aquaculture is being used to meet the continued oyster demand, especially by applying newer genomics techniques, such as inducing polyploidy.14 Note that triploid oysters grow faster and produce higher yields relative to diploids due to partial sterility, higher heterozygosity, and different energy allocations for growth and gametogenesis,15 and can be marketable year-round.16 Moreover, triploid oysters appear to be less sensitive to environmental changes and stressors. According to Qin et al. 2019, the survival rate of triploid oysters was higher than the diploid oysters in warmer months, suggesting that the triploid oysters are more stable with environmental variations. Moreover, triploid oysters have a lower mortality rate in the face of stressors leading to mortality outbreak, including physiological stress associated with gonadal maturation, pathogens, pollutants, aquaculture practices, and elevated temperature.17 Given these advantages, triploid oysters account for most of the commercially produced oysters in the US.
Regardless of ploidy, filter feeding behavior fosters the colonization of the oyster’s nutrient-rich mucosa and digestive organs with a plethora of diverse estuarine microbiota-which are either permanently associated with the bivalve oyster, referred to as the autochthonous bacterial communities,18,19,20 or can be transient, called as the allochthonous bacteria-communities that are ingested through filter feeding and simply making their way through the bivalves gills and digestive organs. The autochthonous “core” microbiota can forge a mutually beneficial symbiotic relationship with their bivalve host organism; however, a universal “core” pertaining to host-microbe interactions remains unclear.21 Furthermore, it also remains to be known whether the triploid oysters, due to their larger size, filter a higher biomass of bacteria from the water column and accumulate larger concentrations and/or diversity of beneficial or pathogenic mortality inducing bacteria, thus represented with a different “core” microbiome compared to the diploids. To this end, De Deckar et al.22 reported a higher susceptibility of triploid oysters to the pathogenic Vibrio bacteria relative to their diploid counterparts.
It was Colwell and Liston that first suggested the existence of a defined commensal flora in oysters dating back to 1960.23 Zurel et al.24 have shown a conserved seasonal association between the Chama-associated oceanospirillales group (CAOG) of bacteria with oysters, likely representing a symbiotic association. Trabal et al.25 reported symbiotic host-bacteria relationships during different growth phases of two oyster species- Crassostrea gigas and Crassostrea corteziensis. Such symbionts may assist in the digestion processes, as has been demonstrated in the larvae of Crassostrea gigas26 and may also supply the bivalve host with vitamins and amino acids that serve as growth factors-as shown in the Pacific vesicomyid clam- Calyptogena magnifica.27 Moreover, certain symbiotic bacteria can even protect their host from pathogens by either producing antimicrobial agents, or by growing in high densities that prevents colonization by other strains.28 More recently, Sakowski et al.29 discovered that up to 33% of the microbiota from the extrapallial fluid, responsible for shell formation, were autochthonous to C. virginica. Interestingly, the extrapallial fluid-associated microbiota were reported to be rich in functions related to dissimilatory nitrate reduction, nitrogen fixation, nitrification, and sulfite reduction. Banker and Vermeij30 reported that sulfate reduction bacteria (SRB) play significant roles in calcification, but they may not be the only bacteria involved in this process. Moreover, the sulfate and nitrate reduction were proposed to have a synergistic effect on calcium carbonate precipitation and thus likely impacting shell formation.29 It was also previously shown by Braissant et al.31 that SRBs can produce exopolymeric substances (EPS) which can serve as a site for CaCO3 precipitation, and that the calcifying ability can be enhanced by an increase in alkalinity due to the reduction in sulfate ions. Furthermore, most calcifying bacteria are also actively involved in nitrate reduction.32 In this regard, beneficial oyster microbiota likely enhances their host health, thus promoting growth and longevity. To further tease out the transient (allochthonous) oyster microbiota from the resident ones, a flexibility score (FS) was recently developed, which showed that the gill tissues harbor a diverse range of resident bacteria.18
The major bacterial phyla of the eastern oysters are as follows: Proteobacteria, Cyanobacteria, Bacteroidia, Mollicutes, Bacteroidetes, Tenericutes and Firmicutes.29,33,34,35,36,37,38,39,40,41 A consensus on the genus level “core” and possibly the resident or autochthonous microbiome of eastern oysters is lacking, but the following bacteria, in addition to Vibrio species, have been identified to be dominant groups: Mycoplasma, Pseudoalteromonas, Burkholderia, Bacteroides, Lactobacillis, Acetobacter, Allobaculum, Ruminococcus, Nocardia, and Oceanospirillales.18,42 It is noteworthy that the bacterial communities within the eastern oyster and its specific niches, such as the mantle fluid, gills, digestive system, gut, extrapallial fluid, and hemolymph have been found to be distinctly different than the surrounding water or sediment communities.18,20,29,34,37 In one previous study conducted on the wild type and farmed eastern oysters, we found Cyanobacteria accounting for 50–75% of the total microbial communities, based on 16S rRNA amplicon metagenomics.43 In another microcosm-based study related to the Deepwater Horizon oil spill, Pseudomonas spp. were found to be the dominant oyster-associated bacteria.43,44,45,46 More recently, our metagenomics survey of the eastern oysters indicated the dominance of Lactobacillus, Burkholderia, Bradyrhizobium, Afipia, and Delfitia bacterial groups with lower representations of Psychrobacter spp. Additionally, Mycoplasma, which has been previously suggested as one of the autochthonous bacteria in eastern oysters, was also identified (unpublished data).
One major ecosystem service associated with the oysters relates to enhancing water quality by the utilization of the dissolved nitrogen by both oysters and their microbial communities. Specifically, N assimilated into oyster shells and tissues is removed from the estuarine systems via harvesting or burial.47 Oyster feces and pseudofeces contribute organic material to sediments, potentially enhancing dentrification processes. Denitrification is a four-step reduction of nitrate (NO3−) to N2 via nitrite (NO2-), NO and N2O and eventually to nitrogen gas.29,48 At the genetic level, denitrifying bacteria harbor genes in operonic clusters that encode functions for the reduction of nitrate (narG/napA), nitrite (nirS/nirK), nitric oxide (norB/norC), and nitrous oxide (nosZ).49
The above stated background led us to the following overarching question - what are those suits of environmentally relevant biogeochemical functions, such as nitrogen cycling, that are performed by the eastern-oyster associated microbiome communities? To address this, we applied shallow shotgun sequencing (SSS) based metagenomics, which is a reliable method to understand both taxonomic and functional gene shifts in complex environmental samples.50 To augment findings obtained from metagenomics, we applied the recently developed method of “Quantitative Microbial Elemental Cycling (QMEC)”, a high-throughput quantitative PCR (HT-qPCR) technique for estimating microbial gene copy numbers engaged in the critical steps of carbon (C), nitrogen (N), phosphorus (P), and sulfur (S) cycling as well as methane metabolism.51 Given that the commercially grown eastern oysters are triploid variants that originated from the wild-type diploids, and that their microbially mediated functions likely shift with time, both diploid and triploid eastern oysters were collected monthly over an annual growth period to: 1) conduct shotgun metagenomics and identify native autochthonous microbial groups; 2) evaluate the microbial gene functional shifts occurring within the microbiome; and 3) evaluate microbially mediated CNPS biogeochemical cycling by applying the QMEC method.
Results and discussion
Characterization of sampled oysters and quality control on shotgun metagenome sequences
At the start of the growing period, diploid oysters ranged from 21 to 35 mm in height and 1–3 g in weight; triploid oyster heights were 12–27 mm and weights were 0.5–1 g. After one year of growth, diploids were 85–95 mm in height and 54–91 g in weight, and triploids were 71–105 mm in height and 75–110 g in weight (cultured oysters are considered harvestable when height >76 mm).
For the metagenomic analysis, 10 diploid and 10 triploid oysters were collected at each time point and pooled separately to generate duplicates A and B. Shallow shotgun sequences (SSS) obtained from n = 6 each of diploid and triploid oysters, collected in duplicates (labeled as A or B), yielded over 113.2 million raw reads and 98.3 quality-filtered reads (Figure SI-1). Around 34.3 (34.8%) and 0.5 million (0.51%) of the quality-filtered reads were assigned to the oyster host (Crassostrea virginica genome v3.0 (Accession number GCF_002022765.2) and the human genome (Genome Reference Consortium Human ref. 37), which were removed prior to further processing.
One goal of this study was to examine the eastern oyster microbiome over an annual growth cycle with oysters collected every month, rather than using random grab samples, which are appropriate only to provide a quick snapshot of bacterial communities but are not sensitive in the assessment of the eastern oyster’s autochthonous microbiome communities. Once the “core” microbiome of the eastern oyster is better understood, specific studies can be conducted to identify those microbial groups that are specific to beneficial functions such as maintenance of oyster growth, overall health, and productivity. To this end, regardless of seasons or ploidy, the eastern oysters were found to be predominantly colonized by bacteria, as shown by 93.4% of sequences that were annotated to the bacterial domain, followed by eukaryotes (5.64%), archaea (0.76%) and viruses (0.25%), respectively. Among bacteria, a total of 36 phyla were identified across all the samples with the top 5 phyla represented by Pseudomonadota (58%; former Proteobacteria); Bacillota (11%; former Firmicutes; Bacteroidota (7%; former Bacteroidetes); Cyanobacteria (6%) and Actinomycetota (3%; former Actinobacteria), respectively. Pseudomonadota are metabolically and ecologically diverse and are commonly found in many environments, including animal hosts; some proteobacterial groups are also pathogenic. These findings are in line with other previous studies, which show the dominance of proteobacterial lineages in oysters.29,36,40,43,52,53,54 Pseudomonadota also likely play important functional roles in the eastern oysters, such as nitrogen cycling.9,38,48 Furthermore, proteobacterial members can also metabolize cellulose and agar-major components of the oyster’s phytoplanktonic diet, and hence colonize bivalve gastrointestinal tracts,25 providing nutrients and bioactive growth factors to their oyster hosts.19,24,39,55,56,57
Among proteobacterial members, the gamma-proteobacterial class was the most abundant, as also shown by,36 followed by alpha- and delta proteobacteria. These proteobacterial groups are typically found in high numbers in marine environments, and are likely concentrated within the oysters due to their filter-feeding activity.25,36,58,59 Also, gamma-proteobacteria have been frequently detected in both healthy or diseased shellfish including oysters28 and as endosymbionts from the gills or gonads of different bivalve species.60,61 Therefore, gamma-proteobacteria are likely to be autochthonous to oysters,62 given their ubiquity with oysters and their surrounding environment.29,62
As shown in Figure 1A, the top 3 gamma-proteobacterial genera included Psychrobacter (34%); Pseudomonas (3.2%); and Pseudoalteromonas (2.7%). Besides these gamma-proteobacteria, cyanobacterial Synechococcus spp., from class Cyanophyceae was the second most abundant bacteria (3.6%). These bacterial genera likely constitute the “core” microbiomes in the eastern oysters analyzed in this study, using the MicrobiomeAnalyst pipeline, at a threshold of 10% sample prevalence and 0.01% relative abundance (Figure 1B). The top 10 bacterial “core” genera were Psychrobacter, Synechococcus, Pseudomonas, Clostridium, Pseudoalteromonas, Mycoplasma, Bacillus, Staphylococcus, Vibrio and Cyanobium, (Figure 1B). Psychrobacter represented 6 out of the dominant 10 species found in these eastern oysters, including P. sp. P11F6; P. sp. 28M-43; P. sp. P11G3 (data not shown).
Figure 1.
