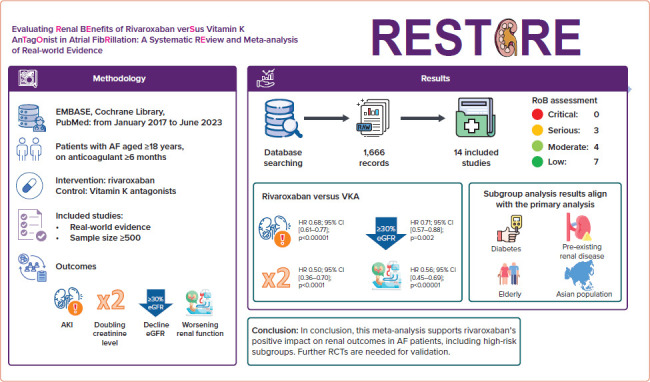
Abstract
Background:
AF is a global health concern, with systemic complications including renal dysfunction. This systematic review and meta-analysis compares the effects of rivaroxaban, a Factor Xa inhibitor, and vitamin K antagonists (VKAs) on renal outcomes in AF patients.
Methods:
The study protocol is registered in PROSPERO (ID: CRD42023462756). We systematically searched the PubMed, Embase and Cochrane Library databases from 1 January 2017 to 30 June 2023 for real-world studies comparing the effects of rivaroxaban and VKAs on renal outcomes in AF patients, including acute kidney injury, a .30% decrease in estimated glomerular filtration rate, doubling of serum creatinine and worsening renal function. Subgroup analyses targeted diabetes, pre-existing kidney disease, the elderly (age .65 years) and Asian populations. The risk of bias was assessed used the Robins-I tool. HRs and 95% CIs were synthesised through a random-effects model. Two sensitivity analyses were performed, using a fixed-effects model and excluding conference abstracts.
Results:
We identified 1,666 records. After screening, 14 studies comparing rivaroxaban and VKAs were included. Rivaroxaban exhibited superiority over VKAs in preventing: acute kidney injury (HR 0.68; 95% CI [0.61.0.77]; p<0.00001); a .30% decrease in estimated glomerular filtration rate (HR 0.71; 95% CI [0.60.0.84]; p<0.0001); doubling of serum creatinine (HR 0.50; 95% CI [0.36.0.70]; p<0.0001); and worsening renal function (HR 0.56; 95% CI [0.45.0.69]; p<0.00001). Subgroup and sensitivity analyses consistently confirmed rivaroxaban’s favourable effects on renal outcomes in diabetes, pre-existing kidney disease, the elderly and Asian populations.
Conclusion:
Our findings support the preference of rivaroxaban over VKAs for renal outcomes in AF. The findings endorse rivaroxaban as the preferred anticoagulant to mitigate renal complications, offering clinicians valuable insights for tailored strategies.
Keywords: Rivaroxaban, AF, acute kidney injury, renal failure, anticoagulant-related nephropathy
Graphical Abstract: Evaluating Renal Benefits of Rivaroxaban Versus Vitamin K Antagonist in Atrial Fibrillation: A Systematic Review and Meta-analysis of Real-world Evidence.
AF is a significant global public health concern, affecting an estimated 60 million individuals worldwide in 2019.[1] In addition to the well-established risk of stroke, there has been increasing recognition of systemic comorbidities and complications associated with AF, including deterioration of renal function, which have garnered attention and have been addressed in clinical guidelines.[2–4] AF is associated with an elevated risk of impaired renal function, which can occur through several mechanisms, including decreased renal blood flow due to activation of the renin–angiotensin–aldosterone system and the formation of microthrombi, leading to renal microinfarction.[5–7] Furthermore, there is concern over the prolonged usage of oral anticoagulants in patients with AF, particularly vitamin K antagonists (VKAs), which accelerate the progression of renal disease.[8]
The effect of direct oral anticoagulants on kidney function has recently gained attention, particularly through the real-world data.[9] The renal benefits of direct oral anticoagulants, particularly rivaroxaban and dabigatran, are acknowledged in AF management guidelines.[2] However, owing to its high degree of renal excretion, dabigatran is less favoured for patients with renal impairment.[4,10] In contrast, rivaroxaban is considered a preferred option for patients with renal impairment or underlying concerns about impaired renal function due, in part, to its reduced susceptibility to changes in kidney function.[4,10] In addition, the potential benefits of rivaroxaban on kidney function further support its preferred use in this patient group.[4,10] Nonetheless, assessment of the effects of rivaroxaban on renal outcomes relative to VKAs has predominantly relied on individual real-world evidence studies, with a paucity of comprehensive assessment. Therefore, a systematic review and meta-analysis are warranted to provide more certain evidence of the effects of rivaroxaban on renal outcomes in AF patients, particularly among subpopulations at elevated risk of unfavourable renal events.
The aim of this systematic review and meta-analysis was to provide a comprehensive comparative assessment of the effects of rivaroxaban and VKAs on renal outcomes in AF patients. This encompasses evaluation of acute kidney injury (AKI) and deterioration of renal function. The analysis includes subgroup analyses that focus on specific high-risk populations, such as the elderly (age ≥65 years), individuals with diabetes and those with pre-existing renal conditions, thus enabling a more nuanced evaluation of the data. Moreover, the analysis provides valuable insights into the effects of rivaroxaban and VKAs in the Asian population.
Methods
Protocol and Registration
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[11] The protocol was collaboratively drafted by three authors (SHV, HPM, PPD) and was comprehensively reviewed and approved by all authors. The protocol has been registered and published on PROSPERO (ID: CRD42023462756).
