Abstract
Objective:
Robotic-assisted minimally invasive esophagectomy accounts for a growing proportion of esophagectomies, potentially due to improved technical capabilities simplifying the challenging aspects of standard minimally invasive esophagectomy. However, there is limited evidence directly comparing both operations. The objective is to evaluate the short-term and long-term outcomes of robotic-assisted minimally invasive esophagectomy in comparison with the minimally invasive esophagectomy approach for patients with esophageal cancer over a 7-year period at a high-volume center. The primary end points of this study were overall survival and disease-free survival. Secondary end points included operation-specific morbidity, lymph node yield, readmission status, and in-hospital, 30-day, and 90-day mortality.
Methods:
Patients who underwent robotic-assisted minimally invasive esophagectomy or standard minimally invasive esophagectomy over a 7-year period were identified from a prospectively maintained database. Inclusion criteria were patients with stage I to III disease, operations performed past the learning curve, and no evidence of scleroderma or cirrhosis. A 1:3 propensity match (robotic-assisted minimally invasive esophagectomy:minimally invasive esophagectomy) for multiple clinical covariates was performed to identify the final study cohort. Perioperative outcomes were compared between the 2 operations.
Results:
A total of 734 patients undergoing minimally invasive esophagectomy (n = 630) or robotic-assisted minimally invasive esophagectomy (n = 104) for esophageal cancer were identified. After exclusions and matching, a total cohort of 246 patients undergoing robotic-assisted minimally invasive esophagectomy (n = 65) or minimally invasive esophagectomy (n = 181) were identified. There was no difference in overall survival (P = .69) or disease-free survival (P = .70). There were no significant differences in rates of major morbidity: pneumonia (17% vs 17%, P = .34), chylothorax (8% vs 9%, P = .95), recurrent laryngeal nerve injury (0% vs 1.5%, P = 1), anastomotic leak (5% vs 4%, P = .49), intraoperative complications (9% vs 8%, P = .73), or complete resection rates (99% vs 96%, P = .68). There was no difference in in-hospital (P = .89), 30-day (P = .66) or 90-day mortality (P = .73) between both cohorts. The robotic-assisted minimally invasive esophagectomy cohort yielded a higher median lymph node harvest in comparison with the minimally invasive esophagectomy cohort (32 vs 29, P = .02).
Conclusions:
Robotic-assisted minimally invasive esophagectomy may improve lymphadenectomy in patients undergoing esophagectomy for cancer. Minimally invasive esophagectomy and robotic-assisted minimally invasive esophagectomy are otherwise associated with similar mortality, morbidity, and perioperative outcomes. Further prospective study is required to investigate whether improved lymph node resection may translate to improved oncologic outcomes.
Keywords: esophageal malignancy, lymphadenectomy, minimally invasive esophagectomy, Robotic-Assisted Minimally Invasive Esophagectomy
Graphical Abstract
We compared perioperative outcomes between 2 approaches to esophagectomy: RAMIE and MIE.
Esophageal cancer is the 18th leading cause of cancer and comprises 1% of all new cancer cases in the United States. The estimated incidence in 2021 by the Surveillance, Epidemiology, and End Results Cancer Database is that 19,260 people will be diagnosed, with an estimated 15,530 deaths.1 Prompt diagnosis and response to treatment are the existing challenges for managing patients with esophageal cancer. A total of 20% of patients present with early-stage disease, whereas 40% of patients present with locoregional disease.2 Multimodal therapy has been advocated to achieve the highest chance of curative success in patients with locoregional disease. Despite poor survival (19.9%) at 5 years, it is important to identify patients with early-stage or locoregional disease and treat aggressively.1 Presently, patients are provided with several treatment options, based on their comorbidities, frailty index, extent of disease, and performance metrics.
Esophagectomy is a cornerstone of multimodal therapy in selected patients with locally advanced disease.3 Current approaches include open esophagectomy (OE), minimally invasive esophagectomy (MIE), and Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE).4
OE has been associated with a higher incidence of complications, resulting in significant morbidity and mortality. Postoperative morbidity of OE includes pneumonia (13%), recurrent laryngeal nerve palsy (6%), and anastomotic leakage (4%), with pulmonary complications being the most significant factor contributing to postoperative mortality. Several studies report in-hospital mortality between 1% and 9%, and as high as 29% with OE.5,6
Since its inception in the 1990s, the MIE approach has improved on morbidity compared with OE, while maintaining optimal oncologic outcomes. This approach has been championed by our group,3 resulting in a mortality risk of 1% to 2%, reduced blood loss, less pain, decreased hospital stay, and decreased overall morbidity. However, there are significant technical challenges of the conventional MIE.7,8 Although MIE shows improvements over OE, 2-dimensional views, suboptimal ergonomic positioning, and restricted range of motion are inherent challenges of the MIE approach contributing to the long learning curve.9 The emergence of the RAMIE approach has significantly improved these intrinsic technical limitations in MIE through enhanced visualization and dexterity, tremor filtering, and self-assisting capabilities, while shortening the learning curve.10–14 The growing popularity of the RAMIE approach has become increasingly widespread because of these technical modifications while also maintaining sound oncological results, noninferior perioperative outcomes, and patient safety.15–18
The Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus MIE for resectable esophageal adenocarcinoma trial, an ongoing study, is a randomized controlled trial that compares patients undergoing RAMIE (n = 109) or MIE (n = 109) for resectable esophageal adenocarcinoma.19 The study’s primary objective is to study the total number of resected abdominal and mediastinal lymph nodes specified per lymph node station. There is otherwise relatively limited literature highlighting the outcome differences between the RAMIE and MIE approaches.20,21
We conducted this retrospective study to evaluate the short-term and long-term outcomes of RAMIE in comparison with the MIE approach for patients with esophageal cancer over a 7-year period at a high-volume center.
MATERIALS AND METHODS
Study Design
This retrospective cohort study included patients with esophageal cancer who underwent MIE and RAMIE. Patients were staged according to the 8th edition of American Joint Committee on Cancer and the International Union for Cancer control TNM system for esophageal cancer. This study was approved by the University of Pittsburgh Institutional Review Board (Number: STUDY2005005S, approved September 30, 2020). Informed written consent was waived by the Institutional Review Board/Research Ethics Board.
Patients
A total of 734 patients with clinical stage T1a-T4, N0–3, M0 underwent elective RAMIE (n = 104) or MIE (n = 630) with curative intent over a 7-year period (2014–2021). Inclusion and exclusion criteria are displayed in Figure 1. All patients with isolated malignancy of the gastric cardia were excluded. All operations were performed by experienced esophageal surgeons past the learning curve of these operations. Greater than 20 RAMIE cases were performed independently per surgeon for RAMIE data inclusion. We previously described the technical aspects of the RAMIE.12,22,23 Video reference can be viewed in the description by Okusanya and colleagues.24
FIGURE 1.
