Abstract
Background
Thrombin-activatable fibrinolysis inhibitor (TAFI) is a plasma zymogen that provides a molecular link between coagulation and fibrinolysis. Studies have shown that the presence of glycosaminoglycans accelerates TAFI activation by plasmin and stabilizes activated TAFI (TAFIa).
Objectives
We aimed to define the elements of TAFI structure that allow these effects.
Methods
Based on crystallographic studies and homology to heparin-binding proteins, we performed mutagenesis of surface-exposed charged residues on TAFI that putatively constitute heparin-binding sites. We determined heparin binding, kinetics of activation by plasmin in the presence or absence of heparin, thermal stability, and antifibrinolytic potential of each variant.
Results
Mutagenesis of Lys211 and Lys212 did not impair heparin binding but affected the ability of TAFI to be activated by plasmin. Mutagenesis of Lys306 and His308 did not impair heparin binding, but mutation of His308 had a severe negative effect on TAFI/TAFIa function. Mutation of Arg320 and Lys324 in combination markedly decreased heparin binding but had no effect on heparin-mediated acceleration of TAFI activation by plasmin while somewhat decreasing TAFIa stabilization by heparin. Mutagenesis of Lys327 and Arg330 decreased (but did not eliminate) heparin binding while decreasing the ability of heparin to accelerate plasmin-mediated TAFI activation, stabilize TAFIa, and increase the antifibrinolytic ability of TAFIa. A quadruple mutant of Arg320, Lys324, Lys327, and Arg330 completely lost heparin-binding ability and stabilization of the enzyme by heparin.
Conclusion
Basic residues in the dynamic flap of TAFIa define a functionally relevant heparin-binding site, but additional heparin-binding sites may be present on TAFI.
Keywords: carboxypeptidase B2, fibrinolysis, glycosaminoglycans, heparin, kinetics, mutagenesis, plasmin
Essentials
-
•
The anticoagulant drug heparin regulates thrombin-activatable fibrinolysis inhibitor (TAFI).
-
•
TAFI’s role is to prevent blood clot breakdown, and this is enhanced by binding of heparin.
-
•
We determined the sites on TAFI to which heparin binds to mediate these effects.
-
•
Heparin-like substances are present at wound sites, where they may stabilize clots through TAFI.
1. Introduction
Thrombin-activatable fibrinolysis inhibitor (TAFI; also known as procarboxypeptidase U and procarboxypeptidase B2) is a plasma zymogen that provides a molecular link between the coagulation and fibrinolytic cascades [1,2] as well as between coagulation and inflammation [3]. Activated TAFI (TAFIa) possesses basic carboxypeptidase activity and thus removes carboxyl-terminal lysine and arginine residues from protein and peptide substrates. Accordingly, TAFIa downregulates fibrinolysis by removing from partially degraded fibrin the carboxyl-terminal lysine and arginine residues that mediate positive feedback in the fibrinolytic cascade [4,5]. TAFIa can also regulate pericellular plasmin formation by removal of carboxyl-terminal lysine residues from cellular plasminogen receptors [6], thereby regulating cell migration [7] and possibly other inflammatory responses. Indeed, deficiency of TAFI in mouse models influences a wide spectrum of inflammatory and fibrotic disorders [3,8].
Two mechanisms exist to regulate the elaboration of TAFIa: control of the rate of TAFI activation and control of the rate of TAFIa inactivation. TAFIa is formed through cleavage at Arg92 by thrombin, thrombin in complex with the endothelial cell cofactor thrombomodulin (TM), or plasmin [[9], [10], [11]]. Thrombin is a relatively poor activator of TAFI, but binding of thrombin to TM increases the catalytic efficiency (kcat/KM) of TAFI activation by 1250-fold [10]. It has been shown that the presence of glycosaminoglycans (GAGs), such as heparin, is able to accelerate TAFI activation by plasmin [11] such that the catalytic efficiency of the reaction is only ∼10-fold lower than that exhibited by thrombin-TM. A different study found that other anionic compounds such as polyphosphate and sodium dodecyl sulfate were able to accelerate plasmin- and thrombin-mediated TAFI activation [12].
With respect to TAFIa inactivation, the enzyme has the unusual property of being metastable [13]: its activity spontaneously decays with a half-life of 8 to 15 minutes at body temperature in the absence of any further proteolytic cleavage [14]. Biophysical and structural studies have attributed the loss of activity to profound structural changes in the enzyme [[14], [15], [16], [17]]. Interestingly, GAGs stabilize TAFIa, causing a 2.3-fold increase in its half-life at 25 °C from 74 to 170 minutes [11]. However, the elements of TAFI structure that mediate the effects of GAGs on TAFI activation and TAFIa stability are unknown.
In a report on the crystal structure of bovine TAFI, Anand et al. [17] described 22 bound sulfate molecules in the model. These bound sulfates may represent potential GAG-binding sites and were clustered in 3 regions: near the so-called “dynamic flap” on the enzyme domain where it interfaces with the activation domain, at the top of the activation domain, and within the active site cleft. The site in the dynamic flap seems to be the most reasonable possibility to account for the effects of GAGs: following activation of TAFI, heparin bound to the activation domain would not affect the stability of TAFIa, and heparin bound in the active site cleft would prevent catalytic activity by blocking substrate access [17]. A putative GAG-binding site was identified by Mao et al. [11] through sequence homology with heparin-binding sequences in GAG-binding proteins.
Although plasmin is a weaker activator of TAFI than thrombin-TM, it is a relevant activator. A study by Mishra et al. [18] in vitro using monoclonal antibodies that inhibit plasmin-mediated TAFI activation showed that plasmin is a relevant TAFI activator during clot formation and lysis in the context of plasma clot lysis assays. Furthermore, when the monoclonal antibody MA-TCK26D6—which inhibits TAFI activation specifically by plasmin—was injected into a mouse thromboembolism model, decreased fibrin deposition in the lungs was observed, indicating an acceleration of fibrinolysis [19]. Injection of the same antibody into Apoe−/− mice in an angiotensin II-mediated abdominal aortic aneurysm model decreased mortality in the mice through an effect on the early stages of aneurysm development [20]. Plasmin-mediated TAFI activation may dominate at sites of vascular injury, which expose an abundance of GAGs present in the extracellular matrix [11]. Additionally, as these injury sites would lack TM, the presence of GAGs could play a significant role in stabilizing the clot and attenuating inflammation by promoting plasmin-mediated TAFI activation and TAFIa stability [11].
In this study, we constructed recombinant TAFI variants containing mutations in the “dynamic flap” instability region as well as the region spanning Trp210-Ser221, which are 2 sites predicted to interact with heparin. We assessed the ability of these variants to bind heparin, measured their kinetics of activation by plasmin in the presence or absence of heparin, and determined their thermal stabilities and antifibrinolytic potential.
2. Methods
2.1. Construction of TAFI variants
Mutagenesis was carried out using the QuikChange mutagenesis kit (Stratagene) according to the manufacturer’s instructions. The mutagenic primers used are shown in Table 1. In all cases, the template for the mutagenesis was TAFI-plasmid name, which contains human CPB2 complementary DNA, which encodes threonine at positions 147 and 325 [14]. The TAFI-R320A/K324A/K327A/R330A was created by successive mutagenesis reactions first using the R320A/K324A variant as the template to create R320A/K324A/K327A followed by using the product of this reaction as the template to create the quadruple mutant. The presence of the mutations was verified by DNA sequence analysis.
Table 1.
