Abstract
Background
Preoperative chemotherapy (CT) or chemoradiotherapy (CRT) show survival benefits in patients with locally advanced esophageal squamous cell carcinoma (ESCC); however, ESCC patients still have a dismal prognosis. We conducted two phase-II, single-armed clinical trials to assess the potential benefits, efficacy, feasibility, and safety of esophagectomy after combining preoperative CT or CRT and neoadjuvant programmed cell death protein 1 (PD-1) inhibitors in the treatment of ESCC.
Methods
Patients were included with histologically confirmed ESCC (clinical stage II–IVA according to the American Joint Committee on Cancer 8th staging system) from two phase-II, single-arm trials (NCT04506138 and NCT03940001). Patients underwent two doses of intravenous PD-1 inhibitor (either camrelizumab or sintilimab) every 3 weeks, combined with two cycles of either CT or CRT. The primary endpoint of the study was the safety and short-term outcomes of esophagectomy as measured by the risk of developing complications within 30 days, after the combination of preoperative PD-1 inhibitor and CT or CRT Secondary endpoint was to evaluate the pCR rates (pT0N0), primary tumor pCR rates (pT0), operation time, postoperative stay, and 30-day mortality rate between both groups. Results between both groups were compared using a multivariable log-binomial regression model to obtain the adjusted relative risk ratios (RRs).
Results
Between May 2019 and June 2022, 55 patients were included. All patients completed neoadjuvant therapy. Age, sex, performance status, clinical stage, histologic subtype, procedure type, operative time, and blood loss volume were similar between the two groups. The primary tumor pCR rates were 52.9% in the nICRT group and 21.6% in the nICT group (P=0.03), while the postoperative pCR rates were 41.2% in the nICRT group and 21.6% in the nICT group (P=0.19). The minimally invasive surgery rates were 89.2% (33/37) in the nICT group and 94.1% (16/17) in the nICRT group. The risk of developing pulmonary, anastomotic, or other complications were similar between the two groups.
Conclusions
Esophagectomy was safe after the addition of the PD-1 inhibitor to preoperative CT or CRT in ESCC neoadjuvant therapies. Follow-up and the exploratory endpoints, including biomarkers analyses, are ongoing.
Keywords: Esophagectomy, neoadjuvant chemotherapy and immunotherapy (nCT and immunotherapy), neoadjuvant chemoradiotherapy and immunotherapy (nCRT and immunotherapy), esophageal squamous cell carcinoma (ESCC), clinical trials
Highlight box.
Key findings
• Esophagectomy is safe and feasible following neoadjuvant immunotherapy combined with chemotherapy (nICT) and neoadjuvant immunotherapy combined with chemoradiotherapy (nICRT) for locally advanced esophageal squamous cell carcinoma (ESCC). Specifically, we found a higher primary tumor pathological complete response (pCR) rate in the nICRT group than the nICT group without significant increase in postoperative morbidity and mortality.
What is known, and what is new?
• Neoadjuvant chemoradiotherapy, or chemotherapy, improves survival in patients with locally advanced esophageal cancer.
• Our preliminary results demonstrate that esophagectomy remain safe and feasible following nICT and nICRT for locally advanced esophageal cancer. The primary tumor pCR rate was higher in the nICRT group than the nICT group. Postoperative morbidity and mortality were similar in patients treated with nICT and nICRT.
What is the implication, and what should change now?
• This is the first study to describe surgical outcomes after nICT or nICRT for locally advanced ESCC. Our results will contribute to ongoing research study design on optimal neoadjuvant immunotherapy strategies for resectable squamous esophageal cancer.
Introduction
Esophageal cancer is the seventh most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality worldwide (1). The incidence of esophageal cancer in East Asia is high, and according to the latest data, the incidence in China accounts for more than 50% of all cases worldwide (1). In China, over 90% of esophageal cancers are esophageal squamous cell carcinomas (ESCCs), and as many as 30–50% of patients have locally advanced disease when diagnosed.