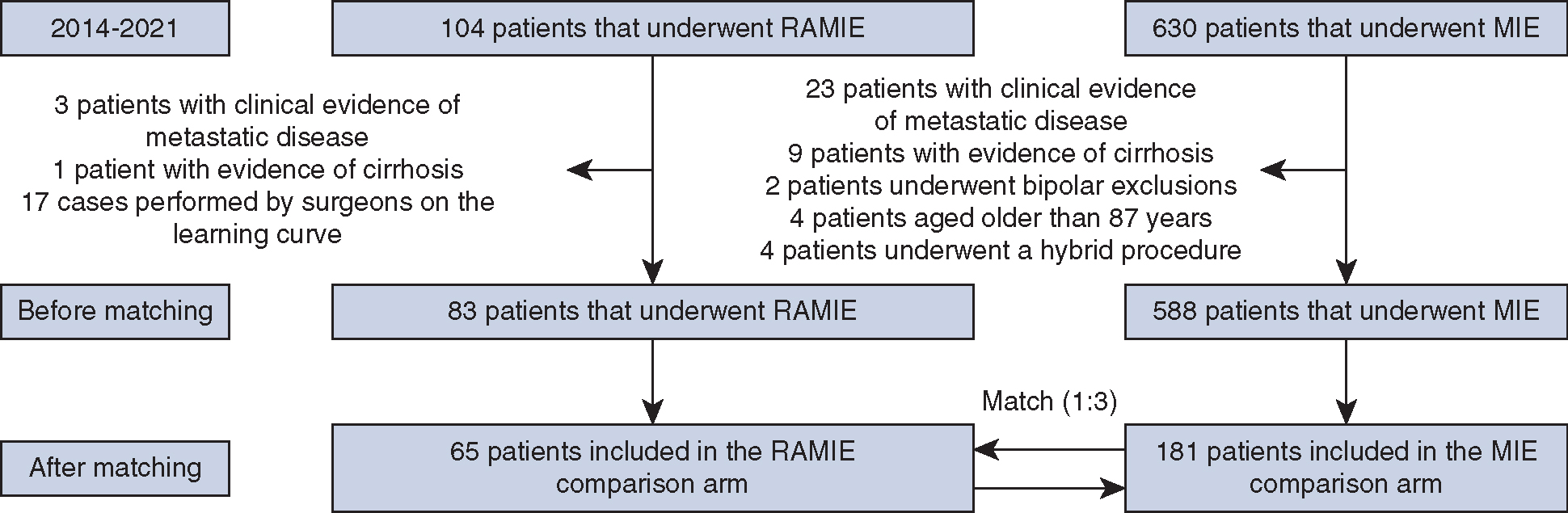
Methodology approach for the propensity match. RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; MIE, minimally invasive esophagectomy.
End Points
The primary end points of this study were overall survival (OS) and disease-free survival (DFS). OS was calculated from the date of surgery to the date of death or last follow-up. DFS was calculated from the date of surgery to recurrence, death related to disease, or last date of follow-up. An anastomotic leak is classified on the basis of evidence of saliva, stool, bile or positive amylase per chest drainage, radiographic sign of leak (barium esophagram or computed tomography scan), or endoscopic assessment of dehiscence.25 Anastomotic leak classifications for this study were used according to the Pittsburgh leak scale: grade 1: radiographic leak only, requiring no intervention; grade 2: leak (<10% of circumference) requiring cervical or percutaneous drainage; grade 3: disruption of anastomosis (10%–50% circumference) with perianastomotic abscess and associated pleural or mediastinal collection thoracoscopic surgery or thoracotomy; and grade 4: gastric tip necrosis with anastomotic separation (>50% circumference).25
Secondary end points included operation-specific perioperative morbidity (pneumonia, atrial arrhythmia, chylothorax, recurrent laryngeal nerve paralysis, and anastomotic leakage). Intraoperative complications, duration of the operation, completeness of resection, lymph node harvest status, and latency period between induction therapy and surgery were recorded. Additionally, we recorded 30-day readmission, hospitalization interval, in-hospital mortality, and mortality at 30 days and 90 days.
Analysis
To ensure that both the treatment and control groups were balanced before analyses, a propensity score match was used. A propensity score is the probability of use of an intervention compared with nonuse.26 Baseline variables comprising the logistic regression model included age, body mass index, clinical tumor stage, clinical T category, Eastern Cooperative Oncology Group Performance Status, Charlson Comorbidity index, gastroesophageal reflux disease, American Society of Anesthesiology score, clinical N category, neoadjuvant treatment, drug use, gender, family history, and smoking status (Tables 1 and 2). The propensity score was then used to perform a 3:1 match with the MIE and RAMIE groups. A greedy matching algorithm was used to find the best possible matches, and the matching caliper was set at 0.2 of the standard deviation of the logit of the PS.27,28 Standardized mean differences are shown in Tables 1 and 2. Values less than 0.1 are indicative of a good balance between the 2 groups.
TABLE 1.