Primer sequences for mutagenesis of thrombin-activatable fibrinolysis inhibitor.
| Variant | Primer sequence |
|---|---|
| TAFI-K306A | 5′-CCTATACACGAAGTAAAAGCGCAGACCATGAGGAACTGTCTC-3′ |
| TAFI-H308F | 5′-ACACGAAGTAAAAGCAAAGACTTTGAGGAACTGTCTCTAGTAGC-3′ |
| TAFI-K212A | 5′-GTTATGACTACTCATGGAAAGCGAATCGAATGTGGAGAAAG-3′ |
| TAFI-K211Q/K212Q | 5′-GTGGACGGTTATGACTACTCATGGCAACAGAATCGAATGTGGAGAAAGAACCG-3′ |
| TAFI-R320A | 5′-AGTAGCCAGTGAAGCAGTTGCTGCTATTGAGAAAACTAGT-3′ |
| TAFI-K324A | 5′-AGCAGTTCGTGCTATTGAGGCAACTAGTAAAAATACCAGG-3′ |
| TAFI-R320A/K324A | 5′-ACTGTCTCTAGTAGCCAGTGAAGCAGTTGCTGCTATTGAGGCAACTAGTAAAAATACC-3′ |
| TAFI-K327A | 5′-TTCGTGCTATTGAGAAAACTAGTGCAAATACCAGGTATACACATGGCC-3′ |
| TAFI-R330A | 5′-GAAAACTAGTAAAAATACCGCGTATACACATGGCCATGGC-3′ |
| TAFI-K327A/R330A | 5′-TTCGTGCTATTGAGAAACTAGTGCAAATACCGCGTATACACATGGCCATGGCTCAG-3′ |
| TAFI-R320A/K324A/K327A/R330A | 5′-TGAAGCAGTTGCTGCTATTGAGGCAACTAGTGCAAATACC-3′a 5′-GCTAATGAGGCAACTAGTGCAAATACCGCGTATACACATG-3′a |
Altered nucleotides are bolded and underlined.
TAFI, thrombin-activatable fibrinolysis inhibitor.
These primers were used in successive reactions as described in the text.
2.2. Expression and purification of recombinant TAFI variants
Recombinant TAFI variants were expressed in BHK cells and purified by immunoaffinity chromatography as previously described [21]. The purity and activatability by thrombin-TM of each variant were verified by sodium dodecyl sulfate-polyacrylamide gele electrophoresis followed by staining with Imperial Stain (Millipore Sigma) (Supplementary Figure S1).
2.3. Binding of TAFI to heparin
A 1-mL heparin-agarose column (containing approximately 900 μg [125 U] of heparin) was prepared by equilibrating the column with 10 column volumes of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered saline/Tween 80 (HBST)—20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid pH 7.4, 150 mM, 0.01% [v/v] Tween 80 (Millipore Sigma)). TAFI (0.25 μg) was applied to the column, after which a wash was performed (0.2 column volumes of HBST × 12) followed by elution (0.2 column volumes of HBST containing 193 U/mL unfractionated heparin (grade I-A from porcine intestinal mucosa; Sigma-Aldrich). The TAFI content in each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gele electrophoresis and Western blotting using a polyclonal antibody raised against human TAFI (Affinity Biologicals). Densitometry of the resultant images was performed using AlphaView SA software (ProteinSimple; version 3.4.0.0). The percentage of input TAFI that was bound by the column was calculated by summing the densities of the TAFI bands in the buffer + heparin fractions (ie, BOUND) and dividing this value by the sum of the densities of the TAFI bands in all the lanes (UNBOUND plus BOUND).
2.4. Kinetics of activation of TAFI variants by plasmin
Activation of TAFI variants was performed as described [21] in the presence or absence of unfractionated heparin (200 U/mL) at 21 °C for 12 minutes. The concentration of plasmin used was 25 nM, and Plummer’s inhibitor (DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid; EMD Millipore; 150 μM) was included to protect TAFIa from further cleavage by plasmin. Rates of TAFI activation were assessed by Western blot analysis as previously described [21]. The data were fit to the Michaelis–Menten equation (SigmaPlot 11.0, SPSS Inc). Representative blots and standard curves are shown in Supplementary Figure S2.
2.5. Thermal stability of the TAFIa variants
TAFI variants (1 μM) were activated by incubation with thrombin (25 nM), TM (100 nM), and CaCl2 (5 mM) in HBST at 21 °C for 15 minutes. Thrombin activity was then quenched by the addition of Phenylalanyl-prolyl-arginyl chloromethyl ketone (PPAck; Calbiochem) (to 1 μM). Residual activity in timed aliquots incubated at 37 °C was determined using the synthetic substrate anisolylazoformylarginine (AAFR; Bachem), as previously described [21], with or without the addition of unfractionated heparin (312 U/mL final) after activation. The amount of residual TAFIa activity relative to the initial residual TAFIa activity was plotted as a function of time, and the data were fit by nonlinear regression to the first-order exponential decay equation (SigmaPlot 11.0). The first-order decay constant k was obtained and used to determine the half-life of each TAFI variant.
2.6. Barium-adsorbed TAFI-deficient plasma
Barium-adsorbed plasma was prepared as described previously [9] and was subsequently made TAFI-deficient (TdBAP) by passing it repeatedly over a 1-mL MA-T4E3-Sepharose column [22] at room temperature. Aliquots of the plasma were then used for an in vitro clot lysis assay (in the absence of added TAFI) in the presence or absence of 10 nM TM [23]. The plasma was considered to be TAFI-deficient when no difference was observed between clot lysis times in the presence or absence of TM.
2.7. Clot lysis assays
All clot lysis assays were performed in a final volume of 100 μL in microtiter plates at 37 °C. The TdBAP was diluted 1:3 in HBST and supplemented with synthetic phosphatidylcholine/phosphatidylserine vesicles (20 μM) as well as different concentrations of TAFI variants either as zymogen (0-100 nM) or as TAFIa (0-50 nM; following activation by thrombin-TM and inactivation of the thrombin with PPAck, as described above). The mixtures also contained no unfractionated heparin or 1 of 2 different concentrations (25 or 100 U/mL) of unfractionated heparin. Clotting was initiated by addition of the assay mixtures into the wells of microtiter plates containing small, separated aliquots of batroxobin (0.5 μg/mL), CaCl2 (5 mM), and tissue plasminogen activator (2 nM). Clot lysis was monitored by the change in turbidity of each reaction at 405 nm in a SpectraMax Plus 384 plate reader (Molecular Devices), and the time to 50% lysis was determined graphically from the midpoint between maximum and minimum turbidities of the clots.
2.8. Statistical analysis
Statistical analyses were performed with the use of GraphPad Prism, version 8.3.1 (GraphPad Software). Comparisons between different variants for a given assay parameter were performed by 1-way analysis of variance using a Tukey post hoc analysis. Differences between individual variants in the absence or presence of heparin were determined using an unpaired, 2-tailed Student’s t-test. Statistical significance was assumed at P < .05.
3. Results
3.1. Construction, expression, and purification of TAFI variants
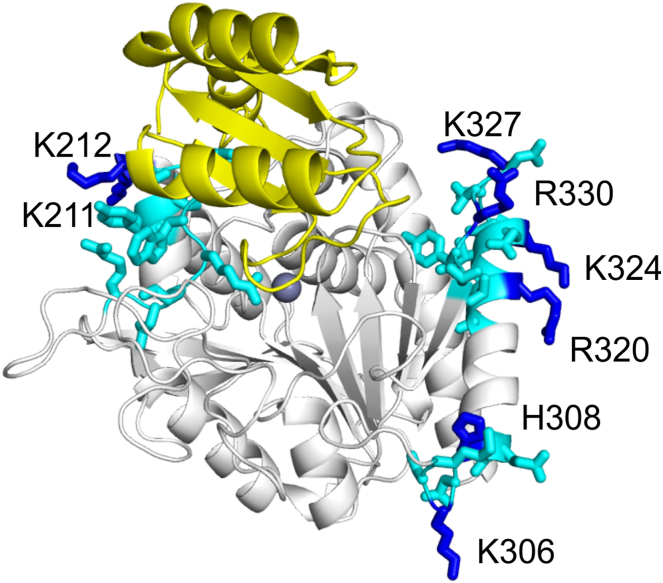
To identify the GAG interaction site(s) on TAFI, we expressed TAFI variants containing mutations of residues that are predicted to interact with heparin in the instability region as well as in the region spanning Trp210-Ser221. The latter region was hypothesized to function as a heparin-binding sequence based on homology with heparin-binding proteins [11]. Specifically, this region contains 2 repeats of the –XBBXBX– consensus, where B = Lys or Arg and X = hydrophobic amino acids. In the bovine crystal structure of TAFI, however, only 1 out of 22 sulfate molecules was bound to the Trp210-Ser221 region at Lys212 [17]. We therefore mutated Lys212 and the neighboring Lys211. None of the other basic residues in this region are surface-exposed (Figure 1). The variant with both lysines mutated to Ala did not express, and so the 2 lysines were together mutated to Gln.