Currently, neoadjuvant chemotherapy (nCT) or neoadjuvant chemoradiotherapy (nCRT) plus esophagectomy is recommended as the standard treatment for locally advanced, operable ESCC. However, due to the high rate of local or distant recurrence, the long-term survival of nCT or nCRT plus esophagectomy for ESCC is still unsatisfactory (2-4). Therefore, the establishment of new and effective treatment strategies is crucial to further improve the long-term survival of ESCC patients.
In recent years, immune checkpoint blockade therapy has revolutionized the treatment paradigm of multiple advanced cancers (5-10). ESCC has a very high tumor mutational burden and high programmed death-1 ligand expression (PD-L1), demonstrating its potential sensitivity to immune checkpoint inhibitors (ICIs) (11-17).
In a study of postoperative adjuvant therapy for locally advanced esophageal cancer, nivolumab was shown to improve disease-free survival after esophagectomy (18). Several other prospective clinical studies have investigated the safety and efficacy of preoperative neoadjuvant immunotherapy combined with chemotherapy (nICT) or neoadjuvant immunotherapy combined with chemoradiotherapy (nICRT) and reported satisfactory outcomes after the addition of programmed cell death protein 1 (PD-1) inhibitors to the neoadjuvant regimen (19-22). However, few studies have investigated the safety of surgery following the neoadjuvant treatment with ICIs in esophageal cancer, and to date, most such studies have only focused on the toxicity and tolerability of ICIs (23,24). Further, to date, no published studies have compared the safety and efficacy of esophagectomy after different neoadjuvant immunotherapy modalities.
In this article, we share our experience on the safety and feasibility of esophagectomy after nICT or nICRT for locally advanced ESCC. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-295/rc).
Methods
Patient selection and study design
We initiated two non-randomized, single-arm, single-center, phase-II trials to investigate the efficacy, feasibility, and safety of the combination of preoperative chemotherapy (CT) or chemoradiotherapy (CRT) and PD-1 inhibitor in the treatment of ESCC. Patients underwent esophagectomy after neoadjuvant therapy completion between May 2019 and June 2022 at the Zhejiang Cancer Hospital, China.
To be eligible for these two clinical studies, patients had to meet the following inclusion criteria: have histologically confirmed, potentially curable ESCC with cT1N1–3M0 or cT2–4aN0–3M0 (American Joint Committee on Cancer 8th staging system); have no metastatic cervical lymph nodes; not have undergone prior therapy for any cancer; be aged 18–75 years; have normal organ function; and have adequate pulmonary and cardiac function. Patients were excluded from the study if they had an immunodeficiency disease, were receiving ongoing systemic immunosuppressive therapy with either corticosteroids (>10 mg daily prednisolone equivalent) or other immunosuppressive drugs, had an infectious disease, had a clinically significant concurrent cancer, were unable to undergo gastric tube reconstruction after esophagectomy, or were hypersensitive to albumin paclitaxel and carboplatin drugs.
These two studies were conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent for their enrollment in the studies, and the studies were approved by the Ethics Committee of the Zhejiang Cancer Hospital, China (Nos. IRB-2019-38 and IRB-2020-183).
Neoadjuvant treatment protocols
nICT group
The enrolled patients received two doses of intravenous PD-1 inhibitor (camrelizumab, at a dose of 200 mg) every 3 weeks, with two cycles of CT simultaneously. The detailed regimen included albumin paclitaxel (100 mg per square meter of body-surface area) on days 1 and 8, and carboplatin targeted at an area under the curve (AUC) of 5 mg per mL per minute on day 1. Changes in tumor size were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (25). For patients who successfully received two cycles of neoadjuvant therapy, surgeons evaluated the suitability of esophagectomy. The surgery was performed 3–5 weeks after the second dose of CT.
nICRT group
Sintilimab was administered on days 1 and 22 of the neoadjuvant therapy intravenously at a dose of 200 mg. The CT regimen included carboplatin (AUC: 2 mg per mL per minute) and paclitaxel (50 mg per square meter of body-surface area), which were administered intravenously on days 1, 8, 15, 22, and 29. Radiotherapy was performed by means of external-beam radiation, and was started on day 1 of CT. A total radiation dose of 41.4 Gy was given by 23 fractions, with 5 fractions per week, and 1.8 Gy per fraction (26). Surgery was performed 6–8 weeks after the end of radiotherapy.