Baseline characteristics of unmatched variables
| Total |
MIE |
RAMIE |
|||
|---|---|---|---|---|---|
| n = 671 | n = 588 | n = 83 | Prematched SMD | SMD | |
|
| |||||
| Variables | n (%) or median (p25, p75) | ||||
| Age, y | 66.0 (59, 72) | 66.0 (59, 72) | 67 (60, 72) | 0.003 | 0.050 |
| BMI | 28.7 (25, 32.1) | 28.3 (25.1, 32.1) | 28.2 (24.6, 31.9) | 0.032 | 0.001 |
| Clinical stage | |||||
| I, IB, IC | 85 (14.9) | 81 (16.4) | 4 (5.2) | 0.312 | 0.041 |
| II, IIA, IIB | 120 (21.0) | 105 (21.2) | 15 (19.5) | 0.006 | 0.038 |
| III, IIIA, IIIB IIIC | 321 (56.1) | 270 (54.6) | 51 (66.2) | 0.315 | 0.053 |
| IV, IVA | 42 (7.3) | 35 (7.1) | 7 (9.1) | 0.096 | 0.003 |
| Clinical T category | |||||
| T1, T1a, T1b | 81 (13.9) | 77 (15.3) | 4 (5.1) | 0.293 | 0.062 |
| T2 | 105 (18.1) | 95 (18.9) | 10 (12.7) | 0.118 | 0.034 |
| T3 | 387 (66.6) | 323 (64.3) | 64 (81.0) | 0.482 | 0.027 |
| T4, T4a, T4b | 8 (1.4) | 7 (1.4) | 1 (1.3) | 0.001 | 0.050 |
| ECOG performance status | |||||
| 0 | 156 (23.4) | 130 (22.3) | 26 (31.3) | 0.209 | 0.048 |
| 1 | 505 (75.7) | 448 (76.7) | 57 (68.7) | 0.169 | 0.036 |
| 3 | 2 (0.3) | 2 (0.3) | 0 (0.0) | 0.083 | 0.105 |
| Charlson Comorbidity Index | 1.0 (0, 2) | 1.0 (0, 2) | 1.0 (0, 2) | 0.017 | 0.020 |
| GERD | |||||
| Yes | 407 (61.8) | 360 (62.3) | 47 (58.0) | 0.087 | 0.040 |
| ASA | |||||
| 2 | 40 (6.2) | 34 (6.1) | 6 (7.2) | 0.059 | 0.060 |
| 3 | 546 (85.1) | 474 (84.8) | 72 (86.8) | 0.167 | 0.030 |
| 4 | 56 (8.7) | 51 (9.41) | 5 (6.0) | 0.102 | 0.020 |
| Clinical N category | |||||
| N0 | 228 (36.8) | 205 (38.2) | 23 (27.7) | 0.155 | 0.030 |
| N1 | 204 (32.9) | 166 (30.9) | 38 (45.8) | 0.370 | 0.080 |
| N2 | 142 (22.9) | 125 (23.3) | 17 (20.5) | 0.019 | 0.050 |
| N3 | 46 (7.4) | 41 (7.6) | 5 (6.0) | 0.038 | 0.040 |
| Neoadjuvant treatment | |||||
| CRT | 322 (49.3) | 272 (47.6) | 50 (61.0) | 0.283 | 0.040 |
| CT | 143 (22.4) | 125 (21.9) | 21 (25.6) | 0.096 | 0.050 |
| None | 185 (28.3) | 174 (30.5) | 11 (13.4) | 0.406 | 0.003 |
| Clinical M category | 627 (99.2) | 544 (99.1) | 83 (100.0) | 0.136 | 0.107 |
| Drug use | |||||
| Never | 575 (93.0) | 501 (92.8) | 74 (94.9) | 0.118 | 0.070 |
| Previously | 19 (3.1) | 18 (3.3) | 1 (1.3) | 0.073 | 0.070 |
| Yes | 24 (3.9) | 21 (3.9) | 3 (3.9) | 0.100 | 0.100 |
| Gender | |||||
| Male | 564 (84.2) | 495 (84.2) | 14 (16.8) | 0.028 | 0.080 |
| Family history | |||||
| Yes | 35 (5.5) | 29 (5.2) | 6 (7.5) | 0.094 | 0.080 |
| Smoking status | |||||
| Never | 176 (26.3) | 159 (27.1) | 17 (20.5) | 0.028 | 0.080 |
| Current | 424 (63.3) | 369 (62.9) | 55 (66.3) | 0.073 | 0.010 |
| Past | 70 (10.5) | 58 (10.1) | 11 (13.3) | 0.100 | 0.004 |
MIE, Minimally invasive esophagectomy; RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; SMD, standardized mean difference; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; GERD, gastroesophageal reflux disease; ASA, American Society of Anesthesiology; CRT, cardiac resynchronization therapy; CT, computed tomography.
TABLE 2.
Baseline characteristics for matched variables
| Total |
MIE |
RAMIE |
|||
|---|---|---|---|---|---|
| n = 246 | n = 181 | n = 65 | SMD | P value | |
|
| |||||
| Variables | n (%) or median (p25, p75) | ||||
| Age, y | 67.0 (60, 72) | 67.0 (61, 72) | 66.3 (60, 70) | 0.05 | .67 |
| BMI | 27.9 (25.1, 31.2) | 27.8 (25.2, 30.7) | 28.4 (24.8, 31.9) | 0.001 | .78 |
| Clinical stage | 0.03 | .99 | |||
| I, IB, IC | 17 (96.9) | 13 (7.2) | 4 (6.2) | ||
| II, IIA, IIB | 52 (21.1) | 39 (21.6) | 13 (20.0) | ||
| III, IIIA, IIIB IIIC | 162 (65.9) | 118 (65.2) | 44 (67.7) | ||
| IV, IVA | 15 (6.1) | 11 (6.1) | 4 (6.2) | ||
| Clinical T category | 0.003 | .96 | |||
| T1, T1a, T1b | 18 (7.3) | 14 (7.7) | 4 (6.2) | ||
| T2 | 32 (13.0) | 23 (12.7) | 9 (13.9) | ||
| T3 | 191 (77.6) | 140 (77.4) | 51 (78.5) | ||
| T4, T4a, T4b | 5 (2.0) | 4 (2.2) | 1 (1.5) | ||
| ECOG performance status | 0.07 | .70 | |||
| 0 | 68 (27.6) | 49 (27.1) | 19 (29.2) | ||
| 1 | 177 (72.0) | 131 (72.4) | 46 (70.1) | ||
| 3 | 1 (0.4) | 1 (0.6) | 0 (0.0) | ||
| Charlson Comorbidity Index | 1.0 (0, 2) | 1.0 (0, 2) | 1.0 (0, 1) | 0.02 | .72 |
| GERD | .85 | ||||
| Yes | 150 (61.0) | 111 (61.3) | 39 (60.0) | 0.04 | |
| ASA score | .87 | ||||
| 2 | 21 (8.5) | 16 (8.8) | 5 (7.7) | 0.06 | |
| 3 | 207 (84.2) | 151 (83.4) | 56 (86.2) | 0.03 | |
| 4 | 18 (7.3) | 14 (7.7) | 4 (6.2) | 0.02 | |
| Clinical N category | .94 | ||||
| N0 | 78 (31.2) | 59 (32.6) | 19 (29.2) | 0.03 | |
| N1 | 113 (45.9) | 81 (44.8) | 32 (49.2) | 0.08 | |
| N2 | 43 (17.5) | 32 (17.7) | 11 (16.9) | 0.05 | |
| N3 | 12 (4.9) | 9 (5.0) | 3 (4.6) | 0.04 | |
| Neoadjuvant treatment | .77 | ||||
| CRT | 141 (57.3) | 102 (56.4) | 39 (60.0) | 0.04 | |
| CT | 60 (24.4) | 44 (24.3) | 16 (24.6) | 0.05 | |
| None | 45 (18.3) | 35 (19.3) | 10 (15.4) | 0.003 | |
| Drug use | .13 | ||||
| Never | 234 (95.2) | 172 (95.0) | 62 (95.4) | 0.07 | |
| Previously | 3 (1.2) | 2 (1.1) | 1 (1.5) | 0.07 | |
| Yes | 9 (3.7) | 7 (3.9) | 2 (3.1) | 0.1 | |
| Gender | .12 | ||||
| Male | 207 (84.2) | 154 (85.1) | 53 (81.5) | 0.08 | |
| Family history | .21 | ||||
| Yes | 12 (4.9) | 8 (4.4) | 4 (6.2) | 0.08 | |
| Smoking status | .67 | ||||
| Never | 57 (23.2) | 43 (23.8) | 14 (21.5) | 0.08 | |
| Current | 165 (67.1) | 122 (67.4) | 43 (66.2) | 0.01 | |
| Past | 24 (9.8) | 16 (8.8) | 8 (12.3) | 0.004 | |
| Tumor type | .75* | ||||
| Adenocarcinoma | 181 (73.9) | 133 (73.9) | 48 (73.9) | ||
| Squamous cell carcinoma | 18 (7.4) | 12 (6.7) | 6 (9.2) | ||
Successful 1:3 match between RAMIE versus MIE based on baseline demographic characteristics. Values below 0.1 indicate that there is a good balance between the 2 groups at baseline. MIE, Minimally invasive esophagectomy; RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; SMD, standardized mean difference; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; GERD, gastroesophageal reflux disease; ASA, American Society of Anesthesiology; CRT, cardiac resynchronization therapy; CT, computed tomography.
