Abstract
This manuscript describes the Advanced Breast Cancer (ABC) international consensus guidelines updated at the last two ABC international consensus conferences (ABC 6 in 2021, virtual, and ABC 7 in 2023, in Lisbon, Portugal), organized by the ABC Global Alliance. It provides the main recommendations on how to best manage patients with advanced breast cancer (inoperable locally advanced or metastatic), of all breast cancer subtypes, as well as palliative and supportive care. These guidelines are based on available evidence or on expert opinion when a higher level of evidence is lacking. Each guideline is accompanied by the level of evidence (LoE), grade of recommendation (GoR) and percentage of consensus reached at the consensus conferences. Updated diagnostic and treatment algorithms are also provided. The guidelines represent the best management options for patients living with ABC globally, assuming accessibility to all available therapies. Their adaptation (i.e. resource-stratified guidelines) is often needed in settings where access to care is limited.
Keywords: ABC, Advanced, Metastatic, Breast cancer, Guidelines, Consensus
1. Introduction
Since its first edition, in 2011, the Advanced Breast Cancer (ABC) International Consensus Conference has established itself as the major international conference for advanced breast cancer. It was created to address the fear and isolation of patients with ABC and the urgent need to change their outcomes. Unique characteristics of the ABC guidelines are the central role taken by patients with ABC and its truly global reach.
The conference's primary goal is the development of international consensus guidelines for the management of advanced breast cancer, known as the ABC Guidelines. These guidelines are based on the most up-to-date evidence and can be used to guide treatment decision making in many different health care settings globally, with the necessary adaptations due to differences in access to care. Throughout the years, these guidelines have been endorsed by several international and European organizations, and many organizations around the world have adapted these guidelines to their country specific environments, particularly in terms of accessibility of treatment modalities. The conference and guidelines started as a pioneering project from the European School of Oncology (ESO) [1] and from its 2nd to 5th edition [[2], [3], [4], [5]], it was developed in collaboration with the European Society of Medical Oncology (ESMO). From 2021, both conference and guidelines started being organized by the ABC Global Alliance, an independent non-profit multi-stakeholder organization dedicated exclusively to improve and extend the lives of women and men living with ABC worldwide, that currently has more than 200 members in over 90 countries worldwide [6]. The ABC conference also aims to be a forum to analyze and discuss the latest scientific updates in the field, to identify research priorities based on the most important areas of unmet needs, as well as influence policy makers, regulatory and funding bodies, and ultimately improve standards of care, survival, and quality of life for all patients living with ABC worldwide. We strongly believe that health professionals working closely together with patients and advocates and with the strong support of the media can raise awareness and strongly lobby in favor of this often underserved and forgotten group of patients.
In the ABC guidelines, advanced breast cancer is defined as comprising both inoperable locally advanced breast cancer (LABC) and metastatic breast cancer (MBC), which includes both distant recurrent disease and stage IV at diagnosis or de novo MBC. Advanced/metastatic breast cancer remains a largely incurable disease, but important advances have occurred leading to an increase in the median overall survival (OS) from 2 to 3 years in the early 2000's to five or more years in patients with Human Epidermal Growth Factor Receptor 2 (HER2) positive disease and those with estrogen positive/HER2 negative ABC [[7], [8]]. This improvement in outcome is best achievable if a patient has access to high quality multidisciplinary care, innovative systemic therapies, high quality pathology and imaging, and radiotherapy, in a setting where there is attention to high-quality international guidelines. Unfortunately, inequalities in access to care are a major hurdle and lead to substantial differences in outcomes, not only between countries but also within each country.
Due to the COVID-19 pandemic, the 6th International Consensus Conference for Advanced Breast Cancer (ABC 6) was held virtually on 4th–6th November 2021 and brought together around 1.000 participants from 67 countries. The 7th International Consensus Conference for Advanced Breast Cancer (ABC 7) was held again in person, in Lisbon, Portugal, from 9th to November 11, 2023, and was attended by 1200 participants from 89 countries, including health professionals, patients, advocates, and journalists. The ABC 6 and 7 guidelines are endorsed by several international oncology organizations, such as Arbeitsgemeinschaft Gynäkologische Onkologie e.V. (AGO), European Cancer Organization (ECO), European Oncology Nursing Society (EONS), European School of Oncology (ESO), European Society for Radiotherapy and Oncology (ESTRO), European Society of Breast Cancer Specialists (EUSOMA), St. Paul Course and Senologic International Society (SIS)/International School of Senology (SIS) and have official representation from American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), Advanced Breast Cancer New Zealand (ABC NZ) and GECOPERU. The ABC 6 and 7 conferences were endorsed or run under the auspices from Arbeitsgemeinschaft Gynäkologische Onkologie e.V. (AGO), European Cancer Organization (ECO), (European Oncology Nursing Society (EONS), European School of Oncology (ESO), European Society for Surgical oncology (ESSO), European Society for Radiotherapy and Oncology (ESTRO), Global Breast Cancer Conference, the Organization of European Cancer Institutes (OECI), Senologic International Society (SIS)/International School of Senology (ISS) and Union for International Cancer Control (UICC), and held with the support from Breast Cancer Research Foundation (BCRF) and Susan G. Komen for the Cure.
This manuscript summarizes the guidelines developed at ABC 6 and 7. Each guideline statement is accompanied by the level of evidence (LoE), grade of recommendation (GoR), percentage of consensus reached at the conference, and supporting references. When available, the ESMO-MCBS (version 1.1) score is also added [9]. These guidelines are based on available evidence and on expert opinion when evidence is lacking. They represent the best management options for ABC patients globally, assuming access to all available therapies. Adaptation of these guidelines is often needed in settings where access to care is limited.
2. Methodology
As the ABC 6 conference was held virtually, the methodology followed in previous ABC consensus guidelines was adapted. Before the ABC 6 conference, initial guidelines statements on the management of ABC were prepared based on available published and presented data. These statements were circulated and intensively discussed among all 46 ABC 6 panel members by email. A final pre-conference set of guidelines was voted through an online confidential system. The guidelines were then presented and discussed during the live virtual consensus session of ABC 6. Required changes in the wording were made following the live discussion. Statements included under the supportive and palliative care section were not voted on but were discussed and unanimously agreed upon by email (100 % consensus agreement). For ABC 7, since it was held again face-to-face, the usual methodology was followed: before the conference, preliminary new recommendation statements on the management of ABC were prepared based on available published data. These recommendations were circulated to all 44 ABC 7 panel members by email for comments and corrections on content and wording. A final set of recommendations was presented, discussed, and voted upon during the consensus session of ABC 7. Additional changes in the wording of statements were made during the session. For both ABC 6 and ABC 7, all panel members were required to vote on all questions, but members with a potential conflict of interest or who did not feel comfortable answering the question (e.g. due to lack of expertise in a particular field), were instructed to vote ‘abstain’.
Two additional statements (on capivasertib and on datopotamab deruxtecan) were developed after the ABC 7 conference, due to presentation of important data and Food and Drug Administration (FDA) approval of capivasertib; these statements were circulated for revision and voted by all panel members by email. Statements related to the management of side effects and difficult symptoms, included under the supportive and palliative care section, were not voted on during the consensus sessions, but were discussed and unanimously agreed by email, and are therefore considered to have 100 % consensus agreement. As usual, guidelines statements from previous ABC consensus that did not require update or only minor changes were not re-voted but were reviewed and approved by all panel members by email.
The current manuscript presents all ABC guidelines recommendations currently approved, listed per subject. Only the new and updated recommendations voted during the ABC 6 and 7 consensus sessions are discussed in detail. We refer the reader to the previous ABC manuscripts for the detailed explanation of the other guidelines [[1], [2], [3], [4], [5]]. Supplementary table 1, describes the LoE and GoR system used [10]. The percentage of consensus was calculated as ratio of “yes” over total number of votes. Slides with all ABC guidelines statements are available online at http://www.abc-lisbon.org/ and at Supplementary material.
2.1. Section I: ABC definitions
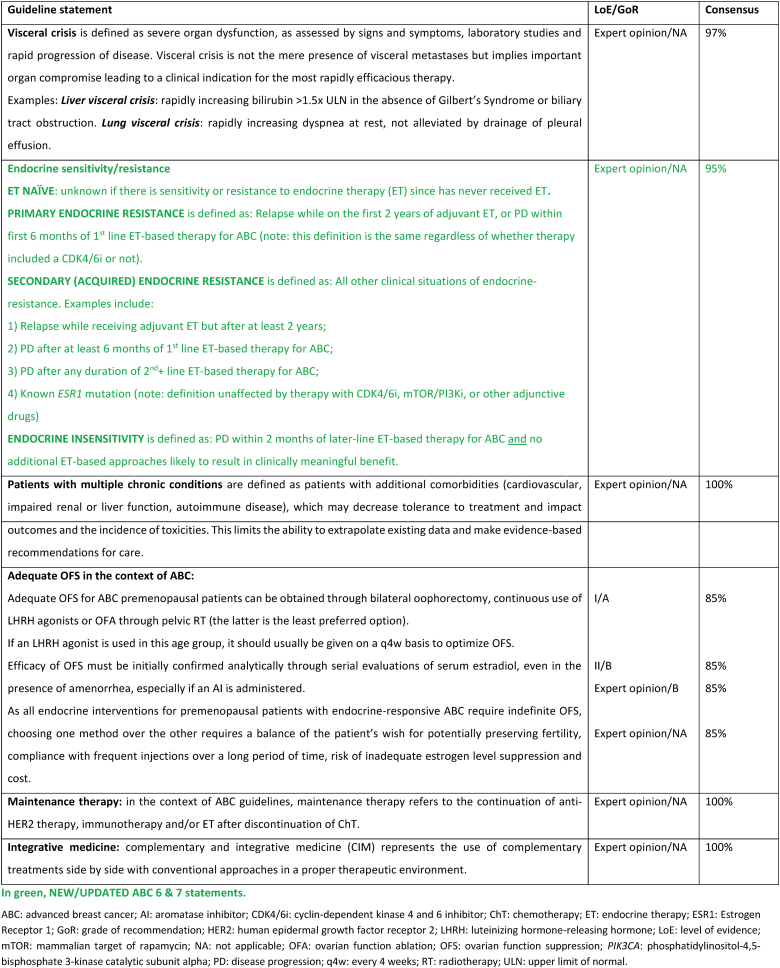
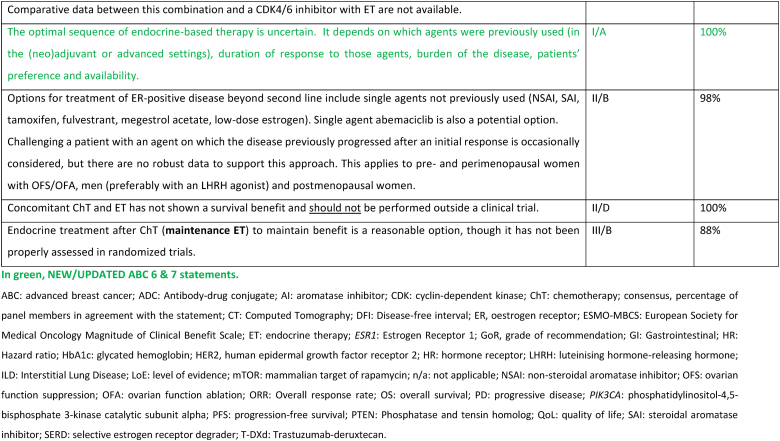
2.2. New endocrine resistance definition
Clinical definitions of endocrine resistance are mostly important for clinical trials to promote comparisons between populations that are as similar as possible. They are less relevant for clinical practice and for treatment decisions, since sensitivity and resistance are a continuum and the exact level of resistance of a given tumor is difficult to ascertain with certainty.
Endocrine-naïve populations are defined as populations where it is not known if there is sensitivity or resistance to ET, since the patient has never previously received this treatment. In practical terms, these cases are considered ET-sensitive, until proven otherwise. Prior exposure to endocrine agents often leads to some degree of resistance. The updated definitions are broad and simplified but attempt to group tumors by response, as well as account for varying lengths of prior adjuvant therapy. Primary endocrine resistance is defined as relapse while on the first 2 years of adjuvant ET, or progressive disease within first 6 months of 1st line ET-based therapy for ABC, while on ET (regardless of CDK4/6 inhibitors use). Secondary endocrine resistance is defined as other clinical situations, including relapse while receiving, but after 2 years’ adjuvant ET-based therapy, progressive disease after at least 6 months of 1st line ET-based therapy for ABC, progressive disease after any duration of 2nd or subsequent lines of ET-based therapy for ABC; and known ESR1 mutation (definition unaffected of receipt of CDK4/6 or mTOR/PI3K inhibitors, or other adjunctive drugs).
Resistance to adjunctive drugs does not equal to resistance to endocrine therapy. Resistance to adjunctive drugs can be de novo or acquired and its mechanisms are numerous. To the current knowledge, no such mechanism is known to affect ET decisions. It is therefore the opinion of the ABC consensus panel that the use of adjunctive drugs, such as CDK 4/6 or PIK3CA inhibitors, and the duration of such treatment does not contribute to or affect the definitions of endocrine sensitivity.
Endocrine insensitivity is defined as progression within 2 months of later-line ET-based therapy for ABC, and the absence of additional ET-based approaches likely to result in clinically meaningful benefit. Of all four situations described, this is the one with the biggest impact on clinical decision-making.
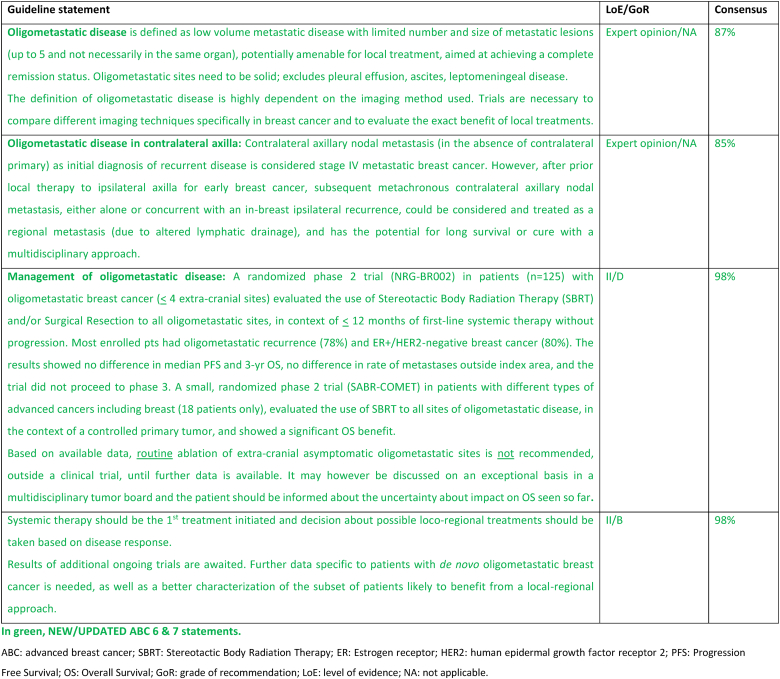
2.3. Section II: Oligometastatic disease
Oligometastatic disease (OMD) is defined as low volume metastatic disease, with a limited number and size of metastatic lesions (usually up to 5 though not necessarily in the same organ) [11]. OMD sites need to be solid; pleural effusion, ascites and leptomeningeal disease are excluded due to their diffuse nature. OMD limited to 1–3 metastases is associated with a more favorable 10-year overall survival [12].
The definition of OMD is highly dependent on the imaging method used. Modern imaging modalities outperform standard imaging modalities such as bone scintigraphy and computed tomography (CT), which are still often the standard of care in many practices [13,14]. Currently, the most effective diagnostic techniques are 18F-fluorodeoxyglucose (18-FDG) positron emission tomography/computed tomography (PET/CT) and whole-body-magnetic resonance imaging (MRI) with diffusion-weighted sequences [15,16]. The optimal imaging work-up may be different according to the primary cancer histology and molecular subtype [17,18]. Metastases from invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) have different preferential tropisms and target organs in their metastatic spread, as well as different metabolic behaviors, resulting in dedicated imaging strategies [19,20]. It is crucial to confirm the presence of malignant disease through a biopsy.
Per definition, OMD is potentially amenable for local ablative treatment (also called metastasis directed treatment), aimed at achieving a complete remission status. The hypothesis behind this approach is that ablating the apparent disease could delay further seeding of other metastatic lesions. The most commonly technique used to treat bone or lung metastasis is stereotactic ablative body radiotherapy (SABR or SBRT). Surgical resection and radiofrequency ablation (RFA) have also been evaluated for liver lesions [21].
Currently, available data does not support the impact of local therapies on overall survival, therefore it cannot be recommended in routine clinical practice. It may be considered, in highly selected cases, after a careful multidisciplinary team discussion and shared decision with the patient, balancing potential gains and risks and explaining the lack of evidence regarding its impact on survival.
Observational data and phase 2 trials [22,23] raised the possibility of a benefit from metastasis directed treatment. The SABR-COMET randomized phase 2 trial, enrolled multiple tumor types including only 18 breast cancer patients, showed long term benefit in OS and PFS in patients with controlled primary tumors and up to five metastases [24], although at the cost of more toxicity [25]. More recently, the randomized NRG-BR002 phase 2 trial (n = 125) compared standard of care systemic therapy (SOC) with or without metastasis directed treatments (SBRT and/or surgical resection) for oligometastatic breast cancer with ≤4 extracranial lesions based on standard imaging, with controlled loco-regional disease and <12 months of initial systemic therapy. After 72 pre-specified events, the study did not show a benefit for the experimental arm, neither in the primary endpoint (PFS) nor in any of the secondary endpoints (OS, new metastases outside the index area or PFS by baseline circulating tumor cells). There were fewer new metastases inside the index area in the ablative arm at 7 % compared to 29 % for SOC [26]. This trial has some limitations: 79 % of cases were ER+/HER2 negative ABC, 78 % oligo-recurrent disease, baseline imaging had limited sensitivity and there was an imbalance in ET use (83 % SOC vs 68 % ablative arm).
The results of several other ongoing breast cancer specific phase 3 trials will provide further data on the impact of metastatic directed treatment on survival and determine if there are patients who may benefit from this approach [27]. At this time, standard of care first-line systemic therapies remain the recommended approach.
Of note, in patients with prior clinical benefit from CDK4/6 inhibitor plus aromatase inhibitors (AI), the possibility to delay a change in systemic therapy by use of SBRT, including the possibility of subsequent SBRT, for up to 5 sites of oligo-progressive disease was investigated in the AVATAR phase 2 trial (n = 32) with a median time until progression not amenable to further SBRT of 10.4 months [28]. The impact on survival was not reported. Although these results are interesting, more data are necessary before it can be recommended for routine clinical practice.
In situations of oligometastatic disease as well as initially inoperable locally advanced disease, it is very important to communicate with the patient regarding the decision taken at the multidisciplinary tumor board, regarding the duration of treatment proposed (continuous as in multi-metastatic disease or more limited in time, with duration of treatment more similar to the early setting).
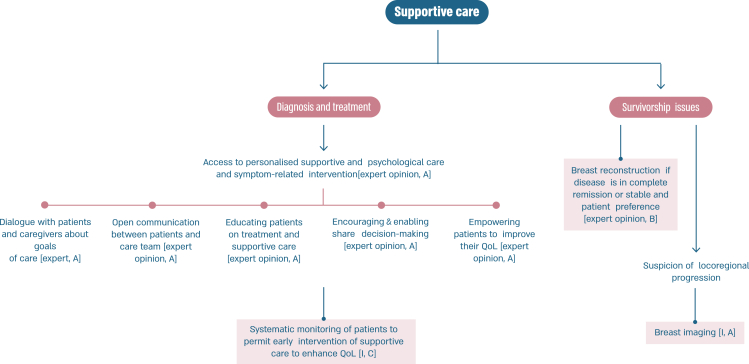
2.4. Section III: General guidelines (see Fig. 5)
Fig. 5.
ABC follow-up and supportive care.a.
Legend: ABC, advanced breast cancer; QoL, quality of life. a Throughout the cancer pathway, adequate information should be provided to the patient.
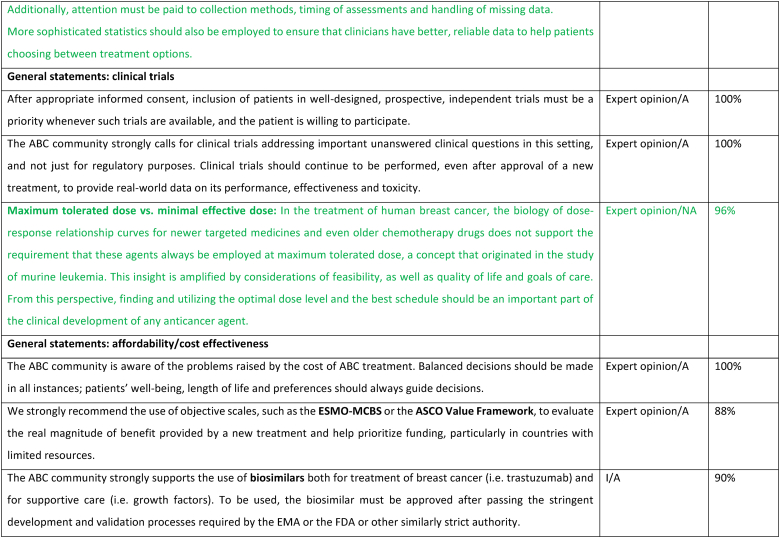
2.5. Maximum tolerated dose vs minimum effective dose
A fundamental paradigm in medical oncology has long been that higher dose levels of cytotoxic drugs kill more cancer cells and that this results in benefit to the patient. This concept derived from the study of murine transplanted leukemia—the log-kill hypothesis [29]. One flaw in the extrapolation of these observations to human disease is that the experimental work used leukemia cell eradication as the desired result, rather than maximum time of disease control in settings in which cure was not possible. Another is that clinical solid tumors follow sigmoid growth kinetics rather than the exponential patterns seen in transplantable mouse leukemias [30]. Sigmoid growth might be the result of cancer self-seeding, stem cell kinetics, or a combination of factors [31]. But the net result is that dose-schedule relations favor frequent administration of moderate dose levels (increased density) over more widely spaced higher levels (dose level escalation) [32]. This has been proven in the post-operative breast cancer adjuvant setting by the results of prospective trials, which also challenged the paradigm of the general superiority of simultaneous, and hence more toxic, multi-agent combinations [33]. Furthermore, in preparation for the first of these trials—CALGB 9741—several studies demonstrated that higher dose levels do not convey advantages over more moderate levels in the treatment of advanced disease [[34], [35], [36]]. Subsequent trials extended these ideas into the design of less-toxic, at least equally efficacious schedules of paclitaxel and the oral cytotoxic drug capecitabine [[37], [38], [39], [40]]. Optimal dose-scheduling of newer anti-cancer drugs, especially antibody-drug conjugates, might also favor less toxic, optimally timed, lower dose level approaches [41]. When duration of disease control, thereby maximizing clinical benefit, is the goal, the relevance of the maximum tolerated dose log-kill paradigm must be questioned. This concept has been further challenged with new agents and studies of new therapies should consider minimum effective dose to allow quality of life to be maintained, to provide less toxicity and to maintain efficacy of the treatments.
2.6. Attention to drug interactions
Several drug interactions have been recognized and in particular drugs that potentiate QTc prolongations or that may interfere with efficacy. Numerous drugs may prolong QTc if given with ribociclib including tamoxifen [42]. In most situations the panel suggested using alternative agents; in the example of ribociclib and tamoxifen, using an alternative endocrine therapy or CDK4/6 inhibitor. Clinicians are advised to order ECGs and to work with their pharmacists or online tools to ensure there are no drug interactions when prescribing. Proton-pump inhibitors (PPIs) may decrease the circulating levels of CDK4/6 inhibitors although the true impact on clinical benefit is still unknown [43,44]. Emerging data suggest a potential impact of antibiotics on the microbiome and consequent compromise of immune-checkpoint inhibitors efficacy [45]. The effect of steroid in the mechanism and activity of checkpoint inhibitors remains an area of debate and contradictory evidence [46].
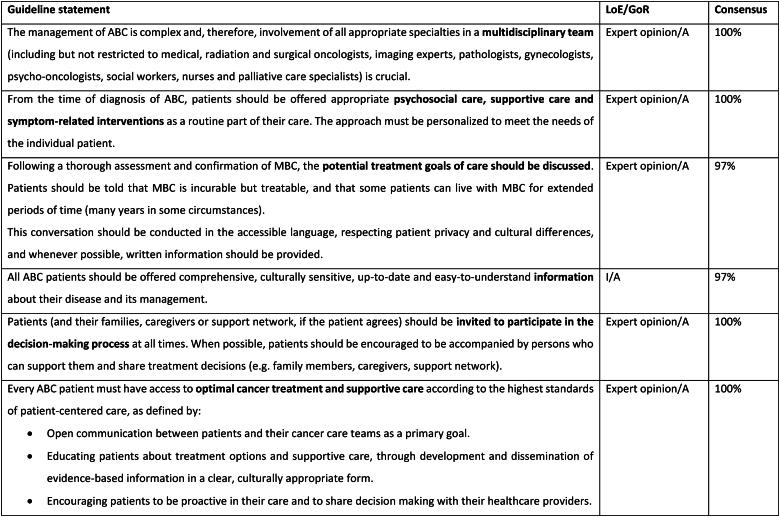
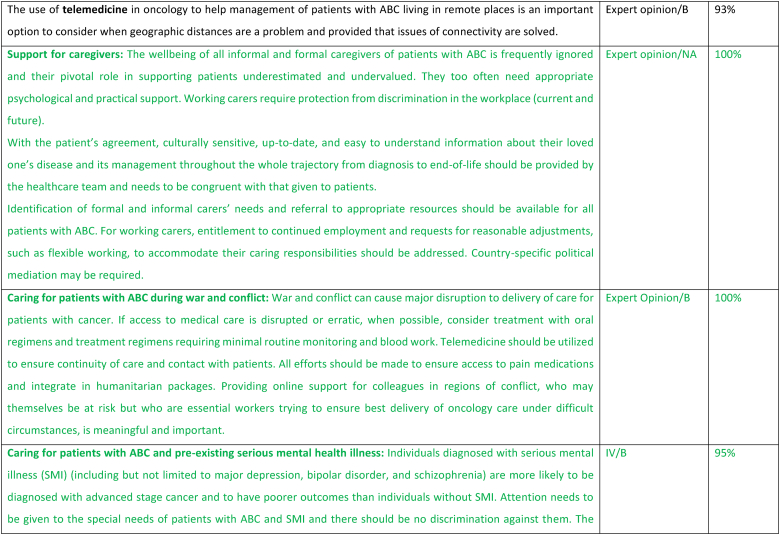
2.7. Support for caregivers
The care of patients with ABC optimally involves caregivers, particularly as the disease progresses and their importance must be acknowledged, both in terms of providing emotional as well as physical and practical support. Health care providers need to recognize the roles as well as the needs of the caregivers and enable them to perform their work more effectively by providing easy access to information about both the disease as well as the goals and treatment of the individual patient. The wellbeing of all informal and formal caregivers of patients with ABC is frequently ignored but they also often need appropriate psychological and practical support [47,48]. Working carers require protection from discrimination in the workplace (current and future). Working as a team with the caregivers through the trajectory of the patient's journey with ABC is fundamental.