Surgery protocols
Minimally invasive esophagectomy (MIE), right transthoracic open esophagectomy (OE), or hybrid approaches (a combination of video-assisted thoracoscopy and laparotomy) with a total two- or three-field lymphadenectomy were performed. A gastric tube was used to reconstruct the digestive tract after esophagectomy. Transesophageal or left thoracic esophagectomy was not performed because of the limited lymph node dissection capacity of the above methods, especially for the lymph nodes along the bilateral recurrent laryngeal nerve. To ensure the success of the surgeries, all the operations were conducted by experienced attending surgeons, who had each conducted more than 100 esophagectomies.
Outcome measures
The postoperative complications were divided into the following four categories: pulmonary complications, including pneumonia, pleural effusion, respiratory failure requiring reintubation, and pulmonary embolism; anastomotic complications, including leak, dehiscence, and fistula; cardiac complications, including arrhythmia, pericardial effusion, and myocardial infarction; and other complications including chyle leak, deep venous thrombosis, wound infection, hematological toxicity, and recurrent laryngeal nerve injury. A severe surgical complication was defined as grade ≥3 toxicity, in accordance with the Clavien-Dindo classification system (27). If a patient had multiple complications in the same category of complications, the more severe complication was recorded. Operative time was measured from incision to wound closure. Postoperative hospital stay was defined as the number of hospitalized days from the day of operation to the day of leaving our hospital. Pathological complete response (pCR) was defined as no evidence of residual tumor cells. The primary endpoint was the risk of 30-day complications. The secondary endpoints included the pathological response, operation time, postoperative stay, and 30-day mortality rate.
Statistical analysis
Patient characteristics are presented as either the median and interquartile range, or the frequency and percentage. The nICT and nICRT groups were compared using either a Wilcoxon rank-sum test (continuous variables) or Fisher’s exact test (categorical variables). The relative risk ratios (RRs) of the 30-day perioperative outcomes for the nICT vs. nICRT were estimated using a multivariable log-binomial regression model, with adjustment for a set of predefined clinical factors, including age, gender, smoking, drinking status, clinical stage, and minimally invasive approach. Adjusted RRs were estimated only for outcomes with ≥8 events. A two-sided P value <0.05 was considered statistically significant. The statistical analyses were performed using SPSS Statistics (version 25, IBM, Armonk, NY, USA).
Results
Patient characteristics
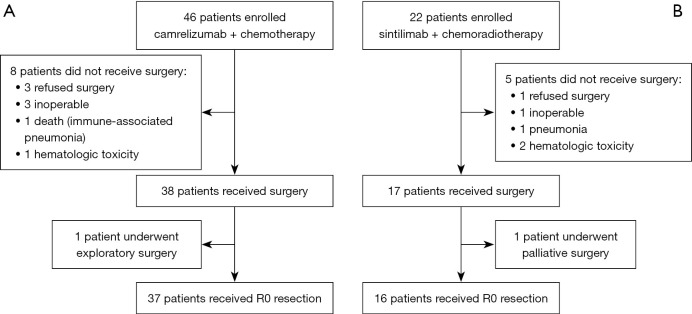
The nICT group comprised 46 patients, of whom, 38 received surgery [37 underwent complete (R0) resection and one underwent exploratory surgery] (Figure 1A). The nICRT group comprised 22 patients, of whom 17 received surgery (16 underwent R0 resection and one received palliative surgery) (Figure 1B). Patient characteristics are summarized in Table 1. Interestingly, the distribution of age, gender, performance status, clinical stage, tumor location, smoking, and drinking history were similar in these two groups. In both groups, there were more male patients than female patients, and a higher percentage of patients were aged between 50 and 75 years, in clinical stage 3, and had squamous cell carcinoma.
