Abstract
Rapid analysis of multiple food allergens is required to confirm the appropriateness of food allergen labelling in processed foods. This study aimed to develop a rapid and reliable method to simultaneously detect trace amounts of seven food allergenic proteins (wheat, buckwheat, milk, egg, crustacean, peanut, and walnut) in processed foods using LC-MS/MS. Suspension-trapping (S-Trap) columns and on-line automated solid-phase extraction were used to improve the complex and time-consuming pretreatment process previously required for allergen analysis using LC-MS/MS. The developed method enabled the simultaneous detection of selected marker peptides for specific proteins derived from seven food ingredients in five types of incurred samples amended with trace amounts of allergenic proteins. The limit of detection values of the method for each protein were estimated to be <1 mg/kg. The developed analytical approach is considered an effective screening method for confirming food allergen labelling on a wide range of processed foods.
Keywords: LC-MS/MS, Peptide marker, Solid-phase extraction, Food allergen, Detection method, Processed food
Highlights
-
•
A method was developed for simultaneous LC-MS/MS detection of 7 food allergens.
-
•
The S-Trap column and on-line SPE system enable simple and rapid measurements.
-
•
The method was validated using 5 incurred samples containing 7 allergenic proteins.
-
•
The developed analytical approach is applicable to a wide variety of processed foods.
1. Introduction
Food allergies are immunologic reactions caused by ingestion of foods containing allergens that cause symptoms, and even a very small amount (e.g., a few milligrams of protein) can cause serious allergic reactions, such as anaphylactic shock. In recent years, the number of individuals with food allergies has increased worldwide and now represents an international health problem (Özdemir, Sato, Yanagida, & Ebisawa, 2023). Therefore, in 1999, the Codex Alimentarius Commission established labelling guidelines for raw materials that cause food allergies. The current guidelines list eight raw materials for which labelling is recommended: gluten-containing grains, shellfish, eggs, fish, peanuts and soybeans, milk, nuts, and sulphites at a concentration of ≥10 mg/kg (Codex Alimentarius Commission, 2018). More recently, the Codex Committee on Food Labelling (CCFL47) suggested that sesame should replace soybeans as a raw material to be labelled (Codex Alimentarius Commission, 2023).
It is important to provide food ingredient information on packaging so that consumers with a food allergy can make appropriate choices when buying food to avoid unexpectedly triggering a food allergy. In Japan, some labelling of allergen-inducing ingredients has been mandatory or recommended since 2002, and currently, labelling is required for eight ingredients (egg, milk, wheat, buckwheat, peanuts, shrimp, crab, and walnut) (Government of Japan, 2023). Validated analytical methods for specified allergenic ingredients are necessary to confirm the adequacy of allergen labelling of processed foods. Methods generally used for this purpose include enzyme-linked immunosorbent assay (ELISA), lateral flow immunoassay (immunochromatography), polymerase chain reaction (PCR), and Western blotting (Akiyama & Adachi, 2021; Albarrak & Al-Sobayil, 2024; Köppel, Stadler, Lüthy, & Hübner, 1998; Miyazaki et al., 2019; Ross, Salentijn, & Nielen, 2019; Saito et al., 2019).
The official approach for testing specified allergenic ingredients in Japan involves initial screening using an ELISA, and if the target protein is detected at a concentration of ≥10 mg/kg, PCR (for wheat, buckwheat, peanuts, shrimp, crab, and walnut) or Western blotting (for milk and egg) should be performed for qualitative confirmation (Akiyama & Adachi, 2021). However, as ELISA, immunochromatography, and Western blotting use antibodies, cross-reactivity with components other than the target raw material may occur, leading to false-positive results (Akiyama & Adachi, 2021; Saito et al., 2019). Furthermore, the sensitivity of PCR methods may be low for some heat-processed foods due to extraction of an inadequate amount of DNA for testing (Linacero, Sanchiz, Ballesteros, & Cuadrado, 2020). ELISA and PCR methods generally target a single protein or gene sequence, which makes it difficult to simultaneously detect multiple food allergens. Moreover, considerable time, effort, and expense are required to analyse a single food sample for multiple allergens using these methods in order to check for unintended allergen contamination in foods.
To overcome these disadvantages, various methods for analysing food allergens using high-performance liquid chromatography–tandem quadrupole mass spectrometry (LC-MS/MS) have recently been developed (Fallahbaghery, Zou, Byrne, Howitt, & Colgrave, 2017; Schalk, Koehler, & Scherf, 2018; Croote, Braslavsky, & Quake, 2019; Henrottin et al., 2023; Lexhaller, Colgrave, & Scherf, 2019; Huang, Zhu, Feng, Zhang, & Zhang, 2020; Ma et al., 2020; Ramachandran, Yang, & Downs, 2020; Wang et al., 2021; Xiong, Parker, Boo, & Fielder, 2021; Li et al., 2022; Neils, Broadbent, Bose, Anderson, & Colgrave, 2022; Henrottin et al., 2023; Yang et al., 2024). Our research group has also previously reported on methods for simultaneous detection of wheat and buckwheat (Seki et al., 2021), or walnut and almond (Torii et al., 2023) in processed foods using LC-MS/MS. In these LC-MS–based methods, proteins extracted from a food source are digested into peptides using proteases such as trypsin, chymotrypsin or lysyl-endopeptidase, and peptides in which the sequence matches that of the target ingredient are detected by mass spectrometry. LC-MS/MS methods are often used to determine the amino acid sequences of target proteins available in public proteome databases. Using the multiple reaction monitoring (MRM) mode of the mass spectrometer, it becomes possible to simultaneously detect peptides derived from multiple allergen proteins with high specificity.
However, LC-MS/MS analyses generally require 4–24 h for protease digestion of proteins extracted from food samples, and as processed foods contain many non-protein matrix components, such as lipids, salts, pigments, and polysaccharides, solid-phase extraction is often required to pre-purify and concentrate the target peptides, a process that complicates the analysis. In shotgun proteomic analyses, in which proteins in blood or cells are the analytical targets, several simple spin-column–type pre-treatment kits have been developed (Templeton et al., 2023). These kits enable protein digestion and peptide purification using only centrifugation. However, many of these tools are very expensive and not applicable to food samples, making their use impractical for routine analysis of food allergens. Therefore, simpler and more rapid pre-treatment methods applicable to food allergen analysis using LC-MS/MS are needed.
The present study evaluated the suspension-trapping (S-Trap) column, which is used as a spin column for pretreatment in proteomics studies, as a simple and rapid sample preparation tool for the LC-MS/MS analysis of allergens in foods. The S-Trap method uses surfactants such as sodium dodecyl sulphate (SDS) for protein extraction and removes matrix components, including the surfactants, from the sample solution through centrifugation before protease digestion (Zougman, Selby, & Banks, 2014). In addition, proteins trapped on filters in the column are efficiently degraded by proteases, such that digestion can be completed in 1 h. The maximum amount of total protein that can be processed on an S-Trap column is approximately 10 mg per sample, making it applicable to food samples. Additionally, S-Trap columns are less expensive than other similar products. The S-trap method has already been used extensively in proteomics studies, and reports indicate that it enables the identification of an equivalent or greater number of proteins than existing pre-treatment methods such as urea extraction, ultrafiltration, phase transfer surfactant (PTS), and single-pot solid-phase–enhanced sample preparation (SP3) (Hughes et al., 2019). However, there have been no previous reports describing application of the S-Trap method to the analysis of food allergens using LC-MS/MS. Therefore, we hypothesized that the S-Trap column would be a rapid and simple pretreatment tool for food allergen analysis using LC-MS/MS and validated the method using processed foods.
In this study, we developed a rapid screening method using LC-MS/MS combined with an S-Trap column to test for seven food allergens (wheat, buckwheat, milk, egg, crustacean [shrimp and crab], peanut, and walnut) for which labelling is mandatory on processed foods in Japan. We also developed an automated and more-sensitive method to analyse multiple food samples by combining the S-Trap with an on-line automated solid-phase extraction (on-line SPE) system. Five different incurred samples with the addition of seven ingredients were prepared to assess the validity of the developed analytical method, and the results were compared with those obtained using ELISA. In addition, the applicability of the developed method was confirmed using commercially available processed foods.
