Abstract
Background
One of the most important adverse effects of anthracyclines is cardiotoxicity. A well‐informed decision on the use of anthracyclines in the treatment of childhood cancers should be based on evidence regarding both antitumour efficacy and cardiotoxicity. This review is the second update of a previously published Cochrane review.
Objectives
To compare antitumour efficacy (survival and tumour response) and cardiotoxicity of treatment including or not including anthracyclines in children with childhood cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6), MEDLINE (1966 to July 2013) and EMBASE (1980 to July 2013). In addition, we searched reference lists of relevant articles and conference proceedings, the International Society for Paediatric Oncology (SIOP) (from 2002 to 2012) and American Society of Clinical Oncology (ASCO) (from 2002 to 2013). We have searched for ongoing trials in the ISRCTN register and the National Institute of Health register (both screened August 2013) (http://www.controlled‐trials.com).
Selection criteria
Randomised controlled trials (RCTs) comparing treatment of any type of childhood cancer with and without anthracyclines and reporting outcomes concerning antitumour efficacy or cardiotoxicity.
Data collection and analysis
Two review authors independently performed the study selection, risk of bias assessment and data extraction. Analyses were performed according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We identified RCTs for seven types of tumour, acute lymphoblastic leukaemia (ALL) (three trials; 912 children), Wilms' tumour (one trial; 316 children), rhabdomyosarcoma and undifferentiated sarcoma (one trial; 413 children), Ewing's sarcoma (one trial; 94 children), non‐Hodgkin lymphoma (one trial; 284 children), hepatoblastoma (one trial; 255 children) and acute myeloid leukaemia (AML) (one trial; 394 children). All studies had methodological limitations. For ALL no evidence of a significant difference in antitumour efficacy was identified in the meta‐analyses, but in most individual studies there was a suggestion of better antitumour efficacy in patients treated with anthracyclines. For both Wilms' tumour and Ewing's sarcoma a significant difference in event‐free and overall survival in favour of treatment with anthracyclines was identified, although for Wilms' tumour the significant difference in overall survival disappeared with long‐term follow‐up. For rhabdomyosarcoma and undifferentiated sarcoma, non‐Hodgkin lymphoma and hepatoblastoma no difference in antitumour efficacy between the treatment groups was identified. The same was true for AML, with the exception of overall survival in a post hoc analysis in a subgroup of patients with relapsed core binding factor (CBF)‐AML in which patients treated with anthracyclines did better. Clinical cardiotoxicity was evaluated in four RCTs; no significant difference between the treatment groups was identified, but in all individual studies there was a suggestion of a lower rate of clinical cardiotoxicity in patients who did not receive anthracyclines. None of the studies evaluated asymptomatic cardiac dysfunction. No RCTs were identified for other childhood cancers.
Authors' conclusions
At the moment no evidence from RCTs is available which underscores the use of anthracyclines in ALL. However, 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. For Wilms' tumour, rhabdomyosarcoma and undifferentiated sarcoma, Ewing's sarcoma, non‐Hodgkin lymphoma, hepatoblastoma and AML only one RCT was available for each type and, therefore, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in these tumours. For other childhood cancers no RCTs were identified and therefore no conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in these tumours.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Infant, Newborn; Anthracyclines; Anthracyclines/adverse effects; Anthracyclines/therapeutic use; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/adverse effects; Antibiotics, Antineoplastic/therapeutic use; Bone Neoplasms; Bone Neoplasms/drug therapy; Heart Diseases; Heart Diseases/chemically induced; Hepatoblastoma; Hepatoblastoma/drug therapy; Kidney Neoplasms; Kidney Neoplasms/drug therapy; Leukemia, Myeloid, Acute; Leukemia, Myeloid, Acute/drug therapy; Liver Neoplasms; Liver Neoplasms/drug therapy; Lymphoma, Non‐Hodgkin; Lymphoma, Non‐Hodgkin/drug therapy; Neoplasms; Neoplasms/drug therapy; Precursor Cell Lymphoblastic Leukemia‐Lymphoma; Precursor Cell Lymphoblastic Leukemia‐Lymphoma/drug therapy; Randomized Controlled Trials as Topic; Sarcoma; Sarcoma/drug therapy; Wilms Tumor; Wilms Tumor/drug therapy
Plain language summary
Treatment with or without anthracycline chemotherapy for childhood cancer
Anthracyclines are used in the treatment of different types of childhood cancer. Unfortunately, one of the most important adverse effects of anthracyclines is damage to the heart. This can become manifest not only during treatment but also years after the end of treatment. A well‐informed decision on the use of anthracyclines in the treatment of different types of childhood cancer should be based on the available evidence on both the antitumour effects of anthracyclines and the risk of damage to the heart.
This systematic review focused on randomised studies evaluating the antitumour effects of anthracycline therapy. The authors found that at the moment no high quality evidence is available which shows that the use of anthracyclines has an increased antitumour effect in acute lymphoblastic leukaemia (ALL) as compared to treatment without anthracyclines, but there was some suggestion that this might be the case. Further high quality studies are needed to provide a definitive conclusion. For Wilms' tumour, rhabdomyosarcoma and undifferentiated sarcoma, Ewing's sarcoma, non‐Hodgkin lymphoma, hepatoblastoma and acute myeloid leukaemia (AML) the review authors found only limited data and were unable to draw conclusions. Also, there were no data for other childhood cancers. More high quality research is needed. At the moment there are five ongoing or unpublished randomised studies evaluating the use of anthracyclines in the following types of childhood cancer, hepatoblastoma, ALL (two studies), rhabdomyosarcoma, and Wilms' tumour.
Background
Anthracyclines, like doxorubicin, daunorubicin and epirubicin, have gained widespread use in the treatment of numerous childhood cancers, both solid tumours and haematological malignancies. Nearly 60% of children diagnosed with a malignancy receive anthracyclines as part of their treatment.
Unfortunately, one of the most important side effects of anthracyclines is cardiotoxicity (that is damage to the heart), which has been known since their introduction (Lefrak 1973). The damage can become manifest in patients as either clinical heart failure (Von Hoff 1979) or asymptomatic cardiac dysfunction (Lipshultz 2005). Asymptomatic cardiac dysfunction includes various cardiac abnormalities diagnosed with different diagnostic methods, like echocardiography, nuclear angiography, cardiac biopsy or cardiac markers, in asymptomatic patients. Anthracycline‐induced cardiotoxicity is a widely prevalent problem in children; the incidence of clinical heart failure has been reported to be as high as 16% 0.9 to 4.8 years after treatment (Kremer 2002a) and the prevalence of asymptomatic cardiac dysfunction has been reported to be more than 57% at a median of 6.4 years after treatment (Kremer 2002b). The risk of anthracycline‐induced cardiotoxicity is dose‐dependent. In a cohort study of 830 children a cumulative anthracycline dose of 300 mg/m² or more produced an eight‐fold higher risk of clinical heart failure as compared to lower doses (less than 300 mg/m²) (Van Dalen 2006). The consequences of anthracycline‐induced cardiotoxicity are extensive. It can lead to long‐term side effects, causing severe morbidity and reduced quality of life. The cardiotoxicity involves long‐term treatment and thus high medical costs and it causes premature death. The excess mortality due to cardiac disease is eight‐fold higher than expected for long‐term survivors of childhood cancer compared to the normal population (Mertens 2001).
If anthracycline therapy does not have an added value with regard to tumour response and survival compared to treatment without anthracyclines, it should not be used in treatment protocols for childhood cancer. As a result, anthracycline‐induced cardiotoxicity would not be an issue. Although ample evidence supports the antileukaemic activity of anthracyclines administered as a single drug, data supporting anthracycline use in modern multi‐drug combinations, which now constitute the mainstay of current acute lymphoblastic leukaemia (ALL) treatments, are lacking. It is unclear if the use of anthracyclines improves the outcome (Messinger 1999). Also, in a randomised controlled trial (RCT) in children with advanced stage non‐lymphoblastic non‐Hodgkin's lymphoma, the addition of daunorubicin to treatment with COMP (intrathecal arabinofuranosyl cytidine (ARA‐C), cyclophosphamide, vincristine, methotrexate and prednisone) did not improve the prognosis; children treated with daunorubicin had an event‐free survival of 57%, whereas in children treated without daunorubicin the event‐free survival was 55% (no significant difference) (Sposto 2001).
This is the second update of the first systematic review evaluating the state of the evidence on the use of anthracyclines in the treatment of childhood cancer.
Objectives
Primary objective:
to compare survival in children with any type of malignancy receiving anthracyclines as part of their treatment with survival in children not receiving anthracyclines as part of their treatment.
Secondary objectives:
to compare tumour response in both treatment groups;
to compare cardiotoxicity in both treatment groups.
Methods
Criteria for considering studies for this review
Types of studies
RCTs comparing treatment of childhood cancer with and without anthracyclines.
Types of participants
Children (aged 0 to 18 years at diagnosis) with any type of malignancy at any stage. RCTs including both children and adults were only eligible for inclusion in this review if the majority of participants were children and the maximal age of the participants did not exceed 30 years.
Types of interventions
Treatment with and without anthracyclines. Therapy other than anthracyclines (that is chemotherapy, cardioprotective interventions, radiotherapy or surgery, or a combination) should have been the same in both treatment groups. Timing of different aspects of the treatment may have differed between the study groups, but the cumulative doses of therapy other than anthracyclines should not have differed more than 25% between the study groups. Furthermore, prior treatment should have been comparable in both treatment groups.
Types of outcome measures
Primary outcomes
Survival (overall survival and event‐free survival as defined by the authors of the original study)
Secondary outcomes
Tumour response (as defined by the authors of the original study)
Anthracycline‐induced cardiotoxicity (i.e. clinical heart failure (as defined by the authors of the original study) or asymptomatic cardiac dysfunction (defined as either histological abnormalities according to the Billingham score (Billingham 1978) on myocardial biopsies or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography))
Search methods for identification of studies
The following electronic databases have been searched: The Cochrane Central Library of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6), MEDLINE in PubMed (from 1966 to July 2013), and EMBASE in Ovid (from 1980 to July 2013). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text word terms) are detailed in the appendices (Appendix 1, Appendix 2, Appendix 3).
Information about trials not registered in CENTRAL, MEDLINE or EMBASE, either published or unpublished, was located by searching the reference lists of relevant articles and review articles. We also scanned the conference proceedings of the International Society for Paediatric Oncology (SIOP) (from 2002 to 2012) and American Society of Clinical Oncology (ASCO) (from 2002 to 2013), if available electronically and otherwise by handsearching. We have searched for ongoing trials in the ISRCTN register and the National Institute of Health register (both screened August 2013) (http://www.controlled‐trials.com). Language restrictions were not imposed.
Data collection and analysis
Selection of studies
For the original version of the review, after employing the search strategy described previously, initial screening of identified references was performed by one review author. Case reports, studies only including adults, studies in which all patients received anthracyclines, and review articles were excluded. Identification of studies meeting the inclusion criteria from the remaining references was undertaken by two review authors working independently. Any study which seemed to meet the inclusion criteria on the grounds of the title or abstract, or both, was obtained in full for closer inspection. Again, for the original version of the review, initial screening was performed by one review author who excluded case reports, studies only including adults, studies in which all patients received anthracyclines, and review articles. The remaining full text articles were evaluated by two independent review authors. For both updates of the review, two independent review authors performed all steps of the study identification process (that is no initial screening by one review author). Details of the reasons for exclusion of any study considered for the review were clearly stated. Discrepancies between review authors were resolved by consensus or if that was not possible by third party arbitration.
Data extraction and management
Two review authors independently performed the data extraction using standardised forms. Data on the following items were extracted:
(1) Study design.
(2) Risk of bias items.
(3) Participants, including: a. age; b. sex; c. type of tumour; d. stage of disease; e. primary tumour or recurrence; f. prior treatment; g. number of patients entering the trial; h. number of patients randomised; i. number of patients excluded (with reasons); j. number of patients evaluable (for each outcome).
(4) Interventions, including: a. type of anthracycline; b. cumulative anthracycline dose; c. anthracycline peak dose (defined as the maximal dose received in one week); d. anthracycline infusion duration; e. other treatment, including: i. chemotherapy (agent and cumulative dose), ii. radiotherapy (location and cumulative dose), iii. surgery (location and procedure), iv. cardioprotective interventions (method).
(5) Outcome measures.
(6) Length of follow‐up.
Discrepancies between review authors were resolved by consensus. No third party arbitration was needed.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included RCTs. For this second update we used the most recent recommendations of the Childhood Cancer Group (that is selection bias, performance bias, detection bias (for each outcome separately), attrition bias (for each outcome separately), reporting bias (where 'all expected outcomes' was defined as reporting on both overall survival and cardiotoxicity and at least one of the following outcomes: event‐free survival or tumour response) and other bias). We used the 'risk of bias' items and definitions of low risk, unclear risk and high risk as described in the module of the Cochrane Childhood Cancer Group (Kremer 2008), which is based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All RCTs (including those already included in earlier versions of the review) were scored using the new 'risk of bias' items. Discrepancies between authors were resolved by discussion. No third party arbitration was needed. The risk of bias in the included studies was taken into account in the interpretation of the review's results.
Data synthesis
Data were entered into RevMan and analysed according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). Dichotomous variables were related to risk using the relative risk or risk ratio (RR). If possible, data were extracted by allocation intervention, irrespective of compliance with the allocated intervention, in order to allow an 'intention‐to‐treat' (ITT) analysis. If this was not possible, this was stated and we performed an 'as treated' analysis. We assessed heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is the I² statistic. If there was evidence of substantial heterogeneity (I² > 50%) (Higgins 2009) this was reported. Studies for which pooling of results was not possible were summarised descriptively. We used a random‐effects model for the estimation of treatment effects throughout the review. All results were presented with the corresponding 95% confidence interval (CI). For the assessment of survival, we used the generic inverse variance function of RevMan to combine logs of the hazard ratios (HRs). We used Parmar's method if HRs had not been explicitly presented in the study (Parmar 1998). Data were analysed separately for different types of tumour and, if possible, also for different stages of disease and different histological subtypes. When a particular outcome was not evaluated in more than 50% of the patients of a study, due to the associated high risk of attrition bias we did not report the results of this outcome measure. For all outcomes for which pooled analyses were possible we performed sensitivity analyses for all risk of bias criteria separately. We excluded studies with a high risk of bias and studies for which the risk of bias was unclear and compared the results of the studies with a low risk of bias with the results of all available studies. The risk of bias in the studies included in the analyses was taken into account in the interpretation of the results of the review. We were not able to construct a funnel plot to graphically ascertain the existence of publication bias. As a rule of thumb, tests for funnel plot asymmetry should be used only when there are at least 10 studies included in a meta‐analysis. When there are fewer studies the power of the test is too low to distinguish chance from real asymmetry (Higgins 2009). Since only a maximum of three trials could be included in the separate meta‐analyses, we did not construct funnel plots. For outcomes where only one study was available, we were unable to calculate a RR if one of the treatment groups experienced no events and the Fischer’s exact test was used instead; this option is not available in Revman and therefore we used http://graphpad.com/quickcalcs/contingency2/.
Results
Description of studies
Running the searches in the electronic databases of CENTRAL, MEDLINE in PubMed and EMBASE in Ovid (in January 2007) yielded a total of 3277 references. Initial screening excluded 987 references based on them being case reports, review articles, studies only including adults, or studies in which all patients received anthracyclines. Of the 2290 remaining references 135 studies were assessed in full. Of the 67 articles remaining after initial screening, we included a total of seven articles which fulfilled all the criteria for this review. A total of 128 articles were excluded after assessing the full text articles, for reasons described in the Characteristics of excluded studies table. The remaining 2155 references were excluded based on the title or abstract, or both, since they were not a RCT, were laboratory studies, were animal studies, did not include children with cancer, or there was a difference in treatment other than anthracyclines between the treatment groups.
Running the searches for the update in CENTRAL, MEDLINE in PubMed and EMBASE in Ovid (in March 2010) yielded a total of 1032 new references. Following screening of the titles, abstracts, or both, 1000 references which clearly did not meet all criteria for considering studies for this review were excluded. We obtained 32 articles in full, of which a single article fulfilled all the criteria for considering studies for this review (Perilongo 2009). The other 31 articles were excluded for reasons described in the Characteristics of excluded studies table.
Running the searches for the second update in CENTRAL, MEDLINE in PubMed and EMBASE in Ovid (in July 2013) yielded a total of 1167 new references. Following screening of the titles, abstracts, or both, 1151 references which clearly did not meet all criteria for considering studies for this review were excluded. We obtained 16 articles in full (five of these were only available as a conference proceeding), of which a single article fulfilled all the criteria for considering studies for this review (Kaspers 2013). Thirteen articles were excluded for reasons described in the Characteristics of excluded studies table; two studies have not been published in full yet (see Characteristics of studies awaiting classification table).
Scanning the reference lists of relevant articles and reviews did not identify any additional eligible studies. We did identify three ongoing trials during the original review. At the time of the first update one ongoing trial identified in the original version of this review was published in full text and identified in the update of the electronic database searches (Perilongo 2009); this trial was thus removed from the Characteristics of ongoing studies table. At the time of the second update it became clear that the SIOP‐2001 trial was closed and preliminary results had been presented as a conference proceeding (identified in the second update of the electronic database searches as described above); this trial was thus removed from the Characteristics of ongoing studies table. Eleven other studies (nine from the original version and two from the first update) were added to the Characteristics of excluded studies table.
By scanning the conference proceedings of SIOP and ASCO for the original version, we identified one study (described in two abstracts) that had not been published in full yet and was awaiting further assessment during the original search. At the time of the updates this study is still not published in full (see the Characteristics of studies awaiting classification table); no other additional eligible studies were identified during the updates.
By scanning the ongoing trials databases for the original version we identified three additional ongoing trials (see the Characteristics of ongoing studies table); no other additional eligible studies were identified during the updates but it became clear that the ISRCTN94206677 and the NCT00186966 trials were in fact the same study and that the study was published in full text and identified in the second update of the electronic database searches (Kaspers 2013); these publications were thus removed from the Characteristics of ongoing studies table.
Finally, during the first update an expert in the field provided us with long‐term follow‐up data (Green 2004) of one of the included studies (D'Angio 1981).
In summary, after the second update the total number of included RCTs was nine. We also identified two ongoing studies and three studies that have not been published in full yet and are awaiting further assessment.
Characteristics of the included studies are summarised below; for more information we refer to the Characteristics of included studies table.
The total number of patients included in the nine identified RCTs was 2668: 1318 children received no anthracyclines, whereas 1350 did receive anthracyclines. In three studies children were diagnosed with ALL (Eden 1991; Van der Does 1975; Van der Does 1989); and in one with acute myeloid leukemia (AML) (Kaspers 2013). In the other five studies they were diagnosed with a solid tumour: Wilms' tumour (D'Angio 1981), rhabdomyosarcoma or undifferentiated sarcoma (Maurer 1988), Ewing's sarcoma (Nesbit 1990), non‐Hodgkin lymphoma (Sposto 2001) or hepatoblastoma (Perilongo 2009). In four studies patients were treated with daunorubicin (Eden 1991; Sposto 2001; Van der Does 1975; Van der Does 1989). In all these studies the cumulative daunorubicin dose actually received by the patients was not mentioned, but according to protocol patients should have received 90 to 350 mg/m². The peak anthracycline dose (that is the maximal dose received in one week) ranged from 25 to 90 mg/m². Infusion durations were not mentioned. In four studies patients were treated with doxorubicin (D'Angio 1981; Maurer 1988; Nesbit 1990; Perilongo 2009). In all these studies the cumulative doxorubicin dose actually received by the patients was not mentioned, but according to protocol patients should have received either (maximal) 300 or 420 mg/m². The peak anthracycline dose (that is the maximal dose received in one week) was either 25 or 60 mg/m². Infusion durations were not mentioned in three studies, in the other study it was 30 mg/m2/24 hours. In the final study patients were treated with daunoxome, that is liposomally entrapped daunorubicin (Kaspers 2013). The cumulative daunoxome dose actually received by the patients was not mentioned, but according to protocol patients should have received 180 mg/m2. The peak anthracycline dose (that is the maximal dose received in one week) was 180 mg/m2. The infusion duration was not mentioned.
Risk of bias in included studies
See the risk of bias section of the Characteristics of included studies table and Figure 1 for the exact scores per included study.
1.
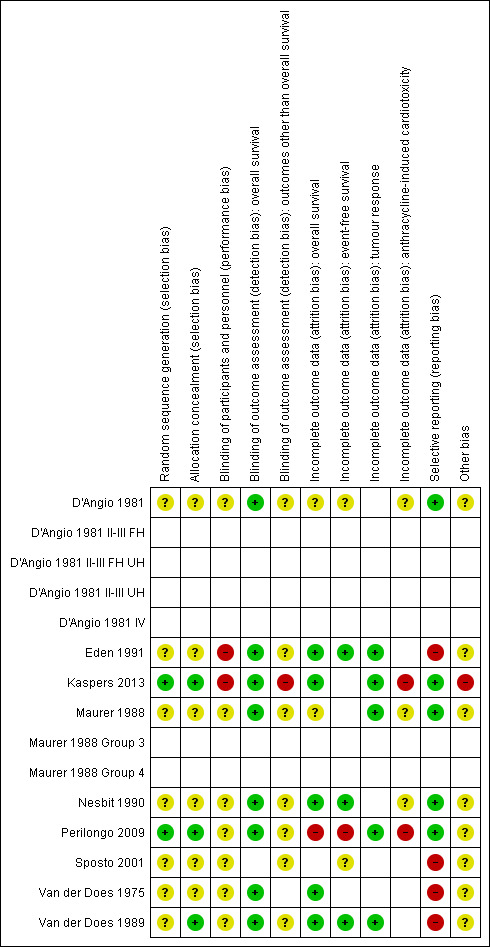
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For evaluating selection bias we have assessed the random sequence generation and the allocation concealment. The risk of selection bias was low in two studies (22%) (Kaspers 2013; Perilongo 2009), while in seven studies (78%) it was unclear (D'Angio 1981; Eden 1991; Maurer 1988; Nesbit 1990; Sposto 2001; Van der Does 1975; Van der Does 1989). For the latter study only the random sequence generation was unclear and the allocation concealment was adequate; for the other six studies both items were unclear.
Blinding
For evaluating performance bias we have assessed the blinding of participants and personnel. In two studies (22%) there was a high risk of bias (Eden 1991; Kaspers 2013), while in seven studies (78%) it was unclear (D'Angio 1981; Maurer 1988; Nesbit 1990; Perilongo 2009; Sposto 2001; Van der Does 1975; Van der Does 1989).
For evaluating detection bias we have evaluated the blinding of outcome assessors for all separate outcomes, with the exception of overall survival since for that outcome blinding is not relevant and the risk of detection bias was thus automatically judged as low for all eight studies (100%) evaluating this outcome (Eden 1991; Kaspers 2013; Maurer 1988; Nesbit 1990; Perilongo 2009; Van der Does 1975; Van der Does 1989). Six studies evaluated event‐free survival; in all studies (100%) the risk of detection bias was unclear (D'Angio 1981; Eden 1991; Nesbit 1990; Sposto 2001; Perilongo 2009; Van der Does 1989). Five studies evaluated tumour response; in four studies (80%) the risk of detection bias was unclear (Eden 1991; Maurer 1988; Perilongo 2009; Van der Does 1989) while in one study (20%) the risk was judged to be high (Kaspers 2013). Five studies evaluated cardiotoxicity; in four studies (80%) the risk of detection bias was unclear (D'Angio 1981; Maurer 1988; Nesbit 1990; Perilongo 2009) while in one study (20%) the risk was judged to be high (Kaspers 2013).