Figure 1.
Consort diagram. (A) nICT group; (B) nICRT group. nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy.
Table 1. Baseline demographics and clinical characteristics of patients in nICT and nICRT groups.
| Characteristics | nICT (n=38) | nICRT (n=17) | P value |
|---|---|---|---|
| Age (years), median [range] | 63.3 [50–74] | 62.8 [52–75] | 0.79 |
| Male, n (%) | 36 (94.7) | 13 (76.5) | 0.07 |
| Smoking history, n (%) | 28 (73.7) | 8 (47.1) | 0.07 |
| Drinking history, n (%) | 30 (78.9) | 9 (52.9) | 0.06 |
| Tumor location, n (%) | 0.61 | ||
| Proximal third | 2 (5.3) | 0 | |
| Middle third | 14 (36.8) | 6 (35.3) | |
| Distal third | 22 (57.9) | 11 (64.7) | |
| Clinical T stage, n (%) | 0.04 | ||
| cT2 | 0 | 2 (11.8) | |
| cT3 | 37 (97.4) | 13 (76.5) | |
| cT4a | 1 (2.6) | 2 (11.8) | |
| Clinical N stage, n (%) | 0.08 | ||
| N0 | 11 (28.9) | 2 (11.8) | |
| N1 | 20 (52.6) | 7 (41.2) | |
| N2 | 6 (15.8) | 8 (47.1) | |
| N3 | 1 (2.6) | 0 | |
| Clinical stage, n (%) | 0.52 | ||
| II | 11 (28.9) | 3 (17.6) | |
| III | 25 (65.8) | 12 (70.6) | |
| IVA | 2 (5.3) | 2 (11.8) | |
| Performance status (ECOG), n (%) | 0.47 | ||
| 0 | 29 (76.3) | 15 (88.2) | |
| 1 | 9 (23.7) | 2 (11.8) | |
nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group.
Toxicities of neoadjuvant therapy
In the nICT group, 8 patients (8/46, 17.4%) did not receive surgery, 3 patients (6.5%) refused surgery, 3 patients (6.5%) were inoperable, 1 patient (2.2%) had grade 5 pneumonitis, and 1 patient (2.2%) had hematologic toxicity. In the nICRT group, 5 patients (5/22, 22.7%) did not receive surgery, 1 patient (4.5%) refused surgery, 1 patient (4.5%) was inoperable, 1 patient (4.5%) had pneumonitis, and 2 patients (9.1%) had hematologic toxicity.
The most common treatment-related adverse event (TRAE) during neoadjuvant therapy was hematological toxicity. The incidence of hematologic toxicities was 91.3% (42/46) in the nICT group and 100% (22/22) in the nICRT group. In addition to hematological toxicity, grade III or higher TRAEs included rash (1/46, 2.2%), pneumonitis (1/46, 2.2%), herpes zoster infection (1/46, 2.2%) in the nICT group, and esophagitis (4/22, 18.2%) and pneumonitis (1/22, 4.5%) in the nICRT group.
Surgical outcomes
The surgical details and pathologic outcomes are summarized in Table 2. The median operation time was 289 minutes in the nICT group and 262 minutes in the nICRT group (P=0.27). The median volume of blood loss was similar in the two groups (100 vs. 100 mL, P=0.65). All patients in the nICRT group underwent three-incision esophagectomy, and 2 (5.4%) patients in the nICT group underwent Ivor-Lewis esophagectomy. Most of the patients in the two groups underwent MIE or hybrid MIE (HMIE) (89.2% in nICT group, 94.1% in the nICRT group), 1 (5.9%) patient in the nICRT group converted to open surgery due to local invasion, and 4 (10.8%) patients in the nICT group underwent open surgery. The number of lymph nodes dissected and postoperative hospital stay were similar in the two groups. The pCR was 21.6% (8/37) in the nICT group and 41.2% (7/17) in the nICRT group (P=0.19). The primary tumor pCR was 21.6% (8/37) in the nICT group and 52.9% (9/17) in the nICRT group (P=0.03) (Figure 2). Thirty-day mortality was only observed in 1 (5.9%) patient in the nICRT group due to postoperative anastomotic leakage and pulmonary infection, which eventually led to death from septic shock on the 9th postoperative day.