Taxonomy and dendogram analysis of the eastern oyster’s microbiome using shallow shotgun metagenomics analysis
Shown are the bacterial genera-level taxonomic groups identified in diploid eastern oyster vs. triploids, sampled in duplicates (each time point is labeled as A or B), over an annual cycle. Specifically, shown are (A), metagenome sequences grouped as originating from either diploid or triploid oysters, regardless of sampled time; (B) core microbiomes identified in the eastern oyster, regardless of ploidy or sampled seasons; (C) dendrogram analysis conducted on bacterial genus-level data obtained from diploid vs. triploid populations with group 1 containing diploids and group 2 containing triploids; (D) dendrogram analysis conducted on samples binned as a function of sampled time period and ploidy was ignored; (E) dendrogram analysis conducted on samples grouped as a function of collection time period: group 1 (December 2016); group 2 (January, 2017); group 3 (February, 2017); group 4 (April, 2017); group 5 (May, 2017); group 6 (July, 2017); group 7 (August, 2017), respectively.
Psychrobacter are known to typically thrive in colder marine ecosystems (Bowman, 2006), such as glacial ice,63 sea ice,64 permafrost,65 as well as found associated with deep sea animal host species e.g., sponge, porpoise, crab, krill, seal, and cyanobacterial mats.66,67,68,69 However, warmer niches have also been found to be colonized by Psychrobacter spp. including humans, lambs, and fecal materials from pigeons.66,68 In a recent genomic and phenotypic comparison of an extensive cohort of Psychrobacter spp. Welter et al.66 showed that the Psychrobacter can be delineated into two growth profiles: the “flexible ecotype” (FE) group that could grow in temperatures between 4°C and 37°C, and the “restricted ecotype” (RE) group that grew between 4°C and 25°C temperature. The dominant Psychrobacter spp. in this study are closely related to those isolated from marine Arctic environments, indicating that the eastern oyster surveyed in this study likely harbor the RE Psychrobacter ecotypes. Proliferation of psychrotrophic bacteria that create conditions for seafood spoilage by hydrolysis of proteins and lipids in oyster tissues, producing malodorous compounds and toxic byproducts,70 can be a cause for concern. Beneficial probiotic properties of Psychrobacter have also been reported in some studies, such as enhancing growth and survival of shrimp larvae,71 and by enhancing autochthonous microbiomes and protection from bacterial pathogens (e.g., Vibrio sp.) in Atlantic cod.72,73,74 Therefore, it may be possible that Psychrobacter spp. are beneficial to eastern oysters in a similar manner. Interestingly, it has been reported that oyster processing, including refrigeration, storage, and depuration at 10°C, caused a shift in the predominant Vibrio species that are characteristic of freshly harvested oysters, toward psychrotrophic bacteria consisting of Pseudomonas spp., Aeromonas spp., Shewanella spp., and Psychrobacter spp..75 Therefore, further research on the dominance of Psychrobacter species in eastern oysters should be conducted to evaluate whether these species provide benefit(s) to their oyster host or represent an underexplored agent of oyster spoilage processes.
In this regard, some species of Psychrobacter, mainly P. sanguinis, P. phenylpyruvicus, P. faecalis, and P. pulmonis are known to cause opportunistic infections in mammals,76,77 which needs to be further investigated. Human infections from consuming raw or undercooked shellfish containing a higher abundance of Psychrobacter species has not been demonstrated, nor has the predominance of Psychrobacter been shown for eastern oysters prior to this work. Given that the Psychrobacter identified in the eastern oysters in this study appear mainly to be the RE ecotypes, it is likely that they can mount mammalian infections that require warmer temperatures, however, this aspect remains unclear. As stated before, genomic analysis has recently suggested that Psychrobacter ecotypes likely evolved from a mesophilic common ancestor, which presents a unique opportunity to delineate the evolutionary mechanisms of the eastern oyster associated Psychrobacter species as a function of their estuarine habitats.
When oysters were compared over different time points, a decline in bacterial abundances was observed from April to August, representing the warmer season. For example, proteobacterial abundances were predominant at an average of 97% between December 2016 to February 2017) but declined to 36% from April to August 2017. Along these lines, it was recently shown for the Australian Pacific oysters (Crassostrea gigas) that temperature strongly correlated to their microbiomes and that microbial diversity increased in winter relative to summer timepoint.43 Besides, temperature is one of the factors that influences the survival rate of oysters with high temperature leading to decrease thermal tolerance, reduced growth, survival, and filtration rates.78 Moreover, elevated temperature leads to oyster spawning and spawned oysters are highly vulnerable to temperature stress.79 Other stressors such as increased ocean acidification and salinity equally impact the strength, reproduction, growth, and oysters’ mortality78). However, it is highly likely that low temperature favored the observed increase of the psychrotrophic Psychrobacter species in this study (Figure 1B). Interestingly, dendrogram analysis conducted on bacterial metagenomes revealed less affiliation with regards to ploidy; conversely, seasonal changes appeared to have a stronger influence in structuring the oyster microbiome (Figures 1C–1E). Specifically, when metagenomes were grouped as originating from either diploid or triploid oysters (Figure 1C), a total of 6 clusters representing different time periods were observed. The general trend was that the metagenomes from the colder months- December, January, and February, clustered together, as did those from the warmer months of April, May, July and August. When sequences were grouped as a function of sampled time and ploidy was ignored, 5 clusters emerged, and again, the season exerted a stronger influence in driving the bacterial communities, relative to ploidy (Figure 1D). As a third option, when samples were grouped as a function of the sampled time, 6 clusters were observed which clearly shows seasonal impacts to be in the major factor structuring the oyster-associated microbiomes, when compared with ploidy (Figure 1E). This finding is noteworthy because most studies on the eastern oysters have been conducted using samples collected at random times during the year,35,37,38,39,40 and even those that were samples on a spatiotemporal basis focused on pathogen dynamics such as Vibrio and their occurrence in the eastern oysters.
The other two main bacterial genera identified were Synechococcus and Pseudomonas spp. (Figure 1A and 1B). Synechococcus spp. are commonly found in estuarine waters and have also been reported from the digestive gland C. gigas, as well as connective tissue, mantle, and gonad of oysters.80 Our previous studies along with several others have demonstrated pseudomonads to be a major bacterial group in the oysters.44,45,46 Notably, some pseudomonads can be opportunistic pathogens and have been found in spoiled oysters.1,53,81 To provide further insights into the metabolic traits of Pseudomonas species from the eastern oysters, we conducted whole genome sequencing (WGS), comparative genomic analysis and BIOLOG-based physiological assessments on isolates obtained from the eastern oysters.82 Interestingly, strains that were isolated from the mantle fluid [(P. stutzeri (MF28)] and tissues [(P. alcaligenes (OT69)], were metabolically versatile relative to those isolates obtained from the overlaying oyster bed water column [(P. aeruginosa (WC55)]. Specifically, P. aeruginosa (WC55) preferred polymers over other carbon sources, while P. stutzeri (MF28) responded better to carbohydrates, while P. alcaligenes (OT69) showed similar metabolic responses to both polymers and carbohydrates, respectively. Additional studies are required to ascertain whether the eastern oyster associated Pseudomonas spp. provide a beneficial service to the host, such as the bioremediation of marine pollutants and providing growth promoting vitamins or mediate spoilage via their pathogenic functions.
Though not the main purpose of this study, we also analyzed the archaeal members in the eastern oyster shotgun metagenomes. Archaea are a small fraction of the microbial communities in oysters,18 if not present at all.36 Similarly in our study, archaea were represented at only 0.76% in the taxonomic annotation; with dominance of Euryarchaeota (41%) and Thaumarchaeota (24%), respectively (data not shown). The oyster-associated archaeal genera mainly belong to Methanosarcina (12%) and Nitrosopumilus (10%). Based on the underrepresentation of archaeal communities, it is likely that conditions for the proliferation of this group, such as anaerobiosis, were likely not present in the oysters analyzed in this study.
Alpha and beta-diversity analysis
The bacterial genus level data were statistically evaluated using Shannon’s index for differences potentially driven by seasons and ploidy using alpha and beta diversity analysis. Samples were assigned to 7 different groups based on the collection dates as stated earlier, and some low sequence samples were removed from the analysis. Regardless, this represents a good snapshot of the oyster-associated microbiomes over an annual cycle. Shannon’s diversity, shown in Figure 2A, was significantly higher at later sampling timepoints but bacterial diversity in diploids and triploids was not different, possibly due to low replication (although p-value for genotypes was 0.056). Alpha diversity analysis revealed that warmer months had increased diversity relative to colder months (Figure 2B). It is widely accepted that warmer temperatures increase marine bacterial abundances,83 which goes well with our observation of enhanced bacterial biomass in the oyster samples collected in the warmer season.
Figure 2.
Microbiome diversity analysis of the eastern oyster, across ploidy and growth time points
Diversity analysis for each month is represented by a unique color identified in diploid eastern oyster vs. triploid populations, sampled in duplicates (each time point is labeled as A or B), over an annual cycle. Shown are (A), Shannon’s diversity analysis, which was significantly higher at later sampling timepoints. No differences between genotypes (diploids and triploids) were found, probably due to low replication, though p-value for genotypes was 0.056; (B) alpha diversity calculated from taxonomic data; warmer months were higher in diversity relative to the colder months.
Beta diversity, which is the diversity between different samples and significance testing was also conducted as shown in Figure 3. Similar to the alpha diversity analysis, beta diversity also revealed that the microbiomes were more similar in oysters sampled in the colder months of December-February than those sampled in warmer months (Figure 3A and 3B), as has also been recently shown for Australian oysters and mussels.34 Microbiomes did not differ between diploid and triploid oysters despite the relatively larger size of the triploid oysters, as stated earlier Note that only few studies are available to compare how ploidy impacts oysters, mostly on mortality, yields, growth rates, and susceptibility to diseases.22,84 Studies on other organisms, especially fish,85 found that triploids contained significantly higher cultivable gut microbiota levels, especially Pseudomonas spp., Pectobacterium carotovorum, Psychrobacter spp., Bacillus spp., and Vibrio spp.
Figure 3.
Ordination of bacterial diversity at the genus level, across ploidy and growth time points
Shown are (A), non-metric multidimensional scaling (NMDS) plot of the beta diversity using Bray-Curtis dissimilarities; (B) PCoA PERMANOVA plot of the beta diversity using Bray-Curtis dissimilarities.
Gene functional analysis of the eastern-oyster microbiomes over an annual cycle
Functional genes annotated from the oyster shotgun metagenome sequences were organized into the SEED hierarchy (Figure 4). At broad levels (levels 1 and 2), functional profiles were very similar; smaller differences were found at lower levels (data not shown). Some differences were apparent at subsystems related to bacterial respiration and they tended to increase in later timepoints; a trend similar to the taxonomic assessment, where the latter warmer timepoints revealed a higher abundance and diversity of oyster associated microbiota. The major metabolic functions identified across oyster samples belonged to respiration and metabolism of carbohydrates, RNA, and protein.
Figure 4.
Bacterial functional genes annotated from the eastern oyster metagenome sequences
Shown are functional gene annotations at SEED functional hierarchy levels (levels 1 and 2), which were very similar. Smaller differences were found at lower levels. Some differences were apparent at subsystems related to bacterial respiration, they tended to increase in later timepoints.
We also conducted a permutational multivariate analysis to test for differences in taxonomic composition and gene function over an annual cycle in diploid and triploid oysters (Table SI-1). This showed that taxonomic profiles changed significantly over time, with little or no significant differences observed between genotypes (ploidy). However, post-hoc tests did not find significant differences between timepoints, which can however be due to the low number of replicates. Because we did not find differences between genotypes, we pooled diploids and triploids for the general model; differences among sampling dates explained around 56% of the variation of taxonomic profiles; thus, ploidy was an insignificant factor relative to time-points. Similar to the taxonomic data, no significant differences were found for the gene functional data over the annual time-period in the sampled oysters. Perhaps if a sampling design is followed to tease out the oyster-associated autochthonous “core” microbiomes relative to the allochthonous microbiota, we would have observed a similar finding to Sakowski et al.29 which showed significant enrichment (p < 0.05) in KEGG based gene functions within the autochthonous microbiomes of the eastern oysters. It was interesting to note that Sakowski et al.29 found genes associated with N-cycling, such as dissimilatory nitrate reduction, nitrogen fixation, and nitrification pathways in the autochthonous relative to the allochthonous bacterial community.