Incomplete outcome data
For evaluating attrition bias we have assessed incomplete outcome data for all separate outcomes. Eight studies evaluated overall survival; in five studies (63%) there was a low risk of attrition bias (Eden 1991; Kaspers 2013; Nesbit 1990; Van der Does 1975; Van der Does 1989), in one study (12%) there was a high risk of attrition bias (Perilongo 2009) and in two studies (25%) the risk of attrition bias was unclear (D'Angio 1981; Maurer 1988). Six studies evaluated event‐free survival; in three studies (50%) there was a low risk of attrition bias (Eden 1991; Nesbit 1990; Van der Does 1989), in one study (17%) there was a high risk of attrition bias (Perilongo 2009) and in two studies (33%) the risk of attrition bias was unclear (D'Angio 1981; Sposto 2001). Five studies evaluated tumour response; in all studies (100%) the risk of attrition bias was low (Eden 1991; Kaspers 2013; Maurer 1988; Perilongo 2009; Van der Does 1989). Five studies evaluated cardiotoxicity; in two studies (40%) the risk of attrition bias was high (Kaspers 2013; Perilongo 2009) while in three studies (60%) it was unclear (D'Angio 1981; Maurer 1988; Nesbit 1990).
Selective reporting
For evaluating reporting bias we have assessed selective reporting. We defined 'all expected outcomes' as reporting on both overall survival and cardiotoxicity and at least one of the following outcomes: event‐free survival or tumour response. In five studies (56%) we judged the risk of reporting bias to be low (D'Angio 1981; Kaspers 2013; Maurer 1988; Nesbit 1990; Perilongo 2009) while in four studies (44%) it was judged to be high (Eden 1991; Sposto 2001; Van der Does 1975; Van der Does 1989).
Other potential sources of bias
For evaluating other potential sources of bias we have assessed the following items: block randomisation in unblinded trials, baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment, age, sex, prior cardiac dysfunction), difference in length of follow‐up between treatment arms, and inappropriate influence of funders. In one study (11%) there was a high risk of other bias (Kaspers 2013) while in the other eight studies (89%) the risk was unclear (D'Angio 1981; Eden 1991; Maurer 1988; Nesbit 1990; Perilongo 2009; Sposto 2001; Van der Does 1975; Van der Does 1989). For a more detailed description of all the different items see the risk of bias section of the Characteristics of included studies table.
Effects of interventions
Not all articles allowed data extraction for all endpoints (see the Characteristics of included studies table for a more detailed description of the extractable endpoints of each article).
Overall survival
(See Figure 2)
2.
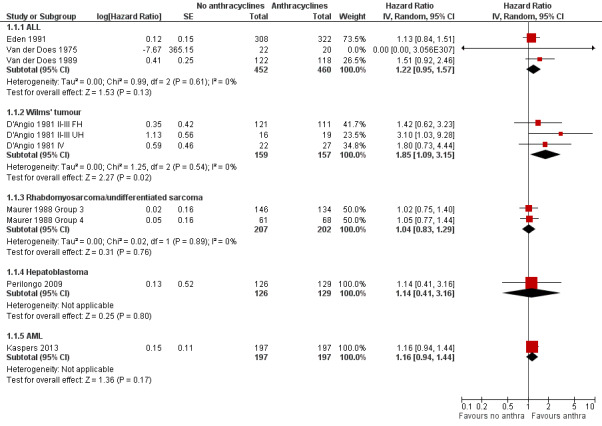
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.1 Overall survival (Parmar's method was used to obtain the necessary data for the meta‐analyses).
ALL
Data on overall survival could be extracted from three trials with a total of 912 patients (Eden 1991; Van der Does 1975; Van der Does 1989). Parmar's method was used to obtain the necessary data for the meta‐analysis. The HR showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.22, 95% CI 0.95 to 1.57, P = 0.13). No heterogeneity was detected (I2 = 0%).
Wilms' tumour
Data on overall survival could be extracted from one trial with a total of 316 patients (D'Angio 1981). Data were presented separately for patients with stage II or III disease with favourable histology, stage II or III with unfavourable histology, and stage IV disease. Parmar's method was used to obtain the necessary data for the analysis. The combination of all patients showed a significant difference in favour of treatment including anthracyclines (HR 1.85, 95% CI 1.09 to 3.15, P = 0.02). No heterogeneity was detected (I2 = 0%). For patients with stage II or III disease with favourable histology and patients with stage IV disease the analyses showed no significant difference between treatment not including and treatment including anthracyclines. However, the analysis of patients with stage II or III with unfavourable histology showed a significant difference in favour of treatment including anthracyclines (HR 3.10, 95% CI 1.03 to 9.28, P = 0.04).
Long‐term follow‐up data of this study have been published (Green 2004; D'Angio 1981 II‐III FH UH; D'Angio 1981 IV) on 275 of 316 patients: 227 patients with stage II or III disease with favourable or unfavourable histology (as opposed to D'Angio 1981 in which data were presented separately for favourable and unfavourable histology) and 48 patients with stage IV disease. The length of follow‐up was not mentioned but at least some of the patients had a follow‐up of 16 years. See Figure 3 for the long‐term follow‐up data. In contrast to the earlier results, the long‐term follow‐up data showed no significant difference between treatment groups (HR 1.27, 95% CI 0.77 to 2.11, P = 0.34). No heterogeneity was detected (I2 = 0%). The long‐term follow‐up data also showed no significant difference between treatment groups for patients with stage II or III disease with favourable or unfavourable histology and for patients with stage IV disease. These results are in line with the earlier data; in D'Angio 1981 the overall survival of patients with stage II or III disease with favourable or unfavourable histology combined was not significantly different between treatment groups (HR 1.92, 95% CI 0.91 to 4.04, P = 0.09, I2 = 19%; data not shown in the figures). Please note that it was not possible to perform an ITT analysis: in the stage IV group 20 patients were included in the no anthracycline group and 28 in the anthracycline group, as opposed to the original data where 22 patients were randomised to the anthracycline group and 27 to the non‐anthracycline group.
3.
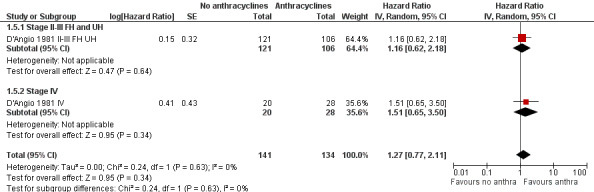
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.5 Overall survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the analyses).
Rhabdomyosarcoma and undifferentiated sarcoma
Data on overall survival could be extracted from one trial with a total of 413 patients (Maurer 1988). Data were presented for patients in clinical groups III and IV separately. Parmar's method was used to obtain the necessary data for the analysis. The combination of both clinical groups showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.04, 95% CI 0.83 to 1.29, P = 0.76). The same was true for each clinical group separately. No heterogeneity was detected (I2 = 0%).
Ewing's sarcoma
Overall survival was evaluated in one trial (Nesbit 1990). Only some of the patients included in this trial were eligible for inclusion in this review (N = 94) and, unfortunately, not all data needed for a correct analysis of overall survival in only the eligible patients were provided in the article. Therefore, we provided descriptive results of overall survival in only the eligible patients. There was evidence of a significant advantage in overall survival for patients treated with anthracyclines as compared to patients treated without anthracyclines (P = 0.02).
Non‐Hodgkin lymphoma
Overall survival could not be evaluated since we were not able to reliably extract the data needed to use Parmar's method for the assessment of this outcome from this study (Sposto 2001).
Hepatoblastoma
Overall survival was evaluated in one trial with a total of 255 patients (Perilongo 2009). Parmar's method was used to obtain the necessary data for the analysis. The HR showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.14, 95% CI 0.41 to 3.16, P = 0.80). Please note that it was not possible to perform an ITT analysis: 12 randomised patients were excluded (seven lacked proper documentation, five had wrong diagnosis; it was unclear to which treatment group these patients were randomised).
AML
Overall survival was evaluated in one trial with a total of 394 patients (Kaspers 2013). Parmar's method was used to obtain the necessary data for the analysis. The HR showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.16, 95% CI 0.94 to 1.44, P = 0.17).
Event‐free survival
(See Figure 4)
4.
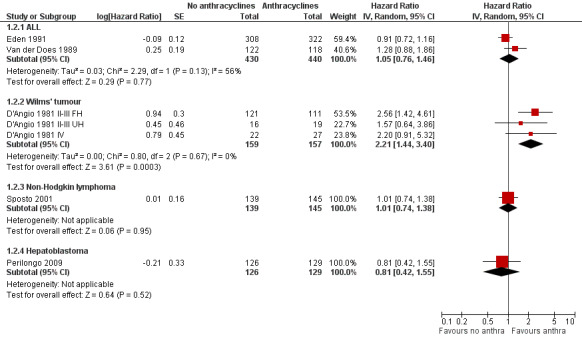
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.2 Event‐free survival (Parmar's method was used to obtain the necessary data for the meta‐analyses).
ALL
Data on event‐free survival could be extracted from two trials with a total of 870 patients (Eden 1991; Van der Does 1989). Parmar's method was used to obtain the necessary data for the meta‐analysis. The HR showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.05, 95% CI 0.76 to 1.46, P = 0.77). However, unexplained heterogeneity was detected (I2 = 56%). In the study of Van der Does 1975 no information on event‐free survival was provided.
Wilms' tumour
Data on event‐free survival could be extracted from one trial with a total of 316 patients (D'Angio 1981). Data were presented separately for patients with stage II or III disease with favourable histology, stage II or III with unfavourable histology, and stage IV disease. Parmar's method was used to obtain the necessary data for the analysis. The combination of all patients showed a significant difference in favour of treatment including anthracyclines (HR 2.21, 95% CI 1.44 to 3.40, P = 0.0003). No heterogeneity was detected (I2 = 0%). The analysis of patients with stage II or III disease with favourable histology also showed a significant difference in favour of treatment including anthracyclines (HR 2.56, 95% CI 1.42 to 4.61, P = 0.002). However, for patients with stage II or III disease with unfavourable histology and patients with stage IV disease the analyses showed no significant difference between treatment not including and treatment including anthracyclines.
Long‐term follow‐up data of this study have been published (Green 2004; D'Angio 1981 II‐III FH UH; D'Angio 1981 IV) on 275 of 316 patients: 227 patients with stage II or III disease with favourable or unfavourable histology (as opposed to D'Angio 1981, data were not presented separately for favourable and unfavourable histology) and 48 patients with stage IV disease. The length of follow‐up was not mentioned but at least some of the patients had a follow‐up of 16 years. See Figure 5 for the long‐term follow‐up data, which also showed a significant difference in favour of treatment including anthracyclines (HR 1.72, 95% CI 1.09 to 2.72, P = 0.02). No heterogeneity was detected (I2 = 0%). For the different stages or histologies the results of the long‐term follow‐up data were also in line with the earlier data, that is a significant difference in favour of treatment including anthracyclines for patients with stage II or III disease with favourable or unfavourable histology (for the long‐term follow‐up: HR 1.80, 95% CI 1.04 to 3.12, P = 0.04; for the earlier follow‐up: HR 2.21, 95% CI 1.35 to 3.62, P = 0.002, I2 = 0%; data not shown in the figures); and no significant difference between treatment groups for patients with stage IV disease. Please note that it was not possible to perform an ITT analysis: in the stage IV group 20 patients were included in the no anthracycline group and 28 in the anthracycline group, as opposed to the original data where 22 patients were randomised to the anthracycline group and 27 to the non‐anthracycline group.
5.
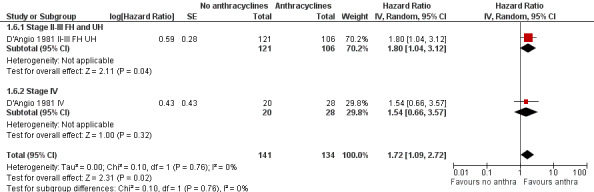
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.6 Event‐free survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the analyses).
Rhabdomyosarcoma and undifferentiated sarcoma
Event‐free survival could not be evaluated since we were not able to reliably extract the data needed to use Parmar's method for the assessment of this outcome from this study (Maurer 1988).
Ewing's sarcoma
Event‐free survival was evaluated in one trial (Nesbit 1990). Only some of the patients included in this trial were eligible for inclusion in this review and, unfortunately, not all data needed for a correct analysis of event‐free survival in only these patients were provided in the article. Therefore, we provide descriptive results of event‐free survival in only the eligible patients. There was evidence of a significant advantage in event‐free survival for patients treated with anthracyclines as compared to patients treated without anthracyclines (P = 0.01).
Non‐Hodgkin lymphoma
Data on event‐free survival could be extracted from one trial with a total of 284 patients (Sposto 2001). Parmar's method was used to obtain the necessary data for the analysis. The HR showed no significant difference between treatment not including and treatment including anthracyclines (HR 1.01, 95% CI 0.74 to 1.38, P = 0.95).
Hepatoblastoma
Event‐free survival was evaluated in one trial with a total of 255 patients (Perilongo 2009). Parmar's method was used to obtain the necessary data for the analysis. The data showed no significant difference between treatment not including and treatment including anthracyclines (HR 0.81, 95% CI 0.42 to 1.55, P = 0.52). Please note that it was not possible to perform an ITT analysis: 12 randomised patients were excluded (seven lacked proper documentation, five had wrong diagnosis; it was unclear to which treatment group these patients were randomised).
AML
No information on event‐free survival was provided (Kaspers 2013).
Tumour response
(See Figure 6)
6.
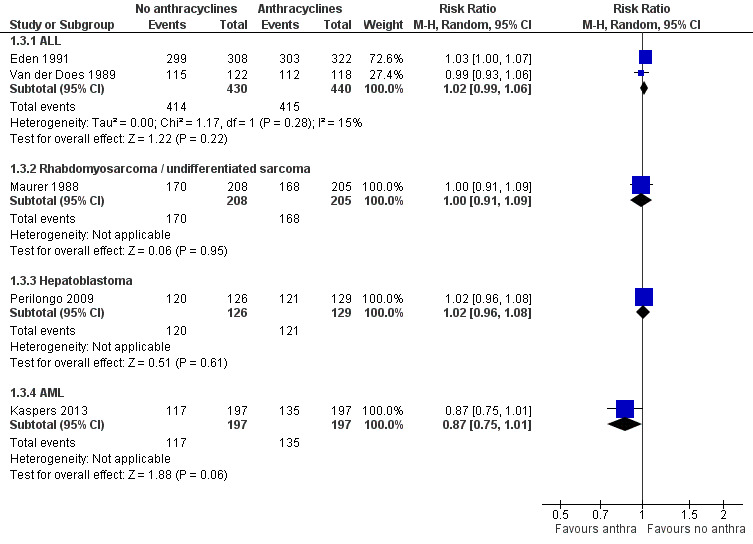
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.3 Tumour response.
Please note that due to the nature of this outcome (that is the number of patients with a remission) a high event rate is favourable. Therefore, in the figures of the analyses, 'favours anthracyclines' is on the left and 'favours no anthracyclines' is on the right, as opposed to the figures of the other analyses.
ALL
Data on tumour response (defined as the number of patients in complete remission) could be extracted from two studies with a total of 870 patients (Eden 1991; Van der Does 1989). The meta‐analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 1.02, 95% CI 0.99 to 1.06, P = 0.22). No substantial heterogeneity was detected (I2 = 15%). We excluded the study of Van der Does 1975 from this analysis since no data on tumour response was provided separately in either treatment group.
Wilms' tumour
No information on tumour response was provided (D'Angio 1981).
Rhabdomyosarcoma and undifferentiated sarcoma
Data on tumour response (defined as the number of patients in complete or partial remission) could be extracted from one trial with a total of 413 patients (Maurer 1988). The analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 1.00, 95% CI 0.91 to 1.09, P = 0.95).
Ewing's sarcoma
No information on tumour response was provided (Nesbit 1990).
Non‐Hodgkin lymphoma
No information on tumour response was provided (Sposto 2001).
Hepatoblastoma
Tumour response (defined as complete surgical resection, that is resection of all tumour sites on the basis of surgical findings and on postsurgical imaging) was evaluated in one trial with a total of 255 patients (Perilongo 2009). The analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 1.02, 95% CI 0.96 to 1.08, P = 0.61). Please note that it was not possible to perform an ITT analysis: 12 randomised patients were excluded (seven lacked proper documentation, five had wrong diagnosis; it was unclear to which treatment group these patients were randomised).
AML
Tumour response (that is complete response after two courses defined as 5% or fewer leukaemic blasts in bone marrow with signs of normal haematopoiesis and of regeneration of normal peripheral blood cell production (platelets > 50 x 109/L without transfusions, neutrophils > 1.0 x 109/L) and no leukaemic cells in the peripheral blood or anywhere else) was evaluated in one trial with a total of 394 patients (Kaspers 2013). The analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 0.87, 95% CI 0.75 to 1.01, P = 0.06).
Cardiotoxicity
(See Figure 7)
7.
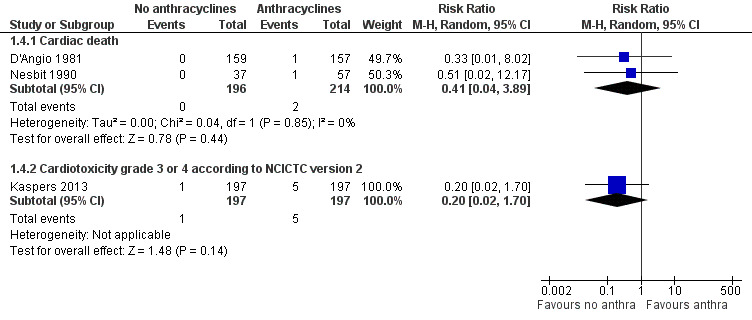
Forest plot of comparison: 1 No anthracyclines versus anthracyclines, outcome: 1.4 Clinical cardiotoxicity.
Cardiac death
Data on cardiac death could be extracted from two trials with a total of 410 patients with Wilms' tumour or Ewing's sarcoma (D'Angio 1981; Nesbit 1990). The meta‐analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 0.41, 95% CI 0.04 to 3.89, P = 0.44). No heterogeneity was detected (I2 = 0%).
Congestive heart failure
Data on congestive heart failure could be extracted from one trial with a total of 413 patients with rhabdomyosarcoma and undifferentiated sarcoma (Maurer 1988). Since in one of the treatment groups there were no events (0 out of 208 children in the group without anthracyclines experienced congestive heart failure as opposed to 1 out of 205 children in the group with anthracyclines) we were unable to calculate a RR, so we used the Fischer’s exact test instead. The analysis showed no significant difference between treatment not including and treatment including anthracyclines (Fischer's exact P = 0.50).
Asymptomatic cardiac dysfunction
We could collect data on asymptomatic cardiac dysfunction from one trial with a total of 255 patients with hepatoblastoma (Perilongo 2009). However, due to the high risk of attrition bias (this outcome was evaluated in only 49% of the patients), results of this study were not reported.
Grade 3 or 4 acute cardiotoxicity according to National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2
Data on grade 3 or 4 acute cardiotoxicity according to the NCI CTC Criteria version 2 could be extracted from one trial with a total of 394 patients with AML (Kaspers 2013). The analysis showed no significant difference between treatment not including and treatment including anthracyclines (RR 0.20, 95% CI 0.02 to 1.70, P = 0.14).
In the studies of Eden 1991; Sposto 2001; Van der Does 1975 and Van der Does 1989 no (reliable) information on cardiotoxicity was provided.
Sensitivity analyses for the used quality criteria
The results of the sensitivity analyses were consistent among the trials and did not differ from the overall analyses.
Discussion
Anthracycline‐induced cardiotoxicity is a considerable and serious problem, causing severe morbidity and mortality. With the current improved cancer survival rates, the problem of late‐onset cardiotoxicity is increasing. The risk of developing heart failure remains a lifelong threat, especially to children who have a long life‐expectancy after successful antineoplastic treatment. If anthracycline therapy does not have an added value with regard to tumour response and survival compared to treatment without anthracyclines, it should not be used in treatment protocols for childhood cancer. As a result anthracycline‐induced cardiotoxicity would not be an issue. This is the second update of the first systematic review evaluating the current state of evidence on the use of anthracyclines in the treatment of childhood cancer. Only RCTs were included since it is widely recognized that a RCT is the only study design which can be used to obtain unbiased evidence on the use of anthracyclines, provided that the design and execution are adequate.
We could identify RCTs for seven types of tumour, ALL, Wilms' tumour, rhabdomyosarcoma and undifferentiated sarcoma, Ewing's sarcoma, non‐Hodgkin lymphoma, hepatoblastoma, and AML. Either the use of doxorubicin or (liposomally entrapped) daunorubicin was evaluated.
For ALL three trials were identified, all evaluating the use of daunorubicin. Our meta‐analysis of these three trials showed no evidence of a significant difference in overall survival between the treatment groups. Our meta‐analysis of two trials also showed no evidence of a significant difference in event‐free survival between the treatment groups (unexplained heterogeneity was detected). However, a long‐term cardiac follow‐up study of one of these studies (Van der Does 1989) mentioned that the five‐year and 10‐year event‐free survival of patients treated with anthracyclines were significantly better than for patients treated without anthracyclines (P = 0.047 and P = 0.038, respectively) (Rammeloo 2000). Our meta‐analysis of two trials showed no evidence of a significant difference in tumour response (defined as the number of patients in complete remission) between the treatment groups. Please note that the reason that no significant difference between the treatment groups was identified could be due to the fact that the numbers of patients included in these studies were too small to detect a difference between the treatment groups (that is low power). Also, the length of follow‐up could be too short to detect a significant difference between the treatment groups. In most individual studies there is some suggestion of better survival in patients treated with anthracyclines. It should be noted that all these RCTs are performed in a different treatment era and not all RCTs stated the risk group(s) of included children. Nowadays most children with ALL are cured (Pieters 2008), while in the studies included in this review approximately 70% of the children survived.
For Wilms' tumour one trial was identified, evaluating the use of doxorubicin. Our analysis of all patients included in this trial showed a significant difference in overall survival in favour of treatment including anthracyclines as compared to treatment without anthracyclines (HR 1.85, 95% CI 1.09 to 3.15, P = 0.02). However, when patients with different stages of disease and different tumour histologies were analysed separately, this result was confirmed only in patients with stage II or III disease with unfavourable histology (HR 3.10, 95% CI 1.03 to 9.28, P = 0.04). For patients with stage II or III disease with favourable histology and patients with stage IV disease there was no evidence of a significant difference in overall survival between the treatment groups. However, with long‐term follow‐up (that is the exact length of follow‐up was unclear, but at least some of the patients had a follow‐up of 16 years), the overall result changed from a significant difference in favour of treatment with anthracyclines into no significant difference between the treatment groups. A possible explanation could be the mortality caused by different late effects (Mertens 2001; Reulen 2010). Our analysis of all patients included in this trial showed a significant difference in event‐free survival in favour of treatment including anthracyclines as compared to treatment without anthracyclines (HR 2.21, 95% CI 1.44 to 3.40, P = 0.0003). However, when patients with different stages of disease and different tumour histologies were analysed separately, this result was confirmed only in patients with stage II or III disease with favourable histology (HR 2.56, 95% CI 1.42 to 4.61, P = 0.002). For patients with stage II or III disease with unfavourable histology and patients with stage IV disease there was no evidence of a significant difference in event‐free survival between the treatment groups. The results of event‐free survival did not change with long‐term follow‐up (HR 1.72, 95% CI 1.09 to 2.72, P = 0.02). No information on tumour response was provided and, therefore, no conclusions can be made regarding this outcome. Please note that the reason that a significant difference between the treatment groups was not found for all stages of disease and tumour histologies could be due to the fact that the numbers of patients included in these studies were too small to detect a difference between the treatment groups (that is low power). The direction of the results of the different stages of disease and different tumour histologies were the same as the overall result.