Search strategy
The data search was conducted across three databases, namely PubMed, EMBASE and the Cochrane Library, from 1 January 2017 to 30 June 2023. The search used the following keywords and Medical Subject Headings (MeSH): ‘atrial fibrillation’, ‘rivaroxaban’, ‘acute kidney injury’, ‘≥30% decreases in estimated glomerular filtration rate’, ‘doubling of the serum creatinine level’, ‘progression to end-stage renal disease’, ‘requirement for haemodialysis’ and ‘need for a kidney transplant’. Our review was restricted to records published in the English language. Additional details of the search strategy are provided in Supplementary Table 1.
Inclusion and Exclusion Criteria
Real-world evidence studies that compared the effects of rivaroxaban and VKAs on desired renal outcomes in patients with AF were included in the analysis. Studies with a limited total patient sample size (<500 patients) and inadequate anticoagulation duration (<6 months) were excluded from the analysis. Two authors (HVS, PPD) conducted the data search in accordance with a predetermined protocol. They screened the records, identified eligible studies and extracted data. In case of disagreements, resolution was sought through consultation with all authors, with any remaining issues decided by a third author (HPM).
Outcomes
Four distinct kidney-related outcomes were assessed: AKI; a ≥30% decreasing in the estimated glomerular filtration rate (eGFR); doubling of the serum creatinine concentration; and worsening renal function, defined as a composite outcome encompassing kidney failure, progression to end-stage renal disease (eGFR ≤15 ml/min/1.73 m2) and the need for haemodialysis or a kidney transplant.
In addition to the pooled analysis, we conducted subgroup analyses on predefined outcomes. These subgroups comprised patients with diabetes, those with pre-existing kidney disease, the elderly (age ≥65 years) and Asian populations.
We used the ROBINS-I tool to assess the risk of bias. Review Manager (RevMan), a web-based platform for reviews, was used to facilitate the assessment. Two independent reviewers (HVS, PPD) evaluated bias risk, resolving disagreements through consultation with all authors, with any remaining issues decided by a third author (HPM).
Statistical Analysis
Data synthesis encompassed the use of HRs and corresponding 95% CIs as the primary effect size estimates. We used a random-effects model to account for potential heterogeneity among studies. To determine the relative importance of individual study effect size estimates, the inverse variance method was used. Heterogeneity was assessed using the Χ[2] test (with p<0.05 indicating potential heterogeneity) and the ∣2 statistic (which quantifies the percentage of total variation across studies due to heterogeneity). ∣2 values of 25%, 50% and 75% are interpreted as indicating low, moderate and high heterogeneity, respectively. In addition, the amount of heterogeneity was quantified using the τ2 statistic. Data analysis was conducted using the web-based RevMan, which is a universally recognised platform for conducting systematic reviews and meta-analyses.
Sensitive Analysis
Sensitivity analyses were performed to evaluate the robustness of the results and to assess the effects of methodological choices. In our Model 1 sensitivity analysis, we evaluated the effects of diabetes, pre-existing renal disease, Asian ethnicity and dosing strategy on the effects of rivaroxaban on renal outcomes to ascertain the robustness of our findings across these diverse patient subgroups. In Model 2, conference abstracts were excluded due to data limitations.
Results
Search Results and Study Characteristics
We initially identified 1,666 records from the PubMed, EMBASE and the Cochrane Library databases. Screening of titles and abstracts resulted in the exclusion of 1,564 records: 153 duplicates and 1,413 that were not relevant. Of the 102 records subjected to full text review, 86 were excluded for various reasons. Thus, 14 studies that met the inclusion criteria were selected for analysis. A flow chart of the selection process is shown in Figure 1.
Figure 1: Selection Process.
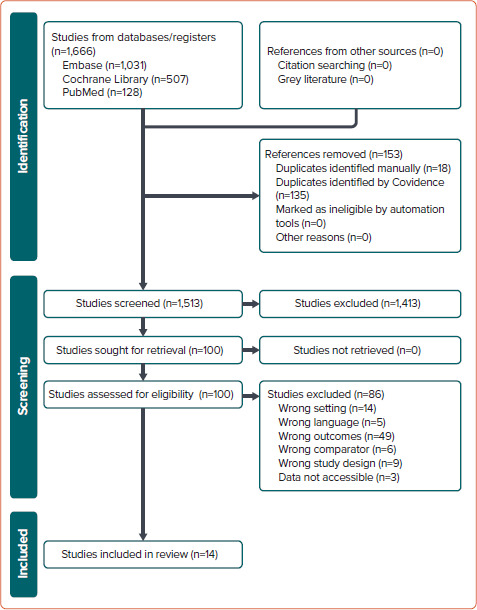
A comprehensive analysis was conducted on 14 studies, involving a total cohort of 418,201 patients with AF.[9,12–24] Of these 418,201 patients, 142,494 received rivaroxaban and 196,337 received VKAs. The study conducted by Klil-Drori et al. lacks information regarding the specific number of patients using rivaroxaban and VKAs.[20] Thirteen of the 14 studies included in the analysis were observational and retrospective in nature, with the study by Kreutz et al. standing out as the sole prospective observational study.[21] Importantly, four of the 14 studies were presented in abstract form at a conference.[12,13,20,21]
Most of the studies included in the analysis were well designed and used methods that matched clinical characteristics and potential confounders. The method used to address potential confounders across the studies varied. Specifically, 12 of 14 studies used propensity score weighting, with the most common being inverse probability of treatment weighting (IPTW).[9,12–16,18,19,21–24] González et al. used Cox proportional hazards regression to adjust for confounding factors.[17] Notably, the study conducted by Klil-Drori et al. did not provide information as to the methods used to adjust for confounding factors.[20]
Details of the included studies are provided in Supplementary Table 2.
Clinical Outcomes
Acute Kidney Injury
Compared with VKAs, the use of rivaroxaban in AF patients was linked to a significant reduction in the risk of AKI (HR 0.68; 95% CI [0.61–0.77]; p<0.00001; ∣2=86%; Figure 2A).