Differential abundance of taxa and gene functions
Differential abundance testing of the bacterial taxa (top 20) and genes functions identified at the subsystem level 3 were investigated as a function of two factors-sampling timepoint and genotype (ploidy). As shown in Figures 5A and 5B, A general linear model showed several bacterial groups were significantly different when sampling began during the colder months, compared to the latter warmer months, including Psychrobacter and Synechococcus species-the two most abundant genera identified in this study (Figure 1A and 1B). Such seasonal trends in the Pacific oysters and mussels gut microbiomes have recently been published by Akter et al.29 Interestingly, Psychrobacter species were differentially abundant in both diploids as well as triploid oysters, when analyzed separately (Figure 5B and 5C). Differential analysis of gene function data revealed several metabolic functions declining at latter timepoints (Figure 5D), which is counterintuitive because the bacterial taxonomic abundances increased in the mid and latter timepoints, as suggested by diversity analysis (Figure 2B). Only four gene functions were differentially abundant in diploid oysters (Figure 5E); conversely, this was not the case in triploids, where several gene abundances were differentially abundant (Figure 5F). It is unclear as to why more taxa and gene functions were differentially abundant in the triploids relative to the diploid oysters.
Figure 5.
Differentially abundant taxa (genus level) and gene functions (subsystem level 3) identified from the eastern oyster metagenomes across ploidy and growth time points
Shown are (A) differentially abundant taxa using a general linear model; (B) differentially abundant taxa in diploid oysters; (C) differentially abundant taxa in triploid oysters; (D) differentially abundant gene functions using a general linear model; (E) differentially abundant gene functions in diploid oysters; (C) differentially abundant gene functions in triploid oysters, respectively.
Statistical correlations between bacterial taxa and gene functions
The top 10 bacterial taxa at the genus level and gene functions from SEED subsystem level 1 were analyzed statistically (Figure 6). Dendrogram analysis confirmed that eastern oyster microbiomes clustered more tightly as a function of sampling date and not from differences in ploidy (Figure 6A). Seven clusters were observed in the dendrogram plot with one outlier (sample from Dec 05, 2016, diploids) and ploidy seems insignificant relative to the sampling date for bacterial community structure in this study. The Non-metric Multi-Dimensional Scaling plots (NMDS) (Figure 6B) at a similarity at 75% and 95% revealed interesting correlations between bacterial top 10 genera and SEED subsystem level 1 features. Specifically, at the 95% similarity threshold, Psychrobacter, a likely member of the “core” microbiome regardless of date or ploidy status, correlated strongly to gene functions of central metabolism, DNA metabolism, and carbohydrates (Figure 6B). Other taxa that were within the 95% similarity index threshold were Moraxella, Pseudoalteromonas, Brochothrix, and Halomonas, and these correlated to gene functions of cofactors, vitamins, prosthetic groups, pigments; cell division and cell cycle; cell wall and capsule; amino acids and derivatives; dormancy and sporulation, and clustering-based subsystems. The other taxa that correlated at the 95% threshold were Vibrio and Acinetobacter with no affiliation to any of the top 10 gene functions. Pseudomonas, Shewanella, and Flavobacterium were not significantly correlated to other taxa or gene functions (Figure 6B). At a threshold of 75% similarity, all the above stated top 10 bacterial genera and gene functions were correlated. Based on this, it is likely that Psychrobacter species perform significant functional roles in the eastern oyster and thus, further studies to assess the role of this genus within the broader context of the oyster microbiome and how it intersects with oyster health and productivity are thus suggested from this study.
Figure 6.
Statistical correlations between the top 10 bacteria (genus level) and SEED subsystems (level 1) from the eastern oyster metagenomes across ploidy and growth time points
Shown plotted as a dendrogram analysis are (A), or a Non-metric Multi-Dimensional Scaling plot (NMDS) (B), respectively, where similarity at 75% and 95% is shown.
Metagenome assembly, phylogenetic, and average nucleotide identity analysis
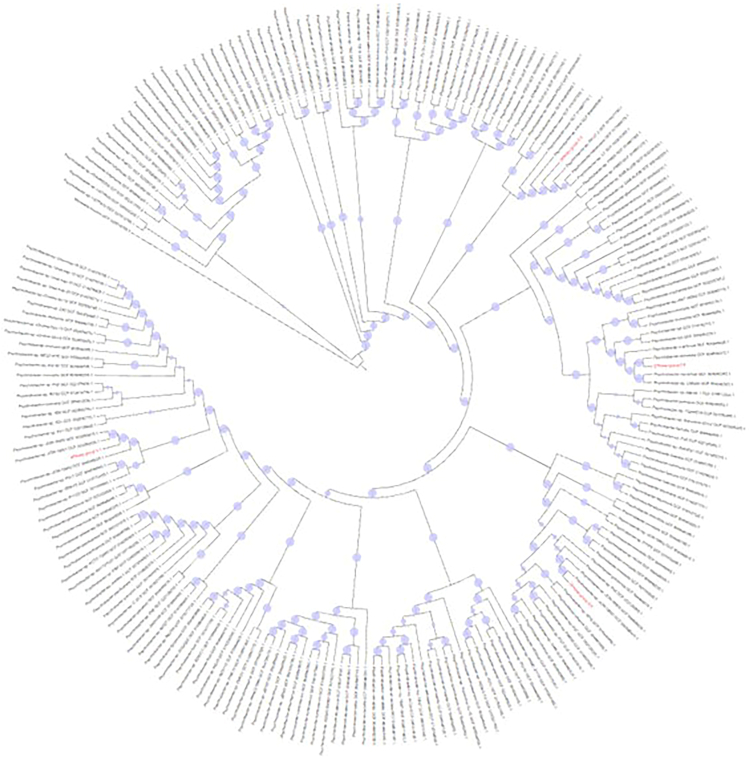
To better characterize the dominant species, metagenome assembly was performed, followed by phylogenetic analysis of select MAGs (metagenome-assembled genomes). Eleven MAGs were obtained, six of which remained after filtering based on completeness as measured using BUSCO (Table 1). Four of the six were classified as Psychrobacter (Figure 7), one as Pseudoalteromonas and one as belonging to the same family as COTS27, a bacterial symbiont of the crown-of-thorns starfish.86 The MAGs consist of many contigs (M = 591.5, SD = 213.4) and exhibit a modest level of completeness (M = 51.6% SD = 22.2%). The MAG SPAdes-group-0.1.fa displays a higher level of duplicated genes (8.1%), which could reflect the presence of genes from more than one species or issues with the assembly. Phylogenetic analysis of inferred protein sequences was performed to compare the Psychrobacter MAGs to each other and to Psychrobacter RefSeq assemblies available in the NCBI Assembly database. The resulting maximum likelihood tree supports that the four Psychrobacter MAGs are each most closely related to separate Psychrobacter RefSeq assemblies, and not part of a single lineage.
Table 1.
Metagenome-assembled genomes (MAG) reconstructed from the eastern oysters in this study are reported in the table
| MAG | Taxonomy (family; genus) | Contig number | Total length bp | BUSCO % complete (single copy, duplicated, fragmented) |
|---|---|---|---|---|
| SPAdes-group-0.1.fa | Moraxellaceae; Psychrobacter | 893 | 3004744 | 41.2 (22.6, 8.1, 10.5) |
| SPAdes-group-0.4.fa | Moraxellaceae; Psychrobacter | 474 | 1479441 | 38.7 (30.6, 0, 8.1) |
| SPAdes-group-0.5.fa | Moraxellaceae; Psychrobacter | 455 | 1873903 | 47.6 (38.7, 0, 8.9) |
| SPAdes-group-0.7.fa | Alteromonadaceae; Pseudoalteromonas | 745 | 4536590 | 77.4 (75.8, 0.8, 0.8) |
| SPAdes-group-0.8.fa | Moraxellaceae; Psychrobacter | 665 | 1695251 | 25 (16.1, 0, 8.9) |
| SPAdes-group-0.11.fa | COTS27; _ | 317 | 1427112 | 79.9 (70.2, 0, 9.7) |
Metagenome-assembled genomes (MAG) based taxonomy and completeness from the eastern-oyster derived shallow shotgun metagenomic data.
Figure 7.
Maximum likelihood tree of Psychrobacter MAG proteomes obtained from this study
Shown are the Psychrobacter MAG proteomes (red labels) relative to RefSeq Psychrobacter proteomes from NCBI (black labels). Bootstrap support from 100 replicates is indicated using blue circles.
To complement the phylogenetic analysis, whole-genome average nucleotide identity (ANI) was calculated between each Psychrobacter MAGs and all Psychrobacter RefSeq genomes (Table 2). MAGs SPAdes-group-0.4.fa and SPAdes-group-0.5.fa exhibit high ANI (98%) with their respective matches, and the similarity involves most of the MAG sequence (97%). These ANI values are characteristic of intra-species relationships.87 SPAdes-group-0.8.fa also showed high ANI with its top match (97%) but the similarity involved just 71% of the MAG. SPAdes-group-0.1.fa exhibited a lower ANI (88%) with its top match, which is lower than the intra-species level (>95%),87 and the similarity involved 71% of the MAG. Together these results further support the presence of multiple Psychrobacter species within the eastern-oyster microbiome. Additional metagenomic sequencing to provide more complete, higher-quality assemblies should help further resolve the relationships between these potentially novel isolates or species and existing Psychrobacter isolates and help clarify their potential role(s) in the oyster holobiont.
Table 2.
Average nucleotide identities (ANI) matches of top MAGs from this study
| MAG | Highest ANI | Percentage of MAG aligned as orthologous matches | RefSeq organism name | RefSeq assembly accession |
|---|---|---|---|---|
| SPAdes-group-0.1.fa | 88.03 | 70.82 | Psychrobacter sp. KH172YL61 | GCF_007182895.1 |
| SPAdes-group-0.4.fa | 98.32 | 97.22 | Psychrobacter sp. JCM18903 | GCF_904846635.1 |
| SPAdes-group-0.5.fa | 98.45 | 97.08 | Psychrobacter celer | GCF_904845945.1 |
| SPAdes-group-0.8.fa | 96.61 | 70.58 | Psychrobacter maritimus | GCF_904846345.1 |
Top ANI matches for Psychrobacter MAGs reconstructed from the eastern oyster derived shallow shotgun metagenomic data.
Denitrification genes in Psychrobacter metagenome assembled genomes and similar RefSeq genomes
The denitrification process is performed by a diverse range of microorganisms, especially under oxygen depleted conditions, with the complete reduction of NO3− to N2 performed via a four-step process, involving the membrane-bound NO3−reductases (narG) and/or the periplasmic napA genes, the NO2− reductases NirK (copper-containing) or NirS (containing cytochrome cd1) encoded by nirK and nirS; the NO reductases cNor (cytochrome c dependent) or qNor (quinol-dependent) encoded by cnor and qnor; and the N2O reductase NosZ.