For rhabdomyosarcoma and undifferentiated sarcoma one trial was identified, evaluating the use of doxorubicin. Our analysis of all patients included in this trial showed no evidence of a significant difference in overall survival between the treatment groups. When patients in different clinical groups (that is clinical groups III and IV) were analysed separately, again no evidence of a significant difference between the treatment groups was identified. It was not possible to evaluate event‐free survival in this study and, therefore, no conclusions can be made regarding this outcome. Our analysis showed no significant difference in tumour response (defined as the number of patients in complete or partial remission) between the treatment groups.
For Ewing's sarcoma one trial was identified, evaluating the use of doxorubicin. Descriptive results of overall survival and event‐free survival identified evidence of a significant advantage for patients treated with anthracyclines as compared to patients treated without anthracyclines (P = 0.02 and P = 0.01, respectively). No information on tumour response was provided and, therefore, no conclusions can be made regarding this outcome.
For non‐Hodgkin lymphoma one trial was identified, evaluating the use of daunorubicin. It was not possible to evaluate overall survival in this study and, therefore, no conclusions can be made regarding this outcome. Our analysis of event‐free survival showed no evidence of a significant difference between the treatment groups. No information on tumour response was provided and, therefore, no conclusions can be made regarding this outcome.
For hepatoblastoma one trial was identified, evaluating the use of doxorubicin. No significant difference in overall survival, event‐free survival and tumour response (defined as complete surgical resection, that is resection of all tumour sites on the basis of surgical findings and on postsurgical imaging) was identified between treatment including and treatment not including anthracyclines.
For AML one trial was identified, evaluating the use of daunoxome, that is liposomally entrapped daunorubicin. No significant difference in overall survival and tumour response (defined as complete response after two courses) was identified between treatment including and treatment not including anthracyclines. No information on event‐free survival was provided and, therefore, no conclusions can be made regarding this outcome. In addition to overall survival for all randomised patients, this study reported overall survival in a subgroup of patients with relapsed core binding factor (CBF)‐AML (that is t(8:21) or inv(16)). In patients randomised to treatment without anthracyclines (n = 34) overall survival at seven years was 58%, while in patients randomised to treatment with anthracyclines (n = 36) it was 82%. Since this was a post hoc analysis it was not included in the results of this review. The authors clearly stated that since this was a post hoc analysis this finding needs to be confirmed.
Please note that the reason that no significant difference between treatment groups was identified in patients with rhabdomyosarcoma and undifferentiated sarcoma, non‐Hodgkin lymphoma, hepatoblastoma and AML could be due to the fact that the numbers of patients included in these studies were too small to detect a difference between the treatment groups (that is low power). Also, the length of follow‐up could be too short to detect a significant difference between the treatment groups.
As mentioned earlier, one of the most serious adverse effects of anthracycline treatment is cardiotoxicity. Therefore, we did not only evaluate the antitumour efficacy of treatment with and without anthracyclines but also the occurrence of cardiotoxicity in both treatment groups. Our meta‐analysis of two trials evaluating cardiac death showed no significant difference between the treatment groups. The same was true for our analysis of one trial evaluating congestive heart failure and our analysis of one trial evaluating acute grade 3 or 4 cardiotoxicity (according to the NCI CTC version 2). One study provided information on asymptomatic cardiac dysfunction but due to the high risk of attrition bias (this outcome was evaluated in only 49% of the patients) results of this study were not included in this systematic review. Please note that the reason that no significant difference between the treatment groups was identified could be due to the fact that the numbers of patients included in these studies were too small to detect a difference between the treatment groups (that is low power). Also, the length of follow‐up could be too short to detect a significant difference between the treatment groups. There is some suggestion of a lower rate of clinical cardiotoxicity in patients who were not treated with anthracyclines.
Although there is only a small amount of data on the occurrence of anthracycline‐induced cardiotoxicity available from RCTs, both clinical and asymptomatic anthracycline‐induced cardiac damage has been evaluated in many non‐randomised studies. These studies show that anthracycline‐induced cardiotoxicity is a widely prevalent problem in children. The incidence of clinical heart failure has been reported to be as high as 16% at 0.9 to 4.8 years after treatment (Kremer 2002a) and the prevalence of asymptomatic cardiac dysfunction has been reported to be more than 57% at a median of 6.4 years after treatment (Kremer 2002b). The incidence of anthracycline‐induced cardiotoxicity, both clinical and asymptomatic, seems to increase with a longer follow‐up period (Green 2001; Kremer 2002b; Van Dalen 2006). For example, in a cohort study of 830 children with different types of tumour the estimated risk of anthracycline‐induced clinical heart failure increased with time from 2% at two years after the first dose of anthracyclines to 5.5% at 15 years after the first dose of anthracyclines (Van Dalen 2006). In three of the four studies included in this review that adequately evaluated cardiotoxicity the length of follow‐up was not mentioned, but it is likely that in all studies the follow‐up was relatively short. In the other study the median follow‐up was four years, but only acute cardiotoxicity was assessed. We did not include data on long‐term cardiac follow‐up from the included RCTs in this review because they included only data on some of the randomised patients and as a result the presence of selection bias could not be ruled out in these studies. Furthermore, in most long‐term follow‐up studies data for patients eligible for inclusion in our review could not be separated from results of patients ineligible for our review. However, in the study of Rammeloo 2000, which was a long‐term cardiac follow‐up study of Van der Does 1989, no late cardiac damage was demonstrated in 90 of the 136 eligible ALL survivors. The minimal follow‐up was 11 years after the last dose of anthracycline therapy. The age at diagnosis ranged from 1.2 to 14.9 years; at the time of the study their age ranged from 14.7 to 31.3 years. It should be noted that in this RCT patients who were randomised to treatment with anthracyclines received a relatively low cumulative anthracycline dose (that is according to the protocol 100 mg/m2 of daunorubicin) and that the occurrence of anthracycline‐induced cardiotoxicity is dose‐dependent (Green 2001; Kremer 2002a; Van Dalen 2006; Von Hoff 1979). However, it is important not to forget that although the risk of anthracycline‐induced clinical heart failure is significantly increased with a cumulative anthracycline dose of 300 mg/m² or more (Van Dalen 2006), both clinical and asymptomatic cardiac dysfunction can occur with a lower cumulative anthracycline dose (Lipshultz 2005; Van Dalen 2006). The fact that the patients in the study of Rammeloo 2000 did not develop cardiac damage at the time of the study does not exclude the possibility that anthracycline‐induced cardiotoxicity will become visible as they become older.
Just as the occurrence of anthracycline‐induced cardiotoxicity is dose‐dependent, it is possible that the cumulative anthracycline dose patients received influenced the antitumour efficacy of treatment. The exact cumulative anthracycline dose was not mentioned in any of the studies, but according to the different protocols the cumulative anthracycline dose ranged from 90 to 420 mg/m2. It should be noted that in the AML study and two of the three ALL studies patients received a relatively low cumulative anthracycline dose, that is either 180 mg/m2 (Kaspers 2013), 90 mg/m2 (Eden 1991) or 100 mg/m2 (Van der Does 1989). However, despite these low cumulative doses, in all studies there was still a suggestion of better antitumour efficacy with anthracycline therapy.
Patient age can be an important prognostic factor for the antitumour efficacy of treatments for different types of tumour (Biondi 2000; Gratias 2008; Pieters 2008). For example, in ALL infants aged less than one year or children aged 10 years or older have a worse outcome than children aged between one and nine years at diagnosis (Biondi 2000; Pieters 2008). Patient's age can also be a prognostic factor for the occurrence of cardiotoxicity (Kremer 2002a; Kremer 2002b). Unfortunately, due to a lack of useful data, these factors could not be evaluated in this review and, therefore, no conclusions can be made regarding age as a prognostic factor for these outcomes.
The risk of bias in the included studies varied. In most studies bias could not be ruled out due to lack of reporting. However, at the moment this is the best available evidence from RCTs evaluating treatment with and without anthracyclines in children with cancer. With regard to performance bias it should be noted that due to the nature of the interventions, blinding of care providers and patients was virtually impossible.
In this review we tried to only perform intention‐to‐treat (ITT) analyses, since they provide the most realistic and unbiased answer to the question of clinical effectiveness (Lachin 2000; Lee 1991). However, for the long‐term results of D'Angio 1981 (Green 2004) and for Perilongo 2009 an ITT analysis was not possible and, therefore, we performed an as‐treated analysis.
Eligible RCTs were identified for only seven types of tumour. No appropriate studies were found for other childhood cancers and, therefore, no conclusions can be made regarding the use of anthracyclines in the treatment of these tumours. It should be noted that in this review RCTs including both children and adults were only eligible for inclusion if the majority of participants were children, and the maximal age of the participants did not exceed 30 years. It is possible that there might be evidence on antitumour efficacy and cardiotoxicity of treatment with and without anthracyclines from studies including both children and adults (for examples see the Characteristics of excluded studies table).
We are awaiting the results of the currently ongoing studies and studies presented as abstracts during a conference on the use of anthracyclines for the following childhood cancers: hepatoblastoma (N = 1), ALL (N = 2), rhabdomyosarcoma (N = 1) and Wilms' tumour (N = 1).
Authors' conclusions
Implications for practice.
Anthracycline‐induced cardiotoxicity is a serious and widely prevalent problem in children treated for childhood cancer. Therefore, if anthracycline therapy does not have an added value with regard to antitumour efficacy and adverse effects compared to treatment without anthracyclines, it should not be used in treatment protocols for childhood cancer.
ALL
At the moment no evidence from RCTs is available which underscores the use of anthracyclines in ALL. However, it should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'; the fact that no significant difference in favour of treatment with anthracyclines was identified in this review can be the result of low power, a too short follow‐up period, or the use of low cumulative anthracycline doses. Based on the currently available evidence, we are not able to favour treatment with or without anthracyclines in patients with ALL.
Wilms' tumour
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with a Wilms' tumour. A significant difference in survival in favour of the use of anthracyclines was identified in this study (especially for patients with stage II and III disease) but this finding should be confirmed in other RCTs. Also, it should be kept in mind that with long‐term follow‐up the result of the analysis of all available patients changed from a significant difference in overall survival in favour of treatment with anthracyclines into no significant difference between the treatment groups.
Rhabdomyosarcomaand undifferentiated sarcoma
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with a rhabdomyosarcoma and undifferentiated sarcoma. No difference in antitumour efficacy between treatment with and treatment without anthracyclines was identified, but this finding should be confirmed in other RCTs.
Ewing's sarcoma
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with Ewing's sarcoma. A significant difference in survival in favour of the use of anthracyclines was identified in this study, but this finding should be confirmed in other RCTs.
Non‐Hodgkin lymphoma
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with a non‐Hodgkin lymphoma. No difference in antitumour efficacy was identified between treatment with and without anthracyclines, but this finding should be confirmed in other RCTs.
Hepatoblastoma
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with a hepatoblastoma. No difference in antitumour efficacy between treatment with and without anthracyclines was identified, but this finding should be confirmed in other RCTs.
AML
Since only one RCT was identified, no definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients with AML. No difference in antitumour efficacy between treatment with and treatment without anthracyclines was identified (with the exception of overall survival in a post hoc analysis in a subgroup of patients with relapsed CBF‐AML in which patients treated with anthracyclines had a better survival) but this finding should be confirmed in other RCTs.
Other childhood cancers
For other childhood cancers no RCTs were identified and, therefore, no conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients diagnosed with these malignancies.
Implications for research.
ALL, Wilms' tumour, rhabdomyosarcoma and undifferentiated sarcoma, Ewing's sarcoma, non‐Hodgkin lymphoma, hepatoblastoma, and AML
Future trials on the use of anthracyclines in patients with these types of tumour should be performed in homogeneous study populations with a long‐term follow‐up using valid outcome definitions (including antitumour efficacy and cardiotoxicity). Different risk groups, different cumulative anthracycline doses, and the age of the patients should be taken into account. It might be feasible to start these RCTs in children with unfavourable prognostic factors. The number of included patients should be sufficient to obtain the power needed for the results to be reliable. We are awaiting the results of the ongoing studies and the studies presented as abstracts during a conference, for patients with ALL, Wilms' tumour, hepatoblastoma and rhabdomyosarcoma. Also, it will be very interesting to examine long‐term survival data from the already performed RCTs. The performance of an individual patient data (IPD) analysis is another possibility to assess the antitumour efficacy of treatment with and without anthracyclines for these childhood cancers.
Other childhood cancers
No RCTs were identified for other childhood cancers. Therefore, before definitive conclusions can be made about the antitumour efficacy of treatment with or without anthracyclines in patients diagnosed with other malignancies, high quality RCTs need to be undertaken. Again, it might be feasible to start these RCTs in children with unfavourable prognostic factors. Also, these RCTs should be performed in homogeneous study populations with a long‐term follow‐up using valid outcome definitions (including antitumour efficacy and cardiotoxicity). Different risk groups, different cumulative anthracycline doses, and the age of the patients should be taken into account. The number of included patients should be sufficient to obtain the power needed for the results to be reliable. The performance of an IPD analysis is another possibility to assess the antitumour efficacy of treatment with and without anthracyclines for these childhood cancers.
What's new
| Date | Event | Description |
|---|---|---|
| 11 June 2014 | New citation required and conclusions have changed | Summary of most important changes in the update: The search for eligible studies was updated to July 2013. One new randomised controlled trial (RCT) addressing children with acute myeloid leukemia was included (this type of tumour was not addressed in the earlier versions of this review). For the risk of bias assessment we used the most recent recommendations of the Childhood Cancer Group. All RCTs (including those already included in earlier versions of the review) were scored using the new risk of bias criteria. |
| 11 June 2014 | New search has been performed | The search for eligible studies was updated to July 2013. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 30 August 2010 | New citation required and conclusions have changed | Summary of most important changes in the update: The search for eligible studies was updated to March 2010. One new randomized controlled trial addressing children with hepatoblastoma was included (this type of tumour was not addressed in the original version of the review). Furthermore, long‐term follow‐up data of the RCT addressing children with Wilms' tumour were included in the update. For overall survival the results changed from a significant difference in favour of treatment with anthracyclines into no significant difference between the treatment groups. |
| 30 August 2010 | New search has been performed | The search for eligible studies was updated to March 2010. |
Acknowledgements
Leontien Kremer and Elvira van Dalen, the Co‐ordinating Editors of the Childhood Cancer Group, are co‐authors of this review and therefore they could not act as the Co‐ordinating Editors for this review. Rob Pieters (department of Paediatric Oncology of the Sophia Children's Hospital, Rotterdam, the Netherlands) was willing to take on this task for the original version of the review, for which we would like to thank him. For the updates, Marianne van de Wetering (department of Paediatric Oncology of the Emma Children's Hospital, Amsterdam, the Netherlands) was willing to take on this task, for which we are very grateful. Also, we would like to thank Edith Leclercq, the Trials Search Co‐ordinator of the Childhood Cancer Group, for running the search strategy in the different databases and providing us with the titles and abstracts of the searches, Lieke Feijen for screening some of the identified titles and abstracts of the second update, Valentina Gracchi for translating an Italian article, and Vasya Vlassov and Boris Liberov for translating Russian articles, Szymon Skoczen for providing additional information on his article, Daniel Green for providing the reference with the long‐term follow‐up data of one of the included studies and, finally, the Foundation of Paediatric Cancer Research (SKK), the Netherlands and Stichting Kinderen Kankervrij (KIKA), the Netherlands for the financial support which made it possible to perform this systematic review. For survival analyses, the hazard ratio and associated statistics were calculated using an Excel spreadsheet developed by Matthew Sydes and Jayne Tierney of the MRC Clinical Trials Unit, London, the United Kingdom. the editorial base of the Cochrane Childhood Cancer Group is funded by Stichting Kinderen Kankervrij (KiKa).
Appendices
Appendix 1. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
(1) For anthracyclines the following text words have been used:
Anthracyclines OR anthracycline antibiotics OR doxorubicin OR adriamycin OR epirubicin OR idarubicin OR daunorubicin OR rubidomycin OR daunoxome OR myocet OR caelyx OR doxil
(2) For children the following text words have been used:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy
(3) For survival the following text words have been used:
survival OR survival rate OR survival rates OR cumulative survival rate OR cumulative survival rates OR survivorship OR mean survival time OR mean survival times OR survival time OR surviv* OR median survival time OR median survival times OR overall survival OR survival analysis OR survival analyses OR disease‐free survival OR disease free survival OR event‐free survival OR event‐free survivals OR event free survival OR progression‐free survival OR progression free survival OR progression‐free survivals OR event‐free OR event free OR progression free OR progression‐free OR time to progression OR treatment outcome OR treatment effectiveness OR treatment efficacy OR neoplasm recurrence OR neoplasm recurrences OR disease‐free survivals OR disease free survivals OR event free survivals OR progression free survivals OR treatment failure
Final search: 1 AND 2 AND 3
The search was performed in All Text.
[*=1 or more characters]
Appendix 2. Search strategy for MEDLINE
(1) For anthracyclines the following MeSH headings and text words have been used: anthracyclines OR anthracyclin* OR anthracycline antibiotics OR antibiotics, anthracycline OR 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR IMI 30 OR IMI30 OR IMI‐30 OR idarubicin hydrochloride OR hydrochloride, idarubicin OR NSC 256439 OR NSC‐256439 OR NSC256439 OR idarubicin OR idarubic* OR 4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR hydrochloride, epirubicin OR farmorubicin OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942 OR epirubicin OR epirubic* OR adriablastine OR adriblastin OR adriablastin OR adriamycin OR DOX‐SL OR DOX SL OR doxorubicin hydrochloride OR hydrochloride doxorubicin OR doxorubic* OR adriamyc* OR dauno‐rubidomycine OR dauno rubidomycin OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR hydrochloride, daunorubicin OR daunorubic* OR rubidomyc* OR NSC‐82151 OR NSC 82151 OR NSC82151 OR daunoxome OR daunosom* OR doxil OR caelyx OR liposomal doxorubicin OR doxorubicin, liposomal OR myocet OR doxorubicin OR daunorubicin
(2) For children the following MeSH headings and text words have been used: infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
(3) For survival the following MeSH headings and text words have been used: survival OR survival rate OR rate, survival OR rates, survival OR survival rates OR cumulative survival rate OR cumulative survival rates OR rate, cumulative survival OR rates, cumulative survival OR survival rate, cumulative OR survival rates, cumulative OR survivorship OR mean survival time OR mean survival times OR survival time, mean OR survival times, mean OR time, mean survival OR times, mean survival OR survival time OR surviv* OR median survival time OR median survival times OR survival time, median OR survival times, median OR time, median survival OR times, median survival OR overall survival OR analysis, survival OR analyses, survival OR survival analysis OR survival analyses OR disease‐free survival OR disease free survival OR survival, disease‐free OR disease‐free survivals OR survival, disease free OR survivals, disease‐free OR event‐free survival OR event‐free survivals OR event free survival OR survival, event‐free OR survivals, event‐free OR progression‐free survival OR progression free survival OR progression‐free survivals OR survival, progression‐free OR survivals, progression‐free OR event‐free OR event free OR progression free OR progression‐free OR time to progression OR treatment outcome OR treatment effectiveness OR treatment efficacy OR neoplasm recurrence OR neoplasm recurrences
(4) For randomized controlled trials the following MeSH headings and text words have been used:
In the original version of the review: randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:NoExp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT humans [mh]) (Higgins 2005).
For the updates of the review: randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) AND humans[mh] (Higgins 2009).