2.8. Caring for patients with ABC during war and conflict
During times of war and conflict, significant interruptions to health care delivery pose an immense challenge in cancer care delivery for patients with cancer, as well as care providers. Conflict and war may impact cancer care delivery for multiple reasons including (i) shortages in health care staff (ii) diversion of health care resources to the injured (iii) interruption in drug supplies (iv) challenges in accessing health care facilities for both patients and staff (v) exacerbation of mental health issues including anxiety, depression, loneliness and isolation (vi) displacement of individuals to other regions distancing them from their healthcare and possibly not having medical summaries of their medical history (vii) delays in diagnosis and interruption of screening (viii) interruption in food supply, possibly famine (ix) poverty because of interruption to employment and income (x) increase risk of infection and communicable diseases (xi) destruction of essential civilian infrastructure including roads and hospitals (xii) loss of family structure and carers [[49], [50], [51]]. Under these circumstances, preference should be given to oral therapies and therapies that require minimal blood work. Additionally, all efforts should be made to ensure a sustained supply in and access to essential medications including pain medication as an integral part of humanitarian aid. Telemedicine may be a preferred option for patient care when telecommunications have not been interrupted. Cross-border collaborations with neighboring countries not affected by the conflict and when circumstances permit, mobile clinics, may aid access to and provision of care [51]. In addition to the impact on oncology patients, the health care providers may also be impacted by the conflict and may themselves be in danger [51]. Providing online support for colleagues in regions of conflict, who may themselves be at risk but who are essential workers trying to ensure the best delivery of oncology care under difficult circumstances, is meaningful and important.
2.9. Caring for patients with ABC and pre-existing serious mental health illness
Serious mental illness (SMI) may include major depression, schizophrenia, bipolar disorder, and substance abuse disorders. It is well established that individuals with SMI have lower uptake of cancer screening [52,53], as well as significant risk factors for cancer incidence, including smoking and higher incidence of obesity. Individuals with SMI are more likely to be diagnosed with advanced disease from the outset and to have poorer outcomes. Research has shown that only 50 % of breast oncologists take into consideration and address the SMI when providing care for patients with breast cancer [54]. Notably, patients with SMI have been shown to be less-likely to receive guideline-based breast cancer care [55]. All efforts should be made to incorporate the psychiatric health care team and the patient's carers in order to optimize compliance and health care delivery. Particular attention needs to be given to drug-drug interactions between the psychiatric medications and oncology drugs, and any necessary changes in the psychiatric medication must be coordinated with the patient's psychiatrist. Under certain circumstances, steroids and medicinal cannabis use should be minimized to avoid triggering episodes of mania and psychosis and the treating psychiatrist should be consulted before use of these medications, particularly in individuals with bipolar disorder and schizophrenia.
2.10. Access of patients with ABC to intensive care units (ICU)
Efforts aimed at understanding and improving the quality of care for patients with ABC must consider that high quality care requires an understanding of and facilitation of individual patient preferences. While some patients might be interested in extending life as a primary goal, others may prioritize specific domains of functioning or life goals, or general quality of life. Patient autonomy is a fundamental principle here, which should be considered in the context of the clinical situation and societal constraints. The COVID-19 pandemic served as a natural experiment as many life and death decisions had to be made, especially in the beginning of the pandemic when resources (such as ventilators) were scarce. In many countries, cancer patients in general and metastatic cancer patients in particular were often considered at the bottom of the priority list for access to ventilators and ICUs. Some types of advanced setting may be extremely indolent or well controlled for many years and others may have more deleterious trajectories such that the benefits of intensive treatment for a complication or unrelated co-morbidity for a given patient must be considered in that context. For example, a patient with indolent metastatic breast cancer who gets an infectious disease should generally be treated similarly to a patient who doesn't have breast cancer given that the patient may have a several-year life expectancy otherwise. In contrast, heroic measures to treat a life-threatening comorbidity in a patient whose cancer has not been under control for some time or that is a complication of their cancer may be less worthwhile for that patient. While both governmental and non-governmental organizations have considered versions of cancer patients' bill of rights [[56], [57], [58], [59]], limited attention to date has been paid to end of life care in this regard. Fortunately, a number of initiatives have focused on optimizing communication among patients with advanced cancer including breast cancer patients.
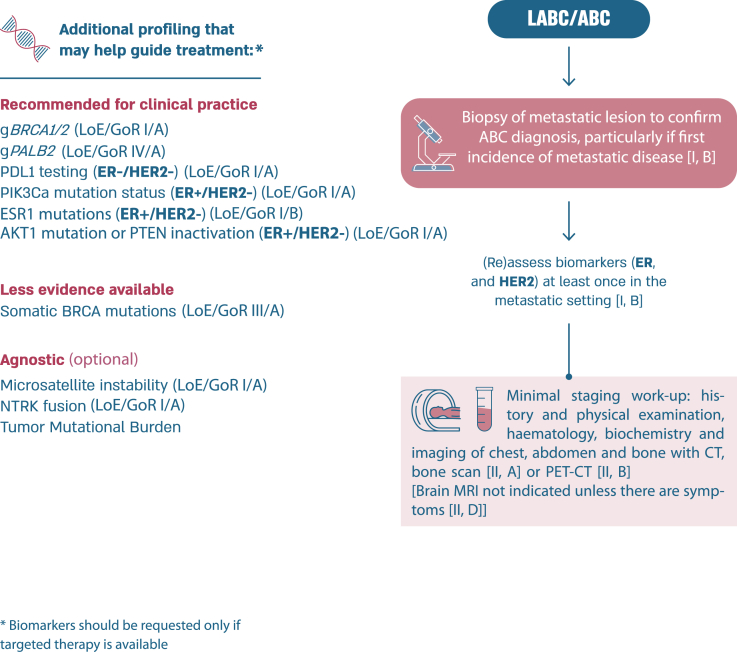
2.11. Section IV: assessment and treatment general guidelines (see Fig. 1)
Fig. 1.
ABC diagnostic work-up and staging.
Legend: ABC, advanced breast cancer; CT, computed tomography; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; LABC, locally advanced breast cancer; MRI, magnetic resonance imaging; PET, positron emission tomography.
2.12. Biopsy guidelines
All patients with newly diagnosed lesions should have a biopsy, if clinically feasible, to confirm the presence of breast cancer and to assess the subtype of the recurrence.
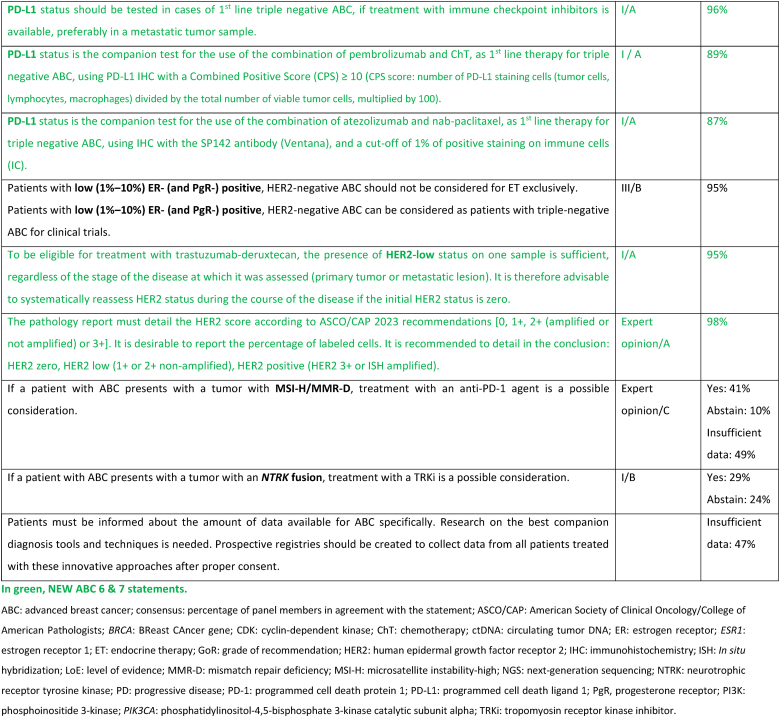
Biological markers (especially ER and HER2) should be reassessed at least once in the metastatic setting, even in case of a bone-only metastatic presentation. In this case, the oncologist must communicate with the interventional radiologist and the pathologist so that the decalcification of the bone biopsy is not carried out in an acidic solution but in EDTA which preserves the antigenic sites for immunohistochemistry and nucleic acids for in situ hybridization or molecular biology [60]. If a bone lesion is the biopsy site, it is preferable to biopsy a mixed lytic and sclerotic lesion rather than a pure sclerotic area to increase the chance of retrieving adequate high-quality cells to assess. In choosing the site it should be an active site on PET imaging or by history, safe to biopsy, and appropriate for testing (see liver biopsies and PD-L1 testing below). In cases where there is discordance in response between sites, more than one area should be biopsied and fully tested to obtain a clear picture of the biology of the disease and plan treatment accordingly.
In cases of discordance between the primary tumor and the metastatic site(s), the first step is to revise the full case, if possible with double-reading, and in some cases, consider re-biopsy.
The value of PR expression in the metastatic setting is limited and reserved only for confirmation of triple negative status. ER-/HER2-/PR + ABC is rare and data from early breast cancer show that they may not be responsive to endocrine treatment. As a clinical trial addressing this issue is difficult to undertake, we recommend considering therapies for triple negative ABC [61]. If an HER2 positive tumor becomes HER2-negative at re-biopsy, the result should be questioned. It is necessary to check by ISH, to re-check the HER2 status of the initial tumor for possible intratumoral heterogeneity, and finally not to hesitate to repeat HER2 testing in case of new progression [62,63].
PD-L1 expression should be tested in cases of first-line triple-negative ABC if treatment with immune checkpoint inhibitors is available, as a companion test for either the combination of pembrolizumab and chemotherapy, with a PD-L1 assay (with 22C3 antibody) and a combined score of 10 or more (CPS score) [[65], [66]] or the combination of atezolizumab and nab-paclitaxel, with SP-142 antibody (Ventana) and a score of 1 % or more positive immune cells (IC score) [67]. If possible, consider avoiding PD-L1 determination in liver biopsy. Due to the general lack of immune cells in this organ, the PD-L1 status is consistently lower in liver biopsy as compared to other metastatic sites [68,69].
2.13. “Treatment holidays”
The aim of any treatment for a patient with ABC must be not only to add quantity to life, but also quality, allowing the patient to continue to build and achieve life projects. With this goal in mind, and to be able to enjoy life to the full without the constraints or side-effects of the treatments, some patients ask for “treatment holidays”. This notion should be understood as a period of surveillance without treatment. It is neither a definitive cessation of treatment, nor simply a lengthening of the interval between two courses of treatment. The response to this request must consider two parameters to adapt it to the risk of disease progression: the level of disease control at the time the decision is made and the biological ABC sub-type. The development of treatments has now made it possible to achieve not only more responses, but also more complete responses, and for longer periods. This occurs more often at the start of treatment for metastatic disease [70]. It is particularly true for HER2-positive ABC with the addition of anti-HER2 agents, and for ER-positive ABC with the addition of CDK4/6 inhibitors [71]. HER2-positive ABC currently have the highest expected rate of complete and durable remissions, and one of the longest life expectancies in the metastatic setting [72]. There are some retrospective data analyzing “treatment holidays” in HER2-positive ABC. It seems that these are safer to consider in cases of complete and durable remission (>2 years) and perhaps in younger populations [73]. In ER+/HER2 negative ABC, early progression (<12 months) on a CDK4/6 inhibitor regimen is a strong clinical marker of a less favorable outcome [74]. If side-effects are not the main reason for “treatment holidays”, some form of maintenance therapy could be the preferred option, to be discussed with the patient [75]. In the case of triple-negative ABC, even though certain targeted treatments can achieve significant benefits, only a very small proportion of patients experience long-term control of their disease (>12–18 months). Under these conditions, it is difficult to define criteria for “treatment holidays”, and this decision must be made based on patient demand and clinical judgement, and should be limited in time.
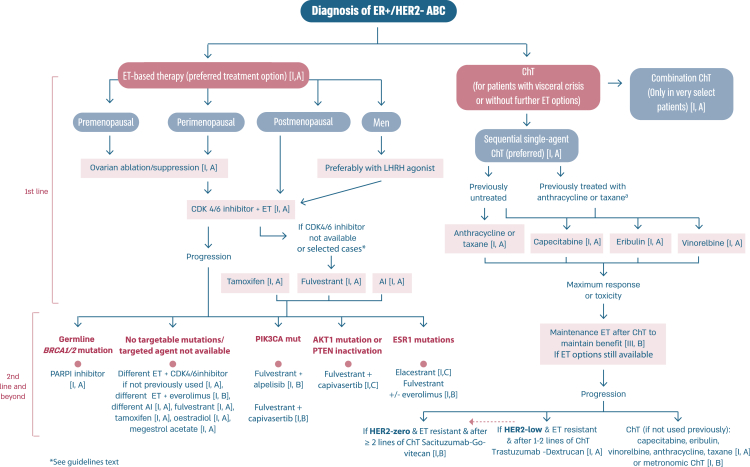
2.14. Section V: ER-positive/HER2-negative (luminal-like) ABC (see Fig. 2)
Fig. 2.
Treatment of ER-positive/HER2-negative ABC
Legend: ABC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin-dependent kinase; DFI, disease-free interval; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; PIK3CA, phosphatidylinositol-4,5-biphosphate 3-kinase catalytic subunit alpha. a Rechallenge with a taxane or anthracycline is possible if cumulative dose not reached and DFI ≥12 months.
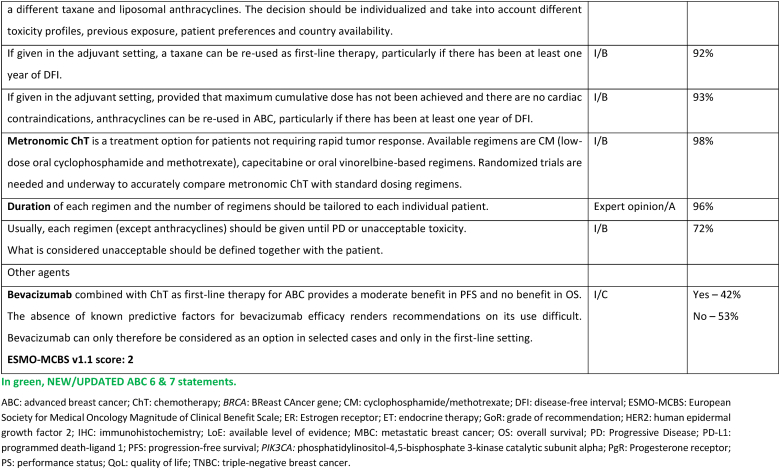
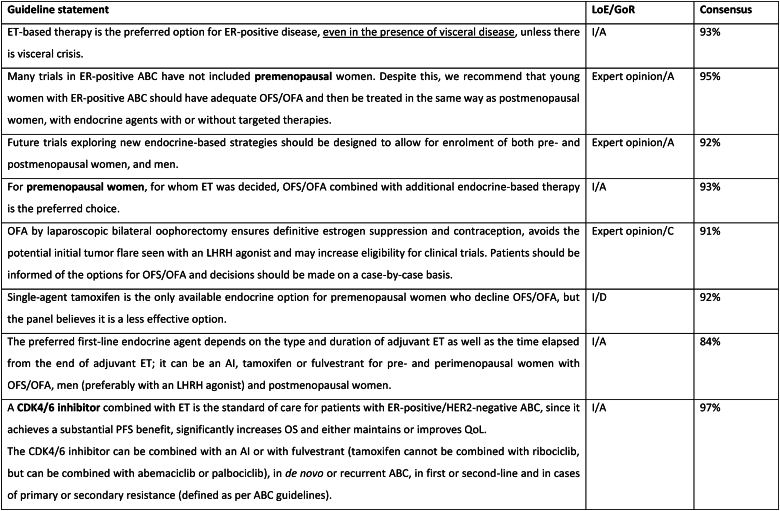
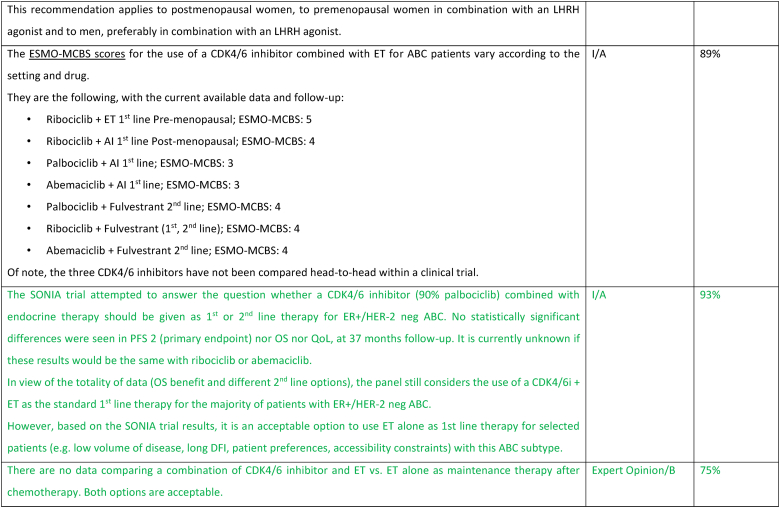
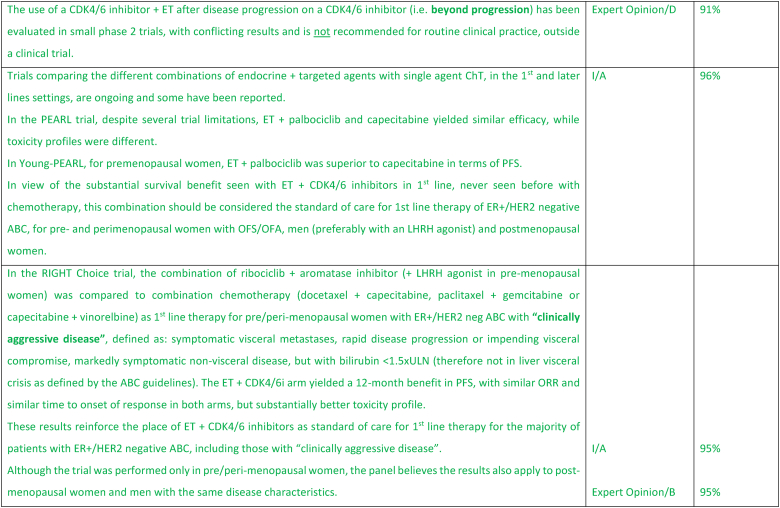
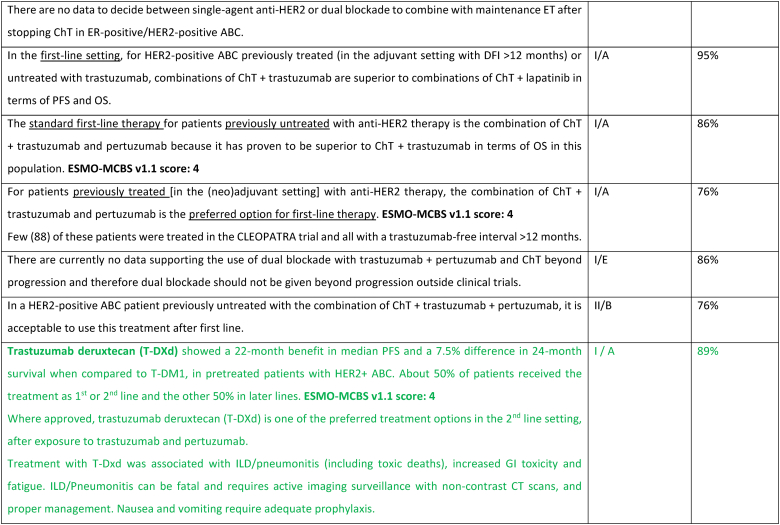
The treatment of ER-positive/HER2 negative ABC has seen several advances in recent years [[76], [77], [78], [79]]. Ribociclib combinations have shown statistically significant and clinically meaningful benefit in overall survival as well as progression free survival [80], both in pre and postmenopausal women and men. Other CDK4/6 inhibitors remain options, based on patient comorbidities, tolerance, availability. After ABC7, results of the final OS analysis from MONARCH 3 were presented, showing a numerical improvement of 13.1 months that did not reach statistical significance [81]. Studies comparing capecitabine to hormonal therapy and CDK4/6 inhibitors did not show a benefit for the early introduction of this chemotherapy [82,83]. Furthermore, the RIGHT Choice trial showed superiority in terms of PFS and tolerability for ribociclib plus ET, when compared with combination chemotherapy, in a patient population presenting with high disease burden [84]. The definition of visceral crisis in the RIGHT Choice trial was not according to the ABC 5 Guidelines since bilirubin could not be above 1.5 times the ULN, per inclusion criteria.
The role of continuing a CDK4/6 inhibitor beyond progression, either switching the endocrine backbone or switching to another CDK4/6 inhibitor, has been evaluated in three small phase 2 trials, with somewhat different outcomes: the MAINTAIN, PACE and PALMIRA trials [[85], [86], [87]]. In the MAINTAIN trial, both CDK4/6 inhibitor and ET were switched upon progression, leading to a small improvement in PFS [86]. In the PACE and PALMIRA trials, only ET was switched upon progression, failing to prove beneficial. In the PACE trial, a third arm was included with the addition of avelumab, a PD-L1 inhibitor, showing a PFS benefit, although not statistically significant [85]. Due to these conflicting results and weak evidence, continuing a CDK4/6 inhibitor upon progression is not recommended, outside a clinical trial. Results from phase 3 trials, such as the postMONARCH study, are still awaited [88].
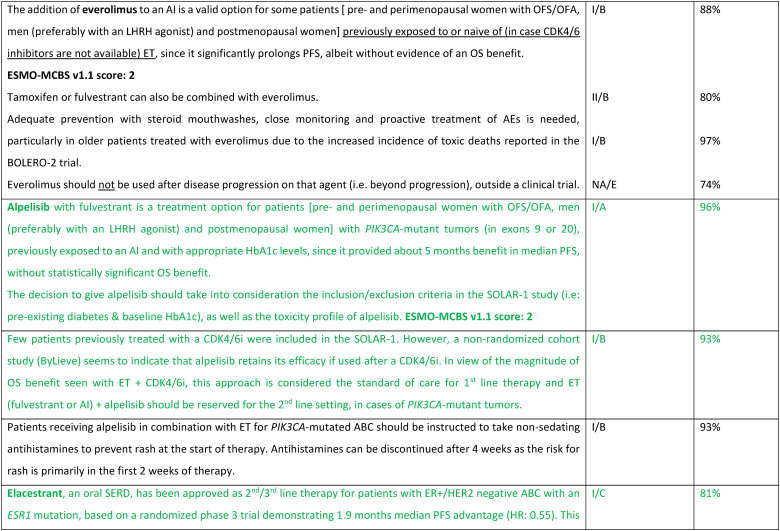
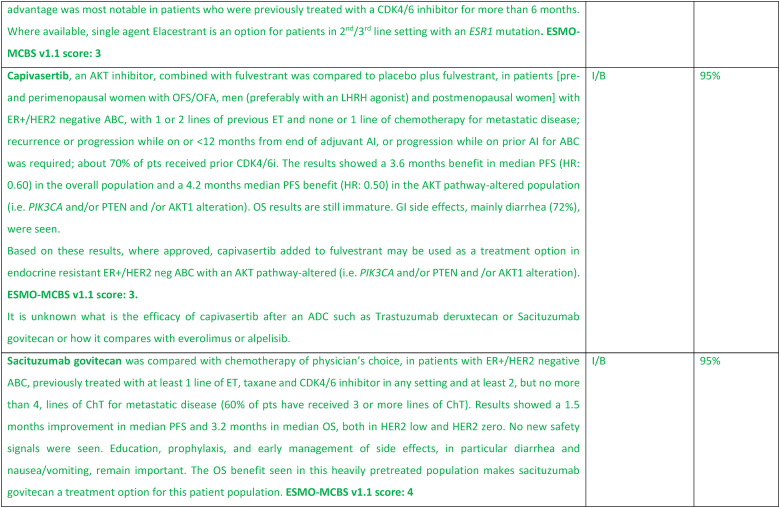
While the optimal sequencing of treatments following progression on ET + CDK4/6 inhibitor remains to be defined, several options may be considered, favoring a sequential use of endocrine-based therapies, considering patient comorbidities, preferences, and emergence of potential targetable alterations. Alpelisib plus fulvestrant is an option for patients whose tumors harbor PIK3CA mutations, as 2nd line therapy, based on the results of the randomized SOLAR-1 study [89] and the non-randomized BYLieve study [90]. The latter provided relevant data on the benefit of alpelisib after prior exposure to CDK4/6 inhibitors, for pre-menopausal women and in combination with an AI. The ongoing phase 3 EPIK-B5 trial will better define the role of this combination upon progression to CDK4/6 inhibitors [91]. In the CAPItello-291 phase 3 trial, in particular in tumors exhibiting PIK3CA/AKT1/PTEN alterations, capivasertib with fulvestrant showed improved PFS and was approved following progression on at least one ET-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant ET [92].