Sequence-based identification of denitrification genes in the Pyschrobacter MAGs indicates the presence of norB in SPAdes-group-0.8.fa (Table 3). Given the incomplete nature of the MAGs, the search for denitrification genes was extended to the top ANI match for each MAG, under the assumption that the more complete RefSeq genomes would provide a more comprehensive view of the denitrification gene repertoire. This search of the highly similar genomes revealed the presence of narH and narI for RefSeq genome GCF_904846635.1 (the top ANI match for SPAdes-group-0.4.fa), and the presence of narH, narI, nirK, and norB for RefSeq genome GCF_904846635.1 (the top ANI match for SPAdes-group-0.8.fa). Other denitrification genes, especially for complete denitrification, such as nosZ could not be identified based on the MAG approach. Deeper sequencing depths and coverages will likely enhance our understanding on the metabolic repertoire of Psychrobacter spp., associated with eastern oysters.
Table 3.
Denitrification genes identified in Psychrobacter MAGs obtained from this study are shown in the table
| MAG or RefSeq genome | Number of query proteins | Denitrification genes |
|---|---|---|
| SPAdes-group-0.1.fa | 2452 | – |
| SPAdes-group-0.4.fa | 1133 | – |
| SPAdes-group-0.5.fa | 1482 | – |
| SPAdes-group-0.8.fa | 1429 | norB (WP_201628849.1) |
| GCF_007182895.1 | 2585 | – |
| GCF_904846635.1 | 2815 | narH (WP_055125087.1), narI (WP_045447153.1) |
| GCF_904845945.1 | 2433 | – |
| GCF_904846345.1 | 2565 | narH (WP_201628839.1), narI (WP_201562832.1), nirK (WP_227670896.1), norB (WP_201628849.1) |
Shown are the denitrification genes from the Psychrobacter MAGs reconstructed from the eastern oyster derived shallow shotgun metagenomic data and similar RefSeq genomes.
aThe NCBI RefSeq accession of the top BLAST hit in the RefSeq protein database is provided for norB in SPAdes-group-0.8.fa. For the other genes in this column the NCBI RefSeq accession is provided for the corresponding protein sequence encoded by the RefSeq genome.
Quantitative microbial elemental cycling analysis
When QMEC was used on genomic DNA extracted from homogenized diploid and triploid oysters, a total of 71 genes responsible for CNPS cycling amplified successfully. Note that we did not separate the bacterial biomass from the oyster tissue homogenates, which likely caused PCR inhibition from the overrepresented oyster DNA/mucous and consequently, gene copy abundance/g tissue from only 8 samples covering 3 sampling timepoints yielded usable data (Figures 8A–8D). Regardless, this is the first application of QMEC to understanding biogeochemical cycling in eastern oysters. Relative abundances of CNPS genes were higher in samples collected in December 2016 but did not vary much except in samples collected on April 12, 2017, which seems to be an outlier. PCA conducted on absolute and relative CNPS gene copy numbers revealed differences between diploid and triploid samples but not the sampling time points (Figure 8E and 8F), which was not in line with the taxonomic data-which suggested that season but not ploidy was the stronger of the two factors in structuring oyster microbiomes. However, it may not be prudent to compare shotgun and qPCR data.
Figure 8.
Quantitative microbial element cycling (QMEC) analysis from the eastern oyster over ploidy and time points
Shown are A, relative abundance of carbon (C); B, nitrogen (N), C, phosphorus (P) and sulfur (S) cycling genes using QMEC analysis. Also shown are principal component analysis (PCA) of absolute (E) and relative (F) gene copy numbers that indicated ploidy to have a significant impact relative to sampling time points.
Among the carbon cycling genes, C-fixation was the most abundant process followed by methane production and oxidation (Figure 9A). Carbon fixation has been found to be an enriched gene function in the mucus microbiomes associated with corals88 as well as the microbiome of the Black-Lipped Pearl Oyster.60 Psychrobacter spp. were abundant in the coral mucus study, and it is likely that they are important in carbon cycling processes.88,89 Methane formation (methanogenesis) and oxidation has also been shown to be driven by macrofaunal organisms, such as the bivalve Limecola balthica and the polychaete Marenzelleria arctia.90 Furthermore, symbiotic associations between methane-producing archaeal communities and bivalves have also been previously reported90; these processes were also identified in the tested eastern oysters, albeit at lower levels. Among the tested 22 nitrogen cycling genes, denitrification was the highest followed by ammonification (Figure 9B). Note that denitrification in oysters can contribute significantly in N transformations in pelagic regions,91 as well as in oyster reefs, aquaculture sites, and their sediments.92,93,94 In fact, it is the oyster-associated denitrifier microbiota that contribute to cycling reactive N into N2 gas9,38 via three pathways: (1) through increasing organic matter deposits in sediments, (2) hosting denitrifying bacteria within gills and/or digestive organs and/or (3) facilitating habitat for other filter-feeding macrofaunal communities.9 Organic phosphorus (P) mineralization and sulfur (S) oxidation were the major pathways in P and S biogeochemical cycling processes (Figure 9C and 9D).
Figure 9.
Major biogeochemical pathways identified in the eastern oyster related to C-fixation, organic phosphorus (P) mineralization and sulfur (S) oxidation
Shown are A, carbon (C); B, nitrogen (N), C, phosphorus (P) and D, sulfur (S) cycling relative gene abundances when QMEC analysis was performed on the diploid and triploid eastern oysters.
Among the denitrification genes encoding for nitrite reductase (nirS or nirK) or nitrous oxide reductase (nosZ), nosZII was the most dominant (Figure 10A). Under anaerobic conditions, denitrification occurs as a stepwise reduction of nitrate (NO3-) to either nitrous oxide (N2O) or dinitrogen gas (N2). Complete denitrification takes place via the reduction of nitric oxide (NO) to N2O and then the reduction of N2O to N2 gas. The two microbially mediated enzymes facilitating the denitrification process are nitric oxide reductase (NOR) and nitrous oxide reductase (N2OR). Based on genotypic studies, nosZ gene has been subdivided into clade I (nosZI) or clade II (nosZII),95,96 with the latter dominant in most habitats including eastern oysters. Clade I nosZ gene containing bacteria mainly belong to Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacterial, but clade II nosZ gene has been identified from a variety of bacterial taxa, such as Deltaproteobacteria, Bacteroidetes, and Gemmatimonadetes,95,96,97 respectively.
Figure 10.
Major biogeochemical pathways identified in the eastern oyster related to N-cycling
Shown are copy number of nitrite and nitrate reductase denitrification genes such as (A), nirK, nirS, nosZ; (B) napA, narG, nasA, when QMEC analysis was performed on the diploid and triploid eastern oysters and (C), heatmap of denitrification genes plotted along with the 16S for comparisons.
More recently, it was found that autochthonous “core” microbiomes within eastern oysters were significantly (p < 0.05) enriched with 933 KEGG based gene functions relative to the 700 gene functions associated with the allochthonous oligotypes.29 The autochthonous oligotypes were also significantly enriched for several N-cycling genes, such as for dissimilatory nitrate reduction, nitrogen fixation, and nitrification pathways relative to the allochthonous oligotypes. To this end, Arfken et al., 2017 showed that an increase in denitrification rates correlated positively to an increase in the gene abundances of nosZI, but this trend did not hold for the more dominant nosZII. This suggests that oyster-associated microbes possessing the nosZI genes may be more significant to denitrification processes than those that possess the nosZII genes. However, other reports have shown that nosZII denitrifiers are dominant over the nosZI denitrifiers in a variety of different ecosystems,96,97,98 including the eastern oysters.38 This could be because the nosZII gene renders ecophysiological diversity relative to the nosZI gene,95 suggesting that denitrifier microbiota in oysters are likely far more diverse than previously known. Moreover, in this study, the nasA, narG, and napA genes were also observed (Figure 10B), along with the with dominant narG gene. It is known that the NarG encodes for membrane-bound nitrate reductase,99 which has previously been found to be high in sediments from oyster farms100 and appears to be dominant in eastern oysters, regardless of ploidy. The higher abundances of nosZII and narG, relative to 16S genes in eastern oysters using the QMEC method are shown in Figure 10C.
In conclusion, overall, this study coupled the use of shallow shotgun metagenomics and high-throughput qPCR (HT-qPCR) to enhance our understanding on the resident “core” or autochthonous microbial communities within the eastern oysters. Regardless of ploidy and season, the eastern oysters were found to be predominantly colonized by bacteria-the Psudomonadota phylum and Psychrobater genus. Furthermore, the diversity analysis revealed that the eastern oyster microbiome did not differ on ploidy, but there were significant differences due to the sampling time. It is likely that the Psychrobacter genus is representative of the eastern oyster’s “core” microbiome due to the predominance and stable numbers of this psychotrophic bacteria over an annual growth cycle. We also found strong positive correlations of gene functions associated with central metabolism, DNA metabolism and carbohydrate metabolism with Psychrobacter spp. Additionally, the metagenome-assembled genomes (MAGs) also confirmed the presence of multiple Psychrobacter genomes harboring denitrification genes - norB, narH, narI, nirK, and norB, thus providing evidence for this genus to be taxonomically and functionally relevant to the eastern oysters. Further assessment of microbially mediated biogeochemical processes was provided by the application of QMEC technique which revealed predominance of carbon (C) and nitrogen (N) cycling genes relative to the phosphorous and sulfur cycling genes, with no specific trends related to ploidy. Interestingly, the nosZII clade was dominant relative to the nosZI clade, thus indicative that this process is likely responsible for the eastern oyster’s role in depleting dissolved nitrogen via denitrification processes.
Limitations of the study
The taxonomic preponderance of the Psychrobacter genus in the eastern oysters from this study are largely based on shallow shotgun sequencing and therefore, future studies using deeper sequencing will likely lead to a comprehensive understanding of the eastern oyster’s “core” microbiomes. This will likely pave the way for improving oyster productivity and their ecosystem functions via the manipulation of their autochthonous microbial communities. Further extension of this study should be conducted to delineate whether Psychrobacter species are beneficial or detrimental to oyster health and associated ecosystem services, including the biogeochemical cycling of nutrients. Given the central role microbiomes play in oysters and other bivalve species, it is imperative to establish a universally accepted standard in defining the “core”,21 and by inference, the resident or autochthonous bacterial species for the eastern oysters; studies in this direction are also thus warranted.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Diploid and Triploid Oysters | This study | N/A |
| Critical commercial assays | ||
| Qiagen MagAttract PowerSoil DNA KF kit | https://www.qiagen.com/us/products/ | Cat. No./ID: 27100-4-EP |
| LightCycler 480 SYBR Green I Master | Roche Life Science(https://lifescience.roche.com/global/en/products/) | Cat # 04707516001 |
| Deposited data | ||
| Shallow shotgun metagenomic sequences | This paper | NCBI: PRJNA858118, Biosample #SAMN29671270 to SAMN29671291 and SRA#SRS13888819 |
| Experimental models: Organisms/strains | ||
| Crassostra virginica | Aquaculture research lease of the Wakulla Environmental Institute located in Oyster Bay | Taxonomic Serial Number (TSN):79872 |
| Software and algorithms | ||
| Fast QC v0.11.5 | Babraham Bioinformatics101 | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) |
| Cutadapt v2.6 | Martin et al.102 | https://doi.org/10.14806/ej.17.1.200 |
| Trimmomatic v0.36 | Bolger et al.103 | https://doi.org/10.1093/bioinformatics/btu170 |
| Komplexity v0.3.6 | Clarke et al.104 | https://doi.org/10.1186/s40168-019-0658-x |
| Kraken2 | Wood et al.105 | https://doi.org/10.1186/s13059-019-1891-0 |
| Super-Focus | Silva et al.106 | https://doi.org/10.1093/bioinformatics/btv584 |
| nf-core/MAG pipeline v2.1.1 | Krakau et al.107 | https://doi.org/10.1093/nargab/lqac007 |
| Fastp v0.20.1 | Chen et al.108 | https://doi.org/10.1093/bioinformatics/bty560 |
| SPAdesv3.15.3 | Prjibelski et al.109 | https://doi.org/10.1002/cpbi.102 |
| Meta BAT2 v2.15 | Kang et al.110 | https://doi.org/10.7717/peerj.7359 |
| BUSCO v5.1.0 | Simao et al.111 | https://doi.org/10.1093/bioinformatics/btv351 |
| Quast v5.0.2 | Gurevich et al.112 | https://doi.org/10.1093/bioinformatics/btt086 |
| GT DB-TK v1.5.0 | Chaumeil et al.113 | https://doi.org/10.1093/bioinformatics/btz848 |
| Prokka v1.14.6 | Seemann.114 | https://doi.org/10.1093/bioinformatics/btu153 |
| PhylophlAn v3.0.67 | Asnicar et al.115 | https://doi.org/10.1038/s41467-020-16366-7 |
| Rax ML v1.33 | Stamatakis et al.116 | https://doi.org/10.1093/bioinformatics/btu033 |
| FastANI v1.33 | Jain et al.87 | https://doi.org/10.1038/s41467-018-07641-9 |
| NCycDB | Tu et al.117 | https://doi.org/10.1093/bioinformatics/bty741 |
| DIAMOND v2.0.14.152 | Buchfink et al.118 | https://doi.org/10.1038/s41592-021-01101-x |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Dr. Ashvini Chauhan (ashvini.chauhan@famu.edu).