Table 2. Surgical characteristics of patients in nICT and nICRT groups.
| Characteristics | nICT (n=37)† | nICRT (n=17) | P value |
|---|---|---|---|
| Surgery time (min), median | 289 | 262 | 0.27 |
| Blood loss (mL), median | 100 | 100 | 0.65 |
| Operative approaches, n (%) | 0.99 | ||
| MeKeown | 35 (94.6) | 17 (100.0) | |
| Ivor-Lewis | 2 (5.4) | 0 | |
| Minimally invasive technique, n (%) | 0.35 | ||
| MIE | 28 (75.7) | 11 (64.7) | |
| HMIE | 5 (13.5) | 5 (29.4) | |
| OE | 4 (10.8) | 1 (5.9) | |
| Lymph nodes removed, n | 23 | 16 | 0.05 |
| pCR, n (%) | 8 (21.6) | 7 (41.2) | 0.19 |
| Primary tumor pCR, n (%) | 8 (21.6) | 9 (52.9) | 0.03* |
| Length of hospital stay (days) | 11 | 14 | 0.16 |
| 30-day mortality, n (%) | 0 | 1 (5.9) | 0.31 |
†, one patient in the nICT group underwent open-close surgery, with no resection, so it is not included in the postoperative analysis; *, P<0.05. nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy; MIE, minimally invasive esophagectomy; HMIE, hybrid minimally invasive esophagectomy; OE, open esophagectomy; pCR, pathological complete response.
Figure 2.
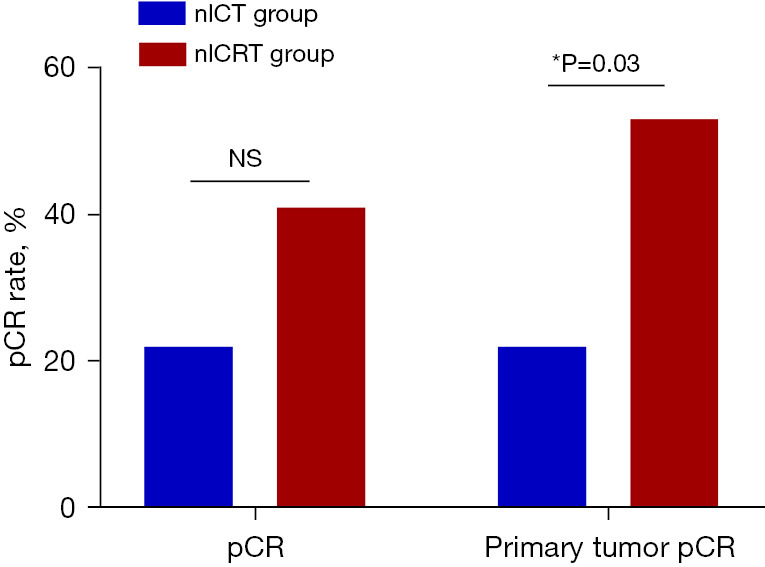
pCR rates between two groups. *, P<0.05. pCR, pathological complete response; NS, not significant; nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy.
Postoperative complications
The postoperative complications within 30 days are summarized in Table 3. The incidence of anastomotic and cardiac complications was similar in both groups. Anastomotic complications occurred in 6 patients (16.2%) in the nICT group and 3 patients (17.6%) in the nICRT group. All the anastomotic complications were severe, requiring further drainage or placement of a gastroscopic fistula drainage tube, as well as antibiotics. The incidence of pulmonary and other complications was numerically higher in the nICRT group than the nICT group, but the difference was not statistically significant difference (Figure 3). In terms of the pulmonary complications, two of eight in the nICT group and two of five in the nICRT group were considered severe, requiring either reintervention, critical care management, or both. After adjustment for age, gender, smoking and drinking status, clinical stage, and the minimally invasive approach, the addition of neoadjuvant radiotherapy did not significantly increase the risk of developing anastomotic [RR: 0.98; 95% confidence interval (CI): 0.757–1.277; P=0.897], pulmonary (RR: 0.90; 95% CI: 0.634–1.279; P=0.56), or other complications (RR: 0.73; 95% CI: 0.473–1.112; P=0.14) in the nICRT group. As only two patients in the nICT group and one in the nICRT group had cardiac complications, the RRs could not be calculated.
Table 3. Thirty-day complications of patients in nICT and nICRT groups.
| Postoperative events | nICT (n=37)† | nICRT (n=17) | P value | Adjusted RR (95% CI) | Adjusted P value |
|---|---|---|---|---|---|
| Anastomotic leakage, n (%) | 6 (16.2) | 3 (17.6) | >0.99 | 0.98 (0.757–1.277) | 0.90 |
| Major anastomotic leakage | 6 (16.2) | 3 (17.6) | >0.99 | 0.98 (0.757–1.277) | 0.90 |
| Pulmonary, n (%) | 8 (21.6) | 5 (29.4) | 0.78 | 0.90 (0.634–1.279) | 0.56 |
| Major pulmonary | 2 (5.4) | 2 (11.8) | 0.79 | – | – |
| Cardiac, n (%) | 2 (5.4) | 1 (5.9) | >0.99 | – | – |
| Major cardiac | 0 (0.0) | 1 (5.9) | 0.69 | – | – |
| Other, n (%) | 7 (18.9) | 7 (41.2) | 0.16 | 0.73 (0.473–1.112) | 0.14 |
| Major other | 4 (10.8) | 4 (23.5) | 0.42 | 0.94 (0.794–1.112) | 0.47 |
†, one patient in the nICT group underwent open-close surgery, with no resection, so it is not included in the postoperative analysis. Other: other complications included chyle leak, deep venous thrombosis, wound infection, hematological toxicity and recurrent laryngeal nerve injury. nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy; RR, risk ratio; CI, confidence interval.
Figure 3.
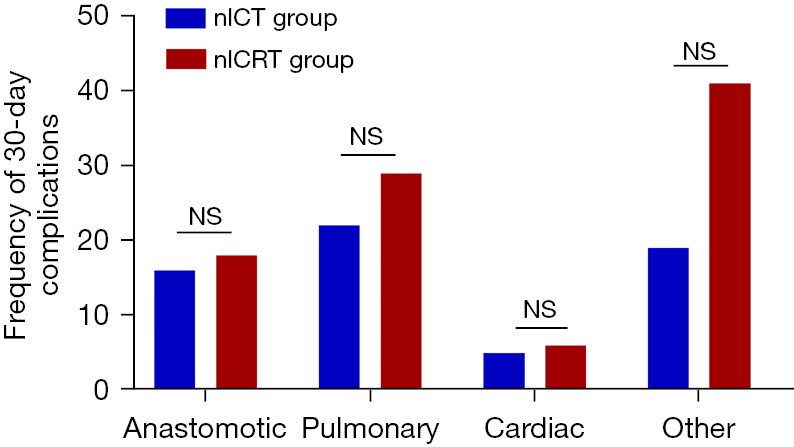
Frequency of 30-day complications between two groups. NS, not significant; nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy.
Discussion
In this study, we assessed the feasibility and safety of esophagectomy after a combination of PD-1 inhibitor and preoperative CT or CRT in ESCC in two clinical trials. According to our preliminary data, there were no statistically significant differences between the nICT and nICRT groups in terms of the surgery-related data and postoperative complications. Immune-related adverse events were relatively infrequent and also did not significantly increase the postoperative complications. ICIs combined with CT have been proven to be safe and feasible in previous large sample-sized studies (14,28), and the addition of radiotherapy did not result in a significantly increased risk of postoperative complications or mortality.