The new generation of anti-estrogen therapies, the oral selective estrogen receptor degraders/downregulators (SERDs) were developed to try to overcome some of the mechanisms of endocrine resistance, especially acquired ESR1 mutations, as well as to address limitations of current ET, such as intramuscular administration of fulvestrant and the agonist activity of tamoxifen. So far, only one of these agents, elascestrant, has been approved based on the results of the phase 3 EMERALD trial [93], which showed a small increase in PFS when compared to fulvestrant, after treatment with a CDK4/6 inhibitor. The magnitude of benefit was somewhat higher in tumors harboring an ESR1 mutation.
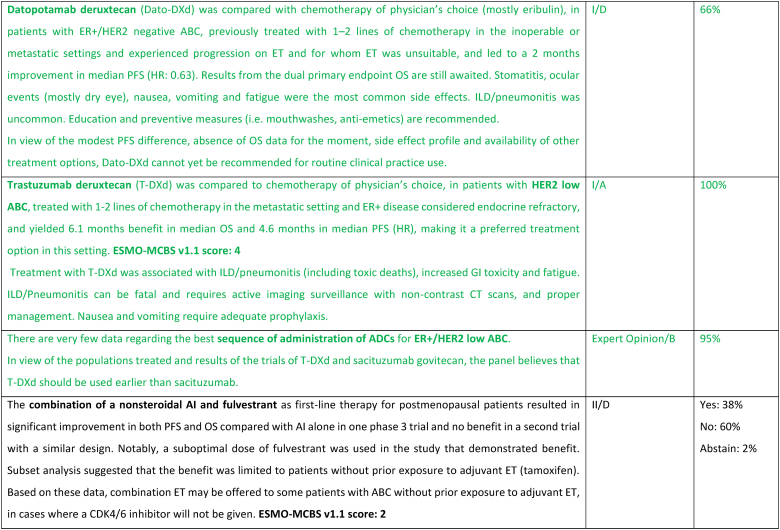
A new treatment option for this ABC subtype are ADCs, namely trastuzumab-deruxtecan (T-DXd) and sacituzumab govitecan. For patients with ER-positive/HER2 low tumors (89 % of the trial population), the DESTINY-Breast04 trial established T-DXd as one of the preferred treatment options [94], as compared to mono-chemotherapy of physician's choice, in disease considered endocrine-refractory by the trial (not exactly according to the ABC definition), in view of the substantial benefit in OS (about 6 months) and PFS. These benefits need to be balanced against associated toxicity, with two adverse events of interest occurring more frequently with T-DXd: left ventricular dysfunction, largely asymptomatic, and interstitial lung disease, emphasizing the need for close monitoring and early interventions to prevent serious complications. For patients with ER-positive/HER2 negative tumors considered endocrine-resistant by the trial (not exactly according to the ABC definition), sacituzumab govitecan lead to improved PFS and OS (3,3 months) in the phase 3 TROPiCS-02 trial [95,96], over mono-chemotherapy of physician's choice. So far, no head-to-head comparisons of T-DXd and sacituzumab govitecan were performed and no robust data exist regarding optimal sequencing of these agents. Given the magnitude of benefit of T-DXd in this ABC subtype, the panel recommends the use of this agent, when indicated, earlier than SG.
Datopotamab deruxtecan, a TROP2-directed ADC, showed modest improvement in PFS over standard ChT in the TROPION-Breast01 trial [97] (PFS 6.9 months vs 4.9 months) and OS data is not yet mature. These results and the fact that approval has not yet been granted, leads the panel to not recommend, at the present moment, this drug for use in routine clinical practice.
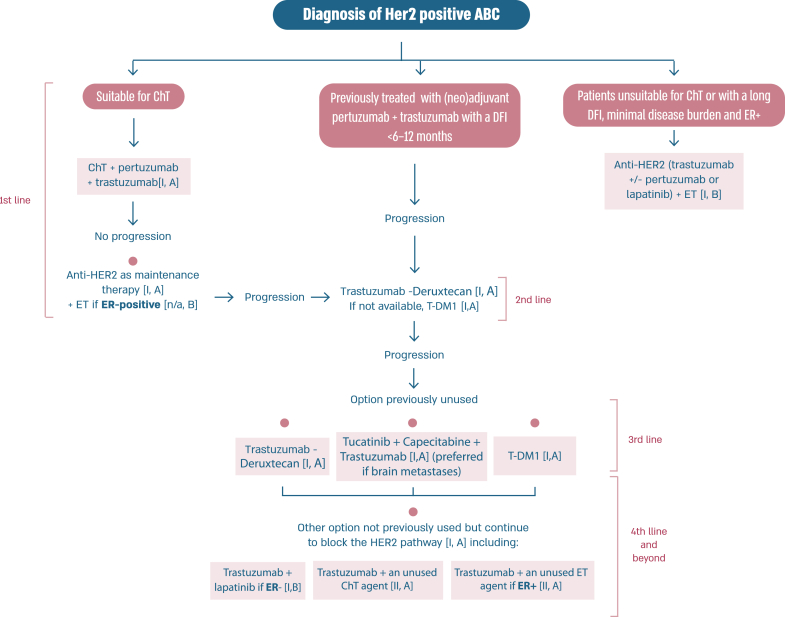
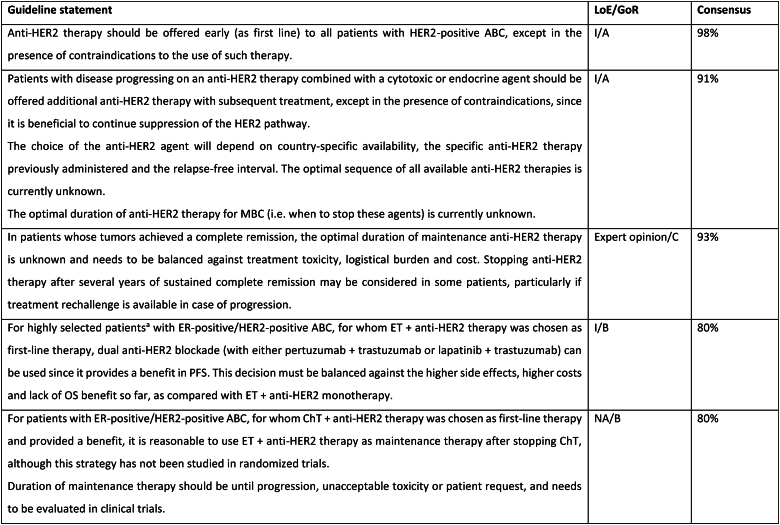
2.15. Section VI: HER2-positive ABC (see Fig. 3)
Fig. 3.
Treatment of HER2-positive ABC
Legend: ABC, advanced breast cancer; ChT, chemotherapy; DFI, disease-free interval; ER, oestrogen receptor; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; T-DM1, trastuzumab-emtansine.
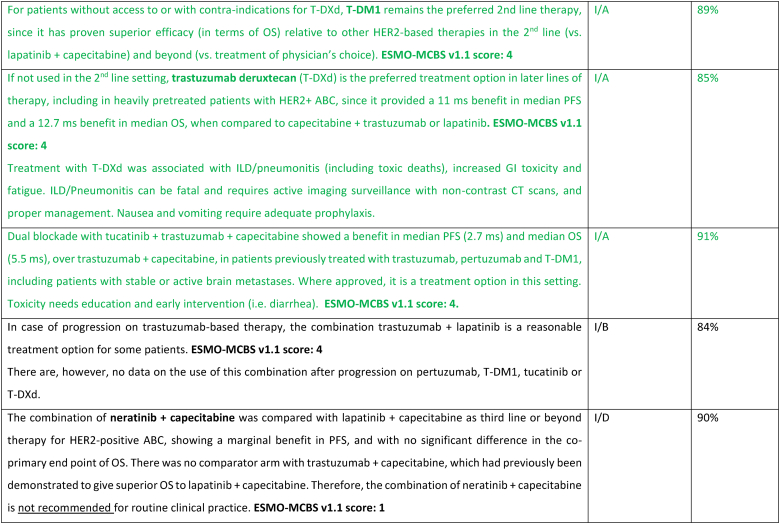
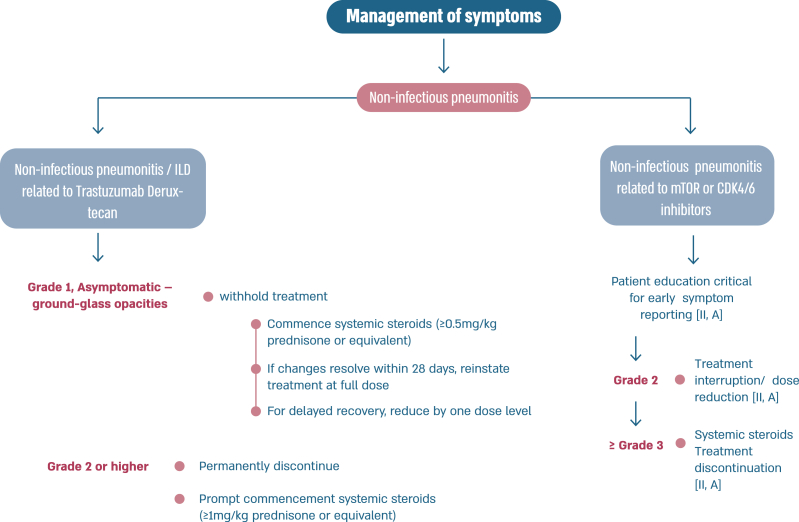
In the last few years, several new agents have demonstrated activity against advanced HER2-positive breast cancer and have been incorporated in the treatment algorithms [98,99]. T-DXd was evaluated in the large phase 2 study DESTINY-Breast01 study [100], for heavily pretreated patients (median 6 lines, range 2–27 lines including trastuzumab and T-DM1), yielding a response rate of 62.0 %, a median PFS of 19.4 months (95 % CI, 14.1–25.0) and a median OS of 29.1 months (95 % CI 24.6–36.1 months) [101]. T-DXd was associated with 15.8 % risk of interstitial lung disease (ILD)/pneumonitis, fatal in 2.7 % of cases, which needs appropriate and rapid diagnosis and treatment [101]. In the phase 3 study DESTINY-Breast02, T-DXd was compared to capecitabine combined with trastuzumab or lapatinib, in heavily pretreated patients with HER2 overexpressing ABC and showed 10.9 months benefit in PFS and 12.7 months benefit in OS. In this trial there were 4 toxic deaths, two of which due to ILD [102]. In the phase 3 trial DESTINY-Breast03, T-DXd was compared to T-DM1, the previous standard 2nd line therapy, in taxane- and trastuzumab-pretreated patients [64], and yielded a PFS improvement of 22 months, with median OS still not reached [104]. T-DXd was associated with 10.5 % of ILD events, but no grade 4 or grade 5 cases, showing that adequate monitoring and prompt management are crucial. For the safe utilization of this compound in current clinical practice, both active surveillance and education of patients and health care professionals are crucial to facilitate rapid diagnosis and management of ILD [103].
Tucatinib, a highly selective inhibitor of the HER2 tyrosine kinase, was tested in combination with capecitabine and trastuzumab in a population of patients with HER2-positive ABC pretreated with trastuzumab, pertuzumab and T-DM1 and reported an improvement in PFS (median 7.8 months vs 5.6 months) and OS (median 21.9 months and 17.4 months), compared to capecitabine-trastuzumab-placebo, in the HER2CLIMB-01 study [105,106]. Importantly, a similar benefit was observed in patients with brain metastases, including active brain metastases, a unique group of patients included in this trial. The experimental arm had increased toxicity, mostly diarrhea and elevated aminotransferase levels of grade 3 or higher, but this did not lead to treatment discontinuation nor significant impact on quality of life.
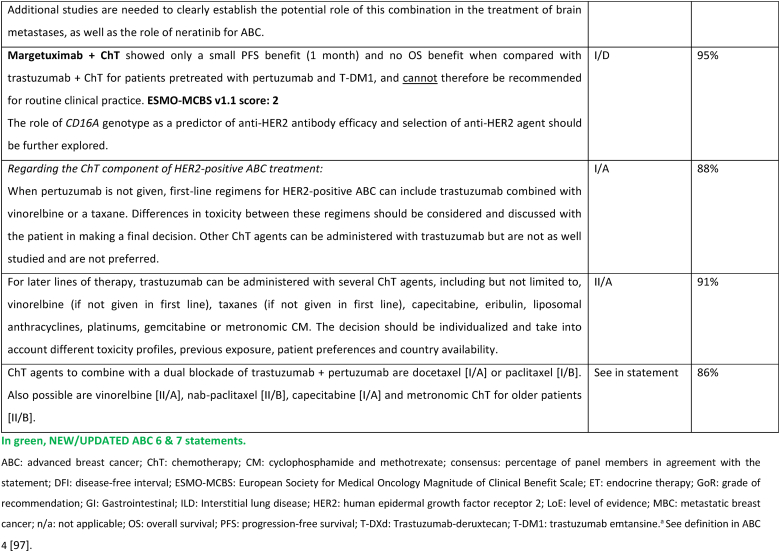
Two additional agents have not demonstrated clinically meaningful benefit in trials of pretreated HER2-positive ABC patients and are therefore not recommended for clinical practice by the ABC panel. Margetuximab was compared to trastuzumab (both combined with chemotherapy of physician's choice) and resulted in a 0.9 month PFS difference [107]. The potential role of CD16A genotype as a predictor of the type of anti-HER2 antibody efficacy was explored and deserves further evaluation. Neratinib was compared to lapatinib, both agents in combination with capecitabine, in the NALA trial, and provided a small reduction in the risk of disease progression of 24 %, a marginal difference in PFS and no impact on overall survival (co-primary endpoint) [108]. Furthermore, the NALA study has the important limitation of not having a comparator arm with trastuzumab + capecitabine, which was previously shown to provide superior OS to lapatinib + capecitabine [109].
In resource limited regions or countries, pyrotinib represents a treatment option that can be less expensive and, where regulatory approved, can be considered for treatment of patients with HER2-positive ABC [110,111].
2.16. Section VII: Triple negative ABC (see Fig. 4)
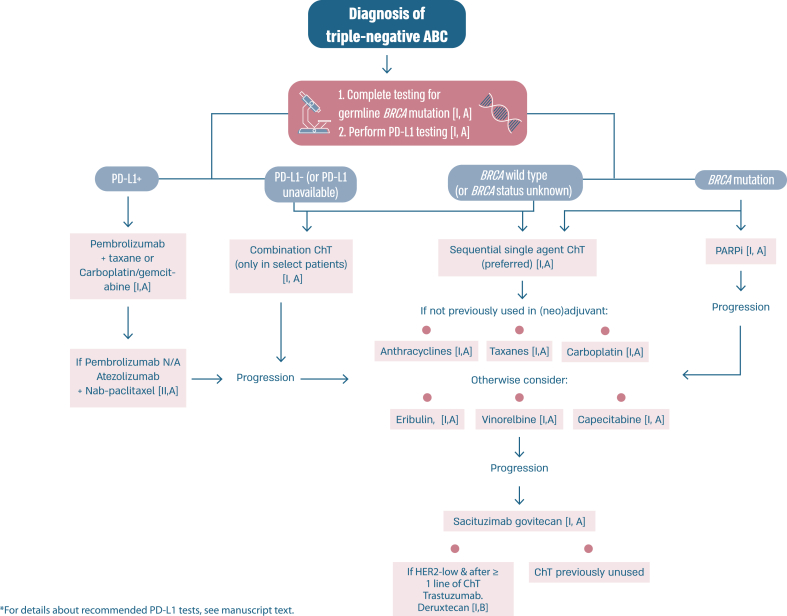
Fig. 4.
Treatment of triple-negative ABC.
Legend: ABC, advanced breast cancer; ChT, chemotherapy; PARPI, poly-adenosine diphosphate ribose polymerase inhibitor; PD-L1, programmed death-ligand 1; N/A, not available.
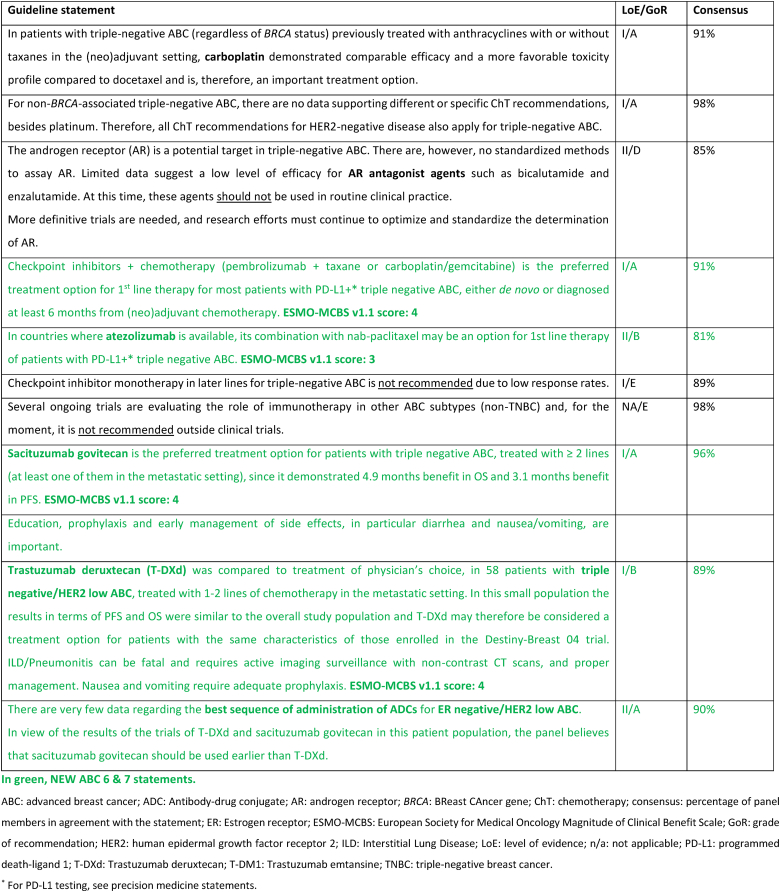
After years of little progress in the treatment of triple negative (TN) ABC, new agents are showing promise. In the KEYNOTE -355 trial [65,66] the addition of pembrolizumab to chemotherapy with paclitaxel, nab-paclitaxel or carboplatin/gemcitabine showed a significant benefit for patients with tumors that were Combined Positive Score (CPS) >=10 who were either de novo Stage IV or who had relapsed more than 6 months after adjuvant therapy with an OS of 23.0 months in the pembrolizumab–chemotherapy group and 16.1 months in the placebo–chemotherapy group [66]. The panel approved this regimen as the treatment of choice for first line TN ABC meeting eligibility criteria. There was discussion about the uncertainty for those with tumors that are CPS 1–10 and where more data are needed. It was recognized that in some countries atezolizumab is an option that can be considered in combination with nab-paclitaxel as there was benefit in PFS seen in the IMPASSION130 study [112], although OS results are controversial and the IMPASSION131 trial [113] with paclitaxel was negative. Sacituzumab govitecan has shown to offer a PFS and OS benefit for later lines of therapy following the results of the ASCENT study [114]. The benefit was independent of existence or not of low HER2 expression. With proper attention to toxicity, especially gastrointestinal, hematological and fatigue, this agent is relatively well tolerated. Results from the DESTINY-Breast04 trial have created a new therapeutic option for patients with ER-negative/HER2 low ABC, despite the fact that only 11 % of the trial population had this ABC subtype. The benefit of T-DXd, compared to standard chemotherapy options, was evaluated in an exploratory analysis in this sub-population and was similar to the whole trial population (median PFS 8.5 months vs 2.9 months, respectively) (71).
Similarly to what was discussed above for ER-positive/HER2-negative ABC, also for triple negative ABC no head-to-head comparisons of T-DXd and SG were performed and almost no data exist regarding optimal sequencing of these agents. Analyzing the totality of the data and in view of the higher level of evidence brought by the ASCENT trial, the panel recommends the use of SG earlier than T-DXd for triple negative ABC. It remains unclear if cross-resistance exists since both these ADCs include a topoisomerase I inhibitor.
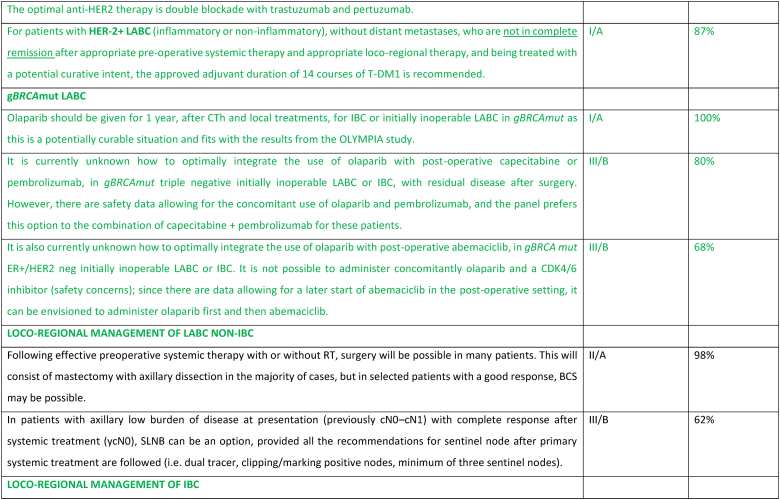
2.17. Section VIII: Hereditary ABC
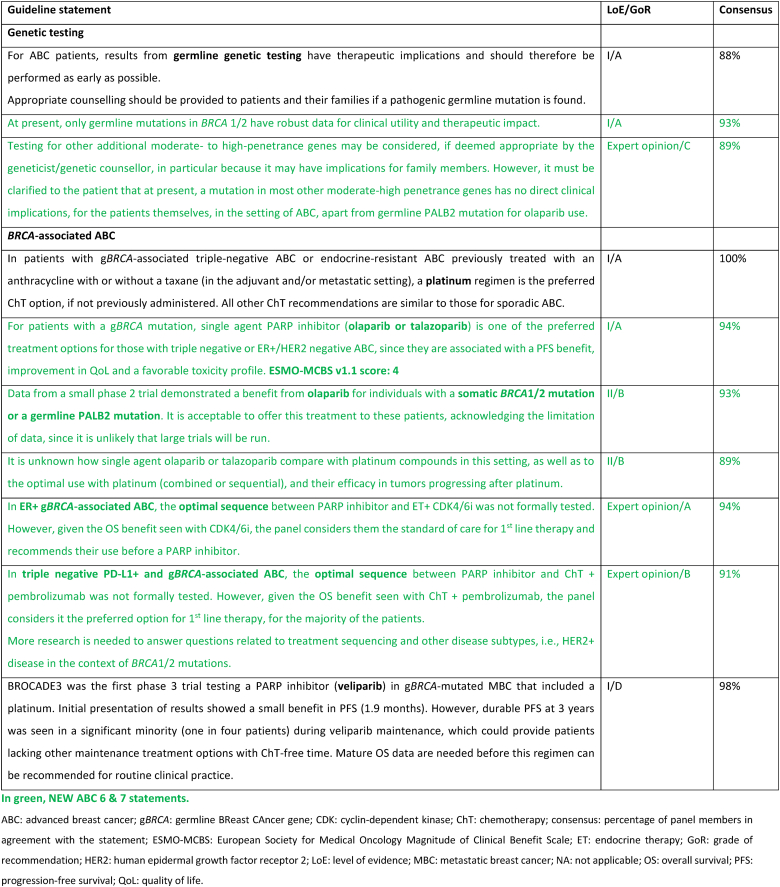
Germline genetic testing for a pathogenic variant in BRCA1/2 has therapeutic implications and should be performed as early as possible for any patient who would be eligible for a PARP inhibitor, all patients with triple-negative breast cancer, males with breast cancer and those meeting international/national guidelines for genetic testing for a hereditary cancer syndrome [115,116]. At present, pathogenic variants in other hereditary breast cancer associated syndromes do not impact choice of systemic therapy in ABC, outside of a clinical trial setting apart from a germline pathogenic variant in PALB2. Amongst women with a pathogenic variant in BRCA1/2, PARP inhibitors have consistently demonstrated superiority over standard single-agent chemotherapy (not including platinum agents) in both the OlympiaAD and EMBRACA studies that evaluated the efficacy of olaparib and talazoparib, respectively [[117], [118], [119], [120], [121], [122]]. In both studies, patients receiving a PARP inhibitor had a significantly higher response rate, PFS and quality of life. The BROCADE study was the first phase 3 study in ABC comparing the addition of a PARP inhibitor to a platinum containing regimen, with a treatment protocol of paclitaxel and carboplatin with or without veliparib for gBRCA associated ABC. The study demonstrated a PFS benefit favoring the veliparib arm, with a median PFS of 14.5 compared to 12.6 months, with a suggestion of sustained response at two and three years favoring the veliparib arm that was receiving maintenance veliparib [123]. In light of the significant toxicity of combination chemotherapy (with or without veliparib), for the time being veliparib combined with chemotherapy is not recommended in the ABC setting.