Materials availability
This study did not generate new unique reagents or materials.
Data and code availability
Standardized shallow shotgun metagenomics sequences have been deposited at NCBI and are publicly available. Accension numbers are listed in the key resources table.
This paper does not report original codes.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact.
Method details and bioinformatics
Sample collection, DNA Extraction and Library Preparation for Next-Generation Sequencing (NGS), DNA Sequencing, Quality Control Analysis, Data Curation, and Sequence Processing, HT-qPCR Quantitative Microbial Element Cycling (QMEC), Metagenome Assembled Genomes (MAGs), Phylogenetic Analysis of Binned Metagenomes, Average Nucleotide Identity (ANI) Analysis of MAGs, Identification of Denitrification Genes in MAGs and RefSeq Genomes, Shallow Shotgun Metagenome Submission: Metagenomic Sequence Accession Numbers.
Sample collection, experimental model, and study participant details
Cultured C. virginica were collected from the aquaculture research lease of the Wakulla Environmental Institute located in Oyster Bay [30.0342702, −84.3558844], near Panacea, Florida. Oysters were grown in plastic cages, suspended from lines attached to pilings at depths of 1–2 m (Australian Adjustable Long-Line System; https://seapa.com.au/farming-systems/). At each sampling time from December 2016-August 2017, representative of the warm and cold seasons in North Florida (Panacea), diploid and triploid oysters grown in adjacent cages were sampled monthly from the Wakulla Environmental Institute Research Aquaculture lease site in Oyster Bay, near Panacea Florida, stored in Ziploc bags over ice for ∼45 min for their transportation from the site to the FAMU laboratory, where they were immediately stored in a −80°C freezer until being processed for DNA analysis. Oysters were measured using digital calipers to ensure only adults are harvested, by measuring the shell height as the maximum distance from umbo (hinge) to lip (commissure) (per Galtsoff, P. S. 1964. The American Oyster Crassostrea virginica Gmelin (Fishery Bulletin, v. 64. United States Government Printing Office, Washington, D. C.). For DNA extraction 6 diploid and 6 triploid oysters were thawed, shucked, and pooled by ploidy for each monthly sampled time point. Duplicate samples were then aliquoted and used for sequencing.
DNA extraction and library preparation for next-generation sequencing
Prior to the opening of bivavles, each oyster was first externally rinsed with sterile water and scrubbed thoroughly to remove the shell biofilm. They were then opened with a shucking knife at the hinge where the adductor muscle is located joining the two shells. Once opened, the oyster’s mantle fluid was drained, and the entire oyster was collected. Pooled (n = 6) each of diploid and triploid oysters, from each time point, were then homogenized thoroughly using the Oster brand kitchen blender and processed for DNA extraction.
At each time, 250 mg of homogenized oyster tissues were used to extract DNA in duplicate, labeled as A and B, using the Qiagen MagAttract PowerSoil DNA KF kit (Formerly MOBio PowerSoil DNA Kit). DNA quality was evaluated visually via gel electrophoresis and quantified using a Qubit 3.0 fluorometer (Thermo-Fischer, Waltham, MA, USA). Libraries were then prepared by MicrobiomeInsights Inc. (https://microbiomeinsights.com/) using an Illumina Nextera library preparation kit using their in-house protocol (Illumina, San Diego, CA, USA).
DNA sequencing, quality control analysis, data curation, and sequence processing
Paired-end sequencing (150 bp × 2) was done on a NextSeq 500 in medium-output mode. Shotgun metagenomic sequence reads were processed with the Sunbeam pipeline. Initial quality evaluation was done using FastQC v0.11,101 followed by adapter removal, read trimming, low-complexity-reads removal, and host-sequence removals. Adapter removal was done using cutadapt v2.6102 and trimming was done with Trimmomatic v0.36103 using custom parameters (leading 3, trailing 3, sliding window 4:15, minimum length 36). Low-quality sequences were detected with Komplexity v0.3.6104 and discarded from further analysis. High-quality reads were mapped to the human genome (Genome Reference Consortium Human ref. 37) and to the Crassostrea virginica genome v3.0 (Accession number GCF_002022765.2); matching sequences were removed (labeled as host in Figure SI-1), along with low quality reads (marked as low quality in Figure SI-1), respectively. Among the high-quality reads, six sequenced samples were considered outliers because they contained a variable number of host reads. These samples, represented by n = 6 pooled oysters included triploid oysters from January (010717 Triploid A and 010717 Triploid B), Diploid B oysters from May (051117), Diploid A oysters from December 2016 (120516), and both duplicate triploids from December 2016 (120516 Triploid A, and 120516 Triploid B), respectively (note that samples are labeled based on their collection date and ploidy). To retain the high quality read samples, we subsampled five of them to the median value of reads for the non-outliers (6768 reads). Sample 051117 Diploid B had a similar number of reads to the other samples, so it was not subsampled. The remaining reads, marked as retained in Figure SI-1, were taxonomically classified using Kraken2105 with the minikraken v1 database. Functional profiles were not subsampled, and the five outliers were removed from the downstream bioinformatics analysis. For functional profiling, high-quality (filtered) reads were aligned against the SEED database via translated homology search and annotated to subsystems, or functional levels, 1–3 using Super-Focus.106
High-throughput- quantitative microbial element cycling
Based on high-throughput qPCR (HT-qPCR), a recently developed method called QMEC [Quantitative Microbial Elemental Cycling (QMEC)], has the potential to simultaneously detect and quantify biogeochemical cycling genes for C, N, P and S, thus. providing a deeper understanding on these microbially mediated processes.51 Briefly, a total of 72 primer sets were used to quantify DNA extracted from homogenized oyster samples. The primer sets targeted the 16S ribosomal RNA (rRNA) gene, and 35 carbon cycling genes (C), 22 nitrogen cycling genes (N), 9 phosphorus cycling genes (P) and 5 sulfur cycling genes (S). Amplification for each sample was conducted in triplicates, using 100 nL containing (final concentration) 1 × Light Cycler 480 SYBR Green I Master Mix (Roche Inc., USA), nuclease free PCR-grade water, 1–3 ng μL−1 DNA template, and 1 μM each of forward and reverse primers. High-throughput qPCR (HT-qPCR) was performed by the Smart Chip Real-time PCR system (Takara Bio USA, Inc.). A non-template negative control was set for each primer set. The thermal cycle curve conditions included an initial denaturation at 95°C for 10 min with 40 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. The melting curve analysis was automatically generated by the software, and the qPCR results were analyzed using Smart Chip qPCR software. Samples with efficiencies beyond the range of 1.7–2.3 or an r2 under 0.99 were discarded. A threshold cycle (Ct) 31 was used as the detection limit. All three replicates that amplified successfully were used for further data analysis. The relative gene copy number was calculated as described by Looft et al.107 using following equation.
where CT refers to HT-qPCR results, 31 refers to the detection limit.
Absolute 16S rRNA copy numbers were determined by the standard curve (SC) method of quantification using the Roche 480 system. Each 20 μL qPCR consisted of 10 μL 2× Light Cycle 480 SYBR Green I Master (Roche Applied Sciences), 1 μM each primer, 2–6 ng DNA as template and enough nuclease-free PCR-grade water. The thermal cycle consisted of 5 min initial enzyme activation at 95°C, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension 72°C for 20 s. A plasmid control containing a cloned and sequenced 16S rRNA gene fragment (6.69 × 108 copies per microliter) was used to generate five-point calibration curves from 10-fold dilutions. All qPCRs were performed in technical triplicates with non-template reactions serving as a negative control.
Metagenome assembled genomes
Metagenomes were assembled, binned into MAGs (metagenome-assembled genomes), and annotated using the nf-core/mag pipeline v2.1.1.108 Raw reads from 22 samples were used as input. Reads were trimmed using fastp v0.20.1109 and assembled using SPAdes v3.15.3.110 Metagenomic contigs were grouped into draft genomes (i.e., binned into MAGs) using MetaBAT 2 v2.15.111 Bin quality was assessed using BUSCO v5.1.0112 and Quast v5.0.2.113 Bins with a level of completeness less than 15% (based on BUSCO results) were excluded from further analysis. Taxonomic classification of bins was performed using GTDB-Tk v1.5.0.114 Bins were annotated using Prokka v1.14.6.115
Phylogenetic analysis of binned metagenomes
Prokka-derived protein sequences from four metagenomic bins classified as Psychrobacter were used to construct a phylogenetic tree, using PhyloPhlAn v3.0.67.116 215 RefSeq Psychrobacter proteomes available in the NCBI Assembly database were downloaded and included in the analysis, as well as a Moraxella lincolnii protein set (GCF_002014765.1), for use as an outgroup. The “phylophlan” database of 400 universal marker genes was used, and a diversity setting of “medium” was specified. RAxML v 8.2.12117 was used to generate a maximum likelihood tree with bootstrap values from 100 replicates.
Average nucleotide identity analysis of metagenome assembled genomes
Whole-genome similarity between each Psychrobacter MAG and all NCBI RefSeq Psychrobacter genomes was assessed by ANI, and calculated using FastANI v1.33.87 For the ANI analysis all RefSeq Pyschrobacter assemblies were compared to each MAG. A separate ANI result was generated for each comparison, providing a total of 993 ANI values. For each MAG the highest ANI was identified and that RefSeq sequence is reported. No minimum ANI threshold was used in this process.
Identification of denitrification genes in metagenome assembled genomes and RefSeq genomes
Prokka-derived proteins for Psychrobacter MAGs and protein sequences from NCBI for similar Psychrobacter RefSeq genomes (based on ANI) were analyzed for denitrification genes using NCycDB.118 Searching was performed against the NCyc_100.faa database using DIAMOND v2.0.14.152.119 The following genes/proteins were assessed: napA (Periplasmic nitrate reductase NapA), napB (Cytochrome c-type protein NapB), narG (Nitrate reductase), narH (Nitrate reductase), narI (Nitrate reductase gamma subunit), nirK (Nitrite reductase), nirS (Nitrite reductase), norB (Nitric oxide reductase subunit B), norC (Nitric oxide reductase subunit C), nosZ (Nitrous-oxide reductase). NCycDB results were compared to Prokka and NCBI annotations, and additional BLAST searches against the NCBI RefSeq and UniProtKB/Swiss-Prot databases were performed to resolve conflicting functional assignments.