Figure 2: Comparison of Rivaroxaban and Vitamin K Antagonists in the Incidence of Acute Kidney Injury Among AF Patients.
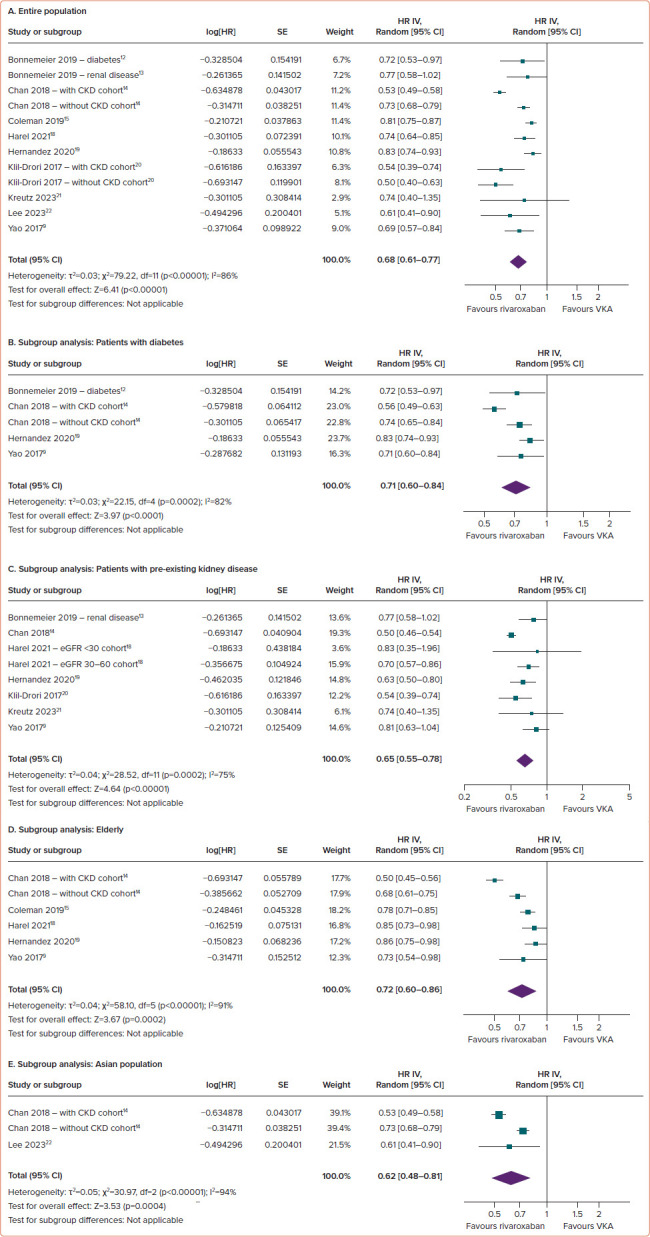
(A) Entire population. (B–E) Subgroup analysis: patients with diabetes (B), patients with pre-existing kidney disease (C), the elderly (D) and the Asian population (E). CKD = Chronic kidney disease; eGFR = estimated glomerular filtration rate; VKA = vitamin K antagonist.
The results of prespecified subgroup analyses (diabetes, pre-existing kidney disease, the elderly and Asian populations) consistently highlighted the superiority of rivaroxaban over VKAs with regard to AKI outcomes across the various subgroups. Specifically, rivaroxaban was associated with a significantly reduced risk of AKI in individuals with diabetes (HR 0.71; 95% CI [0.60–0.84]; p<0.0001; ∣2=82%; Figure 2B), pre-existing kidney disease (HR 0.65; 95% CI [0.55–0.78]; p<0.00001; ∣2=75%; Figure 2C), the elderly (HR 0.72; 95% CI [0.60–0.86]; p=0.0002; ∣2=91%; Figure 2D) and the Asian population (HR 0.62; 95% CI [0.48–0.81]; p=0.0004; ∣2=94%; Figure 2E).
Decrease (≥30%) in Estimated Glomerular Filtration Rate
Compared with VKAs, the use of rivaroxaban in AF patients was linked to a significant reduction in the risk of a ≥30% decreased in eGFR (HR 0.71; 95% CI [0.57–0.88]; p=0.002; ∣2=95%; Figure 3A).
Figure 3: Comparison of Rivaroxaban and Vitamin K Antagonists in Preventing a ≥30% Decrease in Estimated Glomerular Filtration Rate among AF Patients.
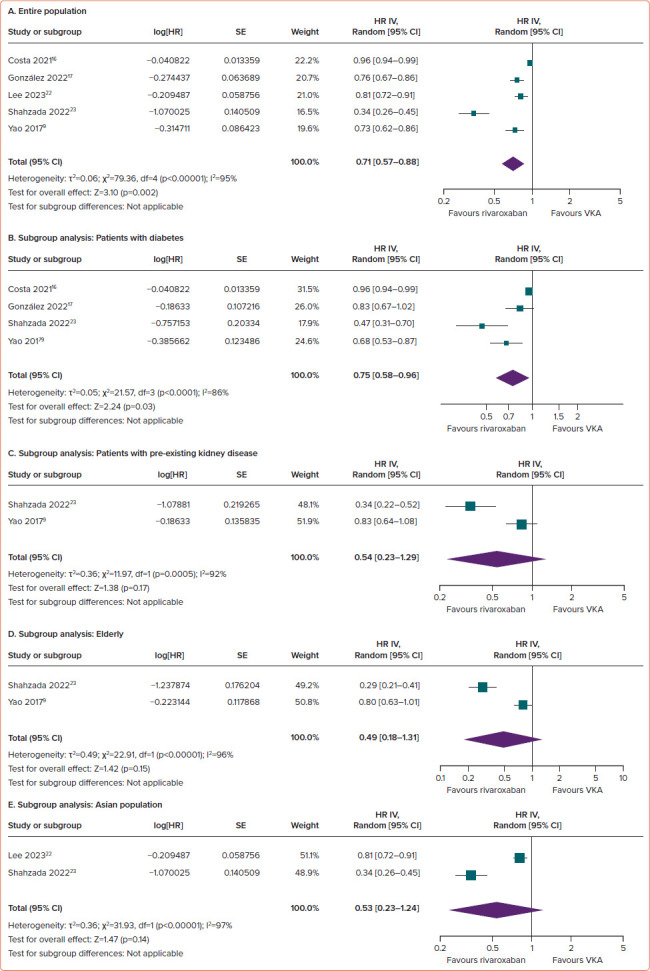
(A) Entire population. (B–E) Subgroup analysis: patients with diabetes (B), patients with pre-existing kidney disease (C), elderly (D) and the Asian population (E). VKA = vitamin K antagonist.
In subgroup analyses, rivaroxaban significantly reduced the risk of risk of a ≥30% decrease in eGFR in individuals with diabetes (HR 0.75; 95% CI [0.58–0.96]; p=0.03; ∣2=86%; Figure 3B). For the remaining three subgroups, there was a trend towards a positive effect of rivaroxaban on declining eGFR outcomes; however, the differences versus VKAs did not reach statistical significance, with HRs of 0.54 (95% CI [0.23–1.29]; p=0.17; ∣2=92%; Figure 3C) for pre-existing kidney disease, 0.49 (95% CI [0.18– 1.31]; p=0.15; ∣2=96%; Figure 3D) for the elderly and 0.53 (95% CI [0.23– 1.24]; p=0.14; ∣2=97%; Figure 3E) for the Asian population.
Doubling of the Serum Creatinine Concentration
Compared with VKAs, the use of rivaroxaban in AF patients was associated with a reduced risk of doubling of the serum creatinine concentration (HR 0.50; 95% CI [0.36–0.70]; p<0.0001; ∣2=50%; Figure 4A).
Figure 4: Comparison of Rivaroxaban and Vitamin K Antagonists on the Risk of Serum Creatinine Levels Doubling Among AF Patients.
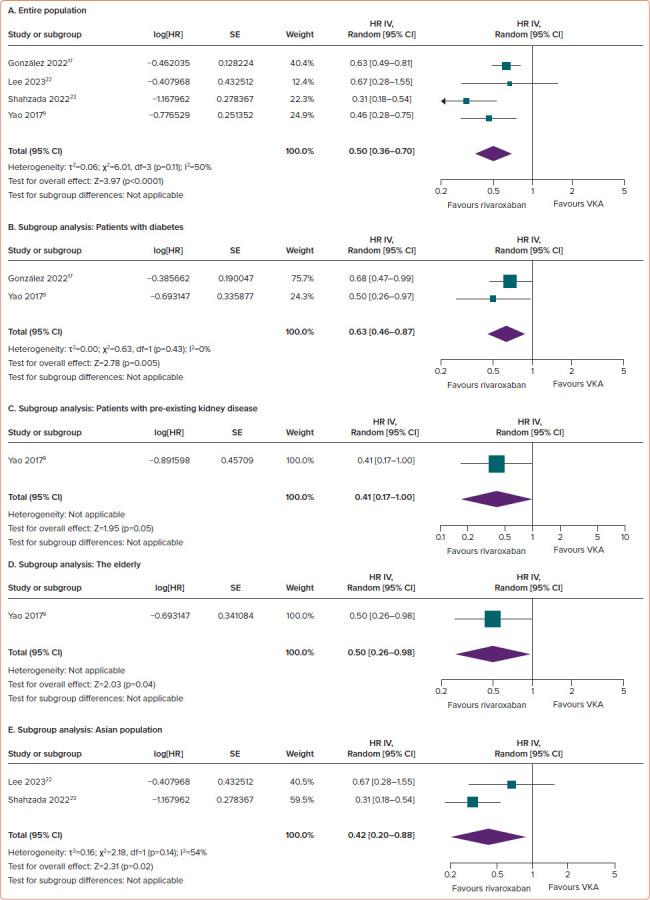
(A) Entire population. (B–E) Subgroup analysis: patients with diabetes (B), patients with pre-existing kidney disease (C), the elderly (D) and the Asian population (E). VKA = vitamin K antagonist.
Consistent results across all subgroups underscore the superiority of rivaroxaban over VKAs in terms of increasing serum creatinine concentrations. Rivaroxaban significantly reduced the risk of doubling of serum creatinine concentrations in individuals with diabetes (HR 0.63; 95% CI [0.46–0.87]; p=0.005; ∣2=0%; Figure 4B), pre-existing kidney disease (HR 0.41; 95% CI [0.17–1.00]; p=0.05; ∣2 not applicable; Figure 4C), the elderly (HR 0.50; 95% CI [0.26–0.98]; p=0.04; ∣2 not applicable; Figure 4D) and the Asian population (HR 0.42; 95% CI [0.20–0.88]; p=0.02; ∣2=54%; Figure 4E).
Worsening Renal Function
Compared with VKAs, the use of rivaroxaban in AF patients was associated with a reduced risk of worsening renal function (HR 0.56; 95% CI [0.45– 0.69]; p<0.00001; ∣2=89%; Figure 5A).
Figure 5: Comparison of Rivaroxaban and Vitamin K Antagonists on the Risk of Worsening Renal Function among AF Patients.