Final search: 1 AND 2 AND 3 AND 4
[*=1 or more characters; tiab= title or abstract; pt=publication type; sh=subheading; mh= mesh heading; mh:NoExp=mesh heading without explosion; tw= text word]
Appendix 3. Search strategy for EMBASE
(1) For anthracyclines the following Emtree terms and text words have been used:
1. exp ANTHRACYCLINE ANTIBIOTIC AGENT/ or exp ANTHRACYCLINE/ or exp ANTHRACYCLINE DERIVATIVE/ 2. (anthracycline or anthracyclines).mp. 3. anthracyclin$.mp. 4. doxorubicin.mp. or exp DOXORUBICIN DERIVATIVE/ or exp DOXORUBICIN/ 5. adriamycin.mp. 6. exp DAUNORUBICIN DERIVATIVE/ or daunorubicin.mp. or exp DAUNORUBICIN/ 7. rubidomycin.mp. 8. epirubicin.mp. or exp EPIRUBICIN/ 9. exp IDARUBICIN DERIVATIVE/ or exp IDARUBICIN/ or idarubicin.mp. 10. (doxorubic$ or adriamyc$ or daunorubic$ or rubidomyc$ or epirubic$ or idarubic$).mp. 11. (daunoxome or doxil or caelyx or myocet).mp. 12. or/1‐10
(2) For children the following Emtree terms and text words have been used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
(3) For survival the following Emtree terms and text words have been used:
1. (survival or survival rate or survival rates).mp. 2. (cumulative survival rate or cumulative survival rates).mp. 3. survivorship.mp. 4. (mean survival time or mean survival times).mp. 5. (survival time or surviv$).mp. 6. (median survival time or median survival times).mp. 7. overall survival.mp. 8. (survival analysis or survival analyses).mp. 9. (disease‐free survival or disease free survival).mp. 10. (disease‐free survivals or disease free survivals).mp. 11. (event‐free survival or event‐free survivals or event free survival or event free survivals).mp. 12. (progression‐free survival or progression free survival or progression‐free survivals or progression free survivals).mp. 13. (survival period or survival probability).mp. 14. (event‐free or event free or progression free or progression‐free).mp. 15. (time to progression or treatment outcome or treatment effectiveness or treatment efficacy).mp. 16. (neoplasm recurrence or neoplasm recurrences).mp. 17. (cancer recurrence or cancer recurrences or cancer recidive or cancer remission).mp. 18. (therapy outcome or therapeutic efficacy).mp. 19. or/1‐18 20. SURVIVAL RATE/ or SURVIVAL/ or SURVIVAL TIME/ 21. Treatment Outcome/ 22. Cancer survival/ or Cancer Recurrence/ 23. or/19‐22 24. 19 or 23
(4) For randomized controlled trials the following Emtree terms and text words have been used:
In the original version of the review (based onHiggins 2005):
1. Clinical Trial/ 2. Controlled Study/ 3. Randomized Controlled Trial/ 4. Double Blind Procedure/ 5. Single Blind Procedure/ 6. Comparative Study/ 7. RANDOMIZATION/ 8. Prospective Study/ 9. PLACEBO/ 10. Phase 2 Clinical Trial/ 11. phase 3 clinical study.mp. 12. phase 4 clinical study.mp. 13. Phase 3 Clinical Trial/ 14. Phase 4 Clinical Trial/ 15. or/1‐14 16. allocat$.mp. 17. blind$.mp. 18. control$.mp. 19. placebo$.mp. 20. prospectiv$.mp. 21. random$.mp. 22. ((singl$ or doubl$ or trebl$ or tripl$) and (blind$ or mask$)).mp. 23. (versus or vs).mp. 24. (randomized controlled trial$ or randomised controlled trial$).mp. 25. controlled clinical trial$.mp. 26. clinical trial$.mp. 27. or/16‐26 28. Human/ 29. Nonhuman/ 30. ANIMAL/ 31. Animal Experiment/ 32. or/29‐31 33. 32 not 28 34. (15 or 27) not 33
For the updates of the review (based onHiggins 2009):
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8
Final search: 1 AND 2 AND 3 AND 4
[mp= title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; $=1 or more characters; ti,ab=title or abstract; sh=subheading; /=Emtree term]
Data and analyses
Comparison 1. No anthracyclines versus anthracyclines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (Parmar's method was used to obtain the necessary data for the meta‐analysis) | 10 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 ALL | 3 | 912 | Hazard Ratio (Random, 95% CI) | 1.22 [0.95, 1.57] |
| 1.2 Wilms' tumour | 3 | 316 | Hazard Ratio (Random, 95% CI) | 1.85 [1.09, 3.15] |
| 1.3 Rhabdomyosarcoma/undifferentiated sarcoma | 2 | 409 | Hazard Ratio (Random, 95% CI) | 1.04 [0.83, 1.29] |
| 1.4 Hepatoblastoma | 1 | 255 | Hazard Ratio (Random, 95% CI) | 1.14 [0.41, 3.16] |
| 1.5 AML | 1 | 394 | Hazard Ratio (Random, 95% CI) | 1.16 [0.94, 1.44] |
| 2 Event‐free survival (Parmar's method was used to obtain the necessary data for the meta‐analysis) | 7 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 ALL | 2 | 870 | Hazard Ratio (Random, 95% CI) | 1.05 [0.76, 1.46] |
| 2.2 Wilms' tumour | 3 | 316 | Hazard Ratio (Random, 95% CI) | 2.21 [1.44, 3.40] |
| 2.3 Non‐Hodgkin lymphoma | 1 | 284 | Hazard Ratio (Random, 95% CI) | 1.01 [0.74, 1.38] |
| 2.4 Hepatoblastoma | 1 | 255 | Hazard Ratio (Random, 95% CI) | 0.81 [0.42, 1.55] |
| 3 Tumour response | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 ALL | 2 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.99, 1.06] |
| 3.2 Rhabdomyosarcoma / undifferentiated sarcoma | 1 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| 3.3 Hepatoblastoma | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 3.4 AML | 1 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.01] |
| 4 Clinical cardiotoxicity | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cardiac death | 2 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.04, 3.89] |
| 4.2 Cardiotoxicity grade 3 or 4 according to NCICTC version 2 | 1 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.02, 1.70] |
| 5 Overall survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the meta‐analysis) | 2 | 275 | Hazard Ratio (Random, 95% CI) | 1.27 [0.77, 2.11] |
| 5.1 Stage II‐III FH and UH | 1 | 227 | Hazard Ratio (Random, 95% CI) | 1.16 [0.62, 2.18] |
| 5.2 Stage IV | 1 | 48 | Hazard Ratio (Random, 95% CI) | 1.51 [0.65, 3.50] |
| 6 Event‐free survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the meta‐analysis) | 2 | 275 | Hazard Ratio (Random, 95% CI) | 1.72 [1.09, 2.72] |
| 6.1 Stage II‐III FH and UH | 1 | 227 | Hazard Ratio (Random, 95% CI) | 1.80 [1.04, 3.12] |
| 6.2 Stage IV | 1 | 48 | Hazard Ratio (Random, 95% CI) | 1.54 [0.66, 3.57] |
1.1. Analysis.
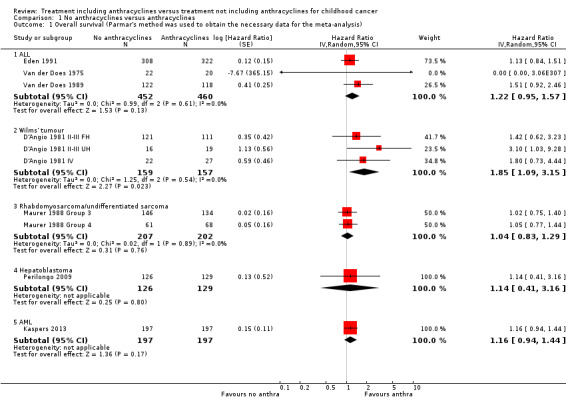
Comparison 1 No anthracyclines versus anthracyclines, Outcome 1 Overall survival (Parmar's method was used to obtain the necessary data for the meta‐analysis).
1.2. Analysis.
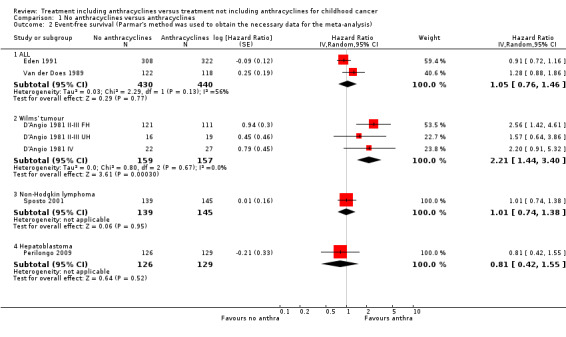
Comparison 1 No anthracyclines versus anthracyclines, Outcome 2 Event‐free survival (Parmar's method was used to obtain the necessary data for the meta‐analysis).
1.3. Analysis.
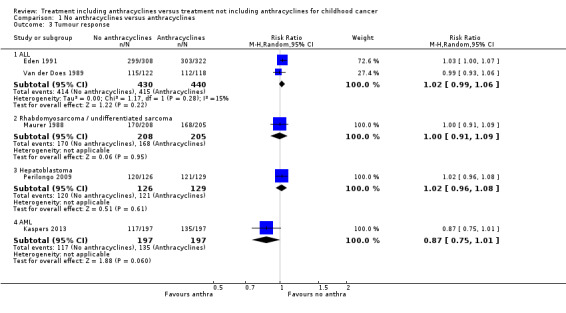
Comparison 1 No anthracyclines versus anthracyclines, Outcome 3 Tumour response.
1.4. Analysis.
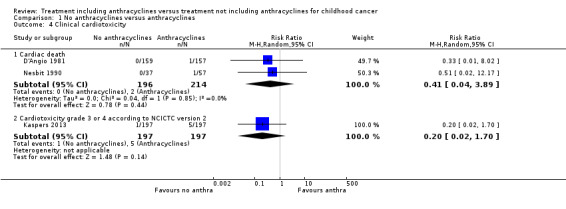
Comparison 1 No anthracyclines versus anthracyclines, Outcome 4 Clinical cardiotoxicity.
1.5. Analysis.
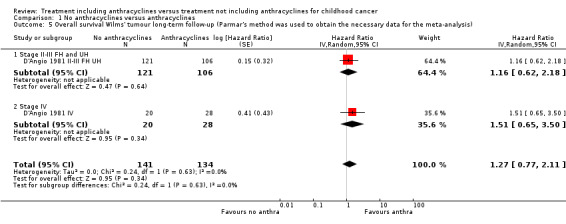
Comparison 1 No anthracyclines versus anthracyclines, Outcome 5 Overall survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the meta‐analysis).
1.6. Analysis.
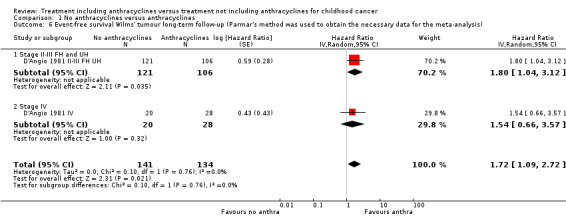
Comparison 1 No anthracyclines versus anthracyclines, Outcome 6 Event‐free survival Wilms' tumour long‐term follow‐up (Parmar's method was used to obtain the necessary data for the meta‐analysis).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
D'Angio 1981.
| Methods | Method of randomisation not clear (patients were stratified by institution, age and risk group) | |
| Participants | 316 children (age nm; 151 boys and 165 girls) with Wilms' tumour (stage II, III or IV; primary disease) No prior treatment Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without doxorubicin (N = 159) versus chemotherapy including doxorubicin (N = 157) Cumulative doxorubicin dose nm (according to protocol 300 mg/m2); peak dose (i.e. the maximal dose received in one week) 60 mg/m2; infusion duration nm All patients underwent radiotherapy (dose adjusted to age; location adjusted to stage of disease) All patients underwent surgery (radical nephrectomy) No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as time from surgery to death without regard to cause) Event‐free survival (defined as the length of time between initial surgery and the earliest detection of abdominal recurrence, distant metastasis or death (whether or not tumour related)) Cardiotoxicity (clinical heart failure defined as cardiac death) |
|
| Notes | Length of follow‐up nm Age in treatment and control group nm Long‐term follow‐up data of this study have been published (Green 2004) on 275 of 316 patients: 227 patients with stage II or III disease with favourable or unfavourable histology (as opposed to D'Angio 1981 where data were presented for favourable and unfavourable histology separately) and 48 patients with stage IV disease. The length of follow‐up was nm but at least part of the patients had a follow‐up of 16 years. Since in the stage IV group 20 patients were included in the no anthracycline group and 28 in the anthracycline group, as opposed to the original data where 22 patients were randomised to the anthracycline group and 27 to the non‐anthracycline group, intention‐to‐treat analyses were not possible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival and cardiotoxicity |
| Incomplete outcome data (attrition bias): overall survival | Unclear risk | It was not clear if all participants were included in the analyses |
| Incomplete outcome data (attrition bias): event‐free survival | Unclear risk | It was not clear if all participants were included in the analyses |
| Incomplete outcome data (attrition bias): anthracycline‐induced cardiotoxicity | Unclear risk | It was not clear if all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | There was no protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (unclear if age, sex and prior cardiac dysfunction were balanced between treatment arms; no prior cardiotoxic treatment) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (the study was supported by a US Public Health Service Grant and by the National Institutes of Health, but no information on the influence of funders was provided) |
D'Angio 1981 II‐III FH.
| Methods | See D'Angio 1981 | |
| Participants | Subgroup of patients from D'Angio 1981: 232 children with stage II or III disease with favourable histology (age and sex nm). For further information: see D'Angio 1981 | |
| Interventions | Chemotherapy without doxorubicin (N = 121) versus chemotherapy including doxorubicin (N = 111). For further information: see D'Angio 1981 | |
| Outcomes |
Overall survival (defined as time from surgery to death without regard to cause) Event‐free survival (defined as the length of time between initial surgery and the earliest detection of abdominal recurrence, distant metastasis or death (whether or not tumour related)) |
|
| Notes | See D'Angio 1981 | |
D'Angio 1981 II‐III FH UH.
| Methods | See D'Angio 1981. | |
| Participants | Subgroup of patients from D'Angio 1981 with stage II or III disease with favourable (N = 232) or unfavourable histology (N = 35) (age and sex nm). For further information: see D'Angio 1981 II‐III FH and D'Angio 1981 II‐III UH | |
| Interventions | Chemotherapy without doxorubicin (N = 137) versus chemotherapy including doxorubicin (N = 130). For further information: See D'Angio 1981 | |
| Outcomes |
Overall survival (defined as time from surgery to death without regard to cause) Event‐free survival (defined as the length of time between initial surgery and the earliest detection of abdominal recurrence, distant metastasis or death (whether or not tumour related)) |
|
| Notes | See D'Angio 1981 | |
D'Angio 1981 II‐III UH.
| Methods | See D'Angio 1981 | |
| Participants | Subgroup of patients from D'Angio 1981: 35 children with stage II or III disease with unfavourable histology (age and sex nm). For further information: see D'Angio 1981 | |
| Interventions | Chemotherapy without doxorubicin (N = 16) versus chemotherapy including doxorubicin (N = 19). For further information: see D'Angio 1981 | |
| Outcomes |
Overall survival (defined as time from surgery to death without regard to cause) Event‐free survival (defined as the length of time between initial surgery and the earliest detection of abdominal recurrence, distant metastasis or death (whether or not tumour related)) |
|
| Notes | See D'Angio 1981 | |
D'Angio 1981 IV.
| Methods | See D'Angio 1981 | |
| Participants | Subgroup of patients from D'Angio 1981: 49 children with stage IV disease (age and sex nm). For further information: see D'Angio 1981 | |
| Interventions | Chemotherapy without doxorubicin (N = 22) versus chemotherapy including doxorubicin (N = 27). For further information: see D'Angio 1981 | |
| Outcomes |
Overall survival (defined as time from surgery to death without regard to cause) Event‐free survival (defined as the length of time between initial surgery and the earliest detection of abdominal recurrence, distant metastasis or death (whether or not tumour related)) |
|
| Notes | See D'Angio 1981 | |
Eden 1991.
| Methods | Method of randomisation not clear | |
| Participants | 630 children (age nm: an inclusion criterion for this study was age between 0 and 14 years; 337 boys and 293 girls) with acute lymphoblastic leukaemia (stage nm; nm if primary disease or relapse) Prior treatment nm Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without daunorubicin (N = 308) versus chemotherapy including daunorubicin (N = 322) Cumulative daunorubicin dose nm (according to protocol 90 mg/m2); peak dose (i.e. the maximal dose received in one week) 90 mg/m2; infusion duration nm. Patients received cranial irradiation No surgery No cardioprotective interventions |
|
| Outcomes |
Overall survival (definition nm) Event‐free survival (defined as time to relapse or death; patients who died without going into remission were counted as having an event on day 1) Tumour response (definition nm; we assumed that the number of total remitters provided in the article was the number of complete remissions) |
|
| Notes | Length of follow‐up nm Age in treatment and control group nm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | High risk | Clinicians were "bound to record therapy given" and were thus not blinded to treatment. No information on blinding of participants was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival and tumour response |
| Incomplete outcome data (attrition bias): overall survival | Low risk | It was stated that all patients were included in the analyses |
| Incomplete outcome data (attrition bias): event‐free survival | Low risk | It was stated that all patients were included in the analyses |
| Incomplete outcome data (attrition bias): tumour response | Low risk | It was stated that all patients were included in the analyses |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it), but not all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (for all four items it was unclear if balanced between treatment arms) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (no information provided) |
Kaspers 2013.
| Methods | Block randomisation (block size 4) with a central interactive computerized system (stratified by study group and time to relapse on a 1:1 basis) | |
| Participants | 394 children (age 0‐19 years; 231 boys and 163 girls) with acute myeloid leukaemia (non‐FAB type M3; stage nm; first relapse or primary refractory disease). Prior treatment yes (heterogenous first‐line treatment, but all groups applied cytarabine‐based treatment combined with an anthracycline and allogeneic stem cell transplant was used in a limited number of patients; no further information provided) No prior cardiac dysfunction (patients with symptomatic cardiac disease and/or left ventricular shortening fraction < 29% were excluded) |
|
| Interventions | Re‐induction chemotherapy without daunoxome (=liposomally entrapped daunorubicin; N = 197) versus chemotherapy including daunoxome (N = 197) Cumulative daunoxome dose nm (according to protocol 180 mg/m2); peak dose (i.e. the maximal dose received in one week) 180 mg/m2; infusion duration nm Cranial irradiation was not recommended No surgery No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as time from study enrolment untill last follow‐up or death from any cause) Tumour response (complete response (after 2 courses) defined as 5% or less leukemic blasts in bone marrow with signs of normal haematopoiesis and of regeneration of normal peripheral blood cell production (platelets > 50x109/L without transfusions, neutrophils > 1.0x109/L) and no leukaemic cells in the peripheral blood or anywhere else) Cardiotoxicity (grade 3 or 4 acute toxicity according to National Cancer Institute Common Toxicity Criteria version 2) |
|
| Notes | Median length of follow‐up 4 years Age in treatment group ranged from 0 to 19 years (median 10 years); age in the control group ranged from 0 to 19 years (median 9 years) Re‐induction chemotherapy was followed by allogeneic stem cell transplant if available (different conditioning regimens); otherwise patients received consolidation chemotherapy (different schedules). An inclusion criterium for this systematic review was that therapy other than anthracyclines should have been the same in both treatment groups; timing of different aspects of the treatment could differ between the treatment groups, but the cumulative doses of therapy other than anthracyclines should not differ more than 25% between the treatment groups. Although this was not completely clear for this study, it was stated that treatment was well‐balanced between the treatment groups and therefore we gave this study the benefit of the doubt and judged it to be eligible for inclusion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed using a central interactive computerised system |
| Allocation concealment (selection bias) | Low risk | Block randomisation was performed using a central interactive computerised system |
| Blinding of participants and personnel (performance bias) | High risk | It was an open label study |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | Since this was an open label study, outcome assessors were not blinded. However, blinding of outcome assessors is not applicable for overall survival, so we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | High risk | It was an open label study |
| Incomplete outcome data (attrition bias): overall survival | Low risk | Intention‐to‐treat analyses were performed |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Intention‐to‐treat analyses were performed |
| Incomplete outcome data (attrition bias): anthracycline‐induced cardiotoxicity | High risk | 7% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | There was no protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | High risk |
Block randomisation in unblinded trials: yes (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (age and sex were balanced between treatment arms; no prior cardiac dysfunction; unclear if prior cardiotoxic treatment was balaned between the treatment arms) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (there was no outside funding for the trial and daunoxome was not provided free of charge, but two authors had a consultant/advisory role at the pharmaceutical company which delivered daunoxome and where compensated for this) |
Maurer 1988.
| Methods | Method of randomisation not clear (patients randomised within each clinical group) | |
| Participants | 413 children (age nm: an inclusion criterion for this study was age < 21 years; 240 males and 173 females) with rhabdomyosarcoma or undifferentiated sarcoma (clinical group III or IV; primary disease) No prior treatment Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without doxorubicin (N = 208) versus chemotherapy including doxorubicin (N = 205) Cumulative doxorubicin dose nm (according to protocol 300 mg/m2); peak dose (i.e. the maximal dose received in one week) 60 mg/m2; infusion duration nm All patients received radiotherapy to the primary lesion (dose adjusted to age); in case of pulmonary metastases patients received pulmonary irradiation No surgery No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as time from start of treatment to death) Tumour response (complete remission defined as complete disappearance of all signs and symptoms of disease; partial remission defined as at least 50% reduction in gross disease in widest diameter) Cardiotoxicity (clinical heart failure defined as congestive heart failure). |
|
| Notes | Length of follow‐up nm Exact age in treatment and control group nm, but it was balanced between treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for tumour response and cardiotoxicity |
| Incomplete outcome data (attrition bias): overall survival | Unclear risk | It was not clear if all participants were included in the analyses |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Only 1% of participants were not evaluable for this outcome |
| Incomplete outcome data (attrition bias): anthracycline‐induced cardiotoxicity | Unclear risk | It was not clear if all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | There was no protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (unclear if prior cardiotoxic treatment was balanced between treatment arms; age, sex, prior cardiac dysfunction were balanced between treatment arms) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (the study was supported by US Public Health Service Grants, but no information on the influence of funders was provided) |
Maurer 1988 Group 3.
| Methods | See Maurer 1988 | |
| Participants | Subgroup of patients from Maurer 1988: 280 children in clinical group III (age and sex nm). The exact number of patients randomised to this subgroup is unclear. For further information: see Maurer 1988 | |
| Interventions | Chemotherapy without doxorubicin (N = 146) versus chemotherapy including doxorubicin (N = 134). For further information: see Maurer 1988 | |
| Outcomes | Overall survival (defined as time from start of treatment to death) | |
| Notes | See Maurer 1988 | |
Maurer 1988 Group 4.
| Methods | See Maurer 1988 | |
| Participants | Subgroup of patients from Maurer 1988: 129 children in clinical group IV (age and sex nm). The exact number of patients randomised to this subgroup is unclear. For further information: see Maurer 1988 | |
| Interventions | Chemotherapy without doxorubicin (N = 61) versus chemotherapy including doxorubicin (N = 68). For further information: see Maurer 1988 | |
| Outcomes | Overall survival (defined as time from start of treatment to death) | |
| Notes | See Maurer 1988 | |
Nesbit 1990.
| Methods | Method of randomisation not clear (patients were randomised on a 2 to 3 patient basis to intervention and control group) | |
| Participants | 94 children (age nm; 56 boys and 38 girls) with non‐metastatic Ewing's sarcoma of the bone (primary disease) No prior treatment Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without doxorubicin (N = 37) versus chemotherapy including doxorubicin (N = 57) Cumulative doxorubicin dose nm (according to protocol maximal 420 mg/m2); peak dose (i.e. the maximal dose received in one week) 60 mg/m2; infusion duration nm Radiotherapy to primary lesion (dose adjusted to age) Part of the patients underwent surgical resection of the lesion: 3 in the intervention group and 19 in the control group No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as time from start of treatment to death) Event‐free survival (defined as time from start of treatment to first evidence of local recurrence or metastatic disease; patients dying before evidence of relapse were counted as failures) Cardiotoxicity (clinical heart failure defined as death of irreversible cardiac failure) |
|
| Notes | Length of follow‐up nm Age in treatment and control group nm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival and cardiotoxicity |
| Incomplete outcome data (attrition bias): overall survival | Low risk | All patients were included in the analyses |
| Incomplete outcome data (attrition bias): event‐free survival | Low risk | All patients were included in the analyses |
| Incomplete outcome data (attrition bias): anthracycline‐induced cardiotoxicity | Unclear risk | It was unclear if outcome data were available for all patients |
| Selective reporting (reporting bias) | Low risk | There was no protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items and methods of the study) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (unclear if age and prior cardiac dysfunction were balanced between treatment arms; no prior cardiotoxic treatment; sex distribution was rather similar in both treatment arms) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (the study was supported by a Public Health Service Grant awarded by the National Cancer Institute, department of Health and Human Services, but no information on the influence of funders was provided) |
Perilongo 2009.
| Methods | Randomisation by the United Kingdom Children's Cancer Study Group Data Centre (using the minimization method) | |
| Participants | 255 patients (age 0 to 11.2 years; 155 males and 100 females) with standard risk hepatoblastoma (standard risk was defined as a PRETEXT classification I, II or III with no evidence of extra‐hepatic disease; during the trial the protocol was amended to exclude patients with an alpha‐fetoprotein level of less than 100 ng/mL, in view of mounting evidence of a poor outcome in these patients; primary disease) No prior treatment Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without doxorubicin (N = 126) versus chemotherapy including doxorubicin (N = 129) Cumulative doxorubicin dose nm (according to protocol maximal 300 mg/m2); peak dose (i.e. the maximal dose received in one week) 60 mg/m2; infusion duration 30 mg/m2/24 hours No radiotherapy Radical surgery of the tumour was attempted in all patients No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as the interval between diagnosis and death from any cause or last contact) Event‐free survival (defined as the interval between diagnosis and disease progression, relapse or death, whichever occurred first) Tumour response (defined as complete surgical resection, i.e. resection of all tumour sites on the basis of surgical findings and on postsurgical imaging) Cardiotoxicity (asymptomatic cardiac dysfunction defined as a left ventricular shortening fraction of < 30%; test to determine left ventricular shortening fraction nm) |
|
| Notes | Length of follow‐up nm Age in treatment group ranged from 0 to 11.2 years (median 1 year); age in the control group ranged from 0.02 to 11.1 years (median 1.3 years) A total of 267 patients were randomized, but 12 patients were excluded (7 lacked proper documentation; 5 had wrong diagnosis; it was unclear to which treatment group these patients were randomized). As a result, intention‐to‐treat analyses were not possible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using the minimization method |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the United Kingdom Children's Cancer Study Group Data Centre |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival, tumour response and cardiotoxicity |
| Incomplete outcome data (attrition bias): overall survival | High risk | 38% of participants lost to follow‐up |
| Incomplete outcome data (attrition bias): event‐free survival | High risk | 36% of participants lost to follow‐up |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Only 1.6% of participants lost to follow‐up |
| Incomplete outcome data (attrition bias): anthracycline‐induced cardiotoxicity | High risk | 51% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | There was no protocol mentioned in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: not applicable (block randomization was not used) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (age, sex and prior cardiotoxic treatment were balanced between treatment arms; for prior cardiac dysfunction this was unclear) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (no information provided) |
Sposto 2001.
| Methods | Method of randomisation not clear (patients were stratified according to histology (large cell versus non‐large cell) and principal disease site (abdomen versus other) | |
| Participants | 284 patients (age nm: an inclusion criterion for this study was age < 21 years; 197 males and 87 females) with non‐lymphoblastic Non‐Hodgkin lymphoma (stage I to IV; nm if primary disease or relapse) Prior treatment nm Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without daunorubicin (N = 139) versus chemotherapy including daunorubicin (N = 145) Cumulative daunorubicin dose nm (according to protocol 350 mg/m2); peak dose (i.e. the maximal dose received in one week) 50 mg/m2; infusion duration nm Craniospinal radiotherapy if CNS disease at diagnosis, parameningeal disease, or isolated CNS relapse (dose depending on reason to give radiotherapy) No surgery No cardioprotective interventions |
|
| Outcomes | Event‐free survival (defined as the minimal time from study entry to failure to induce remission, disease progression, disease relapse, the occurrence of secondary malignant disease or death from any cause; isolated CNS relapses as first event for which treatment was specified in the protocol are not counted as events in this definition although subsequent CNS relapses or persistent CNS disease were counted as events) | |
| Notes | Length of follow‐up nm Exact age in treatment and control group nm, but it was balanced between treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival |
| Incomplete outcome data (attrition bias): event‐free survival | Unclear risk | Unclear if all patients were included in the analyses |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it), but not all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (age and sex were balanced between treatment arms; for prior cardiotoxic treatment and prior cardiac dysfunction this was unclear) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (the study was supported by the Division of Cancer Treatment, National Cancer Institution, National Institutes of Health and the Department of Health and Human Services, but no information on the influence of funders was provided) |
Van der Does 1975.
| Methods | Method of randomisation not clear | |
| Participants | 42 children (age nm: an inclusion criterion for this study was age between 1 and 14 years; sex nm) with acute lymphocytic leukaemia (stage nm; primary disease) No prior treatment Prior cardiac dysfunction unclear |
|
| Interventions | Chemotherapy without daunorubicin (N = 22) versus chemotherapy including daunorubicin (N = 20) Cumulative daunorubicin dose nm (according to protocol maximal 300 mg/m2); peak dose (i.e. the maximal dose received in one week) 30 mg/m2; infusion duration nm No radiotherapy or surgery No cardioprotective interventions |
|
| Outcomes | Overall survival (definition nm) | |
| Notes | Length of follow‐up for all patients maximal 18 months Age in treatment and control group nm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Incomplete outcome data (attrition bias): overall survival | Low risk | All patients were included in the analyses |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it), but not all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (unclear if age, sex and prior cardiac dysfunction were balanced between treatment arms; no prior cardiotoxic treatment) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (the study was supported by Ministerie van Volksgezondheid en Milieuhygiëne, but no information on the influence of funders was provided) |
Van der Does 1989.
| Methods | Randomisation by the DCLSG Central Office (patients were stratified by institution and sex) | |
| Participants | 240 children (age 0 to 15 years; 119 boys and 121 girls) with standard risk acute lymphocytic leukaemia (nm if primary disease or relapse) Prior treatment nm Prior cardiac dysfunction nm |
|
| Interventions | Chemotherapy without daunorubicin (N = 122) versus chemotherapy including daunorubicin (N = 118) Cumulative daunorubicin dose nm (according to protocol 100 mg/m2); peak dose (i.e. the maximal dose received in one week) 25 mg/m2; infusion duration nm. Patients achieving complete remission within 6 weeks of the start of induction chemotherapy underwent cranial irradiation (dose adjusted to age) in combination with intrathecal methotrexate and prednisone No surgery No cardioprotective interventions |
|
| Outcomes |
Overall survival (defined as time from diagnosis to time of death) Event‐free survival (defined as time from diagnosis to induction failure, relapse, death in remission, or the occurrence of a second tumour) Tumour response (complete remission defined as < 5% blast cells and normal hematopoiesis in the bone marrow without evidence of disease at any other site) |
|
| Notes | Median follow‐up for all patients 64 months (range 34 to 87 months) Age in treatment group ranged from 0 to 15 years; age in the control group ranged from 1 to 15 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that randomisation was peformed by the DCLSG Central Office, but no further information on the methods of randomization was provided |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the DCLSG Central Office |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): outcomes other than overall survival | Unclear risk | No information on blinding of outcome assessors was provided for event‐free survival and tumour response |
| Incomplete outcome data (attrition bias): overall survival | Low risk | All patients were included in the analyses |
| Incomplete outcome data (attrition bias): event‐free survival | Low risk | All patients were included in the analyses |
| Incomplete outcome data (attrition bias): tumour response | Low risk | All patients were included in the analyses |
| Selective reporting (reporting bias) | High risk | There was no protocol mentioned in the manuscript (and we did not search for it), but not all expected outcomes were reported |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (see information provided at earlier associated risk of bias items) Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex and/or prior cardiac dysfunction): unclear (age and sex were balanced between treatment arms; this was unclear for prior cardiotoxic treatment and prior cardiac dysfunction) Difference in length of follow‐up between treatment arms: unclear (not reported) Inappropriate influence of funders: unclear (no information provided) |
nm: not mentioned; DCLSG: Dutch Childhood Leukemia Study Group; CNS: central nervous system; FH: favourable histology; UH: unfavourable histology; FAB: French‐American‐British
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvegard 1989a | Including adults aged 30 years or more |
| Alvegard 1989b | Double publication of Alvegard 1989a |
| Alvegard 1990 | Including adults aged 30 years or more |
| Anonymous 1987 | Adults aged 30 years or more |
| Antillon 2008 | No RCT (2 different protocols) |
| Antman 1984a | Including adults aged 30 years or more |
| Antman 1984b | Including adults aged 30 years or more |
| Antman 1985 | Double publication of Antman 1984a |
| Antman 1987 | Including adults aged 30 years or more |
| Antman 1990 | Review (no eligible studies) |
| Aur 1972 | No RCT; all patients received anthracyclines |
| Bacci 1989 | No RCT; including adults aged 30 years or more |
| Balwierz 2013 | No randomization to treatment with and without anthracyclines; consecutive trials; all patients received anthracyclines |
| Barr 1992 | No RCTs; all patients received anthracyclines |
| Bellani 1978 | No RCT; all patients received anthracyclines; including adults aged 30 years or more |
| Berman 1989 | Comparison of two types of anthracycline analogues; adults aged 30 years or more |
| Bernthal 2012 | Difference in treatment other than anthracyclines between study groups; possibly all patients received anthracyclines; possibly including adults aged 30 years or more |
| Bessho 1994 | No RCT |
| Biondi 2006 | No RCT |
| Birch 1986 | Including adults aged 30 years or more |
| Blakely 2003 | Subgroup of patients from different RCTs; comparison of chemotherapy and surgery versus only surgery |
| Bradford 1998 | No RCT |
| Breslow 2004 | Subgroup of patients from different RCTs |
| Brouwer 2007 | Follow‐up study of Van der Does 1989, but only 23/240 patients (9.5%) included; no survival data presented |
| Burnett 2006 | All patients received anthracyclines |
| Caceres 1978 | No RCT; preliminary results of Zaharia 1986 |
| Canter 2007 | No RCT; including adults aged 30 years or more |
| Carlsen 1989 | No RCT |
| Castellino 2008 | All patients received anthracyclines |
| Chau 2003 | No RCT; adults aged 30 years or more |
| Chessells 1992 | All patients received anthracyclines |
| Chessells 2002 | No randomisation between treatment with and without anthracyclines |
| Craft 1993 | Review (no eligible studies) |
| Creutzig 2005 | Review (no eligible studies) |
| Creutzig 2006 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines |
| Crist 1995 | No data provided for only randomised patients |
| Culbert 1991 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines |
| D'Angio 1989 | More than a 25% difference in cumulative doses of agents other than anthracyclines between study groups |
| De Bernardi 2003 | No RCTs; all patients received anthracyclines |
| De Bernardi 2009 | No RCT; treatment with and without anthracyclines not evaluated |
| Dluzniewska 2003 | No RCT; all patients received anthracyclines |
| Dobashi 2006 | Unclear if RCT; including adults aged 30 years or more; all patients received anthracyclines |
| Dunst 1998 | Commentary on ineligible study |
| Eilber 1988 | All patients received anthracyclines; including adults aged 30 years or more |
| Einhorn 1981 | Including adults aged 30 years or more |
| Elomaa 1993 | Review (no eligible studies) |
| Etcubanas 1984 | No RCT |
| Evans 1985 | Subgroup of patients from different RCT |
| Evans 1991 | Subgroup of patients from different RCTs |
| Fink 1990 | No RCT; all patients received anthracyclines |
| Frappaz 2002 | No randomisation between treatment with and without anthracyclines; only poor responders received anthracyclines |
| Fukuoka 1994 | Adults aged 30 years or more; all patients received anthracyclines |
| Gallegos‐Castorena 2009 | No RCTs (3 different protocols); all patients received anthracyclines |
| Garbes 1987 | Most likely no RCT; including adults aged 30 years or more |
| Gaynon 1993 | Difference in treatment other than anthracyclines between study groups |
| Gelderblom 2011 | All patients received anthracyclines |
| Glanzmann 1998 | No RCT; difference in treatment other than anthracyclines between study groups; including adults aged 30 years or more |
| Green 1994 | Subgroup of patients from different RCTs |
| Green 1996 | Subgroup of patients from different RCTs |
| Green 1999 | Subgroup of patients from different RCTs |
| Grier 1990 | Most likely no RCT; all patients received anthracyclines |
| Grundy 2012 | No RCT |
| Gururangan 2000 | Review (one study eligible for inclusion: published full text as Sposto 2001) |
| Hainsworth 1985 | Unclear if RCT; including adults aged 30 years or more |
| Halazun 1974 | Unclear if treatment other than anthracyclines was the same in both treatment groups; no survival data presented |
| Harms 2000 | No RCT; review (no eligible studies) |
| Hayashi 2008 | Unclear if RCT; including adults aged 30 years or more; results treatment with and without anthracyclines were not presented |
| Hays 1988 | Subgroup of patients from different RCT |
| Henze 1989 | Review (no eligible studies) |
| Hitchcock‐Bryan 1986 | After the initial randomisation to treatment with and without anthracyclines all patients received anthracyclines; outcomes were measured at the time all patients received anthracyclines; update of Sallan 1977 |
| Hodgson 2003 | No RCT; including adults aged 30 years or more |
| Holland 1971 | Difference in treatment other than anthracyclines between study groups |
| Humphrey 1975 | No data on any of the eligible outcomes provided |
| Iarussi 2003 | No RCT |
| Ihde 1977 | No RCT; including adults aged 30 years or more |
| Isu 1992 | No RCT |
| Iwenofu 2008 | No RCT; including adults aged 30 years or more |
| Jaffe 1981 | No RCT |
| Janka‐Schaub 1988 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines |
| Kalapurakal 2010 | Included patients from different studies; no randomisation between treatment with and without anthracyclines |
| Karachunskii 2007 | Double publication of Karachunskiy 2008 |
| Karachunskiy 2008 | All patients received anthracyclines |
| Kaspers 2010 | Conference proceeding of Kaspers 2013 |
| Kazanowska 2006 | No RCT; all patients received anthracyclines |
| Khattab 2008 | No RCT; all patients received anthracyclines |
| Kinsella 1991 | No RCT; including adults aged 30 years or more |
| Kobe 2008 | Including adults aged 30 years or more; all patients received anthracyclines |
| Komp 1976 | Some patients were randomised to treatment with and without anthracyclines; unclear if treatment other than anthracyclines was the same in that subgroup; results treatment with and without anthracyclines were not presented |
| Konopka 1989 | Most likely no RCT; difference in treatment other than anthracyclines between study groups; including adults aged 30 years or more |
| Kuleva 2008 | No RCT |
| Kurrle 1988 | All patients received anthracyclines; including adults aged 30 years or more |
| Lager 2006 | Review (no eligible studies) |
| Li 2006 | No RCT; all patients received anthracyclines |
| Lilleyman 1997 | No RCT |
| Lindberg 1977 | Review (no eligible studies) |
| Link 1986 | Difference in treatment other than anthracyclines between study groups |
| Madej 1987 | Most likely no RCT; including adults aged 30 years or more |
| Madon 1985 | Review (no eligible studies) |
| Mahajan 2008 | No RCT; including adults aged 30 years or more; all patients received anthracyclines |
| Mahmoud 1993 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines |
| Maiakova 1986 | No RCT |
| Malpas 1974 | Unclear if RCT; including adults aged 30 years or more |
| Marcus 1987 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines; including adults aged 30 years or more |
| Matsuzaki 2001 | No RCT |
| Maurer 1981 | Preliminary report of Maurer 1988 |
| Meisel 1999 | Subgroup of patients from different RCTs |
| Meza 2006 | No data on patients randomised to treatment with and without anthracyclines presented; subgroup of patients included in Crist 1995 |
| Miser 1993 | Review (no eligible studies) |
| Moricke 2008 | All patients received anthracyclines |
| Muus 1993 | No RCT; including adults aged 30 years or more |
| Nachman 1993 | Review (no eligible studies) |
| Nishimura 1983 | Unclear if RCT; including adults aged 30 years or more |
| Ochiai 1993 | Review |
| Oh 2006 | No RCT; including adults aged 30 years or more |
| Okamura 1987 | No RCT |
| Omura 1985 | Adults aged 30 years or more |
| Ortega 1991 | Subgroup of patients from different RCTs; data not presented for patients receiving or not receiving anthracyclines |
| Paulino 1996 | Probably no RCT; difference in treatment other than anthracyclines between study groups |
| Paulino 2003 | No RCT; including adults aged 30 years or more |
| Pavlovsky 1981 | No RCT; including adults aged 30 years or more |
| Pawson 2001 | No RCT; including adults aged 30 years or more |
| Peeters 2009 | No RCT |
| Perez 1981 | Including adults aged 30 years or more |
| Pfreundschuh 2008 | Including adults aged 30 years or more; all patients received anthracyclines |
| Picci 1997 | No RCT; including adults aged 30 years or more |
| Pontz 1981 | Most likely no RCT; difference in treatment other than anthracyclines between study groups |
| Pratt 1981 | No RCT |
| Pratt 1993 | Review (no eligible studies) |
| Pritchard‐Jones 2011 | Double publication of SIOP2001 |
| Pritchard‐Jones 2012 | No randomisation to treatment with and without anthracyclines |
| Quattrin 1975 | Review (no eligible studies) |
| Rai 1981 | No randomisation to treatment with and without anthracyclines; all patients received anthracyclines; including adults aged 30 years or more |
| Rammeloo 2000 | Subgroup of patients from Van der Does 1989 |
| Raney 1983 | Subgroup of patients from different RCT |
| Raney 1987 | No randomisation to treatment with and without anthracyclines |
| Raney 1988 | Subgroup of patients from different RCT |
| Raney 1990 | Subgroup of patients from different RCTs |
| Raza 2008 | No RCT; including adults aged 30 years or more |
| Rees 1990 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines; including adults aged 30 years or more |
| Ritchey 1994 | Subgroup of patients from different RCT |
| Ritter 1990 | No RCT; all patients received anthracyclines |
| Roy 2005 | No randomisation between treatment with and without anthracyclines |
| Sakic 2006 | All patients received anthracyclines |
| Sallan 1977 | After the initial randomisation to treatment with and without anthracyclines all patients received anthracyclines; preliminary results of Hitchcock‐Bryan 1986 |
| Salodof MacNeil 2010 | No original research; commentary (no eligible studies) |
| Schaison 1992 | Review (no eligible studies) |
| Scherrer 1994 | Probably no RCT; all patients received anthracyclines; including adults aged 30 years or more |
| Schmits 2001 | Review (no eligible studies) |
| Seibel 2008 | All patients received anthracyclines |
| Silverman 2000 | Review (no eligible studies) |
| Skoczen 2006 | No RCT |
| Smithson 1982 | No RCT; case report |
| Sotnikov 1989 | Most likely no RCT; difference in treatment other than anthracyclines between study groups; including adults aged 30 years or more |
| Spears 1992 | No RCT |
| Spreafico 2008 | No RCT |
| Sramkova 2013 | No RCT; historical controls |
| Stary 2003 | No randomisation between treatment with and without anthracyclines |
| Steinherz 1993 | All patients received the same agents; only the order and mode of administration were different between study groups |
| Steinherz 1996 | Double publication of Gaynon 1993 |
| Steinherz 1998 | No RCT; difference in treatment other than anthracyclines between study groups |
| Tarella 2001 | No RCT; difference in treatment other than anthracyclines between study groups; including adults aged 30 years or more |
| Taylor 2006 | Including adults aged 30 years or more |
| Tefft 1978 | Preliminary report of Evans 1985 |
| Thirugnanam 2009 | No RCT; including adults aged 30 years or more |
| Thomas 1988 | Subgroup of patients from different RCT; no data on survival presented |
| Toft 2013 | No RCT; all patients received anthracyclines |
| Tournade 1993 | More than a 25% difference in cumulative doses of agents other than anthracyclines between study groups |
| Tsuchiya 1998 | No RCT; difference in treatment other than anthracyclines between study groups; including adults aged 30 years or more |
| Van der Does 1988 | Double publication of Van der Does 1989 |
| Vinogradova 2008 | Unclear if RCT; including adults aged 30 years or more (Russian article, no further information sought) |
| Virchis 2004 | No RCT; including adults aged 30 years or more |
| Vora 2010 | No randomisation to treatment with and without anthracyclines |
| Vose 1994 | Probably no RCT; all patients received anthracyclines; including adults aged 30 years or more |
| Watts 2002 | No RCT |
| Weinstein 1992 | Review (no eligible studies) |
| Wilimas 1988 | No RCT |
| Willemze 1982 | No RCT; all patients received anthracyclines; including adults aged 30 years or more |
| Willnow 1986 | No RCT; all patients received anthracyclines |
| Zaharia 1986 | No RCT; update of Caceres 1978 |
| Zdrahalova 2011 | Double publication of Kaspers 2013 |
| Zimmermann 2012 | No randomisation to treatment with and without anthracyclines |
| Zintl 1987 | No randomisation between treatment with and without anthracyclines; all patients received anthracyclines |
| Zittoun 1992 | Difference in treatment other than anthracyclines between study groups; all patients received anthracyclines; including adults aged 30 years or more |
RCT: randomised controlled trial; SIOP: International Society for Paediatric Oncology
Characteristics of studies awaiting assessment [ordered by study ID]
FRALLE 2000‐A.
| Methods | Method of randomisation not clear |
| Participants | 524 children (aged 1 to 9 years; sex nm) with standard‐risk B‐cell precursor acute lymphoblastic leukaemia with a good marrow response at day 21 (M1). Prior therapy not mentioned; prior cardiac dysfunction not mentioned |
| Interventions | Treatment including (N = 247) or not including (N = 248) daunorubicin |
| Outcomes | No difference in efficacy and toxicity between the study groups at a follow‐up of 31 months (unclear if this is a mean or median; range 3 to 59 months) |
| Notes | This study has not been published in full text (October 2013), but has been presented at the SIOP conference 2006 |
SIOP2001.
| Methods | Method of randomisation not clear (stratified by participating group and tumour stage) |
| Participants | 583 children with intermediate risk stage II or III Wilms' tumour (aged 6 months to 18 years) |
| Interventions | Postoperative chemotherapy including (N=291) or not including (N=292) doxorubicin (total dose 250 mg/m2) |
| Outcomes | No difference in 2 year EFS and 5 year OS between the study groups at a median follow‐up of 39 months |
| Notes | This study has not been published in full text (October 2013), but has been presented at the SIOP conference 2011 |
Von Stackelberg 2011.
| Methods | Method of randomisation not clear |
| Participants | 420 children with relapsed acute lymphoblastic leukaemia (aged 18 years or less) |
| Interventions | Unclear from the current information: patients might have received anthracyclines in both treatment groups (N=210 in each group) and if not, there might be a difference in treatment other than anthracyclines between study groups |
| Outcomes | No difference in 5 year EFS, 5 year OS and treatent related mortality between the treatment groups, significantly less relapses and mucositis, but significantly more hematological toxicity in the patients who definitely received anthracyclines (idarubicin) |
| Notes | This study has not been published in full text (October 2013), but has been presented at the ASH conference 2011 It is not yet clear if this study is eligible for inclusion in this review |
EFS: event‐free survival; OS: overall survival; SIOP: International Society for Paediatric Oncology; ASH: America Society of Hematology
Characteristics of ongoing studies [ordered by study ID]
COG AHEP0531.
| Trial name or title | Not mentioned |
| Methods | Method of randomisation not clear |
| Participants | Children with intermediate risk hepatoblastoma |
| Interventions | Cisplatin, vincristine, 5‐fluorouracil with and without doxorubicin |
| Outcomes | Not mentioned |
| Starting date | Not mentioned |
| Contact information | Not mentioned |
| Notes | ‐ |
NCT00379457.
| Trial name or title | A protocol for nonmetastatic rhabdomyosarcoma [RMS‐2005] |
| Methods | Method of randomisation not clear (patients are stratified according to risk group and participating country) |
| Participants | Children with high‐risk rhabdomyosarcoma (maximal age 20 years) |
| Interventions | Ifosfamide, vincristine, dactinomycin with or without doxorubicin |
| Outcomes | Response rate, survival and toxicity |
| Starting date | June 2006 |
| Contact information | Study chairs Gianni Bisogno, Meriel Jenney, Joern Treuner and Hans Merks |
| Notes | It is unclear from the current information if the cumulative doses of agents other than anthracyclines differ less than 25% between study groups. |
Differences between protocol and review
For the second update we used the most recent recommendations of the Childhood Cancer Group for the assessment of risk of bias in the included studies. All RCTs (including those already included in earlier versions of the review) were scored using the new 'risk of bias' items. Also, since performing the original review and the first update of this review, the Childhood Cancer Group has adjusted some of its recommendations regarding analyses: when for a particular outcome only one study is available and there are no events in one of the treatment groups, it is impossible to calculate an adequate risk ratio using the RevMan software, instead the Fischer's exact test should be used. We have adjusted this where necessary.