Shallow shotgun metagenome submission: metagenomic sequence accession numbers
The shallow shotgun metagenomic sequences obtained from this study are available under NCBI accession # PRJNA858118, Biosample #SAMN29671270 to SAMN29671291 and SRA#SRS13888819 to SRS13888833(https://www.ncbi.nlm.nih.gov/bioproject/PRJNA858118/).
-
(1)Data Transformation and Statistical Analysis
-
○Summary of Data Transformation
-
○Statistical Analysis
-
○
Summary of data transformation
All qPCR data was normalized into relative and absolute abundance. The relative abundance was calculated by normalizing the relative gene copy of each C N, P, and S-cycling genes with its relative 16S copy number (equation I).51 The absolute abundance was calculated by multiplying the 16S copy number with the relative abundance of each C, N, P, and S-cycling genes and dividing the product with its respective DNA concentration (equation II).51 The calculated absolute abundance was then normalized into copies/g oyster among samples (equation III),51 which was used to evaluate the total CNPS genes in each sample.
Statistical analysis
Averages and standard deviations were calculated in Microsoft Excel. Statistical visualizations were done in R and Origin statistical software. Heatmaps, correlation, hierarchical clustering analysis, and were done on R studio (https://www.R-project.org/) using pheatmap, vegan, corrplot, and ggplot 2. The stacked bar charts were plotted in Origin (https://www.originlab.com/).
Negative binomial models (DESEq2 R package) were used for the differential abundance testing of taxonomic and subsystem level features. Changes due to sampling timepoint differences after controlling for genotype differences (ploidy) were analyzed; because we did not notice major differences between genotypes by PERMANOVA, we used all samples together even though several timepoints were missing for the diploid samples. We plotted the top 20 groups for each test, based on their adjusted p-values, which were calculated using the likelihood ratio test (LRT). The critical p-value (alpha value) were used to compare the adjusted p-values (=0.01) of the multiple independent groups, unless otherwise stated.
Furthermore, the abundance of the top 10 bacterial taxa and gene functions were averaged to produce a single dataset for each sampling time point representing diploids or triploid samples which was then imported into the Primer-E software suite (version 6.1.18; PRIMER-E, Ivybridge, United Kingdom). Normalization of data was performed using the log (X+1) pre-treatment function in the Primer-E software and after transformation, a Bray-Curtis similarity matrix was generated, and non-metric multidimensional scaling plot (NMDS) was obtained using parameters of Kruskal stress formula1 and minimum stress 0.01. Dendrogram analysis was performed based on the group average option using Primer-E software.
Acknowledgments
This work was mainly supported by a National Science Foundation (NSF) award (#1901371). Other funding also partly supported this research, including Cooperative Agreement NA11SEC4810001 between the National Oceanic and Atmospheric Administration’s Educational Partnership Program for Minority Serving Institutions and the Environmental Cooperative Science Center at Florida A&M University, NSF award #2200615 and three US Department of Energy (DOE) projects, that included the Minority Serving Institution Partnership Program (MSIPP) (task order agreements #0000403081, #0000403082, and #0000456318). We thank B. Ballard and A. Wynn form the Wakulla Environmental Institute for supplying oysters and assisting with field collections. We also appreciate Deborah Keller and Erich Martin for providing photos of our study site located in Florida. Technical help provided by Drs. Meenakshi Agarwal, Rajneesh Jaswal, and Rajesh S. Rathore is also much appreciated.
Author contributions
AC, AP, CHJ, and MM contributed to conception and design of the study. AP organized the database and performed the statistical analysis. AP wrote the first draft of the article. AC, AP, MM, PS, JQS, YZ, XYZ, CC, and CHJ wrote sections of the article. All authors contributed to article revision, read, and approved the submitted version.
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Published: June 6, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110193.
Supplemental information
References
- 1.Cruz-Romero M.C., Kerry J.P., Kelly A.L. Fatty acids, volatile compounds and colour changes in high-pressure-treated oysters (Crassostrea gigas) Innov. Food Sci. Emerg. Technol. 2008;9:54–61. doi: 10.1016/j.ifset.2007.05.003. [DOI] [Google Scholar]
- 2.Chen H., Wang M., Yang C., Wan X., Ding H.H., Shi Y., Zhao C. Bacterial spoilage profiles in the gills of Pacific oysters (Crassostrea gigas) and Eastern oysters (C. virginica) during refrigerated storage. Food Microbiol. 2019;82:209–217. doi: 10.1016/j.fm.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 3.National Marine Fisheries Service. 2020. https://www.st.nmfs.noaa.gov/st1/commercial/landings/annual_landings.html
- 4.Fisheries and Agriculture Organisation of the United Nations [FAO] FAO; 2018. The State of World Fisheries and Aquaculture 2018. Meeting the Sustainable Development Goals.https://www.fao.org/family-farming/detail/en/c/1145050 [Google Scholar]
- 5.Prapaiwong N., Wallace R.K., Arias C.R. Bacterial loads and microbial composition in high pressure treated oysters during storage. Int. J. Food Microbiol. 2009;131:145–150. doi: 10.1016/j.ijfoodmicro.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Burge C.A., Mark Eakin C., Friedman C.S., Froelich B., Hershberger P.K., Hofmann E.E., Petes L.E., Prager K.C., Weil E., Willis B.L., et al. Climate change influences on marine infectious diseases: implications for management and society. Ann. Rev. Mar. Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- 7.Chesapeake Bay Foundation Oyster Facts Sheet. 2003. https://www.cbf.org/about-the-bay/chesapeake-wildlife/eastern-oysters/oyster-fact-sheet.html
- 8.Bricker S.B., Grizzle R.E., Trowbridge P., Rose J.M., Ferreira J.G., Wellman K., Zhu C., Galimany E., Wikfors G.H., Saurel C., et al. Bioextractive Removal of Nitrogen by Oysters in Great Bay Piscataqua River Estuary, New Hampshire, USA. Estuaries Coast. 2020;43:23–38. doi: 10.1007/s12237-019-00661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayvazian S., Mulvaney K., Zarnoch C., Palta M., Reichert-Nguyen J., McNally S., Pilaro M., Jones A., Terry C., Fulweiler R.W. Beyond Bioextraction: The Role of Oyster-Mediated Denitrification in Nutrient Management. Environ. Sci. Technol. 2021;55:14457–14465. doi: 10.1021/acs.est.1c01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippini G., Dafforn K.A., Bugnot A.B. Shellfish as a bioremediation tool: A review and meta-analysis. Environ. Pollut. 2023;316 doi: 10.1016/j.envpol.2022.120614. [DOI] [PubMed] [Google Scholar]
- 11.Wilberg M.J., Livings M.E., Barkman J.S., Morris B.T., Robinson J.M. Overfishing, disease, habitat loss, and potential extirpation of oysters in upper Chesapeake Bay. Mar. Ecol. Prog. Ser. 2011;436:131–144. doi: 10.3354/meps09161. [DOI] [Google Scholar]
- 12.Hemraj D.A., Bishop M.J., Hancock B., Minuti J.J., Thurstan R.H., Zu Ermgassen P.S.E., Russell B.D. Oyster reef restoration fails to recoup global historic ecosystem losses despite substantial biodiversity gain. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abp8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby M.X. Fishing down the coast: Historical expansion and collapse of oyster fisheries along continental margins. Proc. Natl. Acad. Sci. USA. 2004;101:13096–13099. doi: 10.1073/pnas.0405150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y., Zhang Y., Mo R., Zhang Y., Li J., Zhou Y., Ma H., Xiao S., Yu Z. Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oysters Crassostrea hongkongensis in southern China. Aquaculture. 2019;507:108–118. doi: 10.1016/j.aquaculture.2019.04.017. [DOI] [Google Scholar]
- 15.Bodenstein S., Callam B.R., Walton W.C., Rikard F.S., Tiersch T.R., La Peyre J.F. Survival and growth of triploid eastern oysters, Crassostrea virginica, produced from wild diploids collected from low-salinity areas. Aquaculture. 2023;564 doi: 10.1016/j.aquaculture.2022.739032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollier D. Tasty Mutants: The Invention of the Modern Oyster. In: Atl. 2014. https://www.theatlantic.com/technology/archive/2014/09/todays-oysters-are-mutants/380858/
- 17.Gagnaire B., Soletchnik P., Madec P., Geairon P., Le Moine O., Renault T. Diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg), reared at two heights above sediment in Marennes-Oleron Basin, France: Difference in mortality, sexual maturation and hemocyte parameters. Aquaculture. 2006;254:606–616. doi: 10.1016/j.aquaculture.2005.10.008. [DOI] [Google Scholar]
- 18.Unzueta-Martínez A., Welch H., Bowen J.L. Determining the Composition of Resident and Transient Members of the Oyster Microbiome. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.828692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero J., García-Varela M., Laclette J.P., Espejo R.T. Bacterial 16S rRNA gene analysis revealed that bacteria related to Arcobacter spp. constitute an abundant and common component of the oyster microbiota (Tiostrea chilensis) Microb. Ecol. 2002;44:365–371. doi: 10.1007/s00248-002-1063-7. [DOI] [PubMed] [Google Scholar]
- 20.Lokmer A., Goedknegt M.A., Thieltges D.W., Fiorentino D., Kuenzel S., Baines J.F., Wegner K.M. Spatial and Temporal Dynamics of Pacific Oyster Hemolymph Microbiota across Multiple Scales. Front. Microbiol. 2016;7:1367. doi: 10.3389/fmicb.2016.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shade A., Handelsman J. Beyond the Venn diagram: the hunt for a core microbiome. Environ. Microbiol. 2012;14:4–12. doi: 10.1111/j.1462-2920.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 22.De Decker S., Normand J., Saulnier D., Pernet F., Castagnet S., Boudry P. Responses of diploid and triploid Pacific oysters Crassostrea gigas to Vibrio infection in relation to their reproductive status. J. Invertebr. Pathol. 2011;106:179–191. doi: 10.1016/j.jip.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Colwell R.R., Liston J. Microbiology of shellfish. Bacteriological study of the natural flora of Pacific oysters (Crassostrea gigas) Appl. Microbiol. 1960;8:104–109. doi: 10.1128/am.8.2.104-109.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurel D., Benayahu Y., Or A., Kovacs A., Gophna U. Composition and dynamics of the gill microbiota of an invasive Indo-Pacific oyster in the eastern Mediterranean Sea. Environ. Microbiol. 2011;13:1467–1476. doi: 10.1111/j.1462-2920.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- 25.Trabal Fernández N., Mazón-Suástegui J.M., Vázquez-Juárez R., Ascencio-Valle F., Romero J. Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during commercial production. FEMS Microbiol. Ecol. 2014;88:69–83. doi: 10.1111/1574-6941.12270. [DOI] [PubMed] [Google Scholar]
- 26.Prieur D., Mevel G., Nicolas J.L., Plusquellec A., Vigneulle M. Interactions between bivalve molluscs and bacteria in the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1990;28:277–352. [Google Scholar]