There are no data assessing optimal treatment sequencing of PARP inhibitors with other subtype-specific therapies. Thus, for patients with a pathogenic variant in BRCA1/2 and ER-positive/HER2 negative ABC the panel recommends commencing with first line ET + CDK4/6 inhibitor before the use of a PARP inhibitor, due to significant OS benefit seen with this combination. For patients with a pathogenic variant in BRCA1/2 and PD-L1+ triple negative ABC, the panel recommends commencing with immunotherapy + ChT and using a PARP inhibitor as a subsequent line of therapy. In patients with PD-L1 negative triple negative ABC, a PARP inhibitor should be offered as an option for first line therapy.
A small phase 2 study demonstrated a benefit from olaparib in patients harboring a germline pathogenic variant in PALB2 and in those with somatic mutations in BRCA1/2 [124]. Although the study is small, it is unlikely that there will be further larger studies. Thus, based on this limited data, the panel supports offering olaparib in these cases.
Further studies are needed to clarify the value of PARP inhibitors in platinum-resistant disease, as well as their value compared with platinum compounds.
2.18. Section IX: precision medicine (see Fig. 1)
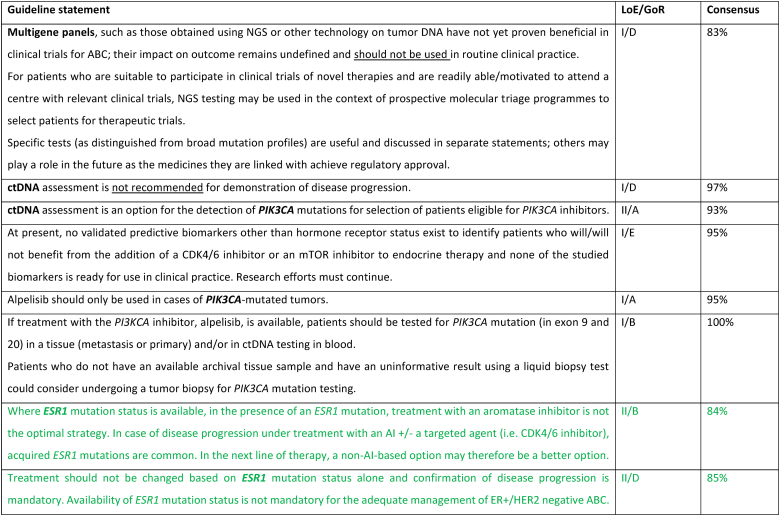
Acquisition of ESR1 mutations, frequent in patients with ABC previously treated with aromatase inhibitors (20%–40 %) is one of the mechanisms of resistance to endocrine therapies with some evidence that tumors with this mutation respond less well overall to endocrine treatments, not just to aromatase inhibitors [125,126]. There are encouraging but limited data in the PADA-1 trial [127], showing PFS benefit of a switch from letrozole to fulvestrant in combination with palbociclib, in case of a rising circulating ESR1 mutation in ctDNA detected in sequential liquid biopsies, without tumor progression. The ABC panel considers that additional data are needed to change therapy based solely on ESR1 mutation status, and that confirmation of disease progression is mandatory. Although knowledge of ESR1 status is not mandatory for the management of a patient with ABC, if this technology is available and feasible, it may guide towards a non-aromatase inhibitor therapeutic strategy [128]. The ESCAT scale for ESR1 mutations is Tier II-a [129]. The ongoing SERENA-6 trial, with a similar design, is using the next-generation oral SERD camizestrant (NCT04964934) [130].
Intrinsic subtyping by PAM50 has recently identified the presence of Luminal A, Luminal B, HER2-enriched and Basal-like tumors within HR+/HER2 negative ABC [[131], [132], [133]]. Of note, 15 % of HR+/HER2 negative ABC are HER2-enriched and 5 % Basal-like. Intrinsic subtype in HR+/HER2 negative ABC is a strong and consistent prognostic biomarker of PFS and OS following endocrine-based therapy, including endocrine therapy and CDK4/6 inhibitors [131,134,135]. From a predictive perspective, Basal-like disease is associated with a lack of benefit from endocrine therapy and CDK4/6i [131]. The predictive value of intrinsic subtype is currently being evaluated in the phase 3 HARMONIA clinical trial (NCT05207709).
Targeting low levels of HER2 expression has reshaped the treatment paradigm for approximately half of patients with ABC. Therefore, correctly stating low levels of HER2 expression in pathology reports, in the cases of tumors traditionally defined as “HER2-negative” (HER2 1+ or 2+ without amplification at in situ hybridization testing) is essential, since it provides the opportunity for treatment of ABC with potent, novel, HER2-directed agents [[136], [137], [138], [139]]. Currently approved for the treatment of pretreated patients with HER2-low ABC is trastuzumab-deruxtecan, based on the results of the DESTINY-Breast04 phase 3 trial [94].
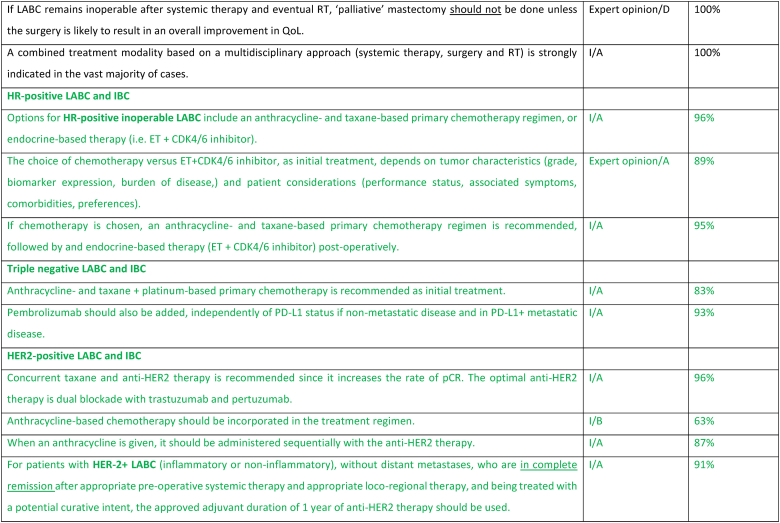
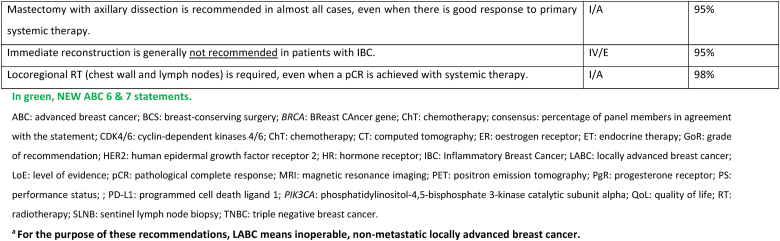
2.19. Section X: LABCa (inflammatory and non-inflammatory) and inflammatory ABC (IBC) (see Fig. 1)
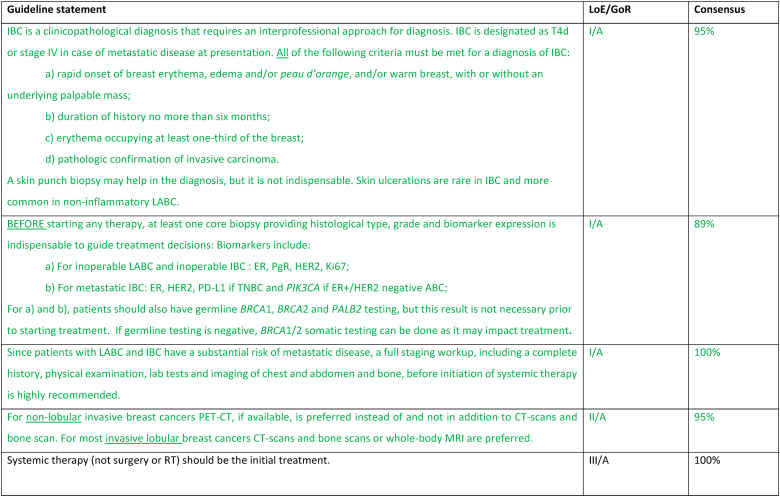
Most patients who present with unresectable LABC, inflammatory or non-inflammatory, nonmetastatic disease should initiate treatment with primary systemic therapy. Upfront staging and biopsy are mandatory. 18-FDG PET-CT is the preferred imaging for the staging of all subtypes except invasive lobular cancers [140], as it is more sensitive and may up-stage inflammatory (IBC) and locally advanced (LABC) cancers in up to 52 % of cases, detecting 1/3 more metastases [141]. The choice of systemic treatment depends on the pathological features of the disease, therapeutic goals, comorbidities, and patient's choice, as biology predicts response to neo-adjuvant treatments [142]. For HER2-positive subtype, treatment should include dual blockade (pertuzumab and trastuzumab) as 40 % of patients included in the NeoSphere study had LABC or IBC [143]. For triple-negative subtype, treatment with pembrolizumab plus chemotherapy according to the KEYNOTE-522 regimen is the recommended treatment since approximately 25 % of included patients had LABC or IBC [144]. For ER+/HER2-negative subtype, the initial systemic treatment may be anthracycline- and taxane-based chemotherapy or ET + CDK4/6 inhibitor; the choice between these two options should be based on disease and patient characteristics.
If the disease is rendered resectable, systemic therapy should be followed by surgery, radiation therapy, and adjuvant treatment accordingly to residual disease, including T-DM1 for HER2 positive cancers [145] or olaparib for patients with germline BRCA1-or BRCA2-mutations [146]. The concomitant use of olaparib with immunotherapy in the post-operative setting for patients with TNBC and without pathologic complete response may be considered, based on existing safety data [147]. If the disease remains unresectable, consideration should be given to treating all sites of the original tumor extension with radiation, including a boost to the area of residual disease. In locally advanced non-inflammatory breast cancer, breast conservation, if possible after neoadjuvant systemic treatment, has loco-regional recurrence rates at 5–10 years similar to mastectomy. Breast reconstruction after mastectomy was not associated with higher rates of local recurrence nor worst OS, in large retrospective studies [148,149]. Sentinel node biopsy in N0 patients at presentation or targeted axillary dissection in N1 converted to N0 after treatment can be used, employing the same rules as used in early breast cancer. In patients with N2/N3 disease at presentation and in IBC there is no evidence to support any surgical procedure other than axillary lymph node dissection, even in cases of good response due to the high rate of false negatives. Clinical experience suggests that most durable remissions can be expected with an elective radiation dose up to an equivalent of 50 Gy to regions with a high likelihood of bearing subclinical disease and a boost up to 60–76 Gy to all sites of macroscopic disease. In unresectable cases where radiation is the first local treatment, regular evaluations during the course of radiation are advised, to select patients that might become amenable for resection after 45–50 Gy. Interesting reports have been published on combined radiation and ChT such as cisplatin, 5-FU, docetaxel or vinorelbine. Further evaluation of the benefit of combining radiation with a PARP inhibitor is ongoing in a prospective trial in patients with LABC or metastatic TNBC and in non-responders to primary ChT [[150], [151], [152]].
2.20. Section XI: Specific populations
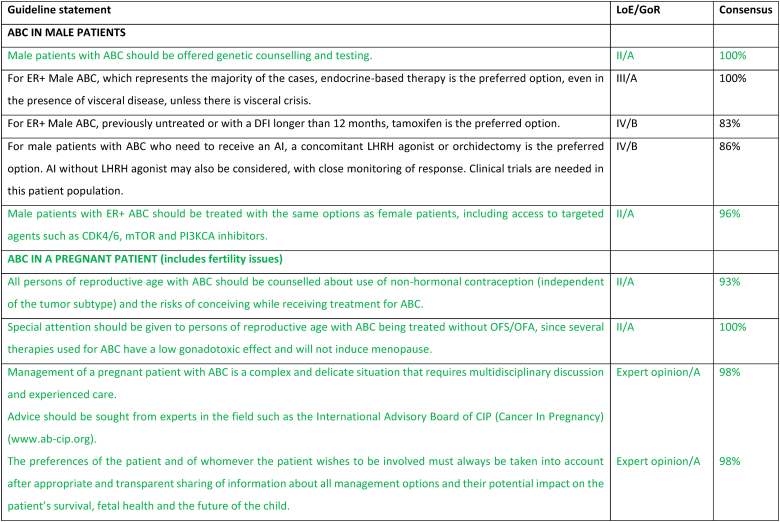
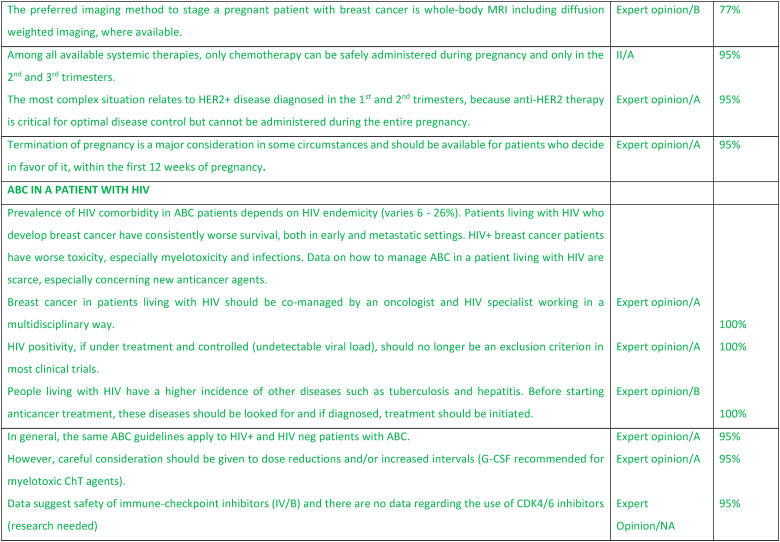
2.21. ABC in a pregnant patient (includes fertility issues)
Amongst young patients with ABC, issues of fertility and contraception are often overlooked. Additionally, pregnancy during ABC is a very complex issue. The desire for pregnancy amongst women with ABC can pose a challenge for health care providers both in terms of medical management and psychosocial management. Moreover, there are times when the medical team may not agree with the patient's choices. All patients with ABC need to be counseled about the need for effective non-hormonal contraception, irrespective of subtype, with a clear communication about the risks for the mother and the fetus of pregnancy while on treatment. Notably, it should be emphasized that for patients not receiving OFS, many therapies are not gonadotoxic and will not induce menopause [153,154]. In terms of fertility preservation, all women of reproductive age (irrespective of disease stage) should be counselled about the impact of cancer therapies on their fertility and the availability of fertility preservation techniques [153,154]. For the patient with ABC this discussion needs to be balanced and presented in the context of the diagnosis of an incurable disease and the need to constantly be on therapy with a clear explanation that interruption of therapy to conceive would likely endanger the mother by preventing much needed treatment for disease control and compromise prolongation of survival. The question of future pregnancy will be an increasing clinical challenge as women with ABC live longer in particular for those with prolonged clinical remissions. If ABC is suspected during pregnancy, the preferred imaging modality for staging is whole-body MRI including diffusion-weighted sequences, where available. If not available, then a combination of non-contrast MRI of the axial skeleton (full-spine and pelvic bones), MRI of the liver including diffusion-weighted sequences, and low-dose chest computed tomography (with abdominal shielding) are suggested. Of note, the safety of the imaging methods depends on gestational age of pregnancy, with some methods being safe with shielding (e.g., chest X-ray or chest CT early in pregnancy). Ultrasound, namely breast or abdominal ultrasound, is safe anytime during pregnancy.
For patients diagnosed with ABC while pregnant, the treatment approach will depend on the trimester at diagnosis, disease subtype and patient preference. The preferences of the patient and of whomever the patient wishes to involve must always be considered after an appropriate and transparent sharing of information about all management options and their potential impact on the patient's survival, fetal health and the future of the child. Termination should be readily available to women who favor this approach and should be discussed in particular for women who are diagnosed in the first 12 weeks of pregnancy when no systemic therapy is considered safe. During the second and third trimester certain chemotherapy agents (anthracyclines, paclitaxel, cyclophosphamide) can be safely administered – the preferred regimens with the most robust safety data would be an anthracycline or paclitaxel [155,156]. Targeted therapies (including but not limited to anti-HER2 agents, ADCs, PARP inhibitors and CDK4/6 inhibitors), immunotherapy and endocrine therapy are contraindicated during pregnancy, because of established risk to the fetus or absence of safety data [155,156]. The greatest challenge is for patients with HER2+ ABC for whom anti-HER2 therapy would need to be delayed until after delivery, potentially compromising patient outcome – the extent of disease and week of pregnancy play a major factor in decision making about continuation or termination of pregnancy in this case. Noteworthy, prematurity is a significantly greater risk factor for impaired cognitive development in the exposed offspring than chemotherapy exposure [157]. Assuming the pregnancy is to be continued and there is no impending danger to the mother's life, the optimal timing for delivery is after week 37. The pregnant patient should be managed by a multi-disciplinary team, preferably in a tertiary, experienced center [153]. Advice should be sought from experts in the field such as the International Advisory Board of CIP (Cancer In Pregnancy) (www.ab-cip.org).
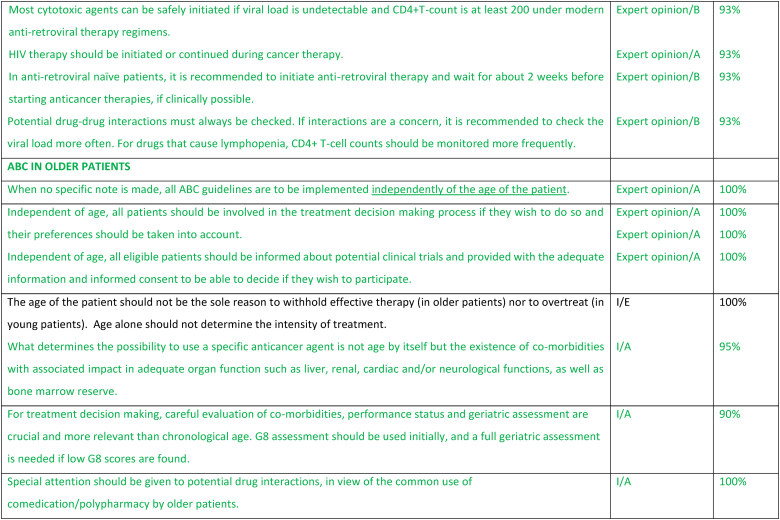
2.22. ABC in a patient with HIV
The incidence of breast cancer is similar for people living with HIV or without HIV, but people living with HIV are usually diagnosed at an earlier age and have a worse survival [158]. Cancer incidence is rising in HIV-endemic regions [159]: widespread use of antiretroviral therapy (ART) has turned HIV into a chronic condition, with latent immunosuppression leading to a higher incidence of non-AIDS defining cancers as breast cancer. HIV leads to a higher risk of chemotherapy-induced myelotoxicity and infections, leading to more dose reductions, lower relative dose intensities, and worse outcomes [160]. Even when equivalent relative dose intensities of ChT can be obtained with the use of granulocyte-colony stimulating factors (G-CSF), the pathological complete response rate is lower in people living with HIV on neoadjuvant ChT [161]. This may be due to exhaustion of the tumor infiltrating T cells [162]. More research is needed on breast cancer in people living with HIV, with inclusion in clinical trials, in particular if the viral load is low. In general, the same ABC guidelines apply to people living with HIV. ChT can be safely initiated if viral load is undetectable and CD4+ T-count is above 200. As there is an increased risk of myelotoxicity under ChT, G-CSF should be recommended, especially to avoid dose reductions or delays. And given the higher incidence (or relapse) of tuberculosis and hepatitis B under ChT, these infections should be screened upfront, and treatment should be initiated when detected. Patient with ABC and HIV should be co-managed by an oncologist and HIV specialist, in a multidisciplinary way. If a patient is ART naïve, ART must be initiated as soon as possible as it will improve outcomes [163]. To manage the initial ART related side-effects, cancer treatment may be delayed by 1–2 weeks. If a patient is already under ART, it is important to continue ART and check for potential drug-drug interactions. Most 1st-line ART drugs do not interfere with most anticancer drugs. If interactions can lead to decreased activity of ART drugs, the evolution of the HIV viral load must be checked more often, and ART treatment adapted if viral load increases. And If CD-4 count goes down due to ChT whilst maintaining a stable viral load, opportunistic infections must be monitored. Some data suggest safety of immune-checkpoint inhibitors [164] but there are no data regarding CDK4/6 inhibitors’ safety in these patients.
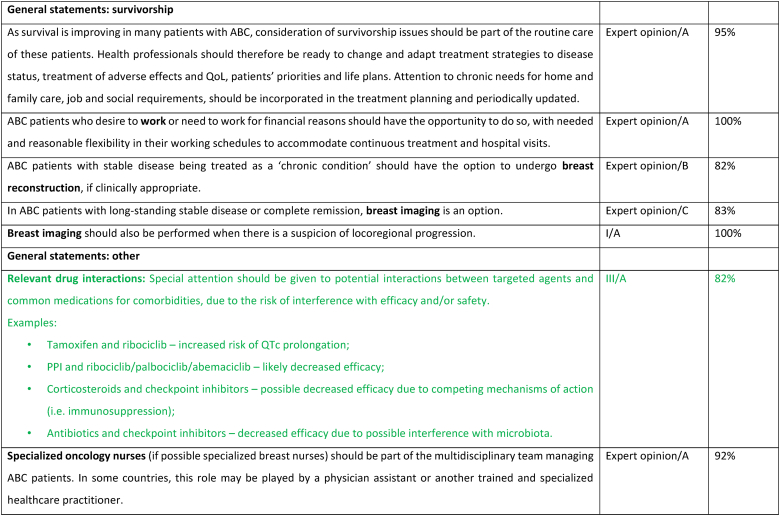
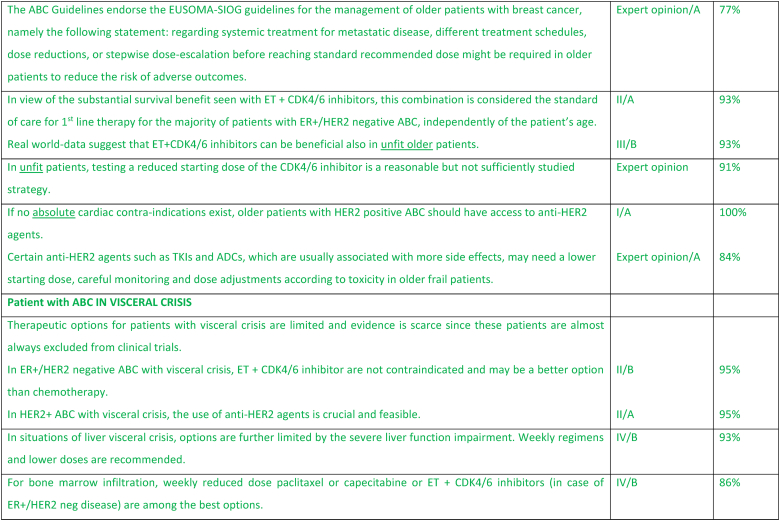
2.23. ABC in older patients
Age is a major risk factor of breast cancer and with life expectancy increasing, the incidence of breast cancer among older women is expected to increase. The underrepresentation of the older population in clinical trials and the heterogeneity of health status of these patients represent major challenges for an evidence-based management of older patients with ABC and may explain the poorer outcomes reported in this population [165]. It is now clearly apparent that chronological age by itself is neither a criterion to exclude older patients with ABC from active and innovative strategies, nor a reason to prevent their participating in clinical trials [166,167]. It is exceptional to see a solely age-dependent treatment effect in fit selected older patients included in clinical studies. Safety and treatment adherence might be an issue [168]. For this reason, a proper evaluation of the health status of older patients with ABC, starting with a frailty screen, or when it is possible, with a comprehensive geriatric assessment should be at the basis of treatment decision making [166,169]. Polypharmacy is common, especially in unfit patients, and therefore special attention should be given to potential interactions when prescribing anticancer agents [170,171]. Real-world data indicate that up to 70 % of older patients with ABC are at potential risk of frailty [172]. Consequently, the SIOG–EUSOMA recommendations on the management of older patients with breast cancer consider that different treatment schedules, dose reductions, or stepwise dose-escalation before reaching standard recommended dose might be required in to reduce the risk of adverse outcomes [173]. Testing a reduced starting dose in unfit patients, even if quite a common and reasonable procedure in clinical practice, is a strategy which needs adequate studies [166,174]. In patients with ER+/HER2 negative ABC, 1st line ET + CDK4/6 inhibitor is considered the standard, with evidence of benefit also in unfit older patients [166,172]. Access to anti-HER2 therapy should be provided, in the absence of cardiac contraindications, to older patients with HER2+ ABC [174]. The EUSOMA-SIOG recommendations about treatment “personalization” refer mainly to ChT but can be extrapolated to new treatments for which data on unfit patients are not yet available. As with patients of all ages, older patients with ABC should be involved in the decision-making process and their preferences taken into account [173].