Tumor type was a demographic variable but not a part of the propensity match.
We described the participant characteristics in each of the groups using descriptive statistics (proportions, median, and range). Continuous variables were analyzed using Wilcoxon signed-rank test, and categorical variables were analyzed using chi-square or Fisher exact tests. SAS version 9.2 (SAS Institute, Inc) was used for the analyses.
RESULTS
A total of 734 patients with operable esophageal malignancy were included in the match, identifying a final study cohort of 246 patients after matching (RAMIE, n = 65; MIE, n = 181). Clinical and demographic variables are presented in Tables 1 and 2.
There was no statistical significance for OS (P = .69) or DFS (P = .70) (Figures 2 and 3). The probability of OS at 5 years was 50% and 40% for RAMIE and MIE, respectively. At 5 years, the probability of DFS was 55% and 25% for RAMIE and MIE, respectively. The median survival after resection was 14.1 months for the overall cohort, 16.4 months for the RAMIE cohort, and 13.8 months for the MIE cohort (P = .49).
FIGURE 2.
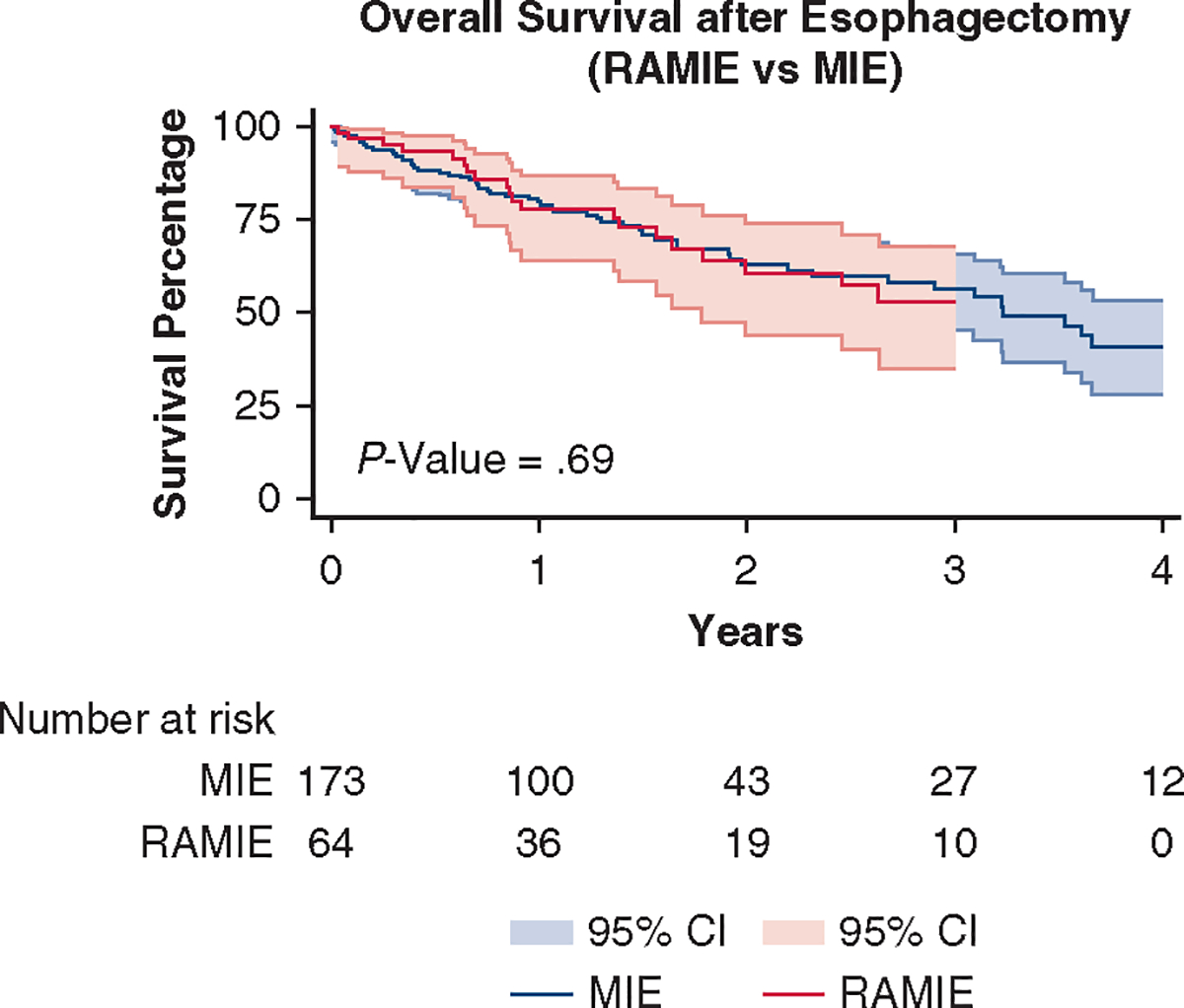
OS was comparable between the 2 surgical approaches (P = .69). RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; MIE, minimally invasive esophagectomy; CI, confidence interval.
FIGURE 3.