- 27.Newton I.L.G., Woyke T., Auchtung T.A., Dilly G.F., Dutton R.J., Fisher M.C., Fontanez K.M., Lau E., Stewart F.J., Richardson P.M., et al. The Calyptogena magnifica chemoautotrophic symbiont genome. Science. 2007;315:998–1000. doi: 10.1126/science.1138438. [DOI] [PubMed] [Google Scholar]
- 28.Pujalte M.J., Ortigosa M., Macián M.C., Garay E. Aerobic and facultative anaerobic heterotrophic bacteria associated to Mediterranean oysters and seawater. Int. Microbiol. 1999;2:259–266. [PubMed] [Google Scholar]
- 29.Sakowski E.G., Wommack K.E., Polson S.W. Oyster Calcifying Fluid Harbors Persistent and Dynamic Autochthonous Bacterial Populations That May Aid in Shell Formation. Mar. Ecol. Prog. Ser. 2020;653:57–75. doi: 10.3354/meps13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banker R., Vermeij G.J. Oyster microbial communities and implications for chalky deposit formation. Hydrobiologia. 2018;816:121–135. doi: 10.1007/s10750-018-3569-0. [DOI] [Google Scholar]
- 31.Braissant O., Decho A.W., Dupraz C., Glunk C., Przekop K.M., Visscher P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology. 2007;5:401–411. doi: 10.1111/j.1472-4669.2007.00117.x. [DOI] [Google Scholar]
- 32.Cacchio P., Ercole C., Contento R., Cappuccio G., Preite Martinez M., Del Gallo M., Lepidi A. Involvement of bacteria in the origin of a newly described speleothem in the gypsum cave of grave grubbo (Crotone, Italy) J. Cave Karst Stud. 2012;74:7–18. doi: 10.4311/2010MB0136R. [DOI] [Google Scholar]
- 33.Hines I.S., Markov Madanick J., Smith S.A., Kuhn D.D., Stevens A.M. Analysis of the core bacterial community associated with consumer-ready Eastern oysters (Crassostrea virginica) PLoS One. 2023;18 doi: 10.1371/journal.pone.0281747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akter S., Wos-Oxley M.L., Catalano S.R., Hassan M.M., Li X., Qin J.G., Oxley A.P. Host Species and Environment Shape the Gut Microbiota of Cohabiting Marine Bivalves. Microb. Ecol. 2023;86:1755–1772. doi: 10.1007/s00248-023-02192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh P., Williams D., Velez F.J., Nagpal R. Comparison of the Gill Microbiome of Retail Oysters from Two Geographical Locations Exhibited Distinct Microbial Signatures: A Pilot Study for Potential Future Applications for Monitoring Authenticity of Their Origins. Appl. Microbiol. 2022;3:1–10. [Google Scholar]
- 36.Pierce M.L., Ward J.E. Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) and the Blue Mussel (Mytilus edulis): Temporal Variation and the Influence of Marine Aggregate-Associated Microbial Communities. mSphere. 2019;4:e00730-19. doi: 10.1128/mSphere.00730-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King G.M., Judd C., Kuske C.R., Smith C. Analysis of stomach and gut microbiomes of the eastern oyster (Crassostrea virginica) from coastal Louisiana, USA. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arfken A., Song B., Bowman J.S., Piehler M. Denitrification potential of the eastern oyster microbiome using a 16S rRNA gene based metabolic inference approach. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevick R.J., Sohn S., Modak T.H., Nelson D.R., Rowley D.C., Tammi K., Smolowitz R., Markey Lundgren K., Post A.F., Gómez-Chiarri M. Bacterial Community Dynamics in an Oyster Hatchery in Response to Probiotic Treatment. Front. Microbiol. 2019;10:1060. doi: 10.3389/fmicb.2019.01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pimentel Z.T., Keith D.-T.,T.R.K., et al. Microbiome Analysis Reveals Diversity and Function of Mollicutes Associated with the Eastern Oyster, Crassostrea virginica. mSphere. 2021;6 doi: 10.1128/mSphere.00227-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banker R.M.W., Coil D. Inoculation With Desulfovibrio sp. Does Not Enhance Chalk Formation in the Pacific Oyster. Front. Mar. Sci. 2020;7 doi: 10.3389/fmars.2020.00407. [DOI] [Google Scholar]
- 42.Britt A., Bernini M., McSweeney B., Dalapati S., Duchin S., Cavanna K., Santos N., Donovan G., O'Byrne K., Noyes S., et al. The effects of atrazine on the microbiome of the eastern oyster: Crassostrea virginica. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauhan A., Wafula D., Lewis D.E., Pathak A. Metagenomic assessment of the eastern oyster-associated microbiota. Genome Announc. 2014;2 doi: 10.1128/genomeA.01083-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas J.C., 4th, Wafula D., Chauhan A., Green S.J., Gragg R., Jagoe C. A survey of deepwater horizon (DWH) oil-degrading bacteria from the Eastern oyster biome and its surrounding environment. Front. Microbiol. 2014;5:149. doi: 10.3389/fmicb.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak A., Stothard P., Chauhan A. Comparative Genomic Analysis of Three Pseudomonas Species Isolated from the Eastern Oyster (Crassostrea virginica) Tissues, Mantle Fluid, and the Overlying Estuarine Water Column. Microorganisms. 2021;9:490. doi: 10.3390/microorganisms9030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chauhan A., Green S., Pathak A., Thomas J., Venkatramanan R. Whole-genome sequences of five oyster-associated bacteria show potential for crude oil hydrocarbon degradation. Genome Announc. 2013;1 doi: 10.1128/genomeA.00802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitousek P.M., Aber J.D., Howarth R.W., Likens G.E., Matson P.A., Schindler D.W., Schlesinger W.H., Tilman D.G. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997;7:737–750. [Google Scholar]
- 48.Ray N.E., Hancock B., Brush M.J., Colden A., Cornwell J., Labrie M.S., Maguire T.J., Maxwell T., Rogers D., Stevick R.J., et al. A review of how we assess denitrification in oyster habitats and proposed guidelines for future studies. Limnol Oceanogr. Methods. 2021;19:714–731. doi: 10.1002/lom3.10456. [DOI] [Google Scholar]
- 49.Zumft W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugli G.A., Ventura M. A breath of fresh air in microbiome science: shallow shotgun metagenomics for a reliable disentangling of microbial ecosystems. Microbiome Res. Rep. 2022;1:8. doi: 10.20517/mrr.2021.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng B., Zhu Y., Sardans J., Peñuelas J., Su J. QMEC: a tool for high-throughput quantitative assessment of microbial functional potential in C, N, P, and S biogeochemical cycling. Sci. China Life Sci. 2018;61:1451–1462. doi: 10.1007/s11427-018-9364-7. [DOI] [PubMed] [Google Scholar]
- 52.Kobiyama A., Ikeo K., Reza M.S., Rashid J., Yamada Y., Ikeda Y., Ikeda D., Mizusawa N., Sato S., Ogata T., et al. Metagenome-based diversity analyses suggest a strong locality signal for bacterial communities associated with oyster aquaculture farms in Ofunato Bay. Gene. 2018;665:149–154. doi: 10.1016/j.gene.2018.04.073. [DOI] [PubMed] [Google Scholar]
- 53.Yu M., Wang X., Yan A. Microbial Profiles of Retail Pacific Oysters (Crassostrea gigas) From Guangdong Province, China. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.689520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S., Young T., Archer S., Lee K., Sharma S., Alfaro A.C. Mapping the Green-Lipped Mussel (Perna canaliculus) Microbiome: A Multi-Tissue Analysis of Bacterial and Fungal Diversity. Curr. Microbiol. 2022;79:76. doi: 10.1007/s00284-021-02758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamiyama T., Yamauchi H., Iwai T., Hamasaki K. Seasonal variations in abundance and biomass of picoplankton in an oyster-farming area of northern Japan. Plankt. Benthos Res. 2009;4:62–71. doi: 10.3800/pbr.4.62. [DOI] [Google Scholar]
- 56.Natrah F.M.I., Bossier P., Sorgeloos P., Yusoff F.M., Defoirdt T. Significance of microalgal–bacterial interactions for aquaculture. Rev. Aquac. 2014;6:48–61. doi: 10.1111/raq.12024. [DOI] [Google Scholar]
- 57.Hernández-Zárate G., Olmos-Soto J. Identification of bacterial diversity in the oyster Crassostrea gigas by fluorescent in situ hybridization and polymerase chain reaction. J. Appl. Microbiol. 2006;100:664–672. doi: 10.1111/j.1365-2672.2005.02800.x. [DOI] [PubMed] [Google Scholar]
- 58.Rappé M.S., Vergin K., Giovannoni S.J. Phylogenetic comparisons of a coastal bacterioplankton community with its counterparts in open ocean and freshwater systems. FEMS Microbiol. Ecol. 2000;33:219–232. doi: 10.1111/j.1574-6941.2000.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 59.Kersters K., De Vos P., Gillis M., et al. In: Introduction to the Proteobacteria BT - The Prokaryotes: Volume 5: Proteobacteria: Alpha and Beta Subclasses. Dworkin M., Falkow S., Rosenberg E., et al., editors. Springer; 2006. pp. 3–37. [Google Scholar]
- 60.Dubé C.E., Ky C.L., Planes S. Microbiome of the Black-Lipped Pearl Oyster Pinctada margaritifera, a Multi-Tissue Description With Functional Profiling. Front. Microbiol. 2019;10:1548. doi: 10.3389/fmicb.2019.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pierce M.L., Ward J.E. Microbial Ecology of the Bivalvia, with an Emphasis on the Family Ostreidae. J. Shellfish Res. 2018;37:793–806. doi: 10.2983/035.037.0410. [DOI] [Google Scholar]
- 62.Worden P.J., Bogema D.R., Micallef M.L., Jeffrey G., Deutscher A.T., Labbate M., Green T.J., King W.L., Liu M., Seymour J.R., Jenkins C. Phylogenomic diversity of Vibrio species and other Gammaproteobacteria isolated from Pacific oysters (Crassostrea gigas) during a summer mortality outbreak. Microb. Genom. 2022;8 doi: 10.1099/mgen.0.000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Y.-X., Yu Y., Liu Y., Li H.-R. Psychrobacter glaciei sp. nov., isolated from the ice core of an Arctic glacier. Int. J. Syst. Evol. Microbiol. 2016;66:1792–1798. doi: 10.1099/ijsem.0.000939. [DOI] [PubMed] [Google Scholar]
- 64.Bowman J.P., Nichols D.S., McMeekin T.A. Psychrobacter glacincola sp. nov., a halotolerant, psychrophilic bacterium isolated from Antarctic sea ice. Syst. Appl. Microbiol. 1997;20:209–215. doi: 10.1016/S0723-2020(97)80067-. [DOI] [Google Scholar]
- 65.Bakermans C., Ayala-del-Río H.L., Ponder M.A., Vishnivetskaya T., Gilichinsky D., Thomashow M.F., Tiedje J.M. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol. 2006;56:1285–1291. doi: 10.1099/ijs.0.64043-0. [DOI] [PubMed] [Google Scholar]
- 66.Welter D.K., Ruaud A., Henseler Z.M., De Jong H.N., van Coeverden de Groot P., Michaux J., Gormezano L., Waters J.L., Youngblut N.D., Ley R.E. Free-Living, Psychrotrophic Bacteria of the Genus Psychrobacter Are Descendants of Pathobionts. mSystems. 2021;6 doi: 10.1128/mSystems.00258-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Apprill A., Miller C.A., Moore M.J., Durban J.W., Fearnbach H., Barrett-Lennard L.G. Extensive core microbiome in drone-captured whale blow supports a framework for health monitoring. mSystems. 2017;2 doi: 10.1128/mSystems.00119-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kämpfer P., Glaeser S.P., Irgang R., Fernández-Negrete G., Poblete-Morales M., Fuentes-Messina D., Cortez-San Martín M., Avendaño-Herrera R. Psychrobacter pygoscelis sp. nov. isolated from the penguin Pygoscelis papua. Int. J. Syst. Evol. Microbiol. 2020;70:211–219. doi: 10.1099/ijsem.0.003739. [DOI] [PubMed] [Google Scholar]
- 69.Bakermans C. Adaptations to marine versus terrestrial low temperature environments as revealed by comparative genomic analyses of the genus Psychrobacter. FEMS Microbiol. Ecol. 2018;94 doi: 10.1093/femsec/fiy102. [DOI] [PubMed] [Google Scholar]