Figure 1.
Mutations of potential heparin-binding sites on thrombin-activatable fibrinolysis inhibitor. The crystal structure of the thrombin-activatable fibrinolysis inhibitor is rendered based on PDB ID 3D66 [16] using Polyview (http://polyview.cchmc.org/polyview3d.html). The activation domain is in yellow. Side chains of residues that were mutated are labeled and shown in blue. Surrounding side chains of note are also shown in cyan (Tyr208 – Lys218; Lys304 – Glu310; Ala318 – Thr332). The active site Zn2+ is shown as a sphere.
In the instability region, there are 3 sulfates bound in the bovine crystal structure [17]. One is bound at His308, Ser312, and Arg313; of these, only His308 is conserved in humans; we therefore mutated this residue as well as the spatially close Lys306 residue. When His308 was mutated to Ala, the protein did not express, so we mutated this residue to Phe. Another sulfate is bound to His326 and Arg327, neither of which is conserved in humans. Therefore, we mutated the Lys at position 324 and the spatially close Arg320 residue. The final sulfate is bound at Arg330. We mutated this residue along with the spatially close Lys327 residue. We created single and double mutated TAFI variants as well as a quadruple TAFI variant comprising the mutated dynamic flap residues. Altogether, eleven variants were constructed (Figure 1, Table 1).
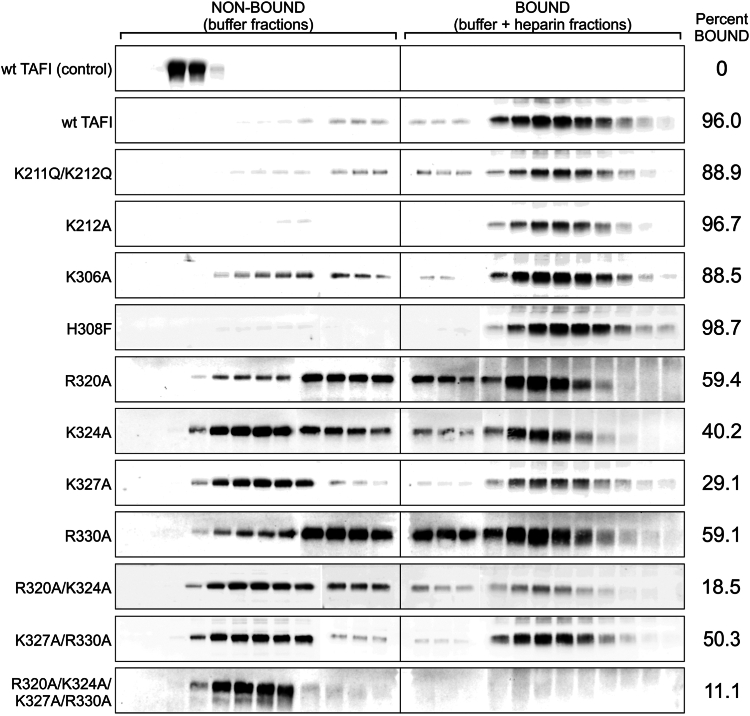
3.2. Binding of TAFI variants to heparin-agarose
We examined the ability of the variants to bind GAGs by utilizing a heparin-agarose column. The protein was applied to the column, and the column was then given a buffer wash followed by elution using a buffer containing heparin. The TAFI content in the fractions was evaluated using Western blot analysis. Wild-type TAFI was eluted almost exclusively in the heparin-containing fractions (Figure 2), demonstrating specific binding of TAFI to heparin immobilized on the column. In a control experiment using an agarose column lacking covalently linked heparin, TAFI eluted completely in the buffer wash (Figure 2), again consistent with specific binding between TAFI and heparin on the column.
Figure 2.
Binding of thrombin-activatable fibrinolysis inhibitor (TAFI) variants to heparin-agarose. Purified recombinant TAFI variants were applied to a heparin-agarose column. The column was washed with 2.4 column volumes of buffer (over 12 fractions) and eluted with 2.4 column volumes of buffer containing heparin (over 12 fractions). Each fraction was subjected to Western blot analysis using a polyclonal anti-TAFI antibody. Densitometry was performed on the blots to assess the fraction of TAFI present in the BOUND (buffer + heparin) fractions (listed to right of blots). A control experiment to evaluate nonspecific binding of TAFI to the column was conducted using the same agarose resin that was not derivatized with heparin (wild-type [wt] TAFI [control]). The data are representative of at least 3 independent experiments.
Variants H308F and K212A displayed similar elution profiles to wild-type, whereas variants K306A and K211Q/K212Q were not as tightly bound compared with wild-type, indicated by the fact that slightly more of the protein was eluted in the wash (Figure 2). The variants K324A, K327A, R320A/K324A, and R320A/K324A/K327A/R330A exhibited the lowest affinity for the heparin-agarose; the majority (approximately 60%-90%) eluted in the wash fractions (Figure 2).
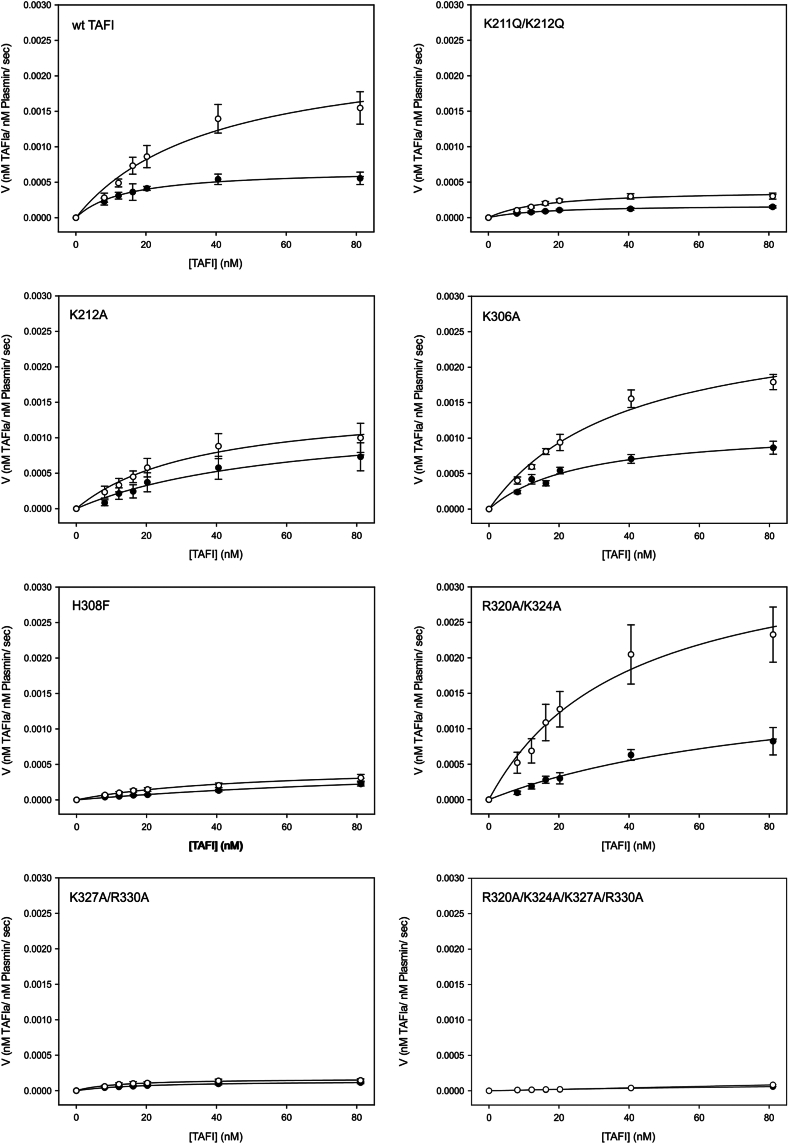
3.3. Kinetics of activation of TAFI variants by plasmin in the absence and presence of heparin
The kinetics of activation of various concentrations of the TAFI variants by plasmin in the absence and presence of heparin were determined (Figure 3). If a reduced accelerative effect of heparin was observed, this would imply that the mutated residues were important for the effect of heparin in this context. The estimated Michaelis–Menten kinetic parameters in the absence and presence of heparin are summarized in Tables 2 and 3, respectively. Our values for kcat and KM are in the same range as was reported by Mao et al. [11] using a similar Western blot-based method (kcat = 0.00044 s-1; KM = 55 nM). We observed a 3.4-fold increase in kcat upon addition of heparin, which compares favorably with the 5.9-fold increase observed by Mao et al. [11]. However, while they observed a decrease in KM upon addition of heparin, we observed an increase, which reduced the increment in catalytic efficiency (kcat/KM) in our data set. Nonetheless, it is clear that heparin has a notable effect on plasmin activation in our hands, most particularly at the highest concentrations of TAFI tested, which correspond to the physiological concentrations of the zymogen [24]. All the TAFI variants, to varying degrees, displayed accelerated activation in the presence of heparin, with K306A and R320A/K324A showing profiles most similar to wild-type (Figure 3). Several of the variants, however, displayed greatly impaired activation by plasmin that was superimposed on the effects of the mutations on acceleration by heparin. In particular, H308F, K211Q/K212Q, K327A/R330A, and R320A/K324A/K327A/R330A showed much reduced rates of activation that were little changed in the presence of heparin. K212A showed an intermediate effect, with moderately reduced rates of activation and a moderate decrease in the effect of heparin (Figure 3). Notably, the rate of plasmin activation of TAFI was unaffected by the pentasaccharide anticoagulant drug fondaparinux (Supplementary Figure S3).