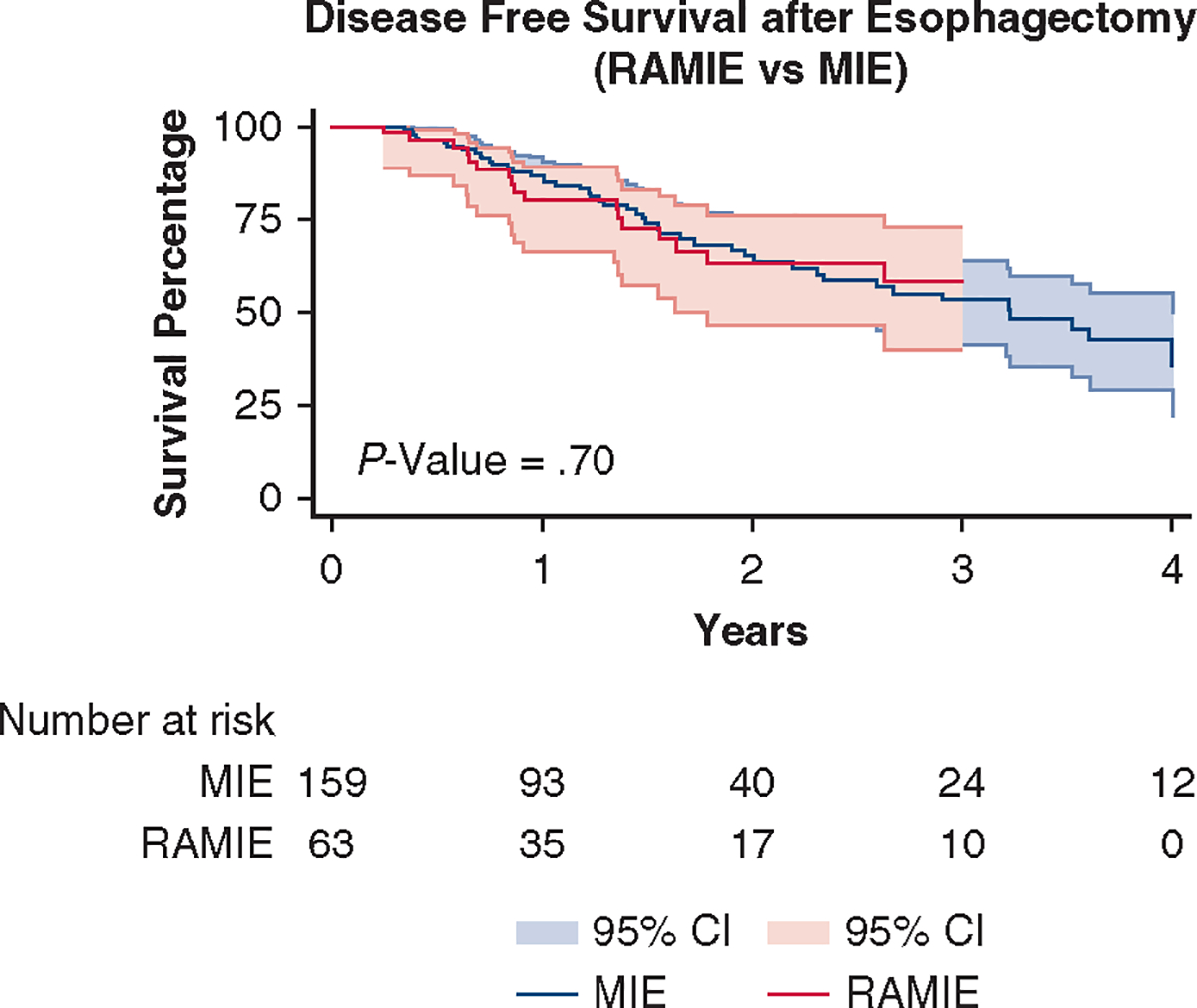
DFS was comparable between the 2 surgical approaches (P = .70). RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; MIE, minimally invasive esophagectomy; CI, confidence interval.
Postoperative morbidity was comparable, with no significant difference among rates of pneumonia (MIE 16.6% vs RAMIE 17.0%, P = .34), atrial arrhythmia (P = .19), chylothorax (P = .95), recurrent laryngeal nerve paralysis (P = 1), and intraoperative complications (P = .73). Anastomotic leak rate was 15.9%, with grade 1 and 2 leaks at 11.8% (P = .14) and grade 3 and higher at 4.1% (P = .49) for the combined approaches (Table E1). Specifically, grade 3 (or greater) anastomotic leakage for each cohort was 3.9% for MIE versus 4.6% for RAMIE (P = .49). There was no statistical difference in in-hospital (3.3%, P = .89), 30-day (2.6%, P = .66) or 90-day mortality (2.6%, P = .73), or length of stay (8 days, P = .31)29 (Table 3).
TABLE 3.
Major morbidity
| Total |
MIE |
RAMIE |
||
|---|---|---|---|---|
| n = 246 | n = 181 | n = 65 | P value | |
|
| ||||
| Variables | n (%) or median (p25, p75) | |||
| Pneumonia | 34 (16.7) | 25 (16.6) | 9 (17.0) | .34 |
| Atrial arrhythmia requiring treatment | 62 (31.3) | 43 (28.9) | 19 (38.8) | .19 |
| Anastomotic leak (grade ≥3) | 10 (4.1) | 7 (3.9) | 3 (4.6) | .49 |
| Chylothorax requiring treatment, n (%) | 16 (8.6) | 12 (8.6) | 4 (8.3) | .95 |
| Recurrent laryngeal nerve paralysis, n (%) | 2 (1.1) | 2 (1.5) | 0 (0.0) | 1 |
| Intraoperative complications | 20 (8.3) | 14 (7.8) | 6 (9.2) | .73 |
There was no statistical difference in major complications between the 2 surgical arms. MIE, Minimally invasive esophagectomy; RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy.
Operative times were comparable (P = .86), and estimated blood loss was 200 mL in both cohorts (P = .19). The latency period between neoadjuvant therapy and surgery was 6.5 and 6 weeks (P = .35) for MIE and RAMIE, respectively. The median intensive care unit length of stay was 3 days for the MIE cohort and 2 days for the RAMIE cohort (P = .86).
R0 resection was achieved in 162 patients (96.4%) in the MIE cohort in comparison with 64 patients (98.5%) in the RAMIE cohort (P = .68) (Table 4). The McKeown approach was performed in 3 patients in the MIE cohort, and all patients in the RAMIE cohort underwent the Ivor Lewis approach. Open abdominal conversion was performed in 9 patients in the MIE cohort and 3 patients in the RAMIE cohort (P = .74). Open thoracic conversion was performed in 6 patients in the MIE cohort and 3 patients in the RAMIE cohort (P = .87).
TABLE 4.