2. Material and methods
2.1. Material and reagents
Trypsin from bovine pancreas (cat. no. T1426, activity ≥10,000 BAEE units/mg protein), iodoacetamide (BioUltra grade), and DL-dithiothreitol (for molecular biology) were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Tris (hydroxymethyl) aminomethane (for biochemistry), urea (for biochemistry), ammonium hydrogencarbonate (for proteomics), sodium dodecyl sulphate (for molecular biology), Tris (2-carboxyethyl) phosphine hydrochloride (for biochemistry), phosphoric acid (HPLC grade), triethylammonium hydrogencarbonate solution (for nucleic acid synthesis), formic acid (LC-MS grade), and acetonitrile (LC-MS grade) were purchased from FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan). Trifluoroacetic acid (HPLC grade) and methanol (LC-MS grade) were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Synthetic peptides (purity ≥95%) were purchased from Greiner Bio-One GmbH (Kremsmünster, Austria). S-Trap™ midi Rapid Universal MS Sample Prep kits were purchased from ProtiFi Technologies (Fairport, NY, USA). ELISA kits (FASTKIT ELISA Ver. III) for wheat, buckwheat, egg, milk, peanut, and walnut were purchased from NH Foods Ltd. (Osaka, Japan). The ELISA kit for crustaceans (FA test EIA-crustacean II ‘NISSUI’) was purchased from Shimadzu Diagnostics Corp. (Tokyo, Japan).
Eight cultivars of wheat (no. 1 Canada Western Red Spring [Canada origin], US Hard Red Winter [USA origin], US Western White [USA origin], Australian Standard White [Australia origin], ‘Yume-Chikara’ [Japan origin], ‘Kita-Honami’ [Japan origin], ‘Siro-Gane’ [Japan origin], and Canada Western Amber Durum [Canada origin]) were kindly provided by Nisshin Flour Milling, Inc. (Tokyo, Japan). Samples of buckwheat (Fagopyrum esculentum, Japan and Chinese origin), pasteurized bovine milk (Japan origin), hen egg (derived from Gallus gallus domesticus ‘White Leghorn’, Japan origin), black tiger prawn (Penaeus monodon, Vietnam origin), peanut (Virginia type of Arachis hypogaea, Japan origin), and walnut (Juglans regia L. ‘Chandler’, USA origin) were obtained from wholesale companies in Japan. Eighteen types of commercial processed foods were purchased at local stores in Fujimino city, Saitama, Japan.
2.2. Design of targeted peptides for detection
The WHO/IUIS Allergen Nomenclature (Pomés et al., 2018) and Allergome database (Mari, Rasi, Palazzo, & Scala, 2009) were used to search for allergenic proteins in the seven food ingredients. Low-molecular-weight glutenin subunit (Tri a 36), high-molecular-weight glutenin subunit (Tri a 26), and γ-gliadin (Tri a 20) were targeted for detection of wheat protein. For detection of buckwheat protein, 13S globulin (Fag e 1) was targeted. α-S1-casein (Bos d 9), α-S2-casein (Bos d 10), and β-lactoglobulin (Bos d 5) were targeted to detect milk protein. Ovalbumin (Gal d 6), ovotransferrin (Gal d 3), lysozyme C (Gal d 4), and vitellogenin (Gal d 6) were targeted to detect egg protein. Tropomyosin of black tiger prawn (Pen m 1) was targeted to detect crustacean protein. For detection of peanut protein, 7S globulin (Ara h 1), 2S albumin (Ara h 2), and 11S globulin (Ara h 3) were targeted, whereas 2S albumin (Jug r 1) and 7S globulin (Jug r 2) were targeted to detect walnut protein. Targeted peptides derived from trypsin-digested proteins were bioinformatically estimated, and the recommended MRM transitions for LC-MS/MS analysis were determined from information obtained from the Skyline software tool, ver. 22.2 (MacLean et al., 2010). Peptides derived from each ingredient protein were analysed using LC-MS/MS, and optimized peptides exhibiting good sensitivity and specificity among the candidate marker peptides were selected. The target peptides selected for detection of the seven ingredient proteins and the optimized MRM transitions, retention time, collision energy are shown in Table 1. For each marker peptide, the product ion with the highest sensitivity is marked with ‘†’ in the table as the quantitation ion.
Table 1.
Target peptides and MRM transitions for seven food allergens analysed in this study.
| Ingredient | Target protein (IUIS name) |
UniProtKB accession no. |
Amino acid sequence of target peptide |
Precursor ion (m/z) and charge state |
Precursor ion (m/z), fragment type and charge state |
Retention time (min) |
Collision energy (V) |
|---|---|---|---|---|---|---|---|
| Wheat | Low-molecular-weight glutenin subunit (Tri a 36) |
B2Y2Q7 | QIPEQSR | 429.3 (+2) | 616.2 (y5, +1)† 308.5 (y5, +2) 519.3 (y4, +1) |
8.5 | −14.1 |
| VFLQQQC[CAM]⁎SPVAMPQSLAR | 687.4 (+3) | 671.4 (y6, +1)† 873.5 (y8, +1) 1069.6(y10, +1) |
12.3 | −22.5 | |||
| High-molecular-weight glutenin subunit (Tri a 26) |
P10388 | SVAVSQVAR | 458.9 (+3) | 730.4 (y7, +1)† 560.3 (y5, +1) 187.1 (b2, +1) |
9.5 | −15.2 | |
| ELQELQER | 522.8 (+2) | 432.2 (y3, +1)† 674.4 (y5, +1) 545.3 (y4, +1) |
9.8 | −17.2 | |||
| IFWGIPALLK | 579.4 (+2) | 897.6 (y8, +1)† 711.5 (y7, +1) |
15.2 | −19.0 | |||
| γ- Gliadin (Tri a 20) |
Q9SYX8 | APFASIVAGIGGQ | 594.3 (+2) | 502.3 (y6, +1)† 927.5 (b10, +1) 814.4 (b9, +1) |
13.4 | −19.5 | |
| Buckwheat | 13S globulin (Fag e 1) |
O23878 | NAILGPR | 370.7 (+2) | 442.3 (y4, +1)† 299.2 (b3, +1) 555.2 (y5, +1) |
10.1 | −12.1 |
| VQVVGDEGR | 479.8 (+2) | 731.3 (y7, +1)† 632.1 (y6, +1) 533.2 (y5, +1) |
9.2 | −15.7 | |||
| ADVFNPR | 409.7 (+2) | 533.2 (y4, +1)† 386.2 (y3, +1) 632.1 (y5, +1) |
10.2 | −13.4 | |||
| GFIVQAR | 395.8 (+2) | 473.3 (y4, +1)† 177.0 (b3, +1) 586.2 (y5, +1) |
10.8 | −13.0 | |||
| Milk | α-S1-casein (Bos d 9) |
P02662 | YLGYLEQLLR | 634.4 (+2) | 249.2 (a2, +1)† 991.3 (y8, +1) 658.4 (y5, +1) |
14.1 | −20.8 |
| FFVAPFPEVFGK | 692.9 (+2) | 920.5 (y8, +1)† 991.5 (y9, +1) 460.8 (y8, +2) |
14.8 | −22.5 | |||
| α-S2-casein (Bos d 10) |
P02663 | NAVPITPTLNR | 598.3 (+2) | 911.3 (y8, +1)† 285.2 (b3, +1) 456.3 (y8, +2) |
11.2 | −19.5 | |
| β-Lactoglobulin (Bos d 5) |
P02754 | TPEVDDEALEK | 623.3 (+2) | 572.8 (y10, +2) 199.2 (b2, +1) 819.4 (y7, +1) |
10.1 | −20.4 | |
| VLVLTDYK | 533.3 (+2) | 853.4 (y7, +1)† 754.4 (y6, +1) 641.1 (y5, +1) |