Figure 3.
Effect of heparin on the kinetics of activation of the thrombin-activatable fibrinolysis inhibitor (TAFI) variants by plasmin. TAFI activation by plasmin (25 nM) was measured at various concentrations of TAFI (8.11-81.1 nM) and in the absence (filled circles) or presence (open circles) of heparin. The rate of TAFI activation was determined by Western blot analysis. The symbols are the means ± SE of the mean of at least 3 independent experiments. The lines are regression lines based on the mean values of kcat and KM obtained from at least 3 independent experiments. TAFIa, activated TAFI. wt, wild-type.
Table 2.
Kinetic parameters for activation of thrombin-activatable fibrinolysis inhibitor variants by plasmin in the absence of heparin.
| Variant | kcat (s−1) | KM (nM) | kcat/KM (μM−1s−1) |
|---|---|---|---|
| wt TAFI | 0.00071 ± 0.00012 | 17.6 ± 5.4 | 0.0516 ± 0.0200 |
| K211Q/K212Q | 0.00019 ± 0.00003 | 17.1 ± 3.6 | 0.0129 ± 0.0020a |
| K212A | 0.00173 ± 0.00051 | 115 ± 38 | 0.0249 ± 0.0107 |
| K306A | 0.00123 ± 0.00012 | 28.0 ± 3.8 | 0.0454 ± 0.0046 |
| H308F | 0.00051 ± 0.00016 | 90.2 ± 18.8 | 0.0053 ± 0.0006a |
| R320A | 0.00049 ± 0.00003 | 50.1 ± 9.9 | 0.0115 ± 0.0022a |
| K324A | 0.00049 ± 0.00007 | 37.4 ± 5 | 0.0144 ± 0.0022a |
| K327A | 0.00307 ± 0.00180a | 320 ± 205a | 0.0109 ± 0.0017a |
| R330A | 0.00020 ± 0.00003 | 83.4 ± 29.6 | 0.0039 ± 0.001a |
| R320A/K324A | 0.00188 ± 0.00058 | 91.0 ± 17.2 | 0.0198 ± 0.0024 |
| K327A/R330A | 0.00014 ± 0.00002 | 20.2 ± 3.6 | 0.0075 ± 0.0009a |
| R320A/K324A/K327A/R330Ab | 0.00018 | 167 | 0.0011 |
Data are the means ± SE of the mean of 3 to 10 independent experiments.
TAFI, thrombin-activatable fibrinolysis inhibitor; wt, wild-type.
Significantly different from wt TAFI (P < .05; 1-way analysis of variance).
n = 1.
Table 3.
Kinetic parameters for activation of thrombin-activatable fibrinolysis inhibitor variants by plasmin in the presence of heparin.
| Variant | kcat (s−1) | KM (nM) | kcat/KM (μM−1s−1) |
|---|---|---|---|
| wt TAFI | 0.00243 ± 0.00036a | 39.3 ± 2.4a | 0.0622 ± 0.0095 |
| K211Q/K212Q | 0.00042 ± 0.00004a,b | 19.0 ± 3.4 | 0.0247 ± 0.0028a,b |
| K212A | 0.00154 ± 0.00040 | 31.2 ± 8.1 | 0.0536 ± 0.0131 |
| K306A | 0.00279 ± 0.00020a | 40.1 ± 1.6a | 0.0698 ± 0.0047a |
| H308F | 0.00053 ± 0.00009b | 52.2 ± 8.1 | 0.0113 ± 0.0019a,b |
| R320A | 0.00165 ± 0.00029a | 41.0 ± 6.9 | 0.0442 ± 0.0104a |
| K324A | 0.00132 ± 0.00015a | 33.6 ± 10.8 | 0.0511 ± 0.0070a |
| K327A | 0.00160 ± 0.00038 | 83.4 ± 42.5 | 0.0354 ± 0.0111 |
| R330A | 0.00138 ± 0.00032a | 135 ± 25b | 0.0110 ± 0.0019a,b |
| R320A/K324A | 0.00364 ± 0.00058 | 41.1 ± 5.0 | 0.0927 ± 0.0201a |
| K327A/R330A | 0.00018 ± 0.00003b | 14.6 ± 6.6 | 0.0160 ± 0.0052 |
| R320A/K324A/K327A/R330Ac | 0.00192 | 179 | 0.0011 |
Data are the means ± SE of the mean of 3 to 10 independent experiments.
TAFI, thrombin-activatable fibrinolysis inhibitor; wt, wild-type.
Significantly different from in the absence of heparin (P < .05; unpaired 2-tailed t-test).
Significantly different from wt TAFI (P < .05; 1-way analysis of variance).
n = 1.