Perioperative outcomes
| Total |
MIE |
RAMIE |
||
|---|---|---|---|---|
| n = 246 | n = 181 | n = 65 | P value | |
|
| ||||
| Variables | n (%) or median (p25, p75) | |||
| Estimated blood loss (mL) (median) | 200 | 200 | 200 | .19 |
| Total lymph node removed (median) | 30 (23, 37) | 29 (22, 36.5) | 32 (25, 39) | .02 |
| Completeness of resection | .68 | |||
| R0 resection | 226 (97.0) | 162 (96.4) | 64 (98.5) | |
| R1 | 6 (2.6) | 5 (3.0) | 1 (1.5) | |
| R2 | 0 | |||
| Total operative time (min) | 621.5 (561, 695) | 625 (557, 696) | 614 (562, 673) | .86 |
| 30-d readmission | 46 (20) | 33 (19.8) | 13 (20.6) | .46 |
| Length of stay (d) (median) | 8 (7, 14) | 9 (7, 14) | 8 (7, 13.5) | .31 |
| In-hospital mortality | 8 (3.3) | 6 (3.3) | 2 (3.1) | .89 |
| 30-d mortality | 6 (2.6) | 4 (2.4) | 2 (3.1) | .66 |
| 90-d mortality | 6 (2.6) | 4 (2.4) | 2 (3.2) | .73 |
Perioperative outcomes were compared between the 2 surgical approaches. The lymph node yield was higher in the RAMIE group in comparison with the MIE group (P = .02). MIE, Minimally invasive esophagectomy; RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy.
Tumor type was comparable (P = .75) (Tables 1 and 2), but the number of harvested lymph nodes was statistically different between the 2 cohorts, with a higher median lymph node yield in the RAMIE cohort (32 lymph nodes) than in the MIE cohort (29 lymph nodes) (P = .02) (Table 4).
DISCUSSION
In this study, we compared perioperative and survival outcomes in propensity-matched RAMIE (n = 65) and MIE (n = 181) cohorts. We concluded that perioperative morbidity, DFS, and OS are comparable between RAMIE and MIE.
Our conclusion does not widely vary from previous comparative studies of RAMIE and MIE. Zhang and colleagues30 performed a propensity score–matched analysis between MIE and RAMIE and demonstrated comparable early outcomes between the 2 approaches. The group matched for demographics, American Society of Anesthesiology score, tumor location and size, and pathological stage. Sarkaria and colleagues31 compared OE (n = 164) and RAMIE (n = 64), and concluded there was equivalent R0 resection (97.2% vs 96.9%), but reduced morbidity. Reduced blood loss (250 vs 350 mL, P < .001), pulmonary sequalae (14% vs 34%, P = .014), infectious complication (17.2% vs 38%, P = .029), and intensive care unit admissions (P = .03) were found in the RAMIE arm. Mortality was unchanged at 30 or 90 days between the 2 groups. van der Sluis and colleagues21 compared OE (n = 56) and RAMIE (n = 56), showing favorable outcomes for RAMIE in comparison with OE, particularly significantly reduced blood loss (400 vs 568 mL, P < .001) and a lower percentage of pulmonary complications (32% vs 58%, P = .005), infectious complications (4% vs 14%, P = .09), and cardiac complications (22% vs 47%, P = .006). In addition, a lower mean postoperative pain (visual analog scale, 1.86 vs 2.62; P < .001) was reported. Short- and long-term oncological outcomes were comparable at a medium follow-up of 40 months, as well as 30-day and 90-day mortality. Quality of life studies have also shown improved patient-reported outcomes and quality of life metrics (Functional Assessment of Cancer Therapy-Esophageal and Esophageal Cancer Subscale scores) and less pain severity with the RAMIE approach in comparison with OE.32
Our study revealed a significant improvement in lymph node harvest in the RAMIE arm. A median of 32 lymph nodes were harvested in the RAMIE cohort in comparison with a median of 29 removed lymph nodes in the MIE cohort (P = .02). The potential role of increased lymph node harvest as an indicator of locoregional tumor control and OS is largely undetermined. Our group identified improved survival in increased lymph node yield (>15 nodes, n = 537) in patients with a pathological complete response after induction therapy and esophagectomy.33 Peyre and colleagues34 concluded that the lymph node yield was an independent predictor of survival in a study of 2302 patients after esophagectomy for esophageal malignancy. Furthermore, a meta-analysis by Visser and colleagues35 including 26 studies in a pooled analysis demonstrated a benefit of increased lymph node yield on OS (hazard ratio, 0.81; 95% confidence interval, 0.74–0.87; P < .01) and DFS (hazard ratio, 0.72; 95% confidence interval, 0.62–0.84; P < .01). The Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus MIE for resectable esophageal adenocarcinoma trial is an ongoing randomized trial that will compare MIE and RAMIE for operable esophageal malignancy. The authors postulate a higher lymph node harvest in the RAMIE approach in comparison with the MIE approach, but this has yet to be confirmed.19 Weksler and Sullivan36 showed similar median survival and 90-day survival but higher lymph node yield in the RAMIE cohort in comparison with the MIE cohort in an unmatched study. Espinoza-Mercado and colleagues37 performed a 1:1 propensity match with RAMIE, MIE, and OE, and showed no statistically significance in OS and 30-day or 90-day mortality among the 3 cohorts. Lymph node yield was higher in the minimally invasive approaches when compared with OE, but similar between MIE and RAMIE (6 nodes and RAMIE: 17 nodes, P = .18).
The propensity-matched study by van der Werf and colleagues38 showed no difference in 3-year survival after comparing patients with less than versus greater than 15 harvested lymph nodes (n = 992 for each cohort). Furthermore, after retrieval of at least 10, 20, or 30 lymph nodes, there was no improvement in survival compared with patients with fewer lymph nodes. However, increased lymph node harvest (≥15 lymph nodes) was associated with more accurate pathological staging. Several of these studies did not specify the surgical approach.
Study Limitations
Although partially mitigated through propensity matching, our study is subject to the limitations of a retrospective analysis, which include time and selection bias, and nonprospective or nonrandomized data collection. Furthermore, our study was conducted in a high-volume academic center of excellence with significant experience in these operations, the results of which may or may not be translatable to other clinical practice settings.
CONCLUSIONS
Our study suggests that RAMIE may improve lymph node retrieval over MIE in patients undergoing esophagectomy for cancer (Figure 4). The morbidity, mortality, and perioperative outcomes otherwise appear to be comparable. Additional studies are needed to determine any putative impact on cancer-specific survival and recurrence rates potentially due to improved lymphadenectomy in these operations.
FIGURE 4.