11.6 | −17.5 | |||
| Egg | Ovalbumin (Gal d 6) |
P01012 | LTEWTSSNVMEER | 791.4 (+2) | 951.4 (y8, +1)† 1052.5 (y9, +1) 1238.5 (y10, +1) |
11.3 | −25.9 |
| GGLEPINFQTAADQAR | 844.4 (+2) | 666.3 (y12, +2)† 1331.7 (y12, +1) 1121.5 (y10, +1) |
12.0 | −27.6 | |||
| ELINSWVESQTNGIIR | 930.0 (+2) | 1017.5 (y9, +1)† 1116.6 (y10, +1) 888.5 (y8, +1) |
13.1 | −30.4 | |||
| Ovotransferrin (Gal d 3) |
P02789 | TDERPASYFAVAVAR | 551.6 (+3) | 416.3 (y4, +1)† 515.4 (y5, +1) 586.4 (y6, +1) |
11.8 | −15.1 | |
| Lysozyme C (Gal d 4) |
P00698 | GTDVQAWIR | 523.3 (+2) | 673.4 (y5, +1)† 545.3 (y4, +1) 887.5 (y7, +1) |
11.7 | −17.1 | |
| Vitellogenin (Gal d 6) |
P87498 | NIPFAEYPTYK | 671.8 (+2) | 508.3 (y4, +1)† 1115.5 (y9, +1) 558.3 (y9, +2) |
12.1 | −22.0 | |
| LPLSLPVGPR | 524.8 (+2) | 468.3 (y9, +2)† 725.4 (y7, +1) |
12.9 | −17.2 | |||
| Crustacean | Tropomyosine (Pen m 1) |
A1KYZ2 | FLAEEADR | 475.7 (+2) | 690.2 (y6, +1)† 261.2 (b2, +1) 619.3 (y5, +1) |
10.0 | −15.6 |
| LAMVEADLER | 573.6 (+2) | 732.3 (y6, +1)† 831.4 (y7, +1) 1033.6 (y9, +1) |
11.5 | −18.8 | |||
| IVELEEELR | 565.3 (+2) | 213.2 (b2, +1)† 917.5 (y7, +1) 675.3 (y5, +1) |
11.7 | −18.5 | |||
| IQLLEEDLER | 629.4 (+2) | 242.2 (b2, +1)† 1016.5 (y8,+1) 903.4 (y7, +1) |
12.1 | −20.6 | |||
| Peanut | 7S globulin (Ara h 1) |
P43238 | QINQNLR | 435.5 (+2) | 530.1 (y4, +1)† 629.2 (y5, +1) |
9.9 | −14.3 |
| DLAFPGSGEQVEK | 688.8 (+2) | 300.2 (b3, +1)† 930.6 (y9, +1) 229.1 (b2, +1) |
11.3 | −22.5 | |||
| 2S albumin (Ara h 2) |
Q6PSU2 | NLPQQC[CAM]⁎GLR | 543.3 (+2) | 429.8 (y7, +2)† 858.4 (y7, +1) 633.3 (y5, +1) |
9.8 | −17.8 | |
| 11S globulin (Ara h 3) |
O82580 | TANDLNLLILR | 628.2 (+2) | 741.5 (y6, +1)† 1083.6 (y9,+1) 854.6 (y7, +1) |
13.5 | −20.6 | |
| Walnut | 2S albumin (Jug r 1) |
P93198 | QQQQQGLR | 493.3 (+2) | 345.2 (y3, +1)† 473.3 (y4, +1) 601.3 (y5, +1) |
7.5 | −16.1 |
| GEEMEEMVQSAR | 698.3 (+2) | 316.1 (b3, +1)† 461.2 (y4, +1) 820.4 (y7, +1) |
10.6 | −22.9 | |||
| 7S globulin (Jug r 2) |
Q9SEW4 | SPDQSYLR | 483.2 (+2) | 781.4 (y6, +1)† 538.3 (y4, +1) 666.4 (y5, +1) |
9.7 | −15.8 | |
| ATLTLVSQETR | 609.8 (+2) | 620.3 (y5, +1)† 719.4 (y6, +1) 832.5 (y7, +1) |
11.1 | −20.0 | |||
†, Quantifier ion (no mark indicates qualifier ion).
⁎, Cysteine residue methyl-carbamated by iodoacetamide.
2.3. Sample preparation for LC-MS/MS analysis
Two sample preparation methods were compared for LC-MS/MS analysis combined with an on-line automated solid-phase extraction system, namely, the S-Trap method and a previously reported conventional method using urea buffer (‘Urea method’) (Torii et al., 2023).
Sample preparation using the S-Trap midi spin column was carried out according to the vendor's protocol, with some modifications. Food samples were ground using a food processor, and a portion of the ground sample (1.0 g) was solubilized in 10 mL of 100 mM Tris-HCl buffer (pH 7.5) containing 5% (w/v) SDS and 50 mM TCEP hydrochloride. To extract and denature proteins, the sample solution was boiled at 95 °C in a water bath for 10 min, after stirring with a vortex mixer. After boiling, the sample was centrifuged at 2000 ×g for 10 min (S700FR, KUBOTA Corp., Ltd., Tokyo, Japan), and 0.5 mL of the supernatant was recovered. To alkylate cysteine residues in proteins, 50 μL of 500 mM iodoacetamide solution was added to the supernatant and incubated at 47 °C for 15 min under light-shielded conditions. Next, 50 μL of 12% (v/v) phosphoric acid and 3.5 mL of S-Trap buffer (90% [v/v] methanol and 0.1 M triethylammonium bicarbonate, pH 7.6) were added to form colloidal protein particulate. The entire protein solution was then transferred onto the S-Trap midi column and centrifuged at 2000 ×g for 10 min, and the column was washed with an additional 3 mL of S-trap buffer by centrifugation at 2000 ×g for 5 min. Next, 350 μL of 1% (w/v) trypsin solution (with 50 mM ammonium hydrogen carbonate, pH 8.0) was added to the column, and bound proteins were digested at 47 °C for 60 min. After digestion, trypsin was deactivated and the peptides eluted from the column by addition of 150 μL of 20% (v/v) acetonitrile containing 1% (v/v) formic acid followed by centrifugation at 2000 ×g for 5 min. Eluted peptides were stored in low-absorption polypropylene vials (GL Sciences Inc., Tokyo, Japan). The peptides were purified and concentrated using the on-line automated solid-phase extraction system under the conditions described below and then subjected to continuous LC-MS/MS analysis.
A urea method was used, with some modifications from our previous studies (Seki et al., 2021; Torii et al., 2023). Ground food sample (1.0 g) was solubilized in 10 mL of 100 mM Tris-HCl buffer (pH 8.5) containing 4 M urea and 0.1 M dithiothreitol. To extract proteins, the sample solution was incubated at 37 °C in a water bath for 3 h. After incubation, the sample was centrifuged at 2000 ×g for 10 min, and then 1 mL of the supernatant was collected. Cysteine residues in proteins were alkylated by addition of 200 μL of 4% (w/v) iodoacetamide and 4 mL of 50 mM ammonium hydrogen carbonate and incubation at 37 °C for 1 h under light-shielded conditions. The proteins were digested for 16 h with 100 μL of 1% (w/v) trypsin solution at 37 °C. Digestion was terminated by the addition of 50 μL of trifluoroacetic acid (TFA). The resultant mixture was centrifuged at 2000 ×g for 10 min. The sample was desalted and purified using a solid-phase extraction mini column (Oasis HLB vac cartridge 150 mg/6 mL, Waters, Milford, MA, USA). The entire resulting supernatant was then loaded onto a column pre-conditioned with 5 mL of methanol followed by 5 mL of water. After sample loading, the column was washed twice with 5 mL of 0.5% (v/v) TFA. The sample was eluted twice with 5 mL of 70% (v/v) acetonitrile each time. The eluate was concentrated using a vacuum evaporator (NVC-2100, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and then dissolved in 1 mL of 5% (v/v) acetonitrile containing 0.1% (v/v) formic acid. The samples were stored in low-adsorption polypropylene vials until injection into the LC-MS/MS system from the HPLC autosampler.