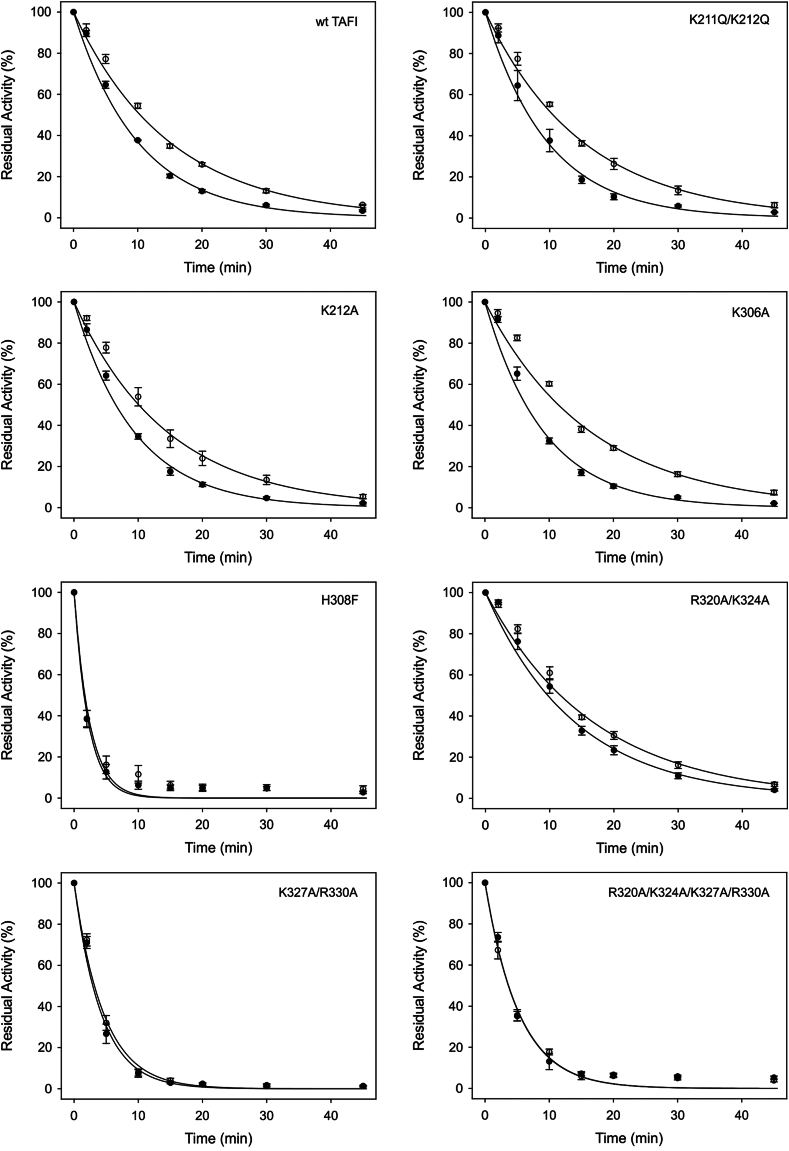
3.4. Thermal stabilities of the TAFI variants in the absence and presence of heparin
Mao et al. [11] previously established that the half-life of TAFIa at 25 °C was increased from 74 minutes to 170 minutes in the presence of heparin. The thermal stability of each variant in the presence and absence of heparin was determined to examine whether this stabilizing effect is also observed at 37 °C. Half-lives of each variant were calculated from the resultant first-order decay rate constants (Table 4). The half-life of wild-type TAFIa was increased 1.5-fold by heparin. In the presence of lower heparin concentrations (100 U/mL and 200 U/mL final), a similar increase in the half-life of wild-type TAFIa was observed (data not shown). In addition, fondaparinux had no effect on the half-life (Supplementary Figure S3). Many of the variants had a different stability to wild-type in the absence of heparin, and this is not unexpected given the results of previous mutagenesis studies [14,25]. However, H308F, K327A, R330A, K327A/R330A, and R320A/K324A/K327A/R330A each showed decreases in the degree to which they were stabilized by heparin. The variant R320A/K324A had an increased half-life compared with wild-type in the absence of heparin but a smaller extent of stabilization in the presence of heparin (Figure 4, Table 4).
Table 4.
Intrinsic stability of thrombin-activatable fibrinolysis inhibitor variants in the absence and presence of heparin.
| Variant | Half-life (min) |
Fold increase | |
|---|---|---|---|
| Minus heparin | Plus heparin | ||
| wt TAFI | 6.9 ± 0.1 | 10.4 ± 0.2a | 1.5 |
| K211Q/K212Q | 6.7 ± 0.6 | 10.6 ± 0.5a | 1.6 |
| K212A | 6.5 ± 0.3 | 10.1 ± 1.0a | 1.5 |
| K306A | 6.4 ± 0.2 | 11.4 ± 0.4a | 1.8 |
| H308F | 1.6 ± 0.2b | 1.7 ± 0.1b | 1.1 |
| R320A | 6.3 ± 0.2 | 9.9 ± 0.3a | 1.6 |
| K324A | 8.3 ± 0.2 | 9.3 ± 0.1a | 1.1 |
| K327A | 6.9 ± 0.7 | 8.2 ± 0.5 | 1.2 |
| R330A | 3.2 ± 0.2b | 5.1 ± 0.3a,b | 1.6 |
| R320A/K324A | 9.7 ± 0.6b | 11.7 ± 0.5a | 1.2 |
| K327A/R330A | 2.9 ± 0.1b | 3.2 ± 0.2b | 1.1 |
| R320A/K324A/K327A/R330A | 3.6 ± 0.3b | 3.7 ± 0.1b | 1.0 |
Data are the means ± SE of the mean of 3 to 5 independent experiments performed in triplicate.
TAFI, thrombin-activatable fibrinolysis inhibitor; wt, wild-type.
Significantly different from in the absence of heparin (P < .05; unpaired 2-tailed t-test).
Significantly different from wt TAFI (P < .05; 1-way analysis of variance).
Figure 4.
Effect of heparin on the stabilities of the activated thrombin-activatable fibrinolysis inhibitor (TAFIa) variants. Each variant was quantitatively activated by incubation with thrombin-thrombomodulin. After quenching of the thrombin, the TAFIa (100 nM) was incubated at 37 °C in the absence (filled circles) or presence (open circles) of heparin, and timed aliquots were removed and placed on ice. The residual TAFIa activity was measured using a TAFIa substrate and is expressed relative to the TAFIa activity present before incubation at 37 °C. The symbols are the means ± SE of the mean of at least 3 independent experiments. The lines are regression lines based on the mean first-order rate constants for TAFIa decay obtained from at least 3 independent experiments. wt TAFI, wild-type thrombin-activatable fibrinolysis inhibitor.
The AAFR hydrolysis data at time = 0 were used to estimate the relative “specific activity” of each TAFIa variant compared with wild-type TAFIa (Table 5). H308F, R330A, and R320A/K324A/K327A/R330A all had a significantly lower activity than wild-type TAFIa, while R320A/K324A had a higher activity.
Table 5.
Relative activities of thrombin-activatable fibrinolysis inhibitor variants.
| Variant | Relative activitya |
|---|---|
| wt TAFI | 100 ± 18.6b |
| K211Q/K212Q | 89.9 ± 8.0 |
| K212A | 109.3 ± 5.6 |
| K306A | 115.0 ± 7.6 |
| H308F | 15.7 ± 6.1b |
| R320A | 100.6 ± 16.4 |
| K324A | 104.5 ± 19.2 |
| K327A | 98.1 ± 2.7 |
| R330A | 42.4 ± 4.3b |
| R320A/K324A | 138.6 ± 5.0b |
| K327A/R330A | 85.6 ± 7.1 |
| R320A/K324A/ K327A/R330A | 31.0 ± 2.2b |
Data are the means ± SE of the mean of 4 to 10 independent experiments performed in triplicate.
TAFI, thrombin-activatable fibrinolysis inhibitor; wt, wild-type.
Determined as the percentage of the rate of AAFR hydrolysis of wt TAFI.
Significantly different from wt TAFI (P < .05; 1-way analysis of variance).
3.5. Antifibrinolytic potential of TAFI variants
We determined the antifibrinolytic potential of selected variants in the absence and presence of heparin using plasma clot lysis assays. We set up 2 different types of assays in order to separately evaluate the effects of heparin on plasmin-mediated activation and on the stability of TAFIa.