Esophagectomy is undoubtedly the most challenging technique for surgeons. Therefore, perioperative safety and short-term outcomes after new neoadjuvant regimens in patients with esophageal cancer are primary concerns of surgeons. The median operation time and blood loss was similar in the two groups, which indicates that the addition of radiotherapy did not significantly increase surgery feasibility compared to that of the nICT group. Further, based on the experiences of the surgeons in the present study, we would like to hypothesize that tumors tended to adhere more loosely to the surrounding tissues, and were thus easier to remove after neoadjuvant therapies. This was quite different from the condition observed in patients with lung cancer after neoadjuvant ICI therapy, where the tissues surrounding the tumors were more likely to have hilar inflammation and fibrosis, as reported by a phase-I trial examining the use of neoadjuvant nivolumab in patients with resectable non-small cell lung cancer (29). However, in our study, fibrosis in the adventitia of the esophagus was rarely observed, which is similar to the findings of Sihag et al. (23). This suggests that responses to ICIs vary in different cancer types.
Another concern is the difference in the pCR rates between these two immunotherapy regimens. The pCR rate was numerically higher in the nICRT group than the nICT group (41.2% vs. 21.6%, P=0.19), but the difference was not statistically significant. When we focused on the primary tumor pCR rate, we found that the nICRT group had a higher pCR rate, and the difference was statistically significant (52.9% vs. 21.6%, P=0.03), which suggests that the addition of neoadjuvant radiotherapy better controlled the local tumor. However, the pCR rate of the nICRT group was similar with that of the nCRT group in two previous nCRT studies (26,30). Therefore, the long-term survival benefits of nICRT regimens require further investigation and we look forward to the results of phase II/III study EA2174 (No. NCT03604991) which compared patients received nCRT with or without nivolumab.
In the subgroup analysis of the NEOCRTEC 5010 study, the pCR patients had a significantly lower risk of recurrence than the non-pCR patients (15.0% vs. 48.1%, P<0.001) (3). Additionally, other studies have shown that patients with pCR have a higher survival rate, and reported a positive correlation between the response to neoadjuvant treatment and long-term survival regardless of the histology (31-35). In the current neoadjuvant treatment regimen for esophageal cancer, patients in the nICRT group had a higher pCR rate than those in the nCT group (36). However, follow-up research needs to be conducted to determine whether patients with higher pCR rates have a longer survival period.
Kamarajah et al. analyzed the National Cancer Database [2006–2015] and found that of the ESCC patients who underwent neoadjuvant therapy, those in the nCRT group had a higher pCR rate than nCT group (50.9% vs. 30.4%, P<0.001). A statistically significant overall survival (OS) benefit was evident for nCRT (hazard ratio, 0.78; 95% CI: 0.62 to 0.97). The 5-year survival rates for patients who had nCRT and nCT were 45.0% and 38.0%, respectively (P=0.026) (37). However, a study comparing the long-term results between nCRT and nCT have reported that the significantly higher pCR rate in the nCRT group did not lead to longer survival (38). A three-arm phase-III trial (JCOG1109, NExT study) (39) showed that nCRT did not significantly improve OS compared to nICT in the treatment of locally advanced ESCC; however, the nCRT group had the highest pCR rate in this three-arm trial. Considering the different immunotherapy regimens, large-sample long-term follow-up studies need to be conducted to confirm whether improving local control in the nICRT group also improves the long-term survival of patients.
To the best of our knowledge, this is the first study to compare the surgical outcomes between nICT and nICRT for locally advanced ESCC. However, our study had several limitations. First, the above two studies were only conducted at a single center with a relatively small sample size of patients. Second, the patients in the two groups were treated with two different PD-1 inhibitors produced by different companies. However, given the achievements of PD-1 inhibitors in treating advanced esophageal cancer, randomized controlled trials with larger patient sample sizes need to be conducted to determine whether either nICT or nICRT could become a new treatment mode for locally advanced esophageal cancer.
Conclusions
Our preliminary results suggest that esophagectomy may be both safe and feasible following nICT and nICRT for locally advanced esophageal cancer. The primary tumor pCR rate was higher in the nICRT group than the nICT group. Postoperative morbidity and mortality were similar in patients treated with nICT or nICRT. A longer follow-up period and more prospective comparative studies need to be conducted to confirm the long-term clinical outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to thank the patients, nurses, and clinicians for their participation in this study. The abstract of this article has been presented in the ASCO Annual Meeting 2024.
Funding: This study was supported by grants from the Zhejiang Medical and Health Science and Technology Project (Nos. 2020KY481 and 2022RC115), the National Natural Science Foundation of China (Nos. 82202827 and 82272744), and the China Postdoctoral Science Foundation (No. 2022M723603).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. These two studies were conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent for their enrollment in the studies, and the studies were approved by the Ethics Committee of the Zhejiang Cancer Hospital, China (Nos. IRB-2019-38 and IRB-2020-183).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-295/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-295/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-295/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-295/coif). R.C. and M.C. are from the United Laboratory of Frontier Radiotherapy Technology of Sun Yat-Sen University & Chinese Academy of Sciences Ion Medical Technology Co., Ltd., Guangzhou, China. The other authors have no conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Wen J, Yang H, et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer 2020;138:113-21. 10.1016/j.ejca.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956-65. 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020;126:4156-67. 10.1002/cncr.33033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuky J, Balar AV, Castellano D, et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 2020;38:2658-66. 10.1200/JCO.19.01213 [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 14.Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 15.Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022;40:277-288.e3. 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 2022;377:e068714. 10.1136/bmj-2021-068714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 18.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. 10.1056/NEJMoa2032125 [DOI] [PubMed] [Google Scholar]
- 19.Shen D, Chen Q, Wu J, et al. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol 2021;12:1-10. 10.21037/jgo-20-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. 10.1136/jitc-2021-003497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021;144:232-41. 10.1016/j.ejca.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 22.Yin J, Yuan J, Li Y, et al. Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial. Nat Med 2023;29:2068-78. 10.1038/s41591-023-02469-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg 2021;161:836-843.e1. 10.1016/j.jtcvs.2020.11.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong ZN, Gao L, Weng K, et al. Safety and Feasibility of Esophagectomy Following Combined Immunotherapy and Chemotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Matching Analysis. Front Immunol 2022;13:836338. 10.3389/fimmu.2022.836338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. 10.1016/j.jtcvs.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol 2017;43:1607-16. 10.1016/j.ejso.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 32.van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg 2013;100:267-73. 10.1002/bjs.8968 [DOI] [PubMed] [Google Scholar]
- 33.Vallböhmer D, Hölscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg 2010;252:744-9. 10.1097/SLA.0b013e3181fb8dde [DOI] [PubMed] [Google Scholar]
- 34.Xi M, Hallemeier CL, Merrell KW, et al. Recurrence Risk Stratification After Preoperative Chemoradiation of Esophageal Adenocarcinoma. Ann Surg 2018;268:289-95. 10.1097/SLA.0000000000002352 [DOI] [PubMed] [Google Scholar]
- 35.Verlato G, Zanoni A, Tomezzoli A, et al. Response to induction therapy in oesophageal and cardia carcinoma using Mandard tumour regression grade or size of residual foci. Br J Surg 2010;97:719-25. 10.1002/bjs.6949 [DOI] [PubMed] [Google Scholar]
- 36.Jing SW, Qin JJ, Liu Q, et al. Comparison of neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy for esophageal cancer: a meta-analysis. Future Oncol 2019;15:2413-22. 10.2217/fon-2019-0024 [DOI] [PubMed] [Google Scholar]
- 37.Kamarajah SK, Phillips AW, Ferri L, et al. Neoadjuvant chemoradiotherapy or chemotherapy alone for oesophageal cancer: population-based cohort study. Br J Surg 2021;108:403-11. 10.1093/bjs/znaa121 [DOI] [PubMed] [Google Scholar]
- 38.Zhang G, Zhang C, Sun N, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol 2022;148:943-54. 10.1007/s00432-021-03659-7 [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. 10.1093/jjco/hyt061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as