2.4. HPLC and MS/MS conditions
The mobile phases for HPLC analyses were 0.1% (v/v) formic acid/water (solution A) and 0.1% (v/v) formic acid/acetonitrile (solution B). Chromatography was carried out at flow rate of 0.3 mL/min under the following gradient conditions: 1% (v/v) solution B from 0 to 3 min, 50% (v/v) solution B at 16 min, 95% (v/v) solution B at 17 min, 95% (v/v) solution B from 17 to 22 min, and 1% (v/v) solution B from 22 to 30 min. Separation was performed using a Nexera X2 (Shimadzu Corp., Kyoto, Japan) chromatography system with an InertSustain Bio C18 column (2.1 × 150 mm, 3 μm, GL Sciences Inc., Tokyo, Japan). The column oven temperature was set at 50 °C. The autosampler injection volume was 10 μL. Mass spectrometry was performed using an LCMS-8060 spectrometer (Shimadzu Corp.) under the following conditions: scheduled MRM mode; electrospray ionization (ESI positive); interface voltage, 1.0 kV; interface temperature, 250 °C; drying gas, 5 L/min; nebulizer gas, 3 L/min; heating gas, 15 L/min; desolvation temperature, 444 °C; desolvation line temperature, 150 °C; heat block temperature, 200 °C; collision-induced dissociation gas (argon), 300 kPa; conversion dynode voltage, 10 kV; and total dwell time, 1 s. LabSolutions for LCMS software, ver. 5.118 (Shimadzu Corp.), was used for optimization of MRM transitions for each peptide and acquisition of all data. The same software was used to identify peaks in the resulting chromatograms and calculate signal-to-noise (S/N) ratios.
2.5. On-line automated solid-phase extraction conditions
An SPL-W100 on-line automated solid-phase extraction system (AiSTI SCIENCE Co., Ltd., Wakayama, Japan) was connected to the LC-MS/MS column using PEEK tubing, and the system was controlled using SGLI-STUDIO software, ver. 2.5.0.7 (AiSTI SCIENCE Co., Ltd.). A Flash-SPE PBX (styrene-divinylbenzene polymer with hydrophilic surface modification) cartridge was used to purify and concentrate S-Trap samples. The cartridge was pre-conditioned with 200 μL of methanol using the E-nozzle and then conditioned with 300 μL of 1% (v/v) formic acid using the L-nozzle. A total of 100 μL of each S-Trap sample was aspirated from the corresponding vial using the L-nozzle and applied to the solid-phase cartridge at a rate of 2 μL/min. After washing with 100 μL of 1% (v/v) formic acid, the peptides were eluted from the solid-phase cartridge with 70 μL of 25% (v/v) acetonitrile containing 1% (v/v) formic acid using the E-nozzle. The entire eluate was mixed with 225 μL of 0.1% (v/v) formic acid in the Mixing injection Valve System and stored in the sample loop (300 μL). The valve was then switched to the flow position, and the mobile phase was delivered to the HPLC column at a flow rate of 0.3 mL/min.
2.6. Incurred sample preparation
Five incurred food samples (rice porridge, pot-au-feu [soup with sausage and vegetables], tomato sauce, sweet red bean soup, and sweet potato cake) were prepared for method validation. Primary standard powders of seven raw ingredients (wheat, buckwheat, egg, milk, prawn, peanut, and walnut) were prepared according to the method described in the Japanese Food Labelling Standards (Government of Japan, 2023), and each powder was added to incurred samples at a protein concentration of 10 mg/kg (wheat, buckwheat, egg, milk, peanut, and walnut) or 50 mg/kg (prawn). The total protein content of each primary standard powder was determined by the combustion method using a nitrogen and protein analyser (FP928, LECO Corp., St. Joseph, MI, USA). Other ingredients in each incurred sample and cooking process are shown in Supplementary Table 1. Negative controls for each incurred sample were prepared and processed using the same procedure used for spiked samples but without spiking the raw materials.
2.7. Qualitative validation of the method
To evaluate the qualitative performance of the proposed LC-MS/MS method, the five incurred samples were analysed and compared with ELISA. ELISAs were performed according to the instructions for each kit. All samples were analysed in triplicate. Limit of detection (LOD) values for LC-MS/MS analyses of the seven ingredients in each incurred sample were estimated based on the concentration of the respective protein determined by ELISA and the S/N ratio of the LC-MS/MS chromatograms (> 3). For LC-MS/MS analyses, LabSolutions LCMS software, ver. 5.118 (Shimadzu Corp.), was used to identify peaks in the chromatograms and calculate the S/N ratios based on root mean square values.
3. Results and discussion
3.1. Selection of peptides for LC-MS/MS analysis
The main allergenic proteins of each ingredient registered in the Allergen Nomenclature and Allergome databases were targeted in analyses of wheat, buckwheat, milk, egg, crustaceans (shrimp and crab), peanut, and walnut. Low-molecular-weight glutenin subunit (Tri a 36), high-molecular-weight glutenin subunit (Tri a 26), and γ-gliadin (Tri a 20) were targeted as allergenic proteins for wheat; 13S globulin (Fag e 1) was targeted for buckwheat; α-S1-casein (Bos d 9), α-S2-casein, (Bos d 10), and β-lactoglobulin (Bos d 5) were targeted for milk; ovalbumin (Gal d 6), ovotransferrin (Gal d 3), lysozyme C (Gal d 4), and vitellogenin (Gal d 6) were targeted for egg; tropomyosine (Pen m 1) was targeted for crustaceans; 7S globulin (Ara h 1), 2S albumin (Ara h 2), and 11S globulin (Ara h 3) were targeted for peanut; and 2S albumin (Jug r 1) and 7S globulin (Jug r 2) were targeted for walnut. The amino acid sequence of each allergenic protein was obtained from the UniProtKB database, and candidate peptides and MRM transitions resulting from trypsin digestion were determined using Skyline software. Peptides from representative varieties of each raw material were purified using the S-Trap method, followed by LC-MS/MS analysis as described in the Material and Methods.
We previously reported specific marker peptides for wheat and buckwheat (Seki et al., 2021) and walnuts (Torii et al., 2023), whereas other studies have reported marker peptides for the remaining raw materials (Croote et al., 2019; Henrottin et al., 2023; Huang et al., 2020; Li et al., 2022; Nagai, Minatani, & Goto, 2015; Neils et al., 2022; Ogura, Clifford, & Oppermann, 2019; Pilolli et al., 2020; Planque, Arnould, Delahaut, Renard, & Gillard, 2017). Candidate peptides and transitions exhibiting excellent sensitivity and selectivity were selected based on previous reports. Furthermore, peptides for which the specificity was confirmed by searching the UniProt Peptide database (at least four peptides per raw material) were selected as the final markers (Table 1).
The accuracy of the qualitative analysis of processed foods with complex matrices was enhanced by selecting at least two MRM transitions for each marker peptide (Pilolli et al., 2020). Therefore, we selected at least four marker peptides for each of the seven ingredients and established at least two MRM transitions for each peptide. The retention time of each selected marker peptide was confirmed using synthetic peptides of each sequence as standard materials (data not shown), and the mass spectrometry collision energy and ion source parameters were optimized through infusion analysis of each synthetic peptide.
3.2. Comparison of the urea and S-trap methods
The suspension-trapping (S-Trap) method is a spin column–type tool for rapid removal of surfactants, desalting of sample solutions, trypsin digestion, and purification of peptides without solid-phase extraction, and it has been used in many proteomics applications (Duong, Park, & Lee, 2022). In the S-Trap method, a high concentration of surfactant (e.g., 5% SDS) is used to extract proteins from food samples. In this study, the applicability of the S-Trap method for pre-treatment of processed food samples for allergen analysis using LC-MS/MS was confirmed by comparison with the urea method (Seki et al., 2021; Torii et al., 2023), in which proteins are extracted using urea, and the resulting peptides are purified using solid-phase extraction columns.