2.24. Patient with ABC in visceral crisis
Visceral crisis is usually defined as severe organ dysfunction as assessed by signs, symptoms and laboratory studies, resulting from rapid progression of neoplastic disease and indicative of substantial visceral compromise that may serve as an indication for more aggressive therapeutic intervention [175]. The ABC guidelines further clarified visceral crisis as defined in liver as rapidly increasing bilirubin >1.5x ULN in the absence of an obstruction, and in lung as rapidly increasing dyspnea at rest in the absence of pleural effusion [176]. Visceral crisis is not only the presence of visceral metastases but is associated with life-threatening organ compromise requiring rapidly efficacious therapy – generally consisting of single agent or, in select cases, combination chemotherapy with or without targeted agents [177]. Visceral crisis at presentation of metastatic disease is thought to be rare, occurring in less than about 15 % of patients, and more frequently in patients with de novo metastatic and highly proliferative breast cancer subtypes. The treatment of visceral crisis in later lines of therapy must be moderated by goals, toxicity and potential efficacy of available therapies. Treatment options vary by biologic subtype, site of disease, and line of therapy. For patients with ER+/HER2 negative ABC, endocrine maintenance therapy is recommended after disease response or stabilization with ChT [177]. For patients with modest visceral dysfunction, ET + CDK4/6 inhibitor appears to provide superior efficacy to combination ChT with less toxicity, as demonstrated in the RIGHT Choice Trial, although patients with true hepatic visceral crisis were not included [84]. For HER2 positive ABC, the combination of ChT and anti-HER2 monoclonal antibodies can rescue even severe organ dysfunction at initial presentation. Triple negative ABC presents the greatest challenge, with treatment dictated by immune markers. New antibody drug conjugates offer a potential highly effective alternative strategy that is currently under investigation. Treatment of patients with visceral crisis is complicated by lack of data regarding optimal dosing in situations of liver or renal dysfunction, and by the fact that patients with visceral disease are almost always excluded from clinical trials. Lower doses of weekly paclitaxel or nab-paclitaxel, platinum compounds, and other single agent ChT have been evaluated in patients with visceral crisis without a clearly most effective regimen, and significant variation in line of therapy and organ involvement [178]. The use of the correct definition of visceral crisis in clinical trials and in practice is critical. With new and highly effective therapies, the concept of impending visceral crisis needs to be re-visited, as evidenced by the data from the RIGHT Choice Trial, and will be addressed in a future update of the ABC guidelines.
2.25. Section XII: Specific sites of metastases
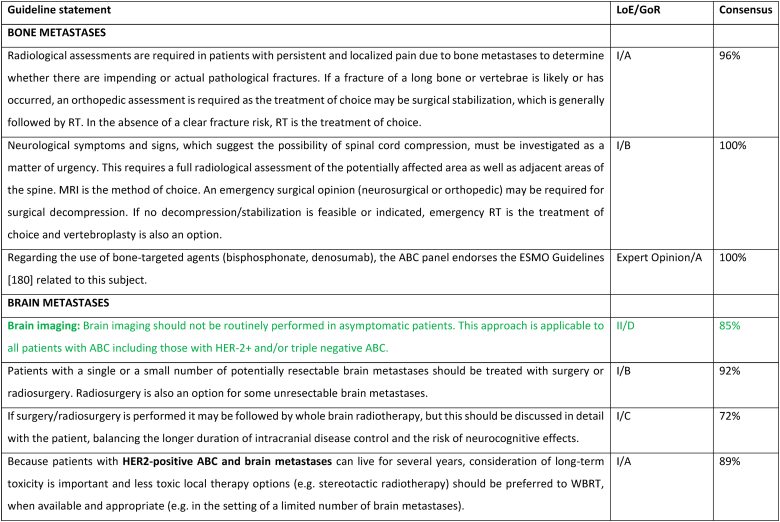
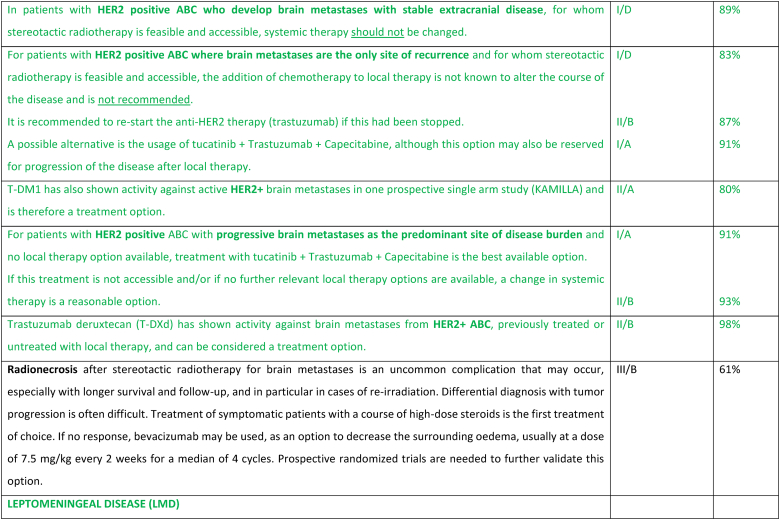
2.26. Brain metastases
Clinical trials for HER2+ or triple negative ABC generally require baseline brain imaging, however outside of the clinical trial setting, brain imaging is not recommended in asymptomatic patients. The role of brain imaging in routine management of asymptomatic patients is being evaluated in prospective clinical trials such as NCT04030507. Large randomized clinical trials evaluating local therapies in patients with brain metastases include patients with brain metastases from a variety of cancer types. The incidence of brain metastases in breast cancer patients is increasing mainly due to improved systemic therapies resulting in more durable control of extracranial metastatic disease and prolonged survival. The management of breast cancer brain metastases is challenging, even more so with the continued advancement of local and highly effective systemic therapies. Treatment of brain metastases should be based on multidisciplinary team discussions and a shared decision with the patient, considering the risks and benefits, aiming to prolong survival while maintaining quality of life. Strategies for graded prognostic assessment of brain metastasis from breast cancer have been proposed to help decision making [179]. For most patients, a metastases-directed initial ablative strategy including surgery and/or radiation therapy is preferred, especially when the metastatic burden is limited [180]. Surgical resection can be both diagnostic and informative in terms of providing histopathological confirmation of the tumor type and biomarkers, since changes might have occurred [181]. Surgical resection is often considered the preferred approach for lesions in the posterior fossa, where even minor volume changes (from e.g., edema) may result in a significant increase in symptoms. Stereotactic brain radiation therapy or stereotactic radiosurgery (SRS) should be the preferred local treatment option for most patients if they have a good performance status and metastatic disease without an indication for surgery. Although multiple lesions are often treated, the total volume and number must allow for effective and safe SRS [180]. Following SRS, if there are increased neurologic symptoms and/or increased local radiologic effects, it may be difficult to distinguish between local tumor progression versus radio-necrosis. Either may respond to steroids. In recent years, whole brain radiotherapy (WBRT) has fallen out of favor as the preferred strategy due to the concerns over cognitive impairment when anticipated survival is more than a few months, as well as the increasing availability of SRS. In the HER2Climb trial, patients with HER2+ ABC, who had received several lines of anti-HER2 therapies, were randomly assigned to tucatinib or placebo, combined with trastuzumab and capecitabine [106,182]. In the cohort of 291 patients (47.5 %) with brain metastases at baseline (including active brain metastases) the estimated 1-year PFS was 24.9 % (95 % CI, 16.5–34.3) in the tucatinib arm versus 0 % in the placebo arm, and the median PFS was 7.6 months versus 5.4 months. The adverse event profile was acceptable. In the subsequent OS analysis, in the baseline brain metastases cohort, the hazard ratio favored the tucatinib arm (HR 0.60; 95 % CI 0.444–0.81) [106]. Thus, a tucatinib-based regimen is a suitable therapy even in heavily pre-treated patients with HER2+ ABC. T-DM1 did not reduce the frequency of CNS recurrence in the post-neoadjuvant setting in the Katherine trial [183]. However, T-DM1 has been tested in patients with HER2+ ABC in a phase 3b single-arm study, where 398 patients had brain metastases at start of therapy [184]. All patients had received prior HER2-targeted systemic therapy, 6 % prior pertuzumab, and 56 % had also received prior brain radiation therapy. Results showed complete response and/or partial response in 21 % of the patients, and an additional 21 % had stable disease lasting minimum 6 months with a median PFS and OS of 5.5 and 18.9 months, respectively. T-DXd was compared to T-DM1 in the DESTINY-Breast03 Trial in previously treated HER2+ ABC [104]. T-DXd showed a significant improvement in PFS (HR 0.28; 95 % CI 0.22–0.37), with subgroup analysis supporting PFS benefit in those with baseline brain (n = 114) metastases (HR 0.38; 95 % CI 0.23–0.64). Encouraging data were also presented from the small subgroup with asymptomatic brain metastases (n = 24) treated with T-DXd in the DESTINY-Breast01 phase 2 trial, with a median PFS of 18.1 months (95 % CI, 6.7–18.1 months) [185]. A pooled analysis of patient with brain metastases (n = 148) treated with T-DXd in the DESTINY--Breast01, 02 and 03 trials, showed an intracranial response rate of 45 % and median CNS-PFS of 12.3 months in treated/stable BM and 18.5 months in untreated/active BM [186].
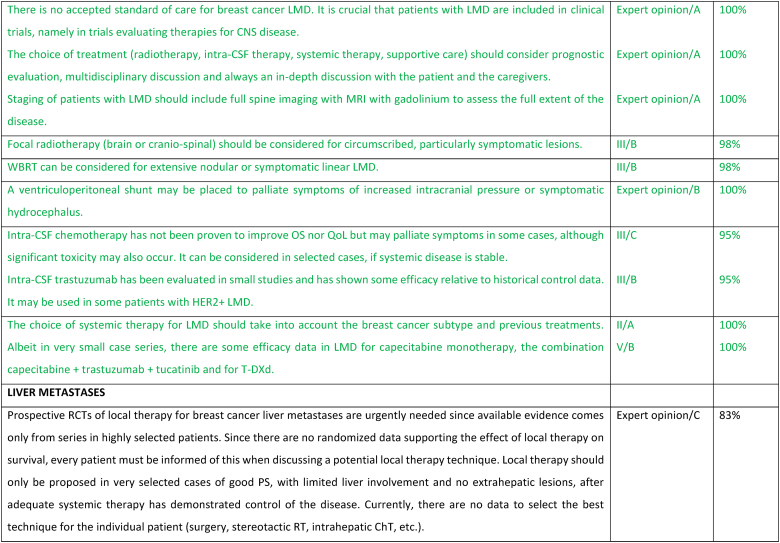
Leptomeningeal disease (LMD) is an aggressive complication of ABC with tumor cells infiltrating the leptomeninges, subarachnoid space, and CSF [187]. LMD is confirmed by positive CSF cytology or can be considered as probable (typical neurological signs and symptoms plus typical neuroimaging findings) or possible (atypical clinical and typical neuroimaging findings) [188,189]. LMD can be further classified into four subtypes based on MRI appearance: type A (typical linear MRI abnormalities), type B (nodular disease only), type C (both linear and nodular disease) and type D (no MRI abnormalities except possibly hydrocephalus). LMD occurs in the presence of CNS metastases in 43%–52 % of cases, and with extra-CNS metastases in 85%–88 % of cases [190,191]. The median time from the diagnosis of breast cancer to LMD is approximately 2.5–5.0 years [192,193]. Risk factors associated with the shortest LMD onset include TNBC subtype and lobular tumor histology [[193], [194], [195], [196], [197], [198], [199]]. LMD is usually associated with rapid neurological decline, reduction in QoL and limited life expectancy [188,194,[200], [201], [202], [203]]. Therefore, treatment is aimed at improving or stabilizing neurological symptoms and QoL.
Treatment options for LMD include systemic and intrathecal pharmacotherapy as well as local radiotherapy. Given the limited data and usually poor prognosis, the choice of treatment (radiotherapy, intra-CSF therapy, systemic therapy, supportive care) should consider prognostic evaluation, multidisciplinary discussion and always an in-depth discussion with the patient and the caregivers. To evaluate treatment options, staging of patients with LMD should include both brain and full spine imaging with MRI with gadolinium to assess the full extent of the disease. Intra-CSF chemotherapy has not been proven to improve OS nor QoL but may palliate symptoms in some cases, although significant toxicity may also occur [[204], [205], [206], [207], [208]]. In an analysis from the real-world Epidemiological Strategy and Medical Economics (ESME) database, among 312 patients who received intra-CSF chemotherapy for LMD, median OS after LMD diagnosis was 5.1 months in HR+/HER2 negative, 5.6 months in HER2+ and 3.0 months in TN ABC disease [195]. Intra-CSF trastuzumab has been evaluated in small studies and has shown some efficacy relative to historical control data, with a favorable toxicity profile. It may be used in some patients with HER2+ LMD [202,209]. A ventriculoperitoneal shunt may be placed to palliate symptoms of increased intracranial pressure or symptomatic hydrocephalus. WBRT may be used in selected cases for symptomatic relief in patients with extensive nodular or symptomatic linear LMD or with coexisting CNS metastases, although it has not been proven to prolong survival [188,210]. Focal radiotherapy (brain or cranio-spinal) should be considered for circumscribed, particularly symptomatic lesions [211]. A recent randomized phase 2 study compared two techniques of radiotherapy, each targeting different volumes; the results suggested that proton craniospinal irradiation could improve survival without serious toxicity compared with local standard radiotherapy in patients with LMD. Therefore, this study may question the best radiotherapy method for patients with LMD [212]. As this technique is not available in most countries and given the limited data available, there was no voting on the topic. To date, there are a lack of high-quality clinical trial data supporting the use of specific systemic therapies in LMD despite some case series and retrospective cohort studies [213]. Patients, including those with a preserved general performance status at diagnosis of LMD, are often excluded from clinical trials in breast cancer; this is presumably due to the risk of rapidly progressing disease and short life expectancy [214,215]. Consequently, the results from clinical trials generally do not provide an accurate reflection of real-world clinical practice. It is crucial that patients with LMD are included in clinical trials, namely in trials evaluating therapies for CNS disease. The choice of systemic therapy for LMD should consider the breast cancer subtype and previous treatments. Systemic regimens with reported benefit include capecitabine, platinum and platinum-based combinations, anthracyclines and endocrine-based therapy [213]. Albeit in very small case series, there are some efficacy data in LMD for capecitabine monotherapy, the combination capecitabine + trastuzumab + tucatinib [105,216] and for T-DXd [217].
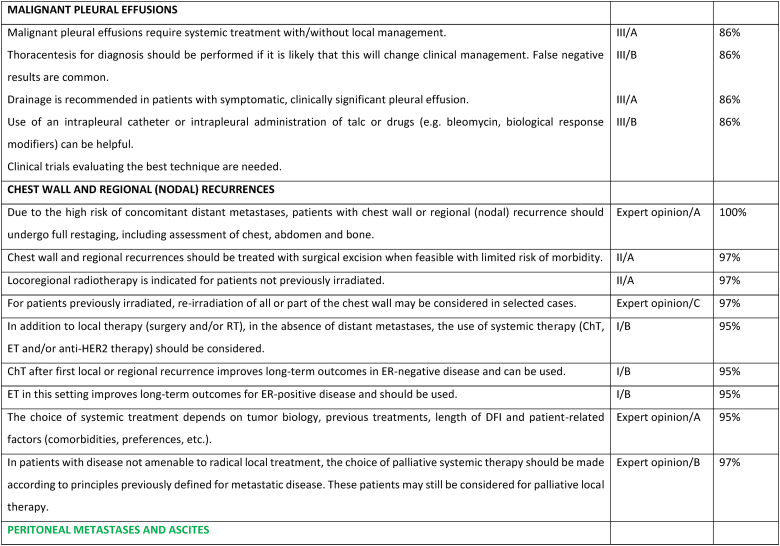
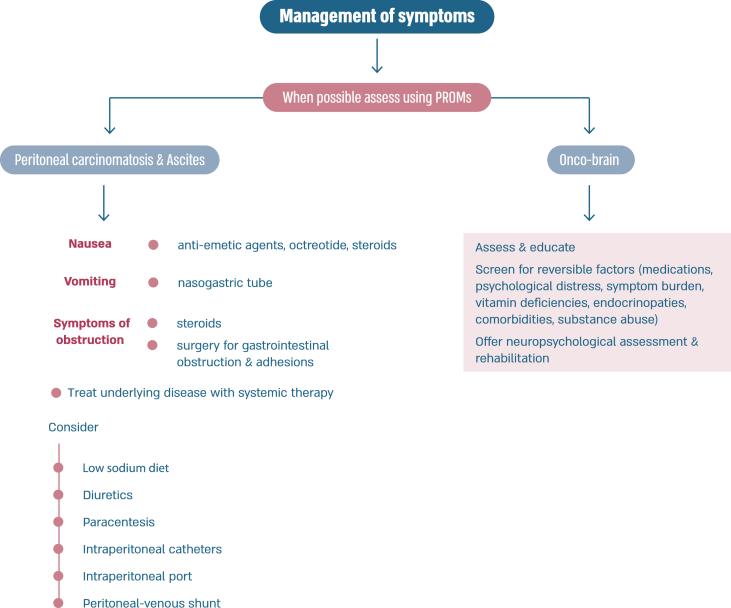
Peritoneal carcinomatosis is more common in patients with invasive lobular carcinoma [218], usually represents advanced stages of disease, has a negative impact on quality of life, and confers a poor prognosis [219]. Patients may present with non-specific symptoms such as abdominal pain, decreased appetite, nausea, vomiting, weight loss, increased abdominal girth. Ascites is present in 50 % of patients. Imaging with ultrasound, CT scan or MRI scans may show peritoneal nodular deposits, thickening of peritoneal folds, diffuse thickening of peritoneum and layer between the bowels and abdominal wall, with or without variable amounts of ascites. Tumor markers and PET/CT scans may be helpful for following disease response. Sensitivity of paracentesis ranges between 40 and 70 % with a higher yield with multiple paracentesis. In some cases, laparoscopy and biopsy may be required for diagnosis [219]. Specific systemic management depends on the subtype of breast cancer. Attention to symptom management is important, namely cachexia (nutrition supplements), fatigue and nausea (anti-emetics such as metoclopramide, 5-HT3 receptor antagonists, neuroleptics, octreotide (somatostatin). Steroids may reduce nausea and alleviate obstructive symptoms. Invasive interventions may include nasogastric tube for vomiting, surgery for gastrointestinal obstructions and adhesions. Ascites management options include low sodium diet, diuretics, paracentesis, intraperitoneal catheters, intraperitoneal port and peritoneal-venous shunt. Palliative paracentesis is an ambulatory procedure usually done under ultrasound guidance and causes relief of symptoms in 90 % of patients. Active and early involvement of palliative care team is crucial [[220], [221], [222]].
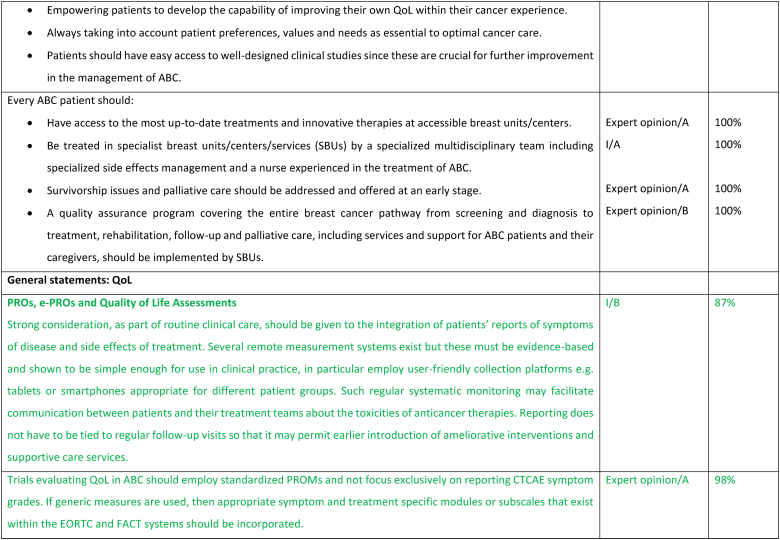
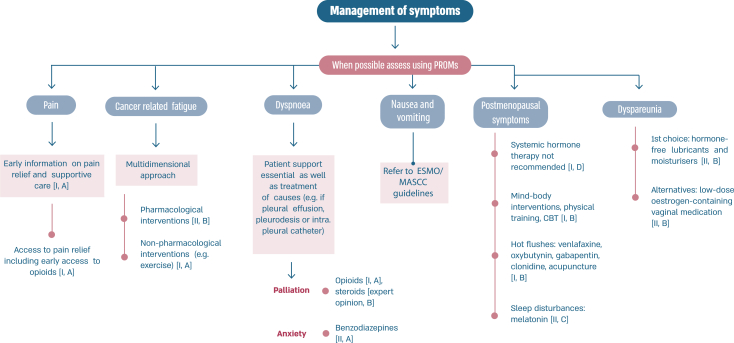
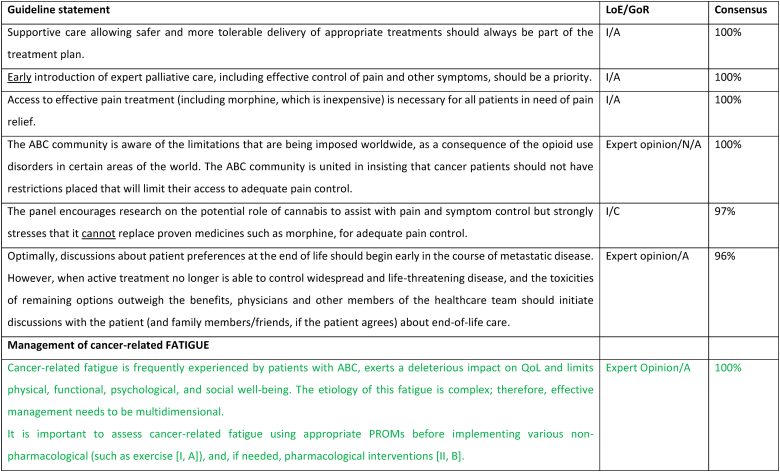
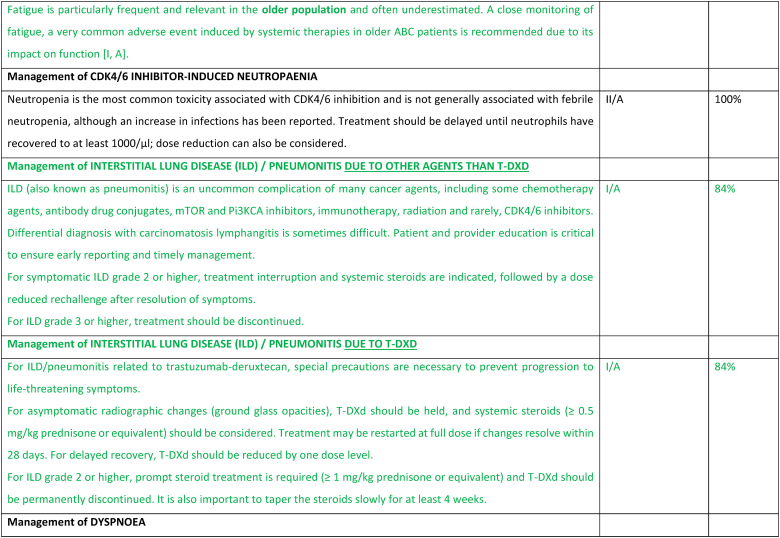
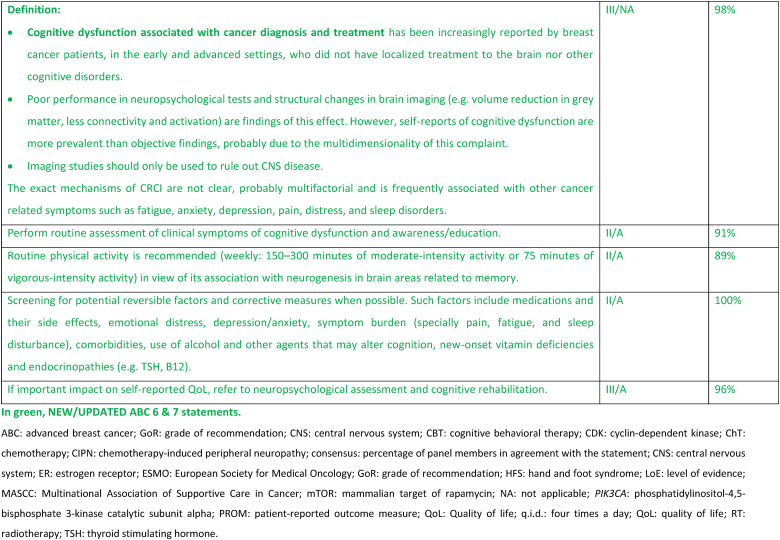
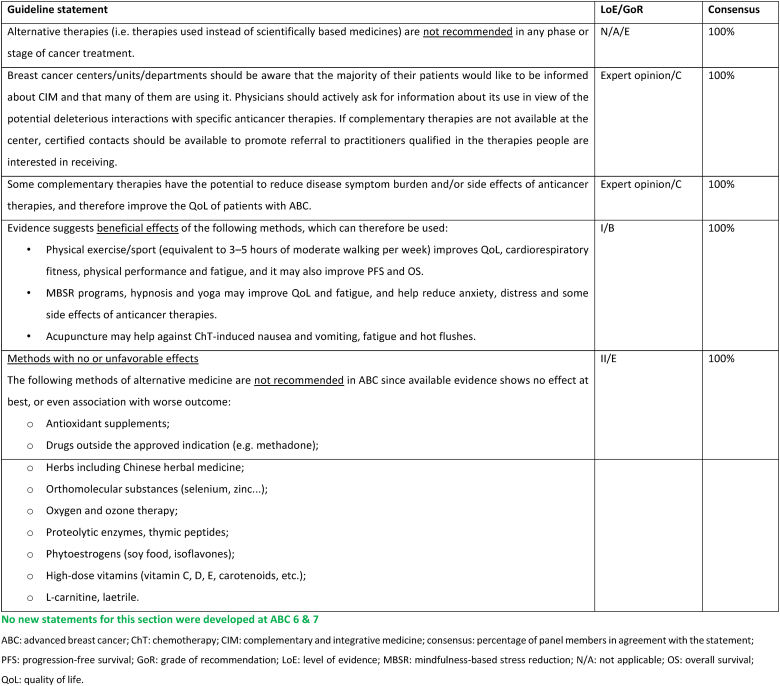
2.27. Section XIII: Supportive and palliative care (see Fig. 6a, Fig. 6b, Fig. 6c, Fig. 6da, b, 6c and 6d)
Fig. 6a.