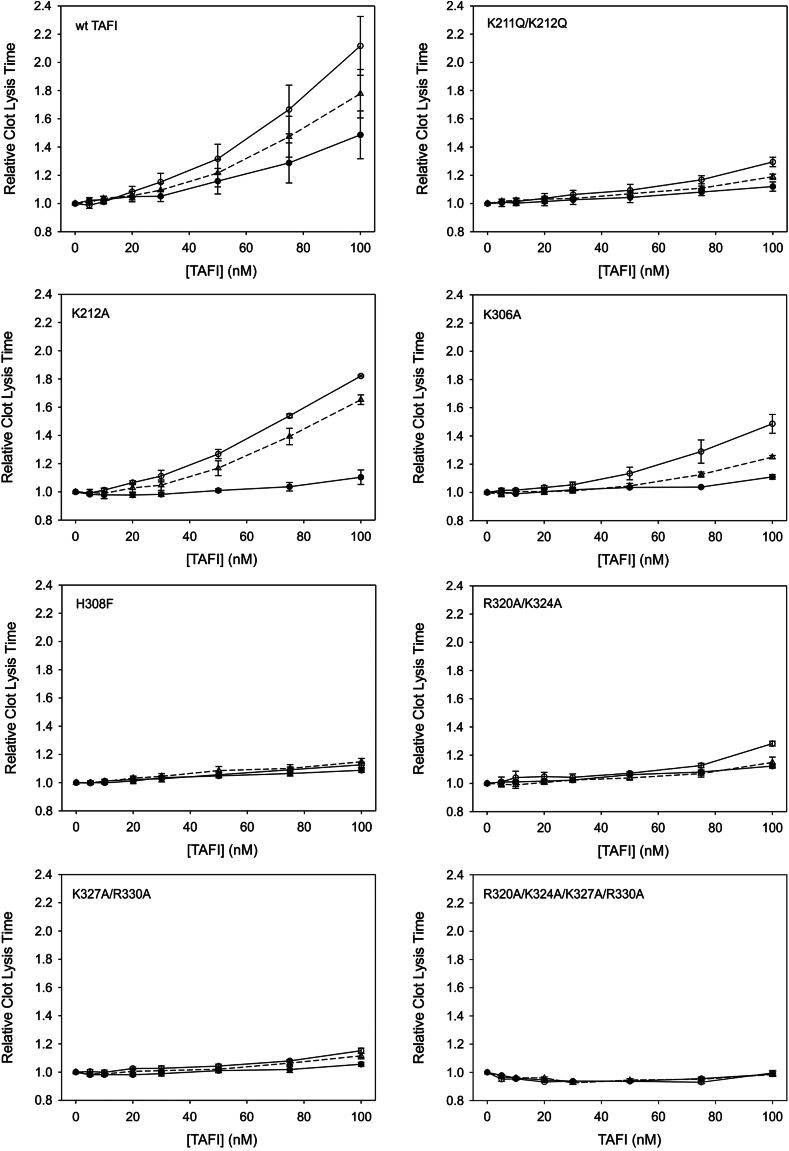
In the first type of lysis assay, the system was manipulated such that the plasmin that was generated during lysis was the sole TAFI activator. This was done by using TdBAP and initiating clot formation using batroxobin. No thrombin would be generated during clot formation or lysis under these conditions, and it was previously shown by Mao et al. [11] that the addition of heparin to stimulate plasmin-mediated TAFI activation was required to observe a TAFI-dependent antifibrinolytic effect. We varied the concentration of recombinant TAFI variants added to the clots and used 2 different concentrations of heparin (Figure 5). Wild-type TAFI exhibited the greatest antifibrinolytic potential with increased heparin concentration, with variants K212A and K306A also having a notable response to heparin. Heparin resulted in a small increase in lysis time at the highest concentrations of K211A/K212A, R320A/K324A, and K327A/R330A, while H308F and R320A/K324A/K327A/R330A showed very little antifibrinolytic effect that was essentially resistant to heparin (Figure 5).
Figure 5.
Effect of heparin on the antifibrinolytic potential of the thrombin-activatable fibrinolysis inhibitor (TAFI) variants. Clots were assembled from barium-adsorbed TAFI-deficient plasma and supplemented with various concentrations of TAFI variants (5-100 nM) in the absence of heparin (filled circles) or in the presence of 25 U/mL (open triangles) or 100 U/mL (open circles) of heparin. Clotting was initiated with batroxobin, and lysis was initiated with tissue plasminogen activator. Clot formation and lysis were allowed to proceed at 37 °C, and lysis was monitored by measurement of absorbance at 405 nm. The time to 50% clot lysis (defined as the midpoint between the maximum and minimum absorbances) was determined and plotted relative to the clot lysis times observed in the absence of TAFI. The data are the means ± SE of the mean of at least 3 independent experiments. wt, wild-type.
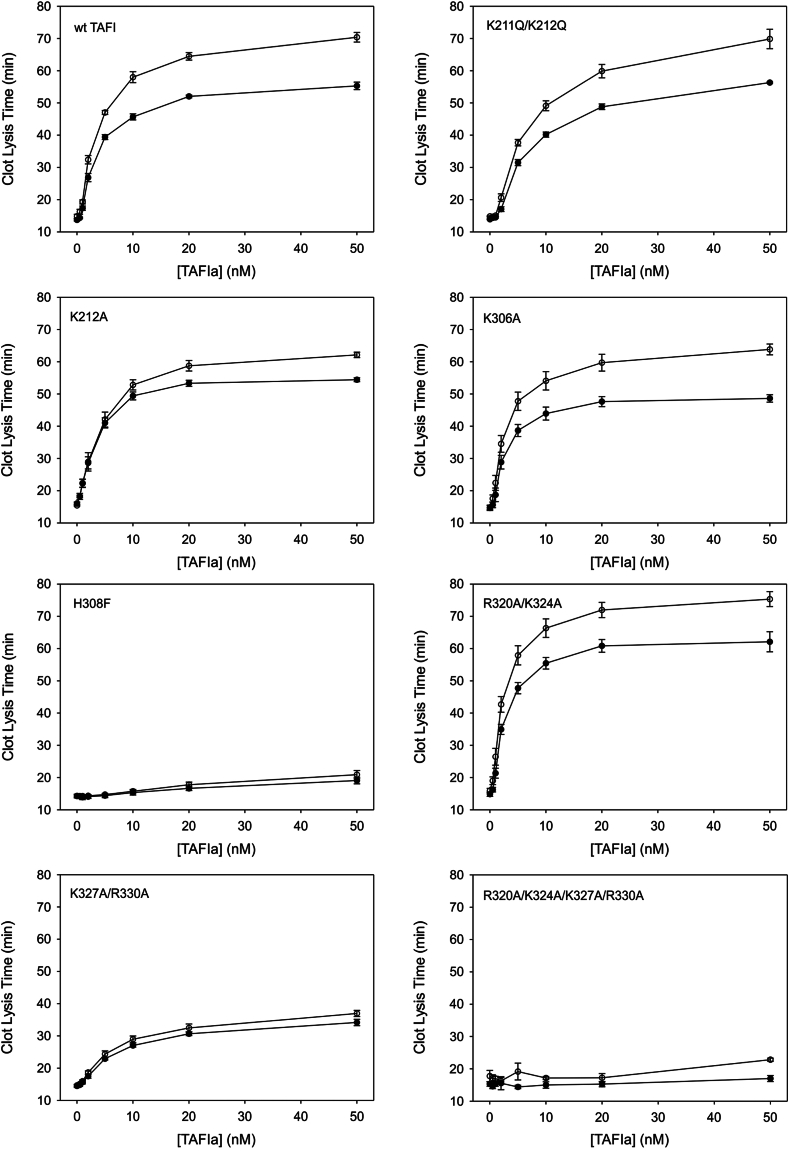
In the second type of clot lysis assay, we activated the TAFI variants using thrombin-TM prior to addition to the clot lysis assay; each variant was activated to a similar extent (Supplementary Figure S1). This allowed us to specifically interrogate the role of heparin-mediated TAFIa stabilization during lysis since the maximal extent of fibrinolysis inhibition (ie, the plateau) is a direct function of the intrinsic stability of TAFIa [14,26]. Wild-type TAFI displayed an increase in stability in the presence of heparin and therefore an increase in the plateau lysis time, as expected (Figure 6). Variants K306A, K211Q/K212Q, and R320A/K324A each exhibited increases in the plateau lysis times due to heparin that were comparable with wild-type TAFIa (Figure 6). K212A showed a moderately decreased increase in plateau lysis time, while lysis time of H308F, K327A/R330A, and R320A/K324A/K327A/R330A were largely resistant to the effects of heparin (Figure 6). Notably, all 3 variants were much less stable than wild-type TAFIa and showed substantially reduced antifibrinolytic potential (ie, plateau lysis time), especially R320A/K324A/K327A/R330A.
Figure 6.