Peptides in samples of each of the seven target ingredients (wheat, buckwheat, milk, egg, crustacean, peanut, and walnut) containing 1000 μg/g protein were purified using the urea and S-Trap methods and then analysed using LC-MS/MS. As shown in Fig. 1, the peak areas of peptides purified using the S-Trap method were significantly greater than those of peptides purified using the urea method, with the exception of peanut peptides. Moreover, when the S-Trap method was combined with automated on-line SPE as described in the Material and Methods, the peak areas of peptides purified from all raw materials using the combined S-Trap and on-line SPE method were significantly greater than those of samples purified using only the urea method or S-Trap method.
Fig. 1.
Peak area comparison for the urea method with off-line solid-phase extraction (SPE), Suspension-Trapping (S-Trap) method without SPE, and S-Trap method with on-line SPE. The analysed target peptides were IFWGIPALLK (m/z 579.4 > 897.6) for wheat, GFIVQAR (m/z 395.8 > 473.3) for buckwheat, FFVAPFPEVFGK (m/z 692.9 > 920.5) for milk, ELINSWVESQTNGIIR (m/z 930.0 > 1017.5) for egg, FLAEEADR (m/z 475.7 > 690.2) for crustacean, NLPQQCGLR (m/z 543.3 > 429.8) for peanut, and ATLTLVSQETR (m/z 609.8 > 620.3) for walnut. Samples containing protein from the seven ingredients at a concentration of 1000 μg/g were analysed using the three methods. Data are presented as the mean (n = 3) ± standard deviation (error bars). Different letters indicate a statistically significant difference (Tukey's multiple comparison test, p < 0.05).
Trypsin selectively degrades peptide bonds on the C-terminal side of lysine and arginine residues in proteins, and the efficiency of digestion can be improved by denaturing the protein, which exposes internal basic amino acid residues to the protein surface due to the change in structure (Zheng & DeMarco, 2017). In the S-Trap method, sample proteins are heated in boiling water with a high concentration of surfactant (5% SDS) and reducing agent (50 mM TCEP-hydrochloride). Phosphoric acid and methanol are added to the extracted protein solution to promote aggregation of the proteins solubilized by SDS, resulting in the formation of a suspension. This suspension is loaded onto an S-Trap column and centrifuged to remove salts and surfactants, and the proteins trapped on the quartz filter in the column are then digested by applying trypsin.
By contrast, the urea method does not involve the use of any surfactants in the extraction solution to avoid diminishing the efficiency of subsequent trypsin digestion, and the concentration of the denaturant urea is set at 4 M. Therefore, we assumed that the rate at which proteins are extracted from the sample and the degree of protein denaturation are lower than with the S-Trap method. In addition, proteins are digested in solution in the urea method, whereas the S-Trap method efficiently digests proteins trapped on the filter using the bioreactor principle and thus provides more efficient digestion. These factors suggest that the S-Trap method is more sensitive than the urea method.
Pretreatment methods for proteomics analyses that allow the use of surfactants include ultrafiltration, PTS, and SP3 (Masuda, Saito, Tomita, & Ishihama, 2009; Wiśniewski, Zougman, Nagaraj, & Mann, 2009; Hughes et al., 2019), but these methods are not ideal for analysing food samples because removal of the surfactant often involves complex procedures or the sample volume is insufficient. Surfactants (e.g., Rapigest™, ProteaseMAX™ Surfactant) that degrade into compounds that do not interfere with LC-MS analyses following addition of acid are available, but these agents are very expensive and thus impractical for routine analysis of food samples. The S-Trap method can be used to easily remove high concentrations of surfactant using only centrifugation, and the method requires only 1 h for trypsin digestion and is relatively inexpensive. In addition, the volume of sample that can be loaded onto the column is thought to be appropriate for the analysis of food samples, making the S-Trap method more sensitive than the conventional urea method. Combining the S-Trap with on-line automated SPE, the peptides in a sample can be concentrated to further increase sensitivity, making this combination a very useful, rapid, and simple pre-treatment approach for LC-MS/MS screening of allergenic proteins in processed foods.
3.3. Optimization of on-line automated SPE
As SPE is a time-consuming step, we applied an on-line automated on-line SPE system to rapidly analyse food allergens in processed foods. The automated SPE system consisted of fully automated solid-phase conditioning, sample solution injection, and solid-phase washing and elution of the target substance via six independent syringes to aspirate six different solvents or sample solutions and three different switching valves. In this system, all of the target components eluted from the solid phase are mixed with the mobile phase in the sample loop (300 μL) before flowing onto and being retained on the HPLC column connected with PEEK tubing.
For purification and concentration of the sample solutions, special small cartridges filled with a few milligrams of solid-phase material are used. The type of solid-phase cartridge and the washing and elution conditions best-suited for the purification and concentration of peptides after trypsin digestion were investigated using this system. A silica-based C18 cartridge (octadecylsilyl, Flash-SPE C18), polymer-based HLB cartridge (hydrophilic-lipophilic-balanced, co-polymer of nitrogen-containing vinyl polymer and styrene-divinylbenzene polymer, Flash-SPE HLB), SDB cartridge (styrene-divinylbenzene copolymer, Flash-SPE BEP), and surface hydrophilic treated SDB cartridge (structure not disclosed by manufacturer, Flash-SPE PBX) were tested, and the Flash-SPE PBX was found to provide optimal retention of peptides exhibiting a wide range of polarity.
Representative chromatograms of analyses using each type of solid-phase cartridge are shown in Supplementary Fig. 1. All of the solid-phase cartridges tested were reversed-phase systems, and the solutions used to elute the peptides from the solid phase exhibited higher intensities of more-hydrophobic peptides (e.g., IFWGIPALLK for wheat and FFVAPFPEVFGK for milk) as the organic solvent ratio was increased. However, in this system, the entire eluate from the solid-phase cartridge is injected onto the HPLC column (C18), and the peak shape for highly hydrophilic peptides (e.g., QIPEQSR for wheat and QQQQQGLR for walnut) deteriorated when the ratio of organic solvent was high. Finally, washing of the solid-phase cartridge with 100 μL of 1% formic acid, elution with 70 μL of 25% acetonitrile containing 1% formic acid, and mixing with 225 μL of 0.1% formic acid as a diluent enabled the simultaneous analysis of marker peptides exhibiting a wide range of polarity while preserving good peak shape.
In the automated SPE approach, the entire sample is loaded from the solid-phase cartridge into the LC-MS, thus eliminating the need for concentration steps using evaporators or centrifugal concentrators. In addition, the system uses very small solid-phase cartridges and only a small volume of solvent. The entire process requires <10 min, which includes automatic cleaning of the flow path. In analyses of processed foods for the presence of food allergens, a large number of various types of samples must be analysed rapidly. Therefore, the combination of on-line automated SPE and LC-MS/MS is considered a useful screening method for confirming the appropriateness of food allergy labelling of processed foods.
3.4. Method validation and LOD
Food allergy labelling of processed foods must be appropriate in order to avoid unexpected exposure of allergic individuals to food allergens. Validated analytical methods are needed to determine whether the food allergy labelling of processed foods is appropriate. However, as processed foods contain a variety of ingredients and are produced using a number of different processes, analytical methods for food allergens must be validated on incurred samples produced using the same processes as commercial processed foods.