Contributions of authors
Elvira van Dalen designed the study and wrote the protocol. She developed the search strategy. She identified the studies meeting the inclusion criteria (both by initial screening and thereafter). She searched for unpublished and ongoing studies. She performed the data extraction and risk of bias assessment of the included studies. She analysed the data and interpreted the results. She wrote and revised the manuscript.
Martine Raphaël identified the studies meeting the inclusion criteria and performed the data extraction and risk of bias assessment of the included studies. She contributed to the interpretation of the results. She critically reviewed the manuscript.
Leontien Kremer critically reviewed the protocol. She acted as third party arbitrator. She contributed to the interpretation of the results. She critically reviewed the manuscript.
Huib Caron critically reviewed the protocol. He contributed to the interpretation of the results. He critically reviewed the manuscript.
All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Foundation of Paediatric Cancer Research (SKK), Netherlands.
Stichting Kinderen Kankervrij (KiKa), Netherlands.
Declarations of interest
None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
D'Angio 1981 {published data only}
- D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 1981;47:2302‐11. [DOI] [PubMed] [Google Scholar]
D'Angio 1981 II‐III FH {published data only}
- D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 1981;47:2302‐11. [DOI] [PubMed] [Google Scholar]
D'Angio 1981 II‐III FH UH {published data only}
- D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 1981;47:2302‐11. [DOI] [PubMed] [Google Scholar]
- Green DM. The treatment of stages I‐IV favorable histology Wilms' tumor. Journal of Clinical Oncology 2004;22:1366‐72. [DOI] [PubMed] [Google Scholar]
D'Angio 1981 II‐III UH {published data only}
- D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 1981;47:2302‐11. [DOI] [PubMed] [Google Scholar]
D'Angio 1981 IV {published data only}
- D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 1981;47:2302‐11. [DOI] [PubMed] [Google Scholar]
- Green DM. The treatment of stages I‐IV favorable histology Wilms' tumor. Journal of Clinical Oncology 2004;22:1366‐72. [DOI] [PubMed] [Google Scholar]
Eden 1991 {published data only}
- Eden OB, Lilleyman JS, Richards S, Shaw MP, Peto J. Results of Medical Research Council Childhood Leukaemia Trial UKALL VIII (report to the Medical Research Council on behalf of the Working Party on Leukaemia in Childhood). British Journal of Haematology 1991;78:187‐96. [DOI] [PubMed] [Google Scholar]
Kaspers 2013 {published data only}
- Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. Journal of Clinical Oncology 2013;31(5):599‐607. [DOI] [PubMed] [Google Scholar]
Maurer 1988 {published data only}
- Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, et al. The Intergroup Rhabdomyosarcoma Study‐I. A final report. Cancer 1988;61:209‐20. [DOI] [PubMed] [Google Scholar]
Maurer 1988 Group 3 {published data only}
- Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, et al. The Intergroup Rhabdomyosarcoma Study‐I. A final report. Cancer 1988;61:209‐20. [DOI] [PubMed] [Google Scholar]
Maurer 1988 Group 4 {published data only}
- Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, et al. The Intergroup Rhabdomyosarcoma Study‐I. A final report. Cancer 1988;61:209‐20. [DOI] [PubMed] [Google Scholar]
Nesbit 1990 {published data only}
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, Vietti TJ, Cangir A, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long‐term follow‐up of the First Intergroup study. Journal of Clinical Oncology 1990;8:1664‐74. [DOI] [PubMed] [Google Scholar]
Perilongo 2009 {published data only}
- Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, et al. Cisplatin versus cisplatin plus doxorubicin for standard‐risk hepatoblastoma. The New England Journal of Medicine 2009;361:1662‐70. [DOI] [PubMed] [Google Scholar]
Sposto 2001 {published data only}
- Sposto R, Meadows AT, Chilcote RR, Steinherz PG, Kjeldsberg C, Kadin ME, et al. Comparison of long‐term outcome of children and adolescents with disseminated non‐lymphoblastic non‐Hodgkin lymphoma treated with COMP or daunomycin‐COMP: A report from the Children's Cancer Group. Medical and Pediatric Oncology 2001;37:432‐41. [DOI] [PubMed] [Google Scholar]
Van der Does 1975 {published data only}
- Does‐van den Berg A, Koning J, Reerink H, Vries JA, Zanen GE. Acute lymphatic leukemia in children in the Netherlands; study ALL I, 1972‐1973; Dutch Childhood Leukemia Study Group [Acute lymfatische leukemie bij kinderen in Nederland; onderzoek ALL I, 1972‐1973; Stichting Nederlandse Werkgroep Leukemie bij Kinderen (SNWLK)]. Nederlands Tijdschrift voor Geneeskunde 1975;119:1445‐51. [PubMed] [Google Scholar]
Van der Does 1989 {published data only}
- Does‐van den Berg A, Wering ER, Suciu S, Solbu G, 't Veer MB, Rammeloo JA, et al. Effectiveness of rubidomycin in induction therapy with vincristine, prednisone, and L‐asparaginase for standard risk childhood acute lymphocytic leukemia: results of a Dutch phase III study (ALL V). A report on behalf of the Dutch Childhood Leukemia Study Group (DCLSG). The American Journal of Pediatric Hematology/Oncology 1989;11:125‐33. [PubMed] [Google Scholar]
References to studies excluded from this review
Alvegard 1989a {published data only}
- Alvegard TA, Sigurdsson H, Mouridsen H, Solheim O, Unsgaard B, Ringborg U, et al. Adjuvant chemotherapy with doxorubicin in high‐grade soft tissue sarcoma: a randomized trial of the Scandinavian Sarcoma Group. Journal of Clinical Oncology 1989;7:1504‐13. [DOI] [PubMed] [Google Scholar]
Alvegard 1989b {published data only}
- Alvegard TA, Berg NO, Ranstam J, Rydholm A, Rooser B. Prognosis in high‐grade soft tissue sarcomas. The Scandinavian Sarcoma Group experience in a randomized adjuvant chemotherapy trial. Acta Orthopaedica Scandinavica 1989;60:517‐21. [DOI] [PubMed] [Google Scholar]
Alvegard 1990 {published data only}
- Alvegard TA, Berg NO, Baldetorp B, Ferno M, Killander D, Ranstam J, et al. Cellular DNA content and prognosis of high‐grade soft tissue sarcoma: the Scandinavian Sarcoma Group experience. Journal of Clinical Oncology 1990;8:538‐47. [DOI] [PubMed] [Google Scholar]
Anonymous 1987 {published data only}
- Anonymous. Randomised comparison of cisplatin with cyclophosphamide/cisplatin and with cyclophosphamide/doxorubicin/cisplatin in advanced ovarian cancer. Gruppo Interegionale Cooperativo Oncologico Ginecologia. Lancet 1987;2:353‐9. [PubMed] [Google Scholar]
Antillon 2008 {published data only}
- Antillon F, Castellanos M, Valverde P, Luna‐Fineman S, Garrido C, Serrato T, et al. Treating pediatric soft tissue sarcomas in a country with limited resources: The experience of the Unidad Nacional de Oncologia Pediatrica in Guatemala. Pediatric Blood and Cancer 2008;51:760‐4. [DOI] [PubMed] [Google Scholar]
Antman 1984a {published data only}
- Antman KH, Amato D, Lerner H, Suit H, Corson J, Wood W, et al. Pooled results of two randomized trials of adjuvant doxorubicin for sarcomas: Eastern Cooperative Oncology Group and Dana‐Farber Cancer Institute/Massachusetts General Hospital Study. In: Jones S, Salmon S editor(s). Adjuvant Therapy of Cancer IV. Orlando: Grune and Stratton, 1984:611‐20. [Google Scholar]
Antman 1984b {published data only}
- Antman K, Suit H, Amato D, Corson J, Wood W, Proppe K, et al. Preliminary results of a randomized trial of adjuvant doxorubicin for sarcomas: lack of apparent difference between treatment groups. Journal of Clinical Oncology 1984;2:601‐8. [DOI] [PubMed] [Google Scholar]
Antman 1985 {published data only}
- Antman KH, Amato D, Lerner H, Suit HD, Corson J, Wood WC, et al. Adjuvant doxorubicin for sarcoma: data from the ECOG and DFCI/MGH studies. Cancer Treatment Symposia 1985;3:109‐15. [Google Scholar]
Antman 1987 {published data only}
- Antman K, Amato D, Pilepich M, Lerner H, Balcerzak S, Borden E, et al. A preliminary analysis of a randomized intergroup (SWOG, ECOG, CALGB, NCOG) trial of adjuvant doxorubicin for soft tissue sarcomas. In: Salmon S editor(s). Adjuvant Therapy of Cancer V. Orlando: Grune and Stratton, 1987:725‐34. [Google Scholar]
Antman 1990 {published data only}
- Antman K, Ryan I, Borden E, Wood WC, Lerner HL, Corson JM, et al. Pooled results from three randomized adjuvant studies of doxorubicin versus observation in soft tissue sarcoma: 10‐year results and review of the literature. In: Salmon S editor(s). Adjuvant Therapy of Cancer VI. Philadelphia: WB Saunders, 1990:529‐44. [Google Scholar]
Aur 1972 {published data only}
- Aur RJ, Verzosa MS, Hustu HO, Simone JV. Response to combination therapy after relapse in childhood acute lymphocytic leukemia. Cancer 1972;30:334‐8. [DOI] [PubMed] [Google Scholar]
Bacci 1989 {published data only}
- Bacci G, Pignatti G, Dallari D, Mercuri M, Bertoni F, Bacchini P, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for telangiectatic osteogenic sarcoma of the extremities. Journal of Chemotherapy 1989;1:190‐6. [DOI] [PubMed] [Google Scholar]
Balwierz 2013 {published data only}
- Balwierz W, Pawinska‐Wasikowska K, Klelawka T, Czogala M, Matysiak M, Fic‐Sikorska B, et al. Development of treatment and clinical results in childhood acute myeloid leukemia in Poland. Magazine of European Medical Oncology 2013;6:54‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 1992 {published data only}
- Barr RD, DeVeber LL, Pai KM, Andrew M, Halton J, Cairney AE, et al. Management of children with acute lymphoblastic leukemia by the Dana‐Farber Cancer Institute protocols. An update of the Ontario experience. American Journal of Pediatric Hematology/Oncology 1992;14:136‐9. [DOI] [PubMed] [Google Scholar]
Bellani 1978 {published data only}
- Bellani FF, Gasparini M, Gennari L, Fontanillas L, Bonadonna G. Adjuvant treatment with adriamycin in primary operable osteosarcoma. Cancer Treatment Reports 1978;62:279‐81. [PubMed] [Google Scholar]
Berman 1989 {published data only}
- Berman E, Raymond V, Gee T, Kempin SJ, Gulati S, Andreeff M, et al. Idarubicin in acute leukemia: results of studies at Memorial Sloan‐Kettering Cancer Center. Seminars of Oncology 1989;16:30‐4. [PubMed] [Google Scholar]
Bernthal 2012 {published data only}
- Bernthal NM, Federman N, Eilber FR, Nelson SD, Eckardt JJ, Eilber FC, et al. Long‐term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high‐grade, operable osteosarcoma. Cancer 2012;118(23):5888‐93. [DOI] [PubMed] [Google Scholar]
Bessho 1994 {published data only}
- Bessho F, Kinumaki H, Yokota S, Hayashi Y, Kobayashi M, Kamoshita S. Liver function studies in children with acute lymphocytic leukemia after cessation of therapy. Medical and Pediatric Oncology 1994;23:111‐5. [DOI] [PubMed] [Google Scholar]
Biondi 2006 {published data only}
- Biondi A, Rizzari C, Valsecchi MG, Lorenzo P, Arico M, Basso G, et al. Role of treatment intensification in infants with acute lymphoblastic leukemia: results of two consecutive AIEOP studies. Haematologica 2006;91:534‐7. [PubMed] [Google Scholar]
Birch 1986 {published data only}
- Birch R, Williams S, Cone A, Einhorn L, Roark P, Turner S, et al. Prognostic factors for favorable outcome in disseminated germ cell tumors. Journal of Clinical Oncology 1986;4:400‐7. [DOI] [PubMed] [Google Scholar]
Blakely 2003 {published data only}
- Blakely ML, Shamberger RC, Norkool P, Beckwith JB, Green DM, Ritchey ML. Outcome of children with cystic partially differentiated nephroblastoma treated with or without chemotherapy. Journal of Pediatric Surgery 2003;38:897‐900. [DOI] [PubMed] [Google Scholar]
Bradford 1998 {published data only}
- Bradford WB, Jose BO, Butler D, Lindberg RD, Paris K, Spanos WJ, et al. Rhabdomyosarcoma in children‐‐a ten year review. The Journal of the Kentucky Medical Association 1998;96:399‐402. [PubMed] [Google Scholar]
Breslow 2004 {published data only}
- Breslow NE, Ou SS, Beckwith JB, Haase GM, Kalapurakal JA, Ritchey ML, et al. Doxorubicin for favorable histology, Stage II‐III Wilms tumor: results from the National Wilms Tumor Studies. Cancer 2004;101:1072‐80. [DOI] [PubMed] [Google Scholar]
Brouwer 2007 {published data only}
- Brouwer CA, Gietema JA, Berg MP, Bink‐Boelkens MT, Elzenga NJ, Haaksma J, et al. Low‐dose anthracyclines in childhood Acute Lymphoblastic Leukemia (ALL): no cardiac deterioration more than 20 years post‐treatment. Journal of Cancer Survivorship 2007;1:255‐60. [DOI] [PubMed] [Google Scholar]
Burnett 2006 {published data only}
- Burnett AK, Wheatley K, Goldstone AH, Stevens R, Hann I, Hills RK. Long‐term result of the MRC AML10 trial. Clinical Advances in Hematology and Oncology 2006;4:445‐51. [PubMed] [Google Scholar]
Caceres 1978 {published data only}
- Caceres E, Zaharia M, Moran M, Tejada F. Adjuvant whole‐lung radiation with or without adriamycin treatment in osteogenic sarcoma. Cancer Treatment Reports 1978;62:297‐9. [PubMed] [Google Scholar]
Canter 2007 {published data only}
- Canter RJ, Qin L‐X, Downey RJ, Brennan MF, Singer S, Maki RG. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft‐tissue sarcoma of the extremity: a retrospective analysis. Cancer 2007;110:2050‐60. [DOI] [PubMed] [Google Scholar]
Carlsen 1989 {published data only}
- Carlsen NL, Schroeder H, Bro PV, Hesselbjerg U, Jensen KB, Nielsen OH, et al. Response to multimodal treatment in advanced neuroblastomas. Factors associated with complete remission induction, and factors associated with prolonged survival. Anticancer Research 1989;9:837‐44. [PubMed] [Google Scholar]
Castellino 2008 {published data only}
- Castellino SM, Alonzo TA, Buxton A, Gold S, Lange BJ, Woods WG. Outcomes in childhood AML in the absence of transplantation in first remission ‐ Children's Cancer Group (CCG) studies 2891 and CCG 213. Pediatric Blood and Cancer 2008;50:9‐16. [DOI] [PubMed] [Google Scholar]
Chau 2003 {published data only}
- Chau I, Jones R, Cunningham D, Wotherspoon A, Maisey N, Norman AR, et al. Outcome of follicular lymphoma grade 3: is anthracycline necessary as front‐line therapy?. British Journal of Cancer 2003;89:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chessells 1992 {published data only}
- Chessells JM, Bailey CC, Richards S. MRC UKALL X. The UK protocol for childhood ALL: 1985‐1990. Leukemia 1992;6:157‐61. [PubMed] [Google Scholar]
Chessells 2002 {published data only}
- Chessells JM, Harrison G, Richards SM, Gibson BE, Bailey CC, Hill FG, et al. Failure of a new protocol to improve treatment results in paediatric lymphoblastic leukaemia: lessons from the UK Medical Research Council trials UKALL X and UKALL XI. British Journal of Haematology 2002;118:445‐55. [DOI] [PubMed] [Google Scholar]
Craft 1993 {published data only}
- Craft AW, Burgers JM. The European Osteosarcoma Intergroup (E.O.I.) studies 1980‐1991. Cancer Treatment and Research 1993;62:279‐86. [PubMed] [Google Scholar]
Creutzig 2005 {published data only}
- Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long‐term results in paediatric patients treated in four consecutive AML‐BFM trials. Leukemia 2005;19:2030‐42. [DOI] [PubMed] [Google Scholar]
Creutzig 2006 {published data only}
- Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony‐stimulating factor in children and adolescents with acute myeloid leukemia: results of AML‐BFM 98. Journal of Clinical Oncology 2006;24:4499‐506. [DOI] [PubMed] [Google Scholar]
Crist 1995 {published data only}
- Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, et al. The Third Intergroup Rhabdomyosarcoma Study. Journal of Clinical Oncology 1995;13:610‐30. [DOI] [PubMed] [Google Scholar]
Culbert 1991 {published data only}
- Culbert SJ, Shuster JJ, Land VJ, Wharam MD, Thomas PR, Nitschke R, et al. Remission induction and continuation therapy in children with their first relapse of acute lymphoid leukemia. A Pediatric Oncology Group study. Cancer 1991;67:37‐42. [DOI] [PubMed] [Google Scholar]
D'Angio 1989 {published data only}
- D'Angio GJ, Breslow N, Beckwith JB, Evans A, Baum H, DeLorimier A, et al. Treatment of Wilms' tumor. Results of the Third National Wilms' Tumor Study. Cancer 1989;64:349‐60. [DOI] [PubMed] [Google Scholar]
De Bernardi 2003 {published data only}
- Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero di Montezemolo L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high‐dose protocols adopted by the Italian Co‐Operative Group for Neuroblastoma. Journal of Clinical Oncology 2003;21:1592‐601. [DOI] [PubMed] [Google Scholar]
De Bernardi 2009 {published data only}
- Bernardi B, Gerrard M, Boni L, Rubie H, Canete A, Cataldo AD, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. Journal of Clinical Oncology 2009;27:1034‐40. [DOI] [PubMed] [Google Scholar]
Dluzniewska 2003 {published data only}
- Dluzniewska A, Balwierz W, Balcerska A, Chybicka A, Dobaczewski G, Kowalczyk J, et al. Efficacy of idarubicin in the treatment of childhood acute non‐lymphoblastic leukemia: report of Polish Pediatric Leukemia/Lymphoma Study Group. Przeglad Lekarski 2003;60 Suppl 5:17‐21. [PubMed] [Google Scholar]
Dobashi 2006 {published data only}
- Dobashi N, Yamaguchi Y, Asai O, Yano S, Osawa H, Yahagi Y, et al. Intensifying daunorubicin in induction for patients with core binding factor leukemia. International Journal of Hematology 2006;84:463‐4. [DOI] [PubMed] [Google Scholar]
Dunst 1998 {published data only}
- Dunst J. Therapy results of the British ET‐1 study for Ewing's sarcoma. Strahlentherapie und Onkologie 1998;174:289‐90. [DOI] [PubMed] [Google Scholar]
Eilber 1988 {published data only}
- Eilber FR, Giuliano AE, Huth JF, Morton DL. A randomized prospective trial using postoperative adjuvant chemotherapy (adriamycin) in high‐grade extremity soft‐tissue sarcoma. American Journal of Clinical Oncology 1988;11:39‐45. [DOI] [PubMed] [Google Scholar]
Einhorn 1981 {published data only}
- Einhorn LH, Williams SD, Troner M, Birch R, Greco FA. The role of maintenance therapy in disseminated testicular cancer. The New England Journal of Medicine 1981;305:727‐31. [DOI] [PubMed] [Google Scholar]
Elomaa 1993 {published data only}
- Elomaa I. An update of Scandinavian studies of osteosarcoma. Cancer Treatment and Research 1993;62:293‐8. [DOI] [PubMed] [Google Scholar]
Etcubanas 1984 {published data only}
- Etcubanas E, Hustu O, Sklar M, Pratt C. Influence of different treatment modalities on the curability of childhood rhabdomyosarcoma of the head or neck. European Paediatric Haematology & Oncology 1;1984:239‐44. [Google Scholar]
Evans 1985 {published data only}
- Evans R, Nesbit M, Askin F, Burgert O, Cangir A, Foulkes M, et al. Local recurrence, rate and sites of metastases, and time to relapse as a function of treatment regimen, size of primary and surgical history in 62 patients presenting with non‐metastatic Ewing's sarcoma of the pelvic bones. International Journal of Radiation Oncology, Biology, Physics 1985;11:129‐36. [DOI] [PubMed] [Google Scholar]
Evans 1991 {published data only}
- Evans AE, Norkool P, Evans I, Breslow N, D'Angio GJ. Late effects of treatment for Wilms' tumor. A report from the National Wilms' Tumor Study Group. Cancer 1991;67:331‐6. [DOI] [PubMed] [Google Scholar]
Fink 1990 {published data only}
- Fink FM, Gadner H, Grumayer ER, Kardos G, Revesz T, Urban C, et al. Treatment of childhood acute nonlymphocytic leukemia: cooperative Austrian‐Hungarian study AML‐IGCI‐84. Haematology and Blood Transfusion 1990;33:233‐6. [DOI] [PubMed] [Google Scholar]
Frappaz 2002 {published data only}
- Frappaz D, Perol D, Michon J, Berger C, Coze C, Bernard JL, et al. The LMCE5 unselected cohort of 25 children consecutively diagnosed with untreated stage 4 neuroblastoma over 1 year at diagnosis. British Journal of Cancer 2002;87:1197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukuoka 1994 {published data only}
- Fukuoka M, Masuda N, Takada M, Kodama N, Kawahara M, Furuse K. Dose‐intensive chemotherapy in extensive‐stage small cell lung cancer. Seminars in Oncology 1994;21:43‐7. [PubMed] [Google Scholar]
Gallegos‐Castorena 2009 {published data only}
- Gallegos‐Castorena S, Medina‐Sanson A, Gonzalez‐Ramella O, Sanchez‐Zubieta F, Martinez‐Avalos A. Improved treatment results in Mexican children with acute myeloid leukemia using a Medical Research Council (MRC)‐acute myeloid leukemia 10 modified protocol. Leukemia and Lymphoma 2009;50:1132‐7. [DOI] [PubMed] [Google Scholar]
Garbes 1987 {published data only}
- Garbes ID, Gomez GA, Han T, Henderson ES. Salvage chemotherapy for advanced Hodgkin's disease. Medical and Pediatric Oncology 1987;15:45‐8. [DOI] [PubMed] [Google Scholar]
Gaynon 1993 {published data only}
- Gaynon PS, Steinherz PG, Bleyer WA, Ablin AR, Albo VC, Finklestein JZ, et al. Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow‐up report of the Childrens Cancer Group Study CCG‐106. Journal of Clinical Oncology 1993;11:2234‐42. [DOI] [PubMed] [Google Scholar]
Gelderblom 2011 {published data only}
- Gelderblom H, Jinks RC, Sydes M, Bramwell VH, Glabbeke M, Grimer RJ, et al. Survival after recurrent osteosarcoma: data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. European Journal of Cancer 2011;47(6):895‐902. [DOI] [PubMed] [Google Scholar]
Glanzmann 1998 {published data only}
- Glanzmann C, Kaufmann P, Jenni R, Hess OM, Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin's disease. Radiotherapy & Oncology 1998;46:51‐62. [DOI] [PubMed] [Google Scholar]
Green 1994 {published data only}
- Green DM, Breslow NE, Beckwith JB, Moksness J, Finklestein JZ, D'Angio GJ. Treatment of children with clear‐cell sarcoma of the kidney: a report from the National Wilms' Tumor Study Group. Journal of Clinical Oncology 1994;12:2132‐7. [DOI] [PubMed] [Google Scholar]
Green 1996 {published data only}
- Green DM, Breslow NE, Evans I, Moksness J, D'Angio GJ. Treatment of children with stage IV favorable histology Wilms tumor: a report from the National Wilms Tumor Study Group. Medical and Pediatric Oncology 1996;26:147‐52. [DOI] [PubMed] [Google Scholar]
Green 1999 {published data only}
- Green DM, Hyland A, Chung CS, Zevon MA, Hall BC. Cancer and cardiac mortality among 15‐year survivors of cancer diagnosed during childhood or adolescence. Journal of Clinical Oncology 1999;17:3207‐15. [DOI] [PubMed] [Google Scholar]
Grier 1990 {published data only}
- Grier HE, Gelber RD, Clavell LA, Camitta BM, Link MP, Garcea MJ, et al. Intensive sequential chemotherapy for children with acute myelogenous leukemia. Haematology and Blood Transfusion 1990;33:193‐7. [DOI] [PubMed] [Google Scholar]
Grundy 2012 {published data only}
- Grundy PE, Green DM, Dirks AC, Berendt AE, Breslow NE, Anderson JR, et al. Clinical significance of pulmonary nodules detected by CT and Not CXR in patients treated for favorable histology Wilms tumor on national Wilms tumor studies‐4 and ‐5: a report from the Children's Oncology Group. Pediatric Blood and Cancer 2012;59(4):631‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gururangan 2000 {published data only}
- Gururangan S, Sposto R, Cairo MS, Meadows AT, Finlay JL. Outcome of CNS disease at diagnosis in disseminated small noncleaved‐cell lymphoma and B‐cell leukemia: a Children's Cancer Group study. Journal of Clinical Oncology 2000;18:2017‐25. [DOI] [PubMed] [Google Scholar]
Hainsworth 1985 {published data only}
- Hainsworth JD, Williams SD, Einhorn LH, Birch R, Greco FA. Successful treatment of resistant germinal neoplasms with VP‐16 and cisplatin: results of a Southeastern Cancer Study Group trial. Journal of Clinical Oncology 1985;3:666‐71. [DOI] [PubMed] [Google Scholar]
Halazun 1974 {published data only}
- Halazun JF, Wagner HR, Gaeta JF, Sinks LF. Daunorubicin cardiac toxicity in children with acute lymphocytic leukemia. Cancer 1974;33:545‐54. [DOI] [PubMed] [Google Scholar]
Harms 2000 {published data only}
- Harms D, Janka‐Schaub GE. Co‐operative study group for childhood acute lymphoblastic leukemia (COALL): long‐term follow‐up of trials 82, 85, 89 and 92. Leukemia 2000;14:2234‐9. [DOI] [PubMed] [Google Scholar]
Hayashi 2008 {published data only}
- Hayashi K, Tsuchiya H, Yamamoto N, Takeuchi A, Tomita K. Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. International Orthopaedics 2008;32:63‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 1988 {published data only}
- Hays DM, Shimada H, Raney RB Jr, Tefft M, Newton W, Crist WM, et al. Clinical staging and treatment results in rhabdomyosarcoma of the female genital tract among children and adolescents. Cancer 1988;61:1893‐903. [DOI] [PubMed] [Google Scholar]
Henze 1989 {published data only}
- Henze G, Fengler R, Hartmann R, Dopfer R, Gobel U, Graf N, et al. Chemotherapy for bone marrow relapse of childhood acute lymphoblastic leukemia. Cancer Chemotherapy & Pharmacology 1989;24:S16‐9. [DOI] [PubMed] [Google Scholar]
Hitchcock‐Bryan 1986 {published data only}
- Hitchcock‐Bryan S, Gelber R, Cassady JR, Sallan SE. The impact of induction anthracycline on long‐term failure‐free survival in childhood acute lymphoblastic leukemia. Medical and Pediatric Oncology 1986;14:211‐5. [DOI] [PubMed] [Google Scholar]
Hodgson 2003 {published data only}
- Hodgson DC, Tsang RW, Pintilie M, Sun A, Wells W, Crump M, et al. Impact of chest wall and lung invasion on outcome of stage I‐II Hodgkin's lymphoma after combined modality therapy. International Journal of Radiation Oncology, Biology, Physics 2003;57:1374‐81. [DOI] [PubMed] [Google Scholar]
Holland 1971 {published data only}
- Holland JF. E Pluribus Unum: Presidential address. Cancer Research 1971;31:1319‐29. [PubMed] [Google Scholar]
Humphrey 1975 {published data only}
- Humphrey GB, Fernbach DJ, Razek AA, Stuckey WJ, Komp D, George S. Evaluation of daunorubicin (NSC‐82151) and methotrexate (NSC‐740) in combination as a remission maintenance regimen in the treatment of acute leukemia. Cancer Chemotherapy Reports 1975;59:395‐9. [PubMed] [Google Scholar]
Iarussi 2003 {published data only}
- Iarussi D, Indolfi P, Pisacane C, Casale F, Martino V, Fusco A, et al. Comparison of left ventricular function by echocardiogram in patients with Wilms' tumor treated with anthracyclines versus those not so treated. The American Journal Of Cardiology 2003;92:359‐61. [DOI] [PubMed] [Google Scholar]
Ihde 1977 {published data only}
- Ihde DC, Kane RC, Cohen MH, McIntire KR, Minna JD. Adriamycin therapy in American patients with hepatocellular carcinoma. Cancer Treatment Report 1977;61:1385‐7. [PubMed] [Google Scholar]
Isu 1992 {published data only}
- Isu K, Yamawaki S, Usui M. A group study of neoadjuvant chemotherapy for osteosarcoma ‐ Results and problems of T‐12 protocol. Journal of the Japanese Orthopaedic Association 1992;66:S9‐S10. [Google Scholar]
Iwenofu 2008 {published data only}
- Iwenofu OH, Lackman RD, Staddon AP, Goodwin DG, Haupt HM, Brooks JS. Phospho‐S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Modern Pathology 2008;21:231‐7. [DOI] [PubMed] [Google Scholar]
Jaffe 1981 {published data only}
- Jaffe N, Link MP, Cohen D, Traggis D, Frei E III, Watts H, et al. High‐dose methotrexate in osteogenic sarcoma. National Cancer Institute Monograph 1981:201‐6. [PubMed] [Google Scholar]
Janka‐Schaub 1988 {published data only}
- Janka‐Schaub GE, Winkler K, Gobel U, Graubner U, Schwenger M, Haas RJ, et al. First results of the CO ALL‐85 cooperative study for high‐risk patients with acute lymphatic leukemia. Klinische Padiatrie 1988;200:171‐6. [DOI] [PubMed] [Google Scholar]
Kalapurakal 2010 {published data only}
- Kalapurakal JA, Li SM, Breslow NE, Beckwith JB, Ritchey ML, Shamberger RC, et al. Intraoperative spillage of favorable histology Wilms tumor cells: influence of irradiation and chemotherapy regimens on abdominal recurrence. A report from the National Wilms Tumor Study Group. International Journal of Radiation Oncology, Biology, Physics 2010;76:201‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karachunskii 2007 {published data only}
- Karachunskii AI, Miakova NV, Rumiantseva I, Timakov AM, Makhortykh TZ, Fechina LG, et al. The results of a multicenter trial of acute lymphoblastic leukemia treatment on ALL‐MB 91/ALL‐BFM 90m in children: analysis of efficacy and toxicity. Terapevticheskii Arkhiv 2007;79:19‐26. [PubMed] [Google Scholar]
Karachunskiy 2008 {published data only}
- Karachunskiy A, Herold R, Stackelberg A, Miakova N, Timakow A, Mahortih T, et al. Results of the first randomized multicentre trial on childhood acute lymphoblastic leukaemia in Russia. Leukemia 2008;22:1144‐53. [DOI] [PubMed] [Google Scholar]
Kaspers 2010 {published data only}
- Kaspers G, Zimmermann M, Reinhardt D, Gibson B, Tamminga R, Aleinikova O, et al. Central Nervous System (CNS) involvement in pediatric relapsed acute myeloid leukemia: Results and lessons from study relapsed AML 2001/01. Blood (Annual Meeting of the American Society of Hematology). 2010:116 (abstract 184).
Kazanowska 2006 {published data only}
- Kazanowska B, Reich A, Wrobel G, Balcerska A, Balwierz W, Bodalski J, et al. Metastatic soft tissue sarcomas in children ‐ A remaining challenge for paediatric oncology. A retrospective multicenter study from the Polish Pediatric Solid Tumors' Group. Nowotwory 2006;56:517‐62. [Google Scholar]
Khattab 2008 {published data only}
- Khattab TM, Atra AA, Elimam NA, Kassar A, Zayed A, Baothman A. Improved outcome of children with acute myeloid leukemia treated on 2 consecutive protocols. Saudi Medical Journal 2008;29:776‐7. [PubMed] [Google Scholar]
Kinsella 1991 {published data only}
- Kinsella TJ, Miser JS, Waller B, Venzon D, Glatstein E, Weaver‐McClure L, et al. Long‐term follow‐up of Ewing's sarcoma of bone treated with combined modality therapy. International Journal Of Radiation Oncology, Biology, Physics 1991;20:389‐95. [DOI] [PubMed] [Google Scholar]
Kobe 2008 {published data only}
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first‐line chemotherapy in advanced‐stage Hodgkin lymphoma. Blood 2008;112:3989‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komp 1976 {published data only}
- Komp DM, George SL, Falletta J, Land VJ, Starling KA, Humphrey GB, et al. Cyclophosphamide‐asparaginase‐vincristine‐prednisone induction therapy in childhood acute lymphocytic and nonlymphocytic leukemia. Cancer 1976;37:1243‐7. [DOI] [PubMed] [Google Scholar]
Konopka 1989 {published data only}
- Konopka L, Pawelski S. Results of three polychemotherapy programs in non‐Hodgkin's lymphomas. Journal of Chemotherapy 1989;1:1259‐62. [PubMed] [Google Scholar]
Kuleva 2008 {published data only}
- Kuleva SA, Anishkin MI, Kolygin BA. A comparison of two risk‐adapted regimens of therapy for Hodgkin's disease in children and adolescents. Voprosy Onkologii 2008;54:53‐8. [PubMed] [Google Scholar]
Kurrle 1988 {published data only}
- Kurrle E, Ehninger G, Freund M, Heil G, Hoelzer D, Link H, et al. A multicentre study on intensive induction and consolidation therapy in acute myelogenous leukaemia. Blut 1988;56:233‐6. [DOI] [PubMed] [Google Scholar]
Lager 2006 {published data only}
- Lager JJ, Lyden ER, Anderson JR, Pappo AS, Meyer WH, Breitfeld PP. Pooled analysis of phase II window studies in children with contemporary high‐risk metastatic rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Journal of Clinical Oncology 2006;24:3415‐22. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li CK, Chik KW, Ha SY, Lee ACW, Yuen HL, Ling SC, et al. Improved outcome of acute lymphoblastic leukaemia treated by delayed intensification in Hong Kong children: HKALL 97 study. Hong Kong Medical Journal 2006;12:33‐9. [PubMed] [Google Scholar]
Lilleyman 1997 {published data only}
- Lilleyman JS, Gibson BE, Stevens RF, Will AM, Hann IM, Richards SM, et al. Clearance of marrow infiltration after 1 week of therapy for childhood lymphoblastic leukaemia: clinical importance and the effect of daunorubicin. The Medical Research Council's Working Party on Childhood Leukaemia. British Journal of Haematology 1997;97:603‐6. [DOI] [PubMed] [Google Scholar]
Lindberg 1977 {published data only}
- Lindberg RD, Murphy WK, Benjamin RS, Sinkovics JG, Martin RG, Romsdahl MM, et al. Adjuvant chemotherapy in the treatment of primary soft tissue sarcomas: a preliminary report. Management of Bone and Soft Tissue Tumors. Chicago: Book Medical Publishers, 1977:343‐52. [Google Scholar]
Link 1986 {published data only}
- Link MP, Goorin AM, Miser AW. The effect of adjuvant chemotherapy on relapse‐free survival in patients with osteosarcoma of the extremity. The New England Journal of Medicine 1986;314:1600‐6. [DOI] [PubMed] [Google Scholar]
Madej 1987 {published data only}
- Madej G, Siedlecki P, Meyza J. Preliminary evaluation of the effectiveness of a modified treatment program in patients with malignant testicular neoplasms. Polski Tygodnik Lekarski 1987;42:836‐40. [PubMed] [Google Scholar]
Madon 1985 {published data only}
- Madon E, Pastore G, Cordero Di Montezemolo L, Brach del Prever A, Lanino E. Italian multicenter experience in the treatment of neuroblastoma and soft tissue sarcoma. Minerva Pediatrica 1985;37:603‐10. [PubMed] [Google Scholar]
Mahajan 2008 {published data only}
- Mahajan A, Woo SY, Kornguth DG, Hughes D, Huh W, Chang EL, et al. Multimodality treatment of osteosarcoma: radiation in a high‐risk cohort. Pediatric Blood and Cancer 2008;50:976‐82. [DOI] [PubMed] [Google Scholar]
Mahmoud 1993 {published data only}
- Mahmoud HH, Rivera GK, Hancock ML, Krance RA, Kun LE, Behm FG, et al. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. The New England Journal of Medicine 1993;329:314‐9. [DOI] [PubMed] [Google Scholar]
Maiakova 1986 {published data only}
- Maiakova SA, Makhonova LA. Consolidating chemotherapy in the treatment program of acute lymphoblastic leukemia in children. Vestnik Akademii Meditsinskikh Nauk SSSR 1986:15‐7. [PubMed] [Google Scholar]
Malpas 1974 {published data only}
- Malpas J, Mathe G, Hayat M. Comparison of the effects of cytosine arabinoside, cytosine arabinoside and thioguanine, and cytosine arabinoside and daunorubicin in acute myeloid leukemia. Bulletin du Cancer 1974;61:411‐8. [PubMed] [Google Scholar]
Marcus 1987 {published data only}
- Marcus RE, Catovsky D, Prentice HG, Newland AC, Chessells JM, Stevens RF, et al. Intensive induction and consolidation chemotherapy for adults and children with acute myeloid leukaemia (AML) joint AML trial 1982‐1985. Haematology and Blood Transfusion 1987;30:346‐51. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2001 {published data only}
- Matsuzaki A, Ishii E, Nagatoshi Y, Eguchi H, Koga H, Yanai F, et al. Long‐term outcome of treatment with protocols AL841, AL851, and ALHR88 in children with acute lymphoblastic leukemia: results obtained by the Kyushu‐Yamaguchi Children's Cancer Study Group. International Journal of Hematology 2001;73:369‐77. [DOI] [PubMed] [Google Scholar]
Maurer 1981 {published data only}
- Maurer HM. The Intergroup Rhabdomyosarcoma Study: update, November 1978. National Cancer Institute Monograph 1981;56:61‐8. [PubMed] [Google Scholar]
Meisel 1999 {published data only}
- Meisel JA, Guthrie KA, Breslow NE, Donaldson SS, Green DM. Significance and management of computed tomography detected pulmonary nodules: a report from the National Wilms Tumor Study Group. International Journal of Radiation Oncology, Biology, Physics 1999;44:579‐85. [DOI] [PubMed] [Google Scholar]
Meza 2006 {published data only}
- Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. Journal of Clinical Oncology 2006;24:3844‐51. [DOI] [PubMed] [Google Scholar]
Miser 1993 {published data only}
- Miser JS, Pritchard DJ, Rock MG, Shives TC, Gilchrist GS, Smithson WA, et al. Osteosarcoma in adolescents and young adults: new developments and controversies. The Mayo Clinic studies. Cancer Treatment and Research 1993;62:333‐8. [PubMed] [Google Scholar]
Moricke 2008 {published data only}
- Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, et al. Risk‐adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL‐BFM 95. Blood 2008;111:4477‐89. [DOI] [PubMed] [Google Scholar]
Muus 1993 {published data only}
- Muus P, Donnelly P, Schattenberg A, Linssen P, Minderman H, Dompeling E, et al. Idarubicin‐related side effects in recipients of T‐cell‐depleted allogeneic bone marrow transplants are schedule dependent. Seminars of Oncology 1993;20:47‐52. [PubMed] [Google Scholar]
Nachman 1993 {published data only}
- Nachman J, Sather HN, Buckley JD, Gaynon PS, Steinherz PG, Tubergen DG, et al. Young adults 16‐21 years of age at diagnosis entered on Children's Cancer Group acute lymphoblastic leukemia and acute myeloblastic leukemia protocols: Results of treatment. Cancer 1993;71:3377‐85. [DOI] [PubMed] [Google Scholar]
Nishimura 1983 {published data only}
- Nishimura H, Yamada T, Hagio Y, Inoue T, Tsunawaki A, Yakushiji M, et al. Treatment of advanced ovarian cancer with cis‐dichlorodiammine platinum (CDDP) as single agent or CDDP in combination with adriamycin (ADM). Gan To Kagaku Ryoho 1983;10:938‐43. [PubMed] [Google Scholar]
Ochiai 1993 {published data only}
- Ochiai K. Strategy for the ovarian cancer treatment. Japanese Journal of Cancer and Chemotherapy 1993;20:2454‐60. [PubMed] [Google Scholar]
Oh 2006 {published data only}
- Oh SY, Ryoo BY, Kim WS, Kim K, Lee J, Kim HJ, et al. Nodal marginal zone B‐cell lymphoma: Analysis of 36 cases. Clinical presentation and treatment outcomes of nodal marginal zone B‐cell lymphoma. Annals of Hematology 2006;85:781‐6. [DOI] [PubMed] [Google Scholar]
Okamura 1987 {published data only}
- Okamura J, Ikuno Y, Yamada S, Tasaka H, Nibu K, Ikeda N, et al. Results of treatment in children with diffuse large cell lymphoma. Rinsho Ketsueki 1987;28:1365‐9. [PubMed] [Google Scholar]
Omura 1985 {published data only}
- Omura GA, Blessing JA, Major F, Lifshitz S, Ehrlich CE, Mangan C, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 1985;3:1240‐5. [DOI] [PubMed] [Google Scholar]
Ortega 1991 {published data only}
- Ortega JA, Wharam M, Gehan EA, Ragab AH, Crist W, Webber B, et al. Clinical features and results of therapy for children with paraspinal soft tissue sarcoma: A report of the intergroup Rhabdomyosarcoma study. Journal of Clinical Oncology 1991;9:796‐801. [DOI] [PubMed] [Google Scholar]
Paulino 1996 {published data only}
- Paulino AC, Wilimas J, Marina N, Jones D, Kumar M, Greenwald C, et al. Local control in synchronous bilateral Wilms tumor. International Journal of Radiation Oncology, Biology, Physics 1996;36:541‐8. [DOI] [PubMed] [Google Scholar]
Paulino 2003 {published data only}
- Paulino AC, Bauman N, Simon JH, Nguyen TX, Ritchie JM, Tannous R. Local control of parameningeal rhabdomyosarcoma: outcomes in non‐complete responders to chemoradiation. Medical and Pediatric Oncology 2003;41:118‐22. [DOI] [PubMed] [Google Scholar]
Pavlovsky 1981 {published data only}
- Pavlovsky S. Argentinian Group for the Treatment of Acute Leukemia: our experience in the treatment of acute lymphoblastic leukemias. Sangre 1981;26:845‐54. [PubMed] [Google Scholar]
Pawson 2001 {published data only}
- Pawson R, Potter MN, Theocharous P, Lawler M, Garg M, Yin JA, et al. Treatment of relapse after allogeneic bone marrow transplantation with reduced intensity conditioning (FLAG ± Ida) and second allogeneic stem cell transplant. British Journal of Haematology 2001;115:622‐9. [DOI] [PubMed] [Google Scholar]
Peeters 2009 {published data only}
- Peeters J, Meitert J, Paulides M, Beck JD, Langer T. Late effects surveillance system after childhood cancer in Germany, Austria and parts of Switzerland‐update 2009. Strahlentherapie und Onkologie 2009;185 Suppl 2:5‐7. [DOI] [PubMed] [Google Scholar]
Perez 1981 {published data only}
- Perez CA, Tefft M, Nesbit ME Jr, Burgert EO Jr, Vietti TJ, Kissane J, et al. Radiation therapy in the multimodal management of Ewing's sarcoma of bone: report of the Intergroup Ewing's Sarcoma Study. National Cancer Institute Monograph 1981:263‐71. [PubMed] [Google Scholar]
Pfreundschuh 2008 {published data only}
- Pfreundschuh M, Zwick C, Zeynalova S, Duhrsen U, Pfluger KH, Vrieling T, et al. Dose‐escalated CHOEP for the treatment of young patients with aggressive non‐Hodgkin's lymphoma: II. Results of the randomized high‐CHOEP trial of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group (DSHNHL). Annals of Oncology 2008;19:545‐52. [DOI] [PubMed] [Google Scholar]
Picci 1997 {published data only}
- Picci P, Bacci G, Ferrari S, Mercuri M. Neoadjuvant chemotherapy in malignant fibrous histiocytoma of bone and in osteosarcoma located in the extremities: analogies and differences between the two tumors. Annals of Oncology 1997;8:1107‐15. [DOI] [PubMed] [Google Scholar]
Pontz 1981 {published data only}
- Pontz BF, Gutjahr P. Aggressive cytostatic treatment of osteogenic sarcoma in childhood. Deutsche Medizinische Wochenschrift 1981;106:200‐7. [DOI] [PubMed] [Google Scholar]
Pratt 1981 {published data only}
- Pratt CB, Hustu HO, Kumar AP, Johnson WW, Ransom JL, Howarth CB, et al. Treatment of childhood rhabdomyosarcoma at St. Jude Children's Research Hospital, 1962‐‐78. National Cancer Institute Monograph 1981:93‐101. [PubMed] [Google Scholar]
Pratt 1993 {published data only}
- Pratt CB, Meyer WH, Rao BN, Parham DM, Fleming ID. Osteosarcoma studies at St. Jude Children's Research Hospital from 1968 through 1990. Cancer Treatment and Research 1993;62:323‐6. [DOI] [PubMed] [Google Scholar]
Pritchard‐Jones 2011 {published data only}
- Pritchard‐Jones K, Graf N, Bergeron C, Camargo B, Heuvel M, Sandstedt B, et al. Doxorubicin can be safely omitted from the treatment of stage II/III, Intermediate risk histology Wilms tumour ‐ Results of the siop WT 2001 randomised trial, on behalf of the SIOP Renal Tumours Study Group. European Journal of Cancer (European Multidisciplinary Cancer Congress). 2011:S284‐5.