ABC Symptom control.
Legend: ABC, advanced breast cancer; ESMO, European Society for Medical Oncology; MASCC, Multinational Association of Supportive Care in Cancer; PROM, patient-reported outcome measure; CBT, cognitive behavioural therapy. For ESMO/MASCC guideline please refer to [226].
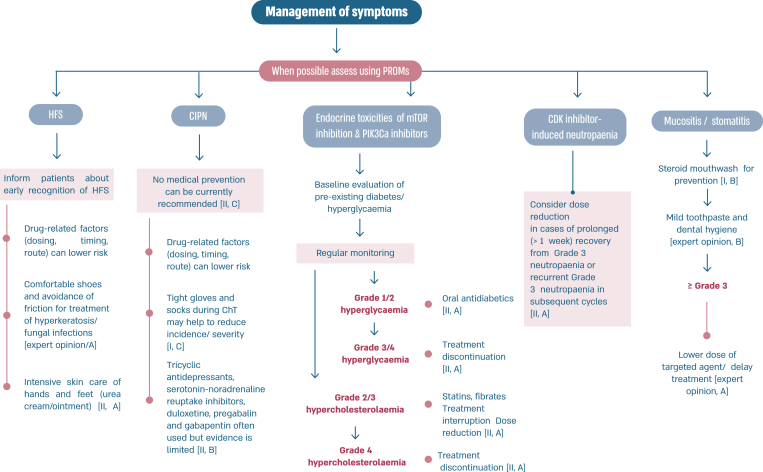
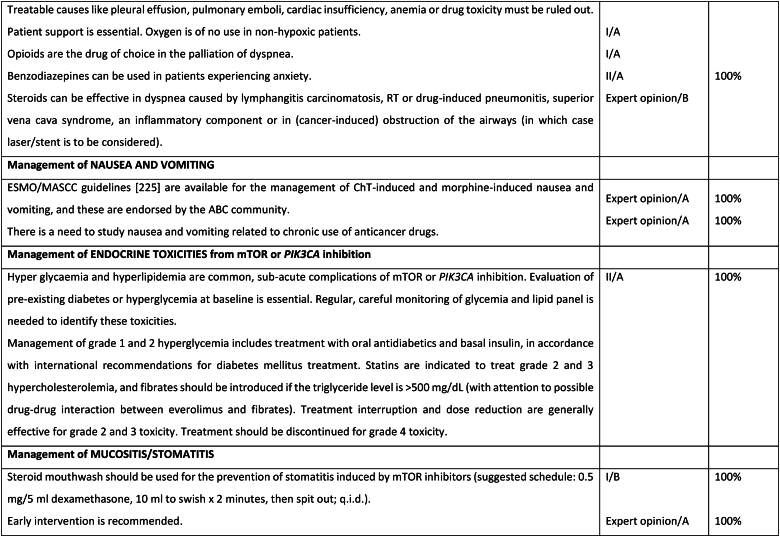
Fig. 6b.
ABC Symptom control.
Fig. 6c.
ABC Symptom control.
Legend: ABC, advanced breast cancer; CIPN, chemotherapy-induced peripheral neuropathy; HFS, hand and foot syndrome; mTOR, mammalian target of rapamycin; PROM, patient-reported outcome measure; CBT, cognitive behavioural therapy.
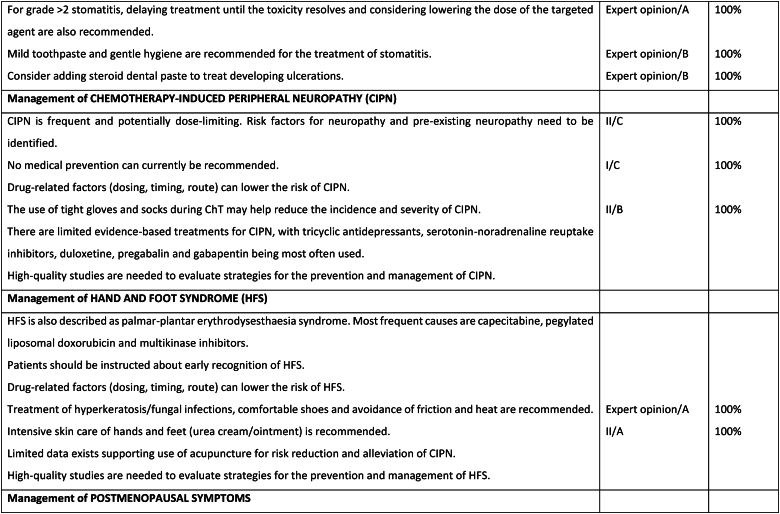
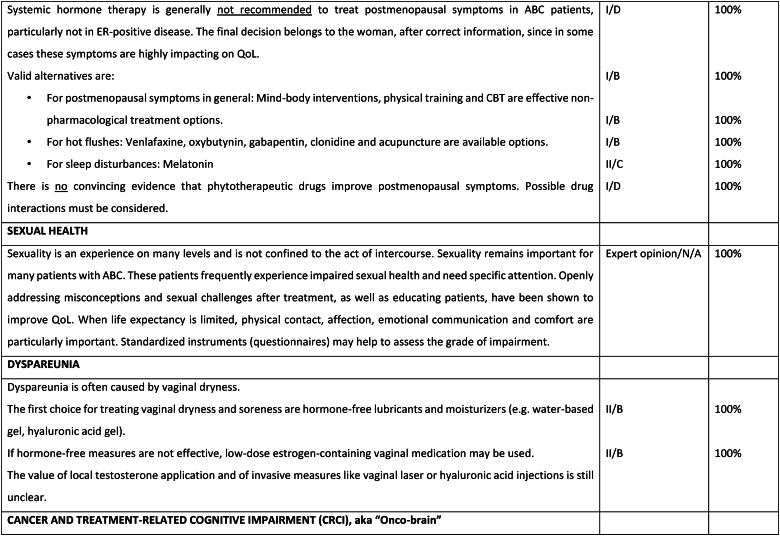
Fig. 6d.
ABC Symptom control.
Legend: ABC, advanced breast cancer; ILD, interstitial lung disease; mTOR, mammalian target of rapamycin; CDK, cyclin-dependent kinase.
Interstitial lung disease (ILD), defined as inflammation that, untreated, results in eventual fibrosis of the lung interstitium, is an uncommon complication associated with hundreds of drugs and numerous drug classes as well as radiation or combinations of radiation and drugs [223]. In the treatment of breast cancer, ILD has been associated with antibody drug conjugates, mTOR inhibitors, HER-2/EGFR targeted oral tyrosine kinase inhibitors, PD-1 or PD-L1 inhibitors, radiation therapy and rarely, CDK4/6 inhibitors [224]. The key differential diagnosis is lymphangitic carcinomatosis, or an acute or subacute infection. Risk is increased in patients with a prior history of pneumonitis, and in those with Asian ethnicity. Additional risk factors are being evaluated [223]. Grading of ILD is provided by the NCITC [225]: grade 1 is defined as abnormalities on imaging, such as ground glass opacities (GGO) without symptoms; patients with grade 2 ILD have moderate symptoms with medical intervention indicated and limiting activities of daily living; grade 3 is associated with severe symptoms requiring oxygen; grade 4 ILD is life threatening with urgent intervention such as intubation and grade 5 is death. In general, grade 1 ILD requires close observation; for grade 2 ILD treatment should be withheld, and systemic steroids are indicated. Cautious retreatment when symptoms have resolved, usually with dose reduction, can be considered. However, with T-DXd, stricter criteria must be employed to avoid death from progressive ILD. Patients treated with T-DXd should have chest CT imaging no longer than at 12 weeks intervals during the first year of treatment. With asymptomatic GGO, drug should be held, and it is recommended that steroids at a dose of ≥0.5 mg/kg prednisone or equivalent be instituted. T-DXd can be restarted at full-dose if radiographic changes resolve within 28 days. If the GGO take longer than 28 days to resolve, T-DXd should be restarted with one dose reduction. Patients with symptomatic ILD, regardless of oxygenation should permanently discontinue treatment with T-DXd and steroids at a dose of >1 mg/kg prednisone or equivalent should be promptly instituted. Early diagnosis of ILD and close adherence to guidelines can prevent mortality from progressive respiratory dysfunction [103].
Cancer-relative cognitive impairment (CRCI), also called onco-brain describes the experience of cognitive complains associated with cancer treatments, such as impairments in short-term and working memory, attention, executive functions and/or processing speed, in patients with non-CNS cancers [227]. Objective findings include poor performance in neuropsychological tests and structural changes in brain imaging (i.e., volume reduction in grey matter, less connectivity and activation). Cognitive complaints are usually subtle or moderate, and most frequently related to ChT. However, other treatments, such as ET, targeted agents, and immunotherapy may also have an impact in cognitive function [228,229]. Most studies describing CRCI were conducted in the early breast cancer setting. CRCI is multifactorial and closely associated with cancer related symptoms such as fatigue, anxiety, depression, pain, and distress [227,229]. This calls for the need to assess the contributing factors in patients with ABC, and most importantly, to encourage studies on how to comprehensively assess all these factors (psychosocial, physical, treatment related) in this population. Such a tool would allow a more appropriate use of efficacious known interventions, as well to test new ones in this setting. Validated PROMs are available to assess subjective cognitive function. The EORTC QLQ-C30 questionnaire that incorporates a cognitive function domain has been the most frequently used tool [230]. The Functional Assessment of Cancer Therapy-Cognitive Function questionnaire (FACT-Cog) is another available PRO tool that has been mainly used in the clinical research setting [231]. If symptoms of cognitive disfunction are present, contributing factors should be assessed, and corrected and/or optimized, including medications and their side effects, emotional distress, depression/anxiety, symptom burden (especially pain, fatigue and sleep disturbance), comorbidities, use of alcohol and other agents that may alter cognition, new-onset vitamin deficiencies and endocrinopathies (e.g., TSH, B12) [227,232]. If all the above have already been assessed and optimized, a referral for a neuropsychological specialist should be considered if cognitive dysfunction is creating an ongoing impact on QoL. The assessment should be directed to determining objective cognitive function and eligibility for cognitive rehabilitation programs [227,233]. Physical exercise is the most efficacious intervention tested, based on the biological rational of its association with neurogenesis in brain areas related to memory [234,235]. Current guidelines for cancer survivors recommend routine physical activity, more specifically, 150 min of moderate-intensity activity or 75 min of vigorous-intensity activity, weekly [232]. Small sample size studies are available in the advanced breast cancer setting concerning the benefits of physical activity [236,237]. Tailored exercise programs are urgently needed to find suitable exercise protocols and to determine the benefit of physical exercise on QoL in this setting.
2.28. Section XIV: integrative medicine
| Guideline statement | LoE/GoR | Consensus |
|---|---|---|
| Alternative therapies (i.e. therapies used instead of scientifically based medicines) are not recommended in any phase or stage of cancer treatment. | N/A/E | 100 % |
| Breast cancer centers/units/departments should be aware that the majority of their patients would like to be informed about CIM and that many of them are using it. Physicians should actively ask for information about its use in view of the potential deleterious interactions with specific anticancer therapies. If complementary therapies are not available at the center, certified contacts should be available to promote referral to practitioners qualified in the therapies people are interested in receiving. | Expert opinion/C | 100 % |
| Some complementary therapies have the potential to reduce disease symptom burden and/or side effects of anticancer therapies, and therefore improve the QoL of patients with ABC. | Expert opinion/C | 100 % |
Evidence suggests beneficial effects of the following methods, which can therefore be used:
|
I/B | 100 % |
|
Methods with no or unfavorable effects The following methods of alternative medicine are not recommended in ABC since available evidence shows no effect at best, or even association with worse outcome:
|
II/E | 100 % |
ABC: advanced breast cancer; ChT: chemotherapy; CIM: complementary and integrative medicine; consensus: percentage of panel members in agreement with the statement; PFS: progression-free survival; GoR: grade of recommendation; LoE: level of evidence; MBSR: mindfulness-based stress reduction; N/A: not applicable; OS: overall survival; QoL: quality of life.
3. Conclusions and future directions
The ABC consensus guidelines provide an invaluable guide to help healthcare providers and patients in treatment decision-making for advanced/metastatic breast cancer. They are also a powerful lobbying and advocacy tool to fight for the best available care for all patients living with this disease worldwide.
Clinical implementation of guidelines is often limited by inequalities in access to cancer care. In some regions of the world, adaptation of these guidelines is necessary and the ABC Global Alliance (https://www.abcglobalalliance.org) remains available to help with this complex endeavour. It is crucial to note that, even when access to the latest and most expensive medicines is limited, a patient-centred approach, with careful balance between efficacy and toxicity, aiming at the longest survival with the best possible quality of life is not only achievable but also of paramount importance, and often cost-effective.
The level of evidence of each guideline is directly related to the quality of the research on the topic. Clinical trials in the field of advanced/metastatic breast cancer continue to exclude important sub-populations of patients, often the ones with the greatest unmet needs, and remain focused on endpoints that, albeit with some value, do not have the potential to dramatically change the outcomes of patients living with ABC. Only aiming higher, at improved survival and better quality of life, we will be able to transform this incurable disease into a chronic or even potentially curable one.
Funding
No funding has been received for the preparation of these guidelines.
Declaration of competing interest
Matti S. Aapro: Consultant for Accord Pharmaceuticals, Amgen, BMS, Celgene, Clinigen Group, Daiichi Sankyo, Eisai Co.Ltd, Eli Lilly, Genomic Health (Exact Sciences), G1 Therapeutics, Inc., GlaxoSmithKline (GSK), Helsinn Healthcare SA, Hospira (Pfizer), Johnson & Johnson, Merck, Merck Serono (Merck KGaA), Mundipharma International Limited, Novartis, Pfizer, Pierre Fabre, Roche, Sandoz, Tesaro (GSK), Teva Pharmaceuticals Industries Ltd., Vifor Pharma. Received honoraria for lectures at symposia of Accord Pharmaceuticals, Amgen, Astellas, Bayer HealthCare Pharmaceuticals (Schering), Biocon, Boeringer Ingelheim, Cephalon, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eisai Co., Ltd., Dr Reddy's Laboratories, Genomic Health (Exact Sciences), Glenmark Pharmaceuticals Limited, GSK, Helsinn Healthcare SA, Hospira (Pfizer), Ipsen, Janssen Biotech, Kyowa Kirin Group, Merck, Merck Serono (Merck KGaA), Mundipharma International Limited, Novartis, Pfizer, Pierre Fabre, Roche, Sandoz, Sanofi, Tesaro (GSK), Taiho Pharmaceutical, Teva Pharmaceutical Industries Ltd., Vifor Pharma. Grant/Research supports: Amgen, Eisai, Genomic Health (Exact Sciences), Helsinn, Hospira, Novartis, Merck, Mundipharma, Pfizer, Roche, Sandoz, Tesaro, Teva, Vifor. Jyoti Bajpai: Institutional financial interests for conducting research: Eli Lilly, MSD, Novartis, Roche, Paxman Coolers Ltd, Samsung Bioepis co. Ltd, Sun Pharma. Carlos H. Barrios: Receipt of grants/research supports: (to the institution) Nektar, Pfizer, Polyphor, Amgen, Daiichi Sankyo, Sanofi, Exelixis, Regeneron, Novartis, GSK, Janssen, OBI Pharma, Lilly, Seagen, Roche, BMS, MSD, Merck Serono, AstraZeneca, Novocure, Aveo Oncology, Takeda, TRIO, PharmaMar, Celgene, PPD, Syneos Health, Labcorp, ICON, IQVIA, Parexel, Nuvisan, PSI, Worldwide, Gilead Sciences, Bayer, Servier. Receipt of honoraria or consultation fees: Advisory Boards and Consulting: Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, MSD, AstraZeneca, Zodiac, Lilly, Sanofi, Daiichi. Stock shareholder: Ownership or stocks: Tummi, MEDSir. Jonas Bergh: Receipt of grants/research supports: Research grants from Amgen, AstraZeneca, Bayer, Merck, Pfizer, Roche & Sanofi-Aventis to Karolinska Institutet and/or University Hospital. No personal payments. Other support: Payment for a chapter in UpToDate on breast cancer prediction to Asklepios Medicin HB. Laura Biganzoli: Personal financial interests (Honoraria, consultancy or advisory role): Amgen, AstraZeneca, Boehringer-Ingelheim, Daiichi-Sankyo, Eisai, Exact Sciences, Gilead, Lilly, Menarini, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, SeaGen.
Institutional financial interests: Celgene, Genomic Health, Novartis. Travel grants: AstraZeneca, Daiichi-Sankyo. Fatima Cardoso: Receipt of honoraria or consultation fees: Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, Gilead, GlaxoSmithKline, Iqvia, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seagen, Teva, Touchime. Maria-Joao Cardoso: Receipt of honoraria or consultation fees: AstraZeneca, Merck-Sharp, Novartis and Roche. Lisa A. Carey: Other support: Research funding (institution): NanoString Technologies, Seagen, Veracyte, AstraZeneca. Uncompensated relationships: Lilly, Seagen, Novartis, Genentech/Roche, GlaxoSmithKline. Mariana Chavez Mac Gregor: Receipt of grants/research supports: BCRF, Susan G Komen. Receipt of honoraria or consultation fees: Astra Zeneca, Pfizer, Lilly, Roche, Merck. Javier Cortés: Receipt of grants/research supports: Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies. Receipt of honoraria or consultation fees: Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp&Dohme, Daiichi Sankyo, Astrazeneca. Stock shareholder: MedSIR, Nektar Pharmaceuticals, Leuko (relative). Other support: Research funding to the Institution: Roche, Ariad pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F.Hoffman-La Roche, Guardanth health, Merck Sharp&Dohme, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London. Travel, accommodation, expenses: Roche, Novartis, Eisai, pfizer, Daiichi Sankyo, Astrazeneca, Gilead. Patents: Pharmaceutical Combinations of a Pi3k Inhibitor and A Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A. ISSUED. Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés.US 2019/0338368 A1. LICENSED. Giuseppe Curigliano: Receipt of grants/research supports: Merck. Receipt of honoraria or consultation fees: Merck, Lilly, Pfizer, Daichi Sankyo, Seagen, Novartis, Roche, Astra Zeneca, Ellipsis. Participation in a sponsored speakers' bureau: Seagen, Novartis, Lilly, Pfizer, Daichii Sankyo. Rebecca A. Dent: Receipt of grants/research supports: AstraZeneca, Roche. Receipt of honoraria or consultation fees: AstraZeneca, DKSH, Eisai, Merck, Novartis, Pfizer, Roche. Nagi S. El Saghir: Receipt of honoraria or consultation fees: Roche, Novartis, Lilly. Alexandru Eniu: Receipt of honoraria or consultation fees: Astra Zeneca, Daiichi Sankyo, Gilead, Janssen, MSD, Novartis, SeaGen. Receipt of grants/research supports: AstraZeneca. Full or part-time employment: European School of Oncology (ESO). Lesley Fallowfield: Receipt of grants/research supports: Novartis, Bristol-Myers Squibb, Lilly. Receipt of honoraria or consultation fees: Voluntis, Genomic Health, NanoString Technologies, Novartis, Pfizer, MSD, Novartis, Abbvie, Clovis Oncology, Puma Biotechnology, AstraZeneca, Takeda, Genomic Health/Exact Sciences, Lilly, Seagen, Roche. Sandra X. Franco Millan: Receipt of honoraria or consultation fees: Novartis, Pfizer, Eli Lilly. Participation in a sponsored speakers' bureau: Novartis, Pfizer, Eli Lilly. Karen Gelmon: Receipt of grants/research supports: Pfizer, AstraZeneca. Receipt of honoraria or consultation fees: Pfizer, Lilly, Novartis, AstraZeneca, Merck, Gilead, Seagen. Jenny Gilchrist: Receipt of honoraria or consultation fees: Eli-Lilly, AstraZeneca, Novartis, Pfizer, MSD, Gilead, Juniper Biologics. Joseph Gligorov: Receipt of grants/research supports: Eisai, Exact Science, Guardant, Roche Genentech. Receipt of honoraria or consultation fees: Astra Zeneca, Daiichi, Eisai, Evapharma, Exact Science, Gilead, Guardant Lilly, Merck, Novartis, Onxeo, Pfizer, Roche Genentech, Seattle Genetics. Participation in a sponsored speakers' bureau: Eisai, Eva Pharm, Novartis, Roche Genentech, Seattle Genetics. Other support (please specify): Institutional: Institut Universitaire de Cancérologie AP-HP Sorbonne Université; French breast cancer guidelines St Paul. Nadia Harbeck: Receipt of grants/research supports: all to institution. Receipt of honoraria or consultation fees: AstraZeneca, Daiichi-Sankyo, Gilead, Lilly, MSD, Novartis, PierreFabre, Pfizer, Roche, Sandoz, Seagen, Viatris, Zuelligpharma. Participation on a sponsored speakers' bureau: EPG Communication, MEDSCAPE, Springer. Stock shareholder: WSG minority ownership. Spouse/Partner: Consulting for WSG. Ranjit Kaur: Receipt of grants/research supports: Novartis, Roche, Pfizer, Astrazeneca. Receipt of honoraria or consultation fees: Novartis, Roche, Pfizer, Astrazeneca. Participation in a sponsored speakers' bureau: Novartis, Roche, Pfizer, Astrazeneca. Belinda Kiely: Receipt of honoraria or consultation fees: Speakers fees: Novartis, Eisai. Advisory boards: Roche, Gilead, Novartis. Other support (please specify): meeting registrations fees: Novartis, Pfizer, MSD. Sung-Bae Kim: Receipt of grants/research supports: Novartis, Sanofi-Aventis, and DongKook Pharm Co. Receipt of honoraria or consultation fees: Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, OBI pharma, Beigene and Daiichi-Sankyo. Stock shareholder: Genopeaks. Smruti Koppikar: Receipt of honoraria or consultation fees: Eli Lilly, Novartis, Cipla, Roche. Participation in a sponsored speakers' bureau: Eli Lilly, Novartis, Cipla, Roche. Marion Kuper-Hommel: Receipt of honoraria or consulting fees: Astra Zeneca. Ginny Mason: Participation in a sponsored speakers' bureau: Novartis. Volkmar Mueller: Receipt of grants/research supports: Institutional research support from Novartis, Roche, Seagen, Genentech, Astra Zeneca. Receipt of honoraria or consultation fees: Speaker honoraria: Astra Zeneca, Daiichi-Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead, Pierre Fabre. Consultancy honoraria from Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, DaiichiSankyo, Eisai, Lilly, Sanofi, Seagen, Gilead, Stemline, ClinSol. Other support (please specify): Travel grants: Roche, Pfizer, Daiichi Sankyo, Gilead. Claire Myerson: Receipt of honoraria or consultation fees: Novartis Pharma. Silvia Neciosup: Receipt of grants/research supports: Roche, Pfizer. Participation in a sponsored speakers' bureau: Tecnofarma, AZ, Roche, Pfizer. Larry Norton: Receipt of honoraria or consultation fees: Blackrock QLS Advisors, Cold Spring Harbor Laboratory, UNC Breast Spore EAB Meeting, Codagenix Scientific Advisory Board Meeting, Martell Diagnostic Laboratories, Inc., Celgene, Agenus, Immix Biopharma, Inc. Shinji Ohno: Participation in a sponsored speakers' bureau: Chugai, Lilly, MSD, Nippon Kayaku. Shani Paluch-Shimon: Receipt of grants/research supports: Pfizer. Receipt of honoraria or consultation fees: Pfizer, Lily, Novartis, Astra Zeneca, Roche, MSD, Gilead, Rhenium/Exact Sciences, Stemline, Daichi Sankyo. Participation in a sponsored speakers' bureau: Pfizer, Lily, Novartis, Astra Zeneca, Roche, MSD, Gilead. Ann H. Partridge: Uptodate Royalties. Aleix Prat: Receipt of grants/research supports: Boehringer, Novartis, Roche, Nanostring, Sysmex Europa GmbH, Medica Scientia inno. Research, SL, Celgene, Astellas and Pzifer. Receipt of honoraria or consultation fees: Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardan Health, Peptomyc and Lilly, Nanostring Technologies and Daiichi Sankyo. Other support: Clinical trials: Boehringer, Lilly, Roche, Novartis, Amgen and Daiichi Sankyo. Hope S. Rugo: Receipt of grants/research supports: Astellas Pharma Inc.; AstraZeneca; Daiichi Sankyo, Inc.; F. Hoffmann-La Roche AG/Genentech, Inc.; Gilead Sciences, Inc.; GlaxoSmithKline; Lilly; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; Pfizer; Pionyr Immunotherapeutics; Sermonix Pharmaceuticals Inc.; Taiho Oncology, Inc. and Veru Inc. Receipt of honoraria or consultation fees: Puma, NAPO, Blueprint, and Scorpion Therapeutics Daichi Sankyo. Elzbieta Senkus: Receipt of honoraria or consultation fees: AstraZeneca, Curio Science, Egis, Eli Lilly, Gilead, high5md, MSD, Novartis, Pfizer, Roche, Swixx. Other support: travel support: AstraZeneca, Egis, Gilead, Novartis, Roche. Contracted research: Amgen, AstraZeneca, Eli Lilly, Gilead, Novartis, OBI Pharma, Roche, Samsung, Seagen. Medical writing: AstraZeneca, Eli Lilly. Royalties: Springer. Sandra M. Swain: Receipt of grants/research supports: Kailos Genetics, Genentech/Roche. Receipt of honoraria or consultation fees: Athenex, AstraZeneca, Biotheranostics, Natera, Exact Sciences, Lilly, Merck, Genentech/Roche, Sanofi, Daiichi Sankyo, Molecular Templates, Napo Pharmaceuticals. Stock shareholder: SEAGEN. Other support: CO- PI of INAVO 122 Genentech/Roche with in-kind travel to investigator meetings, Travel related expenses in kind Sanofi and Daiichi Sankyo. Peter Vuylsteke: Receipt of grants/research supports: UICC grant. Receipt of honoraria or consultation fees: Roche, Novartis and MSD. Theresa Wiseman: Receipt of grants/research supports: Receiver of SPCC Pfizer grant. Binghe Xu: Receipt of grants/research supports: sponsored research to my institution from Roche, AstraZeneca and Pfizer. Receipt of honoraria or consultation fees: advisory or consultancy fees from Novartis and Roche. Participation in a sponsored speakers' bureau: speakers’ bureau fees from AstraZeneca, Pfizer, Roche, Eisai.