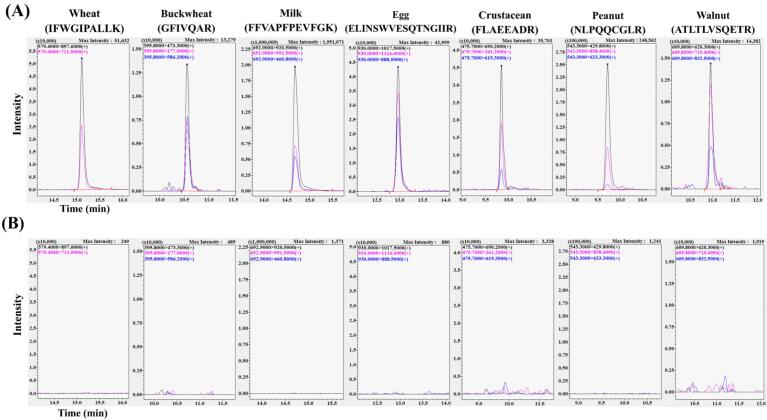
To validate the established analytical method in this study, five incurred samples (rice porridge, pot-au-feu, tomato sauce, sweet red bean soup, and sweet potato cake) containing seven potential allergens at a final protein concentration of 10 mg/kg or 50 mg/kg were prepared and analysed using the LC-MS/MS and ELISA methods. Table 2 shows the results of analyses to detect the seven potential allergens using the LC-MS/MS and ELISA methods for each incurred sample. Representative chromatograms of tomato sauce analysed using the LC-MS/MS method are shown in Fig. 2. Using the LC-MS/MS method, ingredients other than crustacean (prawn) protein were detected in all incurred samples to which protein equivalent to 10 mg/kg was added. Prawn protein was detected in all incurred samples containing added protein equivalent to 50 mg/kg. (See Fig. 3.)
Table 2.
LC-MS/MS and ELISA results for incurred samples.
| Incurred sample | Addition of seven allergens | Wheat |
Buckwheat |
Milk |
Egg |
Crustacean (Prawn, 10 mg/kg) |
Crustacean (Prawn, 50 mg/kg) |
Peanut |
Walnut |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | LC-MS/MS | ELISA | ||
| Rice porridge | yes | + | 11.9 | + | 16.3 | + | 8.9 | + | 5.6 | N.D. | 1.5 | + | 72.7 | + | 8.2 | + | 15.6 |
| no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Pot-au-feu | yes | + | 11.0 | + | 9.9 | + | 6.1 | + | 6.1 | N.D. | N.D. | + | 75.6 | + | 12.1 | + | 14.9 |
| no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Tomato sauce | yes | + | 14.5 | + | 8.2 | + | 6.0 | + | 5.5 | N.D. | N.D. | + | 31.3 | + | 5.5 | + | 9.5 |
| no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Sweet red bean soup | yes | + | 1.2 | + | 8.4 | + | 7.9 | + | 6.0 | N.D. | 1.1 | + | 70.3 | + | 8.2 | + | 13.2 |
| no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Sweet potato cake | yes | + | 10.7 | + | 11.3 | + | 8.5 | + | 6.7 | N.D. | 1.2 | + | 69.2 | + | 9.5 | + | 10.9 |
| no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
+, Peak detected (S/N > 3); N.D., peak not detected (less than detection limit).
Fig. 2.
Representative LC-MS/MS chromatograms (extracted ion chromatograms) of an incurred sample (tomato sauce) analysed using the S-Trap method with on-line SPE. Tomato sauce with (A) and without (B) seven raw ingredients added (wheat, buckwheat, egg, milk, crustacean [prawn], peanut, and walnut) and heated in an autoclave at 120 °C for 20 min. The concentration of protein in six of the ingredients (wheat, buckwheat, egg, milk, peanut, and walnut) in the sauce was 10 mg/kg. Crustacean (prawn) protein was added to the sauce at a concentration of 50 mg/kg.
Fig. 3.
Representative LC-MS/MS chromatograms (extracted ion chromatograms) of commercial processed foods (sweet bread) analysed using the S-Trap method with on-line SPE. Wheat, milk, egg, and peanut were labelled on the package of this product as food allergens.
The LOD of the seven ingredients in each incurred sample was estimated using the LC-MS/MS method based on the concentration of each protein determined using the respective ELISA and the S/N ratio of the LC-MS/MS chromatograms. The average LOD values for the seven ingredients calculated in LC-MS/MS analyses (n = 3) of five different incurred samples were 0.23 ± 0.09 mg/kg for wheat (IFWGIPALLK peptide [m/z 579.4 > 897.6]), 0.58 ± 0.19 mg/kg for buckwheat (GFIVQAR peptide [m/z 395.8 > 473.3]), 0.12 ± 0.07 mg/kg for milk (FFVAPFPEVFGK peptide [m/z 692.9 > 920.5]), 0.27 ± 0.12 mg/kg for egg (ELINSWVESQTNGIIR peptide [m/z 930.0 > 1017.5]), 1.03 ± 0.46 mg/kg for crustacean (prawn, FLAEEADR peptide [m/z 475.7 > 690.2]), 0.35 ± 0.10 mg/kg for peanut (NLPQQC[CAM]GLR peptide [m/z 543.3 > 429.8]), and 0.52 ± 0.19 mg/kg for walnut (ATLTLVSQETR peptide [m/z 609.8 > 620.3]). The threshold for food allergy labelling of these seven ingredients in Japan is 10 mg/kg, and the detection limit using the proposed LC-MS/MS method is thus considered sufficiently sensitive to serve as a food allergen screening method. The five incurred samples contained a variety of food ingredients and were prepared using various heating processes, such as boiling, baking, and retort pasteurization. The results of this study demonstrate that the analytical method is useful for screening a variety of processed foods for food allergens.
In ELISA testing of the incurred samples with shrimp protein equivalent to 10 mg/kg, the value was approximately 1 mg/kg and could not be detected using the LC-MS/MS method. These results were considered due to the very low efficiency of the extraction of shrimp-derived proteins from the incurred samples in both the ELISA and LC-MS/MS methods. The CCFL previously suggested a reference dose for crustacean of 200 mg total protein from the allergen in guidelines on the use of precautionary allergen labelling (Codex, 2023). However, the threshold concentration according to Japan's food allergy labelling system is 10 mg/kg, and it will thus be necessary in the future to prepare more appropriate incurred samples in order to investigate the extraction efficiency of crustacean proteins in more detail. Moreover, the quantitative analytical accuracy of the LC-MS/MS method also needs to be verified in the future and compared with that of the ELISA method.
3.5. Applicability to processed foods
We examined the applicability of the developed detection method to the analysis of different types of processed foods, including pre-heated products such as bakery items or retort pasteurized products. Eighteen different commercial processed foods were selected based on labelling information on the package. Six of these products did not contain any of the seven target ingredients and were used as negative controls. All ingredients labelled on each product are listed in Supplementary Table 2. The analytes for the developed LC-MS/MS method were prepared using the S-Trap method with on-line SPE.
Table 3 shows the measurement results obtained using the LC-MS/MS method along with the allergen labelling on each product. In all of the processed foods, the peaks of target peptides derived from the seven ingredients detected using the proposed method were consistent with the labelling of each product. That is, the proposed method was able to accurately determine the presence or absence of allergenic proteins derived from the seven tested ingredients (wheat, buckwheat, milk, eggs, shellfish, peanuts, and walnuts) in processed foods, as indicated on their food labelling. Therefore, the results of this study indicate that the proposed method is adequate for the analysis of various processed foods.
Table 3.