Effect of heparin on the antifibrinolytic potential of the activated thrombin-activatable fibrinolysis inhibitor (TAFIa) variants. TAFI was quantitatively activated by incubation with thrombin-thrombomodulin, and the resultant TAFIa (at 0.5-50 nM) was included in clots assembled from barium-adsorbed TAFI-deficient plasma and either lacking (filled circles) or containing (open circles) 100 U/mL of heparin. Clotting was initiated with batroxobin, and lysis was initiated with tissue plasminogen activator. Clot formation and lysis were allowed to proceed at 37 °C, and lysis was monitored by measurement of absorbance at 405 nm. The time to 50% clot lysis (defined as the midpoint between the maximum and minimum absorbances) was determined. The data are the means ± SE of the mean of at least 3 independent experiments. wt TAFI, wild-type thrombin-activatable fibrinolysis inhibitor.
4. Discussion
It has been reported that GAGs, such as heparin, accelerate activation of TAFI by plasmin and stabilize TAFIa [11]. However, the number and location of the heparin-binding sites on TAFI that mediate these effects are unknown. Here, we show that the quadruple mutant, R320A/K324A/K327A/R330A, consisting of the combined mutations of the residues in the instability region essentially eliminated heparin binding, which signifies the importance of this region for interaction with heparin. Mutation of Lys327 and Arg330 alone not only reduced heparin binding but also attenuated the effect of heparin on plasmin-mediated TAFI activation and TAFIa stability, and thus, these residues on the dynamic flap represent a functional heparin-binding site.
The binding of the single and double variants to the heparin-agarose column indicated that mutations in the instability region of TAFI result in a reduced affinity for heparin since at least half of the protein eluted in the wash fractions. We do not believe that our findings reflect subfractions of the protein that are or are not capable of binding heparin since if we pool the TAFI in the nonbinding fraction and reapply it to the column, a similar proportion does not bind to the column (data not shown).
Although the K306A variant displays slightly reduced apparent affinity for heparin, we do not observe this reflected in the functional properties of the protein, as heparin normally accelerated its activation by plasmin and stabilized its enzyme form. It does appear to be less potent in inhibiting fibrinolysis than wild-type TAFI in both the presence and absence of heparin, however. This may be a consequence of the slightly reduced stability of the enzyme in the absence of heparin. The H308F variant binds heparin normally but is activated very poorly by plasmin, is far less stable than wild-type TAFIa, and displays a substantially reduced specific activity. Accordingly, it has almost no antifibrinolytic effect under any of the conditions we employed. In fact, it could be argued that its apparent resistance to the effects of heparin reflects its essential lack of functionality. Therefore, it can be concluded that Lys306 and His308 do not constitute a heparin-binding site.
Mutation of Lys211 and/or Lys212 does not significantly impact the heparin affinity of TAFI. It also does not notably alter the stability of the TAFIa variants or the effect of heparin on stability. Differences were observed in the extent of plasmin activation, however. K212A has a much increased KM value compared with wild-type, whereas K211Q/K212Q has a much reduced kcat value compared with wild-type, although the catalytic efficiency of both variants is increased by heparin to a similar extent. These effects are reflected in the reduced potency of the respective variants in lysis assays where the antifibrinolytic effect is sensitive to plasmin-mediated TAFI activation (Figure 5). Overall, the data with these variants are more consistent with them influencing activation by plasmin than affecting heparin binding.
To date, there are few studies characterizing the plasmin-TAFI interaction. A report by Mishra et al. [18] identified a monoclonal antibody that specifically inhibits plasmin-mediated TAFI activation, MA-TCK11A9, with the major epitope binding residues Lys268, Ser272, and Arg276. The MA-TCK22G2 and MA-TCK27A4 antibodies were found to inhibit plasmin and thrombin-mediated activation and plasmin, thrombin, and thrombin-TM activation, respectively. The residues important for binding MA-TCK22G2 are Thr147 and Ala148, and MA-TCK27A4 additionally binds Phe113 [18]. None of these residues are close to Lys211/212. However, Lys211/212 is on the same side of the protein as Arg92, suggesting that they may support plasmin-mediated activation of TAFI by binding to the lysine-binding kringles in plasmin. In support of this idea, neither Lys211 nor Lys212 affects thrombin-mediated TAFI activation either in the presence or absence of TM [27].
The mutations in the instability region (dynamic flap) of TAFI had the greatest effect on TAFI binding to heparin. The weakest binding was observed with the quadruple mutant, R320A/K324A/K327A/R330A. However, this mutant exhibited significantly decreased extent of plasmin activation and therefore is unlikely to have an antifibrinolytic effect with or without heparin, as was observed. The second weakest binding was seen with the double mutant R320A/K324A. Interestingly, this variant exhibited normal activation by plasmin and acceleration of that activation by heparin and in fact shows a higher TAFIa activity than wild-type. These results were not reflected in clot lysis assays with plasmin as the activator, where inhibition of fibrinolysis was very much impaired compared with wild-type. The reason for this observation is not clear. Although the R320A/K324A enzyme was stabilized to a lesser extent by heparin, clot lysis assays using preactivated TAFIa showed that heparin could raise the plateau lysis time of this enzyme. Interestingly, while an R320Q variant is very unstable compared with wild-type [14], the R320A variant was comparable in stability with wild-type and the K324A enzyme was more stable compared with wild-type, which explains why the double mutant R320A/K324A studied here is actually slightly more stable than wild-type and has a correspondingly higher plateau lysis time in the absence of heparin.
Mutation of Lys327 and Arg330 provides the strongest evidence for the existence of a functional heparin-binding site that influences both TAFI activation and TAFIa inactivation. The K327A/R330A double mutant abolishes the increase in kcat for plasmin activation seen in response to heparin, although this is in the context of a profound impairment in plasmin-mediated activation. This effect is reflected in the clot lysis assays with plasmin as the activator; the double mutant showed a much reduced response to the addition of heparin and a substantially diminished antifibrinolytic effect. The K327A single mutant possessed similar enzyme stability to wild-type in the absence of heparin but a lower extent of stabilization with heparin. The R330A single mutant also results in a decreased stabilization of the enzyme, while the K327A/R330A double mutant is both very unstable compared with wild-type and completely resistant to stabilization by heparin. This is reflected in the much lower plateau lysis times of the double mutant along with near-complete resistance to the effect of heparin.
The basic residues in the dynamic flap thus all contribute to heparin-binding affinity while contributing varying degrees of impairment of regulation by heparin. This defines the dynamic flap region as the major heparin-binding site in TAFI/TAFIa. It is possible that Arg320, Lys324, Lys327, and Arg330 are all involved in binding and that the site accounts for the effects on both plasmin-mediated activation and stability. However, mutation of Arg320 and Lys324 does not appear to affect plasmin-mediated activation, suggesting that this effect can be decoupled from effects on stability. There is also evidence for additional binding sites of no functional significance. Indeed, the K306A mutation results in reduced heparin binding but has no impact on plasmin-mediated activation or TAFIa stability. Interestingly, however, the lack of effect of fondaparinux on these parameters indicates that an extended interaction involving more than 5 glycosidic residues is required, evoking the idea of cooperative roles of more than one heparin-binding site on TAFI/TAFIa.
Altogether, the results of this study shed new light on the nature of the TAFI-heparin interaction. Connections can be made between impaired heparin binding, reduced effects of heparin on activation or stabilization, and changes in antifibrinolytic potential. The data suggest that TAFI possesses multiple heparin-binding sites, which may contribute to the different functions of heparin in regulating TAFI activity. It has been hypothesized that heparin and heparan sulfate proteoglycans present in the glycocalyx and exposed at wound sites could serve to promote TAFI activation by plasmin in situ as well as stabilize TAFIa, thereby augmenting the antifibrinolytic effects and wound-healing actions of TAFI locally [11,28]. Conversely, the impact of therapeutic heparins (albeit not fondaparinux) on TAFI activation and TAFIa stability might antagonize the anticoagulant activity of these agents.
Acknowledgments
Funding
This work was supported by a grant (# 05571-2017 RGPIN) from the Natural Sciences and Engineering Research Council of Canada.
Ethics statement
No artificial intelligence (AI) or AI-assisted technologies have been used in the creation of this article.