Elizabeth Bergsten-Nordström, Alberto Costa, Runcie C. W. Chidebe, Prudence A. Francis, Xichun Hu, Renate Haidinger, Leonor Matos, Shirley A. Mertz, Birgitte V. Offersen, Olivia Pagani, Eva Schumacher-Wulf, Daniel A. Vorobiof, Frédéric E. Lecouvet, and Eric P. Winer reported no significant relationships. William J. Gradishar, George W. Sledge and Christoph Thomssen have not submitted a disclosure of interests’ form.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103756.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Annals of Oncology. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017;28:16–33. doi: 10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Annals of Oncology. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Annals of Oncology. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.abcglobalalliance.org/
- 7.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P, Cameron DA, André F, Arteaga CL, Zarate JP, Chakravartty A, Taran T, Le Gac F, Serra P, O’Shaughnessy J. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med. 2022 Mar 10;386(10):942–995. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 9.Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard J-Y, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 10.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33:139–144. doi: 10.1086/321805. [DOI] [PubMed] [Google Scholar]
- 11.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 12.Steenbruggen T.G., Schaapveld M., Horlings H.M., Sanders J., Hogewoning S.J., Lips E.H., et al. Characterization of Oligometastatic Disease in a Real-World Nationwide Cohort of 3447 Patients With de Novo Metastatic Breast Cancer. JNCI Cancer Spectr. 2021;5:pkab010. doi: 10.1093/jncics/pkab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.deSouza N.M., Liu Y., Chiti A., Oprea-Lager D., Gebhart G., Van Beers B.E., et al. Strategies and technical challenges for imaging oligometastatic disease: recommendations from the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2018;91:153–163. doi: 10.1016/j.ejca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Yang H.-L., Liu T., Wang X.-M., Xu Y., Deng S.-M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 15.Terao M., Niikura N. Diagnosis of oligometastasis. Transl Cancer Res. 2020;9:5032–5037. doi: 10.21037/tcr.2020.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmin M., Makris A., Joshi P.V., Ah-See M.-L., Woolf D., Padhani A.R. The addition of whole-body magnetic resonance imaging to body computerised tomography alters treatment decisions in patients with metastatic breast cancer. Eur J Cancer. 2017;77:109–116. doi: 10.1016/j.ejca.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Press D.J., Miller M.E., Liederbach E., Yao K., Huo D. De novo metastasis in breast cancer: occurrence and overall survival stratified by molecular subtype. Clin Exp Metastasis. 2017;34:457–465. doi: 10.1007/s10585-017-9871-9. [DOI] [PubMed] [Google Scholar]
- 18.van Maaren M.C., de Munck L., Strobbe L.J.A., Sonke G.S., Westenend P.J., Smidt M.L., et al. Ten-year recurrence rates for breast cancer subtypes in The Netherlands: a large population-based study. Int J Cancer. 2019;144:263–272. doi: 10.1002/ijc.31914. [DOI] [PubMed] [Google Scholar]
- 19.Bhaludin B.N., Tunariu N., Koh D.-M., Messiou C., Okines A.F., McGrath S.E., et al. A review on the added value of whole-body MRI in metastatic lobular breast cancer. Eur Radiol. 2022;32:6514–6525. doi: 10.1007/s00330-022-08714-6. [DOI] [PubMed] [Google Scholar]
- 20.Vaz S.C., Woll J.P.P., Cardoso F., Groheux D., Cook G.J.R., Ulaner G.A., et al. Joint EANM-SNMMI guideline on the role of 2-[18F]FDG PET/CT in no special type breast cancer. Eur J Nucl Med Mol Imag. 2024 doi: 10.1007/s00259-024-06696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairhurst K., Leopardi L., Satyadas T., Maddern G. The safety and effectiveness of liver resection for breast cancer liver metastases: a systematic review. Breast. 2016;30:175–184. doi: 10.1016/j.breast.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Chalkidou A., Macmillan T., Grzeda M.T., Peacock J., Summers J., Eddy S., et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021;22:98–106. doi: 10.1016/S1470-2045(20)30537-4. [DOI] [PubMed] [Google Scholar]
- 23.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 24.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrow S., Palma D.A., Olson R., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET): extended long-term outcomes. Int J Radiat Oncol Biol Phys. 2022;114:611–616. doi: 10.1016/j.ijrobp.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Chmura S.J., Winter K.A., Woodward W.A., Borges V.F., Salama J.K., Al-Hallaq H.A., et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer ( NCT02364557) J Clin Oncol. 2022;40:1007. doi: 10.1200/JCO.2022.40.16_suppl.1007. [DOI] [Google Scholar]
- 27.Pasquier D., Bidaut L., Oprea-Lager D.E., deSouza N.M., Krug D., Collette L., et al. Designing clinical trials based on modern imaging and metastasis-directed treatments in patients with oligometastatic breast cancer: a consensus recommendation from the EORTC Imaging and Breast Cancer Groups. Lancet Oncol. 2023;24:e331–e343. doi: 10.1016/S1470-2045(23)00286-3. [DOI] [PubMed] [Google Scholar]
- 28.David S.P., Siva S., Bressel M., Tan J., Hanna G., Alomran R.K., et al. Stereotactic ablative body radiotherapy (SABR) for oligoprogressive ER-positive breast cancer (AVATAR): a phase II prospective multicenter trial. Int J Radiat Oncol Biol Phys. 2023;117:e6. doi: 10.1016/j.ijrobp.2023.08.033. [DOI] [Google Scholar]
- 29.Skipper H.E., Schabel F.M.J., Wilcox W.S. Experimental evaluation of potential anticancer agents. XIII. ON the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1–111. [PubMed] [Google Scholar]
- 30.Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48:7067–7071. [PubMed] [Google Scholar]
- 31.Norton L. Cancer stem cells, self-seeding, and decremented exponential growth: theoretical and clinical implications. Breast Dis. 2008;29:27–36. doi: 10.3233/bd-2008-29104. [DOI] [PubMed] [Google Scholar]
- 32.Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol. 1997;24:S10–S13. S10-10. [PubMed] [Google Scholar]
- 33.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440–1452. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher B., Anderson S., DeCillis A., Dimitrov N., Atkins J.N., Fehrenbacher L., et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 35.Citron M.L., Berry D.A., Cirrincione C., Hudis C., Winer E.P., Gradishar W.J., et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemi. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 36.Budman D.R., Berry D.A., Cirrincione C.T., Henderson I.C., Wood W.C., Weiss R.B., et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 37.Winer E.P., Berry D.A., Woolf S., Duggan D., Kornblith A., Harris L.N., et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol. 2004;22:2061–2068. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Cadoo K.A., Gajria D., Suh E., Patil S., Theodoulou M., Norton L., et al. Decreased gastrointestinal toxicity associated with a novel capecitabine schedule (7 days on and 7 days off): a systematic review. NPJ Breast Cancer. 2016;2 doi: 10.1038/npjbcancer.2016.6. [DOI] [Google Scholar]
- 39.Gajria D., Gonzalez J., Feigin K., Patil S., Chen C., Theodoulou M., et al. Phase II trial of a novel capecitabine dosing schedule in combination with lapatinib for the treatment of patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2012;131:111–116. doi: 10.1007/s10549-011-1749-y. [DOI] [PubMed] [Google Scholar]
- 40.Traina T.A., Theodoulou M., Feigin K., Patil S., Tan K.L., Edwards C., et al. Phase I study of a novel capecitabine schedule based on the Norton-Simon mathematical model in patients with metastatic breast cancer. J Clin Oncol. 2008;26:1797–1802. doi: 10.1200/jco.2007.13.8388. [DOI] [PubMed] [Google Scholar]
- 41.Ferraro E., Drago J.Z., Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 2021;23:84. doi: 10.1186/s13058-021-01459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samant T.S., Huth F., Umehara K., Schiller H., Dhuria S.V., Elmeliegy M., et al. Ribociclib drug-drug interactions: clinical evaluations and physiologically-based pharmacokinetic modeling to guide drug labeling. Clin Pharmacol Ther. 2020;108:575–585. doi: 10.1002/cpt.1950. [DOI] [PubMed] [Google Scholar]
- 43.Lee J.-E., Kwon S.-H., Kwon S., Jung H.-I., Nam J.H., Lee E.-K. Concomitant use of proton pump inhibitors and palbociclib among patients with breast cancer. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Çağlayan D., Koçak M.Z., Geredeli Ç., Tatlı A.M., Göksu S.S., Eryılmaz M.K., et al. The effect of concomitant use of proton pump inhibitors with CDK 4/6 inhibitors on survival in metastatic breast cancer. Eur J Clin Pharmacol. 2023;79:243–248. doi: 10.1007/s00228-022-03435-7. [DOI] [PubMed] [Google Scholar]
- 45.Eng L., Sutradhar R., Niu Y., Liu N., Liu Y., Kaliwal Y., et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: a population-based study. J Clin Oncol. 2023;41:3122–3134. doi: 10.1200/JCO.22.00074. [DOI] [PubMed] [Google Scholar]
- 46.Gaucher L., Adda L., Séjourné A., Joachim C., Chaby G., Poulet C., et al. Impact of the corticosteroid indication and administration route on overall survival and the tumor response after immune checkpoint inhibitor initiation. Ther Adv Med Oncol. 2021;13 doi: 10.1177/1758835921996656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shilling V., Starkings R., Jenkins V., Cella D., Fallowfield L. Development and validation of the caregiver roles and responsibilities scale in cancer caregivers. Qual Life Res. 2019;28:1655–1668. doi: 10.1007/s11136-019-02154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shilling V., Matthews L., Jenkins V., Fallowfield L. Patient-reported outcome measures for cancer caregivers: a systematic review. Qual Life Res. 2016;25:1859–1876. doi: 10.1007/s11136-016-1239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mired D., Johnson S., Tamamyan G. Cancer disparities in war-torn and post-war regions. Nat Rev Cancer. 2020;20:359–360. doi: 10.1038/s41568-020-0274-x. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed Y. Enhancing cancer care amid conflict: a proposal for optimizing oncology services during wartime. JCO Glob Oncol. 2023 doi: 10.1200/GO.23.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caglevic C., Rolfo C., Gil-Bazo I., Cardona A., Sapunar J., Hirsch F.R., et al. The armed conflict and the impact on patients with cancer in Ukraine: urgent considerations. JCO Glob Oncol. 2022;8 doi: 10.1200/GO.22.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy K.A., Stone E.M., Presskreischer R., McGinty E.E., Daumit G.L., Pollack C.E. Cancer screening among adults with and without serious mental illness: a mixed methods study. Med Care. 2021;59:327–333. doi: 10.1097/MLR.0000000000001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodhead C., Cunningham R., Ashworth M., Barley E., Stewart R.J., Henderson M.J. Cervical and breast cancer screening uptake among women with serious mental illness: a data linkage study. BMC Cancer. 2016;16:819. doi: 10.1186/s12885-016-2842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin K.E., Park E.R., Shin J.A., Fields L.E., Jacobs J.M., Greer J.A., et al. Predictors of disruptions in breast cancer care for individuals with schizophrenia. Oncol. 2017;22:1374–1382. doi: 10.1634/theoncologist.2016-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kisely S., Alotiby M.K.N., Protani M.M., Soole R., Arnautovska U., Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psycho Oncol. 2023;32:651–662. doi: 10.1002/pon.6120. [DOI] [PubMed] [Google Scholar]
- 56.Marron J.M., Joffe S., Jagsi R., Spence R.A., Hlubocky F.J. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J Clin Oncol. 2020;38:2201–2205. doi: 10.1200/JCO.20.00960. [DOI] [PubMed] [Google Scholar]
- 57.Hantel A., Marron J.M., Casey M., Kurtz S., Magnavita E., Abel G.A. US state government crisis standards of care guidelines: implications for patients with cancer. JAMA Oncol. 2021;7:199–205. doi: 10.1001/jamaoncol.2020.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crisis Standards of Care Committee . 2020. Crisis standards of care: planning guidance for the COVID-19 pandemic. Executive office of health and human services: the commonwealth of Massachusetts. [Google Scholar]
- 59.https://www.europeancancer.org/2-standard/66-european-code-of-cancer-practice [n.d].
- 60.Schrijver W.A.M.E., van der Groep P., Hoefnagel L.D., Ter Hoeve N.D., Peeters T., Moelans C.B., et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol. 2016;29:1460–1470. doi: 10.1038/modpathol.2016.116. [DOI] [PubMed] [Google Scholar]
- 61.Kunc M., Biernat W., Senkus-Konefka E. Estrogen receptor-negative progesterone receptor-positive breast cancer - “Nobody's land” or just an artifact? Cancer Treat Rev. 2018;67:78–87. doi: 10.1016/j.ctrv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Franchet C., Djerroudi L., Maran-Gonzalez A., Abramovici O., Antoine M., Becette V., et al. [2021 update of the GEFPICS’ recommendations for HER2 status assessment in invasive breast cancer in France] Ann Pathol. 2021;41:507–520. doi: 10.1016/j.annpat.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Van Poznak C., Somerfield M.R., Bast R.C., Cristofanilli M., Goetz M.P., Gonzalez-Angulo A.M., et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2015;33:2695–2704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortés J., Kim S.-B., Chung W.-P., Im S.-A., Park Y.H., Hegg R., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 65.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.-A., Yusof M.M., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 66.Cortes J., Rugo H.S., Cescon D.W., Im S.-A., Yusof M.M., Gallardo C., et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217–226. doi: 10.1056/NEJMoa2202809. [DOI] [PubMed] [Google Scholar]
- 67.Schmid P., Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA. IMpassion130 Investigators. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020 Jan;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. Epub 2019 Nov 27. PMID: 31786121. [DOI] [PubMed] [Google Scholar]
- 68.Brasó-Maristany F., Paré L., Chic N., Martínez-Sáez O., Pascual T., Mallafré-Larrosa M., et al. Gene expression profiles of breast cancer metastasis according to organ site. Mol Oncol. 2022;16:69–87. doi: 10.1002/1878-0261.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rozenblit M., Huang R., Danziger N., Hegde P., Alexander B., Ramkissoon S., et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broglio K.R., Berry D.A. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonotto M., Gerratana L., Iacono D., Minisini A.M., Rihawi K., Fasola G., et al. Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncol. 2015;20:719–724. doi: 10.1634/theoncologist.2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haji F., Hurvitz S.A. Can women with HER2-positive metastatic breast cancer Be cured? Clin Breast Cancer. 2021;21:526–531. doi: 10.1016/j.clbc.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Kaplan H.G., Malmgren J.A., Guo B., Atwood M.K. Trastuzumab therapy duration in HER2-positive de novo metastatic breast cancer: 1999-2018. Breast Cancer Res Treat. 2022;195:171–180. doi: 10.1007/s10549-022-06678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Llombart-Cussac A. RF01-03 parsifal-long: extended follow-up of hormone receptor-positive/HER2-negative advanced breast cancer patients treated with fulvestrant and palbociclib vs. letrozole and palbociclib in the PARSIFAL study. Cancer Research. 2024;84(9_Supplement) doi: 10.1158/1538-7445.SABCS23-RF01-03. RF01-03-RF01-03. [DOI] [Google Scholar]
- 75.Ren W., Yu Y., Hong H., Wang Y., Gao Q., Chen Y., et al. Clinical evidence of chemotherapy or endocrine therapy maintenance in patients with metastatic breast cancer: meta-analysis of randomized clinical trials and propensity score matching of multicenter cohort study. Cancer Res Treat. 2022;54:1038–1052. doi: 10.4143/crt.2021.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moy B., Rumble R.B., Carey L.A. Chemotherapy and targeted therapy for endocrine-pretreated or hormone receptor-negative metastatic breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023;41:1318–1320. doi: 10.1200/JCO.22.02807. [DOI] [PubMed] [Google Scholar]
- 77.Burstein H.J., DeMichele A., Fallowfield L., Somerfield M.R., Henry N.L., Henry N.L., et al. Endocrine and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer—capivasertib-fulvestrant: ASCO rapid recommendation update. J Clin Oncol. 2024;42:1450–1453. doi: 10.1200/JCO.24.00248. [DOI] [PubMed] [Google Scholar]
- 78.Burstein H.J., DeMichele A., Somerfield M.R., Henry N.L. Testing for ESR1 mutations to guide therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023;41:3423–3425. doi: 10.1200/JCO.23.00638. [DOI] [PubMed] [Google Scholar]
- 79.Burstein H.J., Somerfield M.R., Barton D.L., Dorris A., Fallowfield L.J., Jain D., et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finn R.S., Rugo H.S., Dieras V.C., Harbeck N., Im S.-A., Gelmon K.A., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses. J Clin Oncol. 2022;40:LBA1003. doi: 10.1200/jco.2022.40.17_suppl.lba1003. LBA1003. [DOI] [Google Scholar]
- 81.Goetz M. SABCS; 2023. Monarch 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy in patients with HR+, HER2- advanced breast cancer. Abstract #: 1643629. n.d. [Google Scholar]
- 82.Park Y.H., Kim T.-Y., Kim G.M., Kang S.Y., Park I.H., Kim J.H., et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20:1750–1759. doi: 10.1016/S1470-2045(19)30565-0. [DOI] [PubMed] [Google Scholar]
- 83.Martin M., Zielinski C., Ruiz-Borrego M., Carrasco E., Turner N., Ciruelos E.M., et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32:488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Lu Y.-S., Mahidin E.I.B.M., Azim H., Eralp Y., Yap Y.-S., Im S.-A., et al. Final results of RIGHT Choice: ribociclib plus endocrine therapy vs combination chemotherapy in premenopausal women with clinically aggressive HR+/HER2− advanced breast cancer. J Clin Oncol. 2024 May;21 doi: 10.1200/JCO.24.00144. JCO2400144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayer E.L., Ren Y., Wagle N., Mahtani R., Ma C., DeMichele A., et al. Abstract GS3-06: GS3-06 palbociclib after CDK4/6i and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated er+/HER2- metastatic breast cancer. Cancer Res. 2023;83 doi: 10.1158/1538-7445.SABCS22-GS3-06. GS3-06-GS3-06. [DOI] [Google Scholar]
- 86.Kalinsky K., Accordino M.K., Chiuzan C., Mundi P.S., Sakach E., Sathe C., et al. Randomized phase II trial of endocrine therapy with or without ribociclib after progression on cyclin-dependent kinase 4/6 inhibition in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: MAINTAIN trial. J Clin Oncol. 2023;41:4004–4013. doi: 10.1200/JCO.22.02392. [DOI] [PubMed] [Google Scholar]
- 87.Llombart-Cussac A., Medioni J., Colleoni M., Ettl J., Schmid P., Macpherson I., et al. 387TiPPalbociclib rechallenge in hormone receptor (HR)[+]/HER2[-] advanced breast cancer (ABC). PALMIRA trial. Ann Oncol. 2019;30 doi: 10.1093/annonc/mdz242.082. [DOI] [Google Scholar]
- 88.Kalinsky K., Layman R.M., Kaufman P.A., Graff S.L., Bianchini G., Martin M., et al. postMONARCH: a phase 3 study of abemaciclib plus fulvestrant versus placebo plus fulvestrant in patients with HR+, HER2-, metastatic breast cancer following progression on a CDK4 & 6 inhibitor and endocrine therapy. J Clin Oncol. 2022;40:TPS1117. doi: 10.1200/JCO.2022.40.16_suppl.TPS1117. TPS1117. [DOI] [Google Scholar]
- 89.André F., Ciruelos E.M., Juric D., Loibl S., Campone M., Mayer I.A., et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32:208–217. doi: 10.1016/j.annonc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 90.Rugo H.S., Lerebours F., Ciruelos E., Drullinsky P., Ruiz-Borrego M., Neven P., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 91.De Laurentiis M., Costa L., Gligorov J., Knop A., Senkus-Konefka E., García-Sáenz J.A., et al. EPIK-B5: a phase III, randomized study of alpelisib (ALP) plus fulvestrant (FUL) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), PIK3CA-mutated advanced breast cancer (ABC) progressing on/after. J Clin Oncol. 2022;40:TPS1109. doi: 10.1200/JCO.2022.40.16_suppl.TPS1109. TPS1109. [DOI] [Google Scholar]
- 92.Turner N.C., Oliveira M., Howell S.J., Dalenc F., Cortes J., Gomez Moreno H.L., et al. Capivasertib in hormone receptor-positive advanced breast cancer. N Engl J Med. 2023;388:2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bidard F.-C., Kaklamani V.G., Neven P., Streich G., Montero A.J., Forget F., et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40:3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rugo H.S., Bardia A., Marmé F., Cortes J., Schmid P., Loirat D., et al. Primary results from TROPiCS-02: a randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician's choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. J Clin Oncol. 2022;40:LBA1001. doi: 10.1200/JCO.2022.40.17_suppl.LBA1001. LBA1001. [DOI] [Google Scholar]
- 96.Rugo H.S., Bardia A., Marmé F., Cortés J., Schmid P., Loirat D., et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1423–1433. doi: 10.1016/S0140-6736(23)01245-X. [DOI] [PubMed] [Google Scholar]
- 97.Bardia A., Jhaveri K., Im S.-A., Pernas Simon S., De Laurentiis M., Wang S., et al. LBA11 Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC)<strong>:</strong> Primary results from the randomised phase III TROPION-B. Ann Oncol. 2023;34:S1264. doi: 10.1016/j.annonc.2023.10.015. 5. [DOI] [Google Scholar]
- 98.Giordano S.H., Franzoi M.A.B., Temin S., Anders C.K., Chandarlapaty S., Crews J.R., et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J Clin Oncol. 2022;40:2612–2635. doi: 10.1200/JCO.22.00519. [DOI] [PubMed] [Google Scholar]
- 99.Ramakrishna N., Anders C.K., Lin N.U., Morikawa A., Temin S., Chandarlapaty S., et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40:2636–2655. doi: 10.1200/JCO.22.00520. [DOI] [PubMed] [Google Scholar]
- 100.Modi S., Saura C., Yamashita T., Park Y.H., Kim S.-B., Tamura K., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2019;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saura C., Modi S., Krop I., Park Y.H., Kim S.-B., Tamura K., et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: updated survival results from a phase II trial (DESTINY-Breast01)☆. Ann Oncol. 2024;35:302. doi: 10.1016/j.annonc.2023.12.001. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.André F., Hee Park Y., Kim S.-B., Takano T., Im S.-A., Borges G., et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401:1773–1785. doi: 10.1016/S0140-6736(23)00725-0. [DOI] [PubMed] [Google Scholar]
- 103.Rugo H.S., Bianchini G., Cortes J., Henning J.-W., Untch M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hurvitz S.A., Hegg R., Chung W.-P., Im S.-A., Jacot W., Ganju V., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 105.Lin N.U., Murthy R.K., Abramson V., Anders C., Bachelot T., Bedard P.L., et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-Positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 2023;9:197–205. doi: 10.1001/jamaoncol.2022.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curigliano G., Mueller V., Borges V., Hamilton E., Hurvitz S., Loi S., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33:321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Rugo H.S., Im S.-A., Cardoso F., Cortés J., Curigliano G., Musolino A., et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saura C., Oliveira M., Feng Y.-H., Dai M.-S., Chen S.-W., Hurvitz S.A., et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38:3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pivot X., Manikhas A., Żurawski B., Chmielowska E., Karaszewska B., Allerton R., et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33:1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 110.Xu B., Yan M., Ma F., Hu X., Feng J., Ouyang Q., et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351–360. doi: 10.1016/S1470-2045(20)30702-6. [DOI] [PubMed] [Google Scholar]
- 111.Ma F., Yan M., Li W., Ouyang Q., Tong Z., Teng Y., et al. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): randomised, double blind, multicentre, phase 3 trial. BMJ. 2023;383 doi: 10.1136/bmj-2023-076065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emens L., et al. LBA16 - IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2020;31(suppl_) [Google Scholar]
- 113.Miles D., Gligorov J., André F., Cameron D., Schneeweiss A., Barrios C., et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 114.Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 115.Sessa C, Balmaña J, Bober SL, Cardoso MJ, Curigliano G, Domchek SM, Evans DG, Fischerova D, Harbeck N, Kuhl C, Lemley B, Levy-Lahad E, Lambertini M, Ledermann JA, Loibl S, Phillips KA, Paluch-Shimon S. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2023 Jan;34(1):33–47. doi: 10.1016/j.annonc.2022.10.004. Epub 2022 Oct 25. PMID: 36307055. [DOI] [PubMed] [Google Scholar]
- 116.Tung N.M., Boughey J.C., Pierce L.J., Robson M.E., Bedrosian I., Dietz J.R., et al. Management of hereditary breast cancer: American society of clinical oncology, American society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol. 2020;38:2080–2106. doi: 10.1200/JCO.20.00299. [DOI] [PubMed] [Google Scholar]
- 117.Hu C., Hart S.N., Gnanaolivu R., Huang H., Lee K.Y., Na J., et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 119.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robson M., Ruddy K.J., Im S.-A., Senkus E., Xu B., Domchek S.M., et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer. 2019;120:20–30. doi: 10.1016/j.ejca.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hurvitz S.A., Gonçalves A., Rugo H.S., Lee K.-H., Fehrenbacher L., Mina L.A., et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncol. 2020;25:e439–e450. doi: 10.1634/theoncologist.2019-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robson M.E., Tung N., Conte P., Im S.-A., Senkus E., Xu B., et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diéras V., Han H.S., Kaufman B., Wildiers H., Friedlander M., Ayoub J.-P., et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:1269–1282. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 124.Tung N.M., Robson M.E., Ventz S., Santa-Maria C.A., Nanda R., Marcom P.K., et al. Tbcrc 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38:4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 125.Wang J., Xu B., Cai L., Song Y., Kang L., Sun T., et al. 235P Efficacy and safety of first-line therapy with fulvestrant or exemestane for postmenopausal ER+/HER2- advanced breast cancer patients after adjuvant nonsteroidal aromatase inhibitor treatment: a randomized, open-label, multicenter study. Ann Oncol. 2021;32:S461–S462. doi: 10.1016/j.annonc.2021.08.518. [DOI] [Google Scholar]