LC-MS/MS results for commercial processed foods and food allergen labelling of the products.
| Processed food⁎ | Allergen labelling on package |
LC-MS/MS analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Buckwheat | Milk | Egg | Crustacean | Peanut | Wheat | Buckwheat | Milk | Egg | Crustacean | Peanut | Walnut | |
| Rice cracker | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Meat sauce | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Rice seasoning powder | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Curry powder | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Premixed rice flour | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Corn soup powder | no | no | no | no | no | no | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Premixed flour for galette | no | yes | no | yes | no | no | N.D. | + | N.D. | + | N.D. | N.D. | N.D. |
| Buckwheat cookie | yes | yes | no | yes | no | no | + | + | N.D. | + | N.D. | N.D. | N.D. |
| Buckwheat bun | yes | yes | yes | yes | no | no | + | + | + | + | N.D. | N.D. | N.D. |
| Shrimp rice cracker | yes | no | yes | no | yes (shrimp) | no | + | N.D. | + | N.D. | + | N.D. | N.D. |
| Peanut with soybean paste | yes | no | yes | yes | no | yes | + | N.D. | + | + | N.D. | + | N.D. |
| Cereal bar | yes | no | no | yes | no | no | + | N.D. | N.D. | + | N.D. | N.D. | + |
| Sweet bread | yes | no | yes | yes | no | yes | + | N.D. | + | + | N.D. | + | N.D. |
| Salad dressing | yes | no | no | yes | no | no | + | N.D. | N.D. | + | N.D. | N.D. | + |
| Deep-fried shrimp | yes | no | yes | yes | yes (shrimp) | no | + | N.D. | + | + | + | N.D. | N.D. |
| Pasta sauce (tomato cream) | yes | no | yes | yes | yes (shrimp, crab) | no | + | N.D. | + | + | + | N.D. | N.D. |
| Pasta sauce (carbonara) | yes | no | yes | yes | no | no | + | N.D. | + | + | N.D. | N.D. | N.D. |
| Instant noodle | yes | no | yes | yes | yes (crab) | no | + | N.D. | + | + | + | N.D. | N.D. |
+, Peak detected; N.D., peak not detected (less than detection limit).
⁎, Ingredients labelled on the package are listed in the Supplementary Table.
By applying the proposed analytical method to other raw ingredients, it will be possible to simultaneously detect additional food allergens, making the proposed method a useful analytical tool for rapidly confirming the accuracy of allergen labelling on processed foods. Further studies focusing on the development of an LC-MS/MS method that enables the simultaneous detection of all potential food allergens from a variety of processed foods are anticipated. Furthermore, Japanese food labelling standards require that shrimp and crab (both of which are crustaceans) must be separately labelled on packages; therefore, it is necessary to select marker peptides that enable clear distinction between shrimp and crab.
4. Conclusions
We developed a method for the rapid screening of seven food allergens using LC-MS/MS combined with an S-Trap on-line automated SPE system. Target peptides digested from proteins of seven ingredients (wheat, buckwheat, milk, egg, crustacean, peanut, and walnut) were analysed using the LC-MS/MS method and determined with excellent sensitivity and selectivity. The on-line automated SPE system was optimized for the purification of food allergen peptides obtained by trypsin digestion. Utilizing the S-Trap, a convenient and rapid spin column tool for proteomics, sample preparation for LC-MS/MS analyses could be accomplished in <2 h and provided improved target peptide peak intensities compared with the conventional urea-based method.
Using the proposed LC-MS/MS method, selected marker peptides for seven food ingredients could be simultaneously detected in five different types of incurred samples to which trace amounts of allergenic proteins were added. The method's LOD values for each protein were estimated at <1 mg/kg. The developed analytical approach would be an effective simultaneous screening method for confirming the accuracy of food allergen labelling on a wide range of processed foods.
CRediT authorship contribution statement
Akira Torii: Writing – original draft, Validation, Methodology, Formal analysis, Data curation. Ryoichi Sasano: Writing – review & editing, Methodology. Yoshiki Ishida: Writing – review & editing, Project administration, Conceptualization. Kosuke Nakamura: Writing – review & editing. Rie Ito: Writing – review & editing. Yusuke Iwasaki: Writing – review & editing. Ken Iijima: Writing – review & editing, Project administration, Conceptualization. Hiroshi Akiyama: Writing – review & editing, Writing – original draft, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. We are very grateful to Akio Ozawa and Fumihiro Arakawa of Research Center for Food Safety and Quality of NH Foods, Ltd., for preparing incurred samples and performing ELISAs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101558.
Contributor Information
Akira Torii, Email: torii.akira@nisshin.com.
Yusuke Seki, Email: seki.yusuke.qg@nisshin.com.
Ryoichi Sasano, Email: sasano@aisti.co.jp.
Yoshiki Ishida, Email: ishida.yoshiki@nisshin.com.
Kosuke Nakamura, Email: kosnakamura@nihs.go.jp.
Rie Ito, Email: rie-ito@hoshi.ac.jp.
Yusuke Iwasaki, Email: iwasaki@hoshi.ac.jp.
Ken Iijima, Email: iijima.ken@nisshin.com.
Hiroshi Akiyama, Email: h-akiyama@hoshi.ac.jp.
Appendix A. Supplementary data
Supplementary material
Data availability
The authors do not have permission to share data.
References
- Akiyama H., Adachi R. Japanese food allergy-labeling system and comparison with the international experience; detection and thresholds. Food Safety. 2021;9:101–116. doi: 10.14252/foodsafetyfscj.D-21-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarrak S.M., Al-Sobayil F.A. Evaluation of protective proteins and cytokines in milk and serum of healthy camels during the first two months postpartum. Int. J. Vet. Sci. 2024;13(4):401–406. doi: 10.47278/journal.ijvs/2023.111. [DOI] [Google Scholar]
- Codex Alimentarius Commission 2018. http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B1-1985%252FCXS_001e.pdf General standard for the labelling of prepackaged foods, Labelling of prepackeaged foods, CODEX STAN 1–1985. (last accessed on Jan. 31, 2024)
- Codex Alimentarius Commission Report of the forty-seventh session of the CODEX COMMITTEE on food labelling. 2023. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-714-47%252FFINAL%252520REPORT%252FREP23_FLe.pdf (last accessed on Jan. 31, 2024)
- Croote D., Braslavsky I., Quake S.R. Addressing complex matrix interference improves multiplex food allergen detection by targeted LC–MS/MS. Analytical Chemistry. 2019;91:9760–9769. doi: 10.1021/acs.analchem.9b01388. [DOI] [PubMed] [Google Scholar]
- Duong V.A., Park J.M., Lee H. A review of suspension trapping digestion method in bottom-up proteomics. Journal of Separation Science. 2022;45(16):3150–3168. doi: 10.1002/jssc.202200297. [DOI] [PubMed] [Google Scholar]
- Fallahbaghery A., Zou W., Byrne K., Howitt C.A., Colgrave M.L. Comparison of gluten extraction protocols assessed by LC-MS/MS analysis. Journal of Agricultural and Food Chemistry. 2017;65:2857–2866. doi: 10.1021/acs.jafc.7b00063. [DOI] [PubMed] [Google Scholar]
- Government of Japan Consumer Affairs Agency Food Labeling Act. 2023. https://www.caa.go.jp/policies/policy/food_labeling/food_labeling_act/assets/food_labeling_cms201_230629_02.pdf (in Japanese, last accessed on Jan. 31, 2024)
- Henrottin J., Piolli R., Huet A.C., Poucke C.C.V., Nitride C., Loose M.D.…Monaci L. Optimization of a sample preparation workflow based on UHPLC-MS/MS method for multi-allergen detection in chocolate: An outcome of the ThRAll project. Food Cont. 2023;143 doi: 10.1016/j.foodcont.2022.109256. [DOI] [Google Scholar]
- Huang X., Zhu Z., Feng H., Zhang Q., Zhang H. Simultaneous determination of multi-allergens in surimi products by LC-MS/MS with a stable isotope-labeled peptide. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126580. [DOI] [PubMed] [Google Scholar]