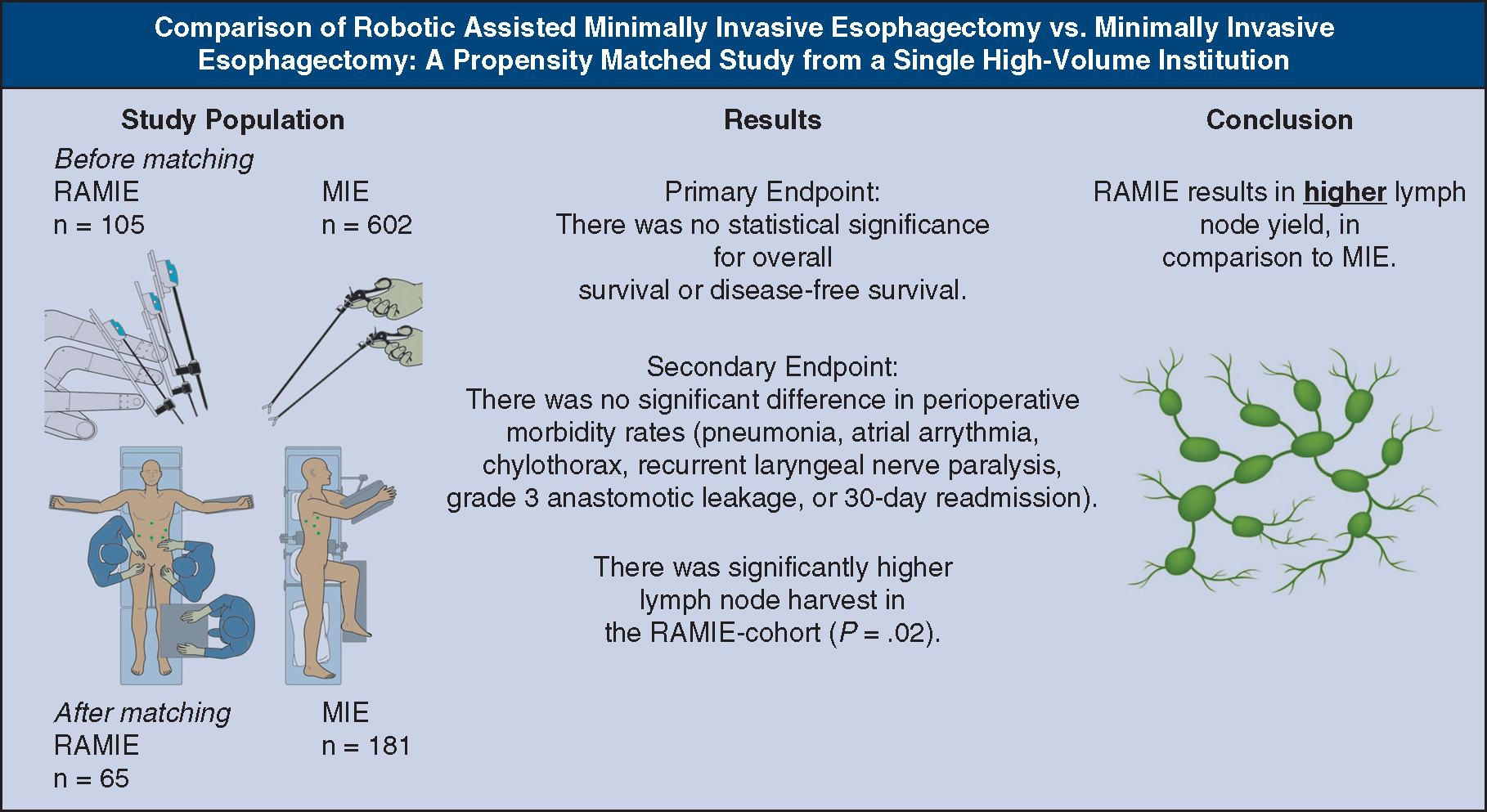
The increased lymph node yield harvest in the RAMIE cohort in comparison with the MIE cohort. RAMIE, Robotic-Assisted Minimally Invasive Esophagectomy; MIE, minimally invasive esophagectomy.
Supplementary Material
CENTRAL MESSAGE
Robotic-assisted MIE (RAMIE) yields a higher lymph node harvest than conventional MIE with comparable morbidity and mortality rates.
PERSPECTIVE
Minimally invasive techniques for esophagectomy have been adopted to reduce associated morbidity in comparison with the open approach. The technical challenges of the traditional MIE are diminished with RAMIE, but comparative outcomes are limited. The RAMIE approach has been shown to be safe with comparable short-term and long-term outcomes compared with the conventional MIE approach.
Acknowledgments
Institutional Review Board Number: STUDY2005005S. Approved September 30, 2020. Informed written consent was waived by the Institutional Review Board/Research Ethics Board.
The project described was supported by the Department of Cardiothoracic Surgery at UPMC and the National Institutes of Health through Grant Number UL1TR001857.
Footnotes
Conflict of Interest Statement
J.D.L. is a recipient of grants from the University of Texas SWMC and Anpac Tech of USA; receives nonfinancial support from Covidien as a speaker; and has equity interest in Intuitive Surgical Inc, Proctor and Gamble, and Cigna Corp. I.S.S. receives honorariums from Intuitive Surgical Inc, On Target Laboratories, Cambridge Medical Robotics, and Auris Medical. None of the authors received any financial awards, property, or equity in exchange for the completion of this manuscript. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Read at the 48th Annual Meeting of theWestern Thoracic Surgical Association, Koloa, Hawaii, June 22-25, 2022.
References
- 1.Surveillance, Epidemiology, and End Results Program . Cancer of the esophagus–cancer stat facts. Accessed September 27, 2022. https://seer.cancer.gov/statfacts/html/esoph.html2022
- 2.Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Azuma M, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. 10.1097/SLA.0b013e3182590603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witek TD, Melvin TJ, Luketich JD, Sarkaria IS. Open, minimally invasive, and robotic approaches for esophagectomy: what is the approach algorithm? Thorac Surg Clin. 2020;30:269–77. 10.1016/j.thorsurg.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol. 2016;14:304. 10.1186/s12957-016-1062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javidfar J, Bacchetta M, Yang JA, Miller J, D’Ovidio F, Ginsburg ME, et al. The use of a tailored surgical technique for minimally invasive esophagectomy. J Thorac Cardiovasc Surg. 2012;143:1125–9. 10.1016/j.jtcvs.2012.01.071 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NT, Follette DM, Wolfe BM, Schneider PD, Roberts P, Goodnight JE Jr. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135:920–5. 10.1001/archsurg.135.8.920 [DOI] [PubMed] [Google Scholar]
- 8.Müller-Stich BP, Probst P, Nienhüser H, Fazeli S, Senft J, Kalkum E, et al. Meta-analysis of randomized controlled trials and individual patient data comparing minimally invasive with open oesophagectomy for cancer. Br J Surg. 2021;108:1026–33. 10.1093/bjs/znab278 [DOI] [PubMed] [Google Scholar]
- 9.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus laparoscopic versus open gastrectomy: a meta-analysis. J Gastric Cancer. 2013;13:136–48. 10.5230/jgc.2013.13.3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sodergren MH, Darzi A. Robotic cancer surgery. Br J Surg. 2013;100:3–4. 10.1002/bjs.8972 [DOI] [PubMed] [Google Scholar]
- 11.Köckerling F Robotic vs. standard laparoscopic technique–what is better? Front Surg. 2014;1:15. 10.3389/fsurg.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin. 2014;24:211–22. 10.1016/j.thorsurg.2014.02.010. vii. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Li B, Hua R, Zhang X, Jiang H, Sun Y, et al. , Written on behalf of the AMETSCG. Assessment of quality outcomes and learning curve for robot-assisted minimally invasive McKeown esophagectomy. Ann Surg Oncol. 2021;28:676–84. 10.1245/s10434-020-08857-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkaria IS, Rizk NP, Finley DJ, Bains MS, Adusumilli PS, Huang J, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg. 2013;43:e107–15. 10.1093/ejcts/ezt013 [DOI] [PubMed] [Google Scholar]
- 15.van der Sluis PC, Ruurda JP, Verhage RJ, van der Horst S, Haverkamp L, Siersema PD, et al. Oncologic long-term results of robot-assisted minimally invasive thoraco-laparoscopic esophagectomy with two-field lymphadenectomy for esophageal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S1350–6. 10.1245/s10434-015-4544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg. 2013;145:90–6. 10.1016/j.jtcvs.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 17.Sarkaria IS, Rizk NP, Grosser R, Goldman D, Finley DJ, Ghanie A, et al. Attaining proficiency in robotic-assisted minimally invasive esophagectomy while maximizing safety during procedure development. Innovations (Phila). 2016;11:268–73. 10.1097/IMI.0000000000000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingma BF, Grimminger PP, van der Sluis PC, van Det MJ, Kouwenhoven EA, Chao YK, et al. Worldwide techniques and outcomes in Robot-Assisted Minimally Invasive Esophagectomy (RAMIE): results from the Multicenter International Registry. Ann Surg. 2022;276:e386–92. 10.1097/sla.0000000000004550 [DOI] [PubMed] [Google Scholar]
- 19.Tagkalos E, van der Sluis PC, Berlth F, Poplawski A, Hadzijusufovic E, Lang H, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer. 2021;21:1060. 10.1186/s12885-021-08780-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Li B, Yi J, Hua R, Chen H, Tan L, et al. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE Trial. Ann Surg. 2022;275:646–53. [DOI] [PubMed] [Google Scholar]
- 21.van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg. 2019;269:621–30. 10.1097/sla.0000000000003031 [DOI] [PubMed] [Google Scholar]
- 22.Ekeke CN, Luketich JD, Sarkaria IS. Robotic-assisted minimally invasive esophagectomy. Ann Esophagus. 2020;4:20–34. 10.21037/aoe-20-34 [DOI] [Google Scholar]
- 23.Witek TD, Brady JJ, Sarkaria IS. Technique of robotic esophagectomy. J Thorac Dis. 2020;13:6195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okusanya OT, Sarkaria IS, Hess NR, Nason KS, Sanchez MV, Levy RM, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg. 2017;6:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuchert MJ, Abbas G, Nason KS, Pennathur A, Awais O, Santana M, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery. 2010;148:831–8; discussion 8–40. 10.1016/j.surg.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 26.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63. 10.7326/0003-4819-127-8_part_2-199710151-00064 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biomed J. 2009;51:171–84. 10.1002/bimj.200810488 [DOI] [PubMed] [Google Scholar]
- 28.Parsons L Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Poster presented at: The Twenty-Sixth Annual SAS Users Group International Conference; April 22–25, 2001; Long Beach, CA. [Google Scholar]
- 29.Abbas AE, Sarkaria IS. Specific complications and limitations of robotic esophagectomy. Dis Esophagus. 2020;33(Suppl 2):1–9. 10.1093/dote/doaa109 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Han Y, Gan Q, Xiang J, Jin R, Chen K, et al. Early outcomes of robot-assisted versus thoracoscopic-assisted Ivor Lewis esophagectomy for esophageal cancer: a propensity score-matched study. Ann Surg Oncol. 2019;26:1284–91. 10.1245/s10434-019-07273-3 [DOI] [PubMed] [Google Scholar]
- 31.Sarkaria IS, Rizk NP, Goldman DA, Sima C, Tan KS, Bains MS, et al. Early quality of life outcomes after robotic-assisted minimally invasive and open esophagectomy. Ann Thorac Surg. 2019;108:920–8. 10.1016/j.athoracsur.2018.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vimolratana M, Sarkaria IS, Goldman DA, Rizk NP, Tan KS, Bains MS, et al. Two-year quality of life outcomes after robotic-assisted minimally invasive and open esophagectomy. Ann Thorac Surg. 2021;112:880–9. 10.1016/j.athoracsur.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutfi W, Martinez-Meehan D, Dhupar R, Christie N, Sarkaria I, Ekeke C, et al. Higher lymph node harvest in patients with a pathologic complete response after neoadjuvant therapy for esophageal cancer is associated with improved survival. J Surg Oncol. 2020;121:654–61. 10.1002/jso.25846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. 10.1097/SLA.0b013e318188c474 [DOI] [PubMed] [Google Scholar]
- 35.Visser E, Markar SR, Ruurda JP, Hanna GB, van Hillegersberg R. Prognostic value of lymph node yield on overall survival in esophageal cancer patients: a systematic review and meta-analysis. Ann Surg. 2019;269:261–8. 10.1097/sla.0000000000002824 [DOI] [PubMed] [Google Scholar]
- 36.Weksler B, Sullivan JL. Survival after esophagectomy: a propensity-matched study of different surgical approaches. Ann Thorac Surg. 2017;104:1138–46. 10.1016/j.athoracsur.2017.04.065 [DOI] [PubMed] [Google Scholar]
- 37.Espinoza-Mercado F, Imai TA, Borgella JD, Sarkissian A, Serna-Gallegos D, Alban RF, et al. Does the approach matter? Comparing survival in robotic, minimally invasive, and open esophagectomies. Ann Thorac Surg. 2019;107:378–85. 10.1016/j.athoracsur.2018.08.039 [DOI] [PubMed] [Google Scholar]
- 38.van der Werf LR, Marra E, Gisbertz SS, Wijnhoven BPL, van Berge Henegouwen MI. A propensity score-matched cohort study to evaluate the association of lymph node retrieval with long-term overall survival in patients with esophageal cancer. Ann Surg Oncol. 2021;28:133–41. 10.1245/s10434-020-09142-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