Pritchard‐Jones 2012 {published data only}
- Pritchard‐Jones K, Moroz V, Vujanic G, Powis M, Walker J, Messahel B, et al. Treatment and outcome of Wilms' tumour patients: an analysis of all cases registered in the UKW3 trial. Annals of Oncology 2012;23(9):2457‐63. [DOI] [PubMed] [Google Scholar]
Quattrin 1975 {published data only}
- Quattrin N, Montuori R, Girolamo R, Cimino R. Our recent therapeutic experiences in acute leukemia. Minerva Medica 1975;66:2538‐45. [PubMed] [Google Scholar]
Rai 1981 {published data only}
- Rai KR, Holland JF, Glidewell OJ, Weinberg V, Brunner K, Obrecht JP, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood 1981;58:1203‐12. [PubMed] [Google Scholar]
Rammeloo 2000 {published data only}
- Rammeloo LA, Postma A, Sobotka‐Plojhar MA, Bink‐Boelkens MT, Berg A, Veerman AJ, et al. Low‐dose daunorubicin in induction treatment of childhood acute lymphoblastic leukemia: no long‐term cardiac damage in a randomized study of the Dutch Childhood Leukemia Study Group. Medical and Pediatric Oncology 2000;35:13‐9. [DOI] [PubMed] [Google Scholar]
Raney 1983 {published data only}
- Raney RB Jr, Lawrence W Jr, Maurer HM, Lindberg RD, Newton WA Jr, Ragab AH, et al. Rhabdomyosarcoma of the ear in childhood. A report from the Intergroup Rhabdomyosarcoma Study‐I. Cancer 1983;51:2356‐61. [DOI] [PubMed] [Google Scholar]
Raney 1987 {published data only}
- Raney B, Schnaufer L, Ziegler M, Chatten J, Littman P, Jarrett P. Treatment of children with neurogenic sarcoma. Experience at the Children's Hospital of Philadelphia, 1958‐1984. Cancer 1987;59:1‐5. [DOI] [PubMed] [Google Scholar]
Raney 1988 {published data only}
- Raney RB Jr, Tefft M, Maurer HM, Ragab AH, Hays DM, Soule EH, et al. Disease patterns and survival rate in children with metastatic soft‐tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)‐I. Cancer 1988;62:1257‐66. [DOI] [PubMed] [Google Scholar]
Raney 1990 {published data only}
- Raney RB Jr, Crist W, Hays D, Newton W, Ruymann F, Tefft M, et al. Soft tissue sarcoma of the perineal region in childhood. A report from the Intergroup Rhabdomyosarcoma Studies I and II, 1972 through 1984. Cancer 1990;65:2787‐92. [DOI] [PubMed] [Google Scholar]
Raza 2008 {published data only}
- Raza S, Ullah K, Ahmed P, Khan B. Comparison of anthracycline‐based combination chemotherapy with or without all‐trans retinoic acid in acute promyelocytic leukemia. Journal of the College of Physicians and Surgeons‐Pakistan 2008;18:546‐50. [PubMed] [Google Scholar]
Rees 1990 {published data only}
- Rees JK, Gray RG. Remission induction and postremission therapy in acute myelogenous leukemia: British MRC Study. Haematology and Blood Transfusion 1990;33:243‐8. [DOI] [PubMed] [Google Scholar]
Ritchey 1994 {published data only}
- Ritchey ML, Pringle KC, Breslow NE, Takashima J, Moksness J, Zuppan CW, et al. Management and outcome of inoperable Wilms tumor. A report of National Wilms Tumor Study‐3. Annals of Surgery 1994;220:683‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritter 1990 {published data only}
- Ritter J, Creutzig U, Schellong G. Improved treatment results in the myelocytic subtypes FAB M1‐M4 but not in FAB M5 after intensification of induction therapy: results of the German childhood AML studies BFM‐78 and BFM‐83. Haematology and Blood Transfusion 1990;33:185‐92. [DOI] [PubMed] [Google Scholar]
Roy 2005 {published data only}
- Roy A, Bradburn M, Moorman AV, Burrett J, Love S, Kinsey SE, et al. Early response to induction is predictive of survival in childhood Philadelphia chromosome positive acute lymphoblastic leukaemia: results of the Medical Research Council ALL 97 trial. British Journal of Haematology 2005;129:35‐44. [DOI] [PubMed] [Google Scholar]
Sakic 2006 {published data only}
- Sakic M, Berbic‐Fazlagic J. Results of treatment with protocols BFM 90 and BFM 95. Medicinski Arhiv 2006;60:369‐72. [PubMed] [Google Scholar]
Sallan 1977 {published data only}
- Sallan SE, Camitta BM, Frei E III, Furman L, Leavitt P, Bishop Y, et al. Clinical and cytokinetic aspects of remission induction of childhood acute lymphoblastic leukemia (ALL): addition of an anthracycline to vincristine and prednisone. Medical and Pediatric Oncology 1977;3:281‐7. [DOI] [PubMed] [Google Scholar]
Salodof MacNeil 2010 {published data only}
- Salodof MacNeil J. MRC AML 15 trial: similar survival rates from multiple regimens. The Oncology Report 2010:26. [Google Scholar]
Schaison 1992 {published data only}
- Schaison G, Sommelet D, Bancillon A, Perel Y, Leblanc T, Bergeron C, et al. Treatment of acute lymphoblastic leukemia French protocol Fralle 83‐87. Leukemia 1992;6 Suppl 2:148‐52. [PubMed] [Google Scholar]
Scherrer 1994 {published data only}
- Scherrer R, Bettelheim P, Geissler K, Jager U, Knobl P, Kyrle PA, et al. High efficacy of the German multicenter ALL (GMALL) protocol for treatment of adult acute lymphoblastic leukemia (ALL) ‐ a single‐institution study. Annals of Hematology 1994;69:181‐8. [DOI] [PubMed] [Google Scholar]
Schmits 2001 {published data only}
- Schmits R, Glass B, Trumper L, Pfreundschuh M. Therapeutic strategies for aggressive lymphomas: the trials of the DSHNHL. Annals of Hematology 2001;80 Suppl 3:B77‐B83. [DOI] [PubMed] [Google Scholar]
Seibel 2008 {published data only}
- Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high‐risk acute lymphoblastic leukemia: A report from the children's oncology group. Blood 2008;111:2548‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 2000 {published data only}
- Silverman LB, Declerck L, Gelber RD, Dalton VK, Asselin BL, Barr RD, et al. Results of Dana‐Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981‐1995). Leukemia 2000;14:2247‐56. [DOI] [PubMed] [Google Scholar]
Skoczen 2006 {published and unpublished data}
- Skoczen S, Balwierz W, Moryl‐Bujakowska A, Pawinska K, Luszczynska A, Balcerska A, et al. Acute lymphoblastic leukemia in children with initial leucocytosis above 50,000/mm3: summary of treatment results of Polish Pediatric Leukemia/Lymphoma Study Group. Przeglad Lekarski 2006;63:11‐4. [PubMed] [Google Scholar]
Smithson 1982 {published data only}
- Smithson WA, Telander RL, Carney JA. Mesenchymoma of the liver in childhood: five‐year survival after combined‐modality treatment. Journal of Pediatric Surgery 1982;17:70‐2. [DOI] [PubMed] [Google Scholar]
Sotnikov 1989 {published data only}
- Sotnikov VM, Polianskaia AM, Pan'shin GA, Ingberman I, Kaplanskaia IB. Chemoradiation treatment of patients with aggressive non‐Hodgkin's lymphomas in stages I to II of the disease. Terapevticheskii Arkhiv 1989;61:20‐3. [PubMed] [Google Scholar]
Spears 1992 {published data only}
- Spears WT, Morphis II JG, Lester SG, Williams SD, Einhorn LH. Brain metastases and testicular tumors: long‐term survival. International Journal of Radiation Oncology, Biology, Physics 1992;22:17‐22. [DOI] [PubMed] [Google Scholar]
Spreafico 2008 {published data only}
- Spreafico F, Bisogno G, Collini P, Jenkner A, Gandola L, D'Angelo P, et al. Treatment of high‐risk relapsed Wilms tumor with dose‐intensive chemotherapy, marrow‐ablative chemotherapy, and autologous hematopoietic stem cell support: experience by the Italian Association of Pediatric Hematology and Oncology. Pediatric Blood and Cancer 2008;51:23‐8. [DOI] [PubMed] [Google Scholar]
Sramkova 2013 {published data only}
- Sramkova L, Sterba J, Hrstkova H, Mihal V, Blazek B, Timr P, et al. Development of treatment and clinical results in childhood acute myeloid leukemia in the Czech Republic. Magazine of European Medical Oncology 2013;6:41‐5. [Google Scholar]
Stary 2003 {published data only}
- Stary J, Gajdos P, Blazek B, Ptoszkova H, Mihal V, Pospisilova D, et al. Improved results in children with acute lymphoblastic leukemia treated with the ALL‐BFM 90 protocol in the Czech Republic. Casopis Lekary Ceskych 2003;142:404‐9. [PubMed] [Google Scholar]
Steinherz 1993 {published data only}
- Steinherz PG, Redner A, Steinherz L, Meyers P, Tan C, Heller G. Development of a new intensive therapy for acute lymphoblastic leukemia in children at increased risk of early relapse. The Memorial Sloan‐Kettering‐New York‐II protocol. Cancer 1993;72:3120‐30. [DOI] [PubMed] [Google Scholar]
Steinherz 1996 {published data only}
- Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, et al. Cytoreduction and prognosis in acute lymphoblastic leukemia‐the importance of early marrow response: report from the Childrens Cancer Group. Journal of Clinical Oncology 1996;14:389‐98. [DOI] [PubMed] [Google Scholar]
Steinherz 1998 {published data only}
- Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, et al. Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T‐cell phenotype or other poor prognostic features: randomized controlled trial from the Children's Cancer Group. Cancer 1998;82:600‐12. [DOI] [PubMed] [Google Scholar]
Tarella 2001 {published data only}
- Tarella C, Cuttica A, Caracciolo D, Zallio F, Ricca I, Bergui L, et al. High‐dose sequential (HDS) chemotherapy for high‐risk non‐Hodgkin's lymphoma: long‐term analysis and future developments. Annals of Hematology 2001;80 Suppl 3:B123‐6. [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor PR, White JM, Prescott RJ, Angus B, Galloway MJ, Jackson GH, et al. The addition of oral idarubicin to a chlorambucil/dexamethasone combination has a significant impact on time to treatment failure but none on overall survival in patients with low grade non‐Hodgkin's lymphoma: Results of the Scotland and Newcastle Lymphoma Group randomized NHL VIII trial. Leukemia & Lymphoma 2006;47:2321‐30. [DOI] [PubMed] [Google Scholar]
Tefft 1978 {published data only}
- Tefft M, Razek A, Perez C, Burgert EO, Gehan EA, Griffin P, et al. Local control and survival related to radiation dose and volume and to chemotherapy in non‐metastatic Ewing's sarcoma of pelvic bones. International Journal of Radiation Oncology, Biology, Physics 1978;4:367‐72. [DOI] [PubMed] [Google Scholar]
Thirugnanam 2009 {published data only}
- Thirugnanam R, George B, Chendamarai E, Lakshmi KM, Balasubramanian P, Viswabandya A, et al. Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide‐based regimen. Biology of Blood and Marrow Transplantation 2009;15:1479‐84. [DOI] [PubMed] [Google Scholar]
Thomas 1988 {published data only}
- Thomas PR, Tefft M, D'Angio GJ, Norkool P. Acute toxicities associated with radiation in the second National Wilms' Tumor Study. Journal of Clinical Oncology 1988;6:1694‐8. [DOI] [PubMed] [Google Scholar]
Toft 2013 {published data only}
- Toft N, Birgens H, Abrahamsson J, Bernell P, Griškevičius L, Hallböök H, et al. Risk group assignment differs for children and adults 1‐45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL‐2008 protocol. European Journal of Haematology 2013;90(5):404‐12. [DOI] [PubMed] [Google Scholar]
Tournade 1993 {published data only}
- Tournade MF, Com‐Nougue C, Voute PA, Lemerle J, Kraker J, Delemarre JFM, et al. Results of the Sixth International Society of Pediatric Oncology Wilms' tumor trial and study: A risk‐adapted therapeutic approach in Wilms' tumor. Journal of Clinical Oncology 1993;11:1014‐23. [DOI] [PubMed] [Google Scholar]
Tsuchiya 1998 {published data only}
- Tsuchiya H, Tomita K, Mori Y, Asada N, Morinaga T, Kitano S, et al. Caffeine‐assisted chemotherapy and minimized tumor excision for nonmetastatic osteosarcoma. Anticancer Research 1998;18:657‐6. [PubMed] [Google Scholar]
Van der Does 1988 {published data only}
- Does‐van den Berg A, Wering ER, Suciu S, Solbu G, Rammeloo JA, Koning J, et al. Results of treatment of children with acute lymphatic leukemia (ALL) according to the ALL V protocol of the Netherlands Working Group on Leukemia in Children. Tijdschrift voor Kindergeneeskunde 1988;56:61‐6. [PubMed] [Google Scholar]
Vinogradova 2008 {published data only}
- Vinogradova I, Lutsenko IN, Kaplanskaia IB, Vorob'ev IA, Samoilova RS, Gorgidze LA, et al. Efficacy of therapy of different variants of anaplastic large T‐cell lymphomas. Terapevticheskii Arkhiv 2008;80:33‐7. [PubMed] [Google Scholar]
Virchis 2004 {published data only}
- Virchis A, Koh M, Rankin P, Mehta A, Potter M, Hoffbrand AV, et al. Fludarabine, cytosine arabinoside, granulocyte‐colony stimulating factor with or without idarubicin in the treatment of high risk acute leukaemia or myelodysplastic syndromes. British Journal of Haematology 2004;124:26‐32. [DOI] [PubMed] [Google Scholar]
Vora 2010 {published data only}
- Vora AJ, Mitchell C, Goulden N, Richards S. UKALL 2003, a randomised trial investigating treatment reduction for children and young adults with minimal residual disease defined low risk acute lymphoblastic leukaema. Blood (ASH Annual Meeting Abstracts). 2010:116 (abstract 496).
Vose 1994 {published data only}
- Vose JM, Anderson JR, Bierman PJ, Bast M, Weisenburger D, Chan WC, et al. Comparison of front‐line chemotherapy for aggressive non‐Hodgkin's lymphoma using the CAP‐BOP regimens. The Nebraska Lymphoma Study Group. Seminars in Hematology 1994;31:4‐8. [PubMed] [Google Scholar]
Watts 2002 {published data only}
- Watts RG, Hilliard LM, Berkow RL. Tailored chemotherapy for malignant lymphoma arising in the setting of posttransplant lymphoproliferative disorder after solid organ transplantation. Journal of Pediatric Hematology/Oncology 2002;24:622‐6. [DOI] [PubMed] [Google Scholar]
Weinstein 1992 {published data only}
- Weinstein H, Ravindranath Y, Krischer J, Steuber P, Civin C, Gresik M, et al. The impact of early intensive therapy on event‐free survival (EFS) in children with acute myeloid leukemia (AML). Leukemia 1992;6 Suppl 2:52‐4. [PubMed] [Google Scholar]
Wilimas 1988 {published data only}
- Wilimas JA, Douglass EC, Lewis S, Fairclough D, Fullen G, Parham D, et al. Reduced therapy for Wilms' tumor: analysis of treatment results from a single institution. Journal of Clinical Oncology 1988;6:1630‐5. [DOI] [PubMed] [Google Scholar]
Willemze 1982 {published data only}
- Willemze R, Haanen C, Dekker A, Sizoo W, Abels J, Tricot G, et al. Results of a multicentre study of the treatment of acute lymphoblastic leukaemia in adolescents and adults (1978‐1981). The Netherlands Journal of Medicine 1982;25:303‐7. [PubMed] [Google Scholar]
Willnow 1986 {published data only}
- Willnow U, Lindner H, Rothe K, Kamprad F. The results of a prospective study for treatment of rhabdomyosarcoma in children. Kinderarztliche Praxis 1986;54:661‐7. [PubMed] [Google Scholar]
Zaharia 1986 {published data only}
- Zaharia M, Caceres E, Valdivia S, Moran M, Tejada F. Postoperative whole lung irradiation with or without adriamycin in osteogenic sarcoma. International Journal of Radiation Oncology, Biology, Physics 1986;12:907‐10. [DOI] [PubMed] [Google Scholar]
Zdrahalova 2011 {published data only}
- Zdrahalova K, Sedlacek P, Smisek P, Keslova P, Sterba J, Hrstkova H, et al. The relapsed AML 2001/01, 02 study for children with relapsed acute myeloid leukaemia or initially resistant disease has improved outcome [Lecebna studie relapsed AML 2001/01, 02 pro deti s relapsem akutni myeloidni leukemie nebo primarne rezistentni nemoci zlepsila jejich nadeji na vyleceni]. Transfuze a Hematologie Dnes 2011;17(3):113‐21. [Google Scholar]
Zimmermann 2012 {published data only}
- Zimmermann C, Pötschger U, Amann G, Horcher E, Dieckmann K, Lakatos K, et al. Results of children with renal tumors treated in the Austrian‐Hungarian Wilms Tumor Study 1989 and the International Society of Pediatric Oncology (SIOP) 93‐01/GPOH trial in Austria. Magazine of European Medical Oncology 2012;5:289‐95. [Google Scholar]
Zintl 1987 {published data only}
- Zintl F, Plenert W, Malke H. Results of acute lymphoblastic leukemia therapy in childhood with a modified BFM protocol in a multicenter study in the German Democratic Republic. Haematology and Blood Transfusion 1987;30:471‐9. [DOI] [PubMed] [Google Scholar]
Zittoun 1992 {published data only}
- Zittoun R, Liso V, Mandelli F, Rotoli B, Witte T, Gattringer C, et al. Intensive consolidation chemotherapy versus standard consolidation maintenance in acute myelogenous leukemia (AML) in first remission. An EORTC/GIMEMA phase III trial (AML8 B). The EORTC Leukemia Cooperative Group and the GIMEMA Group. Leukemia 1992;6 Suppl 2:76‐7. [PubMed] [Google Scholar]
References to studies awaiting assessment
FRALLE 2000‐A {published data only}
- Baruchel A, Auvrignon A, Auclere M‐F, Perel Y, Mechinaud F, Landman‐Parker J, et al. Childhood standard‐risk B‐cell precursor acute lymphoblastic leukemia (SR‐BCP‐ALL): toxicity and efficacy interim results of the FRALLE 2000‐A protocol (O.093). Pediatric Blood and Cancer 2006;47:380. [Google Scholar]
- Cayuela J‐M, Beldjord K, Preudhomme C, Cave H, Eliahou J‐F, Auclerc M‐F, et al. Minimal residual disease (MRD) in childhood standard‐risk B‐cell precursor acute lymphoblastic leukemia (SR‐BCP‐ALL) at the end of induction (EOI) after a three or four drug induction (O.094). Pediatric Blood and Cancer 2006;47:380‐1. [Google Scholar]
SIOP2001 {published data only}
- Pritchard‐Jones K, Graf N, Bergeron C, Camargo B, Heuvel M, Sandstedt B, et al. Doxorubicin can be safely omitted from the treatment of stage II/III, intermediate risk histology Wilms tumour: Results of the SIOP WT 2001 randomised trial. Pediatric Blood and Cancer (Congress of the International Society of Paediatric Oncology). 2011:741.
Von Stackelberg 2011 {published data only}
- Stackelberg A, Yamanaka J, Escherich G, Eckert C, Ratei R, Hitzler JK, et al. Conventional reinduction/consolidation‐type therapy versus short course high intensity combination chemotherapy as post‐induction treatment for children with relapsed acute lymphoblastic leukemia. Early results of study ALL‐REZ BFM 2002. Blood (Annual Meeting of the American Society of Hematology). 2011:118 (abstract 871).
References to ongoing studies
COG AHEP0531 {published data only}
- Not mentioned. COG AHEP0531. Not mentioned.
NCT00379457 {published data only}
- A protocol for nonmetastatic rhabdomyosarcoma [RMS‐2005]. www.controlled‐trials.com.
Additional references
Billingham 1978
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treatment Reports 1978;62:865‐72. [PubMed] [Google Scholar]
Biondi 2000
- Biondi A, Cimino G, Pieters R, Pui CH. Biological and therapeutic aspects of infant leukemia. Blood 2000;96:24‐33. [PubMed] [Google Scholar]
Gratias 2008
- Gratias EJ, Dome JS. Current and emerging chemotherapy treatment strategies for Wilms tumor in North America. Paediatric Drugs 2008;10:115‐24. [DOI] [PubMed] [Google Scholar]
Green 2001
- Green DM, Grigoriev YA, Nan B, Takashima JR, Norkool PA, D'Angio GJ, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. Journal of Clinical Oncology 2001;19:1926‐34. [DOI] [PubMed] [Google Scholar]
Green 2004
- Green DM. The treatment of stages I‐IV favorable histology Wilms' tumor. Journal of Clinical Oncology 2004;22:1366‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3. Chichester (UK): John Wiley & Sons Ltd, 2006. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kremer 2002a
- Kremer LCM, Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline‐induced clinical heart failure in children: a systematic review. Annals of Oncology 2002;13:503‐12. [DOI] [PubMed] [Google Scholar]
Kremer 2002b
- Kremer LCM, Pal HJH, Offringa M, Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 2002;13:819‐29. [DOI] [PubMed] [Google Scholar]
Kremer 2008
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2008, issue 3.
Lachin 2000
- Lachin JM. Statistical considerations in the intent‐to‐treat principle. Controlled Clinical Trials 2000;21:167‐89. [DOI] [PubMed] [Google Scholar]
Lee 1991
- Lee YJ, Ellenberg JH, Hirtz DG, Nelson KB. Analysis of clinical trials by treatment actually received: is it really an option?. Statistics in Medicine 1991;10:1595‐605. [DOI] [PubMed] [Google Scholar]
Lefrak 1973
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302‐14. [DOI] [PubMed] [Google Scholar]
Lipshultz 2005
- Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. Journal of Clinical Oncology 2005;23:2629‐36. [DOI] [PubMed] [Google Scholar]
Mertens 2001
- Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME Jr, Ruccione K, et al. Late mortality experience in five‐year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. Journal of Clinical Oncology 2001;19:3163‐72. [DOI] [PubMed] [Google Scholar]
Messinger 1999
- Messinger Y, Uckun FM. A critical risk‐benefit assessment argues against the use of anthracyclines in induction regimens for newly diagnosed childhood acute lymphoblastic leukemia. Leukemia & Lymphoma 1999;34:415‐32. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pieters 2008
- Pieters R, Carroll WL. Biology and treatment of acute lymphoblastic leukemia. Pediatric Clinics of North America 2008;55:1‐20. [DOI] [PubMed] [Google Scholar]
Reulen 2010
- Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long‐term cause‐specific mortality among survivors of childhood cancer. JAMA 2010;304:172‐9. [DOI] [PubMed] [Google Scholar]
Van Dalen 2006
- Dalen EC, Pal HJ, Kok WE, Caron HN, Kremer LCM. Clinical heart failure in a cohort of children treated with anthracyclines: a long‐term follow‐up study. European Journal of Cancer 2006;42:3191‐8. [DOI] [PubMed] [Google Scholar]
Von Hoff 1979
- Hoff DD, Layard MW, Basa P, Davis HL Jr, Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin‐induced congestive heart failure. Annals of Internal Medicine 1979;91:710‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Dalen 2007
- Dalen EC, Caron HN, Kremer LCM. Anthracyclines versus no anthracyclines for childhood cancer. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006647] [DOI] [Google Scholar]
Van Dalen 2009
- Dalen EC, Raphaël MF, Caron HN, Kremer LCM. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006647.pub2] [DOI] [PubMed] [Google Scholar]
Van Dalen 2011
- Dalen EC, Raphaël MF, Caron HN, Kremer LCM. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006647.pub3] [DOI] [PubMed] [Google Scholar]