- 126.Brett J.O., Spring L.M., Bardia A., Wander S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85. doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berger F., Marce M., Delaloge S., Hardy-Bessard A.-C., Bachelot T., Bièche I., et al. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patie. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pascual J., Attard G., Bidard F.-C., Curigliano G., De Mattos-Arruda L., Diehn M., et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022;33:750–768. doi: 10.1016/j.annonc.2022.05.520. [DOI] [PubMed] [Google Scholar]
- 129.Condorelli R., Mosele F., Verret B., Bachelot T., Bedard P.L., Cortes J., et al. Genomic alterations in breast cancer: level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) Ann Oncol. 2019;30:365–373. doi: 10.1093/annonc/mdz036. [DOI] [PubMed] [Google Scholar]
- 130.Turner N., Huang-Bartlett C., Kalinsky K., Cristofanilli M., Bianchini G., Chia S., et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol. 2023;19:559–573. doi: 10.2217/fon-2022-1196. [DOI] [PubMed] [Google Scholar]
- 131.Prat A., Chaudhury A., Solovieff N., Paré L., Martinez D., Chic N., et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–1467. doi: 10.1200/JCO.20.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cejalvo J.M., Martínez de Dueñas E., Galván P., García-Recio S., Burgués Gasión O., Paré L., et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res. 2017;77:2213–2221. doi: 10.1158/0008-5472.CAN-16-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aftimos P., Oliveira M., Irrthum A., Fumagalli D., Sotiriou C., Gal-Yam E.N., et al. Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in AURORA, the breast international group (BIG) molecular screening initiative. Cancer Discov. 2021;11:2796–2811. doi: 10.1158/2159-8290.CD-20-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prat A., Brase J.C., Cheng Y., Nuciforo P., Paré L., Pascual T., et al. Everolimus plus exemestane for hormone receptor-positive advanced breast cancer: a PAM50 intrinsic subtype analysis of BOLERO-2. Oncol. 2019;24:893–900. doi: 10.1634/theoncologist.2018-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prat A., Cheang M.C.U., Galván P., Nuciforo P., Paré L., Adamo B., et al. Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol. 2016;2:1287–1294. doi: 10.1001/jamaoncol.2016.0922. [DOI] [PubMed] [Google Scholar]
- 136.Schettini F., Chic N., Brasó-Maristany F., Paré L., Pascual T., Conte B., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7 doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gampenrieder S.P., Rinnerthaler G., Tinchon C., Petzer A., Balic M., Heibl S., et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23:112. doi: 10.1186/s13058-021-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tarantino P., Hamilton E., Tolaney S.M., Cortes J., Morganti S., Ferraro E., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 139.de Calbiac O., Lusque A., Mailliez A., Bachelot T., Uwer L., Mouret-Reynier M.-A., et al. Comparison of management and outcomes in ERBB2-low vs ERBB2-zero metastatic breast cancer in France. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hogan M.P., Goldman D.A., Dashevsky B., Riedl C.C., Gönen M., Osborne J.R., et al. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med. 2015;56:1674–1680. doi: 10.2967/jnumed.115.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Groheux D., Giacchetti S., Delord M., Hindié E., Vercellino L., Cuvier C., et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5–11. doi: 10.2967/jnumed.112.106864. [DOI] [PubMed] [Google Scholar]
- 142.Gentile L.F., Plitas G., Zabor E.C., Stempel M., Morrow M., Barrio A.V. Tumor biology predicts pathologic complete response to neoadjuvant chemotherapy in patients presenting with locally advanced breast cancer. Ann Surg Oncol. 2017;24:3896–3902. doi: 10.1245/s10434-017-6085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gianni L., Pienkowski T., Im Y.-H., Roman L., Tseng L.-M., Liu M.-C., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 144.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 145.von Minckwitz G., Huang C.-S., Mano M.S., Loibl S., Mamounas E.P., Untch M., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 146.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Antonarakis E.S., Park S.H., Goh J.C., Shin S.J., Lee J.L., Mehra N., et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-Unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol. 2023;41:3839–3850. doi: 10.1200/JCO.23.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Newman L.A., Kuerer H.M., Hunt K.K., Ames F.C., Ross M.I., Theriault R., et al. Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann Surg Oncol. 1999;6:671–675. doi: 10.1007/s10434-999-0671-6. [DOI] [PubMed] [Google Scholar]
- 149.Dudley C.M., Wiener A.A., Stankowski-Drengler T.J., Schumacher J.R., Francescatti A.B., Poore S.O., et al. Rates of ipsilateral local-regional recurrence in high-risk patients undergoing immediate post-mastectomy reconstruction (AFT-01) Clin Breast Cancer. 2021;21:433–439. doi: 10.1016/j.clbc.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fernando I.N., Bowden S.J., Herring K., Brookes C.L., Ahmed I., Marshall A., et al. Synchronous versus sequential chemo-radiotherapy in patients with early stage breast cancer (SECRAB): a randomised, phase III, trial. Radiother Oncol. 2020;142:52–61. doi: 10.1016/j.radonc.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meattini I., Becherini C., Caini S., Coles C.E., Cortes J., Curigliano G., et al. International multidisciplinary consensus on the integration of radiotherapy with new systemic treatments for breast cancer: European Society for Radiotherapy and Oncology (ESTRO)-endorsed recommendations. Lancet Oncol. 2024;25:e73–e83. doi: 10.1016/S1470-2045(23)00534-X. [DOI] [PubMed] [Google Scholar]
- 152.Norikazu M., Soo-Jung L., Shoichiro O., Young-Hyuck I., Eun-Sook L., Isao Y., et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2024;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 153.Paluch-Shimon S., Cardoso F., Partridge A.H., Abulkhair O., Azim H.A., Bianchi-Micheli G., et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5) Ann Oncol. 2022;33:1097–1118. doi: 10.1016/j.annonc.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 154.Lambertini M., Peccatori F.A., Demeestere I., Amant F., Wyns C., Stukenborg J.-B., et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines(†) Ann Oncol. 2020;31:1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 155.Amant F., Loibl S., Neven P., Van Calsteren K. Breast cancer in pregnancy. Lancet. 2012;379:570–579. doi: 10.1016/S0140-6736(11)61092-1. [DOI] [PubMed] [Google Scholar]
- 156.Loibl S., Azim H.A.J., Bachelot T., Berveiller P., Bosch A., Cardonick E., et al. ESMO Expert Consensus Statements on the management of breast cancer during pregnancy (PrBC) Ann Oncol. 2023;34:849–866. doi: 10.1016/j.annonc.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 157.Amant F., Vandenbroucke T., Verheecke M., Fumagalli M., Halaska M.J., Boere I., et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373:1824–1834. doi: 10.1056/NEJMoa1508913. [DOI] [PubMed] [Google Scholar]
- 158.Chasimpha S., McCormack V., Cubasch H., Joffe M., Zietsman A., Galukande M., et al. Disparities in breast cancer survival between women with and without HIV across sub-Saharan Africa (ABC-DO): a prospective, cohort study. Lancet HIV. 2022;9:e160–e171. doi: 10.1016/S2352-3018(21)00326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCormack V.A., Febvey-Combes O., Ginsburg O., Dos-Santos-Silva I. Breast cancer in women living with HIV: a first global estimate. Int J Cancer. 2018;143:2732–2740. doi: 10.1002/ijc.31722. [DOI] [PubMed] [Google Scholar]
- 160.Martei Y.M., Narasimhamurthy M., Setlhako D.I., Ayane G., Ralefala T., Chiyapo S., et al. Relative dose intensity and pathologic response rates in patients with breast cancer and with and without HIV who received neoadjuvant chemotherapy. JCO Glob Oncol. 2022;8 doi: 10.1200/GO.22.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nietz S., O'Neil D.S., Ayeni O., Chen W.C., Joffe M., Jacobson J.S., et al. A comparison of complete pathologic response rates following neoadjuvant chemotherapy among South African breast cancer patients with and without concurrent HIV infection. Breast Cancer Res Treat. 2020;184:861–872. doi: 10.1007/s10549-020-05889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fenwick C., Joo V., Jacquier P., Noto A., Banga R., Perreau M., et al. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292:149–163. doi: 10.1111/imr.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.El-Sadr W.M., Lundgren J.D., Neaton J.D., Gordin F., Abrams D., Arduino R.C., et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 164.El Zarif T., Nassar A.H., Adib E., Fitzgerald B.G., Huang J., Mouhieddine T.H., et al. Safety and activity of immune checkpoint inhibitors in people living with HIV and cancer: a real-world report from the cancer therapy using checkpoint inhibitors in people living with HIV-international (CATCH-IT) consortium. J Clin Oncol. 2023;41:3712–3723. doi: 10.1200/JCO.22.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen M.-T., Sun H.-F., Zhao Y., Fu W.-Y., Yang L.-P., Gao S.-P., et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7:9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Biganzoli L., Battisti N.M.L., Wildiers H., McCartney A., Colloca G., Kunkler I.H., et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) Lancet Oncol. 2021;22:e327–e340. doi: 10.1016/S1470-2045(20)30741-5. [DOI] [PubMed] [Google Scholar]
- 167.Sedrak M.S., Freedman R.A., Cohen H.J., Muss H.B., Jatoi A., Klepin H.D., et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA A Cancer J Clin. 2021;71:78–92. doi: 10.3322/caac.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wildiers H., de Glas N.A. Anticancer drugs are not well tolerated in all older patients with cancer. Lancet Healthy Longev. 2020;1:e43–e47. doi: 10.1016/S2666-7568(20)30001-5. [DOI] [PubMed] [Google Scholar]
- 169.Mohile S.G., Dale W., Somerfield M.R., Schonberg M.A., Boyd C.M., Burhenn P.S., et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Williams G.R., Hopkins J.O., Klepin H.D., Lowenstein L.M., Mackenzie A., Mohile S.G., et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline questions and answers. JCO Oncol Pract. 2023;19:718–723. doi: 10.1200/OP.23.00263. [DOI] [PubMed] [Google Scholar]
- 171.Alhumaidi R.M., Bamagous G.A., Alsanosi S.M., Alqashqari H.S., Qadhi R.S., Alhindi Y.Z., et al. Risk of polypharmacy and its outcome in terms of drug interaction in an elderly population: a retrospective cross-sectional study. J Clin Med. 2023;12 doi: 10.3390/jcm12123960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carola E., Pulido M., Falandry C., Paillaud E., Caillet P., Tassy L., et al. First-line systemic treatment with palbociclib in women aged ≥70 years presenting with hormone receptor-positive advanced breast cancer: results from the PALOMAGE program. J Clin Oncol. 2023;41:1018. doi: 10.1200/JCO.2023.41.16_suppl.1018. [DOI] [Google Scholar]
- 173.Biganzoli L., Wildiers H., Oakman C., Marotti L., Loibl S., Kunkler I., et al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 174.Brain E., Caillet P., de Glas N., Biganzoli L., Cheng K., Lago L.D., et al. HER2-targeted treatment for older patients with breast cancer: an expert position paper from the International Society of Geriatric Oncology. J Geriatr Oncol. 2019;10:1003–1013. doi: 10.1016/j.jgo.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 175.https://www.cancer.gov/[n.d].
- 176.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gradishar W.J., Moran M.S., Abraham J., Abramson V., Aft R., Agnese D., et al. NCCN Guidelines® insights: breast cancer, version 4.2023. J Natl Compr Cancer Netw. 2023;21:594–608. doi: 10.6004/jnccn.2023.0031. [DOI] [PubMed] [Google Scholar]
- 178.Benvenuti C., Gaudio M., Jacobs F., Saltalamacchia G., De Sanctis R., Torrisi R., et al. Clinical review on the management of breast cancer visceral crisis. Biomedicines. 2023;11 doi: 10.3390/biomedicines11041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sperduto P.W., Mesko S., Li J., Cagney D., Aizer A., Lin N.U., et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38:3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meattini I., Andratschke N., Kirby A.M., Sviri G., Offersen B.V., Poortmans P., et al. Challenges in the treatment of breast cancer brain metastases: evidence, unresolved questions, and a practical algorithm. Clin Transl Oncol. 2020;22:1698–1709. doi: 10.1007/s12094-020-02333-7. [DOI] [PubMed] [Google Scholar]
- 181.Kaidar-Person O., Meattini I., Jain P., Bult P., Simone N., Kindts I., et al. Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: an international multicenter study. Breast Cancer Res Treat. 2018;167:479–483. doi: 10.1007/s10549-017-4526-8. [DOI] [PubMed] [Google Scholar]
- 182.Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 183.Mamounas E.P., Untch M., Mano M.S., Huang C.-S., Geyer C.E.J., von Minckwitz G., et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol. 2021;32:1005–1014. doi: 10.1016/j.annonc.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 184.Montemurro F., Delaloge S., Barrios C.H., Wuerstlein R., Anton A., Brain E., et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(☆) Ann Oncol. 2020;31:1350–1358. doi: 10.1016/j.annonc.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 185.Jerusalem G.H.M., Park Y.H., Yamashita T., Hurvitz S.A., Modi S., Andre F., et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol. 2021;39:526. doi: 10.1200/JCO.2021.39.15_suppl.526. [DOI] [Google Scholar]
- 186.Hurvitz S.A., Modi S., Li W., Park Y.H., Chung W., Kim S.-B., et al. 377O A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast (DB) -01, -02, and -03. Ann Oncol. 2023;34:S335–S336. doi: 10.1016/j.annonc.2023.09.554. [DOI] [Google Scholar]
- 187.Puri A., Mylavarapu C., Xu J., Patel T.A., S, Teh B., Tremont-Lukats I., et al. Clinical factors and association with treatment modalities in patients with breast cancer and brain metastases who develop leptomeningeal metastases. Breast Cancer Res Treat. 2022;193:613–623. doi: 10.1007/s10549-022-06595-3. [DOI] [PubMed] [Google Scholar]
- 188.Le Rhun E., Weller M., Brandsma D., Van den Bent M., de Azambuja E., Henriksson R., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84–99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 189.Le Rhun E., Devos P., Weller J., Seystahl K., Mo F., Compter A., et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2021;23:1100–1112. doi: 10.1093/neuonc/noaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Glass J.P., Melamed M., Chernik N.L., Posner J.B. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29:1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 191.Posner J.B., Chernik N.L. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 192.Bonneau C., Paintaud G., Trédan O., Dubot C., Desvignes C., Dieras V., et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. doi: 10.1016/j.ejca.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 193.Yust-Katz S., Garciarena P., Liu D., Yuan Y., Ibrahim N., Yerushalmi R., et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neuro Oncol. 2013;114:229–235. doi: 10.1007/s11060-013-1175-6. [DOI] [PubMed] [Google Scholar]
- 194.Abouharb S., Ensor J., Loghin M.E., Katz R., Moulder S.L., Esteva F.J., et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014;146:477–486. doi: 10.1007/s10549-014-3054-z. [DOI] [PubMed] [Google Scholar]
- 195.Carausu M., Carton M., Darlix A., Pasquier D., Leheurteur M., Debled M., et al. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fang W., Huang Y., Han X., Peng J., Zheng M. Characteristics of metastasis and survival between male and female breast cancer with different molecular subtypes: a population-based observational study. Cancer Med. 2022;11:764–777. doi: 10.1002/cam4.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kingston B., Kayhanian H., Brooks C., Cox N., Chaabouni N., Redana S., et al. Treatment and prognosis of leptomeningeal disease secondary to metastatic breast cancer: a single-centre experience. Breast. 2017;36:54–59. doi: 10.1016/j.breast.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 198.Kohler B.A., Sherman R.L., Howlader N., Jemal A., Ryerson A.B., Henry K.A., et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wallace G., Ahmed K., Soyano A., Forsyth P. Changing recognition of breast cancer–related leptomeningeal disease and response to therapy: a retrospective single institution review. J Clin Oncol. 2022;40:2027. doi: 10.1200/JCO.2022.40.16_suppl.2027. [DOI] [Google Scholar]
- 200.Ratosa I., Dobnikar N., Bottosso M., Dieci M.V., Jacot W., Pouderoux S., et al. Leptomeningeal metastases in patients with human epidermal growth factor receptor 2 positive breast cancer: real-world data from a multicentric European cohort. Int J Cancer. 2022;151:1355–1366. doi: 10.1002/ijc.34135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Le Rhun E., Taillibert S., Zairi F., Devos P., Pierret M.F., Dubois F., et al. Clinicopathological features of breast cancers predict the development of leptomeningeal metastases: a case-control study. J Neuro Oncol. 2011;105:309–315. doi: 10.1007/s11060-011-0592-7. [DOI] [PubMed] [Google Scholar]
- 202.Kumthekar P.U., Avram M.J., Lassman A.B., Lin N.U., Lee E., Grimm S.A., et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro Oncol. 2023;25:557–565. doi: 10.1093/neuonc/noac195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zagouri F., Zoumpourlis P., Le Rhun E., Bartsch R., Zografos E., Apostolidou K., et al. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: a metanalysis with meta-regression. Cancer Treat Rev. 2020;88 doi: 10.1016/j.ctrv.2020.102046. [DOI] [PubMed] [Google Scholar]
- 204.Boogerd W., Van Den Bent M.J., Koehler P.J., Heimans J.J., Van Der Sande J.J., Aaronson N.K., et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40:2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 205.Le Rhun E., Wallet J., Mailliez A., Le Deley M.C., Rodrigues I., Boulanger T., et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22:524–538. doi: 10.1093/neuonc/noz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grossman S.A., Finkelstein D.M., Ruckdeschel J.C., Trump D.L., Moynihan T., Ettinger D.S. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:561–569. doi: 10.1200/JCO.1993.11.3.561. [DOI] [PubMed] [Google Scholar]
- 207.Glantz M.J., Jaeckle K.A., Chamberlain M.C., Phuphanich S., Recht L., Swinnen L.J., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid Tumors1. Clin Cancer Res. 1999;5:3394–3402. [PubMed] [Google Scholar]
- 208.Bokstein F., Lossos A., Siegal T. Leptomeningeal metastases from solid tumors. Cancer. 1998;82:1756–1763. doi: 10.1002/(SICI)1097-0142(19980501)82:9<1764::AID-CNCR24>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 209.Oberkampf F., Gutierrez M., Trabelsi Grati O., Le Rhun É., Trédan O., Turbiez I., et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol. 2023;25:365–374. doi: 10.1093/neuonc/noac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shafie R.A.E., Böhm K., Weber D., Lang K., Schlaich F., Adeberg S., et al. Palliative radiotherapy for leptomeningeal carcinomatosis-analysis of outcome, prognostic factors, and symptom response. Front Oncol. 2019;9:1–13. doi: 10.3389/fonc.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wolf A., Kvint S., Chachoua A., Pavlick A., Wilson M., Donahue B., et al. Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J Neurosurg. 2018;128:23–31. doi: 10.3171/2016.10.JNS161036. [DOI] [PubMed] [Google Scholar]
- 212.Yang J.T., Wijetunga N.A., Pentsova E., Wolden S., Young R.J., Correa D., et al. Randomized phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis. J Clin Oncol. 2022;40:3858–3867. doi: 10.1200/JCO.22.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bartsch R., Jerzak K.J., Larrouquere L., Müller V., Le Rhun E. Pharmacotherapy for leptomeningeal disease in breast cancer. Cancer Treat Rev. 2024;122 doi: 10.1016/j.ctrv.2023.102653. [DOI] [PubMed] [Google Scholar]
- 214.Sharma A.E., Corbett K., Soliman H., Sahgal A., Das S., Lim-Fat M.J., et al. Assessment of phase 3 randomized clinical trials including patients with leptomeningeal disease: a systematic review. JAMA Oncol. 2023;9:566–567. doi: 10.1001/jamaoncol.2022.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Niwińska A., Pogoda K., Michalski W., Kunkiel M., Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM) J Neuro Oncol. 2018;138:191–198. doi: 10.1007/s11060-018-2790-z. [DOI] [PubMed] [Google Scholar]
- 216.Stringer-Reasor E.M., O'Brien B.J., Topletz-Erickson A., White J.B., Lobbous M., Riley K., et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast can. J Clin Oncol. 2021;39:1044. doi: 10.1200/JCO.2021.39.15_suppl.1044. [DOI] [Google Scholar]
- 217.Alder L., Trapani D., Bradbury C., Van Swearingen A.E.D., Tolaney S.M., Khasraw M., et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. NPJ Breast Cancer. 2023;9:19. doi: 10.1038/s41523-023-00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Beniey M. Peritoneal metastases from breast cancer: a scoping review. Cureus. 2019;11 doi: 10.7759/cureus.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Runyon B.A., Hoefs J.C., Morgan T.R. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104–1109. doi: 10.1002/hep.1840080521. [DOI] [PubMed] [Google Scholar]
- 220.Arends J., Strasser F., Gonella S., Solheim T.S., Madeddu C., Ravasco P., et al. Cancer cachexia in adult patients: ESMO clinical practice Guidelines(☆) ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Irvin W., Muss H.B., Mayer D.K. Symptom management in metastatic breast cancer. Oncol. 2011;16:1203–1214. doi: 10.1634/theoncologist.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ota K.S., Schultz N., Segaline N.A. Palliative paracentesis in the home setting: a case series. Am J Hosp Palliat Care. 2021;38:1042–1045. doi: 10.1177/1049909120963075. [DOI] [PubMed] [Google Scholar]
- 223.Skeoch S., Weatherley N., Swift A.J., Oldroyd A., Johns C., Hayton C., et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:1–30. doi: 10.3390/JCM7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Law J.W., Campbell A., Weller C., Johanson C., Broome R., Piault E., et al. Epidemiology of interstitial lung disease in patients with metastatic breast cancer at baseline and after treatment with HER2-directed therapy: a real-world data analysis. Breast Cancer Res Treat. 2022;196:603–611. doi: 10.1007/s10549-022-06738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kobayashi H., Naito T., Omae K., Omori S., Nakashima K., Wakuda K., et al. ILD-NSCLC-GAP index scoring and staging system for patients with non-small cell lung cancer and interstitial lung disease. Lung Cancer. 2018;121:48–53. doi: 10.1016/j.lungcan.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 226.Herrstedt J., Clark-Snow R., Ruhlmann C.H., Molassiotis A., Olver I., Rapoport B.L., et al. 2023 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. ESMO Open. 2024;9 doi: 10.1016/j.esmoop.2023.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lange M., Joly F., Vardy J., Ahles T., Dubois M., Tron L., et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019;30:1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Joly F., Castel H., Tron L., Lange M., Vardy J. Potential effect of immunotherapy agents on cognitive function in cancer patients. JNCI: J Natl Cancer Inst. 2020;112:123–127. doi: 10.1093/jnci/djz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ahles T.A., Root J.C. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14:425–451. doi: 10.1146/annurev-clinpsy-050817-084903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Clarijs M.E., Thurell J., Kühn F., Uyl-de Groot C.A., Hedayati E., Karsten M.M., et al. Measuring quality of life using patient-reported outcomes in real-world metastatic breast cancer patients: the need for a standardized approach. Cancers. 2021;13 doi: 10.3390/cancers13102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bell M.L., Dhillon H.M., Bray V.J., Vardy J.L. Important differences and meaningful changes for the functional assessment of cancer therapy-cognitive function (FACT-Cog) J Patient Rep Outcomes. 2018;2 doi: 10.1186/s41687-018-0071-4. [DOI] [Google Scholar]
- 232.Sanft T., Day A., Peterson L., Rodriguez M.A., Ansbaugh S., Armenian S., et al. NCCN Guidelines® insights: survivorship, version 1.2022. J Natl Compr Cancer Netw. 2022;20:1080–1090. doi: 10.6004/jnccn.2022.0052. [DOI] [PubMed] [Google Scholar]
- 233.Fernandes H.A., Richard N.M., Edelstein K. Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support Care Cancer. 2019;27:3253–3279. doi: 10.1007/s00520-019-04866-2. [DOI] [PubMed] [Google Scholar]
- 234.Zimmer P., Baumann F.T., Oberste M., Wright P., Garthe A., Schenk A., et al. Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: a systematic review. BioMed Res Int. 2016;2016 doi: 10.1155/2016/1820954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dittus K.L., Gramling R.E., Ades P.A. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132. doi: 10.1016/j.ypmed.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 237.Ligibel J.A., Giobbie-Hurder A., Shockro L., Campbell N., Partridge A.H., Tolaney S.M., et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122:1169–1177. doi: 10.1002/cncr.29899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.