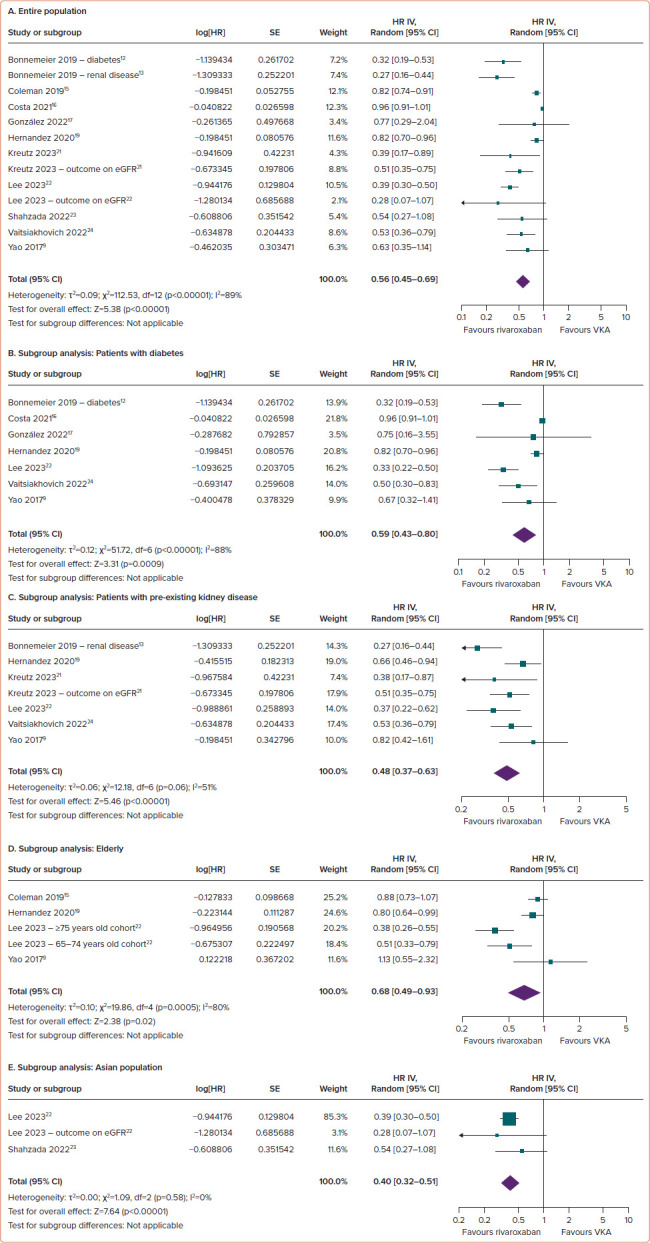
(A) Entire population. (B–E) Subgroup analysis: patients with diabetes (B), patients with pre-existing kidney disease (C), the elderly (D) and the Asian population (E). eGFR = estimated glomerular filtration rate; VKA = vitamin K antagonist.
Consistent results across all subgroups underscore the superiority of rivaroxaban over VKAs in terms of worsening renal function. Rivaroxaban significantly reduced the risk of renal function worsening in individuals with diabetes (HR 0.59; 95% CI [0.43–0.80]; p=0.0009; ∣2=88%; Figure 5B), pre-existing kidney disease (HR 0.48; 95% CI [0.37–0.63]; p<0.00001; ∣2=51%; Figure 5C), the elderly (HR 0.68; 95% CI [0.49–0.93]; p=0.02; ∣2=80%; Figure 5D) and the Asian population (HR 0.40; 95% CI [0.32–0.51]; p<0.00001; ∣2=0%; Figure 5E).
Risk of Bias
Most studies analysed were well designed, using methods to equitably balance clinical characteristics and adjust for confounding factors. Seven studies had a low risk of bias,[9,12,13,16,17,21,23] whereas four studies had a moderate risk.[14,15,19,24] Of the three studies with a serious risk of bias, those conducted by Harel et al. and Lee et al. were identified as having a serious risk of bias in the missing data domain, with the remaining domains showing low risk of bias.[18,22] The study by Klil-Drori et al. lacked clarity in several domains.[20]
Details of the risk of bias assessment are provided in Supplementary Table 3. The funnel plot for assessing publication bias is shown in Supplementary Figure 1.
Sensitive Analyses
We conducted sensitivity analyses to evaluate the robustness of the results and to examine the effects of various methodological choices or assumptions on the overall findings. In Model 1, we assessed the effects of diabetes, renal disease, dosing strategy and race on the effects of rivaroxaban on renal outcomes compared with VKA. In Model 2, we excluded the conference abstracts due to limited data accessibility, particularly the two studies by Bonnemeier et al. because of potential partial overlap in the study populations.[12,13,20,21]
The sensitivity analysis Model 1 revealed that the relative effects of rivaroxaban compared with VKA on renal outcomes were consistent across most examined subgroups. However, a notable exception was observed within the Asian population subgroup, where the beneficial effect of rivaroxaban on worsening renal function was more pronounced than in the non-Asian population, with HRs of 0.40 (95% CI [0.32–0.51]; p<0.00001) for the Asian subgroup and 0.61 (95% CI [0.50–0.75]; p<0.00001) for non-Asian subgroup (pinteraction=0.0007). In Model 2, consistent findings emerged confirming the favourable effects of rivaroxaban relative to VKAs concerning kidney outcomes. Rivaroxaban demonstrated superiority in preventing AKI (HR 0.71; 95% CI [0.61–0.81]; p<0.00001; ∣2=91%), a ≥30% decrease in eGFR (HR 0.71; 95% CI [0.57– 0.88]; p=0.002; ∣2=95%), doubling of the serum creatinine concentration (HR 0.50; 95% CI [0.36–0.70]; p<0.0001; ∣2=50%) and deterioration of renal function (HR 0.89; 95% CI [0.85–0.93]; p<0.00001; ∣2=88%).
The forest plot of sensitive analyses is provided in Supplementary Figure 2 and Supplementary Table 4
Discussion
To the best of our knowledge, this is the first systematic review and metaanalysis comparing rivaroxaban and VKAs on four major renal outcomes, namely AKI, a ≥30% decrease in eGFR, doubling of the serum creatinine concentration and worsening renal function. Anticoagulant-related nephropathy, initially identified as a manifestation of AKI, occurs in patients exposed to excessive anticoagulation with VKAs (international normalized ratio of prothrombin time [INR] >3).[25] AKI may manifest in about one-fifth of patients with supratherapeutic levels of VKAs (INR >3) within a week.[26] Hence, the assessment of incident AKI serves to facilitate a comparative analysis of the effects of rivaroxaban and VKAs on early-stage renal injury. Endpoints of a 30–40% decline in eGFR and doubling of the serum creatinine concentration are endorsed by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for evaluating progression of renal disease in the context of clinical trials.[27] Meanwhile, the endpoint of worsening renal function aims to evaluate the long-term effects of anticoagulation on renal outcomes. The constituent outcomes incorporated into this composite measure align with recommendations from KDIGO, the National Kidney Foundation and the US Food and Drug Administration for conducting clinical trials on nephropathy.[27,28] We believe that using a comprehensive set of clinical endpoints facilitated a thorough comparison of the effects of rivaroxaban and VKAs on renal outcomes in AF patients.
The results of the meta-analysis demonstrated the superiority of rivaroxaban over VKAs across all criteria evaluated. Moreover, we identified specific populations at heightened risk of adverse renal events with anticoagulant use, including the elderly, diabetic patients and those with pre-existing renal disease.[26] Subgroup analyses were conducted on these subpopulations to provide a more nuanced assessment. In addition, the supratherapeutic levels with VKAs emerged as a well-established risk factor for anticoagulation-related kidney injury.[29] Notably, our analysis also revealed thatAsian patients exhibit poor INR control when undergoing anticoagulation, prompting a subgroup analysis for this population.[30] The outcomes of the subgroup analyses consistently support the findings of the primary analysis, providing additional evidence for the renoprotective effects of rivaroxaban compared with VKAs.
Several potential mechanisms of action have been proposed to explain the differences in renal outcomes between rivaroxaban and VKAs. First, there are many pathways linking vascular calcification and arterial stiffness, which are processes correlated with low vitamin K levels, to chronic kidney disease.[31] This may explain the deterioration in renal function observed in patients with AF when treated with VKAs.[8,32] Unlike VKAs, rivaroxaban has no effect on the metabolism of vitamin K and may not adversely affect renal function through this mechanism. Conversely, switching from warfarin to rivaroxaban resulted in a significant decrease in pulse wave velocity, indicating an improvement in vascular stiffness associated with the use of rivaroxaban.[33] Second, in individuals with AF, ischaemic damage and oxidative stress play notable roles in the pathogenesis of renal disease.[7,34] Rivaroxaban exerts renoprotective effects by mitigating oxidative stress, which is a key contributor to kidney damage during ischaemia–reperfusion events. Rivaroxaban significantly reduces levels of malondialdehyde, a marker of oxidative stress, suggests that it helps preserve kidney function by limiting the harmful effects of free radicals and inflammation.[35] By inhibiting Factor Xa, rivaroxaban not only prevents clot formation, but also appears to have anti-inflammatory actions that further protect renal tissues against damage associated with reperfusion after ischaemic episodes.[35] In addition, rivaroxaban inhibits oxidative stress and preserves mitochondrial function in the kidneys. Rivaroxaban has been shown to decrease the production of reactive oxygen species, which are known to contribute to cellular damage and kidney dysfunction.[36] By maintaining the integrity of mitochondrial function, rivaroxaban helps avert cell death pathways that can lead to renal injury.[36] The protective effects of rivaroxaban are dose dependent, with higher doses proving more efficacious in reducing oxidative stress and maintaining mitochondrial health, both of which are critical for kidney function.[36] Third, in the setting of hypertensive renal damage in hypertensive mice overexpressing renin, rivaroxaban significantly reduced albuminuria and attenuated histological changes, such as glomerular hypertrophy and mesangial matrix expansion. Rivaroxaban also inhibited glomerular basement membrane thickening and glomerular hypertrophy, demonstrating antiinflammatory effects.[37] Rivaroxaban exerts protective effects against angiotensin II-induced renal damage and protects against podocyte injury.[37] These protective effects are due, in part, to the inhibition of the protease activated receptor-2 signalling-mediated inflammatory response.[37] In a randomised trial involving patients with chronic kidney disease, rivaroxaban (compared with warfarin) reduced urinary albumin excretion and attenuated tubular injury in individuals with AF and micro-/ macroalbuminuria chronic kidney disease.[38]
The primary limitation of our meta-analysis is the high heterogeneity among studies. The high heterogeneity in outcomes, specifically for AKI, a ≥30% decrease in eGFR and worsening renal function, warrants a multifaceted exploration of potential underlying factors. First, the variability in outcome definitions across the studies included is a salient contributor to heterogeneity. For example, Coleman et al. used International Classification of Diseases, Ninth and Tenth Revision diagnosis codes to define AKI, whereas Harel et al. used a clinical definition encompassing a ≥50% increase in serum creatinine concentration from baseline, an increase of ≥0.3 mg/dl or initiation of acute dialysis.[15,18] The use of a composite definition for worsening renal function in our analysis, which amalgamated different outcomes of advanced stages of kidney injury, may have amplified the heterogeneity. Moreover, the heterogeneity was potentially exacerbated by the diversity in baseline renal function parameters, such as serum creatinine and eGFR, across the studies. Second, there is pronounced heterogeneity within the study populations themselves. The studies included encompass cohorts with distinct clinical characteristics, including (but not limited to) diabetes, pre-existing kidney disease and advanced age. These factors inherently influence renal outcomes and, consequently, may have contributed to the heterogeneity observed. Third, heterogeneity may be influenced by variations in anticoagulation management, including differences in dosing and treatment duration, as well as disparities in study design. We conducted a sensitivity analysis to determine the consistency of the renal outcomes with rivaroxaban. Our findings indicate that the effects of rivaroxaban on renal outcomes remain consistent regardless of patients’ diabetes status, the presence of pre-existing renal disease or the dosing strategy used. Notably, the benefit of rivaroxaban was more pronounced on worsening renal outcomes in the Asian subgroup, which may be attributed to the comparatively poorer INR control commonly reported in Asian populations. INR >3 have been associated with an increased risk of renal injuries in patients with AF, potentially explaining the enhanced efficacy of rivaroxaban in this population.[26,39] The observed high heterogeneity underscores the need for careful consideration when interpreting the pooled outcomes. Robust randomised controlled trials are needed to confirm these results and provide a clearer understanding of the effects under more controlled conditions. Given the high heterogeneity, we used the random-effects model in our meta-analysis, acknowledging and accommodating the diverse methodologies among the studies included. In an effort to curtail the effect of publication bias, we conducted a comprehensive search across multiple databases and deliberately adopted inclusive selection criteria. In addition, we incorporated conference abstracts into our analysis to ensure the inclusion of pertinent but potentially unpublished studies. We excluded three studies due to data inaccessibility.[40–42] However, their inclusion is unlikely to significantly alter the overall results. Conversely, our analysis incorporated four conference abstracts with limited data access. The results of a sensitivity analysis that excluded these four studies closely aligned with those of the primary analysis, reinforcing the robustness of our findings.
In the context of clinical implications, the findings of this systematic review and meta-analysis support the use of rivaroxaban in AF patients at an increased risk of deteriorating renal function. Specifically, these results endorse the use of rivaroxaban in individuals with diabetes, pre-existing kidney disease, the elderly with a heightened risk of renal injury and the Asian population.
Conclusion
In conclusion, this meta-analysis underscores rivaroxaban’s favourable effects on renal outcomes in AF patients. The findings from subgroup analyses of patients with diabetes, pre-existing kidney disease, the elderly and from the Asian population are consistent with the results of the primary analysis. Despite study limitations, these findings support rivaroxaban as the preferred anticoagulant to mitigate renal complications. The results offer clinicians valuable insights for tailored anticoagulation strategies, optimising renal outcomes. Further randomised control trials are warranted to validate the positive effects of rivaroxaban on renal outcomes.
Supplementary Material
Acknowledgments
The authors express their gratitude to Dr Huong Tran, who leads the Medical Affairs Department at Bayer Vietnam, for financial assistance in covering the article processing charges.
References
- 1.Li H, Song X, Liang Y et al. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990– 2019: results from a global burden of disease study, 2019. BMC Public Health. 2022;22:2015. doi: 10.1186/s12889-022-14403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 3.Quang Ho TH, Ton MT, Nguyen VL et al. Selection of nonvitamin K antagonist oral anticoagulant for stroke prevention in atrial fibrillation based on patient profile: perspectives from Vietnamese experts. Part 1. Eur Cardiol. 2023;18:e61. doi: 10.15420/ecr.2023.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ton MT, Quang Ho TH, Nguyen VL et al. Selection of nonvitamin K antagonist oral anticoagulant for stroke prevention in atrial fibrillation based on patient profile: perspectives from Vietnamese experts. Part 2. Eur Cardiol. 2023;18:e62. doi: 10.15420/ecr.2023.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe H, Watanabe T, Sasaki S et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–36. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Iravanian S, Dudley SC. The renin–angiotensin–aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. 2008;5((6 Suppl)):S12–7. doi: 10.1016/j.hrthm.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzarossa AC, Mérola V. Etiology of renal infarction. A systematic review. Rev Med Chil. 2019;147:891–900. doi: 10.4067/S0034-98872019000700891. [in Spanish]. [DOI] [PubMed] [Google Scholar]
- 8.Posch F, Ay C, Stöger H et al. Exposure to vitamin K antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res Pract Thromb Haemost. 2019;3:207–16. doi: 10.1002/rth2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X, Tangri N, Gersh BJ et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–32. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 10.Okumura K, Hori M, Tanahashi N, John Camm A. Special considerations for therapeutic choice of non-vitamin K antagonist oral anticoagulants for Japanese patients with nonvalvular atrial fibrillation. Clin Cardiol. 2017;40:126–31. doi: 10.1002/clc.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnemeier H, Kreutz R, Kloss S et al. AS25-066. Comparative safety and effectiveness of non-vitamin-K oral anticoagulants vs phenprocoumon in patients with non-valvular atrial fibrillation and diabetes – results from the RELOADed study. Eur Stroke J. 2019;4((1 Suppl)):167. [Google Scholar]
- 13.Bonnemeier H, Kreutz R, Kloss S et al. AS25-069. Comparative safety and effectiveness of non-vitamin-K oral anticoagulants vs phenprocoumon in patients with non-valvular atrial fibrillation and renal disease – results from the RELOADed study. Eur Stroke J. 2019;4((1 Suppl)):167. [Google Scholar]
- 14.Chan YH, Yeh YH, Hsieh MY et al. The risk of acute kidney injury in Asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. 2018;265:83–9. doi: 10.1016/j.ijcard.2018.02.075. [DOI] [PubMed] [Google Scholar]
- 15.Coleman CI, Kreutz R, Sood N et al. Rivaroxaban’s impact on renal decline in patients with nonvalvular atrial fibrillation: a US MarketScan claims database analysis. Clin Appl Thromb Hemost. 2019;25:1076029619868535. doi: 10.1177/1076029619868535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa OS, O’Donnell B, Vardar B et al. Kidney, limb and ophthalmic complications, and death in patients with nonvalvular atrial fibrillation and type 2 diabetes prescribed rivaroxaban or warfarin: an electronic health record analysis. Curr Med Res Opin. 2021;37:1493–500. doi: 10.1080/03007995.2021.1947217. [DOI] [PubMed] [Google Scholar]
- 17.González Pérez A, Balabanova Y, Sáez ME et al. Renal decline in patients with non-valvular atrial fibrillation treated with rivaroxaban or warfarin: a population-based study from the United Kingdom. Int J Cardiol. 2022;352:165–71. doi: 10.1016/j.ijcard.2022.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Harel Z, McArthur E, Jeyakumar N et al. The risk of acute kidney injury with oral anticoagulants in elderly adults with atrial fibrillation. Clin J Am Soc Nephrol. 2021;16:1470–9. doi: 10.2215/CJN.05920421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez AV, Bradley G, Khan M et al. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes. 2020;6:301–7. doi: 10.1093/ehjqcco/qcz047. [DOI] [PubMed] [Google Scholar]
- 20.Klil-Drori AJ, Azoulay L, Nie R et al. Comparative risk of acute kidney injury with oral anticoagulant use among patients with nonvalvular atrial fibrillation. Blood. 2017;130((Suppl 1)):700. doi: 10.1182/blood.V130.Suppl_1.700.700. [DOI] [Google Scholar]
- 21.Kreutz RH. Rivaroxaban associates with reduced risk of adverse kidney outcomes in comparison to vitamin K antagonist treatment in a prospective real-world study in patients with non-valvular atrial fibrillation and advanced chronic kidney disease. J Am Coll Cardiol. 2023;81((8 Suppl)):108. doi: 10.1016/S0735-1097(23)00552-1. [DOI] [Google Scholar]
- 22.Lee SR, Choi EK, Park SH et al. Renal outcomes of rivaroxaban compared with warfarin in Asian patients with nonvalvular atrial fibrillation: A nationwide population-based cohort study. Front Cardiovasc Med. 2023;10:1040834. doi: 10.3389/fcvm.2023.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahzada TS, Guo CL, Lee APW. Renal outcomes in Asian patients receiving oral anticoagulants for non-valvular atrial fibrillation. Hong Kong Med J. 2022;28:24–32. doi: 10.12809/hkmj209201. [DOI] [PubMed] [Google Scholar]
- 24.Vaitsiakhovich T, Coleman CI, Kleinjung F et al. Worsening of kidney function in patients with atrial fibrillation and chronic kidney disease: evidence from the real-world CALLIPER study. Curr Med Res Opin. 2022;38:937–45. doi: 10.1080/03007995.2022.2061705. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky SV, Satoskar A, Chen J et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: A report of 9 cases. Am J Kidney Dis. 2009;54:1121–6. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky SV, Nadasdy T, Rovin BH et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–9. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baigent C, Herrington WG, Coresh J et al. Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease – Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;92:297–305. doi: 10.1016/j.kint.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Inker LA, Matsushita K et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–35. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29:2787–93. doi: 10.1681/ASN.2018070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh S, Goto S, Accetta G et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD-AF registry. Int J Cardiol. 2016;223:543–7. doi: 10.1016/j.ijcard.2016.08.236. [DOI] [PubMed] [Google Scholar]
- 31.Shea MK, Booth SL. Vitamin K, vascular calcification, and chronic kidney disease: current evidence and unanswered questions. Curr Dev Nutr. 2019;3:nzz077. doi: 10.1093/cdn/nzz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5:e002665. doi: 10.1161/JAHA.115.002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namba S, Yamaoka-Tojo M, Kakizaki R et al. Effects on bone metabolism markers and arterial stiffness by switching to rivaroxaban from warfarin in patients with atrial fibrillation. Heart Vessels. 2017;32:977–82. doi: 10.1007/s00380-017-0950-2. [DOI] [PubMed] [Google Scholar]
- 34.Linz D, Neuberger HR. Chronic kidney disease and atrial fibrillation. Heart Rhythm. 2012;9:2032–3. doi: 10.1016/j.hrthm.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Özbudak E, Eraldemir FC, Arıkan AA et al. An evaluation of rivaroxaban and clopidogrel in a rat lower extremity ischemia–reperfusion model: an experimental study. Turk Gogus Kalp Damar Cerrahisi Derg. 2019;27:513–20. doi: 10.5606/tgkdc.dergisi.2019.18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samiei F, Sajjadi H, Jamshidzadeh A et al. Contrasting role of concentration in rivaroxaban induced toxicity and oxidative stress in isolated kidney mitochondria. Drug Res (Stuttg) 2019;69:523–7. doi: 10.1055/a-1001-2154. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa H, Shimada M, Narita M et al. Rivaroxaban, a direct Factor Xa inhibitor, ameliorates hypertensive renal damage through inhibition of the inflammatory response mediated by protease-activated receptor pathway. J Am Heart Assoc. 2019;8:e012195. doi: 10.1161/jaha.119.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka A, Suzuki M, Matsunaga K et al. Effect of rivaroxaban on urinary albumin excretion in patients with atrial fibrillation and chronic kidney disease: a randomized trial (X-NOAC). Hypertens Res. 2020;43:571–4. doi: 10.1038/s41440-019-0384-6. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky SV, Collins M, Park E et al. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–6. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun SJ, Lee KH. Effects of new oral anticoagulants on renal outcomes comparing warfarin in patients with non-valvular AF: propensity score matching. J Arrhythm. 2019;35((Suppl 1)):119. doi: 10.1002/joa3.12267. [DOI] [Google Scholar]
- 41.Choi SH, Kim M, Kim H et al. Cardiovascular and renal protective effects of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation. PLoS One. 2022;17:e0275103. doi: 10.1371/journal.pone.0275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WC, Lee PW, Wu PJ et al. The impact on renal function after long-term use of anticoagulants in atrial fibrillation patients. Thromb J. 2021;19:98. doi: 10.1186/s12959-021-00351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