Author contributions
T.T.M. designed the experiments, performed the research, and cowrote the manuscript. M.B.B. designed the experiments and cowrote the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Boffa Senis
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102459
Supplementary material
References
- 1.Foley J.H., Kim P.Y., Mutch N.J., Gils A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J Thromb Haemost. 2013;11(Suppl 1):306–315. doi: 10.1111/jth.12216. [DOI] [PubMed] [Google Scholar]
- 2.Heylen E., Willemse J., Hendriks D. An update on the role of carboxypeptidase U (TAFIa) in fibrinolysis. Front Biosci (Landmark Ed) 2011;16:2427–2450. doi: 10.2741/3864. [DOI] [PubMed] [Google Scholar]
- 3.Colucci M., Semeraro N. Thrombin activatable fibrinolysis inhibitor: at the nexus of fibrinolysis and inflammation. Thromb Res. 2012;129:314–319. doi: 10.1016/j.thromres.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Nesheim M. Thrombin and fibrinolysis. Chest. 2003;124(Suppl):33S. doi: 10.1378/chest.124.3_suppl.33s. 9S. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Boffa M.B., Bajzar L., Walker J.B., Nesheim M.E. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273:27176–27181. doi: 10.1074/jbc.273.42.27176. [DOI] [PubMed] [Google Scholar]
- 6.Redlitz A., Tan A.K., Eaton D.L., Plow E.F. Plasma carboxypeptidases as regulators of the plasminogen system. J Clin Invest. 1995;96:2534–2538. doi: 10.1172/JCI118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaisgood C.M., Schmitt D., Eaton D., Plow E.F. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J Clin Invest. 2002;110:1275–1282. doi: 10.1172/JCI15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morser J., Gabazza E.C., Myles T., Leung L.L. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J Thromb Haemost. 2010;8:868–876. doi: 10.1111/j.1538-7836.2010.03787.x. [DOI] [PubMed] [Google Scholar]
- 9.Bajzar L., Manuel R., Nesheim M.E. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1995;270:14477–14484. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 10.Bajzar L., Morser J., Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–16608. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- 11.Mao S.S., Cooper C.M., Wood T., Shafer J.A., Gardell S.J. Characterization of plasmin-mediated activation of plasma procarboxypeptidase B. Modulation by glycosaminoglycans. J Biol Chem. 1999;274:35046–35052. doi: 10.1074/jbc.274.49.35046. [DOI] [PubMed] [Google Scholar]
- 12.Plug T., Meijers J.C. Stimulation of thrombin- and plasmin-mediated activation of thrombin-activatable fibrinolysis inhibitor by anionic molecules. Thromb Res. 2016;146:7–14. doi: 10.1016/j.thromres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Hendriks D., Scharpé S., van Sande M., Lommaert M.P. Characterisation of a carboxypeptidase in human serum distinct from carboxypeptidase N. J Clin Chem Clin Biochem. 1989;27:277–285. doi: 10.1515/cclm.1989.27.5.277. [DOI] [PubMed] [Google Scholar]
- 14.Boffa M.B., Bell R., Stevens W.K., Nesheim M.E. Roles of thermal instability and proteolytic cleavage in regulation of activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 2000;275:12868–12878. doi: 10.1074/jbc.275.17.12868. [DOI] [PubMed] [Google Scholar]
- 15.Boffa M.B., Wang W., Bajzar L., Nesheim M.E. Plasma and recombinant thrombin-activable fibrinolysis inhibitor (TAFI) and activated TAFI compared with respect to glycosylation, thrombin/thrombomodulin-dependent activation, thermal stability, and enzymatic properties. J Biol Chem. 1998;273:2127–2135. doi: 10.1074/jbc.273.4.2127. [DOI] [PubMed] [Google Scholar]
- 16.Marx P.F., Brondijk T.H., Plug T., Romijn R.A., Hemrika W., Meijers J.C., et al. Crystal structures of TAFI elucidate the inactivation mechanism of activated TAFI: a novel mechanism for enzyme autoregulation. Blood. 2008;112:2803–2809. doi: 10.1182/blood-2008-03-146001. [DOI] [PubMed] [Google Scholar]
- 17.Anand K., Pallares I., Valnickova Z., Christensen T., Vendrell J., Wendt K.U., et al. The crystal structure of thrombin-activable fibrinolysis inhibitor (TAFI) provides the structural basis for its intrinsic activity and the short half-life of TAFIa. J Biol Chem. 2008;283:29416–29423. doi: 10.1074/jbc.M804003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra N., Vercauteren E., Develter J., Bammens R., Declerck P.J., Gils A. Identification and characterisation of monoclonal antibodies that impair the activation of human thrombin activatable fibrinolysis inhibitor through different mechanisms. Thromb Haemost. 2011;106:90–101. doi: 10.1160/TH10-08-0546. [DOI] [PubMed] [Google Scholar]
- 19.Vercauteren E., Emmerechts J., Peeters M., Hoylaerts M.F., Declerck P.J., Gils A. Evaluation of the profibrinolytic properties of an anti-TAFI monoclonal antibody in a mouse thromboembolism model. Blood. 2011;117:4615–4622. doi: 10.1182/blood-2010-08-303677. [DOI] [PubMed] [Google Scholar]
- 20.Bridge K., Revill C., Macrae F., Bailey M., Yuldasheva N., Wheatcroft S., et al. Inhibition of plasmin-mediated TAFI activation may affect development but not progression of abdominal aortic aneurysms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marar T.T., Boffa M.B. Identification of a thrombomodulin interaction site on thrombin-activatable fibrinolysis inhibitor that mediates accelerated activation by thrombin. J Thromb Haemost. 2016;14:772–783. doi: 10.1111/jth.13275. [DOI] [PubMed] [Google Scholar]
- 22.Miah M.F., Boffa M.B. Functional analysis of mutant variants of thrombin-activatable fibrinolysis inhibitor resistant to activation by thrombin or plasmin. J Thromb Haemost. 2009;7:665–672. doi: 10.1111/j.1538-7836.2009.03311.x. [DOI] [PubMed] [Google Scholar]
- 23.Schadinger S.L., Lin J.H., Garand M., Boffa M.B. Secretion and antifibrinolytic function of thrombin-activatable fibrinolysis inhibitor from human platelets. J Thromb Haemost. 2010;8:2523–2529. doi: 10.1111/j.1538-7836.2010.04024.x. [DOI] [PubMed] [Google Scholar]
- 24.Boffa M.B., Koschinsky M.L. Curiouser and curiouser: recent advances in measurement of thrombin-activatable fibrinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clin Biochem. 2007;40:431–442. doi: 10.1016/j.clinbiochem.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Ceresa E., Van de Borne K., Peeters M., Lijnen H.R., Declerck P.J., Gils A. Generation of a stable activated thrombin activable fibrinolysis inhibitor variant. J Biol Chem. 2006;281:15878–15883. doi: 10.1074/jbc.M509839200. [DOI] [PubMed] [Google Scholar]
- 26.Walker J.B., Bajzar L. The intrinsic threshold of the fibrinolytic system is modulated by basic carboxypeptidases, but the magnitude of the antifibrinolytic effect of activated thrombin-activable fibrinolysis inhibitor is masked by its instability. J Biol Chem. 2004;279:27896–27904. doi: 10.1074/jbc.M401027200. [DOI] [PubMed] [Google Scholar]
- 27.Wu C., Kim P.Y., Manuel R., Seto M., Whitlow M., Nagashima M., et al. The roles of selected arginine and lysine residues of TAFI (Pro-CPU) in its activation to TAFIa by the thrombin-thrombomodulin complex. J Biol Chem. 2009;284:7059–7067. doi: 10.1074/jbc.M804745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.te Velde E.A., Wagenaar G.T., Reijerkerk A., Roose-Girma M., Borel Rinkes I.H., Voest E.E., et al. Impaired healing of cutaneous wounds and colonic anastomoses in mice lacking thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. 2003;1:2087–2096. doi: 10.1046/j.1538-7836.2003.00404.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.