- 70.Madigan T.L., Bott N.J., Torok V.A., Percy N.J., Carragher J.F., de Barros Lopes M.A., Kiermeier A. A microbial spoilage profile of half shell Pacific oysters (Crassostrea gigas) and Sydney rock oysters (Saccostrea glomerata) Food Microbiol. 2014;38:219–227. doi: 10.1016/j.fm.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Franco R., Arenal A., Martín L., Martínez Y., Santiesteban D., Sotolongo J., Pimentel E., Carrillo O., Bossier P. Psychrobacter sp. 17-1 enhances growth and survival in early postlarvae of white shrimp, Penaeus vannamei Boone, 1931 (Decapoda, Penaeidae) Crustaceana. 2016;89:1467–1484. doi: 10.1163/15685403-00003595. [DOI] [Google Scholar]
- 72.Caipang C.M.A., Suharman I., Avillanosa A.L., Bargoyo V.T. Host-derived Probiotics for Finfish Aquaculture. IOP Conf. Ser. Earth Environ. Sci. 2020;430 doi: 10.1088/1755-1315/430/1/012026. [DOI] [Google Scholar]
- 73.Makled S.O., Hamdan A.M., El-Sayed A.F.M., Hafez E.E. Evaluation of marine psychrophile, Psychrobacter namhaensis SO89, as a probiotic in Nile tilapia (Oreochromis niloticus) diets. Fish Shellfish Immunol. 2017;61:194–200. doi: 10.1016/j.fsi.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Ramírez C., Rojas R., Romero J. Partial Evaluation of Autochthonous Probiotic Potential of the Gut Microbiota of Seriola lalandi. Probiotics Antimicrob. Proteins. 2020;12:672–682. doi: 10.1007/s12602-019-09550-9. [DOI] [PubMed] [Google Scholar]
- 75.Tokarskyy O., Marshall D.L., Dillon J., Andrews L.S. Long-Term Depuration of Crassostrea virginica Oysters at Different Salinities and Temperatures Changes Vibrio vulnificus Counts and Microbiological Profile. J. Food Prot. 2019;82:22–29. doi: 10.4315/0362-028X.JFP-18-225. [DOI] [PubMed] [Google Scholar]
- 76.Deschaght P., Janssens M., Vaneechoutte M., Wauters G. Psychrobacter isolates of human origin, other than Psychrobacter phenylpyruvicus, are predominantly Psychrobacter faecalis and Psychrobacter pulmonis, with emended description of P faecalis. Int. J. Syst. Evol. Microbiol. 2012;62:671–674. doi: 10.1099/ijs.0.032631-0. [DOI] [PubMed] [Google Scholar]
- 77.Wirth S.E., Ayala-del-Río H.L., Cole J.A., Kohlerschmidt D.J., Musser K.A., Sepúlveda-Torres L.D.C., Thompson L.M., Wolfgang W.J. Psychrobacter sanguinis sp. nov., recovered from four clinical specimens over a 4-year period. Int. J. Syst. Evol. Microbiol. 2012;62:49–54. doi: 10.1099/ijs.0.029058-0. [DOI] [PubMed] [Google Scholar]
- 78.Speights C.J., Silliman B.R., McCoy M.W. The effects of elevated temperature and dissolved ρCO 2 on a marine foundation species. Ecol. Evol. 2017;7:3808–3814. doi: 10.1002/ece3.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y. Oysters and the impacts of climate change - Responsible Seafood Advocate. 2009. https://www.globalseafood.org
- 80.Avila-Poveda O.H., Torres-Ariño A., Girón-Cruz D.A., Cuevas-Aguirre A. Evidence for accumulation of Synechococcus elongatus (Cyanobacteria: Cyanophyceae) in the tissues of the oyster Crassostrea gigas (Mollusca: Bivalvia) Tissue Cell. 2014;46:379–387. doi: 10.1016/j.tice.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Chen H., Liu Z., Wang M., Chen S., Chen T. Characterisation of the spoilage bacterial microbiota in oyster gills during storage at different temperatures. J. Sci. Food Agric. 2013;93:3748–3754. doi: 10.1002/jsfa.6237. [DOI] [PubMed] [Google Scholar]
- 82.Pathak A., Rathore R.S., Chauhan A. In: Prime Archives in Microbiology. 3 rd Edition. Albasha A.M., editor. Vide Leaf; 2022. Comparative Genomic Analysis of Three Pseudomonas Species Isolated from the Eastern Oyster (Crassostrea virginica) [Google Scholar]
- 83.Abreu C.I., Dal Bello M., Bunse C., Pinhassi J., Gore J. Warmer temperatures favor slower-growing bacteria in natural marine communities. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benhaïm D., Leblanc C.A., Horri K., Mannion K., Galloway M., Leeper A., Knobloch S., Sigurgeirsson Ó., Thorarensen H. The effect of triploidy on the performance, gut microbiome and behaviour of juvenile Atlantic salmon (Salmo salar) raised at low temperature. Appl. Anim. Behav. Sci. 2020;229 doi: 10.1016/j.applanim.2020.105031. [DOI] [Google Scholar]
- 85.Cantas L., Fraser T.W.K., Fjelldal P.G., Mayer I., Sørum H. The culturable intestinal microbiota of triploid and diploid juvenile Atlantic salmon (Salmo salar) - a comparison of composition and drug resistance. BMC Vet. Res. 2011;7:71. doi: 10.1186/1746-6148-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wada N., Yuasa H., Kajitani R., Gotoh Y., Ogura Y., Yoshimura D., Toyoda A., Tang S.L., Higashimura Y., Sweatman H., et al. A ubiquitous subcuticular bacterial symbiont of a coral predator, the crown-of-thorns starfish, in the Indo-Pacific. Microbiome. 2020;8:123. doi: 10.1186/s40168-020-00880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain C., Rodriguez-R L.M., Phillippy A.M., Konstantinidis K.T., Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Badhai J., Ghosh T.S., Das S.K. Composition and Functional Characterization of Microbiome Associated with Mucus of the Coral Fungia echinata Collected from Andaman Sea. Front. Microbiol. 2016;7:936. doi: 10.3389/fmicb.2016.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKew B.A., Dumbrell A.J., Daud S.D., Hepburn L., Thorpe E., Mogensen L., Whitby C. Characterization of geographically distinct bacterial communities associated with coral mucus produced by Acropora spp. and Porites spp. Appl. Environ. Microbiol. 2012;78:5229–5237. doi: 10.1128/AEM.07764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonaglia S., Brüchert V., Callac N., Vicenzi A., Chi Fru E., Nascimento F.J.A. Methane fluxes from coastal sediments are enhanced by macrofauna. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang H., Lan W., Li T., Xu Z., Liu W., Pan K. Isotopic Composition Reveals the Impact of Oyster Aquaculture on Pelagic Nitrogen Cycling in a Subtropical Estuary. Water Res. 2020;187 doi: 10.1016/j.watres.2020.116431. [DOI] [PubMed] [Google Scholar]
- 92.Hoellein T.J., Zarnoch C.B., Grizzle R.E. Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hampshire, USA) Biogeochemistry. 2015;122:113–129. doi: 10.1007/s10533-014-0034-7. [DOI] [Google Scholar]
- 93.Mortazavi B., Ortmann A.C., Wang L., Bernard R.J., Staudhammer C.L., Dalrymple J.D., Carmichael R.H., Kleinhuizen A.A. Evaluating the impact of oyster (<I>Crassostrea virginica</I>) gardening on sediment nitrogen cycling in a subtropical estuary. Bull. Mar. Sci. 2015;91:323–341. doi: 10.5343/bms.2014.1060. [DOI] [Google Scholar]
- 94.Smyth A.R., Geraldi N.R., Thompson S.P., Piehler M.F. Biological activity exceeds biogenic structure in influencing sediment nitrogen cycling in experimental oyster reefs. Mar. Ecol. Prog. Ser. 2016;560:173–183. doi: 10.3354/meps11922. [DOI] [Google Scholar]
- 95.Sanford R.A., Wagner D.D., Wu Q., Chee-sanford J.C., Thomas S.H., Cruz-garcía C., Rodríguez G., Massol-Deyá A., Krishnani K.K., Ritalahti K.M., et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci. 2012;109:19709–19714. doi: 10.1073/pnas.1211238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones C.M., Graf D.R.H., Bru D., Philippot L., Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hallin S., Philippot L., Löffler F.E., Sanford R.A., Jones C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018;26:43–55. doi: 10.1016/j.tim.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 98.Orellana L.H., Rodriguez-R L.M., Higgins S., Chee-Sanford J.C., Sanford R.A., Ritalahti K.M., Löffler F.E., Konstantinidis K.T. Detecting nitrous oxide reductase (nosZ) genes in soil metagenomes: method development and implications for the nitrogen cycle. mBio. 2014;5 doi: 10.1128/mbio.01193-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith C.J., Nedwell D.B., Dong L.F., Osborn A.M. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl. Environ. Microbiol. 2007;73:3612–3622. doi: 10.1128/AEM.02894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mara P., Edgcomb V.P., Sehein T.R., Beaudoin D., Martinsen C., Lovely C., Belcher B., Cox R., Curran M., Farnan C., et al. Comparison of Oyster Aquaculture Methods and Their Potential to Enhance Microbial Nitrogen Removal From Coastal Ecosystems. Front. Mar. Sci. 2021;8:1–23. doi: 10.3389/fmars.2021.633314. [DOI] [Google Scholar]
- 101.Babraham Bioinformatics FastQC. 2020. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 102.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 103.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke E.L., Taylor L.J., Zhao C., Connell A., Lee J.J., Fett B., Bushman F.D., Bittinger K. Sunbeam: an extensible pipeline for analyzing metagenomic sequencing experiments. Microbiome. 2019;7:46. doi: 10.1186/s40168-019-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wood D.E., Lu J., Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silva G.G.Z., Green K.T., Dutilh B.E., Edwards R.A. SUPER-FOCUS: a tool for agile functional analysis of shotgun metagenomic data. Bioinformatics. 2016;32:354–361. doi: 10.1093/bioinformatics/btv584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Looft T., Johnson T.A., Allen H.K., Bayles D.O., Alt D.P., Stedtfeld R.D., Sul W.J., Stedtfeld T.M., Chai B., Cole J.R., et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA. 2012;109:1691–1696. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krakau S., Straub D., Gourlé H., Gabernet G., Nahnsen S. nf-core/mag: a best-practice pipeline for metagenome hybrid assembly and binning. NAR Genom. Bioinform. 2022;4 doi: 10.1093/nargab/lqac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prjibelski A., Antipov D., Meleshko D., Lapidus A., Korobeynikov A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinformatics. 2020;70:e102. doi: 10.1002/cpbi.102. [DOI] [PubMed] [Google Scholar]
- 111.Kang D.D., Li F., Kirton E., Thomas A., Egan R., An H., Wang Z. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ. 2019;7 doi: 10.7717/peerj.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 113.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaumeil P.A., Mussig A.J., Hugenholtz P., Parks D.H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–1927. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 116.Asnicar F., Thomas A.M., Beghini F., Mengoni C., Manara S., Manghi P., Zhu Q., Bolzan M., Cumbo F., May U., et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 2020;11:2500. doi: 10.1038/s41467-020-16366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tu Q., Lin L., Cheng L., Deng Y., He Z. NCycDB: a curated integrative database for fast and accurate metagenomic profiling of nitrogen cycling genes. Bioinformatics. 2019;35:1040–1048. doi: 10.1093/bioinformatics/bty741. [DOI] [PubMed] [Google Scholar]
- 119.Buchfink B., Reuter K., Drost H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods. 2021;18:366–368. doi: 10.1038/s41592-021-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Standardized shallow shotgun metagenomics sequences have been deposited at NCBI and are publicly available. Accension numbers are listed in the key resources table.
This paper does not report original codes.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact.