Abstract
Resistant hypertension (RH) is defined as systolic blood pressure (SBP) or diastolic blood pressure (DBP) that remains .140 mmHg or .90 mmHg, respectively, despite an appropriate lifestyle and the use of optimal or maximally tolerated doses of a three-drug combination, including a diuretic. This definition encompasses the category of controlled RH, defined as the presence of blood pressure (BP) effectively controlled by four or more antihypertensive agents, as well as refractory hypertension, referred to as uncontrolled BP despite five or more drugs of different classes, including a diuretic. To confirm RH presence, various causes of pseudo-resistant hypertension (such as improper BP measurement techniques and poor medication adherence) and secondary hypertension must be ruled out. Inadequate BP control should be confirmed by out-of-office BP measurement. RH affects about 5% of the hypertensive population and is associated with increased cardiovascular morbidity and mortality. Once RH presence is confirmed, patient evaluation includes identification of contributing factors such as lifestyle issues or interfering drugs/substances and assessment of hypertension-mediated organ damage. Management of RH comprises lifestyle interventions and optimisation of current medication therapy. Additional drugs should be introduced sequentially if BP remains uncontrolled and renal denervation can be considered as an additional treatment option. However, achieving optimal BP control remains challenging in this setting. This review aims to provide an overview of RH, including its epidemiology, pathophysiology, diagnostic work-up, as well as the latest therapeutic developments.
Keywords: Resistant hypertension, medication adherence, spironolactone, aprocitentan, baxdrostat, renal denervation
Hypertension is one of the most common chronic diseases in the world. Despite the availability of many treatment options, a large proportion of the hypertensive population still fails to achieve long-term blood pressure (BP) control.[1] According to the most recent guidelines of the European Society of Hypertension (ESH), true resistant hypertension (tRH) is defined as systolic BP (SBP) or diastolic BP (DBP) that remains ≥140 mmHg or ≥90 mmHg, respectively, despite appropriate use of lifestyle measures and use of optimal or maximally tolerated doses of a three-drug combination comprising a renin–angiotensin–aldosterone (RAAS) blocker (either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker), a calcium channel blocker and a thiazide/thiazide-like diuretic.[2] This definition of RH also includes those patients taking four or more drugs to maintain BP <140/90 mm Hg, as well as the category of refractory hypertension, referred to as uncontrolled BP despite five or more drugs of different classes, including a diuretic.[3]
The American Heart Association (AHA) and American College of Cardiology (ACC) identify resistant hypertension (RH) at a lower BP threshold (i.e. 130/80 mmHg). The AHA/ACC also propose the term ‘controlled RH’ to indicate the presence of BP effectively controlled by four or more antihypertensive agents.[4,5] According to the latest ESH guidelines, various causes of pseudo-resistant and secondary hypertension must be investigated and ruled out to define tRH. Moreover, inadequate BP control should be confirmed by out-of-office BP measurement.[2] If there are insufficient data on medication dose, adherence to treatment, or out-ofoffice BP levels, pseudo-resistant hypertension cannot be ruled out and RH is defined apparent RH (aRH).[5]
RH is associated with increased cardiovascular (CV) morbidity and mortality. Patients with RH generally require more frequent medical examinations, diagnostic tests and medication, resulting in increased healthcare costs and economic burden.[6,7] Appropriate management of patients with suspected RH is crucial to avoid misdiagnosis and ensure adequate treatment.
The aim of this review is to provide an overview of RH, including epidemiology, pathophysiology, diagnostic procedure and the latest developments in therapeutic strategies.
Epidemiology
The global burden of RH is relevant, with approximately 100–500 million people globally estimated to be affected.[5] Establishing the exact prevalence of RH is challenging. Several factors influence RH prevalence, such as the clinical setting, antihypertensive drugs used, adherence to treatment, method of BP measurement and the definition of target BP.[5,8,9] In a meta-analysis including data from 91 cohort or cross-sectional studies comprising more than 3.2 million patients, the prevalence of RH was about 10% among patients treated for hypertension.[10] In a more recent Italian cohort of patients referred to primary care physicians, >20% of patients with hypertension actually had RH, defined as treatment with three different antihypertensive agents with recorded office BP ≥140/90 mmHg, or patients taking ≥4 medications.[11]
In an analysis of more than 2.4 million individuals in the US, a prevalence of apparent RH of 8.5% was observed, lower than previously reported (12–15%), but with a high burden of comorbidities. Identification of differences in pharmacotherapy between patients with controlled and uncontrolled RH, particularly lower rates of mineralocorticoid receptor antagonist (MRA) use, may help define potential opportunities to improve care and lower CV risk.[12]
After strictly applying the above definition of RH, a reasonable estimate of disease prevalence could be around 5% of the overall population with hypertension.[2] RH prevalence is higher among patients with diabetes and hypertension-mediated organ damage (HMOD), including albuminuria, left ventricular hypertrophy and chronic kidney disease (CKD).[10,13–18] Other clinical conditions significantly associated with RH are the number of antihypertensive drugs used, male sex, older age, obesity, black African origin, low income, depression and a 10-year CV Framingham risk score >20%.[13–15] Obstructive sleep apnoea (OSA) is also very common among patients with RH.[19]
Using data from NHANES, the prevalence of refractory hypertension was reported to be in the range of 0.3% to 0.9% across multiple cycles. Among patients prescribed five or more antihypertensive drugs, the prevalence of refractory hypertension was 34.5%.[20] The demographic and clinical factors associated with refractory hypertension were advancing age, lower household income, black ethnicity, CKD, albuminuria, diabetes, prior stroke and coronary heart disease.
Pathophysiological Mechanisms
The pathophysiology of RH involves an interplay between several neurohumoral factors, including increased sympathetic activity and levels of aldosterone, endothelin-1 and vasopressin.[21–24] These factors induce volume and sodium overload and contribute to increased peripheral vascular resistance, arterial stiffness and HMOD occurrence.[21,25]
Several conditions contribute to such mechanisms (Figure 1). Obesity is an important risk factor for RH development.[14] Findings from NHANES demonstrated that BMI ≥30 kg/m2 approximately doubles the risk of aRH.[26] In particular, visceral adiposity plays a fundamental role in RH occurrence through a variety of mechanisms, including enhanced salt sensitivity, vascular dysfunction and activation of the sympathetic nervous system and RAAS.[27,28] Both reduced physical activity and lower physical fitness are independent risk factors for hypertension, although there is a paucity of data on patients with RH.
Figure 1: Pathophysiological Mechanisms of Resistant Hypertension.
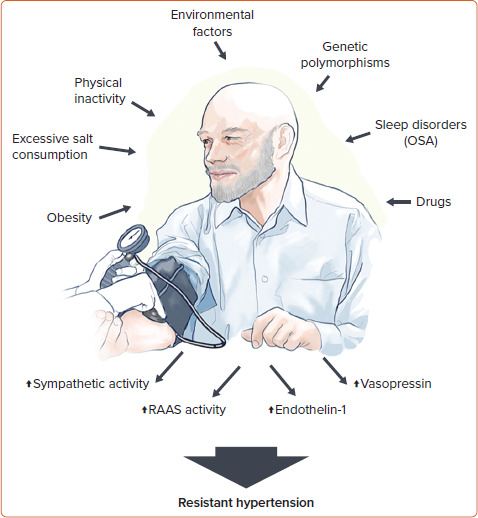
OSA = obstructive sleep apnoea; RAAS = renin–angiotensin–aldosterone system.
In the REGARDS cohort, self-reported inactivity was not predictive of RH.[29] On the other hand, indirect evidence from randomised controlled trials (RCTs) on lifestyle interventions suggests an important role of physical inactivity in the development of the disease.[30,31] Environmental factors may also contribute to the increased risk of RH. Using the NHANES data, Chen et al. recently identified that blood levels of heavy metals, such as lead and cadmium, were associated with increased prevalence of RH.[32]
Several studies have also reported new or exacerbated RH in patients with previous severe acute respiratory syndrome coronavirus 2 infection. The exact mechanism linking such conditions may involve the binding of the spike protein to angiotensin converting enzyme-2 (ACE2) receptors on the cell surface leading to ACE2 downregulation and failure of the counter-regulatory RAAS axis.[33] However, these aspects have yet to be clearly demonstrated.[34]
Sleep is a crucial component of overall health and disruptions in sleep patterns has been associated with an increased risk of hypertension and CV disease as a result of sympathetic overactivity.[35,36] OSA is a common sleep disorder characterised by repetitive episodes of nocturnal breathing cessation due to upper airway collapse. It is a particularly strong risk factor for the development of RH.[19] Recurrent hypoxia occurring in patients with OSA triggers endothelial dysfunction and activates the RAAS and sympathetic systems, leading to systemic inflammation and oxidative stress that contribute to RH and HMOD occurrence.[37,38]
Several classes of drugs can increase BP through various mechanisms, such as increased systemic and renal vasoconstriction, sodium retention and angiotensin biosynthesis. These pharmacological agents include nonsteroidal anti-inflammatory agents, oral contraceptives, immunosuppressive treatments such as cyclosporine and tacrolimus, antineoplastic drugs targeting the vascular endothelial growth factor pathway, steroids and antidepressants.[39–44]
There is limited evidence on RH heritability. Most genetic research on RH has been limited to candidate genes and lack adequate sample sizes.[45–49] One of the genes potentially involved in RH susceptibility is the angiotensinogen (AGT) gene, encoding a protein that is a precursor to angiotensin II.[47] The M235T polymorphism of the AGT gene, in particular, is associated with increased plasma angiotensinogen levels leading to increased risk of hypertension.[50,51] Genetic variants in the adrenergic receptor genes may be also linked to the development of RH.[52] Larger studies in well-characterised individuals with RH are needed to clarify these aspects.
Clinical Implications
Patients with RH have an increased risk of developing HMOD, CKD and premature CV events.[17,18,53–55] In a retrospective study including more than 200,000 patients with hypertension, RH was associated with higher rates of MI, heart failure, stroke, or death over a median of 3.8 years. Differences in CV events were mainly driven by an increased risk of CKD development in patients with RH compared with those with hypertension responsive to treatment.[54] Similar findings were reported by another observational study including over 400,000 subjects.[6] Prospective studies using ambulatory BP monitoring (ABPM) also suggested an increased risk of CV events in patients with RH.[56,57]
In the REGARDS study, uncontrolled aRH was associated with increased risk of coronary heart disease (HR 2.33; 95% CI [1.21–4.48], but not stroke (HR 1.05; 95% CI [0.61–1.81]) or all-cause mortality (HR 1.15; 95% CI [0.91– 1.45]).[58] Interestingly, another study of more than 40,000 patients with RH found that BP control significantly reduced rates of stroke and coronary artery disease, albeit to a lesser extent than in subjects without RH.[59] Compared to patients with hypertension controlled by treatment, patients with uncontrolled RH may, therefore, have worse outcomes regardless of BP control and require closer medical monitoring.
In this regard, patients with refractory hypertension may be at particularly high risk for long-term adverse events. In the CRIC study, despite no significant differences in all-cause mortality, patients with refractory hypertension had a significantly higher risk of CV and renal outcomes than subjects with RH.[60]
Diagnostic Work-up: Ruling out Apparent Resistant and Secondary Hypertension
The diagnostic work-up for patients with suspected RH is shown in Figure 2.
Figure 2: Management of Suspected Resistant Hypertension.
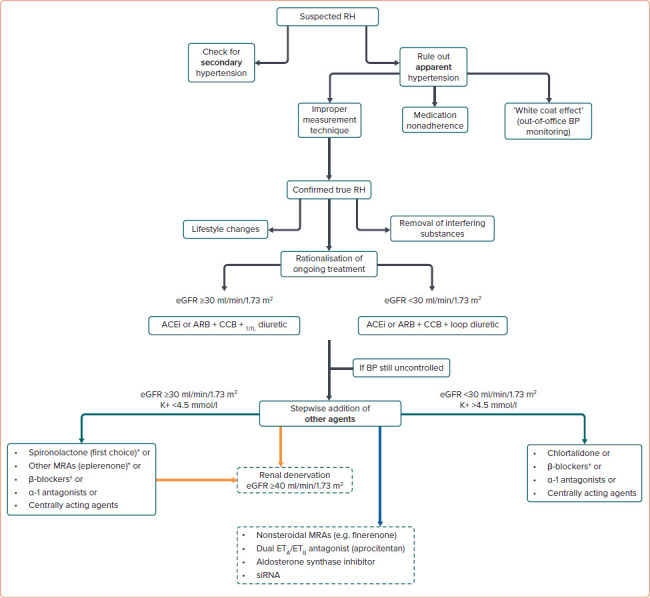
*Caution if eGFR is 30–45 ml/min/1.73 m2. †Can be used earlier at any step if indicated. Current (green arrows), emerging (orange arrows), and potential (blue arrow) pharmacological and non-pharmacological strategies for RH treatment. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; CCB = calcium channel blocker; eGFR = estimated glomerular filtration rate; MRA = mineralocorticoid receptor antagonist; RH = resistant hypertension; siRNA = short interfering RNA; T/TL = thiazide/thiazide-like.
Secondary causes of hypertension must be checked, according to the patient’s medical history and physical examination. Appropriate laboratory or imaging testing may be also warranted, according to the most recent ESH recommendations.[2] Secondary causes account for 5–10% of hypertension cases, particularly primary aldosteronism and atherosclerotic renal artery stenosis.[22,61]
Primary aldosteronism is the most common endocrine cause of secondary hypertension in patients with suspected RH. In a sub-study of PATHWAY-2 trial, a randomised, double-blind crossover trial on patients with RH treated with different classes of antihypertensive agents, including spironolactone, 25% ofthe patients were deemed to have inappropriately high aldosterone concentrations, suggesting that aldosteronism probably goes underdiagnosed in many cases of RH.[62] Atherosclerotic renal artery stenosis is the most common cause of renovascular hypertension. In case of clinical suspicion, renal artery Doppler ultrasound should be performed as first-line imaging, followed by MRI and/or CT angiography to confirm the diagnosis.[63]
Another crucial step is to rule out aRH by excluding improper measurement techniques, medication nonadherence and the ‘white-coat effect’. The patient’s history must be carefully recorded, including lifestyle features, alcohol and dietary sodium intake, concomitant drugs and sleep history. The presence of OSA should be checked, because this condition is frequently involved in the development of hypertension. To estimate the OSA risk, several tools are currently available, including the Berlin Questionnaire, Epworth Sleepiness Scale and the STOP-Bang Questionnaire.[64] The disease should be then confirmed with appropriate tests like in-lab attended sleep study or polysomnography. The nature and dosing of the antihypertensive agents used should be assessed. To confirm RH, drugs should be prescribed above their starting dose and not necessarily at the maximum recommended dose. Once the diagnosis of tRH is confirmed, the patient should be carefully evaluated to identify associated risk factors and HMOD presence.[2]
Errors in BP measurement can account for RH misdiagnosis. Therefore, proper patient preparation, adequate environmental conditions, use of an appropriately sized cuff and the technique of BP measurement are crucial aspects to consider. Accurate BP measurement, both at home and in the office, requires an intentional approach to measurement and some level of training. The use of a standardised office BP methodology is of utmost importance to avoid errors. A recent ESH statement provides recommendations for proper measurement, patient position, device, measurement schedule and interpretation of office BP results.[65] Spurious BP increase associated with brachial artery calcification should be ruled out in this setting, particularly in the elderly and in patients with advanced CKD.[2]
Failure to identify medication nonadherence contributes to overestimating the prevalence of tRH and may have important consequences in clinical research. A recent post hoc analysis by Kario et al. demonstrated that almost half of the participants with RH included in the REQUIRE study exhibited poor adherence to medical treatment.[66] Among these subjects, those receiving catheter-based ultrasound renal denervation displayed unchanged adherence over time, whereas subjects undergoing sham procedure showed a trend towards increased adherence and a significant BP reduction during the study course, possibly explaining the negative findings of the study.[66]
In a meta-analysis of 24 studies including patients with aRH, the mean prevalence of nonadherence was 31%, ranging from 3% to 86%.[67] Similar results were provided by another meta-analysis by Bourque et al.[68] Several direct and indirect tools are currently available, although there is no gold standard.[69] In general, direct methods like witnessed drug intake, the Medication Event Monitoring System and drug screening of urine or blood are much more effective, whereas indirect methods such as questionnaires or pill counts proved to be unreliable.[70]
Pharmacodynamic markers of exposure to drugs, such as bradycardia occurrence on β-blockers, increased urine N-acetyl-seryl-aspartyl-lysyl-proline concentration on angiotensin-converting enzyme inhibitor, increased circulating levels of uric acid on diuretics and renin concentration on diuretics or RAAS blockers, as well as drug-related side-effects, may have limited specificity in this setting.[71] As adherence fluctuates over time and tends to decrease progressively, repeated adherence measurements should be considered.[72]
Out-of-office BP monitoring is generally required to exclude the whitecoat effect and confirm the diagnosis of RH. The white-coat effect is defined as office BP above the target in a patient on at least three antihypertensive drugs but with appropriate levels of out-of-office BP. The latter is measured with 24-hour ABPM. APBM is not only a fundamental tool for diagnosing RH, but it may also play a role in predicting future CV events in subjects with RH.[73] When ABPM is not readily available, home BP monitoring can be considered.[2] Self-measured home SBP has been found to correlate with average daytime SBP assessed by ABPM.[8]
Treatment Options for Resistant Hypertension
Once the diagnosis is established, RH management remains challenging. Effective treatments should combine lifestyle changes and removal of interfering substances, optimisation of ongoing treatment and a sequential introduction of antihypertensive drugs on top of triple therapy.
Further options, such as renal artery denervation, may be considered in selected cases.[2] Given the association with various comorbidities and the need for multiple and complex drug regimes, patients with RH should be referred to a hypertension specialist or even to a specialised hypertension centre if necessary. A dedicated follow-up program is mandatory.[2]
Lifestyle Changes
Growing evidence supports the role of lifestyle changes in RH management. In a recent network meta-analysis, lifestyle interventions were the most effective non-pharmacological treatment in the setting of RH, lowering office SBP by -7.26 mmHg (95% CI [-13.73, -0.8]).
Dietary Approaches to Stop Hypertension (DASH) is a dietary intervention that emphasises the consumption of whole grains, vegetables, fruits and low-fat dairy products, while limiting the intake of saturated fats, processed foods and added sugars. In several trials, this strategy has been shown to be effective in reducing BP in patients with RH.[74] Similar benefits have been observed with a Mediterranean diet.[75]
High sodium intake increases the risk of treatment-RH.[76] However, direct evidence of the benefits of sodium restriction in the RH population is scarce. In a study including 15 patients with RH, self-performed dietary sodium restriction led to a significant reduction in BP and urinary sodium excretion after 2 weeks.[77] In a previous RCT, a low-salt diet reduced office SBP and DBP by 22.7 and 9.1 mmHg, respectively, though the small sample size, the unblinded administration of salt diets and the short duration of dieting periods strongly limit the interpretability of such results.[78]
Regular physical activity improves CV health by reducing inflammation and improving lipid profile.[79] In the setting of RH, both aerobic and resistance physical activity have been found to reduce SBP and DBP.[30,79] In a study of 53 patients with RH, a 12-week program of moderate aerobic exercise reduced ambulatory SBP by 7.1 mmHg compared with usual care.[31] In the TRIUMPH study, a comprehensive lifestyle intervention in which participants received instruction from a nutritionist and exercise in cardiac rehabilitation facilities reduced both office BP and ABPM values while improving biomarkers of CV disease and psychological functioning, compared with standard care.[80,81] In the EnRicH trial, a 12-week moderateintensity aerobic exercise training program led to ambulatory SBP and DBP reduction by 7.1 and 5.7 mmHg, respectively.[31] Compared with usual care, this strategy also lowered central BP while improving angiotensin II and superoxide dismutase circulating levels.[82]
Evidence on the effects of weight loss in patients with RH remains limited. In patients with obesity, glucagon-like peptide-1 agonists modestly lower BP and improve CV risk profile in patients with diabetes or established CV disease.[83–85] In a sub-analysis of the GATEWAY trial, including patients with hypertension and a BMI between 30 and 39.9 kg/m2, RH prevalence significantly decreased following randomisation to bariatric surgery.[86] Interestingly, postoperative BP reduction preceded weight loss in treated patients, suggesting that further changes are responsible for these benefits including improvements in sympathetic overactivity, sodium and water homeostasis and inflammation.[87]
No data are currently available on the effects of avoiding smoking in RH.[88]
In patients with RH and OSA, continuous positive airway pressure (CPAP) therapy has been demonstrated to decrease BP.[37] In a Spanish trial, subjects who benefited most from CPAP treatment were those with uncontrolled baseline BP and more severe OSA-related nocturnal hypoxia.[89] A subsequent sub-analysis of the same study found that reduction in 24-hour BP levels was significant only in patients with a rising profile and no-dipping pattern.[90]
Pharmacological Strategies
According to the latest ESH guidelines, effective pharmacological treatment of RH should combine rationalisation of current medications and the sequential addition of antihypertensive agent to the existing triple therapy.[2] The suggested therapeutic approach for patients with confirmed RH is shown in Figure 2.
To improve ongoing treatment strategies, combination therapies should be used at optimal or maximally tolerated doses while considering patient’s age, indications for specific drug classes, existing comorbidities and risks of drug–drug interactions. Single pill combinations should be preferred to reduce pill burden and improve medication adherence. Increasing the intensity of diuretic therapy may be necessary, particularly in the elderly, in subjects of black African origin or with CKD. In patients with an estimated glomerular filtration rate (eGFR) ≥45 ml/min/1.73 m2 receiving hydrochlorothiazide, the drug dosage can be increased. As hydrochlorothiazide loses its ability to induce predictable natriuresis when eGFR is <45 ml/min/1.73 m2, the drug can be switched to a longer-acting thiazide-like diuretic (e.g. indapamide or chlorthalidone) in such cases. If eGFR is <30 ml/min/1.73 m2, indapamide should not be used, whereas chlorthalidone doses may need to be increased to 50 mg daily. If this strategy is not sufficient, thiazide diuretics may be replaced by loop diuretics (e.g. furosemide, bumetanide, or torsemide).[2] Of note, in a recent RCT on patients with stage 4 CKD (with a mean eGFR of 23.2 ± 4.2 ml/min/1.73 m2), a mean reduction in ambulatory SBP of 10.5 mmHg was reported in patients randomised to chlorthalidone compared with placebo, supporting the efficacy of this drug in severe CKD.[91]
After optimising the ongoing therapy, a stepwise addition of other agents should be considered if BP is still not at goal. Based on the results of the PATHWAY-2 trial and other studies, patients with RH should receive the MRA spironolactone 25–50 mg/day as fourth-line treatment.[92–94] In the ReHOT trial, which randomised spironolactone versus clonidine as the fourth drug in patients with RH, patients on spironolactone displayed a larger decrease in 24 hours SBP and DBP.[95] In a recent network metaanalysis including 24 studies and about 3,000 individuals, among all comparators, spironolactone was the most effective treatment to reduce office SBP (-13.30 mmHg) and 24 hours SBP (-8.46 mmHg) in patients with RH.[96]
Real-world evidence from the US comprising more than 80,000 patients with RH found a non-significant reduction in the rates of stroke and MI in patients taking spironolactone compared to β-blockers as fourth-line treatment, though the risk of hyperkalaemia and kidney function worsening was significantly higher with the former.[97] The risk of hyperkalaemia with spironolactone is a cause for concern, particularly in patients with CKD taking RAAS blockers. According to current guidelines, spironolactone should be used with caution in patients with an eGFR <45 ml/min/1.73 m2 when plasma potassium concentration is >4.5 mmol/l, as these were exclusion criteria in the PATHWAY-2 trial. In patients with heart failure, particularly those with reduced ejection fraction, the drug is recommended in light of the well-established benefits of spironolactone in this clinical setting, although contraindicated with an eGFR <30 ml/ min/1.73 m2.[2,98]
After treatment initiation, plasma potassium and eGFR must be closely monitored, at least annually or at 3 to 6-month intervals thereafter.[2] Given its affinity for other receptors, such as the progesterone and androgen receptors, spironolactone may also be associated with antiandrogenic side-effects, resulting in gynecomastia, breast tenderness and sexual impotence in men and menstrual abnormalities in women.[99] The overall suboptimal tolerability profile of spironolactone may explain its low prescription rate and the low persistence on treatment in real-life settings.
Eplerenone is a more selective MRA that can be used as an alternative to lower BP. However, it is less potent than spironolactone, whereas its cost remains a significant barrier to its widespread use in clinical practice.[100] More selective nonsteroidal MRAs, such as finerenone (approved for the treatment of patients with diabetic CKD), esaxerenone (approved for the treatment of hypertension in Japan) and ocedurenone (undergoing clinical trials for the treatment of RH in patients with CKD) might provide further alternatives to spironolactone in the future.[100,101]
When spironolactone and other MRAs are not tolerated or contraindicated, alternatives include amiloride, which was found to be as effective as spironolactone in reducing BP when used at high dosages (10–20 mg/day), β-blockers like bisoprolol 5–10 mg/day, α-1 antagonists such as doxazosin extended release 4–8 mg/day, and centrally acting agents such as the α-adrenergic receptor agonist clonidine 0.1–0.3 mg twice daily.[62]
In the ReHOT trial, clonidine showed a similar reduction in office and ambulatory BP compared to spironolactone.[95] Conversely, in the PATHWAY-2 trial, bisoprolol and doxazosin reduced BP less effectively than spironolactone, although the difference was small.[92] α-1 antagonists are associated with an increased risk of orthostatic hypotension, syncope, falls and fractures.[102] Furthermore, although controversial, in the ALLHAT trial, doxazosin use was associated with a higher incidence of heart failure compared to chlorthalidone, leading to premature discontinuation of this treatment arm.[103] Clonidine use is also associated with several sideeffects, such as dry mouth, sedation and dizziness.[104] Moreover, the risk of rebound hypertension after drug discontinuation or during periods of nonadherence is a cause for concern. Using a sustained release transdermal formulation may attenuate some of these issues.
Of note, the abrupt cessation/withdrawal of β-blockers, which are used more extensively than clonidine in the hypertensive population, is associated with an even more critical risk of rebound hypertension. This aspect must be carefully considered when prescribing these drugs.
Direct vasodilators, such as hydralazine or minoxidil, should be used with caution, as they may cause severe fluid retention and induce tachycardia through reflex sympathetic activation.[2]
In patients eligible for treatment with sodium-glucose cotransporter 2 inhibitors (SGLT2I), using these agents on top of antihypertensive therapy may have an additional moderate BP-lowering effect.[105] In a post hoc analysis of the EMPA-REG OUTCOME trial, SBP was more frequently controlled with empagliflozin compared to placebo at week 12 in patients with presumed RH (38% versus 26%, respectively), with a mean difference in SBP change of -4.5 (95% CI [-5.9, -3.1]) mmHg between groups. Furthermore, this drug significantly reduced the risk of heart failure leading to hospitalisation, worsening renal function and CV death, both in patients with and without presumed RH.[106] Similar data were found with canagliflozin.[107] However, it should be noted that the above analyses were not prespecified to assess such outcomes and there is insufficient information to determine whether and how many patients with presumed RH had true RH in the studies.
Lastly, in a post hoc analysis of the PARAGON HF trial, sacubitril/valsartan combination reduced BP in patients with RH, despite treatment with at least four antihypertensive drugs including an MRA.[63] Such findings were also reported in a phase II trial of patients with hypertension.[108] However, no data are currently available from clinical studies conducted specifically on patients with RH.
Future Pharmacological Approaches
Several novel antihypertensive agents are being explored for the treatment of RH (Table 1). Endothelin has been implicated in the pathophysiology of hypertension for several years and endothelin receptor antagonists have become an integral part of the treatment of pulmonary arterial hypertension.[109] Aprocitentan is a dual endothelin antagonist directed against endothelin-1 receptors ETA and ETB that may have a future role in RH management, particularly in patients at high risk of hyperkalaemia and renal failure with MRAs.
Table 1: Main Trials on Classical and Novel Pharmacological Agents as Fourth-line Treatment in Patients with Resistant Hypertension.
| Drug Class | Trial Name and Phase | Drug(s) and Comparator(s) | Trial Duration (Weeks) | Main Endpoint | Main Results | Reported Side-effects |
|---|---|---|---|---|---|---|
| MRAs | PATHWAY-2[92] Phase IIII | Spironolactone 25–50 mg versus bisoprolol 5–10 mg versus doxazosin modified release (4–8 mg) versus placebo | 12 | Office SBP change | Spironolactone versus placebo -8.70 mmHg (95% CI [-9.72, -7.69]; p<0.0001), versus doxazosin and bisoprolol -4.26 [-5.13, -3.38]; p<0.0001), and versus doxazosin -4.03 [-5.04, -3.02]; p<0.0001) and versus bisoprolol -4.48 [-5.50, -3.46]; p<0.0001) | Hyperkalaemia (particularly when combined with ACEI/ARB) Anti-androgen effects (gynaecomastia, erectile dysfunction in men; menstrual irregularities in women) |
| Centrally acting drugs | FRESH Phase III | Firibastat 500 mg twice daily versus placebo | 12 | Office SBP change | Firibistat -7.82 mmHg; placebo -7.85 mmHg | Skin allergic reactions: firibistat 5.1%; placebo 0.004% |
| Dual endothelin receptor antagonists | PRECISION[110] Phase III | Aprocitentan 12.5 or 25 mg for 4 weeks → 25 mg for 32 weeks versus placebo | 36 | Office SBP change | Aprocitentan -3.7 (SE 1.3) mmHg (97.5% CI [-6.8, -0.8]; p=0.0042) versus placebo both at the 12.5 and 25 mg doses | Mild to moderate fluid retention in the aprocitentan 25 mg arm leading 7 patients to discontinue treatment |
| Inhibitors of aldosterone synthase | BrigHTN[113] Phase II | Baxdrostat 0.5, 1, or 2 mg versus placebo | 12 | Office SBP change | Baxdrostat 0.5 mg -12.1 mmHg; baxdrostat 1.0 mg -17.5 mmHg; baxdrostat 2 mg -20.3 mmHg; placebo -9.4 mmHg | Hyperkalaemia in 2 patients on baxdrostat (not recurring after stopping the drug) |
| Target-HTN[116] Phase II | Lorundrostat 12.5, 50, or 100 mg once daily, or 12.5 or 25 mg twice daily versus placebo | 8 | Office SBP change | Lorundrostat 100 mg once daily -14.1 mmHg; 50 mg once daily -13.2 mmHg; 25 mg twice daily -10.1 mmHg; 12.5 mg twice daily 13.8 mmHg; 12.5 mg once daily -6.9 mmHg; placebo -4.1 mmHg | Hyperkalaemia in 6 patients on lorundrostat (not recurring after stopping the drug) | |
| RNA interference therapeutic agent inhibiting hepatic angiotensinogen synthesis | KARDIO-1[117] Phase I | Zilebesiran 150, 300 or 600 mg every 6 months versus Zilebesiran 300 mg every 3 months versus placebo | 24 | Mean 24 h BP change | -14.1 mmHg with the 150 mg dose, -16.7 mmHg with the 300 mg dose, and -15.7 mmHg with the 600 mg dose | Transient local injection site reactions Transient rise in serum potassium levels |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; MRA = mineralocorticoid receptor antagonist; SBP = systolic blood pressure.
The PRECISION trial was a multicentre, blinded, randomised, parallel-group, phase III study including patients with uncontrolled RH on triple antihypertensive therapy including a diuretic randomised to aprocitentan versus placebo.[110] Compared with the latter, both 12.5 and 25 mg aprocitentan dosages significantly reduced office SBP at 4 weeks (-3.7 and -3.8 mmHg, respectively), with a sustained effect at week 40. Although well tolerated overall, mild-to-moderate oedema or fluid retention occurred in 9% and 18% of the patients receiving aprocitentan 12.5 and 25 mg, respectively, leading to drug withdrawal in some cases.[23,110]
Baxdrostat (CIN-107) is a highly selective, competitive and potent inhibitor of aldosterone synthase with no effects on the highly homologous enzyme 11β-hydroxylase (CYP11B1), which is responsible for cortisol synthesis.[111,112] The advantage of this drug resides in the possibility of reducing aldosterone synthesis instead of blocking its effects on mineralocorticoid receptors. The phase II BrigHTN showed dose-dependent changes in office SBP of -20.3, -17.5 and -12.1 mmHg in patients randomised to baxdrostat at the dose of 2, 1 and 0.5 mg, respectively. The difference in the change in office SBP observed between the 2-mg group and the placebo group was -11.0 mmHg. No serious adverse events were reported, whereas severe hyperkalaemia was attributed to baxdrostat in two patients.[113] Notably, another phase II study, the HALO trial failed to demonstrate the efficacy of baxdrostat in lowering BP in patients with uncontrolled hypertension.[114] Possible explanations for this discrepancy in terms of outcome include differences in studies duration and baseline clinical characteristics of the patients enrolled, such as BP levels, rates of uncontrolled diabetes and eGFR values.[115] The benefits and safety of baxdrostat thus need to be confirmed in phase III trials over longer periods. The drug is currently undergoing clinical trials in patients with hypertension, hypertension with CKD (NCT05432167) and primary aldosteronism (NCT04605549).
The Target-HTN study also demonstrated the efficacy of lorundrostat in reducing both BP levels and serum aldosterone independently of baseline plasma renin activity, at the higher doses tested, with a remarkable interindividual variability in the in the BP-lowering effect. Like baxdrostat, inhibition of the aldosterone synthase with lorundrostat did not modify cortisol levels.[116]
The use of short interfering RNA (siRNA) to suppress the synthesis of hepatic AGT represents a novel opportunity in the treatment of RH through improved durability and innovative targeting.[117] In the phase I/first-inhumans studies, the first-in-class AGT-targeting siRNA, zilebesiran, was well tolerated and dose-dependently reduced serum AGT levels (by >90%), with sustained effects on BP levels (reduction in 24-hour SBP >10 mmHg by week 8, and lasting 6 months at ≥200 mg dosage), without worsening of renal function.[117]
Renal Denervation
Experimental devices and other interventions are being explored in patients with RH to reduce BP, including baroreflex activation therapy, renal denervation, endovascular baroreflex amplification therapy, carotid body ablation and cardiac neuromodulation therapy. Baroreflex activation therapy, mimicking the natural baroreflex mechanism, stimulates the baroreceptors to send signals to the brain, leading to a reduction in sympathetic nervous system activity and subsequent lowering of BP.[118,119] Renal denervation involves disrupting renal sympathetic nerves to reduce their stimulatory effect on BP. By using catheter-based techniques, nerves in the renal arteries are ablated, leading to decreased sympathetic activity, lower BP, decreased renin activity and increased renal blood flow.
It is important to underline that while these interventions are promising, their effectiveness and safety may vary. Most evidence concerns renal denervation in RH, which is a primary indication for this intervention. The SYMPLICITY HTN-2 trial randomly allocated patients to undergo immediate renal denervation or continue with usual treatment with the possibility of receiving delayed procedure. The study showed that renal denervation is safe, with lasting reduction in BP to 1 year in patients with severe hypertension and SBP >160 mmHg.[120] However, SYMPLICITY HTN-3, the first randomised, sham-controlled trial, failed to demonstrate a significant effect on BP in patients with severe RH.[121] This might be due to several factors, such as a longer history of hypertension, more severe and non-reversible HMOD and arterial stiffness, high failure rates, operator experience and medication nonadherence upon completion of the procedure.
Recent studies, particularly the SPYRAL HTN-OFF MED trial, have highlighted the effectiveness of RDN in reducing both office and 24-hour BP among patients with lower BP values without the use of medications.[122] Additionally, findings from the SPYRAL HTN-ON MED trial underscored significantly lower BP values at the 6-month mark within the renal denervation group, further emphasising its potential benefits with the improved multi-electrode radiofrequency Spyral catheter (Medtronic).[123] A parallel investigation conducted by Azizi et al. affirmed the positive impact of renal denervation in terms of ABPM by employing an innovative ultrasound catheter design and implementing a more stringent medication protocol.[124] In the RADIANCE-HTN Trio study, the authors showcased a noteworthy decrease of daytime ambulatory SBP of 5.8 mmHg in comparison to the control group, indicating a modest yet significant therapeutic advantage.[124]
Following the release of the 2018 European Society of Cardiology and ESH guidelines for the management of hypertension, multiple well-executed sham-controlled trials have been published, underscoring the safety and efficacy of renal denervation in lowering BP.[125] Therefore, renal denervation emerges as an additional viable treatment alternative for adult patients grappling with uncontrolled RH, as indicated by the latest European guidelines stating the potential utility of this strategy in the context of RH with a class II recommendation, provided that the eGFR is >40 ml/min/1.73 m2.[2]
In November 2023, the Food and Drug Administration approved Symplicity Spyral (Medtronic) and Paradise Ultrasound Renal Denervation (Recor Medical) for use in the US.[126,127] Both systems have received CE mark approval for the treatment of hypertension in Europe. RH might not be the optimal target population to validate the efficacy of renal denervation, but it does not negate the potential utility of this strategy in this context. Identifying individuals who are more likely to respond positively to renal denervation, such as those not included in RCTs because of advanced CKD, becomes crucial.[128] In addition, performing such procedures demands operators with proficiency in renal interventions and specialised training. Centres offering renal denervation should possess the capability to address any potential complications.[129]
Conclusion
In the past decade, hypertension research has significantly advanced our understanding, particularly in defining RH. Distinguishing between tRH and aRH has become crucial, as these represent distinct forms of the disease with different management strategies and prognosis. Addressing aRH primarily involves promoting adherence to drug therapy and lifestyle recommendations. While tRH constitutes a smaller percentage of the hypertensive population, certain sub-groups, like those with diabetes or CKD, are at higher risk. These patients present with increased rates of CV and renal complications, warranting aggressive medical surveillance. Spironolactone emerges as the preferred fourth-line treatment for RH.
Ongoing research on novel drugs (NCT04277884) and non-pharmacological treatments including renal denervation might provide further therapeutic options in the coming years.
References
- 1.Myat A, Redwood SR, Qureshi AC et al. Resistant hypertension. BMJ. 2012;345:e7473. doi: 10.1136/bmj.e7473. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Kreutz R, Brunstrom M et al. 2023 ESH guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41:1874–2071. doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 3.Dudenbostel T, Siddiqui M, Oparil S, Calhoun DA. Refractory hypertension: a novel phenotype of antihypertensive treatment failure. Hypertension. 2016;67:1085–92. doi: 10.1161/HYPERTENSIONAHA.116.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/ AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Hypertensio. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM, Calhoun DA, Bakris GL et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim JJ, Bhandari SK, Shi J et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622–32. doi: 10.1038/ki.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champaneria MK, Patel RS, Oroszi TL. When blood pressure refuses to budge: exploring the complexity of resistant hypertension. Front Cardiovasc Med. 2023;10:1211199. doi: 10.3389/fcvm.2023.1211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens. 2014;28:463–8. doi: 10.1038/jhh.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achelrod D, Wenzel U, Frey S. Systematic review and metaanalysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28:355–61. doi: 10.1093/ajh/hpu151. [DOI] [PubMed] [Google Scholar]
- 10.Noubiap JJ, Nansseu JR, Nyaga UF et al. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105:98–105. doi: 10.1136/heartjnl-2018-313599. [DOI] [PubMed] [Google Scholar]
- 11.Romano S, Rigon G, Albrigi M et al. Hypertension, uncontrolled hypertension and resistant hypertension: prevalence, comorbidities and prescribed medications in 228,406 adults resident in urban areas. A population-based observational study. Intern Emerg Med. 2023;18:1951–9. doi: 10.1007/s11739-023-03376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebinger JE, Kauko A. FinnGen, et al. Apparent treatmentresistant hypertension associated lifetime cardiovascular risk in a longitudinal national registry. Eur J Prev Cardiol. 2023;30:960–8. doi: 10.1093/eurjpc/zwad066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 14.Sim JJ, Bhandari SK, Shi J et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–107. doi: 10.1016/j.mayocp.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich) 2014;16:741–5. doi: 10.1111/jch.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveras A, Armario P, Hernandez-Del Rey R et al. Urinary albumin excretion is associated with true resistant hypertension. J Hum Hypertens. 2010;24:27–33. doi: 10.1038/jhh.2009.35. [DOI] [PubMed] [Google Scholar]
- 17.Cuspidi C, Macca G, Sampieri L et al. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens. 2001;19:2063–70. doi: 10.1097/00004872-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Muiesan ML, Salvetti M, Rizzoni D et al. Resistant hypertension and target organ damage. Hypertens Res. 2013;36:485–91. doi: 10.1038/hr.2013.30. [DOI] [PubMed] [Google Scholar]
- 19.Pedrosa RP, Drager LF, Gonzaga CC et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–7. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 20.Buhnerkempe MG, Botchway A, Prakash V et al. Prevalence of refractory hypertension in the United States from 1999 to 2014. J Hypertens. 2019;37:1797–804. doi: 10.1097/HJH.0000000000002103. [DOI] [PubMed] [Google Scholar]
- 21.Dudenbostel T, Acelajado MC, Pisoni R et al. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension. 2015;66:126–33. doi: 10.1161/HYPERTENSIONAHA.115.05449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–47. doi: 10.1146/annurev-med-042711-135929. [DOI] [PubMed] [Google Scholar]
- 23.Airo M, Frishman WH, Aronow WS. New therapy update aprocitentan: an endothelin receptor antagonist for the treatment of drug-resistant systemic hypertension. Cardiol Rev. 2023. [DOI] [PubMed]
- 24.Mendes M, Dubourg J, Blanchard A et al. Copeptin is increased in resistant hypertension. J Hypertens. 2016;34:2458–64. doi: 10.1097/HJH.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 25.Velasco A, Siddiqui M, Kreps E et al. Refractory hypertension is not attributable to intravascular fluid retention as determined by intracardiac volumes. Hypertension. 2018;72:343–9. doi: 10.1161/HYPERTENSIONAHA.118.10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan BM, Zhao Y, Axon RN et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–58. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landsberg L, Aronne LJ, Beilin LJ et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JE, do Carmo JM, da Silva AA et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimbo D, Levitan EB, Booth JN 3rd et al. The contributions of unhealthy lifestyle factors to apparent resistant hypertension: findings from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Hypertens. 2013;31:370–6. doi: 10.1097/HJH.0b013e32835b6be7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pires NF, Coelho-Junior HJ, Gambassi BB et al. Combined aerobic and resistance exercises evokes longer reductions on ambulatory blood pressure in resistant hypertension: a randomized crossover trial. Cardiovasc Ther. 2020;2020:8157858. doi: 10.1155/2020/8157858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes S, Mesquita-Bastos J, Garcia C et al. Effect of exercise training on ambulatory blood pressure among patients with resistant hypertension: a randomized clinical trial. JAMA Cardiol. 2021;6:1317–23. doi: 10.1001/jamacardio.2021.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Zou Y, Leng X et al. Associations of blood lead, cadmium, and mercury with resistant hypertension among adults in NHANES, 1999–2018. Environ Health Prev Med. 2023;28:66. doi: 10.1265/ehpm.23-00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angeli F, Zappa M, Reboldi G et al. The spike effect of acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 vaccines on blood pressure. Eur J Intern Med. 2023;109:12–21. doi: 10.1016/j.ejim.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buso G, Agabiti-Rosei C, Muiesan ML. The relationship between COVID-19 vaccines and increased blood pressure: a word of caution. Eur J Intern Med. 2023;111:27–9. doi: 10.1016/j.ejim.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27:1235–42. doi: 10.1093/ajh/hpu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Garcia MA, Navarro-Soriano C, Torres G et al. Beyond resistant hypertension. Hypertension. 2018;72:618–24. doi: 10.1161/HYPERTENSIONAHA.118.11170. [DOI] [PubMed] [Google Scholar]
- 37.Jehan S, Zizi F, Pandi-Perumal SR et al. Obstructive sleep apnea, hypertension, resistant hypertension and cardiovascular disease. Sleep Med Disord. 2020;4:67–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrini ML, Macedo TA, Castro E et al. Obstructive sleep apnea and hypertension-mediated organ damage in nonresistant and resistant hypertension. Hypertens Res. 2023;46:2033–43. doi: 10.1038/s41440-023-01320-z. [DOI] [PubMed] [Google Scholar]
- 39.Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371:270–3. doi: 10.1016/S0140-6736(08)60137-3. [DOI] [PubMed] [Google Scholar]
- 40.Prentice RL. On the ability of blood pressure effects to explain the relation between oral contraceptives and cardiovascular disease. Am J Epidemiol. 1988;127:213–9. doi: 10.1093/oxfordjournals.aje.a114797. [DOI] [PubMed] [Google Scholar]
- 41.Hoorn EJ, Walsh SB, McCormick JA et al. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. 2012;25:269–75. doi: 10.5301/jn.5000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Kroetz DL. Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol Ther. 2018;182:152–60. doi: 10.1016/j.pharmthera.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costello RE, Yimer BB, Roads P et al. Glucocorticoid use is associated with an increased risk of hypertension. Rheumatol. 2021;60:132–9. doi: 10.1093/rheumatology/keaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thase ME. Effects of venlafaxine on blood pressure: a metaanalysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;59:502–8. doi: 10.4088/jcp.v59n1002. [DOI] [PubMed] [Google Scholar]
- 45.Yugar-Toledo JC, Martin JFV, Krieger JE et al. Gene variation in resistant hypertension: multilocus analysis of the angiotensin 1-converting enzyme, angiotensinogen, and endothelial nitric oxide synthase genes. DNA Cell Biol. 2011;30:555–64. doi: 10.1089/dna.2010.1156. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira-Paula GH, Lacchini R, Coeli-Lacchini FB et al. Inducible nitric oxide synthase haplotype associated with hypertension and responsiveness to antihypertensive drug therapy. Gene. 2013;515:391–5. doi: 10.1016/j.gene.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 47.Lynch AI, Irvin MR, Davis BR et al. Genetic and adverse health outcome associations with treatment resistant hypertension in GenHAT. Int J Hypertens. 2013;2013:578578. doi: 10.1155/2013/578578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontana V, McDonough CW, Gong Y et al. Large-scale gene-centric analysis identifies polymorphisms for resistant hypertension. J Am Heart Assoc. 2014;3:e001398. doi: 10.1161/JAHA.114.001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton-Cheh C, Larson MG, Vasan RS et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hingorani AD, Sharma P, Jia H et al. Blood pressure and the M235T polymorphism of the angiotensinogen gene. Hypertension. 1996;28:907–11. doi: 10.1161/01.hyp.28.5.907. [DOI] [PubMed] [Google Scholar]
- 51.Kolovou V, Lagou E, Mihas C et al. Angiotensinogen (AGT) M235T, AGT T174M and angiotensin-1-converting enzyme (ACE) I/D gene polymorphisms in essential hypertension: effects on ramipril efficacy. Open Cardiovasc Med J. 2015;9:118–26. doi: 10.2174/1874192401509010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wain LV, Verwoert GC, O’Reilly PF et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–11. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Nicola L, Gabbai FB, Agarwal R et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61:2461–7. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 54.Daugherty SL, Powers JD, Magid DJ et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–42. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kario K, Hoshide S, Narita K et al. Cardiovascular prognosis in drug-resistant hypertension stratified by 24-hour ambulatory blood pressure: the JAMP study. Hypertension. 2021;78:1781–90. doi: 10.1161/HYPERTENSIONAHA.121.18198. [DOI] [PubMed] [Google Scholar]
- 56.Tsioufis C, Kasiakogias A, Kordalis A et al. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: a 4-year prospective study. J Hypertens. 2014;32:415–22. doi: 10.1097/HJH.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 57.Pierdomenico SD, Lapenna D, Bucci A et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–8. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Irvin MR, Booth JN 3rd, Shimbo D et al. Apparent treatmentresistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–13. doi: 10.1016/j.jash.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egan BM, Kai B, Wagner CS et al. Blood pressure control provides less cardiovascular protection in adults with than without apparent treatment-resistant hypertension. J Clin Hypertens (Greenwich) 2016;18:817–24. doi: 10.1111/jch.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhnerkempe MG, Prakash V, Botchway A et al. Adverse health outcomes associated with refractory and treatmentresistant hypertension in the chronic renal insufficiency cohort. Hypertension. 2021;77:72–81. doi: 10.1161/HYPERTENSIONAHA.120.15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doroszko A, Janus A, Szahidewicz-Krupska E et al. Resistant hypertension. Adv Clin Exp Med. 2016;25:173–83. doi: 10.17219/acem/58998. [DOI] [PubMed] [Google Scholar]
- 62.Williams B, MacDonald TM, Morant SV et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–75. doi: 10.1016/S2213-8587(18)30071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aboyans V, Ricco JB, Bartelink MEL et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 64.Sarathy H, Salman LA, Lee C, Cohen JB. Evaluation and management of secondary hypertension. Med Clin North Am. 2022;106:269–83. doi: 10.1016/j.mcna.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stergiou GS, Palatini P, Parati G et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–302. doi: 10.1097/HJH.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 66.Kario K, Kai H, Nanto S, Yokoi H. Anti-hypertensive medication adherence in the REQUIRE trial: post-hoc exploratory evaluation. Hypertens Res. 2023;46:2044–7. doi: 10.1038/s41440-023-01333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durand H, Hayes P, Morrissey EC et al. Medication adherence among patients with apparent treatmentresistant hypertension: systematic review and metaanalysis. J Hypertens. 2017;35:2346–57. doi: 10.1097/HJH.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 68.Bourque G, Ilin JV, Ruzicka M et al. Nonadherence is common in patients with apparent resistant hypertension: a systematic review and meta-analysis. Am J Hypertens. 2023;36:394–403. doi: 10.1093/ajh/hpad013. [DOI] [PubMed] [Google Scholar]
- 69.Kably B, Billaud EM, Boutouyrie P, Azizi M. Is there any hope for monitoring adherence in an efficient and feasible way for resistant hypertension diagnosis and follow-up? Curr Hypertens Rep. 2020;22:96. doi: 10.1007/s11906-020-01105-6. [DOI] [PubMed] [Google Scholar]
- 70.Lane D, Lawson A, Burns A et al. Nonadherence in hypertension: how to develop and implement chemical adherence testing. Hypertension. 2022;79:12–23. doi: 10.1161/HYPERTENSIONAHA.121.17596. [DOI] [PubMed] [Google Scholar]
- 71.Kably B, Billaud EM, Derobertmasure A et al. Urine N-acetyl-Ser-Asp-Lys-Pro measurement as a versatile biomarker to assess adherence to angiotensin-converting enzyme inhibitors. J Hypertens. 2022;40:348–55. doi: 10.1097/HJH.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 72.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 73.Mesquita Bastos J, Ferraz L, Pereira FG, Lopes S. Systolic blood pressure and pulse pressure are predictors of future cardiovascular events in patients with true resistant hypertension. Diagnostics (Basel) 2023;13:1837. doi: 10.3390/diagnostics13101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–61. doi: 10.1016/j.numecd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Toledo E, Hu FB, Estruch R et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11:207. doi: 10.1186/1741-7015-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimosawa T. Salt, the renin-angiotensin-aldosterone system and resistant hypertension. Hypertens Res. 2013;36:657–60. doi: 10.1038/hr.2013.69. [DOI] [PubMed] [Google Scholar]
- 77.Hornstrup BG, Hoffmann-Petersen N, Lauridsen TG, Bech JN. Dietary sodium restriction reduces blood pressure in patients with treatment resistant hypertension. BMC Nephrol. 2023;24:274. doi: 10.1186/s12882-023-03333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pimenta E, Gaddam KK, Oparil S et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–81. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pimenta E, Gaddam KK, Oparil S. Mechanisms and treatment of resistant hypertension. J Clin Hypertens. 2008;10:239–44. doi: 10.1111/j.1751-7176.2008.08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blumenthal JA, Hinderliter AL, Smith PJ et al. Effects of lifestyle modification on patients with resistant hypertension: results of the TRIUMPH randomized clinical trial. Circulation. 2021;144:1212–26. doi: 10.1161/CIRCULATIONAHA.121.055329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blumenthal JA, Smith PJ, Mabe S et al. Effects of lifestyle modification on psychosocial function in patients with resistant hypertension: secondary outcomes from the TRIUMPH randomized clinical trial. J Cardiopulm Rehabil Prev. 2024;44:64–70. doi: 10.1097/HCR.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopes S, Mesquita-Bastos J, Garcia C et al. Aerobic exercise improves central blood pressure and blood pressure variability among patients with resistant hypertension: results of the EnRicH trial. Hypertens Res. 2023;46:1547–57. doi: 10.1038/s41440-023-01229-7. [DOI] [PubMed] [Google Scholar]
- 83.Ferdinand KC, White WB, Calhoun DA et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–7. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 84.Maringwa J, Sardu ML, Hang Y et al. Characterizing effects of antidiabetic drugs on heart rate, systolic and diastolic blood pressure. Clin Pharmacol Ther. 2021;109:1583–92. doi: 10.1002/cpt.2130. [DOI] [PubMed] [Google Scholar]
- 85.Giugliano D, Scappaticcio L, Longo M et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. doi: 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schiavon CA, Ikeoka D, Santucci EV et al. Effects of bariatric surgery versus medical therapy on the 24-hour ambulatory blood pressure and the prevalence of resistant hypertension. Hypertension. 2019;73:571–7. doi: 10.1161/HYPERTENSIONAHA.118.12290. [DOI] [PubMed] [Google Scholar]
- 87.Pareek M, Bhatt DL, Schiavon CA, Schauer PR. Metabolic surgery for hypertension in patients with obesity. Circ Res. 2019;124:1009–24. doi: 10.1161/CIRCRESAHA.118.313320. [DOI] [PubMed] [Google Scholar]
- 88.Ozemek C, Tiwari S, Sabbahi A et al. Impact of therapeutic lifestyle changes in resistant hypertension. Prog Cardiovasc Dis. 2020;63:4–9. doi: 10.1016/j.pcad.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez-Garcia MA, Pengo MF. Clinical phenotype of resistant hypertension responders to continuous positive airway pressure treatment: results from the HIPARCO randomized clinical trial. Hypertension. 2021;78:559–61. doi: 10.1161/HYPERTENSIONAHA.121.17364. [DOI] [PubMed] [Google Scholar]
- 90.Pengo MF, Oscullo G, Gomez-Olivas JD et al. Nocturnal BP profile predicts CPAP effect on BP in patients with OSA and resistant hypertension. Chest. 2023;164:1302–4. doi: 10.1016/j.chest.2023.05.021. [DOI] [PubMed] [Google Scholar]
- 91.Agarwal R, Sinha AD, Cramer AE et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385:2507–19. doi: 10.1056/NEJMoa2110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams B, MacDonald TM, Morant S et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsujimoto T, Kajio H. Spironolactone use and improved outcomes in patients with heart failure with preserved ejection fraction with resistant hypertension. J Am Heart Assoc. 2020;9:e018827. doi: 10.1161/JAHA.120.018827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen C, Zhu XY, Li D et al. Clinical efficacy and safety of spironolactone in patients with resistant hypertension: a systematic review and meta-analysis. Med. 2020;99:e21694. doi: 10.1097/MD.0000000000021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krieger EM, Drager LF, Giorgi DMA et al. Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension: the ReHOT randomized study (Resistant Hypertension Optimal Treatment). Hypertension. 2018;71:681–90. doi: 10.1161/HYPERTENSIONAHA.117.10662. [DOI] [PubMed] [Google Scholar]
- 96.Tian Z, Vollmer Barbosa C, Lang H et al. Efficacy of pharmacological and interventional treatment for resistant hypertension – a network meta-analysis. Cardiovasc Res. 2024;120:108–19. doi: 10.1093/cvr/cvad165. [DOI] [PubMed] [Google Scholar]
- 97.Desai R, Park H, Brown JD et al. Comparative safety and effectiveness of aldosterone antagonists versus betablockers as fourth agents in patients with apparent resistant hypertension. Hypertension. 2022;79:2305–15. doi: 10.1161/HYPERTENSIONAHA.122.19280. [DOI] [PubMed] [Google Scholar]
- 98.McDonagh TA, Metra M, Adamo M et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–39. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 99.Manolis AA, Manolis TA, Melita H, Manolis AS. Eplerenone versus spironolactone in resistant hypertension: an efficacy and/or cost or just a men’s issue? Curr Hypertens Rep. 2019;21:22. doi: 10.1007/s11906-019-0924-0. [DOI] [PubMed] [Google Scholar]
- 100.Agarwal R, Kolkhof P, Bakris G et al. Steroidal and nonsteroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–61. doi: 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bakris G, Pergola PE, Delgado B et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD study. Hypertension. 2021;78:74–81. doi: 10.1161/HYPERTENSIONAHA.121.17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hiremath S, Ruzicka M, Petrcich W et al. Alpha-blocker use and the risk of hypotension and hypotension-related clinical events in women of advanced age. Hypertension. 2019;74:645–51. doi: 10.1161/HYPERTENSIONAHA.119.13289. [DOI] [PubMed] [Google Scholar]
- 103.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 104.McComb MN, Chao JY, Ng TMH. Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther. 2016;21:3–19. doi: 10.1177/1074248415587969. [DOI] [PubMed] [Google Scholar]
- 105.Georgianos PI, Agarwal R. Ambulatory blood pressure reduction with SGLT-2 inhibitors: dose-response metaanalysis and comparative evaluation with low-dose hydrochlorothiazide. Diabetes Care. 2019;42:693–700. doi: 10.2337/dc18-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreira JP, Fitchett D, Ofstad AP et al. Empagliflozin for patients with presumed resistant hypertension: A post hoc analysis of the EMPA-REG OUTCOME trial. Am J Hypertens. 2020;33:1092–101. doi: 10.1093/ajh/hpaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye N, Jardine MJ, Oshima M et al. Blood pressure effects of canagliflozin and clinical outcomes in type 2 diabetes and chronic kidney disease: insights from the CREDENCE trial. Circulation. 2021;143:1735–49. doi: 10.1161/CIRCULATIONAHA.120.048740. [DOI] [PubMed] [Google Scholar]
- 108.Ruilope LM, Dukat A, Bohm M et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–66. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 109.Dhaun N, Goddard J, Kohan DE et al. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–9. doi: 10.1161/HYPERTENSIONAHA.108.117366. [DOI] [PubMed] [Google Scholar]
- 110.Schlaich MP, Bellet M, Weber MA et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400:1927–37. doi: 10.1016/S0140-6736(22)02034-7. [DOI] [PubMed] [Google Scholar]
- 111.Bogman K, Schwab D, Delporte ML et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension. 2017;69:189–96. doi: 10.1161/HYPERTENSIONAHA.116.07716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin RE, Aebi JD, Hornsperger B et al. Discovery of 4-aryl-5,6,7,8-tetrahydroisoquinolines as potent, selective, and orally active aldosterone synthase (CYP11B2) inhibitors: in vivo evaluation in rodents and cynomolgus monkeys. J Med Chem. 2015;58:8054–65. doi: 10.1021/acs.jmedchem.5b00851. [DOI] [PubMed] [Google Scholar]
- 113.Freeman MW, Halvorsen YD, Marshall W et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405. doi: 10.1056/NEJMoa2213169. [DOI] [PubMed] [Google Scholar]
- 114.Kumbhani DJ. Efficacy and safety of baxdrostat in patients with uncontrolled hypertension – HALO. 2023. https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2023/03/01/23/34/halo (accessed 12 December 2023)
- 115.Dey S, Frishman WH, Aronow WS. Baxdrostat: an aldosterone synthase inhibitor for the treatment of systemic hypertension. Cardiol Rev 2023. [DOI] [PubMed]
- 116.Laffin LJ, Rodman D, Luther JM et al. Aldosterone synthase inhibition with lorundrostat for uncontrolled hypertension: the Target-HTN randomized clinical trial. JAMA. 2023;330:1140–50. doi: 10.1001/jama.2023.16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Desai AS, Webb DJ, Taubel J et al. Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med. 2023;389:228–38. doi: 10.1056/NEJMoa2208391. [DOI] [PubMed] [Google Scholar]
- 118.Narkiewicz K, Ratcliffe LEK, Hart EC et al. Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci. 2016;1:313–24. doi: 10.1016/j.jacbts.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallbach M, Lehnig LY, Schroer C et al. Effects of baroreflex activation therapy on ambulatory blood pressure in patients with resistant hypertension. Hypertension. 2016;67:701–9. doi: 10.1161/hypertensionaha.115.06717. [DOI] [PubMed] [Google Scholar]
- 120.Esler MD, Krum H, Schlaich M et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–82. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 121.Bhatt DL, Kandzari DE, O’Neill WW et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 122.Townsend RR, Mahfoud F, Kandzari DE et al. Catheterbased renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 123.Kandzari DE, Bohm M, Mahfoud F et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 124.Azizi M, Schmieder RE, Mahfoud F et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, singleblind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 125.Williams B, Mancia G, Spiering W et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 126.Palo Alto, CA, US: Recor Medical, 7 November 2023.: Recor Medical. Recor Medical and Otsuka Medical Devices announce first FDA-approved renal denervation system for the treatment of hypertension.https://www.recormedical.com/recor-medical-and-otsuka-medical-devices-announce-first-fda-approved-renal-denervation-system-for-the-treatment-of-hypertension/ (accessed 26 February 2024) [Google Scholar]
- 127.Medtronic News. Medtronic launches new frontier to treat high blood pressure. 2023. https://news.medtronic.com/fda-approves-medtronic-symplicity-spyral-renal-denervation-system-for-high-blood-pressure-newsroom (accessed 26 February 2024)
- 128.Scalise F, Quarti-Trevano F, Toscano E et al. Renal denervation in end-stage renal disease: current evidence and perspectives. High Blood Press Cardiovasc Prev. 2024;31:7–13. doi: 10.1007/s40292-023-00621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stabile E, Muiesan ML, Ribichini FL et al. Italian Society of Interventional Cardiology (GISE) and Italian Society of Arterial Hypertension (SIIA) consensus document on the role of renal denervation in the management of the difficult to treat hypertension. G Ital Cardiol. 2023;24((Suppl 2)):53S–63S. doi: 10.1714/4101.40995. [in Italian]. [DOI] [PubMed] [Google Scholar]