- Hughes C.H., Moggridge S., Müller T., Sorensen P.H., Morin G.B., Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nature Protocols. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- Köppel E., Stadler M., Lüthy J., Hübner P. Detection of wheat contamination in oats by polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) Zeitschrift Fur Leb. -Untersuchung Und -Forsch. 1998;206:399–403. doi: 10.1007/s002170050281. [DOI] [Google Scholar]
- Lexhaller B., Colgrave M.L., Scherf K.A. Characterization and relative quantitation of wheat, rye and barley gluten protein types by LC-MS/MS. Frontiers in Plant Science. 2019;10:1530. doi: 10.3389/fpls.2019.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li T., Wang Y., Zhang S., Sheng H., Fu L. Liquid chromatography coupled to tandem mass spectrometry for comprehensive quantification of crustacean tropomyosin and arginine kinase in food matrix. Food Cont. 2022;140 doi: 10.1016/j.foodcont.2022.109137. [DOI] [Google Scholar]
- Linacero R., Sanchiz A., Ballesteros I., Cuadrado C. Application of real-time PCR for tree nut allergen detection in processed foods. Critical Reviews in Food Science and Nutrition. 2020;60:1077–1093. doi: 10.1080/10408398.2018.1557103. http://doi [DOI] [PubMed] [Google Scholar]
- Ma X., Li H., Zhang J., Huang W., Han J., Ge Y., Sun J., Chen Y. Comprehensive quantification of sesame allergens in processed food using liquid chromatography-tandem mass spectrometry. Food Cont. 2020;107 doi: 10.1016/j.foodcont.2019.106744. [DOI] [Google Scholar]
- MacLean B., Tomazela D.M., Shulman N., Chambers M., Finney G.L., Frewen B.…MacCoss M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A., Rasi C., Palazzo P., Scala E. Allergen databases: Current status and perspectives. Current Allergy and Asthma Reports. 2009;9:376–383. doi: 10.1007/s11882-009-0055-9. [DOI] [PubMed] [Google Scholar]
- Masuda T., Saito N., Tomita M., Ishihama Y. Unbiased quantitation of Escherichia coli membrane proteome using phase transfer surfactants. Molecular & Cellular Proteomics. 2009;8(12):2770–2777. doi: 10.1074/mcp.M900240-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki A., Watanabe S., Ogata K., Nagatomi Y., Kokutani R., Minegishi Y., Tamehiro N., Sakai S., Adachi R., Hirao T. Real-time PCR detection methods for food allergens (wheat, buckwheat, and peanuts) using reference plasmids. Journal of Agricultural and Food Chemistry. 2019;67:5680–5686. doi: 10.1021/acs.jafc.9b01234. [DOI] [PubMed] [Google Scholar]
- Nagai H., Minatani T., Goto K. Development of a method for crustacean allergens using liquid chromatography/tandem mass spectrometry. Journal of AOAC International. 2015;98(5):1355–1365. doi: 10.5740/jaoacint.14-248. [DOI] [PubMed] [Google Scholar]
- Neils J.L.D., Broadbent J.A., Bose U., Anderson A., Colgrave M.L. Targeted proteomics for rapid and robust peanut allergen quantification. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132592. [DOI] [PubMed] [Google Scholar]
- Ogura T., Clifford R., Oppermann U. Simultaneous detection of 13 allergens in thermally processed food using targeted LC-MS/MS approach. Journal of AOAC International. 2019;102:1316–1329. doi: 10.5740/jaoacint.19-0060. [DOI] [PubMed] [Google Scholar]
- Özdemir P.G., Sato S., Yanagida N., Ebisawa M. Oral immunotherapy in food allergy: Where are we now? Allergy Asthma Immunol Res. 2023;15(2):125–144. doi: 10.4168/aair.2023.15.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilolli R., Nitride C., Gillard N., Huet A.C., Poucke C.V., Loose M.…Monaci L. Critical review on proteotypic peptide marker tracing for six allergenic ingredients in incurred foods by mass spectrometry. Food Research International. 2020;128 doi: 10.1016/j.foodres.2019.108747. [DOI] [PubMed] [Google Scholar]
- Planque M., Arnould T., Delahaut P., Renard P., Gillard N. Liquid chromatography coupled to tandem mass spectrometry for detecting ten allergens in complex and incurred foodstuffs. Journal of Chromatography. A. 2017;1530:138–151. doi: 10.1016/j.chroma.2017.11.039. [DOI] [PubMed] [Google Scholar]
- Pomés A., Davies J.M., Gadermaier G., Hilger C., Holzhauser T., Lidholm J.…Goodman R.E. WHO/IUIS allergen nomenclature: Providing a common language. Molecular Immunology. 2018;100:3–13. doi: 10.1016/j.molimm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran B., Yang C.T., Downs M. Parallel reaction monitoring mass spectrometry method for detection of both casein and whey Milk allergens from a baked food matrix. Journal of Proteome Research. 2020;19(8):2964–2976. doi: 10.1021/acs.jproteome.9b00844. [DOI] [PubMed] [Google Scholar]
- Ross G., Salentijn G.I., Nielen M.W. A critical comparison between flow-throughand lateral flow immunoassay formats for visual and smartphone-based multiplex allergen detection. Biosensors. 2019;9:143. doi: 10.3390/bios9040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito E., Doi H., Kurihara K., Kato K., Aburatani K., Shoji M., Naka Y. The validation of the wheat gluten ELISA kit. Journal of AOAC International. 2019;102:1162–1173. doi: 10.5740/jaoacint.19-0005. [DOI] [PubMed] [Google Scholar]
- Schalk K., Koehler P., Scherf K.A. Quantitation of specific barley, rye, and oat marker peptides by targeted liquid chromatography–mass spectrometry to determine gluten concentrations. Journal of Agricultural and Food Chemistry. 2018;66:3581–3592. doi: 10.1021/acs.jafc.7b05286. [DOI] [PubMed] [Google Scholar]
- Seki Y., Nakamura K., Arimoto C., Kikuchi H., Yamakawa H., Nagai H., Ito T., Akiyama H. Development of a simple and reliable high-performance liquid chromatography–tandem mass spectrometry approach to simultaneously detect grains specified in food allergen labeling regulation on processed food commodities. Journal of Chromatography. A. 2021;1639 doi: 10.1016/j.chroma.2021.461877. [DOI] [PubMed] [Google Scholar]
- Templeton E.M., Pilbrow A.P., Kleffmann T., Pickering J.W., Rademaker M.T., Scott N.J.A.…Lassé M. Comparison of SPEED, S-trap, and in-solution-based sample preparation methods for mass spectrometry in kidney tissue and plasma. International Journal of Molecular Sciences. 2023;24:6290. doi: 10.3390/ijms24076290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii A., Seki Y., Arimoto C., Hojo N., Iijima K., Nakamura K., Ito R., Yamakawa H., Akiyama H. Development of a simple and reliable LC-MS/MS method to simultaneously detect walnut and almond as specified in food allergen labelling regulations in processed foods. Current Research in Food Science. 2023;6 doi: 10.1016/j.crfs.2023.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ge M., Sun L., Ahmed I., Li W., Lin H., Lin H., Li Z. Quantification of crustacean tropomyosin in foods using high-performance liquid chromatography–tandem mass spectrometry method. Journal of the Science of Food and Agriculture. 2021;101:5278–5285. doi: 10.1002/jsfa.11177. [DOI] [PubMed] [Google Scholar]
- Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. Universal sample preparation method for proteome analysis. Nature Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- Xiong W., Parker C.H., Boo C.C., Fielder K.L. Comparison of allergen quantification strategies for egg, milk, and peanut in food using targeted LC-MS/MS. Analytical and Bioanalytical Chemistry. 2021;413:5755–5766. doi: 10.1007/s00216-021-03550-x. [DOI] [PubMed] [Google Scholar]
- Yang S., Lin H., Yang P., Meng J., Abdallarh M.F., Shencheng Y.…Zhang R. Advancing high-throughput MS-based protein quantification: A case study on quantifying 10 major food allergens by LC-MS/MS using a one-sample multipoint external calibration curve. Journal of Agricultural and Food Chemistry. 2024;72(12):6625–6637. doi: 10.1021/acs.jafc.3c08362. [DOI] [PubMed] [Google Scholar]
- Zheng Y.Z., DeMarco M.L. Manipulating trypsin digestion conditions to accelerate proteolysis and simplify digestion workflows in development of protein mass spectrometric assays for the clinical laboratory. Clinical Mass Spectrometry. 2017;6:1–12. doi: 10.1016/j.clinms.2017.10.001. [DOI] [Google Scholar]
- Zougman A., Selby P.J., Banks R.E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics. 2014;14(9):1000–1006. doi: 10.1002/pmic.201300553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The authors do not have permission to share data.